Study on the Kinetics of Zinc Leaching Residue Smelting Reduction
Abstract
1. Introduction
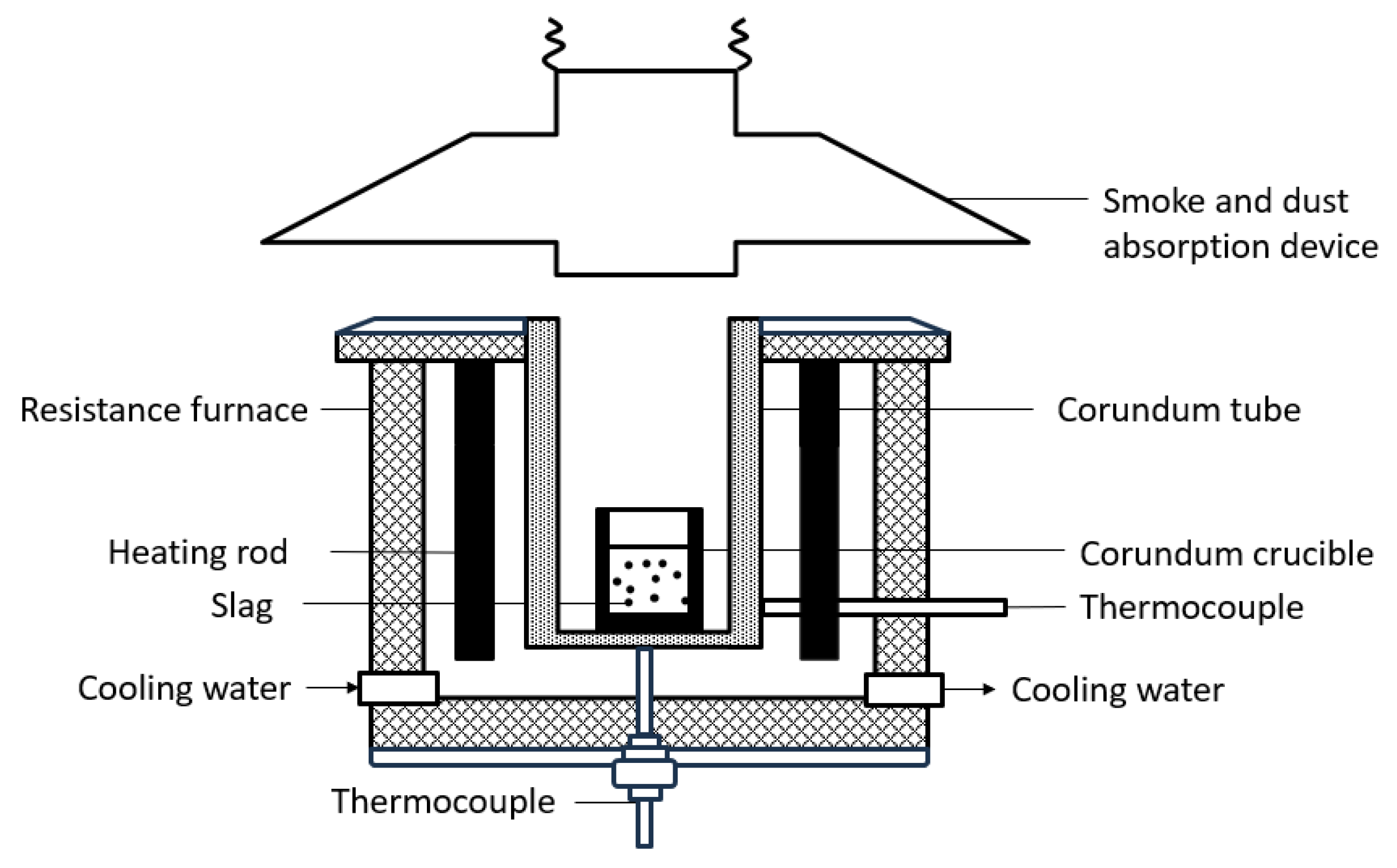
2. Materials and Methods
3. Results and Discussion
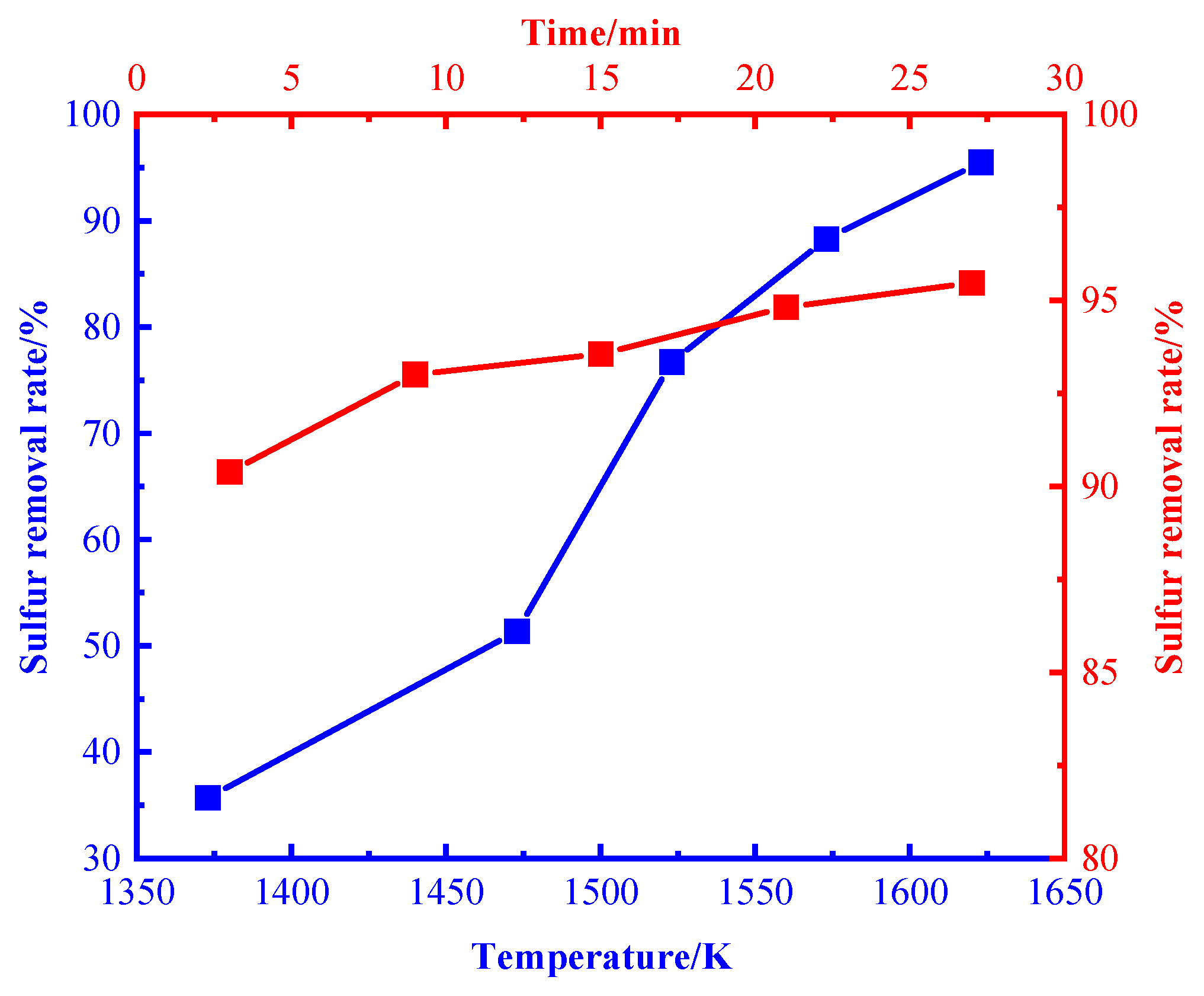
3.1. Desulfurization Process
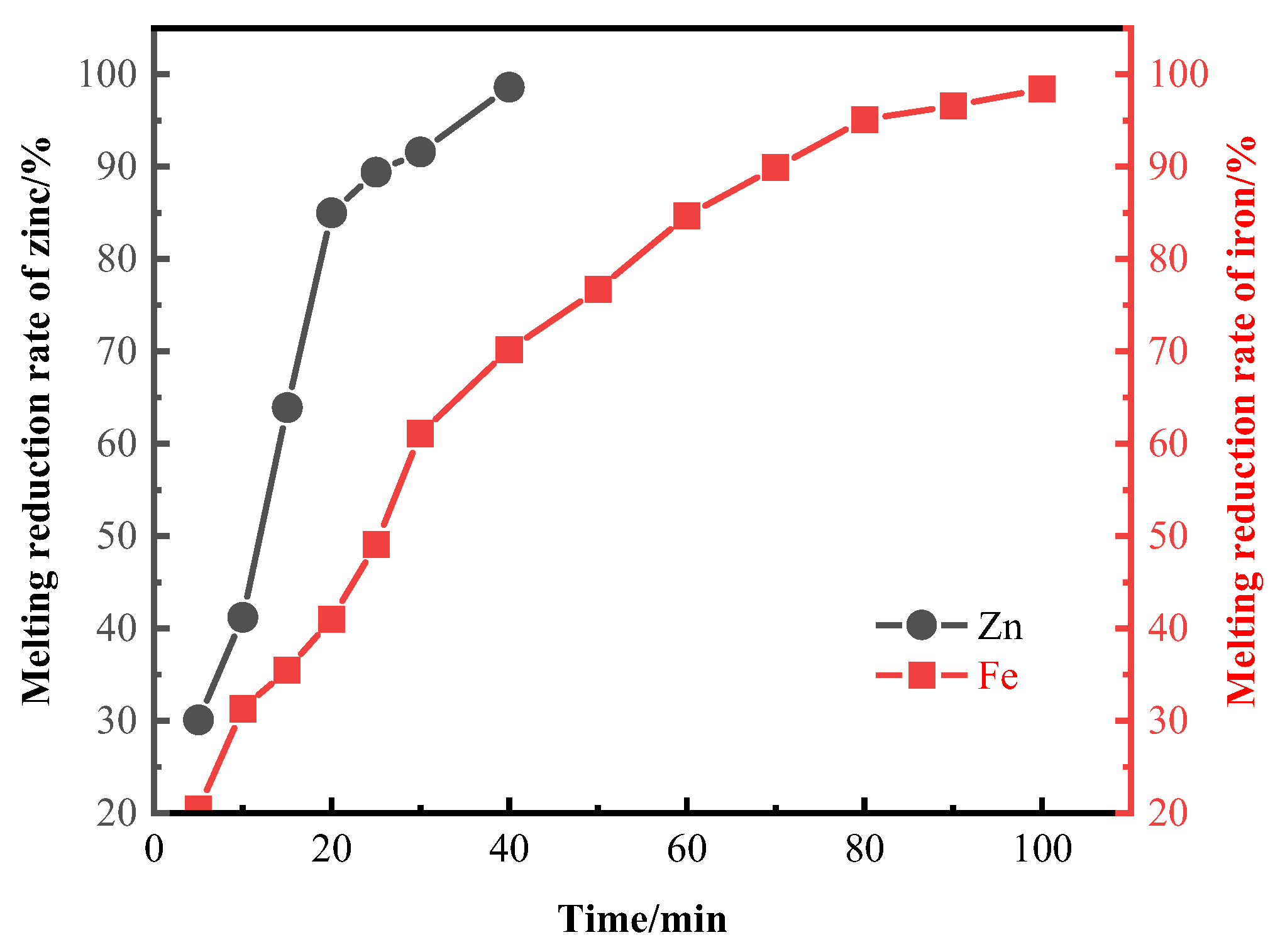
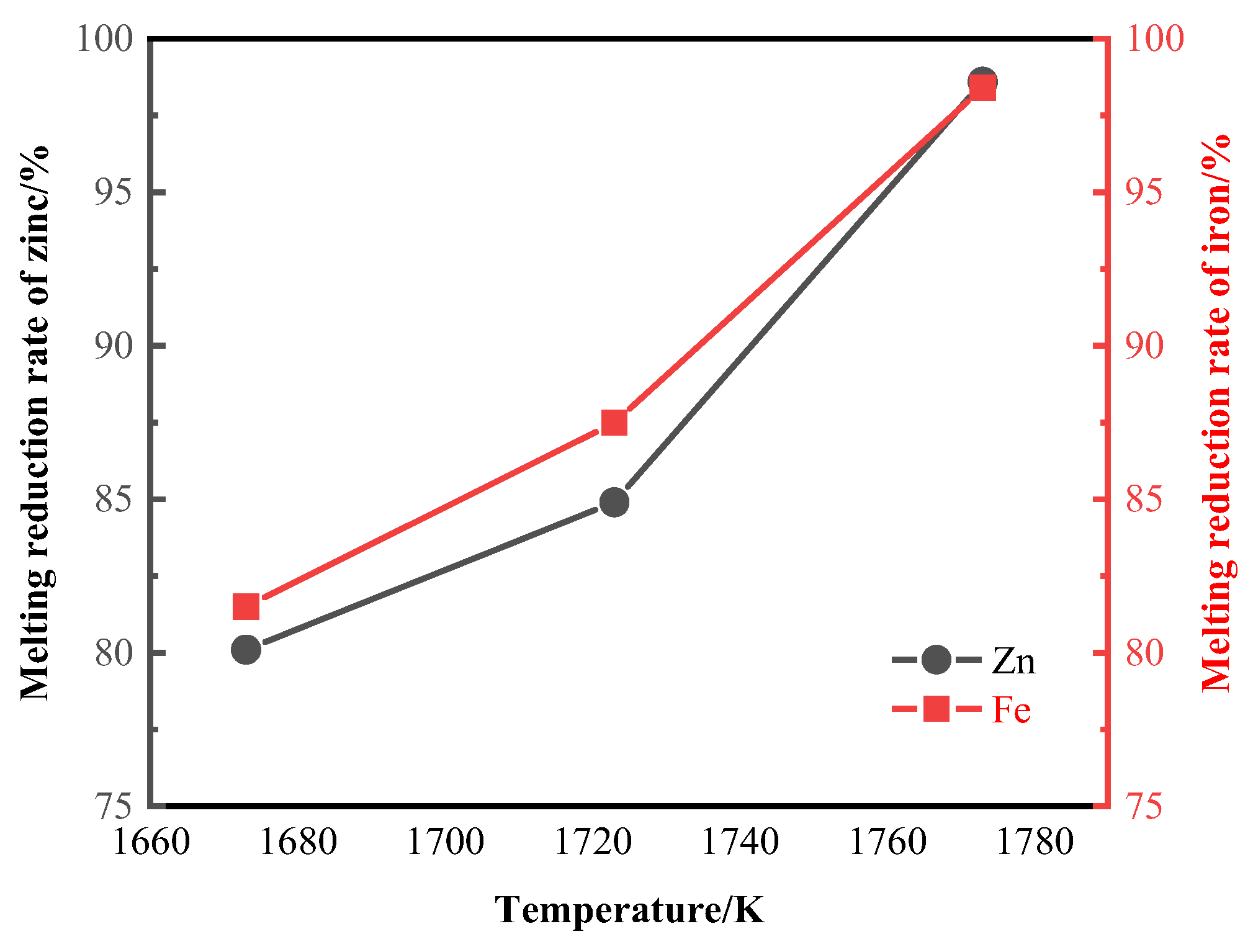
3.2. Smelting Reduction Process
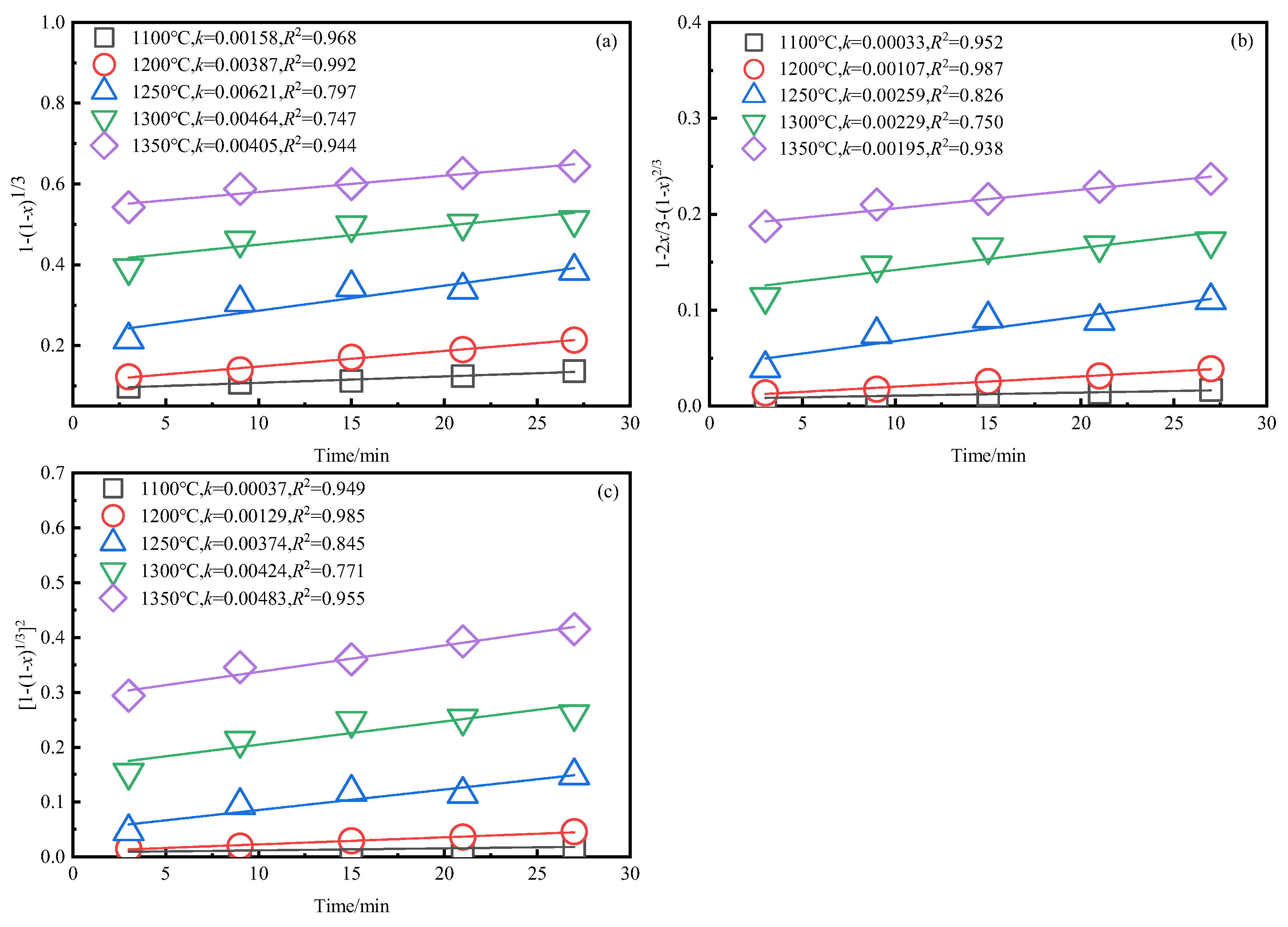
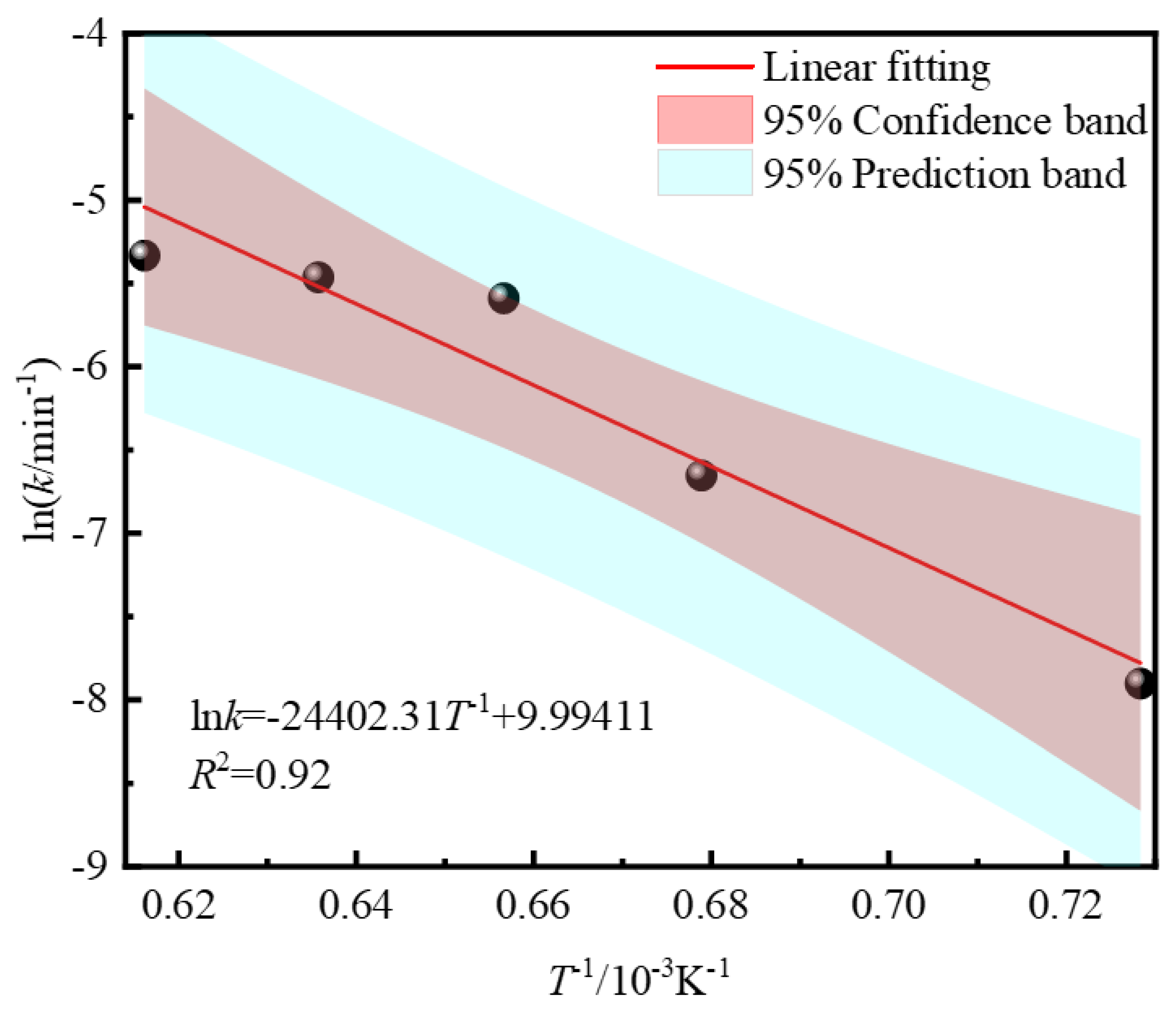
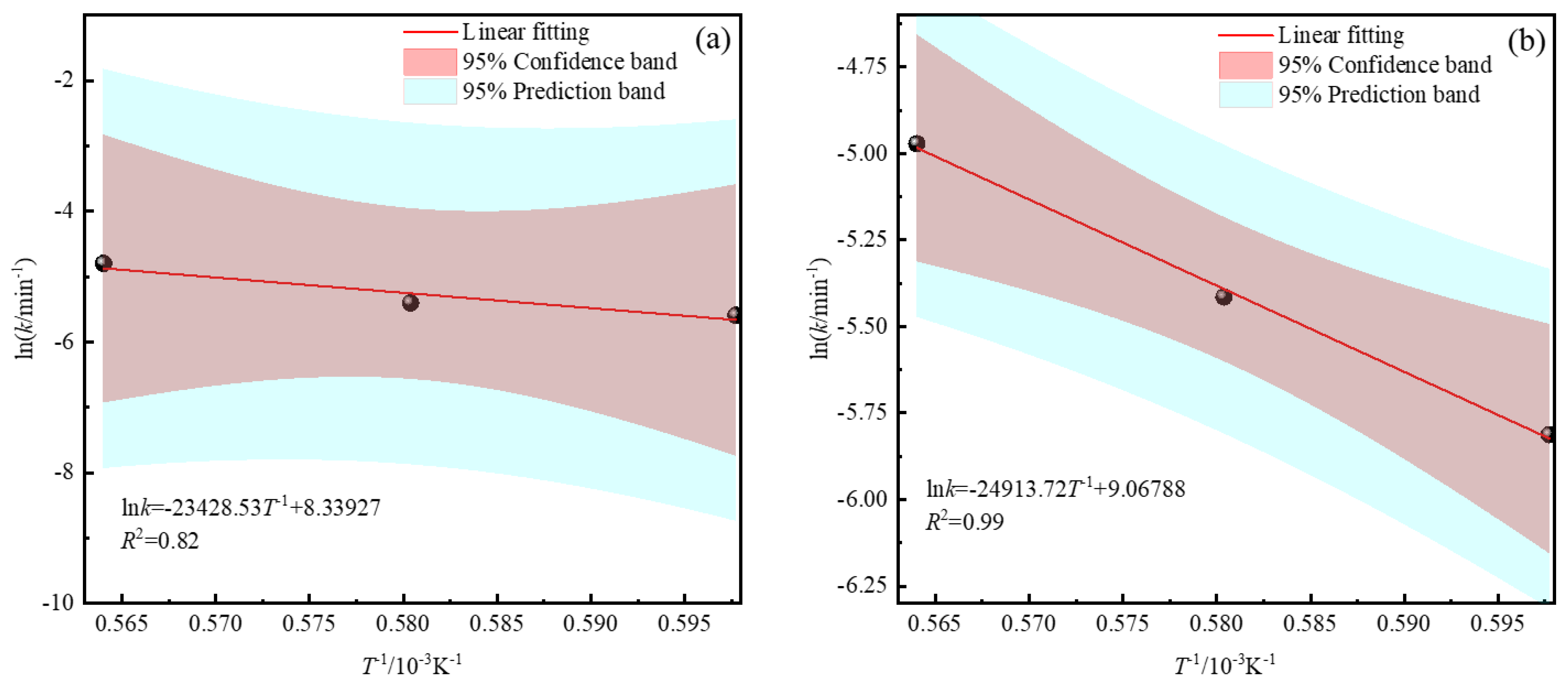
3.3. Kinetics Analysis of Zinc Leaching Residue Desulfurization Process
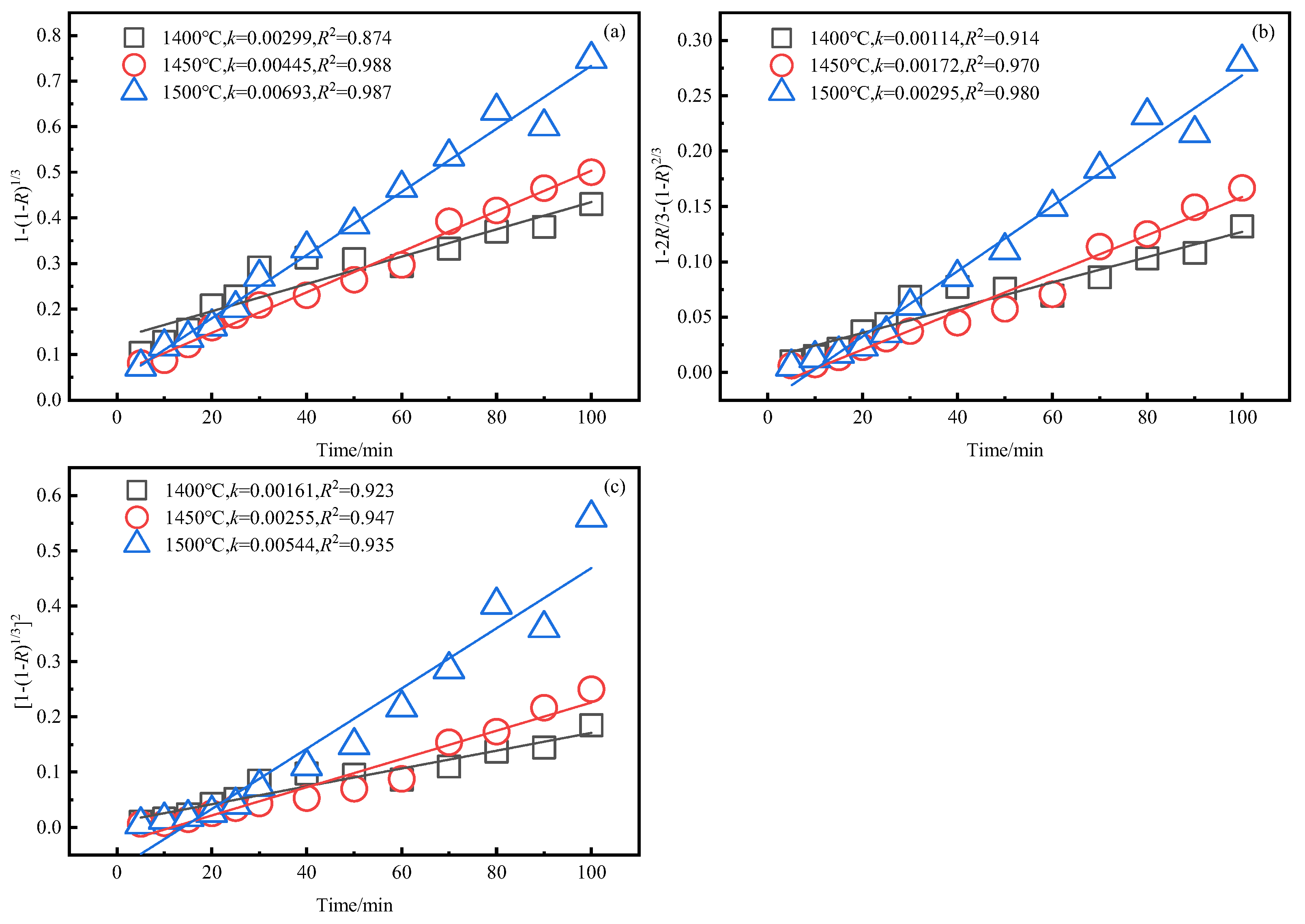
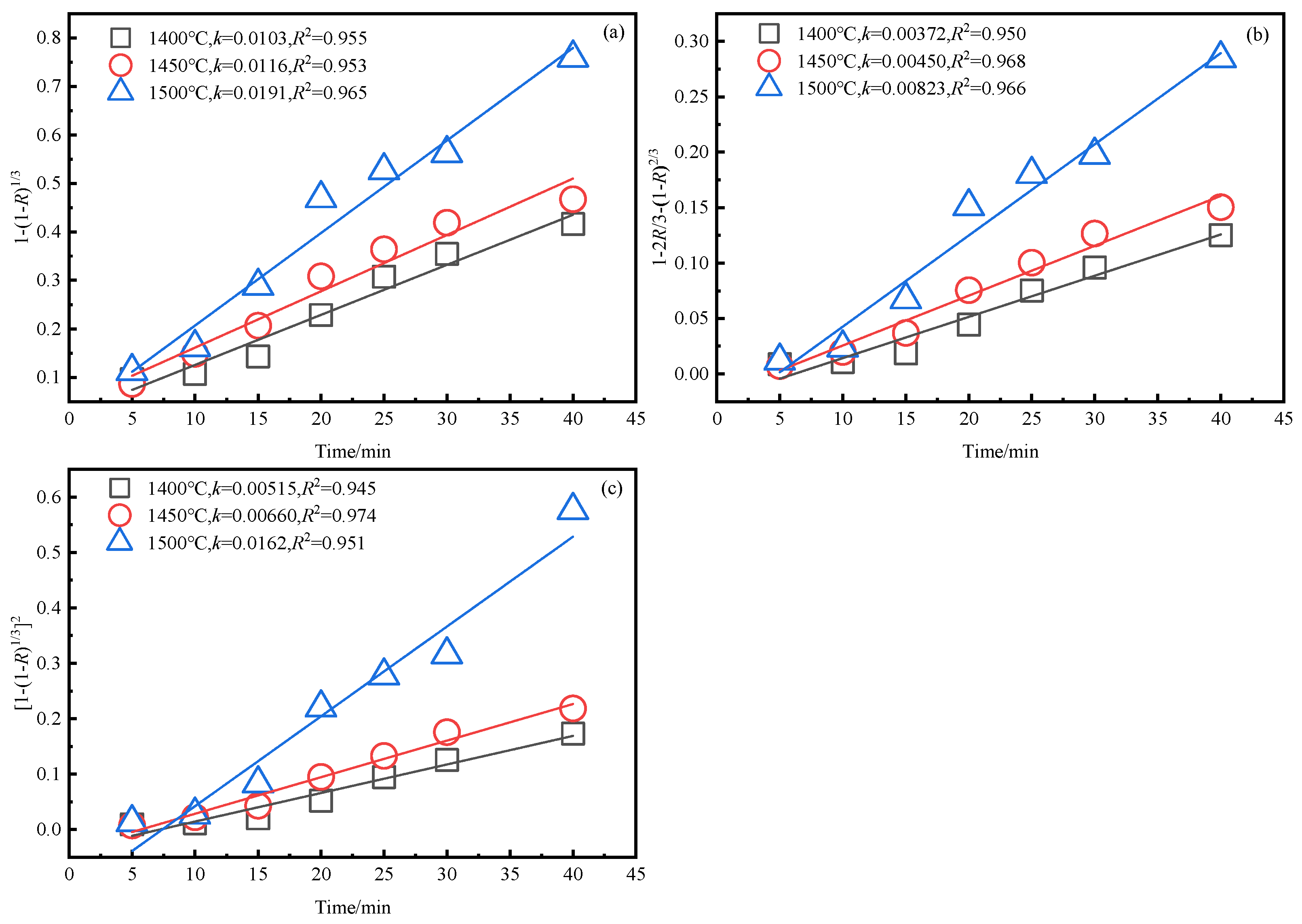
3.4. Kinetics Analysis of Smelting Reduction Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ismael, M.R.C.; Carvalho, J.M.R. Iron recovery from sulphate leach liquors in zinc hydrometallurgy. Miner. Eng. 2003, 16, 31–39. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Gao, W.-C.; Wen, J.-K.; Gan, Y.-G.; Wu, B.; Shang, H. Research progress on valuable metal recovery and comprehensive utilization from zinc leaching residue. Chin. J. Eng. 2020, 42, 1400–1410. [Google Scholar]
- Feng, S. Design of Side Blowing Melting Furnace for Zinc Leaching Residue. Nonferrous Metall. Equip. 2020, 30–32. [Google Scholar]
- Graydon, J.W.; Kirk, D.W. The mechanism of ferrite formation from iron sulfides during zinc roasting. Metall. Trans. B 1988, 19, 777–785. [Google Scholar] [CrossRef]
- Balarini, J.C.; Polli, L.D.O.; Miranda, T.L.S.; Castro, R.M.Z.D.; Salum, A. Importance of roasted sulphide concentrates characterization in the hydrometallurgical extraction of zinc. Miner. Eng. 2008, 21, 100–110. [Google Scholar] [CrossRef]
- Xie, Z.; Jiang, T.; Chen, F.; Guo, Y.; Wang, S.; Yang, L. Phase transformation and zinc extraction from zinc ferrite by calcium roasting and ammonia leaching process. Crystals 2022, 12, 641. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, A.; Jiang, H.; Deng, Y. Magnetic seed-assisted iron recovery from the reductive leaching solution in hydrometallurgical process. Trans. Indian Inst. Met. 2019, 72, 2591–2597. [Google Scholar] [CrossRef]
- Jiang, G.; Peng, B.; Liang, Y.; Chai, L.; Wang, Q.; Li, Q.; Hu, M. Recovery of valuable metals from zinc leaching residue by sulfate roasting and water leaching. Trans. Nonferrous Met. Soc. China 2017, 27, 1180–1187. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Ke, Y.; Luo, Y.; Min, X.; Peng, C.; Li, Y. A novel leaching process of zinc ferrite and its application in the treatment of zinc leaching residue. J. Phys. Conf. Ser. 2024, 2738, 012027. [Google Scholar] [CrossRef]
- Yang, L.J.; Chen, N.C.; Zhong, X.P.; Gao, J.; Lang, Y.X.; Wang, Z.F.; Liu, C.M.; Wu, Z.Y. Factors on leaching zinc and copper from zinc leach residue. Key Eng. Mater. 2014, 633, 169–172. [Google Scholar] [CrossRef]
- Xie, H.; Xiao, X.; Guo, Z.; Li, S. One-stage ultrasonic-assisted calcium chloride leaching of lead from zinc leaching residue. Chem. Eng. Process.—Process Intensif. 2022, 176, 108941. [Google Scholar] [CrossRef]
- Chi, K.H.; Chang, M.B.; Chang, S.H. Measurement of atmospheric PCDD/F and PCB distributions in the vicinity area of Waelz plant during different operating stages. Sci. Total Environ. 2008, 391, 114–123. [Google Scholar] [CrossRef]
- Hung, P.C.; Chi, K.H.; Chen, M.L.; Chang, M.B. Characteristics of dioxin emissions from a Waelz plant with acid and basic kiln mode. J. Hazard. Mater. 2012, 201–202, 229–235. [Google Scholar] [CrossRef]
- Bese, A.V.; Borulu, N.; Copur, M.; Colak, S.; Ata, N.O. Optimization of dissolution of metals from Waelz sintering waste (WSW) by hydrochloric acid solutions. Chem. Eng. J. 2010, 162, 718–722. [Google Scholar] [CrossRef]
- Liang, X.; Ge, Z.; Bo, W. Application of oxygen-enriched side-blowing technology in treating zinc leaching residue in China. J. Phys. Conf. Ser. 2024, 2738, 012023. [Google Scholar] [CrossRef]
- Zhou, A.; Zhang, L.; Zhou, Y.; Li, Y.; Wu, X.; Xia, L.; Liu, Z. Co-smelting process of Pb concentrate and Zn leaching residues with oxygen-rich side blowing furnaces: Industrial application and material balance. JOM 2023, 75, 5833–5846. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Zhan, J.; Li, G.; Zhao, Z.; Hwang, J.Y. A novel technology for the recovery of zinc from the zinc leaching residue by the bottom-blown reduction. Miner. Process. Extr. Metall. Rev. 2020, 42, 380–387. [Google Scholar] [CrossRef]
- Du, Y.; Tong, X.; Xie, X.; Zhang, W.; Yang, H.; Song, Q. Recovery of zinc and silver from zinc acid-leaching residues with reduction of their environmental impact using a novel water leaching flotation process. Minerals 2021, 11, 586. [Google Scholar] [CrossRef]
- Liu, J.; Wen, M.S.; Wu, D.D. Recovery of silver from zinc leach residue by flotation. Adv. Mater. Res. 2012, 1792, 1041–1046. [Google Scholar] [CrossRef]
- Zeng, W.; Hu, X.; Yan, Y.; Chen, B.; Chen, Y.; Tang, C.; Yang, J. Study on the cavitation and dissociation of sulfur from zinc leaching residue. JOM 2024, 76, 1394–1407. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, J.; Yin, Z.; Liu, C.; Chen, Q.; Zhang, P.; Liao, D.; Wang, X. Roasting pretreatment of zinc precipitation slag and zinc leaching residue in wet zinc smelting. Chin. J. Nonferrous Met. 2016, 26, 213–216. [Google Scholar]
- Liang, L.-K.; Che, Y.-C. Metallurgical Thermodynamics and Dynamics; Northeast Institute of Technology Press: Shenyang, China, 1985; pp. 158–182. [Google Scholar]
- Hou, X.; Chou, K.C.; Zhao, B. Reduction kinetics of lead-rich slag with carbon in the temperature range of 1073 to 1473 K. J. Min. Metall. Sect. B Metall. 2013, 49, 201–206. [Google Scholar] [CrossRef]
- Yasushi, S.; Yinhe, S. Study on the kinetics of reduction of molten iron oxide with solid carbon. Trans. Iron Steel Inst. Jpn. 1978, 64, 1797–1799. [Google Scholar]
- Zhou, L.-W. Experimental Study on the Kinetics of Reduction of Feo Containing Slag; Northeastern University: Shenyang, China, 2012. [Google Scholar]

| Factory Address | Zn(%) | Pb(%) | Ag/(G·t−1) | Fe(%) | Cu(%) | Mn(%) |
|---|---|---|---|---|---|---|
| Hunan | 35.99 | 1.73 | - | 15.39 | 0.52 | 0.74 |
| Guangdong | 19.88 | 3.77 | 550 | 24.72 | - | - |
| Yunnan | 24.75 | 0.099 | 97.2 | 25.68 | 1.12 | 0.13 |
| Western Hunan | 3.941 | 6.401 | - | 7.757 | - | 0.46 |
| Shandong | 7.85 | 5.20 | 350 | 9.51 | - | - |
| Inner Mongolia | 3.34 | 6.81 | 600 | 17.04 | 0.18 | - |
| Component | Si | Ca | S | TFe | Zn | Pb |
|---|---|---|---|---|---|---|
| Content (wt.%) | 7.14 | 5.40 | 8.45 | 19.70 | 14.00 | 2.49 |
| Component | Fixed Carbon | Volatile Matter | Ash Compositions | Moisture |
|---|---|---|---|---|
| Content (%) | 83.57 | 5.39 | 9.24 | 1.80 |
| Factor | Parameter | ||||
|---|---|---|---|---|---|
| Temperature/°C | 1100 | 1200 | 1250 | 1300 | 1350 |
| Desulfurization Time/min | 3 | 9 | 15 | 21 | 27 |
| Factor | Temperature/K | Reduction Time/min | W(CaO)/W(SiO2) | Charcoal Ratio |
|---|---|---|---|---|
| Parameter | 1673, 1723, 1773 | 0~100 | 0.9 | 20:3 |
| Component | CaO | PbO | Al2O3 | SiO2 | Fe2O3 | ZnO | Other Components |
|---|---|---|---|---|---|---|---|
| Content (wt.%) | 8.83 | 3.56 | 2.71 | 14.4 | 37.8 | 22.9 | 9.8 |
| Activation Energy Range (kJ.mol−1) | |||
|---|---|---|---|
| Control steps | Reaction is controlled by diffusion | Reaction is jointly controlled | Reaction is controlled by interfacial chemical reactions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhou, M.; Wang, Y.; Du, X.; Zhao, Q. Study on the Kinetics of Zinc Leaching Residue Smelting Reduction. Metals 2025, 15, 1351. https://doi.org/10.3390/met15121351
Wang Z, Zhou M, Wang Y, Du X, Zhao Q. Study on the Kinetics of Zinc Leaching Residue Smelting Reduction. Metals. 2025; 15(12):1351. https://doi.org/10.3390/met15121351
Chicago/Turabian StyleWang, Zihao, Mei Zhou, Yingjiang Wang, Xinwei Du, and Qiuyue Zhao. 2025. "Study on the Kinetics of Zinc Leaching Residue Smelting Reduction" Metals 15, no. 12: 1351. https://doi.org/10.3390/met15121351
APA StyleWang, Z., Zhou, M., Wang, Y., Du, X., & Zhao, Q. (2025). Study on the Kinetics of Zinc Leaching Residue Smelting Reduction. Metals, 15(12), 1351. https://doi.org/10.3390/met15121351






