Study on Combination Mechanism of Pretreatment Layer in Pre-Coated Metal Sheets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Preparation
2.3. Methodology
3. Results
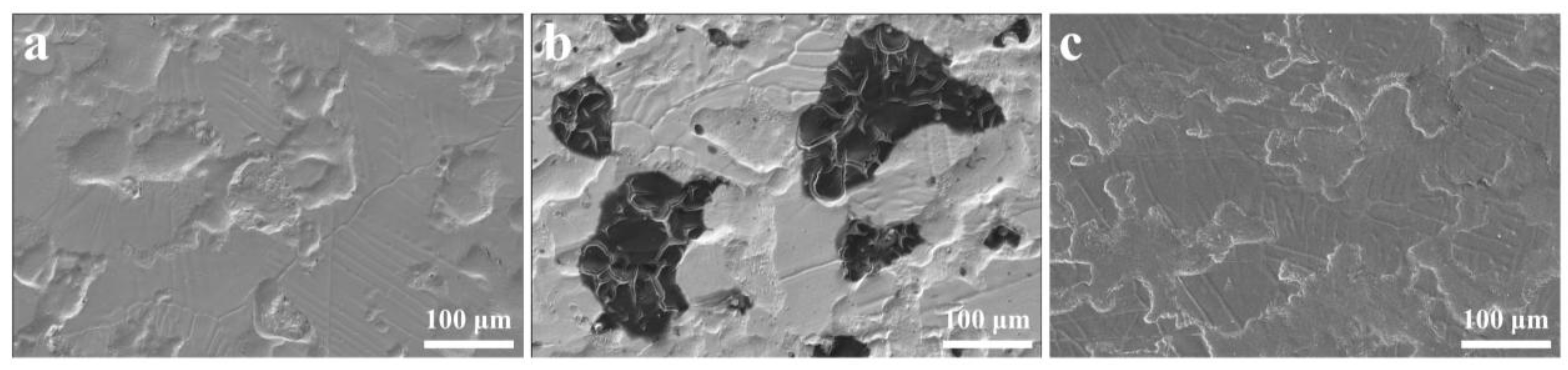
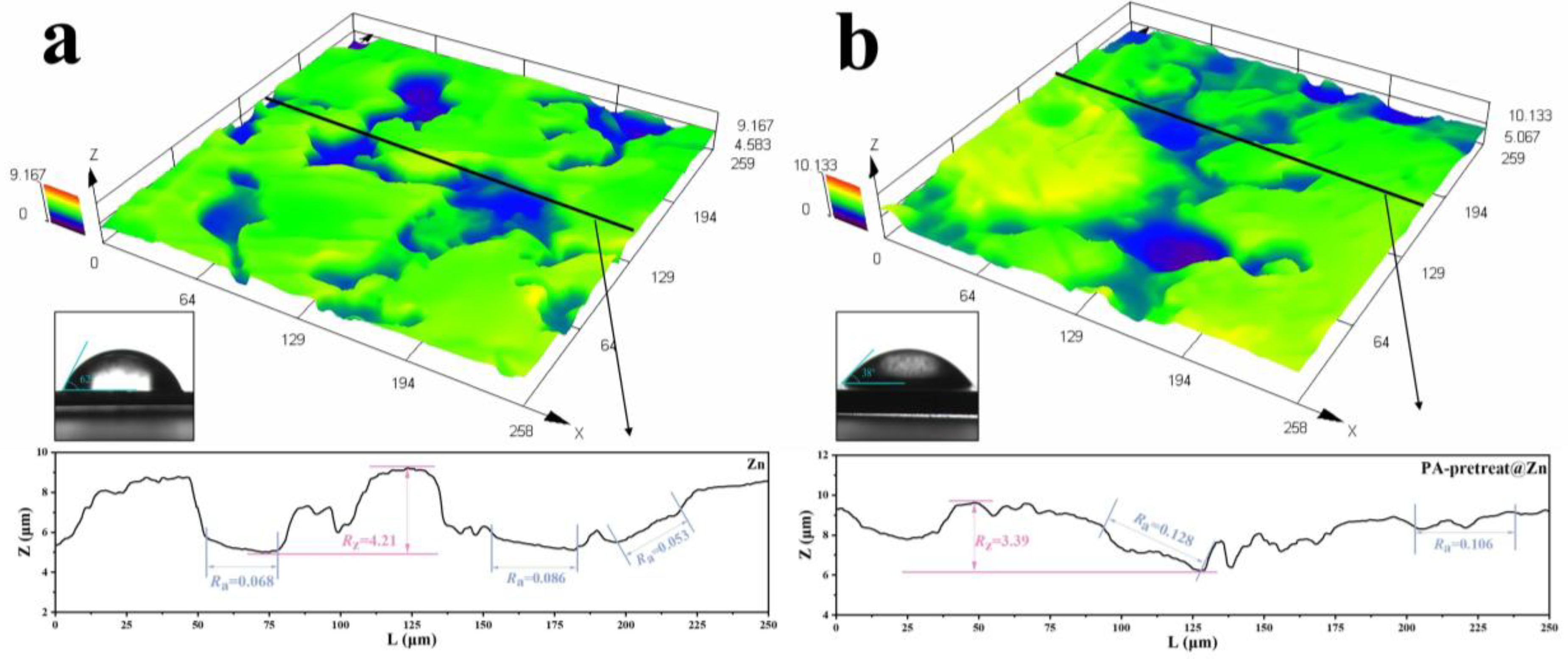
3.1. Surface Characterization
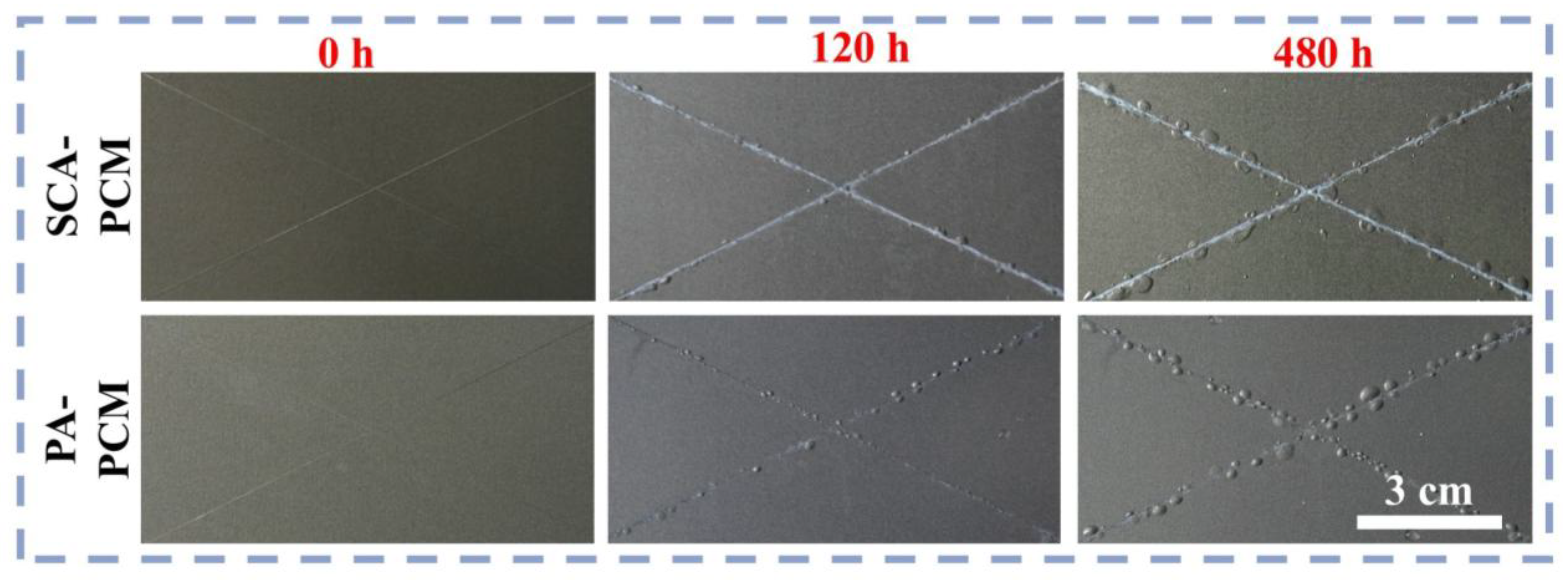
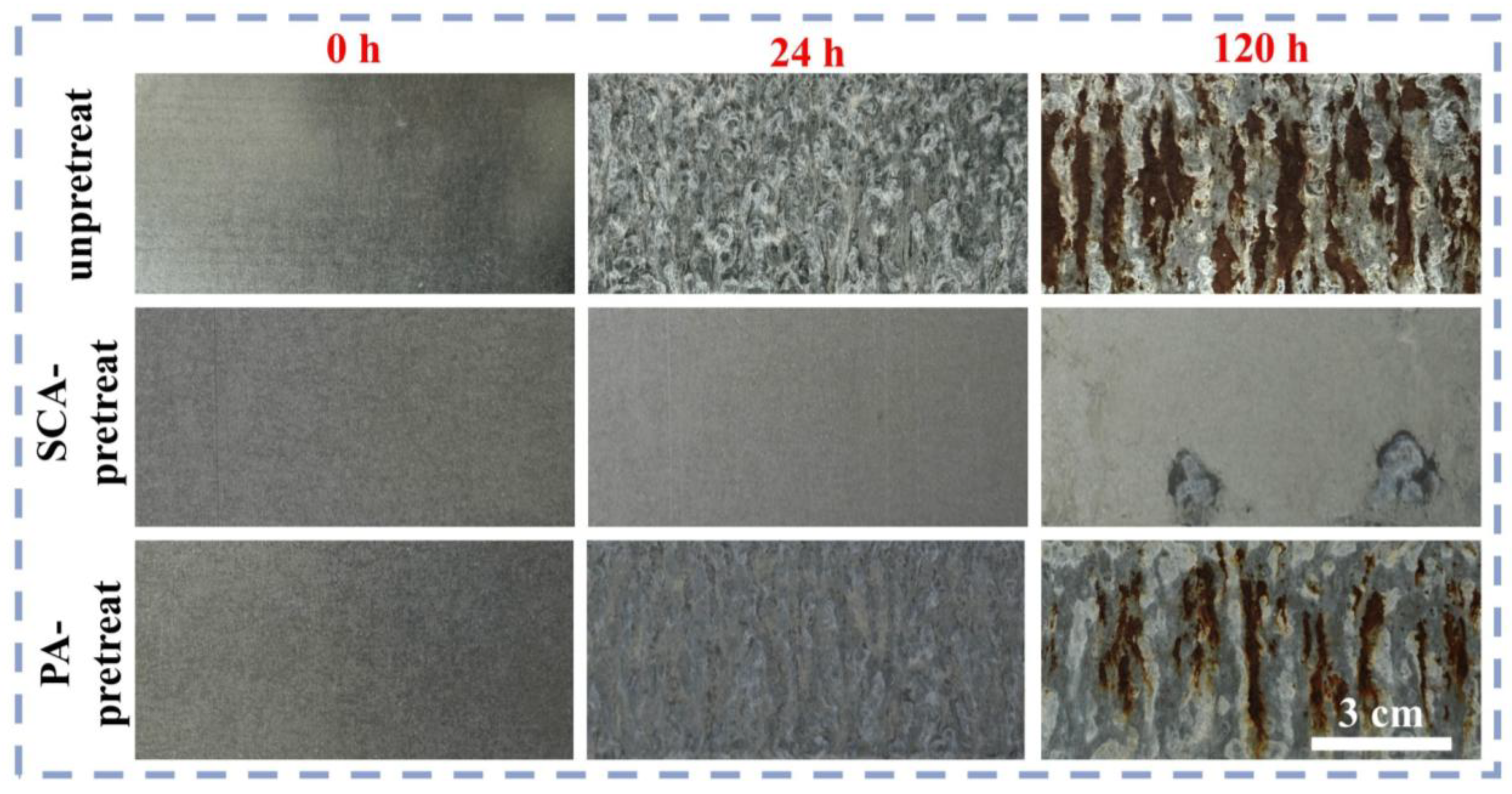
3.2. Anti-Salt-Spray Performance of Coatings
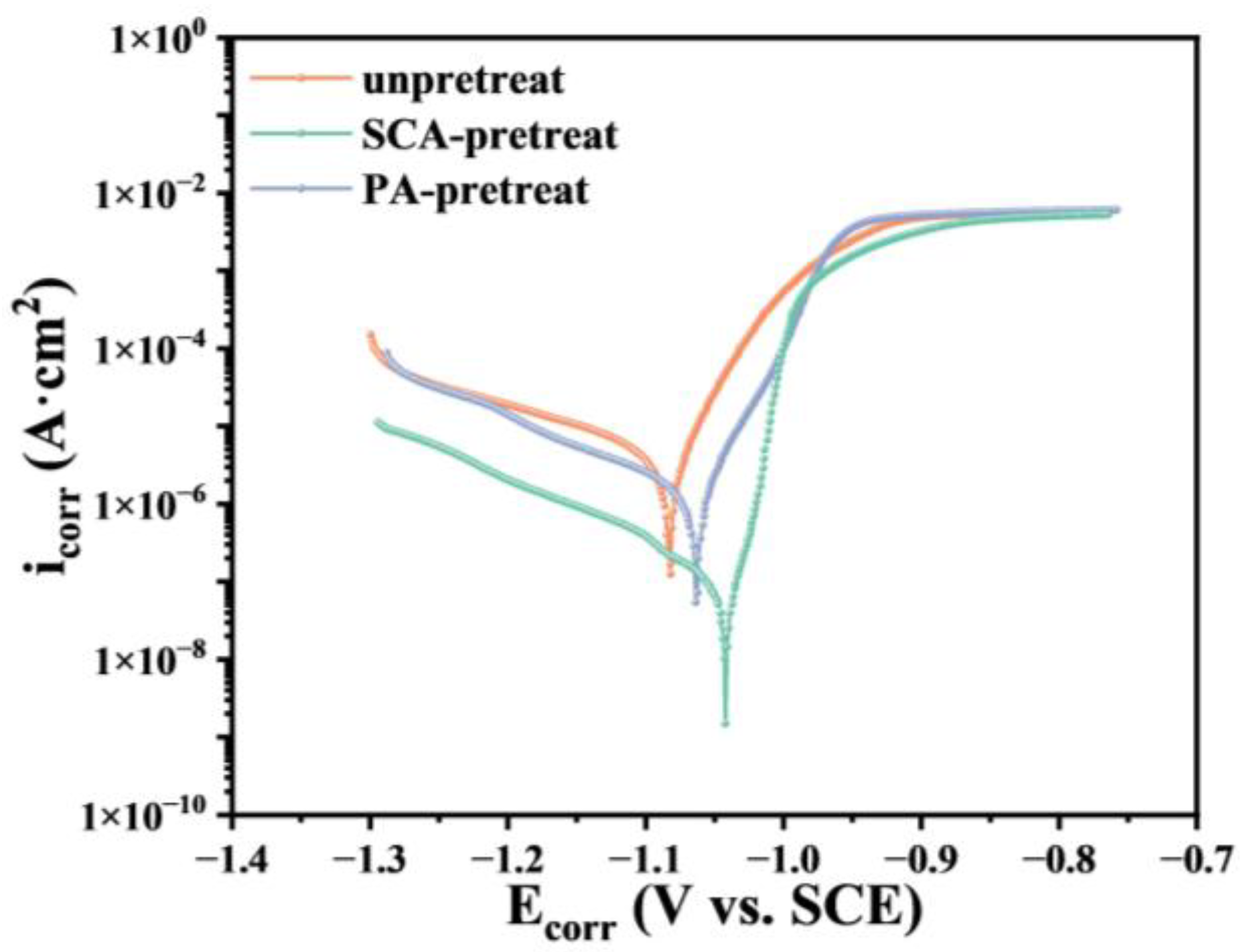
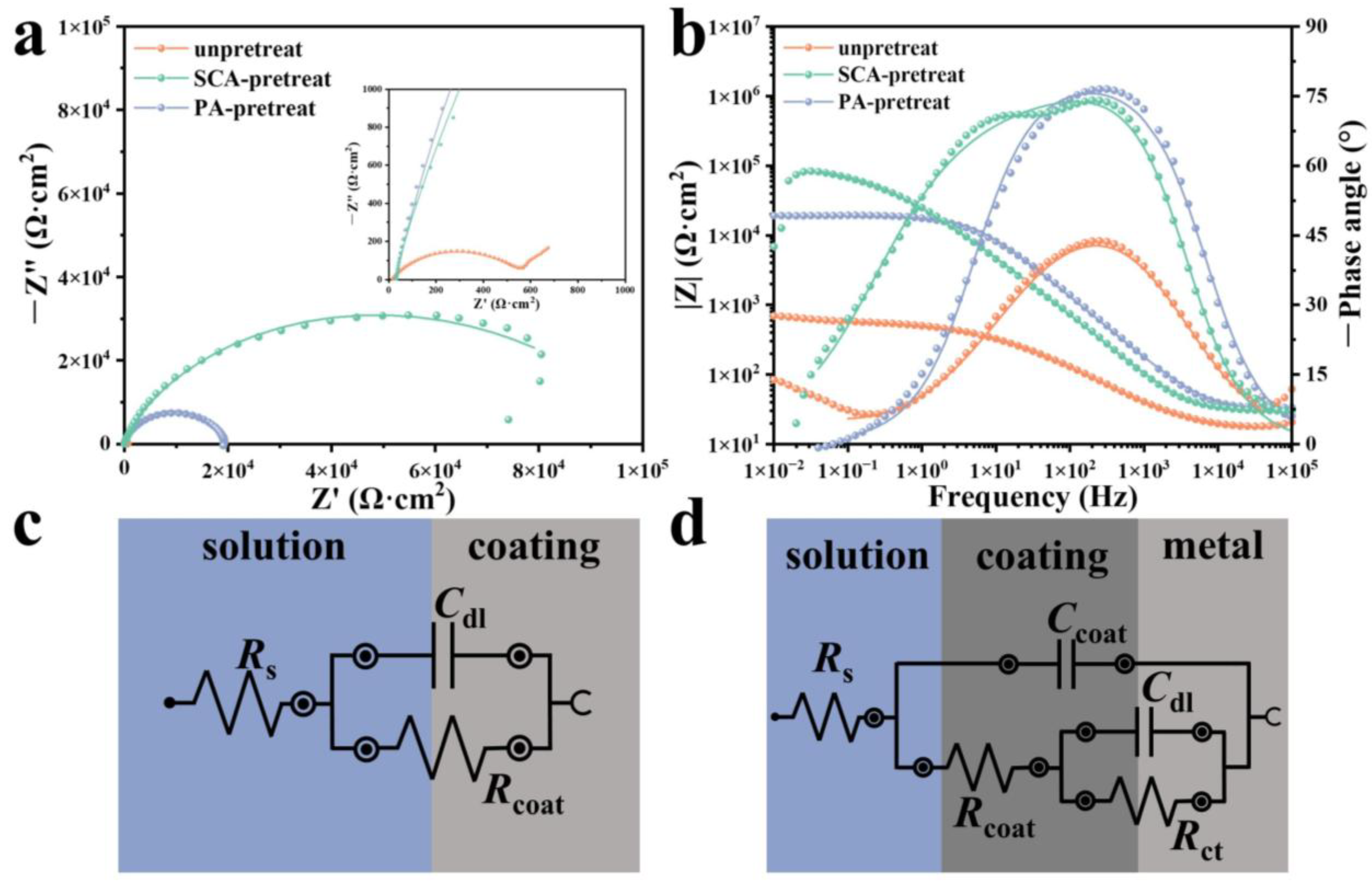
3.3. Corrosion Performance
4. Discussion
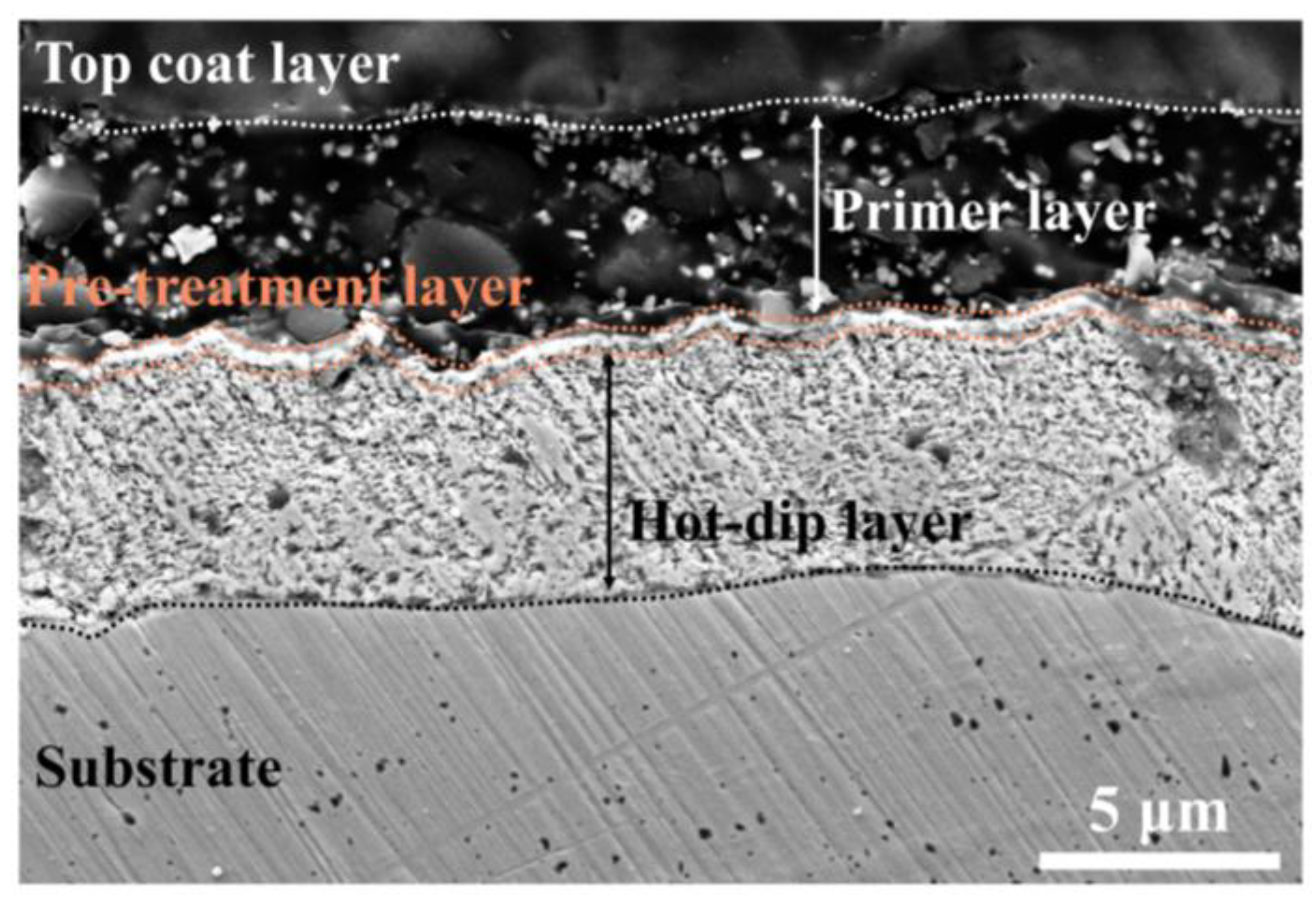
4.1. Cross-Sectional Analysis
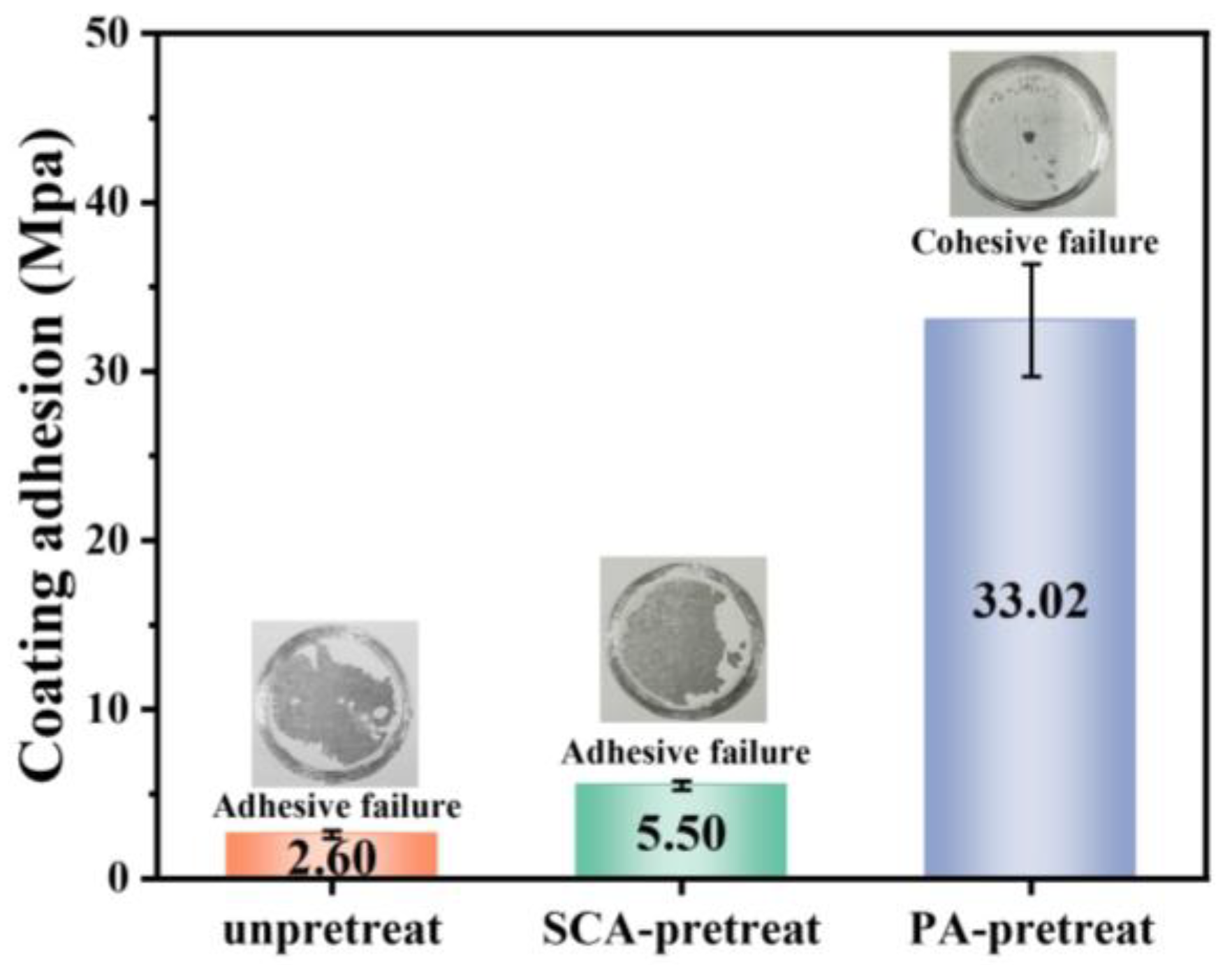
4.2. Effect of Pretreatment on Surface State and Adhesion Strength
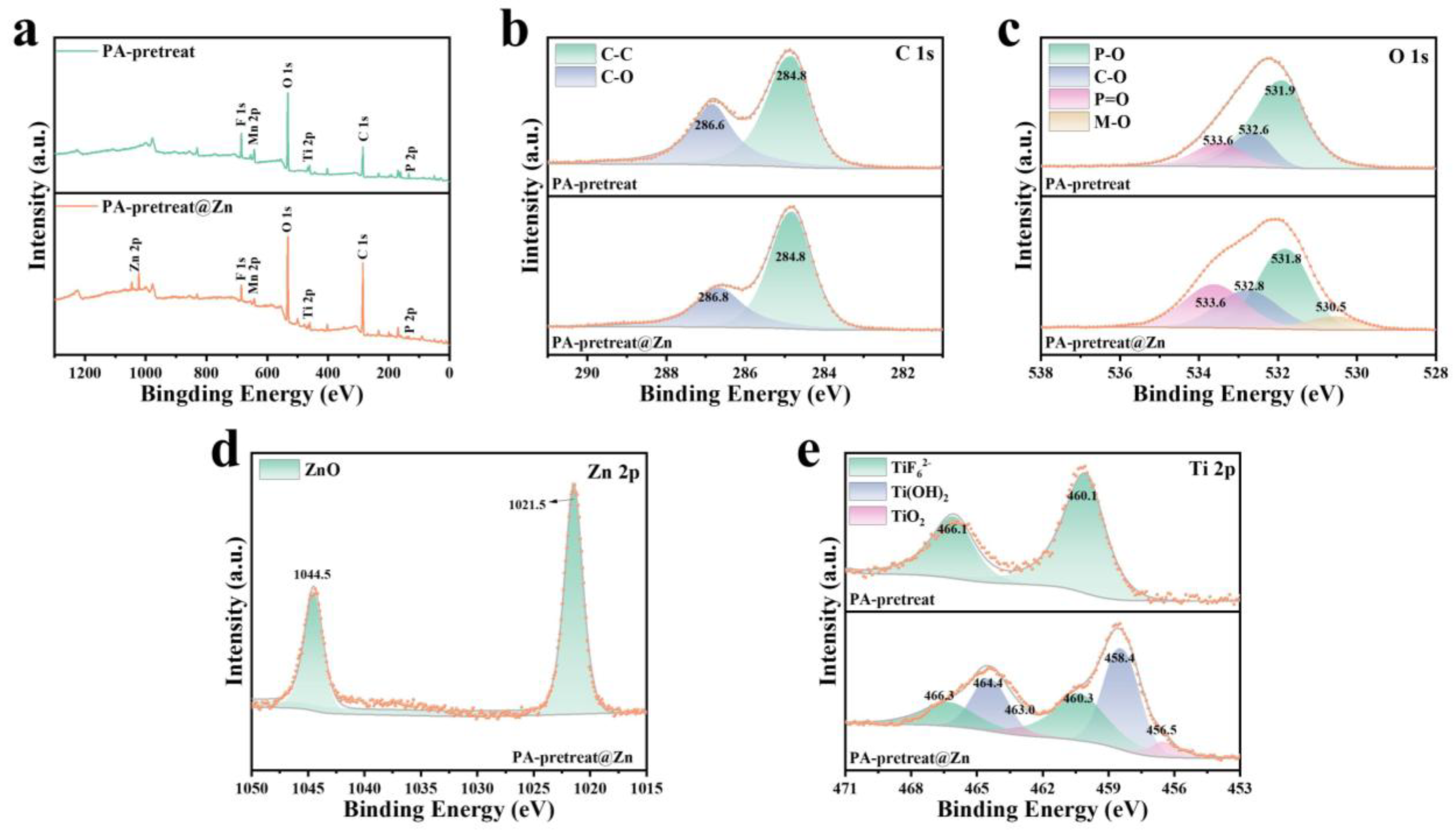
4.3. Mechanism of Film Formation in Pretreatment Layer
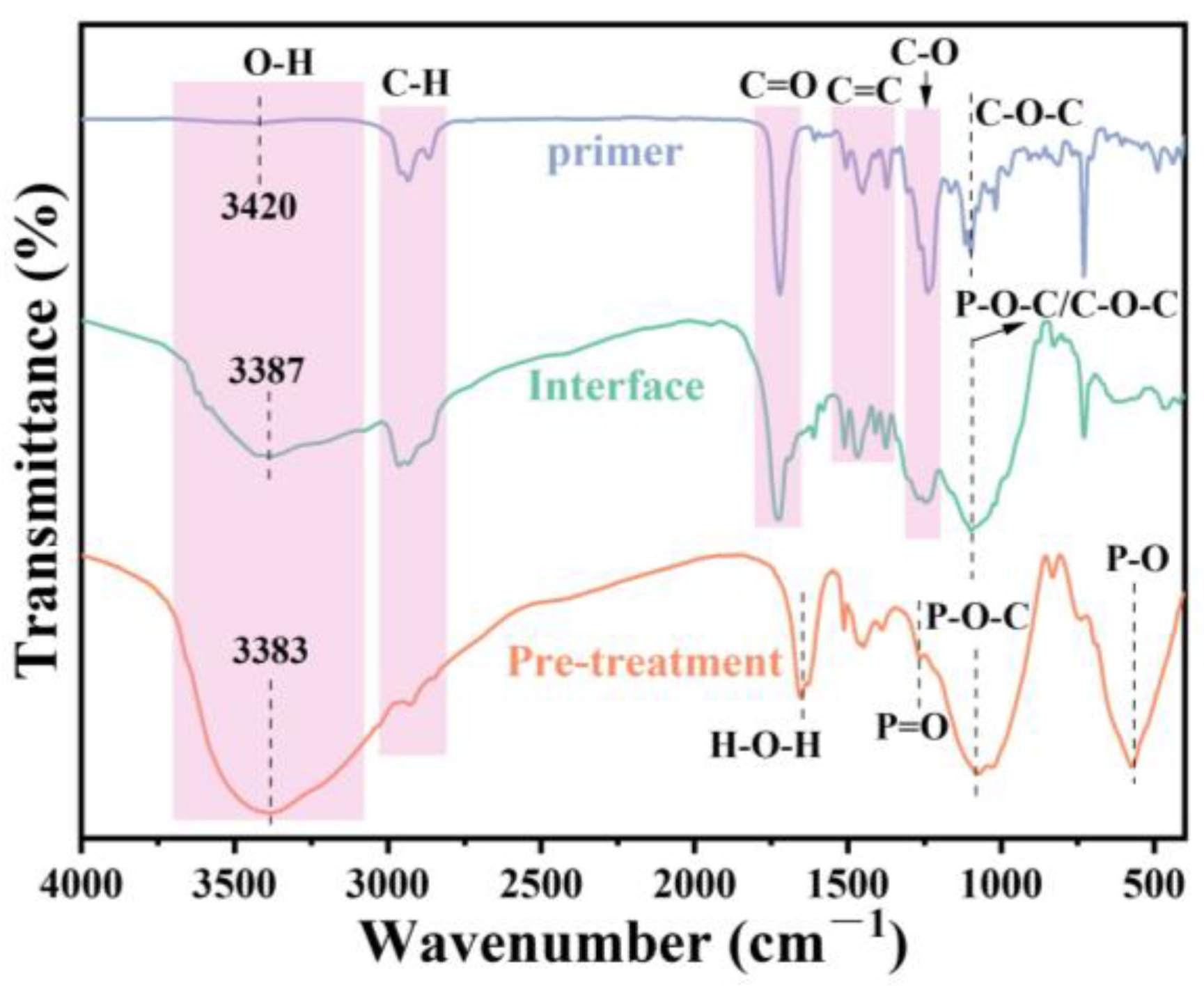
4.4. Interfacial Bonding Mechanism Between Pretreatment and Primer Layers
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Q.; Yang, Q.; Zhang, J.; Ren, F.; Xia, C.; Chen, F. Anti-corrosion properties of bio-inspired surfaces: A systematic review of recent research developments. Mater. Adv. 2024, 5, 2689–2718. [Google Scholar] [CrossRef]
- Sun, H.; Song, B.; Sun, X.; Cui, X.; Liu, Z.; Cong, M.; Sun, M.; Zhu, Z.; Tian, Y.; Liu, S.; et al. Recent Representative Progress of Surface Coating Technology. Chem. Rec. 2025, 25, e202500054. [Google Scholar] [CrossRef] [PubMed]
- Asim, N.; Badiei, M.; Samsudin, N.A.; Mohammad, M.; Razali, H.; Soltani, S.; Amin, N. Application of graphene-based materials in developing sustainable infrastructure: An overview. Compos. Part. B 2022, 245, 110188. [Google Scholar] [CrossRef]
- Yimyai, T.; Crespy, D.; Rohwerder, M. Corrosion-Responsive Self-Healing Coatings. Adv. Mater. 2023, 35, 2300101. [Google Scholar] [CrossRef]
- Lin, W.; Zhao, Y.; Edwards, G.; Guo, Q.; Chen, T.; Song, S.; Heitzmann, M.; Martin, D.; Grøndahl, L.; Lu, M.; et al. Mechanical properties and scratch recovery of nanoclay/polyester composite coatings for pre-coated metal (PCM) sheets. Compos. Part. B 2024, 273, 111217. [Google Scholar] [CrossRef]
- Ueda, K.; Kanai, H.; Amari, T. Formability of polyester/melamine pre-painted steel sheets from rheological aspect. Prog. Org. Coat. 2002, 45, 267–272. [Google Scholar] [CrossRef]
- Golgoon, A.; Aliofkhazraei, M.; Toorani, M.; Moradi, M.H.; Sabour Rouhaghdam, A. Corrosion and wear behavior of alumina-polyester nanocomposite coatings. Polym. Eng. Sci. 2017, 57, 846–856. [Google Scholar] [CrossRef]
- Moon, J.-I.; Lee, Y.-H.; Kim, H.-J. Synthesis and characterization of flexible polyester coatings for automotive pre-coated metal. Prog. Org. Coat. 2012, 73, 123–128. [Google Scholar] [CrossRef]
- Palraj, S.; Selvaraj, M.; Jayakrishnan, P. Effect of phosphate coatings on the performance of epoxy polyamide red oxide primer on galvanized steel. Prog. Org. Coat. 2005, 54, 5–9. [Google Scholar] [CrossRef]
- LeBozec, N.; Picot, P.; LeGac, A.; Thierry, D. Influence of Surface Preparation on the Durability of Repair Coatings for Prepainted Galvanised Steel. Mater. Corros. 2025, 76, 533–541. [Google Scholar] [CrossRef]
- Xing, T.; Ying, L.; Wu, C.; Fu, Z.; Wang, G. Study on the effect of surface tannic acid/silane conversion film on properties of epoxy resin coatings. Anti-Corros. Methods Mater. 2019, 66, 446–453. [Google Scholar] [CrossRef]
- Pan, J.; Tang, X.; Li, Y. Pulse potential method-assisted construction and regulation of a trivalent chromium conversion coating on hot-dip coated steel sheet. Corros. Sci. 2020, 176, 109026. [Google Scholar] [CrossRef]
- Hesamedini, S.; Bund, A. Trivalent chromium conversion coatings. J. Coat. Technol. Res. 2019, 16, 623–641. [Google Scholar] [CrossRef]
- Hesamedini, S.; Bund, A. Formation of Cr(VI) in cobalt containing Cr(III)-based treatment solution. Surf. Coat. Technol. 2018, 334, 444–449. [Google Scholar] [CrossRef]
- Peltier, F.; Thierry, D. Review of Cr-Free Coatings for the Corrosion Protection of Aluminum Aerospace Alloys. Coatings 2022, 12, 518. [Google Scholar] [CrossRef]
- He, W.; Xia, X.; Gao, X.; Yan, R.; Wang, Y.; Ma, H. Corrosion resistance and adhesive performance of a novel phytic acid-triethoxyvinylsilane-zinc hybrid chemical conversion film on steel. Int. J. Adhes. Adhes. 2023, 125, 103430. [Google Scholar] [CrossRef]
- Luo, X.; Li, Y.; Li, S.; Liu, X. Enhancement of Mechanical Properties and Bonding Properties of Flake-Zinc-Powder-Modified Epoxy Resin Composites. Polymers 2022, 14, 5323. [Google Scholar] [CrossRef]
- Awad, K.; Young, S.; Aswath, P.; Varanasi, V. Interfacial adhesion and surface bioactivity of anodized titanium modified with SiON and SiONP surface coatings. Surf. Interfaces 2022, 28, 101645. [Google Scholar] [CrossRef]
- Babu, N.; Zhang, P.; Xian, G. Improving epoxy adhesion with steel adherends using a tannic acid-based additive: Impact on resin properties and interfacial bonding. J. Appl. Polym. Sci. 2023, 140, e53803. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Z.; Qi, Y. Effect of Composite Epoxy Coating on Protective and Bonding Properties to Nickel Aluminum Bronze. Adv. Eng. Mater. 2023, 25, 2201326. [Google Scholar] [CrossRef]
- BS EN ISO 4624:2003; Paints and Varnishes Pull-Off Test for Adhesion. British Standards Institution: London, UK, 2003.
- Kwon, D.-J.; Kim, J.-H.; Kim, Y.-J.; Kim, J.-J.; Park, S.-M.; Kwon, I.-J.; Shin, P.-S.; DeVries, L.K.; Park, J.-M. Comparison of interfacial adhesion of hybrid materials of aluminum/carbon fiber reinforced epoxy composites with different surface roughness. Compos. Part. B 2019, 170, 11–18. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Zhou, W.; Ma, R.; Du, A.; Fan, Y.; Zhao, X.; Cao, X. Improved anti-corrosion behaviour of an inorganic passive film on hot-dip galvanised steel by modified graphene oxide incorporation. Corros. Sci. 2020, 174, 108846. [Google Scholar] [CrossRef]
- Gao, F.; Du, A.; Ma, R.; Lv, C.; Yang, H.; Fan, Y.; Zhao, X.; Wu, J.; Cao, X. Improved corrosion resistance of acrylic coatings prepared with modified MoS2 nanosheets. Colloids Surf. Physicochem. Eng. Asp. 2020, 587, 124318. [Google Scholar] [CrossRef]
- Gou, J.; Wang, G.; Ning, Y.; Guan, L.; Zhang, Y.; Liao, J.; Wang, Y. Preparation and corrosion resistance of chromium-free Zn-Al coatings with two different silane coupling agents. Surf. Coat. Technol. 2019, 366, 1–6. [Google Scholar] [CrossRef]
- Rudawska, A.; Gola, A.; Pizoń, J.; Capała, P.; Wójcik, Ł. Effectiveness of Bonding Steel Elements with Polyester-Coated Paint. Appl. Sci. 2023, 13, 10059. [Google Scholar] [CrossRef]
- Joo, J.; Kang, M.; Moon, H.-S.; Wooh, S.; Lee, J. Design and experimental studies of self-healable anti-corrosion coating: Passivation of metal surfaces by silicone oil impregnated porous oxides. Surf. Coat. Technol. 2020, 404, 126595. [Google Scholar] [CrossRef]
- Yeadon, K.; Lai, E.P.C.; Huang, X.; Song, N. Influence of surface roughness and metal oxide nanoparticles on airframe with icephobic coatings. RSC Appl. Interfaces 2025, 2, 82–93. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, M.; Zhang, B.; Li, H. Effect of surface roughness of electroplating chromium coated steel on bonding strength of polymer coated steel. Polym. Polym. Compos. 2022, 30, 09673911221102128. [Google Scholar] [CrossRef]
- Duan, W.; Fan, Y.; Shu, B.; Liu, Y.; Wan, Y.; Xiao, R.; Xu, J.; Qing, S.; Xiao, Q. The Formation of Phytic Acid–Silane Films on Cold-Rolled Steel and Corrosion Resistance. Metals 2024, 14, 326. [Google Scholar] [CrossRef]
- Banjo, N.; Sasaki, T.T.; Hono, K. Uniform formation of Ti-based conversion coatings on aluminum alloy surfaces by alkaline-acid etch treatment to improve organic coating properties. Appl. Surf. Sci. 2024, 661, 160005. [Google Scholar] [CrossRef]
- Weinert, M.; Gutmann, J.S.; Dornbusch, M. Hydrophobic phytic acid conversion layers for corrosion protection of steel surfaces. J. Coat. Technol. Res. 2024, 21, 703–736. [Google Scholar] [CrossRef]
- Asemani, H.R.; Ahmadi, P.; Sarabi, A.A.; Eivaz Mohammadloo, H. Effect of zirconium conversion coating: Adhesion and anti-corrosion properties of epoxy organic coating containing zinc aluminum polyphosphate (ZAPP) pigment on carbon mild steel. Prog. Org. Coat. 2016, 94, 18–27. [Google Scholar] [CrossRef]
| Ecorro/V | icorro/A·cm−2 | |
|---|---|---|
| Without pretreat | −1.083 | 3.74 × 10−6 |
| SCA-pretreat | −1.043 | 6.54 × 10−8 |
| PA-pretreat | −1.064 | 1.17 × 10−6 |
| Rcoat/Ω·cm2 | Ccoat/μF·cm−2 | Rct/Ω·cm2 | Cdl/μF·cm−2 | |
|---|---|---|---|---|
| Without pretreat | 5.78 × 103 | 1.63 × 104 | - | |
| SCA-pretreat | 2.21 × 103 | 8.89 | 1.03 × 105 | 2.85 × 105 |
| PA-pretreat | 1.87 × 104 | 9.10 | - |
| C-C | C-O | |
|---|---|---|
| PA-pretreat | 59.3% | 40.7% |
| PA-pretreat@Zn | 67.1% | 32.9% |
| P-O | C-O | |
|---|---|---|
| PA-pretreat | 65.0% | 19.6% |
| PA-pretreat@Zn | 41.8% | 22.9% |
| TiF62− | Ti(OH)2 | |
|---|---|---|
| PA-pretreat | 100% | - |
| PA-pretreat@Zn | 40.6% | 51.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Ye, J.; Liao, L.; Dong, X. Study on Combination Mechanism of Pretreatment Layer in Pre-Coated Metal Sheets. Metals 2025, 15, 1245. https://doi.org/10.3390/met15111245
Liu C, Ye J, Liao L, Dong X. Study on Combination Mechanism of Pretreatment Layer in Pre-Coated Metal Sheets. Metals. 2025; 15(11):1245. https://doi.org/10.3390/met15111245
Chicago/Turabian StyleLiu, Changwen, Jinwen Ye, Li Liao, and Xueqiang Dong. 2025. "Study on Combination Mechanism of Pretreatment Layer in Pre-Coated Metal Sheets" Metals 15, no. 11: 1245. https://doi.org/10.3390/met15111245
APA StyleLiu, C., Ye, J., Liao, L., & Dong, X. (2025). Study on Combination Mechanism of Pretreatment Layer in Pre-Coated Metal Sheets. Metals, 15(11), 1245. https://doi.org/10.3390/met15111245






