Influence of a Novel Thermomechanical Processing Route on the Structural, Mechanical, and Corrosion Properties of a Biodegradable Fe-35Mn Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Fe-35Mn Alloy Preparation
2.2. (Micro)Structural Characterization
2.3. Mechanical Properties
2.4. Corrosion Experiments
3. Results and Discussion
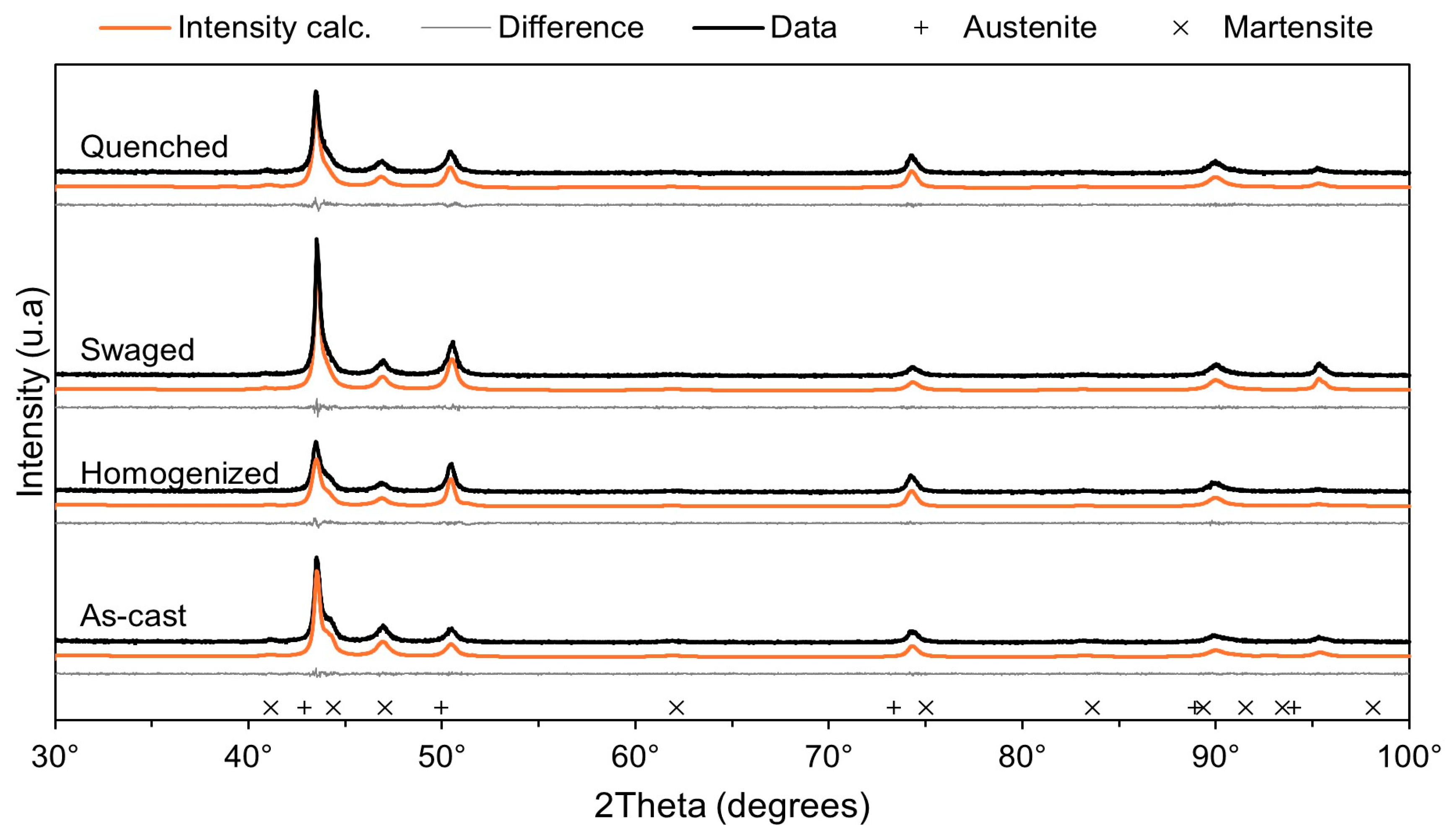
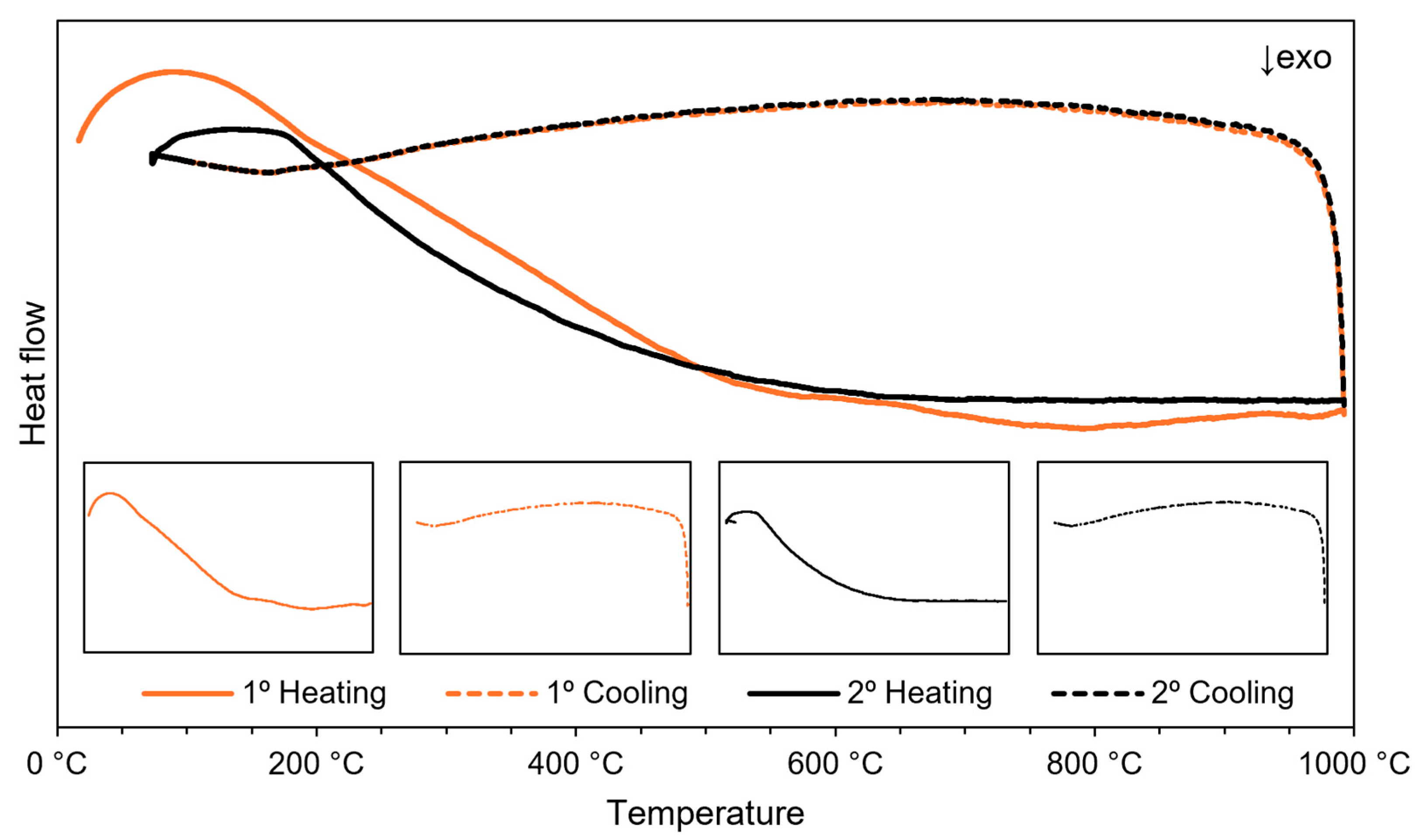
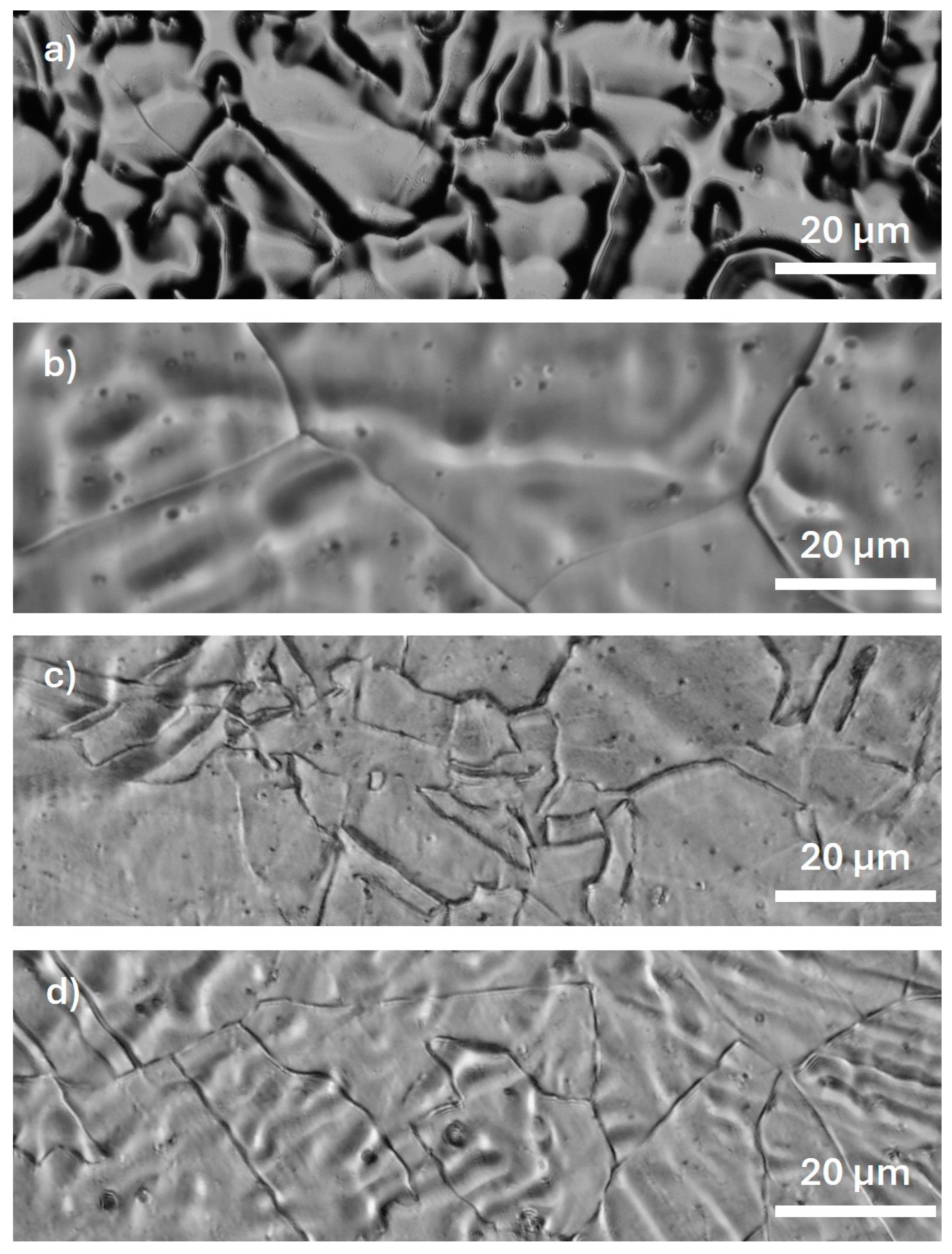
3.1. (Micro)Structural Characterization
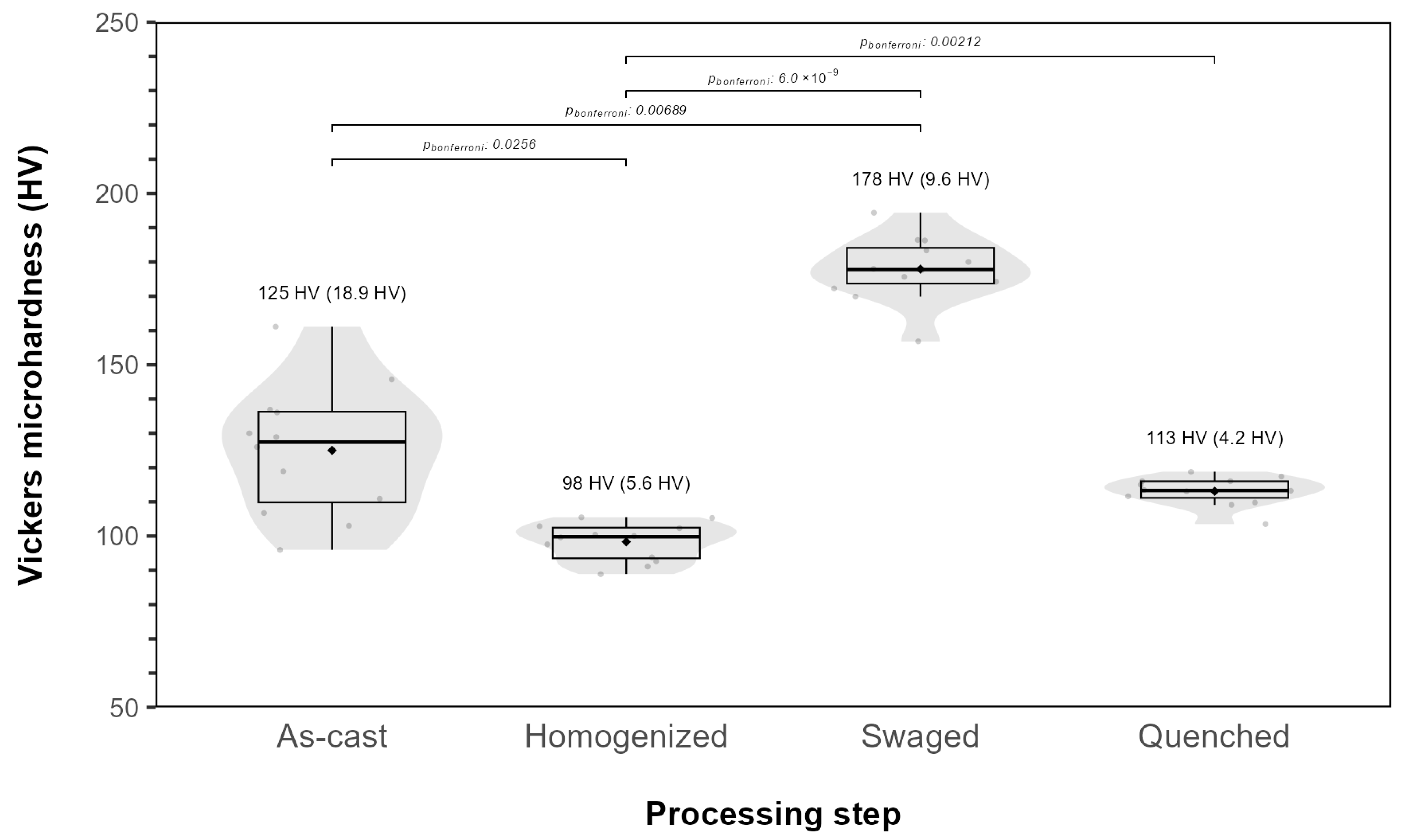
3.2. Mechanical Properties
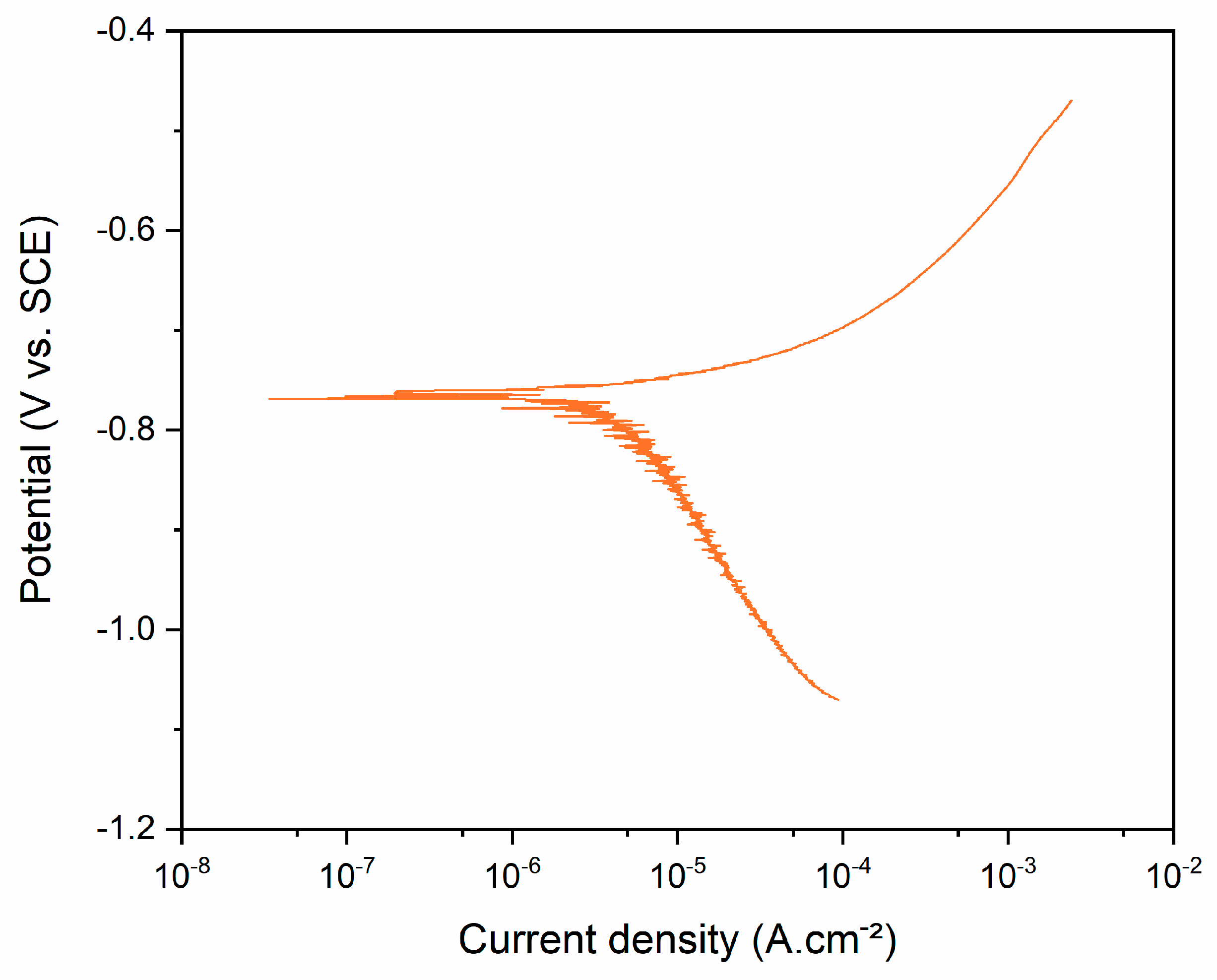
3.3. Corrosion Behavior
4. Conclusions
- The experimental Fe-35Mn alloy was successfully fabricated, and its microstructural characteristics after processing are consistent with the existing literature, showing the formation of a structure predominantly composed of austenite phase, with large, uniformly distributed grains.
- The determined mechanical properties, such as ultimate tensile strength, elongation to failure, yield strength, and Young’s modulus, were similar to or superior to those of annealed pure iron and 316 L stainless steel, which were used as reference standards for evaluating other alloys intended for biomedical applications.
- The processed alloy proved to be more susceptible to corrosion. When compared to pure iron and 316 L stainless steel, the alloy exhibited a lower corrosion potential and an increased current density, suggesting, respectively, lower surface corrosion resistance and a higher corrosion rate. These results underscore the potential of the alloy for use in temporary biomedical devices, such as stents.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dargusch, M.S.; Choudhury, D.; Saha, T.; Pramanik, A.; Tran, P.A.; O’Mullane, A.P.; Brandt, M. Exploring the Role of Manganese on the Microstructure, Mechanical Properties, Biodegradability, and Biocompatibility of Porous Iron-Based Scaffolds. ACS Biomater. Sci. Eng. 2019, 5, 1686–1702. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, Y.; Qin, L. Progress of Biodegradable Metals. Prog. Nat. Sci. Mater. Int. 2014, 24, 414–422. [Google Scholar] [CrossRef]
- Sun, H.; Chen, F.; Chen, Z.-K. Current Status and Outlook on the Clinical Translation of Biodegradable Metals. Mater. Today 2019, 23, 57–71. [Google Scholar] [CrossRef]
- Wang, Z.; Song, J.; Peng, Y. New Insights and Perspectives into Biodegradable Metals in Cardiovascular Stents: A Mini Review. J. Alloys Compd. 2024, 958, 175313. [Google Scholar] [CrossRef]
- Rangel, R.d.C.R.; Rangel, A.L.R.; da Silva, K.B.; Escada, A.L.D.A.; Chaves, J.A.M.; Maia, F.R.; Pina, S.; Reis, R.L.; Oliveira, J.M.; Alves, A.P.R. Characterization of Iron Oxide Nanotubes Obtained by Anodic Oxidation for Biomedical Applications—In Vitro Studies. Materials 2024, 17, 3627. [Google Scholar] [CrossRef]
- Rabeeh, V.P.M.; Hanas, T. Progress in Manufacturing and Processing of Degradable Fe-Based Implants: A Review. Prog. Biomater. 2022, 11, 163–191. [Google Scholar] [CrossRef]
- AlMangour, B.; Sivasankaran, S.; Ammar, H.R.; Almufadi, A.A.; Alsaiari, N. Synthesis, Microstructures, Mechanical, and Corrosion Behavior of FeMn(35−x)–x (Cu, W, and Co)-Based Biodegradable Alloys Prepared by Mechanical Alloying and Selective Laser Melting. Metall. Mater. Trans. A 2023, 54, 3767–3780. [Google Scholar] [CrossRef]
- Kiani, F.; Wen, C.; Li, Y. Prospects and Strategies for Magnesium Alloys as Biodegradable Implants from Crystalline to Bulk Metallic Glasses and Composites—A Review. Acta Biomater. 2020, 103, 1–23. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Hu, T.; Yang, C.; Lin, S.; Yu, Q.; Wang, G. Biodegradable Stents for Coronary Artery Disease Treatment: Recent Advances and Future Perspectives. Mater. Sci. Eng. C 2018, 91, 163–178. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, N.; Dehghan-Manshadi, A.; Venezuela, J.; Bermingham, M.J.; Dargusch, M.S. Fabrication and Properties of Biodegradable Akermanite-Reinforced Fe35Mn Alloys for Temporary Orthopedic Implant Applications. ACS Biomater. Sci. Eng. 2023, 9, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.C.; Aquilina, D.; Paternoster, C.; Vella, D.; Sinagra, E.; Mantovani, D.; Cassar, G.; Wismayer, P.S.; Buhagiar, J. Influence of Cold Rolling on In Vitro Cytotoxicity and Electrochemical Behaviour of an Fe-Mn-C Biodegradable Alloy in Physiological Solutions. Heliyon 2018, 4, e00926. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, H.; Dubé, D.; Mantovani, D. Degradable Metallic Biomaterials: Design and Development of Fe–Mn Alloys for Stents. J. Biomed. Mater. Res. Part A 2010, 93, 1–11. [Google Scholar] [CrossRef]
- Dargusch, M.S.; Venezuela, J.; Dehghan-Manshadi, A.; Johnston, S.; Yang, N.; Mardon, K.; Lau, C.; Allavena, R. In Vivo Evaluation of Bioabsorbable Fe-35Mn-1Ag: First Reports on In Vivo Hydrogen Gas Evolution in Fe-Based Implants. Adv. Healthcare Mater. 2021, 10, 2000667. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, H.; Dubé, D.; Mantovani, D. Developments in Metallic Biodegradable Stents. Acta Biomater. 2010, 6, 1693–1697. [Google Scholar] [CrossRef]
- Dehghan-Manshadi, A.; StJohn, D.H.; Dargusch, M.S. Tensile Properties and Fracture Behaviour of Biodegradable Iron–Manganese Scaffolds Produced by Powder Sintering. Materials 2019, 12, 1572. [Google Scholar] [CrossRef]
- Dehestani, M.; Trumble, K.; Wang, H.; Wang, H.; Stanciu, L.A. Effects of Microstructure and Heat Treatment on Mechanical Properties and Corrosion Behavior of Powder Metallurgy Derived Fe–30Mn Alloy. Mater. Sci. Eng. A 2017, 703, 214–226. [Google Scholar] [CrossRef]
- Mostaed, E.; Vedani, M.; Hashempour, M.; Bestetti, M. Influence of ECAP Process on Mechanical and Corrosion Properties of Pure Mg and ZK60 Magnesium Alloy for Biodegradable Stent Applications. Biomatter 2014, 4, e28283. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, P. Degradable Porous Fe-35wt.% Mn Produced via Powder Sintering from NH4HCO3 Porogen. Mater. Chem. Phys. 2015, 163, 394–401. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, D.; Dai, Y.; Lin, J.; Li, Y.; Wen, C. Microstructure, Mechanical Properties, Degradation Behavior, and Biocompatibility of Porous Fe-Mn Alloys Fabricated by Sponge Impregnation and Sintering Techniques. Acta Biomater. 2020, 114, 485–496. [Google Scholar] [CrossRef]
- Xu, Z.; Hodgson, M.A.; Cao, P. Effect of Immersion in Simulated Body Fluid on the Mechanical Properties and Biocompatibility of Sintered Fe–Mn-Based Alloys. Metals 2016, 6, 309. [Google Scholar] [CrossRef]
- Witusiewicz, V.T.; Sommer, F.; Mittemeijer, E.J. Reevaluation of the Fe-Mn Phase Diagram. J. Phase Equilib. Diffus. 2004, 25, 346–354. [Google Scholar] [CrossRef]
- Nayak, P.; Biswal, A.K.; Sahoo, S.K. Processing and Characterization of Fe-35Mn Biodegradable Metallic Materials. Mater. Today Proc. 2020, 33, 5284–5289. [Google Scholar] [CrossRef]
- Francis, A.; Yang, Y.; Virtanen, S.; Boccaccini, A.R. Iron and Iron-Based Alloys for Temporary Cardiovascular Applications. J. Mater. Sci. Mater. Med. 2015, 26, 1–16. [Google Scholar] [CrossRef] [PubMed]
- da Silva, K.B.; Carobolante, J.P.A.; Rajan, S.S.; Júnior, C.B.; Sabino, R.M.; Seixas, M.R.; Nakazato, R.Z.; Popat, K.C.; Claro, A.P.R.A. Mechanical Properties, Corrosion Behavior, and In Vitro Cell Studies of the New Ti-25Ta-25Nb-5Sn Alloy. Materials 2023, 16, 1970. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, B.; Wu, Y.H.; Zheng, Y.F. Comparative In Vitro Study on Pure Metals (Fe, Mn, Mg, Zn, and W) as Biodegradable Metals. J. Mater. Sci. Technol. 2013, 29, 619–627. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Y.; Liu, J.; Jiang, C.; Li, Y. In Vitro Corrosion Properties and Cytocompatibility of Fe-Ga Alloys as Potential Biodegradable Metallic Materials. Mater. Sci. Eng. C 2017, 71, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Obayi, C.S.; Tolouei, R.; Mostavan, A.; Paternoster, C.; Turgeon, S.; Okorie, B.A.; Obikwelu, D.O.; Mantovani, D. Effect of Grain Sizes on Mechanical Properties and Biodegradation Behavior of Pure Iron for Cardiovascular Stent Application. Biomatter 2016, 6, e959874. [Google Scholar] [CrossRef]
- Liu, R.-Y.; He, R.-G.; Xu, L.-Q.; Guo, S.-F. Design of Fe–Mn–Ag Alloys as Potential Candidates for Biodegradable Metals. Acta Metall. Sin. 2018, 31, 584–590. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Yang, Y.; Pan, H.; He, C.; Qi, F.; Xie, D.; Liang, H. Selective Laser Melted Fe-Mn Bone Scaffold: Microstructure, Corrosion Behavior, and Cell Response. Mater. Res. Express 2020, 7, 015404. [Google Scholar] [CrossRef]
| Concentration (wt %) | |||||
|---|---|---|---|---|---|
| Alloy | Mn | Si | Al | S | Fe |
| Fe-35Mn | 35.18 | 0.15 | 0.08 | 0.07 | Balance |
| Sample | Phase | Content (wt %) | Lattice Parameters (Å) | Volume (A3) | Refinement Factors | |||
|---|---|---|---|---|---|---|---|---|
| a | c | Rwp | GOF | RBragg | ||||
| As-cast | γ | 74.2 | 3.55 | - | 44.99 | 10.74 | 1.03 | 2.81 |
| ε | 25.8 | 2.54 | 4.10 | 23.09 | 1.32 | |||
| Homogenized | γ | 98.1 | 3.56 | - | 45.29 | 11.90 | 1.07 | 4.32 |
| ε | 1.9 | 2.54 | 4.10 | 22.96 | 1.97 | |||
| Swaged | γ | 20.4 | 3.58 | - | 46.20 | 9.94 | 1.03 | 0.953 |
| ε | 79.6 | 2.44 | 4.26 | 22.03 | 1.12 | |||
| Quenched | γ | 96.4 | 3.57 | - | 45.84 | 11.11 | 1.11 | 3.80 |
| ε | 3.6 | 2.55 | 4.10 | 23.18 | 1.97 | |||
| Properties | n | Min | Max | Mean | SD |
|---|---|---|---|---|---|
| Young’s modulus | 3 | 154 GPa | 189 GPa | 171 GPa | 17 GPa |
| Yield strength | 3 | 144 MPa | 457 MPa | 297 MPa | 157 MPa |
| Ultimate tensile strength | 3 | 388 MPa | 636 MPa | 533 MPa | 129 MPa |
| Elongation to failure | 3 | 34% | 46% | 39% | 6% |
| Variable | n | Min | Max | Mean | SD |
|---|---|---|---|---|---|
| Young’s modulus | 5 | 177 GPa | 186 GPa | 182 GPa | 3 GPa |
| Variable | n | Min | Max | Mean | SD |
|---|---|---|---|---|---|
| OCP | 3 | −0.804 V | −0.775 V | −0.785 V | 0.016 V |
| Ecorr | 3 | −0.770 V | −0.760 V | −0.763 V | 0.006 V |
| Icorr | 3 | 3.823 µA·cm−2 | 3.956 µA·cm−2 | 3.889 µA·cm−2 | 0.066 µA·cm−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, K.B.; Carobolante, J.P.A.; Nakazato, R.Z.; Caporalli Filho, A.; Rosifini Alves, A.P. Influence of a Novel Thermomechanical Processing Route on the Structural, Mechanical, and Corrosion Properties of a Biodegradable Fe-35Mn Alloy. Metals 2025, 15, 462. https://doi.org/10.3390/met15040462
da Silva KB, Carobolante JPA, Nakazato RZ, Caporalli Filho A, Rosifini Alves AP. Influence of a Novel Thermomechanical Processing Route on the Structural, Mechanical, and Corrosion Properties of a Biodegradable Fe-35Mn Alloy. Metals. 2025; 15(4):462. https://doi.org/10.3390/met15040462
Chicago/Turabian Styleda Silva, Kerolene Barboza, João Pedro Aquiles Carobolante, Roberto Zenhei Nakazato, Angelo Caporalli Filho, and Ana Paula Rosifini Alves. 2025. "Influence of a Novel Thermomechanical Processing Route on the Structural, Mechanical, and Corrosion Properties of a Biodegradable Fe-35Mn Alloy" Metals 15, no. 4: 462. https://doi.org/10.3390/met15040462
APA Styleda Silva, K. B., Carobolante, J. P. A., Nakazato, R. Z., Caporalli Filho, A., & Rosifini Alves, A. P. (2025). Influence of a Novel Thermomechanical Processing Route on the Structural, Mechanical, and Corrosion Properties of a Biodegradable Fe-35Mn Alloy. Metals, 15(4), 462. https://doi.org/10.3390/met15040462







