Influence of Nd:YAG Laser Melting on an Investment-Casting Co-Cr-Mo Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
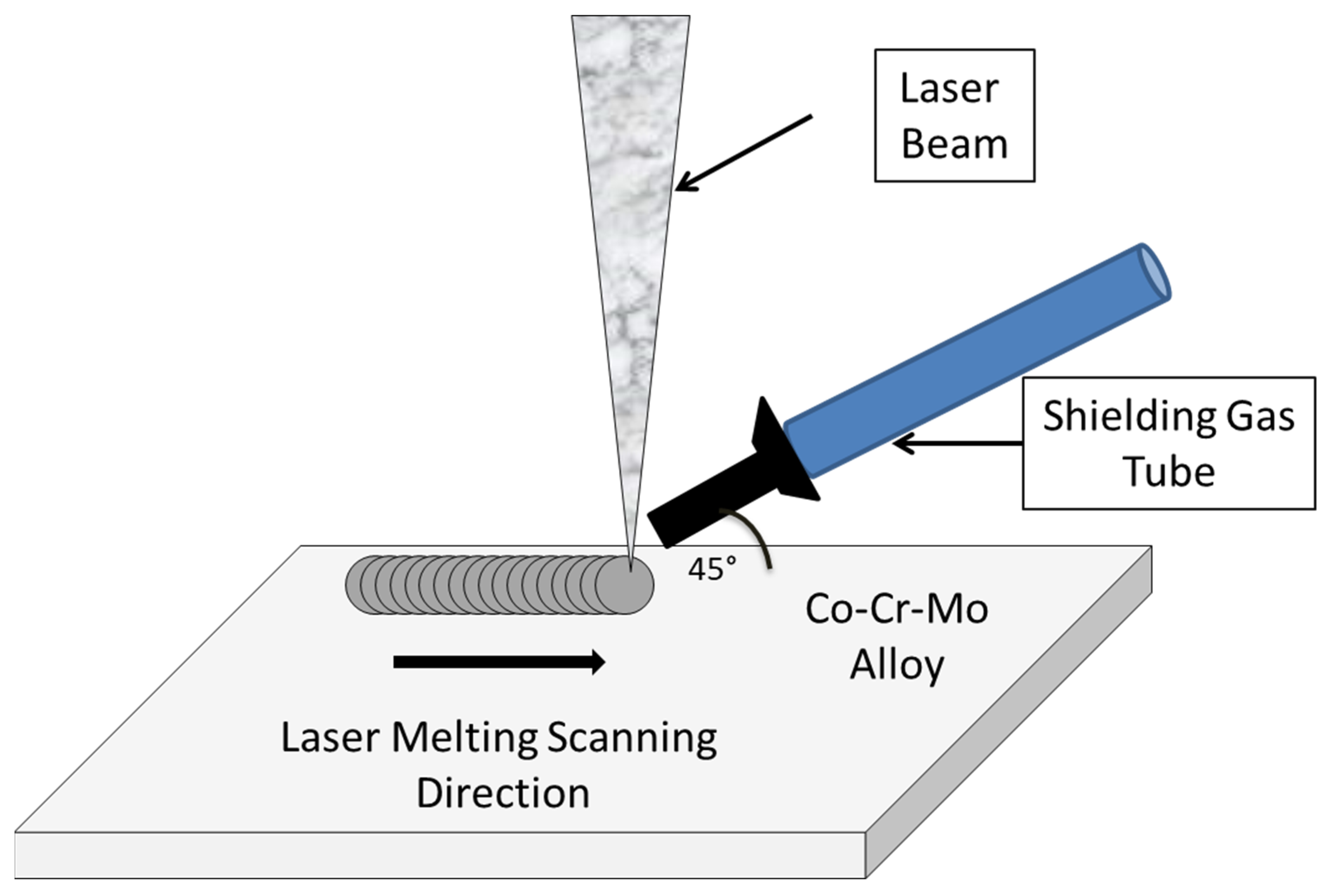
2.2. Nd:YAG Laser Melting Treatment
2.3. Pin-on-Disk Wear Test
2.4. In Vitro Bioactivity Assessment
2.5. Characterization Methods
3. Results and Discussion
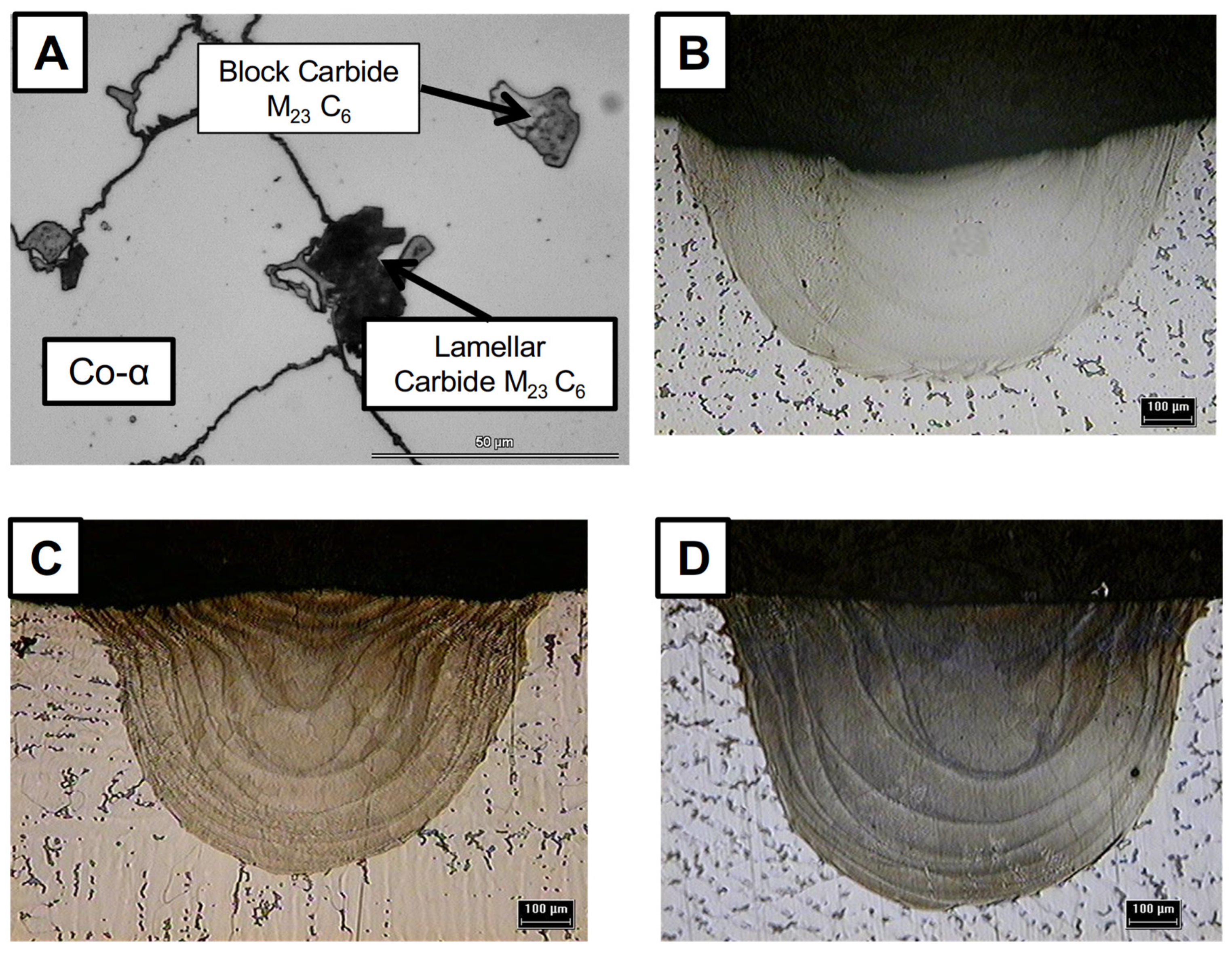
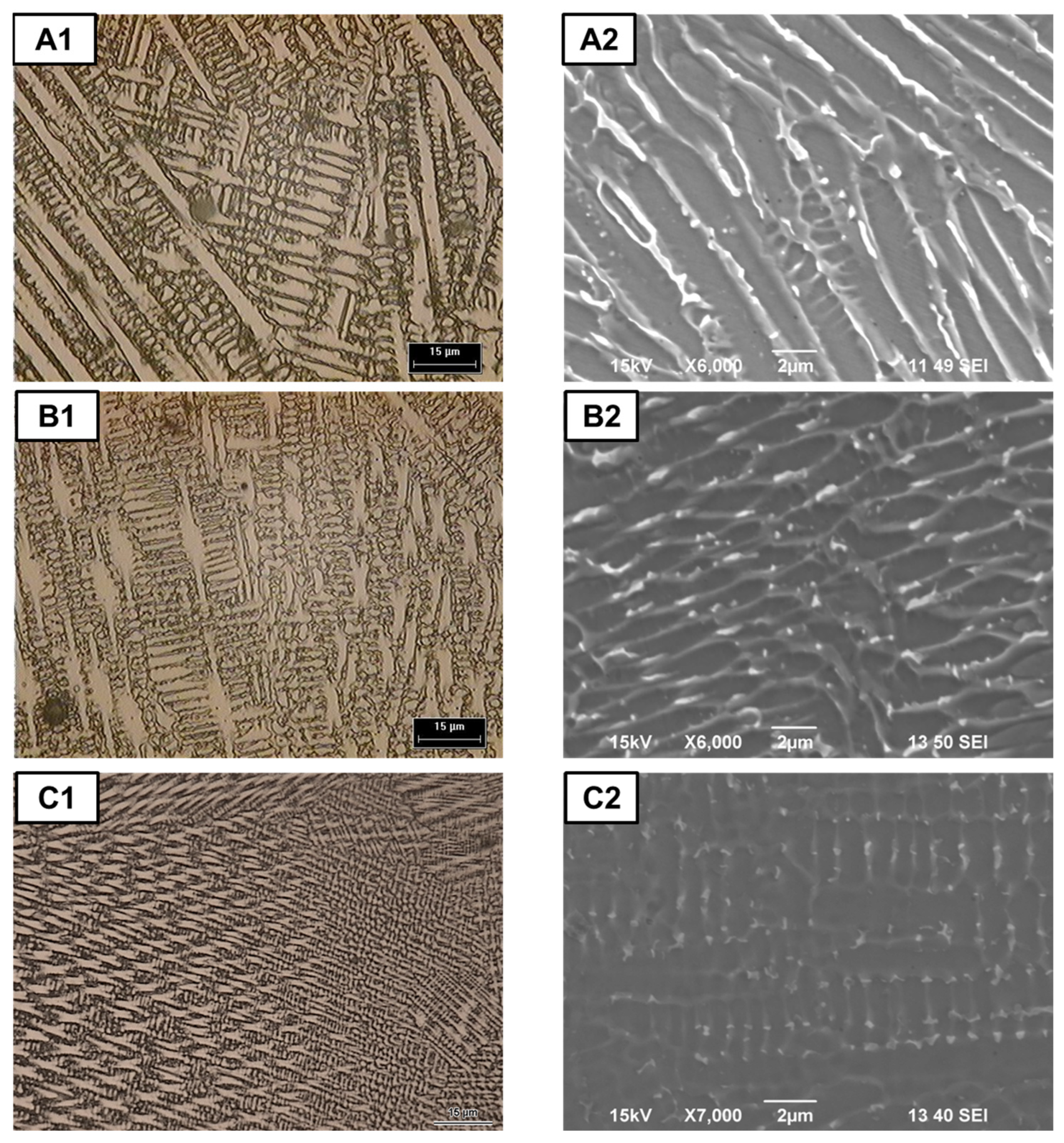
3.1. Macro and Microstructural Analysis
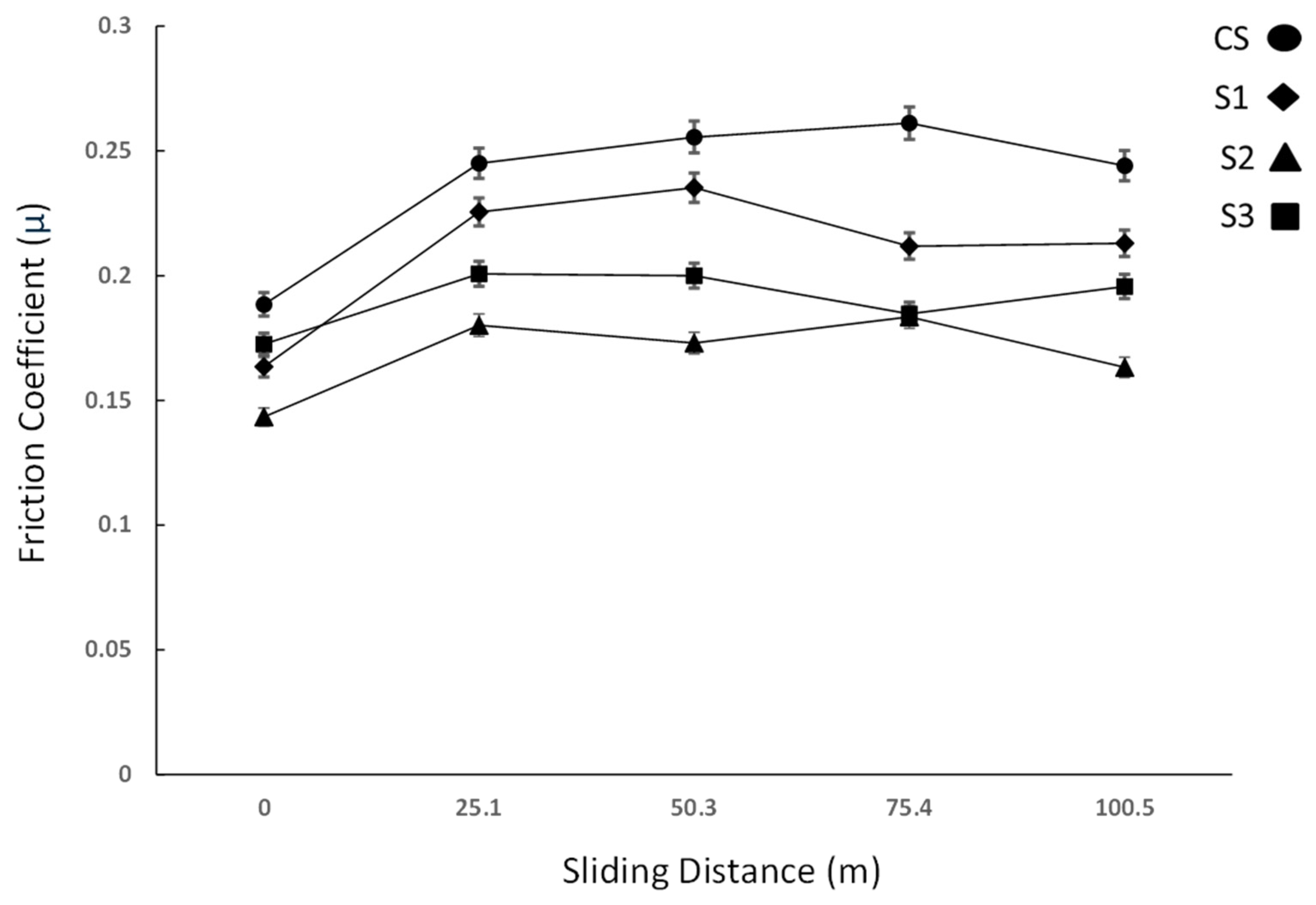
3.2. Microhardness and Wear Test
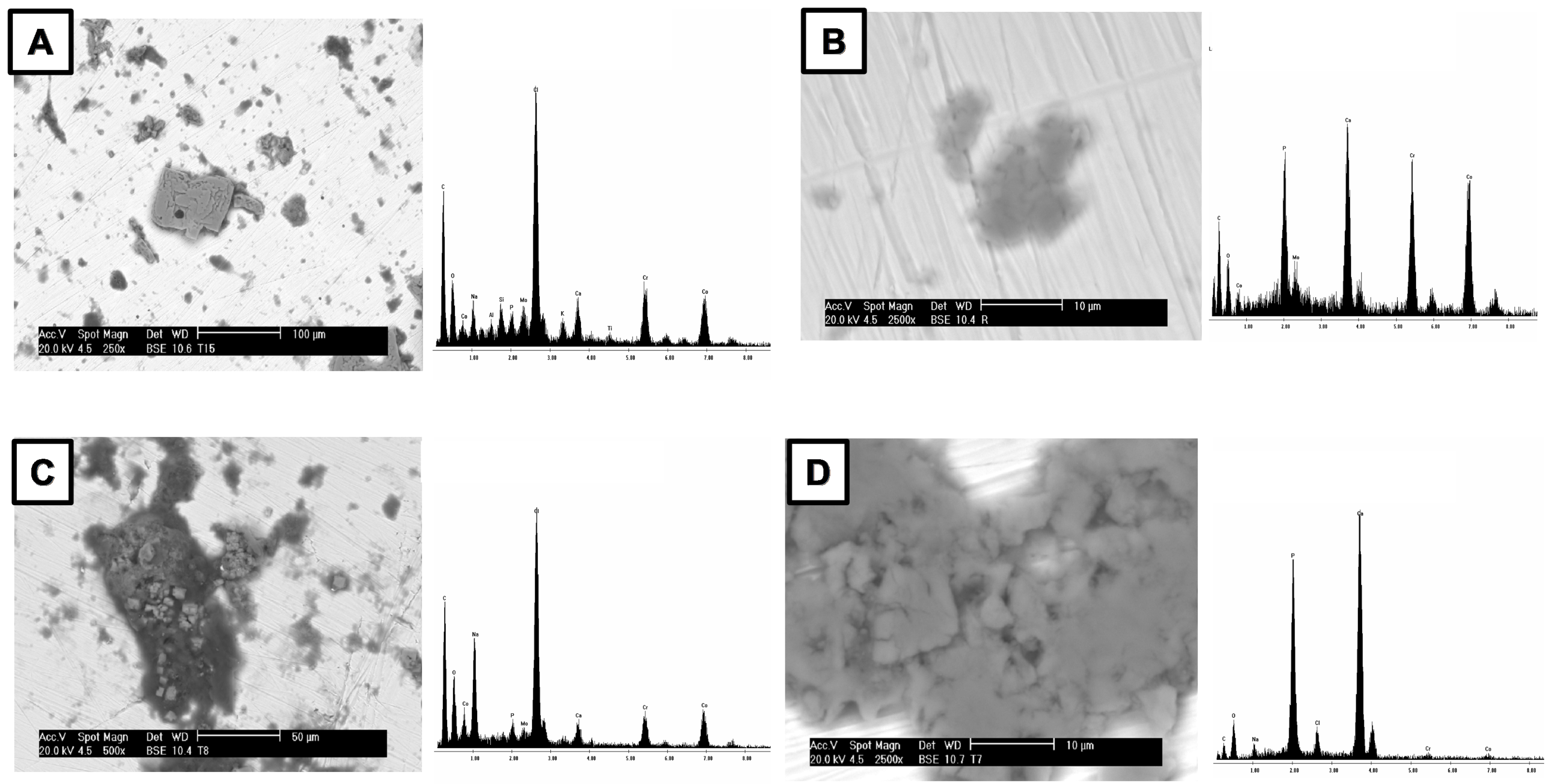
3.3. In Vitro Bioactivity
4. Conclusions
- Nd:YAG laser melting significantly enhances the mechanical properties and preserves the bioactive properties of investment-cast Co-Cr-Mo alloys. The process achieves a refined microstructure, eliminating microstructural defects that serve as stress concentrators, increasing hardness, improving wear resistance, and preserving bioactivity, offering a promising approach for the development of more durable biomedical implants;
- The LM process promotes the dissolution of coarse carbides and their subsequent reprecipitation as a fine carbide network during rapid solidification. The energy directly influences the solidification rate and thermal gradient, thereby further refining both the carbide size and distribution within the solidified metal pool as the pulse energy increases;
- Using an adequate set up of laser parameters such as peak power and pulse duration led to an increase in the microhardness from 325 Vickers to 445 Vickers (approx. 25%), which is correlated to the grain size reduction of 25–35% compared to the untreated sample;
- The exhibited low friction and high wear resistance found in the LM surfaces provides an essential insight into the potential development of novel prostheses with enhanced fatigue properties;
- Laser melting at high energy densities promoted the formation of uniform phase distributions and a homogenized microstructure, which significantly enhanced the wear resistance of the alloy. The process also reduced the wear volume loss by nearly 50% due to the effective microstructure improvement and the elimination of porosities and inhomogeneities. It is important to exercise precise control over variables to achieve the desirable microstructural modifications that enhance both the mechanical properties and the longevity of biomedical implants;
- TIR-ATR analysis of SBF-treated samples revealed P-O absorption bands at 1054, 566, 598, and 600 cm−1, confirming apatite formation and bioactivity. SEM-EDS and calcium consumption analyses further support the hypothesis that all samples are bioactive. Importantly, the laser surface treatment did not compromise their bioactivity. These results demonstrate that surface modification preserves the essential biological properties of the substrates;
- Based on our findings, we recommend the following optimal processing parameters for the Nd:YAG laser melting of Co-Cr-Mo alloys: pulse energy ≈ 37 J, a pulse duration of 7 ms, and a peak power of ≈ 5600 W. These parameters provide the best balance between microstructural refinement, mechanical property enhancement, and the preservation of bioactivity;
- Despite these promising results, the investigation was conducted under controlled laboratory conditions, which may differ from clinical environments. Future research should focus on optimizing laser processing parameters for specific implant geometries, conducting long-term in vivo performance studies to validate biocompatibility and wear resistance, and exploring additional laser surface modification techniques.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CS | Control Sample |
| SEM | Scanning electron microscopy |
| EDX | Energy-dispersive X-ray |
| ATR-IR | Attenuated total reflectance infrared spectroscopy |
| LM | Laser melting |
| S1 | Sample 1 |
| S2 | Sample 2 |
| S3 | Sample 3 |
| Nd:YAG | Neodymium-doped Yttrium Aluminum Garnet |
| P | Penetration depth |
| W | Fusion zone width |
| P:W | Penetration–width aspect ratio |
| µ | Friction coefficient |
References
- Gittens, R.A.; Schwartz, Z.; Smith, T.J.; Boyan, B.D. Implant osseointegration and the role of microroughness and nanostructures: Lessons for spine implants. Acta Biomater. 2014, 10, 2907–2921. [Google Scholar] [CrossRef] [PubMed]
- Vidyasagar, K.E.C.; Kalyanasundaram, D. Enhanced corrosion resistance of CoCrMo by laser-based surface modification. Surf. Eng. 2024, 40, 100–111. [Google Scholar] [CrossRef]
- Yıldırım, M.; Keleş, A. Production of Co–Cr–Mo biomedical alloys via investment casting technique. Turk. J. Electromech. Energy 2018, 3, 87–94. [Google Scholar]
- Pattnaik, S.; Karunakar, D.B.; Jha, P.K. Developments in investment casting process—A review. J. Mater. Process. Technol. 2012, 212, 2332–2348. [Google Scholar] [CrossRef]
- Connors, J.P.; Stelzer, J.W.; Garvin, P.M.; Wellington, I.J.; Solovyova, O. The Role of the Innate Immune System in Wear Debris-Induced Inflammatory Peri-Implant Osteolysis in Total Joint Arthroplasty. Bioengineering 2022, 9, 764. [Google Scholar] [CrossRef]
- Öztürk, O.; Çolak, İ.; Eroglu, A.E. Metal ion release from nitrogen ion implanted CoCrMo orthopedic implant material. Mater. Sci. Eng. C 2014, 45, 1–7. [Google Scholar] [CrossRef]
- Ganjali, M.; Yazdanpanah, A.; Mozafari, M. Laser Deposition of Nano Coatings on Biomedical Implants. In Emerging Applications of Nanoparticles and Architecture Nanostructures; Elsevier: Amsterdam, The Netherlands, 2018; pp. 235–254. [Google Scholar] [CrossRef]
- Ghayad, I.M.; Girgis, N.N.; Ghanem, W.A. Laser surface treatment of metal implants: A review article. J. Metall. Eng. 2015, 4, 48–56. [Google Scholar] [CrossRef]
- Wang, D.; Wu, S.; Yang, Y.; Huang, S.; Luo, Z. Effects of laser polishing on surface microstructure and corrosion resistance of additive manufactured CoCr alloys. Appl. Surf. Sci. 2018, 443, 167–175. [Google Scholar] [CrossRef]
- Liu, D.; Kong, D. Effects of Laser Remelting on Microstructure and Tribological Properties of Plasma–Sprayed Fe45Mn35Co10Cr10 High–Entropy Alloy Coating. Surf. Coat. Technol. 2023, 474, 129202. [Google Scholar] [CrossRef]
- Lohberger, B.; Stuendl, N.; Glaenzer, D.; Rinner, B.; Donohue, N.; Lichtenegger, H.C.; Ploszczanski, L.; Leithner, A. CoCrMo surface modifications affect biocompatibility, adhesion, and inflammation in human osteoblasts. Sci. Rep. 2020, 10, 1682. [Google Scholar] [CrossRef]
- Walker, J.C.; Cook, R.B.; Murray, J.W.; Clare, A.T. Pulsed electron beam surface melting of CoCrMo alloy for biomedical applications. Wear 2013, 301, 250–256. [Google Scholar] [CrossRef]
- Ju, J.; Zhou, Y.; Kang, M.; Wang, J. Optimization of process parameters, microstructure, and properties of laser cladding Fe-based alloy on 42CrMo steel roller. Materials 2018, 11, 2061. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.W.; Lee, J.H.; Joo, S.-H.; Kim, R.E.; Kim, H.S.; Moon, J. Effect of laser surface treatment on microstructural evolution and mechanical properties of a Co–Cr–Fe–Ni–Mo medium–entropy alloy. Mater. Sci. Eng. A 2025, 922, 147617. [Google Scholar] [CrossRef]
- Li, D.; Zhou, Y.; Chen, P.; Li, C.; Zhang, J.; Li, Z.; Zhou, Z.; Li, M.; Xu, Y. Polylactic Acid/Calcium Silicate Composite Scaffold Fabricated by Selective Laser Sintering with Coordinated Regulation of Bioactivity Induction, Degradation, and Mechanical Enhancement. J. Polym. Environ. 2025, 33, 1778–1791. [Google Scholar] [CrossRef]
- Pakieła, W.; Tański, T.; Brytan, Z.; Chladek, G.; Pakieła, K. The impact of laser surface treatment on the microstructure, wear resistance and hardness of the AlMg5 aluminum alloy. Appl. Phys. A 2020, 126, 231. [Google Scholar] [CrossRef]
- Furlani, M.R.; de Carvalho, S.M.; de Lima, M.S.F. Effect of laser surface hardening on a low carbon steel substrate wear and hardness. arXiv 2021, arXiv:2111.13183. [Google Scholar] [CrossRef]
- Rapheal, G.; Kumar, S.; Blawert, C.; Dahotre, N.B. Wear-resistant thin films of MRI-230D-Mg alloy using plasma-driven electrolytic oxidation. arXiv 2017, arXiv:1705.00116. [Google Scholar] [CrossRef]
- Acharya, S.; Soni, R.; Suwas, S.; Chatterjee, K. Additive manufacturing of Co–Cr alloys for biomedical applications: A concise review. J. Mater. Res. 2021, 36, 3746–3760. [Google Scholar] [CrossRef]
- Hedberg, Y.S.; Qian, B.; Shen, Z.; Virtanen, S.; Wallinder, I.O. In vitro biocompatibility of CoCrMo dental alloys fabricated by selective laser melting. Dent. Mater. 2014, 30, 525–534. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Buciumeanu, M.; Costa, E.; Alves, N.; Gasik, M.; Silva, F.S.; Miranda, G. Multi-material Ti6Al4V & PEEK cellular structures produced by selective laser melting and hot pressing: A tribocorrosion study targeting orthopedic applications. J. Mech. Behav. Biomed. Mater. 2019, 89, 54–64. [Google Scholar] [CrossRef]
- Rezayat, M.; Besharatloo, H.; Mateo, A. Investigating the Effect of Nanosecond Laser Surface Texturing on Microstructure and Mechanical Properties of AISI 301LN. Metals 2023, 13, 2021. [Google Scholar] [CrossRef]
- Research and Markets. Microelectronic Medical Implants Market Report: Trends, Forecast and Competitive Analysis to 2030; ID: 6007972; Lucintel: Dallas, TX, USA, 2024. [Google Scholar]
- Mantrala, K.M.; Das, M.; Balla, V.K.; Rao, C.S.; Rao, V.V.S. Laser-deposited CoCrMo alloy: Microstructure, wear, and electrochemical properties. J. Mater. Res. 2014, 29, 2021–2027. [Google Scholar] [CrossRef]
- ASTM F0075-23; Standard Specification for Castings, Wear-Resistant, Chromium Alloy (Class I and Class II). ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM G99-23; Standard Test Method for Wear and Friction Testing with a Pin-on-Disk or Ball-on-Disk Apparatus. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM F732-17; Standard Test Method for Wear Testing of Polymeric Materials Used in Total Joint Prostheses. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM E384-22; Standard Test Method for Microindentation Hardness of Materials. ASTM International: West Conshohocken, PA, USA, 2022.
- Engel, S.L. Laser Welding Technology Learning Manual; HDE Technologies, Inc.: Elk Grove, CA, USA, 2016; ISBN 9780997766394. [Google Scholar]
- Majumdar, J.D.; Manna, I. Laser-Assisted Fabrication of Materials; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3642280290. [Google Scholar]
- Steen, W.M.; Mazumder, J. Laser Material Processing, 4th ed.; Springer: London, UK, 2010; ISBN 978-1-84996-062-5. [Google Scholar] [CrossRef]
- Ion, J.C. Laser Processing of Engineering Materials: Principles, Procedure and Industrial Application; Butterworth-Heinemann: Oxford, UK, 2005; ISBN 978-0750660794. [Google Scholar]
- Ready, J.F. LIA Handbook of Laser Materials Processing; Laser Institute of America, Magnolia Publishing: Orlando, FL, USA, 2001. [Google Scholar]
- Li, B.; Chen, J.; Zhang, Y. A Universal Theoretical Model for Thermal Accumulation in Materials Induced by Repetitive Pulsed Laser Irradiation. Int. J. Laser Sci. 2019, 1, 53–71. [Google Scholar] [CrossRef]
- Chen, G.; Gu, X.; Bi, J. Numerical Analysis of Thermal Effect in Aluminum Alloy by Repetition Frequency Pulsed Laser. Optik 2016, 127, 6371–6378. [Google Scholar] [CrossRef]
- Taylor, R.N.J.; Waterhouse, R.B. A Study of the Ageing Behavior of a Cobalt Based Implant Alloy. J. Mater. Sci. 1983, 18, 3265–3280. [Google Scholar] [CrossRef]
- Hsu, H.C.; Lian, S.S. Wear Properties of Co–Cr–Mo–N Plasma-Melted Surgical Implant Alloy. J. Mater. Process. Technol. 2003, 138, 231–235. [Google Scholar] [CrossRef]
- Saldivar-García, A.J.; López, H.F. Microstructural Effects on the Wear Resistance of Wrought and As-Cast Co-Cr-Mo-C Implant Alloys. J. Biomed. Mater. Res. A 2005, 74A, 269–274. [Google Scholar] [CrossRef]
- Huang, P.; López, H.F. Tribological Behavior of Cast and Wrought Co-Cr-Mo Implant Alloys. Mater. Sci. Technol. 1999, 15, 1324–1329. [Google Scholar] [CrossRef]
- Montero-Ocampo, C.; Talavera, M.; López, H.F. Effect of Alloy Preheating on the Mechanical Properties of As-Cast Co-Cr-Mo-C Alloys. Metall. Mater. Trans. A 1999, 29A, 611–620. [Google Scholar] [CrossRef]
- King, W.E.; Anderson, A.T.; Ferencz, R.M.; Hodge, N.E.; Kamath, C.; Khairallah, S.A. Laser Powder Bed Fusion Additive Manufacturing of Metals; Physics, Computational, and Materials Challenges. Appl. Phys. Rev. 2015, 2, 041304. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive Manufacturing of Metallic Components—Process, Structure and Properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Han, T.; Chen, J.; Wei, Z.; Qu, N.; Liu, Y.; Yang, D.; Zhao, S.; Lai, Z.; Jiang, M.; Zhu, J. Effect of cooling rate on microstructure and mechanical properties of AlCrFe₂Ni₂ medium entropy alloy fabricated by laser powder bed fusion. J. Mater. Res. Technol. 2023, 25, 4063–4073. [Google Scholar] [CrossRef]
- Zhao, C.; Jia, X.; Zhao, Q.; Ma, H.; Yu, Z.; Zhang, Z. Research Progress on the Effect of Surface Texture on the Friction Properties of CoCrMo Alloys. Compos. Interfaces 2024, 31, 1269–1290. [Google Scholar] [CrossRef]
- Takaichi, A.; Suyalatu, T.; Nakamoto, N.; Joko, N.; Nomura, N.; Tsutsumi, Y.; Migita, S.; Doi, H.; Kurosu, S.; Chiba, A.; et al. Microstructures and mechanical properties of Co-29Cr-6Mo alloy fabricated by selective laser melting process for dental applications. J. Mech. Behav. Biomed. Mater. 2013, 21, 67–76. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Sampaio, M.; Carvalho, O.; Pinto, E.; Alves, N.; Silva, F.S. Tribological behavior of Ti6Al4V cellular structures produced by Selective Laser Melting. J. Mech. Behav. Biomed. Mater. 2017, 69, 128–134. [Google Scholar] [CrossRef]
- Redey, S.A.; Razzou, S.; Rey, C.; Bernache-Assollant, D.; Leroy, G.; Nordin, M.; Cournot, G. Osteoclast adhesion and activity on synthetic hydroxyapatite, carbonated hydroxyapatite and calcium carbonate: Relationship to surface energies. J. Biomed. Mater. Res. 1990, 24, 163–177. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphates in Nature, Biology and Medicine. Materials 2009, 3, 3510–3606. [Google Scholar] [CrossRef]
- Román, J.; Padilla, S.; Vallet-Regi, M. Sol-gel glasses as precursors of bioactive glass ceramics. Chem. Mater. 2003, 15, 798–806. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium Phosphates in Biomedical Applications: Materials for the Future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]


| C | S | Mn | Si | P | Cr | Ni | Mo | W | Fe | Co | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ASTM F75/2023 standard | 0.35 | 0.004 | 1.00 | 1.00 | 0.005 | 27.00–30.00 | 1.00 | 5.00–7.00 | 0.04 | 0.75 | Bal |
| max. | max. | max. | max. | max. | max. | max. | max. | ||||
| Cast samples | 0.23 | 0.002 | 0.21 | 0.66 | 0.0014 | 29.18 | 0.43 | 5.42 | 0.02 | 0.38 | Bal |
| Peak Power (watts) | Pulse Width (ms) | Pulse Energy (J) | |
| Sample 1 | 4875 | 5 | 24.37 |
| Sample 2 | 5250 | 6 | 31.5 |
| Sample 3 | 5650 | 7 | 39.37 |
| Microhardness (Vickers) | |
|---|---|
| CS | 325.2 |
| Sample 1 | 416.5 |
| Sample 2 | 435.8 |
| Sample 3 | 445.6 |
| Sample | Wear Rate × 10−4 (mm³/Nm) |
|---|---|
| CS | 1.77 |
| S1 | 1.40 |
| S2 | 1.02 |
| S3 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cepeda Rodríguez, F.; Muñiz Valdez, C.R.; Ortiz Cuellar, J.C.; Martínez Villafañe, J.F.; Galindo Valdés, J.S.; Pérez Medina, G.Y. Influence of Nd:YAG Laser Melting on an Investment-Casting Co-Cr-Mo Alloy. Metals 2025, 15, 385. https://doi.org/10.3390/met15040385
Cepeda Rodríguez F, Muñiz Valdez CR, Ortiz Cuellar JC, Martínez Villafañe JF, Galindo Valdés JS, Pérez Medina GY. Influence of Nd:YAG Laser Melting on an Investment-Casting Co-Cr-Mo Alloy. Metals. 2025; 15(4):385. https://doi.org/10.3390/met15040385
Chicago/Turabian StyleCepeda Rodríguez, Francisco, Carlos Rodrigo Muñiz Valdez, Juan Carlos Ortiz Cuellar, Jesús Fernando Martínez Villafañe, Jesús Salvador Galindo Valdés, and Gladys Yerania Pérez Medina. 2025. "Influence of Nd:YAG Laser Melting on an Investment-Casting Co-Cr-Mo Alloy" Metals 15, no. 4: 385. https://doi.org/10.3390/met15040385
APA StyleCepeda Rodríguez, F., Muñiz Valdez, C. R., Ortiz Cuellar, J. C., Martínez Villafañe, J. F., Galindo Valdés, J. S., & Pérez Medina, G. Y. (2025). Influence of Nd:YAG Laser Melting on an Investment-Casting Co-Cr-Mo Alloy. Metals, 15(4), 385. https://doi.org/10.3390/met15040385








