Combined Effect of the Sc/Zr Ratio and Mg Concentration on the Intergranular Corrosion Resistance of Al–Mg–Sc–Zr Alloys: A Case of Cast Alloys and Ultrafine-Grained Alloys
Abstract
1. Introduction
1.1. Brief Review
1.2. Aims and Objectives of This Study
2. Materials and Methods
3. Results
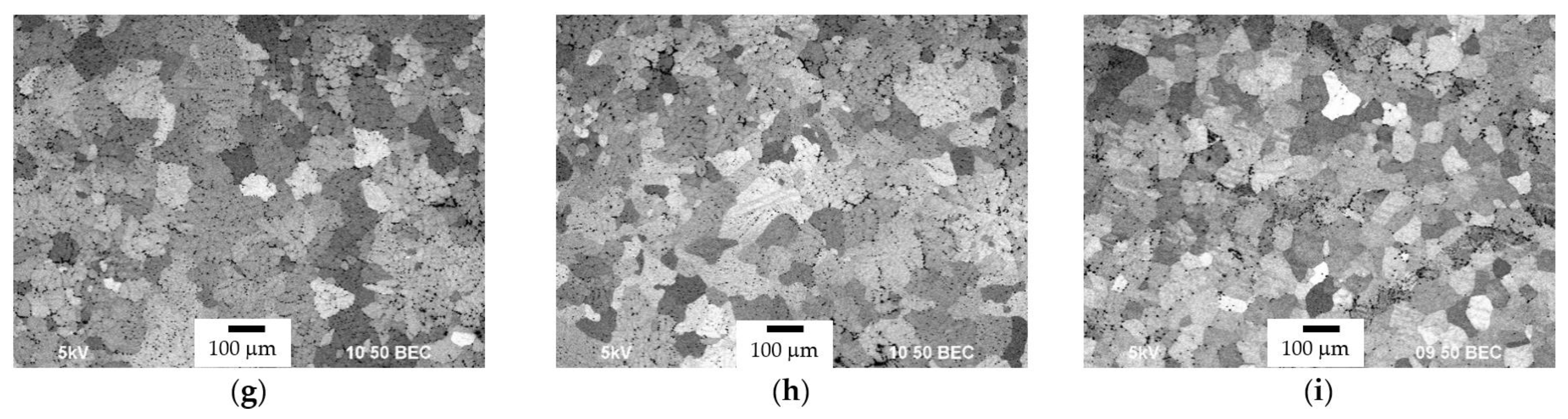
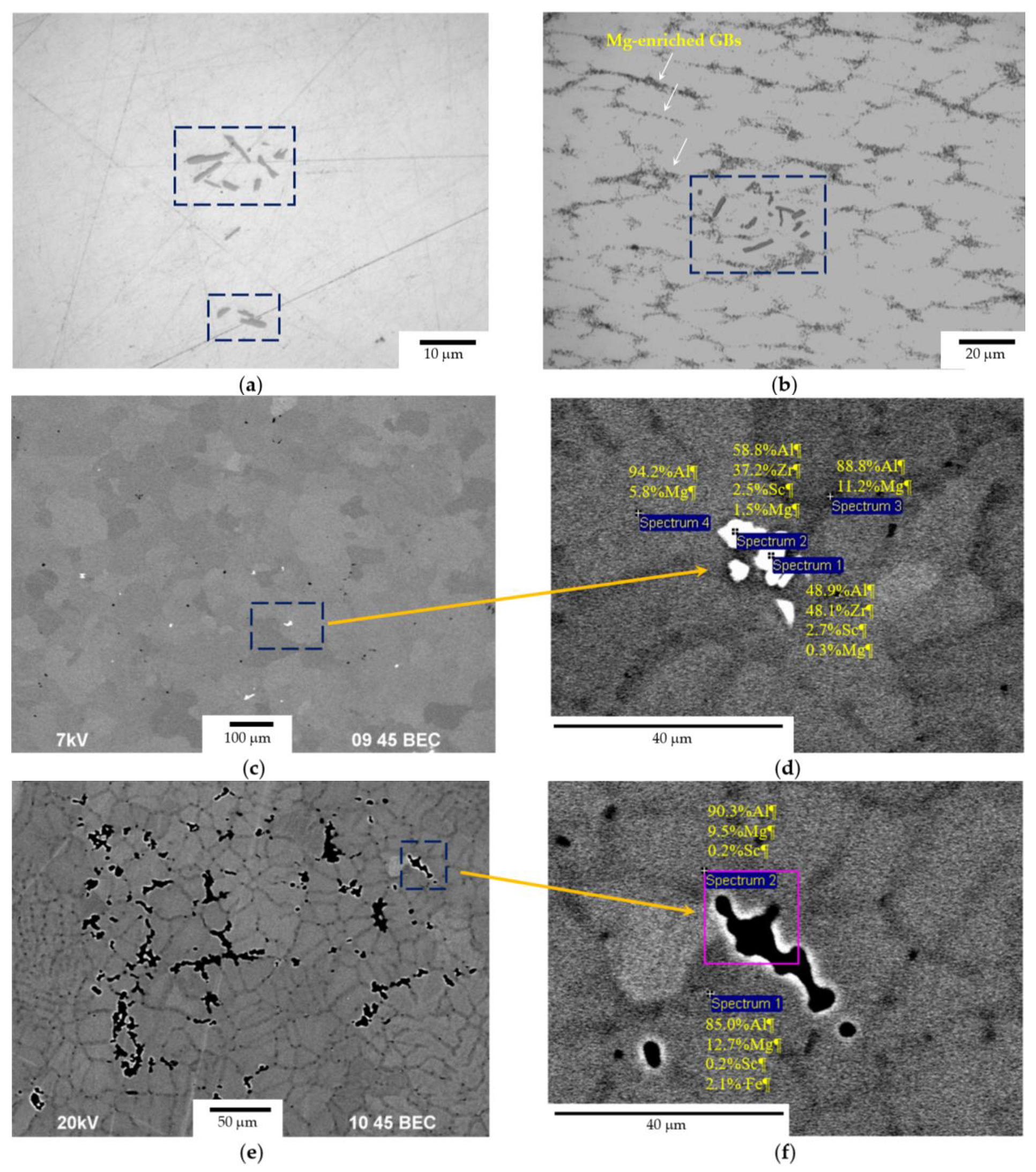
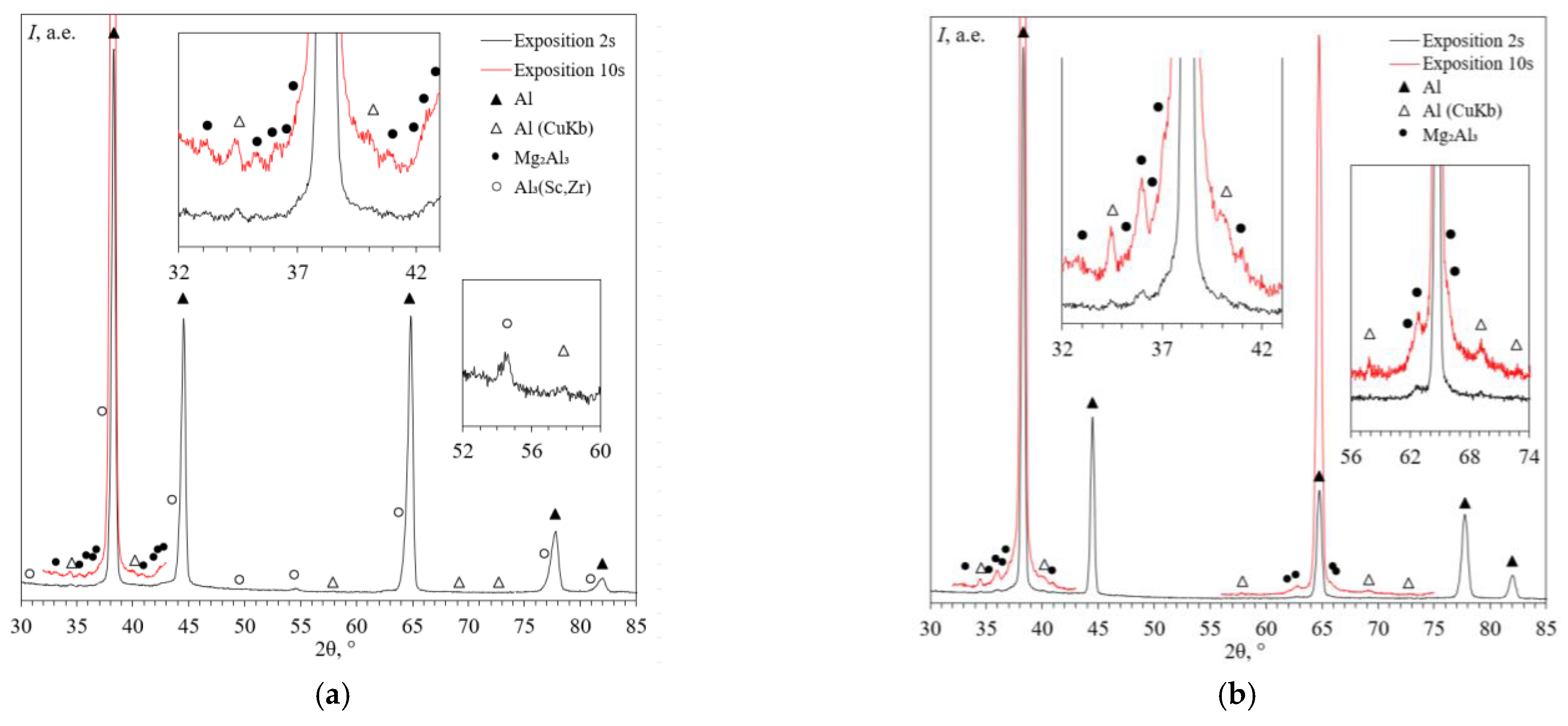
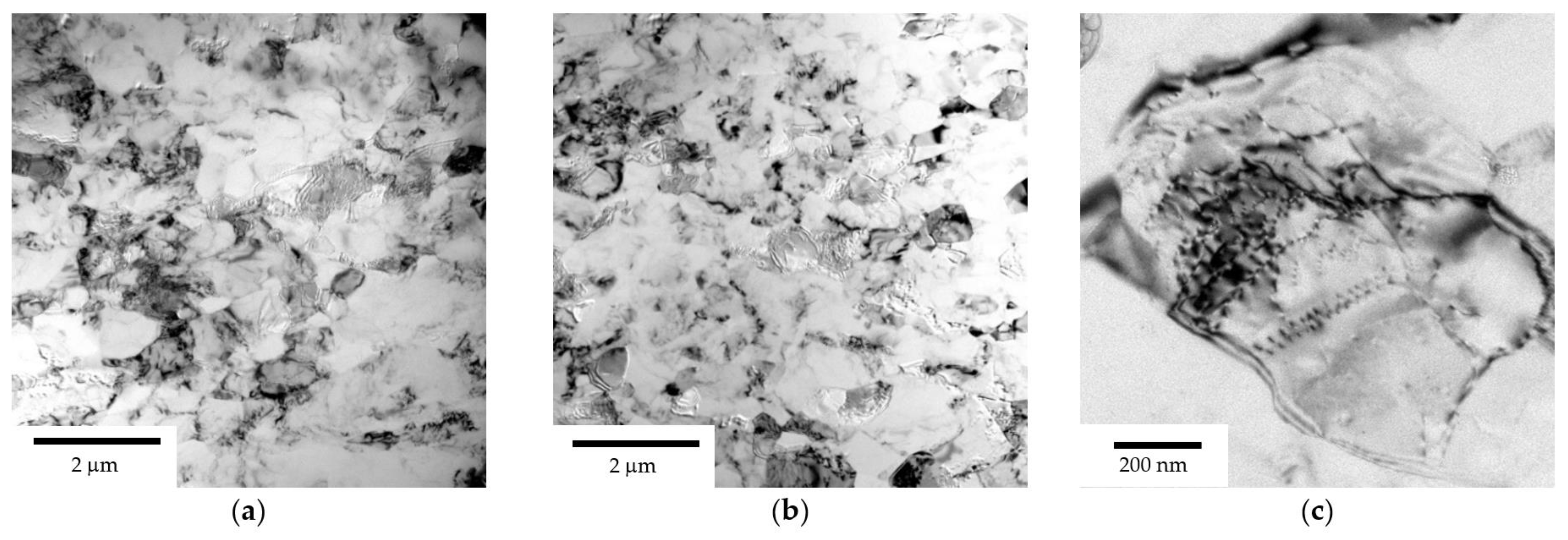
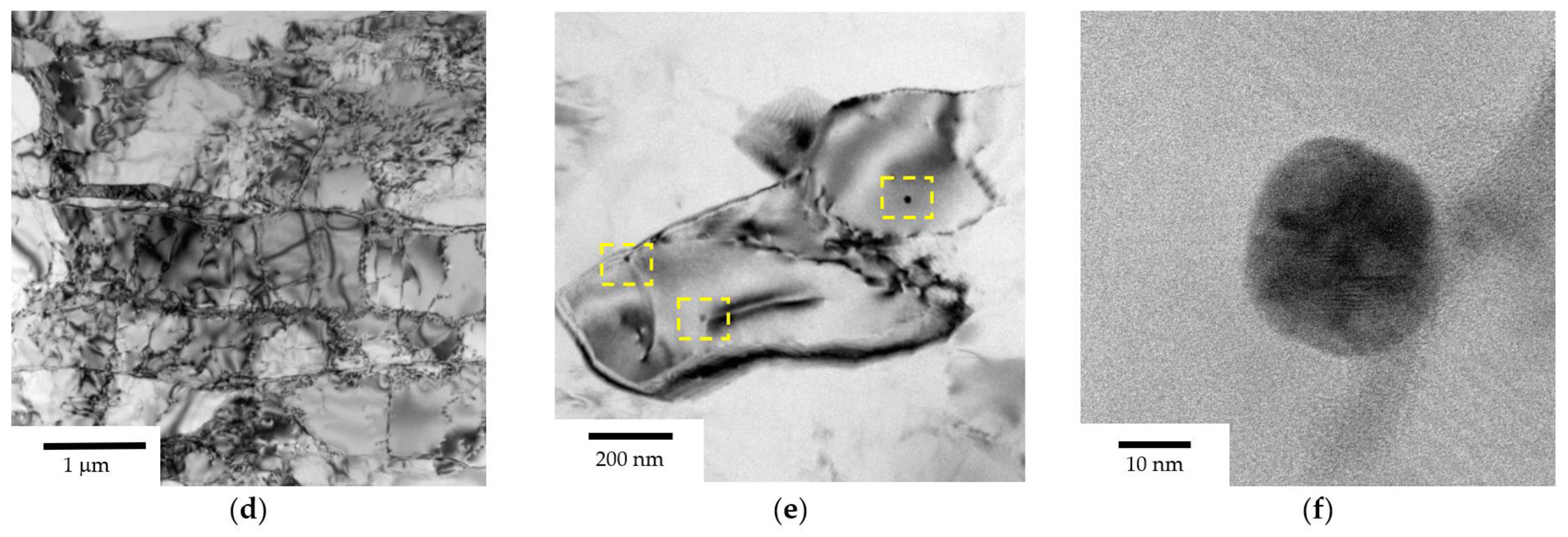
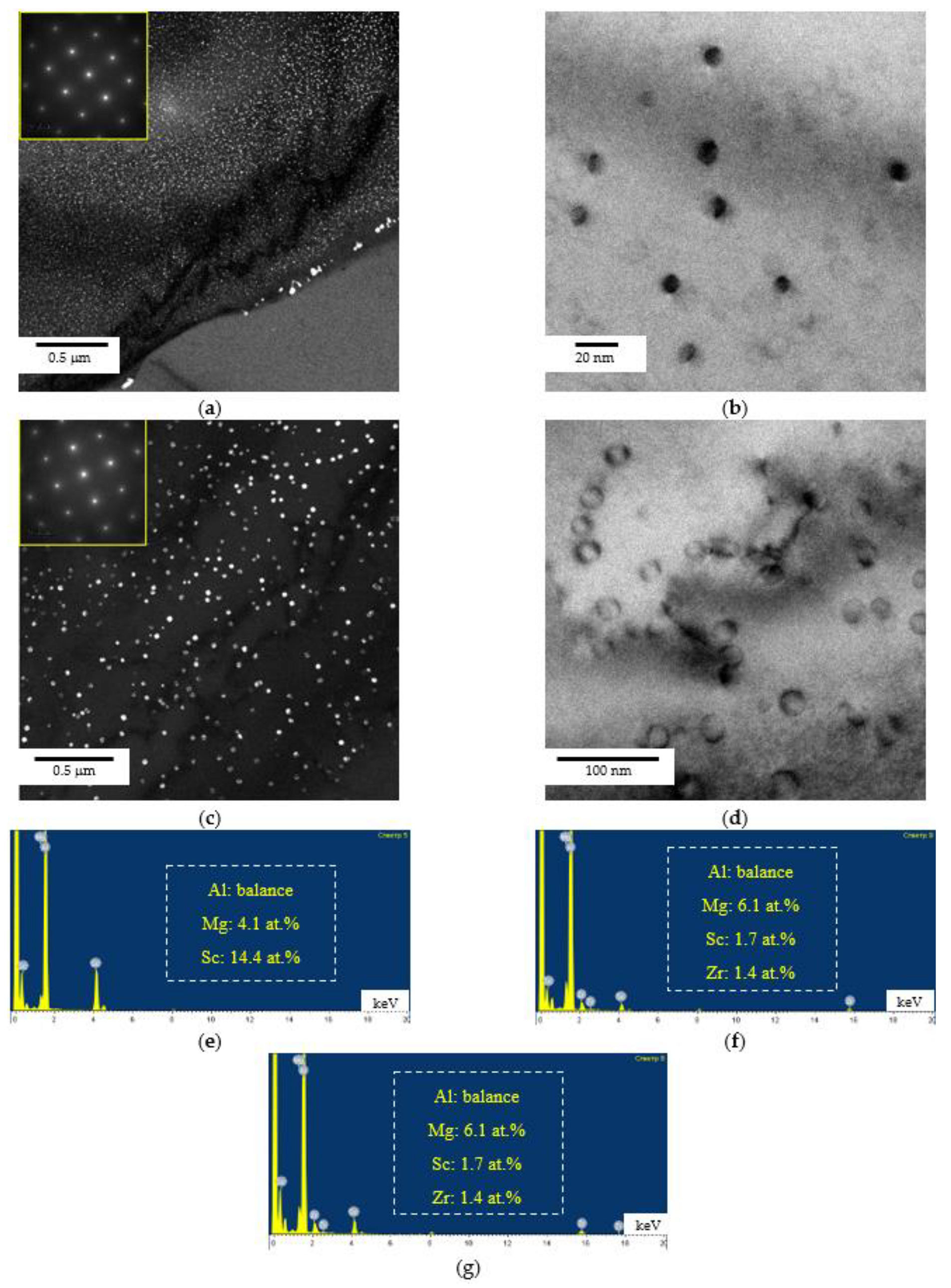
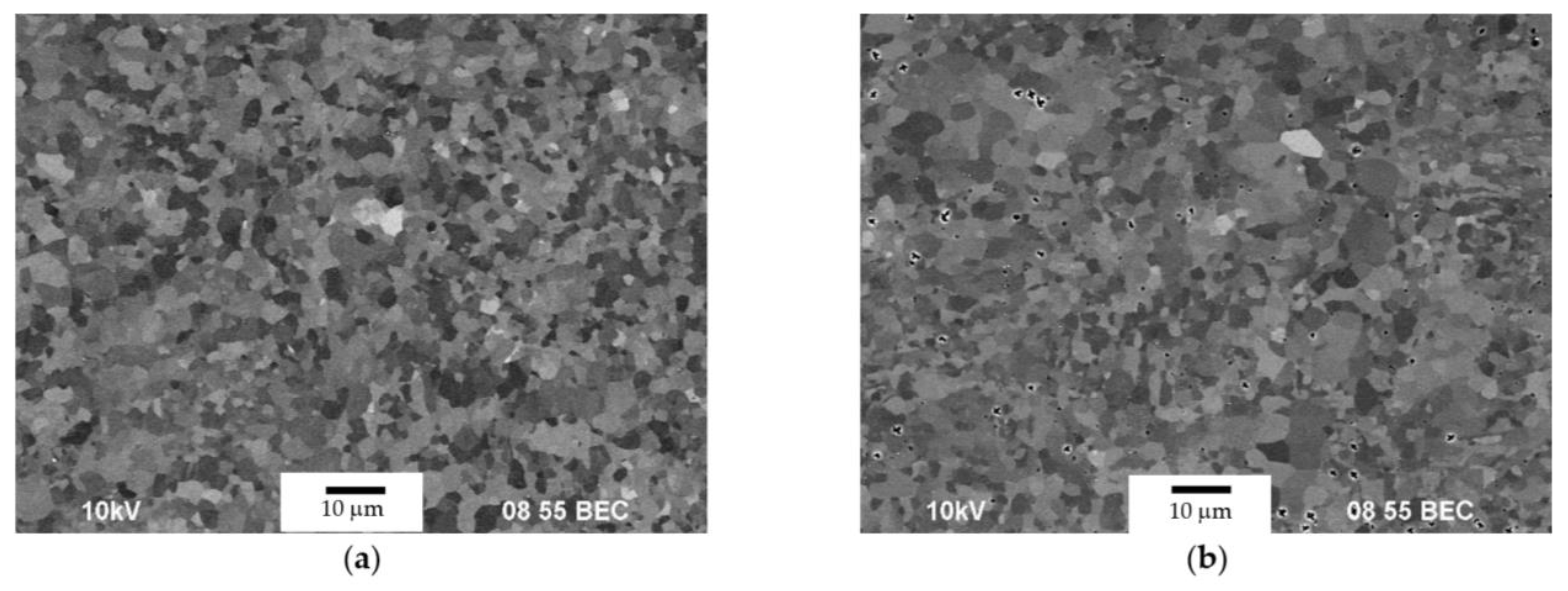
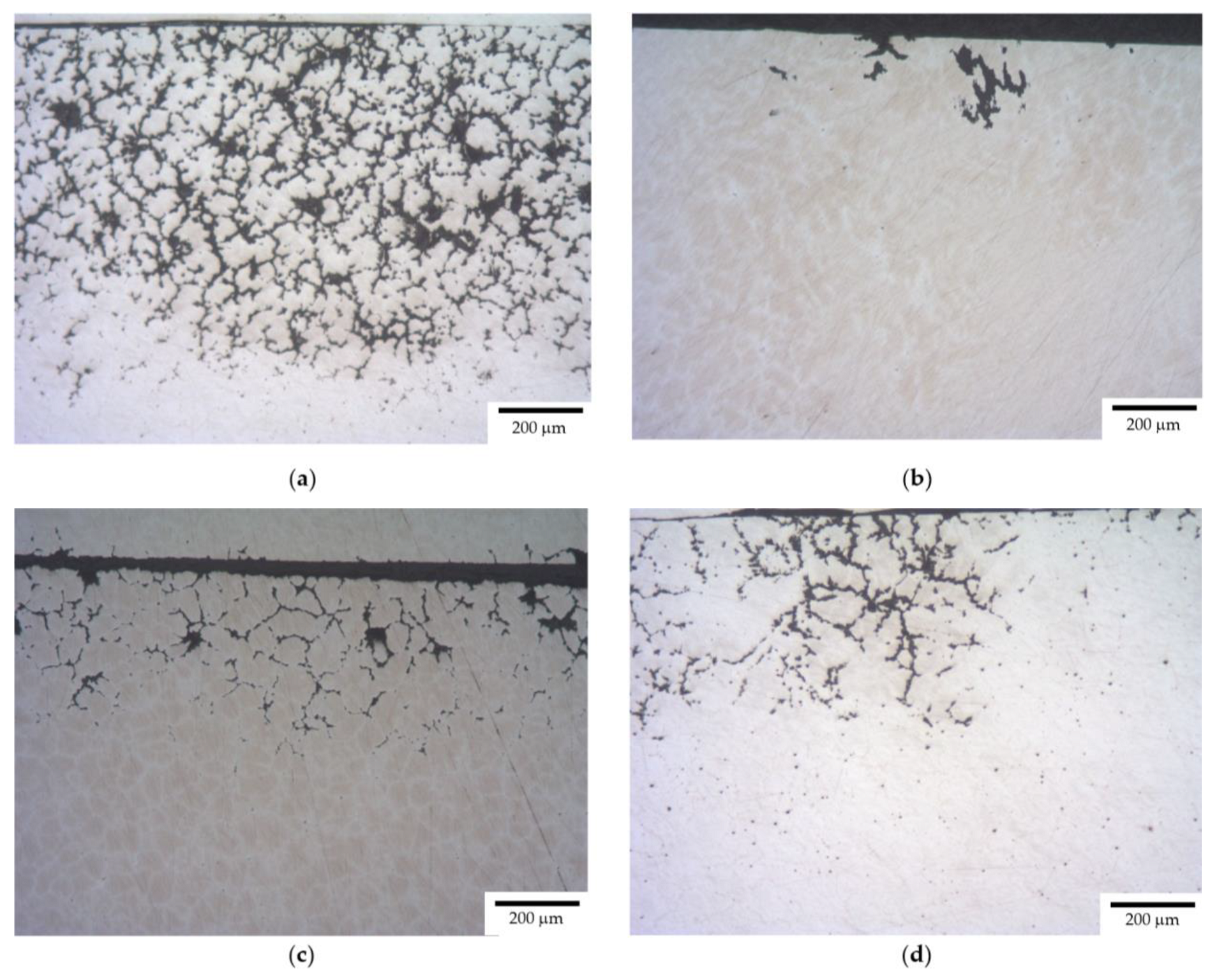
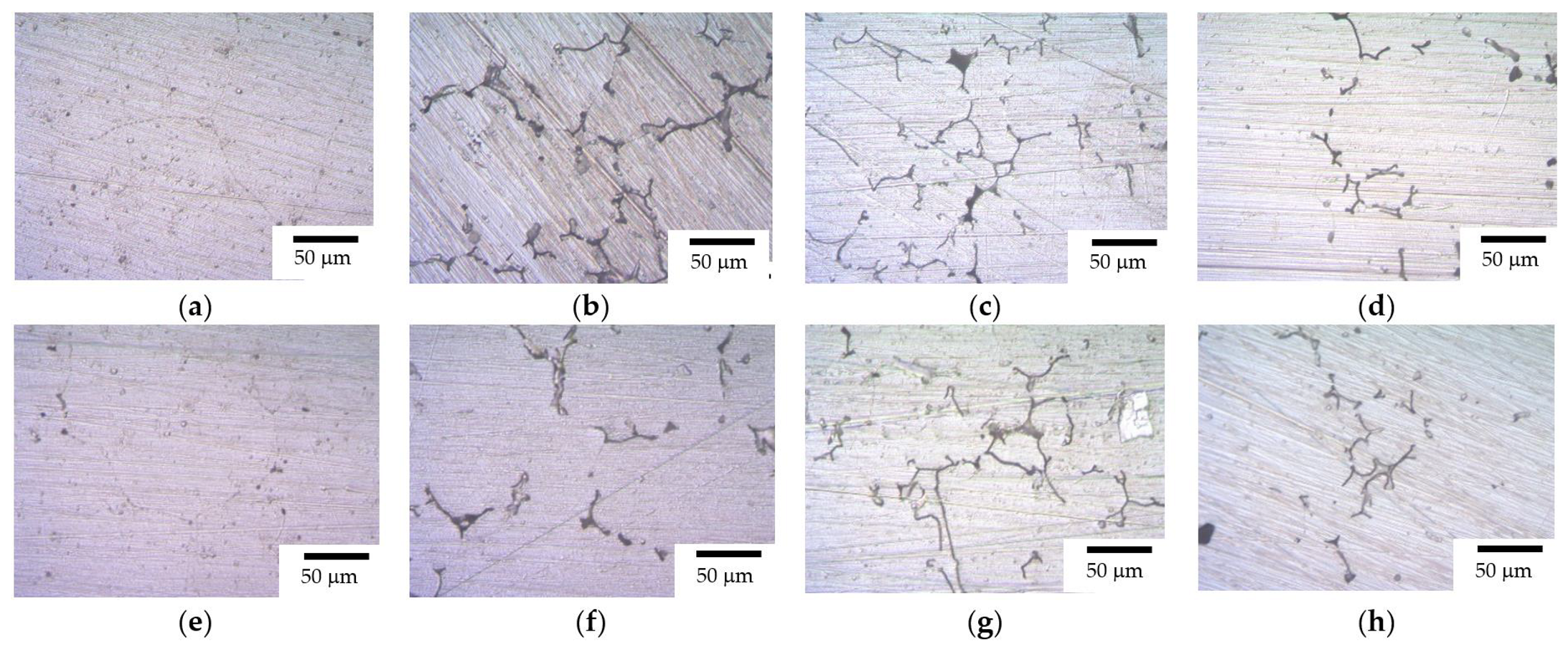
3.1. Microstructure of Alloys
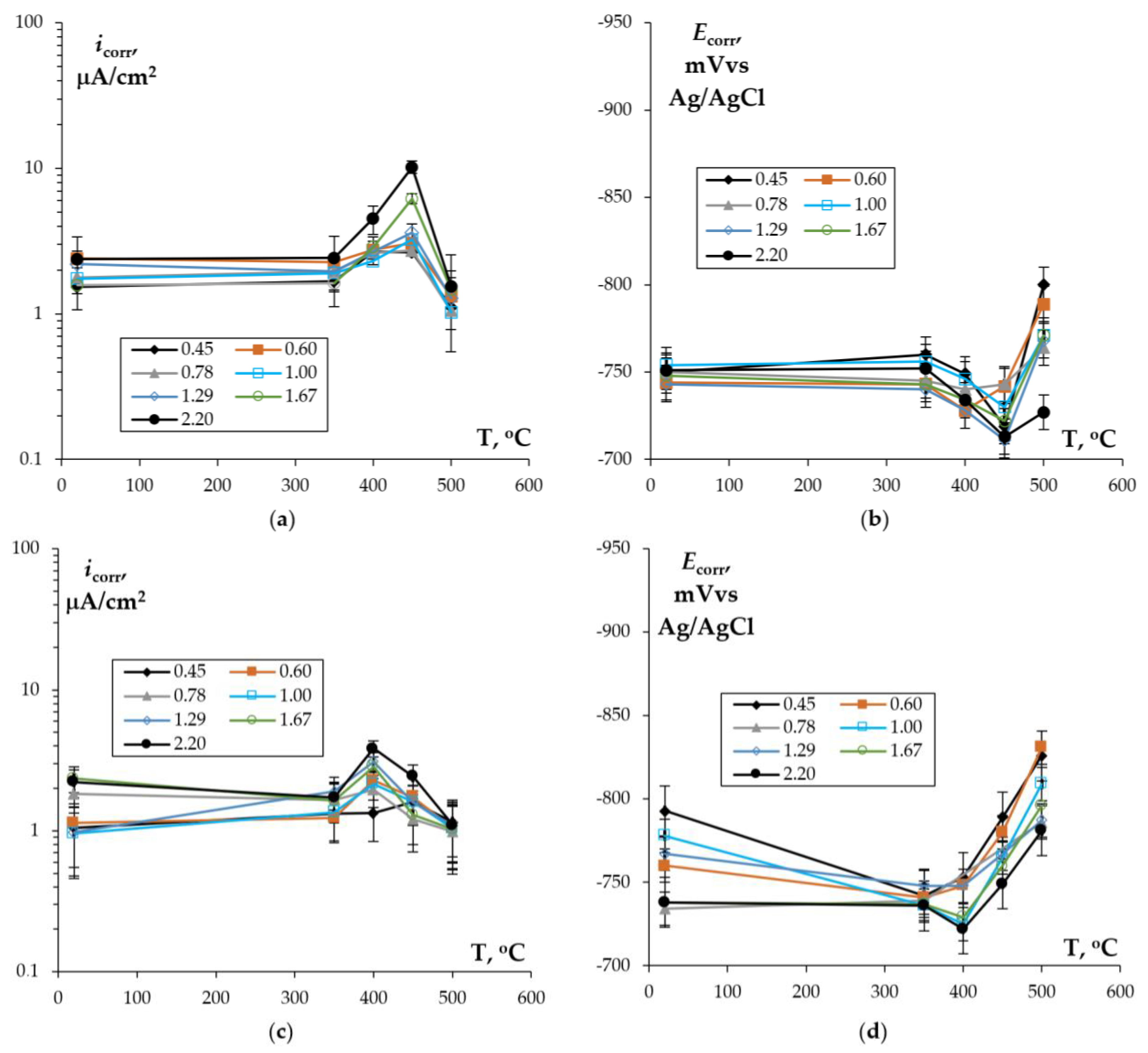
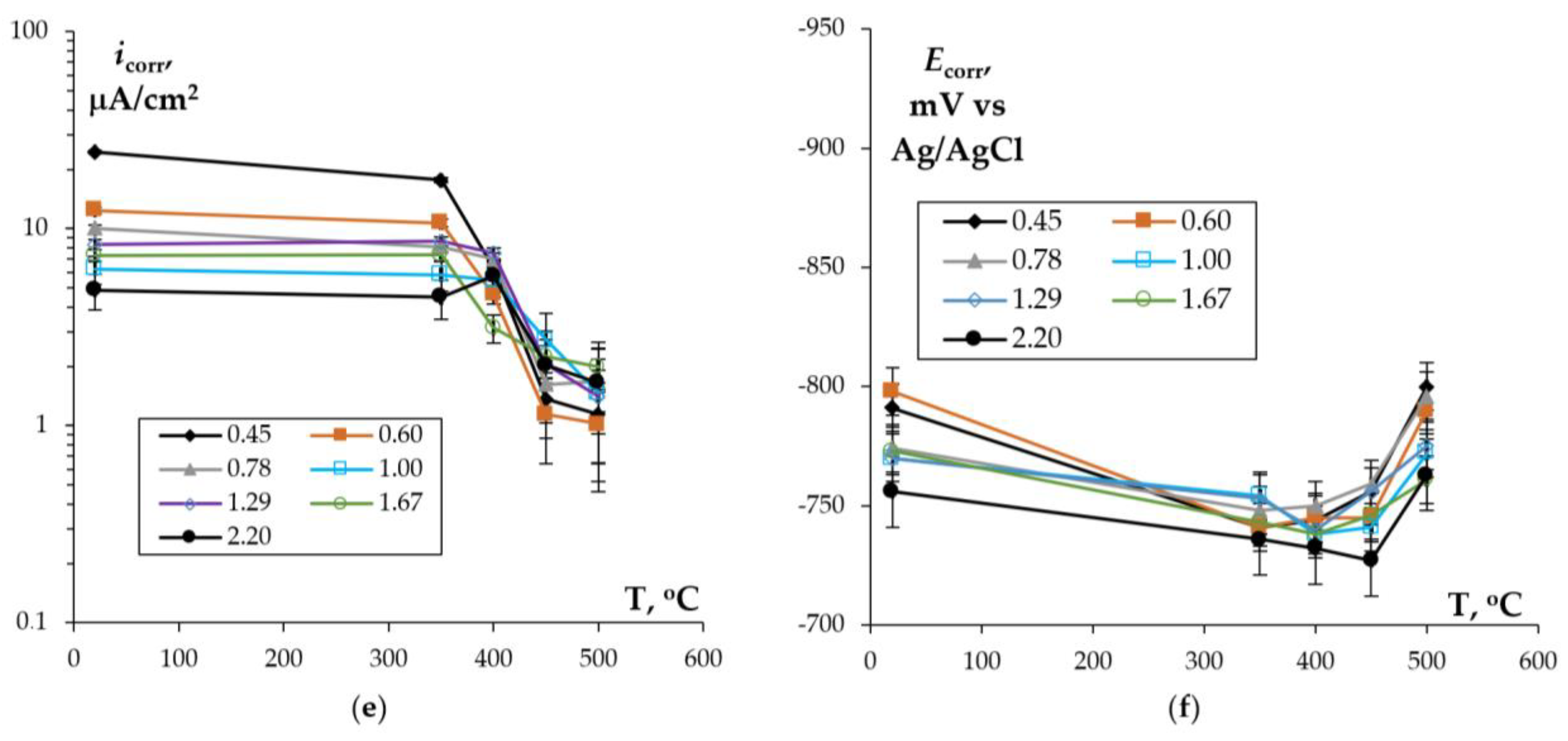
3.2. Corrosion Tests
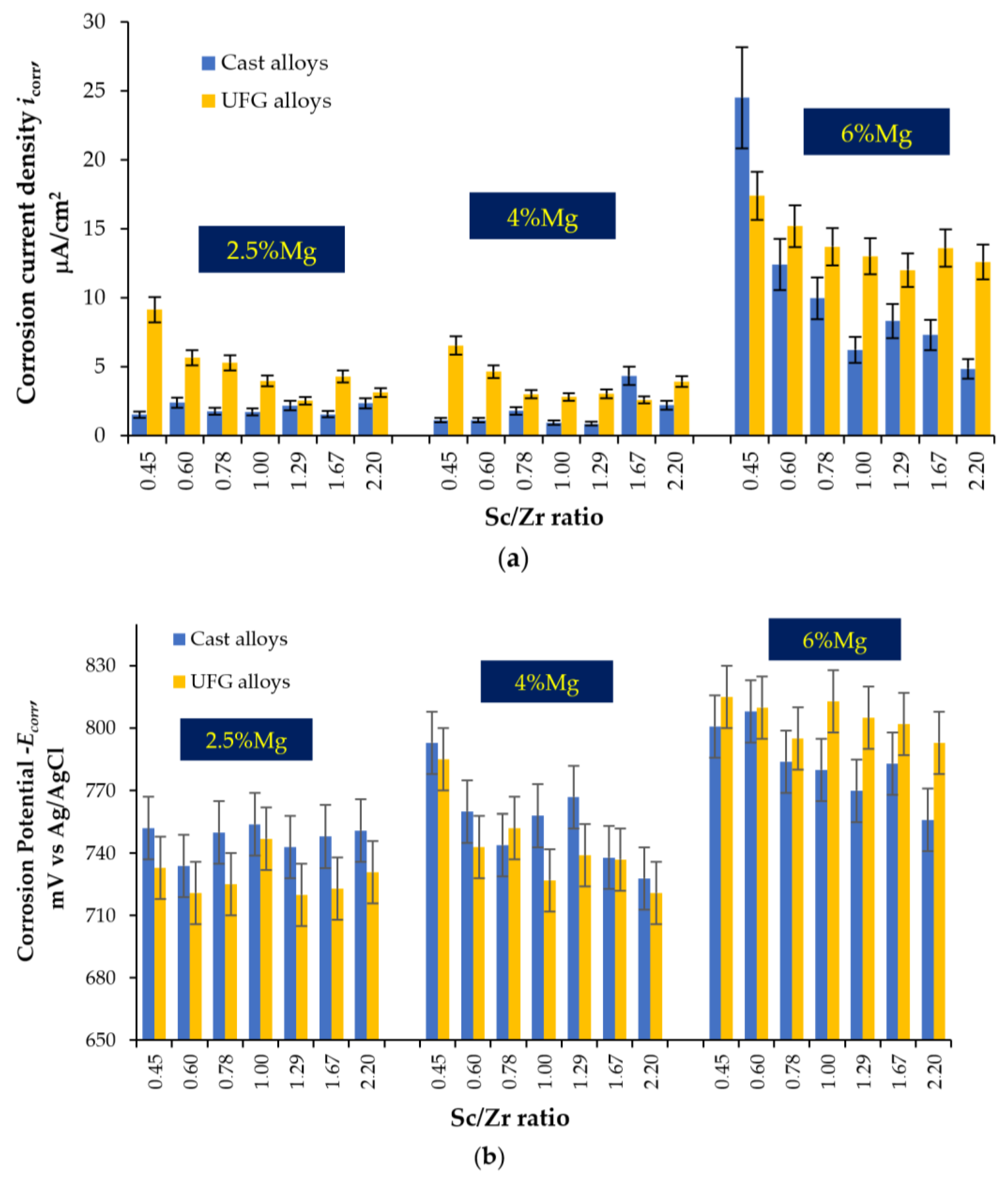
3.2.1. Effect of Mg Concentration and Sc/Zr Ratio: Unannealed Alloys
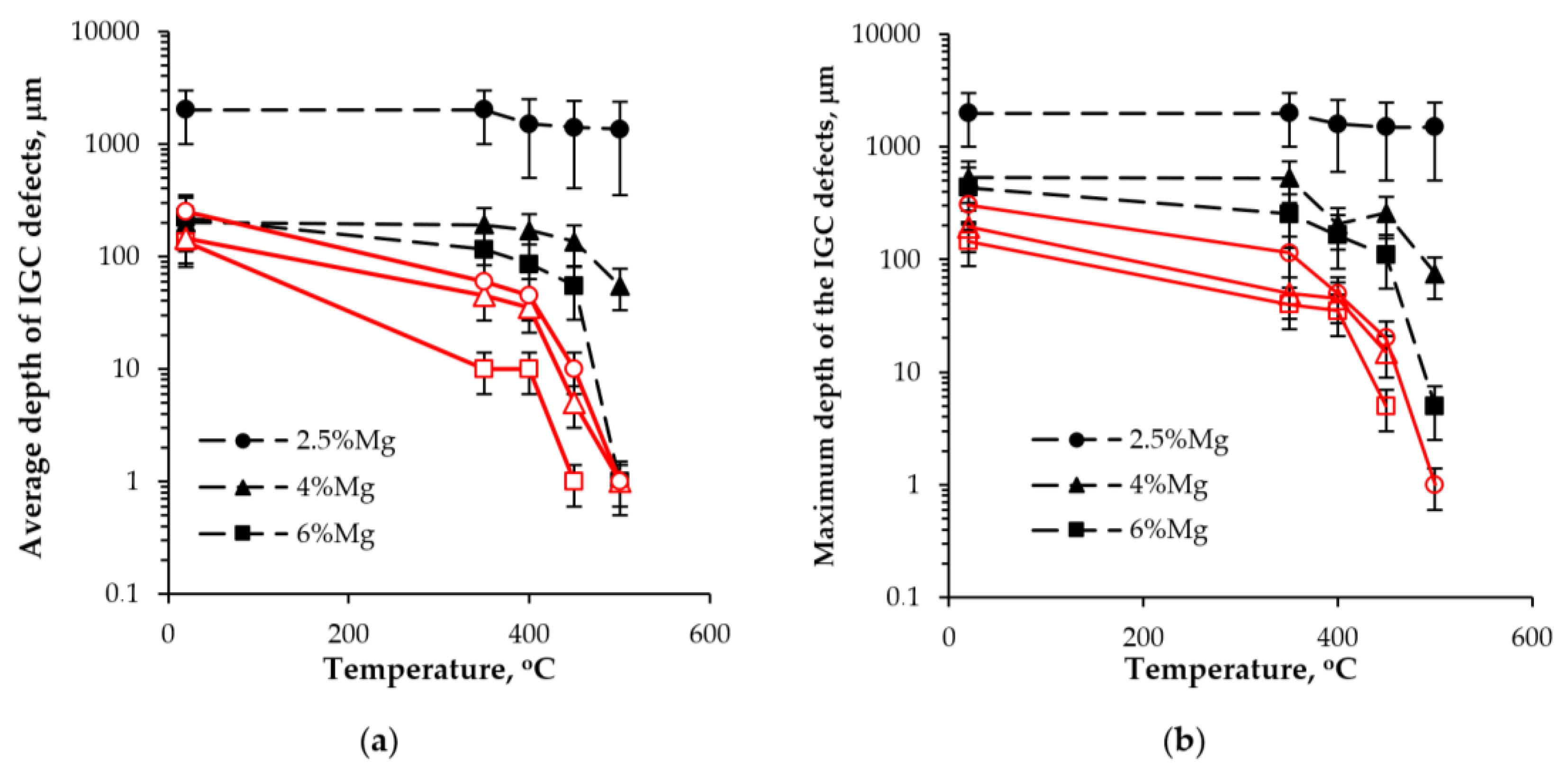
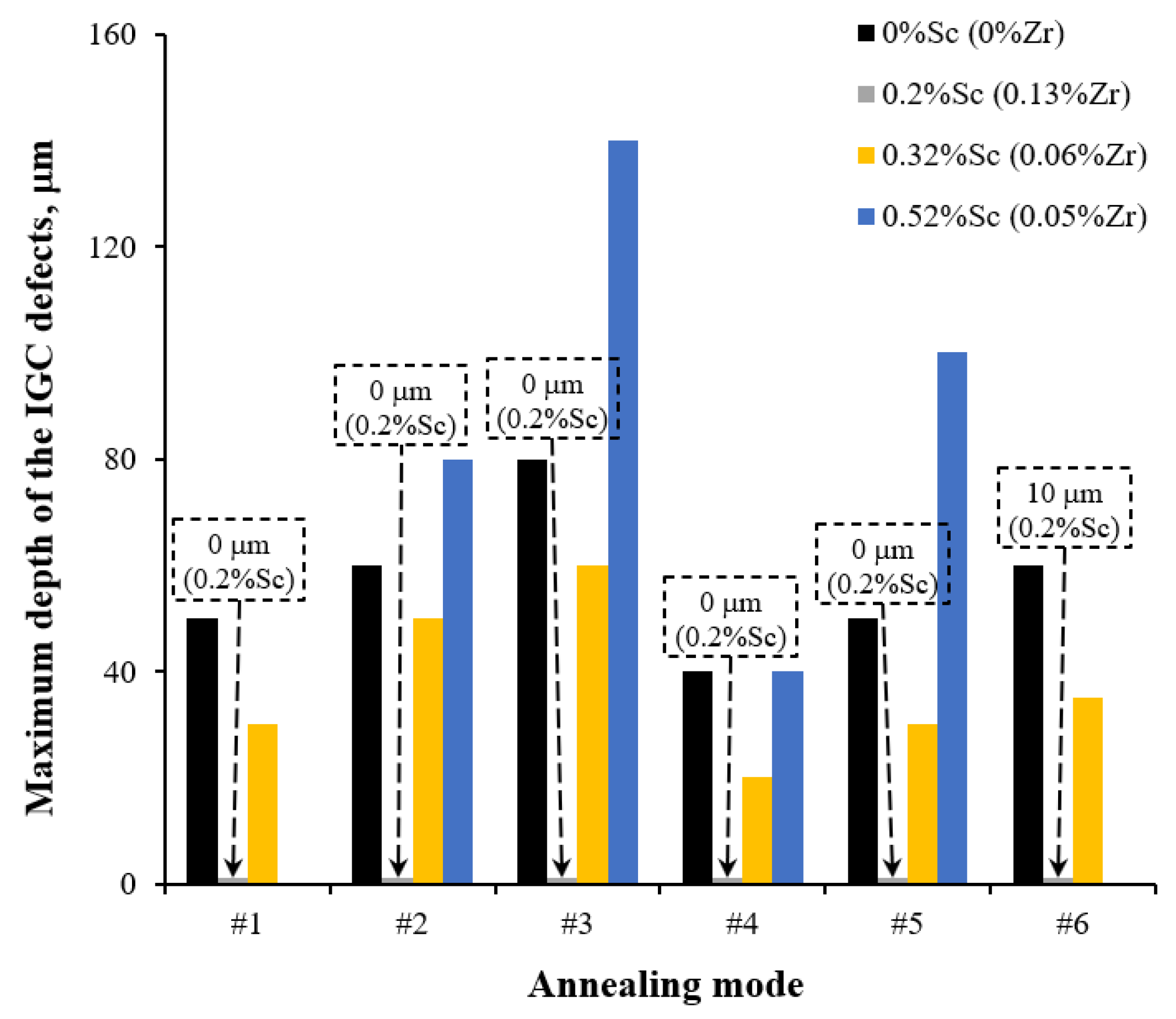
3.2.2. Effect of Annealing
4. Discussion
4.1. Features of Structure and Corrosion Behavior of Cast and UFG Alloys
- (i)
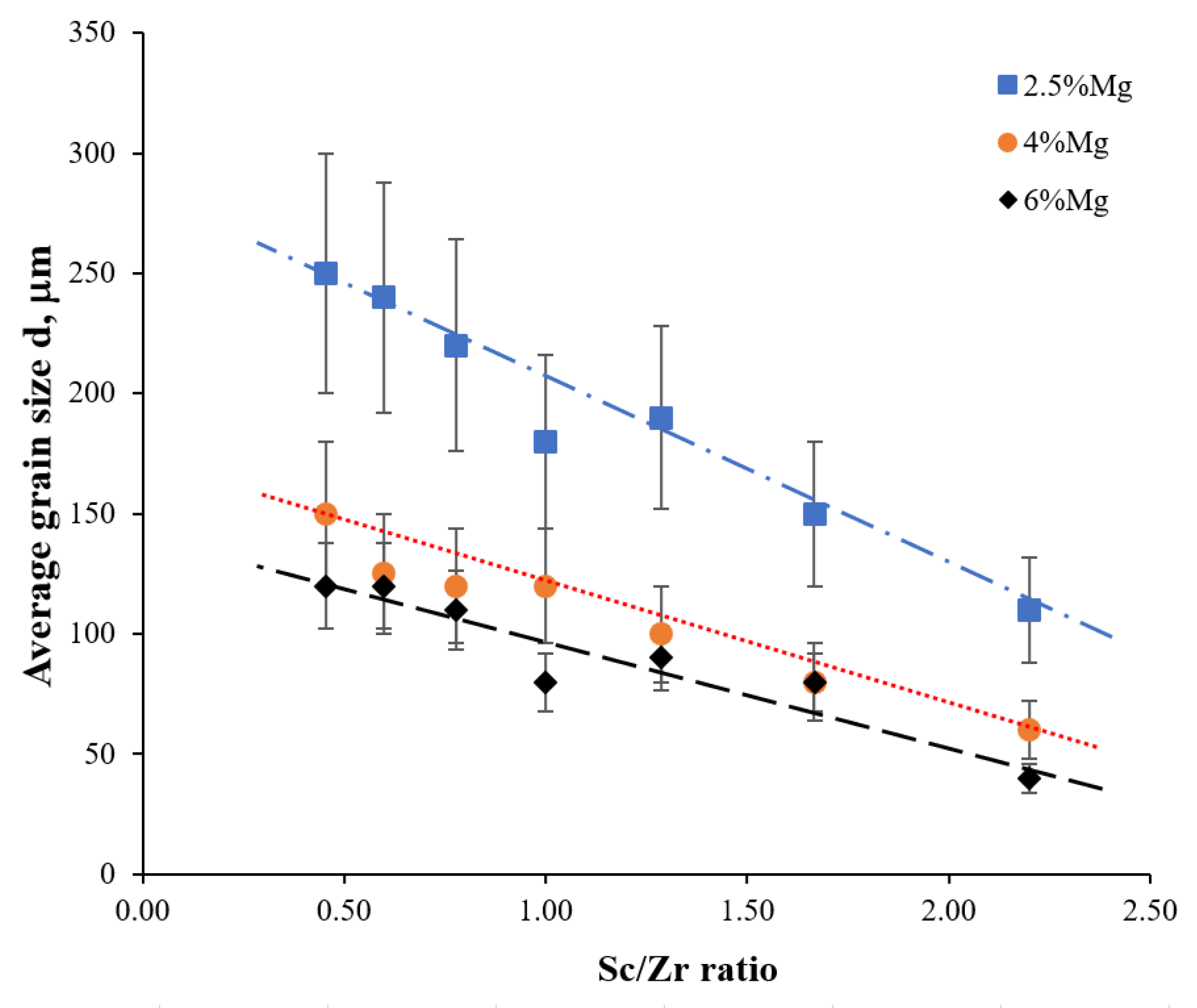
- The cast alloys have a non-uniform macrostructure. There are large dendrites, several millimeters long, located along the sample edges. There are small equiaxial grains in the central sample parts. An increase in the Mg concentration leads to an increase in the fraction of small equiaxial grains and a decrease in the average grain size. Primary Al3(Sc,Zr) particles act as the structure modifiers of Al alloys. Thus, an increase in the Sc/Zr ratio leads to a decrease in the average grain size. Large primary β-phase particles are observed along the GBs and in dendrites formed during cooling the ingots. Increasing the Mg concentration leads to an increase in the area of the GBs occupied by the primary β-phase particles.
- (ii)
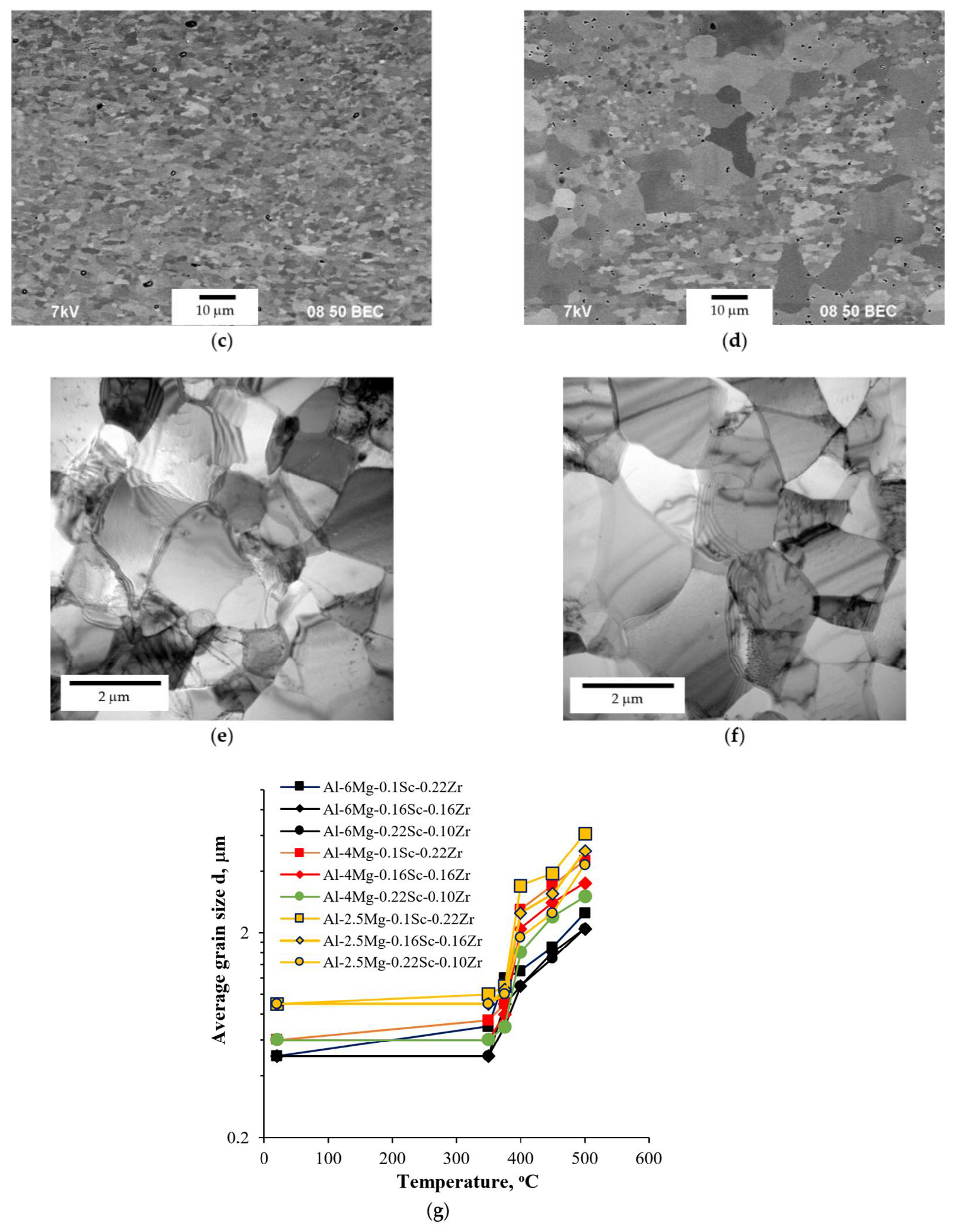
- Warm (250–275 °C) severe plastic deformation under ECAP leads to the formation of a homogeneous UFG microstructure. An increase in the Mg concentration from 2.5 to 6% leads to a slight decrease in the grain size from 0.6–0.8 μm to 0.3–0.5 μm, as well as to an increase in the microhardness and SER of the alloys.
- (iii)
- Heating the cast alloys up to 300–350 °C leads to the precipitation beginning of the secondary particles, particularly, Al3Sc particles, in the alloys with high Sc content. In the alloys with increased Zr content, the formation of the following three types of particles was observed: Al3Sc, Al3(Sc0.5Zr0.5), and submicron Al3Zr fan-shaped particles released near the GBs by the discontinuous precipitation mechanism. The formation of the UFG microstructure leads to a 50–100 °C decrease in the onset temperature of the secondary particle release depending on the Sc/Zr ratio. The secondary Al3(Sc,Zr) particles can be the formation centers of the secondary β-phase particles. A decrease in the Mg concentration leads to an increase in the volume fraction of the secondary Al3(Sc,Zr) particles but does not affect their formation nature. At annealing temperatures above 450 °C, partial diffusion-controlled dissolution of the β-phase particles was observed.
- (iv)
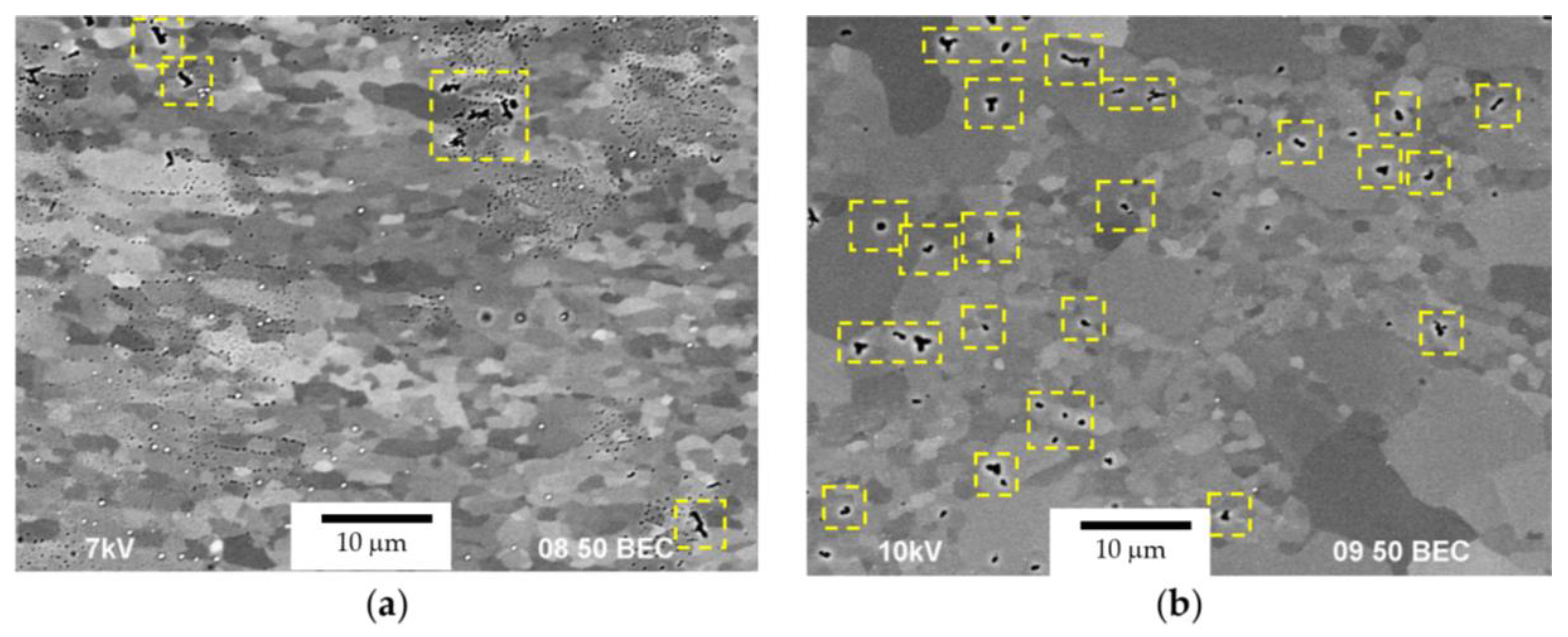
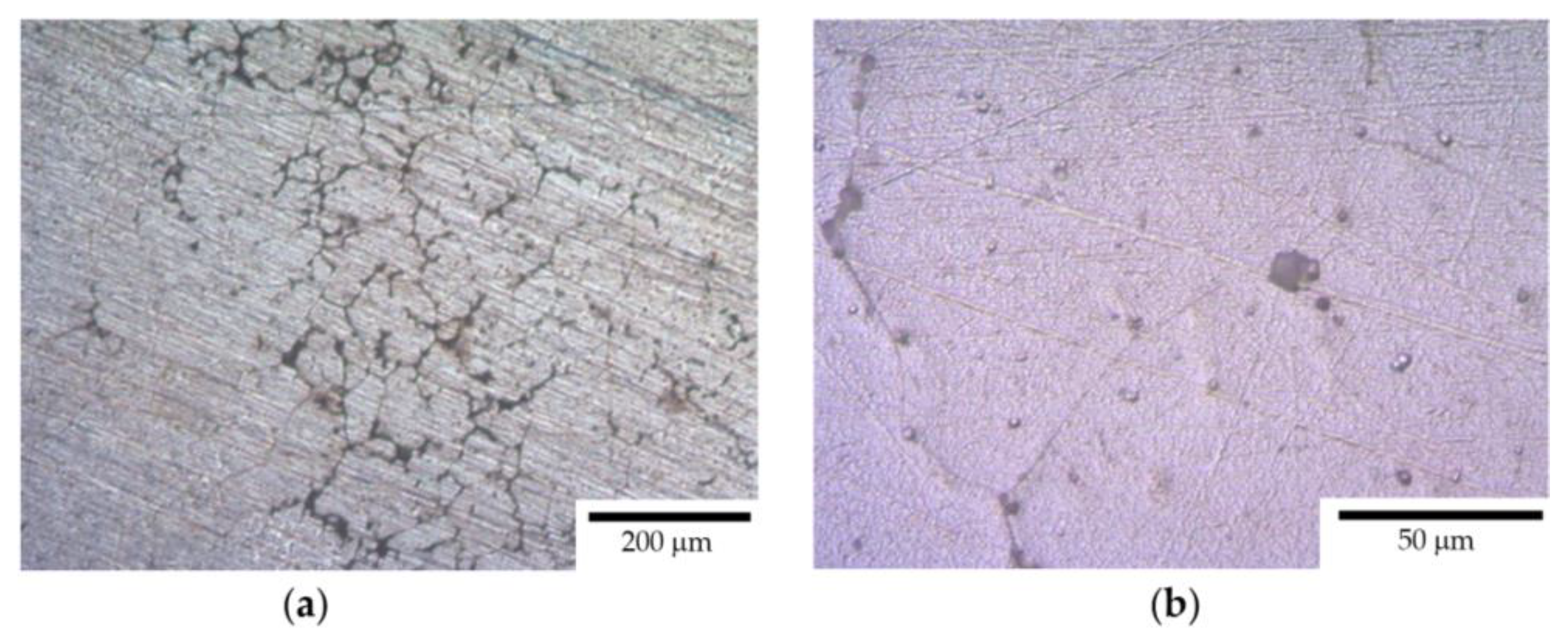
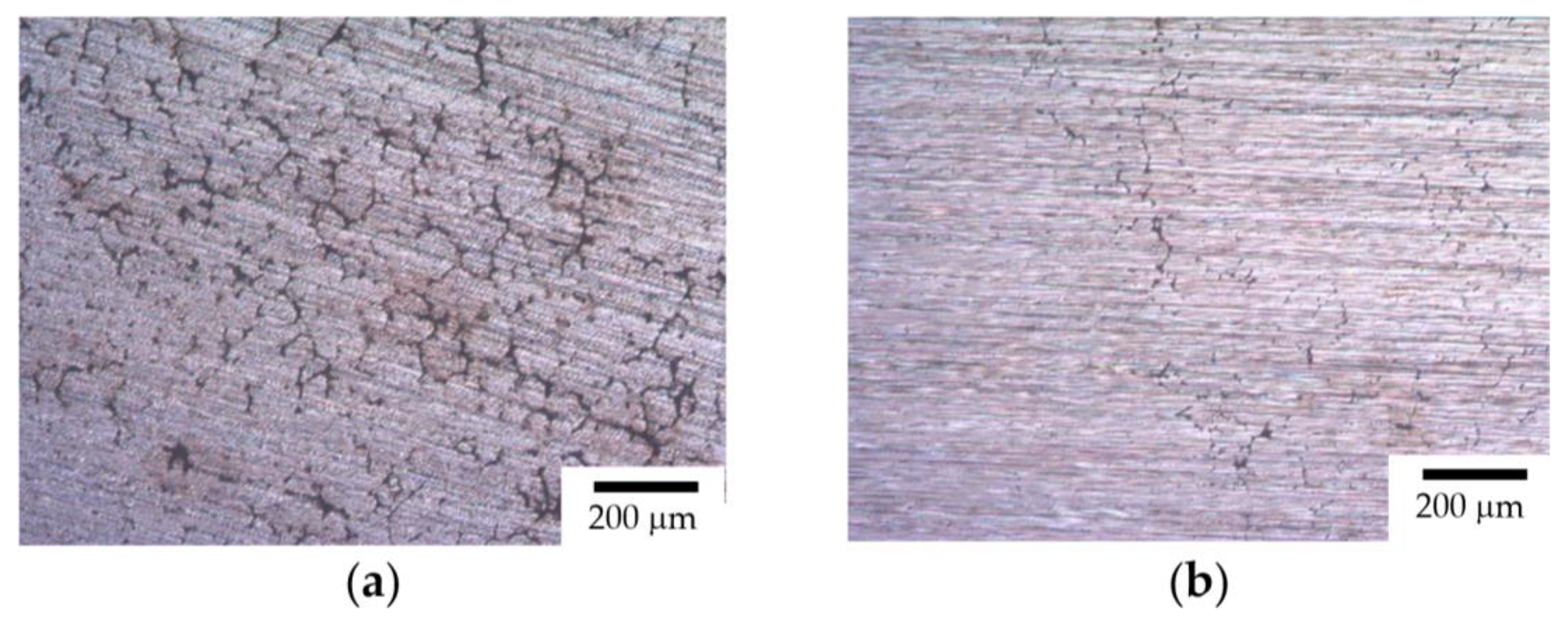
- After the corrosion testing, two types of corrosion defects were observed on the surfaces of the cast alloy samples. Type I—large, wide IGC defects associated with the destruction of primary β-phase particles located along the boundaries of dendrites formed at the stage of crystallization of the Al alloy, and Type II—fine IGC defects associated with the GBs formed at the last stage of crystallization (the central parts of the ingots). The most probable reason for the formation of Type II defects is the effect of solid-phase wetting of aluminum GBs by β-phase particles [76,77,78]. A discussion of the nature of this effect can be found in a series of articles [79,80].
- (v)
- The cast Al–Mg–Sc–Zr alloys tested in an aqueous solution of 3% NaCl + 0.3% HCl show an increased tendency to IGC. The main cause of the IGC of the cast Al–Mg–Sc–Zr alloys is the formation of primary β-phase particles during cooling the ingots. An increase in the Mg concentration leads to a decrease in the depth of the IGC defects and an increase in the corrosion rate during the electrochemical studies (an increase in the corrosion current density icorr). An increase in the Sc/Zr ratio leads to a decrease in the depth of the IGC defects, but the scale of this effect is smaller than the one of Mg on the IGC depth. An increase in the Sc/Zr ratio leads to a decrease in icorr in the electrochemical studies.
- (vi)
- ECAP results in a slight decrease in the depth of the IGC defects and a significant increase in the corrosion current density icorr. Metallographic studies show that ECAP does not affect the size and fraction of large Type I defects (at Mg = const, Sc/Zr = const), but leads to an increase in the fraction of fine Type II defects. The effect of the nature of the Mg concentration and the Sc/Zr ratio on the corrosion resistance of the UFG Al alloys was similar to the effect of these factors on the corrosion behavior of the cast alloys.
- (vii)
- Annealing leads to a decrease in the depth of IGC defects in the cast and UFG alloys. The dependence of icorr(T) of the cast and UFG alloys with 2.5% Mg has a three-stage character with a maximum. The maximum values of icorr were observed after annealing at 450 °C. An increase in the Mg concentration leads to a decrease in the increase scale in icorr. Metallographic studies of the annealed sample surfaces show that in the temperature range from 300 to 450 °C, an increase in the volume and size of fine Type II defects is observed. Annealing at above 450 °C leads to a decrease in the size of both large Type I defects and fine Type II ones. The nature of the annealing effect on the corrosion resistance of UFG alloys is similar to that of the cast alloys.
4.2. Effect of Microstructure on Corrosion Resistance of Al–Mg–Sc–Zr Alloys
4.2.1. Effect of Mg
4.2.2. Effect of Sc/Zr Ratio
4.2.3. Effect of Annealing: Synergistic Effect of Mg and Sc/Zr Ratio
4.3. Summary of the Analysis Results—Concluding Remarks
5. Conclusions
- (1)
- After the corrosion tests in an aqueous solution of 3% NaCl + 0.3% HCl, two types of corrosion defects were observed on the surfaces of the cast Al–Mg–Sc–Zr alloy samples. Type I—large, wide IGC defects associated with corrosion destruction of the primary β-phase particles located along the dendrite boundaries. Type II—fine IGC defects associated with the grain boundaries, which are covered by thin layers of β-phase due to the effect of solid-phase wetting. The fractions of the defect depend on the Mg concentration, Sc/Zr ratio, grain sizes, and annealing regimes.
- (2)
- The main reason for the IGC defects in the cast Al–Mg–Sc–Zr alloys is the formation of the primary β-phase particles during the cooling of the ingots. An increase in the Mg concentration leads to a decrease in the depth of the IGC defects and an increase in the corrosion rate during the electrochemical tests (an increase in the corrosion current density icorr). An increase in the Sc/Zr ratio leads to a decrease in the depth of the IGC defects, but the scale of this effect is smaller than the one of Mg. An increase in the Sc/Zr ratio leads to a decrease in icorr in the electrochemical test.
- (3)
- ECAP leads to a slight decrease in the depth of the IGC defects and a significant increase in the corrosion current density icorr. The effect of the nature of the Mg concentration and the Sc/Zr ratio on the corrosion resistance of the UFG Al alloys is similar to that of the cast alloys.
- (4)
- The dependence of icorr on the annealing temperature of the cast and UFG alloys with 2.5% and 4% Mg has a three-stage character with a maximum value. The origin of the increase in the corrosion current density during heating of the Al–Mg–Sc–Zr alloys is the release of the secondary β-phase particles on the secondary Al3(Sc,Zr) particles and an increase in the proportion of grain boundaries wetted by β-phase. Decreasing icorr at elevated annealing temperatures can be attributed to the dissolution of β-phase particles.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEs | alloying elements |
| ECAP | equal channel Angular pressing |
| EDS | energy dispersion (analysis) |
| GB | grain boundary |
| GBs | grain boundaries |
| HAGBs | high-angle grain boundaries |
| IGC | intergranular corrosion |
| RT | room temperature |
| SEM | scanning electron microscopy |
| SER | specific electrical resistivity |
| SPD | severe plastic deformation |
| TEM | transmission electron microscopy |
| UFG (alloy) | ultra-fine-grained (alloy) |
References
- Filatov, Y.A.; Yelagin, V.I.; Zakharov, V.V. New Al-Mg-Sc alloys. Mater. Sci. Eng. A 2000, 280, 97–101. [Google Scholar] [CrossRef]
- Davydov, V.G.; Rostova, T.D.; Zakharov, V.V.; Filatov, Y.A.; Yelagin, V.I. Scientific principles of making an alloying addition of scandium to aluminium alloys. Mater. Sci. Eng. A 2000, 280, 30–36. [Google Scholar] [CrossRef]
- Avtokratova, E.; Sitdikov, O.; Markushev, M.; Linderov, M.; Merson, D.; Vinogradov, A. The processing route towards outstanding performance of the severely deformed Al-Mg-Mn-Sc-Zr alloy. Mater. Sci. Eng. A 2021, 806, 140818. [Google Scholar] [CrossRef]
- Malopheyev, S.; Kulitskiy, V.; Kaibyshev, R. Deformation structure and strengthening mechanisms in an Al-Mg-Sc-Zr alloy. J. Alloys Compd. 2017, 698, 957–966. [Google Scholar] [CrossRef]
- Vinogradov, A.; Washikita, A.; Kitagawa, K.; Kopylov, V.I. Fatigue life of fine-grained Al-Mg-Sc alloys produced by Equal-Channel Angular Pressing. Mater. Sci. Eng. A 2003, 349, 318–326. [Google Scholar] [CrossRef]
- Furukawa, M.; Utsunomiya, A.; Matsubara, K.; Horita, Z.; Langdon, T.G. Influence of magnesium on grain refinement and ductility in a dilute Al–Sc alloy. Acta Mater. 2001, 49, 3829–3838. [Google Scholar] [CrossRef]
- Avtokratova, E.; Sitdikov, O.; Markushev, M.; Mulyukov, R. Extraordinary high-strain rate superplasticity of severely deformed Al–Mg–Sc–Zr alloy. Mater. Sci. Eng. A 2012, 538, 386–390. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the relationship between grain size and corrosion rate of metals. Scr. Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Ralston, K.D.; Fabijanic, D.; Birbilis, N. Effect of grain size on corrosion of high purity aluminum. Electrochem. Acta 2011, 56, 1729–1736. [Google Scholar] [CrossRef]
- Segal, V.M.; Beyerlein, I.J.; Tome, C.N.; Chuvil’deev, V.N.; Kopylov, V.I. Fundamentals and Engineering of Severe Plastic Deformation; Nova Science Publishers: New York, NY, USA, 2010. [Google Scholar]
- Minoda, T.; Yoshida, H. Effect of grain boundary characteristics of intergranular corrosion resistance of 6061 aluminum alloy extrusion. Metall. Mater. Trans. A 2002, 33, 2891–2898. [Google Scholar] [CrossRef]
- Lervik, A.; Wenner, S.; Lunder, O.; Marioara, C.D.; Holmestand, R. Grain boundary structures and their correlation with intergranular corrosion in an extruded Al-Mg-Si-Cu alloy. Mater. Charact. 2020, 170, 110695. [Google Scholar] [CrossRef]
- Bałkowiec, A.; Michalski, J.; Matysiak, H.; Kurzydlowski, K.J. Influence of grain boundaries misorientation angle on intergranular corrosion in 2024-T3 aluminium. Mater. Sci-Pol. 2011, 29, 305–311. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Nilsson, J.O.; Dong, Z.; Cai, C. Corrosion behaviour of AA6082 Al-Mg-Si alloy extrusion: Recrystallized and non-recrystallized structures. Corros. Sci. 2018, 144, 163–171. [Google Scholar] [CrossRef]
- Sinyavskii, V.S.; Ulanova, V.V.; Kalinin, V.D. On the mechanism of intergranular corrosion of aluminum alloys. Prot. Met. 2004, 40, 481–490. [Google Scholar] [CrossRef]
- Sauvage, X.; Ganeev, A.; Ivanisenko, Y.; Enikeev, N.; Muraskin, M.; Valiev, R. Grain boundary segregation in UFG alloys produced by Severe Plastic Deformation. Adv. Eng. Mater. 2012, 14, 968–974. [Google Scholar] [CrossRef]
- Sauvage, X.; Enikeev, N.; Valiev, R.; Nasedkina, Y.; Murashkin, M. Atomic-scale analysis of the segregation and precipitation mechanisms in a severely deformed Al-Mg alloy. Acta Mater. 2014, 72, 125–136. [Google Scholar] [CrossRef]
- Buranova, Y.; Kulitskiy, V.; Peterlechner, M.; Moguchea, A.; Kaibyshev, R.; Divinski, S.V.; Wilde, G. Al3(Sc,Zr)-based precipitates in Al–Mg alloy: Effect of severe deformation. Acta Mater. 2017, 124, 210–224. [Google Scholar] [CrossRef]
- Popović, M.; Romhanji, E. Characterization of microstructural changes in an Al-6.8wt.% Mg alloy by electrical resistivity measurements. Mater. Sci. Eng. A 2008, 492, 460–467. [Google Scholar] [CrossRef]
- Bhargava, N.R.M.R.; Samajdar, I.; Ranganathan, S.; Surappa, M.K. Role of cold work and SiC reinforcements on the β′/β precipitation in Al-10 pct Mg alloy. Metall. Mater. Trans. A 1998, 29, 2835–2842. [Google Scholar] [CrossRef]
- Tang, Z.; Jiang, F.; Long, M.; Jiang, J.; Liu, H.; Tong, M. Effect of annealing temperature on microstructure, mechanical properties and corrosion behavior of Al-Mg-Mn-Sc-Zr alloy. Appl. Surf. Sci. 2020, 514, 146081. [Google Scholar] [CrossRef]
- Straumal, B.B.; Baretzky, B.; Mazilkin, A.A.; Phillipp, F.; Kogtenkova, O.A.; Volkov, M.N.; Valiev, R.Z. Formation of nanograined structure and decomposition of supersaturated solid solution during high pressure torsion of Al-Zn and Al-Mg alloys. Acta Mater. 2004, 52, 4469–4478. [Google Scholar] [CrossRef]
- Kogtenkova, O.; Straumal, B.B.; Mazilkin, A.A.; Czeppe, T.; Zięba, P. Phase transformations in the Al-Mg alloys driven by high-pressure torsion. Phys. Status Solidi (B) 2021, 258, 2100210. [Google Scholar] [CrossRef]
- Lyndon, J.A.; Gupta, R.K.; Gibson, M.A.; Birbilis, N. Electrochemical behaviour of the β-phase intermetallic (Mg2Al3) as a function of pH as relevant to corrosion of aluminium–magnesium alloys. Corros. Sci. 2013, 70, 290–293. [Google Scholar] [CrossRef]
- Gupta, R.K.; Zhang, R.; Davies, C.H.J.; Birbilis, N. Influence of Mg content on the sensitization and corrosion of Al-xMg(-Mn) alloys. Corrosion 2013, 59, 1081–1087. [Google Scholar] [CrossRef]
- Zhang, R.; Gupta, R.K.; Davies, C.H.J.; Hodge, A.M.; Tort, M.; Xia, K.; Birbilis, N. The influence of grain size and grain orientation of sensitization in AA5083. Corrosion 2016, 72, 160–168. [Google Scholar] [CrossRef]
- Zhang, R.; Qiu, Y.; Yuanshen, Y.; Birblis, N. A closer inspection of a grain boundary immune to intergranular corrosion in a sensitised Al-Mg alloy. Corros. Sci. 2018, 133, 1–5. [Google Scholar] [CrossRef]
- Chang, J.C.; Chuang, T.H. Stress-corrosion cracking susceptibility of the superplastically formed 5083 aluminum alloy in 3.5 pct NaCl solution. Metall. Mater. Trans. A 1999, 30, 3191–3199. [Google Scholar] [CrossRef]
- Popović, M.; Romhanji, E. Stress corrosion cracking susceptibility of Al-Mg alloy sheet with high Mg content. J. Mater. Proc. Technol. 2002, 125–126, 275–280. [Google Scholar] [CrossRef]
- Scotto D′Antuono, D.; Gaies, J.; Golumbfskie, W.; Taheri, M.L. Grain boundary misorientation dependence of β phase precipitation in an Al-Mg alloy. Scr. Mater. 2014, 76, 81–84. [Google Scholar] [CrossRef]
- Scotto D′Antuono, D.; Gaies, J.; Golumbfskie, W.; Taheri, M.L. Direct measurement of the effect of cold rolling on β phase precipitation kinetics in 5xxx series aluminum alloys. Acta Mater. 2017, 123, 264–271. [Google Scholar] [CrossRef]
- Sun, F.; Nash, G.L.; Li, Q.; Liu, E.; He, C.; Shi, C.; Zhao, N. Effect of Sc and Zr additions on microstructures and corrosion behavior of Al-Cu-Mg-Sc-Zr alloys. J. Mater. Sci. Technol. 2017, 33, 1015–1022. [Google Scholar] [CrossRef]
- Yang, J.; Heckman, N.M.; Velasco, L.; Hodge, A.M. Improve sensitization and corrosion resistance of an Al-Mg alloy by optimization of grain boundaries. Sci. Rep. 2016, 6, 26870. [Google Scholar] [CrossRef]
- Ahmad, Z.; Aleem, A.; Abbas, M. Effect of scandium doping on the corrosion resistance and mechanical behavior of Al-3Mg alloy in neutral chloride solutions. Mater. Sci. Appl. 2011, 2, 244–250. [Google Scholar] [CrossRef]
- Ahmad, Z.; Abdul Aleem, B.J. Effect of nano Al(Sc1−xZrx) precipitates on the mechanical and corrosion behavior of Al-2.5Mg alloys. Mater. Corros. 2011, 62, 335–345. [Google Scholar] [CrossRef]
- Rosalbino, F.; Delsante, S.; Borzone, G.; Scavino, G. Assessing the corrosion resistance of binary Al-Sc alloys in chloride-containing environment. Mater. Corros. 2017, 68, 444–449. [Google Scholar] [CrossRef]
- Tuan, N.Q.; Alves, A.C.; Toptan, F.; Lopes, A.B.; Pinto, A.M.P. The effect of Sc and Yb microalloying additions and aged-hardening heat treatment on corrosion behavior of Al-Mg alloys. Mater. Corros. 2016, 67, 60–71. [Google Scholar] [CrossRef]
- Li, M.; Liu, S.; Wang, X.; Shi, Y.; Pan, Q.; Zhou, X.; Zhang, R.; Birbilis, N. Improved intergranular corrosion resistance of Al-Mg-Mn alloys with Sc and Zr additions. Micron 2022, 154, 103202. [Google Scholar] [CrossRef]
- Lee, S.L.; Chiu, Y.C.; Pan, T.A.; Chen, M.C. Effects of trace amounts of Mn, Zr and Sc on the recrystallization and corrosion resistance of Al-5Mg alloy. Crystals 2021, 11, 926. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, X.; Li, J.; Xiang, S.; Shi, J.; Xu, J.; Sanders, R.E. The influence of Sc and Zr additions on microstructure and corrosion behavior of AA5182 alloy sheet. Corros. Sci. 2022, 199, 110181. [Google Scholar] [CrossRef]
- Forbord, B.; Lefebvre, W.; Danoix, F.; Hallem, H.; Marthinsen, K. Three dimensional atom probe investigation on the formation of Al3(Sc,Zr)-dispersoids in aluminum alloys. Scr. Mater. 2004, 51, 333–337. [Google Scholar] [CrossRef]
- Tolley, A.; Rodmilovic, V.; Dahmen, U. Segregation in Al3(Sc,Zr) precipitates in Al-Sc-Zr alloys. Scr. Mater. 2005, 52, 621–625. [Google Scholar] [CrossRef]
- Fuller, C.B.; Murray, J.L.; Seidman, D.N. Temporal evolution of the nanostructure of Al(Sc,Zr) alloys: Part I—Chemical compositions of Al3(Sc1−xZrx) precipitates. Acta Mater. 2005, 53, 5401–5413. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, Z.; Zhang, G. Nanostructure characteristics of Al3Sc1−xZrx nanoparticles and their effects on mechanical property and SCC behavior of Al-Zn-Mg alloys. Materials 2020, 13, 1909. [Google Scholar] [CrossRef]
- Orthacker, A.; Haberfehlner, G.; Taendl, J.; Poletti, M.C.; Sonderegger, B.; Kothleitner, G. Diffusion-defining atomic-scale spinodal decomposition within nanoprecipitates. Nat. Mater. 2018, 17, 1101–1107. [Google Scholar] [CrossRef]
- Clouet, E.; Laé, L.; Épicier, T.; Lefebvre, W.; Nastar, M.; Deschamps, A. Complex precipitation pathways in multicomponent alloys. Nat. Mater. 2006, 5, 482–488. [Google Scholar] [CrossRef]
- Stroev, A.Y.; Gorbatov, O.I.; Gornostyrev, Y.N.; Korzhavyi, P.A. Ab-initio based modeling of precipitation in Al-(Sc,Zr) alloy. Formation and stability of a core-shell structure. Comp. Mater. Sci. 2023, 218, 111912. [Google Scholar] [CrossRef]
- Zha, M.; Tian, T.; Jia, H.L.; Zhang, H.M.; Wang, H.Y. Sc/Zr ratio-dependent mechanisms of strength evolution and microstructural thermal stability of multi-scale hetero-structured Al-Mg-Sc-Zr alloys. J. Mater. Sci. Technol. 2023, 140, 67–78. [Google Scholar] [CrossRef]
- Rokhlin, L.L.; Bochvar, N.R.; Leonova, N.P. Study of decomposition of oversaturated solid solution in Al-Sc-Zr alloys at different ratio of scandium and zirconium. Inorg. Mater. Appl. Res. 2011, 2, 517–520. [Google Scholar] [CrossRef]
- Li, G.; Zhao, N.; Liu, T.; Li, J.; He, C.; Shi, C.; Liu, E.; Sha, J. Effect of Sc/Zr ratio on the microstructure and mechanical properties of new type of Al-Zn-Mg-Sc-Zr alloys. Mater. Sci. Eng. A 2014, 617, 219–227. [Google Scholar] [CrossRef]
- Chuvil’deev, V.N.; Gryaznov, M.Y.; Shotin, S.V.; Nokhrin, A.V.; Likhnitskii, C.V.; Nagicheva, G.S. Effect of Sc/Zr ratio on superplastic behavior of ultrafine-grained Al-6%Mg alloys. Mater. Sci. Eng. A 2024, 898, 146409. [Google Scholar] [CrossRef]
- Mochugovskiy, A.G.; Mikhaylovskaya, A.V. Comparison of precipitation kinetics and mechanical properties in Zr and Sc-bearing aluminum-based alloys. Mater. Lett. 2020, 275, 128096. [Google Scholar] [CrossRef]
- Mochugovskiy, A.G.; Mikhaylovskaya, A.V.; Zadorognyy, M.Y.; Golovin, I.S. Effect of heat treatment on the grain size control, superplasticity, internal friction, and mechanical properties of zirconium-bearing aluminum-based alloy. J. Alloys Compd. 2021, 856, 157455. [Google Scholar] [CrossRef]
- Mikhaylovskaya, A.M.; Mukhamejanova, A.; Tabachkova, N.Y.; Kishchik, M.S.; Mochugovskiy, A.G. Effect of quasicrystalline phase precipitation on the strengthening and grain refinement of Al-Mg-Mn alloy after multidirectional forging. Mater. Lett. 2025, 384, 138033. [Google Scholar] [CrossRef]
- Cavanaugh, M.K.; Birbilis, N.; Buchheit, R.G.; Bovard, F. Investigating localized corrosion susceptibility arising from Sc containing intermetallics Al3Sc in high strength Al-alloys. Scr. Mater. 2007, 56, 995–998. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G. Investigation and discussion of characteristics for intermetallic phases common to aluminum alloys as a function of solution pH. J. Electrochem. Soc. 2008, 155, C117. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Role of intermetallic phases in localized corrosion of AA5083. Electrochem. Acta 2007, 52, 7651–7659. [Google Scholar] [CrossRef]
- Luo, X.; Fang, H.; Liu, H.; Yan, Y.; Zhu, H.; Yu, K. Effect of Sc and Zr on Al6(Mn,Fe) phase in Al-Mg-Mn alloys. Mater. Transact. 2019, 60, 737–742. [Google Scholar] [CrossRef]
- Yan, J.; Hoge, A.M. Study of β precipitation and layer structure formation in Al 5083: The role of dispersoids and grain boundaries. J. Alloys Compd. 2017, 703, 242–250. [Google Scholar] [CrossRef]
- Ding, Y.; Wu, X.; Gao, K.; Huang, C.; Xiong, X.; Huang, H.; Wen, S.; Nie, Z. The influence of stabilization treatment on long-term corrosion resistance and microstructure in Er and Zr containing 5083 aluminum alloy. Mater. Charact. 2020, 161, 110143. [Google Scholar] [CrossRef]
- Yang, Y.K.; Allen, T. Direct visualization of β phase causing intergranular forms of corrosion in Al-Mg alloys. Mater. Charact. 2013, 80, 76–85. [Google Scholar] [CrossRef]
- Zhu, Y.; Cullen, D.A.; Kar, S.; Free, M.L.; Allard, L.F. Evaluation of Al3Mg2 precipitates and Mn-rich phase in aluminum-magnesium alloy based on Scanning Transmission Electron Microscopy imaging. Metall. Mater. Trans. A 2012, 43, 4933–4939. [Google Scholar] [CrossRef]
- Ding, Y.; Gao, K.; Xiong, X.; Huang, H.; Wen, S.; Wu, X.; Nie, Z.; Shao, R.; Huang, C.; Guo, S.; et al. High corrosion resistance and strain hardening of high Mg Al-alloy with Er and Zr by using a new reverse stabilization process. Scr. Mater. 2019, 171, 26–30. [Google Scholar] [CrossRef]
- Synyavskii, V.S.; Val’kov, V.D.; Titkova, E.V. The Effect of Scandium and Zirconium Additions on Corrosion Properties of Al-Mg Alloys. Prot. Met. 1998, 34, 549–555. Available online: https://elibrary.ru/item.asp?id=13300299 (accessed on 11 March 2025). (In Russian).
- Leibner, M.; Vlach, M.; Kodetová, V.; Kudrnová, H.; Veselý, J.; Zikmund, S. Effect of deformation on evolution of Al3(Er,Zr) precipitates in Al-Er-Zr-based alloy. Mater. Charact. 2022, 186, 111781. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Wang, M. Microstructures and corrosion resistances of hypoeutectic Al-6.5Si-0.45Mg casting alloy with addition of Sc and Zr. Mater. Chem. Phys. 2022, 276, 125321. [Google Scholar] [CrossRef]
- Wloka, J.; Virtanen, S. Influence of scandium on the pitting behaviour of Al-Zn-Mg-Cu alloys. Acta Mater. 2007, 55, 6666–6672. [Google Scholar] [CrossRef]
- Ryabov, D.K.; Kolobnev, N.I.; Samohvalov, S.V. Effect of scandium addition on mechanical properties and corrosion resistance of medium strength Al-Zn-Mg-Cu alloy. Mater. Sci. Forum 2014, 794–796, 241–246. [Google Scholar] [CrossRef]
- Kannan, M.B.; Raja, V.S. Influence of heat treatment and scandium addition on the electrochemical polarization behavior of Al-Zn-Mg-Cu-Zr alloy. Metall. Mater. Trans. A 2007, 38, 2843–2852. [Google Scholar] [CrossRef]
- Kozlova, N.A.; Nokhrin, A.V.; Chuvil’deev, V.N.; Shadrina, Y.S.; Bobrov, A.A.; Chegurov, M.K. The effect of a Sc:Zr ratio on the corrosion resistance of cast Al-Mg alloys. Phys. Metals Metallogr. 2024, 125, 851–861. [Google Scholar] [CrossRef]
- Russian National Standard GOST 4784-2019; Aluminium and Wrought Aluminium Alloys. Grades. Interstandard: Moscow, Russia, 2019.
- Russian National Standard GOST 9.021-74; Unified System of Corrosion and Ageing Protection. Aluminium and Aluminium Alloys. Accelerated Test Methods for Intercrystalline Corrosion. Gosstandart: Minsk, Belarus, 1975.
- Russian National Standard GOST 9.908-85; Unified System of Corrosion and Ageing Protection. Metals and Alloys. Methods for Determination of Corrosion and Corrosion Resistance Indices. Gosstandart: Minsk, Belarus, 1987.
- Orlova, T.S.; Latynina, T.A.; Murashkin, M.Y.; Chabanais, F.; Rugutti, L.; Lefebvre, W. Effects of Mg on strengthening mechanisms in ultrafine-grained Al-Mg-Zr alloy. J. Alloys Compd. 2021, 859, 157775. [Google Scholar] [CrossRef]
- Shamtko, O.A.; Usov, Y.V. Structure and Properties of Metals and Alloys. Electrical and Magnetic Properties of Metals; Naukova Dumka: Kiev, Russia, 1987. (In Russian) [Google Scholar]
- Straumal, B.B.; Baretzky, B.; Kogtenkova, O.A.; Straumal, A.B.; Sidorenko, A.S. Wetting of grain boundaries in Al by the solid Al3Mg2 phase. J. Mater. Sci. 2010, 45, 2057–2061. [Google Scholar] [CrossRef]
- Kogtenkova, O.A.; Zieba, P.; Czeppe, T.; Litynska-Dobrzynska, L.; Straumal, B.B.; Nekrasov, A.N. Wetting of grain boundaries by the second solid phase in Al-based alloys. Bull. Russ. Acad. Sci. Phys. 2013, 77, 1386–1390. [Google Scholar] [CrossRef]
- Kogtenkova, O.; Straumal, B.; Korneva, A.; Czeppe, T.; Wierzbicka-Miernik, A.; Faryna, M.; Zięba, P. Grain boundary complexions and phase transformations in Al- and Cu-based alloy. Metals 2019, 9, 10. [Google Scholar] [CrossRef]
- Straumal, B.; Lepkova, T.; Korneva, A.; Gerstein, G.; Kogtenkova, O.; Gornakova, A. Grain boundary wetting by the second solid phase: 20 years of history. Metals 2023, 13, 929. [Google Scholar] [CrossRef]
- Straumal, B.B.; Mazilkin, A.A.; Baretzky, B. Grain boundary complexions and pseudopartial wetting. Curr. Opin. Solid State Mater. Sci. 2016, 20, 247–256. [Google Scholar] [CrossRef]
- Karkina, L.E.; Karkin, I.N.; Kuznetsov, A.R.; Razumov, I.K.; Korzhavyi, P.A.; Gornostyrev, Y.N. Solute-grain boundary interaction and segregation formation in Al: First principles calculations and molecular dynamics modeling. Comp. Mater. Sci. 2016, 112, 18–26. [Google Scholar] [CrossRef]
- Straumal, B.B.; Kulagin, R.; Klinger, L.; Rabkin, E.; Straumal, P.B.; Kogtenkova, O.A.; Baretzky, B. Structure refinement and fragmentation of precipitates under severe plastic deformation: A review. Materials 2022, 15, 601. [Google Scholar] [CrossRef]
- Nikulin, I.; Kipelova, A.; Malopheyev, S.; Kaibyshev, R. Effect of second phase particles on grain refinement during equal-channel angular pressing of an Al-Mg-Mn alloy. Acta Mater. 2012, 60, 487–497. [Google Scholar] [CrossRef]
- Fujikawa, S.I. Impurity diffusion of scandium in aluminium. Defect Diffus. Forum 1997, 143–147, 115–120. [Google Scholar] [CrossRef]
- Kerkove, M.A.; Wood, T.D.; Sanders, P.G.; Kampe, S.L.; Swenson, D. The diffusion coefficient of scandium in dilute aluminum-scandium alloys. Metall. Mater. Trans. A 2014, 45, 3800–3805. [Google Scholar] [CrossRef]
- Fuller, C.B.; Seidman, D.N. Temporal evolution of the nanostructure of Al(Sc,Zr) alloys: Part II-coarsening of Al3(Sc1−xZrx) precipitates. Acta Mater. 2005, 53, 5415–5428. [Google Scholar] [CrossRef]
- Asta, M.; Ozolins, V.; Woodward, C. A first-principles approach to modeling alloy phase equilibria. JOM 2001, 53, 16–19. [Google Scholar] [CrossRef]
- Vo, N.Q.; Dunand, D.C.; Seidman, D.N. Atom probe tomographic study of a friction-stir-processed Al-Mg-Sc alloy. Acta Mater. 2012, 60, 7078–7089. [Google Scholar] [CrossRef]
- Marquis, E.A.; Seidman, D.N.; Astra, M.; Woodward, C. Composition evolution of nanoscale Al3Sc precipitates in an Al-Mg-Sc alloy: Experiments and computations. Acta Mater. 2006, 54, 119–130. [Google Scholar] [CrossRef]
- Mikhaylovskaya, A.V.; Kishchik, M.S.; Kotov, A.D.; Tabachkova, N.Y. Grain refinement during isothermal multidirectional forging due to β-phase heterogenization in Al-Mg-based alloys. Mater. Lett. 2022, 321, 132412. [Google Scholar] [CrossRef]
- Mikhaylovskaya, A.V.; Mochugovskiy, A.G.; Levchenko, V.S.; Tabachkova, N.Y.; Mufalo, W.; Pornoy, V.K. Precipitation behavior of L12 Al3Zr phase in Al-Mg-Zr alloy. Mater. Charact. 2018, 139, 30–37. [Google Scholar] [CrossRef]
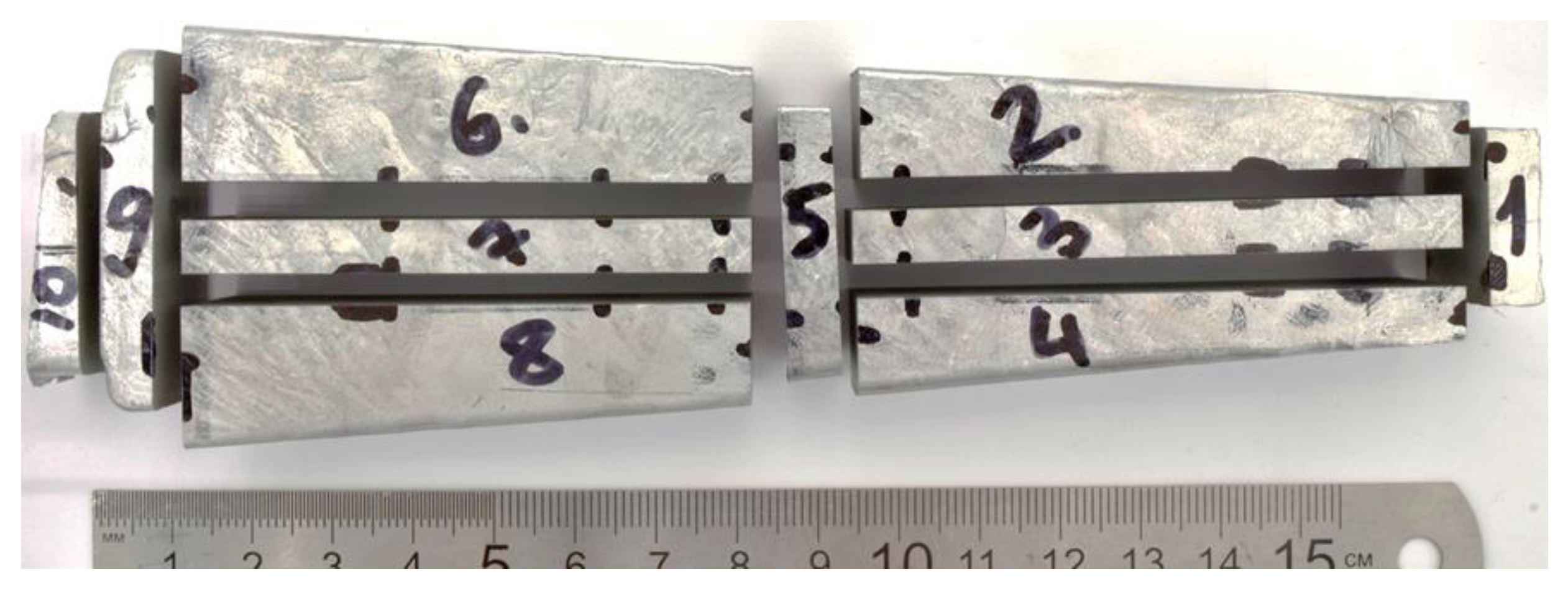
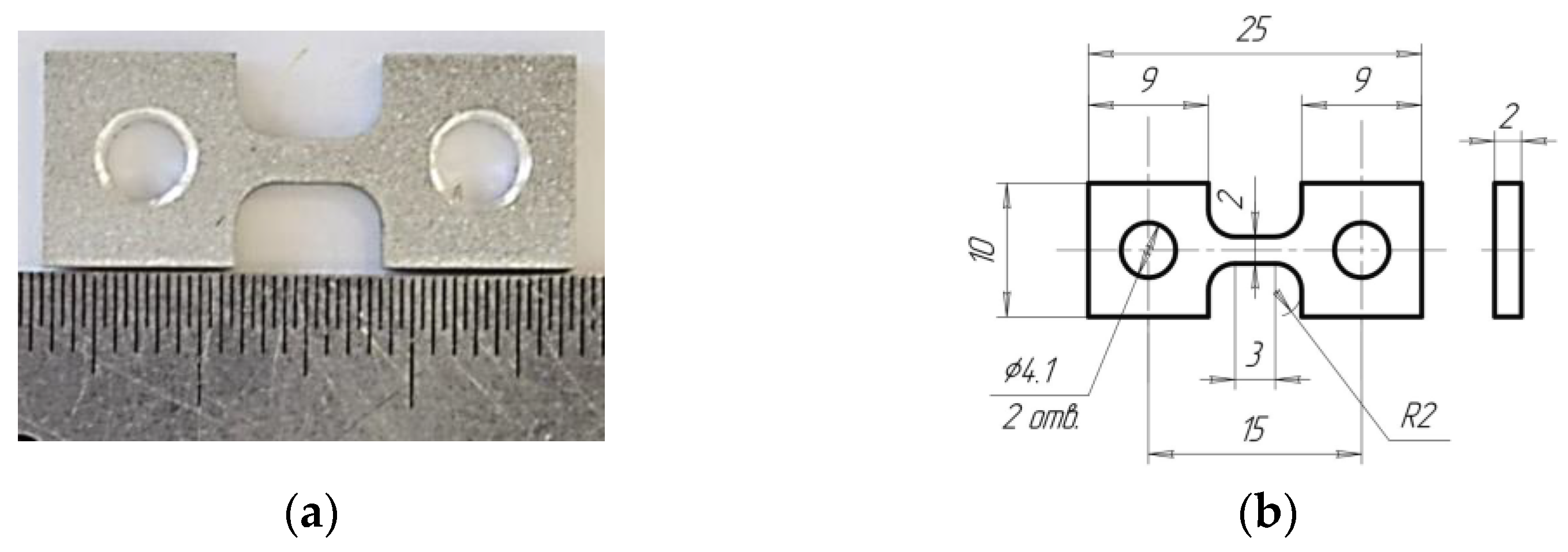




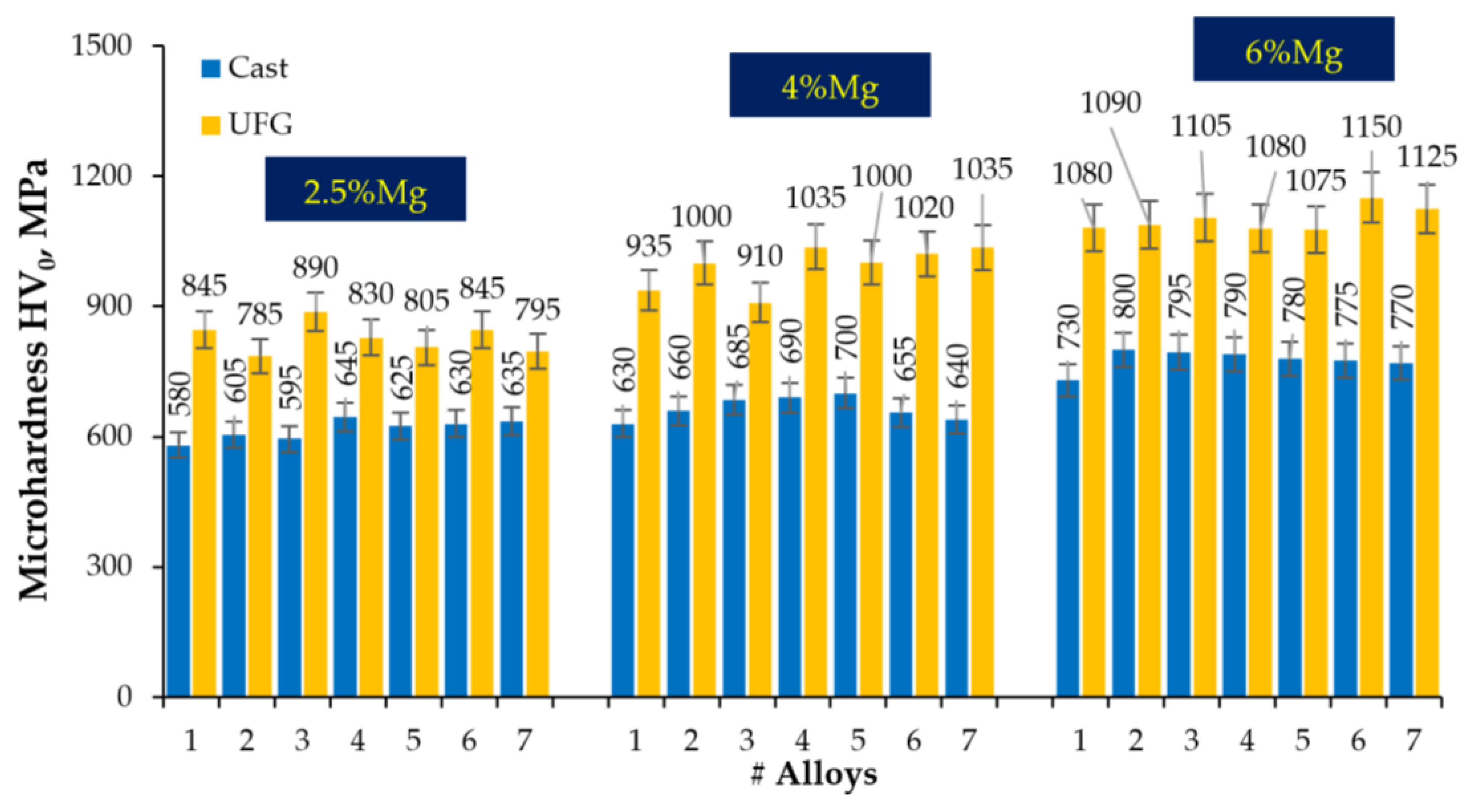
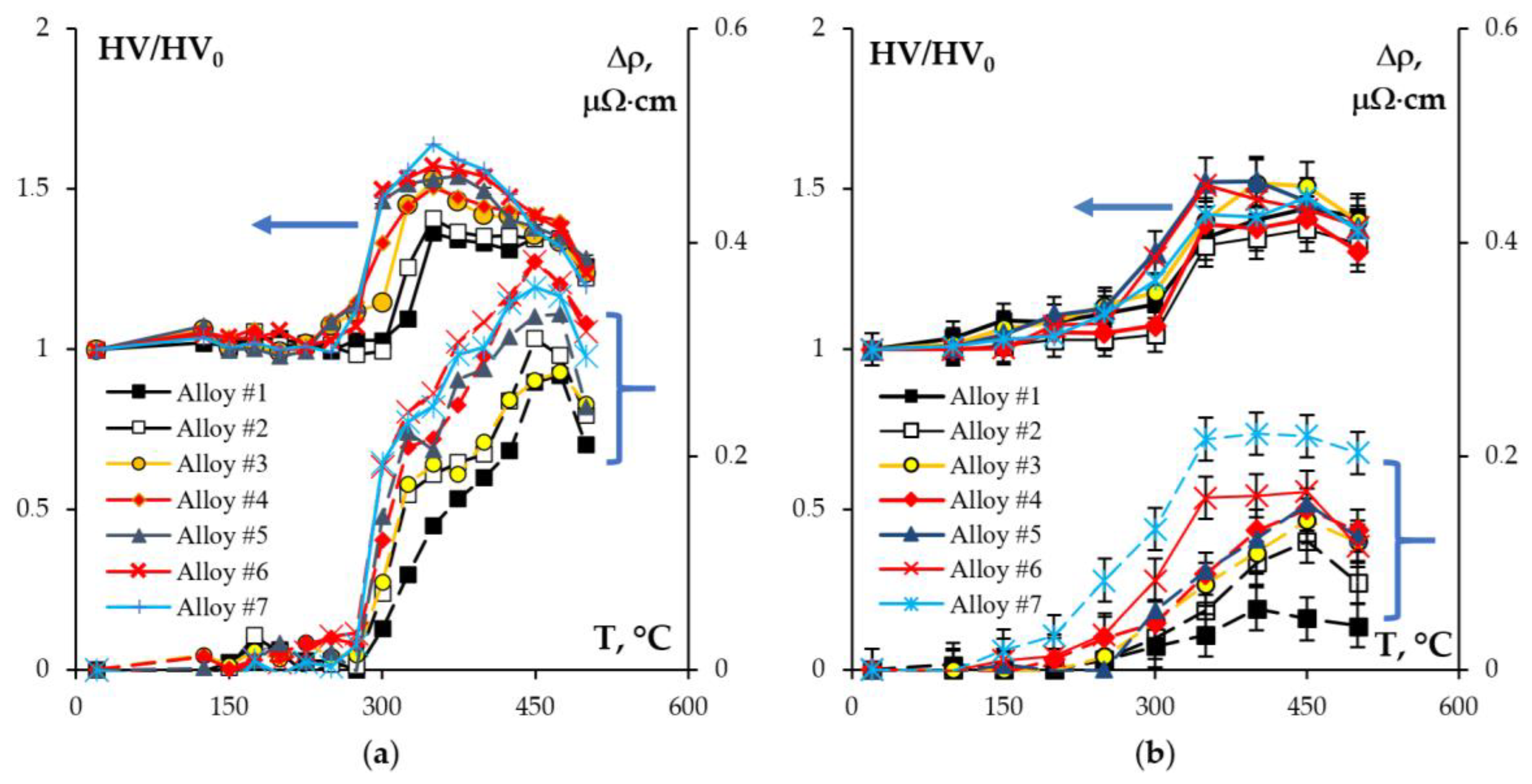
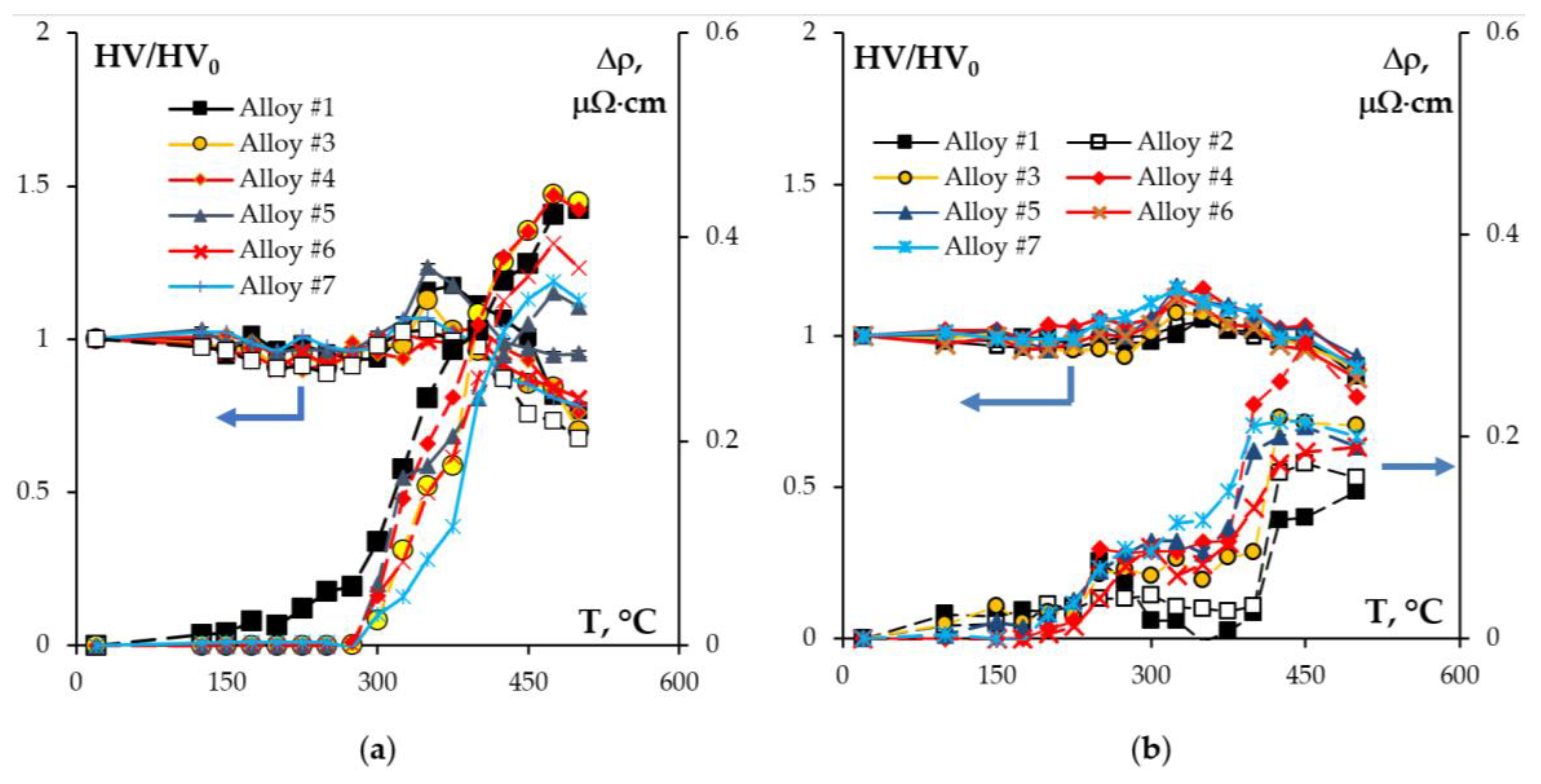
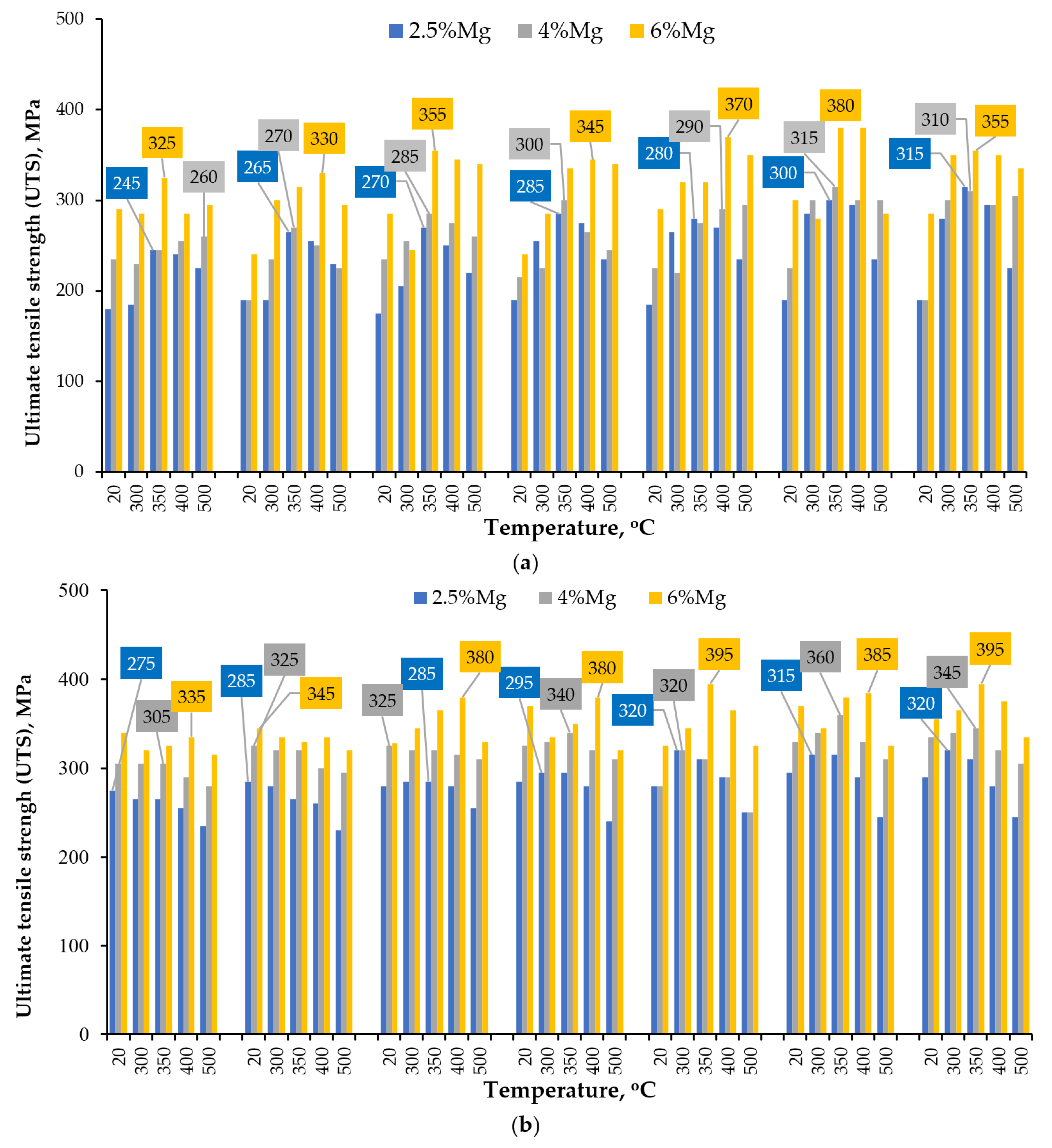


| Series | Alloy # | AEs Concentration, % wt. | |||||
|---|---|---|---|---|---|---|---|
| Mg | Sc | Zr | Sc + Zr | Sc/Zr | |||
| Estimated | Actual | ||||||
| 1 | 1-2.5 | 2.5 | 0.10−0.013 | 0.22−0.025 | 0.32−0.038 | 0.45 | 0.45 |
| 1-4.0 | 4.0 | ||||||
| 1-6.0 | 6.0 | ||||||
| 2 | 2-2.5 | 2.5 | 0.12−0.015 | 0.20−0.022 | 0.32−0.037 | 0.60 | 0.59 |
| 2-4.0 | 4.0 | ||||||
| 2-6.0 | 6.0 | ||||||
| 3 | 3-2.5 | 2.5 | 0.14−0.018 | 0.18−0.02 | 0.32−0.038 | 0.78 | 0.76 |
| 3-4.0 | 4.0 | ||||||
| 3-6.0 | 6.0 | ||||||
| 4 | 4-2.5 | 2.5 | 0.16−0.02 | 0.16−0.021 | 0.32−0.041 | 1.00 | 1.01 |
| 4-4.0 | 4.0 | ||||||
| 4-6.0 | 6.0 | ||||||
| 5 | 5-2.5 | 2.5 | 0.18−0.02 | 0.14−0.011 | 0.32−0.031 | 1.29 | 1.24 |
| 5-4.0 | 4.0 | ||||||
| 5-6.0 | 6.0 | ||||||
| 6 | 6-2.5 | 2.5 | 0.20−0.011 | 0.12−0.012 | 0.32−0.023 | 1.67 | 1.75 |
| 6-4.0 | 4.0 | ||||||
| 6-6.0 | 6.0 | ||||||
| 7 | 7-2.5 | 2.5 | 0.22−0.015 | 0.10−0.004 | 0.32−0.019 | 2.20 | 2.14 |
| 7-4.0 | 4.0 | ||||||
| 7-6.0 | 6.0 | ||||||
| SER, μΩ·cm | ||||||
|---|---|---|---|---|---|---|
| Sample Zone (see Figure 1) | Al-6%Mg-0.22%Sc-0.10%Zr | Al-6%Mg-0.16%Sc-0.16%Zr | Al-6%Mg-0.10%Sc-0.22%Zr | |||
| Center | Edge | Center | Edge | Center | Edge | |
| #1 (bottom of the ingot) | 6.34 ± 0.02 | 6.49 ± 0.14 | 6.34 ± 0.02 | 6.44 ± 0.04 | 6.31 ± 0.02 | 6.42 ± 0.05 |
| #5 (center of the ingot) | 6.23 ± 0.04 | 6.47 ± 0.07 | 6.46 ± 0.11 | 6.56 ± 0.07 | 6.19 ± 0.07 | 6.38 ± 0.04 |
| #9 (top of the ingot) | 6.22 ± 0.03 | 6.40 ± 0.05 | 6.31 ± 0.10 | 6.47 ± 0.06 | 6.20 ± 0.08 | 6.34 ± 0.05 |
| #10 (remainder) | 6.15 ± 0.04 | 6.33 ± 0.05 | 6.23 ± 0.09 | 6.49 ± 0.06 | 6.17 ± 0.07 | 6.38 ± 0.05 |
| Al–2.5%Mg–Sc–Zr Alloys | ||||||||
| Alloy # | 1-2.5 | 2-2.5 | 3-2.5 | 4-2.5 | 5-2.5 | 6-2.5 | 7-2.5 | |
| ρ0, μΩ·cm | Cast | 4.50 ± 0.04 | 4.59 ± 0.03 | 4.37 ± 0.03 | 4.62 ± 0.03 | 4.55 ± 0.02 | 4.54 ± 0.03 | 4.50 ± 0.03 |
| UFG | 4.54 ± 0.04 | 4.58 ± 0.04 | 4.55 ± 0.03 | 4.52 ± 0.03 | 4.56 ± 0.03 | 4.55± 0.04 | 4.49 ± 0.04 | |
| ∆ρmax, μΩ·cm | Cast | 0.30 | 0.33 | 0.35 | 0.38 | 0.34 | 0.39 | 0.38 |
| UFG | 0.42 | 0.44 | 0.46 | 0.44 | 0.35 | 0.40 | 0.36 | |
| ρth, μΩ·cm | 4.59 | 4.60 | 4.60 | 4.61 | 4.61 | 4.62 | 4.62 | |
| Al–4%Mg–Sc–Zr alloys | ||||||||
| Alloy # | 1-4.0 | 2-4.0 | 3-4.0 | 4-4.0 | 5-4.0 | 6-4.0 | 7-4.0 | |
| ρ0, μΩ·cm | Cast | 5.25 ± 0.04 | 5.37 ± 0.04 | 5.34 ± 0.05 | 5.36 ± 0.05 | 5.37 ± 0.05 | 5.34 ± 0.04 | 5.39 ± 0.06 |
| UFG | 5.41 ± 0.03 | 5.43 ± 0.04 | 5.43 ± 0.04 | 5.43 ± 0.03 | 5.49 ± 0.04 | 5.48 ± 0.05 | 5.52 ± 0.05 | |
| ∆ρmax, μΩ·cm | Cast | 0.25 | 0.35 | 0.33 | 0.32 | 0.44 | 0.35 | 0.40 |
| UFG | 0.37 | 0.37 | 0.35 | 0.33 | 0.26 | 0.26 | 0.24 | |
| ρth, μΩ·cm | 5.30 | 5.31 | 5.31 | 5.32 | 5.32 | 5.33 | 5.33 | |
| Al–6%Mg–Sc–Zr alloy | ||||||||
| Alloy # | 1-6.0 | 2-6.0 | 3-6.0 | 4-6.0 | 5-6.0 | 6-6.0 | 7-6.0 | |
| ρ0, μΩ·cm | Cast | 6.27 ± 0.04 | 6.30 ± 0.04 | 6.30 ± 0.05 | 6.32 ± 0.05 | 6.33 ± 0.06 | 6.34 ± 0.04 | 6.35 ± 0.05 |
| UFG | 6.31 ± 0.03 | 6.28 ± 0.03 | 6.38 ± 0.04 | 6.51 ± 0.04 | 6.40 ± 0.04 | 6.39 ± 0.04 | 6.38 ± 0.04 | |
| ∆ρmax, μΩ·cm | Cast | 0.10 | 0.16 | 0.16 | 0.17 | 0.17 | 0.17 | 0.22 |
| UFG | 0.11 | 0.15 | 0.21 | 0.30 | 0.22 | 0.18 | 0.22 | |
| ρth, μΩ·cm | 6.49 | 6.49 | 6.50 | 6.50 | 6.51 | 6.51 | 6.52 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuvil’deev, V.; Nokhrin, A.; Kozlova, N.; Shadrina, I.; Bobrov, A.; Kopylov, V.; Komel’kov, A.; Morozkina, E. Combined Effect of the Sc/Zr Ratio and Mg Concentration on the Intergranular Corrosion Resistance of Al–Mg–Sc–Zr Alloys: A Case of Cast Alloys and Ultrafine-Grained Alloys. Metals 2025, 15, 372. https://doi.org/10.3390/met15040372
Chuvil’deev V, Nokhrin A, Kozlova N, Shadrina I, Bobrov A, Kopylov V, Komel’kov A, Morozkina E. Combined Effect of the Sc/Zr Ratio and Mg Concentration on the Intergranular Corrosion Resistance of Al–Mg–Sc–Zr Alloys: A Case of Cast Alloys and Ultrafine-Grained Alloys. Metals. 2025; 15(4):372. https://doi.org/10.3390/met15040372
Chicago/Turabian StyleChuvil’deev, Vladimir, Aleksey Nokhrin, Nataliya Kozlova, Iana Shadrina, Aleksandr Bobrov, Vladimir Kopylov, Andrey Komel’kov, and Ekaterina Morozkina. 2025. "Combined Effect of the Sc/Zr Ratio and Mg Concentration on the Intergranular Corrosion Resistance of Al–Mg–Sc–Zr Alloys: A Case of Cast Alloys and Ultrafine-Grained Alloys" Metals 15, no. 4: 372. https://doi.org/10.3390/met15040372
APA StyleChuvil’deev, V., Nokhrin, A., Kozlova, N., Shadrina, I., Bobrov, A., Kopylov, V., Komel’kov, A., & Morozkina, E. (2025). Combined Effect of the Sc/Zr Ratio and Mg Concentration on the Intergranular Corrosion Resistance of Al–Mg–Sc–Zr Alloys: A Case of Cast Alloys and Ultrafine-Grained Alloys. Metals, 15(4), 372. https://doi.org/10.3390/met15040372







