1. Introduction
Bayan Obo rare earth concentrate (BOREC) is a mixture of bastnaesite and monazite; the ratio of bastnaesite to monazite ranges from 9:1 to 1:1. Therefore, the comprehensive recovery of fluorine and phosphorus resources is of importance for the industrial production of rare earths (RE). However, due to the complexity in the treatment of waste gas and waste residue produced in the decomposition of both high-temperature concentrated sulfuric acid roasting and high concentration of alkali conversion methods, the comprehensive utilization target is far from being reached [
1,
2,
3,
4]. For example, the waste gas produced by the sulfuric acid decomposition method contains fluorine, sulfur, phosphorus, silicon, etc., and its subsequent comprehensive recovery and waste gas treatment are very difficult. The filtrate produced during alkaline decomposition contains both trisodium phosphate and sodium fluoride, and finally, it is treated as wastewater using lime to precipitate fluorine and phosphate, which not only does not recover fluorine and phosphorus resources due to the poor quality of by-products but also produces a large amount of solid waste. Therefore, the efficient recovery of fluorine and phosphorus has always been a difficult point in the comprehensive utilization of BOREC.
In order to realize the efficient leaching and comprehensive utilization of BOREC, some new processes for the decomposition of BOREC have been developed [
4,
5,
6,
7,
8,
9,
10,
11,
12], such as roasting the ore in an inert and oxidative atmosphere followed by a hydrochloric acid countercurrent optimal solution leaching process, an alkali decomposition process, an acid–alkali combined low-temperature decomposition process and an acid–alkali two-step decomposition process. However, due to the complexity of the metallurgical extraction processes of BOREC, there are still many problems, such as the efficiency of mineral decomposition; the recovery rate and quality indicators of rare earth, fluorine, phosphorus and other resources; the saving of acid and alkali consumption in the whole process and the reduction in wastewater and residue [
12].
In contrast, both the single bastnaesite and monazite metallurgical extraction processes are relatively simple, inexpensive and environmentally friendly [
13]. If the bastnaesite and monazite are separated into two concentrates, respectively, and then leached or decomposed by two different processes, it is easy to meet the requirements of the comprehensive utilization of elements. Therefore, many works have been carried out around the beneficiation and separation of bastnaesite and monazite [
13,
14,
15,
16,
17,
18,
19,
20,
21,
22]. For example, Ren et al. [
14] used benzoic acid as a collector and alum as an inhibitor to separate bastnaesite and monazite via flotation under acidic conditions. Alum had a selective flotation effect on monazite but had little effect on the flotation of bastnaesite; Sarvaramini et al. [
15] and Wang et al. [
16,
17] found that the recovery of monazite was approximately 75% when citric acid was used as the inhibitor of bastnaesite, and monoalkyl phosphate was used as the collector. Moreover, the recovery of bastnaesite was only 15% at a citric acid dosage of 50 mg/L, which was favorable for the selective separation of bastnaesite and monazite. However, the separation efficiency and serious chemical contamination make it difficult to meet the requirements of their widespread industrial application [
18,
19,
20,
21,
22,
23].
Therefore, chemical beneficiation linked to wet selective leaching has attracted extensive attention. According to the leaching order of bastnaesite and monazite by acids, selectively leaching bastnaesite from monazite can be realized by tuning the acidity and other conditions. However, although increasing the acid concentration and reaction temperature can improve the leaching efficiency of bastnaesite, the leaching rate of monazite will also increase. Therefore, the selective leaching of bastnaesite at low acid concentrations and low temperatures is the key to solving this technical problem [
24,
25].
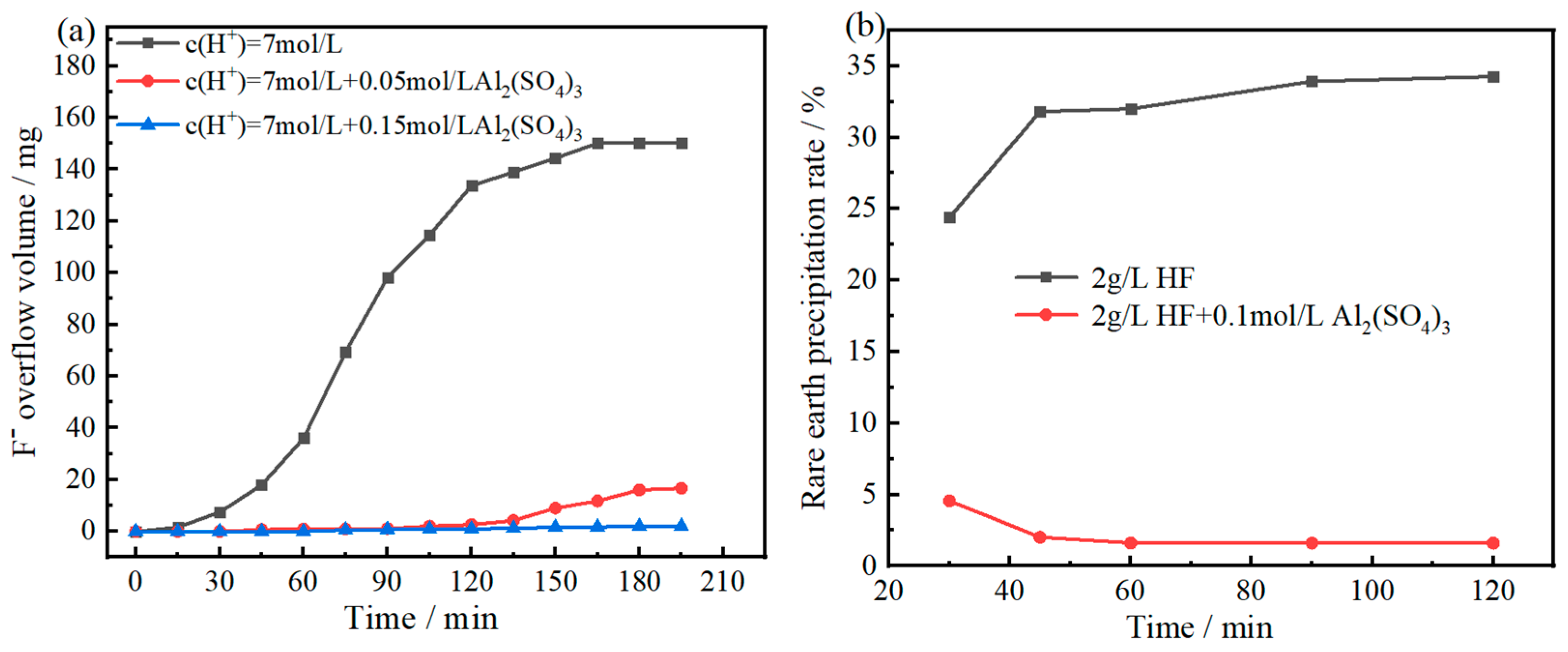
Based on the principle of chemical equilibrium, the leaching of bastnaesite can be enhanced by any reagent and reaction method that can reduce the concentration of hydrogen fluoride (HF) produced during the acid leaching process of bastnaesite. Among them, the formation of fluorine complexes is an effective way. For example, boric acid complex leaching was used to recover F from the bastnaesite leaching solution by preparing the by-product KBF
4 [
26].
In fact, iron, aluminum and boron can form stable complexes with fluorine. In particular, aluminum has a stronger coordination ability toward F [
27]. Li et al. and Zhang et al. studied the separation of bastnasite from monazite by using a solution of HCl-AlCl
3 for leaching bastnasite [
28,
29,
30,
31]. Accordingly, they used aluminum to complex fluorine in bastnaesite to promote the decomposition of BOREC and studied the complex leaching effect of HCl-AlCl
3 and HNO
3-Al(NO
3)
3 systems on BOREC. These methods proved effective in leaching a significant portion of bastnasite and addressed the challenge of dissolving bastnasite. The results show that under the optimal leaching conditions, the HCl-AlCl
3 system can achieve a concentrate leaching rate (CLR) of 74.80% and a rare earth leaching rate (RELR) of 69.08% in BOREC [
28,
29]. The HNO
3-Al(NO
3)
3 system can make the fluorine leaching rate (FLR) reach 97.59% [
30]. They also used microwaves to facilitate the reaction and reduce the amount of HCl by 30%, AlCl
3 by 46.7% and the reaction time by 16.67% under optimal conditions [
31], achieving the separation purpose of bastnaesite from monazite.
Meanwhile, a green process involving selective mineral phase transformation by heating, followed by leaching to separate bastnaesite and monazite in BOREC to facilitate their subsequent decomposition or extraction, was proposed [
13]. Under suitable conditions, bastnasite decomposed into REOF with a leaching efficiency of 93.7%, and monazite remained unchanged with a leaching efficiency of only 3.2%. Furthermore, the content of monazite in the leach residue was 91.2%.
Recently, we studied the leaching of bastnaesite from BOREC in the H
2SO
4-Al
2(SO
4)
3 system, and the results showed that under the optimal conditions, the CLR in BOREC could reach 68.00%, the RELR could reach 66.91%, and the FLR could reach 94.42%. At the same time, N1923 was used to extract rare earth from leachate with an extraction rate of 97.38%, and then a rare earth chloride solution with a ratio of 0.008 for aluminum to rare earth was obtained after stripping with hydrochloric acid, which basically realized the separation of RE from Al [
32]. The fluorine and aluminum in the raffinate are recovered in the form of cryolite, which realizes the recycling of RE, F and Al resources [
33].
It is demonstrated that the leaching capacity of HCl for bastnaesite is higher than that of sulfuric acid, but HCl is easy to volatilize at higher temperatures and produces acid-containing waste gas. Moreover, in HCl media, the separation of REs from Al is more difficult. Although the REs can be recovered via sodium rare earth sulfate (SRES) double salt, the consumption of sulfate is high. Furthermore, the sulfate and calcium ions in the leachate can be precipitated in the form of calcium sulfate (CS) and will remain in the leaching slag with the undecomposed monazite, which will increase the alkali consumption of the subsequent alkaline decomposition of monazite and affect the purity of the trisodium phosphate by-product.
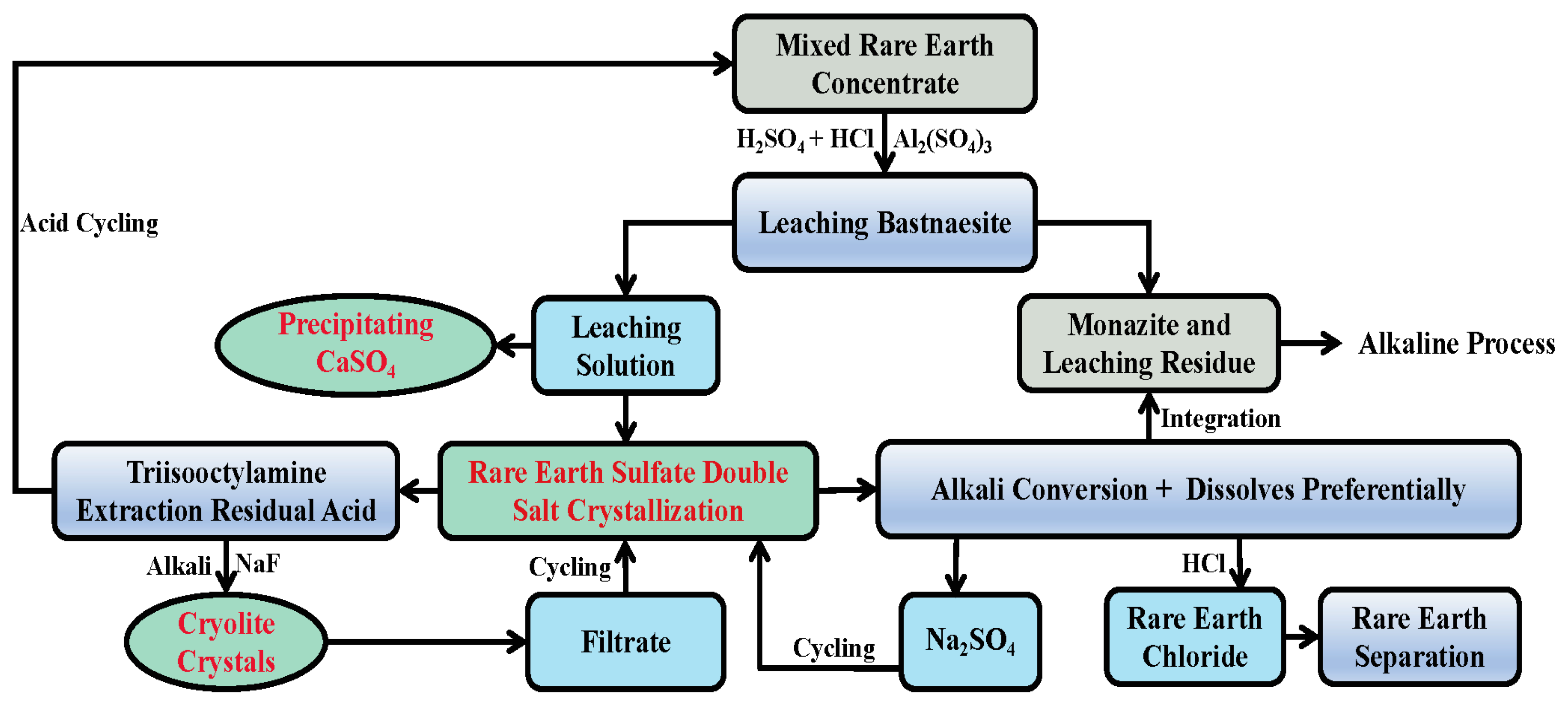
In this study, a small amount of HCl was added to the sulfuric acid and aluminum sulfate (AS) mixture solution to improve the leaching selectivity of bastnaesite. The RE in the leaching solution can be precipitated by sodium sulfate (SS) or extracted by N1923, achieving an efficient separation of REs from Al. Therefore, the effects of the ratio of c(H2SO4) to c(HCl), hydrogen ion concentration, liquid–solid ratio, Al concentration, reaction time, reaction temperature and other factors on the leaching rate (LR) of bastnaesite from BOREC with H2SO4-HCl-Al2(SO4)3 were studied, and the optimal leaching conditions and the kinetic equation of leaching bastnaesite were determined. Subsequently, the process conditions of respectively isolating calcium sulfate, SRES and cryolite were studied. It was found that the addition of HCl in the leaching solution can avoid the precipitation of calcium sulfate in the acid leaching process. Excellent conditions are provided for the recovery of calcium sulfate from the filtered leachate by the addition of seed crystals.
4. Conclusions
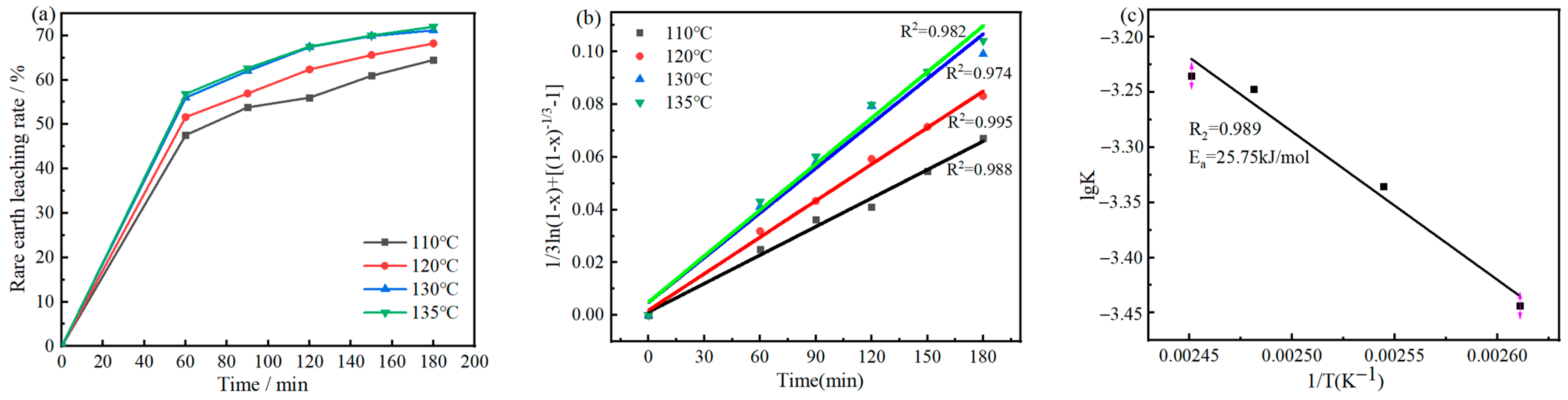
Adding appropriate amounts of HCl into the mixture solution of sulfuric acid and aluminum sulfate can improve the leaching rate and selectivity of bastnaesite, avoid the precipitation of calcium sulfate in the leaching residue during leaching between 115 and 135 °C, preserve the content of monazite and reduce the difficulty of subsequent alkaline decomposition. At the same time, it can also meet the requirements for recovering REs via the sodium rare earth sulfate precipitation method, and the rare earth leaching process follows the shrinking core kinetic model, with an activation energy of 25.75 kJ/mol.
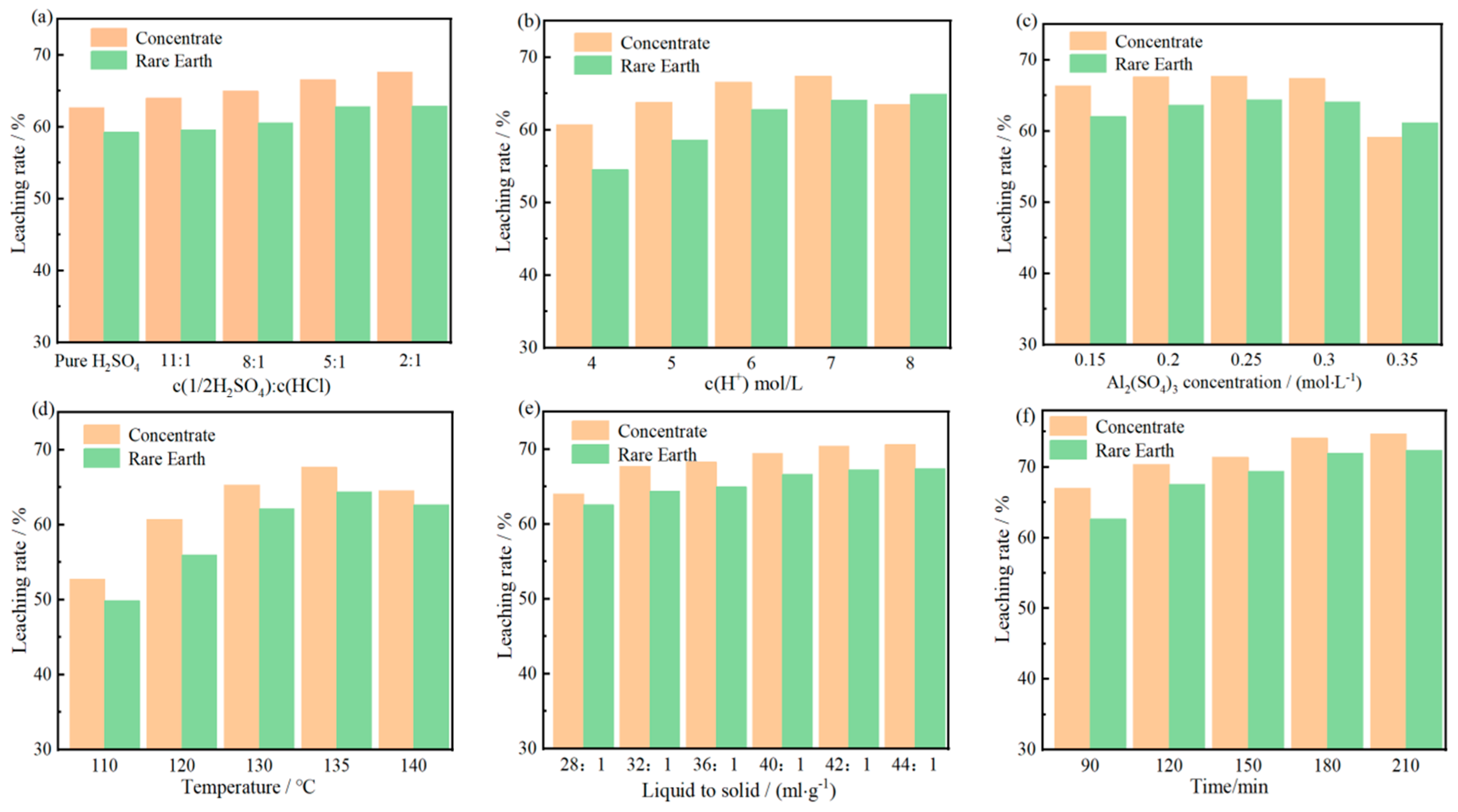
The optimal leaching conditions for bastnaesite in BOREC using the H2SO4-HCl-Al2(SO4)3 mixed leaching system are determined to be c(1/2H2SO4):c(HCl) = 5:1, the liquid–solid ratio 42:1, hydrogen ion concentration 7 mol/L, aluminum sulfate concentration 0.25 mol/L, temperature 135 °C and the time 3 h. At this time, the concentrate leaching rate is 74.08%, and the RE leaching rate is 71.95%.
The main mineral phase of the leaching residue is monazite, which only contains a very small amount of bastnaesite. There are traces of erosion on the surface of the particles, with pores and unevenness, and obvious reaction marks. The contents of Ca and F in the leaching residue decreased significantly, indicating that almost all the Ca and F in BOREC have been leached out; the total F element in the mineral has decomposed by 94.43%. However, the P content in the slag increased significantly, and the decomposition rate of bastnaesite was 96.84%.
The calcium sulfate product obtained by adding 0.1% calcium sulfate seeds has better crystallinity. After stirring at room temperature for 1 h and standing at room temperature for 2 days, the calcium ion precipitation rate is 77.35%. The XDR and XRF determination results confirmed that the obtained product is indeed calcium sulfate with no other impurity peaks. Its purity is 99.22%, and the impurities are mainly REs, with a content of 0.66%.
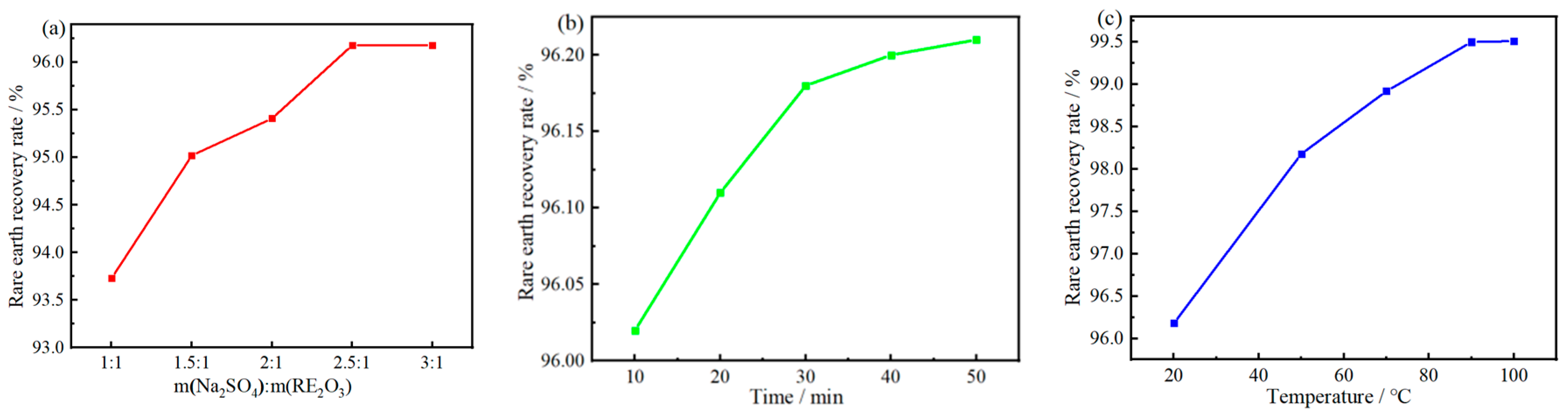
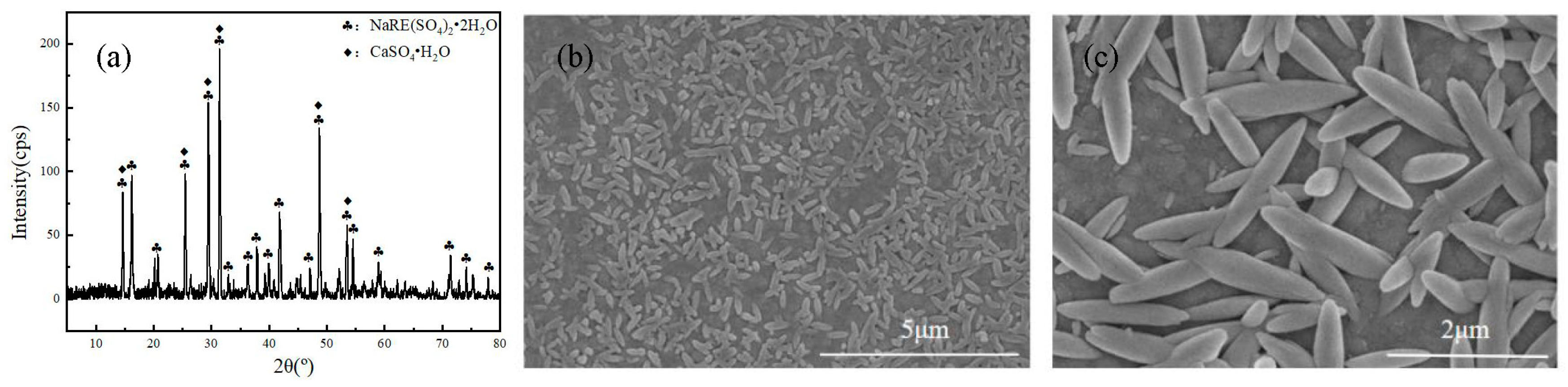
The optimal conditions for the sodium rare earth sulfate precipitation method are reaction time of 30 min, stirring speed of 100 r/min, reaction temperature of 90 °C and m(Na2SO4):m(RE2O3) = 2.5:1 At this time, the RE recovery rate is 99.5%. The XRD, XRF and SEM analysis results show that the purity of the obtained SRES product is 98.47%, with a regular spindle-shaped morphology and relatively uniform particle size below 2 µm.
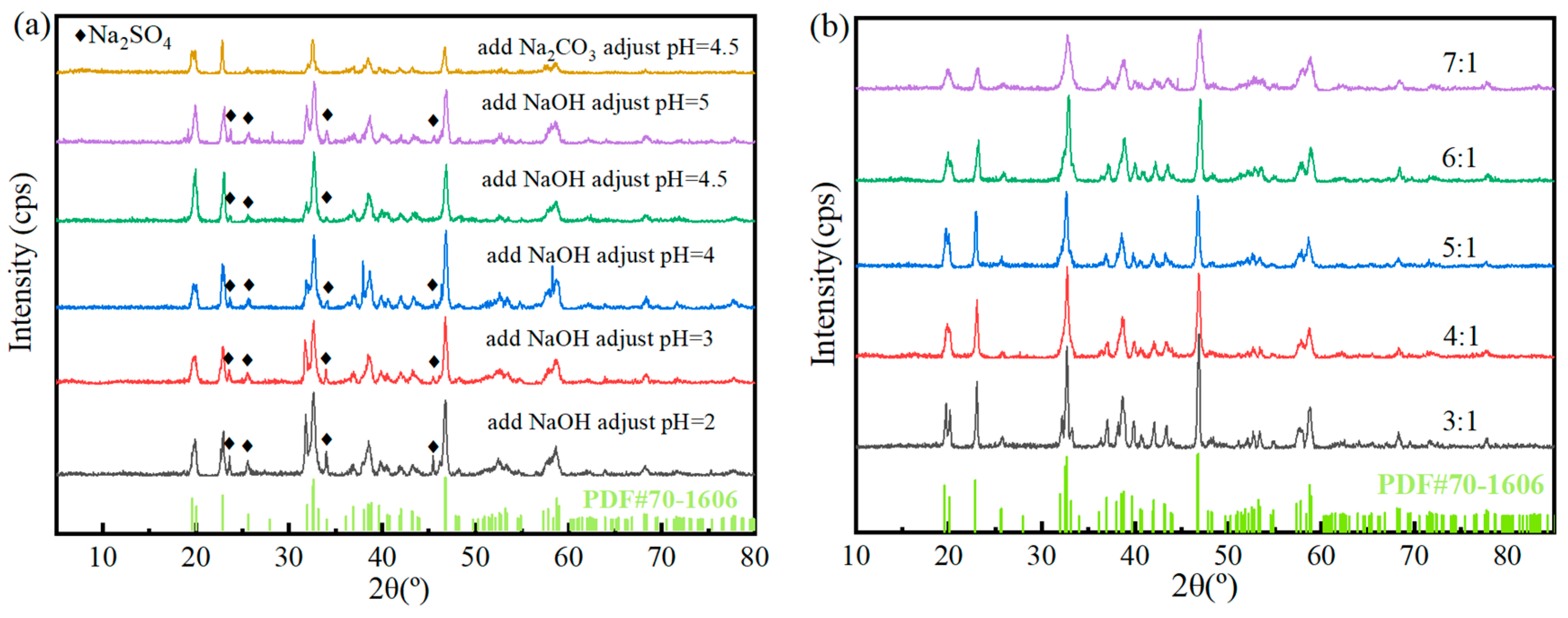
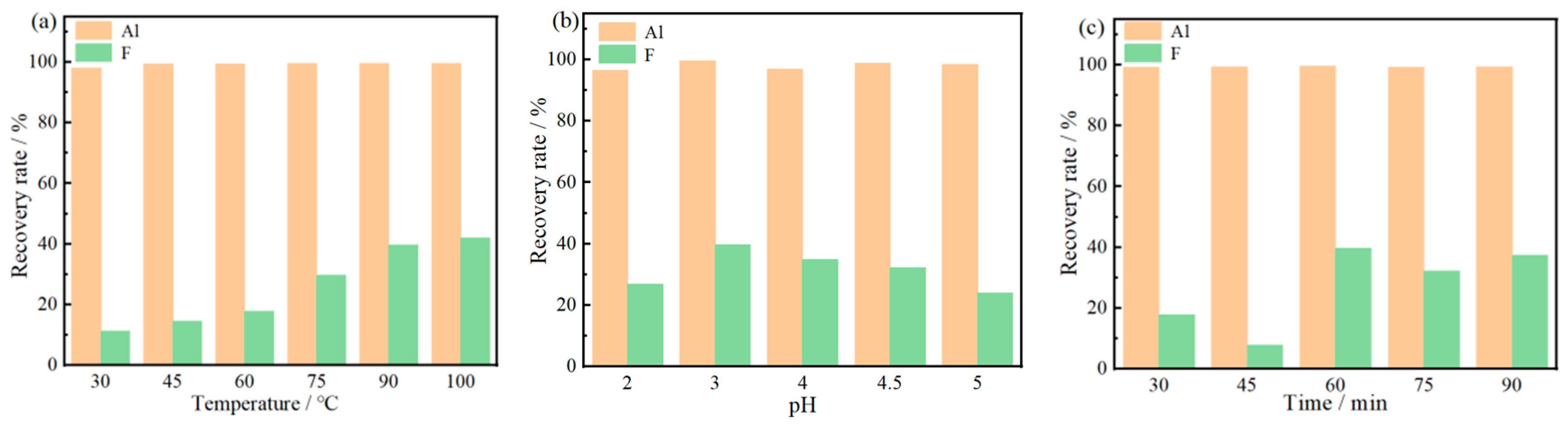
By adding NaF to make the F:Al ratio of 6:1, adjusting the pH to 3 with 1 mol/L sodium carbonate and stirring at 150 r/min for 60 min at 90 °C, the recovery rates of Al and F ions were 99.59% and 39.64%, respectively. Extracting the residual acid from the leachate using triisooctylamine at a phase ratio of 2:1, and then adjusting the pH with sodium carbonate, the extraction rate of acid is 54.53%, which can save 59.3% of sodium carbonate. At this time, the recovery rates of Al and F ions are 99.6% and 62.02%, respectively. The purity of the obtained cryolite is 95.59%, with the main impurity being SO42−. The XRD, XRF and SEM analysis results show that the product is composed of a single cryolite phase with a relatively uniform particle size and a small overall particle size of less than 1 µm.
This study primarily addresses the issues associated with the decomposition of Baotou rare earth ore using concentrated sulfuric acid. Based on the complex leaching method, a more environmentally friendly process flow is proposed. However, further work is still required, mainly including the following points:
Further purification of the obtained rare earth sulfate double salt products, such as through alkali conversion, to ultimately obtain high-purity rare earth products.
Further optimization of the synthesis conditions for cryolite or purification of the cryolite product to meet industrial standards.
Further refinement of the entire process flow to enable the recycling of inputs such as acid and sodium sulfate throughout the process, making the process more environmentally friendly and energy efficient.
Author Contributions
Conceptualization, L.L. and Y.L. (Yanzhu Liu); methodology, L.L., X.Y. and X.H.; software, Y.D. and X.H.; validation, Y.D., X.Y. and X.H.; formal analysis, L.L.; investigation, L.L. and Y.L. (Yanzhu Liu); resources, Y.D.; data curation, L.L. and H.X.; writing—original draft preparation, H.X. and Y.L. (Yanzhu Liu); writing—review and editing, Y.L. (Yanzhu Liu), Y.L. (Yongxiu Li) and Y.D.; visualization, Y.L. (Yongxiu Li); supervision, Y.L. (Yanzhu Liu) and Y.L. (Yongxiu Li); project administration, Y.L. (Yanzhu Liu) and Y.L. (Yongxiu Li); funding acquisition, Y.D. and Y.L. (Yongxiu Li). All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported financially by the State Key Laboratory of Baiyun Obo Rare Earth Resources Research and Comprehensive Utilization (grant number 202172362) and the National Key Research Development Program of China (grant number 2022YFC2905201).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors sincerely thank Fen Nie, Ying Ma, Bingwei Li, Shaohua Zeng, Dongping Li and Jing Li for their support of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BOREC | Bayan Obo rare earth concentrate |
| RELR | rare earth leaching rate |
| FLR | fluorine leaching rate |
| CLR | concentrate leaching rate |
| CS | calcium sulfate |
| SRES | sodium rare earth sulfate |
| AS | aluminum sulfate |
| SS | sodium sulfate |
References
- Li, Y.X.; Zhu, W.C.; Zhang, L.Z.; Liu, Y.Z. Rare Earth Metallurgy and Environmental Protection, 1st ed.; Chemical Industrial Press: Beijing, China, 2024; Volume 1, ISBN 978-7-122-46001-1. [Google Scholar]
- Cheng, S.; Li, W.; Han, Y.; Sun, Y.; Gao, P.; Zhang, X. Recent process developments in beneficiation and metallurgy of rare earths: A review. J. Rare Earths 2024, 42, 629–642. [Google Scholar] [CrossRef]
- Ma, Y.; Li, N.; Wang, Q.W.; Yang, Q.S. Characteristics and current research situation of rare earth resources in Bayan Obo Ore. J. Chin. Soc. Rare Earths 2016, 34, 641–649. [Google Scholar]
- Wang, L.; Huang, X.; Yu, Y.; Zhao, L.; Wang, C.; Feng, Z.; Cui, D.; Long, Z. Towards cleaner production of rare earth elements from bastnaesite in China. J. Clean. Prod. 2017, 165, 231–242. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.; Zhang, D.; Gao, K.; Li, J.; Weng, Z.; Xu, W. Phase change, micro- structure and reaction mechanism during high temperature roasting of high grade rare earth concentrate. J. Rare Earths 2020, 38, 1140–1150. [Google Scholar] [CrossRef]
- Zhao, J.; Pan, F.; Liu, H. An environmental friendly Na2CO3-roasting decomposition strategy for the mixed rare earth concentrate. Sep. Purif. Technol. 2016, 168, 161–167. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Shuai, G.; Long, Z.; Cui, D.; Huang, X. Thermal decomposition and oxidation of bastnaesite concentrate in inert and oxidative atmosphere. J. Rare Earths 2018, 36, 758–764. [Google Scholar] [CrossRef]
- Li, M.; Zhang, D.; Yan, Y.; Gao, K.; Liu, X.; Li, J. Effect of oxidation behavior of cerium during the roasting process on the leaching of mixed rare earth concentrate. Hydrometallurgy 2017, 174, 156–166. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Liu, Z.; Zhang, D.; Gao, K.; Li, M. A novel, clean, closed-loop process for directional recovery of rare earth elements, fluorine, and phosphorus from mixed rare earth concentrate. J. Clean. Prod. 2021, 321, 128784. [Google Scholar] [CrossRef]
- Liu, W.; Feng, X.; Noble, A.; Yoon, R.H. Ammonium sulfate leaching of NaOH- treated monazite. Miner. Eng. 2022, 188, 107817. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.; Zou, D.; Liu, T.; Li, D. Kinetics of nitric acid leaching of cerium from oxidation roasted Baotou mixed rare earth concentrate. J. Rare Earths 2019, 37, 198–204. [Google Scholar] [CrossRef]
- Liu, C.H.; Zhang, H.P.; Luo, B.; Li, L.Q.; Sun, X.Q. A quantitative recovery process of rare earth in bastnaesite leachate for energy saving and emission reduction. Miner. Eng. 2022, 190, 107920. [Google Scholar] [CrossRef]
- He, J.H.; Gao, P.; Yuan, S.; Cheng, S.K.; Ning, J.L.; Zhou, Z.Y.; Sun, Y.S.; Li, W.B. High efficiency separation of bastnaesite (REFCO3) and monazite (REPO4) in mixed rare earth concentrate by heating under N2 and leaching with HCl/AlCl3. Hydrometallurgy 2024, 228, 106338. [Google Scholar] [CrossRef]
- Ren, J.; Song, S.X.; Lopez-Valdivieso, A.; Lu, S.C. Selective flotation of bastnaesite from monazite in rare earth concentrates using potassium alum as depressant. Int. J. Miner. Process. 2000, 59, 237–245. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Azizi, D.; Larachi, F. Hydroxamic acid interactions with solvated cerium hydroxides in the flotation of monazite and bastnasite—Experiments and DFT study. Appl. Surf. Sci. 2016, 387, 986–995. [Google Scholar] [CrossRef]
- Wang, W.W.; Li, E.R.; Peng, Z.K.; Guo, C.L.; Hou, S.C.; Li, Q. Flotation separation of bastnaesite from monazite using depressant dextrin hydrate and its depression mechanism. Miner. Eng. 2023, 200, 108151. [Google Scholar] [CrossRef]
- Wang, W.W.; Peng, Z.K.; Guo, C.L.; Li, Q.; Liu, Y.J.; Hou, S.C.; Jin, H.L. Exploring rare earth mineral recovery through characterization of riebeckite type ore in Bayan Obo. Heliyon 2023, 9, e14060. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.S.; Satur, J.V.; Sanematsu, K.; Laukkanen, J.; Saastamoinen, T. Beneficiation studies of a complex REE ore. Miner. Eng. 2015, 71, 55–64. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, L.H.; Wang, D.H.; Xue, K.; Tian, J. Selective adsorption mechanism of SHMP onto fine fluorite in bastnaesite flotation system. Colloids Surf. A Physicochem. Eng. Aspects 2023, 670, 131527. [Google Scholar] [CrossRef]
- Marion, C.; Li, R.; Waters, K.E. A review of reagents applied to rare-earth mineral flotation. Adv. Colloid Interface Sci. 2020, 279, 102142. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wu, H.Q.; Yang, J.; Tang, Z.; Luo, L.P.; Shu, K.Q.; Xu, Y.B.; Xu, L.H. Selective flotation separation of bastnaesite from calcite using xanthan gum as a depressant. Appl. Surf. Sci. 2020, 512, 145714. [Google Scholar] [CrossRef]
- Yang, Z.R.; Bian, X.; Wu, W.Y. Flotation performance and adsorption mechanism of styrene phosphonic acid as a collector to synthetic (Ce, La)2O3. J Rare Earths 2017, 35, 621–628. [Google Scholar] [CrossRef]
- Cao, Z.; Cao, Y.D.; Qu, Q.Q.; Zhang, J.S.; Mu, Y.F. Separation of bastnäsite from fluorite using ethylenediamine tetraacetic acid as depressant. Miner. Eng. 2019, 134, 134–141. [Google Scholar] [CrossRef]
- Zhou, X.Z.; Li, H.Y.; Liu, Y.Z.; Li, Y.X. Function, energy and environmental orientation in innovation research of rare earth materials. Sci. China Technol. Sci. 2019, 62, 2303–2305. [Google Scholar] [CrossRef]
- Cen, P.; Bian, X.; Liu, Z.; Gu, M.; Wu, W.; Li, B. Extraction of rare earths from bastnaesite concentrates: A critical review and perspective for the future. Miner Eng. 2021, 171, 107081. [Google Scholar] [CrossRef]
- Li, Y.L.; Zou, D.; Chen, J.; Deng, Y.F.; Li, D.Q. Stepwise recovery of cerium and fluorine from bastnaesite: Utilizing complex properties of B-F to obtain high purity CeO2 and KBF4. Sep. Purif. Technol. 2023, 310, 123152. [Google Scholar] [CrossRef]
- Li, Y.Y.; Hu, Q.Y.; Li, H.Y.; Li, J.; Liu, Y.Z.; Zhou, X.Z.; Li, D.P.; Li, Y.X. Rare earths and aluminum: From entanglement to respect with each other. J. Chin. Soc. Rare Earths 2021, 39, 479–489. [Google Scholar]
- Li, M.; Zhang, X.W.; Liu, Z.G.; Wang, M.T.; Liu, J.; Yang, J.P. Mixed rare earth concentrate leaching with HCl–AlCl3 solution. Rare Metals 2013, 32, 312–317. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.W.; Liu, Z.G.; Wang, M.T.; Liu, J.; Yang, J.P. Kinetics of leaching fluoride from mixed rare earth concentrate with hydrochloric acid and aluminum chloride. Hydrometallurgy 2013, 140, 71–76. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Liu, Z.; Hu, Y.; Liu, J.; Yang, J. Separation of Baotou mixed rare earth concentrate by HNO3-Al(NO3)3 solution. J. Chin. Soc. Rare Earths 2013, 31, 588–596. [Google Scholar]
- Zhang, D.; Li, M.; Gao, K.; Li, J.; Yan, Y.; Liu, X. Physical and chemical mechanism underlying ultrasonically enhanced hydrochloric acid leaching of non-oxidative roasting of bastnaesite. Ultrason. Sonochem. 2017, 39, 774–781. [Google Scholar] [CrossRef]
- Li, C.C.; Yang, L.F.; Wang, Y.; Li, H.Y.; Wang, D.S.; Liu, Y.Z.; Zhou, X.Z.; Wang, X.F.; Liu, M.B.; Li, Y.X. Extraction separation of rare earth and aluminum from ion-adsorbed rare earth leaching solution by Primary Amine N1923. J. Chin. Soc. Rare Earths. 2019, 37, 351–360. [Google Scholar]
- Li, J.; Yang, Y.; Tao, W.; Wang, Z.; Yu, J.; Yang, J.; Liu, Z. Study on preparation of aluminum fluoride from cryolite. Mater. Today Commun. 2023, 37, 107440. [Google Scholar] [CrossRef]
- LI, H.P.; Han, M.X.; Hu, G.S.; Wang, B.; Tian, B. Reduction during Rare Earth Extraction and Separation by Induced Crystallization. J. Rare Earths 2021, 42, 93–98. [Google Scholar]
- Lai, F.G.; Guo, H.; Xiao, Y.F.; Xu, Z.F. Research progress on solubility phase diagrams of calcium sulfate in chloride-sulfate solutions. J. Inorg. Chem. Ind. 2018, 50, 16–21. [Google Scholar]
Figure 1.
Process flow diagram of leaching BOREC by H2SO4-HCl-Al2(SO4)3 and the resource recovery.
Figure 2.
Time dependence of HF overflow (a) and the coordination dissolution of REF3 (b).
Figure 3.
Effects of ratio of [H2SO4] to [HCl] (c(H+) = 6 mol/L, AS concentration 0.3 mol/L, liquid–solid ratio 32:1, temperature 135 °C, and time 2 h) (a), [H](c(1/2H2SO4): c(HCl) = 5:1, AS concentration 0.3 mol/L, liquid–solid ratio 32:1, temperature 135 °C, and time 2 h) (b), [Al](c(1/2H2SO4): c(HCl) = 5:1, c(H+) = 7 mol/L, liquid–solid ratio 32:1, temperature 135 °C, and time 2 h) (c), reaction temperature (c(1/2H2SO4): c(HCl) = 5:1, c(H+) = 7 mol/L, liquid–solid ratio 32:1, AS concentration 0.25 mol/L, and time 2 h) (d), liquid–solid ratio (c(1/2H2SO4): c(HCl) = 5:1, c(H+) = 7 mol/L, temperature 135 °C, AS concentration 0.25 mol/L, and time 2 h) (e), and reaction time (c(1/2H2SO4): c(HCl) = 5:1, c(H+) = 7 mol/L, temperature 135 °C, AS concentration 0.25 mol/L, and liquid–solid ratio 42:1) (f) on CLR and RELR.
Figure 4.
XRD patterns (a) and SEM of BOMREC (c,d) and its leaching residue (b,e,f) from H2SO4-HCl-Al2(SO4)3.
Figure 5.
Time dependence of rare earth leaching rate at different temperatures (a) and the relationship between 1/3ln(1 − x) + [(1 − x) −1/3 − 1] and t (b), Arrhenius plot for decomposition rate of RE minerals between 110 °C and 135 °C (c).
Figure 6.
Precipitation rate (a), XRD (b), RE content (c), and electron microscopy images ((d1) CS dihydrate seeds; (d2) room temperature; (d3) ice water bath; (d4) add 0.05% CS seed crystals at room temperature; (d5) add 0.1% seed crystals at room temperature; (d6) add 0.25% seed crystals at room temperature) (d) of CS under different precipitation conditions.
Figure 7.
Effects of SS dosage (the reaction temperature is 20 °C, the reaction time is 30 min) (a), reaction time (m(Na2SO4):m(RE2O3) = 2.5:1, the reaction temperature is 20 °C) (b) and temperature (m(Na2SO4):m(RE2O3) = 2.5:1, the reaction time is 30 min (c) on RE recovery rate.
Figure 8.
XRD (a) and SEM (b,c) images of SRES.
Figure 9.
XRD patterns of cryolite products synthesized under different alkaline pH adjustments (a) and different F:Al ratios (b).
Figure 10.
Effects of reaction temperature (reaction time is 60 min, pH = 3) (a), time (reaction time is 60 min, and temperature is 90 °C) (b), and pH (temperature is 90 °C, pH = 3) (c) on the recovery rate of F and Al.
Figure 11.
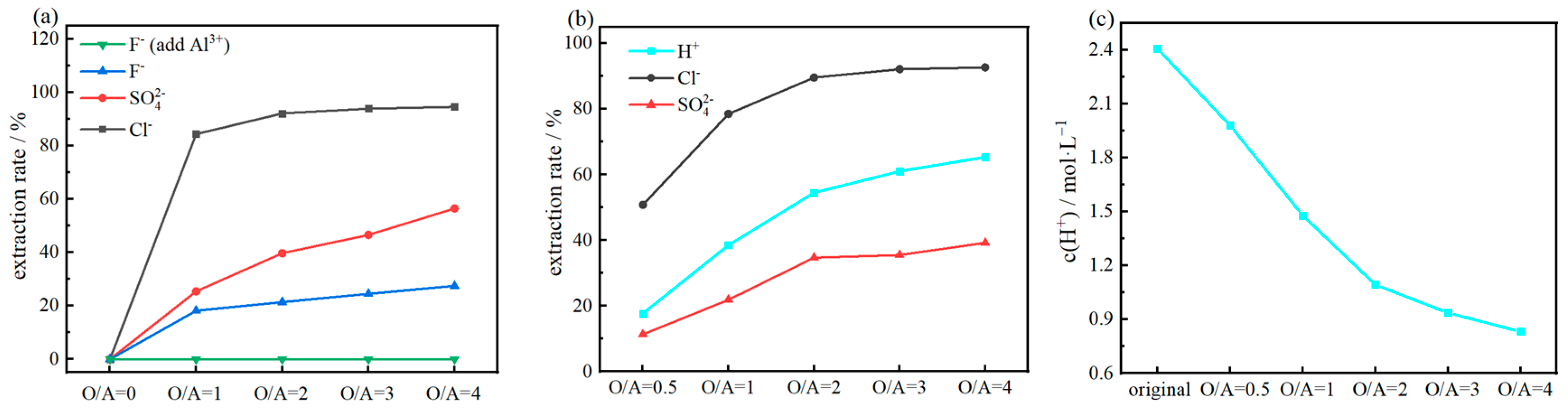
Compares the extraction efficiency of triisooctylamine for different acids (a,b) and changes in hydrogen ion concentration in the extract (c).
Figure 12.
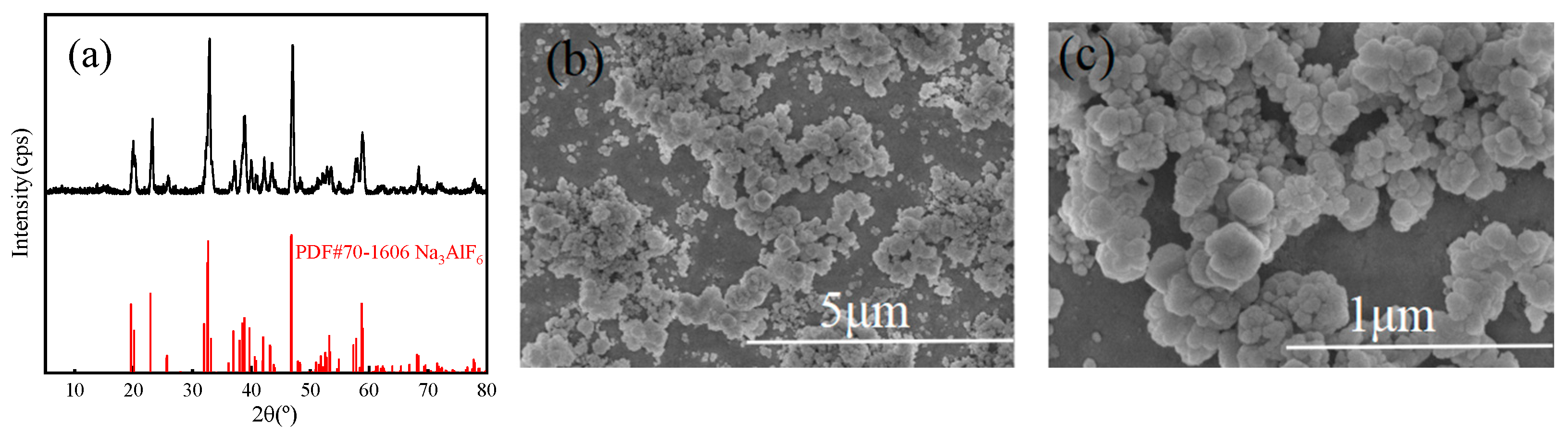
XRD (a) and electron microscopy images (b,c) of cryolite products.
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).