Research Progress of Ternary Cathode Materials: Failure Mechanism and Heat Treatment for Repair and Regeneration
Abstract
1. Introduction
2. Failure Mechanism of Ternary Cathode Materials
2.1. Li/Ni Mixture
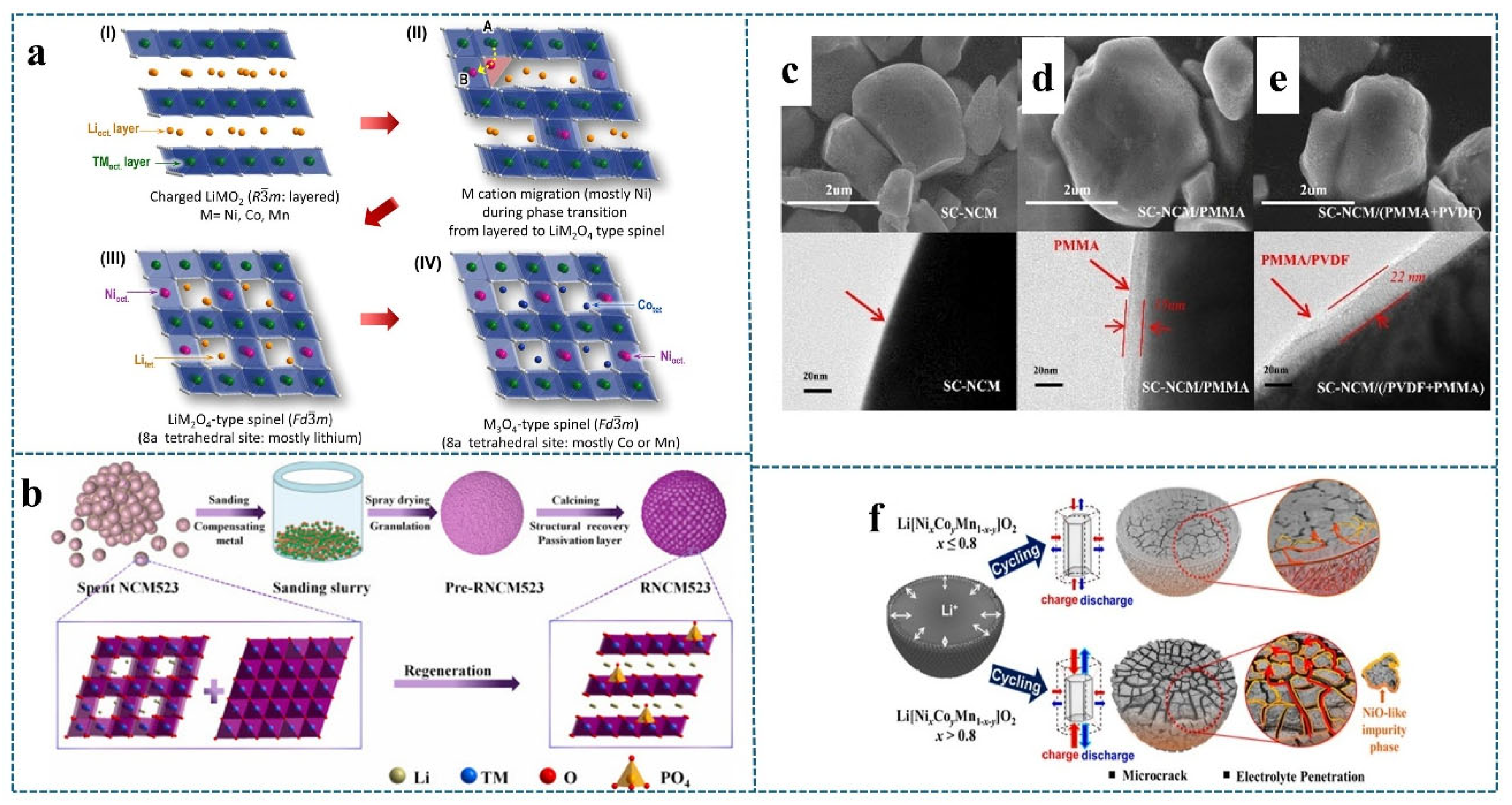
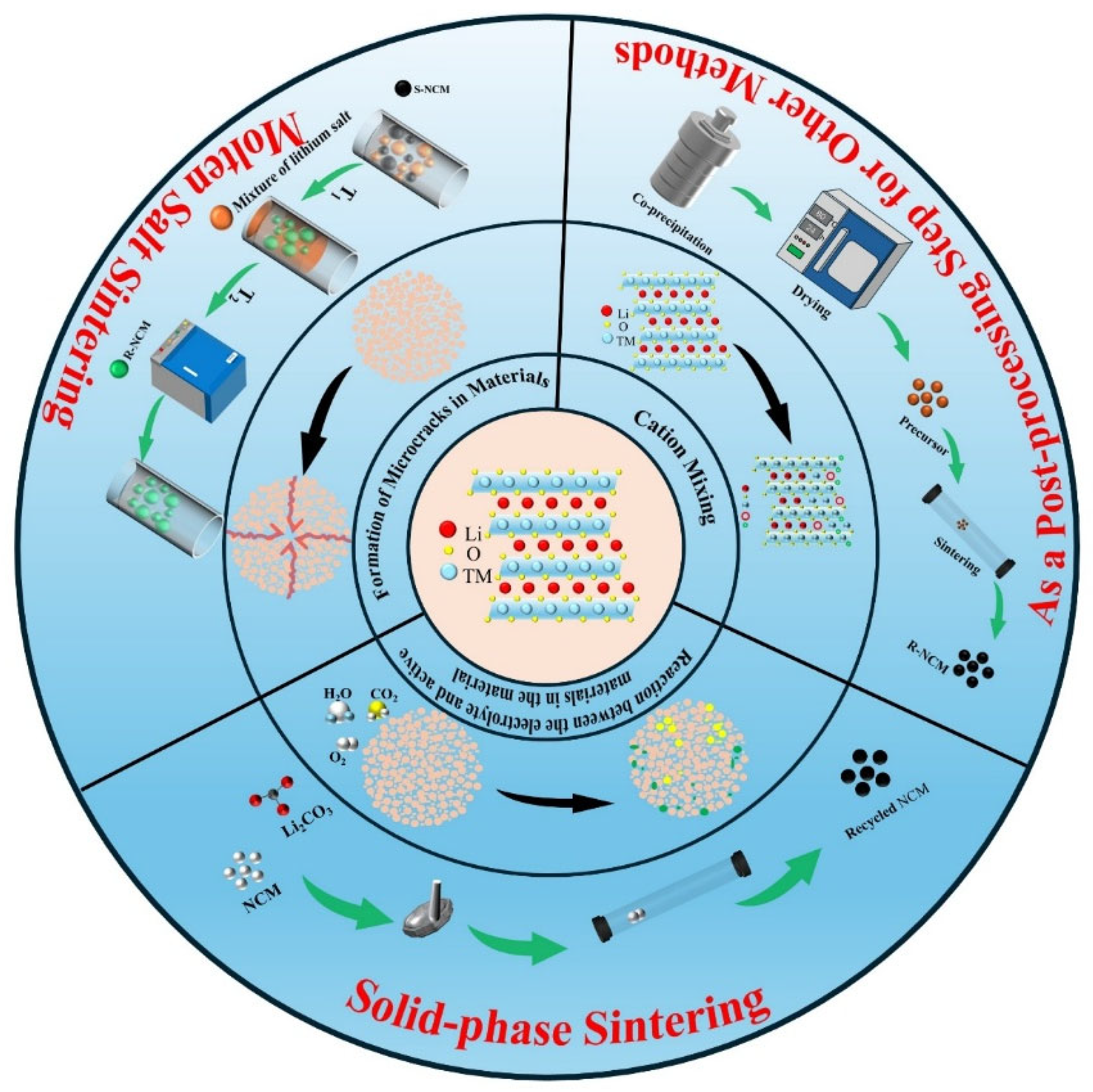
2.2. Formation of Microcracks in Materials
2.2.1. Microcracks Caused by Phase Transitions
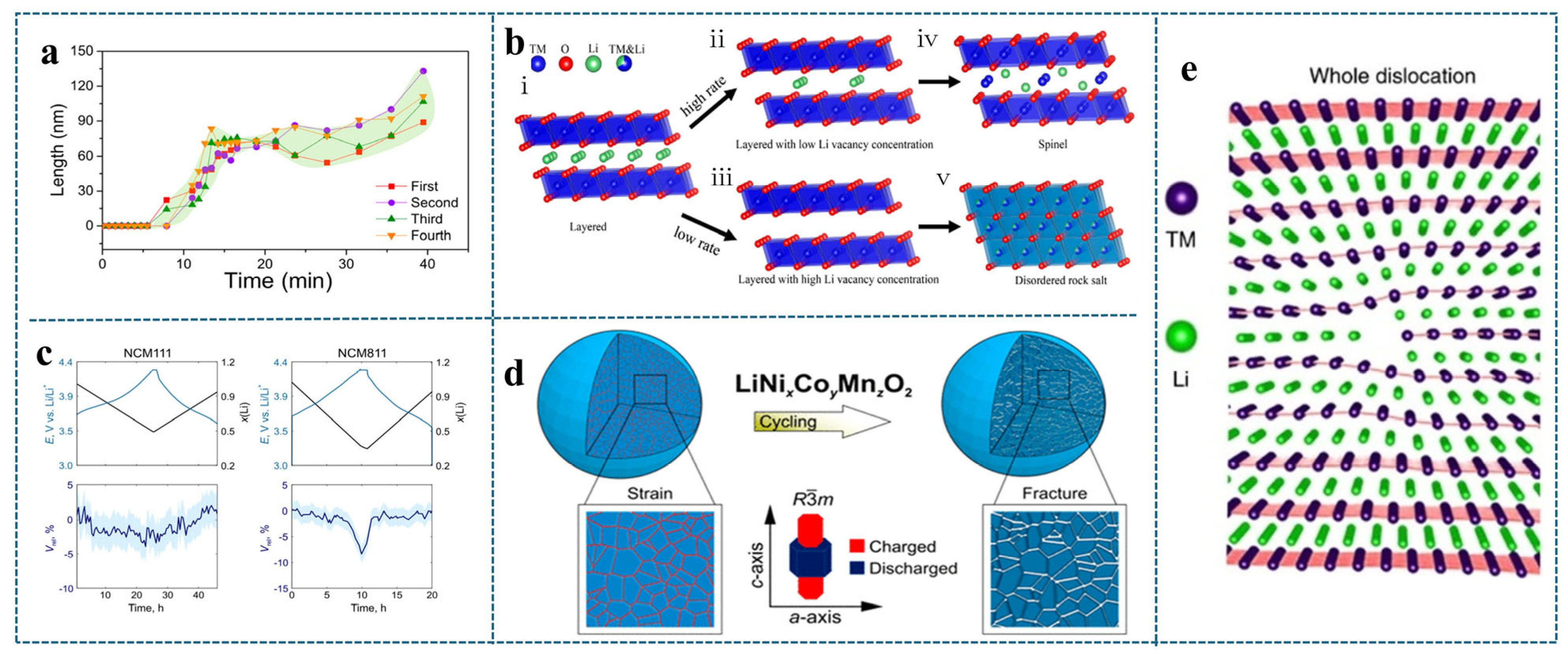
2.2.2. Microcracks Due to Lattice Distortion
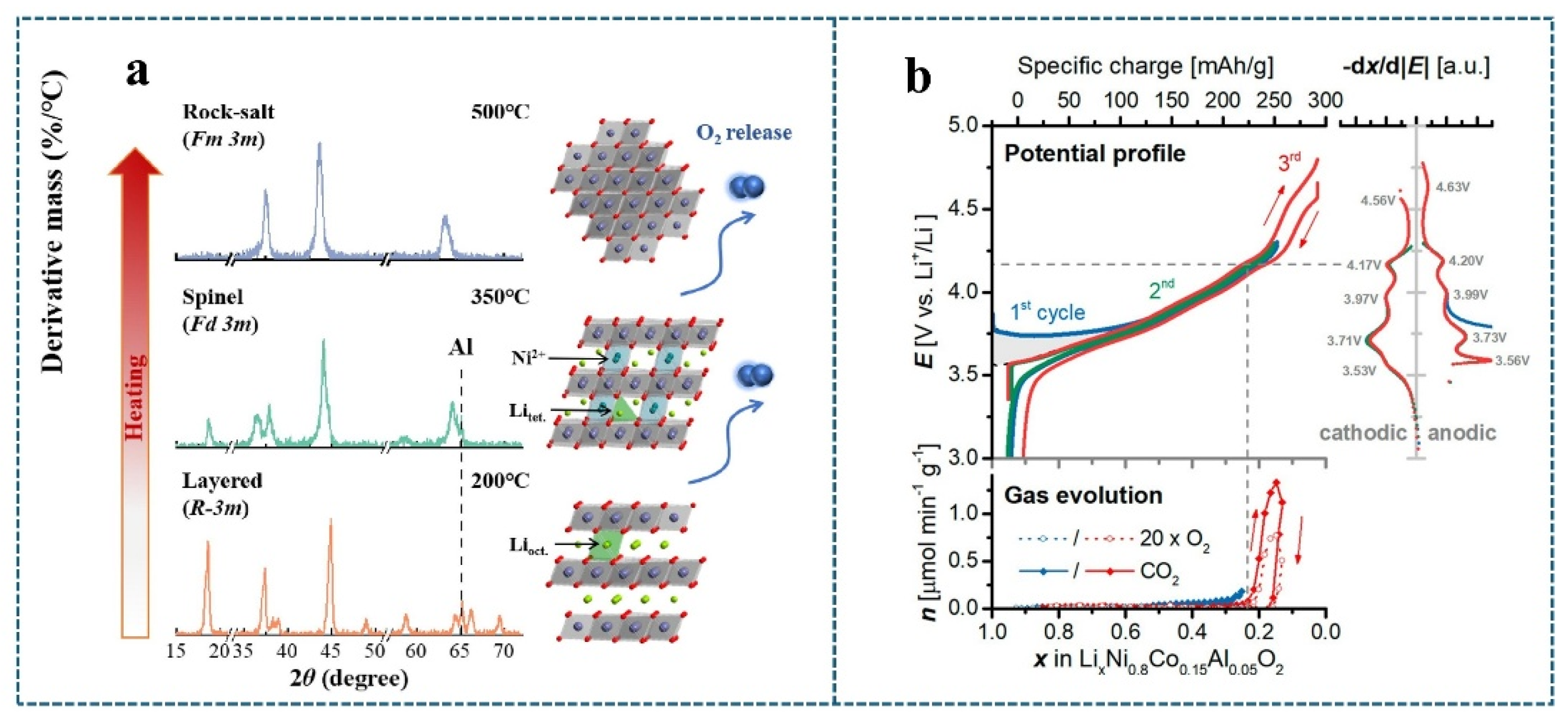
2.2.3. Microcracking Due to Oxygen Release
2.3. Interaction Between the Electrolyte and the Active Component in the Material
3. Progress in the Application of the Heat Treatment Method in Repairing Regenerated Ternary Cathode Materials
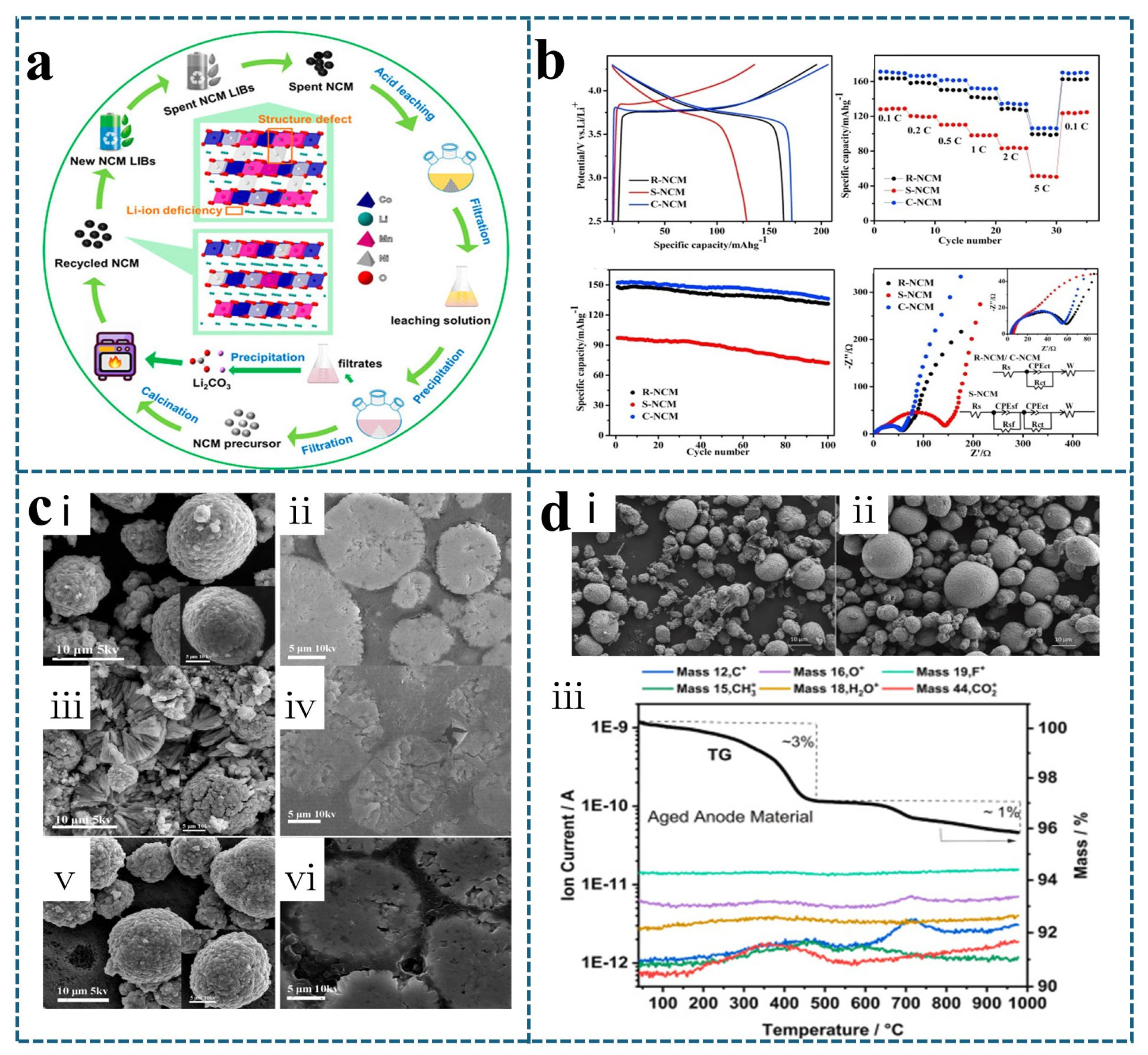
3.1. Regeneration of Failed Ternary Cathode Materials by Heat Treatment Technology
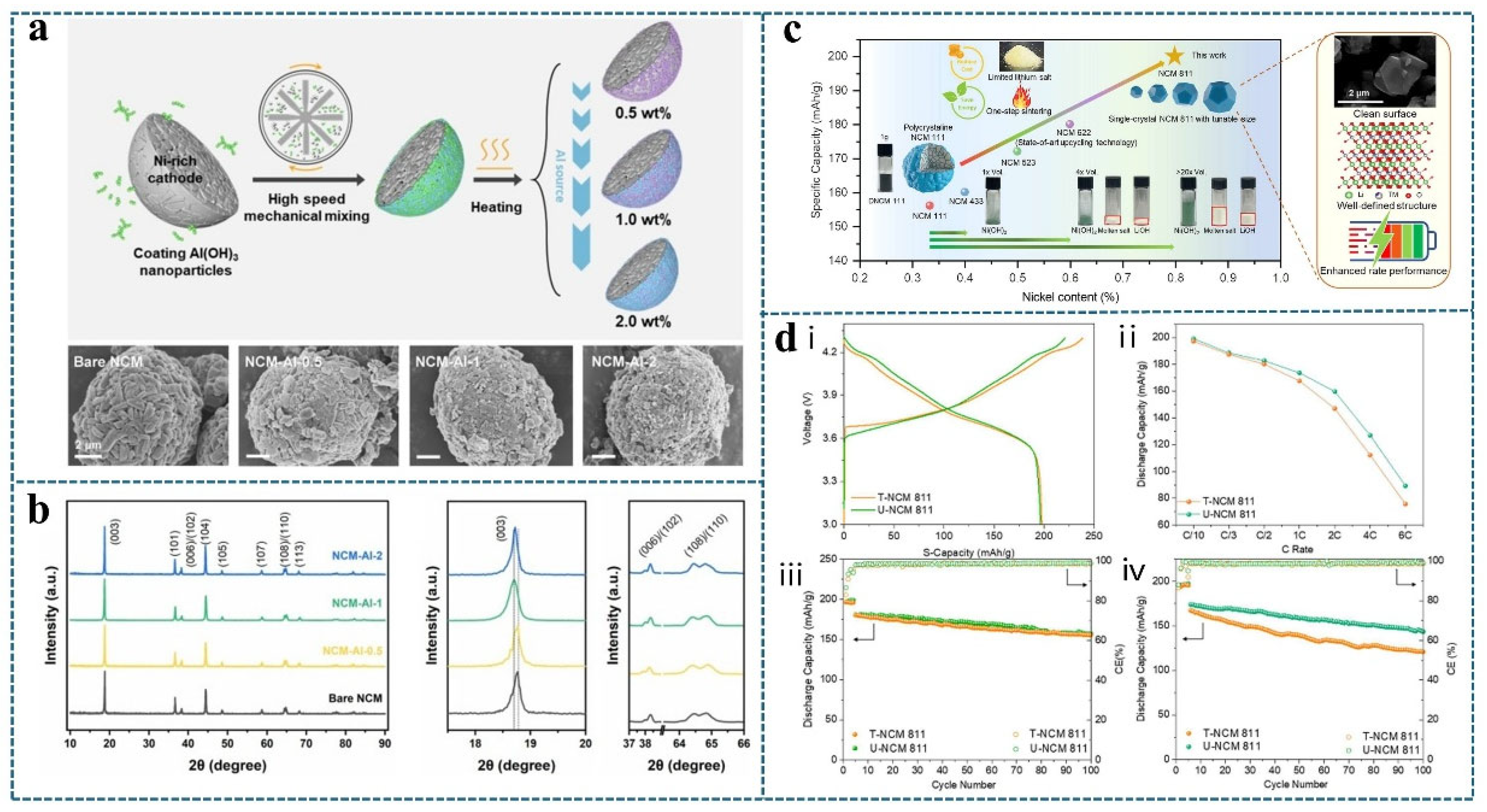
3.2. Synergistic Effects of Heat Treatment Technology, Element Doping, and Surface Coating
3.3. Influence of Heat Treatment Process Parameters on Regenerated Materials
4. Synergistic Effects of Heat Treatment Technology and Other Regeneration Technologies
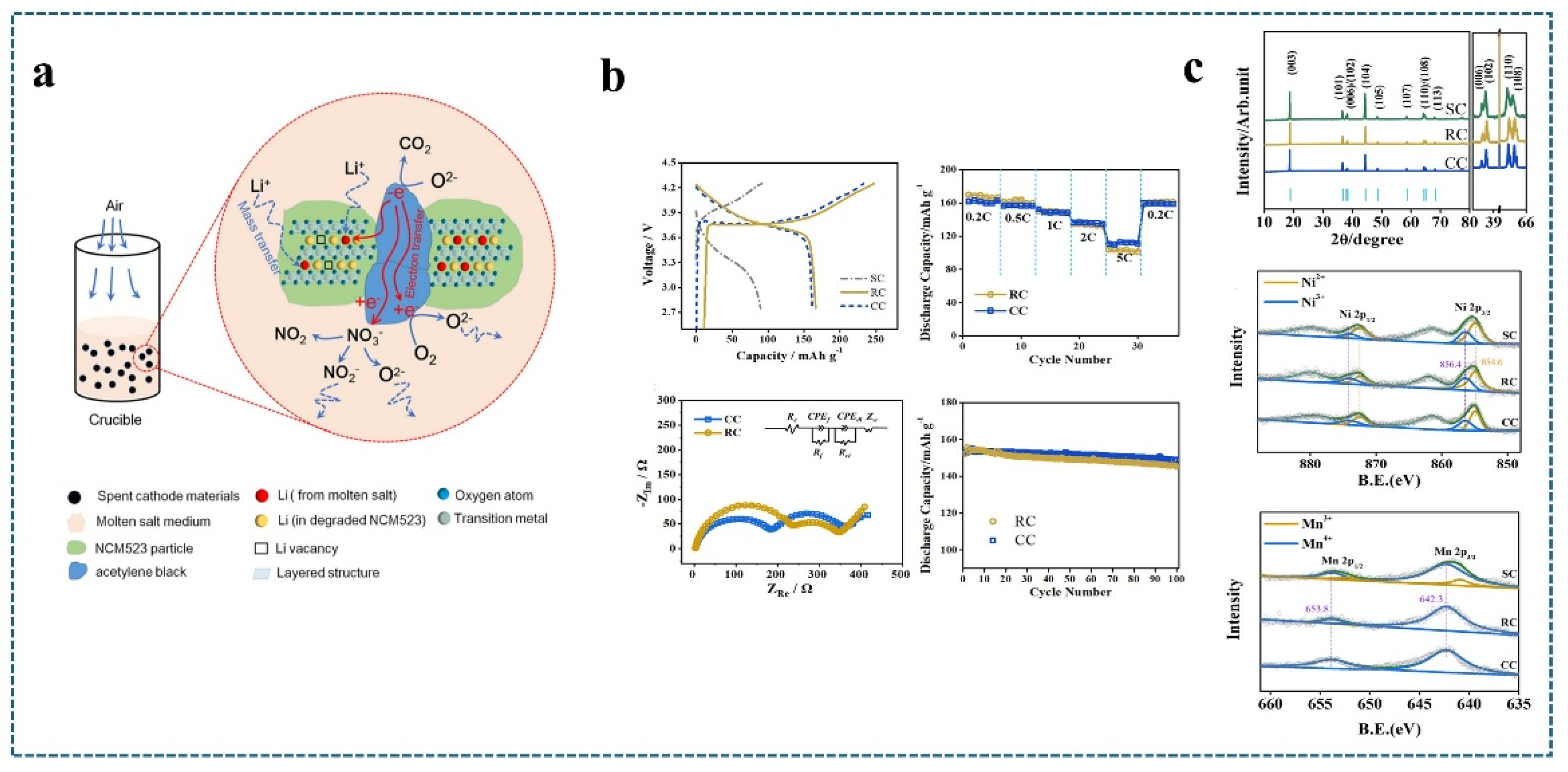
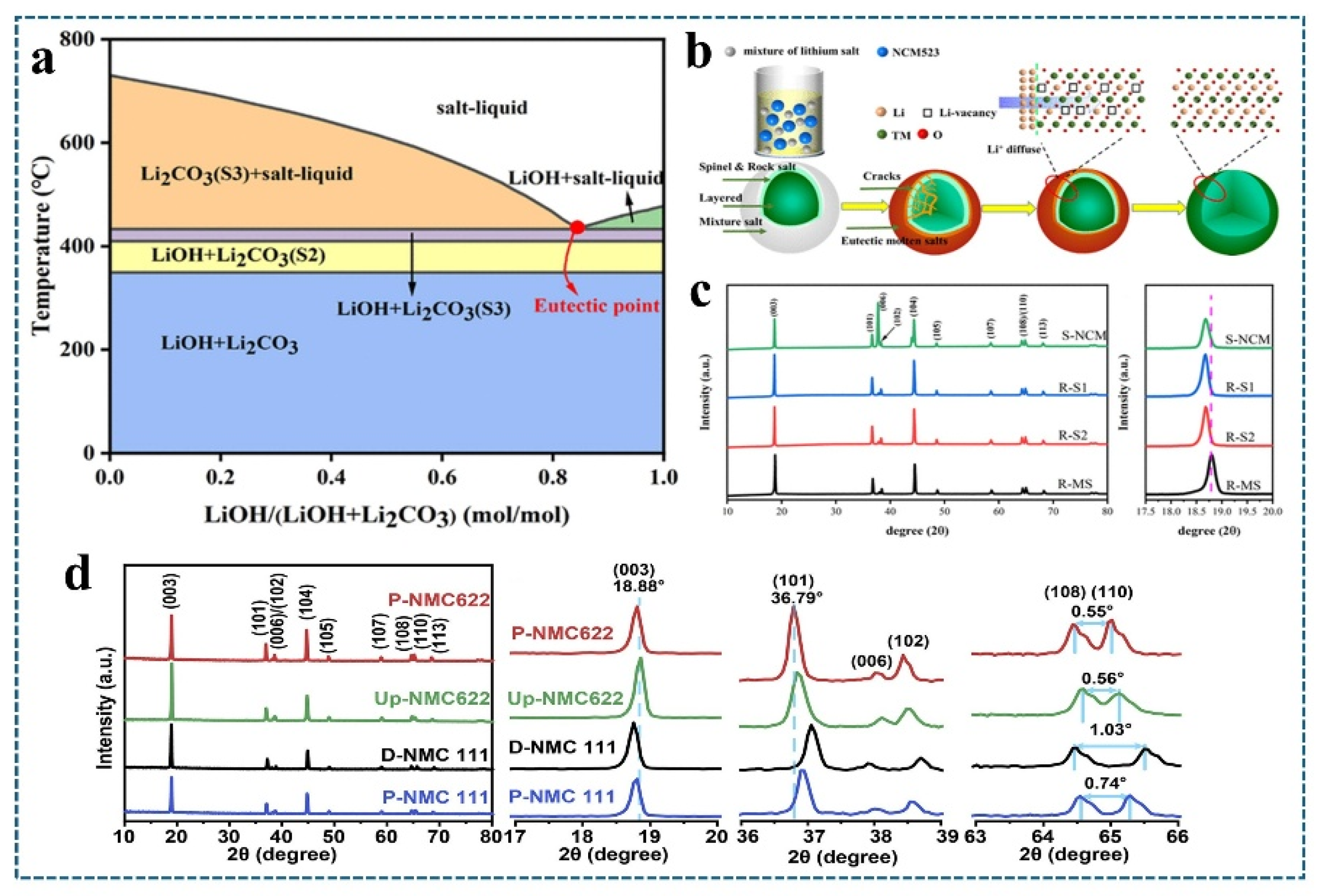
4.1. Combination of Heat Treatment Technology and Molten Salt-Molten Salt Sintering Method
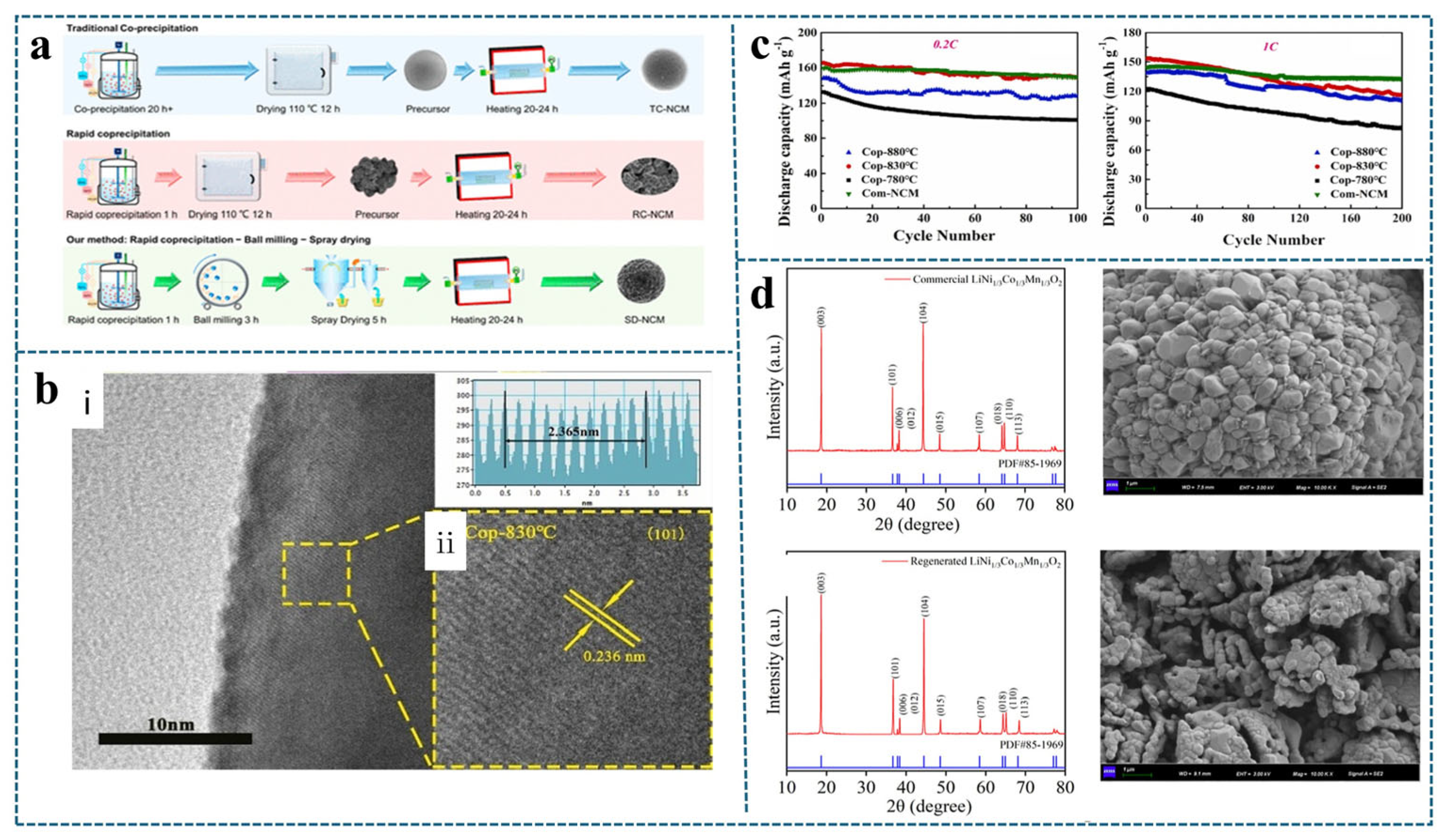
4.2. Combination of Heat Treatment Technology and Co-Precipitation Method
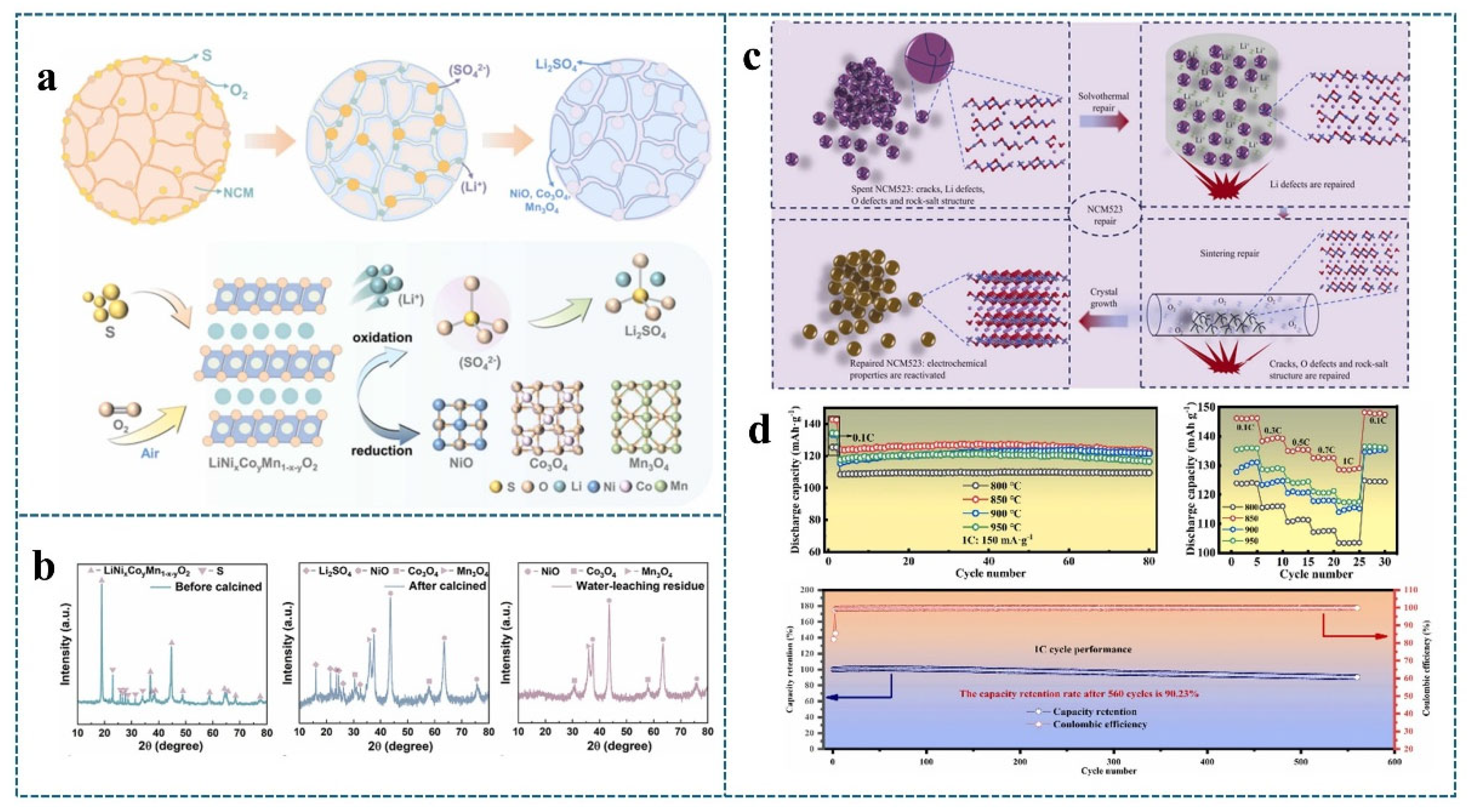
4.3. Combination of Heat Treatment Technology and Hydrothermal Method
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Yu, L.; Liu, X.; Feng, S.; Jia, S.; Zhang, Y.; Zhu, J.; Tang, W.; Wang, J.; Gong, J. Recent progress on sustainable recycling of spent lithium-ion battery: Efficient and closed-loop regeneration strategies for high-capacity layered NCM cathode materials. Chem. Eng. J. 2023, 476, 146733. [Google Scholar] [CrossRef]
- Liu, P.; Mi, X.; Zhao, H.; Cai, L.; Luo, F.; Liu, C.; Wang, Z.; Deng, C.; He, J.; Zeng, G.; et al. Effects of incineration and pyrolysis on removal of organics and liberation of cathode active materials derived from spent ternary lithium-ion batteries. Waste Manag. 2023, 169, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cui, C.; Zhang, X.; Fan, E.; Chen, R.; Wu, F.; Li, L. Closed-loop selective recycling process of spent LiNixCoyMn1-x-yO2 batteries by thermal-driven conversion. J. Hazard. Mater. 2022, 424, 127757. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Qian, G.; Zhao, L.; Yu, Y.; Su, L.; Cai, X.; Zhao, H.; Zuo, Y.; Zhang, Y.; Li, H.; et al. Simultaneous Enhancement of Interfacial Stability and Kinetics of Single-Crystal LiNi0.6Mn0.2Co0.2O2 through Optimized Surface Coating and Doping. Nano Lett. 2020, 20, 8832–8840. [Google Scholar] [CrossRef]
- Li, T.; Yuan, X.-Z.; Zhang, L.; Song, D.; Shi, K.; Bock, C. Degradation Mechanisms and Mitigation Strategies of Nickel-Rich NMC-Based Lithium-Ion Batteries. Electrochem. Energy Rev. 2019, 3, 43–80. [Google Scholar] [CrossRef]
- Niu, H.; Zhang, N.; Lu, Y.; Zhang, Z.; Li, M.; Liu, J.; Zhang, N.; Song, W.; Zhao, Y.; Miao, Z. Strategies toward the development of high-energy-density lithium batteries. J. Energy Storage 2024, 88, 111666. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, J.; Hu, Z.; Cui, G.; Chen, L. Identifying and Addressing Critical Challenges of High-Voltage Layered Ternary Oxide Cathode Materials. Chem. Mat. 2019, 31, 6033–6065. [Google Scholar] [CrossRef]
- Ando, K.; Matsuda, T.; Imamura, D. Degradation diagnosis of lithium-ion batteries with a LiNi0.5Co0.2Mn0.3O2 and LiMn2O4 blended cathode using dV/dQ curve analysis. J. Power Sources 2018, 390, 278–285. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, S.; Liu, B.; He, X.; Deng, J.; Ding, Y. Valuable metals recovery from spent ternary lithium-ion battery: A review. Int. J. Miner. Metall. Mater. 2024, 31, 2556–2581. [Google Scholar] [CrossRef]
- Yu, D.; Huang, Z.; Makuza, B.; Guo, X.; Tian, Q. Pretreatment options for the recycling of spent lithium-ion batteries: A comprehensive review. Miner. Eng. 2021, 173, 107218. [Google Scholar] [CrossRef]
- Larouche, F.; Tedjar, F.; Amouzegar, K.; Houlachi, G.; Bouchard, P.; Demopoulos, G.P.; Zaghib, K. Progress and Status of Hydrometallurgical and Direct Recycling of Li-Ion Batteries and Beyond. Materials 2020, 13, 801. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-n.; Jiang, S.-q.; Li, X.-L.; Yan, S.; Li, L.; Qin, X.-z. Review on the sustainable recycling of spent ternary lithium-ion batteries: From an eco-friendly and efficient perspective. Sep. Purif. Technol. 2024, 348, 127777. [Google Scholar] [CrossRef]
- Hou, D.; Chen, J.; Bai, F.; Meng, F.; Dong, P.; Zhang, C.; Zhang, Y.; Hu, J. Efficient regeneration of waste LiFePO4 cathode material by short process low temperature plasma assisted nitrogen doped technology. J. Power Sources 2024, 613, 234845. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Y.; Xu, S.; Fan, E.; Xue, Q.; Guan, Y.; Wu, F.; Li, L.; Chen, R. Innovative Application of Acid Leaching to Regenerate Li(Ni1/3Co1/3Mn1/3)O2 Cathodes from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 5959–5968. [Google Scholar] [CrossRef]
- Holzer, A.; Wiszniewski, L.; Windisch-Kern, S.; Raupenstrauch, H. Optimization of a Pyrometallurgical Process to Efficiently Recover Valuable Metals from Commercially Used Lithium-Ion Battery Cathode Materials LCO, NCA, NMC622, and LFP. Metals 2022, 12, 1642. [Google Scholar] [CrossRef]
- Sun, Y.-Q.; Fu, W.; Hu, Y.-X.; Vaughan, J.; Wang, L.-Z. The role of tungsten-related elements for improving the electrochemical performances of cathode materials in lithium ion batteries. Tungsten 2021, 3, 245–259. [Google Scholar] [CrossRef]
- Hou, D.; Bai, F.; Dong, P.; Chen, J.; Zhang, Y.; Meng, F.; Zhang, Z.; Zhang, C.; Zhang, Y.; Hu, J. Recent development of low temperature plasma technology for lithium-ion battery materials. J. Power Sources 2023, 584, 233599. [Google Scholar] [CrossRef]
- Sloop, S.E.; Crandon, L.; Allen, M.; Lerner, M.M.; Zhang, H.; Sirisaksoontorn, W.; Gaines, L.; Kim, J.; Lee, M. Cathode healing methods for recycling of lithium-ion batteries. Sustain. Mater. Technol. 2019, 22, e00113. [Google Scholar] [CrossRef]
- Song, L.; Du, J.; Xiao, Z.; Jiang, P.; Cao, Z.; Zhu, H. Research Progress on the Surface of High-Nickel Nickel–Cobalt–Manganese Ternary Cathode Materials: A Mini Review. Front. Chem. 2020, 8, 761. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Q.; Xu, L.; Tian, Y.; Zhao, Z. Enhanced selective separation of valuable metals from spent lithium-ion batteries by aluminum synergistic sulfation roasting strategy. Sep. Purif. Technol. 2024, 345, 127279. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S.; Canbulat, I. Towards a low-carbon society: A review of lithium resource availability, challenges and innovations in mining, extraction and recycling, and future perspectives. Miner. Eng. 2021, 163, 106743. [Google Scholar] [CrossRef]
- Qian, G.; Li, Z.; Meng, D.; Liu, J.-b.; He, Y.-S.; Rao, Q.; Liu, Y.; Ma, Z.-F.; Li, L. Temperature-Swing Synthesis of Large-Size Single-Crystal LiNi0.6Mn0.2Co0.2O2 Cathode Materials. J. Electrochem. Soc. 2021, 168, 010534. [Google Scholar] [CrossRef]
- Guo, Y.; Liao, X.; Huang, P.; Lou, P.; Su, Y.; Hong, X.; Han, Q.; Yu, R.; Cao, Y.-C.; Chen, S. High reversibility of layered oxide cathode enabled by direct Re-generation. Energy Storage Mater. 2021, 43, 348–357. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Lin, J.; Wu, J.; Yang, J.; Wu, F.; Chen, R. Low-Temperature Molten-Salt-Assisted Recovery of Valuable Metals from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 16144–16150. [Google Scholar] [CrossRef]
- Refly, S.; Floweri, O.; Mayangsari, T.R.; Sumboja, A.; Santosa, S.P.; Ogi, T.; Iskandar, F. Regeneration of LiNi1/3Co1/3Mn1/3O2Cathode Active Materials from End-of-Life Lithium-Ion Batteries through Ascorbic Acid Leaching and Oxalic Acid Coprecipitation Processes. ACS Sustain. Chem. Eng. 2020, 8, 16104–16114. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, T.; Li, G.; Wan, T.; Li, M.; Zhang, X.; Li, M.; Su, M.; Dou, A.; Zeng, W.; et al. The surface double-coupling on single-crystal LiNi0.8Co0.1Mn0.1O2 for inhibiting the formation of intragranular cracks and oxygen vacancies. Energy Storage Mater. 2022, 52, 534–546. [Google Scholar] [CrossRef]
- Meng, X.; Hao, J.; Cao, H.; Lin, X.; Ning, P.; Zheng, X.; Chang, J.; Zhang, X.; Wang, B.; Sun, Z. Recycling of LiNi1/3Co1/3Mn1/3O2 cathode materials from spent lithium-ion batteries using mechanochemical activation and solid-state sintering. Waste Manag. 2019, 84, 54–63. [Google Scholar] [CrossRef]
- Yang, C.; Hao, Y.; Wang, J.; Zhang, M.; Song, L.; Qu, J. Research on the facile regeneration of degraded cathode materials from spent LiNi0.5Co0.2Mn0.3O2 lithium-ion batteries. Front. Chem. 2024, 12, 1400758. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, Y.; Luo, W.; Zhang, T.; Wang, T.; Ni, L.; Wang, H.; Zhang, N.; Liu, X.; Zhou, J.; et al. A Universal Molten Salt Method for Direct Upcycling of Spent Ni-rich Cathode towards Single-crystalline Li-rich Cathode. Angew. Chem. Int. Ed. 2023, 62, e202218672. [Google Scholar] [CrossRef]
- Gnutzmann, M.M.; Makvandi, A.; Ying, B.; Buchmann, J.; Lüther, M.J.; Helm, B.; Nagel, P.; Peterlechner, M.; Wilde, G.; Gomez-Martin, A.; et al. Direct Recycling at the Material Level: Unravelling Challenges and Opportunities through a Case Study on Spent Ni-Rich Layered Oxide-Based Cathodes. Adv. Energy Mater. 2024, 26, 2400840. [Google Scholar] [CrossRef]
- Jo, C.-H.; Voronina, N.; Myung, S.-T. Single-crystalline particle Ni-based cathode materials for lithium-ion batteries: Strategies, status, and challenges to improve energy density and cyclability. Energy Storage Mater. 2022, 51, 568–587. [Google Scholar] [CrossRef]
- Jiang, M.; Danilov, D.L.; Eichel, R.A.; Notten, P.H.L. A Review of Degradation Mechanisms and Recent Achievements for Ni-Rich Cathode-Based Li-Ion Batteries. Adv. Energy Mater. 2021, 11, 2103005. [Google Scholar] [CrossRef]
- Wang, L.; Liu, T.; Wu, T.; Lu, J. Strain-retardant coherent perovskite phase stabilized Ni-rich cathode. Nature 2022, 611, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Qu, G.; Huang, Y. Stabilization strategies for high-capacity NCM materials targeting for safety and durability improvements. eTransportation 2023, 16, 100233. [Google Scholar] [CrossRef]
- Li, L.; Liu, M.; Yang, P.; Yuan, W.; Chen, J. Tris(pentafluoro)phenylborane electrolyte additive regulates the highly stable and uniform CEI membrane components to improve the high-voltage behaviors of NCM811 lithium-ion batteries. J. Colloid Interface Sci. 2024, 676, 613–625. [Google Scholar] [CrossRef]
- Song, H.; Lee, J.; Sagong, M.; Jeon, J.; Han, Y.; Kim, J.; Jung, H.G.; Yu, J.S.; Lee, J.; Kim, I.D. Overcoming Chemical and Mechanical Instabilities in Lithium Metal Anodes with Sustainable and Eco-Friendly Artificial SEI Layer. Adv. Mater. 2024, 36, 2407381. [Google Scholar] [CrossRef]
- Wang, S.; Yan, M.; Li, Y.; Vinado, C.; Yang, J. Separating electronic and ionic conductivity in mix-conducting layered lithium transition-metal oxides. J. Power Sources 2018, 393, 75–82. [Google Scholar] [CrossRef]
- Su, Y. Comparative Analysis of Lithium Iron Phosphate Battery and Ternary Lithium Battery. J. Phys. Conf. Ser. 2022, 2152, 012056. [Google Scholar] [CrossRef]
- Xie, H.; Peng, H.; Jiang, D.; Xiao, Z.; Liu, X.; Liang, H.; Wu, M.; Liu, D.; Li, Y.; Sun, Y.; et al. Structures, issues, and optimization strategies of Ni-rich and Co-low cathode materials for lithium-ion battery. Chem. Eng. J. 2023, 470, 144051. [Google Scholar] [CrossRef]
- Ji, H.; Wang, J.; Ma, J.; Cheng, H.-M.; Zhou, G. Fundamentals, status and challenges of direct recycling technologies for lithium ion batteries. Chem. Soc. Rev. 2023, 52, 8194–8244. [Google Scholar] [CrossRef]
- Yang, J.; Hou, M.; Haller, S.; Wang, Y.; Wang, C.; Xia, Y. Improving the Cycling Performance of the Layered Ni-Rich Oxide Cathode by Introducing Low-Content Li2 MnO3. Electrochim. Acta 2016, 189, 101–110. [Google Scholar] [CrossRef]
- Cao, J.; Huang, H.; Qu, Y.; Tang, W.; Yang, Z.; Zhang, W. Construction of a hetero-epitaxial nanostructure at the interface of Li-rich cathode materials to boost their rate capability and cycling performances. Nanoscale 2021, 13, 20488–20497. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xia, X.; Chi, Z.; Yang, Z.; Huang, H.; Chen, Z.; Tang, W.; Wu, G.; Chen, H.; Zhang, W. Preparation of single-crystal ternary cathode materials via recycling spent cathodes for high performance lithium-ion batteries. Nanoscale 2022, 14, 9724–9735. [Google Scholar] [CrossRef] [PubMed]
- Bak, S.-M.; Hu, E.; Zhou, Y.; Yu, X.; Senanayake, S.D.; Cho, S.-J.; Kim, K.-B.; Chung, K.Y.; Yang, X.-Q.; Nam, K.-W. Structural Changes and Thermal Stability of Charged LiNixMnyCozO2 Cathode Materials Studied by Combined In Situ Time-Resolved XRD and Mass Spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef]
- Fan, X.; Tan, C.; Li, Y.; Chen, Z.; Li, Y.; Huang, Y.; Pan, Q.; Zheng, F.; Wang, H.; Li, Q. A green, efficient, closed-loop direct regeneration technology for reconstructing of the LiNi0.5Co0.2Mn0.3O2 cathode material from spent lithium-ion batteries. J. Hazard. Mater. 2021, 410, 124610. [Google Scholar] [CrossRef]
- Luo, Y.-h.; Pan, Q.-l.; Wei, H.-x.; Huang, Y.-d.; Tang, L.-b.; Wang, Z.-y.; He, Z.-j.; Yan, C.; Mao, J.; Dai, K.-h.; et al. Towards Ni-rich layered oxides cathodes with low Li/Ni intermixing by mild molten-salt ion exchange for lithium-ion batteries. Nano Energy 2022, 102, 107626. [Google Scholar] [CrossRef]
- Han, Y.; Heng, S.; Wang, Y.; Qu, Q.; Zheng, H. Anchoring Interfacial Nickel Cations on Single-Crystal LiNi0.8Co0.1Mn0.1O2 Cathode Surface via Controllable Electron Transfer. ACS Energy Lett. 2020, 5, 2421–2433. [Google Scholar] [CrossRef]
- Fan, X.M.; Huang, Y.D.; Wei, H.X.; Tang, L.B.; He, Z.J.; Yan, C.; Mao, J.; Dai, K.H.; Zheng, J.C. Surface Modification Engineering Enabling 4.6 V Single-Crystalline Ni-Rich Cathode with Superior Long-Term Cyclability. Adv. Funct. Mater. 2021, 32, 2109421. [Google Scholar] [CrossRef]
- Sun, H.H.; Ryu, H.-H.; Kim, U.-H.; Weeks, J.A.; Heller, A.; Sun, Y.-K.; Mullins, C.B. Beyond Doping and Coating: Prospective Strategies for Stable High-Capacity Layered Ni-Rich Cathodes. ACS Energy Lett. 2020, 5, 1136–1146. [Google Scholar] [CrossRef]
- Liu, W.; Oh, P.; Liu, X.; Lee, M.J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-Rich Layered Lithium Transition-Metal Oxide for High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457. [Google Scholar] [CrossRef]
- Li, X.; Gao, A.; Tang, Z.; Meng, F.; Shang, T.; Guo, S.; Ding, J.; Luo, Y.; Xiao, D.; Wang, X.; et al. Robust Surface Reconstruction Induced by Subsurface Ni/Li Antisites in Ni-Rich Cathodes. Adv. Funct. Mater. 2021, 31, 2010291. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Chen, X.; Yang, X.; Xiang, F.; Lu, W. Enhancing the stabilities and electrochemical performances of LiNi0.5Co0.2Mn0.3O2 cathode material by simultaneous LiAlO2 coating and Al doping. Electrochim. Acta 2021, 376, 138038. [Google Scholar] [CrossRef]
- Li, S.; Yao, Z.; Zheng, J.; Fu, M.; Cen, J.; Hwang, S.; Jin, H.; Orlov, A.; Gu, L.; Wang, S.; et al. Direct Observation of Defect-Aided Structural Evolution in a Nickel-Rich Layered Cathode. Angew. Chem. Int. Ed. 2020, 59, 22092–22099. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Lin, R.; Xu, R.; Han, L.; Xia, S.; Sokaras, D.; Steiner, J.D.; Weng, T.-C.; Nordlund, D.; Doeff, M.M.; et al. Oxygen Release Induced Chemomechanical Breakdown of Layered Cathode Materials. Nano Lett. 2018, 18, 3241–3249. [Google Scholar] [CrossRef]
- Zou, L.; Zhao, W.; Liu, Z.; Jia, H.; Zheng, J.; Wang, G.; Yang, Y.; Zhang, J.-G.; Wang, C. Revealing Cycling Rate-Dependent Structure Evolution in Ni-Rich Layered Cathode Materials. ACS Energy Lett. 2018, 3, 2433–2440. [Google Scholar] [CrossRef]
- Zhu, J.; Sharifi-Asl, S.; Garcia, J.C.; Iddir, H.H.; Croy, J.R.; Shahbazian-Yassar, R.; Chen, G. Atomic-Level Understanding of Surface Reconstruction Based on Li[NixMnyCo1–x–y]O2 Single-Crystal Studies. ACS Appl. Energ. Mater. 2020, 3, 4799–4811. [Google Scholar] [CrossRef]
- Kim, U.H.; Jun, D.W.; Park, K.J.; Zhang, Q.; Kaghazchi, P.; Aurbach, D.; Major, D.T.; Goobes, G.; Dixit, M.; Leifer, N.; et al. Pushing the limit of layered transition metal oxide cathodes for high-energy density rechargeable Li ion batteries. Energy Environ. Sci. 2018, 11, 1271–1279. [Google Scholar] [CrossRef]
- Xu, G.L.; Liu, X.; Daali, A.; Amine, R.; Chen, Z.; Amine, K. Challenges and Strategies to Advance High-Energy Nickel-Rich Layered Lithium Transition Metal Oxide Cathodes for Harsh Operation. Adv. Funct. Mater. 2020, 30, 2004748. [Google Scholar] [CrossRef]
- Kondrakov, A.O.; Schmidt, A.; Xu, J.; Geßwein, H.; Mönig, R.; Hartmann, P.; Sommer, H.; Brezesinski, T.; Janek, J. Anisotropic Lattice Strain and Mechanical Degradation of High- and Low-Nickel NCM Cathode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2017, 121, 3286–3294. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Gu, M.; Xiao, J.; Zhang, J.-G.; Wang, C.-M. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat. Commun. 2017, 8, 14101. [Google Scholar] [CrossRef]
- Nam, G.W.; Park, N.-Y.; Park, K.-J.; Yang, J.; Liu, J.; Yoon, C.S.; Sun, Y.-K. Capacity Fading of Ni-Rich NCA Cathodes: Effect of Microcracking Extent. ACS Energy Lett. 2019, 4, 2995–3001. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, X.; Xia, S.; Zhang, K.; Wei, C.; Bak, S.; Shadike, Z.; Liu, X.; Yang, Y.; Xu, R.; et al. High-Voltage Charging-Induced Strain, Heterogeneity, and Micro-Cracks in Secondary Particles of a Nickel-Rich Layered Cathode Material. Adv. Funct. Mater. 2019, 29, 1900247. [Google Scholar] [CrossRef]
- Goonetilleke, D.; Sharma, N.; Pang, W.K.; Peterson, V.K.; Petibon, R.; Li, J.; Dahn, J.R. Structural Evolution and High-Voltage Structural Stability of Li(NixMnyCoz)O2Electrodes. Chem. Mat. 2018, 31, 376–386. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, K.-J.; Yoon, C.S.; Sun, Y.-K. Capacity Fading of Ni-Rich Li[NixCoyMn1–x–y]O2 (0.6 ≤ x ≤ 0.95) Cathodes for High-Energy-Density Lithium-Ion Batteries: Bulk or Surface Degradation? Chem. Mat. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen Release and Its Effect on the Cycling Stability of LiNixMnyCozO2(NMC) Cathode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A1361–A1377. [Google Scholar] [CrossRef]
- Liang, C.; Jiang, L.; Wei, Z.; Zhang, W.; Wang, Q.; Sun, J. Insight into the structural evolution and thermal behavior of LiNi0.8Co0.1Mn0.1O2 cathode under deep charge. J. Energy Chem. 2022, 65, 424–432. [Google Scholar] [CrossRef]
- Zhang, S.S. Understanding of performance degradation of LiNi0.80Co0.10Mn0.10O2 cathode material operating at high potentials. J. Energy Chem. 2020, 41, 135–141. [Google Scholar] [CrossRef]
- Mahne, N.; Fontaine, O.; Thotiyl, M.O.; Wilkening, M.; Freunberger, S.A. Mechanism and performance of lithium–oxygen batteries—A perspective. Chem. Sci. 2017, 8, 6716–6729. [Google Scholar] [CrossRef]
- Flores, E.; Vonrüti, N.; Novák, P.; Aschauer, U.; Berg, E.J. Elucidation of LixNi0.8Co0.15Al0.05O2 Redox Chemistry by Operando Raman Spectroscopy. Chem. Mat. 2018, 30, 4694–4703. [Google Scholar] [CrossRef]
- Hatsukade, T.; Schiele, A.; Hartmann, P.; Brezesinski, T.; Janek, J. Origin of Carbon Dioxide Evolved during Cycling of Nickel-Rich Layered NCM Cathodes. J. Alloys Compd. 2018, 10, 38892–38899. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; Li, H.; Kong, X.; Li, X.; Zhao, J. Insight into the Redox Reaction Heterogeneity within Secondary Particles of Nickel-Rich Layered Cathode Materials. ACS Appl. Mater. Interfaces 2021, 13, 27074–27084. [Google Scholar] [CrossRef]
- Yang, T.F.; Lin, P.Y.; Teng, L.T.; Rashidi, S.; Yan, W.M. Numerical and experimental study on thermal management of NCM-21700 Li-ion battery. J. Power Sources 2022, 548, 232068. [Google Scholar] [CrossRef]
- Li, A.; Xin, W.; Wang, Q.; Ai, W.; Han, W.; Yang, C.; Wang, Y.; Du, N.; Liu, C.; Zhang, Y.; et al. Study on an Interpenetrating Artificial SEI for Lithium Metal Anode Modification and Fast Charging Characterization. ACS Appl. Energ. Mater. 2024, 16, 65984–65992. [Google Scholar] [CrossRef]
- Langdon, J.; Manthiram, A. Crossover Effects in Batteries with High-Nickel Cathodes and Lithium-Metal Anodes. Adv. Funct. Mater. 2021, 31, 2010267. [Google Scholar] [CrossRef]
- Xia, S.; Mu, L.; Xu, Z.; Wang, J.; Wei, C.; Liu, L.; Pianetta, P.; Zhao, K.; Yu, X.; Lin, F.; et al. Chemomechanical interplay of layered cathode materials undergoing fast charging in lithium batteries. Nano Energy 2018, 53, 753–762. [Google Scholar] [CrossRef]
- Fan, X.; Hu, G.; Zhang, B.; Ou, X.; Zhang, J.; Zhao, W.; Jia, H.; Zou, L.; Li, P.; Yang, Y. Crack-free single-crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries. Nano Energy 2020, 70, 104450. [Google Scholar] [CrossRef]
- Sun, J.; Cao, X.; Yang, H.; He, P.; Dato, M.A.; Cabana, J.; Zhou, H. The Origin of High-Voltage Stability in Single-Crystal Layered Ni-Rich Cathode Materials. Angew. Chem. Int. Ed. 2022, 61, e202207225. [Google Scholar] [CrossRef]
- Zhang, F.; Lou, S.; Li, S.; Yu, Z.; Liu, Q.; Dai, A.; Cao, C.; Toney, M.F.; Ge, M.; Xiao, X.; et al. Surface regulation enables high stability of single-crystal lithium-ion cathodes at high voltage. Nat. Commun. 2020, 11, 3050. [Google Scholar] [CrossRef]
- Lecompte, M.; Bernard, J.; Calas, E.; Richardet, L.; Guignard, A.; Duclaud, F.; Voyer, D.; Montaru, M.; Crouzevialle, B.; Lonardoni, L.; et al. Experimental assessment of high-energy high nickel-content NMC lithium-ion battery cycle life at cold temperatures. J. Energy Storage 2024, 94, 112443. [Google Scholar] [CrossRef]
- Zhu, H.B.; Ma, J.; Ding, H.H.; Wu, H.H.; Zhang, C.M.; Fang, X.L.; Xuan, H.; Lao, L.; Ni, L.P.; Wang, X.F. Experimental study of capacity fading mechanism in multiple overdischarge on LiNi0.5Co0.2Mn0.3O2&LiMn2O4/graphite lithium-ion batteries. Ceram. Int. 2024, 50, 35537–35548. [Google Scholar]
- Wang, S.; Hu, C.; Yu, R.; Sun, Z.; Jin, Y. Study on low-temperature cycle failure mechanism of a ternary lithium ion battery. RSC Adv. 2022, 12, 20755–20761. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Z.; Sun, M.; Tu, Y.; Duan, X. Separation and Regeneration of LiNi1/3Co1/3Mn1/3O2 Materials from Spent Lithium-Ion Batteries: A Facile Process. ACS Sustain. Chem. Eng. 2023, 11, 11199–11206. [Google Scholar] [CrossRef]
- Siyu, G.; Enhua, D.; Bingguo, L.; Chao, Y.; Yifan, N.; Guangxiong, J.; Wang, C.; Keren, H.; Shenghui, G.; Libo, Z. Eco-friendly closed-loop recycling of nickel, cobalt, manganese, and lithium from spent ternary lithium-ion battery cathodes. Sep. Purif. Technol. 2024, 348, 127771. [Google Scholar] [CrossRef]
- Nam, G.; Hwang, J.; Kang, D.; Oh, S.; Chae, S.; Yoon, M.; Ko, M. Mechanical densification synthesis of single-crystalline Ni-rich cathode for high-energy lithium-ion batteries. J. Energy Chem. 2023, 79, 562–568. [Google Scholar] [CrossRef]
- Chi, Z.; Li, J.; Wang, L.; Li, T.; Wang, Y.; Zhang, Y.; Tao, S.; Zhang, M.; Xiao, Y.; Chen, Y. Direct regeneration method of spent LiNi1/3Co1/3Mn1/3O2 cathode materials via surface lithium residues. Green Chem. 2021, 23, 9099–9108. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, X.; Yin, C.; Li, J. Regeneration of LiNi0.5Co0.2Mn0.3O2 cathode material from spent lithium-ion batteries. Electrochim. Acta 2018, 291, 142–150. [Google Scholar] [CrossRef]
- Mancini, M.; Hoffmann, M.F.; Martin, J.; Weirather-Köstner, D.; Axmann, P.; Wohlfahrt-Mehrens, M. A proof-of-concept of direct recycling of anode and cathode active materials: From spent batteries to performance in new Li-ion cells. Chem. Eng. J. 2024, 595, 233997. [Google Scholar] [CrossRef]
- Shi, J.-L.; Sheng, H.; Meng, X.-H.; Zhang, X.-D.; Lei, D.; Sun, X.; Pan, H.; Wang, J.; Yu, X.; Wang, C.; et al. Size controllable single-crystalline Ni-rich cathodes for high-energy lithium-ion batteries. Natl. Sci. Rev. 2023, 10, nwac226. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, J.; Wang, Q.; Hu, C.; Luo, B.; Liu, Y.; Xiao, Z.; Ou, X. Boosting High-Voltage and Ultralong-Cycling Performance of Single-Crystal LiNi0.5Co0.2Mn0.3O2 Cathode Materials via Three-in-One Modification. Energy Environ. Mater. 2022, 6, e12270. [Google Scholar] [CrossRef]
- Qin, L.; Yu, H.; Jiang, X.; Chen, L.; Cheng, Q.; Jiang, H. All-dry solid-phase synthesis of single-crystalline Ni-rich ternary cathodes for lithium-ion batteries. Sci. China Mater. 2024, 67, 650–657. [Google Scholar] [CrossRef]
- Wu, F.; Shi, Q.; Chen, L.; Dong, J.; Zhao, J.; Wang, H.; Gao, F.; Liu, J.; Zhang, H.; Li, N.; et al. New insights into dry-coating-processed surface engineering enabling structurally and thermally stable high-performance Ni-rich cathode materials for lithium ion batteries. Chem. Eng. J. 2023, 470, 144045. [Google Scholar] [CrossRef]
- Dong, H.; Wang, H.; Qi, J.; Wang, J.; Ji, W.; Pan, J.; Li, X.; Yin, Y.; Yang, S. Single-Crystal Materials Regenerated and Modified by Spent NCM523 as a High-Voltage Stable Cycling Cathode Material. ACS Sustain. Chem. Eng. 2022, 10, 11587–11596. [Google Scholar] [CrossRef]
- Gao, H.; Yan, Q.; Tran, D.; Yu, X.; Liu, H.; Li, M.; Li, W.; Wu, J.; Tang, W.; Gupta, V.; et al. Upcycling of Spent LiNi0.33Co0.33Mn0.33O2 to Single-Crystal Ni-Rich Cathodes Using Lean Precursors. ACS Energy Lett. 2023, 8, 4136–4144. [Google Scholar] [CrossRef]
- Yang, R.-m.; Zhang, Y.-j.; Dong, P.; Zhang, Y.-n. The Effect of Heating Rate on the Structure and Electrochemical Performance of the Li-rich Cathode Material Li1.2Ni0.15Co0.10Mn0.55O2 Prepared Using the Co-precipitation Method. Int. J. Electrochem. Sci. 2018, 13, 8116–8126. [Google Scholar] [CrossRef]
- Tang, X.; Guo, Q.; Zhou, M.; Zhong, S. Direct regeneration of LiNi0.5Co0.2Mn0.3O2 cathode material from spent lithium-ion batteries. Chin. J. Chem. Eng. 2021, 40, 278–286. [Google Scholar] [CrossRef]
- Han, Y.; You, Y.; Hou, C.; Xiao, X.; Xing, Y.; Zhao, Y. Regeneration of Single-Crystal LiNi0.5Co0.2Mn0.3O2 Cathode Materials from Spent Power Lithium-Ion Batteries. J. Electrochem. Soc. 2021, 168, 040525. [Google Scholar] [CrossRef]
- He, S.; Zhou, A.; Jiang, T.; Liu, Z. Recovery of LiNi0.5Mn0.3Co0.2O2 cathode material from spent lithium-ion batteries with oxygen evolution reduction in ammonium sulfate low-temperature molten salt. J. Clean Prod. 2023, 422, 138511. [Google Scholar] [CrossRef]
- Ni, L.; Guo, R.; Deng, W.; Wang, B.; Chen, J.; Mei, Y.; Gao, J.; Gao, X.; Yin, S.; Liu, H.; et al. Single-Crystalline Ni-Rich layered cathodes with Super-Stable cycling. Chem. Eng. J. 2022, 431, 133731. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, M.; Meng, Y.S.; Chen, Z. Ambient-Pressure Relithiation of Degraded LixNi0.5Co0.2Mn0.3O2 (0 < x < 1) via Eutectic Solutions for Direct Regeneration of Lithium-Ion Battery Cathodes. Adv. Energy Mater. 2019, 9, 1400454. [Google Scholar]
- Deng, B.; Zhou, Z.; Wang, W.; Wang, D. Direct Recovery and Efficient Reutilization of Degraded Ternary Cathode Materials from Spent Lithium-Ion Batteries via a Homogeneous Thermochemical Process. ACS Sustain. Chem. Eng. 2020, 8, 14022–14029. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, Y.; Meng, Q.; Zhang, Y.; Dong, P.; Zhang, M.; Yang, X. Direct Regeneration of LiNi0.5Co0.2Mn0.3O2 Cathode from Spent Lithium-Ion Batteries by the Molten Salts Method. ACS Sustain. Chem. Eng. 2020, 8, 18138–18147. [Google Scholar] [CrossRef]
- Ma, T.; Guo, Z.; Shen, Z.; Wu, Q.; Li, Y.; Yang, G. Molten salt-assisted regeneration and characterization of submicron-sized LiNi0.5Co0.2Mn0.3O2 crystals from spent lithium ion batteries. J. Alloy. Compd. 2020, 848, 156591. [Google Scholar] [CrossRef]
- He, J.; Fei, Z.; Meng, Q.; Dong, P.; Zhang, Y.; Li, Q. Preparation of Ternary Cathode Materials from Spent Lithium Batteries at Low Temperature. Int. J. Electrochem. Sci. 2021, 16, 210342. [Google Scholar] [CrossRef]
- Ma, J.; Wang, J.; Jia, K.; Liang, Z.; Ji, G.; Zhuang, Z.; Zhou, G.; Cheng, H.-M. Adaptable Eutectic Salt for the Direct Recycling of Highly Degraded Layer Cathodes. J. Am. Chem. Soc. 2022, 144, 20306–20314. [Google Scholar] [CrossRef]
- Qin, Z.; Wen, Z.; Xu, Y.; Zheng, Z.; Bai, M.; Zhang, N.; Jia, C.; Wu, H.B.; Chen, G. A Ternary Molten Salt Approach for Direct Regeneration of LiNi0.5Co0.2Mn0.3O2 Cathode. Small 2022, 18, 2106719. [Google Scholar] [CrossRef]
- Wang, T.; Luo, H.; Fan, J.; Thapaliya, B.P.; Bai, Y.; Belharouak, I.; Dai, S. Flux upcycling of spent NMC 111 to nickel-rich NMC cathodes in reciprocal ternary molten salts. iScience 2022, 25, 103801. [Google Scholar] [CrossRef]
- Tong, H.; Lv, H.; Li, Y.; Mao, G.; Yu, W.; Guo, X. Bifunctional Treatment of Spent Ternary Cathode Materials with Improved Electrochemical Performance. ACS Appl. Energ. Mater. 2024, 7, 2816–2824. [Google Scholar] [CrossRef]
- Xing, C.; Gan, M.; Ying, Y.; Zhang, B.; Liu, L.; Ye, J.; Liu, Y.; Zhang, Y.; Huang, H.; Fei, L. Multiscale observations on mechanisms for direct regeneration of degraded NCM cathode materials. Energy Storage Mater. 2024, 65, 103182. [Google Scholar] [CrossRef]
- Huang, B.; Liu, D.; Zhang, L.; Qian, K.; Zhou, K.; Cai, X.; Kang, F.; Li, B. An Efficient Synthetic Method to Prepare High-Performance Ni-rich LiNi0.8Co0.1Mn0.1O2 for Lithium-Ion Batteries. ACS Appl. Energ. Mater. 2019, 2, 7403–7411. [Google Scholar] [CrossRef]
- Ou, H.; Zhang, J.; Shen, A.; Chen, Y.; Wang, C. A simplified method for the recycling of spent lithium-ion batteries via manganese selective recovery by anoxic ammonia leaching and spontaneous precipitation. J. Power Sources 2024, 590, 233799. [Google Scholar] [CrossRef]
- He, T.; Dai, J.; Dong, Y.; Zhu, F.; Wang, C.; Zhen, A.; Cai, Y. Green closed-loop regeneration of ternary cathode materials from spent lithium-ion batteries through deep eutectic solvent. Ionics 2023, 29, 1721–1729. [Google Scholar] [CrossRef]
- Chen, X.; Yang, C.; Yang, Y.; Ji, H.; Yang, G. Co-precipitation preparation of Ni-Co-Mn ternary cathode materials by using the sources extracting directly from spent lithium-ion batteries. J. Alloy. Compd. 2022, 909, 164691. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, H.; Li, J.; Li, J.; Zhuge, X.; Ren, Y.; Ding, Z. A large volume and low energy consumption recycling strategy for LiNi0.6Co0.2Mn0.2O2 from spent ternary lithium-ion batteries. J. Power Sources 2024, 602, 234407. [Google Scholar] [CrossRef]
- Bai, X.; Jiang, Z.; Sun, Y.; Liu, X.; Jin, X.; He, R.; Liu, Z.; Pan, J. Clean Universal Solid-State Recovery Method of Waste Lithium-Ion Battery Ternary Positive Materials and Their Electrochemical Properties. ACS Sustain. Chem. Eng. 2023, 11, 3673–3686. [Google Scholar] [CrossRef]
- Zhou, M.; Shen, J.; Duan, Y.; Zuo, Y.; Xing, Z.; Liu, R. The Le Chatelier’s principle enables closed loop regenerating ternary cathode materials for spent lithium-ion batteries. Energy Storage Mater. 2024, 67, 103250. [Google Scholar] [CrossRef]
- Liang, J.; Chen, R.; Gu, J.-n.; Li, J.; Xue, Y.; Shi, F.; Huang, B.; Guo, M.; Jia, J.; Li, K.; et al. Sustainable recycling of spent ternary lithium-ion batteries via an environmentally friendly process: Selective recovery of lithium and non-hazardous upcycling of residue. Chem. Eng. J. 2024, 481, 148516. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Z.; Zou, J.; Pan, T.; Li, P.; Yu, G.; Wang, X.; Wang, S.; Zhang, J. A mild and efficient closed-loop recycling strategy for spent lithium-ion battery. J. Hazard. Mater. 2024, 474, 134794. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, X.; Zheng, Y.; Wang, Q.; Liu, J.; Xu, S. Boosting efficient and low-energy solid phase regeneration for single crystal LiNi0.6Co0.2Mn0.2O2 via highly selective leaching and its industrial application. Chem. Eng. J. 2023, 451, 139039. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, X.; Yu, W.; Shang, Z.; Yang, Y.; Xu, S. Solvothermal strategy for direct regeneration of high-performance cathode materials from spent lithium-ion battery. Nano Energy 2024, 120, 109145. [Google Scholar] [CrossRef]
- Karati, A.; Gargh, P.P.; Paul, S.; Das, S.; Shrotriya, P.; Nlebedim, I.C. Materials recovery from NMC batteries with water as the sole solvent. J. Environ. Manag. 2024, 366, 121710. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Xiao, J.; Zhang, Y.; Dong, P.; Meng, Q.; Zhang, M. Restoring Surface Defect Crystal of Li-Lacking LiNi0.6Co0.2Mn0.2O2 Material Particles toward More Efficient Recycling of Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2021, 9, 16997–17006. [Google Scholar] [CrossRef]
- Jia, K.; Wang, J.; Zhuang, Z.; Piao, Z.; Zhang, M.; Liang, Z.; Ji, G.; Ma, J.; Ji, H.; Yao, W.; et al. Topotactic Transformation of Surface Structure Enabling Direct Regeneration of Spent Lithium-Ion Battery Cathodes. J. Am. Chem. Soc. 2023, 145, 7288–7300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, H.; Liu, J.; Li, X.; Zhang, Y. Simple Preparation of Co3O4 with a Controlled Shape and Excellent Lithium Storage Performance. Int. J. Electrochem. Sci. 2020, 15, 2894–2902. [Google Scholar] [CrossRef]
- Xu, P.; Yang, Z.; Yu, X.; Holoubek, J.; Gao, H.; Li, M.; Cai, G.; Bloom, I.; Liu, H.; Chen, Y.; et al. Design and Optimization of the Direct Recycling of Spent Li-Ion Battery Cathode Materials. ACS Sustain. Chem. Eng. 2021, 9, 4543–4553. [Google Scholar] [CrossRef]
- Chan, K.H.; Malik, M.; Azimi, G. Direct recycling of degraded lithium-ion batteries of an electric vehicle using hydrothermal relithiation. Mater. Today Energy 2023, 37, 101374. [Google Scholar] [CrossRef]
- Wang, T.; Luo, H.; Bai, Y.; Li, J.; Belharouak, I.; Dai, S. Direct Recycling of Spent NCM Cathodes through Ionothermal Lithiation. Adv. Energy Mater. 2020, 10, 2001204. [Google Scholar] [CrossRef]
- Fan, M.; Chang, X.; Guo, Y.-J.; Chen, W.-P.; Yin, Y.-X.; Yang, X.; Meng, Q.; Wan, L.-J.; Guo, Y.-G. Increased residual lithium compounds guided design for green recycling of spent lithium-ion cathodes. Energy Environ. Sci. 2021, 14, 1461–1468. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, Y.; Chen, L.; Lu, Y.; Bao, L.; He, T.; Wang, J.; Chen, R.; Tan, J.; Wu, F. Pre-oxidizing the precursors of Nickel-rich cathode materials to regulate their Li+/Ni2+ cation ordering towards cyclability improvements. J. Power Sources 2018, 396, 734–741. [Google Scholar] [CrossRef]
- Xing, C.; Da, H.; Yang, P.; Huang, J.; Gan, M.; Zhou, J.; Li, Y.; Zhang, H.; Ge, B.; Fei, L. Aluminum Impurity from Current Collectors Reactivates Degraded NCM Cathode Materials toward Superior Electrochemical Performance. ACS Nano 2023, 17, 3194–3203. [Google Scholar] [CrossRef]
- Ding, W.; Bao, S.; Zhang, Y.; Xin, C.; Chen, B.; Li, J.; Liu, B.; Xia, Y.; Hou, X.; Xu, K. Sustainable regeneration of high-performance cathode materials from spent lithium-ion batteries through magnetic separation and coprecipitation. J. Clean Prod. 2024, 438, 140798. [Google Scholar] [CrossRef]
- Xing, L.; Lin, S.; Yu, J. Novel Recycling Approach to Regenerate a LiNi0.6Co0.2Mn0.2O2 Cathode Material from Spent Lithium-Ion Batteries. Ind. Eng. Chem. Res. 2021, 60, 10303–10311. [Google Scholar] [CrossRef]

| Waste Material | Regeneration Method | Failed Material Capacity/Regenerative Capacity | Magnification | Increase Efficiency | Reference |
|---|---|---|---|---|---|
| NCM523 | Acid leaching + calcination | 29 mAh g−1/147 mAh g−1 | 1C | 83% | Ref [28] |
| NCM111 | Mechanical activation + calcination | 75 mAh g−1/165 mAh g−1 | 0.2C | 54.5% | Ref [27] |
| NCM | Solid sintering | 175 mAh g−1/191.1 mAhg −1 | 0.1C | 8.4% | Ref [90] |
| NCM111 | Leaching + precipitation + sintering | −/134.3 mAh g−1 | 0.5C | - | Ref [82] |
| NCM111 | Solid sintering | −/173 mAh g−1 | 1C | - | Ref [93] |
| NCM111 | Molten salt sintering | 120 mAh g−1/150 mAh g−1 | 1C | 20% | Ref [106] |
| NCM111 | Direct calcination | 80 mAh g−1/129.1 mAh g−1 | 0.5C | 37% | Ref [85] |
| NCM111 | Hot lithium of ionic liquid | 100 mAh g−1/145 mAh g−1 | 1C | 31% | Ref [126] |
| NCM811 | Sol-gel method | −/180 mAh g−1 | 1C | - | Ref [26] |
| NCM811 | Coprecipitation + sintering | −/190 mAh g−1 | 1C | - | Ref [76] |
| NCM622 | Peroxidation sintering | 90 mAh g−1/153.82 mAh g−1 | 1C | 41% | Ref [121] |
| NCM811 | Molten salt sintering | −/187.2 mAh g−1 | 1C | - | Ref [29] |
| NCM523 | Solid sintering | −/157.7 mAh g−1 | 0.5C | - | Ref [127] |
| NCM811 | Pre-oxidation +sintering | −/180 mAh g−1 | 1C | - | Ref [128] |
| NCM523 | Molten salt process | 120 mAh g−1/150.6 mAh g−1 | 1C | - | Ref [107] |
| NCM523 | Molten salt process | 100 mAh g−1/160 mAh g−1 | 0.5C | 37.5% | Ref [105] |
| NCM523 | Molten salt process | −/159 mAh g−1 | 1C | - | Ref [99] |
| NCM523 | Molten salt process | 120 mAh g−1/146.3mAh g−1 | 1C | Ref [101] | |
| NCM523 | Molten salt process | −/152.5 mAh g−1 | 1C | - | Ref [102] |
| NCM523 | Molten salt method for Al doping | 45 mAh g−1/158.6 mAh g−1 | 1C | Ref [129] | |
| NCM523 | Molten salt process | −/160 mAh g−1 | 0.2C | - | Ref [100] |
| NCM523 | Molten salt process | 20 mAh g−1/150 mAh g−1 | 1C | 71.5% | Ref [104] |
| NCM523 | Hydrothermal method + molten salt sintering | 60 mAh g−1/140 mAh g−1 | 1C | 57% | Ref [23] |
| NCM523 | Coprecipitation + calcination | −/173.9 mAh g−1 | 0.2C | - | Ref [130] |
| NCM622 | Leaching + precipitation + sintering | −/181 mAh g−1 | 0.1C | - | Ref [131] |
| NCM111 | Leaching + precipitation + sintering | −/164.9 mAh g−1 | 0.2C | - | Ref [25] |
| NCM1.51.57 | Hydrothermal method + sintering | 99 mAh g−1/150.7 mAh g−1 | C/3 | 34% | Ref [125] |
| NCM111 | Hydrothermal method + short annealing time | 130 mAh g−1/150.4 mAh g−1 | C/3 | 13.3% | Ref [124] |
| NCM622 | Hydrothermal method + sintering | 87 mAh g−1/153.82 mAh g−1 | 1C | 43% | Ref [121] |
| NCM111 | Leaching + sintering | −/200 mAh g−1 | 0.5C | - | Ref [82] |
| NCM111 | Leaching + precipitation + sintering | −/160 mAh g−1 | 0.5C | - | Ref [114] |
| NCM523 | Leaching + roasting | −/156.9 mAh g−1 | 0.5C | - | Ref [115] |
| NCM | Leaching + roasting | 85 mAh g−1/144.3 mAh g−1 | 1C | 40.9% | Ref [117] |
| NCM622 | Leaching + roasting | −/152.2 mAh g−1 | 1C | - | Ref [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Zhang, C.; Hu, J. Research Progress of Ternary Cathode Materials: Failure Mechanism and Heat Treatment for Repair and Regeneration. Metals 2025, 15, 552. https://doi.org/10.3390/met15050552
Wu T, Zhang C, Hu J. Research Progress of Ternary Cathode Materials: Failure Mechanism and Heat Treatment for Repair and Regeneration. Metals. 2025; 15(5):552. https://doi.org/10.3390/met15050552
Chicago/Turabian StyleWu, Tingting, Chengxu Zhang, and Jue Hu. 2025. "Research Progress of Ternary Cathode Materials: Failure Mechanism and Heat Treatment for Repair and Regeneration" Metals 15, no. 5: 552. https://doi.org/10.3390/met15050552
APA StyleWu, T., Zhang, C., & Hu, J. (2025). Research Progress of Ternary Cathode Materials: Failure Mechanism and Heat Treatment for Repair and Regeneration. Metals, 15(5), 552. https://doi.org/10.3390/met15050552





