Design and Performance Evaluation of Sn58Bi/SAC305 Layered Composite Solder for Low-Temperature Applications
Abstract
1. Introduction
2. Materials and Methods
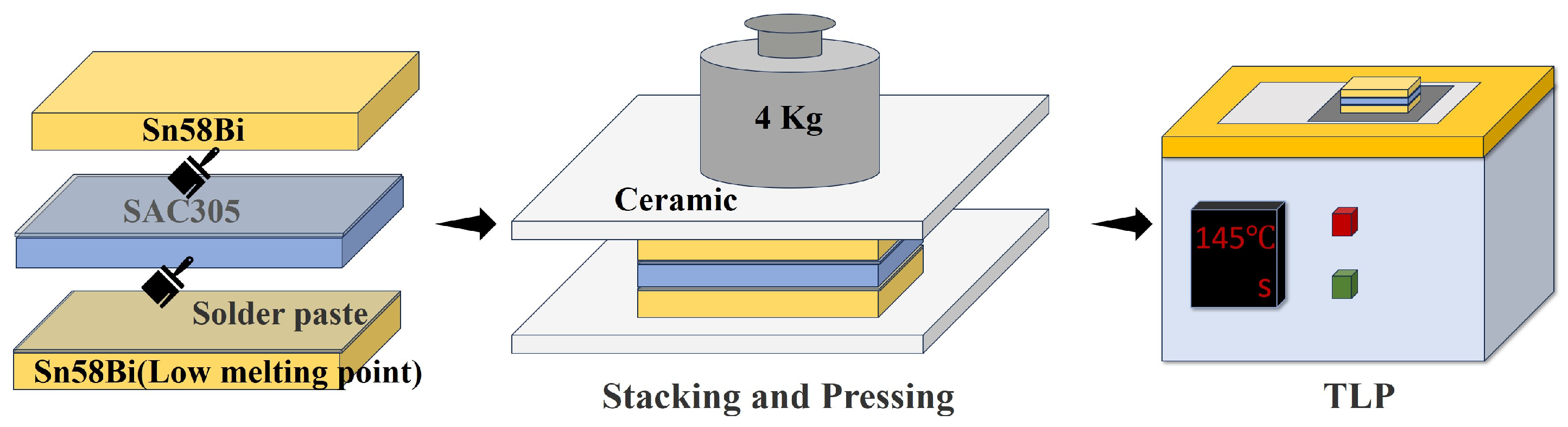
2.1. Design Principles and Fabrication of Sn58Bi/SAC305 Layered Composite Solder
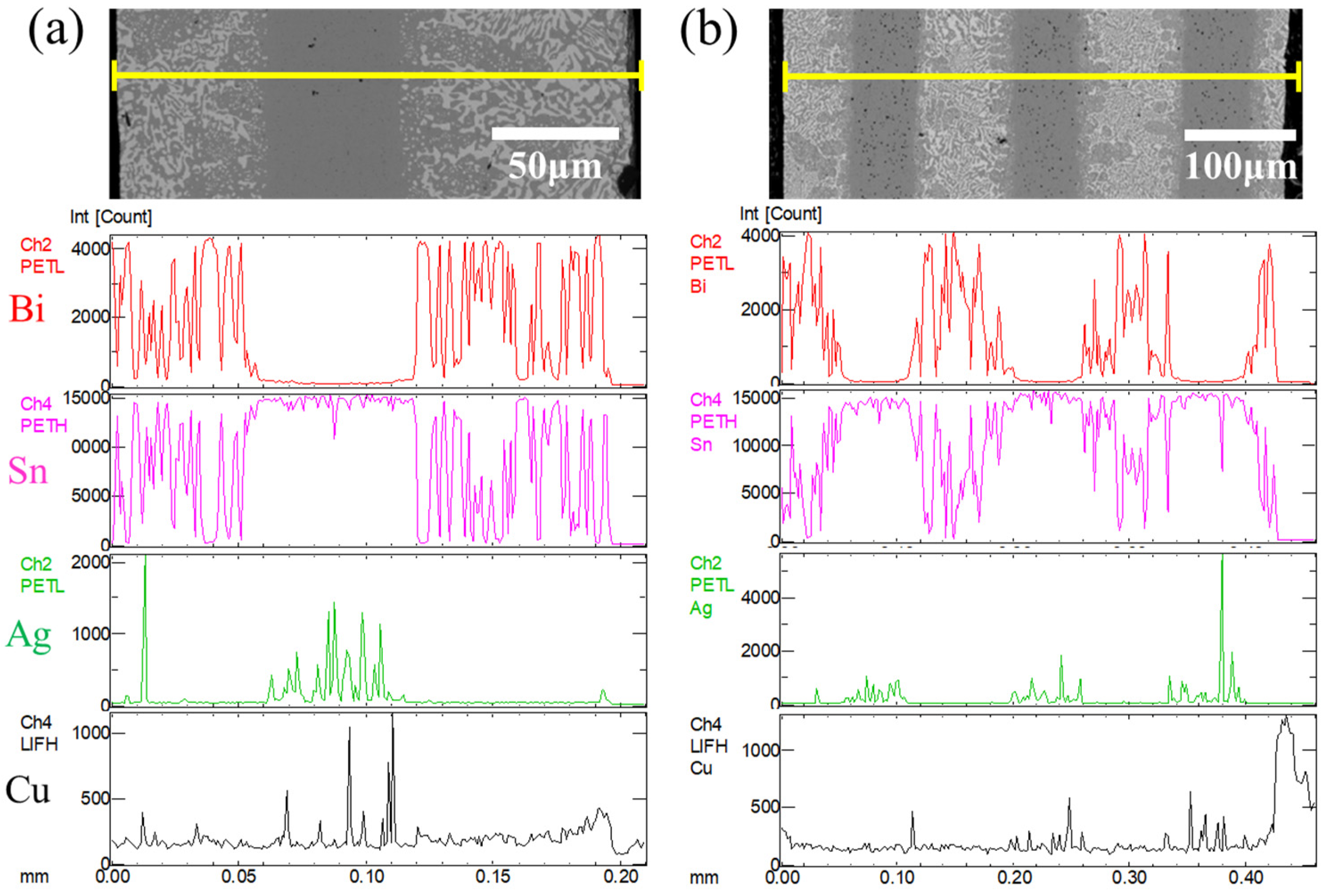
2.2. Microstructural and Elemental Distribution Characterization
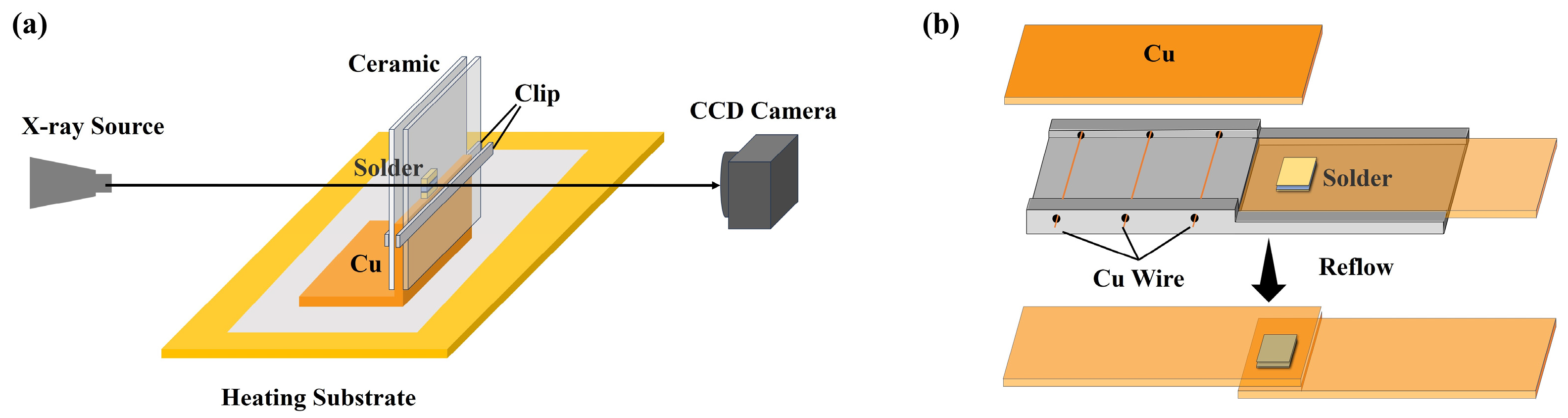
2.3. Soldering Process and Joint Performance Evaluation
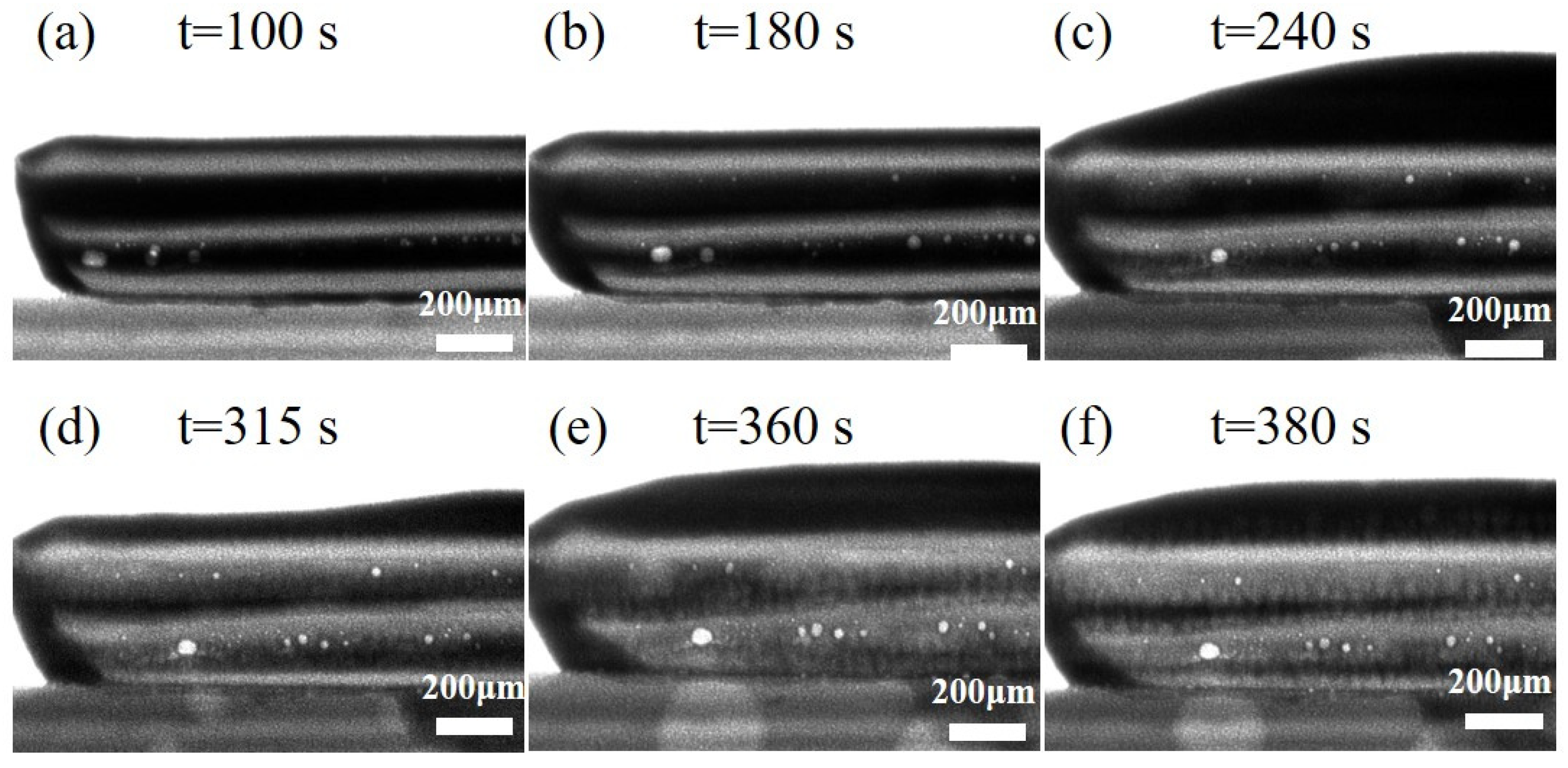
2.4. Synchrotron X-Ray Real-Time Imaging Experiment
2.5. Evaluation of Shear Properties of Solder Joints
3. Results and Discussion
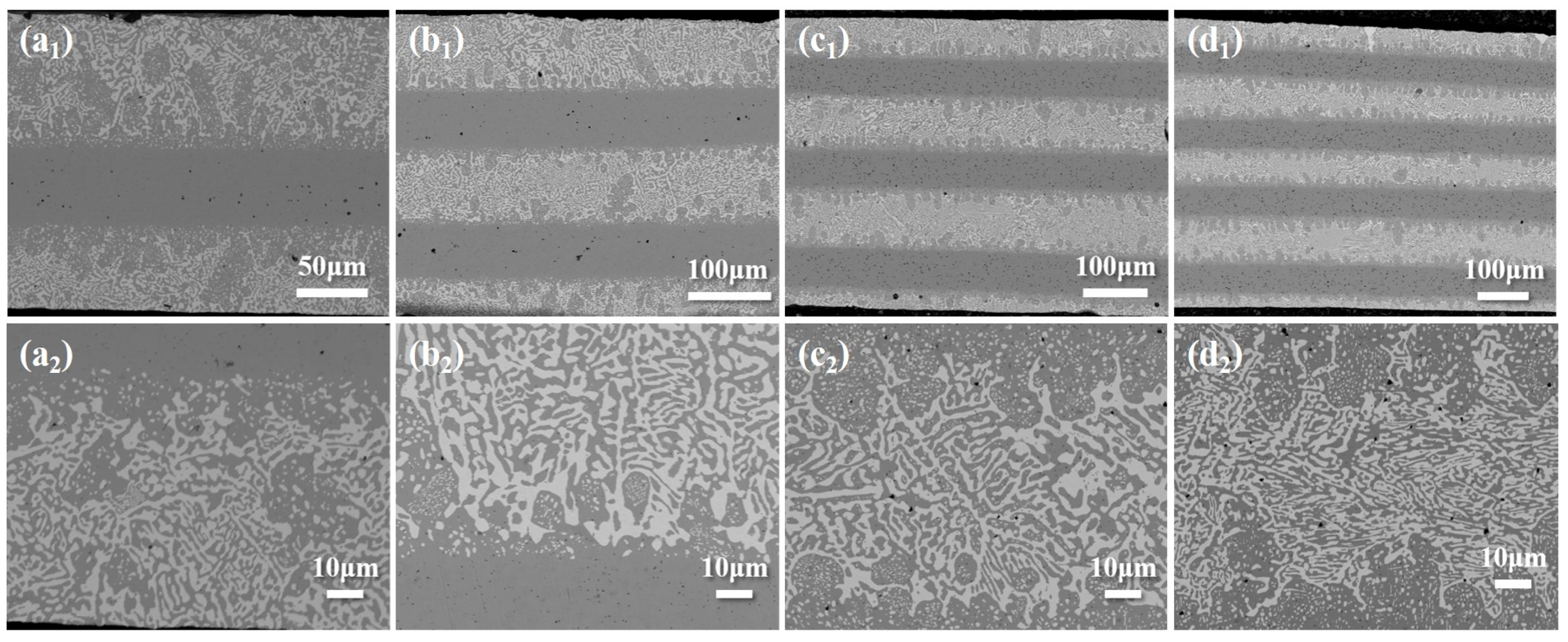
3.1. Microstructure of Layered Composite Solder
3.2. In Situ Observation of Interfacial Fusion Dynamics During Reflow
3.3. Soldering Performance of Layered Composite Solder
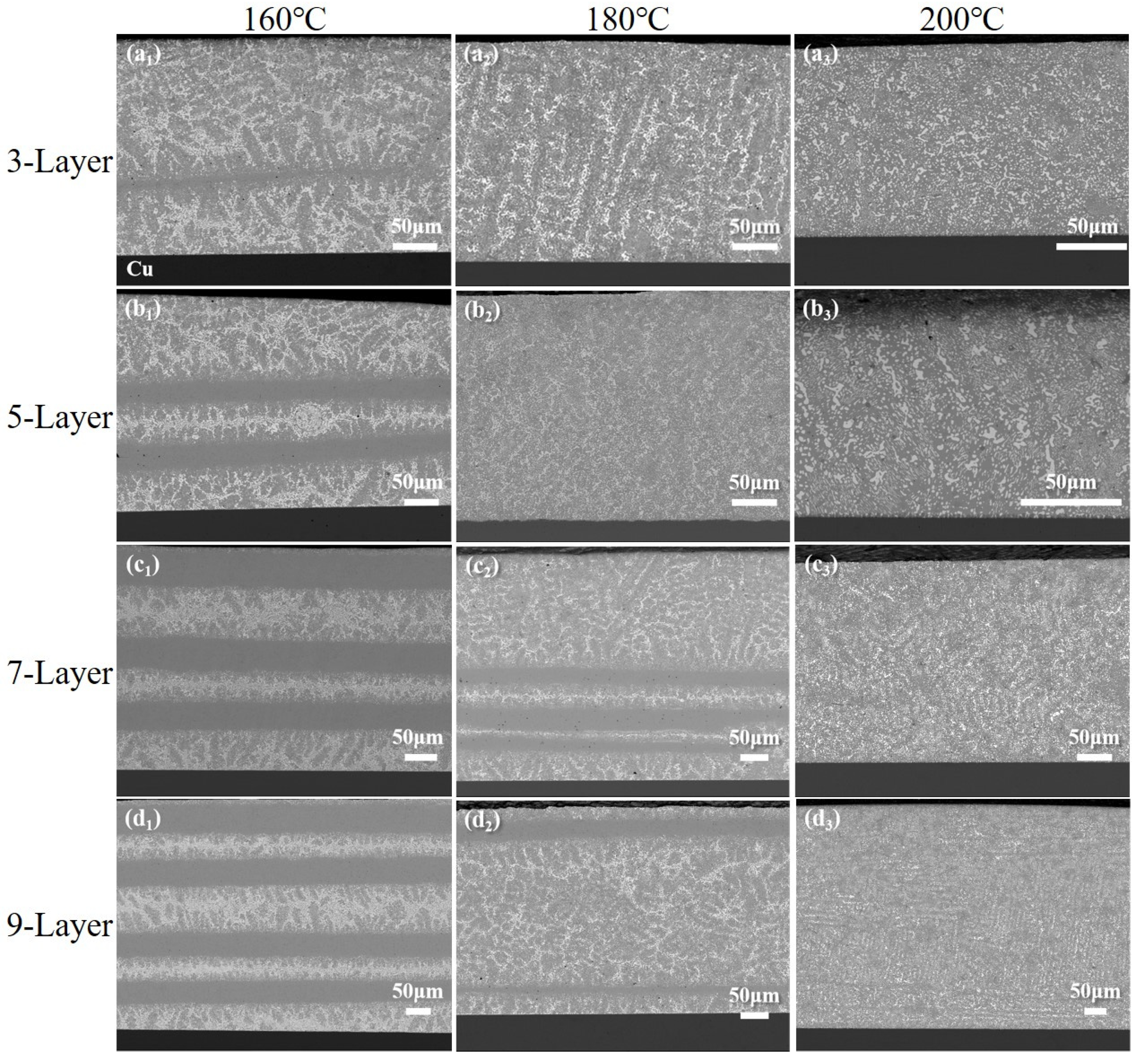
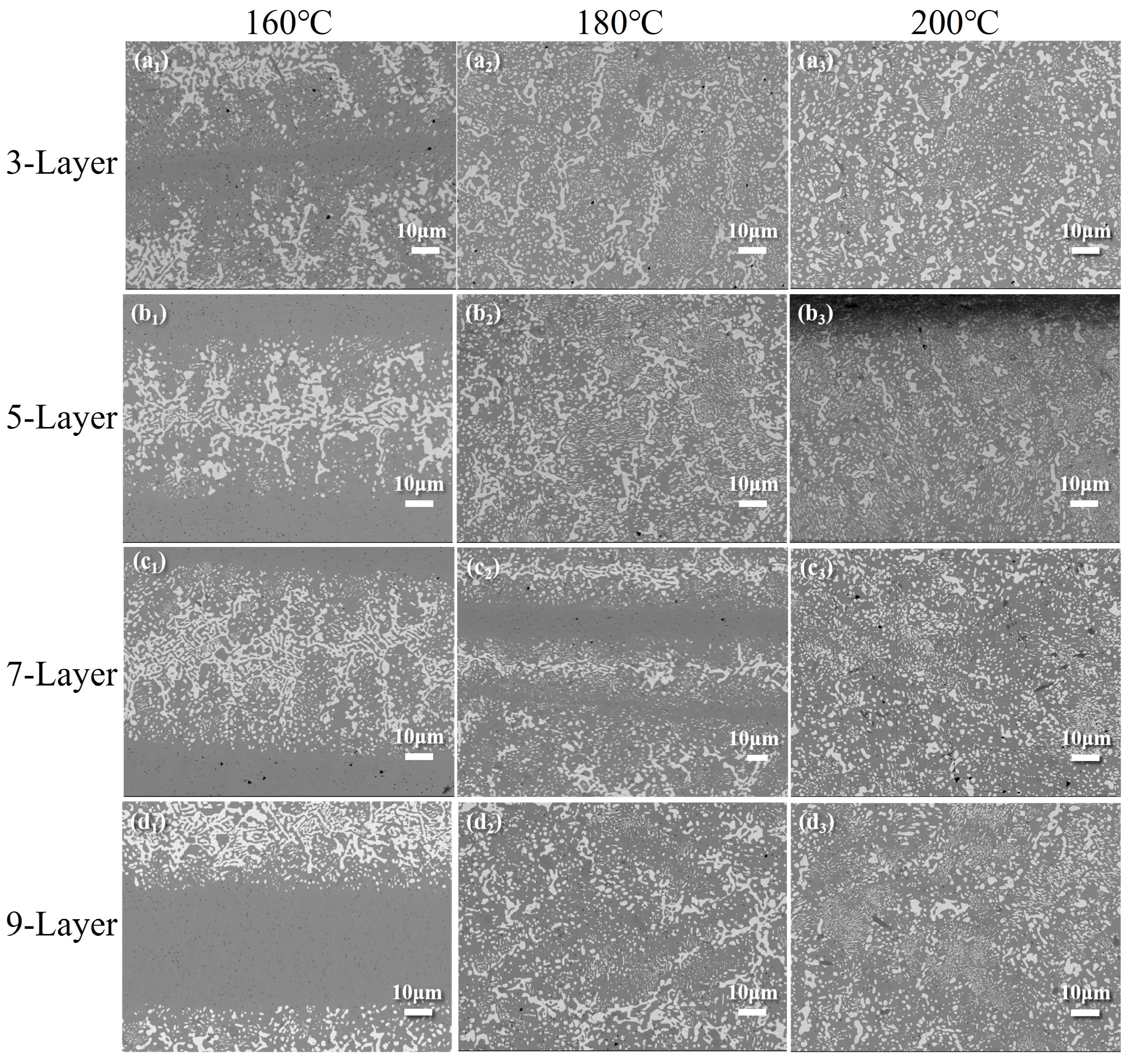
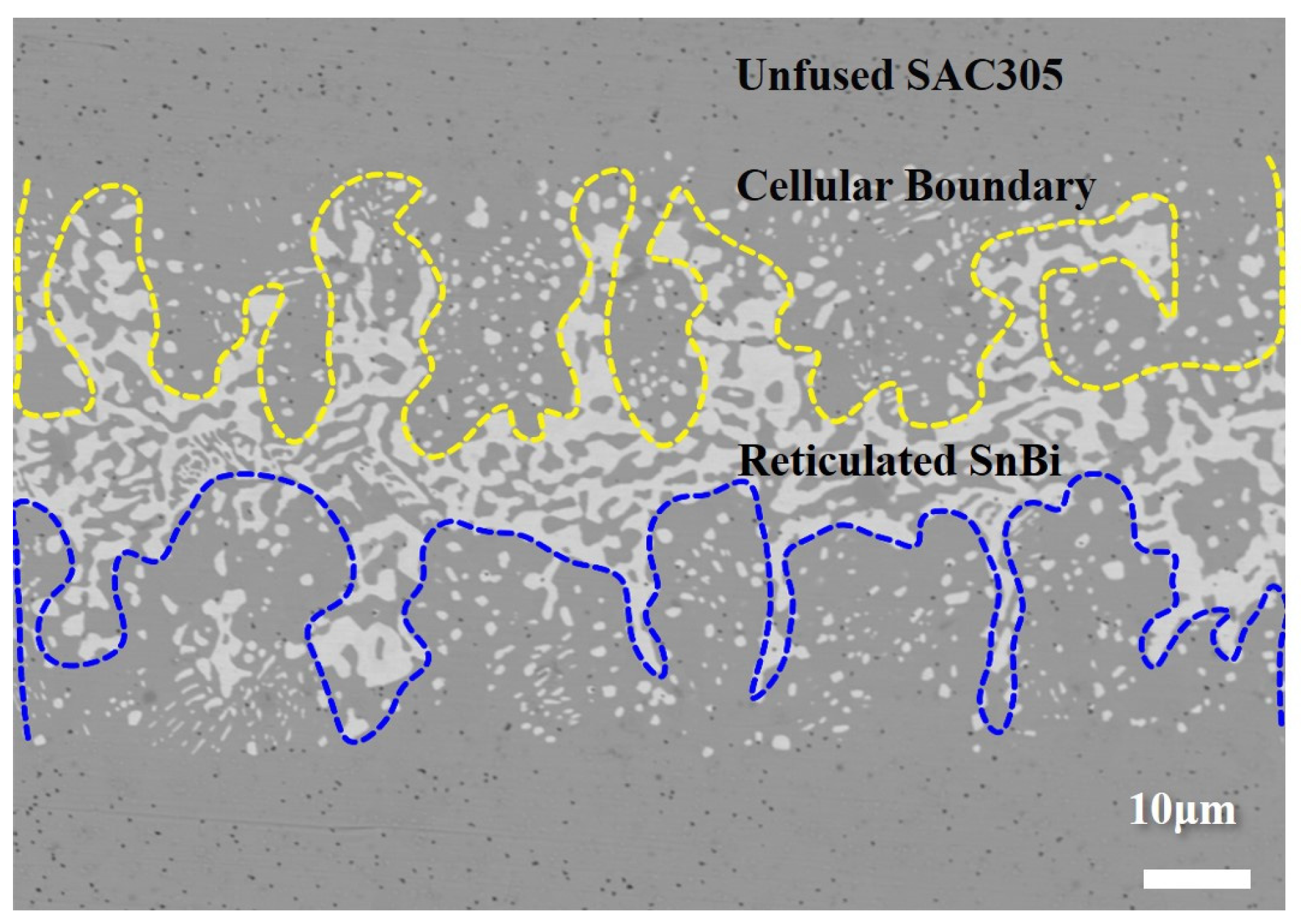
3.3.1. Fusion Behavior of Sn58Bi/SAC305 Layered Solder After Soldering
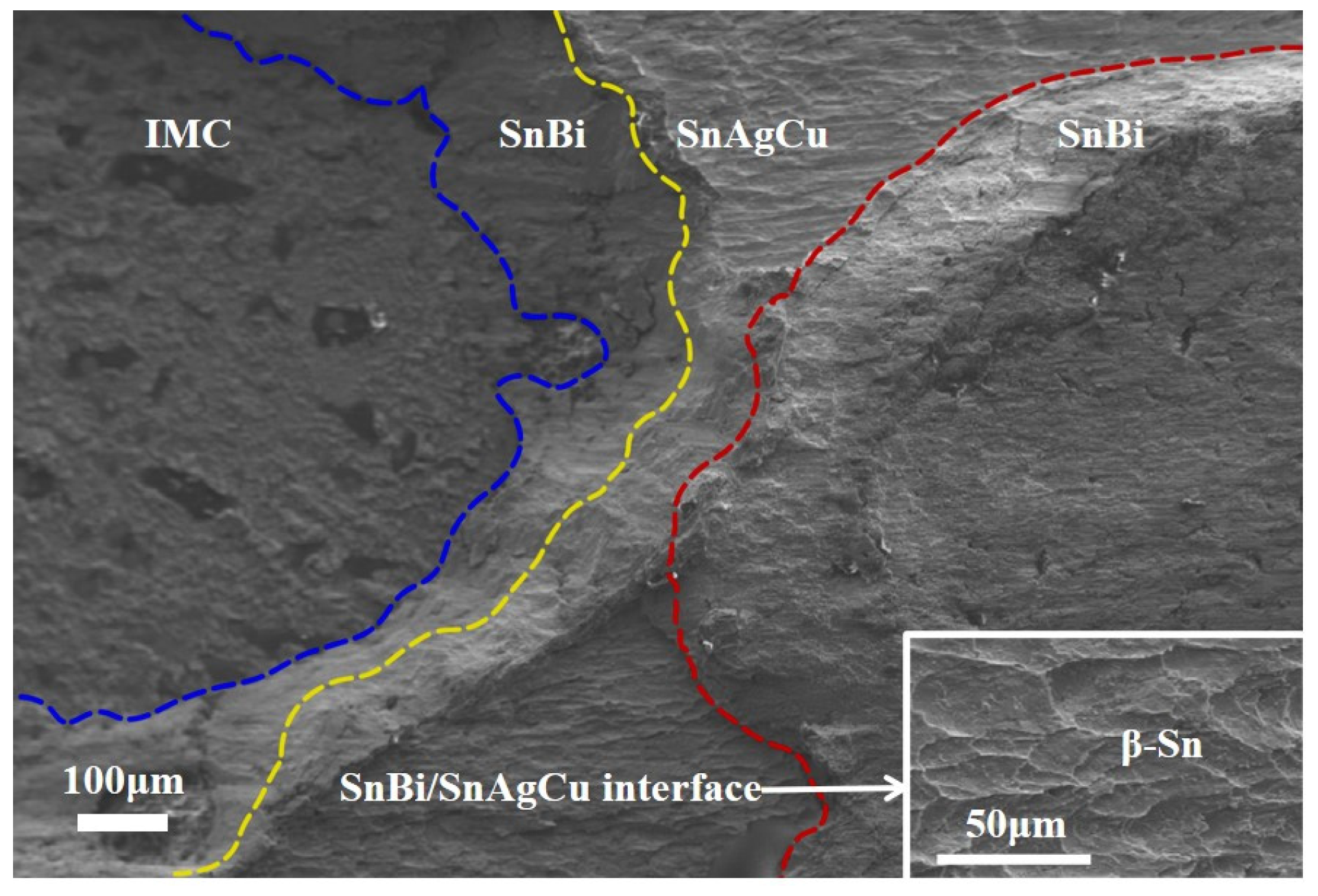
3.3.2. Interface Structure and Bi-Rich Phase Distribution After Soldering
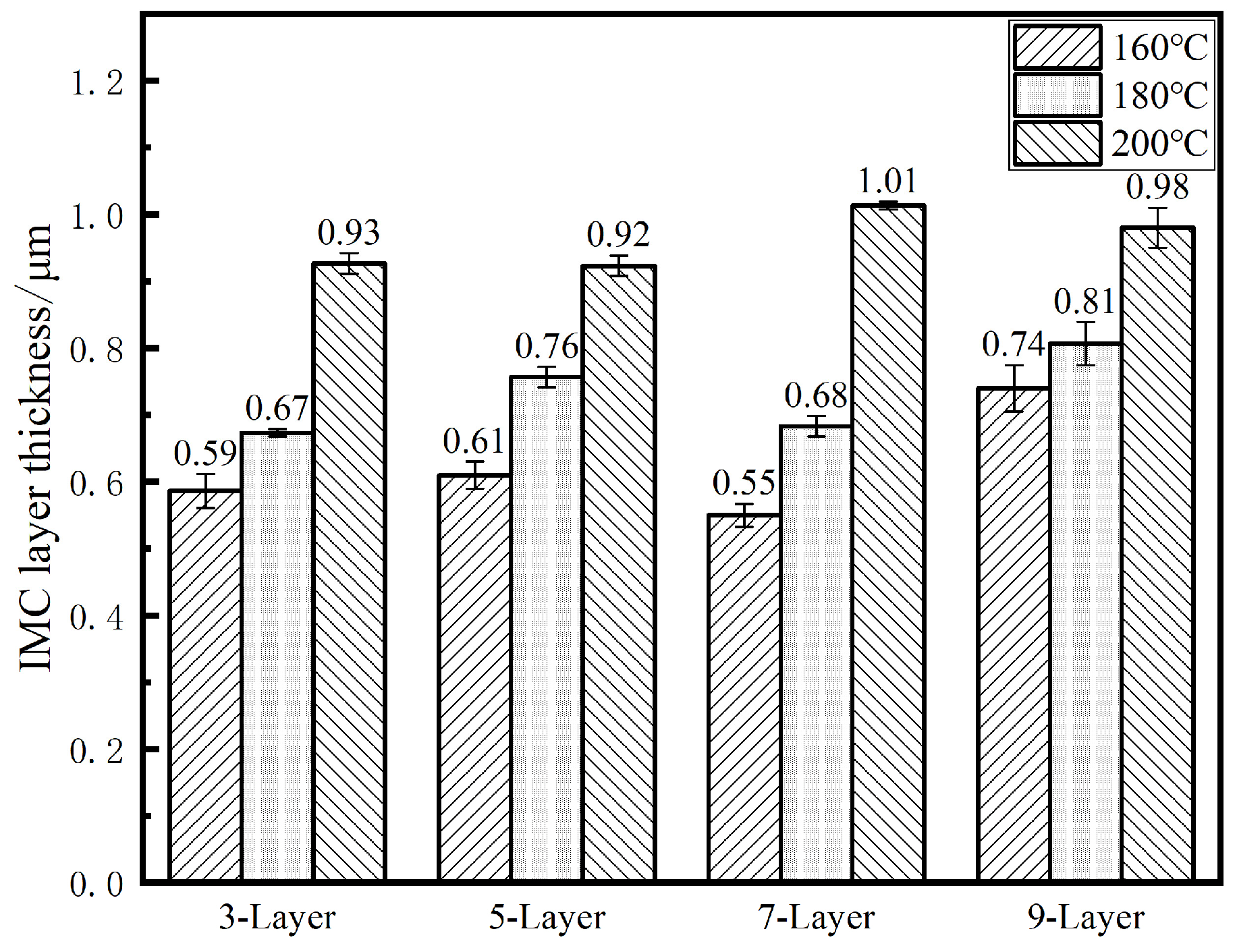
3.3.3. Changes in Interface IMC with Soldering Temperature
3.4. Shear Properties of Layered Composite Solder Joints
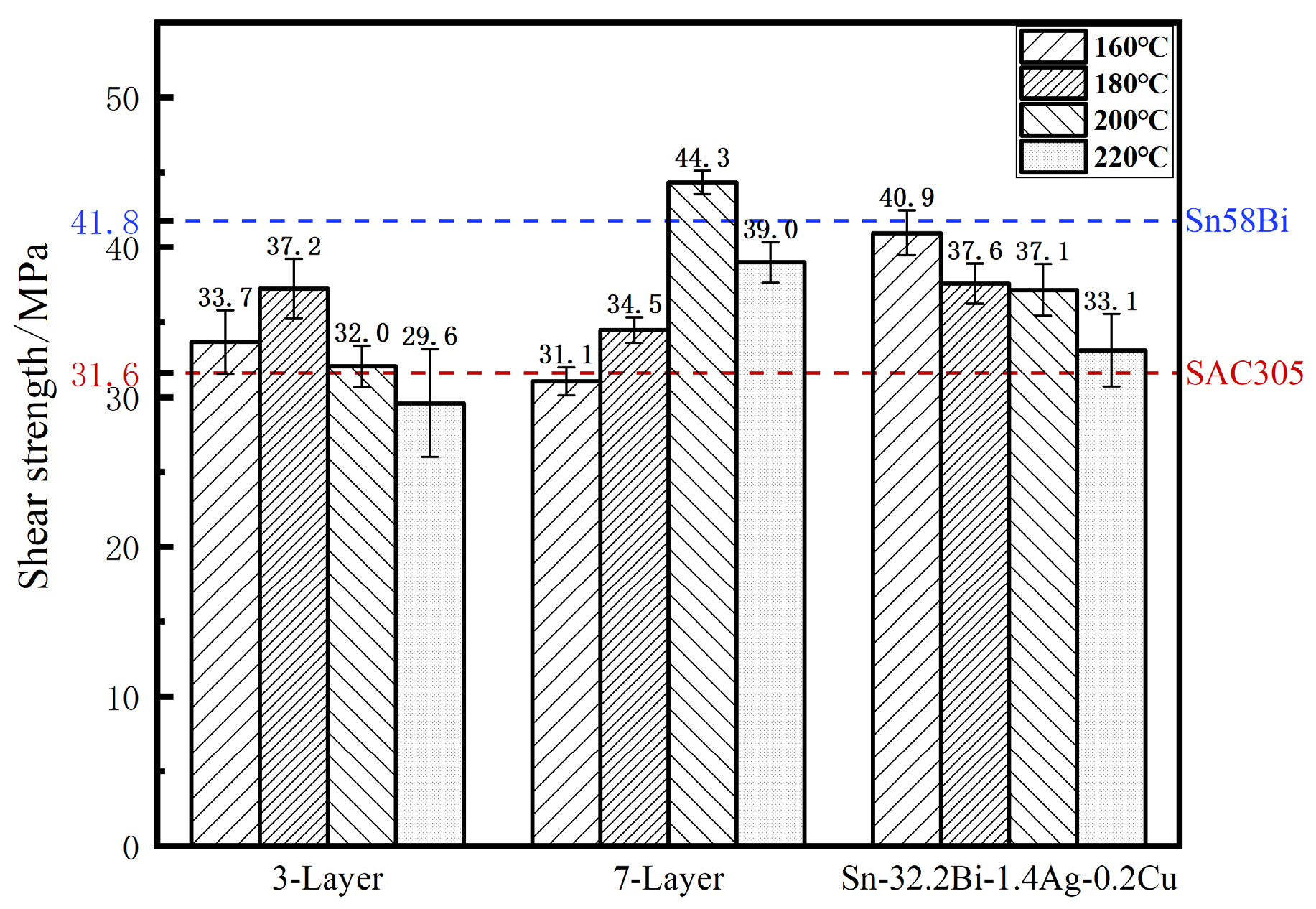
3.4.1. Shear Strength
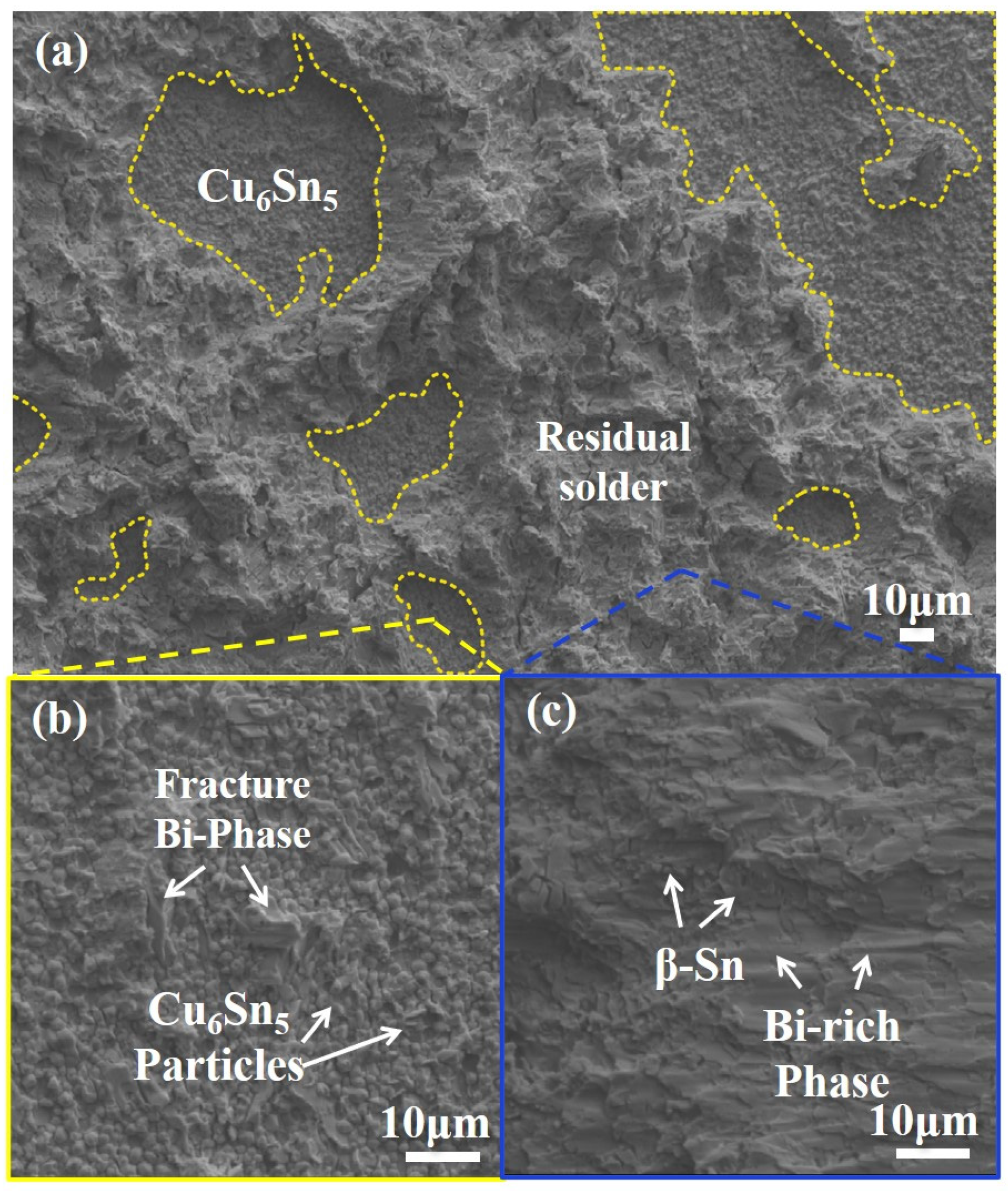
3.4.2. Fracture Morphology
4. Conclusions
- (1)
- The alternating layers of SAC305 and Sn58Bi enable controlled element diffusion during reflow. Bi diffusion into SAC305 reduces segregation, while the partial dissolution of SAC305 into molten Sn58Bi forms a sub-eutectic structure. Synchrotron imaging reveals that cellular interfaces arise from compositional supercooling, driven by localized Bi enrichment and rapid solidification at the solid–liquid boundary.
- (2)
- The fusion degree of layered solder increases with temperature, transitioning from a partially mixed “unfused SAC305 zone + cellular boundary zone + reticulated SnBi zone” (160–180 °C) to a fully homogenized structure (200 °C). The refined distribution of Ag3Sn and Cu6Sn5 phases at elevated temperatures enhances grain boundary strengthening, while excessive IMC growth at 220 °C degrades joint reliability.
- (3)
- Although melt-cast solders perform well at lower temperatures, the 7-layer composite solder reaches a peak shear strength of 44.3 ± 0.8 MPa at 200 °C, slightly surpassing Sn58Bi (41.8 ± 1.1 MPa). This is due to its uniform phase dispersion and optimized IMC thickness, making it suitable for applications that require both low-temperature assembly and high-temperature reliability. Fracture analysis indicates mixed ductile–brittle modes, with crack initiation predominantly at solder/IMC interfaces or heterogeneous layer boundaries.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Y.; Li, X.; Yang, L.; Zhou, W.; Wang, M.; Zhu, W.; Wu, P. Effects of graphene nanosheets addition on microstructure and mechanical properties of SnBi solder alloys during solid-state aging. Mater. Sci. Eng. A 2017, 696, 437–444. [Google Scholar] [CrossRef]
- Suganuma, K. Advances in lead-free electronics soldering. Curr. Opin. Solid State Mater. Sci. 2001, 5, 55–64. [Google Scholar] [CrossRef]
- Sidhu, R.; Chawla, N. Microstructure Characterization and Creep Behavior of Pb-Free Sn-Rich Solder Alloys: Part I. Microstructure Characterization of Bulk Solder and Solder/Copper Joints. Metall. Mater. Trans. A 2008, 39, 340–348. [Google Scholar] [CrossRef]
- Keller, J.; Baither, D.; Wilke, U.; Schmitz, G. Mechanical properties of Pb-free SnAg solder joints. Acta Mater. 2011, 59, 2731–2741. [Google Scholar] [CrossRef]
- Prabhu, K.N.; Deshapande, P.; Satyanarayan. Effect of cooling rate during solidification of Sn–9Zn lead-free solder alloy on its microstructure, tensile strength and ductile–brittle transition temperature. Mater. Sci. Eng. A 2012, 533, 64–70. [Google Scholar] [CrossRef]
- Yagi, S.; Ichitsubo, T.; Matsubara, E.; Yamaguchi, M.; Kimura, H.; Sasamori, K. Interfacial reaction of gas-atomized Sn-Zn solder containing Ni and Cu additives. J. Alloys Compd. 2009, 484, 185–189. [Google Scholar] [CrossRef]
- Yang, W.; Mao, J.; Ma, Y.; Yu, S.; He, H.; Qi, D.; Zhan, Y. Effects of yttrium addition on the microstructure evolution and electrochemical corrosion of Sn–9Zn lead-free solders alloy. Materials 2021, 14, 2549. [Google Scholar] [CrossRef]
- Sundelin, J.J.; Nurmi, S.T.; Lepistö, T.K.; Ristolainen, E.O. Mechanical and microstructural properties of SnAgCu solder joints. Mater. Sci. Eng. A 2006, 420, 55–62. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Z.; Yu, Y. Thermomechanical properties and fatigue life evaluation of SnAgCu solder joints for microelectronic power module application. J. Mater. Res. Technol. 2020, 9, 5955–5967. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Wang, Y.; Tian, C.; Fan, C.; Motozuka, S.; Yu, J. The Effect of Bi Addition on the Electromigration Properties of Sn-3.0Ag-0.5Cu Lead-Free Solder. Metals 2024, 14, 1149. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Huang, Y.; Liu, L.; Zhang, Z. Recent progress on the development of Sn–Bi based low-temperature Pb-free solders. J. Mater. Sci. Mater. Electron. 2019, 30, 3222–3243. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Li, M.L.; Wang, X.; Zhao, M. Enhancement of structure and properties of Sn58Bi solder by AlN ceramic particles. J. Mater. Sci. Mater. Electron. 2022, 19, 2584–2595. [Google Scholar] [CrossRef]
- Chen, L.T.; Chen, C.M. Electromigration study in the eutectic SnBi solder joint on the Ni/Au metallization. J. Mater. Res. 2006, 21, 15–21. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, H.; Sun, F.; Zhang, H.; Kong, X.; Xin, T. Microstructure and mechanical properties of as-reflowed Sn58Bi composite solder pastes. J. Mater. Process. Technol. 2016, 238, 290–296. [Google Scholar] [CrossRef]
- Ye, D.; Du, C.; Wu, M.; Lai, Z. Microstructure and mechanical properties of Sn–xBi solder alloy. J. Mater. Sci. Mater. Electron. 2015, 26, 3629–3637. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, K.; Chen, W.; Wu, J.; Wang, Y. Thermomigration Microstructure and Properties of Ni Nanoparticle-Reinforced Sn58Bi Composite Solder/Cu Solder Joint. Metals 2024, 14, 1420. [Google Scholar] [CrossRef]
- Ren, G.; Collins, M.N. Improved reliability and mechanical performance of Ag microalloyed Sn58Bi solder alloys. Metals 2019, 9, 462. [Google Scholar] [CrossRef]
- Zhang, P.; Xue, S.; Wang, J. New challenges of miniaturization of electronic devices: Electromigration and thermomigration in lead-free solder joints. Mater. Des. 2020, 192, 108726. [Google Scholar] [CrossRef]
- El-Daly, A.A.; El-Taher, A.M.; Gouda, S. Development of new multicomponent Sn–Ag–Cu–Bi lead-free solders for low-cost commercial electronic assembly. J. Alloys Compd. 2015, 627, 268–275. [Google Scholar] [CrossRef]
- Wu, X.; Sun, L.; Liu, Y.; Ye, Z.; Zhao, X.; Liu, Y. Preparation and performance of Sn-based composite solder joints by solid-liquid low-temperature solder bonding technology. J. Mater. Res. Technol. 2023, 24, 6378–6390. [Google Scholar] [CrossRef]
- Suh, J.; Tu, K.; Lutsenko, G.; Gusak, A. Size distribution and morphology of Cu6Sn5 scallops in wetting reaction between molten solder and copper. Acta Mater. 2008, 56, 1075–1083. [Google Scholar] [CrossRef]
- Xu, R.; Liu, Y.; Sun, F. Effect of isothermal aging on the microstructure, shear behavior and hardness of the Sn58Bi/Sn3.0Ag0.5Cu/Cu solder joints. Results Phys. 2019, 15, 102701. [Google Scholar] [CrossRef]
- Zhang, S.; Jing, X.; Chen, J.; Paik, K.; He, P.; Zhang, S. Preparation, characterization and mechanical properties analysis of SAC305-SnBi-Co hybrid solder joints for package-on-package technology. Mater. Charact. 2024, 208, 113624. [Google Scholar] [CrossRef]
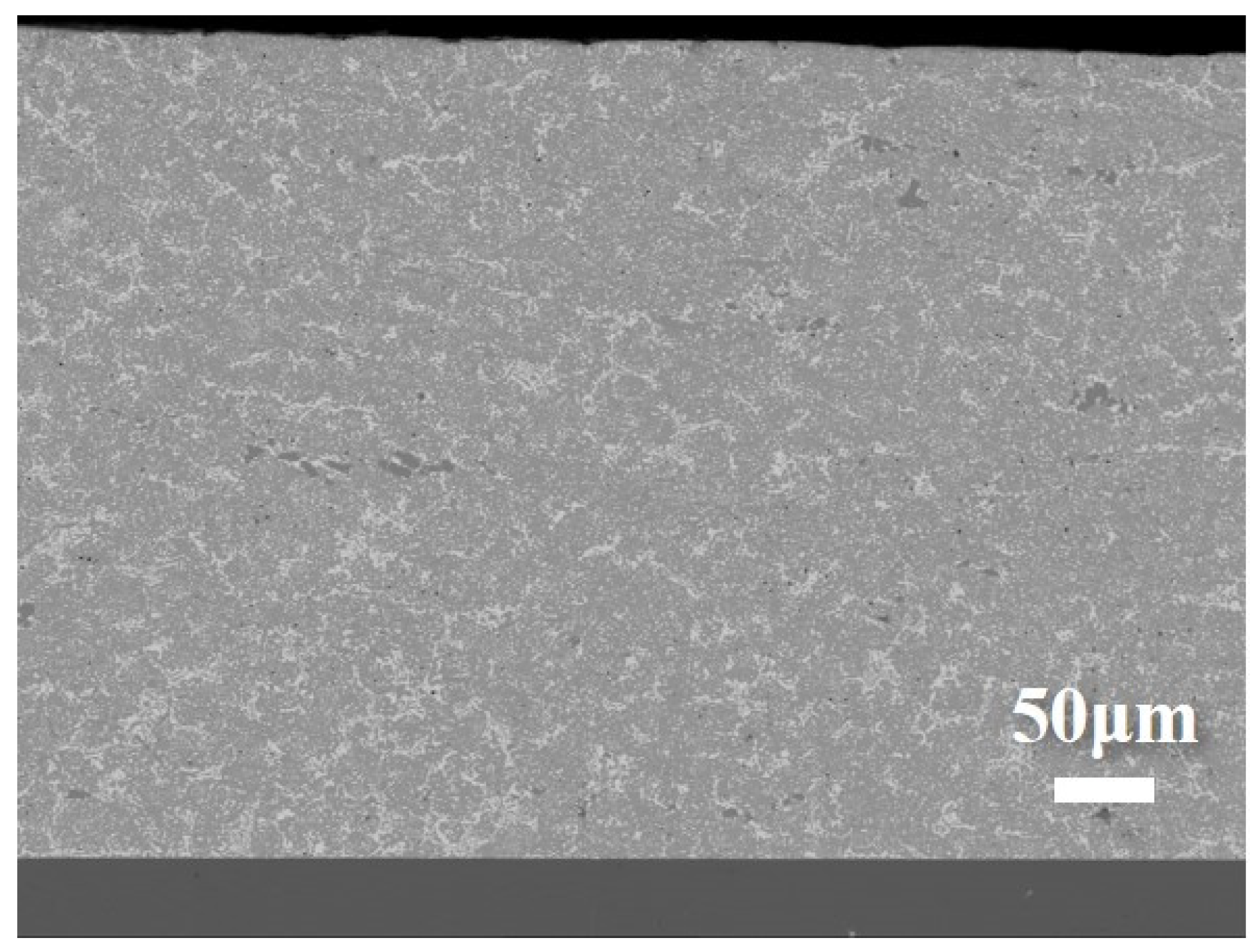

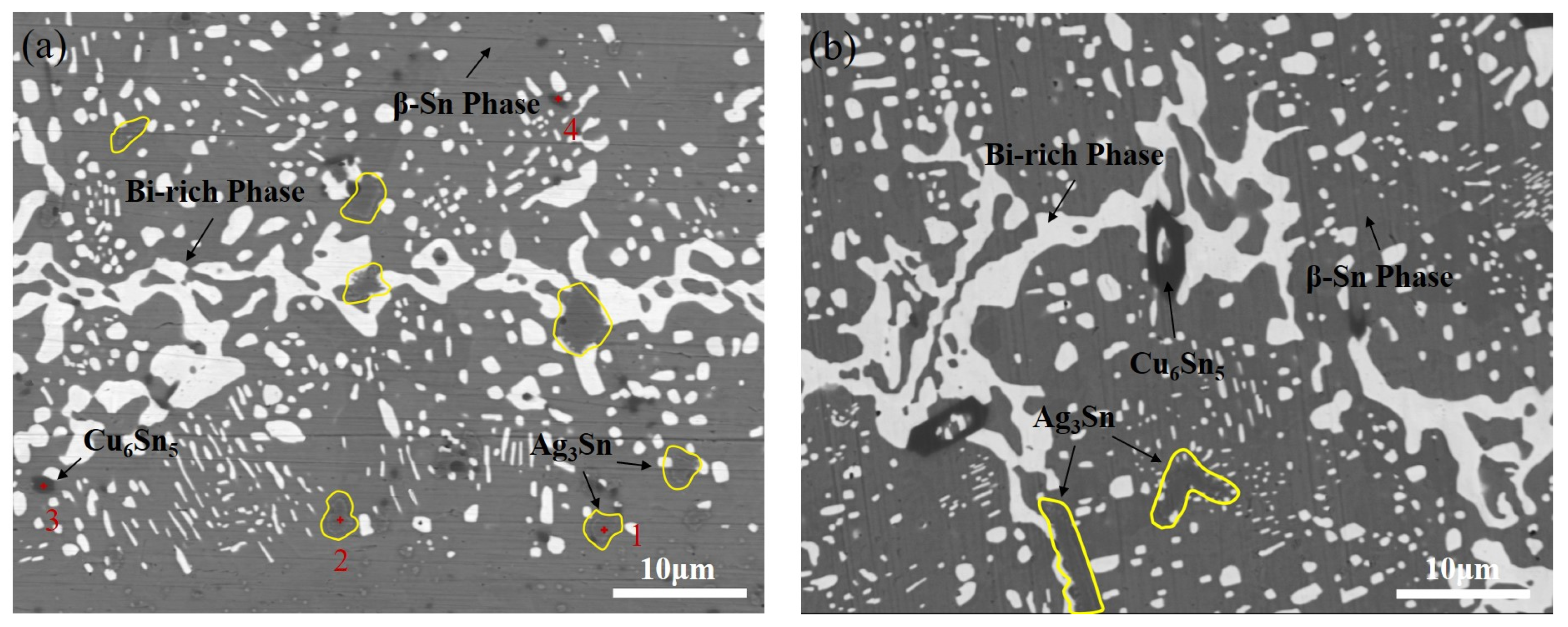

| Element | Sn | Bi | Ag | Cu |
|---|---|---|---|---|
| Mass% | 66.2 | 32.2 | 1.4 | 0.2 |
| Element | Sn | Ag | Cu | Bi |
|---|---|---|---|---|
| 1 | 25.6 | 70.8 | 2.7 | 0.9 |
| 2 | 24.1 | 74.3 | 1.0 | 0.6 |
| 3 | 48.9 | 0.1 | 50.7 | 0.3 |
| 4 | 49.5 | 0.1 | 50.3 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, D.; Li, C.; Yang, W.; Ma, H.; Ma, H.; Wang, Y. Design and Performance Evaluation of Sn58Bi/SAC305 Layered Composite Solder for Low-Temperature Applications. Metals 2025, 15, 185. https://doi.org/10.3390/met15020185
Zhang Z, Zhang D, Li C, Yang W, Ma H, Ma H, Wang Y. Design and Performance Evaluation of Sn58Bi/SAC305 Layered Composite Solder for Low-Temperature Applications. Metals. 2025; 15(2):185. https://doi.org/10.3390/met15020185
Chicago/Turabian StyleZhang, Zhongxu, Dan Zhang, Chenyu Li, Wenlong Yang, Haitao Ma, Haoran Ma, and Yunpeng Wang. 2025. "Design and Performance Evaluation of Sn58Bi/SAC305 Layered Composite Solder for Low-Temperature Applications" Metals 15, no. 2: 185. https://doi.org/10.3390/met15020185
APA StyleZhang, Z., Zhang, D., Li, C., Yang, W., Ma, H., Ma, H., & Wang, Y. (2025). Design and Performance Evaluation of Sn58Bi/SAC305 Layered Composite Solder for Low-Temperature Applications. Metals, 15(2), 185. https://doi.org/10.3390/met15020185






