Abstract
The effect of different SiC doping content on the properties of MoSi2-based composites was analyzed in this study. The MoSi2-SiC composites were fabricated in situ by the SPS technique, utilizing self-synthesized carbon-containing Mo powder and Si powder as raw materials. A two-step sintering process was employed to ensure the formation of a uniform and dense composite structure. The microstructures and mechanical properties of these composites with various compositions were characterized. The results show that the composites were primarily composed of MoSi2, SiC, and a minor proportion of MoSiC phase. The introduction of SiC as a second phase was found to considerably enhance the mechanical properties of the MoSi2 matrix material. In particular, the MoSi2-26mol.%SiC sample exhibited Vickers hardness, fracture toughness, and flexural strength values of 16.1 GPa, 6.7 MPa·m1/2, and 496 MPa, respectively, corresponding to increases of 33%, 24%, and 28% compared to the pure MoSi2 material.
1. Introduction
Molybdenum disilicide (MoSi2) is a borderline intermetallic with both metallic and ceramic characteristics [1,2]. It is widely used in aerospace, high-temperature smelting, electronic components, and refractory materials that require high temperature resistance, excellent oxidation resistance, and good thermal stability [3,4,5]. This can be attributed to several unique properties of MoSi2, including its high melting point, low density, high thermal conductivity, and low coefficient of thermal expansion. Additionally, a protective glass layer is produced on the surface of MoSi2 at high temperatures, which plays the role of hindering oxygen diffusion, so as to maintain a longer service life in high-temperature air environments [6,7,8,9]. However, there are a number of problems with its practical applications, which limit the use of MoSi2 in certain areas with high performance requirements. MoSi2 undergoes the “PEST” phenomenon when exposed to oxygen at moderate temperatures, and is rapidly oxidized to powder and volatilizes, leading to the destruction of the structure of the matrix as well as the degradation of its mechanical properties. Although MoSi2 exhibits good strength at high temperatures, its inherent toughness is poor, especially at low temperatures, and the MoSi2 material is susceptible to brittle fracture in environments subjected to shock loads or load variations [10,11]. Therefore, further research is required to enhance the properties of MoSi2 materials.
The properties of MoSi2 can be improved by a variety of methods, such as doping or alloying, process improvements, and surface coating. Numerous enhancement methods have been proposed by researchers to improve its material properties. These approaches include the incorporation of elements such as metallic W, Al, and Ti, or the addition of reinforcing phases such as carbides, nitrides, and oxides. Furthermore, grain refinement techniques have been employed to control grain size and distribution, thereby enhancing the mechanical properties. Advanced processing techniques have also been utilized to improve the material density and overall mechanical performance [12,13,14,15]. Xu et al. successfully prepared MoSi2/WSi2 solid solution powders with nanostructures by combustion synthesis using a blend of mechanically activated Mo, W, and Si powders [16]. Li et al. prepared (Mo,Ti)Si2 biphasic composite antioxidant coatings on molybdenum alloys by the molten slurry method, and the composite coatings formed during oxidation of the Si-Ti-O composite glass can provide long-term antioxidant protection [17]. Ko et al. synthesized nanoscale MoSi2 and Si3N4 powders via high-energy ball milling using Mo2N and Si powders as raw materials, and then prepared MoSi2-Si3N4 compounds by pulsed-current-activated sintering method, with an average hardness and fracture toughness of 12 GPa and 6 MPa·m1/2, respectively [18]. It is worth noting that SiC, as a strong and high-temperature-resistant material, has a low coefficient of thermal expansion (CTE, 5 × 10−6 K−1) as well as good compatibility with MoSi2 materials [19,20]. Therefore, the addition of SiC to MoSi2 materials can effectively enhance their thermal stability and high-temperature strength, while the composites have a relatively low density, making them advantageous for aerospace and other applications that require lightweighting [21]. Therefore, MoSi2-SiC composites are considered to have broad application prospects and have received extensive attention [22,23]. Chen et al. prepared 10% SiC/MoSi2 composites using in situ pressureless sintering with Mo, Si, and C powders. The flexural strength and fracture toughness of the composites reached 274.5 MPa and 5.5 MPa·m1/2, respectively, equating to an increase of 40.8% and 30.6%, respectively, over the pure MoSi2 material [24]. Dense SiC/MoSi2 nanocomposites with a relative density exceeding 98% were prepared by Peng et al. using reactive hot pressing. The room temperature flexural strength of the 15 vol% SiC/MoSi2 composite was 610 MPa, which represented a 141% increase compared to that of monolithic MoSi2. Additionally, the yield strength at 1200 °C reached 720 MPa [25]. He et al. fabricated SiC/MoSi2 composite coatings on the surface of C/C composites using the chemical vapor infiltration/reaction technique. The composite coatings effectively protected the samples from oxidation at 1500 °C for 80 h, with only a 1.25% mass loss [26]. Overall, the incorporation of SiC effectively strengthens the comprehensive properties of MoSi2-based composites, positioning them as promising candidates for a wider array of high-temperature environments.
To date, various preparation methods have been developed to synthesize MoSi2 matrix composites, including mechanical alloying (MA) [27], hot pressing (HP) [28], plasma spraying [29], spark plasma sintering (SPS) [30], self-propagating high-temperature synthesis (SHS) [31] and other methods. The results of the current study indicate that powder metallurgy techniques can effectively optimize the microstructure of MoSi2 and improve its mechanical properties by adjusting sintering parameters such as the temperature, holding time, and pressure [32]. Notably, SPS is recognized as one of the most advanced powder metallurgy techniques, capable of achieving nearly full densification while maintaining a fine grain structure during the sintering process [33]. In this study, the MoSi2 materials were strengthened by introducing the SiC second phase, and the MoSi2-based composites were prepared by in situ synthesis using SPS equipment using Si powder and self-synthesized carbon-containing Mo powders as raw materials. MoSi2-SiC composites with different SiC contents were successfully produced and analyzed for their microstructure and various mechanical properties.
2. Experimental Procedures
2.1. Sample Fabrication
The experimental raw materials were ammonium molybdate ((NH4)2Mo4O13, Jinduicheng Molybdenum Co., Ltd., Xi’an, China), carbon black (BR193, Tianjin Ebory Chemical Co., Ltd., Tianjin, China), and silicon powder (Si, 99.99% purity, particle size 5 µm, Beijing Xingrongyuan Technology Co., Ltd., Bejing, China). Molybdenum (Mo) powder and carbon-containing Mo powder were first prepared by a two-step reduction method [34]. The detailed preparation process is given as follows.
(NH4)2Mo4O13 was calcined at 500 °C for 4 h to obtain molybdenum trioxide, which was mixed with four different molar ratios of carbon black by ball milling (1:1.9, 1:2.2, 1:2.3, and 1:2.4), and then carbothermally reduced to obtain homogeneous and dispersed pre-reduced Mo powders. Finally, a slight portion of residual molybdenum oxide is removed with hydrogen at the deep deoxygenation stage. The C contents of the four powders obtained were 0.01 wt.%, 1.63 wt.%, 3.19 wt.%, and 4.26 wt.%, respectively. The powder mixtures were prepared by adding Si powder according to the powder compositions, which are listed in Table 1. The powder blends were milled by zirconia balls using a high-energy ball mill for 6 h to ensure that the powder mixtures were homogeneous and dispersed. Anhydrous ethanol was used as the grinding medium with a ball to material ratio of 5:1 and a rotational speed of 200 rpm. After the milling treatment, the mixtures were placed into 20 mm diameter cylindrical graphite molds, and a layer of graphite foil was added between the powders and the molds to avoid sticking during the high-temperature sintering procedure. The sintering process was performed using SPS equipment (FIH-452NP, Fuji Electronic Industrial Co., Ltd., Tokyo, Japan) with a two-step sintering process, first at 1200 °C for 5 min, and then at 1600 °C for 10 min. The uniaxial pressure was maintained at 50 MPa during the sintering process, and the temperature ramping rate was 100 °C/min.

Table 1.
Designations and raw material compositions for different samples.
2.2. Sample Characterization
The sintered circular flake samples were fully sanded with SiC sandpaper to remove the graphite foil on the surface and polished until the sample surface showed a mirror effect. The theoretical densities of the composites with different compositions were calculated based on the densities of MoSi2 (6.28 g/cm3) and SiC (3.2 g/cm3) [35,36]. The actual densities of each group of samples were calculated by Archimedes’ method and the relative densities were further obtained by the aspect index of actual density to theoretical density. The Vickers hardness was measured on the surface of the samples by a digital Vickers hardness tester at a load of 1 kg and a holding pressure of 10 s. Every sample was randomly measured at 10 positions and the data were averaged. The Vickers hardness was calculated as , where f is the applied load in kg and d is the average of the two diagonal lines of the indentation in mm. Fracture toughness (KIC) data were obtained by measuring the crack length produced on the sample surface under a load of 5 kg and calculated according to the formula of Anstis et al. [37]. The sintered samples of different compositions were all machined into rectangles with 12 mm × 4 mm × 3 mm dimensions by a diamond wire cutting machine. Three-point flexural tests were carried out on a universal testing machine to measure the flexural strength with a loading span of 9 mm and a crosshead speed of 0.5 mm/min, and the samples of each composition were tested three times to calculate the mean value. The electrical resistivity was determined by the four-probe method using a multifunctional digital four-probe tester, and the average value was taken from at least three measurements for each sample.
The phase compositions of the composites were analyzed and determined by an X-ray diffractometer (XRD, TTR III, Rigaku Corporation, Tokyo, Japan) with Cu Kα radiation in the 2θ range of 10–90° at a scanning rate of 0.2 s/step and a step size of 0.02 °/step. The microstructures and chemical compositions of the sintered specimen surfaces and sections were analyzed by field emission scanning electron microscopy (FE-SEM, ZEISS SUPRA 55, Oberkochen, Germany) and energy spectroscopy (EDS).
3. Result and Discussion
3.1. Thermodynamics of MoSi2-SiC System
Based on the composition of the Mo-Si-C mixture powders, the following reactions are assumed to take place during the sintering process:
In order to further verify the sintered products, the thermodynamic data of the possible reactions were calculated using HSC Chemistry 6.0 software. The Gibbs free energy changes (∆Gθ) for these reactions at various temperatures are depicted in Figure 1, showing that the reactions proceeded spontaneously during both holding stages. Therefore, the composites obtained using two-stage SPS of Mo-Si-C mixed powders are inferred to be composed of MoSi2, Mo5Si3, and SiC.
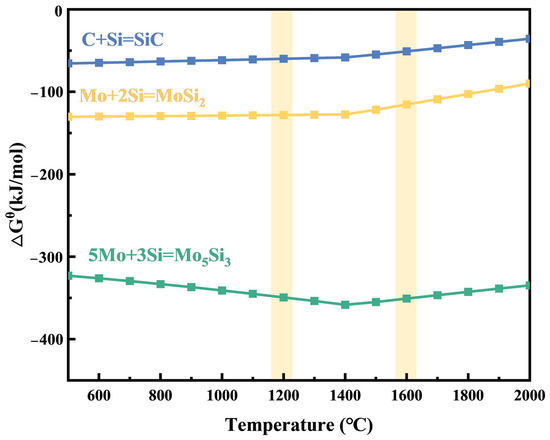
Figure 1.
Temperature dependences of the changes in standard Gibbs free energy for Equations (1)–(3).
3.2. Phase Identification
Figure 2 illustrates the XRD patterns of four groups of samples containing different SiC contents after sintering by SPS. The results indicate that the primary phase in the composites is MoSi2, while the SiC phase becomes more obvious as the C content in the powder samples increases. Additionally, weak diffraction peaks corresponding to Mo5Si3 were observed. Mo5Si3 is a typical by-product in the MoSi2 synthesis process [38,39]. In addition, Hu et al. [40] determined that Mo5Si3C is the equilibrium phase within the Mo-Si-C system, as indicated by the following high-temperature reactions:
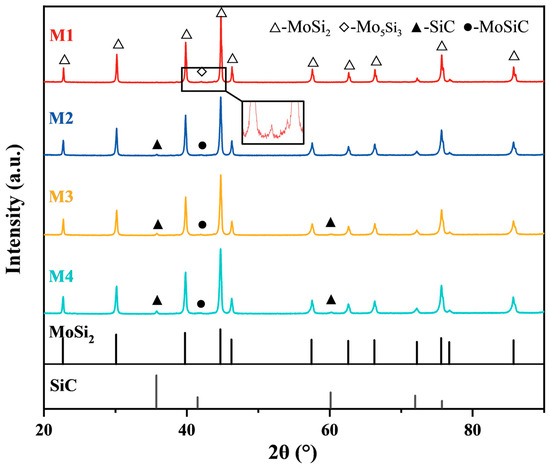
Figure 2.
XRD patterns of the as-sintered MoSi2-SiC composites.
The approximate composition of this phase was found to be Mo4.8Si3C0.6. This product phase has been confirmed by other relevant studies [41,42]. It can be observed that MoSi2 and SiC were primarily generated in the reaction process without the residual raw powders.
3.3. Behavior of Sintering
It is well established that the sintering temperature has an essential role in controlling the microstructure and properties of materials. Therefore, a series of preliminary experiments were conducted by adjusting the sintering temperature (1300 °C–1700 °C) before determining the experimental conditions. The results indicated that at lower sintering temperatures, incomplete reactions of the raw materials and the formation of a porous structure occurred. This is attributed to the slower atomic migration rates and insufficient particle contact, which hindered the formation of sintering necks and reduced the degree of densification. Conversely, higher sintering temperatures promoted excessive grain growth, creating defects and deteriorating the mechanical properties of the material. Additionally, the residual oxides in the samples generate gases during the carbon reduction process at high temperatures, resulting in the formation of numerous pores. When high-temperature sintering was employed directly, rapid densification prevented gas discharge, resulting in the formation of closed pores and consequently increasing the porosity of the sintered samples. In conclusion, the two-step sintering process used in this study offers distinct advantages. In the first step, the material was held at 1200 °C for 5 min to complete the reaction and achieve initial densification, followed by a second step, where the temperature was raised to 1600 °C for 10 min to further enhance the densification and material strength. This approach enabled the gradual optimization of the microstructure, preventing excessive grain growth and sintering inhomogeneity, ultimately producing a more homogeneous and densified material. As shown in Figure 3, the relative densities of the four powders after sintering demonstrate that almost full densification was achieved in all of the samples. The introduction of SiC as a second phase caused a slight reduction in the relative density, attributed to gas evolution during the reduction of SiO2 by C. However, all of the composites achieved relative densities exceeding 98%.
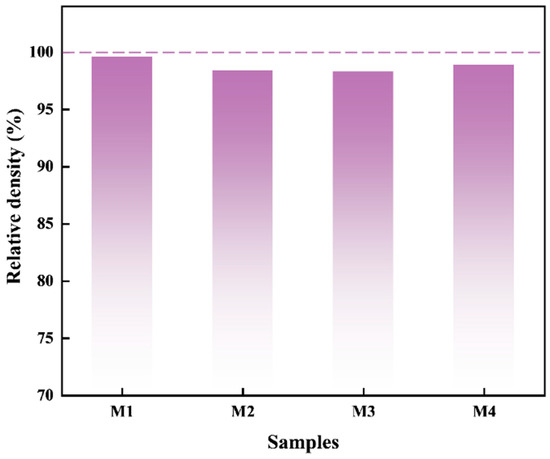
Figure 3.
The effect of different SiC contents on the relative densities of the samples.
Figure 4 shows the temperature and shrinkage displacement curves as a function of the sintering time during the two-step sintering process for the Mo-Si-C system. The shrinkage displacement curves represent the changes in the sample thickness, with positive or negative displacements indicating expansion or contraction, respectively. The entire procedure can be separated into five unique stages. In the initial stage, as the temperature and pressure increase, the displacement remains stable after the mixed powder is gradually compacted. In the second stage, as the temperature continues to rise, diffusion and particle rearrangement occur between the powder particles, leading to the formation of preliminary inter-particle connections. Due to the large volume change before and after the reaction, this corresponds to a rapid contraction of the displacement curve in the figure. In the third stage, the rate of displacement contraction slows down during the holding period at 1200 °C. Until the temperature is further increased, in the fourth stage, the diffusion rate between the particles accelerates, and the reaction becomes more intense. The raw material powder is fully reacted and shows an instantaneous shrinkage of the displacement. During the final holding stage at 1600 °C, the displacement curve remains almost constant and the sintered sample tends to densify completely. During the final densification stage, grain growth occurs. Meanwhile, the particles are more tightly bound and the porosity is reduced. As a result, the sintered samples exhibit high densification degrees, resulting in excellent mechanical properties.
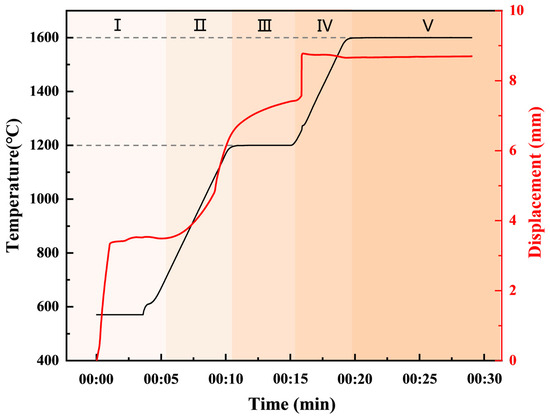
Figure 4.
The displacement and temperature variation curves of the M2 sample vs. time during the two-step sintering process.
3.4. Microstructure of Composites
Figure 5 presents the microstructure of the sintered samples of four various compositions, all of which exhibit a uniform and dense microstructure. The pure MoSi2 sample depicted in Figure 5a consists of a grey matrix phase, a white phase, and a nearly round dark phase. Conversely, the Mo-Si-C system samples shown in Figure 5b–d contain, in addition to the grey matrix phase and some white phase, a significant quantity of black phases. These black phases are characterized by irregular shapes and extensive agglomeration, the causes of which will be discussed in detail below.
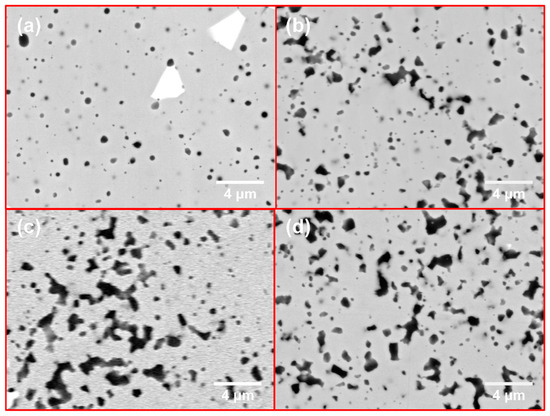
Figure 5.
The morphology images of sintered products: (a) M1; (b) M2; (c) M3; (d) M4.
Figure 6 displays an enlarged view of the sample surface and corresponding EDS results. On the basis of the EDS mapping data, it can be deducted that the grey phase corresponds to the MoSi2 matrix. The white phase, characterized by a high Mo content and low Si content, is likely Mo5Si3, while the darker phase, enriched in oxygen, corresponds to SiO2. Due to the amorphous nature of SiO2, its diffraction peaks are not detected in XRD. Its generation is related to the inevitable adsorption of oxygen on the powder surface in treatment, and, thus, oxidation of the highly activated Si powder produces the SiO2 phase. Furthermore, the reduction in the Si content in certain regions causes the creation of the Mo5Si3 phase.
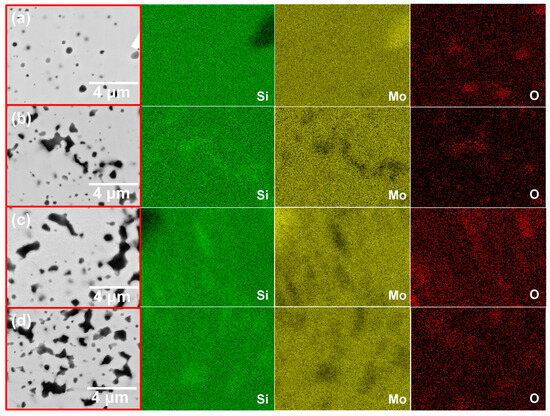
Figure 6.
Enlargement of surface topography and EDS mapping analyses of four samples: (a) M1; (b) M2; (c) M3; (d) M4.
In the Mo-Si-C system samples (Figure 6b–d), besides the MoSi2 matrix and a small amount of MoSiC phase, a considerable number of irregular black phases are observed, primarily caused by the agglomeration of SiC particles. Since the melting point of Si is 1410 °C, the elevated sintering temperature and exothermic reactions during sintering cause Si to reach its molten state, increasing its migration rate [41]. As C particles are much lighter, they transfer simultaneously with Si, leading to the agglomeration of SiC. Moreover, as the C content in the sample increases, the surface morphology of the sintered sample remains largely unchanged, but the proportion of SiC phase increases, with numerous aggregates appearing. This process also mitigates the impact of the brittle SiO2 phase on the mechanical properties of the MoSi2 matrix, as SiO2 at the grain boundaries is reduced by C to form SiC.
3.5. Mechanical Properties
Figure 7 illustrates the Vickers hardness and fracture toughness data for four composite sets with varying C contents. It is evident that the presence of carbon in the samples, which results in the generation of SiC as a second phase, significantly enhances both the hardness and toughness of the MoSi2-SiC composites compared to pure MoSi2 materials. As the proportion of SiC increases, the properties of the composites show further improvement. A comparison between the M1 and M4 samples reveals that the hardness increases from 12.1 GPa to 16.1 GPa, representing a 33% enhancement. This demonstrates that the incorporation of SiC effectively raises the hardness of MoSi2-based composites. Although it is generally expected that an increase in hardness would be associated with a decline in the fracture toughness, the data indicate that the fracture toughness of the samples does not follow this trend. In fact, it appears to increase. Specifically, the fracture toughness of the M4 sample reaches 6.7 MPa·m1/2, which is 24% higher than the pure MoSi2 sample, which exhibits a toughness of 5.4 MPa·m1/2 under similar preparation conditions.
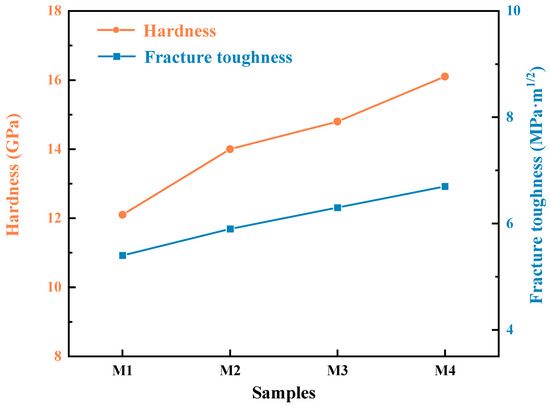
Figure 7.
Hardness (Hv) and indentation fracture toughness (KIC) of as-sintered composites versus SiC content.
The trend of increasing fracture toughness in the composites is further analyzed through a comparison of the crack extension paths observed in the four sets of samples. The cracks and their propagation paths resulting from indentations on the surface of the sintered samples are labeled in Figure 8. The crack pattern in the pure MoSi2 sample is characterized by a relatively flat morphology, with crack bridging occurring during crack extension and no significant deflection observed, as shown in Figure 8a. Crack bridging generates resistance to crack propagation, requiring the crack to surmount a higher energy barrier. In composite materials, cracks may exhibit bending or deflection when encountering particles or phase interfaces, thereby increasing the total crack propagation length [13,43]. In the M2 sample (Figure 8b), SiC exists as a second phase, and both crack bridging and deflection are observed during crack extension. With an increase in the SiC phase content, more phase interfaces are formed, causing cracks to propagate primarily through SiC particles while undergoing deflection. This results in a more tortuous crack path, as shown in Figure 8c,d. Both crack bridging and crack deflection mechanisms play a vital part in increasing the toughness of the material, with their combined effects boosting the crack resistance of the composites and consequently decreasing the risk of brittle fracture.
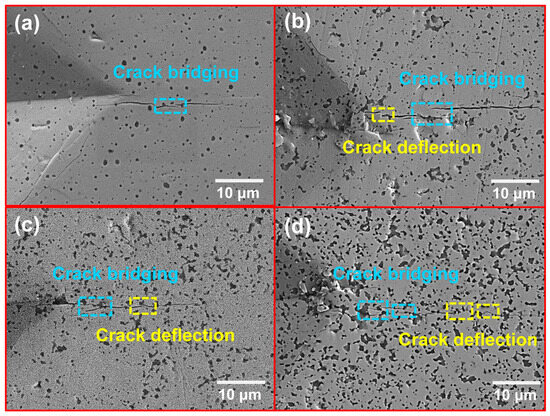
Figure 8.
Crack propagation path of MoSi2 composites with various SiC contents: (a) M1; (b) M2; (c) M3; (d) M4.
Figure 9 illustrates the fracture surface morphology of the sintered sample. Figure 9a presents a typical brittle fracture characterized by a flat, relatively smooth surface with distinct river patterns and cleavage steps. Based on the uniform crack propagation observed Figure 9a, it can be concluded that the fracture mode of the M1 sample is predominantly transgranular, indicating that the sample has relatively poor toughness, which aligns with the known properties of MoSi2 material. In Figure 9b, the introduction of the second-phase SiC in the M2 sample results in plastic deformation, with the fracture surface appearing uneven. Despite this, the fracture mode remains transgranular overall. With increasing SiC contents, as depicted in Figure 9c,d, the fracture surfaces become noticeably irregular and exhibit an uneven morphology. This can be attributed to plastic deformation, stretching, and slip occurring during the fracture process. The fracture surface reveals distinct grain structures, and at this stage, the fracture mode transitions into a mixed intergranular–transgranular fracture, with intergranular fracture being the dominant mechanism. Additionally, as the SiC content increases, a marked reduction in the grain size is observed. This suggests that the SiC second phase plays a crucial role in refining the MoSi2 matrix, thereby improving the mechanical properties of the composite material.
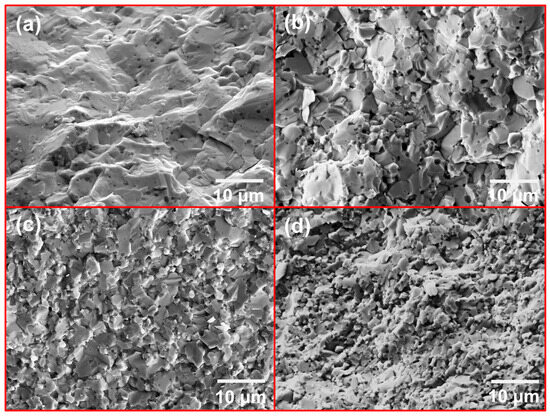
Figure 9.
Fracture morphologies of MoSi2 composites with various SiC contents: (a) M1; (b) M2; (c) M3; (d) M4.
Figure 10 displays the flexural strength of the pure MoSi2 and composites with various SiC contents. The incorporation of SiC considerably enhances the flexural strength of the samples. The flexural strength increases steadily with the SiC content, with the M4 samples achieving a flexural strength of 496 MPa. This improvement is attributed to the synergistic effects of the high densification degree, fracture behavior, microstructure, and finer grain size of the composites.
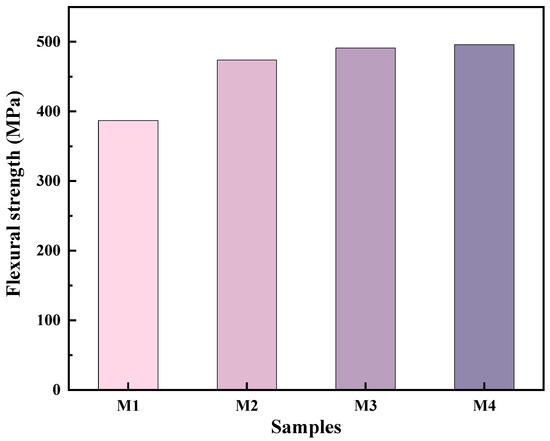
Figure 10.
Flexural strength of as-sintered composites versus SiC content.
3.6. Comparison of Mechanical Properties
The relative density, hardness, fracture toughness, and flexural strength of the sintered samples corresponding to the four different compositions in this study are presented in detail in Table 2. The performance data of the MoSi2-based composites produced by different preparation methods and reinforcing phases in the literature are also quoted and further compared with the M4 samples in this study. Compared with the MoSi2-SiC composites obtained by other preparation methods (e.g., microwave reaction sintering, pressureless sintering, and hot press sintering) listed in the table, the M4 samples have higher densities and superior mechanical properties. The fracture toughness or flexural strength values of the individual cited samples are slightly higher than those of the M4 samples, but the data for other properties are much lower than those of the M4 samples. In addition, the MoSi2-SiC composites in this study also exhibit superior mechanical properties compared to MoSi2-based composites that introduce reinforcing phases other than SiC. It can be concluded that the M4 samples synthesized by the two-step SPS method in this study have better overall properties. Notably, the MoSi2-SiC composites demonstrated an enhanced performance, particularly in terms of both the hardness and toughness, which were simultaneously improved.

Table 2.
Comparison of the mechanical properties of MoSi2-based composite materials prepared in this study and the literature.
3.7. Electrical Properties
The room temperature resistivities of the four groups of sintered samples are detailed in Table 3, where the resistivity of the pure MoSi2 sample is 16.8 µΩ·cm. The resistivity of the samples shows a gradual increase as the SiC content in the samples increases. The resistivity of the composites is influenced by multiple factors, including the additive composition, porosity, grain size, and grain boundaries [50]. SiC, as a semiconductor, exhibits a room temperature resistivity that can be varied over a wide range, typically between 103 and 1019 µΩ·cm for both single-crystal and polycrystalline forms [51]. However, the resistivity of SiC is considerably higher than that of MoSi2 materials. Therefore, the introduction of SiC as a second phase in the MoSi2 matrix leads to an increase in the resistivity of the composite. In addition, as the proportion of the SiC phase increases, more phase interfaces are generated between MoSi2 and SiC, which further increases the resistivity of the composites. In conclusion, the resistivity of the MoSi2-SiC composites is theoretically higher than that of the MoSi2 materials. The room temperature resistivities of the four groups of samples in Table 3 also confirm the effect of the SiC content on the resistivity of the composites. It is observed that the resistivity increases progressively as the molar fraction of SiC increases. Thus, the resistivity of the MoSi2-SiC composites can be effectively tuned by controlling the amount of SiC added.

Table 3.
Electrical resistivity versus SiC content.
4. Conclusions
MoSi2-SiC composites were successfully prepared from carbon-containing Mo powder and Si powder by in situ synthesis, and the influence of various SiC contents on the microstructure and mechanical properties of the composites were analyzed. The sintering process involved a two-step treatment at 1200 °C and 1600 °C to ensure the formation of uniform, dense, and high-performance composites. The incorporation of SiC as a secondary phase into the MoSi2 matrix effectively enhanced the mechanical properties of the composites. Among the samples, the MoSi2-26mol.%SiC composite demonstrated the highest overall performance, exhibiting a relative density of 98.9%, a Vickers hardness of 16.1 GPa, a fracture toughness of 6.7 MPa·m1/2, and a flexural strength of 496 MPa. Additionally, the inclusion of SiC was observed to increase the electrical resistivity of the MoSi2 material.
Author Contributions
Y.-Y.W.: formal analysis, investigation, writing—original draft, writing—review and editing; G.-H.Z.: conceptualization, methodology, resources, supervision, funding acquisition, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors gratefully acknowledge the financial support from the State Key Laboratory of Advanced Metallurgy, University of Science and Technology Beijing, China.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Ramasesha, S.K.; Tantri, P.S.; Bhattacharya, A.K. MoSi2 and MoSi2-Based Materials as Structural Ceramics. Met. Mater. Process. 2000, 12, 181–190. [Google Scholar]
- Tantri, P.S.; Bhattacharya, A.K.; Ramasesha, S.K. Synthesis and properties of MoSi2 based engineering ceramics. J. Chem. Sci. 2001, 113, 633–649. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, S. Insight into the oxidation mechanism of MoSi2: Ab-initio calculations. Ceram. Int. 2018, 44, 19583–19589. [Google Scholar] [CrossRef]
- Cao, L.; Bai, Z.; Huang, J.; OuYang, H.; Li, C.; Wang, B.; Yao, C. Fabrication of gradient C/C-SiC-MoSi2 composites with enhanced ablation performance. Ceram. Int. 2016, 42, 12289–12296. [Google Scholar] [CrossRef]
- Ding, Z.; Brouwer, J.C.; Zhu, J.-N.; Popovich, V.; Hermans, M.J.; Sloof, W.G. Effects of boron addition on the high temperature oxidation of MoSi2 alloys. Scr. Mater. 2023, 234, 115580. [Google Scholar] [CrossRef]
- Yan, J.-h.; Xu, J.-j.; Wang, Y.; Liu, L.-F. Preparation of agglomerated powders for air plasma spraying MoSi2 coating. Ceram. Int. 2015, 41, 10547–10556. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Bai, C. Microstructure and oxidation behavior of Si–MoSi2 functionally graded coating on Mo substrate. Ceram. Int. 2017, 43, 6250–6256. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Liu, L.; Wang, Y. Oxidation and interdiffusion behavior of Niobium substrate coated MoSi2 coating prepared by spark plasma sintering. Appl. Surf. Sci. 2014, 320, 791–797. [Google Scholar] [CrossRef]
- Erfanmanesh, M.; Bakhshi, S.R.; Khajelakzay, M.; Salekbafghi, M. The effect of argon shielding gas at plasma spray process on the structure and properties of MoSi2 coating. Ceram. Int. 2014, 40, 4529–4533. [Google Scholar] [CrossRef]
- Shan, A.; Fang, W.; Hashimoto, H.; Park, Y.-H. Effect of Mg addition on the microstructure and mechanical properties of MoSi2 alloys. Scr. Mater. 2002, 46, 645–648. [Google Scholar] [CrossRef]
- Wen, S.; Sha, J. Improvement of “Pest” Resistance of MoSi2 Intermetallic Compound at 500 °C and 600 °C via Addition of B Fabricated by Spark Plasma Sintering. Oxid. Met. 2019, 92, 243–257. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, J.; Zhuang, S.; Chen, Y.; Gu, S. Effect of WSi2 and Si3N4 contents on the thermal expansion behaviors of (Mo,W) Si2-Si3N4 composites. Ceram. Int. 2017, 43, 2847–2852. [Google Scholar] [CrossRef]
- Nie, X.; Lu, Q. Fracture toughness of ZrO2–SiC/MoSi2 composite ceramics prepared by powder metallurgy. Ceram. Int. 2021, 47, 19700–19708. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, X.; Feng, P.; Li, D.; Yang, T.; Akhtar, F. Synthesis, microstructure and mechanical properties of (Mo,Ti) Si2/Al2O3 composites prepared by thermite-reaction-assisted combustion synthesis. J. Alloys Compd. 2016, 688, 870–877. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, L.; Li, N.; Cheng, D.; Zhang, J.; Yu, S.; Bai, H.; Ma, H. Microstructures and mechanical properties of reactive spark plasma-sintered ZrB2–SiC–MoSi2 composites. Ceram. Int. 2022, 48, 27401–27408. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.; Li, B. Synthesis of MoSi2/WSi2 nanocrystalline powder by mechanical-assistant combustion synthesis method. Int. J. Refract. Met. Hard Mater. 2010, 28, 217–220. [Google Scholar] [CrossRef]
- Li, W.; Fan, J.; Fan, Y.; Xiao, L.; Cheng, H. MoSi2/(Mo,Ti) Si2 dual-phase composite coating for oxidation protection of molybdenum alloy. J. Alloys Compd. 2018, 740, 711–718. [Google Scholar] [CrossRef]
- Ko, I.-Y.; Kang, H.-S.; Doh, J.-M.; Yoon, J.-K.; Shon, I.-J. Properties and densification of nanocrystalline MoSi2–Si3N4 composite from mechanically alloyed powders by pulsed current-activated sintering. J. Alloys Compd. 2010, 502, L10–L13. [Google Scholar] [CrossRef]
- Peng, K.; Han, G.; Li, J.; Wang, L.; Zhang, W.; Wang, W.; Zhang, J. Anti-oxidation properties and phase composition evolution of the MoSi2-HfO2 duplex bond coat in air and steam/oxygen atmosphere at 1450 °C. Surf. Coat. Technol. 2024, 492, 131235. [Google Scholar] [CrossRef]
- Chen, P.; Zhu, L.; Ren, X.; Kang, X.; Wang, X.; Feng, P. Preparation of oxidation protective MoSi2–SiC coating on graphite using recycled waste MoSi2 by one-step spark plasma sintering method. Ceram. Int. 2019, 45, 22040–22046. [Google Scholar] [CrossRef]
- Wang, X.; Gao, X.; Zhang, Z.; Cheng, L.; Ma, H.; Yang, W. Advances in modifications and high-temperature applications of silicon carbide ceramic matrix composites in aerospace: A focused review. J. Eur. Ceram. Soc. 2021, 41, 4671–4688. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Z.; Jin, Z. Electrical resistivity and microstructure of pressureless reactive sintered MoSi2–SiC composite. Mater. Chem. Phys. 2004, 86, 16–20. [Google Scholar] [CrossRef]
- Esmaeily, S.; Kermani, M.; Razavi, M.; Rahimipour, M.R.; Zakeri, M. An investigation on the in situ synthesis–sintering and mechanical properties of MoSi2–xSiC composites prepared by spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2015, 48, 263–271. [Google Scholar] [CrossRef]
- Chen, F.; Xu, J.; Hou, Z. In situ pressureless sintering of SiC/MoSi2 composites. Ceram. Int. 2012, 38, 2767–2772. [Google Scholar] [CrossRef]
- Peng, K.; Yi, M.; Ran, L.; Ge, Y. Reactive hot pressing of SiC/MoSi2 nanocomposites. J. Am. Ceram. Soc. 2007, 90, 3708–3711. [Google Scholar] [CrossRef]
- He, Z.-b.; Li, H.-j.; Shi, X.-h.; Fu, Q.-g.; Heng, W. Formation mechanism and oxidation behavior of MoSi2–SiC protective coating prepared by chemical vapor infiltration/reaction. Trans. Nonferrous Met. Soc. China 2013, 23, 2100–2106. [Google Scholar] [CrossRef]
- Zakeri, M.; Yazdani-Rad, R.; Enayati, M.; Rahimipour, M. Synthesis of nanocrystalline MoSi2 by mechanical alloying. J. Alloys Compd. 2005, 403, 258–261. [Google Scholar] [CrossRef]
- Guo, S.-Q.; Nishimura, T.; Mizuguchi, T.; Kagawa, Y. Mechanical properties of hot-pressed ZrB2–MoSi2–SiC composites. J. Eur. Ceram. Soc. 2008, 28, 1891–1898. [Google Scholar] [CrossRef]
- Wang, C.-C.; Li, K.-Z.; He, D.-Y.; Shi, X.-H. Oxidation behavior of plasma-sprayed MoSi2-Yb2O3 composite coating at 1700 °C. Ceram. Int. 2020, 46, 9538–9547. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, S.; Ye, F.; Ren, X.; Feng, P. Recycling of MoSi2-based industrial solid wastes for the fabrication and high-temperature oxidation behavior of MoSi2–ZrSi2–SiC composite coating. Compos. Part B Eng. 2024, 274, 111281. [Google Scholar] [CrossRef]
- Potanin, A.Y.; Vorotilo, S.; Pogozhev, Y.S.; Rupasov, S.; Lobova, T.; Levashov, E. Influence of mechanical activation of reactive mixtures on the microstructure and properties of SHS-ceramics MoSi2–HfB2–MoB. Ceram. Int. 2019, 45, 20354–20361. [Google Scholar] [CrossRef]
- Cabouro, G.; Chevalier, S.; Gaffet, E.; Grin, Y.; Bernard, F. Reactive sintering of molybdenum disilicide by spark plasma sintering from mechanically activated powder mixtures: Processing parameters and properties. J. Alloys Compd. 2008, 465, 344–355. [Google Scholar] [CrossRef]
- Kermani, M.; Razavi, M.; Rahimipour, M.R.; Zakeri, M. The effect of temperature on the in situ synthesis–sintering and mechanical properties of MoSi2 prepared by spark plasma sintering. J. Alloys Compd. 2014, 585, 229–233. [Google Scholar] [CrossRef]
- Sun, G.-D.; Zhang, G.-H.; Ji, X.-P.; Liu, J.-K.; Zhang, H.; Chou, K.-C. Size-controlled synthesis of nano Mo powders via reduction of commercial MoO3 with carbon black and hydrogen. Int. J. Refract. Met. Hard Mater. 2019, 80, 11–22. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, G.; Li, W.; Zhuang, H.; Zhang, B.; Chen, L. In situ synthesis of SiCW/MoSi2 composite through SPS process. J. Alloys Compd. 2008, 462, 170–174. [Google Scholar] [CrossRef]
- Singh, S.; Bhaskar, R.; Narayanan, K.B.; Kumar, A.; Debnath, K. Development of silicon carbide (SiC)-based composites as microwave-absorbing materials (MAMs): A review. J. Eur. Ceram. Soc. 2024, 44, 7411–7431. [Google Scholar] [CrossRef]
- Anstis, G.; Chantikul, P.; Lawn, B.R.; Marshall, D. A critical evaluation of indentation techniques for measuring fracture toughness: I, direct crack measurements. J. Am. Ceram. Soc. 1981, 64, 533–538. [Google Scholar] [CrossRef]
- Kermani, M.; Razavi, M.; Rahimipour, M.R.; Zakeri, M. The effect of mechanical alloying on microstructure and mechanical properties of MoSi2 prepared by spark plasma sintering. J. Alloys Compd. 2014, 593, 242–249. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Luo, J.; Wang, S.; Liang, B.; Chen, W. Microstructure, properties and toughening mechanisms of MoSi2@ ZrO2 core shell composites prepared by spark plasma sintering. Mater. Charact. 2023, 195, 112510. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, P.; Yan, Y. Microstructures and densification of MoSi2–SiC composite by field-activated and pressure-assisted combustion synthesis. J. Alloys Compd. 2009, 468, 136–142. [Google Scholar] [CrossRef]
- Han, X.-x.; Wang, Y.-l.; Xiong, X.; Heng, L.; Chen, Z.-k.; Wei, S. Microstructure, sintering behavior and mechanical properties of SiC/MoSi2 composites by spark plasma sintering. Trans. Nonferrous Met. Soc. China 2018, 28, 957–965. [Google Scholar] [CrossRef]
- Guan, S.; Liang, H.; Liu, Y.; Lin, W.; He, D.; Peng, F. Production of silicon carbide reinforced molybdenum disilicide composites using high-pressure sintering. Ceram. Int. 2020, 46, 23643–23650. [Google Scholar] [CrossRef]
- Nazari, M.; Shakeri, E.; Mohammadi, M. Fabrication and optimization process of mechanical properties of MoSi2 composites reinforced by carbon nanotubes (CNT) using Taguchi method. Ceram. Int. 2024, 50, 25568–25577. [Google Scholar] [CrossRef]
- Xu, J.; Chen, F.; Tan, F. In-situ preparation of SiC–MoSi2 composite by microwave reaction sintering. Ceram. Int. 2012, 38, 6895–6898. [Google Scholar] [CrossRef]
- Oh, D.Y.; Kim, H.C.; Yoon, J.K.; Shon, I.J. One step synthesis of dense MoSi2–SiC composite by high-frequency induction heated combustion and its mechanical properties. J. Alloys Compd. 2005, 395, 174–180. [Google Scholar] [CrossRef]
- Mitra, R.; Mahajan, Y.; Prasad, N.E.; Chiou, W. Processing—Microstructure—Property relationships in reaction hot-pressed MoSi2 and MoSi2/SiCp composites. Mater. Sci. Eng. A 1997, 225, 105–117. [Google Scholar] [CrossRef]
- Wang, J.; Feng, P.; Niu, J.; Guo, R.; Liu, Z.; Akhtar, F. Synthesis, microstructure and properties of MoSi2–5 vol% Al2O3 composites. Ceram. Int. 2014, 40, 16381–16387. [Google Scholar] [CrossRef]
- Kasraee, K.; Tayebifard, S.A. Mechanical and Oxidation Behavior of MoSi2-40wt.% Ti5Si3 Composites Produced by MASHS and SPS. Ceram. Int. 2024, 51, 1888–1899. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, X.; Feng, P.; Liu, Z.; Niu, J.; Akhtar, F. Synthesis and properties of MoSi2–MoB–SiC ceramics. J. Am. Ceram. Soc. 2016, 99, 1147–1150. [Google Scholar] [CrossRef]
- Kim, Y.-W.; Kim, Y.-H.; Kim, K.J. Electrical properties of liquid-phase sintered silicon carbide ceramics: A review. Crit. Rev. Solid State Mater. Sci. 2020, 45, 66–84. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.Y.; Cho, M.K.; Ahn, J.-P.; Kim, Y.-W. Electrical resistivity at the micron scale in a polycrystalline SiC ceramic. Ceram. Int. 2021, 47, 27100–27106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).