Abstract
With the increasing demand for premium-quality aluminum alloy castings that can be used as safety-critical structural components, as well as the rising urge to utilize sustainable materials during the manufacturing process, novel technologies need to be developed and implemented during the treatment of liquid alloys. Impurity and alloying elements accumulate in recycled aluminum alloys, which frequently results in the formation of coarse intermetallic compound (IMC) particles in the microstructure that have a detrimental effect on the ductility of cast products. One successful approach to alleviate this negative effect relies on affecting the phase selection and refinement of IMC phases. A growing body of literature has shown that the crystallization process of IMCs is affected by the native oxide phases present in the liquid alloys. It has also been demonstrated that by appropriate technologies, harmful oxide inclusion (like oxide bifilms) can be transformed into small-sized oxide particles that can be dispersed throughout the liquid alloy to serve as heterogeneous nucleation sites for different phases. In this way, the adverse effects of oxide inclusions and IMCs are simultaneously mitigated. This contribution aims to review the recent progress of experimental and theoretical work related to intermetallic particle refinement by oxide phases. Emerging technological solutions capable of refining intermetallics through transforming harmful oxide inclusions into numerous, well-dispersed heterogeneous nucleation sites are comprehensively reviewed. Besides analyzing the current state of these techniques, this discussion evaluates their future implications and the potential challenges that may arise in their application and development.
1. Introduction
The development of aluminum alloy casting technologies is critical to the sustainability of the global automotive and aerospace sectors. These industries have seen a rise in interest in casting aluminum alloys in recent years due to their outstanding castability, high strength-to-weight ratio, low manufacturing costs, and suitability for recycling [1,2,3]. Due to the rising share of large body-in-white components (like gigacastings or megacastings) and new, electric vehicle (EV)-specific parts like e-drive and battery pack housings, the average aluminum cast product content in passenger vehicles is expected to rise continuously [4,5,6]. Novel technologies must be developed and put into practice for the treatment and solidification processing of liquid alloys due to the increasing urge to use sustainable materials during the manufacturing process and the growing demand for premium-quality aluminum alloy castings that can be used as safety-critical structural components [7,8]. At present, a significant proportion of high-quality aluminum castings is produced using primary alloys. On the other hand, global aspirations towards sustainability drive the industry to increase the usage of recycled aluminum alloys. Increasing the impurity tolerance of aluminum alloys would take a step forward, achieving the full circulation of Al alloys. The adverse effects of impurity and alloying elements accumulated in recycled aluminum must be alleviated to prevent the functional downgrading of secondary aluminum and minimize the need for diluting with primary alloys [8,9,10].
The increased impurity concentration of secondary alloys generally results in the formation of coarse intermetallic compound (IMC) particles in the microstructure that have a detrimental effect on the ductility of cast products. Fe is generally recognized as one of the most significant impurity elements of casting aluminum alloys due to its strong negative impact on the ductility (and, in this way, the crashworthiness) [11,12,13,14,15,16], castability [17,18,19,20], and susceptibility to porosity formation [21,22,23]. Fe can form numerous different intermetallic phases in aluminum alloys with varying compositions, crystal structures, and morphologies [24,25,26,27,28,29]. The most common Fe-rich intermetallic compounds of casting aluminum alloys include α-Al15 (Fe,Mn)3Si2, α’-Al8Fe2Si, β-Al5FeSi, δ-Al4FeSi2, π-Al8Mg3FeSi6, θ-Al13Fe4, and η-Al6 (Fe,Mn) [14,30]. Besides Fe, other alloying elements are also prone to accumulation during recycling due to their relatively high concentration in certain alloy types and the unfeasibility of incorporating technologies capable of their removal into the recycling processes. Elements like Cu, Mn, and Si are all common alloying elements in certain casting alloys but can be troublesome in others [31]. For example, Si is an essential alloying element for most casting alloy types, but it is regarded as an impurity in Al-Mg-Fe alloys like the Castaduct alloy family by Rheinfelden Alloys GmbH due to the possibility of forming a platelet-like β-Al5FeSi intermetallic compound [32,33,34]. Casting aluminum alloys are generally multicomponent systems with various major and minor alloying elements, as well as impurities [35]. For this reason, a large number of different intermetallic phases can form besides Fe-rich IMCs during solidification, such as Al2Cu in Al-Cu and Al-Si-Cu alloys, Q-Al5Mg8Cu2Si6 in Al-Si-Mg-Cu alloys, and Mg2Si in Al-Mg-Si and Al-Si-Mg alloys [36,37,38,39,40,41]. In addition, the application of grain refiners containing Ti or Zr can lead to the formation of Ti- and/or Zr-containing aluminides, while Sr addition, which is usually made to modify eutectic Si particles, leads to the formation of AlSrSi compounds like Al2SrSi2 [42,43,44,45,46,47].
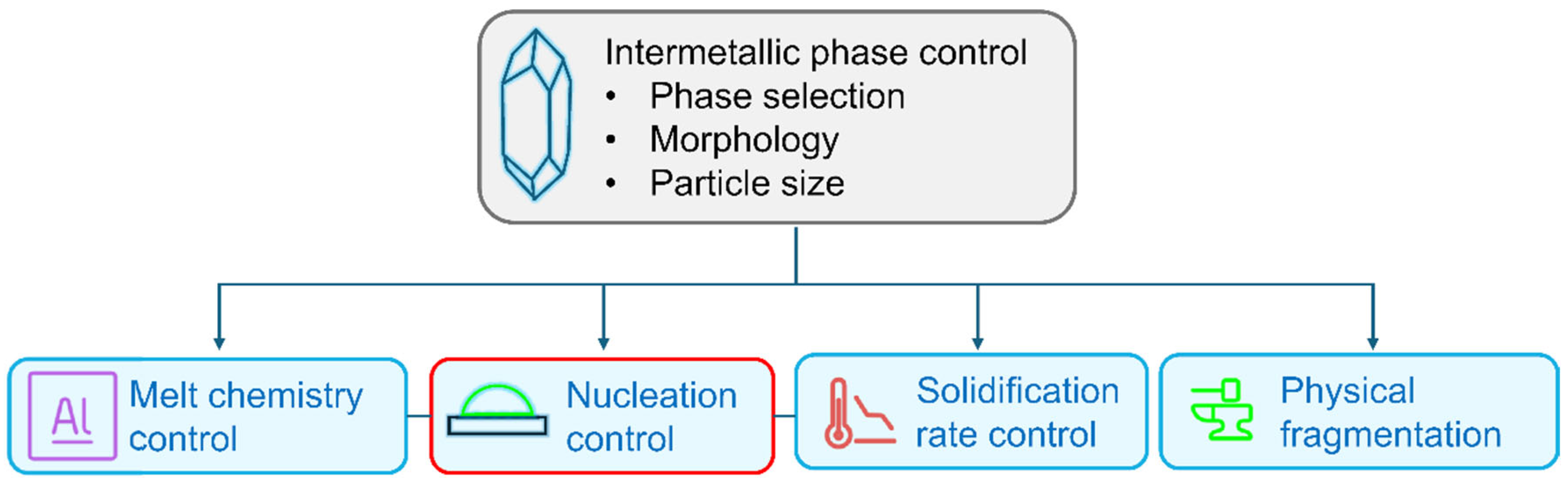
The adverse effects of coarse IMCs on ductility can be minimized through refinement and morphological control. In this way, a higher tolerance of unwanted elements can be achieved, which would allow a more efficient utilization of secondary alloys [48,49]. Figure 1 gives an overview of the currently available methods for controlling the phase selection, morphology, and size of IMC phases in aluminum alloys.

Figure 1.
Summary of the current approaches to controlling intermetallic phase selection, morphology, and size.
In terms of IMC phase control, the vast majority of research has focused on the effect of different alloying elements (melt chemistry control) [50,51,52,53,54,55,56,57,58,59] and the influence of varying solidification rates (solidification rate control) on the crystallization of IMCs [60,61,62,63,64,65,66]. A typical example of this is the adjustment of the appropriate Fe/Mn ratio for a given solidification rate to prevent the formation of platelet-like β-Al5FeSi and bring forth the formation of α-Al15 (Fe,Mn)3Si2 and/or α’-Al8Fe2Si in Si-containing casting alloys [67,68]. As a primary phase, α-Al15 (Fe,Mn)3Si2 and α’-Al8Fe2Si have a compact polyhedral morphology, while as part of a binary eutectic structure, these phases have a Chinese-script morphology [69,70]. By avoiding the formation of brittle, platelet-like β-Al5FeSi, ductility can be remarkably improved [55,56]. In addition to Mn, various alloying elements, such as Cr, V, Mo, B, and Sr, have been found to suppress β-Al5FeSi formation through the stabilization of α- and α’-AlFeSi variants [71,72,73,74]. Technologies like semi-solid metal processing and applying electromagnetic stirring during solidification can provide more homogeneous solute distribution, which can result in refined intermetallic particles [75,76].
The cooling rate and thermal gradient during solidification have a decisive effect on intermetallic phase selection, phase transformations, IMC particle size, and morphology as they affect both nucleation and growth (for this reason, solidification rate control and nucleation control are interconnected in Figure 1) [77]. The nucleation of certain intermetallic phases (such as α-Al15 (Fe,Mn)3Si2) requires a high undercooling due to their high number of constituent elements and complex crystal structure. By changing the thermal conditions of solidification, the kinetic requirements of the crystallization of stable phases may not be fulfilled, which results in the formation of metastable phases [30]. For the prediction of intermetallic phase selection, formation maps can be used, which consider the effect of different cooling rates for a given alloy compositional range [24]. An increasing cooling rate results in reduced intermetallic particle sizes due to the limited time for crystal growth. For this reason, to a certain degree, high cooling rates can mitigate the negative effects that intermetallic phases have on the mechanical properties of castings [78,79].
Physical processing during or after solidification (physical fragmentation in Figure 1) can be successfully used to fragment intermetallic particles. Examples of this approach include severe plastic deformation (SPD) by equal channel angular pressing (ECAP) [80,81], tube channel pressing (TCP) [82], or high-pressure torsion (HPT) [83,84,85]. It has also been shown that combining plastic deformation by cold-rolling with subsequent recrystallization and partial melting (RAP), as well as thixoforming, Fe-containing intermetallic particles of highly alloyed casting alloys like A380 can be effectively refined, resulting in enhanced tensile strength and elongation [86,87]. Applying high-intensity ultrasonic vibration during different stages of solidification can also induce the fragmentation of previously crystallized intermetallic phases by the shock wave pressure exerted upon bubble collapse during acoustic cavitation [88,89,90,91]. Ultrasonic treatment (UST) also provides forced convection in the liquid phase, which affects solute distribution and, in this way, the nucleation and growth of intermetallic compounds (note that ultrasonic processing in the liquid state will be discussed in detail in Section 4.1) [92,93,94].
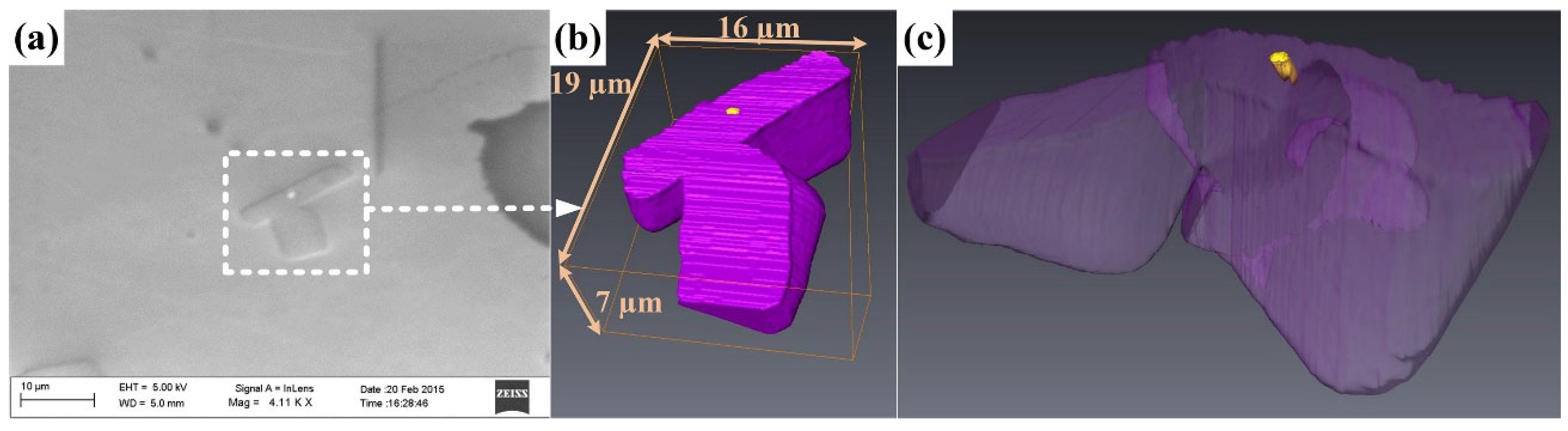
The presence of solid, nonmetallic particles and films in the liquid metal has been found to affect the nucleation and growth of the different phases of metal alloys during solidification [95,96,97,98,99,100,101,102,103,104]. It is now generally understood that by affecting the nucleation process, the phase selection, morphology, and size of intermetallic compounds can be influenced (nucleation control in Figure 1) [30]. A growing number of studies report on the possibility of nonmetallic phases acting as heterogeneous nucleation sites for different IMCs [105,106,107,108,109,110]. For example, Zhao et al. [108] added 0.2% Al-5%Ti-1%B to an Al-6%Cu-2%Fe-1%Si alloy and found TiB2 in α-Al15Fe3 (SiCu)2 IMC particles. In the central region of one of the investigated α-Al15Fe3 (SiCu)2 IMCs, Al4C3, amorphous Al2O3, and crystalline α-Al2O3 were also found. Figure 2a shows the scanning electron microscopic (SEM) image of the α-Al15Fe3 (SiCu)2 phase with the multiphase inclusion in its center, while Figure 2b presents a scanning transmission electron microscopic (STEM) image (made in high-angle annular dark-field imaging (HAADF) mode) of the inclusion. By the application of SEM-based serial ultramicrotomy tomography, Yu et al. [111] found TiB2 clusters embedded in α-Al15 (FeMn)3Si2 (Figure 2c), β-Al5FeSi, and Mg2Si in an AA6082 alloy inoculated with 0.2% Al-5%Ti-1%B.
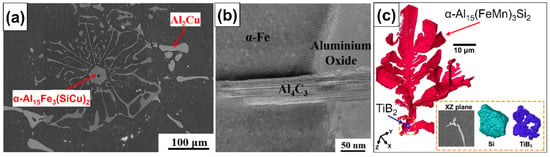
Figure 2.
(a) SEM image of an α-Al15Fe3 (SiCu)2 particle with a multiphase inclusion in its central region. The inclusion consists of TiB2, Al4C3, amorphous Al2O3, and α-Al2O3 phases. (b) HAADF-STEM image of the complex inclusion found in the intermetallic particle presented in (a). Reprinted with permission from Ref. [108]. Copyright 2025 Elsevier. (c) Three-dimensional reconstruction of a dendritic α-AlFeSi compound particle that appears to be initiated by TiB2 particles. Reprinted with permission from Ref. [111] Copyright 2025 Elsevier.
The fact that the growing intermetallics can engulf nonmetallic phases suggests a low interfacial energy between these phases, which is favorable during heterogeneous nucleation. In most cases, the nucleation of intermetallic compounds needs a higher free energy barrier (higher undercooling) to overcome than that of a dilute solid solution because it requires a local chemical composition that allows the formation of stoichiometric compound crystals by the atomic arrangement of multiple alloying elements (for this reason, melt chemistry and nucleation control are connected in Figure 1). In addition, certain intermetallics, like the α-Al15 (Fe,Mn)3Si2, have a complex crystal structure that makes their nucleation process more difficult [112,113]. In the case of Fe-rich IMCs, different types (equilibrium and non-equilibrium) of intermetallics compete for nucleation, and for IMCs with a similar formation temperature, the IMCs that have a smaller nucleation undercooling will nucleate first [30,114]. It was revealed that the competition of different types of IMCs can be influenced by the presence of inoculant particles introduced by grain refiners, suggesting that by providing potent substrates for heterogeneous nucleation, the free energy barrier of the nucleation of certain IMCs can be successfully lowered [115,116]. For example, Zhao et al. [117] found that the addition of 0.2% Al-5%Ti-1%B master alloy to an Al-2%Fe alloy reduced the quantity of primary Al13Fe4 and promoted the crystallization of eutectic Al6Fe, while both types of intermetallic particles were effectively refined. It was reported by Rakhmonov et al. [118] that without grain refiner additions, the presence of Mn, Cr, and V in an AlSi7Cu3Mg alloy was able to suppress the formation of β-Al5FeSi. In contrast, after grain refinement with Al-5%Ti-1%B, β-Al5FeSi became the dominant Fe-containing IMC in the microstructure due to the heterogeneous nucleation of β-Al5FeSi on TiB2 particles.
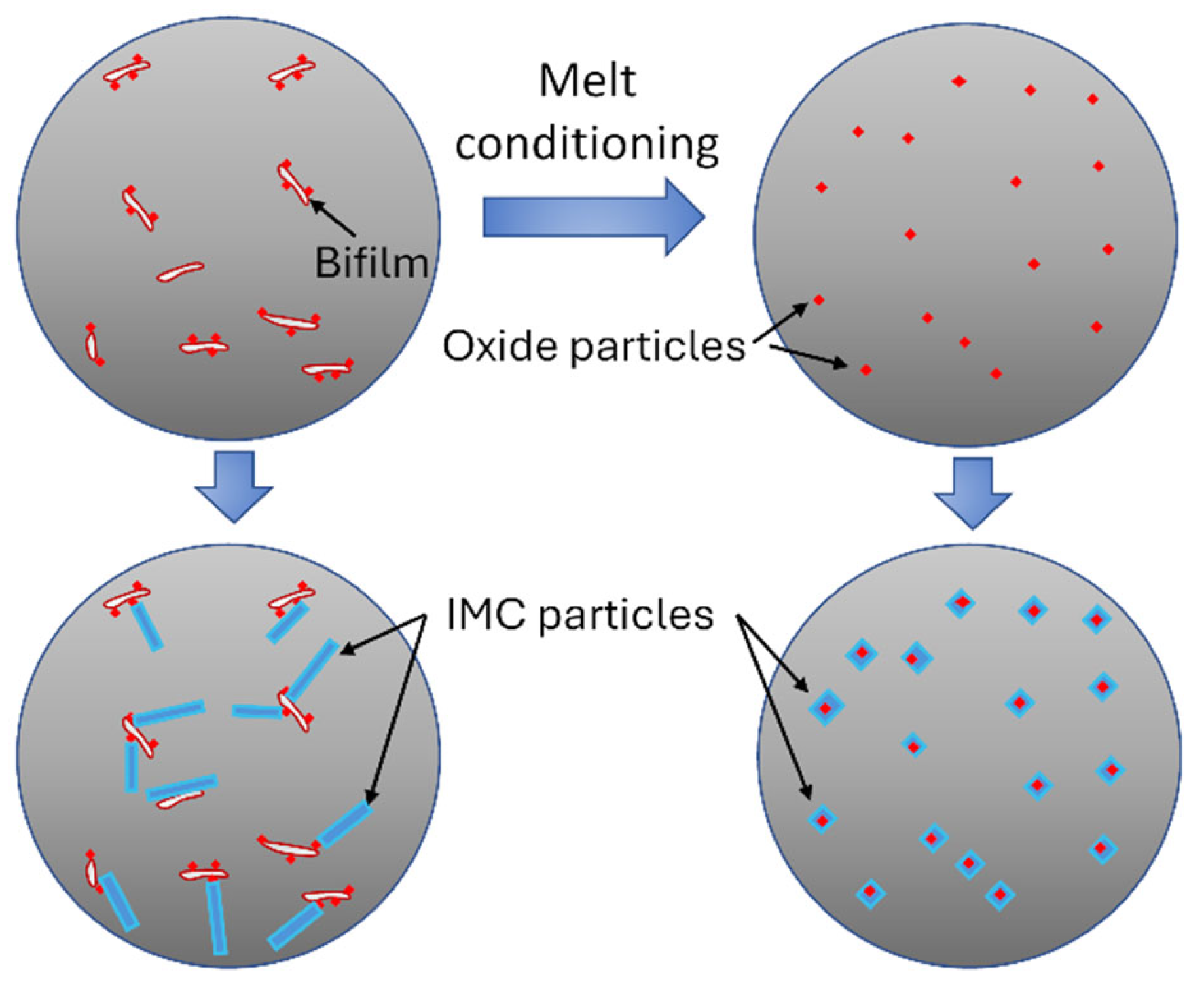
Liquid aluminum alloys have a variety of naturally occurring solid phases (inclusions) that may affect the nucleation and growth of intermetallic compounds [119,120,121,122]. Among them, oxides are the most frequently occurring phases due to the high chemical affinity of aluminum to oxygen. Oxides are generally present in the form of double oxide films (bifilms) or particle agglomerates, which hinders the possibility of refining intermetallic particles due to the non-uniform spatial distribution of heterogeneous nucleation substrates in the melt [123,124,125,126,127]. The latest research results suggest that double oxide films have an overarching effect on the nucleation and growth of various phases, as well as on the microstructural defects of aluminum alloys [128,129,130,131,132]. There are numerous examples of research focusing on the removal of oxide inclusions (including bifilms) from liquid aluminum alloys [133,134,135,136,137]. On the other hand, a new approach of minimizing the adverse effects of oxide inclusions simultaneously with the alleviation of the impact of coarse intermetallics is starting to gain increased attention [127,138,139]. The central concept of this approach is presented in Figure 3.

Figure 3.
Schematic illustration of the concept of refining intermetallics by transforming bifilms and oxide particle agglomerates into a well-dispersed suspension of oxide particles.
A growing body of experimental work shows that the crystallization process of IMCs is affected by the oxide phases present in the liquid alloys. However, a comprehensive review of these results has not been made to date. It has also been demonstrated that by appropriate technologies, harmful oxide inclusion (like oxide bifilms) can be transformed into small-sized oxide particles that can be dispersed throughout the liquid alloy to serve as heterogeneous nucleation sites for different intermetallic phases. For simplicity, Figure 3 presents this mechanism in a system where solidification starts with the crystallization of a primary intermetallic phase. Using this approach holds the potential to mitigate the negative effects of oxide inclusions and IMCs. It can be essential for secondary Al alloys, which are usually contaminated by a higher concentration of oxide inclusions, as well as impurity elements [122]. Although they offer a significant advancement in the field of liquid metal engineering, a profound overview of these technologies is yet to be made. For these reasons, this contribution aims to review the recent progress in the understanding of the role of oxide phases in the crystallization process of intermetallic compounds, as well as technological solutions capable of refining IMCs through the transformation of harmful oxide inclusions into numerous, well-dispersed heterogeneous nucleation sites. Besides analyzing the current state of these techniques, the discussion evaluates their future implications and the potential challenges that may arise in their application and development.
2. Oxides in Casting Aluminum Alloys
To understand the possible role of different oxide phases in the microstructure formation of aluminum alloys, the properties of these oxides should be first investigated. There are multiple reviews [140,141,142,143,144,145] given by different authors that are recommended for a detailed discussion. In this section, we summarize the most important aspects of oxides in Al alloys.
Due to its high affinity for oxygen, Al readily oxidizes both in the solid and liquid states. When an aluminum alloy melt is exposed to air or an atmosphere containing O2 or H2O vapor, an extremely thin, amorphous oxide layer is formed within just a few milliseconds due to the reaction between Al and O2 and/or H2O [141].
The standard enthalpy of reaction values, as well as the standard Gibbs free energy change accompanying the chemical reactions described in the present article, are presented in Table A1 of Appendix A.
Al2O3 has various polymorphic forms, and certain alloying elements like Mg and Sr are also capable of oxidation. For this reason, different melt temperatures, oxidation times, oxidizing atmospheres, and melt chemical compositions may result in the formation of various types of oxide phases [142,146]. In the case of pure Al, within a short time after the formation of the initial, amorphous Al2O3, γ-Al2O3 starts to crystallize at the metal/oxide interface. With time, the crystalline fraction of the oxide layer increases until the complete transformation of the amorphous phase [142]. Bonner [145] reported the presence of γ-Al2O3 nanocrystals (with sizes smaller than 100 nm), even after a 1 min long oxidation process at 750 °C, which suggests that the formation of crystalline oxides on the initially formed oxide layer is a relatively fast process. After an incubation period, γ-Al2O3 transforms into the thermodynamically more stable α-Al2O3. The transformation can be interpreted as a series of phase transitions with several different metastable Al2O3 variants forming during the process. The time needed for phase conversion depends on the temperature, oxidizing atmosphere, chemical composition (impurities), stirring, etc. [141,147]. Although the total conversion of the surface oxide layer into α-Al2O3 may take several hours at normal melt-holding temperatures (650–800 °C), the first appearance of α-Al2O3 can occur even after a few minutes [141,148]. The transformation into α-Al2O3 involves a 24% reduction in the oxide molar volume, which induces tensile stresses in the oxide layer and eventual scale failure (cracking), which enhances the rate of further oxidation [147,149]. Localized high-temperature oxidation and aluminothermic reactions between oxides like SiO2, CuO, Fe2O3, or ZnO and the liquid Al may result in direct α-Al2O3 formation [150,151].
The effect of Mg on the oxidation behavior should be treated in detail as it substantially changes the oxide phase formation and oxidation kinetics, while it is an essential alloying element in a wide variety of casting aluminum alloys. During the oxidation of Mg-containing alloys, Mg oxidizes selectively, as follows:
This is due to the more negative Gibbs free energy change of reaction (3) relative to reaction (1). In addition, Mg tends to segregate to the surface region of the liquid metal during melt holding (or during the melting process), which results in a locally increased Mg concentration relative to the bulk liquid. For these reasons, after the formation of the initial amorphous Al2O3 layer on a Mg-containing alloy, the first crystalline oxide phase that forms on the metal/oxide interface is often MgO [143,152,153]. Mg has a relatively high vapor pressure, which can lead to the evaporation and direct oxidation of Mg (note the possible gaseous state of Mg in reaction (3)) [143,154]. Impey et al. [148,155] reported that during the oxidation of liquid Al-Mg alloys (containing 1–5% Mg) at 750 °C, beneath the initially formed amorphous Al2O3 layer, primary MgO crystals with a size range of 200–500 nm are developed within 5 min of oxidation. These are accompanied by smaller (around 20 nm) secondary MgO particles, which form inside the amorphous oxide film. According to the mentioned authors, the crystallization of primary MgO at the metal/oxide interface is caused by the segregation of Mg atoms to the surface and the diffusion of O2- ions through the oxide film. Secondary MgO nanocrystals are produced by the following secondary reduction reaction:
where represents the amorphous Al2O3 phase in the initial oxide layer. Wu et al. [143] also reported the presence of different-sized MgO crystals beneath the initially formed amorphous oxide film on an Al-0.2%Mg alloy after 5 min of oxidation at 750 °C (Figure 4). They applied anhydrous butanol to extract the surface oxide films of the samples and, in this way, revealed the wet side of the film that was originally in contact with the liquid alloy (Figure 4a). Numerous oxide crystals and intermetallic particles were found on this surface. Energy-dispersive X-ray spectroscopy (EDX) results (Figure 4b) in P1 and P2 suggest the presence of MgO crystals. These have rather different particle sizes: around 1 µm in P1 and less than 400 nm in P2. These findings indicate that MgO crystals can form even in the case of low Mg concentration and that oxide growth is not uniform throughout the oxide film.

Figure 4.
(a) Secondary electron SEM image of an oxide layer extracted from an Al-0.2%Mg alloy after an oxidation time of 5 min at 750 °C. The image shows the surface originally in contact with the liquid alloy. (b) EDX spectra of the three different spots (P1–P3) illustrated in (a). Reprinted with permission from Ref. [143]. Copyright 2025 Elsevier.
Due to the local depletion of Mg or because of the low Mg level of the base alloy, primary MgAl2O4 (spinel) crystals can be formed on the oxide/metal interface after the formation of the initial, amorphous Al2O3 layer [148,155]. In addition, according to Wu et al. [143], the amorphous Al2O3 film can be gradually transformed into a MgAl2O4 layer. Spinel formation has been reported to take place through the following chemical reactions:
As Cao and Campbell [141] reported, for a major fraction of casting aluminum alloys, the formation of MgAl2O4 is thermodynamically favored. For alloys containing Mg at a concentration between 0.005% and 2%, the formation of a crystalline MgAl2O4 layer on the melt surface is to be expected. MgO formation becomes favored for Mg levels above 2%. However, local compositional changes may occur during oxidation due to local segregation or depletion of Mg, which results in oxide films consisting of multiple oxide phases [156,157,158,159].
Different oxidation times result in oxide films with rather different structures and appearances. Figure 5 shows the SEM images of the surface of MgAl2O4 oxide films found on the fracture surface of Al-7%Si-0.5%Cu-0.4%Mg alloy samples. In Figure 5a, a thin, folded oxide film is shown with numerous wrinkles. Based on the width of some of the folds, the thickness of this oxide film is below 1 µm, indicating that the oxidation time of the film was short, i.e., the oxide film is young and was created shortly before the solidification of the sample (most likely during the pouring of the liquid metal). Young oxide films are relatively flexible due to their thinness, and their folds are created by the compressive or deformational strains that occur during the disturbance of the melt surface. Oxide layers progressively thicken over longer oxidation times and become increasingly brittle. Old oxide films can easily crack by the stresses induced by melt flow or the solidification of the metal [141,142,160]. Figure 5b shows a rough, granular oxide film surface with numerous cracks, a common feature of old oxide films. Also, multiple oxide crystals, which cover the dendritic arms of the base metal, are visible. These crystalline features indicate a prolonged oxidation state.

Figure 5.
SEM image of (a) a young oxide film Reprinted from Ref. [134] and (b) an old oxide inclusion found on the fracture surfaces of Al-7%Si-0.5%Cu-0.4%Mg alloy samples Reprinted from Ref. [161].
Oxide films are generally present as double oxide films (bifilms) in liquid aluminum alloys. Surface turbulence during liquid metal processing (melt pouring, transfer, or the casting process, as well as stirring during melting or holding) might cause the surface oxide film to get immersed beneath the liquid metal. This can be either an impingement action between surface films of colliding liquid fronts or a folding action of the surface. In both cases, unbonded inner interfaces are inevitably created by bringing “dry” oxide film surfaces into contact. A significant consequence of these mechanisms is that surface oxide films cannot be entrained into the bulk liquid as single films; they are necessarily double. Between the unbonded oxide layers, bifilms contain entrained air. In this way, they constitute cracks present in the metals, both in the liquid and the solid state [128,142,162]. Besides being detrimental to the mechanical properties of castings by reducing the effective cross-section, the unbonded central interface of bifilms can induce porosity and hot-tear formation during solidification [163,164,165,166]. These adverse effects may be alleviated either by removing double oxide films from the liquid metal or by their transformation into well-dispersed suspensions of oxide particles (Figure 3) [167].
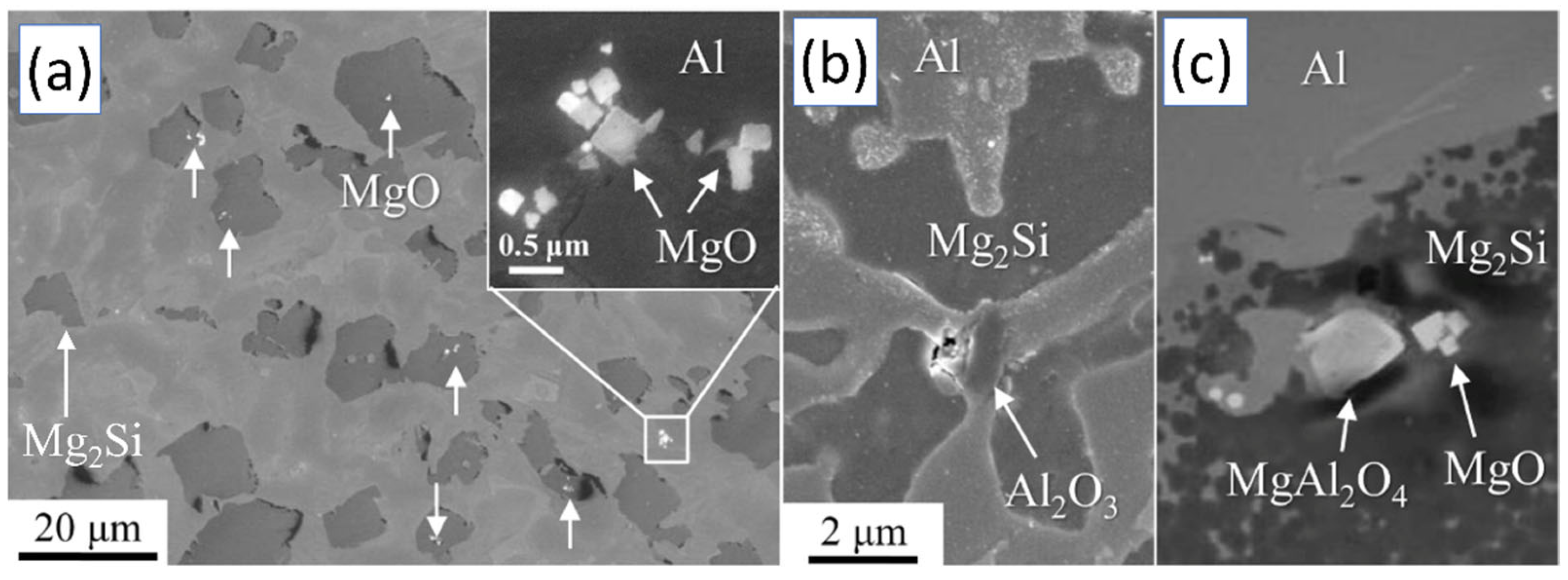
As described above, crystals of various oxide phases may be formed beneath or inside the initial amorphous oxide films early during the oxidation process of liquid aluminum alloys (Figure 4). For this reason, the oxide layers of double oxide films contain a copious amount of oxide crystals (generally nanocrystalline oxides) that are formed through the transformation of the amorphous oxide phase, while larger (in the order of a few µm) crystals are often attached to the oxide layers as a result of nucleation at the metal/oxide interface (Figure 6) [125,127,148]. Figure 6a shows an oxide bifilm with numerous MgAl2O4 particles attached to its oxide layers. The bifilm was found in an Al-0.7%Mg alloy that was melted at 750 °C and was held at 700 °C for four hours [168]. Figure 6b shows another example of a double oxide film containing numerous MgAl2O4 particles that was partly engulfed by an (Al,Si)3Ti intermetallic particle. Note that both Figure 6a,b show secondary electron (SE) SEM images, as this imaging mode proved to be effective in revealing the small-sized oxide particles. Besides being reservoirs of entrained reactive gases (as well as the noble gas content of the air and the hydrogen that diffuses into the inner atmosphere of bifilms), double oxide films can be interpreted as sites of oxide (and, in some cases, nitride [119]) particle agglomeration. As depicted by the schematic illustration in Figure 3, novel melt treatment methods may aim to transform these agglomerations into dispersed particles through the fragmentation of the bifilm defects. However, the properties of bifilms, such as oxidation time, phase composition, size, and gas content, could be crucial in determining how easily this fragmentation can be realized.

Figure 6.
(a) SE-SEM image of a double oxide film found in an Al-0.7%Mg alloy. Numerous sub-micron-sized MgAl2O4 particles are nucleated on the oxide layers. Reprinted with permission from Ref. [168]. Copyright 2025 Elsevier. (b) SE-SEM image of an oxide film with numerous MgAl2O4 particles attached to it in an Al-7%Si-0.7%Mg-0.5%Ti-0.5%Cu alloy. A segment of the oxide film was engulfed by an (Al,Si)3Ti particle. Reprinted from Ref. [169].
Some circumstances may ease the fragmentation process of oxide films. Old oxide films are generally more brittle than young films, and they contain a higher fraction of crystalline oxides that may be dispersed in the liquid metal [141,142]. Certain alloying elements can also change the structure of oxide films and affect how easily oxide particles can be dispersed in the melt. For example, Li et al. [168] reported that Al-Mg alloys with a Mg concentration above 1% contain naturally dispersed discrete MgAl2O4 particles, while MgAl2O4 was only present in the form of oxide films when the Mg level was below 1%. The authors explain this observation with the help of the Pilling–Bedworth ratio (PBR) and the capillary pressure (Pc) between discrete oxide particles and the liquid alloy present in the narrow channels between the particles. PBR can be used for the rough estimation of the stresses induced by the volumetric differences between the oxide formed and the metal consumed during the oxidation process (PBR = volume of oxide/volume of metal) [170,171]. In most cases, the first crystalline oxide formed during the oxidation of liquid Al-Mg alloys is MgO, which has a PBR of 0.73. As this value is below 1, tensile stresses are induced in the oxide layer, resulting in the formation of discrete MgO particles, which transform into MgAl2O4 at a later oxidation stage. According to Li et al. [168], liquid metal is rising in the channels between discrete MgAl2O4 particles, and these channels can be interpreted as capillary tubes. The authors claim that the increased Mg concentration lowers the surface tension of the liquid alloy, which results in lowered Pc, and in this way, the oxide particles become more easily separated. According to the authors of the present review, another possible explanation may be the increased thermodynamic stability and increased phase fraction of MgO in the oxide layer due to the higher Mg concentration. Because of the low PBR ratio, oxide layers with a high MgO fraction become discontinuous, and for this reason, the fragmentation of these layers becomes simpler.
Phase transformations in the oxide layers may also induce stresses that contribute to the fragmentation of oxide films. For example, using high melt temperatures accelerates the γ-Al2O3 →α-Al2O3 transformation, which involves a 24% reduction in the oxide molar volume. This volume change induces tensile stresses in the oxide layer, which facilitates the formation of dispersed oxide particles [147,149]. This was experimentally verified by Wang et al. [127], who found an increased number of dispersed α-Al2O3 oxide particles in commercial-purity Al after increasing the holding temperature to 920 °C.
3. Heterogeneous Nucleation of Intermetallic Particles on Oxides
3.1. The Potency of Oxide Phases to Heterogeneously Nucleate Intermetallics
The mechanism proposed in Figure 3 works only if the grain initiation of the intermetallic phase starts simultaneously at multiple sites throughout the melt. This can be achieved only if the dispersed oxide particles heterogeneously nucleate the given intermetallic phase, and each nucleation event is realized at a similar undercooling. This means that a high number of evenly distributed oxide particles with a similar nucleation potency are needed to achieve the desired refinement effect. During heterogeneous nucleation, atoms of the newly developing solid phase are attached to the energetically most favorable interfacial sites of the nucleant. For this reason, it is generally accepted that the interfacial energy between the nucleant and the new solid crystal has a major impact on the heterogeneous nucleation process [172,173,174]. In the theoretical framework of classical nucleation theory (CNT), it is assumed that during heterogeneous nucleation, the solid nucleus forms a spherical cap on the surface of the solid substrate (Figure 7a). According to the Young equation, the relationship between the interfacial energies between the three phases determines the equilibrium contact angle (θ) [175]:
where [J·m−2], [J·m−2], and [J·m−2] are the interfacial energies of the substrate (nucleant)/liquid metal, the nucleant/solid nucleus, and liquid metal/solid nucleus interfaces, respectively. The free energy barrier for heterogeneous nucleation is highly dependent on the contact angle: by reducing its value (improving the wettability of the substrate by the solid nucleus), the maximum Gibbs energy change accompanying heterogeneous nucleation is lowered; thus, the required undercooling is greatly reduced. To reduce the value of the contact angle, should be as low as possible [172].
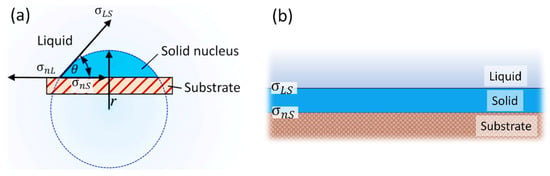
Figure 7.
Depiction of heterogeneous nucleation according to (a) the classical nucleation theory and (b) the athermal nucleation model (Reprinted from Ref. [100]).
As described by Götz et al. [100], the assumption of spherical cap formation and isotropic interfacial energy may be erroneous in the case of highly anisotropic solid phases such as graphite. Similarly, a major fraction of intermetallic compound phases is anisotropic with different interfacial energies for various terminating crystal planes. Also, for the most potent nucleants (°), the spherical cap model yields a nucleus with less than one atomic monolayer thickness [176]. For these reasons, the description of heterogeneous nucleation behavior with CNT is expected to be inaccurate in a majority of cases when intermetallics are nucleated on potent substrates. According to Quested and Greer [177], for potent nucleants, athermal nucleation dominates, which is a deterministic process that is not dependent on time, and the number of nucleation events depends only on the undercooling. Götz et al. [100] derived expressions for a disk-shaped nucleus (to address the anisotropic behavior of graphite), as well as for an athermal nucleation model in which the solid phase forms a monolayer on the substrate surface, which is treated as infinite in the lateral dimensions (Figure 7b). In the case of the athermal nucleation model, a low is also essential to maintain the stability of the solid layer.
Recently, there have been significant advancements in the fundamental understanding of heterogeneous nucleation by Fan and co-workers [178,179,180,181,182], who introduced a new framework for the analysis of early-stage solidification (ESS). ESS is defined as “a solidification process that occurs in the time interval between the onset of melt cooling and the morphological instability of growing solid particles”. In the case of solidifying metallic phases, ESS includes prenucleation, three-layer heterogeneous nucleation, constrained cap formation, grain initiation, and free growth [182]. Although spherical cap formation and spherical growth are questionable for anisotropic intermetallic phases, the general mechanisms of these processes can be expected to be similar regardless of the morphology of the initial solid crystal. Similar to other approaches [183,184,185], in the framework of Fan and co-workers, the nucleation potential of the nucleant is dependent on crystallographic matching between the substrate and the solid. They express heterogeneous nucleation undercooling (, [K]) as a function of lattice misfit (, [%]) [178]:
where is a constant and is calculated as
where [nm] and [nm] are the atomic spacings of the solid and the substrate, respectively.
Extensive molecular dynamics (MD) simulations revealed that the substrate induces a certain atomic ordering in the liquid adjacent to the liquid/substrate interface, even above the nucleation temperature. This phenomenon is called prenucleation, and it provides a precursor for heterogeneous nucleation [180,186]. At the nucleation temperature, heterogeneous nucleation is started by the precursor created by prenucleation. The nucleation process makes progress layer by layer through a structural templating mechanism, and it is completed within three atomic layers (Figure 8), generating a 2D nucleus (a crystal plane of the solid phase). According to Fan and Men [181], further growth (grain initiation) of the solid leads to spherical cap formation, and free growth can only occur by increasing undercooling.

Figure 8.
Schematic representation of the 3-layer heterogeneous nucleation process. Heterogeneous nucleation proceeds layer by layer; the first two layers accommodate lattice misfit through various mechanisms that are dependent on the sign and value of misfit, while the third layer is a crystal plane of the solid phase. Reprinted from Ref. [178].
In terms of interfacial energy change, if we assume that the area of the three atomic layers is the same, the free energy change accompanying heterogeneous nucleation (, [J]) by the mechanism shown in Figure 8 can be expressed as [181]
where is the number of atoms in one atomic layer, and represents the projected area of an atom in the layer. The term is the area covered by the 2D nucleus. For the minimization of and should be low, while should be high. From the viewpoint of intermetallic inoculation, for a given intermetallic phase, is not affected by the nucleant selection. Thus, our goal is to find substrates that have high interfacial energy with the liquid metal but have low interfacial energy with the solid intermetallic phase. In terms of high values, contact angle measurements by the sessile drop method suggest that solid oxides have relatively high interfacial energy with liquid metals [187,188,189,190]. According to Kaptay and Báder [191], the reason for this is that there is only a weak ion-induced dipole interaction between ionic solids (such as most oxides present in liquid aluminum) and liquid metals, resulting in low adhesion energies. For these reasons, low remains the only criterion that should be investigated in detail.
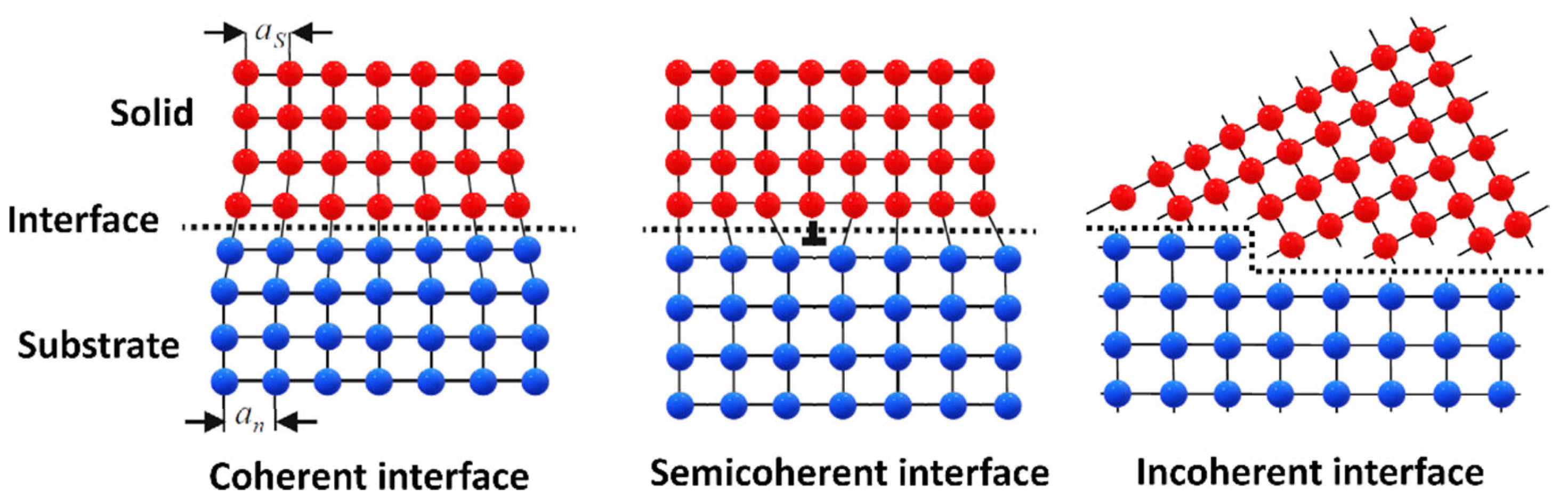
Regardless of the nucleation mechanism, it is generally acknowledged that crystallographic matching of the substrate and the solid nucleus has a major impact on the undercooling requirement of heterogeneous nucleation and grain initiation. Good crystallographic matching (low misfit) is essential in minimizing substrate/solid nucleus interfacial energy ( For this reason, potent substrates are generally searched through crystallographic analysis, such as the edge-to-edge matching model [185,192]. Substrate/solid nucleus interfaces, or generally speaking, solid/solid interfaces, can be classified as coherent, semicoherent, or incoherent interfaces (Figure 9). Coherent interfaces only have slight differences in their interatomic spacings along matching crystallographic planes ( is low). This slight deviation induces strain in the lattice, which increases the interfacial energy of the substrate/solid interface. If the misfit is too high to be accommodated by strain, dislocations are formed, further increasing the value. In this case, the interface is said to be semicoherent. If there is no crystallographic matching between the two solid phases, the interface is incoherent, and becomes the highest [193].
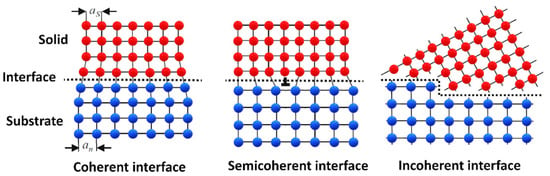
Figure 9.
Schematic illustration of solid/solid interface types. as and an represent the atomic spacing in the solid and the substrate, respectively (Reprinted from Ref. [172]).
According to Fan and Men [178], in the case of the three-layer nucleation mechanism, the first two atomic layers accommodate misfit through various mechanisms that are dependent on the sign and value of misfit. In the case of small negative misfit (−12.5% < < 0%), edge dislocations are formed in the first and screw dislocations in the second layer. If the misfit is positive, but it has a small value (0% < < 12.5%), the first layer is epitaxial to the nucleant, while the second layer accommodates misfit by the formation of vacancies. In the case of large misfits ( > 12.5%), most of the misfit is accommodated by the formation of a coincidence site lattice during prenucleation, and the residual misfit is accommodated by one of the small-misfit mechanisms.
Fan and Men [178] suggest that can be considered as the total energy of the defects present in the three atomic layers, meaning that it is only affected by the crystallographic matching of the substrate and the solid. It should be highlighted, however, that this approach neglects the chemical interactions between the atoms of the substrate and the solid and will be accurate only in systems with similar atomic bond types (for example, nucleation of solid metals on metallic substrates). For this reason, the following general expression can be written for the interfacial energy of semicoherent interfaces ( < 12.5%) [193]:
where [J·m−2] is the surface energy per unit area due to atomic interactions, [J·m−2] is the elastic strain energy per unit area caused by the elastic deformation of the lattice, and is the total energy contribution of defects per unit area. For detailed analysis and modeling of solid/solid interfacial energies, the reader is referred to the works by Kaptay [194,195,196]. In the case of systems with different atomic bond types, the contribution of atomic interactions cannot be neglected. This is the reason why some ionic oxides, such as MgAl2O4 and γ-Al2O3, show good crystallographic matching with aluminum [127], but their heterogeneous nucleation potency for Al is limited. MD simulations of Fang and Fan [197,198,199,200] revealed that ionic oxides of aluminum alloys, such as γ- and α-Al2O3, MgAl2O4, and MgO, are impotent substrates for the nucleation of Al. According to the simulations, this is due to the formation of a terminating Al layer on the surface of the substrate. The Al atoms of this layer are positively charged due to electron transfer to the outmost oxygen ions of the substrate. The terminating Al layer becomes chemically bonded to the substrate and generates an atomically rough surface due to vacancies and displacements vertical to the substrate/solid interface. This roughness weakens the nucleation potential of the oxide substrate due to its weak structural templating ability.
In terms of atomic interactions, it can be expected that intermetallic phases are more compatible with ionic oxides than metallic phases. Chemical bonds in intermetallic compounds are mixtures of metallic, ionic, and covalent bonds. For this reason, the intermetallics possess properties that are intermediate between those of ceramics and metals [201,202]. Based on this, it is a reasonable thought that instead of forming oxide ceramic/liquid metal interfaces, the formation of intermetallics as intermediate phases between the oxide ceramics and liquid metals would be preferable in terms of minimizing . As will be presented in the following chapters, there is an exceptionally high number of cases documented in the literature where oxide phases were found inside various intermetallics. This means that oxides serve as heterogeneous nucleation sites or the growing intermetallics engulf oxide phases. Both mechanisms of oxide incorporation suggest a low interfacial energy between the oxides and the presented intermetallic phases [203].
The potency of various substrates for the heterogeneous nucleation of intermetallic phases can be significantly altered by the interfacial segregation of various solute elements present in the liquid metal [113,116,204]. Lattice misfit can be significantly altered by interfacial segregation, which can enhance and also impede heterogeneous nucleation. In most cases, the nucleation of intermetallic compounds needs a higher undercooling to overcome than that of a dilute solid solution because it requires a local chemical composition that allows the formation of stoichiometric compound crystals by the atomic arrangement of multiple alloying elements. Que et al. [113] revealed that with the aid of modifying AlB2 particles by the interfacial segregation of Fe, the heterogeneous nucleation of α-Al15(Fe,Mn)3Si2 on AlB2 can be enhanced, and the α-Al15(Fe,Mn)3Si2 particles can be refined. In their other work, Que et al. [116] found that the interfacial segregation of Fe and Mn into the (0001) TiB2 interface facilitates the heterogeneous nucleation of Al6(Fe,Mn) on TiB2. Future research on the interfacial segregation of certain elements on oxide/liquid metal interfaces may provide further insight into whether this composition templating phenomenon affects intermetallic nucleation on oxide substrates.
Finally, the effect of substrate size on the heterogeneous nucleation of intermetallics should be analyzed. According to Greer et al. [205], for potent, disk-shaped nucleant particles, the undercooling needed for the free growth (grain initiation) of the crystal , [K]) that heterogeneously nucleated on the substrate is related to the diameter of the nucleant particle (, [m]), as follows:
where [J·m−2] is the interfacial energy between the liquid metal and the solid phase, and [J·K−1·m−3] is the entropy of fusion per unit volume. Based on that, larger substrate particles initiate grains more easily, and smaller ones become active at lower melt temperatures. To achieve the refinement effect depicted in Figure 3, the oxide particles that serve as nucleation substrates for intermetallics should have a narrow size distribution. If this is not achieved, the particles initiate grains at rather different undercoolings, and some of the intermetallic grains have more time to grow, resulting in non-uniform intermetallic size distribution. Oxide particle size-distribution measurements revealed that in untreated, low-Mg liquid alloys, two distinct lognormal curves can be used to describe the distribution due to the presence of two major groups of particles. By increasing the Mg concentration above 2% or utilizing melt treatments such as high-shear melt conditioning (HSMC), the particle sizes will follow a single-peak lognormal distribution, which is suitable for refining intermetallic particles [125].
3.2. Experimental Evidence of Intermetallic Nucleation on Oxide Phases
During microscopic investigations of casting aluminum alloys, intermetallic phases are commonly found to contain crack-like inhomogeneities or particles. The cracking of intermetallics is generally attributed to their brittle nature. On the other hand, Campbell [128,206,207] proposed that these “cracks” are already present in the liquid metal before the start of the solidification in the form of double oxide films, which are preferred heterogeneous nucleation sites for various intermetallic phases. Numerous examples of experimental findings have been published that support the idea of heterogeneous nucleation of intermetallics on bifilms and surface oxide films, as well as individual oxide particles. For example, in our previous studies [208,209,210], it was found that (Al,Si)3Ti crystals nucleate on bifilms, and double oxide film segments became engulfed by the growing intermetallic phase (Figure 10a–d).
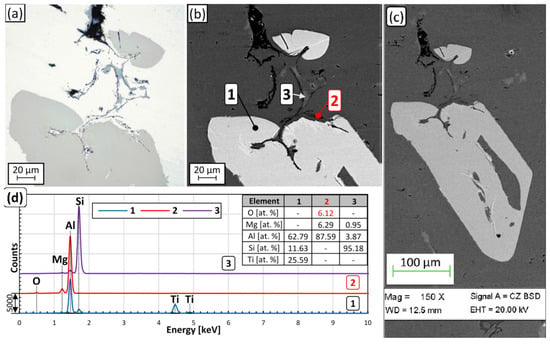
Figure 10.
(a) Optical microscopic and (b,c) SEM images of an entrained double oxide film partially engulfed by (Al,Si)3Ti particles and eutectic Si. The results of the EDS analysis are presented in (d) Reprinted from Refs. [208,210].
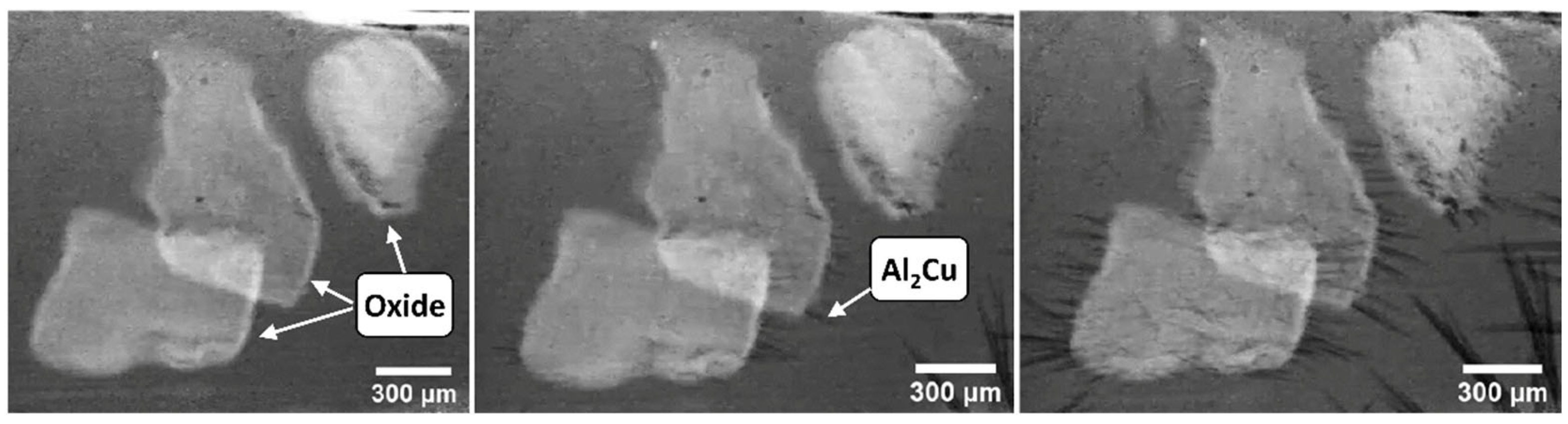
Similar, crack-like double oxide films were found inside α-Al15 (Fe,Mn)3Si2 phases by Cao and Campbell [211,212,213,214], who also found that by the crystallization and subsequent settling of α-Al15 (Fe,Mn)3Si2 in the liquid alloy, a remarkable amount of bifilm defects can be forced to sediment to the bottom region of the melt. Oxide films were also reported to be present inside β-Al5FeSi [17,207] and β-Al7Cu2 (FeMn) [215,216] platelets, but in this case, the oxide films appear to be straightened due to the strongly anisotropic growth of β platelets. Fe-rich intermetallics were also found to be commonly associated with different oxide particles that were introduced into the liquid alloys ex situ [105,217]. Oxide particles were also found inside intermetallics such as Al3Ti [138,218], Al3Zr [219], Al3Sc [220,221,222], and Mg2Si [37,223]. Besides observation in the solidified microstructure, in situ examinations also prove that oxide phases are effective heterogeneous nucleation substrates of intermetallics. With the aid of in situ synchrotron X-ray radiographic observations, Wang et al. [224] found that primary Al2Cu crystals crystallize on the surface of oxide films present in an Al-35%Cu alloy melt (Figure 11). Similarly, Peng et al. [225] used synchrotron radiography to study the interactions of Al8Mn5 intermetallic particles and oxide films present in an AZ91 Mg alloy melt. They found that Al8Mn5 particles heterogeneously nucleated on the floating oxide films, and also, some of the settling Al8Mn5 crystals became trapped in the crumpled oxide films.

Figure 11.
In situ synchrotron X-ray radiography images of Al2Cu crystal formation on the surface of oxide films floating in an Al-35%Cu alloy melt. Reprinted from Ref. [224].
As discussed in Section 3.1, the nucleation potential of the nucleant (oxide) is dependent on crystallographic matching between the substrate (oxide) and the solid (intermetallic). Theoretical calculations, as well as observations with the aid of transmission electron microscopy (TEM), show well-defined orientation relationships (ORs) with low misfit values between several intermetallic compounds and oxide phases. Table 1 compiles the related literature in which intermetallic compound/oxide couples were investigated in terms of crystallographic matching.

Table 1.
Oxide and intermetallic phases that have good crystallographic matching according to experimental or theoretical calculation results.
Based on Table 1, for most (if not all) intermetallic phases, low misfit interfaces can be formed with various oxides, supporting the theory that oxide phases are potent heterogeneous nucleation substrates of intermetallic phases. For example, Cao and Campbell [211] calculated the planar disregistry (a metric introduced by Bramfitt [184] to evaluate crystallographic matching between substrates and solid nuclei) values between various oxides and α-Al15 (Fe,Mn)3Si2 and found that α-, γ-Al2O3, MgAl2O4, and MgO are all potent substrates of α-Al15 (Fe,Mn)3Si2. Wang et al. [139] used the edge-to-edge matching model (E2EM) to evaluate the crystallographic matching between α-Al2O3 and Al3Ti. The E2EM predicted several possible crystallographic orientation relationships between the mentioned phases, and one of them was experimentally verified by analyzing the convergent beam Kikuchi line diffraction patterns obtained during the TEM examinations. Following ultrasonic melt treatment, Jung et al. [226] found Al2O3 particles inside Al3(Zr,Ti) intermetallic grains in an Al-0.27%Zr-0.1%Ti alloy (Figure 12a). One of the oxide particles consisted of γ-Al2O3, which had an incoherent interface with Al3 (Zr,Ti) (Figure 12b), while another oxide particle was multiphase, containing θ-, γ-, and amorphous Al2O3 (Figure 12c). θ-Al2O3 had a semicoherent interface with the intermetallic phase (Figure 12e). Contrary to the individual γ-Al2O3 particle, the γ-Al2O3 phase in the multiphase particle had an excellent lattice matching with Al3 (Zr,Ti) (Figure 12d) with a well-defined orientation relationship (Figure 12f). This indicates that Al3 (Zr,Ti) heterogeneously nucleated on the γ-Al2O3 phase in the multiphase oxide particle.
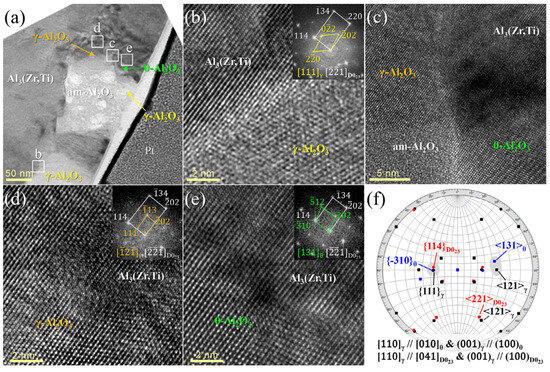
Figure 12.
(a) TEM image of a multiphase oxide particle inside an Al3 (Zr,Ti) crystal; (b–e) HRTEM images and fast Fourier transform (FFT) of the oxide/intermetallic interfaces. (f) Stereographic projections at the poles of [041] D023 (Al3 (Zr,Ti)), [010] θ-Al2O3, and [110] γ-Al2O3. A high degree of coherency can be observed at the γ-Al2O3/Al3 (Zr,Ti) interface in (d). Reprinted with permission from Ref. [226] Copyright 2025 Elsevier.
The experimental results presented in this section support the idea that oxide phases present in liquid aluminum alloys can be potential heterogeneous nucleation substrates of various intermetallic phases of the alloy. Theoretical calculations and experimental observations show good crystallographic matching between various oxides and intermetallics, which results in low interfacial energy, and in this way, the heterogeneous nucleation of these intermetallics can be realized at low undercooling. Based on that, the uniform dispersion of small oxide particles with a narrow size distribution remains the main challenge that needs to be accomplished to realize intermetallic refinement by the mechanism presented in Figure 3.
4. Methods of Melt Conditioning to Gain a Favorable Oxide Dispersion
In this section, those technologies are presented that can transform harmful oxide inclusions into small-sized oxide particles dispersed throughout the liquid alloy to serve as heterogeneous nucleation sites for different intermetallic phases.
4.1. Ultrasonic Melt Treatment
Despite being the subject of academic and industrial research for several decades, ultrasonic melt processing receives continuously increasing attention. This can be attributed to the fact that ultrasonic melt treatment is an environmentally friendly processing technique that is capable of degassing liquid metals, inducing structural refinement during solidification, and dispersing various solid particles evenly in the melt [237,238]. The two most significant physical phenomena that take place during the propagation of high-intensity ultrasound waves are acoustic cavitation and acoustic streaming. During acoustic cavitation, cavitation bubbles are formed, which grow and then collapse implosively. Acoustic streaming means the establishment of a continuous circulation flow propelled by acoustic wave propagation and the pulsation of the cavitation region [89,238,239].
Ultrasonic processing is capable of inducing a remarkable refining effect on intermetallic phases, and it can also influence the phase selection in the case of Fe-rich IMCs [240,241,242]. There are several theories that try to elucidate the governing refinement mechanism, but it is generally acknowledged that the following two mechanisms can be considered to have a significant effect on IMC refinement: (I) cavitation-enhanced nucleation and (II) fragmentation of intermetallic particles induced by cavitation (sonofragmentation) [243]. Sonofragmentation affects solid IMC particles that are present in the cavitation zone, meaning that this mechanism is active below the liquidus temperature of the alloy [88,89,90,91]. In our work, we discuss cavitation-enhanced nucleation with a special focus on the effect of ultrasonication on the oxide inclusions of liquid metals. According to Eskin and Eskin [88], in liquid metals, cavitation is initiated by the heterogeneous nucleation of cavitation bubbles on cavitation nuclei. These nuclei are solid inclusions of liquid metals that are poorly wetted by the melt, such as oxides, nitrides, and carbides. Eskin and Eskin [88] propose that very small pre-existing gas bubbles are attached to oxide inclusions in liquid aluminum alloys due to the molecular hydrogen that precipitates on these poorly wetted solid particles. Based on this approach, small gas volumes present in the form of double oxide films can also serve as initiation sites for acoustic cavitation. Campbell [244] proposes that entrainment defects, such as bifilms and small air bubbles, provide atomically unbonded mesoscale interfaces, which can act as initiation sites for cavitation. However, it is currently not well understood how the cavitation process affects bifilms.
Based on Section 2, double oxide films can be interpreted as oxide particle agglomerates with a small inner gas volume (Figure 6). The inner gas pockets act as initiation sites for acoustic cavitation during ultrasonic processing (Figure 13). During the collapse of cavitation bubbles, extremely high pressures (GPa range) and temperatures (>5000 K) are reached locally, which persist for a very short period of time (microseconds) [245,246]. Besides the dispersion effect of oxides present in the liquid metal, Sun et al. [247] proposed and experimentally verified that ultrasonication induces oxide particle (α- and γ-Al2O3) formation on the liquid Al surface by the fragmentation of the surface oxide film. The authors suggest that oxide film fragmentation is caused by cavitation-induced micro-jet formation. The cavitation-induced oxide film fragmentation process is also supported by other researchers [248,249]. Oxide particles created in this way can be entrained into the bulk liquid by the collapse of cavitation bubbles in the vicinity of the surface and the acoustic streaming effect.
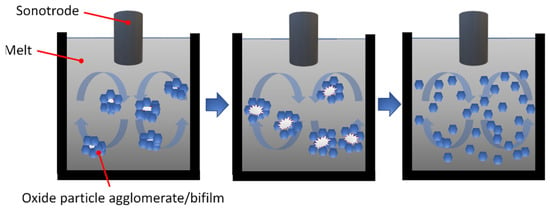
Figure 13.
Schematic illustration of the effect of acoustic cavitation and acoustic streaming on the spatial distribution of oxide particles in liquid alloys (based on Reprinted with permission from Ref. [245]). Copyright 2025 Elsevier.
After ultrasonic melt processing, oxide particles are frequently observed inside different intermetallic phases (for example, see Figure 12). Wang et al. [139,243] applied ultrasonic treatment to an Al-0.4%Ti alloy well above its liquidus temperature and achieved a remarkable refinement of the primary Al3Ti intermetallic phase. During the microstructural investigations, they found α-Al2O3 particles inside the Al3Ti phase particles (Figure 14a–c). Analysis with the edge-to-edge matching model, as well as TEM investigations, showed that there is a good crystallographic matching between the oxide and the Al3Ti phases. According to the authors, the refinement of primary Al3Ti can be attributed to the cavitation-induced deagglomeration of oxide particles and the cavitation-enhanced wetting of α-Al2O3 by the liquid metal. Similar observations were made on Al3Zr [226] and Al3 (Zr,Ti) [231] intermetallic phases. Jeon et al. [233] reported that applying ultrasonication to an Al-11.52%Zn-9.92%Cu-9.61%Si-9.36%Mg-0.01%P alloy modifies the coarse dendritic primary Mg2Si into fine compact particles. With the aid of high-resolution transmission electron microscopy (HRTEM), they identified the nucleant particles of primary Mg2Si to be Mg3P2 when UST was not utilized. On the other hand, when ultrasonic treatment was conducted, different oxide phases were identified as heterogeneous nucleation substrates of primary Mg2Si (Figure 15a–c). Similarly to Wang et al. [139,243], Jeon et al. [233] attributed this observation to the cavitation-induced deagglomeration and cavitation-enhanced wetting of native oxide particles of the liquid metal.
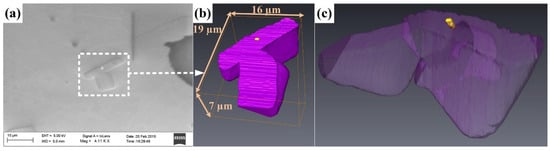
Figure 14.
(a) SEM image of an Al3Ti intermetallic containing an α-Al2O3 particle after ultrasonic melt treatment; (b,c) reconstructed 3D tomographic views of the Al3Ti phase (purple) and the α-Al2O3 particle (yellow). Reprinted from Ref. [139].

Figure 15.
SEM images of (a) MgO, (b) Al2O3, and (c) MgAl2O4 particles found inside Mg2Si compound particles in an Al-11.52%Zn-9.92%Cu-9.61%Si-9.36%Mg-0.01%P alloy after ultrasonic melt treatment. Reprinted with permission from Ref. [233] Copyright 2025 Elsevier.
In summary, experimental evidence shows that primary intermetallic phases can be significantly refined by applying high-intensity ultrasonic vibrations to liquid aluminum alloys. Although the exact interactions of different types of oxide inclusions (such as bifilms) need further investigation, it is evident that ultrasonic melt processing leads to the dispersion of fine oxide particles within the melt, which act as potent heterogeneous nucleation sites for different intermetallic phases.
4.2. Intensive Melt Shearing
Applying intensive shearing (or high-shear melt conditioning; HSMC) on liquid Al and Mg alloys was shown to induce notable microstructural refinement through the transformation of oxide films (and bifilms) into well-dispersed oxide particles with a narrow size distribution, which can act as heterogeneous nucleation sites for primary phases [218,250,251]. Fan et al. [252] describe an apparatus that uses a rotor–stator mixer to realize intensive melt-shearing (Figure 16a–c). The rotor and the stator are made from an inert ceramic material. An electrical motor with speed control drives the rotor, and the rotational speed can change between 1000 and 15,000 rpm. The liquid metal is sheared in the gap between the stator and the rotor, as well as in the stator openings (Figure 16b). By using this setup, the shear rate can reach up to 105 s−1. The centrifugal force created by the high-speed rotation squeezes the liquid metal through the stator holes while an equivalent amount of melt is pushed upward in a pumping action. In this way, macroflow is generated in the liquid volume, which aids distributive mixing [167,253]. Combining HSMC with purging inert gas below the rotor–stator mixer was found to be highly efficient in degassing due to the formation and dispersion of small purging gas bubbles. Besides improving the dispersion of the inert gas bubbles, surface turbulence, which is common during rotary degassing treatments, can be completely avoided by this novel treatment technology [254,255,256]. HSMC was also found to be an effective method in metal–matrix composite (MMC), as well as in semi-solid slurry production [252,257]. The effect of different rotor and stator geometries was extensively studied by modeling fluid flow during HSMC treatments [258,259,260].
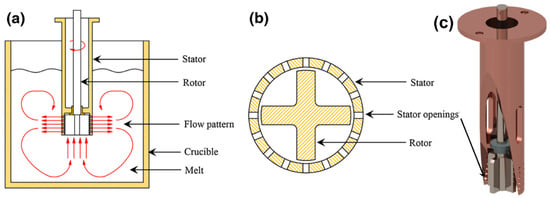
Figure 16.
(a) Transverse cross-sectional illustration of the rotor–stator arrangement used during high-shear melt conditioning; (b) bottom view of the mixer head, which consists of a rotor and a stator with numerous openings; (c) 3D view of the melt conditioning unit with the stator sectioned to allow a better view of the rotor. Reprinted from Refs. [167,255].
The strong shear force exerted by the mixer head is capable of fragmenting oxide films and bifilms, resulting in the formation of fine oxide particles, which are dispersed by the macroflow generated by the mixer. Figure 17 shows examples of oxide inclusions collected by pressurized melt filtration from commercial-purity (CP) Al melts that were held at different temperatures [253]. At 750 °C, a large population of oxide bifilms was collected, with γ-Al2O3 being the main constituent of the oxide films (Figure 17a). After HSMC, numerous small (<1 µm) γ-Al2O3 particles were created by the shearing action of the rotor–stator mixer (Figure 17b). When the holding temperature was increased to 900 °C, oxide stringers containing larger (1–2 µm) α-Al2O3 particles were found in the samples cast from the untreated melt (Figure 17c). The oxide stringers were effectively broken apart and dispersed by the HSMC treatment (Figure 17d) [127,253]. In the case of Al-Mg alloys, Niu et al. [125] found that native oxide particles follow a lognormal size distribution; however, in low-Mg alloys (0.08% and 0.4%), the agglomeration of oxide particles (and their occurrence in oxide bifilms) brings forth inhomogeneous sampling, which results in dual-peak lognormal curves. The HSMC treatment eliminated the dual peaks and resulted in a lowered mean particle diameter and a narrow, uniform oxide particle size distribution. Niu et al. [122] performed an in-depth analysis of the types of inclusions that can be found in recycled A357 alloy melts treated with traditional rotary degassing and HSMC combined with degassing. They found that HSMC was more efficient in reducing the number density of large oxide films, and the treatment resulted in well-dispersed, discontinuous inclusions. Although the treatment could not completely eliminate bifilms, it resulted in a significant microstructural refinement, improved fluidity, and reduced tendency to shrinkage porosity formation.
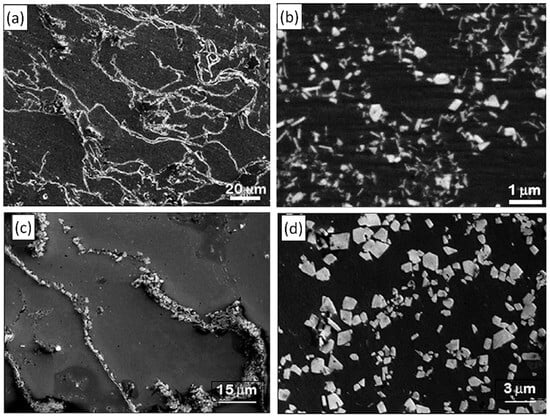
Figure 17.
Secondary electron SEM images of (a) oxide films collected by pressurized filtration from liquid CP Al at 750 °C and (c) 900 °C. (b) γ-Al2O3 and (d) α-Al2O3 particles collected after high-shear melt conditioning. Reprinted with permission from Ref. [253] Copyright 2025 Elsevier.
Besides refining α-Al, HSMC treatment was found to be efficient in the refinement of intermetallic phases [261]. Li et al. [262] found that applying HSMC treatment promoted the crystallization of compact α-Al15 (Fe,Mn)3Si2 over β-Al5FeSi platelets in LM24 alloy samples that were either cast into a TP-1 test mold or made with high-pressure die casting (HPDC). Using HSMC before casting with HPDC resulted in improved average ultimate tensile strength and elongation at fracture values (304 MPa instead of 279.5 MPa and 3.9% instead of 2.6%). The authors contributed the modification of Fe-rich IMCs to the enhanced heterogeneous nucleation effect of MgAl2O4 particles that were effectively dispersed in the liquid metal by the intensive melt shearing. HSMC treatment was also shown to be effective in refining the Fe-rich intermetallics of direct-chill (DC) cast A6063 alloy [263]. Lazaro-Nebreda et al. [48,264] also report on the modification of Fe-rich IMCs by applying HSMC on an A380 alloy melt. It was found that the HSMC treatment reduced the undercooling of primary α-Al15 (Fe,Mn)3Si2 significantly (more than 15 °C reduction regardless of the cooling rate). Because of this, α-Al15 (Fe,Mn)3Si2 becomes more dominant in the microstructure (instead of β-Al5FeSi), but the size of the α-Al15 (Fe,Mn)3Si2 particles becomes slightly larger due to the increased available growth time. β-Al5FeSi platelets, however, became refined significantly: in the case of A380 alloy containing 0.2% Mn and 2% Fe solidified at 0.15 °C/s, the average length of β-Al5FeSi platelets was reduced from 309 ± 109 µm to 224 ± 113 µm. For 1% Mn and 1% Fe concentrations, only α-Al15 (Fe,Mn)3Si2 particles crystallized, and their size was reduced by the HSMC treatment from 137 ± 15 µm to 102 ± 21 µm.
Similarly to ultrasonic melt treatment, intensive melt shearing can provide microstructural refinement without the need for any master alloy addition (such as grain refiners). Combining the treatment with inert gas bubbling provides an alternative to traditional rotary degassing without the need for flux additions and, in this way, enables more economic and sustainable melt processing. Due to the microstructural refinement, HSMC treatment increases the tolerance of the given alloy to impurity elements like Fe and makes it possible to utilize an increased portion of secondary alloys for the production of quality castings. More research is needed on whether this kind of melt processing is capable of eliminating double oxide films completely and how the inner gas atmosphere of bifilms is affected during intensive shearing. Increasing the efficiency of bifilm transformation into oxide particles would enable the production of castings free from porosity and hot tears and with highly increased ductility and fatigue life [265,266].
4.3. Melt Superheating
Increasing liquid metal superheating has a notable effect on the crystallization behavior of certain intermetallic compounds. One of the explanations given identifies the heterogeneous nucleation of the intermetallic compounds on different oxides to be the main mechanism behind this phenomenon. Narayanan et al. [217] showed that by superheating a 319 alloy containing 1% Fe to 850 °C or 900 °C followed by cooling it to a casting temperature of 750 °C and solidifying at high cooling rates (10 °C/s or higher), α-AlFeSi IMC is stabilized, and β-Al5FeSi formation is mostly suppressed. The authors explain this by the transformation of γ-Al2O3 inclusion into α-Al2O3 by increasing the melt temperature to 850 °C or 900 °C and by the ability of these oxides to nucleate β-Al5FeSi. Metallographic observations showed that γ-Al2O3 (regardless of in situ formed or ex situ added) is a potent nucleant for β-Al5FeSi. By the transformation into α-Al2O3, which is not a potent substrate for β-Al5FeSi according to the authors, the nucleation of β-Al5FeSi becomes less feasible, and the undercooling needed for the nucleation of α-AlFeSi is reached at higher cooling rates. Similarly, Ahmad and Marshall [267] investigated various melt temperatures ranging from 710 °C to 1000 °C in the case of two casting Al-Si alloys containing 1.12% and 1.94% Fe. They found that by increasing the superheating temperature, β-Al5FeSi first became refined, and then fine, compact α-Al15 (Fe,Mn)3Si2 particles became dominant. In this case, the casting temperatures of the samples were highly different, so besides the effect of oxide transformation, the influence of higher solidification rates should also be taken into account. Mathew et al. [268] also showed that increasing the casting temperature of an Al-7%Si-2%Fe alloy to 900 °C resulted in finely distributed β-Al5FeSi. Cao and Campbell [213] reported that increasing the melting temperature of an LM9 alloy to 850 °C resulted in refined primary α-Al15 (Fe,Mn)3Si2 particles.
Increasing superheat temperature may ease the dispersion of oxide particles within the liquid metal due to the advanced oxidation state and increased brittleness of the oxide films. Old oxide films are generally more brittle than young films, and they contain a higher fraction of crystalline oxides that may be dispersed in the liquid metal [141,142]. Also, phase transformations in the oxide layers induce stresses that contribute to the fragmentation of oxide films. For example, using high melt temperatures accelerates the γ-Al2O3 →α-Al2O3 transformation, which involves a 24% reduction in the oxide molar volume. This volume change induces tensile stresses in the oxide layer, which facilitates the formation of dispersed oxide particles [147,149]. This was experimentally verified by Wang et al. [127], who found an increased number of dispersed α-Al2O3 oxide particles in commercial-purity Al after increasing the holding temperature to 920 °C. On the other hand, superheating should be combined with a melt treatment method that can induce oxide film fragmentation without the formation of new double oxide film defects in the liquid metal.
Currently, increasing superheat temperatures is not a technologically viable option to induce microstructural refinement due to the drawbacks of this method. Increasing the melt temperature results in a higher hydrogen solubility and a higher loss of alloying elements that can be selectively oxidized. Besides that, the higher energy costs that come with increased melt temperatures should also be taken into account.
4.4. In Situ Oxide Formation by Chemical Reaction
Instead of the transformation of native oxide phases of the alloy melts, finely dispersed oxide particles can be produced by in situ chemical reactions between different additives and the liquid metal. These chemical reactions provide the foundation of the fabrication methods of in situ oxide-reinforced aluminum matrix composites (AMCs). The in situ oxide phase formation either relies on the displacement reaction between the liquid Al (or its reactive alloying elements) and different oxides or on the oxidation of Al by the oxygen released during the decomposition of different substances [151,269,270]. Contrary to ex situ oxide introduction, the in situ generation of oxide phases generally results in a more uniform distribution of oxide particles within the microstructure and stronger interfacial bonding between the matrix and the oxide particles (Figure 18a,b) [151]. Thermite reactions between oxides that are thermodynamically less stable than Al2O3 (represented as , where represents a metallic element such as Cu, Fe, Zn, Ti, Co, Ni, Zr, etc.; and are stoichiometric constants) and liquid Al are generally utilized to produce Al2O3-reinforced AMCs in accordance with the following general reaction [271]:
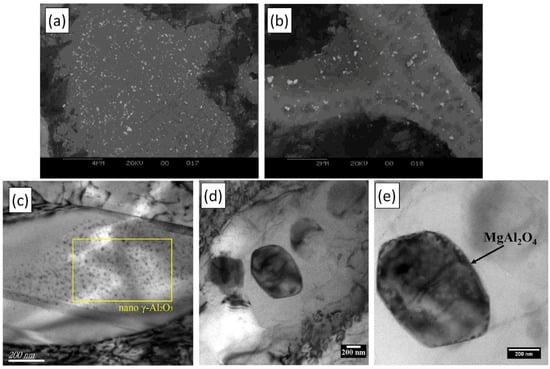
Figure 18.
(a,b) SEM micrographs of α-Al2O3 particles in the (a) α-Al and (b) Al2Cu phases in an AMC. The oxide particles were created through the reaction of CuO and liquid Al. Reprinted with permission from Ref. [272] Copyright 2025 Elsevier. (c) Bright-field TEM image of γ-Al2O3 nanoparticles embedded in an Al9Co2 intermetallic. Reprinted with permission from Ref. [273] Copyright 2025 Elsevier. (d,e) TEM images of MgAl2O4 nanoparticles generated through the reaction of SiO2 and Al-2%Mg liquid alloy. Reprinted with permission from Ref. [274] Copyright 2025 Elsevier.
Some examples of solid sources of oxygen include SiO2 [272], CuO [275], TiO2 [271,276], Co3O4 [273], Fe2O3 [150,277], ZrO2 [278], NiO [279], ZnO [271], CeO2 [280], and B2O3 [281]. As demonstrated by the examples presented in Figure 18a–e, evenly distributed oxide nanoparticles can be generated by in situ reactions. Different approaches can be utilized for the introduction and dispersion of the oxygen source particles. Huang et al. [272] applied uniaxial cold pressing to prepare compacts from CP Al and CuO, as well as from CP Al, CuO, and SiO2 powders. These compacts were then introduced to liquid Al to bring forth the exothermic oxide-forming reactions between CuO and Al, as well as SiO2 and Al. Based on the microstructural investigations, this production method was able to provide even oxide distribution in the α-Al matrix (Figure 18a) and the Al2Cu phase (Figure 18b). Xu et al. [273] utilized the vortex method (vortex formation with a high-speed impeller) under an Ar atmosphere to introduce and disperse Co3O4 particles within an A356 alloy melt. The formation of γ-Al2O3 nanoparticles (Figure 18c) induced remarkable microstructural refinement and increased ultimate tensile strength and elongation values when the concentration of γ-Al2O3 nanoparticles was 0.6 vol%.
In the case of high-Mg alloys, the in situ reactions cause the selective oxidation of Mg. The resulting reaction products are either MgO or MgAl2O4 particles. Thakur et al. [274] combined stir casting and ultrasonic melt treatment to produce Al-MgAl2O4 in situ composite using SiO2 as a solid oxygen source. The size of the resulting MgAl2O4 particles changed by between 0.2 and 5 µm (Figure 18d,e). Sreekumar et al. [282] also applied impeller stirring combined with ultrasonic melt treatment to produce an Al-MgAl2O4 in situ composite with a SiO2 oxygen source. The following chemical reactions can be identified to be relevant when SiO2 is added to high-temperature liquid Al-Mg alloys [283]:
During the introduction of solid oxygen sources, one of the main challenges is to avoid the entrainment of the surface oxide films and the creation of double oxide film defects. If the reactant particles are introduced through the liquid metal surface, the oxygen source particles come into contact with the surface oxide film instead of the liquid metal [142]. In this case, the in situ reactions can only take place if the oxide layers are torn apart or become cracked. For this reason, better reaction efficiency and particle distribution can be achieved if the reactant is injected into the liquid metal with an inert carrier gas (high-purity, dry Ar) [279].
In in situ oxide-reinforced AMCs, microstructural refinement is realized by a high fraction of oxide particles (concentrations of several weight percentages of Al2O3 or MgAl2O4 are common) [151]. However, for the purpose of intermetallic phase refinement (Figure 3), lower addition rates could also be sufficient (a concentration similar to traditional grain refiner particles) to initiate enhanced heterogeneous nucleation of intermetallics. The application of small quantities of in situ oxide-forming reactants to refine intermetallic phases in casting aluminum alloys is currently an unexplored area. Finally, it should also be noted that the dispersion of in situ oxides can only be efficiently achieved by applying technological solutions already described in previous chapters.
4.5. Ex Situ Oxide Addition
Small-sized oxide particles can be added to the liquid alloy in an ex situ manner with the aid of methods such as stir casting (or the “vortex method”) [284], vortex-free stir casting [285,286], injection with inert carrier gas [105], or the addition of pre-synthesized master alloys [276,287]. Ex situ oxides also have the potential to change the crystallization behavior of intermetallic phases. For example, it was reported by Que et al. [228] that when 0.1 wt% MgO nanoparticles were added to an Al–5%Mg–2%Si–1.1%Fe-0.7%Mn alloy, the formation of the primary Al6 (Fe,Mn) phase was enhanced in favor of the primary Al15 (Fe,Mn)3Si2 at a cooling rate of 3.5 K/s. The crystallographic analysis of MgO and Al6 (Fe,Mn) revealed that a small-misfit (0.95%) interface can be formed between the oxide and the metastable Al6 (Fe,Mn), making the heterogeneous nucleation process more feasible.
The main challenges of ex situ oxide additions are achieving uniform particle distribution, realizing strong interfacial bonding between the oxide and the matrix (or the intermetallics of the alloy), and avoiding air and oxide film entrainment during the production process [151]. If oxide particles are introduced through the liquid metal surface, the particles come into contact with the surface oxide film instead of the liquid metal (Figure 19). Particles or particle agglomerates are submerged in the bulk liquid while they are surrounded by an oxide film and significant quantities of air. Because there is no true contact between the liquid metal and the oxide particles, their heterogeneous nucleation effect cannot be utilized. By increasing the native population of undesirable oxide inclusions (such as bifilms) with the application of methods similar to the one presented in Figure 19, any beneficial effects of oxide addition may be lost [288]. In this case, additional processing steps, such as high-shear melt conditioning and ultrasonic melt treatment, may be desirable to break down particle agglomerates and to facilitate the wetting of the particles by the liquid metal.
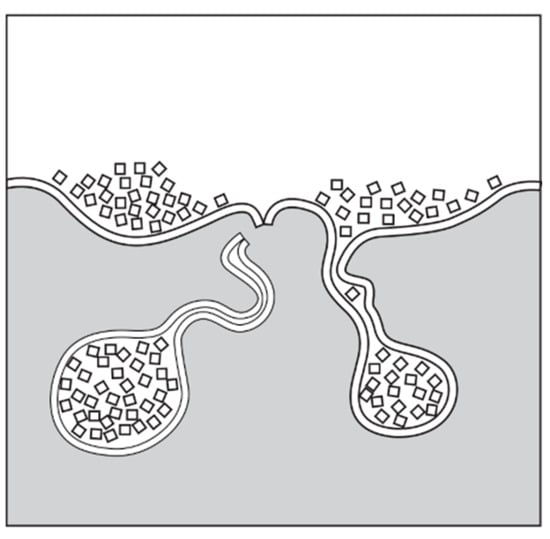
Figure 19.
Schematic representation of particle agglomerate formation during the simultaneous entrainment of the surface oxide film and ex situ particles. Reprinted with permission from Ref. [288]. Copyright 2025 John Campbell. Published by Elsevier Ltd.
5. Conclusions and Outlook
This work aimed to review the recent progress of experimental and theoretical work related to the possibility of influencing intermetallic phase crystallization with the aid of oxide phases. The most important conclusions of the present review, as well as the future aspects and research directions related to oxide-aided intermetallic refinement, are presented in Table 2.

Table 2.
Summary of the main findings and related future challenges.
Based on the results reviewed in this work, for most intermetallic phases occurring in cast Al alloys, common oxide phases (such as Al2O3 variants, MgAl2O4, and MgO) are potent heterogeneous nucleation sites during the solidification of the alloy. This can be attributed to the low interfacial energies between the oxides and the intermetallic phases. By controlling the oxide phase population in liquid alloys, the phase selection, morphology, and size of intermetallic compounds can be influenced by affecting the nucleation process of the intermetallics.
- Oxides are generally present in liquid aluminum alloys in the form of double oxide films that can be interpreted as sites of oxide particle agglomeration. Novel melt treatment methods should aim to transform these agglomerations into well-dispersed particles through the fragmentation of the bifilms. Using this approach holds the potential to simultaneously mitigate the negative effects of oxide inclusions and coarse IMCs.
- Ultrasonic melt processing and intensive melt shearing are sustainable melt treatment technologies that provide microstructural refinement without the need for any additives (master alloys, fluxes, etc.). These treatment methods can increase the tolerance of the given alloy to impurity elements, which enables the utilization of an increased portion of secondary alloys to produce quality castings. However, more research is needed on how ultrasonic melt treatment and intensive melt shearing affects double oxide film defects and whether these processing techniques make it possible to eliminate bifilm defects completely, which would enable the production of castings of unprecedented quality. Research should focus on increasing the efficiency of transforming bifilms into fine oxide particles.
- It should be examined whether the application of small quantities of in situ oxide-forming reactants is capable of refining intermetallic phases in casting aluminum alloys. The minimum quantity of reactants that can refine intermetallics through in situ oxide-forming reactions is currently not known for most aluminum alloy and reactant combinations. Also, combining in situ oxide-forming reactions with melt processing techniques like ultrasonic melt processing and intensive melt shearing can result in increased refining efficiency.
Author Contributions
Conceptualization, G.G.; writing—original draft preparation, G.G.; writing—review and editing, J.E.; visualization, G.G.; supervision, J.E.; funding acquisition, J.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research, Development and Innovation Fund, grant number EKÖP-24-4-I.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Supported by the University Research Scholarship Program of The Ministry for Culture and Innovation from the Source of the National Research, Development and Innovation Fund. This Research topic is supported by the SteelTech-Center Hungary project of the University of Miskolc through providing access to FactSage 8.3 software and its FactPS database.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
The standard enthalpy of reaction values (, [kJ/mol]), as well as the standard Gibbs free energy change accompanying the chemical reactions (, [kJ/mol]) described in the article, are presented in Table A1. The calculations were made with the FactSage 8.3 software and its FactPS database [289] at 700 °C, which is a common liquid metal processing temperature for Al alloys.

Table A1.
Standard enthalpy of reaction and standard Gibbs free energy data for the presented chemical reactions at 700 °C.
Table A1.
Standard enthalpy of reaction and standard Gibbs free energy data for the presented chemical reactions at 700 °C.
| Reaction No. | [kJ/mol] | Notes | |
|---|---|---|---|
| (1) | −1129.07 | −913.56 | α-Al2O3 |
| −1114.20 | −903.51 | γ-Al2O3 | |
| (2) | −950.52 | −787.94 | α-Al2O3 |
| −928.22 | −772.85 | γ-Al2O3 | |
| (3) | −1217.50 | −992.74 | liquid Mg |
| −1483.87 | −1068.21 | Mg vapor | |
| (4) | −132.65 | −118.77 | α-Al2O3 (no data for am-Al2O3) |
| −154.96 | −133.86 | γ-Al2O3 (no data for am-Al2O3) | |
| (5) | −1162.73 | −948.08 | - |
| (6) | −1144.47 | −933.18 | - |
| (7) | −23.11 | −29.44 | α-Al2O3 |
| (15) | −262.24 | −236.55 | β-quartz |
| (16) | −414.93 | −383.78 | β-quartz |
| (17) | −521.41 | −472.12 | β-quartz, α-Al2O3 |
| (18) | −567.63 | −531.00 | β-quartz |
References
- Luo, A.A.; Sachdev, A.K.; Apelian, D. Alloy Development and Process Innovations for Light Metals Casting. Mater. Process. Technol. 2022, 306, 117606. [Google Scholar] [CrossRef]
- Lehmhus, D. Advances in Metal Casting Technology: A Review of State of the Art, Challenges and Trends—Part I: Changing Markets, Changing Products. Metals 2022, 12, 165–166. [Google Scholar]
- Li, S.S.; Yue, X.; Li, Q.Y.; Peng, H.L.; Dong, B.X.; Liu, T.S.; Yang, H.Y.; Fan, J.; Shu, S.L.; Qiu, F.; et al. Development and Applications of Aluminum Alloys for Aerospace Industry. J. Mater. Res. Technol. 2023, 27, 944–983. [Google Scholar] [CrossRef]
- Ducker Aluminum Content in Passenger Vehicles (Europe). 2023. Available online: https://european-aluminium.eu/wp-content/uploads/2023/05/2023_04_Aluminum-Content_Ducker-Study_EA-Public-Summary_190423.pdf (accessed on 1 December 2024).
- Wang, Q.G.; Wang, A.; Coryell, J. Ultra-Large Aluminum Shape Casting: Opportunities and Challenges. China Foundry 2024, 21, 397–408. [Google Scholar] [CrossRef]
- Burggräf, P.; Bergweiler, G.; Kehrer, S.; Krawczyk, T.; Fiedler, F. Mega-Casting in the Automotive Production System: Expert Interview-Based Impact Analysis of Large-Format Aluminium High-Pressure Die-Casting (HPDC) on the Vehicle Production. J. Manuf. Process. 2024, 124, 918–935. [Google Scholar] [CrossRef]
- Raabe, D.; Ponge, D.; Uggowitzer, P.; Roscher, M.; Paolantonio, M.; Liu, C.; Antrekowitsch, H.; Kozeschnik, E.; Seidmann, D.; Gault, B.; et al. Making Sustainable Aluminum by Recycling Scrap: The Science of “Dirty” Alloys. Prog. Mater. Sci. 2022, 128, 100947. [Google Scholar] [CrossRef]
- Nunes, H.; Emadinia, O.; Soares, R.; Vieira, M.F.; Reis, A. Adding Value to Secondary Aluminum Casting Alloys: A Review on Trends and Achievements. Materials 2023, 16, 895. [Google Scholar] [CrossRef]
- Zhu, H.; Xia, C.; Zhang, H.; Zhao, D.; Wang, M.; Wang, H. Design of Non-Heat Treatable High Pressure Die Casting Al Alloys: A Review. J. Mater. Eng. Perform. 2024, 33, 8601–8626. [Google Scholar] [CrossRef]
- Zhan, H.; Zeng, G.; Wang, Q.; Wang, C.; Wang, P.; Wang, Z.; Xu, Y.; Hess, D.; Crepeau, P.; Wang, J. Unified Casting (UniCast) Aluminum Alloy—A Sustainable and Low-Carbon Materials Solution for Vehicle Lightweighting. J. Mater. Sci. Technol. 2023, 154, 251–268. [Google Scholar] [CrossRef]
- Sacinti, M.; Cubuklusu, E.; Birol, Y. Effect of Iron on Microstructure and Mechanical Properties of Primary AlSi7Mg0.3 Alloy. Int. J. Cast. Met. Res. 2017, 30, 96–102. [Google Scholar] [CrossRef]
- Liu, K.; Cao, X.; Chen, X.G. Effects of Iron-Rich Intermetallics on Tensile Deformation of Al-Cu 206 Cast Alloys. Metall. Mater. Trans. B 2015, 46, 1566–1575. [Google Scholar] [CrossRef]
- Ferraro, S.; Timelli, G. Influence of Sludge Particles on the Tensile Properties of Die-Cast Secondary Aluminum Alloys. Metall Mater. Trans. B 2015, 46, 1022–1034. [Google Scholar] [CrossRef]
- Elsharkawi, E.A.; Abdelaziz, M.H.; Doty, H.W.; Valtierra, S.; Samuel, F.H. Effect of β-Al5FeSi and π-Al8Mg3FeSi6 Phases on the Impact Toughness and Fractography of Al–Si–Mg-Based Alloys. Int. J. Metalcast. 2018, 12, 148–163. [Google Scholar] [CrossRef]
- Mathew, J.; Remy, G.; Williams, M.A.; Tang, F.; Srirangam, P. Effect of Fe Intermetallics on Microstructure and Properties of Al-7Si Alloys. JOM 2019, 71, 4362–4369. [Google Scholar] [CrossRef]
- Zahedi, H.; Emamy, M.; Razaghian, A.; Mahta, M.; Campbell, J.; Tiryakioǧlu, M. The Effect of Fe-Rich Intermetallics on the Weibull Distribution of Tensile Properties in a Cast Al-5 Pct Si-3 Pct Cu-1 Pct Fe-0.3 Pct Mg Alloy. Metall. Mater. Trans. A 2007, 38, 659–670. [Google Scholar] [CrossRef]
- Mahta, M.; Emamy, M.; Cao, X.; Campbell, J. Overview of β-Al5FeSi Phase in Al-Si Alloys. Materials Science Research Trends; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2008; pp. 251–271. [Google Scholar]
- Puncreobutr, C.; Lee, P.D.; Kareh, K.M.; Connolley, T.; Fife, J.L.; Phillion, A.B. Influence of Fe-Rich Intermetallics on Solidification Defects in Al-Si-Cu Alloys. Acta Mater. 2014, 68, 42–51. [Google Scholar] [CrossRef]
- Lu, L.; Dahle, A.K. Iron-Rich Intermetallic Phases and Their Role in Casting Defect Formation in Hypoeutectic Al-Si Alloys. Metall. Mater. Trans. A 2005, 36, 819–935. [Google Scholar] [CrossRef]
- Bas, E.N.; Alper, S.; Tuncay, T.; Dispinar, D.; Kirtay, S. Influence of Melt Quality on the Formation of Fe Intermetallic in A360 Alloy. Arch. Foundry Eng. 2022, 22, 53–59. [Google Scholar] [CrossRef]
- Khalifa, W.; Samuel, A.M.; Samuel, F.H.; Doty, H.W.; Valtierra, S. Metallographic Observations of β-AlFeSi Phase and Its Role in Porosity Formation in Al-7%Si Alloys. Int. J. Cast Met. Res. 2006, 19, 156–166. [Google Scholar] [CrossRef]
- Samuel, A.M.; Doty, H.W.; Valtierra, S.; Samuel, F.H. Beta Al5FeSi Phase Platelets-Porosity Formation Relationship in A319.2 Type Alloys. Int. J. Metalcast. 2018, 12, 55–70. [Google Scholar] [CrossRef]
- Taylor, J.A.; Schaffer, G.B.; Stjohn, D.H. The Role of Iron in the Formation of Porosity in Al-Si-Cu-Based Casting Alloys: Part I. Initial Experimental Observations. Metall. Mater. Trans. A 1999, 30, 1643–1650. [Google Scholar]
- Cinkilic, E.; Ridgeway, C.D.; Yan, X.; Luo, A.A. A Formation Map of Iron-Containing Intermetallic Phases in Recycled Cast Aluminum Alloys. Metall. Mater. Trans. A 2019, 50, 5945–5956. [Google Scholar] [CrossRef]
- Taylor, J.A. Iron-Containing Intermetallic Phases in Al-Si Based Casting Alloys. Proc. Mat. Sci. 2012, 1, 19–33. [Google Scholar] [CrossRef]
- Sigworth, G.K.; Donahue, R.J. The Metallurgy of Aluminum Alloys for Structural High-Pressure Die Castings. Int. J. Metalcast. 2020, 15, 1031–1046. [Google Scholar] [CrossRef]
- Becker, H.; Leineweber, A. Dealing with Fe in Secondary Al-Si Alloys Including Metal Melt Filtration. In Multifunctional Ceramic Filter Systems for Metal Melt Filtration; Springer International Publishing: Berlin/Heidelberg, Germany, 2024; pp. 191–213. ISBN 9783031409301. [Google Scholar]
- Belov, N.A.; Aksenov, A.A.; Eskin, D.G. Iron in Aluminium Alloys; CRC Press: Boca Raton, FL, USA, 2002; ISBN 9780429189906. [Google Scholar]
- Allen, C.M.; O’Reilly, K.A.Q.; Cantor, B.; Evans, P.V. Intermetallic Phase Selection in 1XXX Al Alloys. Prog. Mater. Sci. 1998, 43, 89–170. [Google Scholar] [CrossRef]
- Que, Z.; Wang, Y.; Mendis, C.L.; Fang, C.; Xia, J.; Zhou, X.; Fan, Z. Understanding Fe-Containing Intermetallic Compounds in Al Alloys: An Overview of Recent Advances from the LiME Research Hub. Metals 2022, 12, 1677. [Google Scholar] [CrossRef]
- Sigworth, G.K. Refining of Secondary Aluminum: Important Chemical Factors. JOM 2021, 73, 2594–2602. [Google Scholar] [CrossRef]
- Wiesner, S. Cast Alloy. Aluminium Rheinfelden Alloys GmbH. EP3235916B1, 19 April 2016. [Google Scholar]
- Liu, C.; Jiao, X.; Nishat, H.; Akhtar, S.; Wiesner, S.; Guo, Z.; Xiong, S. Characteristics of Fe-Rich Intermetallics Compounds and Their Influence on the Cracking Behavior of a Newly Developed High-Pressure Die Cast Al–4Mg–2Fe Alloy. J. Alloys Compd. 2021, 854, 157121. [Google Scholar] [CrossRef]
- Aluminium Rheinfelden Alloys Castaduct. Available online: https://rheinfelden-alloys.eu/en/alloys/castaduct/ (accessed on 1 December 2024).
- Sigworth, G. Aluminum Casting Alloys and Casting Processes. Alum. Sci. Technol. 2018, 2, 119–142. [Google Scholar] [CrossRef]
- Samuel, E.; Samuel, A.M.; Doty, H.W.; Samuel, F.H. Intermetallics Formation, Hardness and Toughness of A413.1 Type Alloys: Role of Melt and Aging Treatments. Inter. J. Met. 2023, 17, 1095–1113. [Google Scholar] [CrossRef]
- Slyudova, A.; Trudonoshyn, O.; Prach, O.; Lisovskii, V. Morphology and Nucleation of Intermetallic Phases in Casting Al—Mg—Si Alloys. Metallogr. Microstruct. Anal. 2020, 9, 873–883. [Google Scholar] [CrossRef]
- Ji, S.; Wang, Y.U.N.; Watson, D.; Fan, Z. Microstructural Evolution and Solidification Behavior of Al-Mg-Si Alloy in High-Pressure Die Casting. Metall. Mater. Trans. A 2013, 44, 3185–3197. [Google Scholar] [CrossRef]
- Talamantes-Silva, M.A.; Rodríguez, A.; Talamantes-Silva, J.; Valtierra, S.; Colás, R. Characterization of an Al-Cu Cast Alloy. Mater. Charact. 2008, 59, 1434–1439. [Google Scholar] [CrossRef]
- Samuel, E.; Samuel, A.M.; Doty, H.W.; Valtierra, S.; Samuel, F.H. Highlights on the Role of Fe, Sr, and Solidification Time on Porosity Formation in Al–Si Cast Alloys. Int. J. Met. 2024, 1–22. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Ibrahim, M.F.; Samuel, E.; Samuel, A.M.; Samuel, F.H.; Doty, H.W. Assessment of the Effect of Mg Addition on the Solidification Behavior, Tensile and Impact Properties of Al–Si–Cu Cast Alloys. Int. J. Met. 2023, 17, 82–108. [Google Scholar] [CrossRef]
- Fortini, A.; Lattanzi, L.; Merlin, M.; Garagnani, G.L. Comprehensive Evaluation of Modification Level Assessment in Sr-Modified Aluminium Alloys. Inter. J. Met. 2018, 12, 697–711. [Google Scholar] [CrossRef]
- Samuel, E.; Samuel, A.M.; Doty, H.W.; Valtierra, S.; Samuel, F.H.; Samuel, A.M.; Doty, H.W.; Valtierra, S.; Samuel, F.H.; Samuel, E.; et al. Intermetallic Phases in Al–Si Based Cast Alloys: New Perspective. Int. J. Cast Met. Res. 2014, 27, 107–114. [Google Scholar] [CrossRef]
- Ganesh, M.R.S.; Reghunath, N.J.; Levin, M.; Prasad, A.; Doondi, S.; Shankar, K.V. Strontium in Al–Si–Mg Alloy: A Review. Met. Mat. Int. 2021, 28, 1–40. [Google Scholar] [CrossRef]
- Samuel, A.M.; Samuel, E.; Songmene, V.; Samuel, F.H. A Comparative Study of Grain Refining of Al-(7–17%) Si Cast Alloys Using Al-10% Ti and Al-4% B Master Alloys. Materials 2023, 16, 2867. [Google Scholar] [CrossRef]
- Jaffarnia, A.; Ghomashchi, R. AlTiSi Intermetallics: Morphology and Its Role on Nozzle Blockage during Semi-Continous Casting. Inter. J. Met. 2015, 9, 61–68. [Google Scholar] [CrossRef]
- Samuel, A.M.; Mohamed, S.S.; Doty, H.W.; Valtierra, S.; Samuel, F.H. Grain Refining of Al-Si Alloys Using Al-10% Ti Master Alloy: Role of Zr Addition. Int. J. Cast Met. Res. 2019, 32, 46–58. [Google Scholar] [CrossRef]
- Lazaro-Nebreda, J.; Patel, J.B.; Chang, I.T.H.; Stone, I.C.; Fan, Z. Solidification Processing of Scrap Al-Alloys Containing High Levels of Fe. In Proceedings of the IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Bristol, UK, 2019; Volume 529. [Google Scholar]
- Liu, Y.; Luo, L.; Han, C.; Ou, L.; Wang, J.; Liu, C. Effect of Fe, Si and Cooling Rate on the Formation of Fe- and Mn-Rich Intermetallics in Al-5Mg-0.8Mn Alloy. J. Mater. Sci. Technol. 2016, 32, 305–312. [Google Scholar] [CrossRef]
- Kishor, M.; Chopra, K.; Ayyagari, K.P.R. Tackling Fe-Rich Intermetallics in Al-Si Alloy: A Critical Review. Trans. Ind. Inst. Met. 2023, 77, 3031–3036. [Google Scholar] [CrossRef]
- Balasubramani, N.; Moodispaw, M.; Luo, A.A. Controlling the Fe-Intermetallic Phases and Mechanical Properties of Secondary Al-9Si-1Fe Alloy with Cr and Mn Additions. J. Mater. Sci. Technol. 2024, 206, 135–152. [Google Scholar] [CrossRef]
- Kishor, M.; Ram, K.Y.P.; Rao, A.K.P.; Nahid, S.A.G.; Ramavajjala, A.K. Synergistic Effect of Mo and V Addition on Al–Si Alloys Containing High Iron Impurity. Inter. J. Met. 2024, 18, 3538–3548. [Google Scholar] [CrossRef]
- Balasubramani, N.; Moodispaw, M.; Cinkilic, E.; Miao, J.; Luo, A.A. Strontium Effects on the Formation of Iron-Intermetallic Phases in Secondary Al–9Si–0.6Fe Alloys. Metall. Mater. Trans. A 2023, 55, 550–568. [Google Scholar] [CrossRef]
- Song, D.; Zhao, Y.; Jia, Y.; Li, X.; Fu, Y. Synergistic Effects of Mn and B on Iron-Rich Intermetallic Modification of Recycled Al Alloy. J. Mater. Res. Technol. 2023, 24, 527–541. [Google Scholar] [CrossRef]
- Song, D.F.; Zhao, Y.L.; Wang, Z.; Jia, Y.W.; Li, D.X.; Fu, Y.N.; Zhang, D.T.; Zhang, W.W. 3D Fe-Rich Phases Evolution and Its Effects on the Fracture Behavior of Al–7.0Si–1.2Fe Alloys by Mn Neutralization. Acta Metall. Sin. 2022, 35, 163–175. [Google Scholar] [CrossRef]
- Li, Z.; Limodin, N.; Tandjaoui, A.; Quaegebeur, P.; Zhu, X.; Balloy, D. Effect of Fe and Mn Content on the Microstructures and Tensile Behaviour of AlSi7Cu3 Alloy: Thermal Analysis and Tensile Tests. Met. Mat. Int. 2022, 28, 2118–2133. [Google Scholar] [CrossRef]
- Podprocká, R.; Bolibruchová, D. Iron Intermetallic Phases in the Alloy Based on Al-Si-Mg by Applying Manganese. Arch. Foundry Eng. 2017, 17, 217–221. [Google Scholar] [CrossRef]
- Ferraro, S.; Fabrizi, A.; Timelli, G. Evolution of Sludge Particles in Secondary Die-Cast Aluminum Alloys as Function of Fe, Mn and Cr Contents. Mater. Chem. Phys. 2015, 153, 168–179. [Google Scholar] [CrossRef]
- Moustafa, M.A.; Lepage, C.; Samuel, F.H.; Doty, H.W. Metallographic Observations on Phase Precipitation in Strontium-Modified Al-11.7% Si Alloys: Role of Alloying Elements. Int. J. Cast Met. Res. 2003, 15, 609–626. [Google Scholar] [CrossRef]
- Mikel, J.; Arribas, M.; Galarraga, H.; Garcia, M.; Cortazar, D.; Ellero, M.; Girot, F. Effects of Mn Addition, Cooling Rate and Holding Temperature on the Modification and Purification of Iron-Rich Compounds in AlSi10MnMg(Fe) Alloy. Heliyon 2023, 9, e13005. [Google Scholar] [CrossRef]
- Song, D.; Zhao, Y.; Jia, Y.; Li, R.; Zhou, N.; Zheng, K.; Fu, Y.; Zhang, W. Study of the Evolution Mechanisms of Fe-Rich Phases in Al-Si-Fe Alloys with Mn Modification Using Synchrotron X-Ray Imaging. J. Alloys. Compd. 2022, 915, 165378. [Google Scholar] [CrossRef]
- Liu, B.; Ma, C.; Li, L.; Yang, C.; Yu, N. Morphologies and Compositions of α–Al15Fe3Si2-Type Intermetallics in Al–Si–Fe–Mn–Cr Alloys. Inter. J. Met. 2022, 17, 1156–1164. [Google Scholar] [CrossRef]
- Fabrizi, A.; Timelli, G. The Influence of Cooling Rate and Fe/Cr Content on the Evolution of Fe-Rich Compounds in a Secondary Al-Si-Cu Diecasting Alloy. IOP Conf. Ser. Mater. Sci. Eng. 2016, 117, 012017. [Google Scholar] [CrossRef]
- Gorny, A.; Manickaraj, J.; Cai, Z.; Shankar, S. Evolution of Fe Based Intermetallic Phases in Al-Si Hypoeutectic Casting Alloys: Influence of the Si and Fe Concentrations, and Solidification Rate. J. Alloys Compd. 2013, 577, 103–124. [Google Scholar] [CrossRef]
- Ourfali, M.F.; Todd, I.; Jones, H. Effect of Solidification Cooling Rate on the Morphology and Number per Unit Volume of Primary Mg2Si Particles in a Hypereutectic Al-Mg-Si Alloy. Metall. Mater. Trans. A 2005, 36, 1368–1372. [Google Scholar] [CrossRef]
- Shabestari, S.G.; Gruzleski, J.E. The Effect of Solidification Condition and Chemistry on the Formation and Morphology of Complex Intermetallic Compounds in Aluminium—Silicon Alloys. Cast Metals. 1994, 6, 217–224. [Google Scholar] [CrossRef]
- Bolibruchova, D.; Podprocká, R. The Effect of Different Mn/Fe Ratio on Microstructure Alloy Based on Al-Si-Mg. Arch. Foundry Eng. 2019, 19, 15–20. [Google Scholar]
- Belmares-Perales, S.; Castro-Román, M.; Herrera-Trejo, M.; Ramirez-Vidaurri, L.E. Effect of Cooling Rate and Fe/Mn Weight Ratio on Volume Fractions of α-AlFeSi and β-AlFeSi Phases in Al-7.3Si-3.5Cu Alloy. Met. Mat. Int. 2008, 14, 307–314. [Google Scholar] [CrossRef]
- Que, Z.; Wang, Y.; Fan, Z. Formation of the Fe-Containing Intermetallic Compounds during Solidification of Al-5Mg-2Si-0.7Mn-1.1Fe Alloy. Metall. Mater. Trans. A 2018, 49, 2173–2181. [Google Scholar] [CrossRef]
- Que, Z.; Fang, C.; Mendis, C.L.; Wang, Y.; Fan, Z. Effects of Si Solution in θ-Al13Fe4 on Phase Transformation between Fe-Containing Intermetallic Compounds in Al Alloys. J. Alloys Compd. 2023, 932, 167587. [Google Scholar] [CrossRef]
- Jin, L.; Liu, K.; Chen, X.G. Evolution of Fe-Rich Intermetallics in Al-Si-Cu 319 Cast Alloy with Various Fe, Mo, and Mn Contents. Metall. Mater. Trans. B 2019, 50, 1896–1907. [Google Scholar] [CrossRef]
- Eidhed, W. Modification of β-Al5FeSi Compound in Recycled Al-Si-Fe Cast Alloy by Using Sr, Mg and Cr Additions. J. Mat. Sci. Technol. 2008, 24, 45–47. [Google Scholar]
- Santos, J.; Jarfors, A.E.W.; Dahle, A.K. Formation of Iron-Rich Intermetallic Phases in Al-7Si-Mg: Influence of Cooling Rate and Strontium Modification. Metall. Mater. Trans. A 2019, 50, 4148–4165. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, Y.; Huang, G.; Zhang, Z. Effect of B Addition on the Formation of Fe-Rich Phases in Al-Si-Fe Alloys. J. Alloys Compd. 2023, 930, 167426. [Google Scholar] [CrossRef]
- Nafisi, S.; Emadi, D.; Shehata, M.T.; Ghomashchi, R. Effects of Electromagnetic Stirring and Superheat on the Microstructural Characteristics of Al-Si-Fe Alloy. Mat. Sci. Eng. A 2006, 432, 71–83. [Google Scholar] [CrossRef]
- Fan, Z.; Fang, X.; Ji, S. Microstructure and Mechanical Properties of Rheo-Diecast (RDC) Aluminium Alloys. Mat. Sci. Eng. A 2005, 412, 298–306. [Google Scholar] [CrossRef]
- Feng, S.; Liotti, E.; Lui, A.; Wilson, M.D.; Grant, P.S. Nucleation Bursts of Primary Intermetallic Crystals in a Liquid Al Alloy Studied Using in Situ Synchrotron X-Ray Radiography. Acta Mater. 2021, 221, 117389. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Xue, C.; Miao, Y.; Hou, Q.; Meng, Y.; Yang, X.; Li, X. Quantifying the effects of cooling rates on Fe-rich intermetallics in recycled Al-Si-Cu alloys by machine learning. J. Alloys Compd. 2025, 1014, 178718. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, S.; Grant, P.S.; O’Reilly, K.A.Q. Influence of Cooling Rate on the Fe Intermetallic Formation in an AA6063 Al Alloy. J. Alloys Compd. 2013, 555, 274–282. [Google Scholar] [CrossRef]
- Szczygiel, P.; Roven, H.J.; Reiso, O. On the Effect of SPD on Recycled Experimental Aluminium Alloys: Nanostructures, Particle Break-up and Properties. Mat. Sci. Eng. A 2005, 410–411, 261–264. [Google Scholar] [CrossRef]
- Shuai, G.; Zhang, M.; Li, Z.; Valiev, R.Z.; Medvedev, A.E.; Zhang, D.; Elhefnawey, M.; Chen, F.; Li, L. Microstructural Evolution and Superior Properties of Conductive Al–Fe Alloy Processed by ECAP. Int. J. Lightweight Mater. Manuf. 2023, 6, 552–562. [Google Scholar] [CrossRef]
- Farshidi, M.H.; Rifai, M.; Miyamoto, H. Microstructure Evolution of a Recycled Al–Fe–Si–Cu Alloy Processed by Tube Channel Pressing. Int. J. Miner. Metall. Mater. 2018, 25, 1166–1172. [Google Scholar] [CrossRef]
- Okeke, U.; Yilmazer, H.; Sato, S.; Boehlert, C.J. Strength Enhancement of an Aluminum Alloy through High Pressure Torsion. Mat. Sci. Eng. A 2019, 760, 195–205. [Google Scholar] [CrossRef]
- Tang, Y.; Tomita, Y.; Horita, Z. Mechanical Properties and Microstructures of Highly Fe-Containing AlMgSi Alloys Processed by Severe Plastic Deformation under High Pressure. Mater. Trans. 2023, 64, 448–457. [Google Scholar] [CrossRef]
- Duchaussoy, A.; Sauvage, X.; Edalati, K.; Horita, Z.; Renou, G.; Deschamps, A.; De Geuser, F. Structure and Mechanical Behavior of Ultrafine-Grained Aluminum-Iron Alloy Stabilized by Nanoscaled Intermetallic Particles. Acta Mater. 2019, 167, 89–102. [Google Scholar] [CrossRef]
- Shabestari, S.G.; Ghanbari, M. Effect of Plastic Deformation and Semisolid Forming on Iron-Manganese Rich Intermetallics in Al-8Si-3Cu-4Fe-2Mn Alloy. J. Alloys Compd. 2010, 508, 315–319. [Google Scholar] [CrossRef]
- Shabestari, S.G.; Parshizfard, E. Effect of Semi-Solid Forming on the Microstructure and Mechanical Properties of the Iron Containing Al-Si Alloys. J. Alloys Compd. 2011, 509, 7973–7978. [Google Scholar] [CrossRef]
- Eskin, G.I.; Eskin, D.G. Ultrasonic Treatment of Light Alloy Melts, 2nd ed.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2015; ISBN 978-1-4665-7799-2. [Google Scholar]
- Eskin, D.G.; Tzanakis, I. High-Frequency Vibration and Ultrasonic Processing. In Solidification Processing of Metallic Alloys Under External Fields; Eskin, D.G., Mi, J., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 153–194. ISBN 978-3-319-94841-6. [Google Scholar]
- Priyadarshi, A.; Khavari, M.; Subroto, T.; Conte, M.; Prentice, P.; Pericleous, K.; Eskin, D.; Durodola, J.; Tzanakis, I. On the Governing Fragmentation Mechanism of Primary Intermetallics by Induced Cavitation. Ultrason. Sonochem. 2021, 70, 105260. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tzanakis, I.; Eskin, D.; Mi, J.; Connolley, T. In Situ Observation of Ultrasonic Cavitation-Induced Fragmentation of the Primary Crystals Formed in Al Alloys. Ultrason. Sonochem. 2017, 39, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jie, J.; Gao, Y.; Lu, Y.; Li, T. Effects of Ultrasonic Treatment on the Formation of Iron-Containing Intermetallic Compounds in Al-12%Si-2%Fe Alloys. Intermetallics 2013, 42, 120–125. [Google Scholar] [CrossRef]
- Kotadia, H.R.; Qian, M.; Das, A. Microstructural Modification of Recycled Aluminium Alloys by High-Intensity Ultrasonication: Observations from Custom Al–2Si–2Mg–1.2Fe–(0.5,1.0)Mn Alloys. J. Alloys Compd. 2020, 823, 153833. [Google Scholar] [CrossRef]
- Kim, S.B.; Jung, J.G.; Cho, Y.H.; Kim, S.H.; Euh, K.; Lee, J.M. Effect of Ultrasonic Melt Treatment on Solidification Microstructure of Al–5Ti–1B Alloy Containing Numerous Inoculant Particles. Met. Mat. Int. 2021, 28, 1549–1560. [Google Scholar] [CrossRef]
- Kim, J.K.; Rohatgi, P.K. Nucleation on Ceramic Particles in Cast Metal-Matrix Composites. Metall. Mater. Trans. A 2000, 31, 1295–1304. [Google Scholar] [CrossRef]
- Kaptay, G. Interfacial Aspects to Produce Particulate Reinforced Metal Matrix Composites. In Affordable Metal-Matrix Composites for High Performance Applications; Pabdey, A.B., Kendig, K.L., Watson, T.J., Eds.; TMS: Pittsburgh, PA, USA, 2001; pp. 72–99. [Google Scholar]
- Stawarz, M.; Dojka, M. Bifilm Inclusions in High Alloyed Cast Iron. Materials 2021, 14, 3067. [Google Scholar] [CrossRef]
- Xiao, S.; Xiao, Z.; Gao, X.; Zhao, D.; Dai, Z. Synergistic Effects of TiCp on Microstructure Refinement and Mechanical Enhancement in Al-Si-Cu-Ni-Mg Alloys. J. Alloys Compd. 2024, 1006, 176226. [Google Scholar] [CrossRef]
- Stefanescu, D.M.; Alonso, G.; Suarez, R. Recent Developments in Understanding Nucleation and Crystallization of Spheroidal Graphite in Iron-Carbon-Silicon Alloys. Metals 2020, 10, 221. [Google Scholar] [CrossRef]
- Götz, A.; Michels, L.; Akola, J. Density Functional Investigation of the Heterogeneous Nucleation of Graphite on Divalent Metal Oxides and Sulfides. Acta Mater. 2024, 282, 120427. [Google Scholar] [CrossRef]
- Alonso, G.; Stefanescu, D.M.; Bravo, B.; Suárez, R. Graphite Spheroids: The Place Where They Are Born. Inter. J. Met. 2024, 18, 1854–1868. [Google Scholar] [CrossRef]
- Campbell, J. The Pre-Existing Microcrack Population in Metals a Personal Overview. Procedia Struct. Integr. 2023, 43, 234–239. [Google Scholar] [CrossRef]
- Jia, Y.; Song, D.; Zhou, N.; Zheng, K.; Fu, Y.; Shu, D. The Growth Restriction Effect of TiCN Nanoparticles on Al-Cu-Zr Alloys via Ultrasonic Treatment. Ultrason. Sonochem. 2021, 80, 105829. [Google Scholar] [CrossRef]
- Campbell, J. Modification of Al-Si Alloys. Trans. Am. Foundry Soc. 2011, 119, 171–176. [Google Scholar]
- Khalifa, W.; Samuel, F.H.; Gruzleski, J.E.; Doty, H.W.; Valtierra, S. Nucleation of Fe-Intermetallic Phases in the Al-Si-Fe Alloys. Metall. Mater. Trans. A 2005, 36, 1017–1032. [Google Scholar] [CrossRef]
- Lui, A.; Grant, P.S.; Stone, I.C.; O’Reilly, K.A.Q. The Role of Grain Refiner in the Nucleation of AlFeSi Intermetallic Phases During Solidification of a 6xxx Aluminum Alloy. Metall. Mater. Trans. A 2019, 50, 5242–5252. [Google Scholar] [CrossRef]
- Yang, W.; Ji, S.; Zhou, X.; Stone, I.; Scamans, G.; Thompson, G.E.; Fan, Z. Heterogeneous Nucleation of α-Al Grain on Primary α-AlFeMnSi Intermetallic Investigated Using 3D Sem Ultramicrotomy and HRTEM. Metall. Mater. Trans. A 2014, 45, 3971–3980. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, D.; Wang, H.; Li, X.; Chen, L.; Sun, Z.; Wang, Z.; Zhai, T.; Fu, Y.; Wang, Y.; et al. Revealing the Nucleation and Growth Mechanisms of Fe-Rich Phases in Al—Cu—Fe (-Si) Alloys under the Influence of Al—Ti—B. Intermetallics 2022, 146, 107584. [Google Scholar] [CrossRef]
- Wang, W.; Xu, R.; Xiang, L.; Han, Y.; Li, E.; Zhang, S.; Zheng, H. Effect of B4C Nanoparticles Addition on the Refinement of Fe-Rich Phase in ZL108 Alloy. Mater. Des. 2024, 247, 113424. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Timelli, G.; Bonollo, F. Influence of Melt Superheat, Sr Modifier, and Al-5Ti-1B Grain Refiner on Microstructural Evolution of Secondary Al-Si-Cu Alloys. Metall. Mater. Trans. A 2016, 47, 5510–5521. [Google Scholar] [CrossRef]
- Yu, J.M.; Hashimoto, T.; Li, H.T.; Wanderka, N.; Zhang, Z.; Cai, C.; Zhong, X.L.; Qin, J.; Dong, Q.P.; Nagaumi, H.; et al. Formation of Intermetallic Phases in Unrefined and Refined AA6082 Al Alloys Investigated by Using SEM-Based Ultramicrotomy Tomography. J. Mater. Sci. Technol. 2022, 120, 118–128. [Google Scholar] [CrossRef]
- Zhou, Y. Solidification Behaviour of Fe-Rich Intermetallic Compounds in Aluminium Alloys. Ph.D. Thesis, Brunel University, Uxbridge, UK, 2018. [Google Scholar]
- Que, Z.; Wang, Y.; Fan, Z.; Hashimoto, T.; Zhou, X.R. Composition Templating for Heterogeneous Nucleation of Intermetallic Compounds. Sci. Rep. 2024, 14, 8968. [Google Scholar] [CrossRef]
- Kaptay, G. Beyond the Parallel Tangent Method to Predict the Composition of the First Nucleating Phase from Oversaturated Solutions. J. Phase Equilibria Diffus. 2023, 44, 445–455. [Google Scholar] [CrossRef]
- Feng, S.; Liotti, E.; Lui, A.; Wilson, M.D.; Connolley, T.; Mathiesen, R.H.; Grant, P.S. In-Situ X-Ray Radiography of Primary Fe-Rich Intermetallic Compound Formation. Acta Mater. 2020, 196, 759–769. [Google Scholar] [CrossRef]
- Que, Z.; Wang, Y.; Fan, Z.; Hashimoto, T.; Zhou, X. Enhanced Heterogeneous Nucleation of Al6(Fe,Mn) Compound in Al Alloys by Interfacial Segregation of Mn on TiB2 Particles Surface. Mater. Lett. 2022, 323, 132570. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Song, D.; Lin, B.; Shen, F.; Zheng, D.; Xie, C.X.; Sun, Z.; Fu, Y.; Li, R. Nucleation and Growth of Fe-Rich Phases in Al-5Ti-1B Modified Al-Fe Alloys Investigated Using Synchrotron X-Ray Imaging and Electron Microscopy. J. Mater. Sci. Technol. 2021, 80, 84–99. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Timelli, G.; Bonollo, F.; Arnberg, L. Influence of Grain Refiner Addition on the Precipitation of Fe-Rich Phases in Secondary AlSi7Cu3Mg Alloys. Inter. J. Met. 2017, 11, 294–304. [Google Scholar] [CrossRef]
- Wang, F.; Fan, Z. Characterization of AlN Inclusion Particles Formed in Commercial Purity Aluminum. Metall. Mater. Trans. A 2019, 50, 2519–2526. [Google Scholar] [CrossRef]
- Hudson, S.W.; Apelian, D. Inclusion Detection in Molten Aluminum: Current Art and New Avenues for in Situ Analysis. Inter. J. Met. 2016, 10, 315–321. [Google Scholar] [CrossRef]
- Que, Z.; Mendis, C.L. Effects of Native AlN Particles on Heterogeneous Nucleation in an Al-3Fe Alloy. Metall Mater Trans A 2021, 52, 553–559. [Google Scholar] [CrossRef]
- Niu, Z.; Que, Z.; Patel, J.B.; Fan, Z. Assessment and Improvement of Melt Quality of Recycled Secondary A357 Alloy by Application of the High Shear Melt Conditioning (HSMC) Technology. Crystals 2024, 14, 1044. [Google Scholar] [CrossRef]
- Campbell, J. The Consolidation of Metals: The Origin of Bifilms. J. Mater. Sci. 2016, 51, 96–106. [Google Scholar] [CrossRef]
- Campbell, J. An Overview of the Effects of Bifilms on the Structure and Properties of Cast Alloys. Metall. Mater. Trans. B 2006, 37, 857–863. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, S.; Gao, F.; Fan, Z. Nature of Oxides in Al–Mg Alloys. Trans. Indian Inst. Met. 2024, 77, 2929–2933. [Google Scholar] [CrossRef]
- Song, H.; Zhang, L.; Cao, F.; Gu, X.; Sun, J. Oxide Bifilm Defects in Aluminum Alloy Castings. Mater. Lett. 2021, 285, 129089. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.T.; Fan, Z. Oxidation of Aluminium Alloy Melts and Inoculation by Oxide Particles. Trans. Indian Inst. Met. 2012, 65, 653–661. [Google Scholar] [CrossRef]
- Campbell, J. The Mechanisms of Metallurgical Failure: On the Origin of Fracture; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-822411-3. [Google Scholar]
- Campbell, J. Defects in Aluminum Alloy Castings. In Encyclopedia of Aluminum and Its Alloys; Taylor & Francis: Abingdon, UK, 2019; pp. 587–592. ISBN 9781351045636. [Google Scholar]
- Campbell, J. Entrainment Defects. In Encyclopedia of Aluminum and Its Alloys; Taylor & Francis: Abingdon, UK, 2018; pp. 873–892. [Google Scholar]
- Tiryakioglu, M.; Campbell, J. Metal Casting Research: Application to Aluminum Alloy Casting. In Encyclopedia of Aluminum and Its Alloys; Taylor & Francis: Abingdon, UK, 2019; pp. 1456–1460. ISBN 9781351045636. [Google Scholar]
- Campbell, J. A Personal View of Microstructure and Properties of Al Alloys. Materials 2021, 14, 1297. [Google Scholar] [CrossRef]
- Çolak, M.; Kayikci, R.; Dispinar, D. Melt Cleanliness Comparison of Chlorine Fluxing and Ar Degassing of Secondary Al-4Cu. Metall. Mater. Trans. B 2016, 47, 2705–2709. [Google Scholar] [CrossRef]
- Gyarmati, G.; Fegyverneki, G.; Tokár, M.; Mende, T. The Effects of Rotary Degassing Treatments on the Melt Quality of an Al–Si Casting Alloy. Int. J. Met. 2020, 14, 372–380. [Google Scholar] [CrossRef]
- Yüksel, C.; Dispinar, D.; Cigdem, M. An Analytical Approach for the Correlation between Bifilm Index and Tensile Properties of AlSi7Mg0.3 (A356) Aluminum Alloy Cleaned via Rotary Degassing and Different Fluxes. Int. J. Met. 2022, 16, 1615–1627. [Google Scholar] [CrossRef]
- Máté, M.; Tokár, M.; Fegyverneki, G.; Gyarmati, G. The Comparative Analysis of the Inclusion Removal Efficiency of Different Fluxes. Arch. Foundry Eng. 2020, 20, 53–58. [Google Scholar] [CrossRef]
- Li, C.; Li, J.G.; Mao, Y.Z.; Ji, J.C. Mechanism to Remove Oxide Inclusions from Molten Aluminum by Solid Fluxes Refining Method. China Foundry 2017, 14, 233–243. [Google Scholar] [CrossRef]
- Li, H.T.; Xia, M.; Jarry, P.; Scamans, G.M.; Fan, Z. Grain Refinement in a AlZnMgCuTi Alloy by Intensive Melt Shearing: A Multi-Step Nucleation Mechanism. J. Cryst. Growth 2011, 314, 285–292. [Google Scholar] [CrossRef]
- Wang, F.; Eskin, D.; Mi, J.; Connolley, T.; Lindsay, J.; Mounib, M. A Refining Mechanism of Primary Al3Ti Intermetallic Particles by Ultrasonic Treatment in the Liquid State. Acta Mater. 2016, 116, 354–363. [Google Scholar] [CrossRef]
- Hinton, E.M. The Oxidation of Liquid Aluminium and the Potential for Oxides in Grain Refinement of Aluminium Alloys. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2014. [Google Scholar]
- Cao, X.; Campbell, J. Oxide Inclusion Defects in Al-Si-Mg Cast Alloys. Can. Metall. Q. 2005, 44, 435–448. [Google Scholar] [CrossRef]
- Campbell, J. Complete Casting Handbook: Metal Casting Processes, Metallurgy, Techniques and Design, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9780444635099. [Google Scholar]
- Wu, G.; Dash, K.; Galano, M.L.; O’Reilly, K.A.Q. Oxidation Studies of Al Alloys: Part II Al-Mg Alloy. Corros. Sci. 2019, 155, 97–108. [Google Scholar] [CrossRef]
- Wu, G.; Dash, K.; Galano, M.L.; O’Reilly, K.A.Q. Oxidation Studies of Al Alloys: Part I Al-Cu (Liquid Phase) Alloy. Corros. Sci. 2019, 157, 41–50. [Google Scholar] [CrossRef]
- Bonner, S.J. A Microstructural and Kinetic Study of Molten Aluminium Oxidation in Relation to Dross Formation. Ph.D. Thesis, University of Queensland, St. Lucia, Australia, 2015. [Google Scholar]
- Miresmaeili, S.M. Oxidation of Liquid Al-7Si Alloys Containing Strontium and Magnesium. In Proceedings of the 13th International Scientific Conference on Achievements in Mechanical and Materials Engineering, Gliwice-Wisla, Poland, 16–19 May 2005. [Google Scholar]
- Impey, S.A.; Stephenson, D.J.; Nicholls, J.R. Mechanism of Scale Growth on Liquid Aluminium. Mater. Sci. Technol. 1988, 4, 1126–1132. [Google Scholar] [CrossRef]
- Impey, S.; Stephenson, D.; Nicholls, J.R. A Study of the Effect of Magnesium Additions on the Oxide Growth Morphologies on Liquid Aluminium Alloys. In Microscopy of Oxidation; CRC Press: Boca Raton, FL, USA, 1991; pp. 238–244. [Google Scholar]
- Kim, K. Formation of Fine Clusters in High-Temperature Oxidation of Molten Aluminum. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 3650–3660. [Google Scholar] [CrossRef]
- Maity, P.C.; Chakraborty, P.N.; Panigrahi, S.C. Preparation of Al-Al2O3 in-Situ Particle Composites by Addition of Fe2O3 Particles to Pure Al Melt. J. Mater. Sci. Lett. 1997, 16, 1224–1226. [Google Scholar] [CrossRef]
- Rong, X.; Zhao, D.; He, C.; Zhao, N. Review: Recent Progress in Aluminum Matrix Composites Reinforced by in Situ Oxide Ceramics. J. Mater. Sci. 2024, 59, 9657–9684. [Google Scholar] [CrossRef]
- Ritchie, I.M.; Sanders, J.V.; Weickhardt, P.L. Oxidation of a Dilute Aluminum Magnesium Alloy. Oxid. Met. 1971, 3, 91–101. [Google Scholar] [CrossRef]
- Haginoya, I.; Fukusako, T. Oxidation of Molten Al-Mg Alloys. Trans. Jpn. Inst. Met. 1983, 24, 613–619. [Google Scholar]
- Bloch, J.; Bottomley, D.J.; Mihaychuk, J.G.; van Driel, H.M.; Timsit, R.S. Magnesium Surface Segregation and Its Effect on the Oxidation Rate of the (111) Surface of Al-1.45at%Mg. Surf. Sci. 1995, 322, 168–176. [Google Scholar] [CrossRef]
- Impey, S.; Stephenson, D.J.; Nicholls, J.R. The Influence of Surface Preparation and Pretreatments on the Oxidation of Liquid Aluminum and Al-Mg Alloys. In Microscopy of Oxidation; CRC Press: Boca Raton, FL, USA, 1993; pp. 323–337. [Google Scholar]
- Kim, K.H. Formation of Endogenous MgO and MgAl2O4 Particles and Their Possibility of Acting as Substrate for Heterogeneous Nucleation of Aluminum Grains. Surf. Interface Anal. 2015, 47, 429–438. [Google Scholar] [CrossRef]
- Kim, K.H. Detection of a Layer of Al2O3 at the Interface of Al/MgAl2O4 by High-Resolution Observation Using Dual-Beam FIB and TEM. Metallogr. Microstruct. Anal. 2014, 3, 233–237. [Google Scholar] [CrossRef]
- Kim, K.; Green, N.; Griffiths, W. Focused Ion Beam Milling and Imaging: An Advanced Method to Detect Fine Inclusions in Cast Aluminium Alloys. Mater. Sci. Forum 2013, 765, 150–154. [Google Scholar] [CrossRef]
- More, K.L.; Tortorelli, P.F.; Walker, L.R.; Hryn, J.; Krumdick, G. Microstructural Evaluation of Dross Formation on Mg- and Non-Mg-Containing Al Alloys from Industrial Furnaces. Mater. High Temp. 2003, 20, 453–460. [Google Scholar] [CrossRef]
- Bagh, N.T.; Divandari, M.; Shahmiri, M.; Akbarifar, M. Characteristics of Dynamically Formed Oxide Films in Al-Zn Melt. Int. J. Met. 2020, 15, 747–762. [Google Scholar] [CrossRef]
- Gyarmati, G.; Vincze, F.; Fegyverneki, G.; Kéri, Z.; Mende, T.; Molnár, D. The Effect of Rotary Degassing Treatments with Different Purging Gases on the Double Oxide- and Nitride Film Content of Liquid Aluminum Alloys. Metall. Mater. Trans. B 2022, 53, 1244–1257. [Google Scholar] [CrossRef]
- Campbell, J. Stop Pouring, Start Casting. Int. J. Met. 2012, 6, 7–18. [Google Scholar] [CrossRef]
- Tiryakioğlu, T. The Effect of Hydrogen on Pore Formation in Aluminum Alloy Castings: Myth Versus Reality. Metals 2020, 10, 368. [Google Scholar] [CrossRef]
- Yousefian, P.; Tiryakioğlu, M. Pore Formation During Solidification of Aluminum: Reconciliation of Experimental Observations, Modeling Assumptions, and Classical Nucleation Theory. Metall. Mater. Trans. A 2018, 49, 563–575. [Google Scholar] [CrossRef]
- Tiryakioğlu, M.; Yousefian, P. Hot Tear Nucleation During Solidification of Aluminum and Its Alloys. In Encyclopedia of Aluminum and Its Alloys; Totten, M.G.E., Tiryakioğlu, O.K., Eds.; Taylor & Francis: Abingdon, UK, 2019; pp. 1263–1269. ISBN 9781351045636. [Google Scholar]
- Uludağ, M.; Çetin, R.; Dispinar, D. Freezing Range, Melt Quality, and Hot Tearing in Al-Si Alloys. Metall. Mater. Trans. A 2018, 49, 1948–1961. [Google Scholar] [CrossRef]
- Patel, J.B.; Yang, X.; Mendis, C.L.; Fan, Z. Melt Conditioning of Light Metals by Application of High Shear for Improved Microstructure and Defect Control. JOM 2017, 69, 1071–1076. [Google Scholar] [CrossRef]
- Li, H.T.; Wang, Y.; Fan, Z. Mechanisms of Enhanced Heterogeneous Nucleation during Solidification in Binary Al-Mg Alloys. Acta Mater. 2012, 60, 1528–1537. [Google Scholar] [CrossRef]
- Gyarmati, G.; Koncz-Horváth, D.; Karacs, G.; Mende, T. The Interactions of Primary (Al,Si)3Ti Intermetallic Particles and Oxide Phases in a Liquid Al-Si-Mg-Ti Alloy. In Proceedings of the The 8th International Conference on Solidification and Gravity, Miskolc-Lillafüred, Hungary, 2–5 September 2024. [Google Scholar]
- Pilling, N.B.; Bedworth, R.E. The Oxidation of Metals at High Temperatures. J. Inst. Met. 1923, 29, 529–591. [Google Scholar]
- Xu, C.; Gao, W. Pilling-Bedworth Ratio for Oxidation of Alloys. Mater. Res. Innov. 2000, 3, 231–235. [Google Scholar] [CrossRef]
- Stefanescu, D.M. Nucleation and Growth Kinetics—Nanoscale. In Science and Engineering of Casting Solidification; Springer International Publishing: Cham, Switzerland, 2015; pp. 29–59. ISBN 9783319156934. [Google Scholar]
- Fredriksson, H.; Akerlind, U. Nucleation. In Solidification and Crystallization Processing in Metals and Alloys; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 166–200. [Google Scholar]
- Kurz, W.; Fisher, D.J.; Rappaz, M. Fundamentals of Solidification, 5th ed.; Trans Tech Publications Ltd.: Seestrasse, Switzerland, 2023. [Google Scholar]
- Kaptay, G. The Chemical (Not Mechanical) Paradigm of Thermodynamics of Colloid and Interface Science. Adv. Colloid. Interface Sci. 2018, 256, 163–192. [Google Scholar] [CrossRef]
- Cantor, B. Heterogeneous Nucleation and Adsorption. Philos. Trans. Math. Phys. Eng. Sci. 2003, 361, 409–417. [Google Scholar] [CrossRef]
- Quested, T.E.; Greer, A.L. Athermal Heterogeneous Nucleation of Solidification. Acta Mater. 2005, 53, 2683–2692. [Google Scholar] [CrossRef]
- Fan, Z.; Men, H. An Overview on Atomistic Mechanisms of Heterogeneous Nucleation. Metals 2022, 12, 1547. [Google Scholar] [CrossRef]
- Fan, Z.; Men, H.; Wang, Y.; Que, Z. A New Atomistic Mechanism for Heterogeneous Nucleation in the Systems with Negative Lattice Misfit: Creating a 2D Template for Crystal Growth. Metals 2021, 11, 478. [Google Scholar] [CrossRef]
- Men, H.; Fan, Z. Prenucleation Induced by Crystalline Substrates. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2018, 49, 2766–2777. [Google Scholar] [CrossRef]
- Fan, Z.; Men, H. Heterogeneous Nucleation and Grain Initiation on a Single Substrate. Metals 2022, 12, 1454. [Google Scholar] [CrossRef]
- Fan, Z.; Gao, F. Grain Initiation and Grain Refinement: An Overview. Metals 2022, 12, 1728. [Google Scholar] [CrossRef]
- Turnbull, D.; Vonnegut, B. Vonnegut Nucleation Catalysis. Ind. Eng. Chem. 1952, 44, 1292–1298. [Google Scholar] [CrossRef]
- Bramfitt, B.L. The Effect of Carbide and Nitride Additions on the Heterogeneous Nucleation Behavior of Liquid Iron. Metall. Trans. 1970, 1, 1987–1995. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M.; Easton, M.A.; Taylor, J.A. Crystallographic Study of Grain Refinement in Aluminum Alloys Using the Edge-to-Edge Matching Model. Acta Mater. 2005, 53, 1427–1438. [Google Scholar] [CrossRef]
- Men, H.; Fang, C.; Fan, Z. Prenucleation at the Liquid/Substrate Interface: An Overview. Metals 2022, 12, 1704. [Google Scholar] [CrossRef]
- Kaptay, G. Method for Estimating Solid-Solid Interface Energies in Metal-Ceramic Systems: The Aluminum-Silicon Carbide System. Mater. Sci. Forum 1996, 216, 475–484. [Google Scholar] [CrossRef]
- Voigt, C.; Ditscherlein, L.; Werzner, E.; Zienert, T.; Nowak, R.; Peuker, U.; Sobczak, N.; Aneziris, C.G. Wettability of AlSi7Mg Alloy on Alumina, Spinel, Mullite and Rutile and Its Influence on the Aluminum Melt Filtration Efficiency. Mater. Des. 2018, 150, 75–85. [Google Scholar] [CrossRef]
- Fankhänel, B.; Stelter, M.; Voigt, C.; Aneziris, C.G. Interaction of AlSi7Mg with Oxide Ceramics. Adv. Eng. Mater. 2017, 19, 1700084. [Google Scholar] [CrossRef]
- Kaptay, G. Interfacial Criterion of Spontaneous and Forced Engulfment of Reinforcing Particles by an Advancing Solid/Liquid Interface. Metall. Mater. Trans. A 2001, 32, 993–1005. [Google Scholar] [CrossRef]
- Kaptay, G.; Bader, E. Ion-Dipole Adhesion Energy Model for Wettability of Oxide Ceramics by Non-Reactive Liquid Metals. Trans. Join. Weld. Res. Inst. 2001, 30, 55–60. [Google Scholar]
- Liu, Y.; Huang, Y.; Xiao, Z. Study of Orientation Relationship between Al Matrix and Several Typical Inclusions in Al Alloy by Edge-to-Edge Matching Model. J. Mater. Res. 2017, 32, 2092–2099. [Google Scholar] [CrossRef]
- Fredriksson, H.; Åkerlind, U. Solidification and Crystallization Processing in Metals and Alloys; Wiley: Hoboken, NJ, USA, 2012; ISBN 9781119993056. [Google Scholar]
- Kaptay, G. Interfacial Energy of Strained Coherent Interfaces and a New Design Rule to Select Phase Combinations for In Situ Coherent Nanocomposites. Langmuir 2023, 39, 6316–6323. [Google Scholar] [CrossRef]
- Kaptay, G. On the Interfacial Energy of Coherent Interfaces. Acta Mater. 2012, 60, 6804–6813. [Google Scholar] [CrossRef]
- Kaptay, G. A Coherent Set of Model Equations for Various Surface and Interface Energies in Systems with Liquid and Solid Metals and Alloys. Adv. Colloid. Interface Sci. 2020, 283, 102212. [Google Scholar] [CrossRef]
- Fang, C.M.; Fan, Z. Atomic Ordering at the Liquid-Al/MgAl2O4 Interfaces from Ab Initio Molecular Dynamics Simulations. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2020, 51, 6318–6326. [Google Scholar] [CrossRef]
- Fang, C.; Yasmin, S.; Fan, Z. Interfacial Interaction and Prenucleation at Liquid-Al/γ-Al2O3{1 1 1} Interfaces. J. Phys. Commun. 2021, 5, 015007. [Google Scholar] [CrossRef]
- Fang, C.; Fan, Z. Prenucleation at the Liquid-Al/α-Al2O3 and the Liquid-Al/MgO Interfaces. Comput. Mater. Sci. 2020, 171, 109258. [Google Scholar] [CrossRef]
- Fang, C.; Fan, Z. Ab Initio Molecular Dynamics Investigation of Prenucleation at Liquid—Metal/Oxide Interfaces: An Overview. Metals 2022, 12, 1618. [Google Scholar] [CrossRef]
- Askeland, D.R.; Phulé, P.P.; Wright, W.J.; Bhattacharya, D.K. The Science and Engineering of Materials, 7th ed.; Cengage Learning: Boston, MA, USA, 2016. [Google Scholar]
- Russell, A.M. Ductility in Intermetallic Compounds. Adv. Eng. Mater. 2003, 5, 629–639. [Google Scholar] [CrossRef]
- Cui, Y.; King, D.J.M.; Horsfield, A.P.; Gourlay, C.M. Solidification orientation relationships between Al3Ti and TiB2. Acta Mater. 2020, 186, 149–161. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Que, Z.; Fang, C.; Hashimoto, T.; Zhou, X.; Ramasse, Q.M.; Fan, Z. Manipulating Nucleation Potency of Substrates by Interfacial Segregation: An Overview. Metals 2022, 12, 1636. [Google Scholar] [CrossRef]
- Greer, A.L.; Bunn, A.M.; Tronche, A.; Evans, P.V.; Bristow, D.J. Modelling of Inoculation of Metallic Melts: Application to Grain Refinement of Aluminium by Al-Ti-B. Acta Mater. 2000, 48, 2823–2835. [Google Scholar] [CrossRef]
- Campbell, J. The Origin of Griffith Cracks. Metall. Mater. Trans. B 2011, 42, 1091–1097. [Google Scholar] [CrossRef]
- Sahin, U.; Campbell, J.; Brown, D.N.; Griffiths, W.D. Precipitation of Sr-Rich and Fe-Rich Intermetallic Compounds in Al Alloys. Mater. Sci. Technol. 2024, 0, 02670836241302686. [Google Scholar] [CrossRef]
- Gyarmati, G.; Fegyverneki, G.; Kéri, Z.; Molnár, D.; Tokár, M.; Varga, L.; Mende, T. Controlled Precipitation of Intermetallic (Al,Si)3Ti Compound Particles on Double Oxide Films in Liquid Aluminum Alloys. Mater. Charact. 2021, 181, 111467. [Google Scholar] [CrossRef]
- Gyarmati, G.; Bubonyi, T.; Fegyverneki, G.; Tokár, M.; Mende, T. Interactions of Primary Intermetallic Compound Particles and Double Oxide Films in Liquid Aluminum Alloys. Intermetallics 2022, 149, 107681. [Google Scholar] [CrossRef]
- Gyarmati, G. Kettős Oxidhártyák és Ti Tartalmú Vegyületfázisok Kölcsönhatásainak Vizsgálata AlSi7MgCu Ötvözet Esetén. Ph.D. Thesis, University of Miskolc, Miskolc, Hungary, 2024. [Google Scholar]
- Cao, X.; Campbell, J. The Nucleation of Fe-Rich Phases on Oxide Films in Al-11.5Si-0.4Mg Cast Alloys. Metall. Mater. Trans. A 2003, 34, 1409–1420. [Google Scholar] [CrossRef]
- Cao, X.; Campbell, J. Precipitation of Primary Intermetallic Compounds in Liquid Al 11.5Si 0.4Mg Alloy. Int. J. Cast Met. Res. 2000, 13, 175–184. [Google Scholar] [CrossRef]
- Cao, X.; Campbell, J. Effect of Melt Superheating on Convection-Free Precipitation and Sedimentation of Primary α-Fe Phase in Liquid Al-11.5Si-0.4Mg Alloy. Int. J. Cast Met. Res. 2003, 15, 595–608. [Google Scholar] [CrossRef]
- Cao, X.; Campbell, J. Effect of Precipitation and Sedimentation of Primary α-Fe Phase on Liquid Metal Quality of Cast Al-11·1Si-0·4Mg Alloy. Int. J. Cast Met. Res. 2004, 17, 1–11. [Google Scholar] [CrossRef]
- Liu, K.; Cao, X.; Chen, X.G. Precipitation of Iron-Rich Intermetallic Phases in Al-4.6Cu-0.5Fe-0.5Mn Cast Alloy. J. Mater. Sci. 2012, 47, 4290–4298. [Google Scholar] [CrossRef]
- Liu, K.; Cao, X.; Chen, X.G. Formation and Phase Selection of Iron-Rich Intermetallics in Al-4.6Cu-0.5Fe Cast Alloys. Metall. Mater. Trans. A 2013, 44, 682–695. [Google Scholar] [CrossRef]
- Narayanan, L.A.; Samuel, F.H.; Gruzleski, J.E. Crystallization Behavior of Iron-Containing Intermetallic Compounds in 319 Aluminum Alloy. Metall. Mater. Trans. A 1994, 25, 1761–1773. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Zhang, Z.F.; Xia, M.; Li, H.T.; Xu, J.; Granasy, L.; Scamans, G.M. Shear Enhanced Heterogeneous Nucleation in Some Mg- and Al-Alloys. Int. J. Cast. Met. Res. 2009, 22, 318–322. [Google Scholar] [CrossRef]
- Wang, F.; Eskin, D.; Connolley, T.; MI, J. Wei Influence of Ultrasonic Treatment on Formation of Primary Al3Zr in Al–0.4Zr Alloy. Trans. Nonferrous Met. Soc. China 2017, 27, 977–985. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Koe, B.; Du, W.; Wang, M.; Wang, W.; Boller, E.; Rack, A.; Sun, Z.; Da Shu, B.S.; et al. Multiscale Characterization of the Nucleation and 3D Structure of Al3Sc Phases Using Electron Microscopy and Synchrotron X-Ray Tomography. Mater. Charact. 2020, 164, 183135. [Google Scholar] [CrossRef]
- Yan, K.; Chen, Z.W.; Zhao, Y.N.; Ren, C.C.; Lu, W.J.; Aldeen, A.W. Morphological Characteristics of Al3Sc Particles and Crystallographic Orientation Relationships of Al3Sc/Al Interface in Cast Al-Sc Alloy. J. Alloys Compd. 2021, 861, 158491. [Google Scholar] [CrossRef]
- Trudonoshyn, O.; Prach, O. Multistep Nucleation and Multi-Modification Effect of Sc in Hypoeutectic Al-Mg-Si Alloys. Heliyon 2019, 5, e01202. [Google Scholar] [CrossRef] [PubMed]
- Trudonoshyn, O.; Prach, O.; Slyudova, A.; Lisovskii, V. Structure Formation and Multistep Nucleation in CASTING Al-Mg-Si Alloys. Int. J. Cast Met. Res. 2020, 33, 184–193. [Google Scholar] [CrossRef]
- Wang, F.; Eskin, D.; Connolley, T.; Wang, C.; Koe, B.; King, A.; Reinhard, C.; Mi, J. In-Situ Synchrotron X-Ray Radiography Observation of Primary Al2Cu Intermetallic Growth on Fragments of Aluminium Oxide Film. Mater. Lett. 2018, 213, 303–305. [Google Scholar] [CrossRef]
- Peng, L.; Zeng, G.; Su, T.C.; Yasuda, H.; Nogita, K.; Gourlay, C.M. Al8Mn5 Particle Settling and Interactions with Oxide Films in Liquid AZ91 Magnesium Alloys. JOM 2019, 71, 2235–2244. [Google Scholar] [CrossRef]
- Jung, J.G.; Cho, Y.H.; Kim, S.D.; Kim, S.B.; Lee, S.H.; Song, K.; Euh, K.; Lee, J.M. Mechanism of Ultrasound-Induced Microstructure Modification in Al–Zr Alloys. Acta Mater. 2020, 199, 73–84. [Google Scholar] [CrossRef]
- Liu, K.; Cao, X.; Chen, X.G. Solidification of Iron-Rich Intermetallic Phases in Al-4.5Cu-0.3Fe Cast Alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2011, 42, 2004–2016. [Google Scholar] [CrossRef]
- Zhou, Z.P.Q.Y.P.; Fan, Y.W.Z. Effect of MgO on Phase Selection in Al—Mg—Si—Fe—Mn Alloys. Trans. Indian Inst. Met. 2015, 68, 1167–1172. [Google Scholar] [CrossRef]
- Que, Z.; Mendis, C.L. Heterogeneous Nucleation and Phase Transformation of Fe-Rich Intermetallic Compounds in Al-Mg-Si Alloys. J. Alloys Compd. 2020, 836, 155515. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Wei, Q.; He, W.; Song, D.; Sun, Z.; Fu, Y.; Xiao, F.; Shu, D. Tailoring Thermal Conductivity and Microstructure of Al–Fe Alloys by Addition of Sc2O3 and Variable Cooling Rates. J. Mater. Res. Technol. 2024, 32, 2547–2562. [Google Scholar] [CrossRef]
- Zhang, L.; Eskin, D.G.; Miroux, A.G.; Katgerman, L. On the Mechanism of the Formation of Primary Intermetallics under Ultrasonic Melt Treatment in an Al-Zr-Ti Alloy. IOP Conf. Ser. Mater. Sci. Eng. 2011, 27, 012002. [Google Scholar] [CrossRef]
- Hu, J.; Liu, J.; Yang, Y.; Wei, Z.; Li, M.; Liu, G.; Gao, T.; Liu, S. On the Interactions Between y–Al2O3 Nanoparticles and Primary or Eutectic Al2Cu in Hypereutectic Al-Cu Alloy. JOM 2025, 77, 1044–1049. [Google Scholar] [CrossRef]
- Jeon, J.; Lee, S.H.; Kim, S.D.; Mao, Z.; Seidman, D.N.; Kim, K.; Cho, Y.H.; Kim, S.H.; Euh, K.; Lee, J.M.; et al. Ultrasound Alters the Nucleation Pathway of Primary Mg2Si in a Chemically Modified Multicomponent Al–Mg2Si Alloy. J. Alloys Compd. 2024, 1009, 177001. [Google Scholar] [CrossRef]
- Al-Helal, K.; Wang, Y.; Stone, I.; Fan, Z. Effect of Ca Level on the Formation of Silicon Phases during Solidification of Hypereutectic Al-Si Alloys. Mater. Sci. Forum 2013, 765, 117–122. [Google Scholar] [CrossRef]
- Miresmaeili, S.M.; Campbell, J.; Shabestari, S.G.; Boutorabi, S.M.A. Precipitation of Sr-Rich Intermetallic Particles and Their Influence on Pore Formation in Sr-Modified A356 Alloy. Metall. Mat. Trans. A 2005, 36, 2341–2349. [Google Scholar] [CrossRef]
- Chen, Q.I.; Griffiths, W.D. Modification of Double Oxide Film Defects with the Addition of Mo to An Al-Si-Mg Alloy. Metall. Mater. Trans. B 2021, 52, 502–516. [Google Scholar] [CrossRef]
- Eskin, D.G.; Tzanakis, I.; Wang, F.; Lebon, G.S.B.; Subroto, T.; Pericleous, K.; Mi, J. Fundamental Studies of Ultrasonic Melt Processing. Ultrason. Sonochem. 2019, 52, 455–467. [Google Scholar] [CrossRef]
- Jaime, R.F.; Puga, H.; Prokic, M.; Söderhjelm, C.; Apelian, D. Fundamentals of Ultrasonic Treatment of Aluminum Alloys. Int. J. Met. 2024, 18, 2783–2807. [Google Scholar] [CrossRef]
- Barbosa, J.; Puga, H. Ultrasonic Melt Treatment of Light Alloys. Int. J. Metalcasting 2019, 13, 180–189. [Google Scholar] [CrossRef]
- Osawa, Y.; Takamori, S.; Kimura, T.; Minagawa, K.; Kakisawa, H. Morphology of Intermetallic Compounds in Al-Si-Fe Alloy and Its Control by Ultrasonic Vibration. Mater. Trans. 2007, 48, 2467–2475. [Google Scholar] [CrossRef]
- Puga, H.; Costa, S.; Barbosa, J.; Ribeiro, S.; Prokic, M. Influence of Ultrasonic Melt Treatment on Microstructure and Mechanical Properties of AlSi9Cu3 Alloy. J. Mater. Process. Technol. 2011, 211, 1729–1735. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Q.; Wang, K.; Hu, P. Effects of Ultrasonic Treatment on Microstructure and Mechanical Properties of Recycled Al-9Si-1Fe Alloy. Int. J. Met. 2024, 1–13. [Google Scholar] [CrossRef]
- Wang, F.; Eskin, D.; Connolley, T.; Mi, J. Effect of Ultrasonic Melt Treatment on the Refinement of Primary Al3Ti Intermetallic in an Al-0.4Ti Alloy. J. Cryst. Growth 2016, 435, 24–30. [Google Scholar] [CrossRef]
- Campbell, J. Cavitation in Liquid and Solid Metals: Role of Bifilms. Mater. Sci. Technol. 2015, 31, 565–572. [Google Scholar] [CrossRef]
- Lan, J.; Yang, Y.; Li, X. Microstructure and Microhardness of SiC Nanoparticles Reinforced Magnesium Composites Fabricated by Ultrasonic Method. Mater. Sci. Eng. A 2004, 386, 284–290. [Google Scholar] [CrossRef]
- Priyadarshi, A.; Khavari, M.; Subroto, T.; Prentice, P.; Pericleous, K.; Eskin, D.; Durodola, J.; Tzanakis, I. Mechanisms of Ultrasonic De-Agglomeration of Oxides through in-Situ High-Speed Observations and Acoustic Measurements. Ultrason. Sonochem. 2021, 79, 105792. [Google Scholar] [CrossRef]
- Sun, J.; Higashi, K.; Romankov, S.; Yamamoto, T.; Komarov, S. Free Surface Entrainment of Oxide Particles and Their Role in Ultrasonic Treatment Performance of Aluminum Alloys. Ultrason. Sonochem. 2022, 90, 106209. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Huang, L.-W.; Shih, T.-S. Diagnosis of Oxide Films by Cavitation Micro-Jet Impact. Mater. Trans. 2003, 44, 327–335. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Li, X.; Yang, Y.; Chen, P.; Dong, F.; Jiang, R. Possible Effects and Mechanisms of Ultrasonic Cavitation on Oxide Inclusions during Direct-Chill Casting of an Al Alloy. Metals 2018, 8, 814. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Lordan, E.; Wang, Y.; Fan, Z. Improve Mechanical Properties of High Pressure Die Cast Al9Si3Cu Alloy via Dislocation Enhanced Precipitation. J. Alloys Compd. 2019, 785, 1015–1022. [Google Scholar] [CrossRef]
- Czerwinski, F.; Benkel, F.; Birsan, G. Gas-Enhanced Ultrahigh-Shear Mixing: An Application to Molten Aluminum Alloys. Metall. Mater. Trans. B 2020, 51, 1079–1087. [Google Scholar] [CrossRef]
- Fan, Z.; Zuo, Y.B.; Jiang, B. A New Technology for Treating Liquid Metals with Intensive Melt Shearing. Mater. Sci. Forum 2011, 690, 141–144. [Google Scholar] [CrossRef]
- Scamans, G.; Li, H.T.; Nebreda, J.L.; Patel, J.; Stone, I.; Wang, Y.; Yang, X.; Fan, Z. Advanced Casting Technologies Using High Shear Melt Conditioning. In Fundamentals of Aluminium Metallurgy: Recent Advances; Elsevier: Amsterdam, The Netherlands, 2018; pp. 249–277. ISBN 9780081020630. [Google Scholar]
- Lazaro-Nebreda, J.; Patel, J.B.; Lordan, E.; Zhang, Y.; Karakulak, E.; Al-Helal, K.; Scamans, G.M.; Fan, Z. Degassing of Aluminum Alloy Melts by High Shear Melt Conditioning Technology an Overview. Metals 2022, 12, 1772. [Google Scholar] [CrossRef]
- Lazaro-Nebreda, J.; Patel, J.B.; Fan, Z. Improved Degassing Efficiency and Mechanical Properties of A356 Aluminium Alloy Castings by High Shear Melt Conditioning (HSMC) Technology. J. Mater. Process. Technol. 2021, 294, 117146. [Google Scholar] [CrossRef]
- Zhang, Y.; Patel, J.B.; Lazaro-Nebreda, J.; Fan, Z. Improved Defect Control and Mechanical Property Variation in High-Pressure Die Casting of A380 Alloy by High Shear Melt Conditioning. JOM 2018, 70, 2726–2730. [Google Scholar] [CrossRef]
- Himmler, D.; Randelzhofer, P.; Körner, C. Formation Kinetics and Phase Stability of In-Situ Al3Ti Particles in Aluminium Casting Alloys with Varying Si Content. Results Mater. 2020, 7, 100103. [Google Scholar] [CrossRef]
- Lebon, G.S.B.; Patel, J.B.; Fan, Z. Numerical Studies of Batch and Inline High Shear Melt Conditioning Technology Using Different Rotors. Crystals 2022, 12, 1299. [Google Scholar] [CrossRef]
- Lebon, G.S.B.; Lazaro-Nebreda, J.; Patel, J.B.; Fan, Z. Numerical Assessment of In-Line Rotor–Stator Mixers in High-Shear Melt Conditioning (HSMC) Technology. JOM 2020, 72, 4092–4100. [Google Scholar] [CrossRef]
- Tong, M.; Patel, J.B.; Stone, I.; Fan, Z.; Browne, D.J. Identification of Key Liquid Metal Flow Features in the Physical Conditioning of Molten Aluminium Alloy with High Shear Processing. Comput. Mater. Sci. 2017, 131, 35–43. [Google Scholar] [CrossRef]
- Al-Helal, K.; Lazaro-Nebreda, J.; Patel, J.B.; Scamans, G.M. High-Shear de-Gassing and de-Ironing of an Aluminum Casting Alloy Made Directly from Aluminum End-of-Life Vehicle Scrap. Recycling 2021, 6, 66. [Google Scholar] [CrossRef]
- Li, H.T.; Ji, S.; Wang, Y.; Xia, M.; Fan, Z. Effect of Intensive Melt Shearing on the Formation of Fe-Containing Intermetallics in LM24 Al-Alloy. IOP Conf. Ser. Mater. Sci. Eng. 2011, 27, 012075. [Google Scholar] [CrossRef]
- Li, H.T.; Patel, J.B.; Kotadia, H.R.; Fan, Z. Controlling the Formation of Iron-Bearing Intermetallics in Wrought Al Alloys by Melt Conditioned DC (MC-DC) Casting Technology. In Materials Science Forum; Trans Tech Publications Ltd.: Seestrasse, Switzerland, 2015; Volume 828–829, pp. 43–47. [Google Scholar]
- Lazaro-Nebreda, J.; Patel, J.B.; Al-helal, K.; Gao, F.; Stone, I.; Chang, I.T.H.; Scamans, G.M.; Fan, Z. De-Ironing of Aluminium Alloy Melts by High Shear Melt Conditioning Technology: An Overview. Metals 2022, 12, 1579. [Google Scholar] [CrossRef]
- Campbell, J. Intrinsic and Extrinsic Metallurgy. In Shape Casting: 3rd International Symposium 2009; Wiley: Hoboken, NJ, USA, 2009; pp. 3–10. [Google Scholar]
- Campbell, J.; Tiryakioǧlu, M. Fatigue Failure in Engineered Components and How It Can Be Eliminated: Case Studies on the Influence of Bifilms. Metals 2022, 12, 1320. [Google Scholar] [CrossRef]
- Ahmadt, R.; Marshall, R.I. Effect of Superheating on Iron-Rich Plate-Type Compounds in Aluminium-Silicon Alloys. Int. J. Cast Met. Res. 2003, 15, 497–504. [Google Scholar] [CrossRef]
- Mathew, J.; Williams, M.A.; Srirangam, P. Effect of Superheat on Microstructure and Mechanical Properties of Al-7Si-2Fe Alloy. JOM 2024, 76, 464–472. [Google Scholar] [CrossRef]
- Azarniya, A.; Azarniya, A.; Abdollah-zadeh, A.; Madaah Hosseini, H.R.; Ramakrishna, S. In Situ Hybrid Aluminum Matrix Composites: A Review of Phase Transformations and Mechanical Aspects. Adv. Eng. Mater. 2019, 21, 1801269. [Google Scholar] [CrossRef]
- Thakur, A.; Bandhu, D.; Peshwe, D.R.; Mahajan, Y.Y.; Saxena, K.K.; Eldin, S.M. Appearance of Reinforcement, Interfacial Product, Heterogeneous Nucleant and Grain Refiner of MgAl2O4 in Aluminium Metal Matrix Composites. J. Mater. Res. Technol. 2023, 26, 267–302. [Google Scholar] [CrossRef]
- Afkham, Y.; Khosroshahi, R.A.; Rahimpour, S.; Aavani, C.; Brabazon, D.; Mousavian, R.T. Enhanced Mechanical Properties of in Situ Aluminium Matrix Composites Reinforced by Alumina Nanoparticles. Arch. Civ. Mech. Eng. 2018, 18, 215–226. [Google Scholar] [CrossRef]
- Huang, Z.-J.; Yang, B.; Cui, H.; Zhang, J.-S. Study on the Fabrication of Al Matrix Composites Strengthened by Combined In-Situ Alumina Particle and in-Situ Alloying Elements. Mater. Sci. Eng. A 2003, 351, 15–22. [Google Scholar]
- Xu, T.; Li, G.; Xie, M.; Liu, M.; Zhang, D.; Zhao, Y.; Chen, G.; Kai, X. Microstructure and Mechanical Properties of In-Situ Nano γ-Al2O3p/A356 Aluminum Matrix Composite. J. Alloys Compd. 2019, 787, 72–85. [Google Scholar] [CrossRef]
- Thakur, A.; Gupta, R.K.; Udhayabanu, V.; Peshwe, D.R.; Mahajan, Y.Y. Ultrasonic Assisted Reactive Synthesis and Characterization of Al–MgAl2O4 in-Situ Composite. Mater. Chem. Phys. 2023, 297, 127311. [Google Scholar] [CrossRef]
- Yang, B.; Sun, M.; Gan, G.; Xu, C.; Huang, Z.; Zhang, H.; Fang, Z.Z. In Situ Al2O3 Particle-Reinforced Al and Cu Matrix Composites Synthesized by Displacement Reactions. J. Alloys Compd. 2010, 494, 261–265. [Google Scholar] [CrossRef]
- Sreekumar, V.M.; Babu, N.H.; Eskin, D.G. Potential of an Al-Ti-MgAl2O4 Master Alloy and Ultrasonic Cavitation in the Grain Refinement of a Cast Aluminum Alloy. Metall. Mater. Trans. B 2017, 48, 208–219. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Sun, C.; Cheng, Z.; Pang, Z.; Wang, L.; Chen, H.; Liu, N. Hypereutectic Al-Si Matrix Composites Prepared by In Situ Fe2O3/Al System. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2021, 36, 636–643. [Google Scholar] [CrossRef]
- Zhu, H.; Min, J.; Li, J.; Ai, Y.; Ge, L.; Wang, H. In Situ Fabrication of (α-Al2O3+Al3Zr)/Al Composites in an Al-ZrO2 System. Compos. Sci. Technol. 2010, 70, 2183–2189. [Google Scholar] [CrossRef]
- Najarian, A.R.; Emadi, R.; Hamzeh, M. Fabrication of As-Cast Al Matrix Composite Reinforced by Al2O3/Al3Ni Hybrid Particles via in-Situ Reaction and Evaluation of Its Mechanical Properties. Mater. Sci. Eng. B 2018, 231, 57–65. [Google Scholar] [CrossRef]
- Wang, H.; Li, G.; Zhao, Y.; Chen, G. In Situ Fabrication and Microstructure of Al2O3 Particles Reinforced Aluminum Matrix Composites. Mater. Sci. Eng. A 2010, 527, 2881–2885. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, H.; Zhang, D.; Li, C.; Li, J.; Huang, J. Reaction Mechanisms and Tensile Properties of the Composites Fabricated by Al-B2O3 System. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 1024–1029. [Google Scholar] [CrossRef]
- Sreekumar, V.M.; Hari Babu, N.; Eskin, D.G.; Fan, Z. Structure-Property Analysis of in-Situ Al-MgAl2O4 Metal Matrix Composites Synthesized Using Ultrasonic Cavitation. Mater. Sci. Eng. A 2015, 628, 30–40. [Google Scholar] [CrossRef]
- Sreekumar, V.M.; Pillai, R.M.; Pai, B.C.; Chakraborty, M. Synthesis of an Al/MgAl2O4 in Situ Metal Matrix Composite from Silica Gel. J. Am. Ceram. Soc. 2007, 90, 2905–2911. [Google Scholar] [CrossRef]
- Hashim, J.; Looney, L.; Hashmi, J. Metal Matrix Composites: Production by the Stir Casting Method. J. Mater. Process. Technol. 1999, 92–93, 1–7. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Du, Y.H.; Zhang, P. Vortex-Free Stir Casting of Al-1.5 wt% Si-SiC Composite. J. Alloys Compd. 2019, 787, 206–215. [Google Scholar] [CrossRef]
- Lu, Y.; Du, Y.; Zhang, W.; Tan, H.; Zhang, N.; Luo, Y.; Zhang, P. Nano-Al2O3 Particle Incorporated in Al Matrix Composite by Vortex-Free High-Speed Stir Casting. Int. J. Met. 2024, 19, 761–776. [Google Scholar] [CrossRef]
- Li, M.; Gao, T.; Li, C.; Sun, Y.; Wu, Y.; Liu, X. On the Nano–Treating Effect of Al2O3 on the Eutectic Si in Al–Si Alloy. Micron 2023, 168, 103443. [Google Scholar] [CrossRef]
- Campbell, J. Entrainment. In Complete Casting Handbook; Butterworth-Heinemann: Oxford, UK, 2015; pp. 17–90. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage Thermochemical Software and Databases, 2010–2016. CALPHAD 2016, 54, 35–53. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).