Abstract
This work presents a comparative study of five rare earth compounds—Erbium nitrate pentahydrate lll (Er), Neodymium nitrate pentahydrate (Nd), Samarium III Nitrate Hexahydrate (Sm), Yterbium III Chloride Hexahydrate (Yb) and Praseodymium nitrate hexahydrate lll (Pr)—protecting API 5L X70 steel from corrosion in saline medium that uses electrochemical impedance spectroscopy (EIS) and polarization curves (CPs) at different concentrations and in static mode. The results show that Erbium is the best corrosion inhibitor, containing 50 ppm and reaching an inhibition efficiency of about 89%, and similar result was shown by Sm with an IE~87.9%, while the other rare earths (Nd, Yb and Pr) showed a decrease in corrosion protection at the same concentration, since they were below an IE~80%. On the other hand, with the Langmuir model it was possible to describe that the adsorption process of the three rare earths follows a combined physisorption–chemisorption process to protect the metal’s surface. The observed adsorption free energy, ΔG°ads, reaches −38.7 kJ/mol for Er, −34.4 kJ/mol for Nd, and −33.6 kJ/mol for Pr; whereas Sm and Yb have adsorption free energies of −33.9 and −35.0 kJ/mol, respectively. Scanning electron microscopy (SEM) and atomic force microscopy (AFM) further confirmed the formation of a protective film. Their characterization using density functional theory showed the transference of charge from the iron cluster towards the rare earth metal compounds. The adsorption process produced a slightly polarized region of interaction with the metal surface. Also, it was found that the adsorption of the rare earths affected the magnetic properties of the surface of the iron cluster. Quantum chemical descriptors, such as Pearson’s HSAB (Hard and Soft Acids and Bases) descriptors, were useful in predicting the behavior of the flow of electrons between the metal surface and the interacting rare earth ions.
1. Introduction
Corrosion is a prevalent issue in the oil industry that, if not managed effectively, can cause significant damage to installations, pipelines, and equipment [1]. This can lead to serious consequences such as leaks, decreased flow efficiency, and product loss, highlighting the need for effective corrosion management strategies.
Corrosion is a significant financial burden for the oil and gas industry, with estimated annual costs of about USD 100 billion [2]. To combat this, corrosion inhibitors are used as a control measure [3]. These substances, when added in low concentrations, are effective in slowing down the corrosion process [3,4]. There are different types of corrosion inhibitors, including organic [5], inorganic [6], and plant extracts [7]. These inhibitors have different characteristics, such as whether they form a film on the metal’s surface. In particular, plant extracts are natural products and they have the advantages of being environmentally friendly and readily available [8]. However, the disadvantage is they contain mixtures of organic compounds and their ability to protect the surface of the metal depends on different factors.
On the other hand, in the relentless pursuit of effective corrosion protection solutions, rare earth elements have been recognized as potential candidates for corrosion inhibitors for the oil industry [9,10,11]. Rare earth elements are not scarce in the earth’s crust; they are found in practically all rocks, which means they have the characteristic of being commercially available.
Rare earth salts have minimal toxicity and effectiveness in preventing corrosion. Some of them function as cathodic inhibitors, forming oxide/hydroxide layers [12].
Some of the metal surfaces on which they have demonstrated protection against corrosion are magnesium or aluminum alloys and stainless steel [13]. It has been reported that rare earths mixed with chlorides show protection against corrosion as deoxidizers [14]. These elements can act through several mechanisms to prevent corrosion [15], including forming a protective layer on the surface of metals, reacting with corrosive ions, or altering the chemical conditions of the environment [16]. Their effectiveness in these roles underscores their potential for improving the corrosion management strategies used in various industrial applications [17,18]. For example, among the rare earths used as corrosion inhibitors are lanthanum oxide (La2O3) [19], cerium chloride (CeCl3) [20,21], samarium III [22,23], or a combination of rare earths [24], which form a protective film on metal surfaces. In addition to lanthanides, other rare earth elements have also been used to create corrosion inhibitors. For example, gadolinium and yttrium are used in extreme conditions, such as those found in oil wells.
Therefore, the aim of this work is to study the effect, under static conditions, of different concentrations of five rare earth compounds (Figure 1)—(a) Erbium Nitrate pentahydrate III (Er), (b) Neodymium Nitrate pentahydrate (Nd), (c) Praseodymium Nitrate hexahydrate III (Pr), (d) Samarium III Nitrate Hexahydrate (Sm), and (e) Yterbium III Chloride Hexahydrate (Yb)—using a saline medium on API 5L X70 steel.
Figure 1.
Chemical structures of rare earths used as corrosion inhibitors: (a) Er, (b) Nd, (c) Pr, (d) Sm, (e) Yb.
2. Materials and Methods
2.1. Surface Preparation of API 5L X70 Steel
The API 5L X70 steel (wt%) has a nominal chemical composition of C: 0.037; Mn: 1.50; Si: 0.14; S: < 0.003, p < 0.015; Al: 0.03; Nb: 0.09; Cu: 0.27; Cr: 0.26; Ni: 0.16; Ti: 0.010; Ca: 0.0025; N2: 0.0040; and Fe: balance [24].
The API 5L X70 steel pieces were treated with a conventional sanding process and an abrasive paper sandpaper of SiC (grade 80–600) was used. Also, they were rinsed with acetone and distilled water to remove grease and contaminations from the surface. Eventually, the metal surface was dried and its exposure area was 0.76 cm2.
2.2. Inhibitor Preparation
The preparation of the corrosion inhibitors (Aldrich grade, Burlington, MA, USA) was carried out as follows: Initially, the solution of the corrosion inhibitor was prepared using 0.01 M (mol/L), dissolved in ethanol (because they are insoluble in water), of Samarium III Nitrate Hexahydrate (Sm), Erbium III Nitrate pentahydrate (Er), Yterbium III Chloride Hexahydrate (Yb), Neodymium Nitrate Hexahydrate (Nd), or Praseodymium III Nitrate Hexahydrate (Pr). Afterwards, the dilutions of the rare earth inhibitors were evaluated at different concentrations (0, 5, 10, 20, and 50 ppm) in a corrosive solution of 3 wt% NaCl (without detailing the solution, pH = 6.27) at room temperature (25 °C).
2.3. Electrochemical Test
The electrochemical evaluation was conducted on Gill AC potentiostat equipment (ACM instruments, Grange-over-Sands, UK) using a cell and three electrodes: the wor-king electrode was API 5L X70, saturated Ag/AgCl was the reference electrode, and graphite was the counter electrode.
The test sequence was as follows: an open circuit potential (OCP) measurement and then electrochemical impedance spectroscopy (EIS). Initially, the open circuit potential had a stabilization time of ten minutes to achieve a steady state.
The EIS signal amplitude was ±10 mV and the frequency range was 10 kHz to 10 mHz. All tests were performed in triplicate. Finally, a cell identical to that previously described was used to perform potentiodynamic polarization (PP), with a sweep speed of 1 mV/s and overpotentials ranging from −300 mV to +300 mV.
2.4. Surface Characterization
API 5L X70 steel disks were examined by atomic force microscopy (AFM) after being exposed to different corrosion conditions. The samples were analyzed using a Bruker Di-gital Instruments Scanning Probe Microscope (Billerica, MA, USA), in AFM mode, equipped with a NanoScope IIIa (Dallas, TX, USA) controller and an “E” scanner with a scan area of 10 × 10 µm and a 2.5 µm z range. Standard etched silicon AFM probes with a sharp tip at their end were used to scan the sample surface in tapping mode. The scanning was performed in air under normal temperature and pressure conditions. Uncoated AFM probes that were model Tap300-G (BudgetSensors, Watsonville, CA, USA) were selected to explore the surface of the samples due to the rotated shape of their tip, which allows for a good symmetrical visualization of the surface. The tip of the probes has a nominal radius of 10 nm, which ensures good resolution and the reproducibility of the results. All the samples were analyzed using a scan area of 2 × 2 µm, with the z range adjusted for each sample.
2.5. Computational Details
A dispersion-corrected DFT study was carried out to study the structural, energetic, chemical, and electronic changes introduced by the rare earth compounds Er, Yb, and Sm to the steel surfaces. Utilizing a cluster-based approach, which has been assumed through several studies to model the interactions between corrosion inhibitors and iron clusters [25,26], the rare earth salts and the Fe6-D2h iron cluster were examined with the BPW91 functional and 6-311++G(2d,2p) basis set, including dispersion corrections performed by the D2 correction term proposed by Grimme, as implemented in Gaussian 09 D.01. [27] In addition, effective core pseudopotentials, as defined by the Ahlrichs def2-TZVP basis set family [28], were used to model the rare earth elements Er, Yb, and Sm. Solvation effects were also considered through use of the Polarizable Continuum Model (PCM) with a dielectric constant of about ε = 78.3553. Thus, the full method was labelled BPW91-D2/PCM/6-311++G(2d,2p).
The Fe6 cluster was found to have the D2h symmetry point group and high spin multiplicity, M = 21. The initial geometries of the rare earth–Fe6 system were optimized, considering spin multiplicities from M = 17 to 21, to identify stable structures, taking into account zero-point energy (ZPE) corrections. The energy of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) were determined as well. Global parameters, including HOMO-LUMO energy gaps, ionization potential, electron affinity, electronegativity, hardness, and electrophilicity were computed using Pearson’s HSAB theory.
The global electronegativity χ = (I + A)/2, global hardness η = (I − A)/2, and global electrophilicity ω = χ2/2η parameters were obtained. The fraction of electron transfer (ΔN) from the rare earth salts to the bulk iron was determined utilizing standard values for iron’s electronegativity and hardness. In the current work, this parameter was computed as ΔN = (χFe-bulk − χInh) (2(ηFe-bulk + ηInh))−1, where the χFe6 and ηFe6 are assumed to be the absolute electronegativity and hardness of bulk iron, 7 and 0 eV, respectively. Also, χInh and ηInh are the electronegativity and hardness, obtained for the corresponding inhibitor, respectively.
Local parameters such as natural bond orbital (NBO) charges were computed and analyzed. Additionally, HOMO and LUMO frontier orbitals and electrostatic potential (ESP) maps were generated for electron density isosurfaces with a specific electron density of 0.02 a.u.
3. Results and Discussion
3.1. Evaluation at Different Concentrations via OCP (Open Circuit Potential) and EIS
Figure 2 shows the variation in the OCP for the several rare earths used as corrosion inhibitors. It is observed that they reached a steady state at around 1800 s.
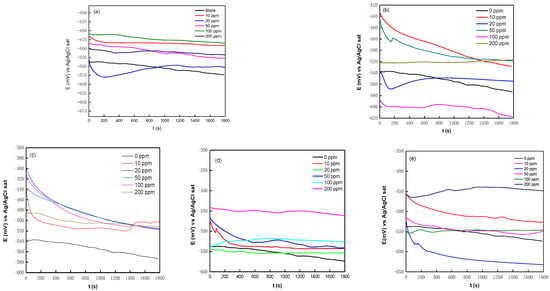
Figure 2.
Effect of immersion time on variation in API 5L X70 steel’s OCP in 3 wt% NaCl at room temperature: (a) Er, (b) Ne, (c) Pr, (d) Sm, and (e) Yb.
Electrochemical impedance spectroscopy (EIS) results for the different rare earth concentrations are shown in Figure 3. For the system with no inhibitor, Figure 3A.1, the semicircle’s diameter reached a Zreal value of ~152.0 Ω cm2, showing one time constant related to the charge transfer resistance [29,30].
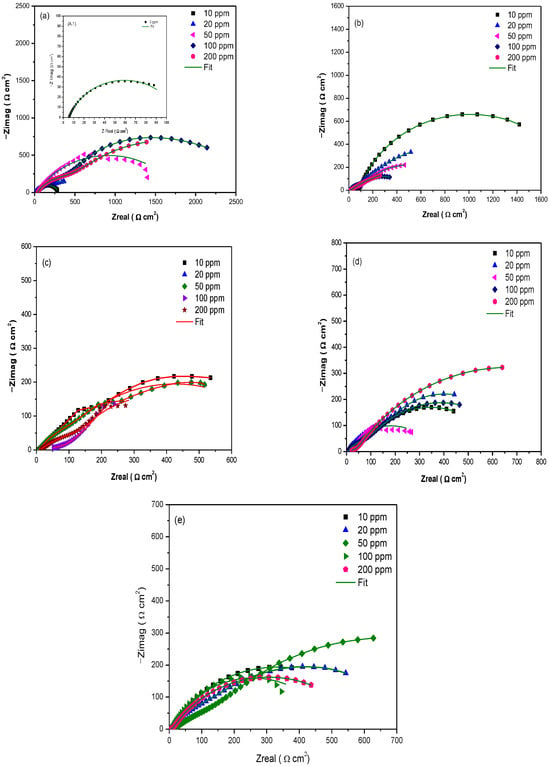
Figure 3.
Nyquist diagrams of rare earths—(a) Er, (b) Nd, (c) Pr, (d) Sm, and (e) Yb—as corrosion inhibitors on API 5L X70 steel immersed in a 3 wt% NaCl solution and (A.1) without inhibitor.
The presence of rare earth elements as corrosion inhibitors (Figure 3a–e) leads to a depression of the semicircle shape, which can be attributed to the existence of two time constants: one associated with the charge transfer resistance and the other with the inhibitor film. These observations are corroborated by Bode plots (Figure 4).
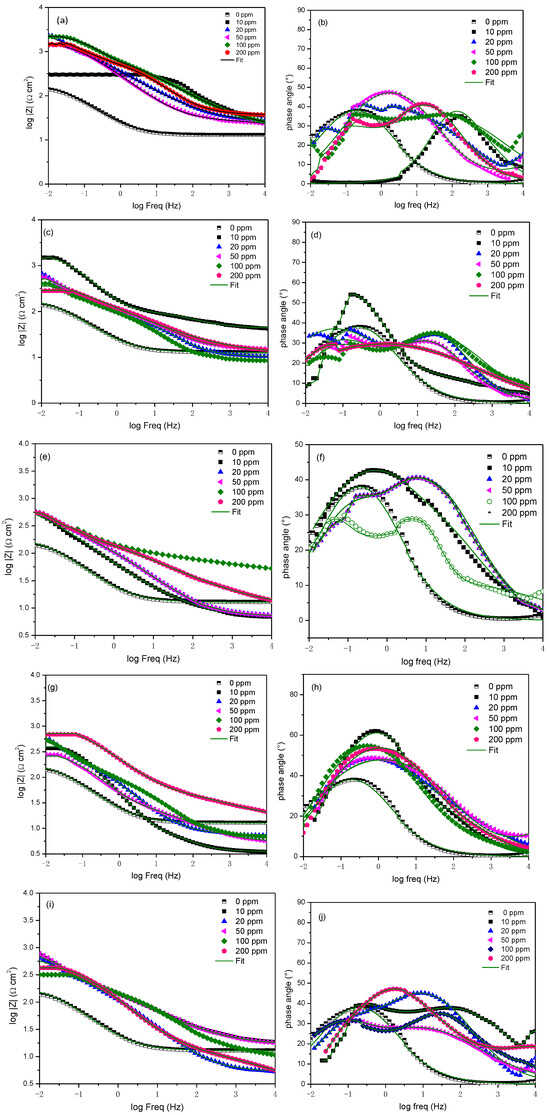
Figure 4.
Bode diagrams of rare earth molecules (a,b) Er, (c,d) Nd, (e,f) Pr, (g,h) Sm, and (i,j) Yb used as corrosion inhibitors on API 5L X70 steel immersed in a 3 wt% NaCl solution.
In general, Figure 3a shows that the Zreal value (Z’) increases with increasing inhibitor concentrations, and at 100 ppm it reached its maximum value, indicating that this condition is the best for protecting the metal surface in terms of the presence of Er.
However, when Ne was evaluated (Figure 3b), it did not show a clear trend of the higher the concentration, the larger the semicircle diameter, with the best behavior of this inhibitor being seen at only 10 ppm, where it reached a value of approximately ~1600 ohm cm2. This can be attributed this compound adsorbing more easily at this concentration [31] as a result of the formation of a protective film on the metal surface.
Then, for the Pr salt (Figure 3c), it was observed that for 10 to 200 ppm the semicircle’s diameter is very similar, and it is maintained with increasing concentrations, which may be associated with adsorption processes occurring at higher concentrations [32]. Figure 3d shows that at 50 ppm, the Nyquist diagram for the Sm inhibitor differs greatly from the others. In this case, the results indicate that this compound offers greater protection at high concentrations (200 ppm), while Yb showed the best protection against corrosion at intermediate concentrations (Figure 3e). Beyond 50 ppm, it appears that the system becomes saturated with the rare earth compounds, which prevents further adsorption on the metal surface.
Finally, the addition of rare earth compounds increased polarization resistance, indicating a protective effect on the metal surface.
The Bode plot results are presented in Figure 4, where it can be observed that the Bode impedance modulus increases with the presence of rare earth elements (Figure 4a,c,e,g,i) as corrosion inhibitors. On the other hand, the phase angle reveals one time constant related to charge transfer resistance when the system is without an inhibitor and, in most cases, the presence of two time constants within the low-to-intermediate frequency range with the presence of a rare earth element. When the phase angle is less than 90°, it is associated with the presence of inhomogeneities on the metal surface. As inhibitors are introduced into the corrosive medium, the phase angle increases, indicating the adsorption of the inhibitor onto the metal surface.
Finally, the similar shape of the semicircles suggests that the inhibition process works via the same mechanism, with two processes involved; one related to charge transfer resistance and the other to the adsorbed inhibitor molecules [33].
The equivalent electrical circuits used to obtain the electrochemical parameters of the Nyquist diagrams are shown in Figure 5a (without an inhibitor), while Figure 5b was used in the calculations quoted below, with the system not controlled solely by charge transfer resistance (a rare earth element is present). They involve the solution resistance (Rs), the charge transfer resistance (Rct), and the adsorbed rare earth molecule’s resistance (Rmol), as well as their respective constant phase elements (CPEs).
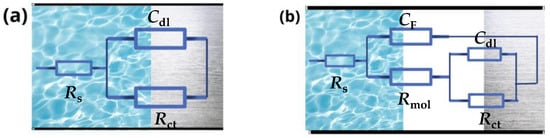
Figure 5.
Equivalent electrical circuits for the interpretation of the results. Rs, Rct, Rmol, Cdl, and CF stand for the solution resistance, the charge transfer resistance, the adsorbed rare earth molecule’s resistance, and the associated constant phase elements, respectively. (a) without inhibitor and (b) with inhibitor.
Equation (1) was used to calculate the inhibition efficiency (IE) of the system [34]:
where Rct blank is the charge transfer resistance without an inhibitor and Rct inhibitor is the charge transfer resistance with an inhibitor.
The electrochemical double-layer capacitance (Cdl) can be calculated using Equation (2) [35]:
where a proportion factor is used to represent the magnitude of the CPE, the phase change is (which can take values of 0 for a resistance, 1 for a capacitance, and −1 for an inductance), Rs is the solution resistance, and Rct is the charge transfer resistance.
CF is the film capacitance and is calculated using [36]
where ϖ’ is the angular frequency at which Z’ reaches its maximum value.
The electrochemical parameters obtained from the five different rare earths are listed in Table 1; in this system, an increase in the charge transfer resistance (Rct) of Rtotal is associated with a decrease in the electrochemical double-layer capacitance (Cdl) and CF. This decrease is caused by the adsorption of the inhibitor, which is associated with the displacement of water molecules, indicating that the surface area exposed to the corrosive medium is decreasing due to the formation of a protective film [37].

Table 1.
Electrochemical parameters of rare earths used in the protection of API 5L X70 steel immersed in a 3 wt% NaCl solution at room temperature.
From the values obtained from Table 1 and according to the parameters and inhibition efficiencies seen with different concentrations of the rare earth inhibitors, the protection they offer as corrosion inhibitors can be confirmed, with Er at 100 ppm being the best. This comparison can be better visualized in Figure 6.
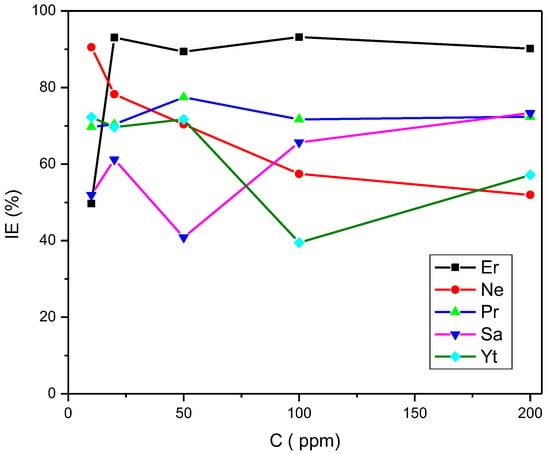
Figure 6.
Variation in the rare earth compounds’ corrosion inhibition efficiency for API 5L X70 steel immersed in a saline medium.
3.2. Evaluation at Different Concentrations by Potentiodynamic Polarization
The polarization curves are shown in Figure 7 and the electrochemical parameters obtained in Table 2 through this technique include the Tafel anodic (βa) and cathodic (βc) slopes, corrosion potential (Ecorr), and corrosion current density (icorr). If the shift in Ecorr exceeds more than 85 mV compared to the Ecorr of the blank, the inhibitor is classified as either cathodic or anodic [38]. Otherwise, it is categorized as a mixed-type inhibitor. Under these parameters, the Nd and Pr inhibitors are classified as mixed-type inhibitors. The Er and Sm are cathodic inhibitors and Yt is anodic.
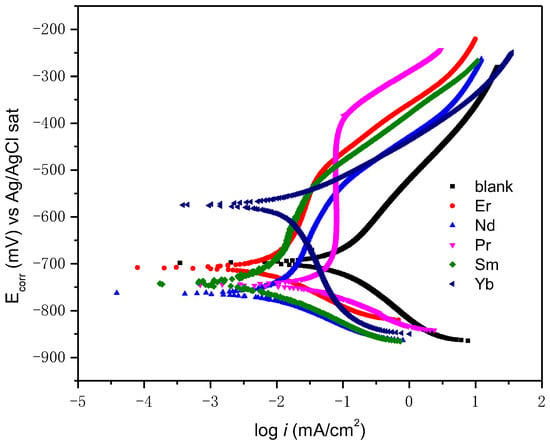
Figure 7.
Potentiodynamic polarization of rare earths as corrosion inhibitors on API 5L X70 steel immersed in a 3 wt% NaCl solution.

Table 2.
Electrochemical parameters from the polarization curves of rare earth compounds used in the protection of API 5L X70 steel immersed in a 3 wt% NaCl solution at room temperature.
The inhibition efficiency (IE) values of the rare earths, determined using the polarization curve technique, were calculated using the following equation [39]:
where icorr0 and icorr are the corrosion current densities for dissolutions without and with an inhibitor.
The electrochemical parameters established using this technique are shown in Table 2, and the icorr value decreased with the presence of rare earth elements, indicating that the compounds evaluated could reduce the corrosion rate of carbon steel. Additionally, the results are consistent with those obtained using the EIS technique.
Rare earth compounds (REs) used as corrosion inhibitors have valence electrons of RE3+. Consequently, they generate a film composed of hydroxides in the cathodic region [14]. It is expected that the reduction of oxygen produces hydroxide ions, leading to a local change in pH.
The formation of RE(OH)3 and RE2O3 in cathode field is as follows:
2 RE3+ + 6 OH− = 2RE(OH)3 ↓ (reaction 1)
RE(OH)3 = RE2O3 + 2 H2O (reaction 2)
After determining the effectiveness of rare earths as corrosion inhibitors, an analysis of the adsorption process of the proposed compounds was carried out. A linear regression was performed to determine the adsorption model that fits, with the Langmuir model being as follows:
where is the inhibitor concentration, is the adsorption constant, and is the surface coverage [40], which was calculated using the following equation:
In the literature, it is mentioned that physical adsorption can be achieved from the Gibbs free energy of adsorption (ΔG°ads), which is calculated using Equation (7).
where R is the ideal gas constant, T is the temperature in Kelvin, and 55.5 is the concentration of water in mol/L.
ΔG°ads = −RTln (55.5 × Kads)
It is worth mentioning that values up to −20 kJ/mol indicate that a physisorption process takes place, while at values above −40 kJ/mol, adsorption occurs chemically [41].
The linear fitting results are shown in Figure 8, where the correlation coefficient is close to 1 (Table 3) with Langmuir isotherm model, which indicates the presence of a rare earth monolayer on the metal surface (Figure 8a,b).
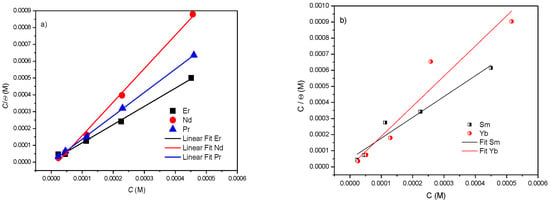
Figure 8.
Thermodynamic analysis of rare earths using the Langmuir model for (a) Er, Nd, Pr and (b) Sm, Yb.

Table 3.
Adsorption parameters using the Langmuir isotherm.
The results shown in Table 3 reveal that the adsorption mechanism of the rare earths on the studied surface in 3% NaCl medium is a combined-type adsorption. In the case of these rare earths, it is closer to 40 kJ/mol, so the adsorption process of these compounds is predominantly chemisorption.
3.3. Scanning Electron Microscopy (SEM) Results
The SEM images demonstrate that on metal exposed to the corrosive medium (Figure 9a), clusters of corrosion products are observed in certain areas. On the contrary, with the presence of Er (Figure 9b) or Yb (Figure 9c), a protective film with more extensive coverage, formed based on the concentration of the inhibitor, is observed.

Figure 9.
SEM images of API 5L X70 steel surface after 24 h of immersion in (a) NaCl, and with an (b) Erbium inhibitor or (c) ytterbium inhibitor applied.
3.4. Atomic Force Microscopy (AFM) Results
Two-dimensional and three-dimensional AFM images are shown in Figure 10. These images illustrate the different roughness behaviors of the inhibitors.
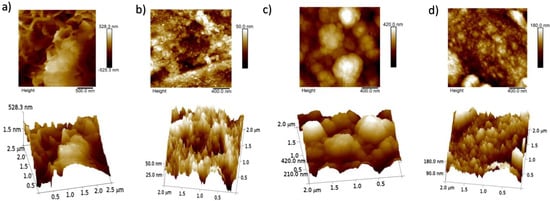
Figure 10.
AFM results of corrosion in (a) NaCl alone and with (b) Er, (c) Sm, and (d) Yb applied as corrosion inhibitors on API 5L X70 steel immersed in a 3 wt% NaCl solution.
Table 4 reports the roughness values of the inhibitors, including both Ra (the arithmetic average of the absolute values of the roughness profile ordinates) and Rq (the root mean square of the roughness profile ordinates). It can be seen that the Ra and Rq values of the samples exposed to the corrosive medium are 143 and 141 nm, respectively.

Table 4.
AFM roughness results due to use of different corrosion inhibitors.
However, after the addition of rare earths (Er, Sm or Yb) into the 3%wt NaCl, the roughness value decreased and these results confirm the efficiency of the inhibition of corrosion granted by the rare earth compounds adsorbed on the metal surface. Indeed, the corrosion decreased in presence of the compounds compared to in the corrosive media alone.
3.5. Computational Results
To complement these experimental evaluations, density functional theory calculations were performed and analyzed for the Yb, Er, and Sm compounds. Thus, ions of Yb3+, Er3+, and Sm3+, and Cl− were modeled at the BPW91-D2/PCM/6-311++G(2d,2p)/ECP level. First of all, their atomic ground states were retrieved from the NIST Atomic Spectra Database version 5.11 [42,43,44,45]. In comparison with spectroscopic measurements of their first ionization energies, the computed values were in full agreement in the case of Yb, Er, and Sm (Table 5), as were their subsequent ionization energies in the case of the constituents of our systems under study: Yb3+, Er3+, and Sm3+.

Table 5.
Multiplicity (M) and ionization energy measured (Iexp) and calculated (Itheo) for the isolated atoms and ions under study.
Recently, the importance of the quantum chemical descriptors described within the Hard-Soft Acid-Base theory in evaluating corrosion inhibition performance has been studied [46]. Consequently, some descriptors were evaluated and included in Table 6. It is noticeable that the massive energy required to remove an additional electron from these trications hinders the donation of charge to the metal surface. Also, the high electron affinity promotes the donation of charges coming from the iron atoms. The above is consistent with the negative value of ΔN and the reversed behavior seen in the case of Cl−. These assumptions were confirmed in the following discussion on the adsorption of the ions of interest onto the Fe6-D2h iron cluster.

Table 6.
Ionization energy, electron affinity, hardness, electronegativity, electrophilicity, and fraction of electrons transferred to the bulk iron for all ions under study.
All the ionic species were set at a minimal distance of 2.5 Å from the bridge, top, and face-centered positions of the Fe6-D2h neutral iron cluster. Spin multiplicities of M = 17, 19, and 21 were considered in all cases. Only, the ground state (GS) configurations, with all their real vibrational frequencies, were characterized (Figure 8). The spin multiplicities of the ground state structures are listed in Table 7.

Table 7.
The spin multiplicity, ion–cluster adsorption energy, orbital energies, and HOMO-LUMO gap of all the systems in their GSs.
From the study of the ions’ nonequivalent initial positions in their GSs, it is noticeable that the equilibrium position depends on the attached ionic species (Figure 11). For instance, Er3+ moved from the bridge position to a face-centered position, with a minimal bond length of about 3.155 Å (Table 7). Similarly, Sm3+, initially set at a top position, preferred to adopt a face-centered conformation with a slightly larger bond length of 3.357 Å. Also, Yb3+ was found located at 3.234 Å from the face of the iron cluster. In contrast, the Cl− ion moved from a face-centered position to a neighboring top position, adopting the shortest bond length among all the systems under study of approximately 2.267 Å (Figure 11).
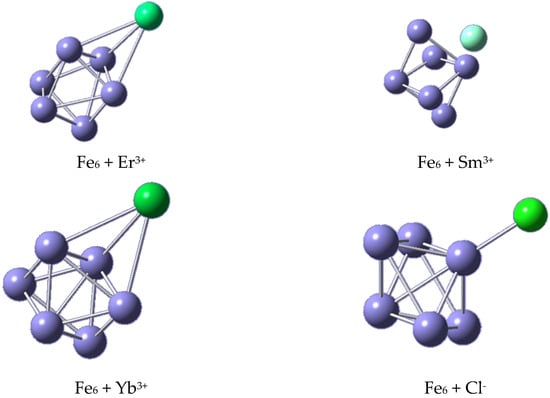
Figure 11.
Ground state structures of all systems under study.
Since all the systems under study were modeled as the interaction of a neutral iron cluster with charged moieties, their electrostatic potential maps and NBO charge distributions were obtained while considering them as interacting systems. It is noticed that Er3+ received charge from the iron cluster, in particular from the closest iron atom (Figure 12). Thus, the erbium was reduced as the result of its final charge of 2.083 e, while −0.043 e was transferred to the neighboring atom. There was a complete rearrangement of the charge, leading to a slightly polarized interface. As a consequence, it is expected that an electrostatic attraction occurs between the ion and the polarized surface. Similarly, the Sa3+ and Yt3+ ions received charge from the Fe6 iron cluster, since their NBO charges were found to be 2.033 and 2.008 e, respectively. Thus, the previous assumptions, made in terms of HSAB descriptors, can be fully contrasted with the results coming from the NBO population analysis, since the flow of electron charge from the iron cluster to the rare earth metals can be explained by their favorable fraction of electrons transferred and electron affinity.
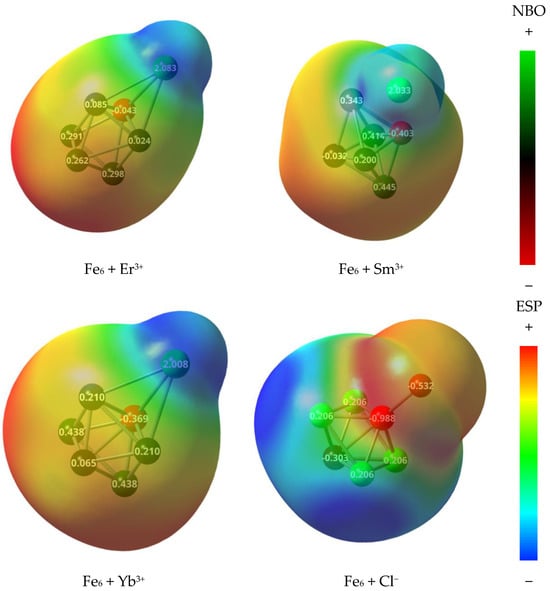
Figure 12.
Electrostatic potential mapped on isosurfaces with electron density of 0.004 a.u. Natural bond orbital charges are also labeled and colored.
To understand the chemical part of the adsorption process, frontier molecular orbitals were plotted for all systems under study (Figure 13). It is noticeable that only some particular systems shared orbitals between the iron cluster and the ionic species, forming bonding orbitals. For instance, the LUMOα of Fe6 + Sm3+ exhibits a σ bonding orbital, similar to the HOMOβ of Fe6 + Er3+. In contrast, the most recurrent contribution emerged as being from the iron cluster, with a mix of bonding and antibonding molecular orbitals due to the overlap of the d orbitals of iron. In the case of the adsorbed rare earth metals, f nonbonding orbitals were exposed.
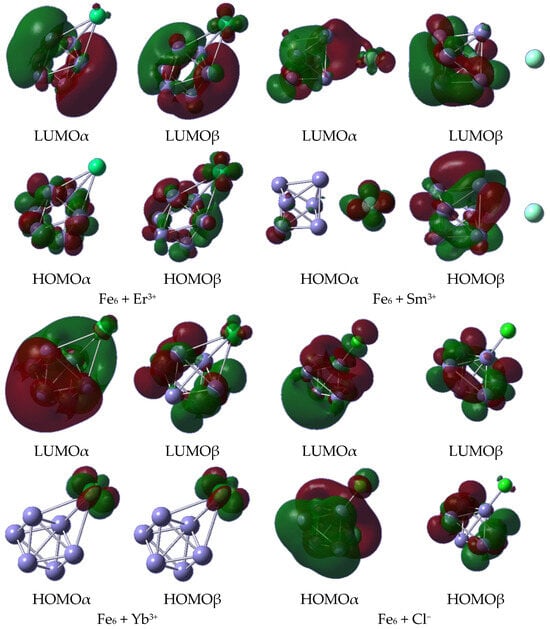
Figure 13.
HOMO and LUMO orbitals, α and β, plotted on isosurfaces with 0.02 a.u.
Finally, the high spin multiplicity obtained after the adsorption of the rare earth metals could be incorrectly assumed to be a minimal perturbation of the total spin of the bare iron cluster. However, to elucidate the distribution of unpaired electrons, the spin density was obtained and is shown in Figure 14. It is noticeable that there is an important contribution of the reduced ion as a consequence of the remaining unpaired electrons. The above is in agreement with the size of the contributions and the spin multiplicity M of free dications or rare earths in their ground state, following the order M (Yb2+) < M (Er2+) < M (Sm2+), since they are singlet, triplet, and septet, respectively. The contribution coming from the chlorine atom can be attributed to the charge transferred from this atom to the iron cluster, leaving an unpaired electron behind but reducing the global spin state to M = 19 by electron pairing. Thus, the adsorption could quench the magnetic properties of the surface iron atoms.
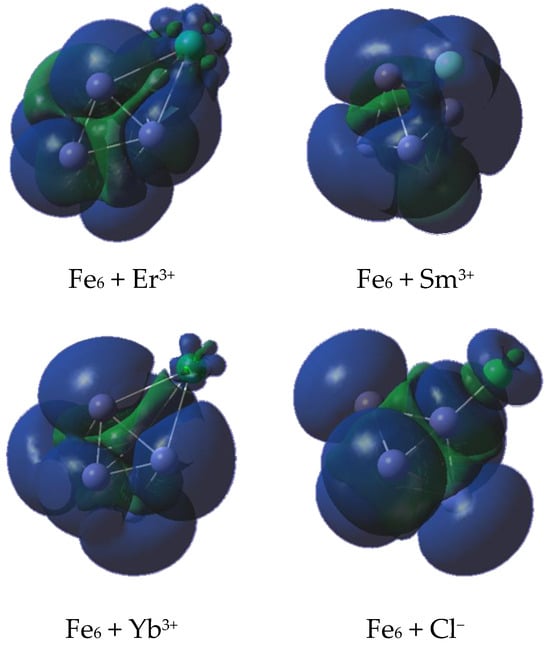
Figure 14.
Spin density on isosurfaces with an electron density of 0.004 a.u. Blue means a spin-up charge, whereas green means a spin-down.
4. Conclusions
Rare earth compounds have gained interest as corrosion inhibitors due to their effectiveness and lower environmental impact compared to traditional inhibitors, as demonstrated in this study. The corrosion inhibition properties of erbium (Er), neodymium (Nd), praseodymium (Pr), ytterbium (Yb), and samarium (Sm) were investigated using the EIS technique and it was found that they can form protective films on metal surfaces, reducing corrosion rates.
The five rare earth compounds have a decreasing order of effectiveness at a concentration of 20 ppm: Er < Nd < Pr < Yb < Sm. Of these, Er is the only one that meets the PEMEX standard for use as a corrosion inhibitor in the oil industry, with an inhibition efficiency of 93%. By means of the polarization curves technique, Nd and Pr are classified as mixed-type inhibitors at the tested concentrations, while Er and Sm act as cathodic inhibitors and Yb is an anodic-type inhibitor. The corrosion current density—and consequently the corrosion rate—decreases with the presence of rare earth compounds, as observed through this technique.
On the other hand, by investigating their adsorption mechanism using the Langmuir model, we see that the adsorption of rare earth elements is combined-type (physisorption and chemisorption), with a predominance of chemisorption. Meanwhile, the SEM images demonstrate a reduction in corrosion products due to the formation of a protective film of the inhibitor. Then, the AFM results demonstrate that the roughness decreases when a rare earth element is added as a corrosion inhibitor.
The theoretical study using DFT evidenced that the physical component of the interaction of the rare earths with the iron surface is due to electrostatic interactions. Also, rare earths could influence the magnetic properties of surface iron metals. Additionally, Pearson’s HSAB descriptors were useful in predicting the direction of the flow of charge between ions and the metal surface.
Author Contributions
Conceptualization, A.E.V., F.J.R.G., S.H.G. and A.M.; methodology, A.E.V., A.M. and M.C.; software, A.E.V., A.M.J. and M.C.; validation, A.E.V., F.J.R.G. and R.O.C.; formal analysis, F.J.R.G., S.H.G., A.M.J., R.O.C. and A.M.; investigation, A.E.V., F.J.R.G. and Ó.A.G.V.; resources, F.J.R.G. and Ó.A.G.V.; data curation, A.E.V. and A.M.; writing—original draft preparation, A.E.V., L.N.P.-G. and A.M.; writing—review and editing, A.E.V., L.N.P.-G., F.J.R.G., A.M.J., R.O.C. and A.M.; supervision, and A.M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors thank the Facultad de Química and the SNII for their appointments. M.C. thanks the Dirección General de Cómputo y de Tecnologías de la Información (DGTIC-UNAM) for providing supercomputing resources on the Miztli supercomputer; Project LANCAD-UNAM-DGTIC-063; and the PAPIIT IN102622 project from DGAPA UNAM.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Askari, M.; Aliofkhazraei, M.; Afroukhteh, S. A comprehensive review on internal corrosion and cracking of oil and gas pipelines. J. Nat. Gas Sci. Eng. 2019, 71, 102971. [Google Scholar] [CrossRef]
- Hussein, A.; Xiao, Y.; Xiao, N.; Wu, B.; Li, H.; Lin, B.; Nie, Z.; Tang, J. Emerging AI technologies for corrosion monitoring in oil and gas industry: A comprehensive review. Eng. Fail. Anal. 2024, 155, 107735. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Chauhan, D.S.; Ansari, F.A. Development of environmentally benign corrosion inhibitors for organic acid environments for oil-gas industry. J. Mol. Liq. 2021, 329, 115514. [Google Scholar] [CrossRef]
- Zu, P.; Zhang, Y.; Zhang, S.; Li, Y.; Hu, L. Excellent corrosion inhibition and lubrication performance of MMT based ionic liquids as ester oil additives. Tribol. Int. 2024, 194, 109473. [Google Scholar] [CrossRef]
- Tian, Y.; Bao, J.; Xie, D.; Wang, B.; Zhang, P.; Zhao, T.; Lei, D. The effects of organic corrosion inhibitor on concrete properties and frost resistance. J. Build. Eng. 2025, 65, 105762. [Google Scholar] [CrossRef]
- Razizadeh, M.; Mahdavian, M.; Ramezanzadeh, B.; Alibakhshi, E.; Jamali, S. Synthesis of hybrid organic–inorganic inhibitive pigment based on basil extract and zinc cation for application in protective construction coatings. Constr. Build. Mater. 2021, 287, 123034. [Google Scholar] [CrossRef]
- Holla, B.R.; Mahesh, R.; Manjunath, H.R.; Anjanapura, V.R. Plant extracts as green corrosion inhibitors for different kinds of steel: A review. Heliyon 2024, 10, e33748. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.; Newaz, K.; Basirun, W.J.; Ali, H.B.M.; Faraj, F.L.; Khan, G.M. Application of Natural Product Extracts as Green Corrosion Inhibitors for Metals and Alloys in Acid Pickling Processes—A review. Int. J. Electrochem. Sci. 2015, 10, 6120–6134. [Google Scholar] [CrossRef]
- Somers, A.E.; Hinton, B.R.W.; De Bruin-Dickason, C.; Deacon, G.B.; Junk, P.C.; Forsyth, M. New, environmentally friendly, rare earth carboxylate corrosion inhibitors for mild Steel. Corros. Sci. 2018, 139, 430–437. [Google Scholar] [CrossRef]
- Peng, Y.; Hughes, A.E.; Deacon, G.B.; Junk, P.C.; Hinton, B.R.W.; Forsyth, M.; Mardel, J.I.; Somers, A.E. A study of rare-earth 3-(4-methylbenzoyl)-propanoate compounds as corrosion inhibitors for AS1020 mild steel in NaCl solutions. Corros. Sci. 2018, 145, 199–211. [Google Scholar] [CrossRef]
- Behrsing, T.; Deacon, G.B.; Junk, P.C. The chemistry of rare earth metals, compounds, and corrosion inhibitors. In Rare Earth-Based Corrosion Inhibitors; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–37. [Google Scholar]
- Hughes, A.E.; Mol, J.M.C.; Cole, I.S. The cost and availability of rare earth-based corrosion inhibitors. In Rare Earth-Based Corrosion Inhibitors; Elsevier: Amsterdam, The Netherlands, 2014; pp. 291–305. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhuang, J.; Yu, Y.; Zeng, X. Research on anti-corrosion property of rare earth inhibitor for X70 steel. J. Rare Earths 2013, 31, 734–740. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Zhang, S.; Zhang, L. Effect of rare earth element yttrium modified inclusion on corrosion properties of stainless steel. Mater. Today Commun. 2024, 39, 109185. [Google Scholar] [CrossRef]
- Bethencourt, M.; Botana, F.J.; Calvino, J.J.; Marcos, M.; Rodríguez-Chacón, M.A. Lanthanide compounds as environmentally-friendly corrosion inhibitors of aluminium alloys: A review. Corros. Sci. 1998, 40, 1803–1819. [Google Scholar] [CrossRef]
- Hossain, M.K.; Rubel, M.H.K.; Akbar, M.A.; Ahmed, M.H.; Haque, N.; Rahman, M.d.F.; Hossain, J.; Hossain, K.M. A review on recent applications and future prospects of rare earth oxides in corrosion and thermal barrier coatings, catalysts, tribological, and environmental sectors. Ceram. Int. 2022, 48, 32588–32612. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, J.; Dong, S.; Zhou, C.; Lü, K.; Xie, Y.; Duan, Z.; Huang, Y.; Chen, T.; Deng, L.; et al. (Gd0.2Ho0.2Er0.2Yb0.2Lu0.2)2Si2O7 and (Sc0.2Ho0.2Er0.2Yb0.2Lu0.2)2Si2O7 high-entropy rare-earth disilicates as promising materials for environmental barrier coatings. Ceram. Int. 2024, 50, 23342–23355. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, Y.; Zuo, Y. Evolution of the corrosion process of AA 2024-T3 in an alkaline NaCl solution with sodium dodecylbenzenesulfonate and lanthanum chloride inhibitors. Appl. Surf. Sci. 2015, 357, 35–744. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Van Vuong, Q.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Effects of Different Drying Methods on Bioactive Compound Yield and Antioxidant Capacity of Phyllanthus amarus. Drying Technol. 2015, 33, 1006–1017. [Google Scholar] [CrossRef]
- Ivušić, F.; Lahodny-Šarc, O.; Ćurković, H.O.; Alar, V. Synergistic inhibition of carbon steel corrosion in seawater by cerium chloride and sodium gluconate. Corros. Sci. 2015, 98, 88–97. [Google Scholar] [CrossRef]
- Palaniappan, N.; Cole, I.S.; Kuznetsov, A.E.; Thomas, K.R.J.; Ruszkowski, P.; Kujawska, M. Samarium functionalized few-layer nano graphene oxide redox behavior, cytotoxicity and corrosion inhibition on Mg AZ31 alloy in 3.5% NaCl environment. J. Mol. Struct. 2023, 1294, 136353. [Google Scholar] [CrossRef]
- Liu, B.; Wang, H.-H.; Zhang, Y.; Yang, Y.; Ren, X.; Du, H.; Hou, L.; Wei, Y.; Song, G. The influence of adding samarium on the microstructure, mechanical performance and corrosion behavior of as-extruded AZ41 alloys. J. Phys. Chem. Solids 2021, 150, 109851. [Google Scholar] [CrossRef]
- Chenghao, L.; Shusen, W.; Naibao, H.; Zhihong, Z.; Shuchun, Z.; Jing, R. Effects of Lanthanum and Cerium Mixed Rare Earth Metal on Abrasion and Corrosion Resistance of AM60 Magnesium Alloy. Rare Met. Mat. Eng. 2015, 44, 521–526. [Google Scholar] [CrossRef]
- Udunwa, D.I.; Onukwuli, O.D.; Nwanonenyi, S.C.; Ude, C.N.; Arukalam, I.O.; Uche, R. Newly synthesized 1-butyl-3-methylimidazolium p-toluenesulfonate ionic liquid for acid corrosion of API 5L X70 steel: Experimental, DFT/MD-simulation, statistical and machine learning predictions. Results Surf. Interfaces 2024, 18, 100398. [Google Scholar] [CrossRef]
- Espinoza-Vázquez, A.; Santiago Cárdenas, L.J.; Galván-Martínez, R.; Miralrio, A.; Castro, M.; Carmona Hernández, A.; Orozco-Cruz, R. Electrochemical evaluation of Trasar trac 102 as a corrosion inhibitor on API 5L X65 steel and theoretical study. J. Electroanal. Chem. 2023, 943, 117599. [Google Scholar] [CrossRef]
- Espinoza Vázquez, A.; González-Olvera, R.; Moreno Cerros, D.; Negrón Silva, G.E.; Figueroa, I.A.; Rodríguez Gómez, F.J.; Castro, M.; Miralrio, A.; Huerta, L. Inhibition of acid corrosion in API 5L X52 steel with 1,2,3-triazole derivatized from benzyl alcohol: Experimental and theoretical studies. J. Mol. Struct. 2021, 1242, 130731. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09, Revision D. 01; Gaussian: Wallingford, CT, USA, 2009. [Google Scholar]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Sun, Q.; Fu, S.; Peng, Y.; Li, P.; Ma, H.; Fang, Z.; Ma, T.; Zhang, R.; Liang, Z.; Li, J. Isoindigo derivatives as some potential corrosion inhibitors for mild steel in hydrochloric acid solution: Synthesis, experimental and theoretical studies. J. Mol. Struc. 2024, 1312, 138480. [Google Scholar] [CrossRef]
- Chen, M.; Chen, S.; Pi, J.; Chen, S.; Wang, Q.; Fu, C. An investigation of modified dialdehyde starch as a highly efficient green corrosion inhibitor for carbon steel in 1 M HCl medium: Synthesis, experimental and theoretical studies. Ind. Crops Prod. 2024, 215, 118534. [Google Scholar] [CrossRef]
- Zhu, J.; Lin, B.; Duan, T.; Lin, H.; Zhang, G.; Zhou, X.; Xu, Y. Zea mays bracts extract as an eco-friendly corrosion inhibitor for steel in HCl pickling solution: Experimental and simulation studies. Arab. J. Chem. 2024, 17, 105895. [Google Scholar] [CrossRef]
- Negi, R.; Thakur, S.; Singh, R.; Kaur, V.; Singh, K. Double-layer protection of stainless steel by using triethylammonium-3-silatranylpropyldithiocarbamate as a corrosion inhibitor: Experimental and computational studies. J. Mol. Struc. 2024, 1309, 138166. [Google Scholar] [CrossRef]
- Song, Z.; Liu, L.; Guo, M.-Z.; Cai, H.; Liu, Q.; Donkor, S.; Zhao, H. Inhibition performance of extract reinforcement corrosion inhibitor from waste Platanus acerifolia leaves in simulated concrete pore solution. Case Stud. Constr. Mater. 2024, 20, e02992. [Google Scholar] [CrossRef]
- Somers, A.E.; Peng, Y.; Chong, A.L.; Forsyth, M.; MacFarlane, D.R.; Deacon, G.B.; Hughes, A.E.; Hinton, B.R.W.; Mardel, J.I.; Junk, P.C. Advances in the development of rare earth metal and carboxylate compounds as corrosion inhibitors for steel. Corros. Eng. Sci. Techn. 2020, 55, 311–321. [Google Scholar] [CrossRef]
- Tan, B.; Liu, Y.; Gong, Z.; Zhang, X.; Chen, J.; Guo, L.; Xiong, J.; Liu, J.; Marzouki, R.; Li, W. Pyracantha fortuneana alcohol extracts as biodegradable corrosion inhibitors for copper in H2SO4 media. J. Mol. Liq. 2024, 397, 124117. [Google Scholar] [CrossRef]
- Yang, H.; Deng, S.; Li, X. Eupatorium adenophorum Spreng root extract as an efficient inhibitor for the corrosion of steel in sulfamic acid solution. Int. J. Electrochem. Sci. 2024, 19, 100790. [Google Scholar] [CrossRef]
- Simović, A.; Milovanović, B.; Etinski, M.; Matović, L.; Bajat, J.B. Innovative hybrid and bifunctional rare earth complexes as corrosion inhibitors for AA2024 Alloy: Electrochemical and surface analysis enhanced by DFT/MD simulation. Appl. Surf. Sci. 2024, 670, 160718. [Google Scholar] [CrossRef]
- Cheng, Z.; Liao, B.; Zhou, Z.; Mai, W.; Chen, Q.; He, J. Exploration on the corrosion inhibition performance of Salvia miltiorrhiza extract as a green corrosion inhibitor for Q235 steel in HCl environment. J. Mater. Res. Technol. 2024, 32, 3857–3870. [Google Scholar] [CrossRef]
- Al-Amiery, A.; Betti, N.; Shaker, L. Exploring the effectiveness of 3-chloro-4-morpholin-4-yl-1,2,5-thiadiazole as an eco-friendly corrosion inhibitor for mild steel in HCl solution: Experimental and DFT analysis. Results Eng. 2024, 24, 103014. [Google Scholar] [CrossRef]
- Tang, J.; Shi, Y.; He, S.; Luo, J.; Liu, Y.; Zhai, K.; Duan, M.; Wang, H.; Xie, J. Study on the corrosion inhibition properties of some quinoline derivatives as acidizing corrosion inhibitors for steel. Int. J. Electrochem. Sci. 2024, 19, 100547. [Google Scholar] [CrossRef]
- Worden, E.F.; Solarz, R.W.; Paisner, J.A.; Conway, J.G. First ionization potentials of lanthanides by laser spectroscopy. J. Opt. Soc. Am. 1978, 68, 52. [Google Scholar] [CrossRef]
- Johnson, D.A.; Nelson, P.G. Lanthanide Ionization Energies and the Sub-Shell Break. Part 2. The Third and Fourth Ionization Energies. J. Phys. Chem. Ref. Data 2017, 46, 013109. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, X.; Zhang, W.; Huang, C.; Shen, L. Shift of the first ionization threshold of Sm atom in electric field. Chin. Opt. 2020, 13, 1385–1400. [Google Scholar] [CrossRef]
- Aymar, M.; Debarre, A.; Robaux, O. Highly excited levels of neutral ytterbium. II. Multichannel quantum defect analysis of odd- and even-parity spectra. J. Phys. B At. Mol. Phys. 1980, 13, 1089–1109. [Google Scholar] [CrossRef]
- Kramida, A.; Ralchenko, Y. NIST Atomic Spectra Database, NIST Standard Reference Database. 1999; p. 78. Available online: https://www.nist.gov/pml/atomic-spectra-database (accessed on 1 June 2024).
- Serrano, A.A.A.; Miralrio, A.; Beltran-Perez, C. Models for predicting corrosion inhibition efficiency of common drugs on steel surfaces: A rationalized comparison among methodologies. Appl. Surf. Sci. Adv. 2024, 22, 100621. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).