Abstract
The microstructure, mechanical properties, corrosion behavior, cytocompatibility, and antibacterial properties of biodegradable Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys with or without pulsed magnetic field treatment during casting were systematically investigated. Mg addition induced the formation of fine Mg2Zn11 precipitated along the matrix grain boundaries. With the increase in Mg content, the precipitation of the Mg2Zn11 phase increased, and the grain size became finer. Pulsed magnetic field treatment exacerbated the occurrence of this phenomenon. Under the combined action of the Mg2Zn11 phase and refined grain size, Zn3Cu0.5Mg alloy with pulsed magnetic field treatment had the best strength–ductility match (σUTS = 181.46 ± 1.06 MPa, δ = 3.95 ± 0.07%), moderate corrosion rate (icorr = 5.69 ± 3.96 μA/cm2), positive cytocompatibility, and antibacterial properties. This study indicated that Zn3Cu0.5Mg alloy with pulsed magnetic field treatment had the greater potential to further improve its properties through subsequent conventional metal-forming processing and severe plastic deformation techniques to meet clinical requirements, compared to existing as-cast Zn alloys.
1. Introduction
Magnesium (Mg)-, iron (Fe)-, and zinc (Zn)-based alloys are considered biodegradable metallic materials for medical applications. However, the rapid degradation of Mg-based alloys in physiological environments can lead to a premature loss of mechanical integrity of the implant [1]. Fe-based alloys with degradation rates significantly below clinical requirements (complete bioabsorption within 12–24 months) may generate fragmentary embolism, as noted with other non-biodegradable implants [2]. Zn has a standard corrosion potential of 0.76 V, which is intermediate between that of Fe (0.44 V) and Mg (2.37 V) [3]. Therefore, in recent years, owing to moderate corrosion rates, the development of Zn-based biodegradable alloys has emerged as a research hotspot in biomedicine. Notably, Zn-Cu alloys have attracted more attention recently for their excellent ductility and strength enhanced by Cu solid solution, grain refinement, and dispersed CuZn5 phase in the η-Zn matrix [4,5,6]. Tang et al. [7] reported that the elongation of as-extruded Zn-4Cu alloy reached 50.6 ± 2.8%, and the ultimate tensile strength reached 270 ± 0.5 MPa. Zn-Cu alloys exhibit moderate in vitro degradation rates (0.02–0.11 mm/year) [8,9]. Additionally, the alloying element Cu is an essential trace element for humans, and Zn-Cu alloys have been reported to stimulate endothelial cell proliferation, enhance angiogenesis, and possess antibacterial properties [7,10]. However, the mechanical properties of Zn-Cu alloys still fail to meet clinical strength standards (ultimate tensile strength (σUTS) > 300 MPa, yield strength (σYS) > 200 MPa [9]).
Mg has a superior strengthening ability for Zn alloys [11,12,13,14]. Pachla et al. [15] reported that the mechanical properties of Zn-xMg (x = 0, 0.5, 1, 1.5 wt.%) alloys continued to improve as the Mg content increased. Su et al. [16] incorporated various amounts (0, 0.5, 1, 2 wt.%) of Mg element into Zn-5Cu alloy. Compared to Zn-5Cu alloy, the tensile strength of Zn-5Cu-2Mg alloy increased by 35% to 216 MPa. The Mg2Zn11 phase precipitates when the content of Mg is below 1.5 wt.% [13]. Dispersing the Mg2Zn11 phase effectively impedes dislocation motion and, thus, improves the strength of Zn-Mg alloys [14]. Additionally, the effect of Mg addition on the corrosion behavior of Zn alloys has been studied by many scholars [17,18]. Jin et al. [17] reported that adding Mg improved the biocompatibility and degrading performance of Zn alloys. Ye et al. [11] demonstrated that the corrosion rate of Zn-Mg alloys varied due to the competition between the positive effect of grain refinement resulting from the addition of Mg and the negative effect of Mg2Zn11 volume fraction increment. Furthermore, Mg is an essential element for humans, which can not only prevent acute thrombosis and regulate vascular dilation [19] but also stimulate bone growth and lower the risk of osteoporosis [20]. Therefore, adding Mg to Zn-Cu alloys is expected to optimize their mechanical properties, corrosion behavior, and biocompatibility.
In recent years, with the successful design of biodegradable Zn alloy compositions, researchers focused on further optimizing the microstructure and enhancing the mechanical properties of these alloys through subsequent conventional metal-forming processing, such as extrusion, drawing, rolling, forging, and severe plastic deformation techniques [4,6,7,10,15,16]. Additionally, bioactive coatings were prepared on the surface of biodegradable Zn alloys through a plasma electrolytic oxidation process, electrodeposition method, or microwave-assisted coating method [21,22] to regulate their corrosion rate and biocompatibility. However, the initial microstructure after solidification during casting, which also plays an important role in determining Zn alloys’ final properties due to structural inheritance, has garnered relatively limited attention. Therefore, employing specialized techniques to control the solidification structure of biodegradable Zn alloys during casting to improve their properties is of great significance.
Pulsed magnetic field treatment is a commonly used and effective method in the field of alloy solidification processing. It can achieve microstructural homogenization, reduce casting defects, and refine the alloy grains. Zhang et al. [23] found that pulsed magnetic field treatment refined the solidification microstructure of Mg-7Zn alloy and reduced the volume fraction of the second phase. The σUTS, σYS, and elongation of Mg-7Zn treated with a 300 V pulsed magnetic field were increased by 40.4%, 38.2%, and 48.3%, respectively, compared to those of the untreated alloy. Li et al. [24] studied the effects of the high-voltage pulsed magnetic field on the microstructure of the Zn-Ag alloys and indicated that it refined the primary ε-AgZn3 dendrites and distributed them homogeneously in the specimens. Li et al. [25] applied a 6 kV pulsed magnetic field to the solidification process of Zn-xCu (x = 1.5, 2.5, 4.0 wt.%) alloys and discussed the structure of the alloys refined due to the convection and Joule heat induced by the pulsed magnetic field in the melt. However, studies on the effect of pulsed magnetic field treatment on biodegradable Zn alloys have primarily focused on microstructures, and their effects on properties have not yet been systematically evaluated. Therefore, this study systematically investigated the effects of Mg addition and pulsed magnetic field treatment on the microstructure, mechanical properties, corrosion behavior, cytocompatibility, and antibacterial property of Zn-3Cu-xMg (x = 0, 0.5, 1 wt%) alloys, aiming to develop an as-cast Zn alloy with improved properties for subsequent conventional metal-forming processing (extrusion, drawing, rolling, or forging) and severe plastic deformation techniques.
2. Materials and Methods
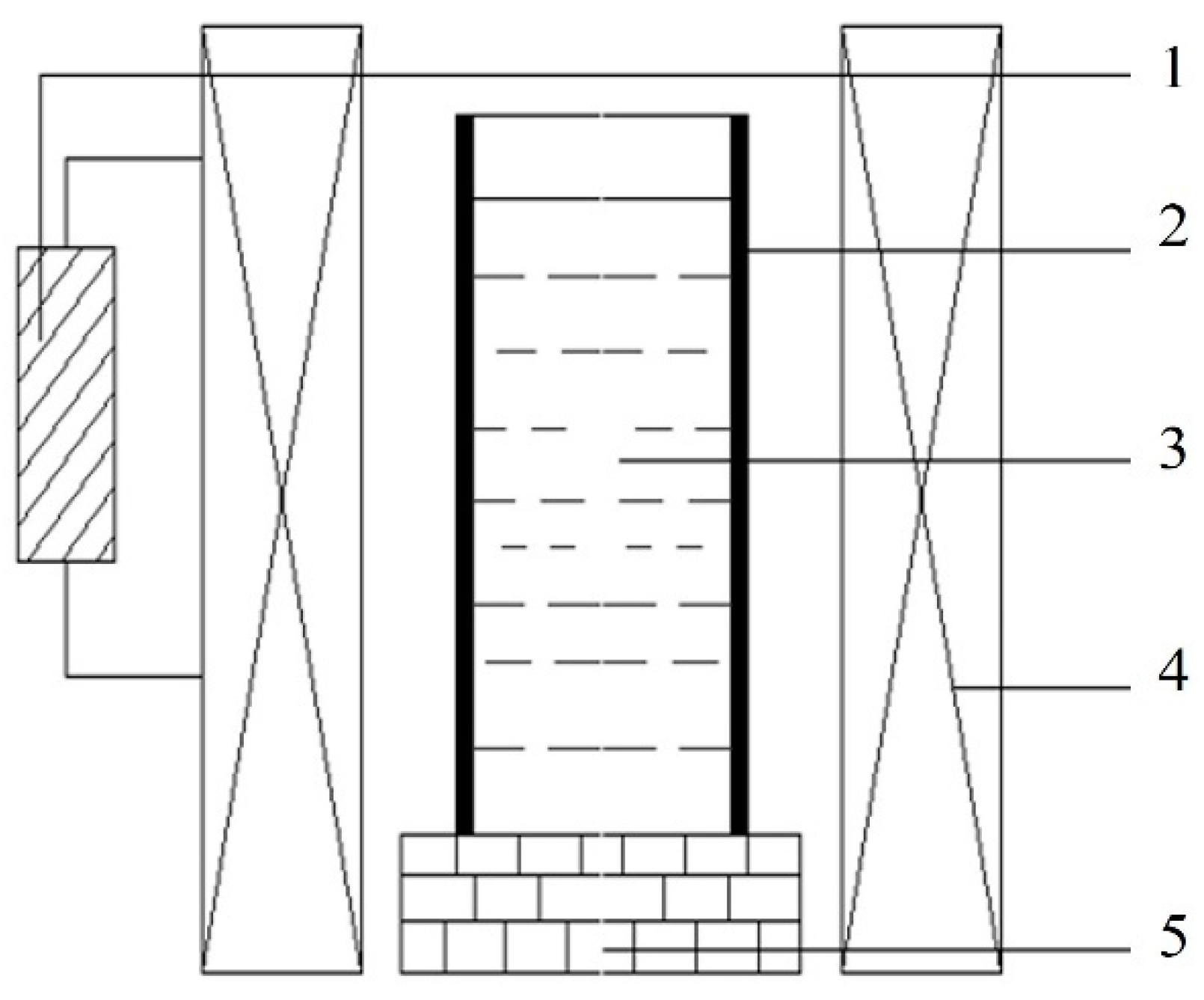
Commercially pure Zn (99.99%), Cu (99.99%), and Mg (99.96%) were used to prepare the as-cast Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys. According to the composition of the Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys, the dried pure metals were placed into a refractory crucible and completely melted through electrical resistance furnace (SG2-5-10, Shanghai real research Electric Furnace Co., Ltd., Shanghai, China). Then, the molten alloy was held at 620 °C for 30 min. During this period, the molten alloy was continuously stirred, and impurities were promptly removed. Subsequently, the molten alloy was poured into a cylindrical graphite mold positioned in the pulsed magnetic field device, as shown in Figure 1. Immediately after pouring, the pulsed magnetic field device was activated until the metal solidified completely. To prevent oxidation of the alloy, the entire process of melting, pouring, and solidification was carried out under a protective gas atmosphere (CO2: 99.5%, SF6: 0.5%).
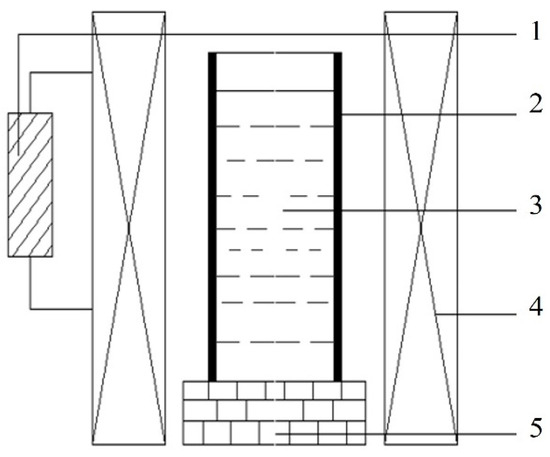
Figure 1.
Schematic diagram of a pulsed magnetic field device. (1—Pulse Generator; 2—Graphite Mold; 3—Melt; 4—Coil; 5—Refractory Brick Base).
The pulsed magnetic field generates periodic pulsed forces within the melt, which combine with electromagnetic forces to form electromagnetic oscillations [26]. By controlling the pulsed voltage and frequency, the magnitude and period of the pulsed forces can be regulated. According to our previous study [27], the microstructure and mechanical properties of Zn-3Cu alloy were explored by setting different pulsed voltages and frequencies. It was demonstrated that when the pulsed voltage was 300 V and the frequency was 5 Hz, Zn-3Cu alloy had the finest and most homogeneous microstructure and the best mechanical properties. Based on this, in the solidification process of this study, an external pulsed magnetic field with a voltage of 300 V and a frequency of 5 Hz was directly applied. For the convenience of subsequent research description, the alloys without pulsed magnetic field treatment were named W-Zn3Cu, W-Zn3Cu0.5Mg, and W-Zn3Cu1Mg, respectively, and the alloys with pulsed magnetic field treatment were named P-Zn3Cu, P-Zn3Cu0.5Mg, and P-Zn3Cu1Mg, respectively.
For the microstructure observation, alloys were sandpapered to 5000 grit and then polished to a mirror finish. The polished section was etched by a solution of 1.0 g oxalic acid, 1.0 mL nitric acid, 1.0 mL acetic acid, and 150 mL distilled water. The elemental distribution on the surface of alloys was characterized using a scanning electron microscope (FEI Apreo, Thermo Fisher, Waltham, MA, USA) equipped with energy dispersive spectroscopy (EDS). Secondary electrons (SE) and backscattered electrons (BSE) were both used to characterize the surface morphology and phase distribution. In addition, the phase composition of alloys was detected by XRD techniques. The XRD scanning range was from 20 to 80°, and the scanning rate was 10°/min.
Tensile tests were conducted on a universal material testing machine (WDW-200D, Shandong Wanchen Testing Machine Co., Ltd, Jinan, China) at a strain rate of 1 mm/min. The dimensions of tensile samples were designed according to GB/T 228.1-2021 [28], with a gauge of Φ5 × 25 mm.
The electrochemical corrosion investigation was carried out in a standard three-electrode electrochemical flask cell with an electrochemical workstation (Reference 600 TM, Gamry Instruments, Inc., Warminster, PA, USA). A saturated calomel electrode (SCE), a platinum sheet, and a sample having dimensions of Φ10 × 2 mm were utilized as the reference, counter, and working electrodes, respectively. The exposed area of the sample was approximately 0.636 cm2 after sealing the edges of sample with silicone. The simulated body fluid (SBF) solution was used in this study, which consisted of NaCl (8.035 g/L), NaHCO3 (0.355 g/L), KCl (0.225 g/L), K2HPO4·3H2O (0.231 g/L), MgCl2·6H2O (0.311 g/L), 1mol/L HCl (39 mL/L), CaCl2 (0.292 g/L), Na2SO4 (0.072 g/L), and finally, the pH value was adjusted to 7.40 using Tris and 1mol/L HCl. After the open circuit potential (OCP) test for 3600 s, the electrochemical impedance spectroscopy (EIS) tests were performed with a sinusoidal voltage signal of 10 mV and in a frequency range of 100 kHz to 0.1 Hz at EOCP. The potentiodynamic polarization (PD) test was performed from −0.2 Vvs OCP to 0.3 Vvs OCP at a scanning rate of 0.5 mV/s. All the experiments were conducted thrice to obtain reproducible results.
Human umbilical vein endothelial cells (HUVECs, KeyGEN Bio TECH, Nanjing, China) were used to assess the cytocompatibility of Zn-3Cu-xMg alloys. The experiment was designed with a negative control group, a positive control group, and experimental groups. Each group had five replicate wells in a 96-well plate. For the negative control group, 100 μL of medium was added to each well without culturing cells. For the positive control group, 100 μL of medium was added to each well to culture 5 × 103 cells. For the experimental group, 100 μL of medium containing 10% extract of the alloy [7] was added to each well to culture 5 × 103 cells. The medium used was RPMI 1640 medium (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco, Langley, OK, USA), penicillin (80 U/mL), and streptomycin (0.08 mg/mL). The extract of the Zn-3Cu-xMg alloys was prepared in advance using a culture medium with an extraction ratio of alloy surface area to medium volume of 1.25 cm2/mL in a humidified atmosphere with 5% CO2 at 37 °C for 3 d. The HUVECs were cultured for 1, 3, and 7 d. At each time point, wells were cleaned with PBS solution, and 100 μL of medium containing 10% CCK-8 solution was added to each well. After that, the cells were cultured at a temperature of 37 °C for 2 h. The absorbance was measured by a plate reader (MuLtiskan GO, Thermo Scientific, Waltham, MA, USA) at 450 nm. The relative growth rate (RGR) is calculated by Formula (1):
where ODAlloys is the optical density of experimental group (ODEG) minus negative control group (ODNC), and ODControl is the optical density of positive control group (ODPC) minus negative control group (ODNC).
RGR (%) = (ODEG − ODNC)/(ODPC − ODNC) × 100% = (ODAlloys/ODControl) × 100%
The antibacterial activity of Zn-3Cu-xMg alloys against Staphylococcus aureus (S. aureus, ATCC6538) was assessed by a plate count method according to ISO 10993-5:2009. The alloys were soaked in 1000 μL of bacterial suspension with a concentration of 106 CFU/mL and incubated at a temperature of 37 °C with 90% relative humidity. After 24 h, alloys with adherent bacteria were transferred to 500 μL of fresh phosphate-buffered saline (PBS) and shaken mechanically by vortex for 1 min. Then, 100 μL of the solution, after vortexing, was spread onto a nutrient agar plate and incubated at 37 °C for 24 h. Thereafter, the number of bacterial colonies on the nutrient agar plate was counted, and the antibacterial rate was calculated. The antibacterial rate is calculated by the Formula (2):
where NCK and Nalloys denote the mean numbers of bacterial colonies on control check and Zn-3Cu-xMg alloys, respectively.
Antibacterial rate (%) = (NCK − Nalloys)/NCK × 100%
All experiments were performed three times, and the data were expressed as mean ± standard deviation. Student t-test was used to analyze the significant difference. * p < 0.05, ** p < 0.01, and *** p < 0.001 were considered statistically significant.
3. Results and Discussion
3.1. Microstructure
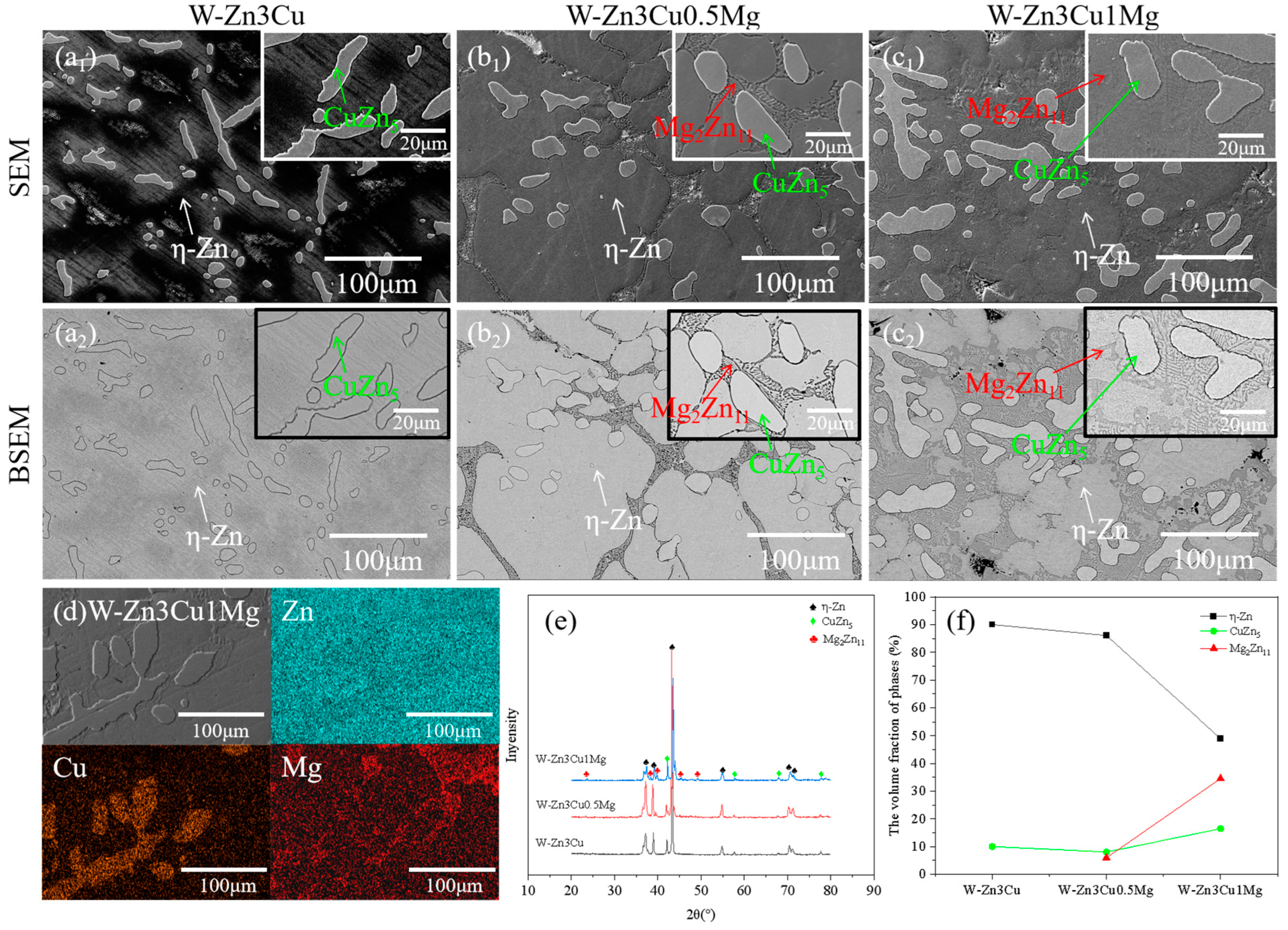
Figure 2(a1–c1) show the SEM images of Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys without pulsed magnetic field treatment, and Figure 2(a2–c2) show the corresponding BSEM images. For W-Zn3Cu, the matrix and about 50 μm dendritic second phase were observed, and after adding Mg, about 5 μm lamellar phases precipitated along the grain boundaries of the matrix. Based on the EDS (Figure 2d) and XRD (Figure 2e) patterns, it could be concluded that the matrix was -Zn, the dendritic second phase was CuZn5, and the lamellar phase was Mg2Zn11. Based on the Zn-Cu binary phase diagram [29], a peritectic reaction Zn + L → CuZn5 occurs at 425 °C. And a eutectic reaction L → Zn + Mg2Zn11 occurs at 364 °C based on the Zn-Mg binary phase diagram [30]. Consequently, during the solidification process, the CuZn5 phase initially precipitated from the melt as the temperature decreased to approximately 425 °C, followed by the precipitation of Mg2Zn11 along the grain boundaries of the matrix at around 364 °C. The XRD (Figure 2e) patterns also show that higher intensity of the peaks of Mg2Zn11 was detected with increasing Mg content. The volume fraction of the phase was statistically analyzed, and the results are shown in Figure 2f. It clearly showed that the volume fraction of the lamellar Mg2Zn11 phase increased with increasing Mg content while that of the -Zn phase decreased. Meanwhile, the volume fraction of the CuZn5 phase first decreased and then increased.
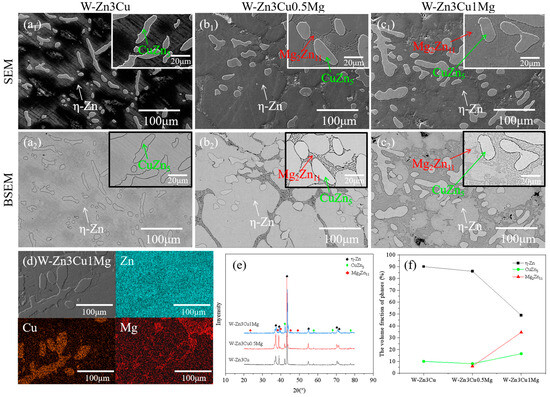
Figure 2.
Microstructure of Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys without pulsed magnetic field treatment: (a1–c1) SEM images; (a2–c2) the corresponding BSEM images; (d) EDS analysis; (e) XRD patterns; (f) phase volume fraction statistics.
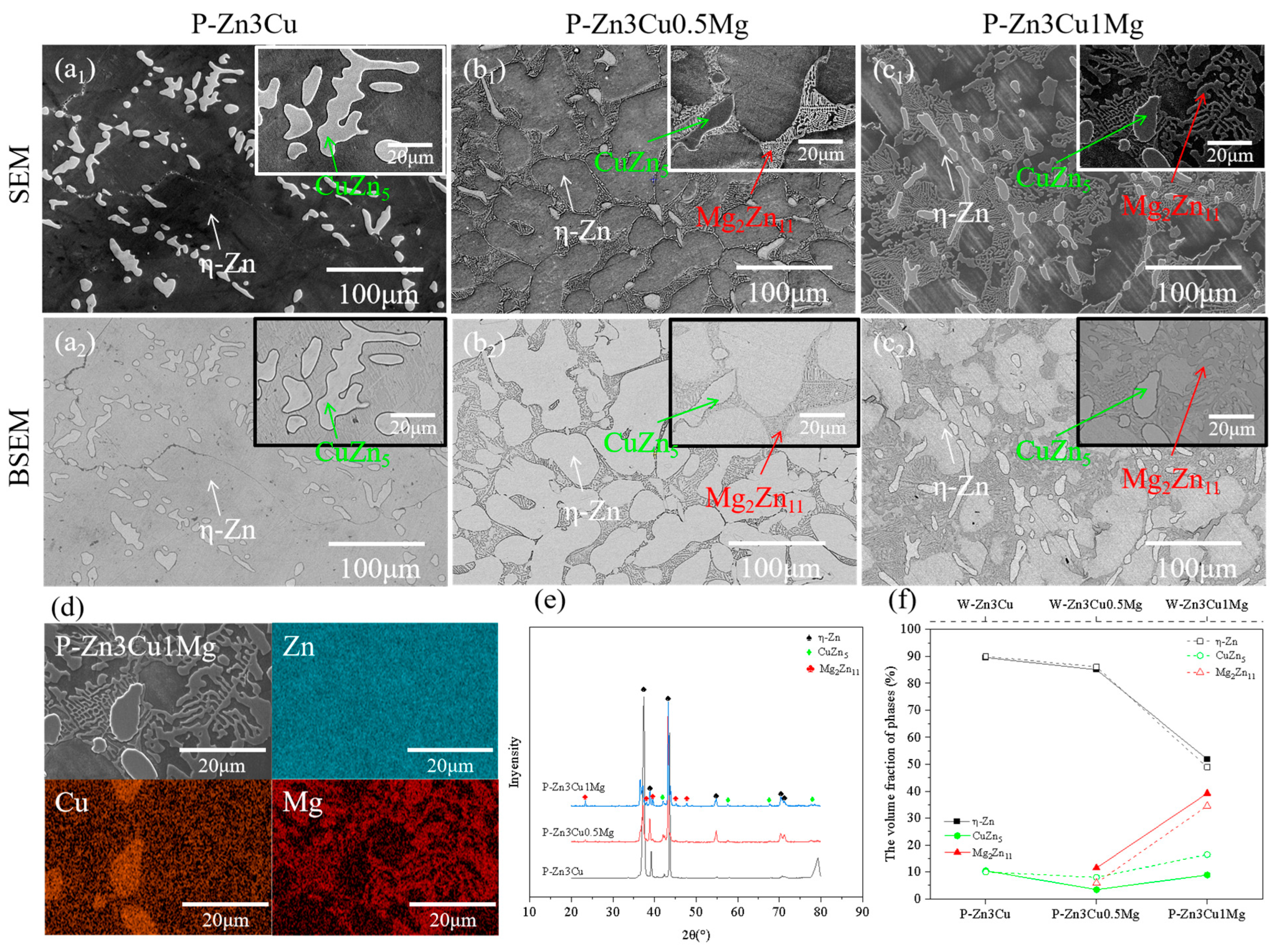
Compared with Figure 2, the SEM, BSEM, EDS, and XRD results in Figure 3 all confirmed that applying a pulsed magnetic field during the solidification process, the phase composition of Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys was the same as that of the alloys without pulsed magnetic field treatment. P-Zn3Cu consisted of the primary -Zn matrix and the dendritic CuZn5 phase. Similarly, P-Zn3Cu0.5Mg and P-Zn3Cu1Mg consisted of the -Zn, CuZn5 phase, and lamellar Mg2Zn11 phase. Statistical analysis of the phase volume fraction for Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys with pulsed magnetic field treatment was performed, with results compared to those of the alloys without pulsed magnetic field treatment (as indicated by the dashed line in Figure 3f). It showed that the pulsed magnetic field had a minimal effect on the volume fraction of -Zn and CuZn5 phase for Zn-3Cu alloy. However, for Zn-3Cu-0.5Mg and Zn-3Cu-1Mg alloys, pulsed magnetic field treatment resulted in the reduced volume fraction of the CuZn5 phase, while that of the Mg2Zn11 phase increased.
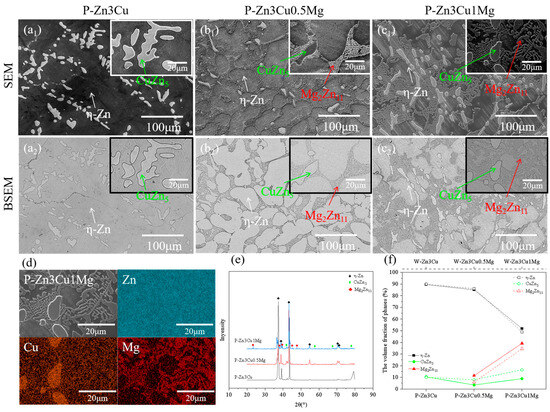
Figure 3.
Microstructure of Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys with pulsed magnetic field treatment: (a1–c1) SEM images; (a2–c2) the corresponding BSEM images; (d) EDS analysis; (e) XRD patterns; (f) phase volume fraction statistics.
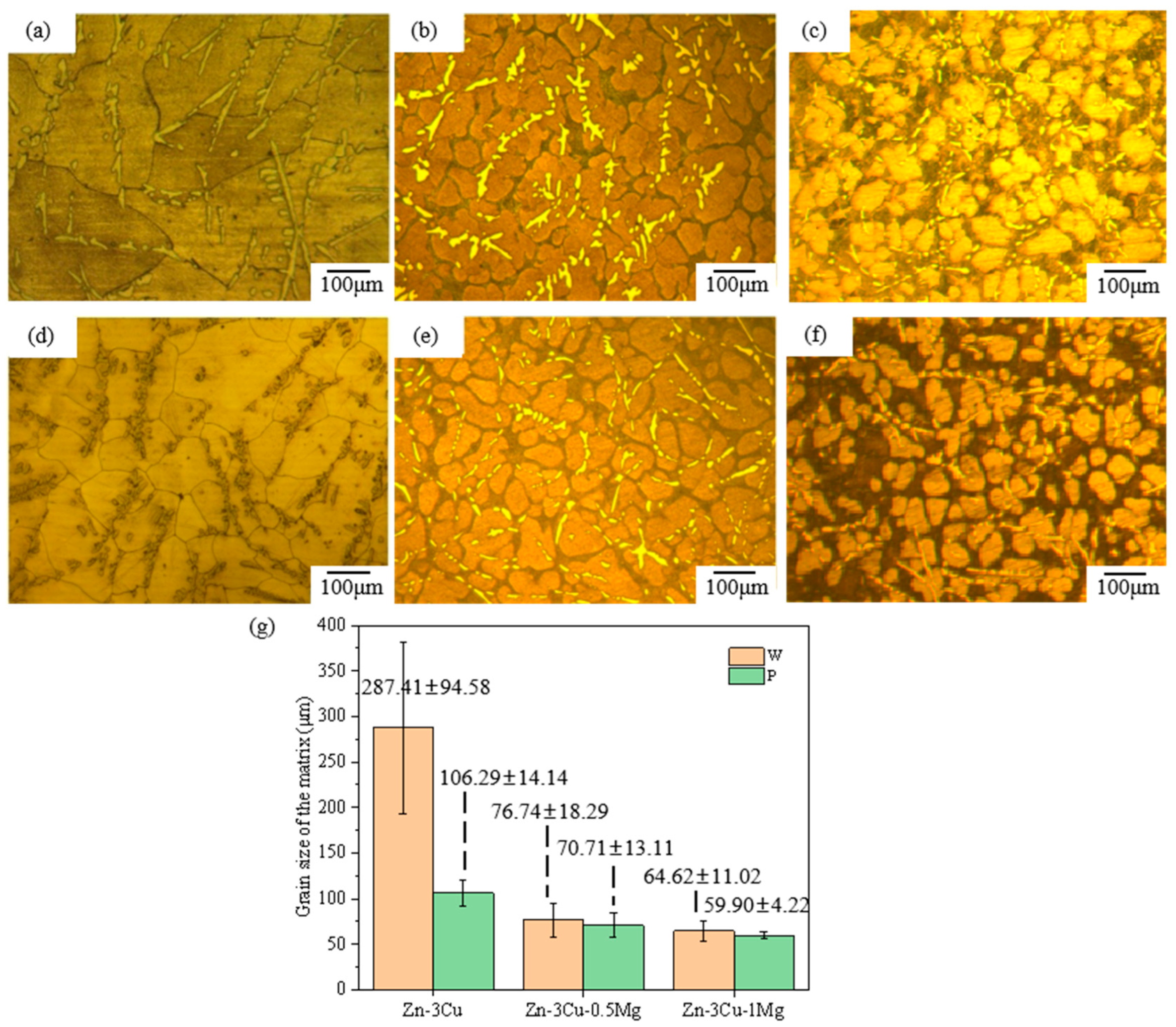
In addition, the SEM and BSEM images in Figure 2 and Figure 3 also indicated that the addition of Mg and pulsed magnetic field treatment affected the grain size of Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys. Figure 4a–f show the optical microstructure (OM) images of Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys with or without pulsed magnetic field treatment. The grain size of alloys was statistically analyzed and was shown in Figure 4g. As the Mg content increased, the grain size gradually decreased, and the pulse magnetic field treatment further refined the grain size. The refinement of grain size was mainly attributed to the addition of Mg, and pulsed magnetic field treatment increased the precipitation of the Mg2Zn11 phase along the grain boundaries (Figure 2 and Figure 3), which impeded the growth of the Zn matrix. And pulsed magnetic fields influenced the nucleation and growth processes of grains during solidification by increasing the number of nucleation sites [31], further refining grain size. Thus, P-Zn3Cu1Mg alloys had the smallest grain size of 59.90 ± 4.22 μm among all the Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys.
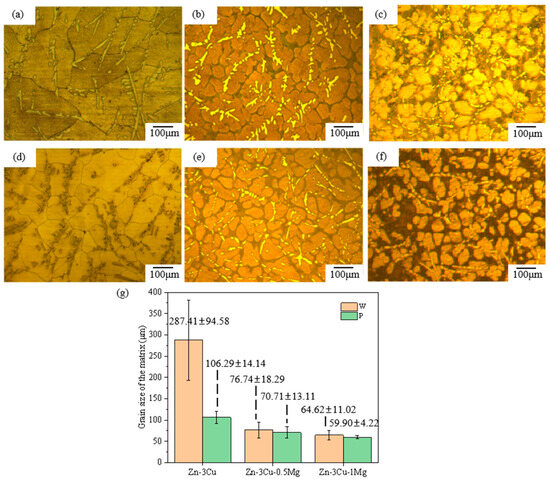
Figure 4.
The optical microstructure (OM) images of Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys: (a) W-Zn3Cu; (b) W-Zn3Cu0.5Mg; (c) W-Zn3Cu1Mg; (d) P-Zn3Cu; (e) P-Zn3Cu0.5Mg; (f) P-Zn3Cu1Mg; (g) grain size statistics.
3.2. Mechanical Properties
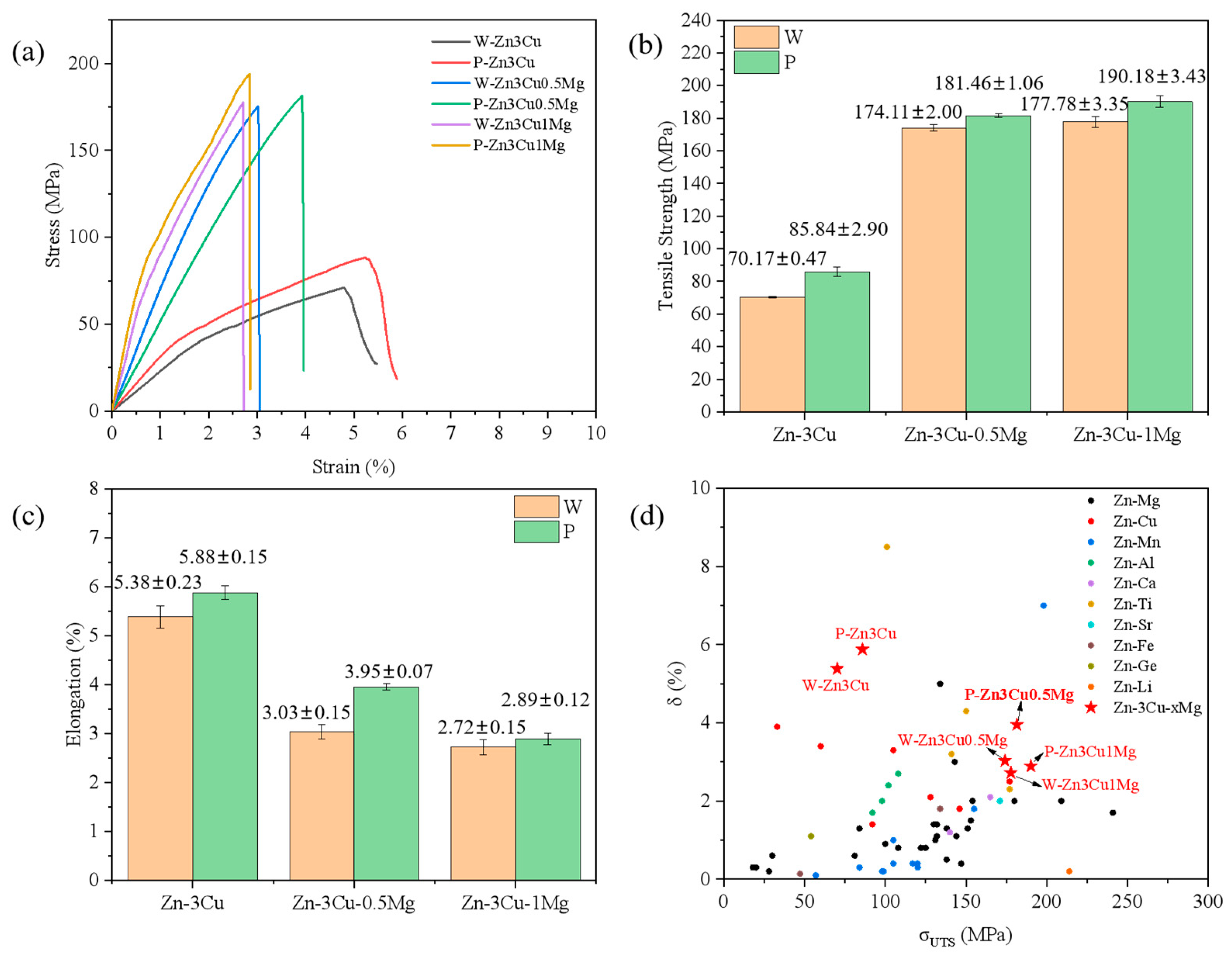
Figure 5a shows the room temperature stress–strain curves of the Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys with or without pulsed magnetic field treatment. All alloys exhibited a rapid decline in the stress–strain curve after reaching the maximum stress, almost with no plastic deformation before fracture. The ultimate tensile strength (σUTS) and elongation (δ) are shown in Figure 5b,c. As the Mg content increased, σUTS gradually increased while δ decreased. The improvement of σUTS could be mainly attributed to the massive precipitation of the Mg2Zn11 phase (Figure 2f and Figure 3f), blocking the dislocation sliding [14] and strengthening the alloys. Massive precipitation of brittle Mg2Zn11 phase also led to the stress concentration, resulting in cracking, thus decreasing the ductility. It also showed that the pulsed magnetic field treatment increased both σUTS and δ of Zn-3Cu-xMg alloys. The improvements in strength and ductility were attributed to the grain refinement [4] after pulsed magnetic field treatment (Figure 4g). Therefore, under the combined action of the Mg2Zn11 phase and refined grain size, P-Zn3Cu0.5Mg alloy had the best strength–ductility match (σUTS = 181.46 ± 1.06 MPa, δ = 3.95 ± 0.07%). Compared with other types of as-cast Zn alloys [32] (Figure 5d), the P-Zn3Cu0.5Mg alloy had the greater potential to further improve its mechanical properties through subsequent conventional metal-forming processing such as extrusion, drawing, rolling, forging, and severe plastic deformation techniques to meet clinical requirements.
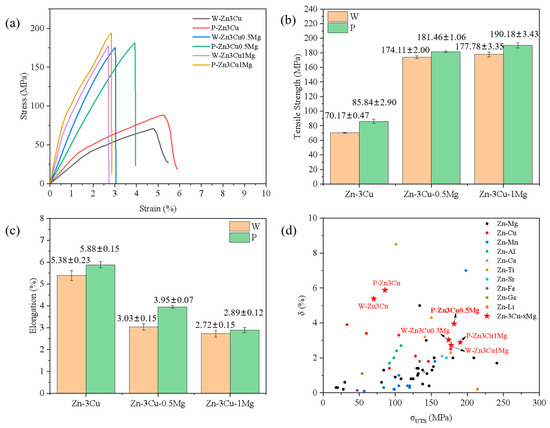
Figure 5.
(a) Stress–strain curves, (b) tensile strength, (c) elongation of Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys, and (d) comparison of tensile properties among different as-cast Zn alloys (the data of as-cast Zn alloys is adapted from Ref. [32]).
3.3. Corrosion Properties
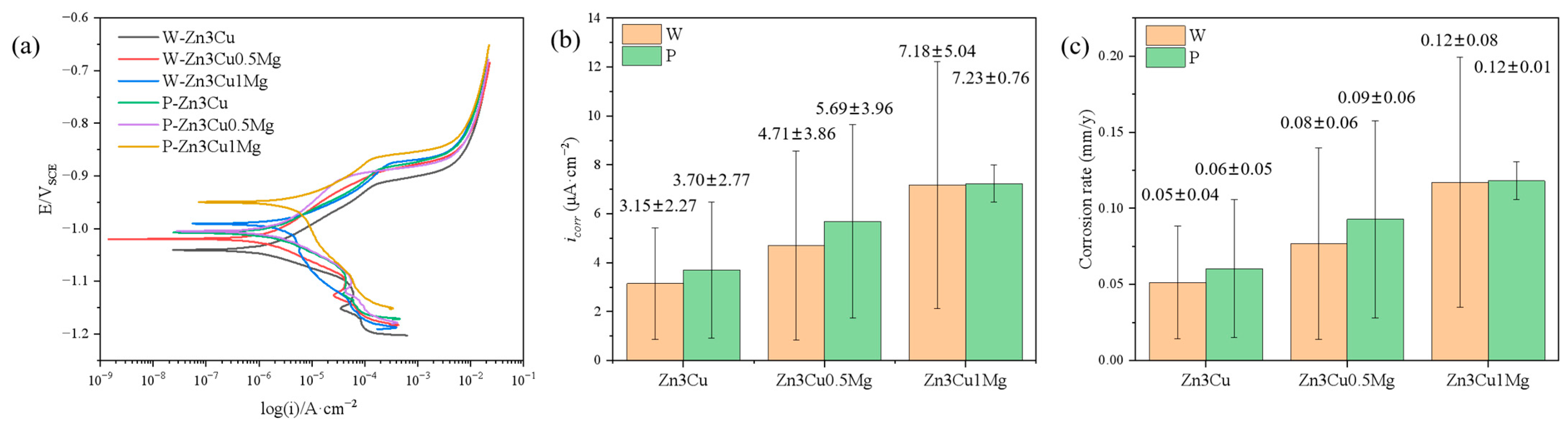
The corrosion behavior of Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys with or without pulsed magnetic field treatment in SBF solution was also evaluated systematically. Potentiodynamic polarization (PD) curves proved that all the alloys showed spontaneously passive behaviors in the SBF solution (Figure 6a). The corrosion current density (icorr) is depicted in Figure 6b. It can be concluded that the value of icorr increased as the Mg content increased, and pulsed magnetic field treatment further increased icorr. The corrosion rate (CR) can be evaluated using ASTM G102-23 [33]:
where EW is the equivalent weight (EW = 32.43 g/eq), and ρ is the density (ρ = 6.5 g/cm3) of the Zn-3Cu-xMg(x = 0, 0.5, 1 wt.%) alloys.
CR = 3.27 × 10−3 × icorr × EW/ρ
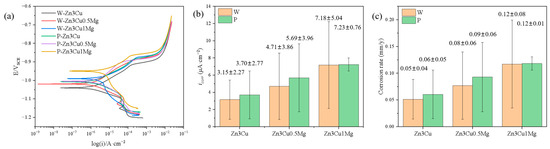
Figure 6.
Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys: (a) potentiodynamic polarization curves; (b) corrosion current density icorr; (c) corrosion rate.
The CR value was calculated and shown in Figure 6c. It indicated that the addition of Mg and pulsed magnetic field treatment both accelerate the corrosion rate of Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys. Precipitate phases influence the corrosion behavior of the material. The Mg2Zn11 phase and -Zn matrix phase could form a micro-battery cell due to the difference in their corrosion potentials [11,16], resulting in galvanic corrosion. In addition, the Mg2Zn11 phase precipitated along the grain boundary of the matrix had a low potential, causing the grain boundaries to be preferentially corroded during corrosion [34]. The addition of Mg and pulsed magnetic field treatment both promoted the precipitation of the Mg2Zn11 phase along grain boundaries (Figure 2f and Figure 3f) and increased the number of grain boundaries (Figure 4g) by refining grain size, thus escalating galvanic corrosion and grain boundary corrosion, accelerating the corrosion rate of Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys.
Although the addition of Mg and pulsed magnetic field treatment accelerated the corrosion rate of Zn-3Cu alloy, these rates remained between 0.02 and 0.11 mm/year [8,9], and were lower than that of Mg alloys (0.42–4.83 mm/year) [35], retaining the characteristic of the moderate degradation rate of Zn alloys.
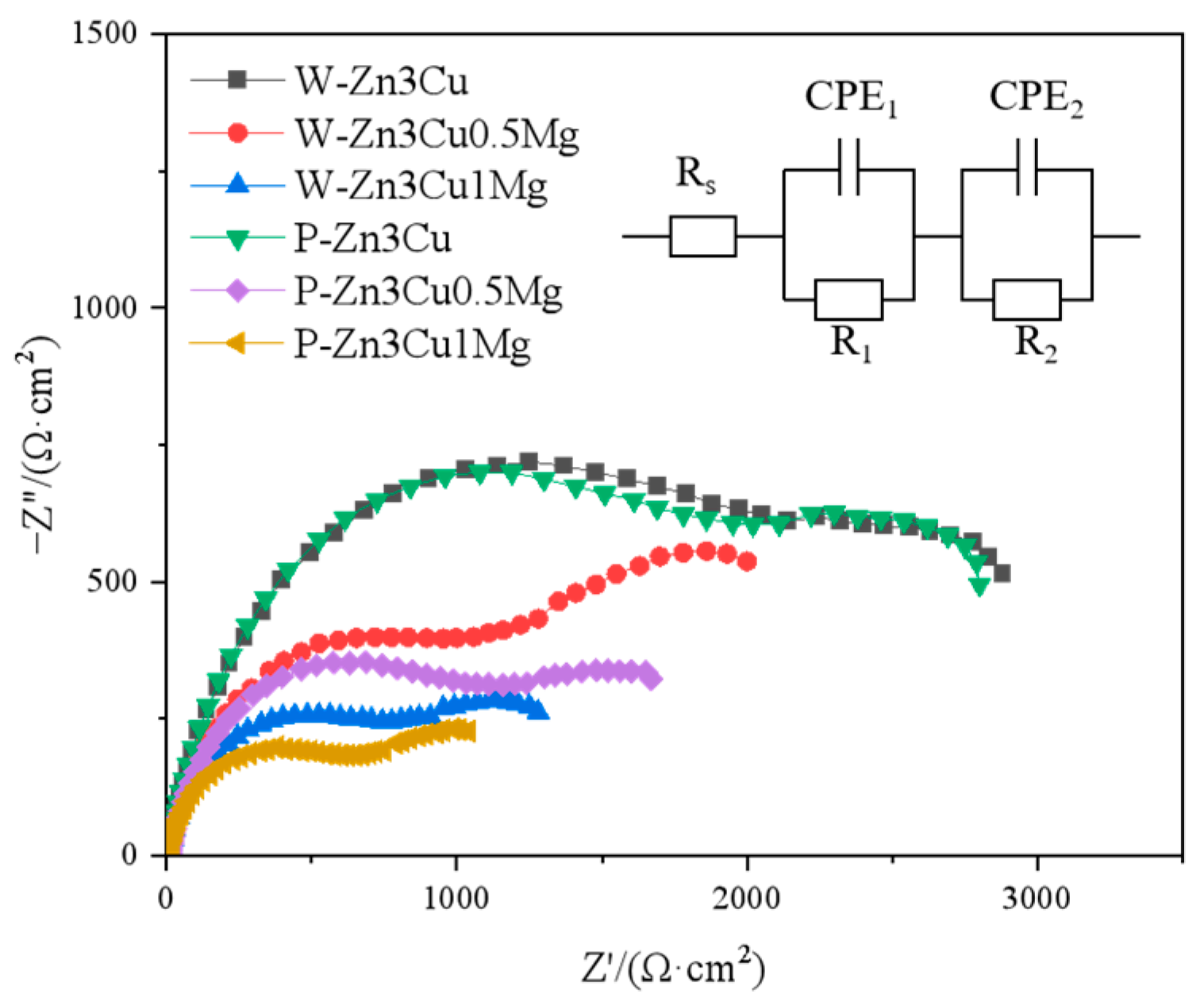
Figure 7 shows that as the Mg content increased, both high-frequency capacitive arc radius (related to the charge transfer) and low-frequency capacitive arc radius (related to the diffusion process) [36] decreased and were even smaller after pulsed magnetic field treatment. Smaller capacitive arc radii indicate poorer corrosion resistance. The Rs(CPE1R1)(CPE2R2) [36] model was used to analyze the EIS spectra, and the results are shown in Table 1, where Rs represents the solution resistance, R1 and CPE1 represent the resistance and capacitance of the charge transfer layer, and R2 and CPE2 represent the resistance and capacitance of the corrosion product layer. It could be seen that both R1 and R2 values decreased in the order of Zn-3Cu > Zn-3Cu-0.5Mg > Zn-3Cu-1Mg, and the pulsed magnetic field treatment also decreased R1 and R2 values, indicating that adding Mg and pulsed magnetic field treatment made the charge transfer easier and the protective performance of corrosion product weaker, accelerating the corrosion rate of Zn-3Cu-xMg alloys.
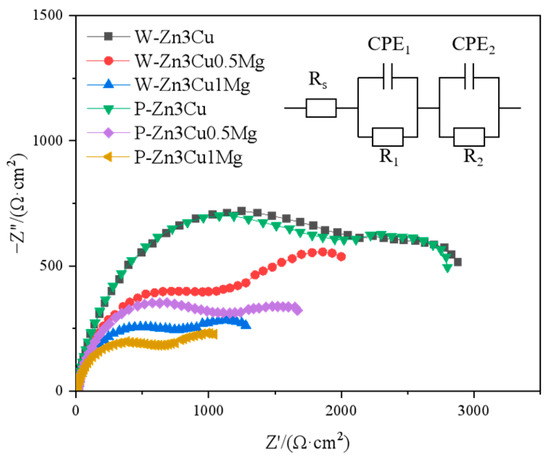
Figure 7.
Nyquist diagrams at OCP of Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys.

Table 1.
Simulated EIS data for Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys.
3.4. Cytocompatibility and Antibacterial Activity
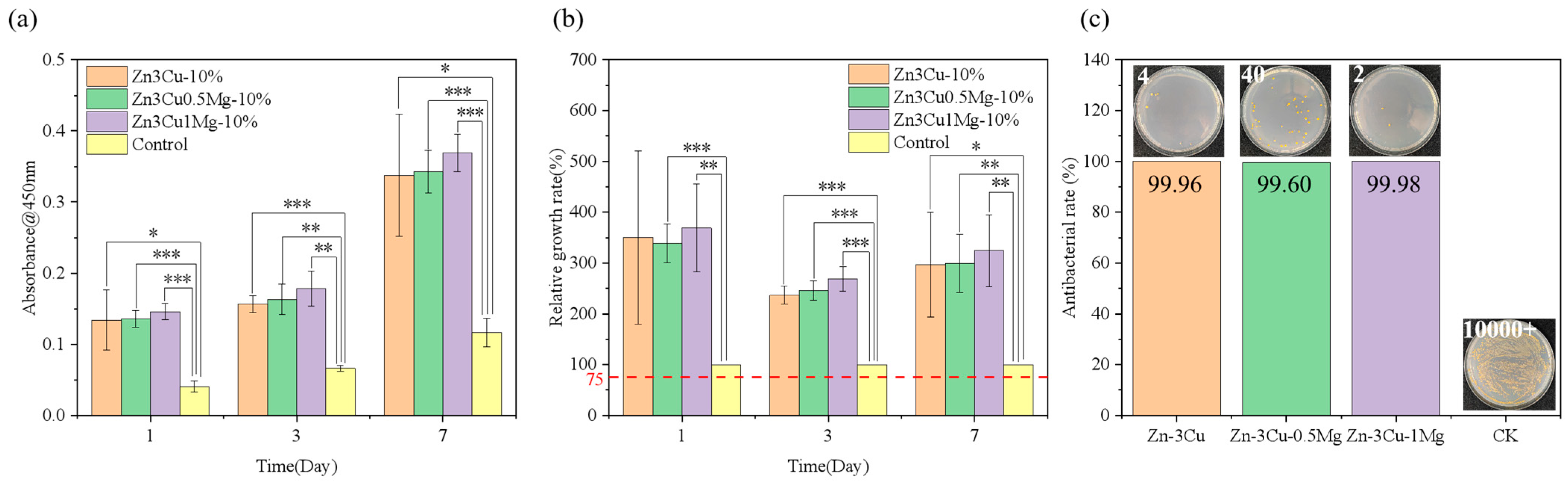
The deficiency in mechanical properties is one of the primary issues faced by Zn alloys at present. Considering the enhancement of strength and the acceleration of corrosion rate in Zn-3Cu-xMg alloys after pulsed magnetic field treatment, their responses to HUVECs and S. aureus were further investigated. Figure 8a,b show that Zn-3Cu-xMg alloys promoted cell proliferation and had no cytotoxicity compared with the control group. As the Mg content increased, the level of cell proliferation increased. Differences in cell viability can be attributed to the ion concentration of the extract, which is closely related to the alloy degradation process. The corrosion rate of Zn-3Cu-xMg alloy accelerated as the Mg content increased, releasing more Zn2+ and Mg2+ ions; thus, biocompatibility was improved. Li et al. [37] reported the in vivo corrosion rates of the Zn-1X (X = Mg, Ca, Sr) alloys as 0.17 mm /y, 0.19 mm/y, and 0.22 mm/y for Zn-1Mg, Zn-1Ca, and Zn-1Sr, respectively. And the radiographs of mice with Zn-1X alloy implants at 0, 1, 2, 3, 4, 6, and 8 weeks showed no inflammation and gas shadows around the implantation site, and all alloys exhibited no cytotoxicity in vivo. The corrosion rates of Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys were 0.06–0.12 mm/y (Figure 6c), lower than those of Zn-1X alloys. It could be concluded that the Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys should not cause cytotoxicity in vivo over extended periods. Figure 8c shows that the antibacterial rates of Zn-3Cu-xMg alloys were all up to 99.9%, owing to the combination of positively charged Zn2+, Cu2+, and Mg2+ ions with the negatively charged thiol, carboxyl, and amino groups on the bacterial membrane, leading to complete enzyme inactivation, protein destruction, and subsequent bacterial death [38].
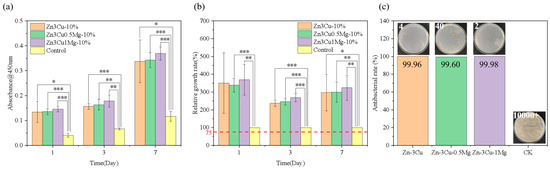
Figure 8.
Zn-3Cu-xMg alloys: (a) cell proliferation; (b) cell relative growth rate; (c) antibacterial rate. * p < 0.05, ** p < 0.01, *** p < 0.001.
4. Conclusions
This study investigated the effects of Mg addition and pulsed magnetic field treatment on the microstructure and properties of as-cast biodegradable Zn-3Cu-xMg (x = 0, 0.5, 1 wt.%) alloys. Mg addition induced the precipitation of fine Mg2Zn11 along matrix grain boundaries. With the increase in Mg content, the precipitation of the Mg2Zn11 phase increased, and the grain size became finer. Pulsed magnetic field treatment exacerbated the occurrence of this phenomenon. Under the combined action of Mg2Zn11 phase and refined grain size, Zn3Cu0.5Mg alloy with pulsed magnetic field treatment had the best strength–ductility match (σUTS = 181.46 ± 1.06 MPa, δ = 3.95 ± 0.07%), moderate corrosion rate (icorr = 5.69 ± 3.96 μA/cm2), cytocompatibility and antibacterial property, which makes it a promising as-cast Zn alloy with improved properties for subsequent conventional metal-forming processing (extrusion, drawing, rolling, or forging) and severe plastic deformation techniques.
Author Contributions
Conceptualization, C.P., L.R. and H.L. (Hui Liu); methodology, H.L. (Houqing Liu); validation, L.S. and H.L. (Houqing Liu); formal analysis, L.S.; investigation, L.S. and H.L. (Houqing Liu); data curation, H.L. (Hui Liu); writing—original draft preparation, L.S.; writing—review and editing, L.S., H.L. (Hui Liu) and H.L. (Houqing Liu); visualization, L.S., H.L. (Hui Liu) and L.R.; supervision, C.P. and L.R.; project administration, H.L. (Hui Liu); funding acquisition, C.P. and L.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (No. 2022YFC2406000), Liaoning Provincial Science and Technology Program—Excellent Youth Fund Program (No. 2023JH3/10200002), National Natural Science Foundation of China (No. 52301308), IMR Innovation fund (No. 2023-PY15), Natural Science Foundation of Jiangxi Provincial Education Department (No. DA202103161).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We would like to express our gratitude to Ke Yang (Shi-changxu Innovation Center for Advanced Materials, Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China; kyang@imr.ac.cn), Hai Wang (Shi-changxu Innovation Center for Advanced Materials, Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China; hwang13s@imr.ac.cn) and Yang Hong (Department of Neurosurgery, Shengjing Hospital, China Medical University, Shenyang 110122, China; hongyangcmu@hotmail.com) for their valuable guidance and assistance in overcoming the challenges encountered during the research work of this project.
Conflicts of Interest
Author Houqing Liu was employed by the company Avic Guizhou Anji Aviation Investment Casting Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Banerjee, P.C.; Al-Saadi, S.; Choudhary, L.; Harandi, S.E.; Singh, R. Magnesium implants: Prospects and challenges. Materials 2019, 12, 136. [Google Scholar] [CrossRef]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; von Schnakenburg, C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 2006, 27, 4955–4962. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef]
- Lin, J.; Tong, X.; Wang, K.; Shi, Z.; Li, Y.; Dargusch, M.; Wen, C. Biodegradable Zn-3Cu and Zn-3Cu-0.2Ti alloys with ultrahigh ductility and antibacterial ability for orthopedic applications. J. Mater. Sci. Technol. 2021, 68, 76–90. [Google Scholar] [CrossRef]
- Lan, C.; Hu, Y.; Wang, C.; Li, W.; Gao, X.; Lin, X. Phase formation and strengthening mechanism of Zn-2 wt%Cu alloy fabricated by laser powder bed fusion. Addit. Manuf. 2024, 85, 104153. [Google Scholar] [CrossRef]
- Tong, X.; Shen, T.; Zhou, X.; Zeng, J.; Tao, J.; Munir, K.; Li, Y.; Huang, S.; Wu, X.; Ma, J.; et al. Biodegradable Zn-Cu-Li alloys with ultrahigh strength, ductility, antibacterial ability, cytocompatibility, and suitable degradation rate for potential bone-implant applications. Smart Mater. Manuf. 2023, 1, 100012. [Google Scholar] [CrossRef]
- Tang, Z.; Niu, J.; Huang, H.; Zhang, H.; Pei, J.; Ou, J.; Yuan, G. Potential biodegradable Zn-Cu binary alloys developed for cardiovascular implant applications. J. Mech. Behav. Biomed. Mater. 2017, 72, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, D.; Liu, B.; Li, N.; Liang, L.; Chen, C.; Zhou, K.; Baker, I.; Wu, H. Microstructural evolution, mechanical properties and corrosion mechanisms of additively manufactured biodegradable Zn-Cu alloys. J. Mater. Sci. Technol. 2024, 186, 142–157. [Google Scholar] [CrossRef]
- Duan, J.; Li, L.; Cao, F.; Suo, Y.; Yang, Q.; Qin, J.; Wang, X.; Yang, Y. An in vitro and in vivo study of biodegradable Zn-Cu-Li alloy with high strength and ductility fabricated by hot extrusion combined with room-temperature ECAP. J. Mater. Res. Technol. 2024, 33, 4226–4242. [Google Scholar] [CrossRef]
- Ye, L.; Huang, H.; Sun, C.; Zhuo, X.; Dong, Q.; Liu, H.; Ju, J.; Xue, F.; Bai, J.; Jiang, J. Effect of grain size and volume fraction of eutectic structure on mechanical properties and corrosion behavior of as-cast Zn-Mg binary alloys. J. Mater. Res. Technol. 2022, 16, 1673–1685. [Google Scholar] [CrossRef]
- García-Mintegui, C.; Córdoba, L.C.; Buxadera-Palomero, J.; Marquina, A.; Jiménez-Piqué, E.; Ginebra, M.-P.; Cortina, J.L.; Pegueroles, M. Zn-Mg and Zn-Cu alloys for stenting applications: From nanoscale mechanical characterization to in vitro degradation and biocompatibility. Bioact. Mater. 2021, 6, 4430–4446. [Google Scholar] [CrossRef] [PubMed]
- Venezuela, J.; Dargusch, M.S. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef]
- Liu, S.; Yao, L.; Wang, Y.; Li, Y.; Jia, Y.; Yang, Y.; Li, N.; Hu, Y.; Kong, D.; Dong, X.; et al. Immunomodulatory hybrid micro-nanofiber scaffolds enhance vascular regeneration. Bioact. Mater. 2019, 21, 464–482. [Google Scholar] [CrossRef] [PubMed]
- Pachla, W.; Przybysz, S.; Jarzębska, A.; Bieda, M.; Sztwiertnia, K.; Kulczyk, M.; Skiba, J. Structural and mechanical aspects of hypoeutectic Zn-Mg binary alloys for biodegradable vascular stent applications. Bioact. Mater. 2021, 6, 26–44. [Google Scholar] [CrossRef]
- Su, L.; Liu, W.; Wang, Y.; Jiang, Y.; Li, Z.; Wang, M.; Liu, G. Corrosion behavior, antibacterial properties and in vitro and in vivo biocompatibility of biodegradable Zn-5Cu-xMg alloy for bone-implant applications. Biomater. Adv. 2024, 165, 214000. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhao, S.; Guillory, R.; Bowen, P.K.; Yin, Z.; Griebel, A.; Schaffer, J.; Earley, E.J.; Goldman, J.; Drelich, J.W. Novel high-strength, low-alloys Zn-Mg (<0.1wt% Mg) and their arterial biodegradation. Mater. Sci. Eng. C 2018, 84, 67–79. [Google Scholar] [CrossRef]
- Vojtěch, D.; Kubásek, J.; Šerák, J.; Novák, P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, A.; Guo, H.; Shen, Y.; Wen, P.; Lin, H.; Xia, D.; Voshage, M.; Tian, Y.; Zheng, Y. Additive manufacturing of Zn-Mg alloy porous scaffolds with enhanced osseointegration: In vitro and in vivo studies. Acta Biomater. 2022, 145, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A.M. Magnesium and osteoporosis: Current state of knowledge and future research directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Ebrahimi, S.; Yarmand, B. In-vitro corrosion and bioactivity behavior of tailored calcium phosphate-containing zinc oxide coating prepared by plasma electrolytic oxidation. Corros. Sci. 2020, 173, 108781. [Google Scholar] [CrossRef]
- Shoeib, M.A.; Abdel-Gawad, S.A. High performance nano hydroxyapatite coating on zinc for biomedical applications. J. Mater. Sci. 2023, 58, 740–756. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, P.H.; Zhou, Q.; Zhan, W.; Jin, F. Effects of pulsed magnetic field on microstructure, mechanical properties and bio-corrosion behavior of Mg-7Zn alloy. Mater. Lett. 2017, 193, 224–227. [Google Scholar] [CrossRef]
- Li, L.; Liang, W.; Ban, C.; Suo, Y.; Lv, G.; Liu, T.; Wang, X.; Zhang, H.; Cui, J. Effects of a high-voltage pulsed magnetic field on the solidification structures of biodegradable Zn-Ag alloys. Mater. Charact. 2020, 163, 110274. [Google Scholar] [CrossRef]
- Li, L.; Liang, W.; Yang, L.; Cao, F.; Sun, K.; Ban, C.; Cui, J. Structure Refinement and Homogenization of Zn-Cu Alloys Induced by a High-Voltage Pulsed Magnetic Field During the Solidification Process. Int. J. Met. 2023, 17, 399–413. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Y.-S.; Sun, M.-L. Microstructure refinement of AZ31 alloy solidified with pulsed magnetic field. Trans. Nonferrous Met. Soc. China 2010, 20, 1685–1690. [Google Scholar] [CrossRef]
- Liu, H.; Peng, C.; Yuan, Y.; Zhou, Q.; Chen, L.; Xu, Y. Effect of pulsed magnetic field on solidification microstructure and properties of Zn-3Cu alloy. Trans. Mater. Heat Treat. 2024, 45, 93–101. Available online: https://link.cnki.net/doi/10.13289/j.issn.1009-6264.2023-0275 (accessed on 5 February 2025).
- GB/T 228.1-2021; Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. Standards Press of China: Beijing, China, 2021.
- Okamoto, H. Comment on Mg-Zn (magnesium-zinc). J. Phase Equilibr. 1994, 15, 129–130. [Google Scholar] [CrossRef]
- Su, Y.; Lin, X.; Wang, M.; Huang, W. Phase and microstructure pattern selection of Zn-rich Zn-Cu peritectic alloys during laser surface remelting. J. Mater. Sci. 2021, 56, 14314–14332. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Zhong, H.; Xu, Z.; Li, L.; Gong, Y.; Miao, X.; Song, C.; Zhai, Q. Comparative Study on the Grain Refinement of Al-Si Alloy Solidified under the Impact of Pulsed Electric Current and Travelling Magnetic Field. Metals 2016, 6, 170. [Google Scholar] [CrossRef]
- Kabir, H.; Munir, K.; Wen, C.; Li, Y. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: Biomechanical and biocorrosion perspectives. Bioact. Mater. 2021, 6, 836–879. [Google Scholar] [CrossRef]
- ASTM G102-23; Standard Practice for Calculation of Corrosion Rates and Related Informationfrom Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2023.
- Afshari, V.; Dehghanian, C. Effects of grain size on the electrochemical corrosion behaviour of electrodeposited nanocrystalline Fe coatings in alkaline solution. Corros. Sci. 2009, 51, 1844–1849. [Google Scholar] [CrossRef]
- Johnston, S.; Shi, Z.; Atrens, A. The influence of pH on the corrosion rate of high-purity Mg, AZ91 and ZE41 in bicarbonate buffered Hanks’ solution. Corros. Sci. 2015, 101, 182–192. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Wu, G.; Zhao, Y.; McKenzie, D.R.; Bilek, M.M.M.; Chu, P.K. Electrochemical corrosion behavior of biodegradable Mg-Y-RE and Mg-Zn-Zr alloys in Ringer’s solution and simulated body fluid. Corros. Sci. 2015, 91, 160–184. [Google Scholar] [CrossRef]
- Li, H.F.; Xie, X.H.; Zheng, Y.F.; Cong, Y.; Zhou, F.Y.; Qiu, K.J.; Wang, X.; Chen, S.H.; Huang, L.; Tian, L.; et al. Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci. Rep. 2015, 5, srep10719. [Google Scholar] [CrossRef]
- Mwaanga, P.; Carraway, E.R.; van den Hurk, P. The induction of biochemical changes in Daphnia magna by CuO and ZnO nanoparticles. Aquat. Toxicol. 2014, 150, 201–209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).