1. Introduction
Copper was one of the first metals used by humanity and is still one of the most widely used metals in modern society. Pure copper is a heavy metal with a density of 8.94 × 10
3 kg/m
3, characterized by a face-centered cubic lattice, with a melting point and resistivity of 1083 °C and 0.0172 μΩ·m, respectively. It has excellent electrical and thermal conductivity, corrosion resistance, and weldability, and it can be made into tubes, rods, wires, plates, and strips. Given its exceptional wear resistance, mechanical properties, formability, cryogenic performance, and non-magnetic character, copper can be applied in various fields, such as energy systems, electronic components, high-performance cabling, machinery manufacturing, shipbuilding, precision instrumentation, and chemical processing [
1,
2,
3,
4,
5,
6,
7,
8,
9].
Copper alloys hold a vital position among non-ferrous metal materials due to their excellent properties. Furthermore, copper and its alloys serve as excellent materials for the tubes that are utilized widely in air conditioning and refrigeration, building plumbing systems, electronics, and aerospace components [
10,
11,
12]. However, with the recent rapid advancements in key technological sectors—such as electronic information systems, electromobility, and sustainable energy solutions—there is a growing demand for copper-based materials with increasingly stringent performance specifications. These requirements include high thermal and electrical conductivity, excellent corrosion resistance, and enhanced mechanical strength at both room temperature and elevated temperatures.
Rare-earth elements (REEs) serve as highly effective microalloying agents due to their typical metallic behaviors and high chemical reactivity, allowing them to interact with nearly all elements except noble gases. As a result, REEs are ideal candidates for improving the microstructural properties of copper and its alloys. They are primarily introduced into copper alloys through alloying and purification processes [
13,
14,
15,
16].
The REEs comprise a group of 17 chemically similar metallic elements, including the 15 lanthanides along with scandium and yttrium, which commonly occur together in geological deposits due to their similar electronic configurations and ionic radii [
17]. It has been over two centuries since the Finnish scientist Johan Gadolin first discovered yttrium—along with other rare-earth elements—in 1794, within a mineral later known as gadolinite, which is found in Sweden. The investigations and applications of REEs in metallic materials have spanned more than eight decades of scientific research and technological advancement. Often treated as the ‘vitamin of industry’, REEs function as highly effective alloying elements that enhance the properties of a wide range of metal systems, including ferrous metals and non-ferrous alloys such as aluminum, magnesium, and copper [
18,
19,
20,
21].
Due to their unique ability to modify and enhance the physical and mechanical properties of copper alloys, REEs have attracted worldwide interest since the middle of the 20th century. In China, research on the applications of rare-earth elements to copper and its alloys began in the early 1960s, with some developments already then implemented in industrial production. REEs play crucial roles in copper alloys, primarily through the strong chemical affinities of the REEs. This effect helps REEs to preferentially interact with interstitial impurities (such as O, S, and H), forming thermally stable compounds. These reaction products can then be effectively removed via slag flotation, thereby purifying the alloy matrix [
22,
23,
24]. Experimental studies have shown that incorporating REEs into copper alloys significantly increases tensile strength and ductility, while also raising hardness—thereby improving cold workability and weldability. REEs contribute to the purification of copper and its alloys by removing impurities, which enhances overall alloy quality. This purification process leads to improved mechanical properties, reduced susceptibility to hot cracking, and increased thermoplasticity, corrosion resistance, thermal conductivity, and electrical conductivity [
25]. High-temperature-stable dispersoids within the matrix of the copper alloy promote grain refinement by serving as affective heterogeneous nucleation sites [
14,
26]. The strong chemical affinities of rare elements allow them to react with impurity phases in copper, forming thermodynamically stable intermetallic compounds with high melting points and diverse morphologies. These newly formed phases significantly alter the natures of existing inclusions [
27,
28].
The unparalleled electrical and thermal conductivity of copper and its alloys is increasingly critical in addressing both current and emerging technological challenges. High-performance copper alloys with superior mechanical strength and excellent electrical conductivity are essential components in a wide range of applications, including electronic devices, robotics, and artificial intelligence. Moreover, copper and copper alloy tubes and plates—valued for their high heat-transfer efficiency—are integral to air conditioning and refrigeration systems, 5G/6G base stations, supercomputing centers, and other advanced technologies. In light of these demands, it is essential to explore the roles of REEs in the development of copper materials that meet the combined requirements of high electrical and thermal conductivity along with mechanical strength.
This review aims to systematically summarize the effects of rare-earth elements on the microstructure, mechanical properties, and corrosion resistance, as well as the processing, fabrication, and service performance, of copper and its alloys. The current research priorities, technological frontiers, and key findings related to rare-earth-enhanced copper systems are comprehensively examined. This paper is expected to provide valuable insights to guide future research and development in this important field. This review provides a comprehensive summarization of the effects of the addition of REEs in Cu-based alloys, with a focus on the multi-scale influence of these REEs. These include (1) microstructural modifications such as impurity removal, grain refinement, and control of recrystallization behavior; (2) property enhancements, encompassing improved mechanical strength, optimized electrical conductivity, and increased corrosion resistance; and (3) practical applications in the fields of advanced engineering. The synthesized insights establish fundamental principles and propose innovative strategies for the design and development of next-generation Cu alloys to meet the evolving demands of modern industry.
4. Influence of Rare Earths on the Mechanical Properties of Copper
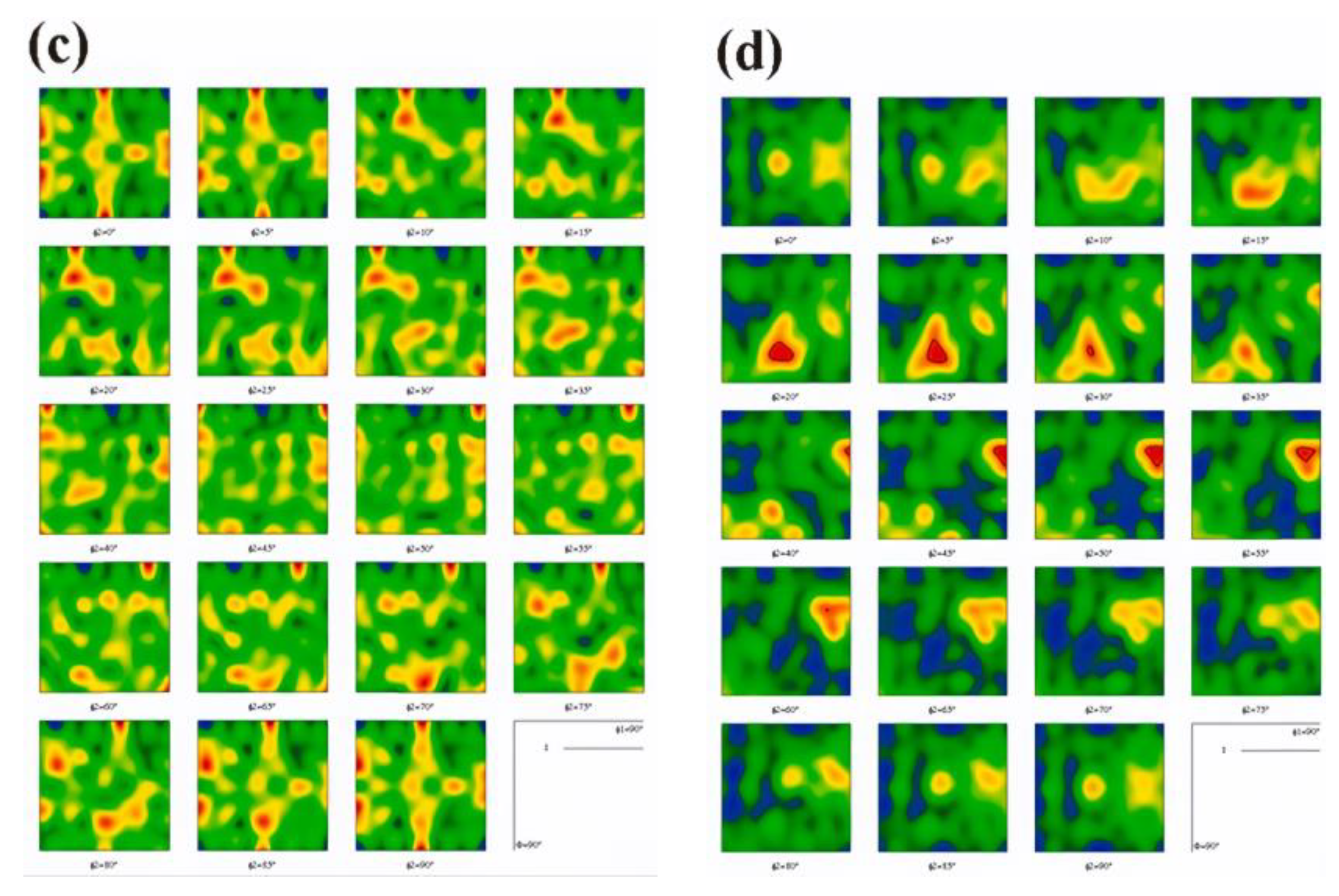
Figure 11 and
Figure 12 illustrate the microstructure and mechanical characteristics of Cu bars/strips produced by vacuum-induction melting (MV) and Y-microalloyed vacuum melting (MMV). Metallographic analysis revealed that primary recrystallization in the tested copper strips initiates at 200 °C for MV copper, as illustrated in
Figure 11a, and at 300 °C for MMV copper containing 0.02% yttrium, as illustrated in
Figure 12a. In both cases, the rolling-induced texture remains largely preserved. Upon annealing at higher temperatures, between 500 to 600 °C, a non-equiaxed, anisotropic microstructure develops, as seen in
Figure 11b,c and
Figure 12b,c. This transformation corresponds to the final stage of primary recrystallization and the onset of normal grain growth. The addition of yttrium notably reduces the alloy’s plastic strain, while slightly enhancing its compressive strength and elastic strain limit [
42].
Isothermal annealing does not yield a fine-grained, equiaxed microstructure in MV-grade copper, even after a 90% total rolling reduction. In contrast, yttrium microalloying significantly modifies the mesostructures, as shown in
Figure 13, leading to improved mechanical strength, as evidenced in
Figure 14. Specially, the addition of 0.02% yttrium promotes increased dislocation density and aggregation, as illustrated in
Figure 13, contributing to the observed enhancement in strength [
29]. The incorporation of Y elevates the recrystallization temperature of Cu, thereby inhibiting dynamic recovery under deformation conditions. This leads to the accumulation of dislocations, which intensifies strain hardening and subsequently diminishes the material’s uniform elongation.
The mechanical properties of the alloy continue to improve with an increase in La content [
46]. The hardness and microstructure of the alloy are strongly influenced by heat treatment. Peak hardness is achieved after annealing at 400 °C for 2 h, while elevated temperatures promote grain growth and soften the material [
47].
The tensile strength enhancement of as-cast pure Cu by means of the addition of La occurs in two distinct stages. Below 0.1 wt.% La, the marginal strength improvement mainly results from solid-solution strengthening, in which dissolved La atoms induce minor lattice distortions in the Cu matrix. A notable enhancement in tensile strength is observed beyond 0.1 wt.% La, which is attributed to the combined effects of grain refinement and precipitation hardening. The addition of La promotes the formation of refined grains and intermetallics of Cu
6La, which impede dislocation motion, thereby strengthening the alloy [
36]. Chen et al. demonstrated that rare-earth elements improved the alloy’s strength and conductivity through grain refinement, homogenized precipitate distribution, and enhanced precipitate–matrix coherency [
42].
Coincidently, the addition of cerium improves the tensile strength of copper via synergistic mechanisms, including solid-solution strengthening, grain refinement, and precipitation strengthening. The optimal strength (182 MPa) is achieved at 0.12 wt.% Ce. Liu et al. investigated the properties of a Cu-0.1%Ag-0.06%Ce alloy and found that the addition of cerium significantly enhances the alloy’s strength and hardness while also improving its resistance to thermal softening [
48]. It was also found the incorporation of cerium promotes the precipitation of nanoscale particles, which contribute to increased hardness in the alloy [
49]. However, the addition of cerium reduces the elongation of copper, with the lowest value, 31.7%, being observed at a cerium content of 0.045 wt.%. SEM-based fracture analysis indicates that cerium promotes the accumulation of second-phase particles near grain boundaries, which contributes to the observed reduction in ductility [
14].
5. Influence of Rare Earths on the Electrical Properties of Copper
Electrical conductivity is one of the most prominent characteristics of copper materials, making them highly sought-after in applications such as electric vehicles, artificial intelligence, and 5G communications. REEs serve as highly effective modifiers for enhancing the conductive properties of copper-based materials [
33]. REEs effectively enhance both the electrical and the thermal properties of copper-based materials through impurity removal. Specifically, REEs react with oxygen and sulfur to form stable compounds (e.g., RE
2O
3, RE
2O
2S), thereby purifying the copper matrix [
33]. For example, La reacts with detrimental impurities (O, S) to form stable intermetallic compounds (e.g., La–O, La–S), reducing impurity scattering and increasing conductivity by ~1–3% IACS compared to La-free alloys [
50]. RE elements enhance conductivity by purifying grain boundaries and immobilizing solute atoms, collectively reducing electron scattering. This complements their strengthening role, enabling high strength–conductivity synergy in Cu alloys [
51].
As shown in the data in
Table 3, after refining with rare earth (RE) composite molten salt, the electrical conductivity of impure copper shows significant improvement. Compared with traditional processes, the introduction of rare-earth elements significantly enhances the deoxidation and impurity-purification capabilities of the melt. RE refining agents can efficiently remove solid oxygen from the copper matrix (such as by forming stable oxides like La
2O
3 and Ce
2O
3), increasing the electrical conductivity of copper materials by 15–22% [
30].
The effects of rare-earth (RE) elements on the electrical properties of copper and its alloys are characterized by the two competing mechanisms that determine the overall conductivity of the material. On one hand, the addition of REEs promotes grain refinement, leading to increased numbers of grain boundaries. Serving as scattering centers for conductive electrons, these boundaries lead to increases in resistivity and decreases in conductivity. On the other hand, RE elements exhibit strong affinities for impurities such as oxygen, sulfur, and other trace elements found in copper. By forming stable compounds with these impurities, the added REEs effectively purify the copper matrix, reducing lattice distortions and enhancing electrical conductivity. These two opposing effects coexist, and the relative influence of each varies with the amount of RE added.
Research indicates that the RE element concentration significantly affects the electrical conductivity of copper-based alloys. For instance, when the addition of La or Ce is approximately 0.02 wt.%, the alloy exhibits the highest electrical conductivity. Below 0.015 wt.% RE, the dominant purification effect enhances electrical conductivity (~2% improvement). Beyond this threshold, grain refinement prevails, reducing conductivity via intensified grain-boundary electron scattering [
30]. It was found that Ce can react with phosphorus (P) to form CeP compounds, reducing the content of solid solution P, and thereby improving the conductivity of copper. However, a slight decrease in conductivity is observed after hot extrusion. The observed effect results from CeP precipitation, decreasing solid-solution phosphorus concentration. During the heat preservation treatment prior to hot extrusion, CeP redissolves into the copper matrix, thereby adversely affecting the conductivity [
52].
Furthermore, the duration of heat treatment significantly influences the electrical properties of RE-microalloyed Cu alloys. Prolonged holding allows sufficient time for RE elements to react with impurities in the copper matrix, leading to effective purification and improved conductivity. However, excessively long holding times can result in the formation of refractory compounds between RE elements and low-melting-point impurities. These compounds disperse throughout the copper matrix, acting as additional scattering centers and degrading the electrical conductivity [
14]. Therefore, optimizing both the RE content and holding time is essential for achieving the desired balance between grain refinement and purification, ultimately maximizing the electrical conductivity of copper and its alloys. Cu-Cr alloys, including Zr-modified variants (e.g., Cu-Cr-Zr), are widely used in electrical and thermal applications such as contact wires and welding electrodes, owing to their high strength and conductivity. This property synergy arises from the limited solubility of Cr in the Cu FCC matrix [
38]. The electrical conductivity of the alloys decreases linearly as the heat treatment temperature rises. The Cu-Cr-La and Cu-Cr-Y alloys exhibit comparable conductivity across temperatures, demonstrating their similar thermal stability [
39].
6. Influence of Rare Earths on the Corrosion Resistance of Copper
Recent studies have demonstrated that rare-earth elements (REEs), such as La, Ce, and Y, significantly influence the corrosion-related behavior of copper. Specifically, lanthanum (La) interacts with impurity elements present in copper, reducing the cathodic active sites. This suppression of the cathodic reaction effectively mitigates electrochemical corrosion, thereby improving the overall corrosion resistance of copper tubes [
53].
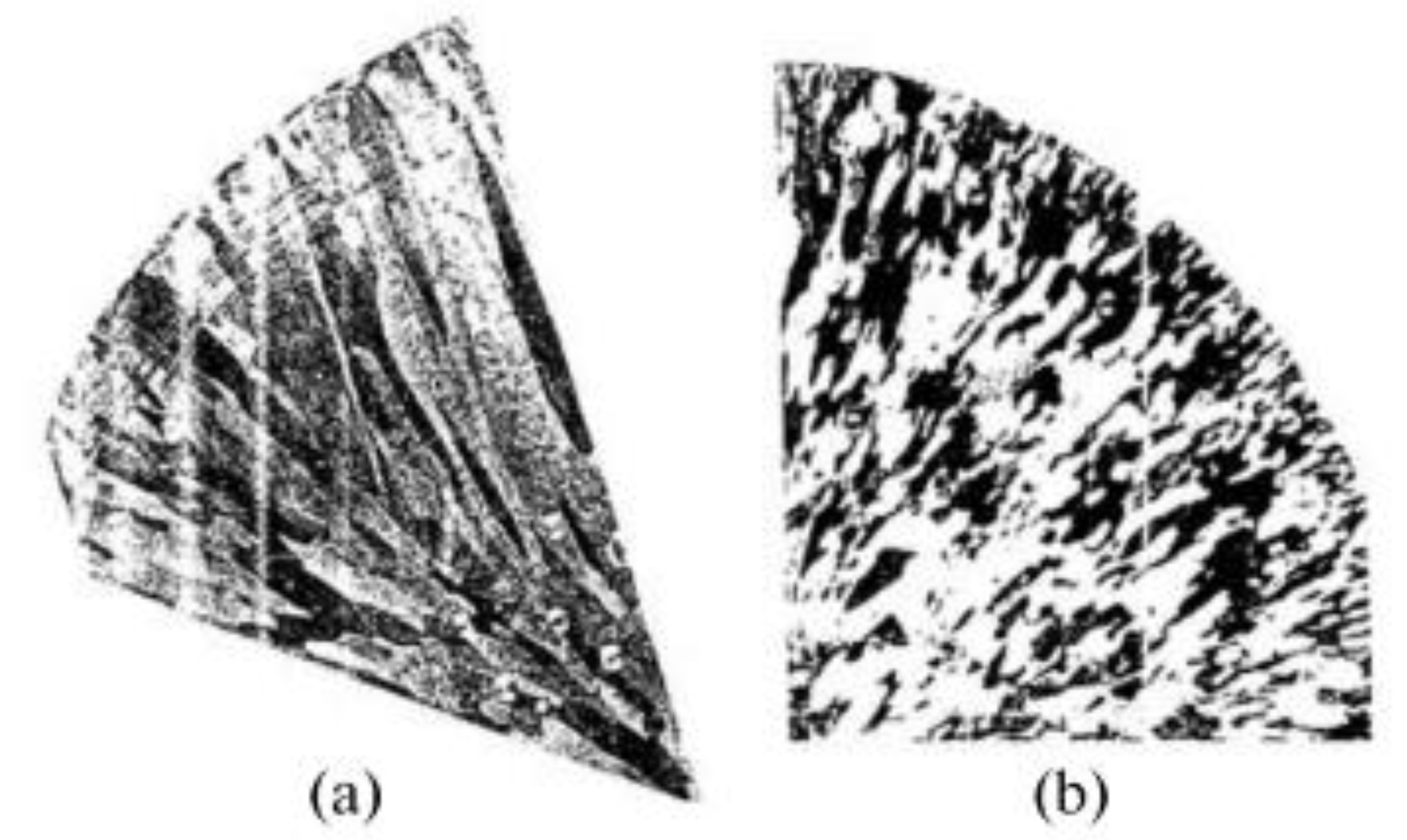
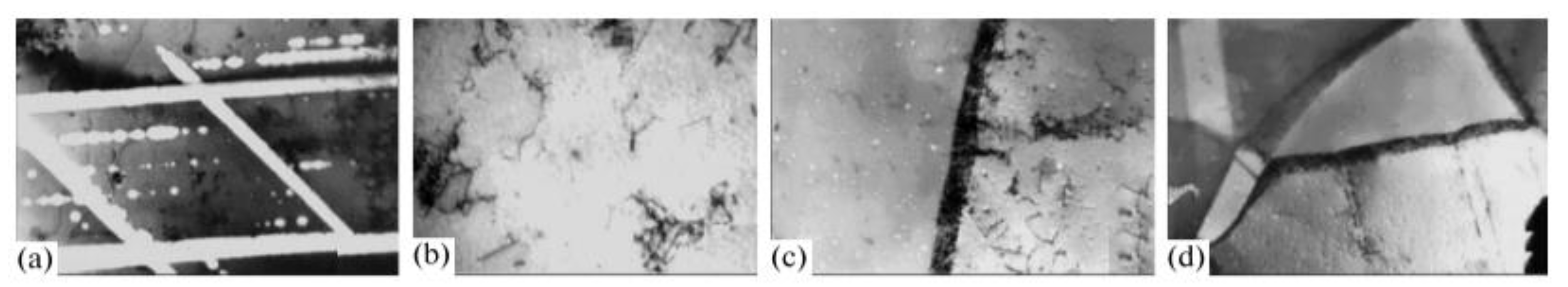
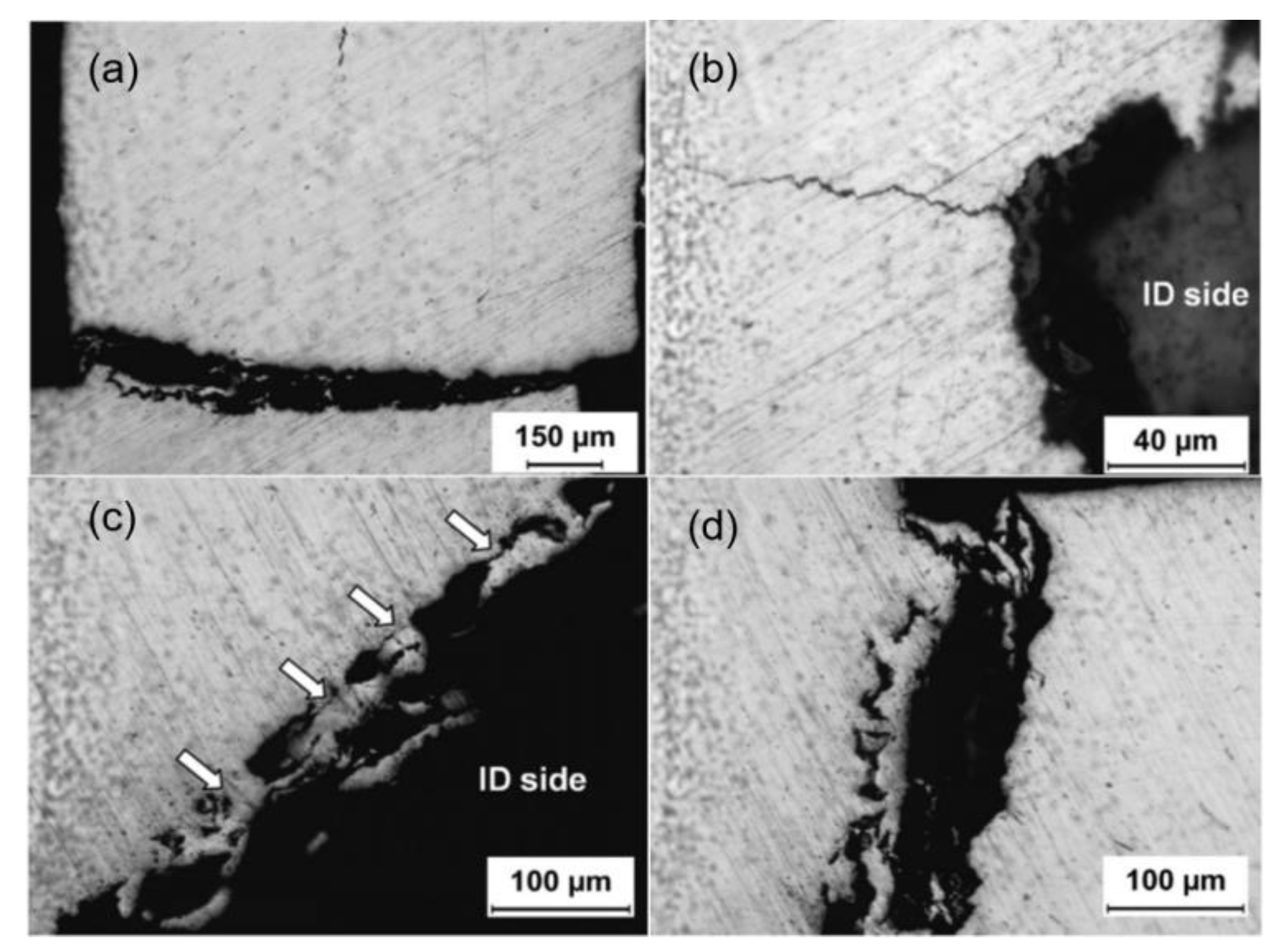
Figure 15a displays an optical micrograph revealing a primary crack propagating through the entire wall thickness of the copper tube. The inner surface adjacent to the fracture region exhibits numerous corrosion pits. Secondary microcracks are observed initiating from these pit features and extending radially through the tube wall.
Figure 15b exhibits a representative thickness-spanning crack originating from an inner surface pit. These secondary cracks, observed exclusively in the bent tube segment adjacent to the primary fracture, result from significant residual stresses concentrated in this region. The synergistic effects of stress concentration at corrosion pits and residual stresses promote crack nucleation and subsequent through-wall propagation.
Beyond the bent region, shallow pits and cracks are still observable on the inner surface of the copper tube. However, the nature of these cracks differs. Instead of the transverse cracks seen in the bent region, these are longitudinal.
Figure 15c displays an optical micrograph obtained from the unbent section of the tube, adjacent to the interior wall and distal to the curved zone. The image exhibits numerous shallow pits interconnected by microcracks (indicated by arrows), forming a networked morphology. Corrosive damage is localized within a narrow band of 40–50 μm depth from the tube’s inner wall. Comparable degradation features, characterized by crack-linked pit clusters, can be observed propagating along the primary fracture path in
Figure 15d. This strongly suggests that this particular corrosion morphology is the primary cause of the copper tube’s failure [
10].
The corrosion resistance of copper in 0.5 mol/L HCl is notably enhanced by the addition of Ce, with an optimal content below 0.069 wt.% for balanced mechanical and anti-corrosion performance. This enhancement stems from Ce’s purifying effect and the development of a compact corrosion-product layer on the surface of the Cu. At a cerium content of 0.020 wt.%, the copper alloy exhibits the lowest corrosion current density and corrosion rate, demonstrating optimal corrosion resistance. However, higher cerium contents lead to an increase in secondary-phase particles, which creates a potential difference with the copper matrix, instead exacerbating micro-galvanic corrosion [
14].
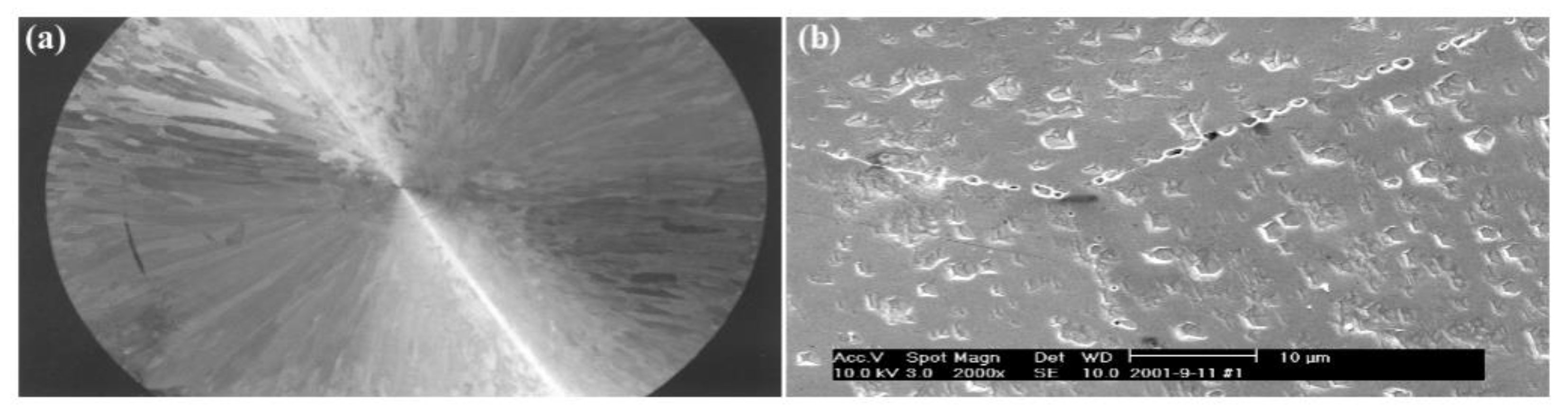
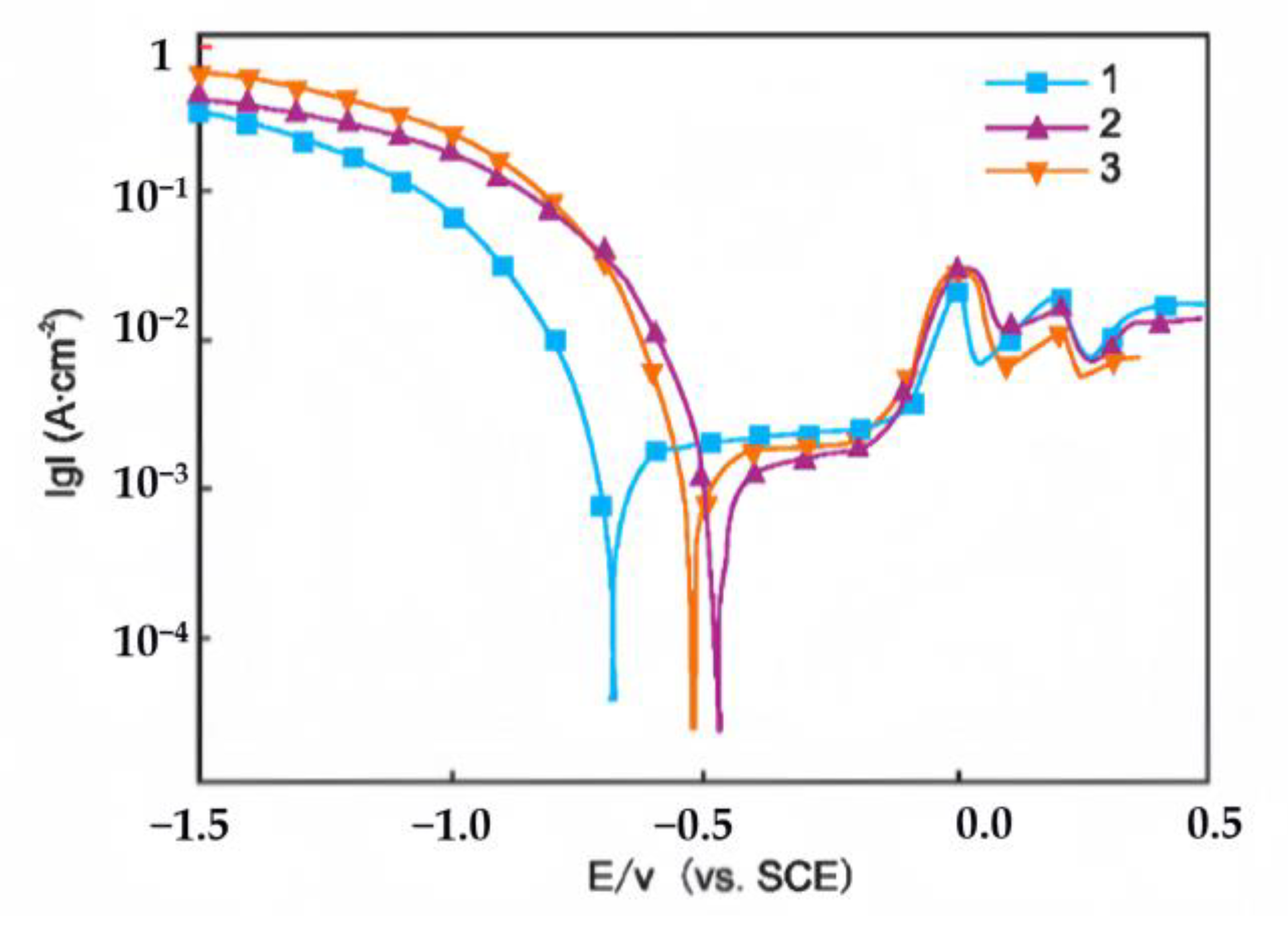
Figure 16 compares the potentiodynamic polarization curves of impure copper in 0.5 mol/L HCl before and after the addition of Y.
Table 4 summarizes the derived electrochemical parameters. The corrosion potential of Y-refined samples (ZY1, ZY2) increases relative to ZY0, indicating enhanced corrosion resistance. Their lower corrosion current densities, consistent with this finding, further confirm the improvement. In contrast, ZY3 exhibits higher corrosion current density despite potential correction, suggesting inferior performance.
The addition of yttrium (Y) enhances the corrosion resistance of impure copper in HCl, with 0.15 wt.% Y demonstrating optimal performance due to its purifying effect, as confirmed by potentiodynamic polarization tests [
14].
Figure 17 and
Table 5 show that the addition of La in horizontally continuous cast (HCC) TP2 copper tubes increases the corrosion potential (E
corr), compared to La-free samples. Additionally, La reduces the corrosion current density (I
corr) and annual corrosion rate (V
A). The cathodic polarization curves shift positively, with E
corr rising from −679.31 mV to −471.91 mV and −521.67 mV. Moreover, I
corr decreases from 432.2 to 398.04 μA·cm
−2 and 417.64 μA·cm
−2, while V
A drops from 10.18 and 9.37 mm·a
−1 to 9.84 and 9.37 mm·a
−1, respectively. These findings indicate that La improves the corrosion resistance of HCC TP2 copper tubes [
54].
As depicted in
Figure 17, the polarization curves exhibit similar morphological characteristics before and after La incorporation, suggesting that the fundamental corrosion mechanisms remain unchanged. The corrosion process is predominantly governed by cathodic reactions, with minimal influence from electrochemical corrosion mechanisms. Commercial copper typically contains trace impurity elements, including O, H, S, Pb, Bi, and Fe, which can form intermetallic compounds or eutectic phases within the copper matrix. These secondary phases possess higher electrochemical potential values than pure copper. They function as cathodic sites in corrosive environments, while the copper matrix acts as the anode, thereby accelerating the electrochemical corrosion process.
Rare earths such as La can react with detrimental impurity elements to become stable compounds, owing to high chemical reactivity. This purification effect results in the following beneficial outcomes: (1) reduction of cathodic active sites, (2) suppression of cathodic reaction kinetics, and (3) consequent decrease of anodic dissolution rate. The mitigation of galvanic corrosion effects through the addition of La significantly enhances the corrosion resistance of HCC TP2 copper tubes [
53].
7. Application
The effects of REEs on purification, microstructural refinement, recrystallization, texture development, mechanical strength, electrical conductivity, and corrosion resistance in copper and its alloys have been demonstrated not only through experimental studies but also in practical industrial applications. The following are several representative examples.
The simultaneous optimization of strength and electrical conductivity remains a central challenge in modern copper alloy development. Traditional copper materials exhibit an inherent property trade-off: high-purity oxygen-free copper (OFHC) achieves excellent conductivity (≥102% IACS) but low yield strength (<100 MPa), while precipitation-strengthened alloys (e.g., Cu-Cr-Zr) attain their 500 MPa strength at the expense of conductivity (40–60% IACS). This fundamental limitation, governed by Matthiessen’s rule, severely restricts copper alloys’ applications in strategic sectors like advanced manufacturing and energy systems. Although novel processing methods (e.g., powder metallurgy, rapid solidification) have been explored, their industrial adoption is hindered by their prohibitive costs (5–8× conventional methods) and limited batch sizes (<100 kg/furnace). The industry has developed an innovative approach to overcome these constraints. It has developed a new preparation technology, namely, “direct smelting + rare earth tellurium microalloying”. This performance breakthrough achieves the synergistic optimization of strength (480–520 MPa) and electrical conductivity (≥90% IACS). Its industrial advantages include the facts that production costs are reduced by 60%, and single-furnace production capacity now reaches 3 to 5 tons. This approach has successfully been applied in key industries such as power transmission (high-speed rail catenary), electronic communication (5G base stations), and the national defense and military industry (electromagnetic gun components). This technology breaks through the limitations of traditional processes and provides a practical and feasible solution for the large-scale production of high-strength and high-conductivity copper alloys [
55].
La addition introduces new phases, including micron-sized LaP
2 and sub-micron LaCu
4P
3, while suppressing the formation of Cu
3P. Nano-scale La (Cu
2P)
2 precipitates form during annealing, contributing to strength but slightly reducing ductility. La improves hot workability and mechanical performance, offering a cost-effective solution for high-strength Cu-Sn-P alloys [
56]. Practical applications have demonstrated that La can be effectively utilized to enhance both the hot workability and the mechanical performance of copper alloys.
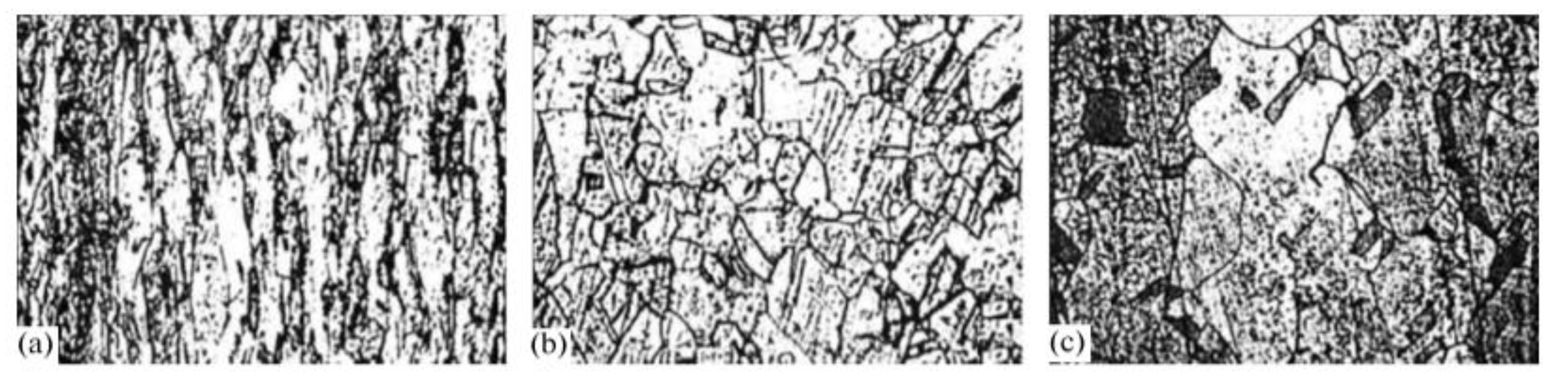
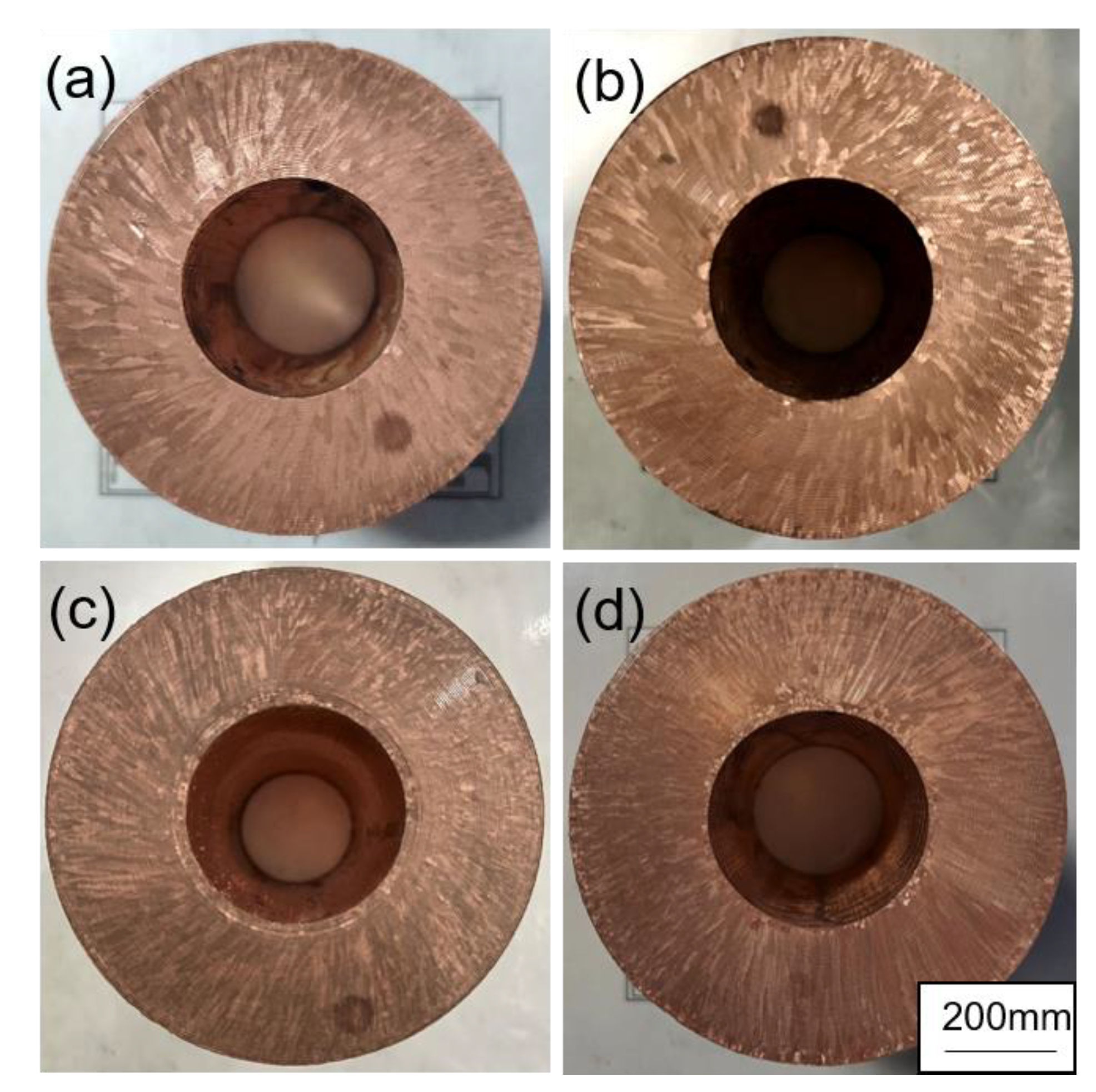
Previous studies have investigated the effects of lanthanum (La) on the casting microstructure of TP2 copper (containing 150~400 ppm phosphorus), as illustrated in
Figure 18. Metallographic analysis reveals that the rare-earth-free slab exhibits a coarse and heterogeneous grain structure. With the addition of 10 ppm rare earths, as depicted in
Figure 18b, the slab shows the phenomenon of refinement. Consequently, the microstructure is characterized by a decrease in grain size and a uniform distribution of grains. This refinement and homogenization effect is even more pronounced when the rare-earth content is further increased, as illustrated in
Figure 18c,d. The results indicate that rare earth also has a significant refining effect on the as-cast microstructure of TP2 copper.
Despite their potential, the application of rare-earth elements in copper and its alloys continues to face notable challenges. Several critical issues may remain to be addressed despite the significant potential of rare-earth elements in enhancing the properties of copper and copper alloys: The intermetallic phases formed with rare-earth elements (REEs) are sometimes brittle, compared to the copper matrix, which can impair deformation compatibility. Additionally, the undesirable aggregation of REE-containing phases may increase microstructural heterogeneity, which can negatively affect the overall performance of the alloy—particularly its ductility. In addition, the incorporation of REEs can introduce processing challenges, as it may increase the strength of copper alloys while reducing their plasticity. Furthermore, it is important to note that REEs—in particular, the heavy rare earths—are expensive, which can raise the overall costs of copper alloy production by approximately 5–20%, based on manufacturing experience. These factors collectively pose limitations to the widespread application of REEs in copper alloys.
Author Contributions
Conceptualization, J.-S.L. and D.-Y.C.; Methodology, D.-Y.C. and S.-W.W.; Software, J.-S.L. and W.-X.Y.; Validation, D.-Y.C. and H.-W.S.; Formal analysis, W.-X.Y. and D.-Y.C.; Investigation, J.-S.L., H.-W.S. and S.-H.Z.; Resources, H.-W.S. and S.-H.Z.; Data curation, D.-Y.C. and S.-W.W.; Writing—original draft preparation, J.-S.L. and W.-X.Y.; writing—review and editing, D.-Y.C. and H.-W.S.; Visualization, D.-Y.C. and H.-W.S.; Supervision, H.-W.S. and S.-H.Z.; Project administration, J.-S.L. and H.-W.S.; Funding acquisition, S.-W.W. and S.-H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by International Partnership Program of Chinese Academy of Sciences (Grant No. 172GJHZ2022054GC).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Lu, L.; Shen, Y.; Chen, X.; Qian, L.; Lu, K. Ultrahigh Strength and High Electrical Conductivity in Copper. Science 2004, 304, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Fang, D.; Bao, C.; Tan, S.; Liu, Y.; Li, F.; You, X.; Tao, J.; Bao, R.; Li, C.; et al. Enhancing mechanical properties of copper matrix composite by adding SiO2 quantum dots reinforcement. Vacuum 2022, 195, 110682. [Google Scholar] [CrossRef]
- Tong, W.; An, Y.; Bao, C.; Fang, D.; Wang, M.; Yi, J. Improving mechanical properties of copper composite by interconnected MoO2 quantum dots. Mater. Sci. Eng. A 2022, 848, 143365. [Google Scholar] [CrossRef]
- Wang, D.; Yan, A.; Liu, Y.; Wu, Z.; Gan, X.; Li, F.; Tao, J.; Li, C.; Yi, J. Interfacial Bonding Improvement through Nickel Decoration on Carbon Nanotubes in Carbon Nanotubes/Cu Composite Foams Reinforced Copper Matrix Composites. Nanomaterials 2022, 12, 2548. [Google Scholar] [CrossRef]
- Luo, H.; Bao, R.; Ma, R.; Liu, J.; Nie, Y.; Yi, J. Preparation and properties of copper matrix composites synergistically strengthened by Al2O3 and CPD. Diam. Relat. Mater. 2022, 124, 108916. [Google Scholar] [CrossRef]
- Zhao, W.M.; Bao, R.; Yi, J.H.; Hou, X.H.; Fang, D.; Liu, C.X. Fabrication of RGO/Cu composites based on electrostatic adsorption. Trans. Nonferrous Met. Soc. China 2020, 30, 982–991. [Google Scholar] [CrossRef]
- Popovich, A.; Sufiiarov, V.; Polozov, I.; Borisov, E.; Masaylo, D.; Orlov, A. Microstructure and mechanical properties of additive manufactured copper alloy. Mater. Lett. 2016, 179, 38–41. [Google Scholar] [CrossRef]
- Peltola, H.; Lindgren, M. Failure analysis of a copper tube in a finned heat exchanger. Eng. Fail. Anal. 2015, 51, 83–97. [Google Scholar] [CrossRef]
- Pan, Z.Y.; Chen, J.B.; Li, J.F. Microstructure and properties of rare earth-containing Cu–Cr–Zr alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 1206–1214. [Google Scholar] [CrossRef]
- Chandra, K.; Kain, V.; Shetty, P.S.; Kishan, R. Failure analysis of copper tube used in a refrigerating plant. Eng. Fail. Anal. 2014, 37, 1–11. [Google Scholar] [CrossRef]
- Mao, X.; Fang, F.; Jiang, J.; Tan, R. Effect of rare earths on corrosion resistance of Cu-30Ni alloys in simulated seawater. J. Rare Earths 2009, 27, 1037–1041. [Google Scholar] [CrossRef]
- Guo, F.A.; Xiang, C.J.; Yang, C.X.; Cao, X.M.; Mu, S.G.; Tang, Y.Q. Study of rare earth elements on the physical and mechanical properties of a Cu–Fe–P–Cr alloy. Mater. Sci. Eng. B 2008, 147, 1–6. [Google Scholar] [CrossRef]
- Ning, Y.T.; Zhang, X.H.; Qin, G.Y. Influence of cerium addition on microstructure and properties of Cu-Ag alloy in situ filamentary composites. J. Rare Earths 2005, 23, 392–398. [Google Scholar]
- Li, H.H.; Sun, X.Q.; Zhang, S.Z.; Zhao, Q.Y.; Wang, G.Z. Application of rare-earth element Y in refining impure copper. Int. J. Miner. Metall. Mater. 2015, 22, 453–459. [Google Scholar] [CrossRef]
- Liu, J.B.; Meng, L.; Zhang, L. Rare earth microalloying in as-cast and homogenized alloys Cu–6wt.% Ag and Cu–24wt.% Ag. J. Alloys Compd. 2006, 425, 185–190. [Google Scholar]
- Li, M.; Hao, H.; Zhang, A.; Song, Y.; Zhang, X. Effects of Nd on microstructure and mechanical properties of as-cast Mg-8Li-3Al alloy. J. Rare Earths 2012, 30, 492–496. [Google Scholar] [CrossRef]
- Klemettinen, L.; Aromaa, R.; Dańczak, A.; O’Brien, H.; Taskinen, P.; Jokilaakso, A. Distribution Kinetics of Rare Earth Elements in Copper Smelting. Sustainability 2020, 12, 208. [Google Scholar] [CrossRef]
- Cai, G.; Li, C. Effects of Ce on Inclusions, Microstructure, Mechanical Properties, and Corrosion Behavior of AISI 202 Stainless Steel. J. Mater. Eng. Perform. 2015, 24, 3989–4009. [Google Scholar] [CrossRef]
- Gao, Z.; Li, H.; Lai, Y.; Ou, Y.; Li, D. Effects of minor Zr and Er on microstructure and mechanical properties of pure aluminum. Mater. Sci. Eng. A 2013, 580, 92–98. [Google Scholar] [CrossRef]
- Gavras, S.; Easton, M.A.; Gibson, M.A.; Zhu, S.; Nie, J.-F. Microstructure and property evaluation of high-pressure die-cast Mg–La–rare earth (Nd, Y or Gd) alloys. J. Alloys Compd. 2014, 597, 21–29. [Google Scholar] [CrossRef]
- Chen, Z.W.; Tang, M.J.; Zhao, K. Effect of rare earth samarium addition on the kinetics of precipitation in Al-Cu-Mn casting alloy. Int. J. Miner. Metall. Mater. 2014, 21, 155–161. [Google Scholar] [CrossRef]
- Otto, F.; Viswanathan, G.B.; Payton, E.J.; Frenzel, J.; Eggeler, G. On the effect of grain boundary segregation on creep and creep rupture. Acta Mater. 2012, 60, 2982–2998. [Google Scholar] [CrossRef]
- Duscher, G.; Chisholm, M.F.; Alber, U.; Rühle, M. Bismuth-induced embrittlement of copper grain boundaries. Nat. Mater. 2004, 3, 621–626. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, S.; Chen, Y.; Li, H.; Liu, J. Effects of La Microalloying on Microstructure Evolution of Pure Copper. Mater. Sci. Forum 2017, 898, 361–366. [Google Scholar] [CrossRef]
- Tenwick, M.J.; Davies, H.A. Enhanced strength in high conductivity copper alloys. Mater. Sci. Eng. 1988, 98, 543–546. [Google Scholar] [CrossRef]
- Liu, H.; Teng, X.; Wu, W.; Xiao, Z.; Wu, X.; Leng, J. Effects of Rare Earth Y Addition on Microstructural and Properties of Pure Copper. Mater. Sci. Forum 2018, 913, 862–869. [Google Scholar] [CrossRef]
- Lin, G.Y.; Li, K.; Feng, D.; Feng, Y.P.; Song, W.Y.; Xiao, M.Q. Effects of La–Ce addition on microstructure and mechanical properties of Al–18Si–4Cu–0.5Mg alloy. Trans. Nonferrous Met. Soc. China 2019, 29, 1592–1600. [Google Scholar] [CrossRef]
- Li, J.; Chang, L.; Li, S.; Zhu, X.; An, Z. Microstructure and properties of as-cast Cu-Cr-Zr alloys with lanthanum addition. J. Rare Earths 2018, 36, 424–429. [Google Scholar] [CrossRef]
- Kamyshanchenko, N.V.; Durykhin, M.I.; Gal’tsev, A.V.; Neklyudov, I.M.; Borts, B.V.; Shevchenko, S.V. Effect of microalloying of copper with yttrium on its structure and mechanical properties. Inorg. Mater. 2011, 47, 1592–1595. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.; Chen, M. Effect of Rare Earth on Microstructure and Property of Refining Impure-Copper. Adv. Mater. Res. 2011, 189–193, 3982–3985. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, J.; Li, H. The Influence of Rare Earth Ce on the Microstructure and Properties of Cast Pure Copper. Materials 2024, 17, 2387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, S.; Song, H.; Cheng, M.; Li, H.; Liu, J. Sudden transition from columnar to equiaxed grain of cast copper induced by rare earth microalloying. Mater. Des. 2016, 91, 314–320. [Google Scholar] [CrossRef]
- Du, T. Physical-chemistry effect of rare earth elements on metallic materials. Acta Metall. Sin. 1997, 33, 69–77. [Google Scholar]
- Qian, S.; Shao, W.; Zhao, Y.; Wan, X.; Zhou, J.; Zhang, Y. Improving uniformity and performance of electroformed copper using rare earth element additives. Int. J. Electrochem. Sci. 2024, 19, 100549. [Google Scholar] [CrossRef]
- Li, H.H.; Liu, X.; Li, Y.; Zhang, S.H.; Chen, Y.; Wang, S.W.; Liu, J.S.; Wu, J.H. Effects of rare earth Ce addition on microstructure and mechanical properties of impure copper containing Pb. Trans. Nonferrous Met. Soc. China 2020, 30, 1574–1581. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, M.; Song, H.; Zhang, S.; Liu, J.; Zhu, Y. Effects of lanthanum addition on microstructure and mechanical properties of as-cast pure copper. J. Rare Earths 2014, 32, 1056–1063. [Google Scholar] [CrossRef]
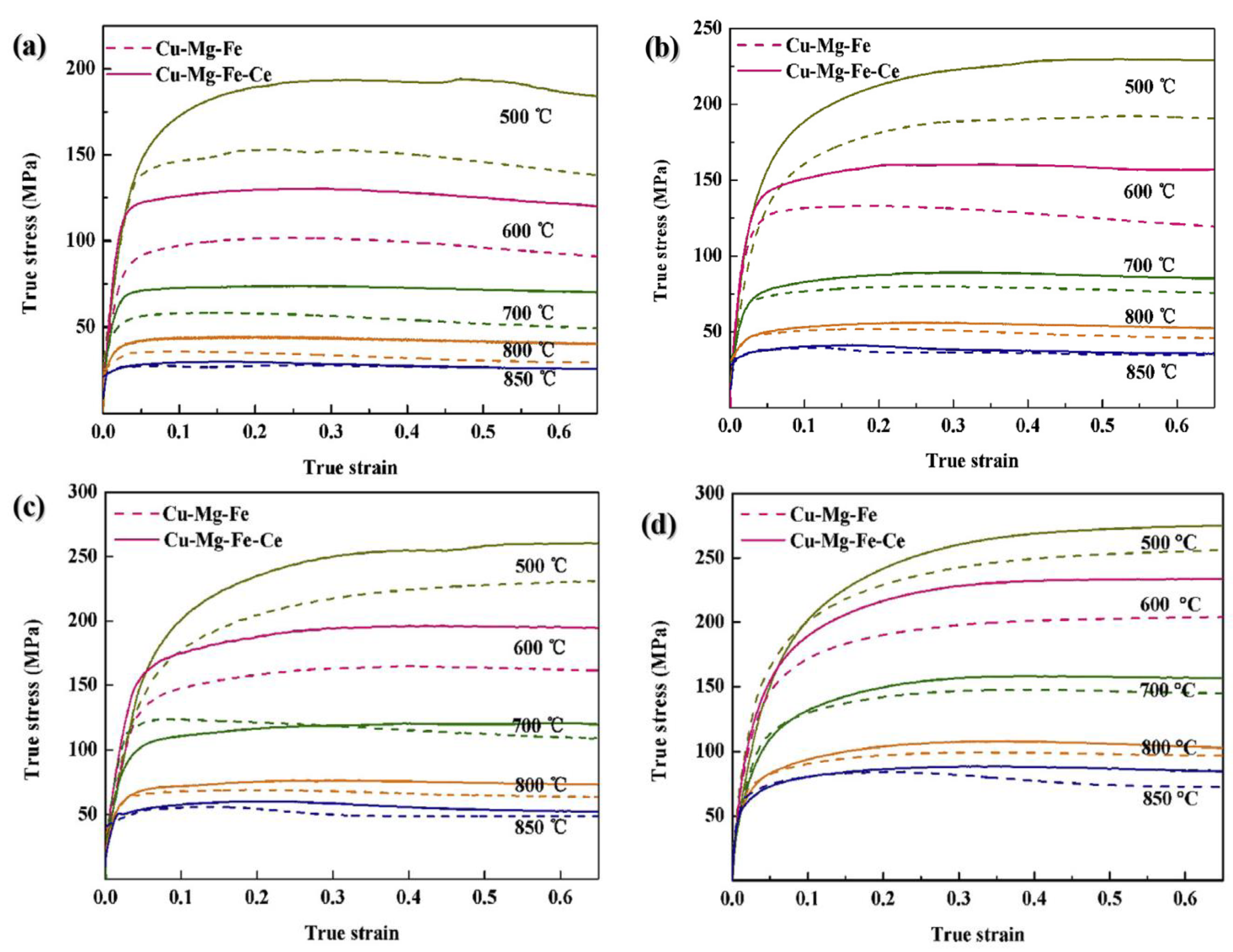
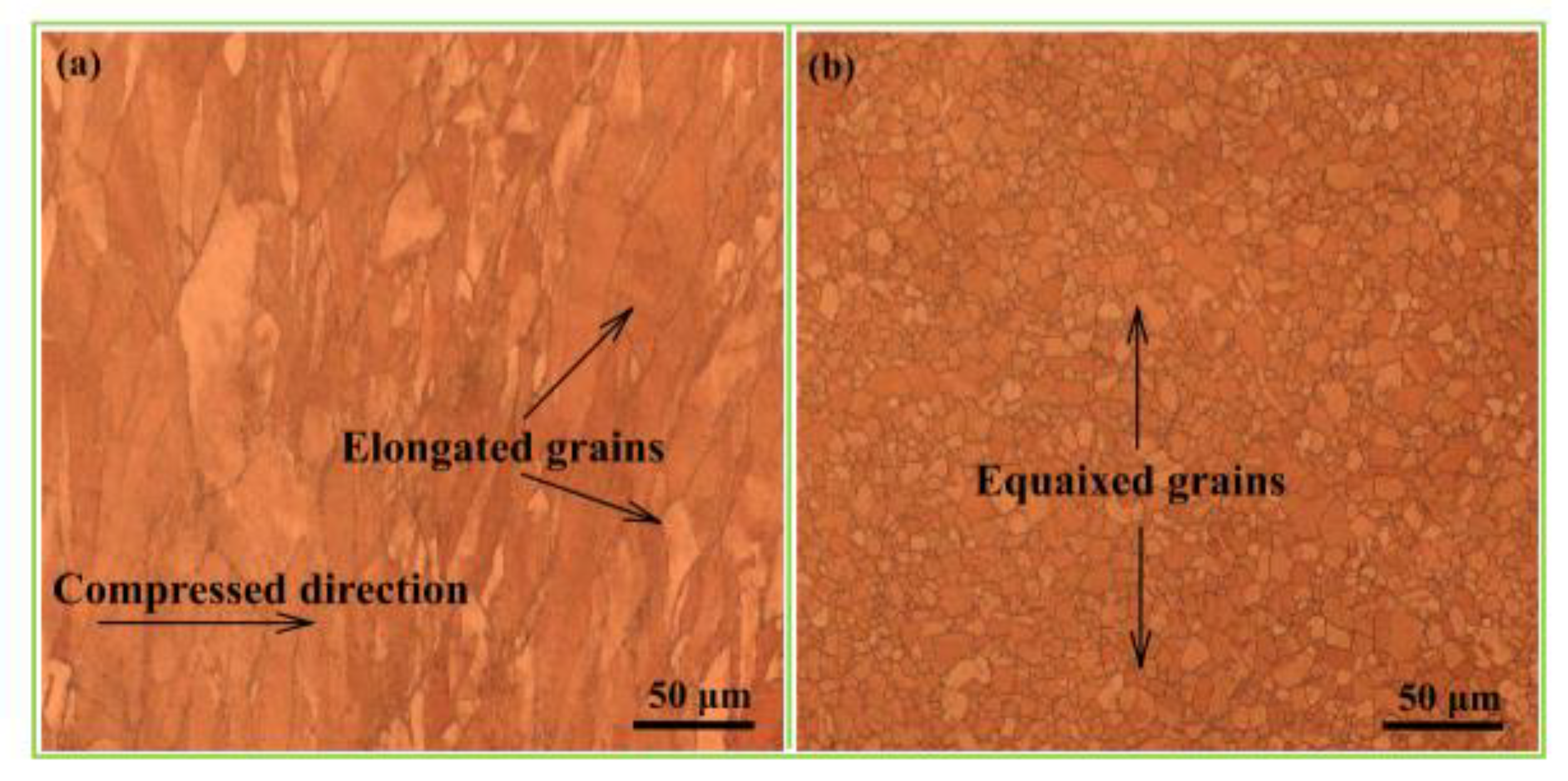
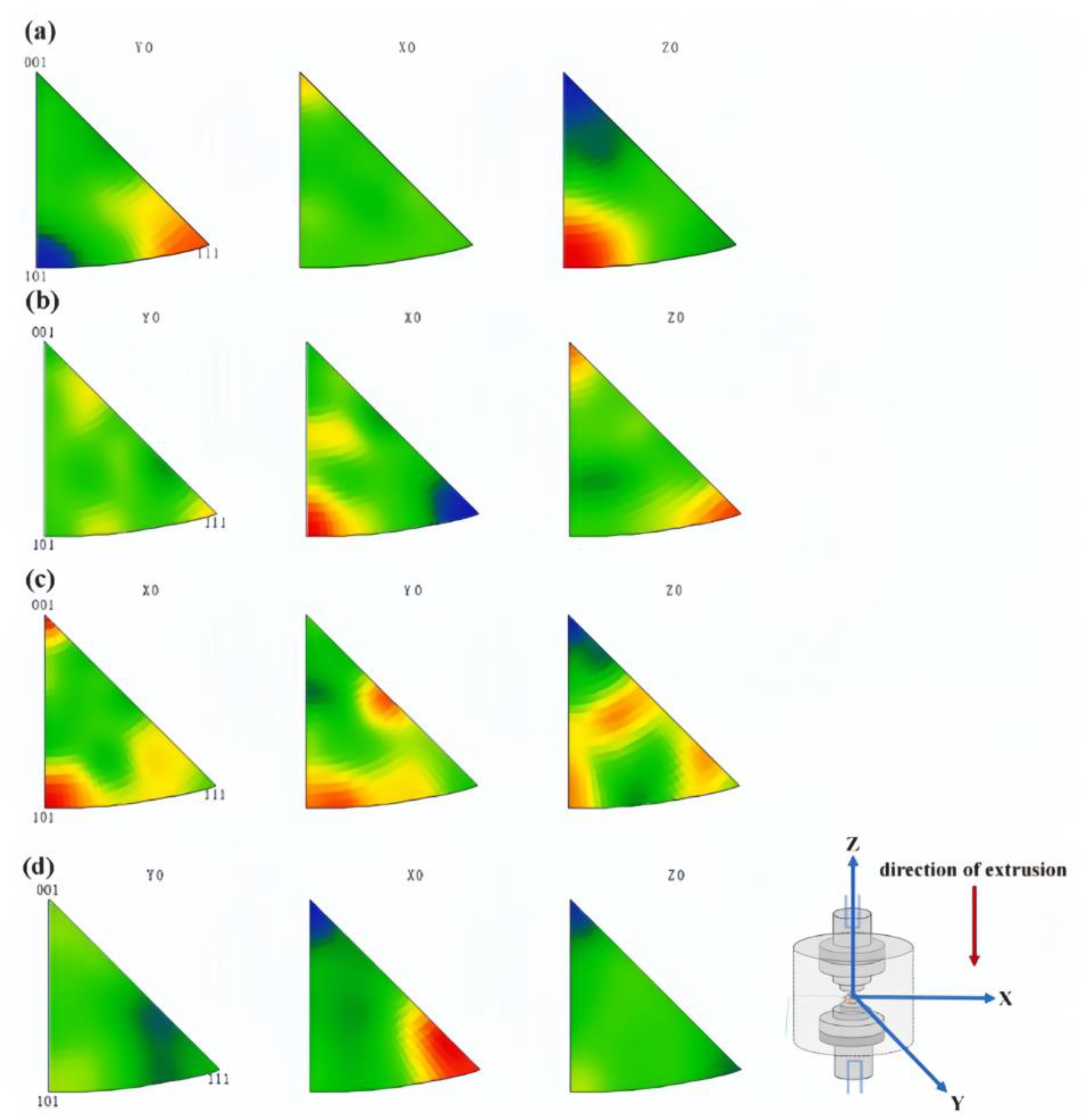
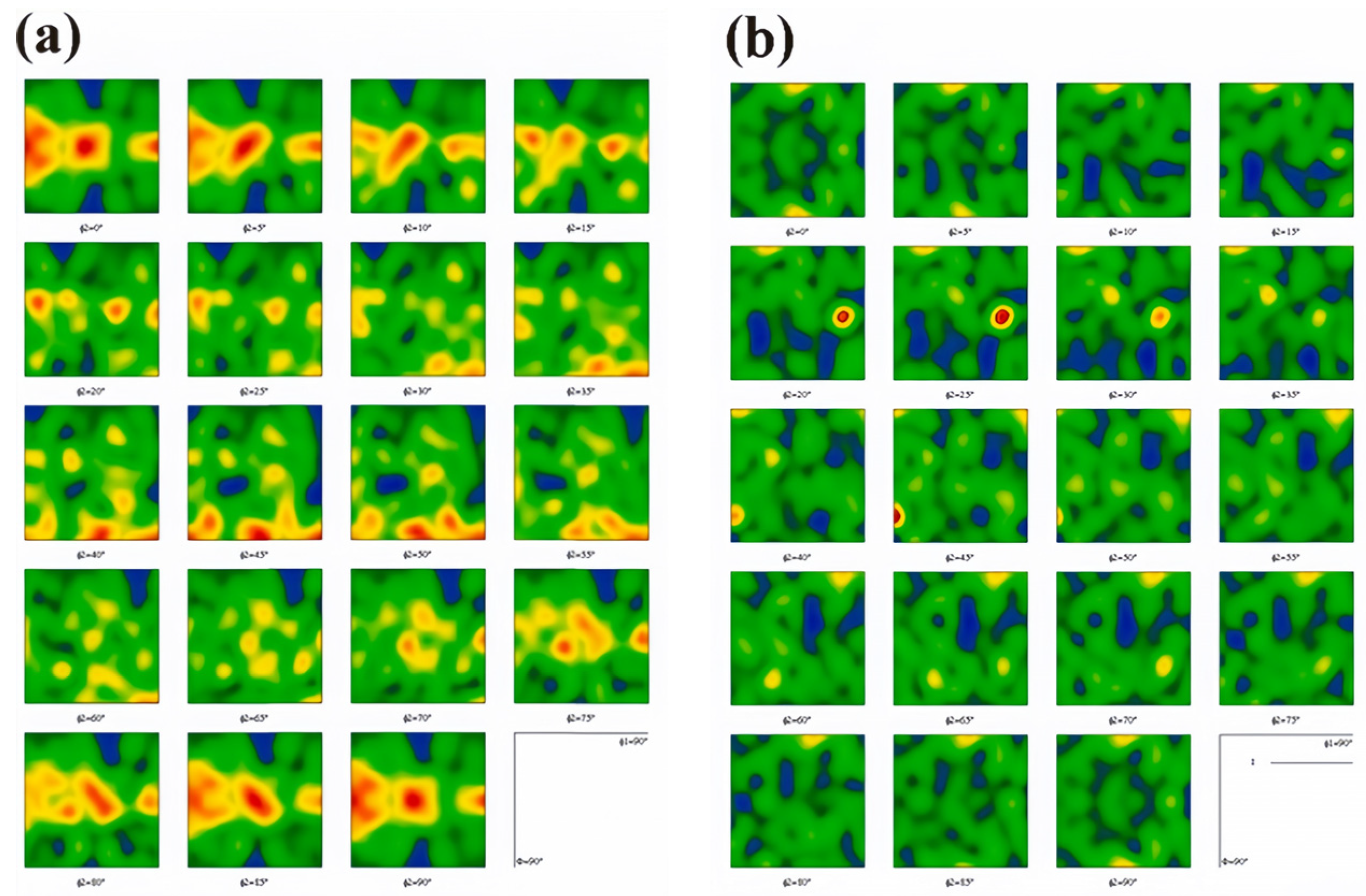
- Wang, B.; Zhang, Y.; Tian, B.; An, J.; Volinsky, A.A.; Sun, H.; Liu, Y.; Song, K. Effects of Ce addition on the Cu-Mg-Fe alloy hot deformation behavior. Vacuum 2018, 155, 594–603. [Google Scholar] [CrossRef]
- Chbihi, A.; Sauvage, X.; Blavette, D. Atomic scale investigation of Cr precipitation in copper. Acta Mater. 2012, 60, 4575–4585. [Google Scholar] [CrossRef]
- Xie, M.; Liu, J.; Lu, X.; Shi, A.; Den, Z.; Jang, H.; Zheng, F. Investigation on the Cu–Cr–RE alloys by rapid solidification. Mater. Sci. Eng. A 2001, 304–306, 529–533. [Google Scholar] [CrossRef]
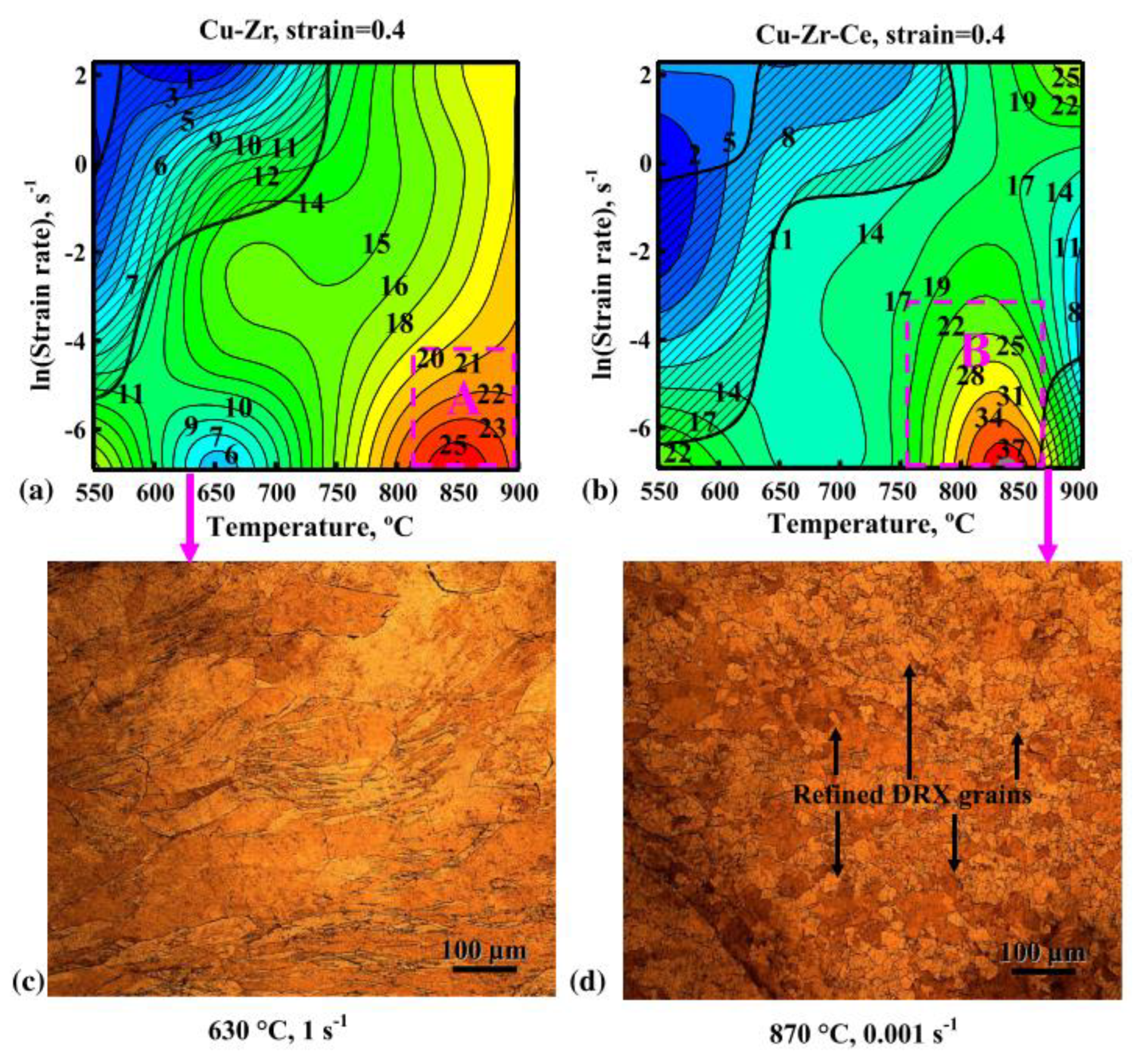
- Zhang, Y.; Sun, H.; Volinsky, A.A.; Wang, B.; Tian, B.; Chai, Z.; Liu, Y.; Song, K. Small Y Addition Effects on Hot Deformation Behavior of Copper-Matrix Alloys. Adv. Eng. Mater. 2017, 19, 1700197. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Tian, B.; Yakubov, V.; An, J.; Volinsky, A.A.; Liu, Y.; Song, K.; Li, L.; Fu, M. Effects of Ce and Y addition on microstructure evolution and precipitation of Cu-Mg alloy hot deformation. J. Alloys Compd. 2019, 781, 118–130. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; He, J.P.; Yao, K.F.; Wei, B.C.; Chen, G.L. Metallographic analysis of Cu–Zr–Al bulk amorphous alloys with yttrium addition. Scr. Mater. 2006, 54, 1351–1355. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Volinsky, A.A.; Wang, B.; Tian, B.; Liu, Y.; Song, K. Constitutive Model for Hot Deformation of the Cu-Zr-Ce Alloy. J. Mater. Eng. Perform. 2018, 27, 728–738. [Google Scholar] [CrossRef]
- Sakai, T.; Jonas, J.J. Overview no. 35 Dynamic recrystallization: Mechanical and microstructural considerations. Acta Metall. 1984, 32, 189–209. [Google Scholar]
- Rohatgi, A.; Vecchio, K.S.; Gray, G.T. The influence of stacking fault energy on the mechanical behavior of Cu and Cu-Al alloys: Deformation twinning, work hardening, and dynamic recovery. Metall. Mater. Trans. A 2001, 32, 135–145. [Google Scholar] [CrossRef]
- Li, J.; Wen, M.; Zhao, Z. Influence of rare earth element la on the performance of copper-based composites. Mater. Today Commun. 2024, 38, 107675. [Google Scholar] [CrossRef]
- Tan, L.K.; Li, Y.; Ng, S.C.; Lu, L. Effects of rare earth additions on structures and properties of rapidly solidified copper alloys. Mater. Sci. Technol. 1999, 15, 169–179. [Google Scholar] [CrossRef]
- Liu, X.B.; Liu, P.; Jia, S.; Tian, B.H. Effects of trace Ce and Cr on properties of Cu-0.1Ag alloy. Heat Treat. Met. 2005, 30, 238–241. [Google Scholar]
- Zhou, M.; Tang, S.; Zhang, Y.; Zheng, X.; Xu, D.; Zhang, Z.; Li, Y.; Fu, L.; Li, X.; Tian, B.; et al. Ce effects on dynamic recrystallization and microstructure of the copper alloy under hot deformation behavior with different strain rate. J. Mater. Res. Technol. 2023, 24, 4888–4903. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, W.; Huang, S.; Chen, H.; Wang, H.; Yang, Z.; Peng, L.; Yang, B. Effect of trace La on microstructure and properties of Cu–Cr–In alloys. Mater. Res. Express 2020, 7, 066506. [Google Scholar] [CrossRef]
- Liu, P.; Kang, B.X.; Cao, X.G.; Huang, J.L.; Gu, H.C. Strengthening mechanisms in a rapidly solidified and aged Cu-Cr alloy. J. Mater. Sci. 2000, 35, 1691–1694. [Google Scholar] [CrossRef]
- Song, J.T.; Fu, Y.D.; Song, K.X.; Zhu, Q.Q.; Peng, X.W.; Hua, Y.X.; Zhou, Y.J.; Guo, X.H.; An, S.Z.; Ma, Y.X. Effect of interaction between P and Ce on properties of copper. Mater. Sci. Technol. 2023, 39, 2966–2975. [Google Scholar] [CrossRef]
- Wu, J.H.; Zhang, S.H.; Chen, Y.; Li, H.H.; Song, H.W.; Cheng, M.; Liu, J.S. Microstructure and properties of TP2 copper tube with La microalloying by horizontal continuous casting. China Foundry 2018, 15, 31–36. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, P.; Ma, C. Microstructures and mechanical properties of Al-Cu-Mn alloy with La and Sm addition. Rare Met. 2012, 31, 332–335. [Google Scholar] [CrossRef]
- Zhou, S.J.; Zhao, B.J.; Zhao, Z.; Jin, X. Application of Lanthanum in High Strength and High Conductivity Copper Alloys. J. Rare Earths 2006, 24, 385–388. [Google Scholar]
- Wang, S.W.; Yu, K.K.; Chen, S.F.; Deng, S.Y.; Song, H.W.; Zhang, S.H. Modificated microstructure and enhanced mechanical properties of Cu-Sn-P alloy with rare earth La addition. Mater. Today Commun. 2024, 39, 109304. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).