Abstract
The Al-Ti-C alloy is the most widely used grain refiner for Al-7Si alloys. However, TiC particles in Al-7Si alloys tend to aggregate and settle, thereby reducing their refinement efficiency. In the present paper, a novel Al-3Ti-4.35La master alloy was developed, and its influence on the stability of TiC particles in Al-7Si alloys was investigated by XRD, SEM, and TEM. The results show that when the Al-TiC alloy is added to the Al-7Si alloy, TiC will accumulate and settle obviously after holding the alloy for a certain time (15 min, 30 min, and 60 min). When the Al-TiC alloy and the Al-3Ti-4.35La master alloy are added to the Al-7Si alloy, the aggregation and settlement of TiC are weakened under the same holding time. Additionally, the refinement effect of TiC is enhanced. The Ti and La elements dissolved by Ti2Al20La in the Al-3Ti-4.35La master alloy are absorbed on the surface of the TiC particles, which improves the wettability between the TiC particles and the aluminum melt, reduces the agglomeration and sedimentation of TiC particles in the aluminum melt, and improves its stability.
1. Introduction
Al-Si casting alloys have garnered extensive application in lightweight automotive structural materials due to their high specific strength, excellent mechanical properties, low manufacturing cost, and easy recycling [1,2,3,4]. However, the large α-Al grains in the microstructure of unrefined Al-Si alloys can significantly reduce the mechanical properties of the alloys. Therefore, adding refiners to Al-Si alloys during casting has become the main means to improve their mechanical properties [5,6,7,8].
Currently, Al-Ti-B is the commonly used refiner [9,10]. However, the Al-Ti-B grain refiner loses its refining ability when Si and Zr elements are added at about 0.2 wt.% [11]. Additionally, the Al-Ti-C grain refiner resists Si and Zr “poisoning” and uses TiC as its primary nucleating particle to refine α-Al [12,13]. TiC particles are about one-third the size of TiB2, which reduces their settling in the aluminum melt and enhances grain refinement [14].
However, the stoichiometric ratio between Ti and C in the TiC crystal structure is not fixed, and a large number of vacancy defects are prone to occur in the lattice or atomic gap, therefore decreasing the stability of TiC [15]. In addition to the structural instability of TiC, studies have shown that Si also significantly impacts TiC particles. When the Si content exceeds 3.5%, the stability of TiC particles will be reduced, and the decomposition of TiC particles will be accelerated [16]. To improve the stability of TiC particles in Al-Si alloys, Nie et al. [17] added B and N elements to TiC particles to form TiCx by regulating the chemical properties of TiC particles. Eskin et al. [18] improved the agglomeration of TiC particles and promoted the nucleation efficiency of TiC by ultrasonic melt treatment technology. Recent research [19,20] has shown that by combining rare earth elements with Al-Ti-B/C, Al-Ti-B/C-RE-refining modifiers can effectively change the coarse grain of Al-Si alloys. Wu et al. [21] proposed that the wettability of liquid aluminum and graphite can be improved by adding a small amount of Ce into molten aluminum. Zhao et al. [22] showed that Ti2Al20RE will release rare earth elements and Ti to the A356 alloy, enriching the surface of TiC particles and improving the stability of TiC particles. Furthermore, the incorporation of the rare earth element La into the Al-Ti-C alloy not only facilitated further grain refinement but also significantly bolstered the material’s mechanical properties [23,24,25]. Despite those findings, the direct evidence regarding the effect of rare earth elements on TiC’s nucleation ability and stability remains insufficient. Furthermore, how to improve the stability of TiC particles by adding rare earth elements, reduce the aggregation and sedimentation of TiC particles, and ensure their even dispersion in Al-Si alloys requires further study.
Based on the aforementioned findings, the Al-3Ti-4.35La master alloy was combined with the Al-7Si and Al-TiC master alloys to investigate the influence of the Al-3Ti-4.35La master alloy on the stability of TiC particles in hypoeutectic Al-7Si alloys. This study analyzed the mechanistic influence of the Al-3Ti-4.35La master alloy on the aggregation and sedimentation behavior of TiC particles in Al-7Si alloys. The current research provides a theoretical basis for the industrial application of Al-TiC master alloys.
2. Experiment
The Al-3Ti-4.35La master alloy and the Al-TiC alloy were prepared by the in situ reaction synthesis method of aluminum melt. For the specific preparation process, see references [11,26]. The detailed compositions of the Al-3Ti-4.35La master alloy, the Al-TiC alloy, and the Al-7Si alloy are shown in Table 1, which were measured by an X-ray spectrometer(Panako Zetium, Breda, The Netherlands).

Table 1.
Alloy composition (wt%).
To study the effect of the Al-3Ti-4.35La master alloy on the aggregation of TiC particles, the Al-7Si alloy, Al-3Ti-4.35La master alloy, and Al-TiC alloy (mass ratio, Al-3Ti-4.35La:Al-TiC:Al-7Si = 1:1:2) were put into a graphite crucible at 750 °C, holding for 15 min. A total of 1wt% C2Cl6 was added for refining and degassing. The alloy was cast into a steel mold (a diameter of 10 mm) with a preheating temperature of 200 °C, as shown in Process Ⅰ of Figure 1. The Al-7Si alloy and Al-TiC alloy (mass ratio, Al-TiC:Al-7Si = 1:2) were prepared as a control group. To further investigate the sedimentation behavior of TiC particles in the hypoeutectic Al-7Si alloy, the alloy according to the above mass ratio is held at 750 °C for 30 min and 60 min, respectively. After refining and degassing, the alloy was cast into a square mold with a preheating temperature of 200 °C, as shown in Process Ⅱ of Figure 1. The sample numbers are shown in Table 2.
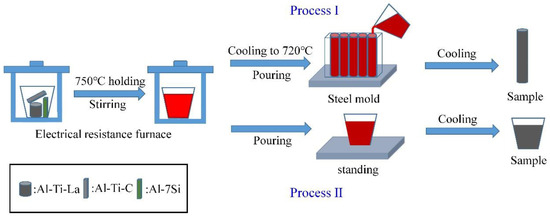
Figure 1.
Flow chart of the interfusion experiment between the Al-3Ti-4.35La master alloy, the Al-7Si alloy, and the Al-TiC alloy.

Table 2.
Sample number and mass ratio of different alloys.
The Al-3Ti-4.35La master alloy, Al-TiC alloy, and Al-7Si alloy by the addition of the Al-3Ti-4.35La master alloy and the Al-TiC alloy were characterized by using a Thermo ARL X-ray diffractometer (XRD, Thermo Fisher Scientific, Waltham, MA, USA) and monochromatic Cu Kα radiation at 2θ angles ranging from 20 to 90°. The microstructure of the alloy was analyzed by a Tescan Mira4 SEM and Xplore 30(Tescan, Brno, Czech Republic). Talos F200X G2 transmission electron microscopy (TEM, Thermo Fisher Scientific, Waltham, MA, USA) with selected electron diffraction (SAED, Thermo Fisher Scientific, Waltham, MA, USA) was used to confirm the crystal structure, and the TEM samples were thinned by double-jet electrodeposition using a mixture of nitric acid.
3. Results and Discussion
3.1. Analysis of Al-TiC and Al-3Ti-4.35La Master Alloys
Figure 2a shows the microstructure of the Al-TiC master alloy, which has a large number of granular particles dispersed in the matrix. Figure 2b shows that the main phases of the Al-TiC master alloy prepared by the in situ reaction method are α-Al and TiC. Figure 2c,d is a larger version of Figure 2a and an EDS analysis. The Ti and C elements are evenly distributed in the granular phase. The ratio of Ti:C in the granular phase is close to 1:1. Combined with an XRD analysis in Figure 2b, TiC particles are abundant in the Al-TiC master alloy.
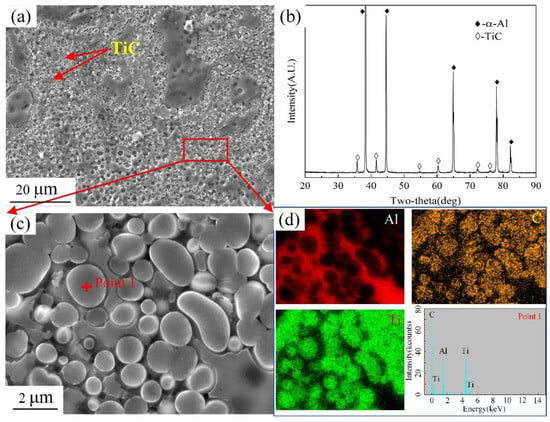
Figure 2.
Microstructure analysis of Al-TiC master alloy: (a) SEM image of Al-TiC master alloy; (b) XRD pattern of Al-TiC master alloy; (c) enlarged view of (a); (d) EDS analysis in (c).
Figure 3a shows the microstructure of the Al-3Ti-4.35La master alloy. A large number of white bulk second phases are distributed in the matrix, and some of the white phases are mixed with gray phases. Additionally, fibrous white structures exist at the grain boundaries. Figure 3b shows the XRD pattern of the Al-3Ti-4.35La master alloy. The main phases of the Al-3Ti-4.35La master alloy are α-Al and Ti2Al20La, which also have a small amount of the Al3Ti and Al11La3 phases. Figure 3c,d is a larger version of Figure 3a and an EDS analysis. Combined with an XRD analysis in Figure 3b, the white blocky, fibrous, and gray phases of the Al-3Ti-4.35La master alloy are Ti2Al20La, Al11La3, and Al3Ti, respectively.
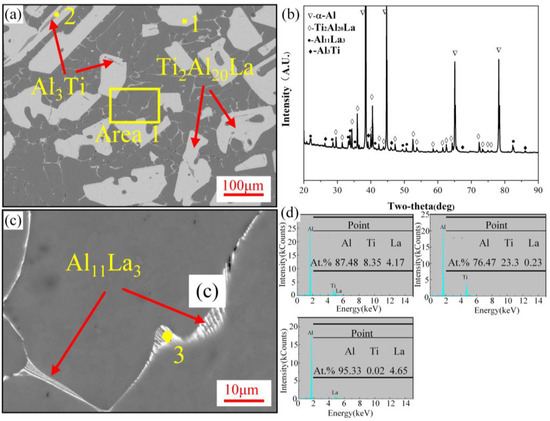
Figure 3.
Microstructure analysis of Al-3Ti-4.35La master alloy: (a) SEM image of Al-3Ti-4.35La master alloy; (b) XRD pattern of Al-3Ti-4.35La master alloy; (c) enlarged view of (a); (d) EDS analysis.
3.2. Influence of Al-3Ti-4.35La Master Alloy on the Aggregation Property of TiC
Figure 4 shows the XRD pattern after adding the Al-TiC master alloy (A0) and adding the Al-TiC and the Al-3Ti-4.35La master alloy (A1) to the Al-7Si alloy at 750 °C for 15 min. The intensity of TiC corresponding to the diffraction peak in the A1 sample is significantly higher than that in the A0 sample. The number of TiC particles in the Al-7Si alloy increases after adding the Al-3Ti-4.35La master alloy.
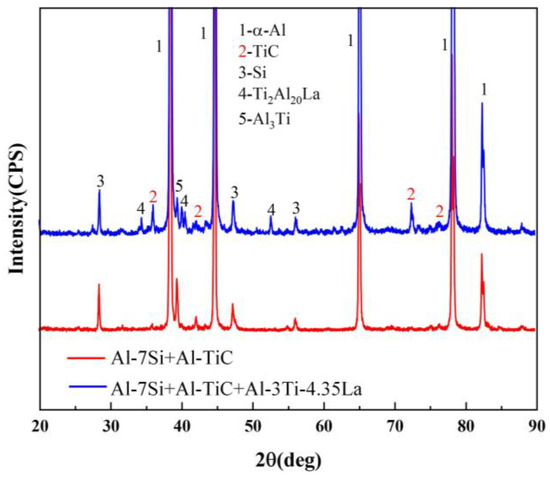
Figure 4.
XRD patterns of adding Al-TiC alloy to Al-7Si alloy (A0) and adding Al-3Ti-4.35 La alloy and Al-TiC alloy to Al-7Si alloy (A1) for a holding time of 15 min.
Figure 5 shows the SEM and EDS analyses of the Al-7Si alloy with the addition of the Al-TiC master alloy (A0). The TiC particles tend to aggregate. This is mainly due to the affinity of the TiC particles themselves and their poor wettability with other particles in the melt [27]. The aggregation of TiC particles will reduce the effective heterogeneous nucleation sites of α-Al, thereby decreasing the refining effect.
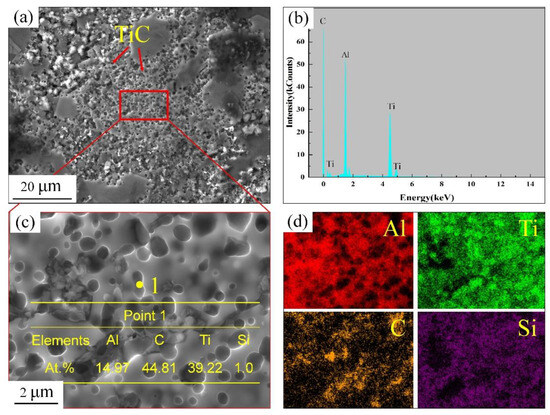
Figure 5.
Microstructure analysis of adding Al-TiC alloy to Al-7Si alloy for a holding time of 15 min, A0 sample: (a) SEM image; (b) EDS analysis; (c) enlarged view of (a); (d) mapping of Al, Ti, C, and La elements in (c).
Figure 6 shows the SEM and EDS analysis of the A1 sample. Figure 6a shows the SEM image of TiC in the Al-7Si alloy after composite addition, and the degree of TiC aggregation is significantly lower than that of the A0 sample. Figure 6b is the enlarged view of Figure 6a, and the EDS analysis is shown in Figure 6c–f. Meanwhile, the EDS results of each point in Figure 6b are shown in Table 3. The results show that the distribution of TiC particles in the A1 sample is strongly correlated with the distribution of free La, and La elements are distributed on the surface of TiC. At the same time, some of the La will combine with Si in the melt to form La-Si intermetallic compounds.
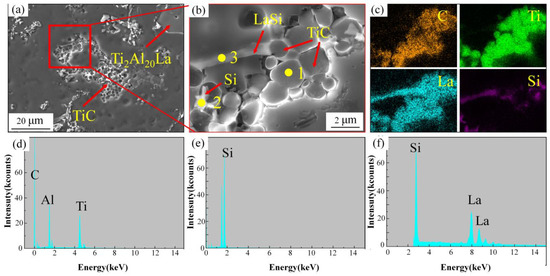
Figure 6.
SEM and EDS results of adding Al-3Ti-4.35 La alloy and Al-TiC alloy to Al-7Si alloy for a holding time of 15 min, A1 sample: (a) SEM images; (b) enlarged view of (a); (c) mapping of elements Al, Ti, C, and La in (b); (d–f) EDS analysis in (b).

Table 3.
Analysis results of EDS points (d–f) in Figure 6.
3.3. Influence of Al-3Ti-4.35La Master Alloy on the Settling Property of TiC
Figure 7 shows the macroscopic structure of A2~A5 samples. In sample A2, which was heated for 30 min, the grain size at the bottom of the sample was significantly smaller than that in the upper middle part of the sample. In sample A3, which is heated for 30 min after adding the Al-3Ti-4.35La master alloy, the grain size at the bottom and the upper middle part tended to be consistent, as shown in Figure 7a,b. The grain distribution of the A4 and A5 samples also has the same rule. When TiC particles exist alone, they are easy to settle to the bottom of the melt. TiC can be used as an effective heterogeneous nucleation site of α-Al, promote α-Al nucleation, and reduce the grain size of α-Al. Due to its sedimentation characteristics, the grain size at the bottom of the sample is smaller than the middle and upper parts. When Ti2Al20La and TiC particles co-exist, the sedimentation of the TiC particles can be effectively reduced so that the grain size distribution in the sample is consistent.

Figure 7.
Macroscopic structure of different samples (a) adding Al-TiC alloy to Al-7Si alloy for a holding time of 30 min, A2 sample; (b) adding Al-3Ti-4.35 La alloy and Al-TiC alloy to Al-7Si alloy for a holding time of 30 min, A3 sample; (c) adding Al-TiC alloy to Al-7Si alloy for a holding time of 60 min, A4 sample; (d) adding Al-3Ti-4.35 La alloy and Al-TiC alloy to Al-7Si alloy for a holding time of 60 min, A5 sample.
Figure 8 shows the XRD analysis results of the bottom region of the A2~A5 samples. After holding for 30min, the diffraction peak (θ = 42.10°) corresponding to TiC particles at the bottom of the sample with the Al-3Ti-4.35La master alloy added is significantly lower than that without the addition. After holding for 60 min, the diffraction peak intensity corresponding to TiC particles at the bottom of the sample with the Al-3Ti-4.35La master alloy is also lower than that without the addition. That is, the Al-3Ti-4.35La master alloy can weaken the sedimentation of TiC particles in Al-Si alloys.
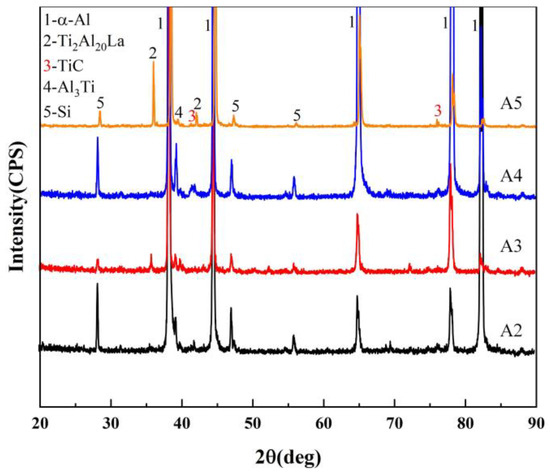
Figure 8.
XRD results of different samples: A2 (adding Al-TiC alloy to Al-7Si alloy for a holding time of 30 min), A3 (adding Al-3Ti-4.35 La alloy and Al-TiC alloy to Al-7Si alloy for a holding time of 30 min), A4 (adding Al-TiC alloy to Al-7Si alloy for a holding time of 60 min), A5 (adding Al-3Ti-4.35 La alloy and Al-TiC alloy to Al-7Si alloy for a holding time of 60 min).
Figure 9 shows the SEM and EDS images of the A2~A5 samples. There is an obvious black precipitate at the bottom of the samples, and the corresponding EDS surface scanning analysis reveals the enrichment of Ti and C elements. Combined with the XRD analysis results in Figure 8, the black precipitate is TiC particles. The precipitation of obvious TiC particles at the bottom of the sample without adding the Al-3Ti-4.35La master alloy is obvious, as shown in Figure 9a,b. However, TiC precipitation in the bottom of the alloy sample significantly decreases after adding the Al-3Ti-4.35La master alloy, as shown in Figure 9c,d.
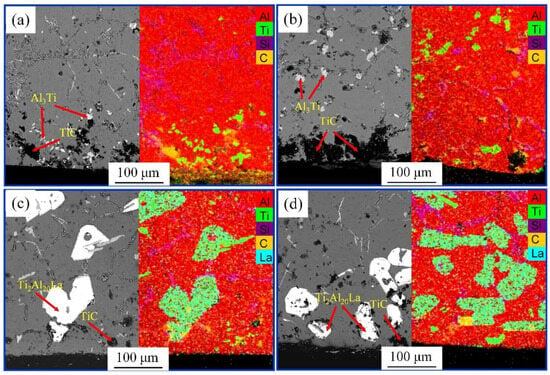
Figure 9.
SEM diagram of sample bottom of different samples: (a) adding Al-TiC alloy to Al-7Si alloy for a holding time of 30 min, A2 sample; (b) adding Al-TiC alloy to Al-7Si alloy for a holding time of 60 min, A4 sample; (c) adding Al-3Ti-4.35 La alloy and Al-TiC alloy to Al-7Si alloy for a holding time of 30 min, A3 sample; (d) adding Al-3Ti-4.35 La alloy and Al-TiC alloy to Al-7Si alloy for a holding time of 60 min, A5 sample.
3.4. Effect of Al-3Ti-4.35La Master Alloy on the Stability of TiC in Al-Si Alloy
From the above analysis, it can be seen that the addition of the Al-3Ti-4.35La master alloy reduces the agglomeration and settlement characteristics of TiC in Al-Si alloys, thus improving the refinement effect of TiC in Al-Si alloys. Studies have shown that the main reason for the decrease in TiC stability in Al-Si alloys [28] is that TiC decomposition occurs, as shown in the following equation:
3TiC + 4Al ⇄ Al4C3 + 3Ti
In this study, Al4C3 was not detected. This is mainly because the increase in Ti concentration in melt could promote the reaction to reverse. TiC particles would not be “poisoned” into Al4C3.
At the same time, the long-term static insulation of the Al-TiC master alloy in the Al-7Si alloy will attenuate the refinement effect [29], which is mainly due to the sedimentation of TiC particles, thereby decreasing the effective nucleation site of α-Al. The sedimentation characteristics of TiC particles in the melt can be described by the Stokes formula [30]:
where υ is the particle sedimentation rate, r is the particle radius, ρ1 is the particle density, ρ2 is the melt density, and μ is the melt viscosity. The density of TiC in the aluminum melt is 4.93 g/cm3, and the density of aluminum melt is about 2.4g/cm3 [11]. Due to the density difference, TiC sedimentation will occur in the aluminum melt. At the same time, TiC particles tend to aggregate into large particles and settle. However, the sedimentation characteristics of TiC particles can be improved when the Al-3Ti-4.35La master alloy is added.
υ = 2r2(ρ1 − ρ2)/9μ
Figure 10 presents the microstructure analysis of TiC in the A1 sample. Figure 10a shows the TEM bright-field image of TiC. The high-resolution image at the interface between TiC and α-Al is shown in Figure 10b, and the inverse Fourier transform image corresponding to region 2 is shown in Figure 10c. The results show that the TiC atoms at the interface between TiC and α-Al are arranged in an orderly manner, with no evidence of atomic distortion. Additionally, the interface between TiC and α-Al exhibits good lattice matching, with a TiC lattice spacing of 0.21 nm, demonstrating that TiC can act as an effective heterogeneous nucleation substrate for α-Al.
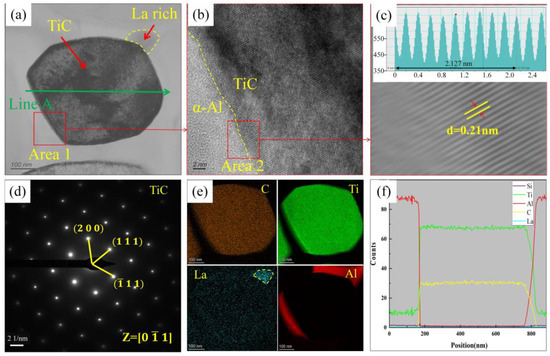
Figure 10.
Microstructure analysis of TiC in the A1 sample (adding Al-3Ti-4.35 La alloy and Al-TiC alloy to Al-7Si alloy for a holding time of 15 min): (a) TEM bright field image of TiC particles; (b) high-resolution map of area 1 in (a); (c) inverse Fourier transformation diagram of region 2 in (b); (d) TiC diffraction spot calibration; (e) mapping of the elements C, Ti, La, and Al in (a); (f) EDS Line scan results of Line A in (a).
Furthermore, there is a rich area of the La element around the TiC particles, and the concentration of Ti around and on the surface of TiC is higher, as shown in Figure 10e,f. The Ti2Al20La phase, which is the main particle in the Al-3Ti-4.35La master alloy, will dissolve in the Al-7Si alloy and release Ti and La elements. The poor wettability between the Ti/Al and TiC particles leads to the formation of a Ti-rich zone around or on the TiC surface, reducing the density difference between the TiC particles and the aluminum melt (Ti density: ~4.51 g/cm3 [11]). Simultaneously, free La elements accumulate on the TiC surface, which can promote the wettability between the TiC particles and the matrix Al. Therefore, the sedimentation phenomenon of the TiC particles is significantly reduced due to the combined effects of the Ti and La elements released by Ti2Al20La.
Ti2Al20La is the primary phase in the Al-3Ti-4.35La master alloy. Although previous studies [23,24,25] have shown that the addition of La improves the wettability between TiC and Al, the specific mechanism remains unclear, particularly whether Ti2Al20La directly influences the stability of TiC particles in the Al-7Si alloys. Figure 11 presents a microstructural analysis of Ti2Al20La in the aluminum melt, with EDS point scanning, area scanning, and line scanning analyses. The results indicate that TiC particles are not observed around Ti2Al20La in the hypoeutectic Al-7Si alloy, suggesting that Ti2Al20La does not directly interact with TiC. This further confirms that the improvement in TiC stability is primarily due to the release of La and Ti elements during the dissolution of Ti2Al20La. Additionally, Si tends to accumulate around Ti2Al20La. The interfacial relationship between Si and Ti2Al20La was analyzed through high-resolution imaging and inverse Fourier transform mapping, as shown in Figure 11b,c. The analysis reveals that the lattice spacings of eutectic Si and Ti2Al20La are 0.17 nm and 0.39 nm, respectively, with well-aligned lattices and no significant atomic distortion regions. This indicates that eutectic Si merely adheres to the periphery of the Ti2Al20La phase without affecting its structural integrity. This discovery provides a valuable reference for further investigation into the modification mechanism of eutectic Si by Ti2Al20La in Al-Si alloys.
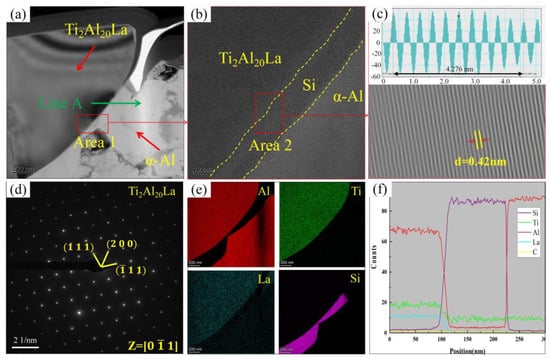
Figure 11.
TEM analysis of Ti2Al20La phase in the A1 sample (adding Al-3Ti-4.35 La alloy and Al-TiC alloy to Al-7Si alloy for a holding time of 15 min): (a) TEM bright field image of Ti2Al20La; (b) high-resolution images at the interface between Ti2Al20La and Si; (c) inverse Fourier transform diagram at the interface between Ti2Al20La and Si; (d) Ti2Al20La diffraction spot calibration; (e) element mapping of Al, Ti, La, and Si in (a); (f) EDS Line scan map of Line A in (a).
Previous studies have shown [31] that Si can accelerate the decomposition of TiC particles at 800 °C. To study the effect of master Si on the stability of TiC in Al-7Si alloys, a detailed structural analysis of the Si-TiC interface was conducted, as depicted in Figure 12. Figure 12a shows the TEM bright field phase of TiC particles and eutectic Si. The interface between Si and TiC is analyzed by high resolution and inverse Fourier transform, as shown in Figure 12b,c. The results show that the atoms are arranged neatly at the interface of Si and TiC with no evidence of atomic distortion. Meanwhile, the lattice fringe spacing is uniform, and the lattice spacing is 0.19 nm. Figure 12e,f show the surface scanning and line scanning results in Figure 12a. Si is only distributed on the surface of TiC, and the Si element distribution is not found inside Ti. Ding et al. [32] calculated the energy required for Si diffusion into the TiC lattice, indicating that Si can spontaneously diffuse into the TiC lattice, thereby enhancing the wettability between TiC and the aluminum matrix. Additionally, Xia et al. [33] reported that the presence of Si particles near TiC particles is a common phenomenon, consistent with our experimental observations. Furthermore, the proximity of Si particles to TiC accelerates the decomposition of TiC, reducing its effectiveness as a grain refiner in Al-7Si alloys. In summary, the presence of Si does not improve the wettability of TiC particles within Al alloys.
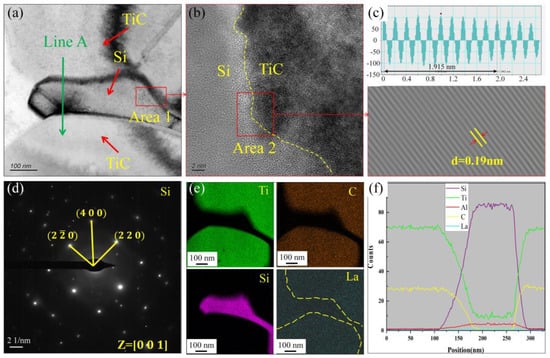
Figure 12.
TEM analysis of the effect of Si on TiC particle in the A1 sample (adding Al-3Ti-4.35 La alloy and Al-TiC alloy to Al-7Si alloy for a holding time of 15 min): (a) TEM bright field images of Si and TiC; (b) high-resolution images at the interface between Si and TiC; (c) inverse Fourier transform diagram at Si and TiC interface; (d) diffraction spot calibration of Si; (e) the mapping of Ti, C, Si, and La elements in (a); (f) EDS Line scan map of Line A in (a).
4. Conclusions
The effect of the Al-3Ti-4.35La master alloy on the agglomeration and sedimentation of TiC in Al-7Si alloys was studied. The main conclusions are as follows:
- (1).
- The dissolution of the Ti2Al20La phase releases La elements into the melt, which accumulate on the surface of TiC particles. This accumulation enhances the interfacial wettability between TiC particles and the Al matrix, thereby reducing the agglomeration tendency of TiC particles. When the Al-3Ti-4.35La master alloy was incorporated into the Al-7Si alloy and held for 15 min, the distribution of TiC particles became more uniform.
- (2).
- The Ti elements released by the dissolution of the Ti2Al20La phase will form a Ti-rich zone around or on the surface of TiC particles. This Ti-rich zone reduces the density difference between the TiC particles and the aluminum melt, thereby decreasing their sedimentation behavior. After holding periods of 30 min and 60 min, the amount of TiC precipitation at the bottom of the sample was notably reduced.
- (3).
- The atomic arrangement at the Si-TiC interface exhibits an ordered structure without any detectable distortion, which demonstrates that Si does not exert a direct influence on the agglomeration and sedimentation behavior of TiC particles in the Al-7Si alloy.
Author Contributions
Conceptualization, L.M.; resources, X.T., H.Y. and J.A.; data curation, J.A., L.M., H.Y. and X.T.; investigation W.D., X.T. and J.A.; methodology, L.M. and W.D.; writing—original draft preparation, L.M., H.Y. and H.Z.; writing—review and editing, W.D. and J.A.; supervision, W.D. and J.A.; project administration, W.D. and H.Z.; funding acquisition, W.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 52161006]. Industrial Support Plan Project of Gansu Provincial Department of Education (2021CYZC-23). Central Guidance for Local Scientific and Technological Development Funding Projects (23ZYQB309, 24ZYQD004). Gansu Provincial Science and Technology Major Project (22ZD6GB019).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tian, H.; Zhao, F.; Ma, D.; Zhao, X.; Meng, J.; Zhang, G.; Wu, F. An Improved Process for Solving the Sintering Problem of Al-Si Alloy Powder Metallurgy. Metals 2024, 14, 1295. [Google Scholar] [CrossRef]
- Xue, L.; Jia, H.; Ma, P.; Song, J.; Zha, M.; Wang, H. Influence of Mg and Cu on precipitation behaviors and mechanical properties of Al–Si alloys. Mater. Sci. Eng. A 2024, 908, 146775. [Google Scholar]
- Callegari, B.; Lima, T.N.; Coelho, R. The Influence of Alloying Elements on the Microstructure and Properties of Al-Si-Based Casting Alloys: A Review. Metals 2023, 13, 1174. [Google Scholar] [CrossRef]
- Sezgin, C.; Hisham, A.; Mattias, T. Effect of Ce addition on microstructure, thermal and mechanical properties of Al-Si alloys. Mater. Today Commun. 2023, 34, 105518. [Google Scholar]
- Sigworth, G. Grain Refinement of Al–Si–Cu Alloys by AlB2 and (Al,Ti)B2. Int. J. Met. 2024, 18, 1. [Google Scholar]
- Samuel, E.; Tahiri, H.; Samuel, A.; Samuel, F. Heterogenous Grain Nucleation in Al-Si Alloys: Types of Nucleant Inoculation. Metals 2024, 14, 271. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, B.; Chen, T.; Wu, J. Achieving superior grain refinement efficiency for Al–Si casting alloys through a novel Al–La–B grain refiner. J. Mater. Res. Technol. 2024, 30, 5. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Z.; Cui, H.; Meng, C.; Bai, P.; Du, Z.; Zhao, X.; Li, J. Study on mechanism of refining and modifying in Al–Si–Mg casting alloys with adding rare earth cerium. Mater. Res. Express 2023, 10, 086511. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Zhan, Y.; Li, L.; Yuan, Z.; Wang, X.; Shan, Q.; Li, Z. Interfacial characteristics of dual-phase Si/TiB2 and its crack initiation mechanism in hypereutectic Al-Si alloys. J. Alloys Compd. 2024, 981, 173748. [Google Scholar]
- Xu, J.; Li, Y.; Ma, K. In-situ observation of grain refinement dynamics of hypoeutectic Al-Si alloy inoculated by Al-Ti-Nb-B alloy. Scr. Mater. 2020, 187, 142. [Google Scholar]
- Vinod, K.; Murty, B.; Chakraborty, M. Development of Al-Ti-C grain refiners and study of their grain refining efficiency on Al and Al-7Si alloy. J. Alloys Compd. 2005, 396, 143. [Google Scholar] [CrossRef]
- Ding, W.; Zhao, X.; Zhao, W.; Guo, T.; Tang, X.; Qiao, J.; Xia, T. Effects of Al–Ti–C–Ce master alloy on microstructure and mechanical properties of hypoeutectic Al–7%Si Alloy. Int. J. Met. 2019, 13, 426. [Google Scholar] [CrossRef]
- Xia, L.; Li, W.; Wei, Z.; Wu, Y.; Liu, X. Achieving further refinement of grain structure and improvement of mechanical properties in Al-12Si-4Cu-2Ni-1Mg alloy by Al-Ti-C-B master alloy addition and deep cryogenic treatment. China Foundry 2024, 22, 75–82. [Google Scholar] [CrossRef]
- Kumar, G.; Murty, B.; Chakraborty, M. Grain refinement response of LM25 alloy towards Al-Ti-C and Al-Ti-B grain refiners. J. Alloys Compd. 2009, 472, 112. [Google Scholar] [CrossRef]
- Yang, H.; Gao, T.; Wang, H.; Nie, J.; Liu, X. Influence of C/Ti stoichiometry in TiCx on the grain refinement efficiency of Al–Ti–C master alloy. J. Mater. Sci. Technol. 2017, 33, 616. [Google Scholar] [CrossRef]
- López, V.; Scoles, A.; Kennedy, A. The thermal stability of Tic particles in an Al7 wt.%Si alloy. Mater. Sci. Eng. A 2003, 356, 316. [Google Scholar] [CrossRef]
- Nie, J.; Wu, Y.; Li, P.; Li, H.; Liu, X. Morphological evolution of TiC from octahedron to cube induced by elemental nickel. CrystEngComm 2012, 14, 2213. [Google Scholar] [CrossRef]
- Barbosa, J.; Puga, H. Ultrasonic melt treatment of light alloys. Int. J. Met. 2018, 13, 180. [Google Scholar] [CrossRef]
- Mahmoud, M.; Zedan, Y.; Samuel, A.; Songmene, V.; Samuel, F. The use of rare earth metals in Al–Si–Cu casting alloys. Int. J. Met. 2022, 16, 535. [Google Scholar] [CrossRef]
- Wang, Y.; Que, Z.; Hashimoto, T.; Zhou, X.; Fan, Z. Mechanism for Si poisoning of Al-Ti-B grain refiners in Al alloys. Metall. Mater. Trans. A 2020, 51, 5743. [Google Scholar] [CrossRef]
- Wang, F.; Hu, M.; Liu, T.; Jiang, B.; Ji, Z. Microstructure of Al-Ti-C master alloy triggered by rare-earth Ce. J. Mater. Res. 2022, 37, 1486. [Google Scholar]
- Zhao, H.; Song, Y.; Li, M. Grain refining efficiency and microstructure of Al-TiC-RE master alloy. J. Alloys Compd. 2010, 508, 206. [Google Scholar]
- Wang, M.; Sun, J.; Meng, Y.; Li, S.; Shou, H.; Zhang, G.; Yin, Z.; Dong, Y.; Zheng, H.; Zhang, Y. Insights into the effects of La on the grain refinement and mechanical properties of Al-Ti-C intermediate alloy and pure Al: A first-principle study and experimental investigation. J. Alloys Compd. 2024, 1002, 175290. [Google Scholar]
- Ding, W.; Liu, X.; Zhao, X.; Chen, T.; Zhang, H.; Zhao, W.; Li, C. Effect of Al-Ti-C-La composite alloy on microstructure and mechanical properties of 6063 aluminum alloy. Preprints 2020, 2020070115. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; Niu, J.; Xu, H.; Ren, X. Enhanced fluidity of ZL205A alloy with the combined addition of Al-Ti-C and La. Materials 2021, 14, 6169. [Google Scholar] [CrossRef]
- Ding, W.; Xu, M.; Gou, L.; Li, L.; Ma, J.; An, J.; Lu, X.; Liu, X.; Li, X. Refinement Mechanism of the Hypoeutectic Al–7Si Alloy by Adding a Novel La-Rich Rare-Earth Grain Refiner. Metall. Mater. Trans. A 2024, 55, 3617. [Google Scholar]
- Birol, Y. Grain refining efficiency of Al–Ti–C alloys. J. Alloys Compd. 2006, 422, 128. [Google Scholar]
- Dong, H.; Guo, Y.; Chen, Y.; Xia, F.; Guo, Q.; Chen, Q. On the mechanism of Si-promoted destabilization of TiCx particles in Al alloys. J. Mater. Sci. Technol. 2023, 165, 17. [Google Scholar]
- Yu, L.; Liu, X. Ti transition zone on the interface between TiC and aluminum melt and its influence on melt viscosity. J. Mater. Process. Technol. 2007, 182, 519. [Google Scholar]
- Ding, W.; Xu, C.; Hou, X. Preparation and synthesis thermokinetics of novel Al-Ti-C-La Composite Master alloys. J. Alloys Compd. 2019, 776, 904–911. [Google Scholar] [CrossRef]
- Weston, K.; Jones, M.; Enel, C. Reaction in Al-Ti-C powders and its relation to the formation and stability of TiC in Al at high temperatures. Scr. Mater. 2000, 42, 1187. [Google Scholar]
- Ding, H.; Liu, X. Influence of Si on stability of TiC in Al melts. Trans. Nonferrous Met. Soc. China 2011, 21, 1465. [Google Scholar]
- Xia, F.; Liang, M.; Gao, X. Instability of in situ TiC particles in an Al-12Si alloy. J. Mater. Res. Technol. 2020, 9, 11361. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).