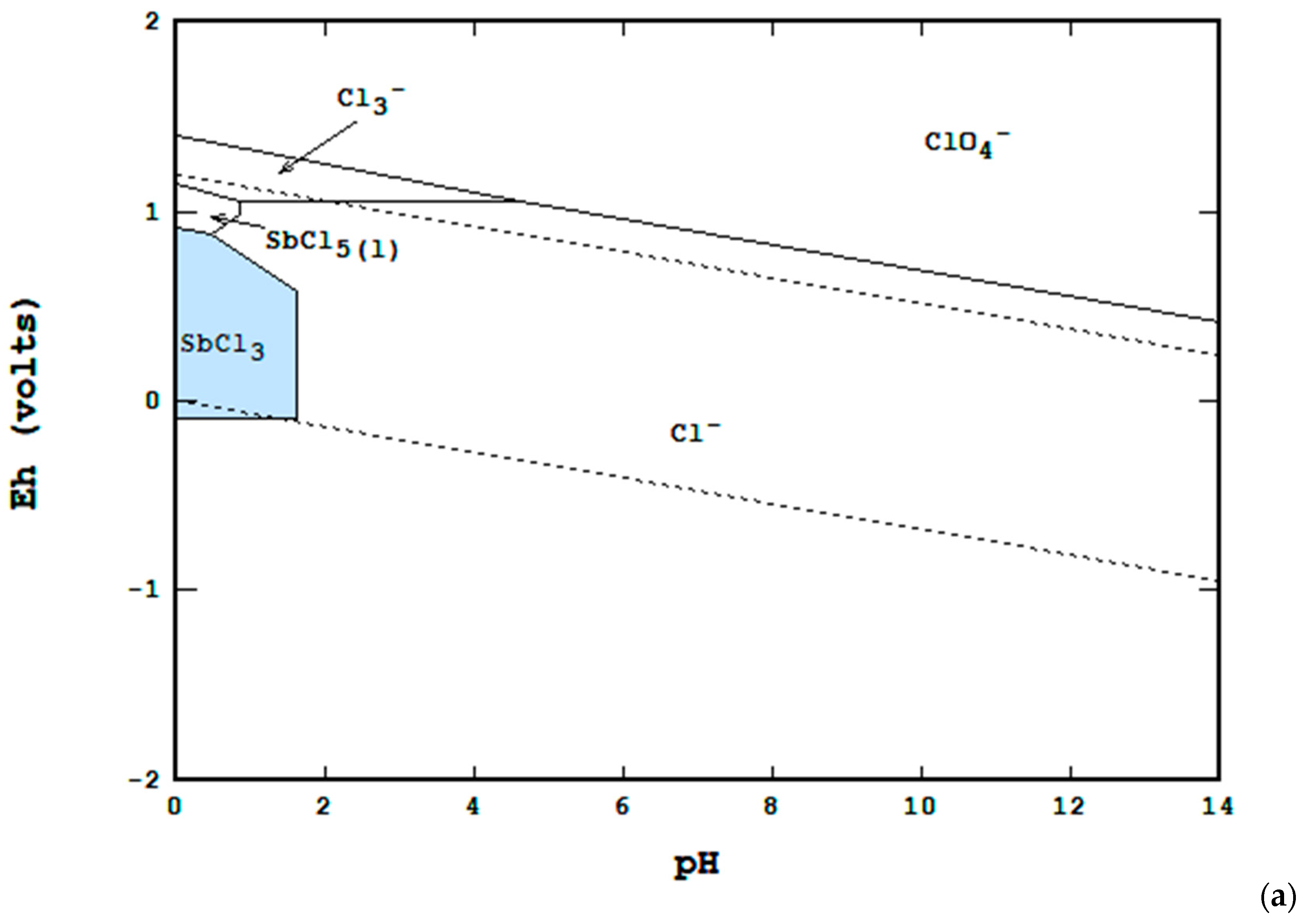
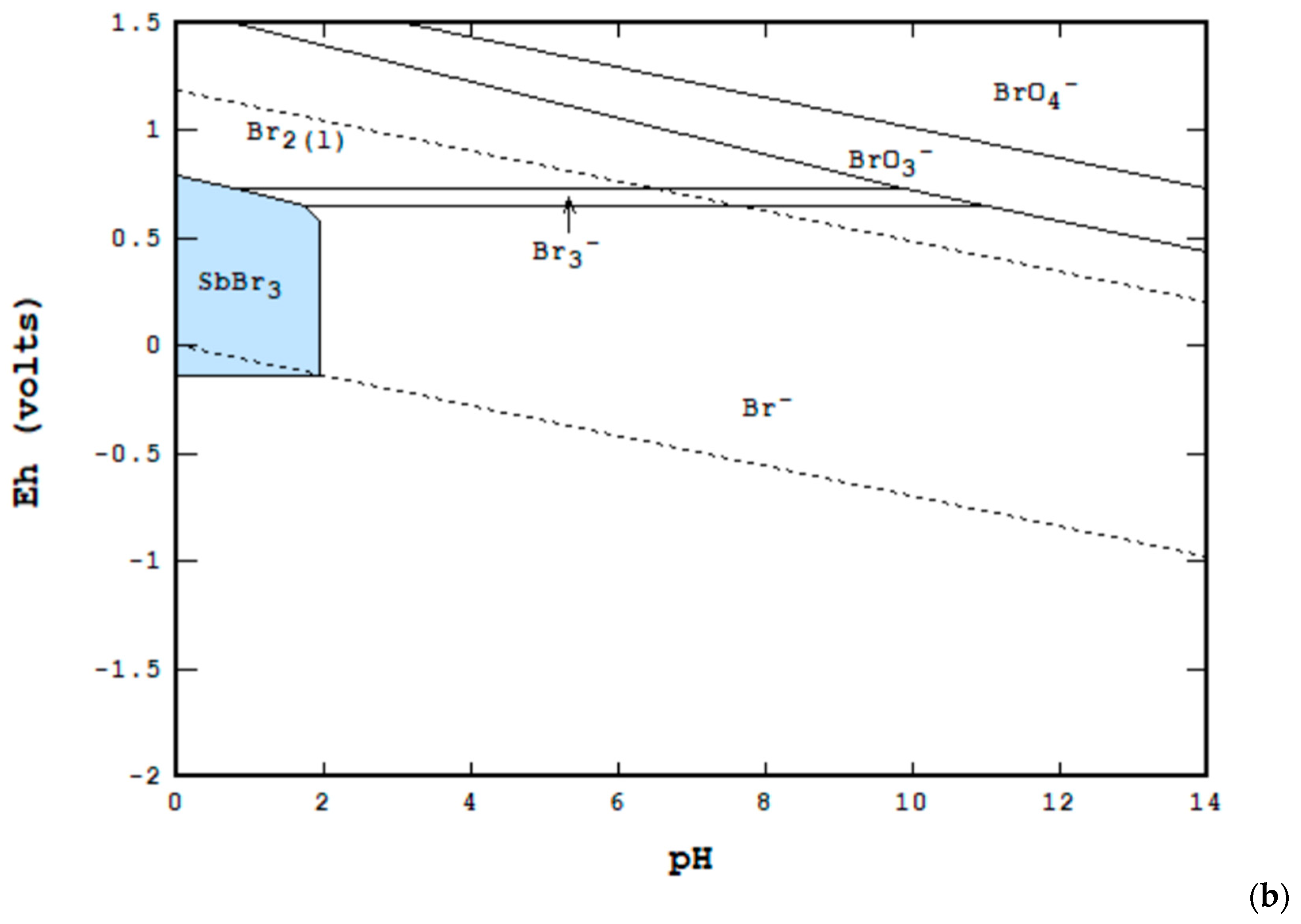
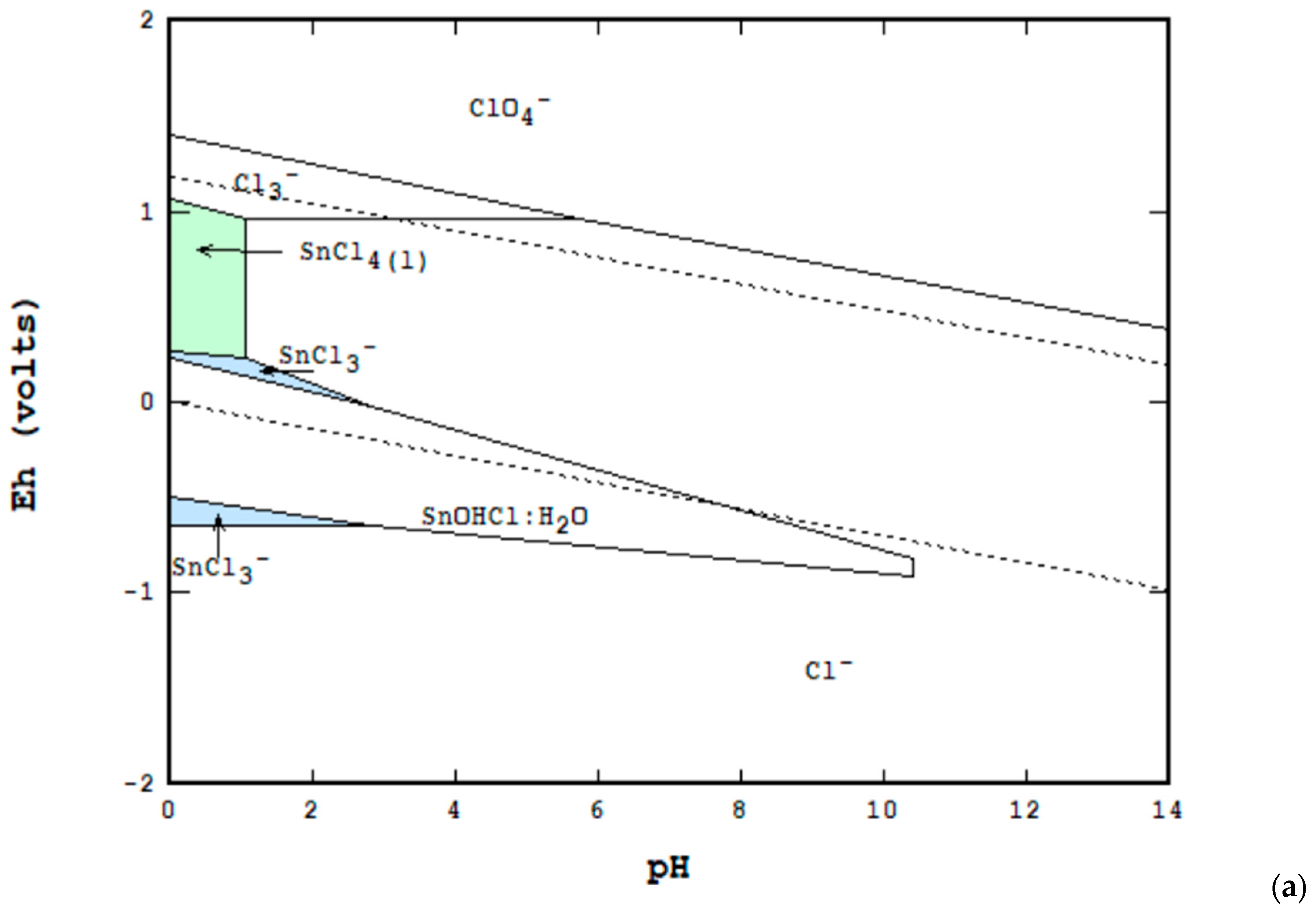
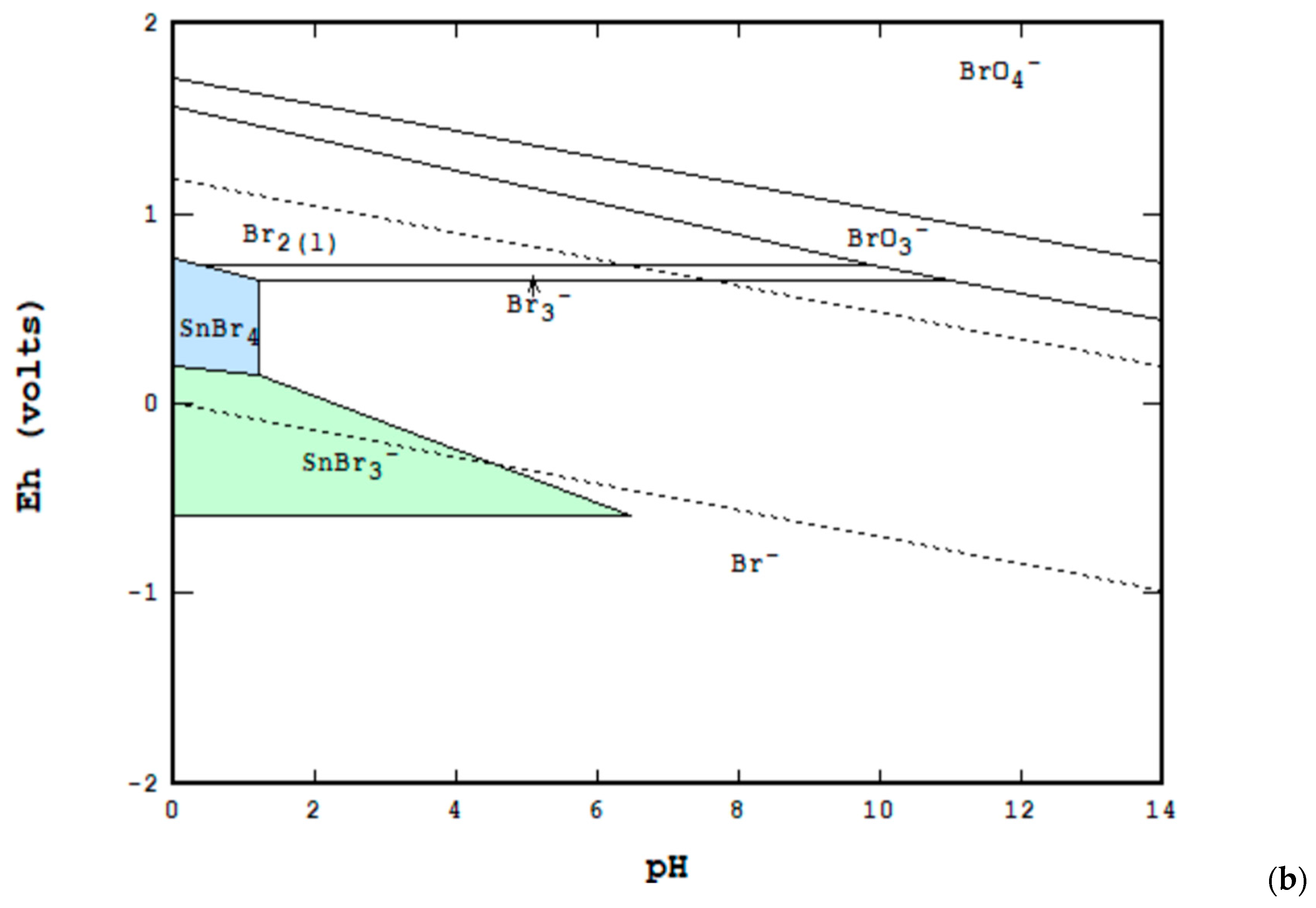
3. Materials and Methods
While developing the procedure, the focus was on testing scenarios that would represent the general behavior of antimony in hydrobromic acid (HBr). The chosen variables were temperature, residence time, acid concentration, and liquid-to-solid ratio (
Table 1). A second set of experiments was designed to determine the effect of hydrobromic acid (HBr) (MilliporeSigma, Burlington, MA, USA) on the sample after initially treating it with nitric acid (HNO
3) (MilliporeSigma, Burlington, MA, USA) to remove part of the lead content (
Table 2). The quantitative analysis of this material prior to treatment is presented in
Table 3.
Additionally, the sample was sent for mineral characterization.
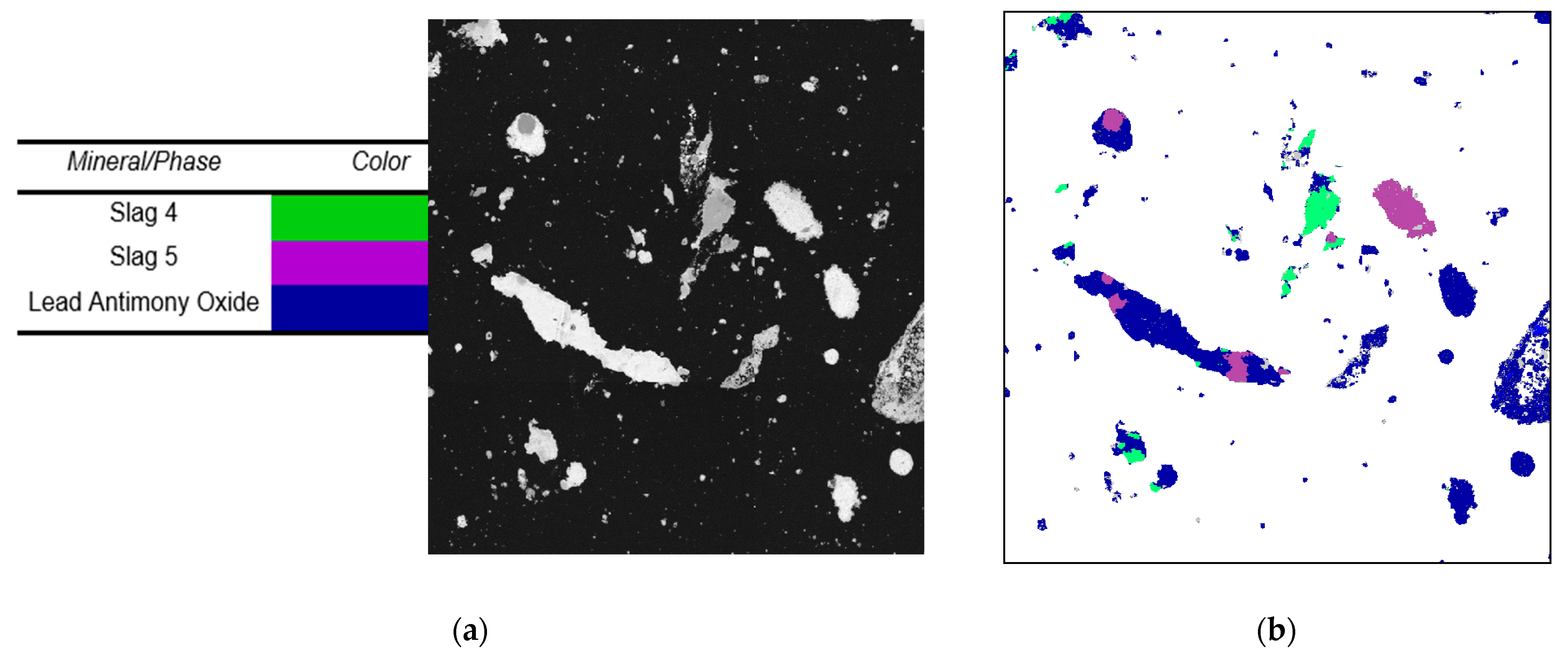
Figure 4 shows the backscatter images received from the SEM and AMICS (Automated Material Identification and Classification System) for the sample. The SEM and AMICS reports received labeled two multi-element phases as slag 4 and 5 due to how complex the samples received were. When comparing the SEM, AMICS, and safety data sheet provided by the supplier, we were able to determine that the phases for lead, tin, and antimony are lead monoxide (PbO), tin oxide (SnO), and antimony trioxide (Sb
2O
3).
Detailed information on the slag phase composition can be found in
Table 4, which allows us to see that most elements are in an oxide phase.
The bulk samples from the lead–acid battery recycling plant were crushed in a Massco single-toggle jaw crusher (Mine & Smelter Supply Co., Denver, CO, USA) for each experiment to a particle size of <2 cm, ground with a Bico VP-1989 ring (BICO Inc., Burbank, CA, USA), puck pulverizer to a particle size < 150 μm, and separated by particle size utilizing a Tyler RX-29 mechanical sieve shaker (W.S. Tyler, Mentor, OH, USA). Then, the samples were carefully split into smaller sample sizes adequate for one leach test. Solutions were prepared for the leaching test. The first leach test solutions were prepared from ACS 48% HBr diluted with di-ionized water. The second leach test solutions were prepared from ACS 68% HNO3 diluted with di-ionized water. The solution was then added to the beaker and heated to the appropriate temperature.
For the first set of experiments, a total volume of 250 milliliters of hydrobromic acid was added to a 500 mL beaker and heated on a hot plate to the appropriate temperature (
Table 1). Additionally, a magnetic stirring bar was used to agitate the solution to 400 RPM, which was monitored throughout the test. Once the temperature had been reached, the appropriate solids were added to the hot solution. The beaker was then sealed from the top and the leaching timer was started. Upon completion, the beaker was removed from the hot plate and the leach slurry was filtered and measured volumetrically while the solids were rinsed with di-ionized water, dried to remove excess moisture, and carefully weighed.
For the second set of experiments, a total volume of 250 milliliters of nitric acid was added to a 500 mL beaker and heated in a hot plate to the appropriate temperature with a magnetic stirring bar to agitate the solution to 400 RPM (
Table 2). Once the temperature had been reached, 10 g of solid feed was added to the hot solution. The beaker was then sealed from the top and the leaching timer for 2 h was started. Once the two hours had passed, the beaker was removed from the hot plate and the leach slurry was filtered and measured volumetrically while the solids were rinsed with di-ionized water, dried to remove excess moisture, and carefully weighed. Once the solids were completely dried, we split the solids to have a representative sample of 0.5 g for elemental analysis while the rest went on to the next acid stage.
To continue the second set of experiments, the same procedure presented in the nitric acid stage was used; however, we changed the acid from nitric acid to hydrobromic acid and the concentrations used.
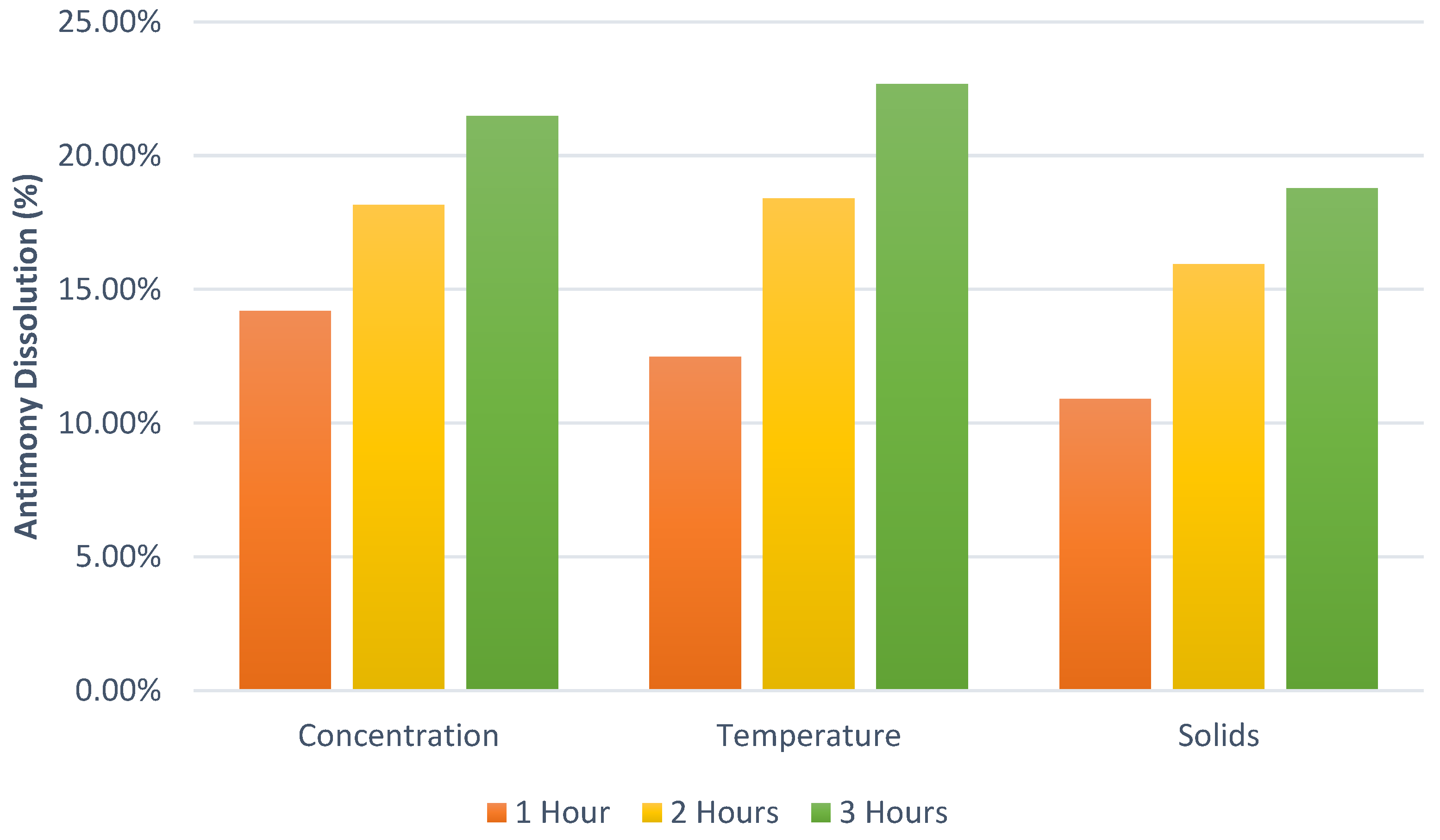
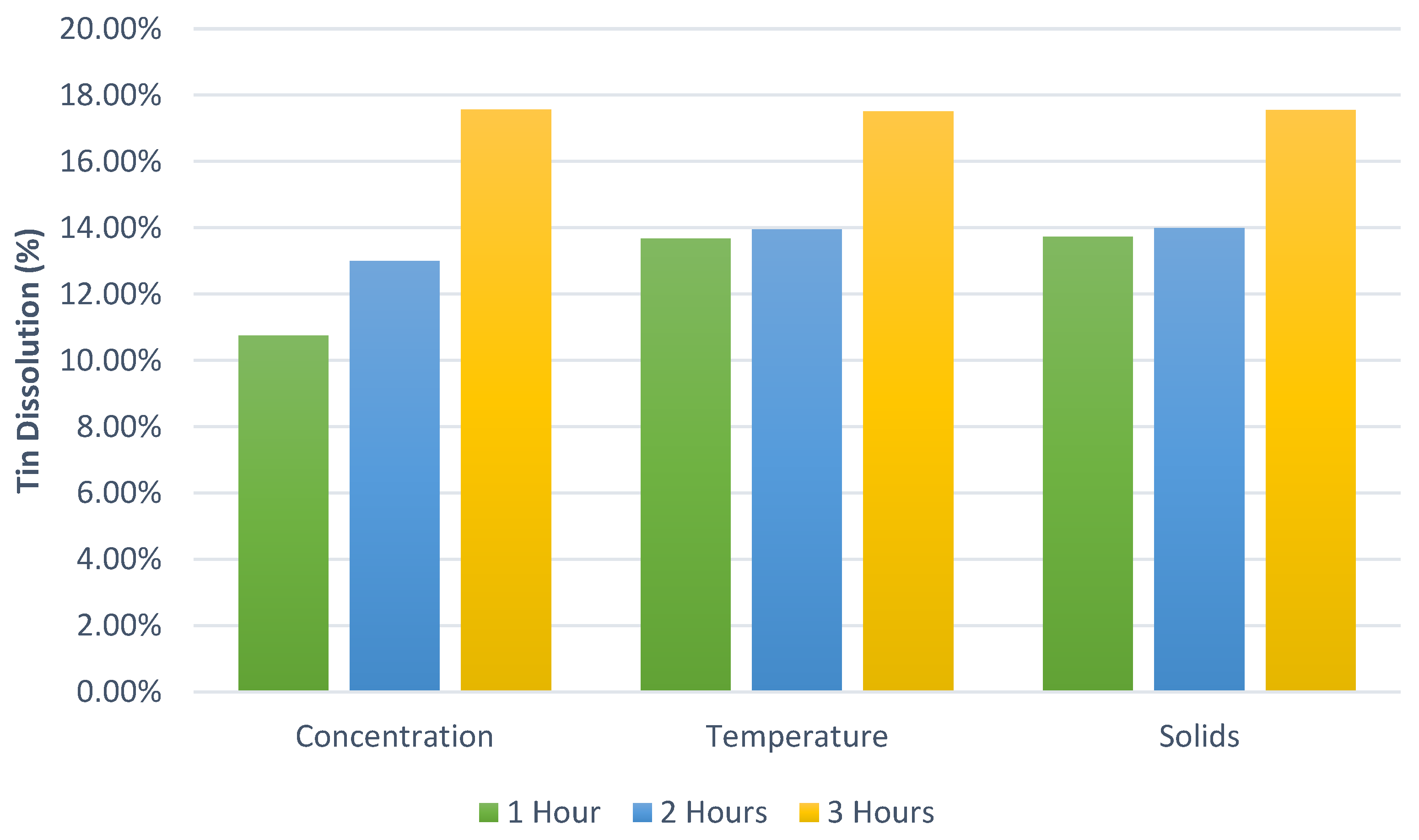
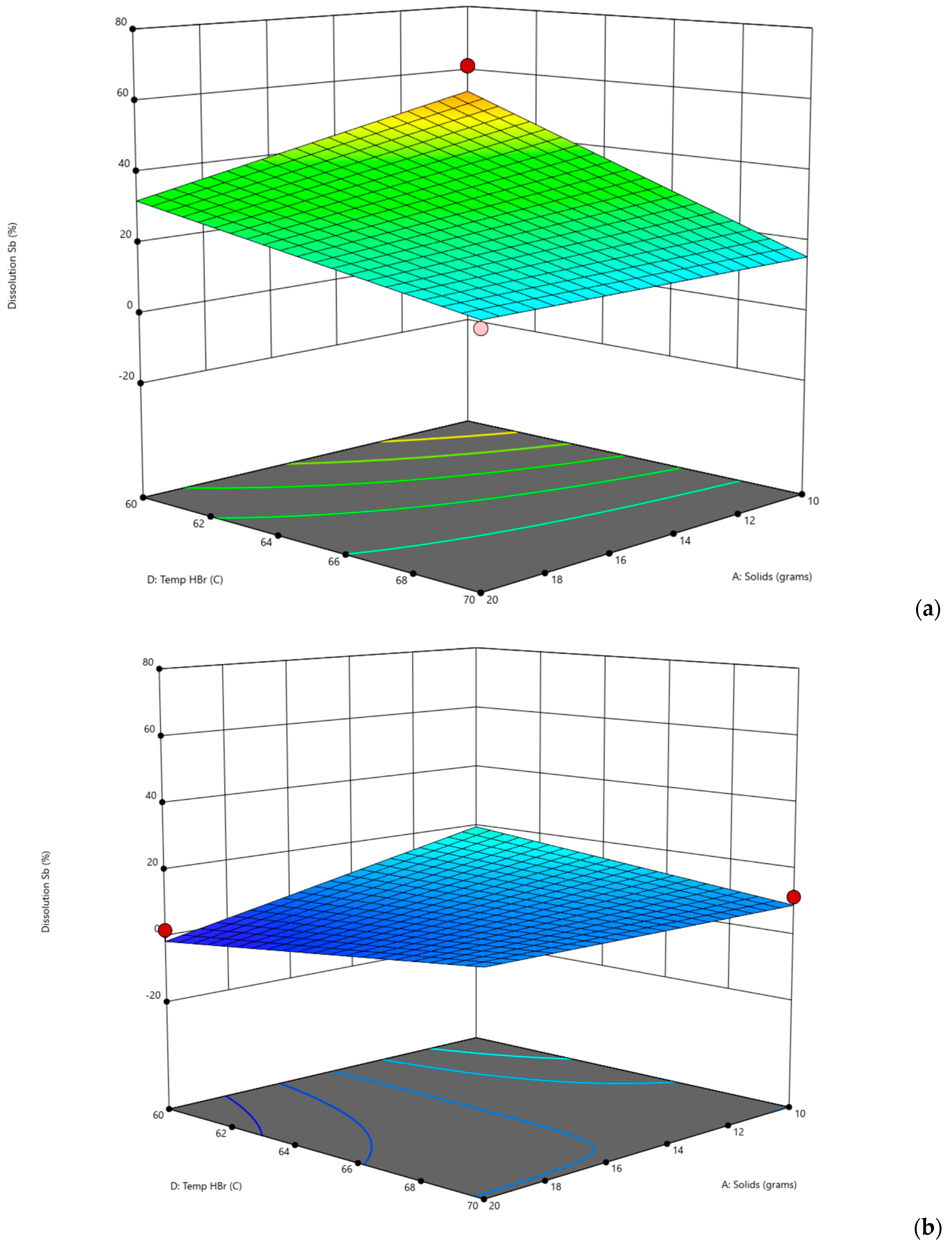
One of the best ways to represent the behavior of antimony in hydrobromic acid was to perform a design of experiment (DoE). The first set of experiments consisted of a total of 40 laboratory experiments (
Table 5). For the DoE, temperatures of 50 °C, 60 °C, and 70 °C were selected to avoid higher temperatures; reaction times of 1, 2, and 3 h were selected for shorter times and test reaction rates; HBr concentrations of 0.5 M, 0.75 M, and 1 M were selected; however, there is a lack of studies with hydrobromic acid as a leaching reagent, for which we decided to use a slightly wider range of concentrations.
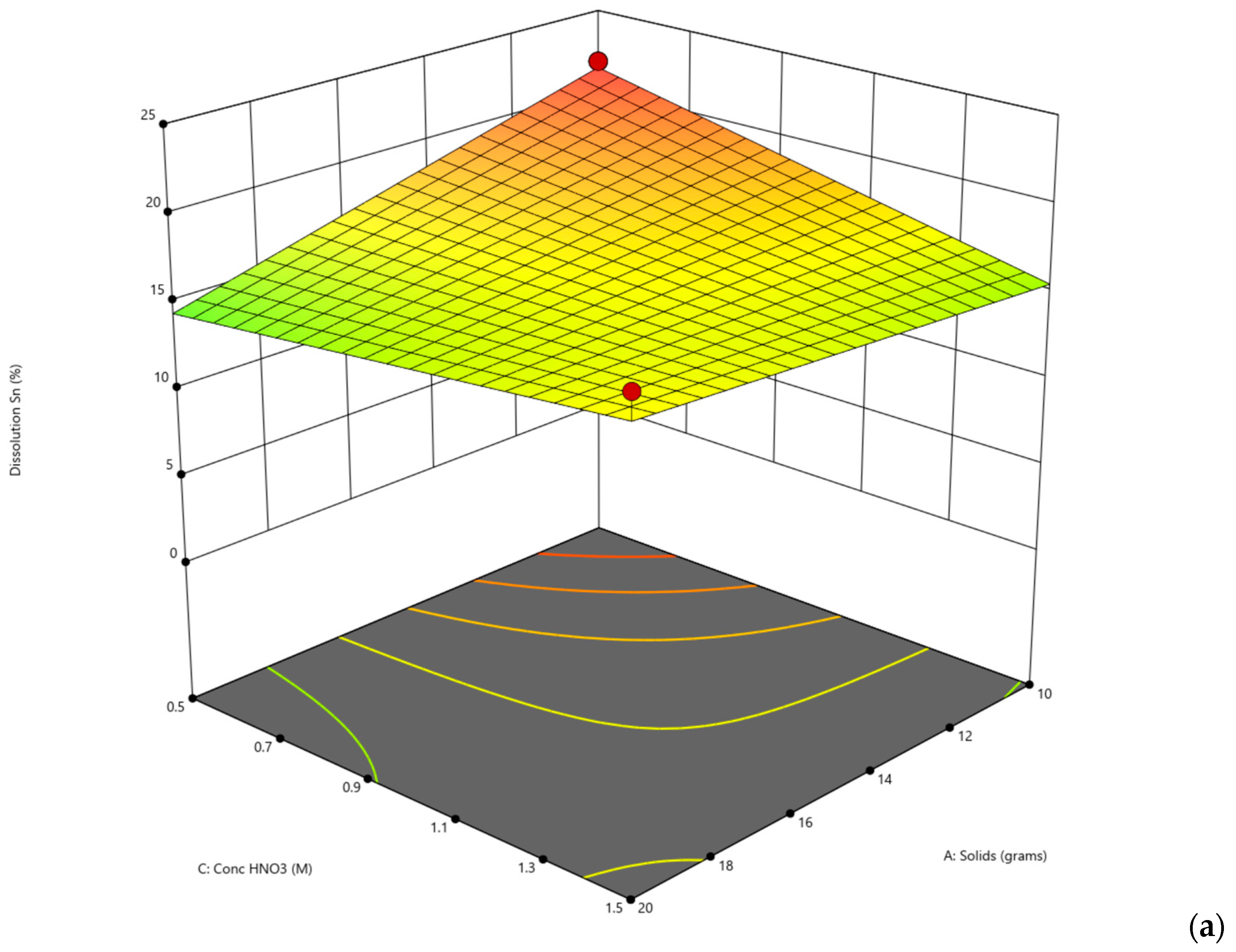
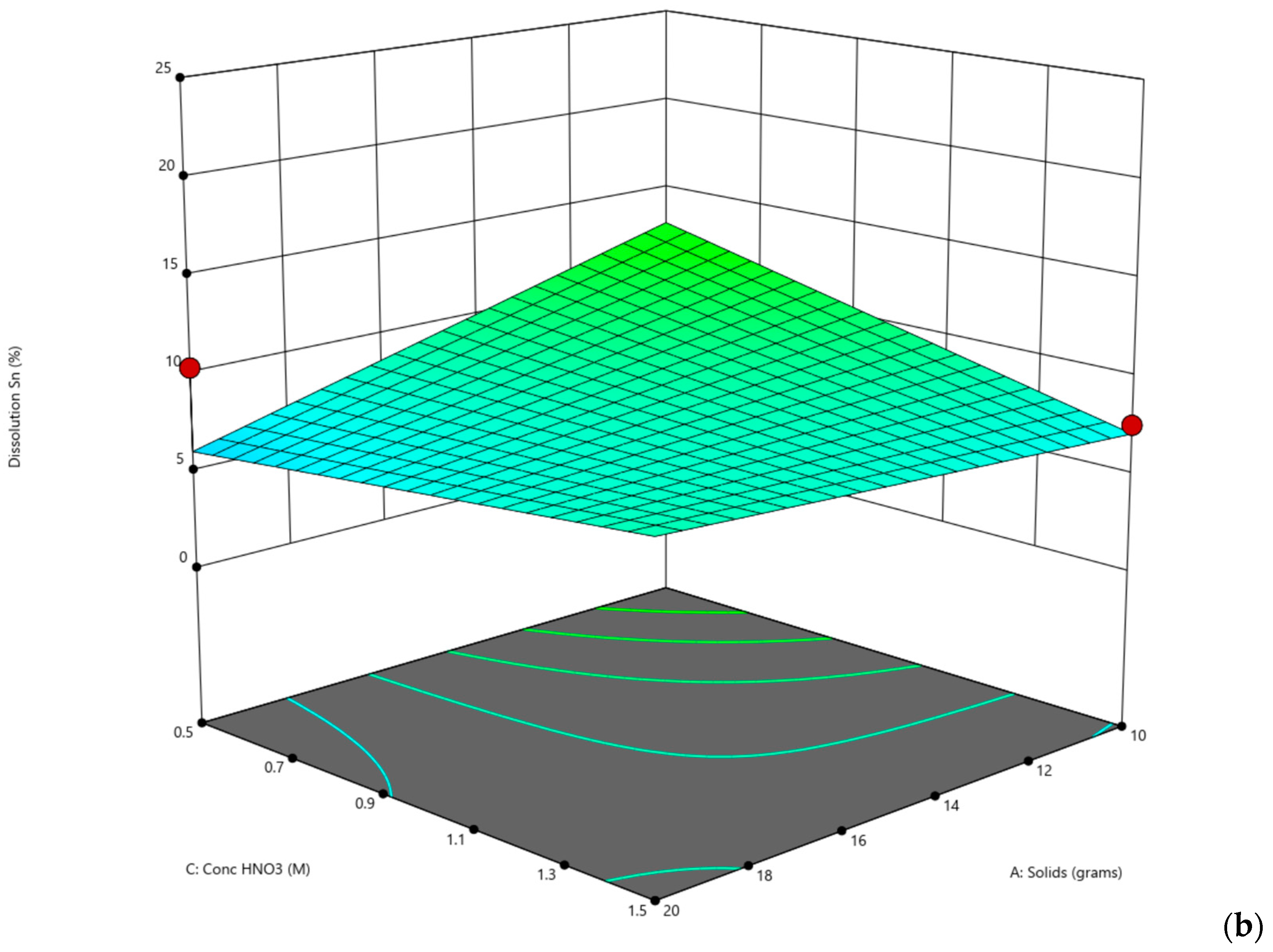
The second set of experiments consisted of a total of 16 laboratory experiments (
Table 6). For the DoE, the level in each variable was reduced from 3 to 2. The nitric acid values for both temperature and concentration were selected from Ichlas’ paper on selective nitric acid leaching [
24]. The hydrobromic acid values for temperature were selected based on the first set of experiments since they yielded better results, while the concentration selected was higher, since not much antimony was dissolved into the solution.