Abstract
Nano Cu precipitation plays a crucial role in significantly improving the performance of the Cu-bearing high-strength low-alloy steel. The final cooling temperature effects the transformation products of austenite during the continuous cooling process, as well as the nano precipitations of steel. This study investigated the microstructure and hardness at different final cooling temperatures (750, 700, 650, 600, 550, and 500 °C) using the MMS-300 thermal simulation experimental machine (Northeastern University, Shenyang, China) and Vickers hardness tester. The changes in microstructure and the phase transformation law of austenite were determined during continuous cooling and then analyzed. The precipitation reaction of nano Cu precipitation during continuous cooling was studied using transmission electron microscopy (TEM), revealing the precipitation state under different final cooling temperature conditions. The results showed that the precipitations led to an increase and then a decrease in the microhardness, and the microhardness reaches its peak at 550 °C. The precipitations changed from spherical to elliptical, and the size gradually increased when the final cooling temperature increased.
1. Introduction
Cu-bearing high-strength low-alloy steel has excellent comprehensive mechanical properties of high yield strength, excellent weldability, and good toughness, which meets the requirements of components in many fields such as bridges, pipelines, and ship hulls [1,2,3,4]. The main purpose of various processing techniques is to achieve excellent strength and toughness. In recent years, in order to improve the weldability and toughness, the carbon content has been kept below 0.08 wt% in Cu-bearing HSLA steels [5]. Adding 1–2 wt% Cu does not reduce weldability and can also form nanoscale Cu precipitates to enhance the matrix. The heating rate, peak temperature, and cooling rate have a significant impact on the balance between strength and toughness [6,7,8]. With the extension of austenitizing temperature and holding time, the grain size of austenite shows an increasing trend. The final cooling temperature has a certain impact on the transformation temperature from austenite to ferrite during continuous cooling, and the cooling rate also has a certain influence on the transformation temperature from austenite to ferrite [9,10]. Therefore, it will result in the refinement of transformation products such as ferrite, bainite, or martensite, as well as martensite/austenite phases, significantly enhancing the strength and toughness of the steel [11,12]. Due to its lower carbon content and incorporation of a certain proportion of Cu, high-strength low-alloy steel containing copper exhibits significant differences in phase transformation microstructure and grain size compared to traditional low-carbon steel [13,14,15,16]. These differences directly determine the mechanical properties of the steel. Therefore, it is particularly important to explore the phase transformation mechanism of ultra-low carbon Cu containing ship structural steel in depth.
In this context, the expansion method is considered an ideal approach for studying the solid-state phase transformation behavior in steel, as it can track, in real time, the changes in sample size or the progress of phase transformation during the heat treatment process. As a traditional experimental method, the expansion method is usually combined with quantitative analysis of microstructure and differential thermal analysis to determine various transformation temperatures in steel [17,18,19]. Based on this, the design of thermomechanical treatment, including treatment under continuous cooling and isothermal conditions, requires a solid theoretical knowledge foundation.
As an element that can stabilize austenite, copper can affect the kinetic mechanism of phase transformation during the transformation from austenite to ferrite, thereby affecting the final formed phase transformation products. The results have shown that the addition of Cu affects the phase transformation process, microstructure, and transformation temperature in Fe Cu alloys. With the decrease in isothermal temperature or the increase in cooling rate, austenite gradually transforms into polygonal ferrite, massive ferrite, bainite, flat noodles martensite, and twin martensite [20]. When multiple alloying elements such as Cu were added, the phase transformation mechanism of composite steel became more complex, and the different phase transformation products of austenite also affected the precipitation kinetics of Cu.
The precipitation of nano rich Cu phase plays a crucial role in high-strength low-alloy steel containing copper, which can significantly improve the performance of the steel [21,22]. Numerous studies have shown that nano rich Cu phases can significantly enhance the strength of steel, and the degree of precipitation enhancement mainly depends on the type, structure, number density, scale, and distribution of precipitation phases. In high-strength low-alloy steel containing copper, the precipitation of Cu was mainly achieved through the aging tempering process [23,24]. However, during the cooling process of medium thick plates, there was a difference in the cooling rate between the surface and core of the steel plate, resulting in inconsistent performance between the surface and core of the steel plate. By promoting the precipitation of Cu rich phase at a slower cooling rate in the center of the steel plate, the unevenness of performance in the thickness direction of the steel plate can be alleviated, providing a new idea for the development of thick specification steel [25]. In summary, the study of the precipitation and precipitation behavior of Cu rich phases during continuous cooling can provide a key theoretical basis for the manufacture of low-cost and high-performance Cu containing steels.
In this article, the microstructure changes of the steel during continuous cooling were analyzed in depth using MMS-300 thermal simulation equipment, transmission electron microscopy (FEI Company, Hillsboro, OR, USA), and Vickers hardness tester, and the regularity of austenite phase transformation during continuous cooling was determined. This study further investigated the precipitation reaction of nanoscale Cu rich phases during continuous cooling, revealing the precipitation state of Cu and its regular influence on the microhardness changes of materials under different final cooling temperature conditions.
2. Materials and Methods
2.1. Materials
In the current study, a Cu-Bearing high-strength low-alloy steel was produced using a vacuum induction furnace (Northeastern University, Shenyang, China) under an argon atmosphere and then cast into an ingot weighing 25 kg. The composition of the experimental steel detected by the chemical analysis method is shown in Table 1. The ingots were forged into billets with a size of 80 mm × 80 mm × 120 mm. The ingots were homogenized at 1473 K for 2 h and then forged into a thickness of 80 mm. After eliminating the surface defects, the ingots were hot-rolled into plates with a thickness of 10 mm (with an initial rolling temperature of 1423 K and finishing temperature greater than 1073 K, and then water-quenched). The reduction schedule was 80 → 54 → 36 → 24 → 17 → 12 → 10 (mm).

Table 1.
Actual chemical composition of experimental steel (mass fraction) %.
2.2. Experimental Process
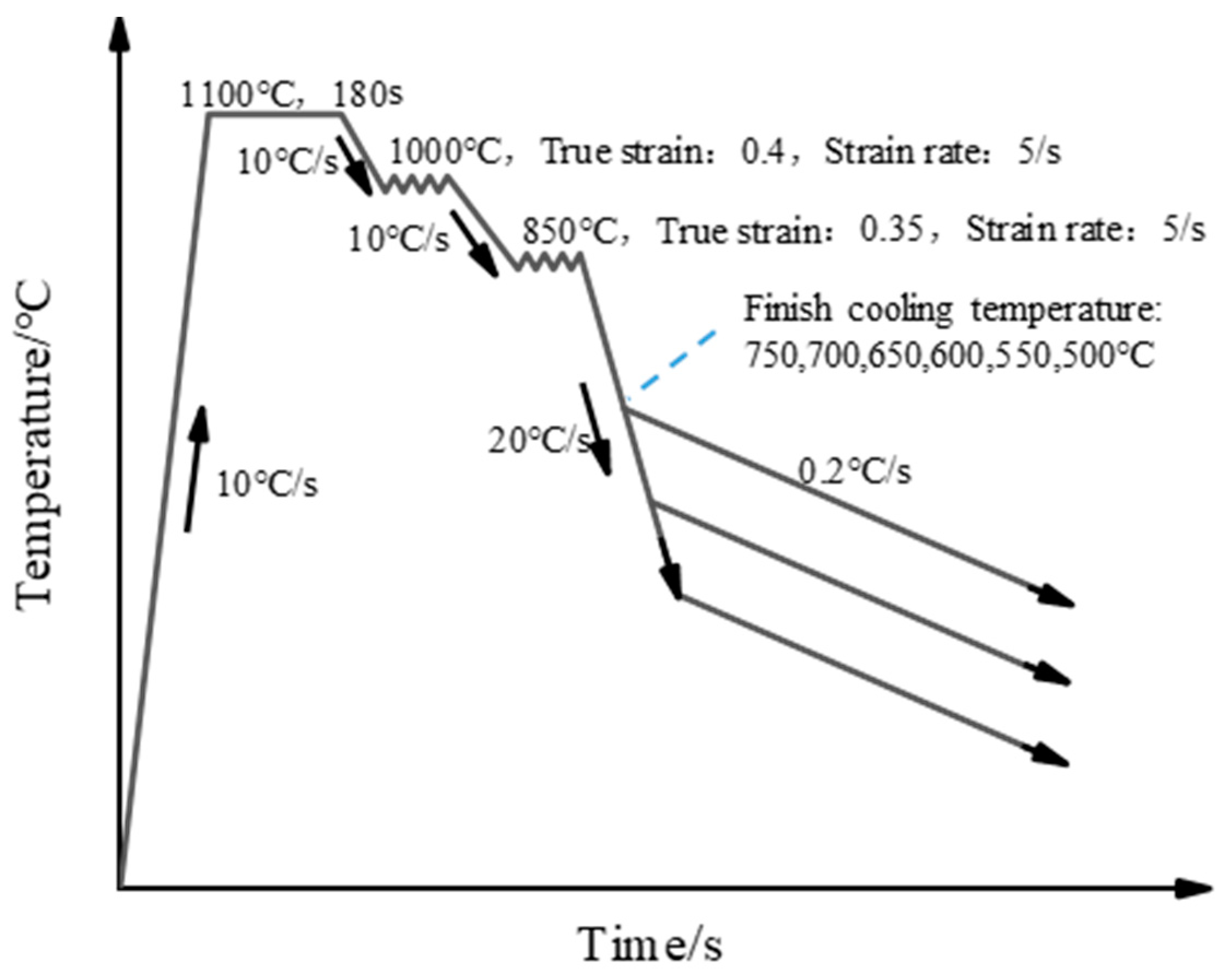
The thermal simulation experiment was conducted in the MMS-300 model dynamic thermo-mechanical simulator (Northeastern University, Shenyang, China). The thermal simulation experiments were cut from the slabs along the rolling direction, and then machined into cylindrical dimensions of ϕ 8 mm × 15 mm. Through the computation of chemical composition and experimental determination of the test steel, the temperatures Ac1 and Ac3 were ascertained to be 769 °C and 834 °C, respectively. A schematic illustration is shown in Figure 1. Specimens were homogenization-treated by a direct resistance heating system at 1100 °C for 180 s, with a heating rate of 10 °C/s, followed by a two-stage deformation. The true strain was 0.4 in the first stage at 1000 °C and 0.35 in the second stage at 850 °C, with a same strain rate of 5 s−1. The compressed samples were rapidly cooled at a rate of 20 °C/s, with final cooling temperatures of 750, 700, 650, 600, 550, and 500 °C, respectively. Finally, the samples were slowly cooled to room temperature at a rate of 0.2 °C/s.
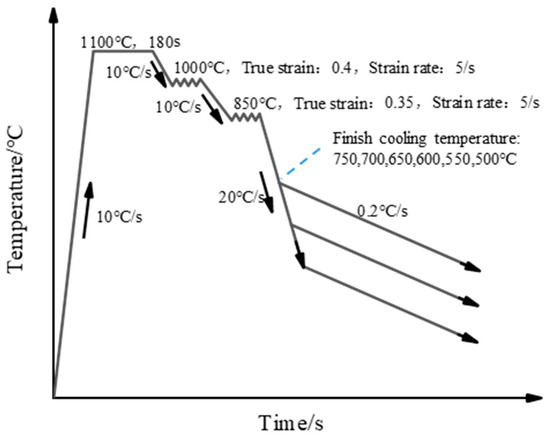
Figure 1.
The schematic illustration of Cu precipitation behavior with different finish cooling temperatures.
2.3. Characterization
Microstructure observations were made by OM (Leica Microsystems GmbH, Wetzlar, Germany), TEM and STEM (FEI Company, Hillsboro, OR, USA). Samples for OM (LEICA DMIRM, Leica Microsystems GmbH, Wetzlar, Germany) were prepared by traditional methods including mechanical polishing and etching in 6% Nital solution. TEM samples were prepared by cutting slices from the samples followed by mechanical grinding to 50 μm using SiC papers. Then, a twin-jet electropolishing was carried out using a mixture of 87.5% absolute ethyl alcohol and 12.5% perchloric acid at −30 °C and potential of 30 V. The thin foils were examined by a FEG-TEM (FEI Tecnai G2 F20, FEI Company, Hillsboro, OR, USA).
3. Results and Disscussion
3.1. The Effect of Final Cooling Temperature on Microstructure
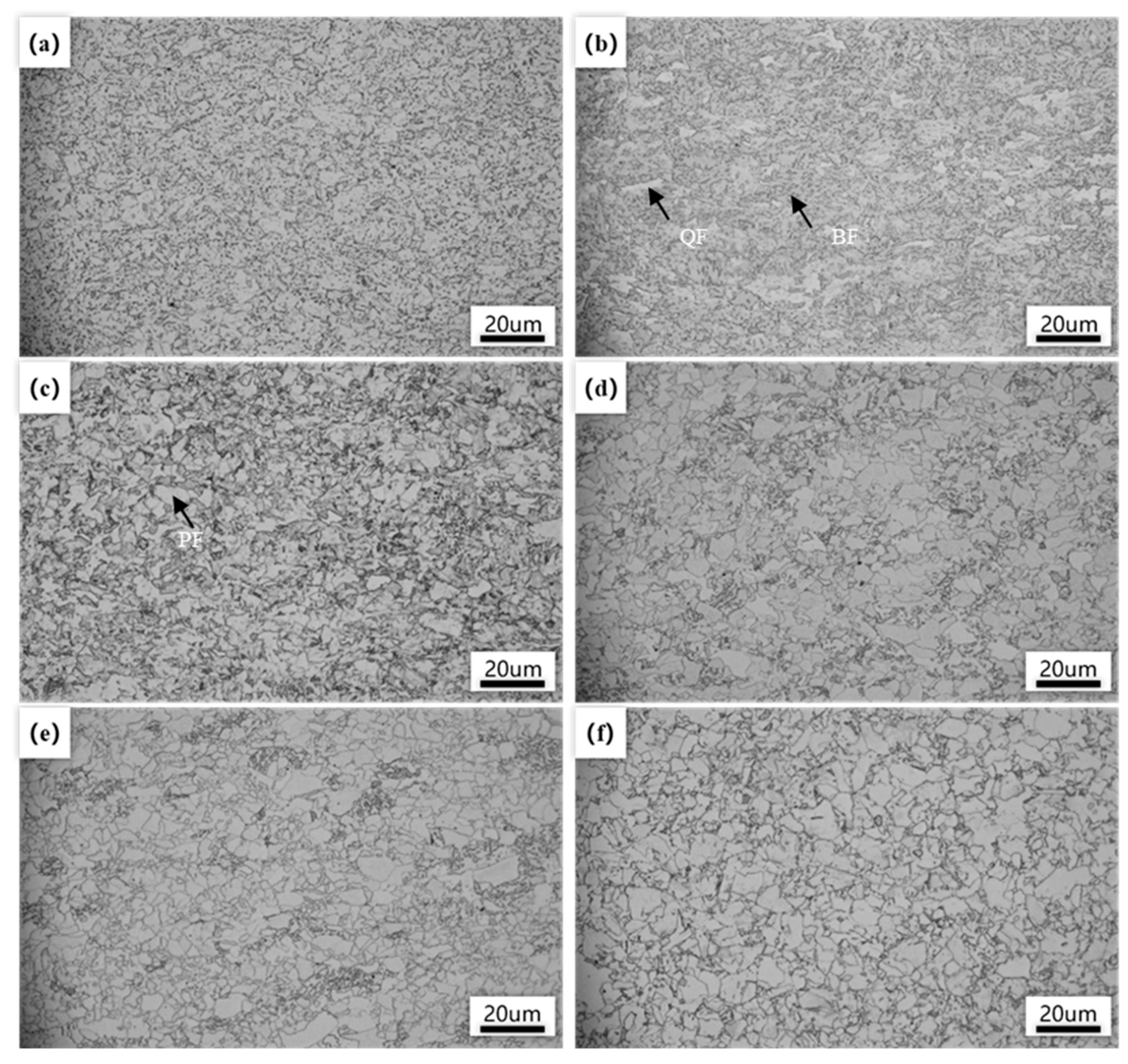
Figure 2 shows the optical microstructure of the experimental steel under different final cooling temperature conditions. It can be seen that, as the final cooling temperature increased, the microstructure of the steel underwent significant changes. In the lower final cooling temperature range (500–600 °C), the experimental steel exhibited a mixed microstructure of polygonal ferrite (PF), quasi-polygonal ferrite (QF), and bainitic ferrite (BF) (Figure 2a–c). At this stage, the diffusion and migration ability of carbon atoms under low temperature conditions were insufficient to form polygonal ferrite, while the generation of bainitic ferrite relied less on atomic migration and more on the non-diffusive transformation of austenite. As the final cooling temperature increased to 550 °C, the diffusion ability of alloy atoms and carbon atoms improved, leading to the transformation of some austenite into quasi-polygonal ferrite through diffusion.
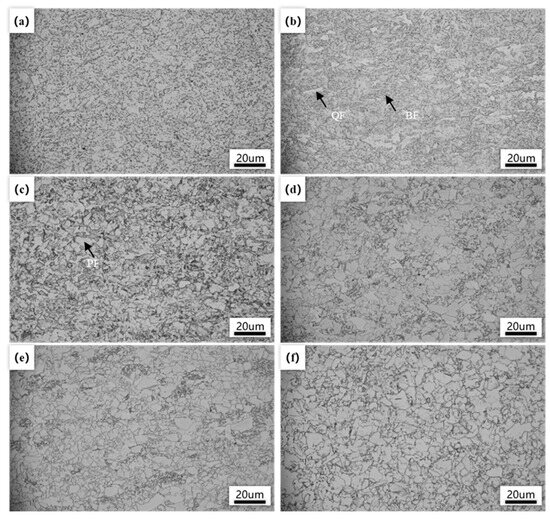
Figure 2.
Optical microstructure of the experimented steel with different finish cooling temperatures. (a) 500 °C; (b) 550 °C; (c) 600 °C; (d) 650 °C; (e) 700 °C; (f) 750 °C.
When further increasing to 600 °C, a significant decrease in the amount of bainite ferrite was observed, and the main structure was composed of quasi-polygonal ferrite, indicating that higher temperatures promote the formation of quasi-polygonal ferrite. This quasi-polygonal ferrite had irregular morphology, varying sizes, and unclear boundaries, indicating that the phase transformation process was more complex under these temperature conditions, involving different degrees of decomposition of austenite. When the final cooling temperature reached 650 °C, the structure of the experimental steel completely transformed into a ferritic structure. At this point, polygonal ferrite nucleated near the original austenite grain boundaries and grew during the subsequent slow cooling process, indicating that the phase transformation mechanism had shifted to diffusion controlled. This change was attributed to the enhanced diffusion ability of the alloy and carbon atoms at higher temperatures, which promoted the uniform growth of ferrite. At 650–750 °C, it was observed that the ferrite grains increased in size with increasing temperature (Figure 2d–f). The elevation in temperature increased the diffusion rate of alloy atoms and carbon atoms. At high temperatures, the diffusion of iron and carbon atoms was accelerated at the interface between austenite and ferrite, and ferrite grains nucleated and expanded rapidly near austenite grain boundaries. As the temperature increased, the expansion rate of ferrite grains was also accelerated. The ferrite grains were capable of rapidly assimilating iron and carbon atoms from austenite, a phenomenon that facilitated their continued growth. When the final cooling temperatures were relatively high, the formation of ferrite was more predominantly governed by diffusion mechanisms. This diffusion-driven process enabled ferrite grains to develop into larger-volume configurations.
3.2. Analysis of Quantity Density of Copper Precipitation
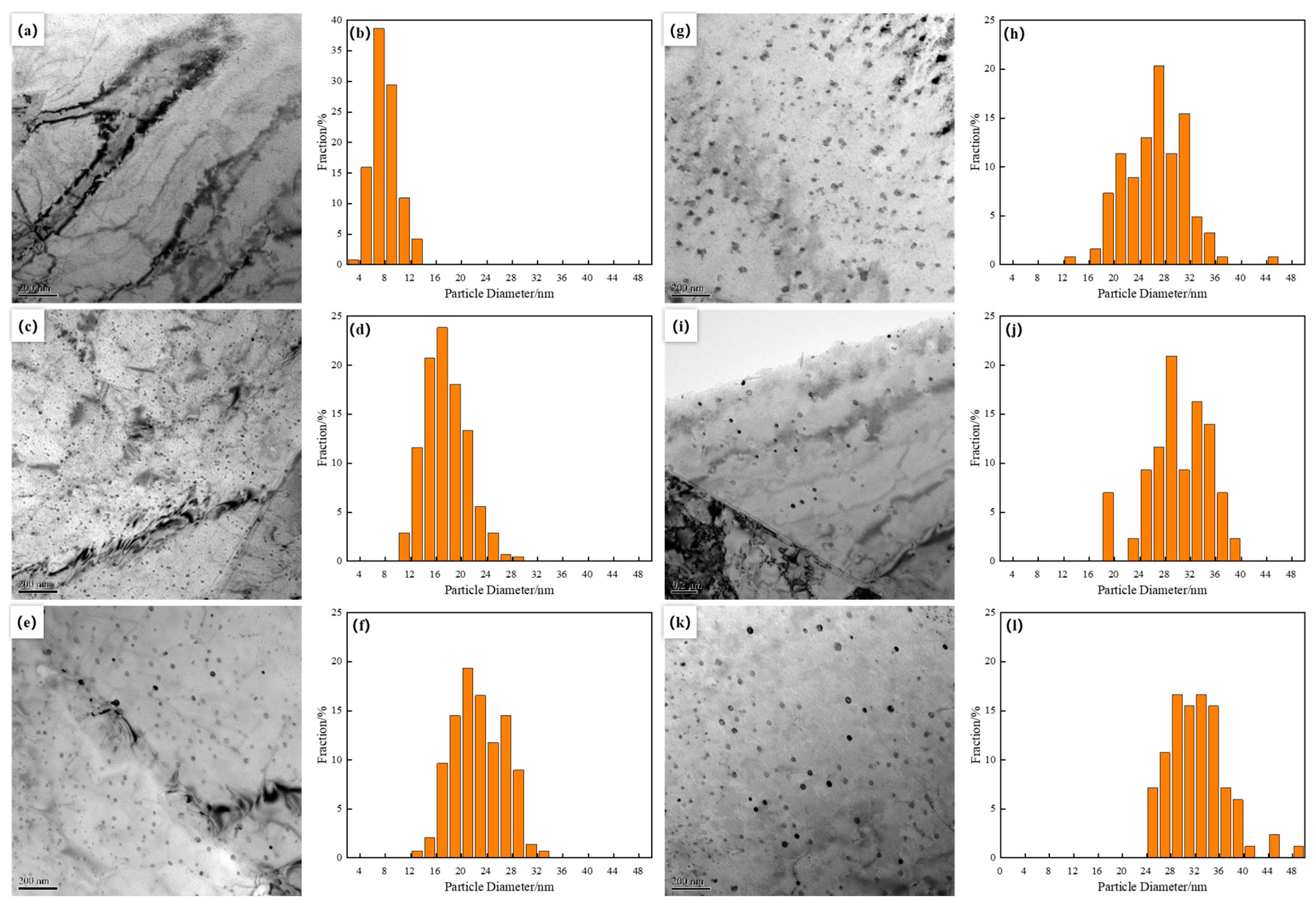
Figure 3 shows the transmission electron microscopy (TEM) morphology of precipitated particles at different final cooling temperatures. The final cooling temperature had a decisive impact on the evolution of the internal microstructure of materials. From the figure, it can be seen that the nano Cu precipitation dispersed and distributed in the ferrite matrix, presenting a spherical morphology with a diameter of less than 10 nm at the final cooling temperature of 500 °C (Figure 3a,b). The statistical average diameter of the particle size was 7.9 nm. At lower final cooling temperatures, the nucleation rate of the precipitated phase was higher, but the growth rate was limited, resulting in the formation of smaller particles. As the final cooling temperature increased to 550 °C, the precipitated particles still remained spherical, but the main distribution range of particle size expanded to 14–18 nm, and the average size increased to 17.5 nm (Figure 3c,d). When the final cooling temperature increased to 600 °C, some of the precipitated particles changed from spherical to ellipsoidal, while most of the particles remained spherical (Figure 3e). The particle size was mainly concentrated in 20–24 nm, with an average particle size of 22.7 nm (Figure 3f). This phenomenon indicated that, as the final cooling temperature further increased, the growth kinetics of the precipitated phase changed, resulting in a transformation of some particle morphology and an increase in size.
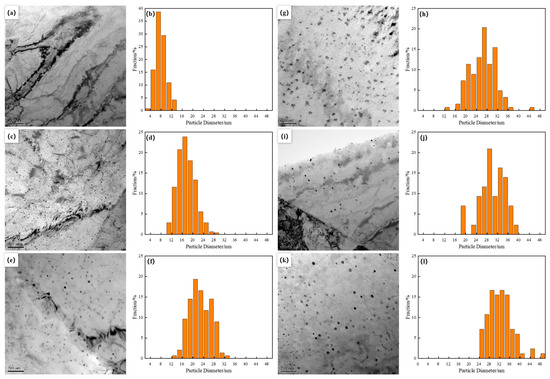
Figure 3.
TEM images and histograms showing size distribution of Cu particles formed by different finish cooling temperatures (a,b) 500 °C; (c,d) 550 °C; (e,f) 600 °C; (g,h) 650 °C; (i,j) 700 °C; (k,l) 750 °C.
When the final cooling temperature further increased to 650 °C, the proportion of ellipsoidal particles increased, and the particle size was mainly distributed between 26–32 nm, with an average size of 26.4 nm (Figure 3g,h). The changes in this stage further confirmed the trend of particle growth and coarsening at high temperatures, as well as the expansion of particle size distribution range caused by inter-particle interactions. When the final cooling temperature increased to 700–750 °C, the majority of precipitated particles were ellipsoidal, and the particle size distribution range and average size did not change much within this temperature range (Figure 3i–l). The main particle size distribution range was 28–36 nm, with average particle sizes of 30 nm and 32.4 nm, respectively. Under these conditions, the particles that precipitated first continuously coarsen and grow by swallowing smaller particles around them, thereby further reducing the precipitation density.
When the final cooling temperature was low, the formation of nano Cu precipitation mainly occurred through solid-state decomposition within the ferrite matrix. At this point, the nucleation rate was relatively high, because the lower temperature slowed down the diffusion rate of atoms, limited the growth of particles after nucleation, and resulted in smaller precipitate sizes. When the final cooling temperature was 500 °C, the size and number of the Cu rich phase were small, and the strengthening effect was weak. At 550–650 °C, the higher temperature increased the diffusion coefficient of atoms, thereby promoting the growth process of precipitated phases. The high temperature provided more thermal energy, making the migration of Cu atoms in the ferrite matrix more active, resulting in the nucleated precipitate phase being able to absorb more Cu rich atoms and grow larger.
As the temperature increased, the morphology of precipitated particles changed from spherical to ellipsoidal, which could be due to differences in diffusion rates in different crystal directions, leading to faster growth of particles in certain directions, and the formation of ellipsoidal shapes. This phenomenon could be explained by the Ostwald ripening theory, which states that, as the system tends to reduce overall energy, smaller particles gradually dissolve while larger particles continue to grow. The effect of this phenomenon was more pronounced under high temperature conditions, as the high diffusion rate at high temperatures accelerates the material transferred from small particles to large particles. When the final cooling temperature raised to 700–750 °C, the Cu rich phase nucleated at higher temperatures and underwent significant growth and coarsening during the subsequent slow cooling process, resulting in a significant decrease in the number of precipitated particles. Due to the dissolution of small particles and the growth of large particles under high temperature conditions. In addition, the interaction between particles at high temperatures, such as aggregation or coalescence, could also reduce the number of observable particles.
3.3. The Effect of Final Cooling Temperature on Hardness
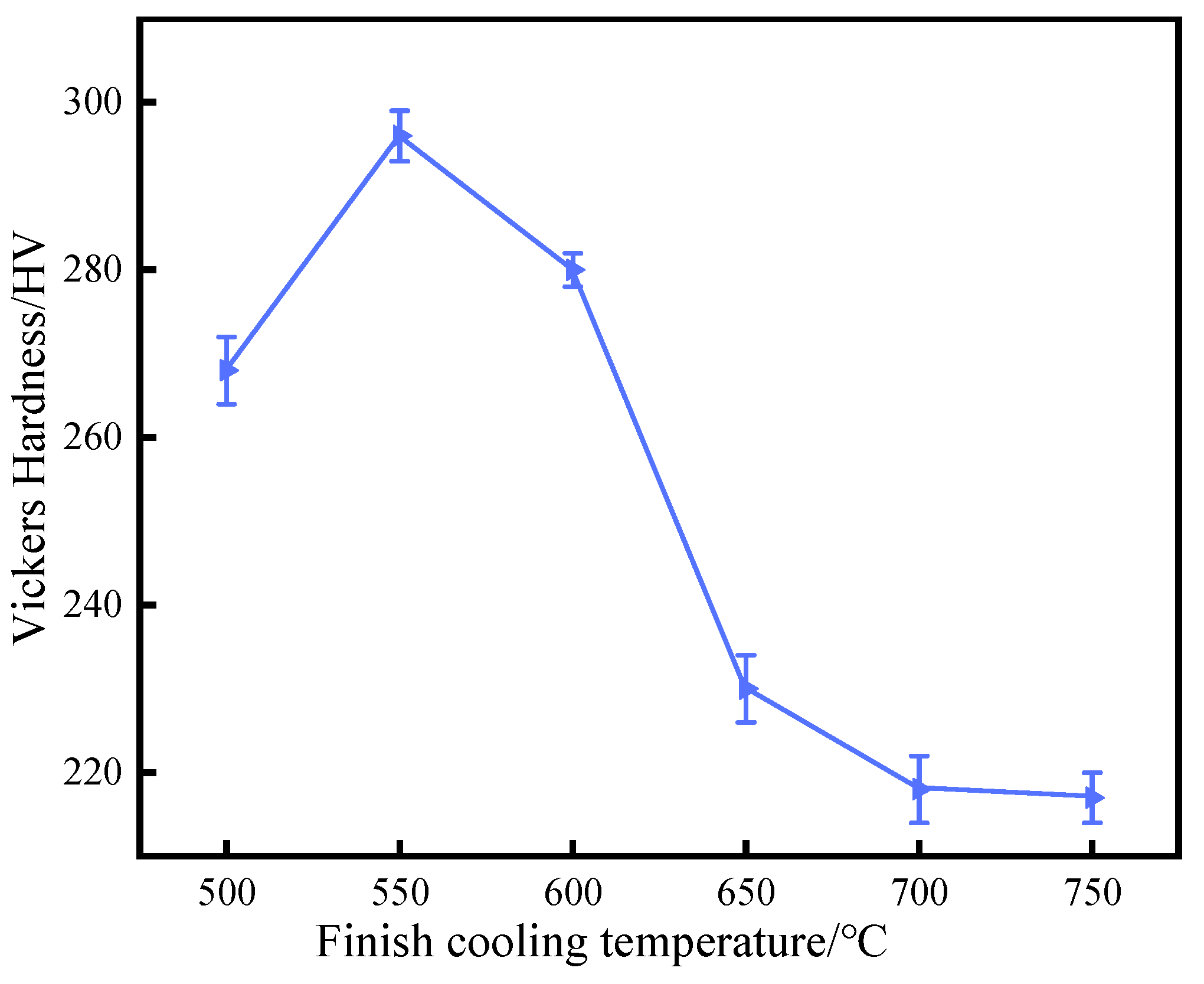
Figure 4 shows the variation trends of the average microhardness of experimental steel at different final cooling temperatures. These show that the final cooling temperature had a significant impact on the microhardness of experimental steel, manifested as an increasing trend followed by a decreasing trend in microhardness as the final cooling temperature increases. When the final cooling temperature was 550 °C, the microhardness reached its peak, and the Vickers hardness of the ferrite was 296 HV. In the final cooling temperature range of 500–600 °C, the main microstructure of the experimental steel was bainite ferrite, which was rapidly cooled at lower temperatures and usually contains small ferrite carbides.
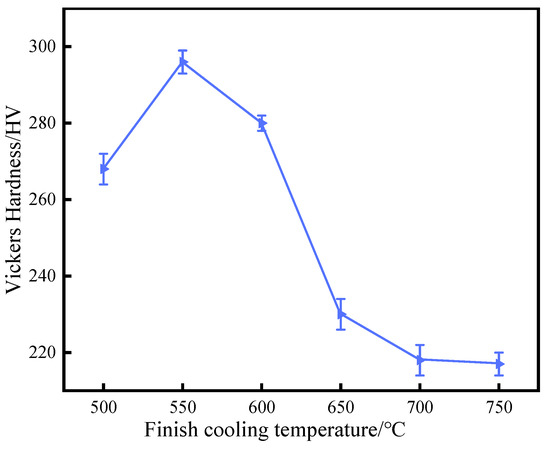
Figure 4.
Variation in Vickers hardness with finish cooling temperature for steel.
The fine structure of bainite ferrite increased the interface area, thereby improving hardness and strength. In addition, the precipitation strengthening of Cu was also a key factor in the improvement in hardness. The nano Cu precipitation can effectively hinder the activity of dislocations and enhance the hardness of the material.
At 650 °C, bainitic ferrite and quasi-polygonal ferrite completely transformed into polygonal ferrite. This transformation led to grain coarsening and redistribution of carbides, resulting in a decrease in hardness. When compared with bainitic ferrite, polygonal ferrite contains a smaller proportion of interfaces and larger-sized grains, thus weakening the strengthening effect brought about by the grain boundaries. As the final cooling temperature increased from 650 °C to 750 °C, the ferrite grains further increased and the nano Cu precipitation coarsed, resulting in a weakening of the effects of fine grain strengthening and precipitation strengthening. The expansion of grain size led to a decrease in the number of grain boundaries per unit volume, weakening the hindering effect of grain boundaries on dislocation movement. At the same time, the coarsening of nano rich Cu phase reduces its ability to hinder dislocation movement, further reducing the microhardness.
On the hardness curve, it shows that, as the final cooling temperature increased, the Vickers hardness of ferrite grains gradually decreased. In summary, in order to obtain sufficient precipitation strengthening, it was necessary to adopt an appropriate final cooling temperature to ensure the formation of a large number of fine dispersed Cu rich phases.
3.4. TEM Analysis of Nano Copper Precipitation
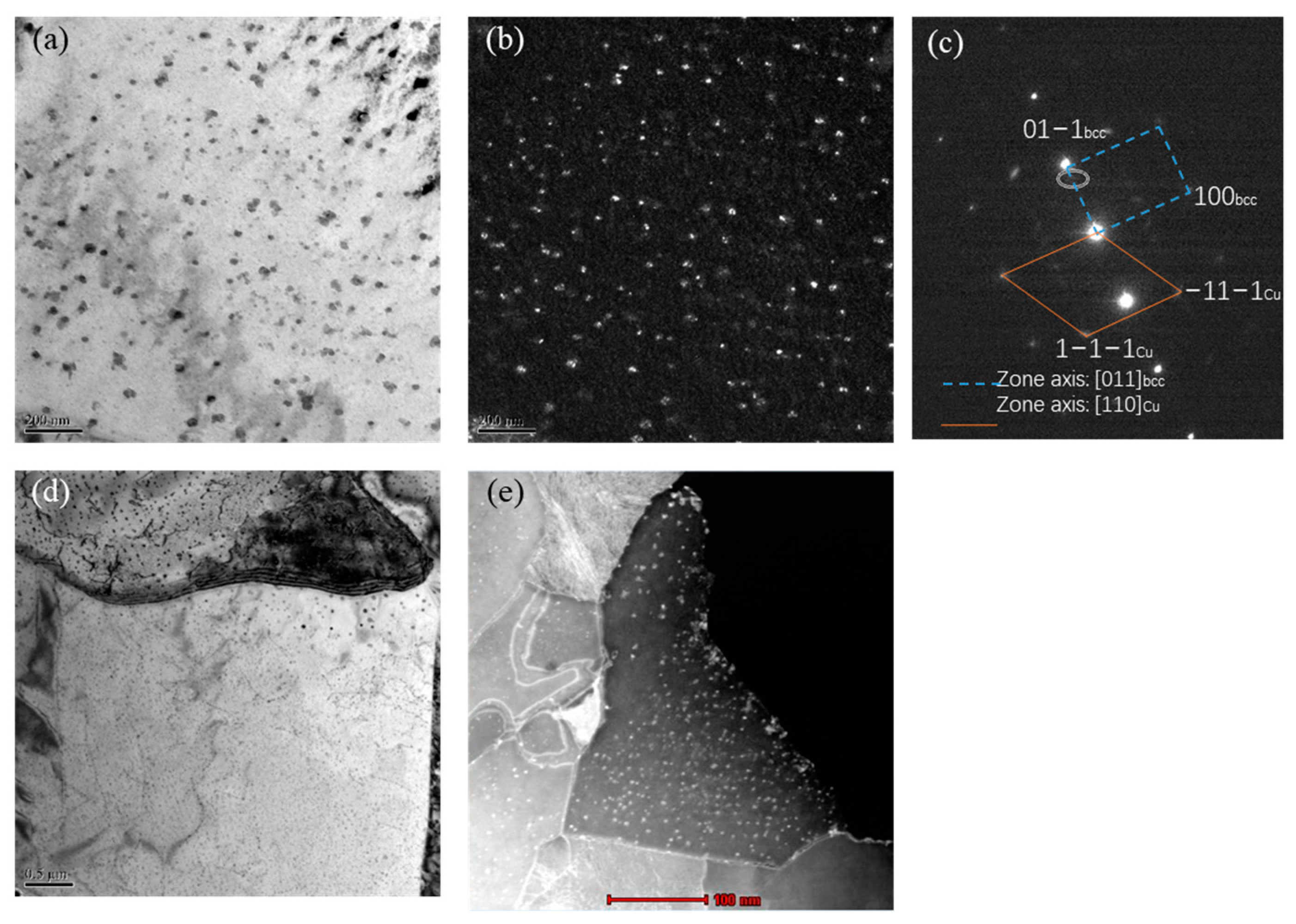
Figure 5 shows the TEM image of the nano Cu precipitation formed at a cooling temperature of 650 °C. Figure 5a,b show the corresponding bright field and dark field images of copper precipitation. Figure 5c shows the selected electron diffraction spot for the precipitation of nano Cu. Through calibration, it can be indexed that the axis of the matrix was [011]bcc. And the precipitation phase was fcc-Cu precipitation with [110]Cu axes. It can be seen that the precipitation of nano Cu presents a clear form of interphase precipitation. The precipitated phases were arranged in the matrix in a certain spacing order. Interphase precipitation had been reported by many scholars, and its formation was related to the cooling rate, as cited in references such as Hungwei Yen’s and Fengqin Ji’s [21,22]. Figure 5d is a TEM image at a low magnification, showing clear interphase precipitation arrangement of nano Cu precipitates. The nano Cu precipitation near the crystal showed a significant growth trend with a dispersed distribution, which was due to the different movement speeds of the interface during the cooling process, resulting in different precipitation sizes and positions. When a large amount of phase precipitation exists, the mechanical properties of experimental steel can be significantly improved. Figure 5e shows the dark field image of nano Cu precipitation in the scanning transmission mode. It can be seen that the interphase precipitation exists in the grains of the matrix, to a certain extent, parallel to the grain boundaries, with uniform size.
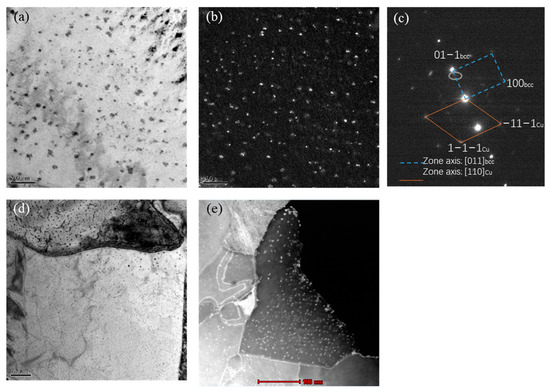
Figure 5.
TEM and STEM images of nano-Cu particles formed at 650 °C cooling temperatures. (a) BF (b) DF (c) SADP (d) TEM (e) STEM.
4. Conclusions
This article investigates the effect of final cooling temperature on the precipitation of nano Cu, and clarifies the differences in the size, quantity, and hardness of copper precipitation at different temperatures. The conclusions drawn are as follows:
- (1)
- As the final cooling temperature increases, the microstructure of the experimental steel gradually transitions from a mixed structure of ferrite and bainite to a fully ferritic structure.
- (2)
- The continuous nucleation and coarsening process of nano Cu precipitation led to an increase and then a decrease in the microhardness of ferrite. When the final cooling temperature was 550 °C, the microhardness reached its peak, and the Vickers hardness of the ferrite was 296 HV.
- (3)
- As the final cooling temperature increased, the shape of the precipitated particles changed from spherical to elliptical, and the particle size gradually increased. At 650 °C, there was a significant amount of interphase precipitation in the Cu-bearing high-strength low-alloy steel
Author Contributions
Conceptualization, H.C. and H.L.; methodology, H.L. and J.L.; investigation, H.C.; resources, J.L.; data curation, H.C. and Z.G.; writing—original draft preparation, H.C. and X.S.; writing—review and editing, H.C. and Y.L.; visualization, Z.G. and Y.L.; supervision, J.L.; project administration, J.L. and X.S.; funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Foundation of Education Bureau of Liaoning Province, China, grant number JYTQN2023056.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors also acknowledge Yu Dong and Na Xiao in the Analytical and Testing Center of Northeastern University for the assistance with data analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ray, P.K.; Ganguly, R.I.; Panda, A.K. Optimization of mechanical properties of an HSLA-100 steel through control of heat treatment variables. Mater. Sci. Eng. A 2003, 346, 122–131. [Google Scholar] [CrossRef]
- Misra, R.D.K.; Jia, Z.; O’Malley, R.; Jansto, S.J. Precipitation behavior during thin slab thermomechanical processing and isothermal aging of copper-bearing niobium-microalloyed high strength structural steels: The effect on mechanical properties. Mater. Sci. Eng. A 2011, 528, 8772–8780. [Google Scholar] [CrossRef]
- Timokhina, I.B.; Hodgson, P.D.; Ringer, S.P.; Zheng, R.K.; Pereloma, E.V. Precipitate characterisation of an advanced high-strength low-alloy (HSLA) steel using atom probe tomography. Scr. Mater. 2007, 56, 601–604. [Google Scholar] [CrossRef]
- Funakawa, Y.; Shiozaki, T.; Tomita, K.; Yamamoto, T.; Maeda, E. Development of high strength hot-rolled sheet steel consisting of ferrite and nanometer-sized carbides. ISIJ Int. 2004, 44, 1945–1951. [Google Scholar] [CrossRef]
- Sun, M.; Xu, Y.; Wang, J. Effect of Aging Time on Microstructure and Mechanical Properties in a Cu-Bearing Marine Engineering Steel. Materials 2020, 13, 3638. [Google Scholar] [CrossRef]
- Yang, X.; Di, X.; Liu, X.; Wang, D.; Li, C.J.M.C. Effects of heat input on microstructure and fracture toughness of simulated coarse-grained heat affected zone for HSLA steels. Mater. Char. 2019, 155, 109818. [Google Scholar] [CrossRef]
- Okamoto, R.; Borgenstam, A.; Ågren, J. Interphase precipitation in niobium-microalloyed steels. Acta Mater. 2010, 58, 4783–4790. [Google Scholar] [CrossRef]
- Tomida, T.; Imai, N.; Yoshida, M.; Fukushima, S. Effect of ultra-fast cooling after rolling in stable austenite region on grain refinement of C-Mn steel. Mater. Sci. Forum 2007, 539–543, 4708–4713. [Google Scholar] [CrossRef]
- Kamikawa, N.; Sato, K.; Miyamoto, G.; Murayama, M.; Sekido, N.; Tsuzaki, K.; Furuhara, T. Stress–strain behavior of ferrite and bainite with nano-precipitation in low carbon steels. Acta. Mater 2015, 83, 383–396. [Google Scholar] [CrossRef]
- Charleux, M.; Poole, W.; Militzer, M.; Deschamps, A. Precipitation behavior and its effect on strengthening of an HSLA-Nb/Ti steel. Metall. Mater. Trans. A 2001, 32A, 1635–1647. [Google Scholar] [CrossRef]
- Xu, C.; Dai, W.J.; Chen, Y.; Qi, Z.X.; Zheng, G.; Cao, Y.D.; Zhang, J.P.; Bu, C.C.; Chen, G. Control of dislocation density maximizing precipitation strengthening effect. J. Mater. Sci. Technol. 2022, 127, 133–143. [Google Scholar] [CrossRef]
- Mulholland, M.D.; Seidman, D.N. Nanoscale co-precipitation and mechanical properties of ahigh-strength low-carbon steel. Acta Mater. 2011, 59, 1881–1897. [Google Scholar] [CrossRef]
- Han, G.; Xie, Z.J.; Li, Z.Y.; Lei, B.; Shang, C.J.; Misra, R.D.K. Evolution of crystal structure of Cu precipitates in a low carbon steel. Mater. Des. 2017, 135, 92–101. [Google Scholar] [CrossRef]
- Wen, Y.R.; Li, Y.P.; Hirata, A.; Zhang, Y.; Fujita, T.; Liu, C.T.; Chiba, A.; Chen, M.W. Synergistic alloying effect on microstructural evolution and mechanical properties of Cu precipitation-strengthened ferritic alloys. Acta Mater. 2013, 61, 7726–7740. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, W.; Liu, Z.; Wang, G. Direct observations on the crystal structure evolution of nano Cu-precipitates in an extremely low carbon steel. Mater. Lett. 2017, 15, 49–52. [Google Scholar] [CrossRef]
- Habibi, H.R. Atomic structure of the Cu precipitates in two stages hardening in maraging steel. Mater. Lett. 2005, 59, 1824–1827. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, L.; Liu, C. Effect of step quenching on microstructures and mechanical properties of HSLA steel. Mater. Sci. Eng. A 2016, 675, 371–378. [Google Scholar] [CrossRef]
- Hwang, G.C.; Lee, S.; Yoo, J.Y.; Choo, W.Y. Effect of direct quenching on microstructure and mechanical properties of copper-bearing high-strength alloy steels. Mater. Sci. Eng. A 1998, 252, 256–268. [Google Scholar] [CrossRef]
- Ghosh, A.; Mishra, B.; Das, S.; Chatterjee, S. An ultra low carbon Cu bearing steel: Influence of thermomechanical processing and aging heat treatment on structure and properties. Mater. Sci. Eng. A 2004, 374, 43–56. [Google Scholar] [CrossRef]
- Yang, X.; Li, C.; Han, J.; Yang, Y.; Ju, Y.; Ba, L.; Wang, C.; Di, X. Effect of welding state on the re-precipitation behavior of Cu-rich and NiAl nanoparticles in HAZ of 1100 MPa grade low carbon ultra-high strength steel. Mater. Sci. Eng. A 2024, 897, 146334. [Google Scholar] [CrossRef]
- Yen, H.; Chen, P.; Huang, C.; Yang, J. Interphase precipitation of nanometer-sized carbides in a titanium-molybdenum-bearing low-carbon steel. Acta Mater. 2011, 59, 6264–6274. [Google Scholar] [CrossRef]
- Ji, F.Q.; Li, C.N.; Tang, S.; Wang, G.D. Interphase precipitation of nanometre-sized carbides in Ti bearing steels. Mater. Res. Innov. 2015, 19, S92–S97. [Google Scholar] [CrossRef]
- Ji, F.Q.; Li, C.N.; Tang, S.; Liu, Z.Y.; Wang, G.D. Effects of carbon and niobium on microstructure and properties for Ti bearing steels. Mater. Sci. Technol. 2014, 31, 695–702. [Google Scholar] [CrossRef]
- Yi, H.; Xu, Y.; Xu, Z.; Liu, Z.; Wang, G. Microstructure and properties of low cost 780 MPa hot-rolled high-strength steel. Mater. Mech. Eng. 2010, 34, 37–39. [Google Scholar]
- Sun, M.; Xu, Y.; Xu, T. Cu Precipitation Behaviors and Microscopic Mechanical Characteristics of a Novel Ultra-Low Carbon Steel. Materials 2020, 13, 3571. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).