Aging Behavior of 10CrNi2Mo3Cu2V Maraging Alloy: Clustering, Precipitation, and Strengthening
Abstract
1. Introduction
2. Experimental Procedure
3. Results and Discussion
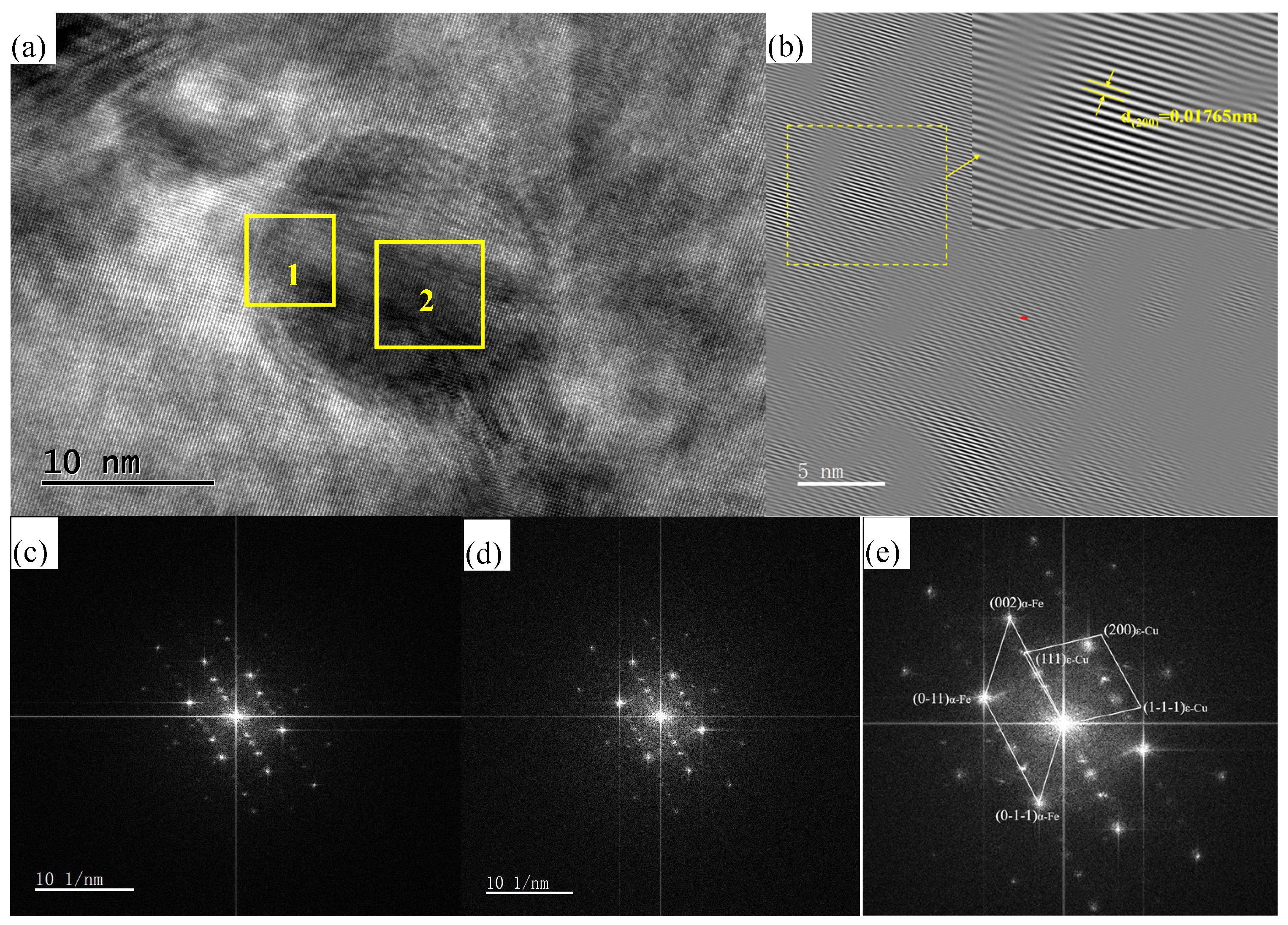
3.1. Precipitation Behavior of Rich Cu Phase
3.2. Crystallographic Relationship Between Cu-Rich Phase and Matrix
3.3. Mechanism of Secondary Strengthening in Copper
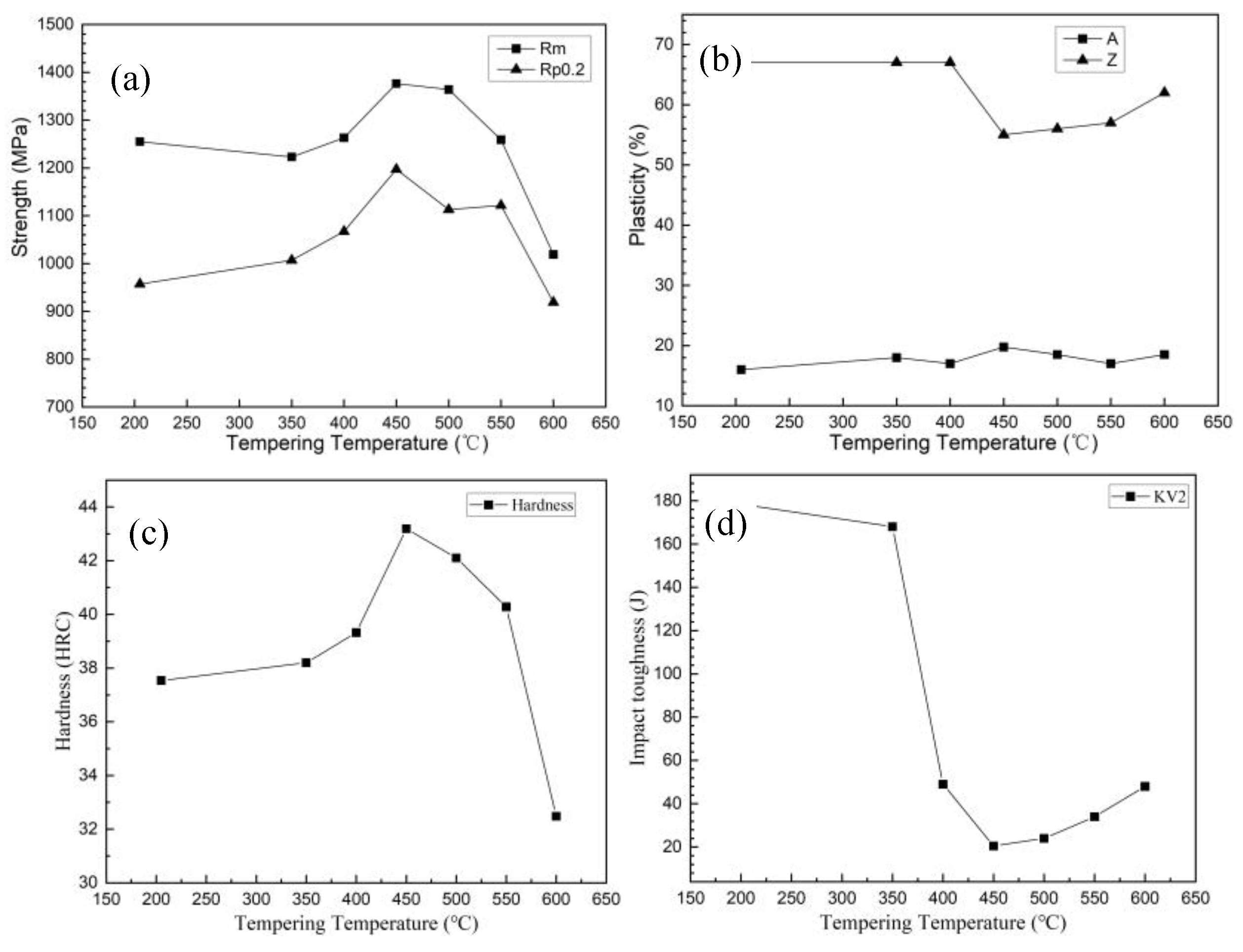
3.3.1. Influence of Copper-Rich Phases on Mechanical Properties
3.3.2. Analysis of the Mechanism Behind Secondary Strengthening in Copper
- (1)
- Chemical strengthening
- (2)
- Coherent strain strengthening
- (3)
- Modulus strengthening
- (4)
- Orowan reinforcement effect
4. Conclusions
- (1)
- Tempering between 350 °C and 450 °C promotes the formation of nanoscale Cu-rich phases, leading to peak strength and hardness at 450 °C. Specifically, the steel reaches a yield strength approximately 240 MPa greater than that at 205 °C after tempering at 450 °C. However, subsequent coarsening of these precipitates at higher temperatures diminishes strength and significantly reduces impact toughness, which reaches its lowest point at 450 °C. This is attributed to the loss of coherency and the facilitation of micro-crack formation at incoherent interfaces. This highlights the critical role of tempering temperature in controlling the Cu-rich phase morphology and, consequently, the mechanical properties.
- (2)
- Analysis of Cu-rich phase size and theoretical calculations indicate that the dislocation cutting mechanism is the primary contributor to strengthening, rather than Orowan bypassing. The APT-measured average particle diameter of 1.46 nm is significantly smaller than the calculated Orowan critical diameter of 6.18 nm. Further, calculations reveal that the modulus-strengthening effect contributes the most to the increase in strength, followed by the coherent strain-strengthening effect, and then the chemical-strengthening effect. This suggests that optimizing the size, distribution, and coherency of Cu-rich precipitates is crucial for maximizing strength.
- (3)
- Theoretical calculations of strength enhancement due to both the cutting mechanism (approximately 121.8 MPa) and Orowan bypassing mechanism (approximately 97.2 MPa) total a predicted yield strength increase of 219 MPa, closely matching the experimentally observed yield strength increase of approximately 240 MPa at 450 °C compared to 205 °C. This agreement validates the accuracy of the models used and supports using these insights to guide future alloy design strategies, such as optimizing composition and heat treatment to tailor precipitate characteristics and achieve desired strength-toughness balance in high-strength steels. Future work should focus on achieving this refined control over Cu-rich phase morphology to improve overall mechanical performance.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rejith, R.; Kesavan, D.; Chakravarthy, P.; Murty, S.N. Bearings for aerospace applications. Tribol. Int. 2023, 181, 108312. [Google Scholar] [CrossRef]
- Hu, X.; Yang, L.; Wei, X.; Wang, H.; Fu, G. Molecular Dynamics Simulation on Nanoindentation of M50 Bearing Steel. Materials 2023, 16, 2386. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, J.; Dong, N.; Tan, H.; Wang, J.; Han, P. Effect of quenching and tempering on microstructure and corrosion resistance of aerospace-bearing steel. Mater. Today Commun. 2024, 39, 108791. [Google Scholar] [CrossRef]
- Zu, H.; He, Z.; He, B.; Tang, Z.; Fang, X.; Cai, Z.; Cao, Z.; An, L. Effect of Metallic Coatings on the Wear Performance and Mechanism of 30CrMnSiNi2A Steel. Materials 2023, 16, 6191. [Google Scholar] [CrossRef] [PubMed]
- Iżowski, B.; Wojtyczka, A.; Motyka, M. Numerical Simulation of Low-Pressure Carburizing and Gas Quenching for Pyrowear 53 Steel. Metals 2023, 13, 371. [Google Scholar] [CrossRef]
- Wei, P.; Bai, P.; Yue, L.; Zhou, X.; Wen, X.; Zhao, H.; Meng, Y.; Tian, Y. Comparing friction behavior and mechanism of three generation bearing steel (GCr15, M50, CSS-42L) paired silicon nitride under ester oil lubrication from room temperature to 350 °C. J. Mater. Res. Technol. 2024, 30, 1354–1367. [Google Scholar] [CrossRef]
- He, Y.; Zeng, X.J.; Li, L.Q.; Li, N.; Niu, J.B.; Ma, X.X. Phase composition and numerical model of low-pressure pulse carburized layer in 15Cr14Co12Mo5Ni2W steel. Surf. Coat. Technol. 2024, 484, 130868. [Google Scholar] [CrossRef]
- Liu, B.F.; Yang, G.; Hua, J.S.; Yang, M.X. Effect of quenching temperature on structure and mechanical properties of AMS 6308 steel. Spec. Steel Technol. 2012, 18, 15. (In Chinese) [Google Scholar]
- Stepanova, N.V.; Mikhalev, R.I.; Tarasova, T.D. Peculiarities of Copper Precipitation in Hypereutectoid Steels. Metallurgist 2023, 66, 1388–1400. [Google Scholar] [CrossRef]
- Goodman, S.R.; Brenner, S.S.; Low, J.R. An FIM-atom probe study of the precipitation of copper from lron-1.4 at. pct copper. Part I: Field-ion microscopy. Metall. Trans. 1973, 4, 2363–2369. [Google Scholar] [CrossRef]
- Maruyama, N.; Sugiyama, M.; Hara, T.; Tamehiro, H. Precipitation and phase transformation of copper particles in low alloy ferritic and martensitic steels. Mater. Trans. 1999, 40, 268–277. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Zhang, X.; Liu, T.; Peng, Y.; Wang, K. Effect of heat treatment on microstructure and mechanical properties of 17-4PH stainless steel manufactured by laser-powder bed fusion. J. Mater. Res. Technol. 2023, 26, 5707–5715. [Google Scholar] [CrossRef]
- Sato, K.; Ihara, T.; Sakurai, H.; Yoshiie, T.; Xu, Q. Comparison of interaction between Cu precipitate and vacancy in Fe using first-principle calculations and empirical N-body potential calculations. Comput. Mater. Sci. 2009, 47, 521–525. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, R. Research progress on coarsening of second phase particles in two-phase systems. Mater. Rev. 2010, 24, 113–115. [Google Scholar]
- Liu, W. Study on the Precipitation and Crystal Structure Evolution of Cu-Rich Phase in Reactor Pressure Vessel Simulated Steel. Ph.D. Thesis, Shanghai University, Shanghai, China, 2011. (In Chinese). [Google Scholar]
- Kajiwara, S. Stacking disorder in martensite with 3R close-packed structure. J. Appl. Crystallogr. 1971, 4, 329–330. [Google Scholar] [CrossRef]
- Zhu, L. Molecular Dynamics Simulation of Early Precipitation Behavior of Rich Cu Clusters in Fe-Cu-Ni Alloys. Master’s Thesis, Shanghai University, Shanghai, China, 2014. (In Chinese). [Google Scholar]
- Holzer, I.; Kozeschnik, E. Computer simulation of the yield strength evolution in Cu- precipitation strengthened ferritic steel. Mater. Sci. Eng. A 2010, 527, 3546–3551. [Google Scholar] [CrossRef]
- Goodman, S.R.; Brenner, S.S.; Low, J.R. An FIM-atom probe study of the precipitation of copper from lron-1.4 at. pct copper. Part II: Atom probe analyses. Metall. Trans. 1973, 4, 2371–2378. [Google Scholar] [CrossRef]
- Ferguson, B.L.; Freborg, A.M.; Li, Z. Residual stress and heat treatment-Process design for bending fatigue strength improvement of carburized aerospace gears. HTM Haerterei-Tech. Mitteilungen 2007, 62, 279–284. [Google Scholar] [CrossRef][Green Version]
- Ferguson, B.L.; Freborg, A.M.; Li, Z. Improving Gear Performance by Intensive Quench. In Proceedings of the 24th ASM Heat Treating Society Conference, Detroit, MI, USA, 17–19 September 2007. [Google Scholar]
- Ferguson, B.L. Residual Stress and Bending Fatigue Strength in Carburized and Quench Hardened Pyrowear 53 Steel Gears. In Proceedings of the International Federation of Heat Treatment and Surface Engineering 2016, Savannah: Proceedings of the 23rd IFHTSE Congress, Savannah, GE, USA, 18–21 April 2016. [Google Scholar]
- Hornbogen, E.; Glenn, R.C. A metallographic study of precipitation of copper from alpha iron. Trans. Metall. Soc. AIME 1960, 218, 1064–1070. [Google Scholar]
- Brown, L.M.; Ham, R.K. Dislocation–particle interactions. In Strengthening Methods in Crystals; Kelly, A., Nicholson, R.B., Eds.; Applied Science Publishers: London, UK, 1965; pp. 9–135. [Google Scholar]
- Russell, K.C.; Brown, L.M. Dispersion strengthening model based on differing elastic-moduli applied to IronCopper system. Acta Metall. Mater. 1972, 20, 969–974. [Google Scholar] [CrossRef]
- Kolli, R.P.; Seidman, D.N. The temporal evolution of the decomposition of a concentrated multicomponent Fe–Cu-based steel. Acta Mater. 2008, 56, 2073–2088. [Google Scholar] [CrossRef]
- Deschamps, A.; Militzer, M.; Poole, W.J. Precipitation kinetics and strengthening of a Fe-0.8 wt% Cu alloy. ISIJ Int. 2001, 41, 196–205. [Google Scholar] [CrossRef]
- Monzen, R.; Jenkins, M.L.; Sutton, A.P. The bcc-to-9R martensitic transformation of Cu precipitates and the relaxation process of elastic strains in an Fe-Cu alloy. Philos. Mag. A 2000, 80, 711–723. [Google Scholar] [CrossRef]
- Benz, R.; Elliott, J.F.; Chipman, J. Thermodynamics of the carbides in the system Fe- Cr-C. Metall. Trans. 1974, 5, 2235–2240. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, G.; Peng, T. Effect of Cu Element on Tempering Resistance of AMS 6308 Steel. Iron Steel 2021, 56, 76–82. (In Chinese) [Google Scholar]
- Ashby, M. The theory of the critical shear stress and work hardening of dispersion-hardened crystals. In Metallurgical Society Conference; Ansell, G.S., Cooper, T.D., Lenel, F.V., Eds.; Gordon and Breach: New York, NY, USA, 1968; Volume 47, pp. 143–205. [Google Scholar]

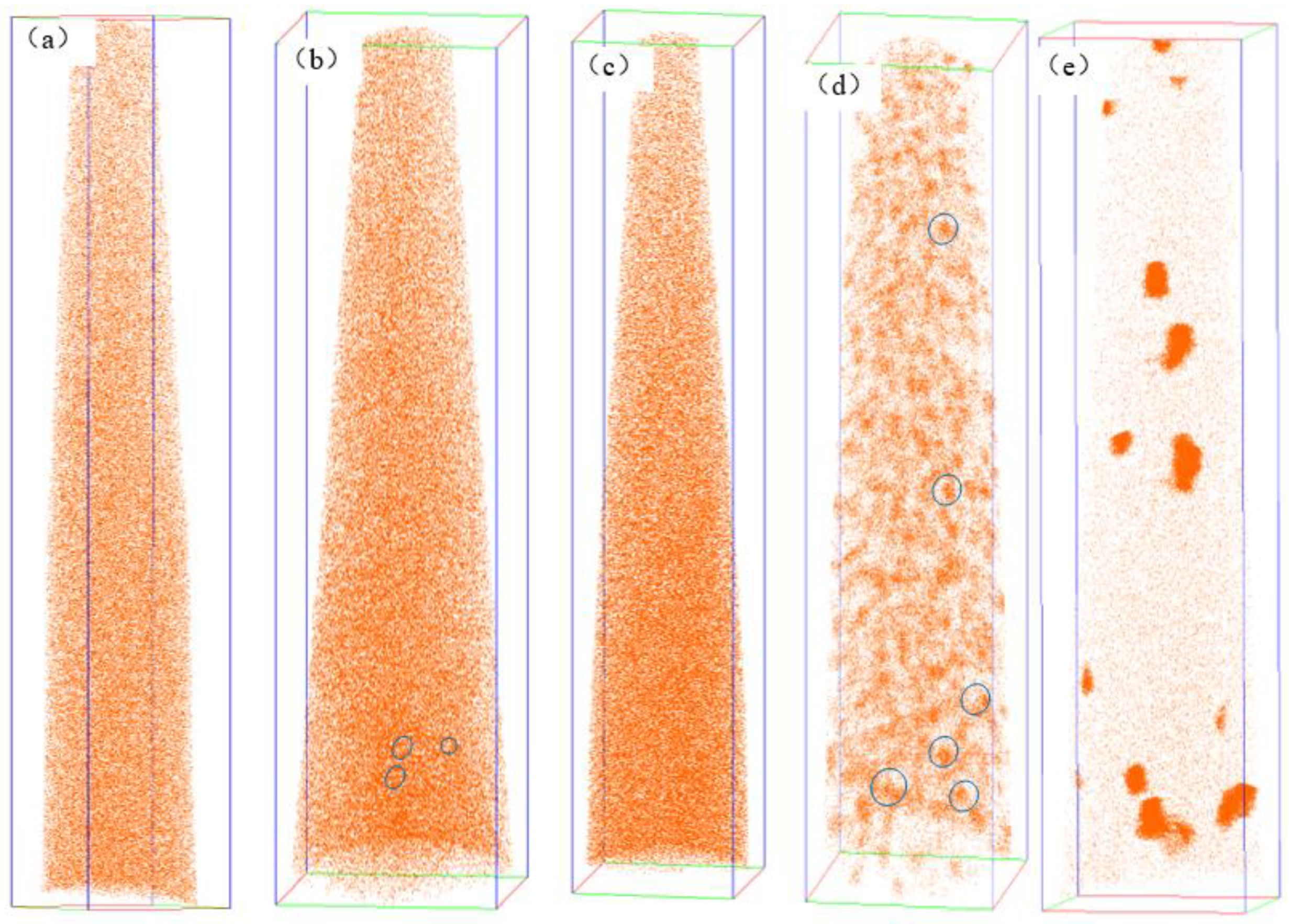
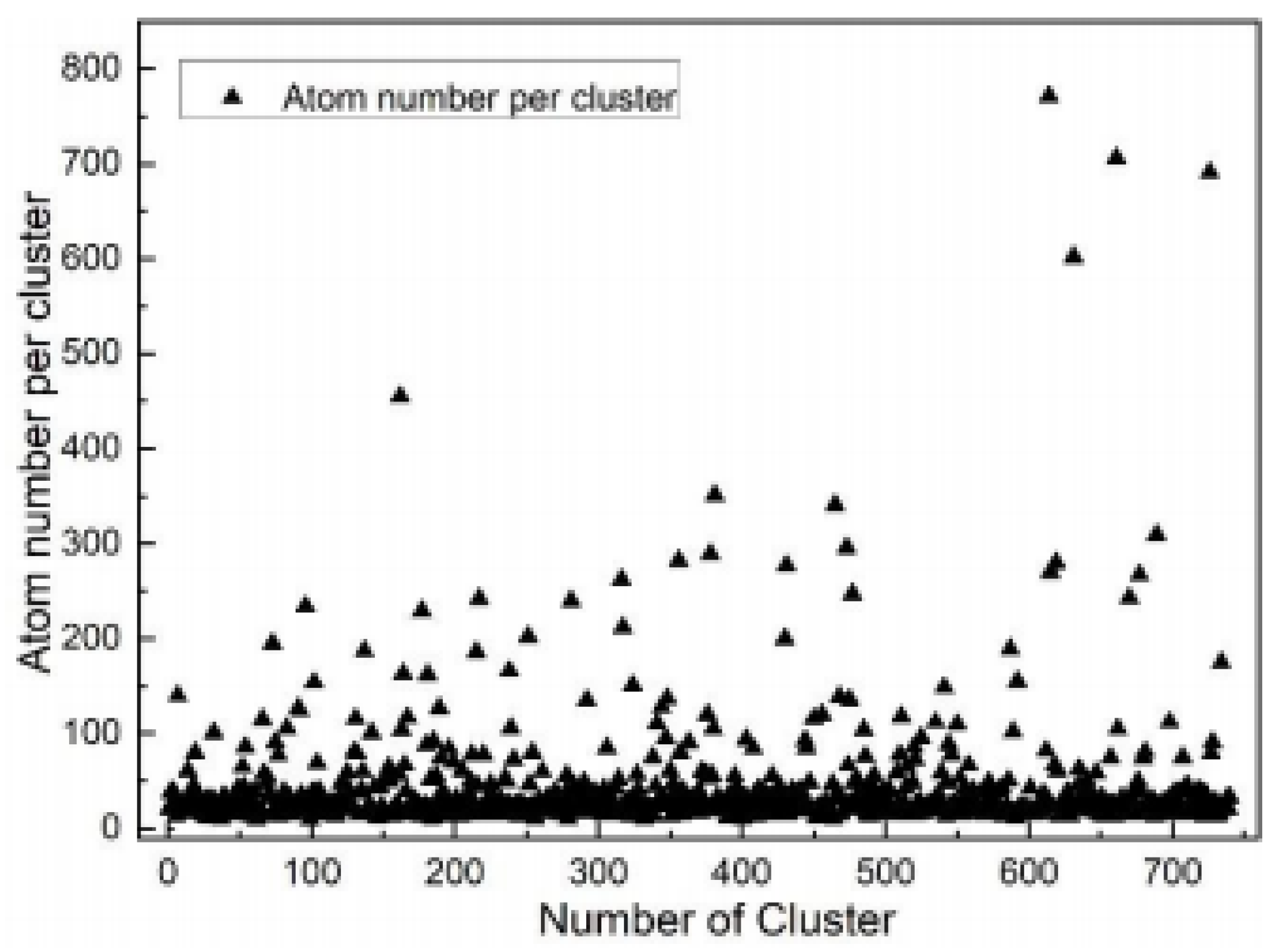
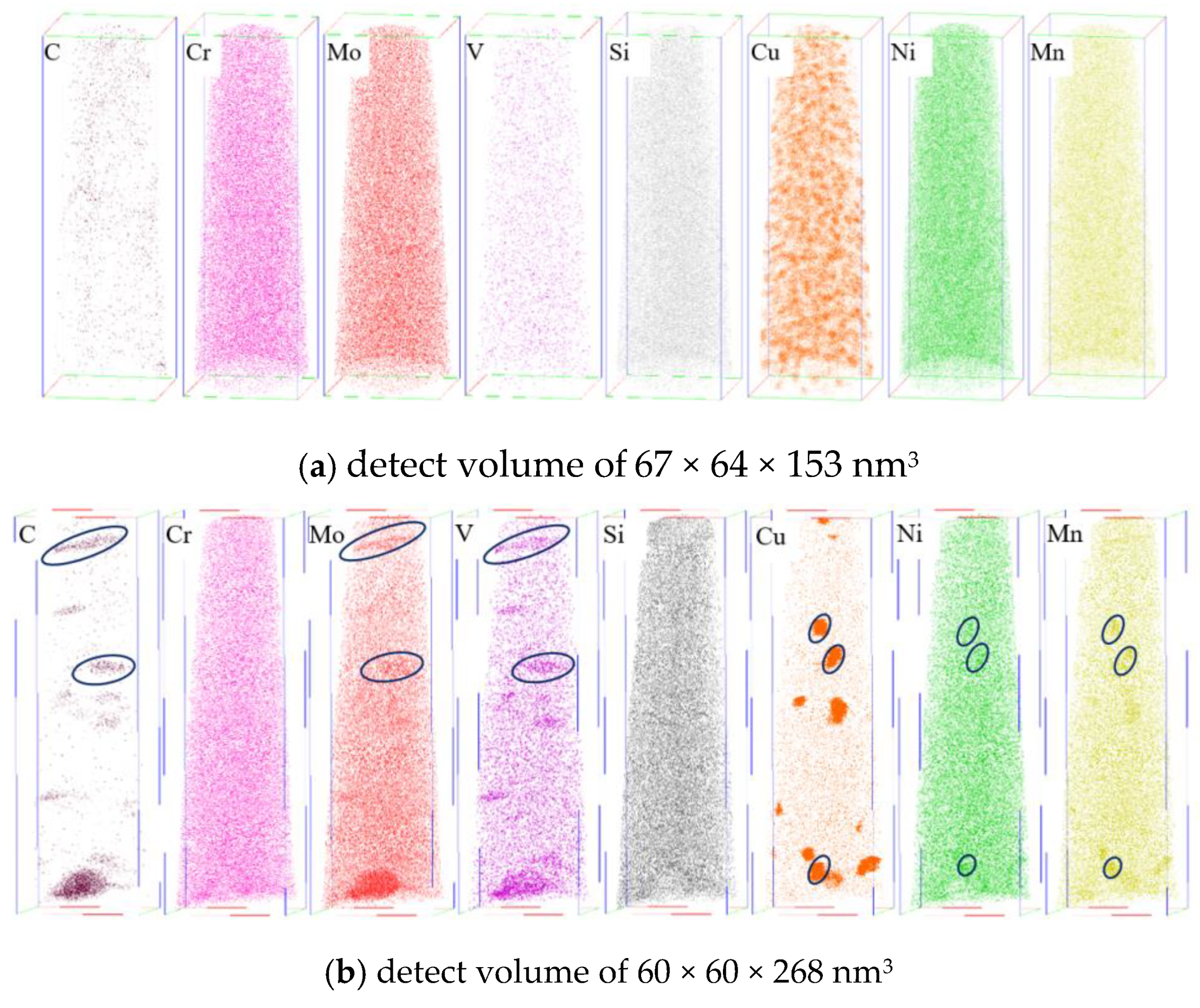
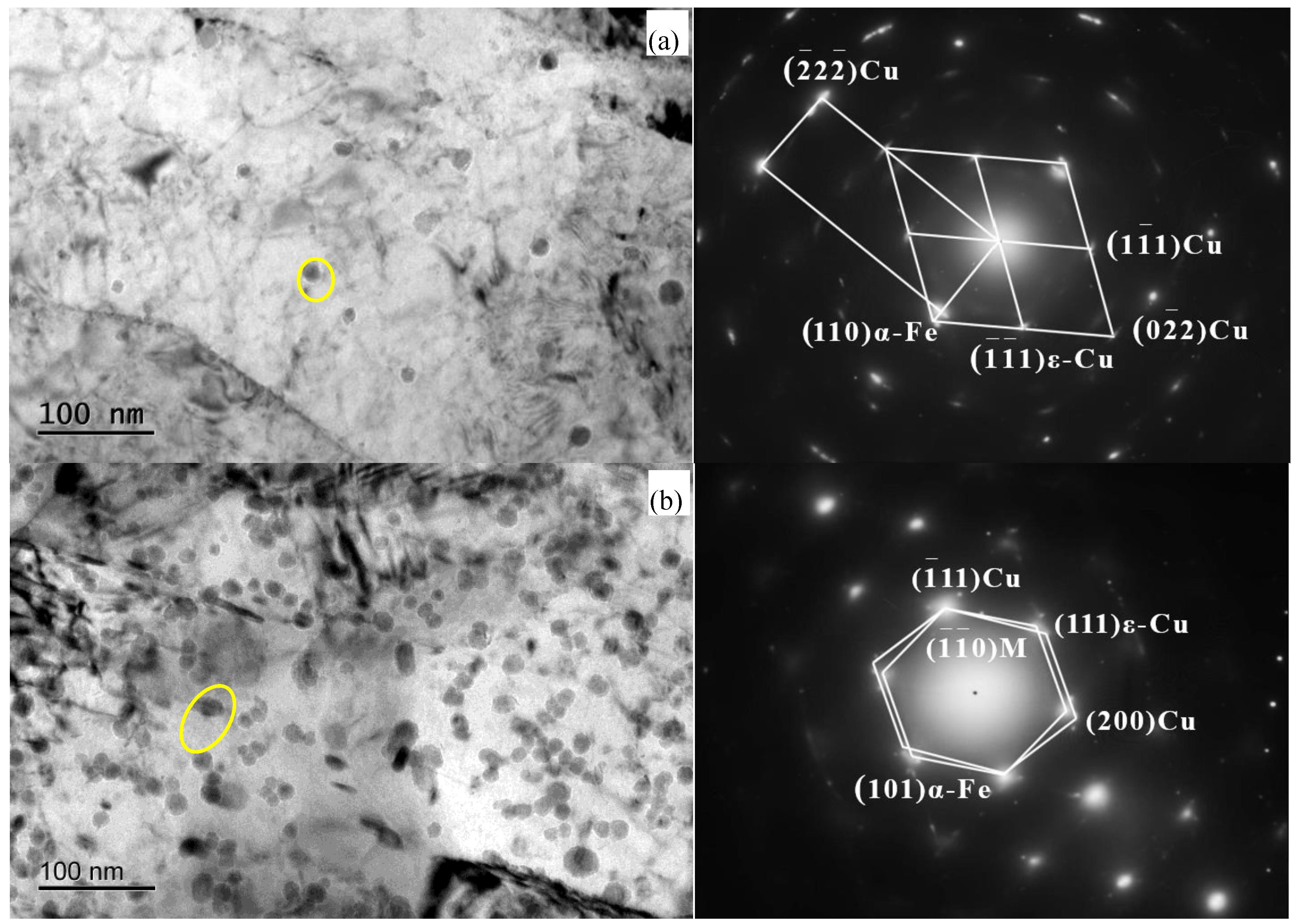

| Element | C | Si | Mn | S | P | Cr | Ni | Mo | V | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 0.10 | 0.90 | 0.35 | 0.001 | 0.005 | 1.00 | 2.18 | 3.26 | 0.09 | 1.90 | balance |
| Chemical strengthening | G (MPa) | M | b (nm) | λ (nm) | γ (J/m2) | σchem (MPa) | |
| 80,000 | 2.75 | 0.248 | 22.88 | 0.22–0.36 | 2460 | 7.9–14.3 | |
| Coherent strain strengthening | G (MPa) | M | b (nm) | λ (nm) | r (nm) | ε | σcoh (MPa) |
| 80,000 | 2.75 | 0.248 | 1.967 × 1024 | 1.4576 | 0.0057 | 37.6 | |
| Modulus strengthening | G (MPa) | M | b (nm) | λ (nm) | Up/Um | -- | σmod (MPa) |
| 80,000 | 2.75 | 0.248 | 22.88 | 0.9823 | -- | 115.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Yang, G.; Gong, Z. Aging Behavior of 10CrNi2Mo3Cu2V Maraging Alloy: Clustering, Precipitation, and Strengthening. Metals 2025, 15, 389. https://doi.org/10.3390/met15040389
Zhao J, Yang G, Gong Z. Aging Behavior of 10CrNi2Mo3Cu2V Maraging Alloy: Clustering, Precipitation, and Strengthening. Metals. 2025; 15(4):389. https://doi.org/10.3390/met15040389
Chicago/Turabian StyleZhao, Jiqing, Gang Yang, and Zhihua Gong. 2025. "Aging Behavior of 10CrNi2Mo3Cu2V Maraging Alloy: Clustering, Precipitation, and Strengthening" Metals 15, no. 4: 389. https://doi.org/10.3390/met15040389
APA StyleZhao, J., Yang, G., & Gong, Z. (2025). Aging Behavior of 10CrNi2Mo3Cu2V Maraging Alloy: Clustering, Precipitation, and Strengthening. Metals, 15(4), 389. https://doi.org/10.3390/met15040389






