The Impact of Ce on the Microstructure and Properties of Weld Metal in Corrosion-Resistant Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Welding Wire Manufacturing
2.2. Welding Process
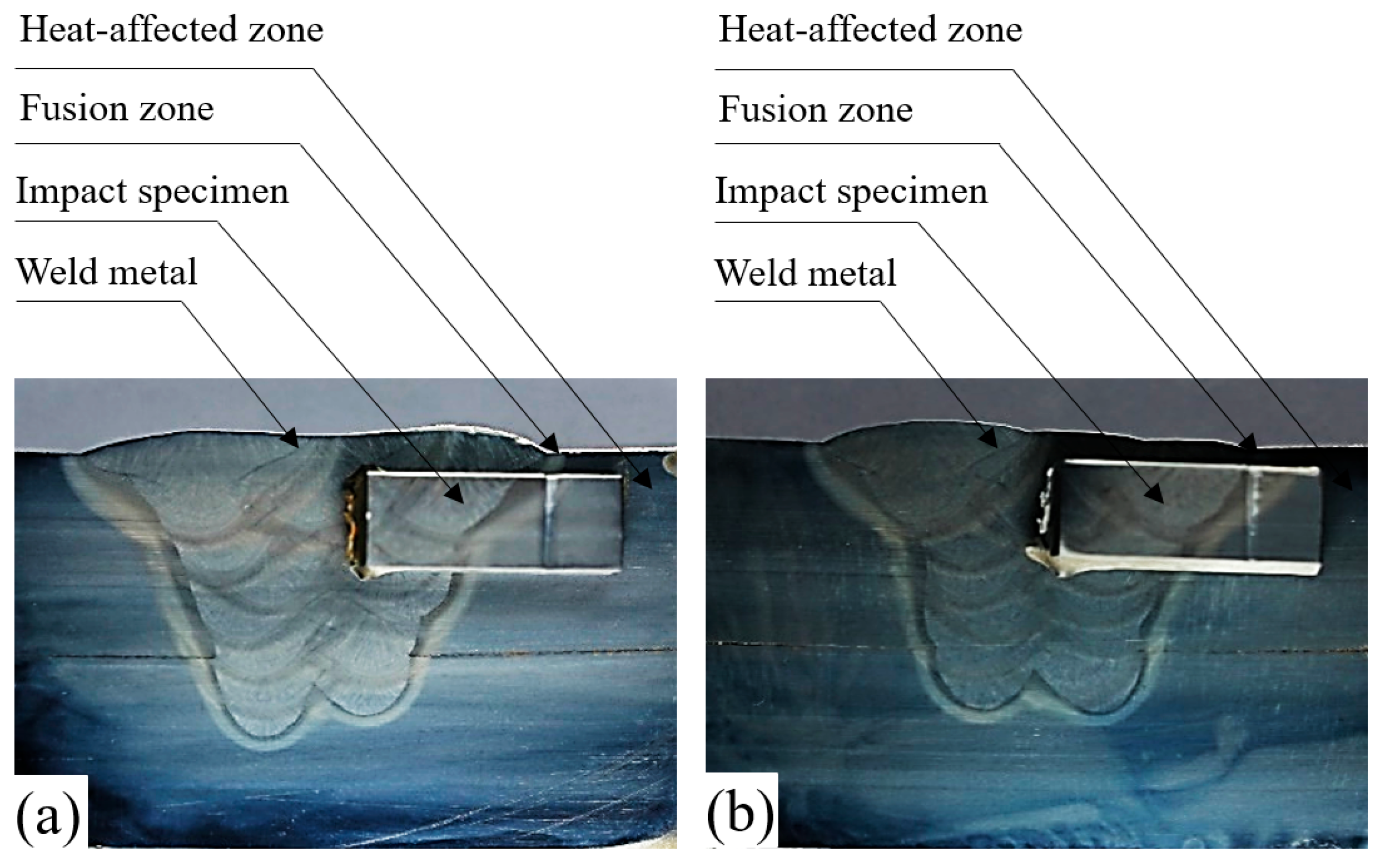
2.3. Experimental Procedure
3. Results
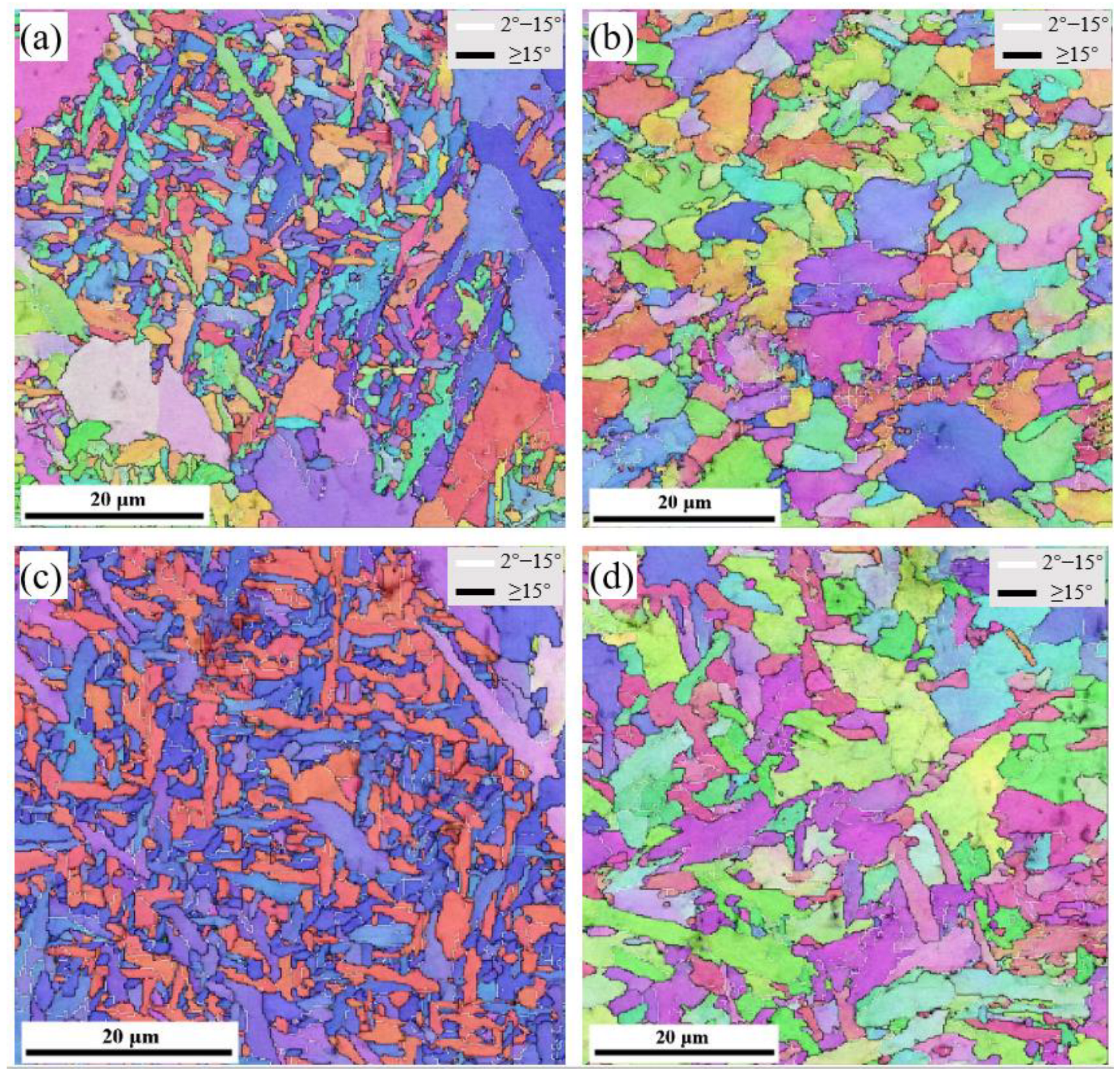
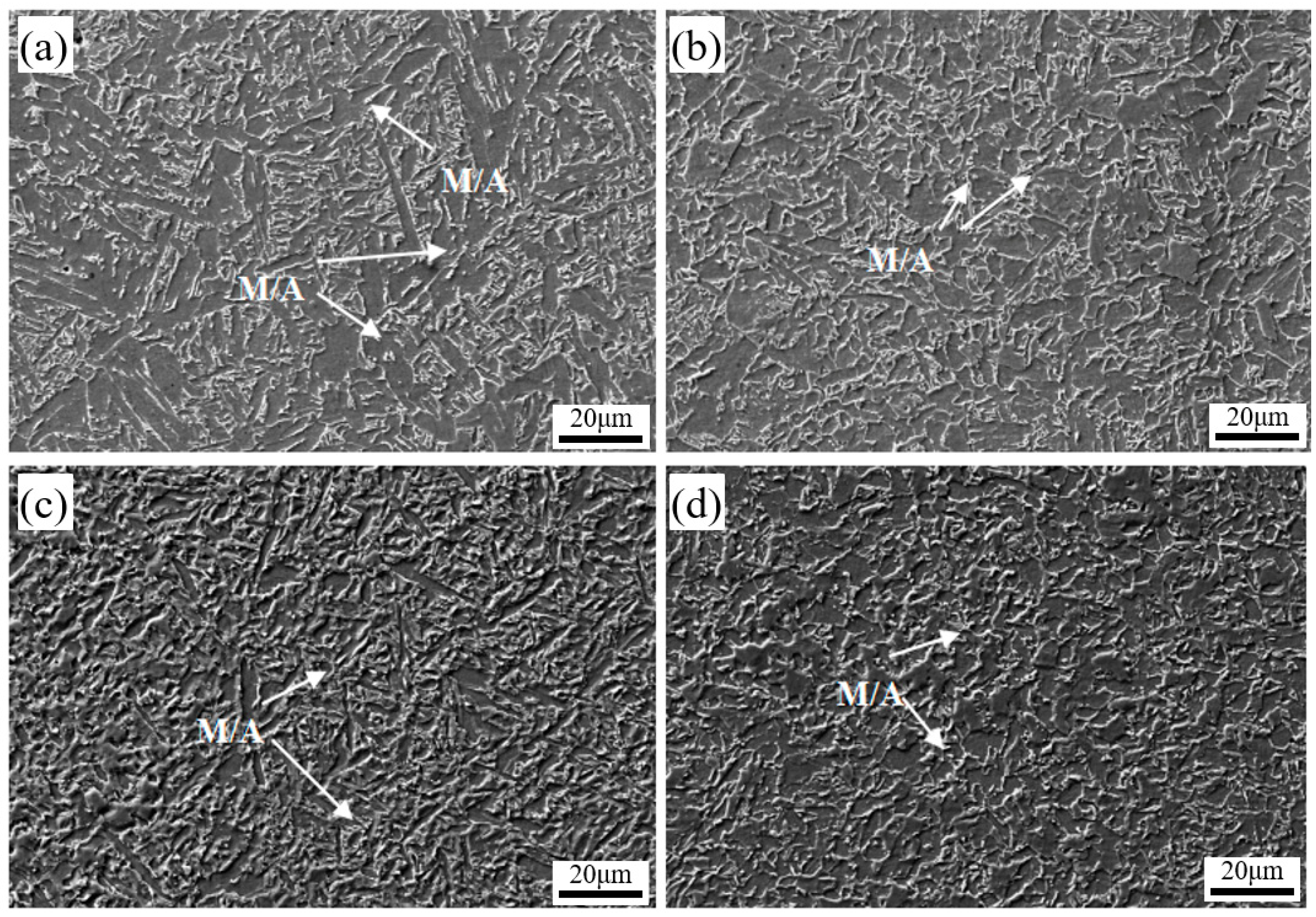
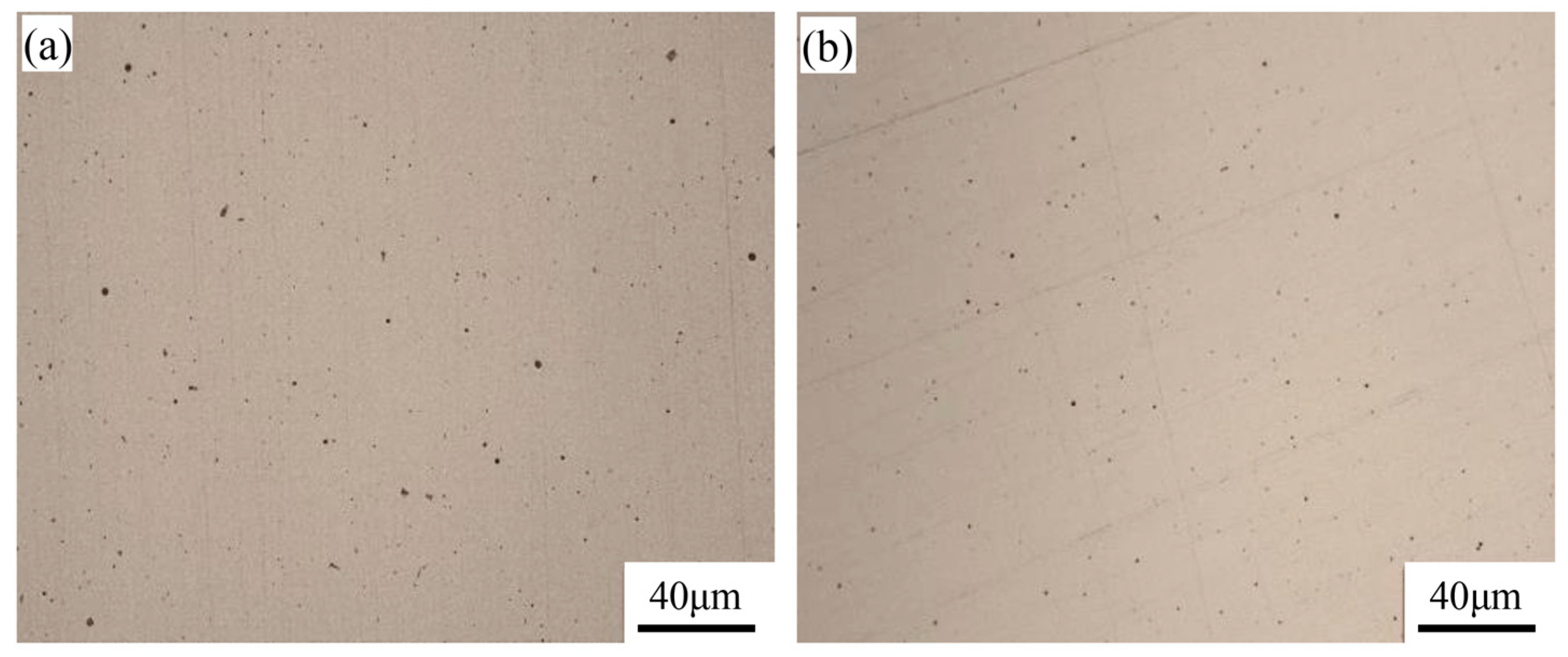
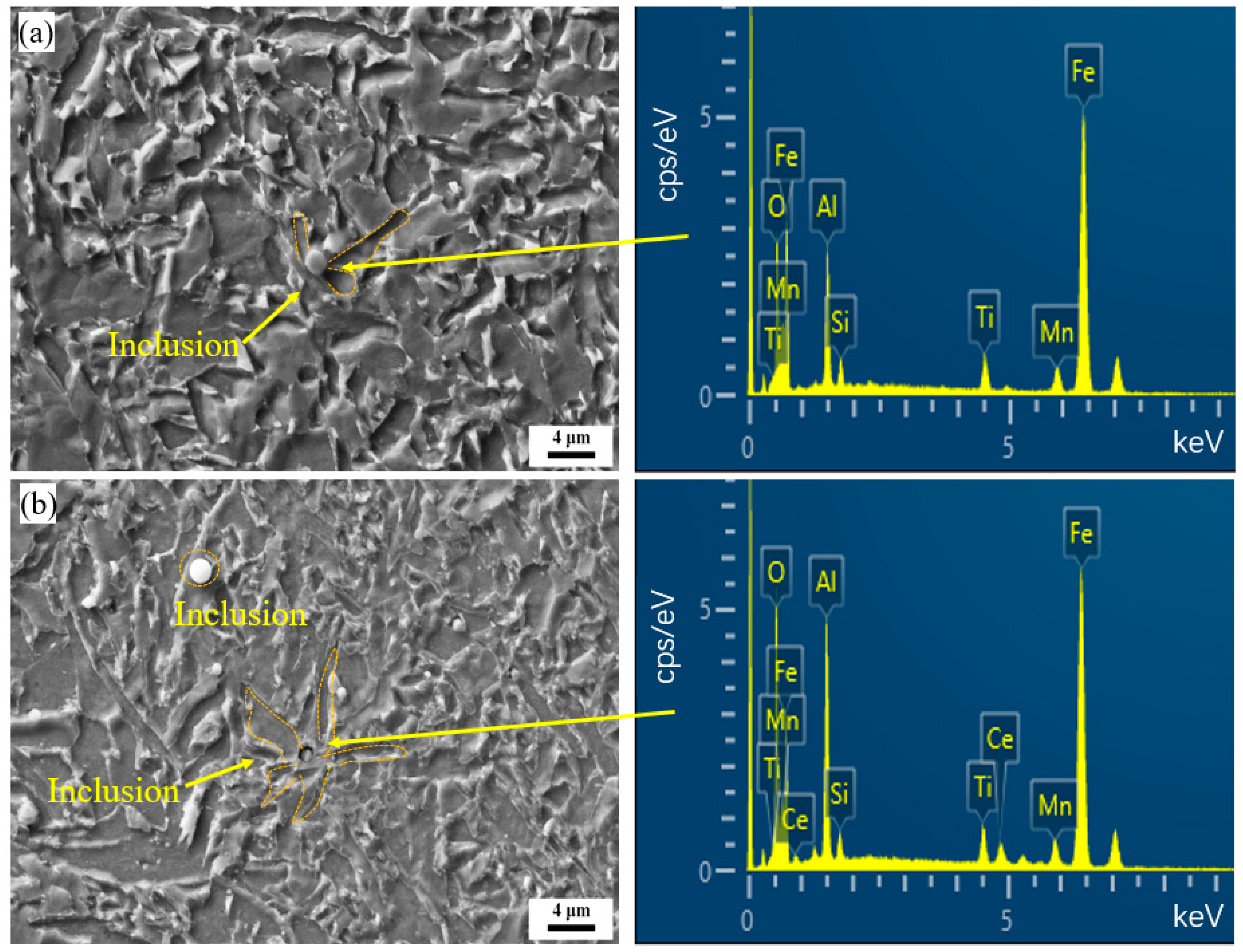
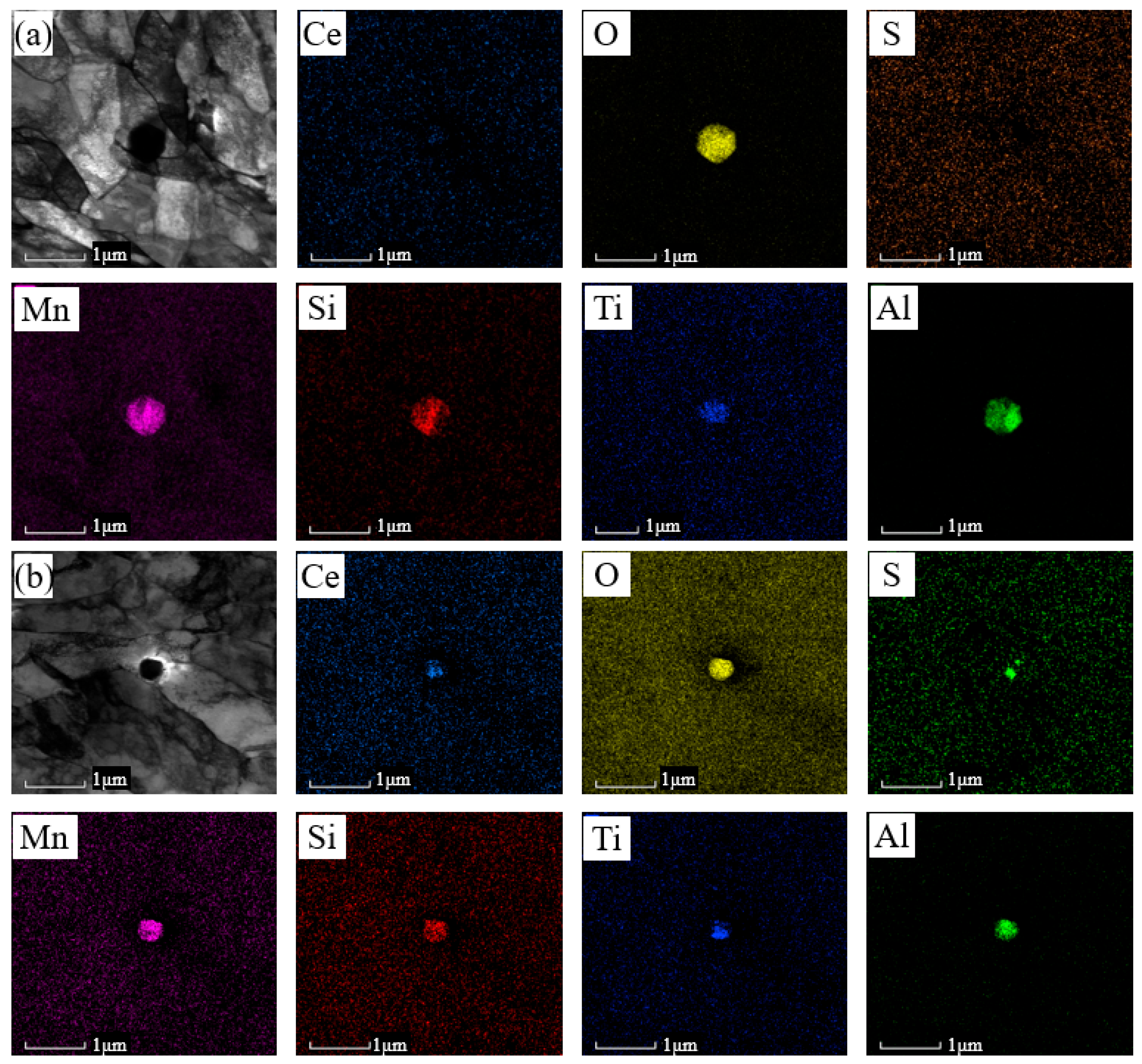
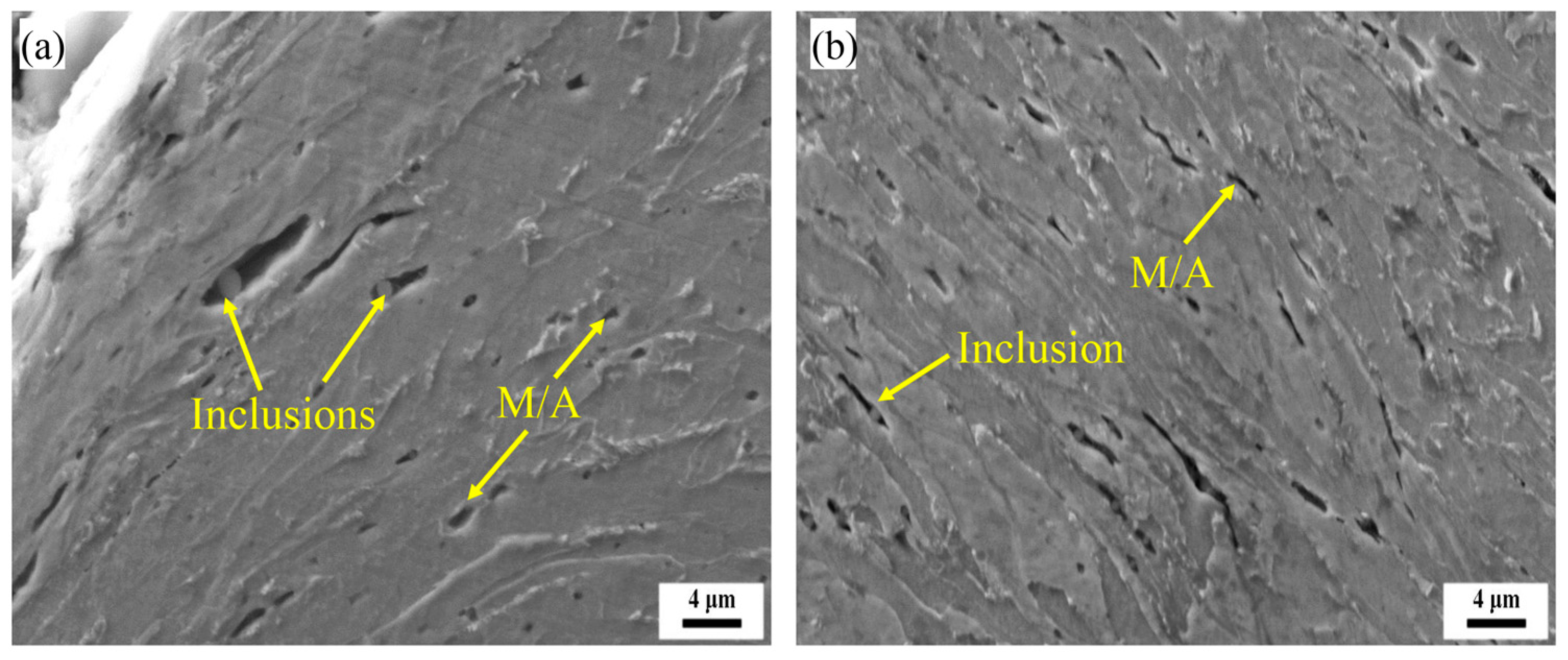
3.1. Microstructural Characterization
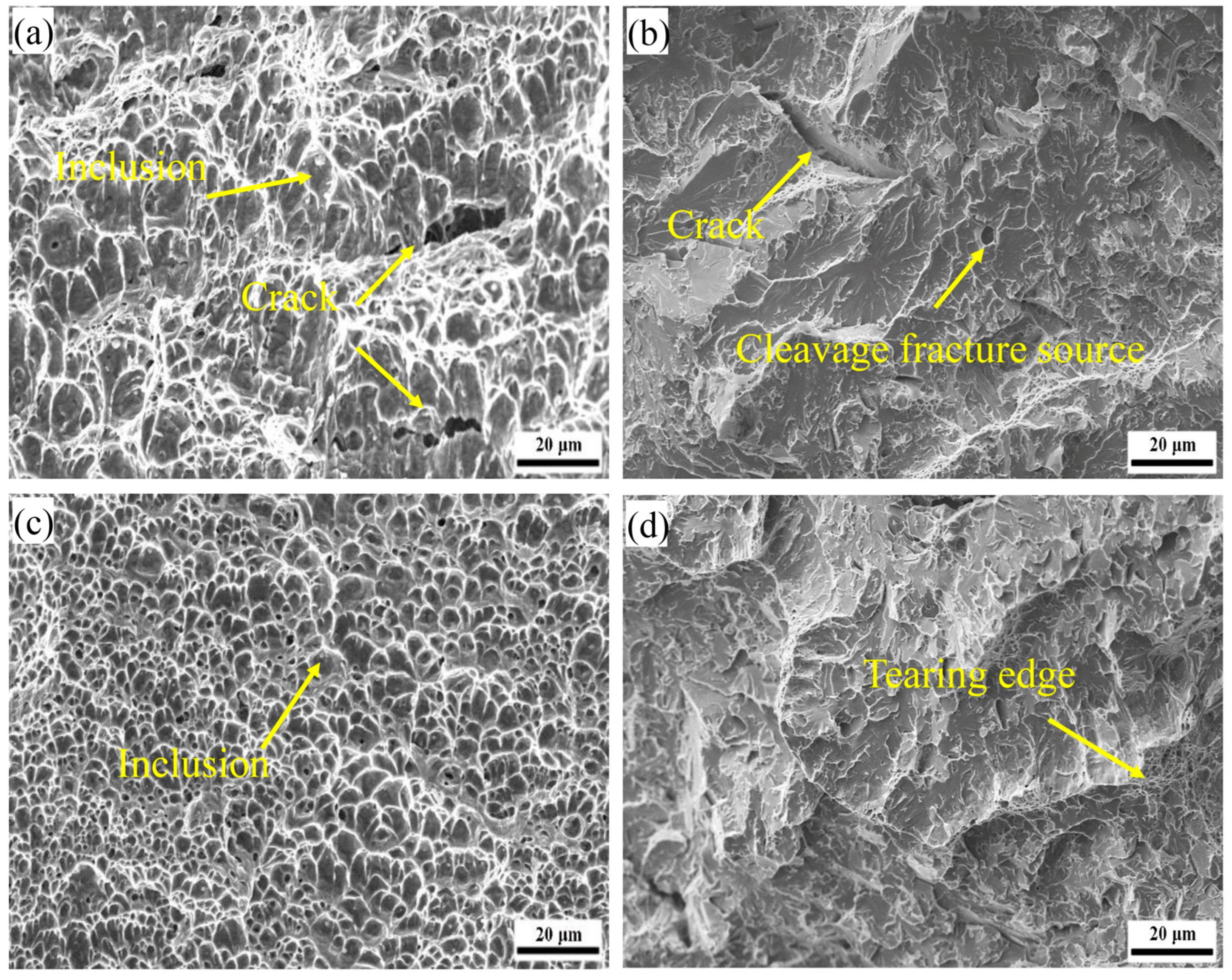
3.2. Impact Properties and Fracture Characterisation of Welds
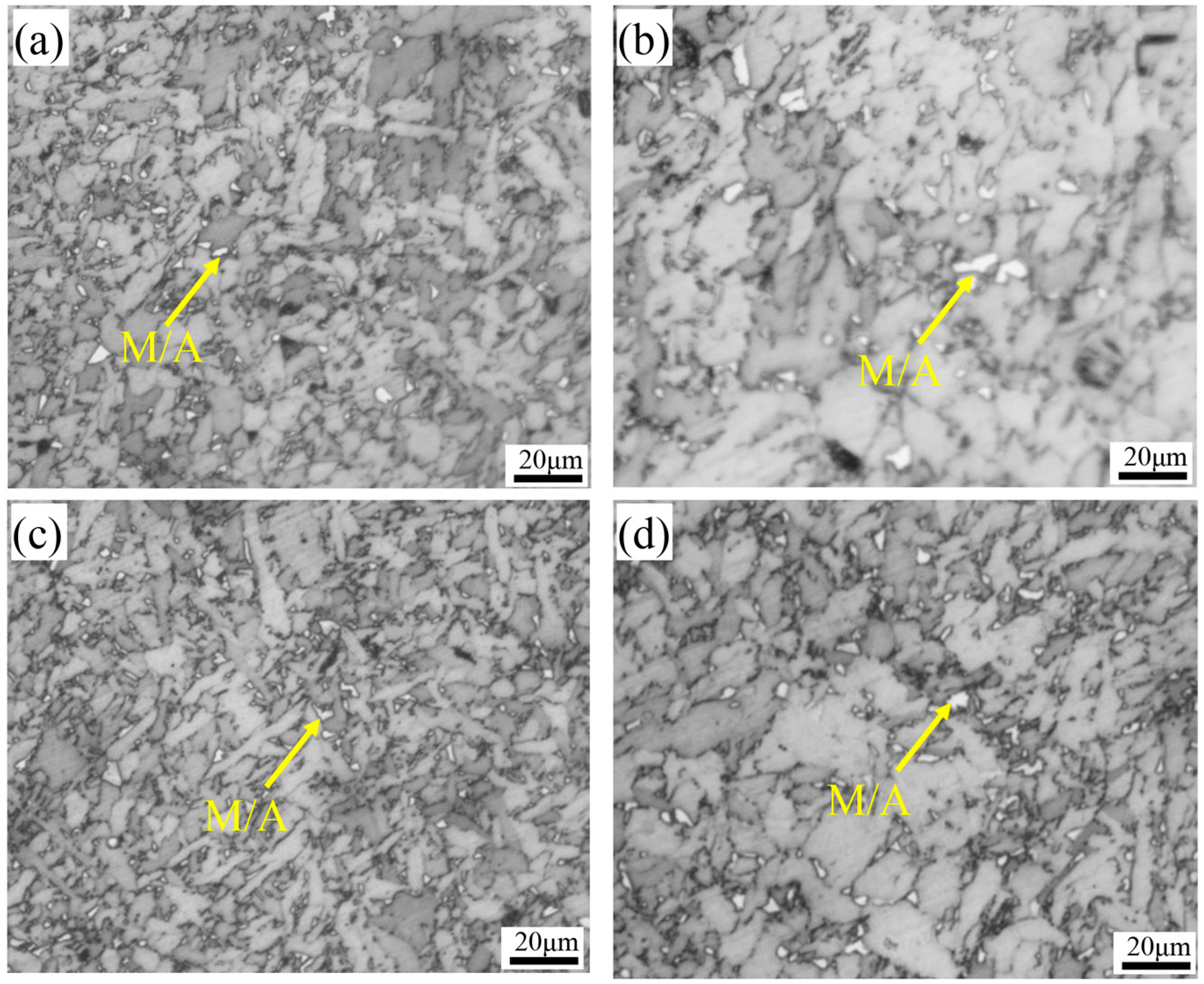
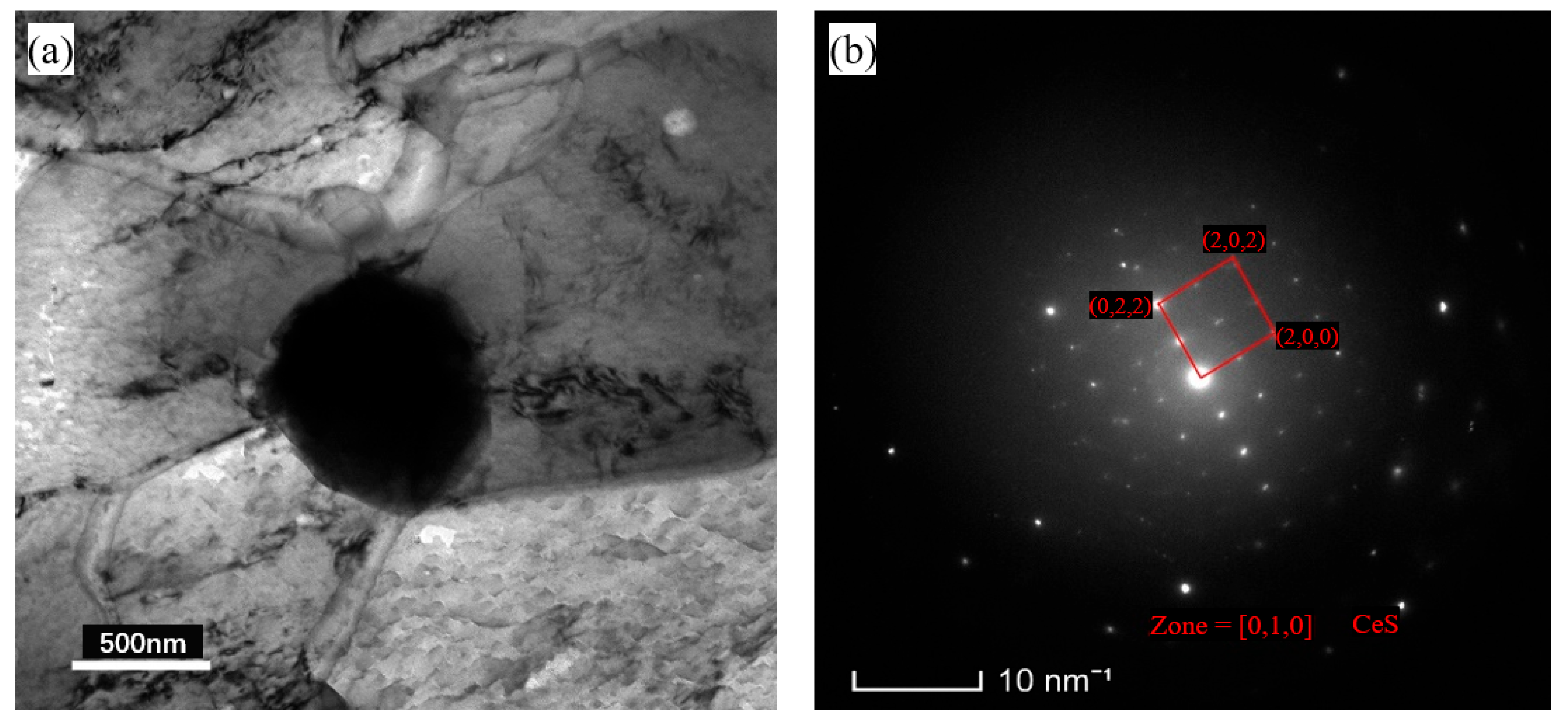
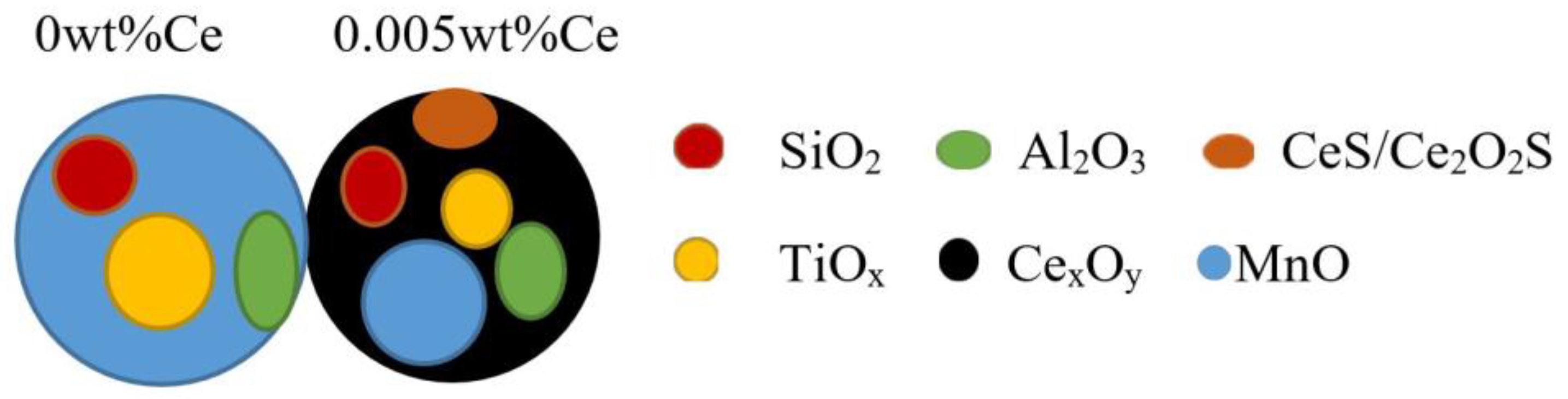
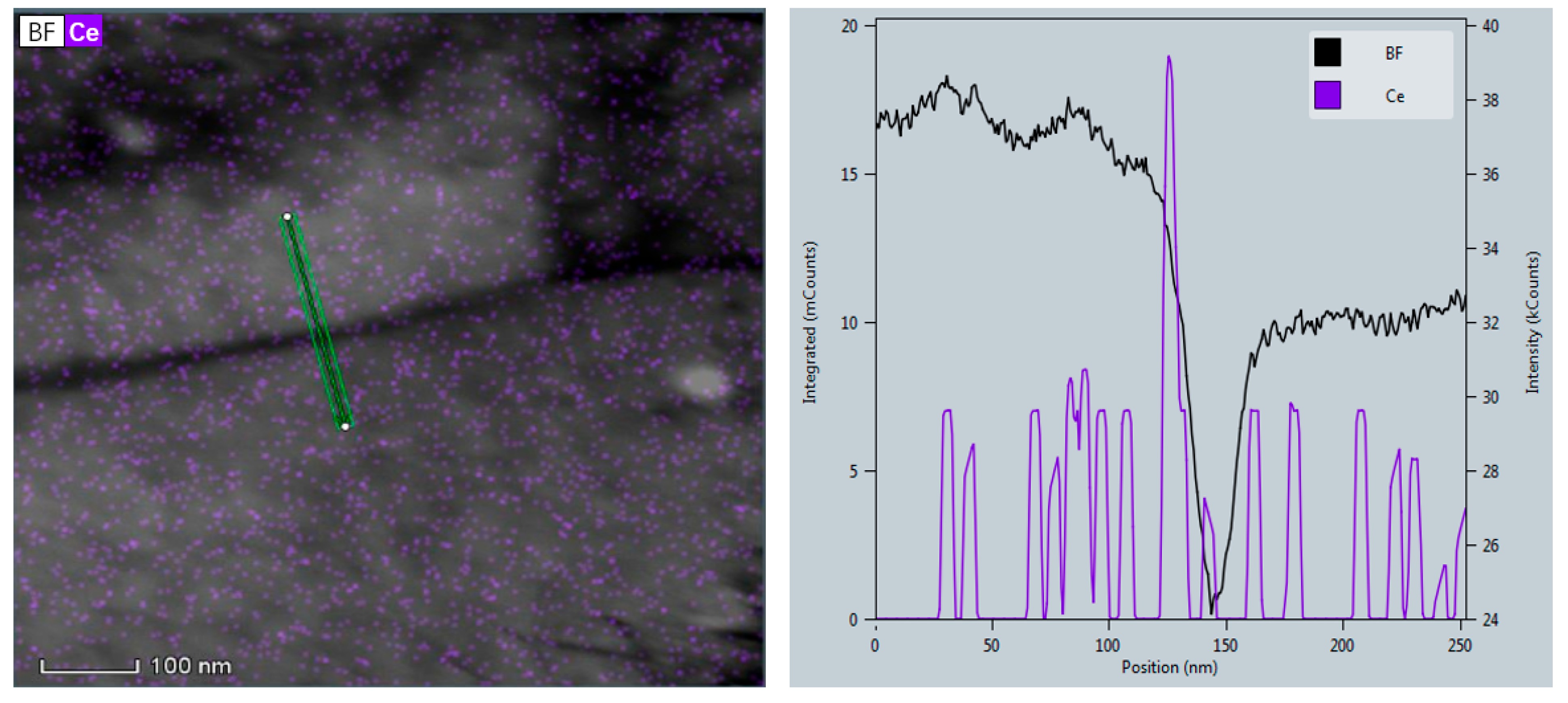
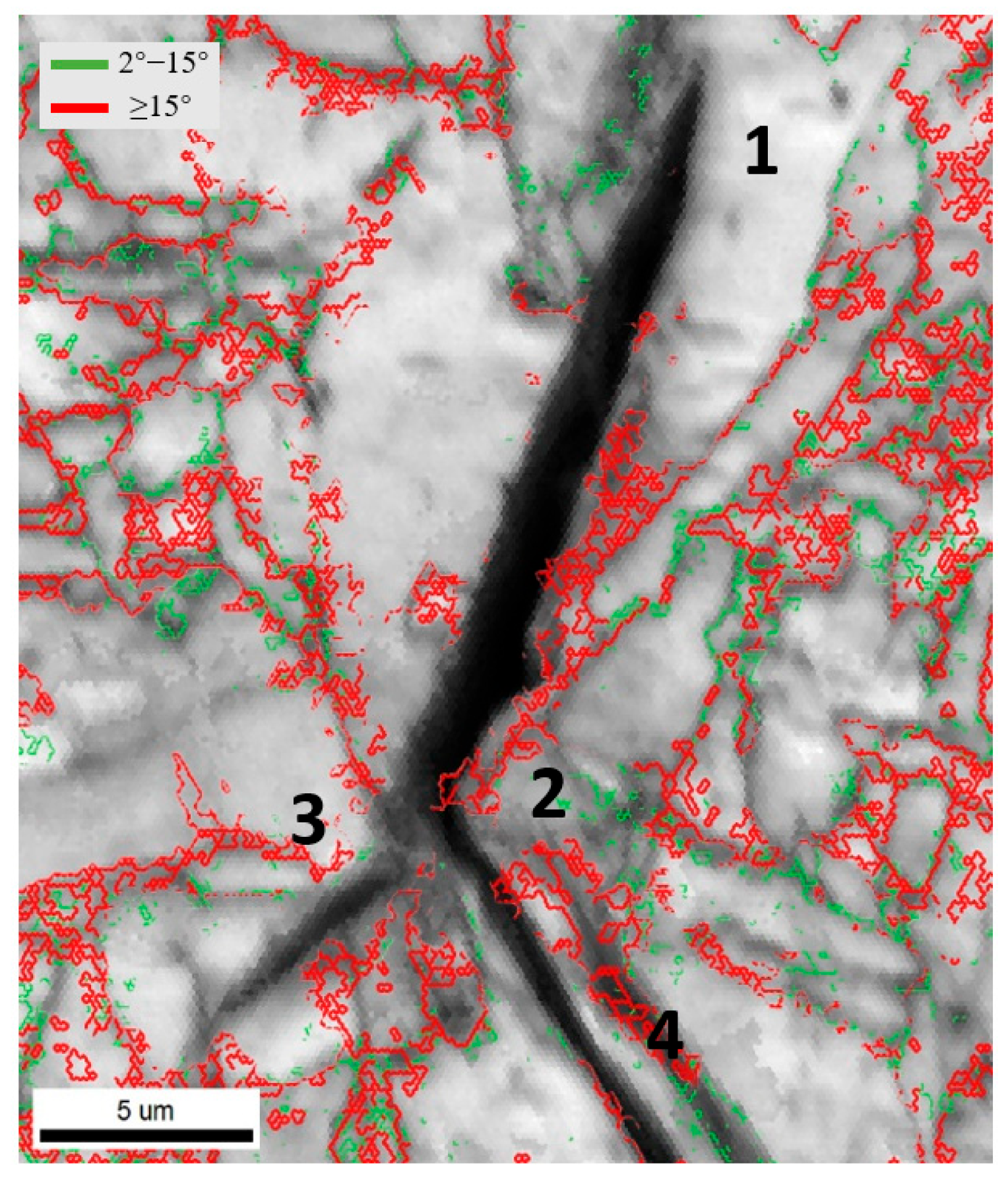
3.3. Microstructural Evolution Analysis
3.4. Impact Fracture Analysis
- —Equivalent diameter of brittle phase structures such as inclusions or M/A components;
- —Young’s modulus of the particle;
- —Stress concentration factor at inclusions;
- —Surface energy of inclusions.
- E—Young’s modulus;
- γ—Effective surface energy at fracture;
- ν—Poisson ratio;
- d0—Microcrack dimensions.
4. Conclusions
- (1)
- The weld metal consists of a columnar crystal zone and a heat-affected zone. The columnar zone mainly contains acicular ferrite, grain boundary ferrite, polygonal ferrite, granular bainite, and M/A constituents, while the heat-affected zone has a coarser microstructure dominated by polygonal and acicular ferrite, granular bainite, and minor M/A constituents.
- (2)
- Increasing Ce from 0 to 0.005 wt% in the weld metal raises the acicular ferrite fraction in the columnar zone and heat-affected zone from 52% and 20% to 83% and 37%, respectively, while reducing polygonal and grain boundary ferrite. Large-angle grain boundaries also increase from 59.2% to 80.1%.
- (3)
- Ce addition refines M/A constituents and inclusions, enhancing critical fracture stress and crack-initiation resistance. The increased acicular ferrite and large-angle grain boundaries improve crack-propagation resistance, resulting in a notable rise in low-temperature (−20 °C) impact energy from 117 J to 197 J.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De Baere, K.; Verstraelen, H.; Willemen, R.; Smet, J.-P.; Tchuindjang, J.T.; Lecomte-Beckers, J.; Lenaerts, S.; Meskens, R.; Jung, H.G.; Potters, G. Assessment of corrosion resistance, material properties, and weldability of alloyed steel for ballast tanks. J. Mar. Sci. Technol. 2017, 22, 176–199. [Google Scholar] [CrossRef]
- Hao, X.; Dong, J.; Wei, J.; Ke, W.; Wang, C.; Xu, X.; Ye, Q. Influence of microstructure of AH32 corrosion resistant steel on corrosion behavior. Acta Met. Sin 2012, 48, 534–540. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, H.; Wang, B.; Tian, Y. Impact of Mo/Ni alloying on microstructural modulation and low-temperature toughness of high-strength low-alloy steel. J. Iron Steel Res. Int. 2024, 31, 1746–1762. [Google Scholar] [CrossRef]
- Li, Y.; Baker, T.N. Effect of morphology of martensite–austenite phase on fracture of weld heat affected zone in vanadium and niobium microalloyed steels. Mater. Sci. Technol. 2010, 26, 1029–1040. [Google Scholar] [CrossRef]
- Honma, Y.; Sasaki, G.; Hashi, K.; Minami, F. Improving the toughness of the weld-heat-affected zone of Cu-containing low-alloy steel for offshore applications by optimizing chemical composition. J. Eng. Mater. Technol. 2021, 143, 011006. [Google Scholar] [CrossRef]
- Fu, W.; Li, C.; Di, X.; Fu, K.; Gao, H.; Fang, C.; Lou, S.; Wang, D. Refinement mechanism of nanoscale Cu-rich precipitates by Mo addition and its effect on strength-toughness of Cu-bearing low carbon high strength steel. Mater. Sci. Eng. A 2022, 849, 143469. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Biswal, S.; Barik, R.K.; Mahato, B.; Ghosh, M.; Mitra, R.; Chakrabarti, D. Comparative interplay of C and Mn on austenite stabilization and low temperature impact toughness of low C medium Mn steels. Mater. Charact. 2024, 208, 113658. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, T.; Chen, Y.; Wang, W.; Wu, W. Effect of CeO2 on microstructures and mechanical properties of welded high-strength steel weld metal. Mater. Res. Express 2021, 8, 016515. [Google Scholar] [CrossRef]
- Wang, C.; Di, X.; Dai, L.; Liu, J.; Han, J.; Yang, X.; Yang, Z.; Cui, S.; Zhang, C.; Ma, Z.; et al. Synchronously improving strength and toughness for pipeline steel weld via cerium adding. Int. J. Press. Vessel. Pip. 2025, 215, 105466. [Google Scholar] [CrossRef]
- NB/T 47014-2011; Welding Procedure Qualification for Pressure Equipment. National Energy Administration: Beijing, China, 2011.
- GB/T 228.1-2021; Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. State Administration for Market Regulation, Standardization Administration of PRC: Beijing, China, 2021.
- GB/T 229-2020; Metallic Materials—Charpy Pendulum Impact Test Method. State Administration for Market Regulation. Standardization Administration of PRC: Beijing, China, 2020.
- Yin, T.W.; Shen, Y.F.; Xue, W.Y.; Jia, N.; Zuo, L. Ce addition enabling superior strength and ductility combination of a low-carbon low-manganese transformation-induced plasticity steel. Mater. Sci. Eng. A 2022, 849, 143474. [Google Scholar] [CrossRef]
- Na, X.; Lei, C.; Luo, W. Microstructure and Mechanical Properties of GMAW+ SAW Joint of Heavy Thickness High Strength Marine Engineering Steel DH36. Silicone Mater. 2022, 36, 615–620+629. [Google Scholar] [CrossRef]
- Liang, J.M.; Tang, D.; Wu, H.B.; Zhang, P.C.; Liu, W.; Mao, H.Y.; Liu, X.T. Corrosion behaviors of Cr low-alloy steel in bottom plate environment of cargo oil tanks. J. South China Univ. Technol. Nat. Sci. 2013, 41, 72–98. [Google Scholar]
- Kozlov, L.J.; Vorobyev, A.P. The role of rare-earth metals in the process of spheroidal graphite formation. Cast Met. 1991, 4, 7–11. [Google Scholar] [CrossRef]
- Jia, S.; Ma, Q.; Hou, Y.; Li, B.; Zhang, H.-S.; Liu, Q.-Y. Changes in microstructure and properties of weld heat-affected zone of high-strength low-alloy steel. J. Iron Steel Res. Int. 2024, 31, 2041–2052. [Google Scholar] [CrossRef]
- Ning, Y.A.N.; Shengfu, Y.U.; Ying, C. In situ observation of austenite grain growth and transformation temperature in coarse grain heat affected zone of Ce-alloyed weld metal. J. Rare Earths 2017, 35, 203–210. [Google Scholar] [CrossRef]
- Liang, G.L.; Yang, S.W.; Wu, H.B.; Liu, X. Microstructure and mechanical performances of CGHAZ for oil tank steel during high heat input welding. Rare Met. 2013, 32, 129–133. [Google Scholar] [CrossRef]
- Li, Y.; Huang, M. A method to calculate the dislocation density of a TWIP steel based on neutron diffraction and synchrotron X-ray diffraction. Acta Met. Sin 2020, 56, 487–493. [Google Scholar]
- Zhang, J.; Li, G.; Wang, H.; Wan, X.; Hu, M.; Cao, Y. Effect of cerium on microstructure and microsegregation behavior of novel cryogenic high-Mn austenitic steel weld metal. Mater. Charact. 2022, 194, 112427. [Google Scholar] [CrossRef]
- Wilson, W.G.; Kay, D.A.R.; Vahed, A. The use of thermodynamics and phase equilibria to predict the behavior of the rare earth elements in steel. JOM 1974, 26, 14–23. [Google Scholar] [CrossRef]
- Khalaj, G.; Yoozbashizadeh, H.; Khodabandeh, A.; Tamizifar, M. Austenite grain growth modelling in weld heat affected zone of Nb/Ti microalloyed linepipe steel. Mater. Sci. Technol. 2014, 30, 424–433. [Google Scholar] [CrossRef]
- Curry, D.A.; Knott, J.F. Effects of microstructure on cleavage fracture stress in steel. Met. Sci. 1978, 12, 511–514. [Google Scholar] [CrossRef]
- Thewlis, G. Effect of cerium sulphide particle dispersions on acicular ferrite microstructure development in steels. Mater. Sci. Technol. 2006, 22, 153–166. [Google Scholar] [CrossRef]
- Wang, G.; Kang, D.; Ye, Y.; Deng, D. Effect of Cr addition on microstructure, mechanical properties, and corrosion behavior of weld metal in weathering steel of high-speed train bogie. Weld. World 2024, 68, 3115–3128. [Google Scholar] [CrossRef]
- Zhang, J.; Li, G.; Wang, H.; Wan, X.; Hu, M.; Cao, Y. Achieving superior cryogenic impact toughness and sufficient tensile properties in a novel high-Mn austenitic steel weld metal via cerium addition. J. Mater. Res. Technol. 2023, 23, 5016–5030. [Google Scholar] [CrossRef]
- Maalekian, M.; Radis, R.; Militzer, M.; Moreau, A.; Poole, W. In situ measurement and modelling of austenite grain growth in a Ti/Nb microalloyed steel. Acta Mater. 2012, 60, 1015–1026. [Google Scholar] [CrossRef]
- Yan, N.; Yu, S.F.; Chen, Y. Inclusions, microstructure and properties of flux copper backing submerged arc weld metal. Sci. Technol. Weld. Join. 2015, 20, 418–424. [Google Scholar] [CrossRef]
- Stone, R.H.V.; Cox, T.B.; Low, J.R.; Psioda, J.A. Microstructural aspects of fracture by dimpled rupture. Int. Met. Rev. 1985, 30, 157–180. [Google Scholar] [CrossRef]
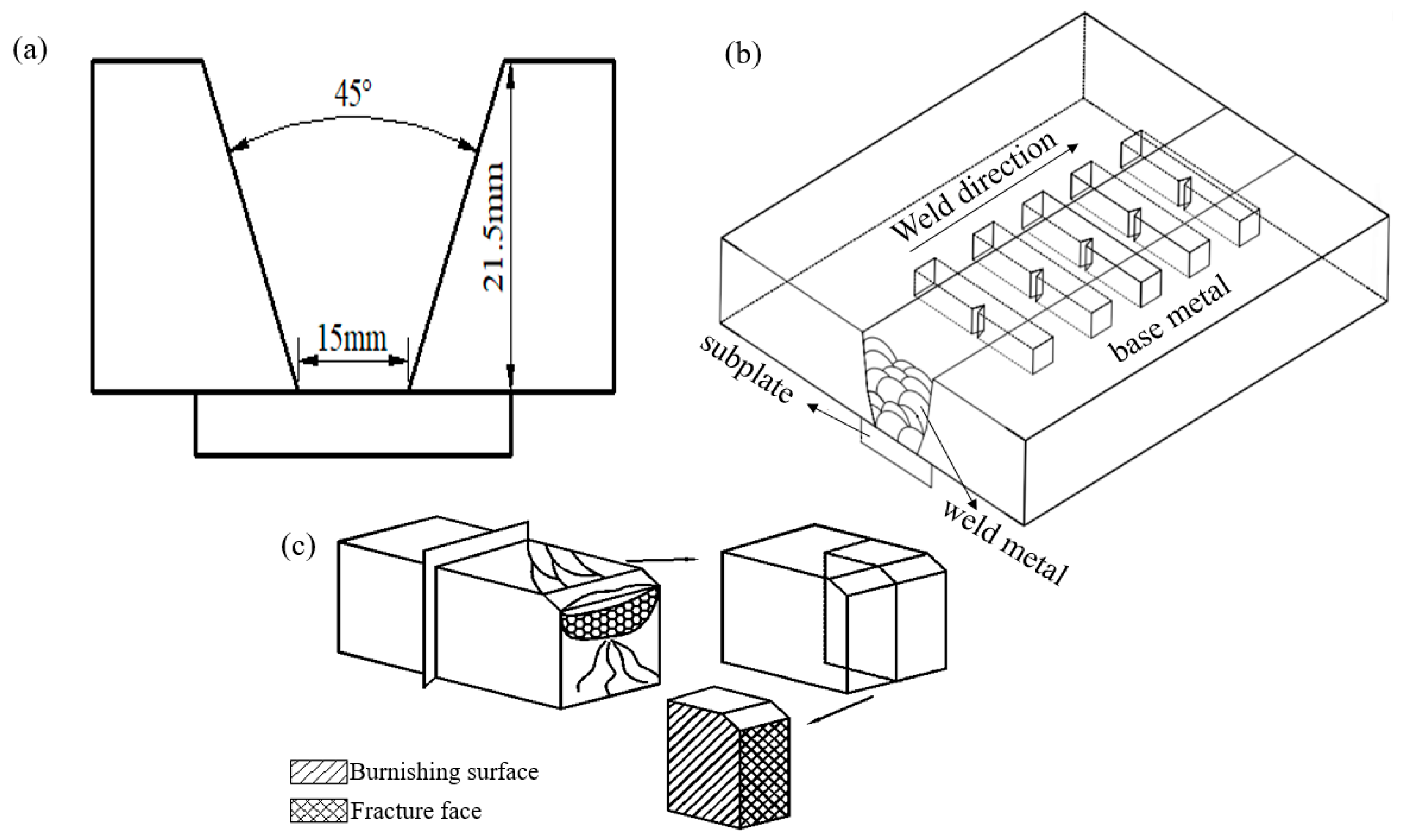



| Welding Wire Type | C | Si | Mn | P | S | Ni | Cu | Mo | Ti | Ce |
|---|---|---|---|---|---|---|---|---|---|---|
| Ce-free | 0.071 | 0.41 | 1.51 | 0.010 | 0.004 | 0.40 | 0.28 | 0.18 | 0.041 | / |
| 14Ce | 0.068 | 0.39 | 1.50 | 0.009 | 0.003 | 0.41 | 0.29 | 0.17 | 0.042 | 0.14 |
| C | Si | Mn | P | S | Nb | V | Ti | Mo | Ni | Cu | Sn | Al | Fe | CEV | Pcm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.088 | 0.31 | 1.38 | 0.009 | 0.002 | 0.02 | 0.042 | 0.02 | 0.13 | 0.21 | 0.20 | 0.02 | 0.036 | Bal. | 0.426 | 0.191 |
| Mg | CaO | SiO2 | TiO2 | Al2O3 | MnO | CaF2 | P | S | Others |
|---|---|---|---|---|---|---|---|---|---|
| 33 | 5 | 14 | 2 | 16 | 2 | 25 | 0.026 | 0.017 | Bal. |
| Interlayer Temperature (°C) | Current (A) | Voltage (V) | Welding Speed (cm/min) | Welding Heat Input (kJ/cm) | Number of Welding Passes |
|---|---|---|---|---|---|
| 120 ± 10 | 600 | 32 | 38 | 30 | 13 |
| Welding Wire Type | C | Si | Mn | P | S | Ni | Cu | Mo | Ti | Ce | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ce-free | 0.061 | 0.38 | 1.47 | 0.011 | 0.004 | 0.40 | 0.28 | 0.18 | 0.016 | / | Bal. |
| 14Ce | 0.063 | 0.41 | 1.49 | 0.010 | 0.003 | 0.41 | 0.29 | 0.17 | 0.018 | 0.005 | Bal. |
| Ce Content in Weld | Columnar Crystal Region (Math.) | Heat-Affected Zone of the Weld Channel | ||||
|---|---|---|---|---|---|---|
| AF | PF | QPF | AF | PF | QPF | |
| Ce-free | 52 | 26 | 12 | 20 | / | 51 |
| 0.005 wt% | 83 | 4 | 8 | 37 | / | 63 |
| Ce Content of Weld | Columnar Crystal Region (Math.) | Heat-Affected Zone of the Weld Channel | ||
|---|---|---|---|---|
| fM/A/% | dM/A/μm | fM/A/% | dM/A/μm | |
| 0 wt.% | 9.2 | 1.15 | 5.3 | 0.75 |
| 0.005 wt.% | 7.8 | 0.87 | 4.2 | 0.65 |
| Ce Content of Weld | Area Proportion/% | Average Diameter/um | Maximum Diameter/um | Area Destiny/mm−2 |
|---|---|---|---|---|
| Ce-free | 0.150 | 0.473 | 2.69 | 4550 |
| 0.005 wt% | 0.169 | 0.431 | 2.48 | 5225 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Qiu, J.; Wang, Q.; Wang, Q. The Impact of Ce on the Microstructure and Properties of Weld Metal in Corrosion-Resistant Steel. Metals 2025, 15, 1289. https://doi.org/10.3390/met15121289
Wang Y, Qiu J, Wang Q, Wang Q. The Impact of Ce on the Microstructure and Properties of Weld Metal in Corrosion-Resistant Steel. Metals. 2025; 15(12):1289. https://doi.org/10.3390/met15121289
Chicago/Turabian StyleWang, Yuwei, Jun Qiu, Qiuming Wang, and Qingfeng Wang. 2025. "The Impact of Ce on the Microstructure and Properties of Weld Metal in Corrosion-Resistant Steel" Metals 15, no. 12: 1289. https://doi.org/10.3390/met15121289
APA StyleWang, Y., Qiu, J., Wang, Q., & Wang, Q. (2025). The Impact of Ce on the Microstructure and Properties of Weld Metal in Corrosion-Resistant Steel. Metals, 15(12), 1289. https://doi.org/10.3390/met15121289





