Optimizing the Leaching Parameters of Asbestos Tailings for Maximizing the Recovery of Critical Metals
Abstract
1. Introduction
- The nature of the minerals to be dissolved;
- The mineralogical site to which the value (or non-desired) metals are associated with, and their readiness to be leached or not;
- Their size, and the grain size distribution of the powder;
- The proportion of solid to acid (solid percentage in % by mass, or L/S ratio in g/mL);
- Acid concentration;
- Leachate washing intensity;
- Physico-chemical conditions such as temperature, pressure, oxidation–reduction state, acidity, saturation index regarding secondary phases;
- Contact conditions between solid and acid: agitation mode, agitation duration.
2. Materials and Methods
2.1. Collection and Preparation of Homogeneous Head-Samples
2.1.1. Preparation of Homogeneous Head-Samples
- Wet method preparation for the first series (W-series);
- Dry method preparation for the second series (D-series);
2.1.2. Preparation of Fibers, Magnetic, Non-Magnetic Fractions and Hand-Picked Grains for the Mineralogical Study
2.2. Methods of Characterization of Samples
2.2.1. Solid Samples
- Particle sizes distribution
- Moisture
- Mineralogy
- Geochemistry
- ○
- Wet chemistry quantification of selected elements and CSM
- ○
- Whole-rock geochemistry by wavelength-dispersive X-ray fluorescence (XRF)
- ○
- Validation of head-subsamples homogeneity
2.2.2. Aqueous Samples
2.3. Leaching Methods
2.3.1. Experimental Protocols
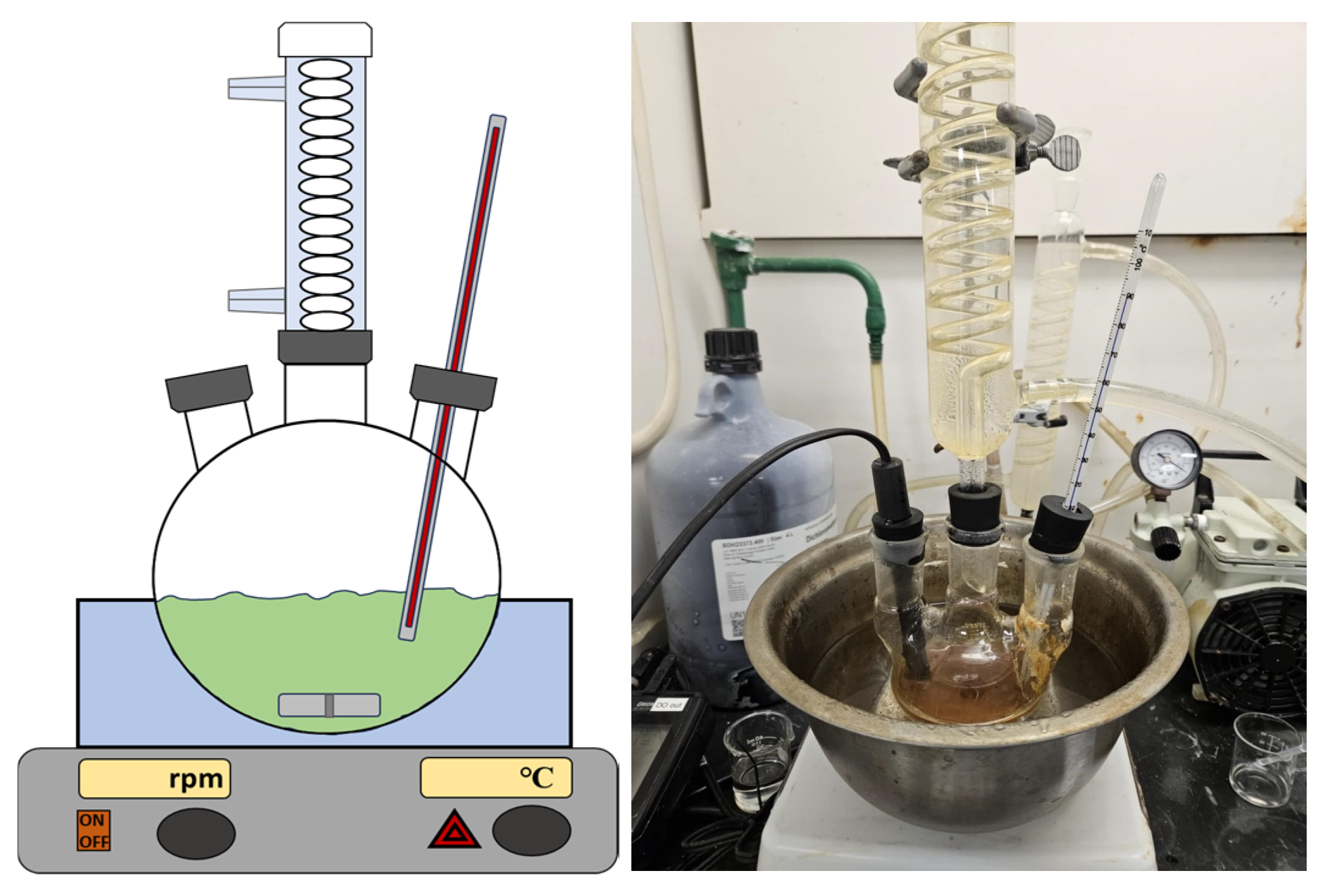
2.3.2. Set-Ups
2.3.3. Reagents
2.3.4. Operating Variables and Conditions
2.4. Design of Experiments (DOE): Modeling of Some Asbestos Tails Leaching Experiments to Optimise Leaching Conditions
| Parameters | Unit | Low Level | High Level |
|---|---|---|---|
| HCl concentration | mol/L (%) | 2 (6.1) | 12 (37) |
| Solid to liquid ratio | % | 10 | 40 |
| Temperature | °C | 20 | 90 |
3. Results and Interpretations
3.1. Characterization of Samples
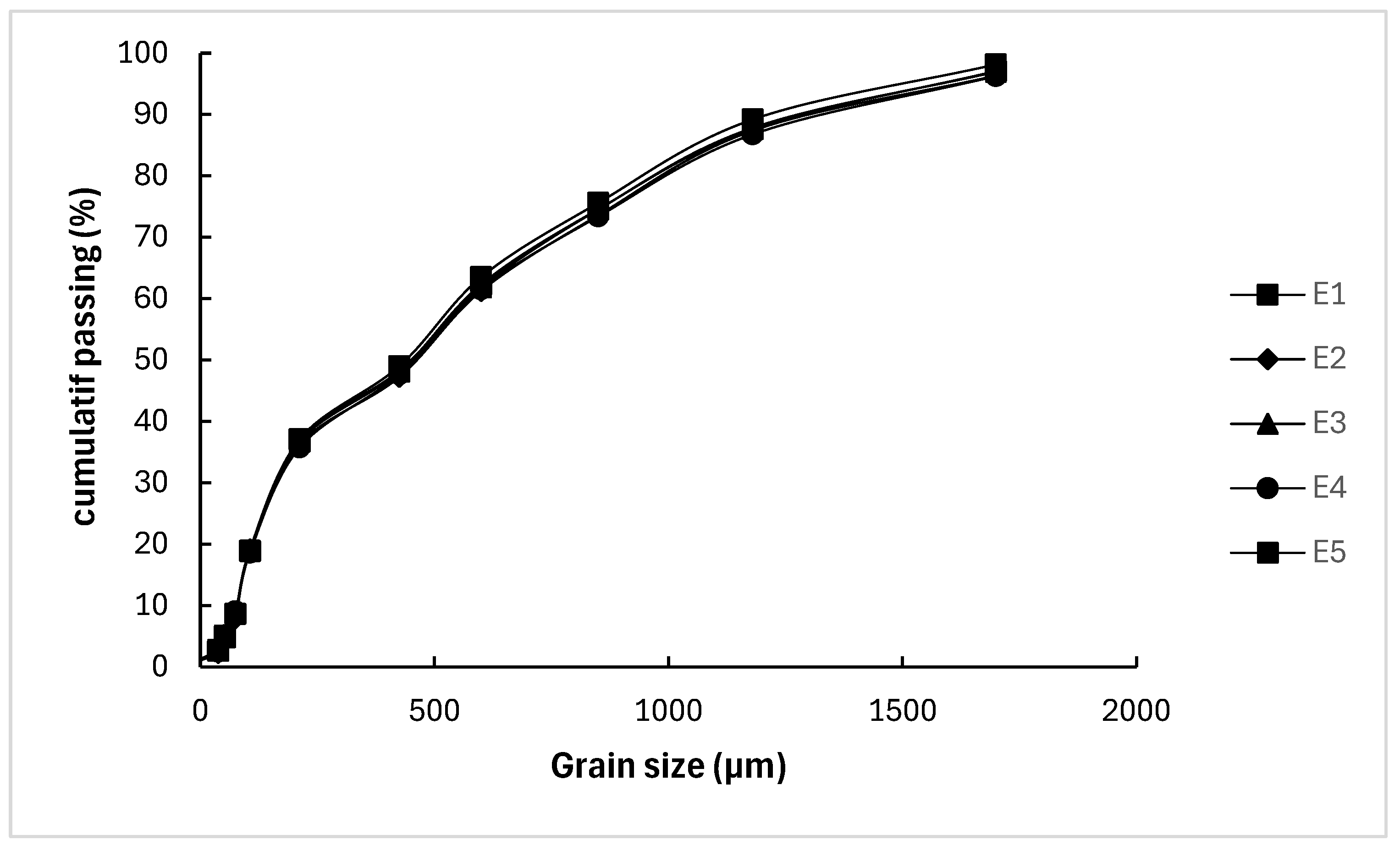
3.1.1. Particle Sizes Distribution (PSD)
3.1.2. Mineralogy
- Inventory of main minerals by X-ray diffraction (XRD) in the head-sample
- Variable Fe2+/Fe3+ ratios in minerals:
3.1.3. Geochemistry of the Head Sample
- Major elements geochemistry by energy-dispersive X-ray fluorescence (XRF)
- Geochemistry of metals followed during leaching by wet preparation and inductively coupled plasma mass spectrometry (ICP-MS)
3.1.4. Check of the Homogeneous Division Quality
3.2. Leaching Experiments and DOE Treatment of Data
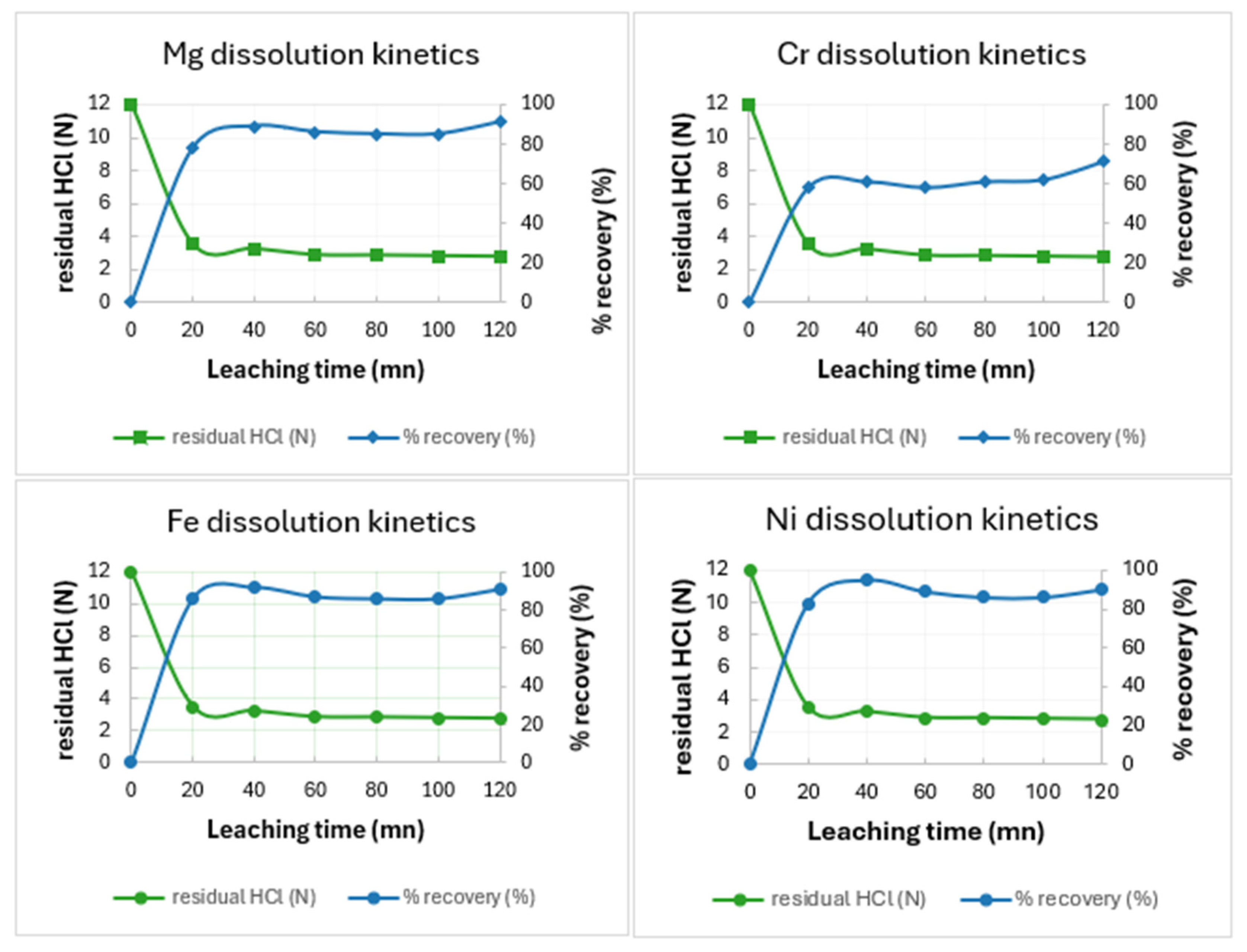
3.2.1. Metals Leached from the W-Series Feeds
3.2.2. Statistical Analysis of Leaching
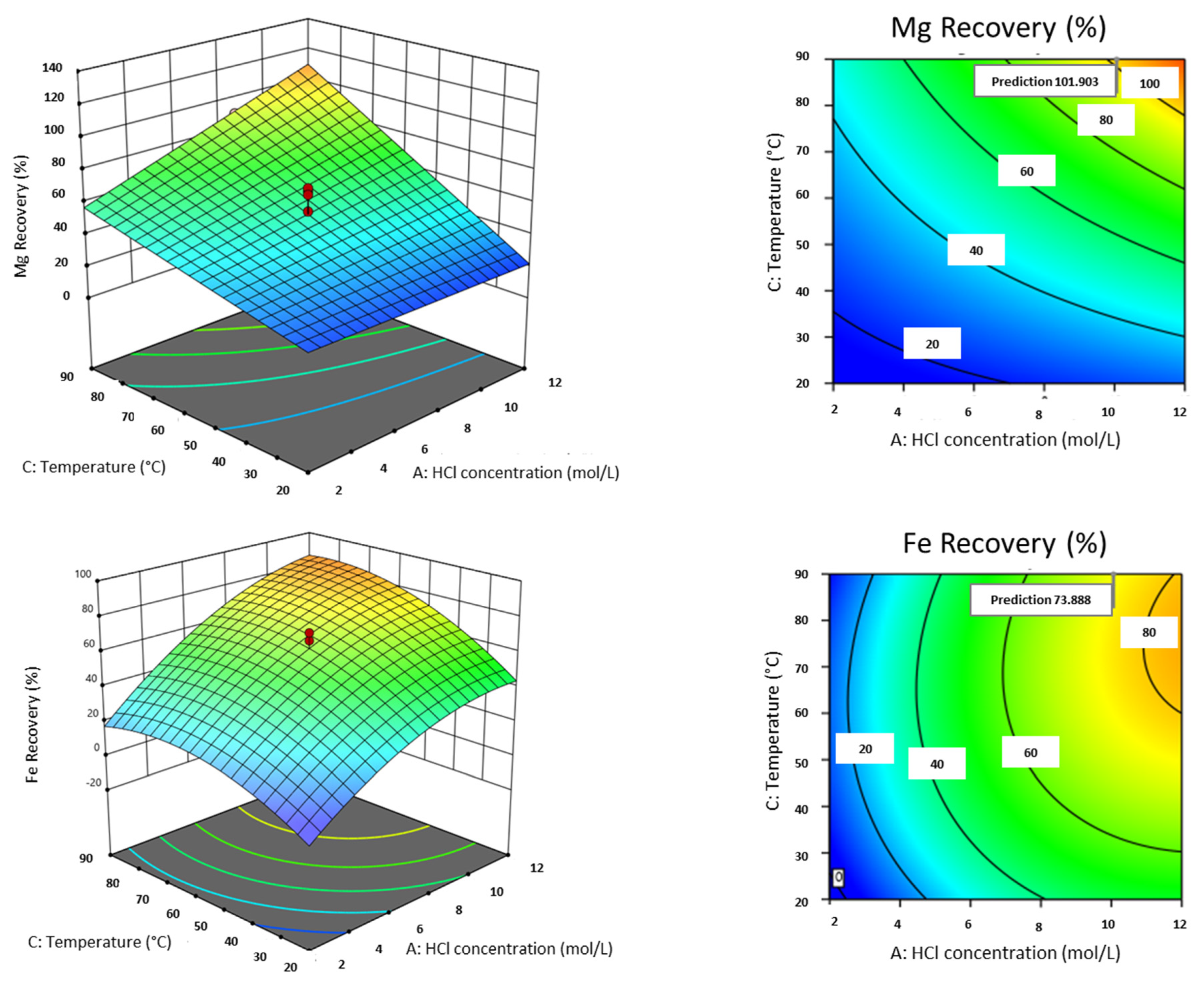
3.2.3. Effect of Individual Factors on Metal Recovery
3.2.4. Analysis of Interactive Factors on Metal Recovery
3.2.5. Behavior of CSM (Cr, Ni, Co, Mn) and Al
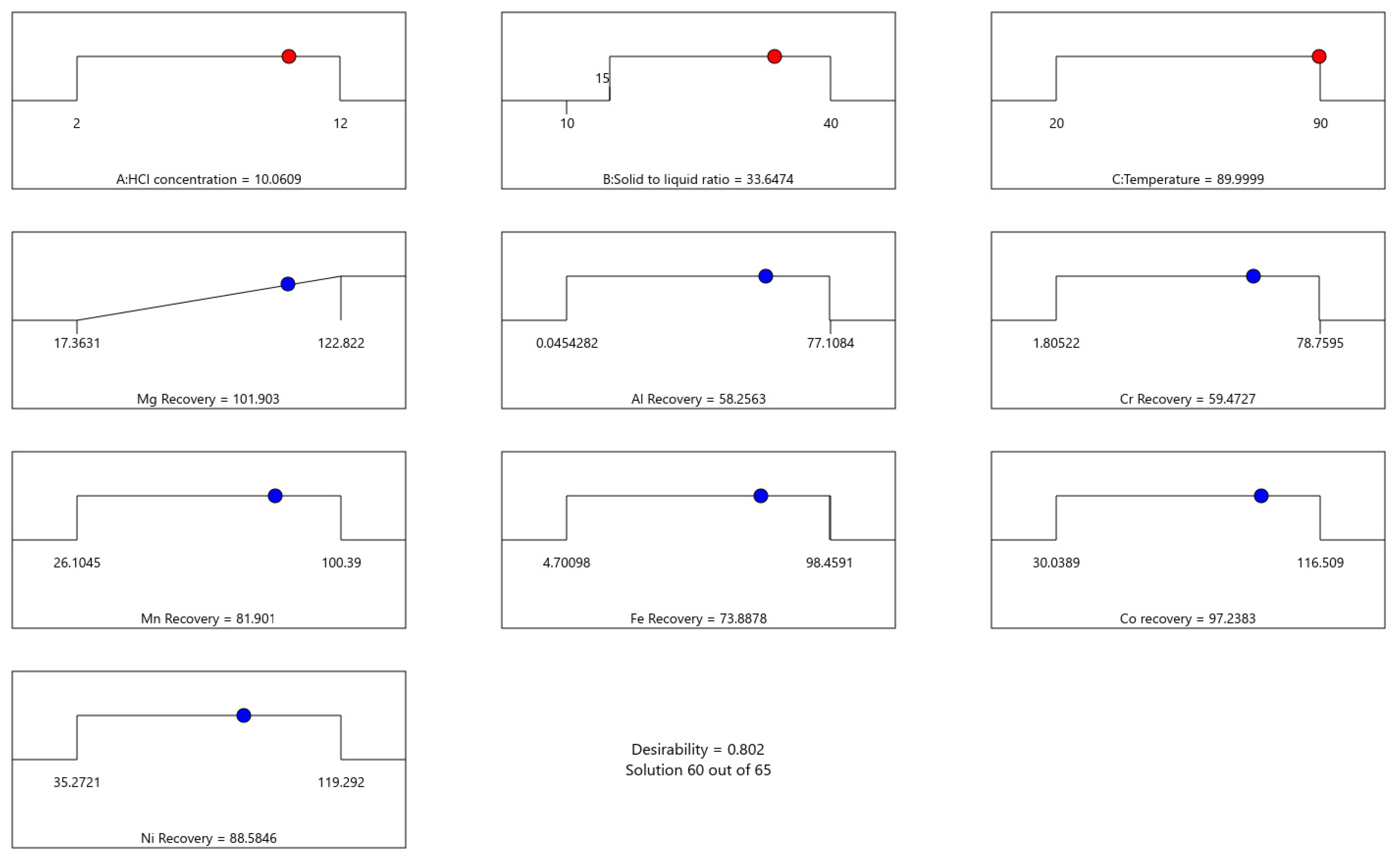
3.2.6. Parameter Optimization
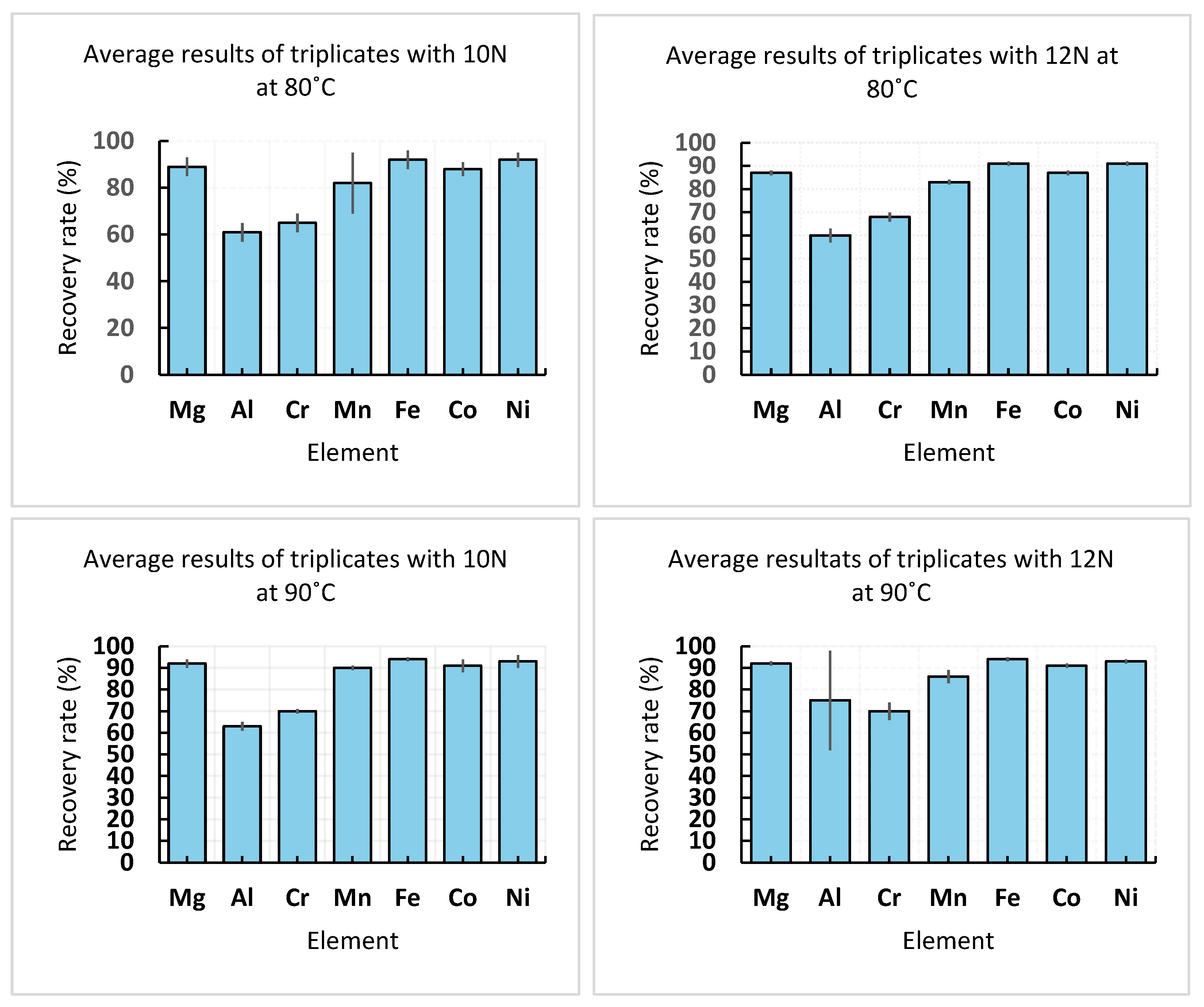
3.3. Experimental Validation and Sensitivity Analysis Around Optimal Experimental Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSM | Critical and strategic metals |
| Mg | Magnesium element |
| Mg0 | Magnesium metal |
| MgO | Magnesium oxide |
| MgCl2 | Magnesium chloride |
| Ni | Nickel element |
| Cr | Chromium element |
| Si | Silicon element |
| SiO2 | Silica |
| DOE | Design of experiments |
| RSM | Response surface methodology |
| CCD | Central composite design |
| XRD | X-Ray Diffraction |
| EPMA | Electron probe microanalysis |
| NIR-SWIR | Near InfraRed/shortwave InfraRed |
| PSD | Particle sizes distribution |
| ACS | American Chemical Society |
| σ | Population standard deviation |
| n | Number of statistical individuals |
| D80 | D stands for diameter of the screen mesh, and the subscript 80 indicates the percent of material which is lower or equal to the indicated diameter |
| CTRI | Technology Center for Industrial Residues |
| IGS | Impact Global Solutions |
Appendix A. Head-Sample Characterization Section
Appendix A.1. Quantitative Inventory of Minerals by XRD Analysis
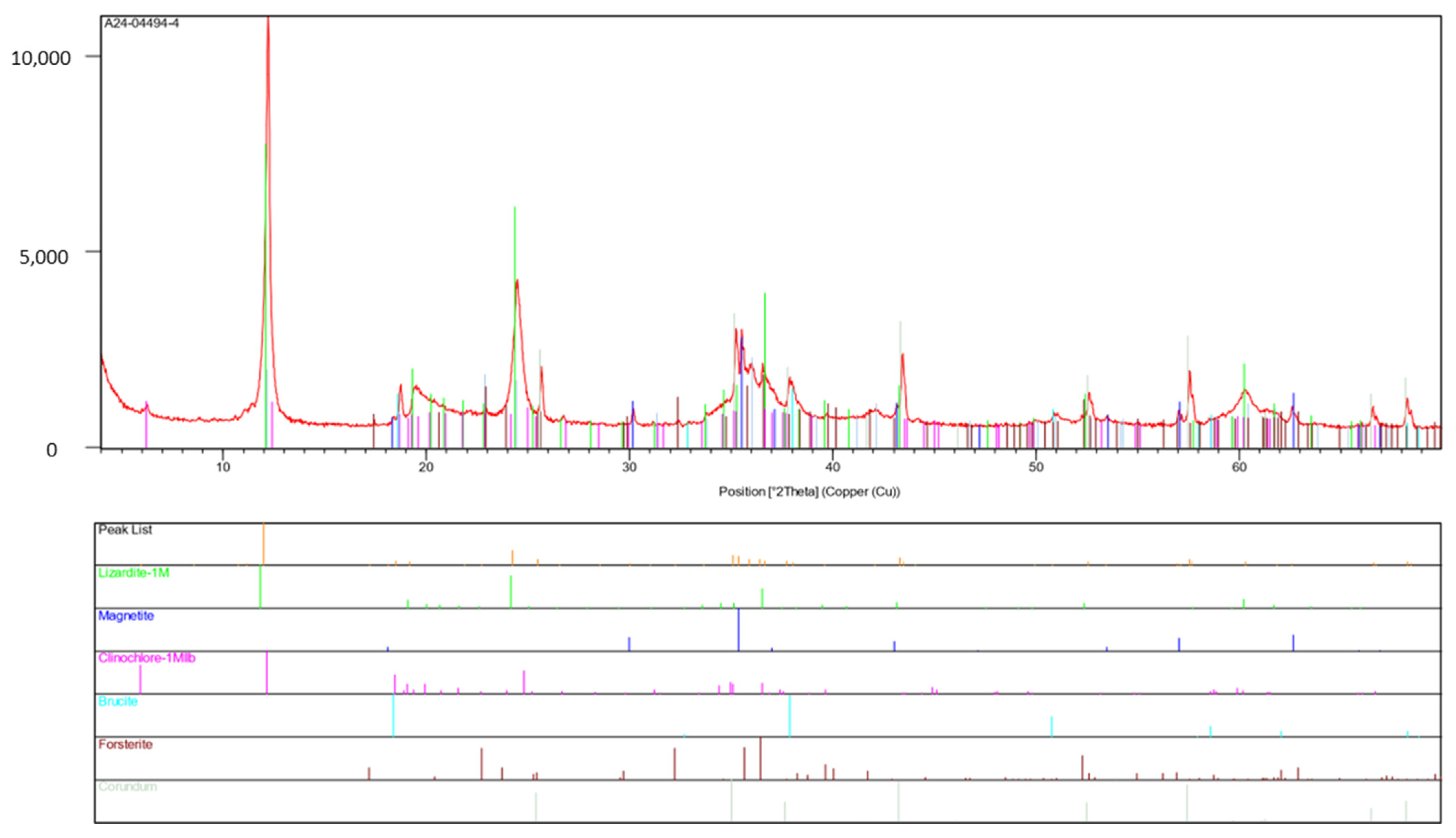
| Identified Minerals by XRD | Recovery of Elements (% by Weight) |
|---|---|
| Serpentine | 44.7 |
| Chlorite | 2.0 |
| Forsterite (Mg-olivine) | 2.0 |
| Brucite (Mg (OH)2) | 1.6 |
| Magnetite (Fe3O4, and other spinels) | 1.1 |
| Quartz (SiO2) | 0.2 |
| Amorphous | 48.4 |
Appendix A.2. Experimental Mineralogical Data Collected During the Present Study
Appendix A.2.1. Introduction to Mössbauer Spectroscopy (MS) and Literature Review of Known Responses of Serpentine Minerals
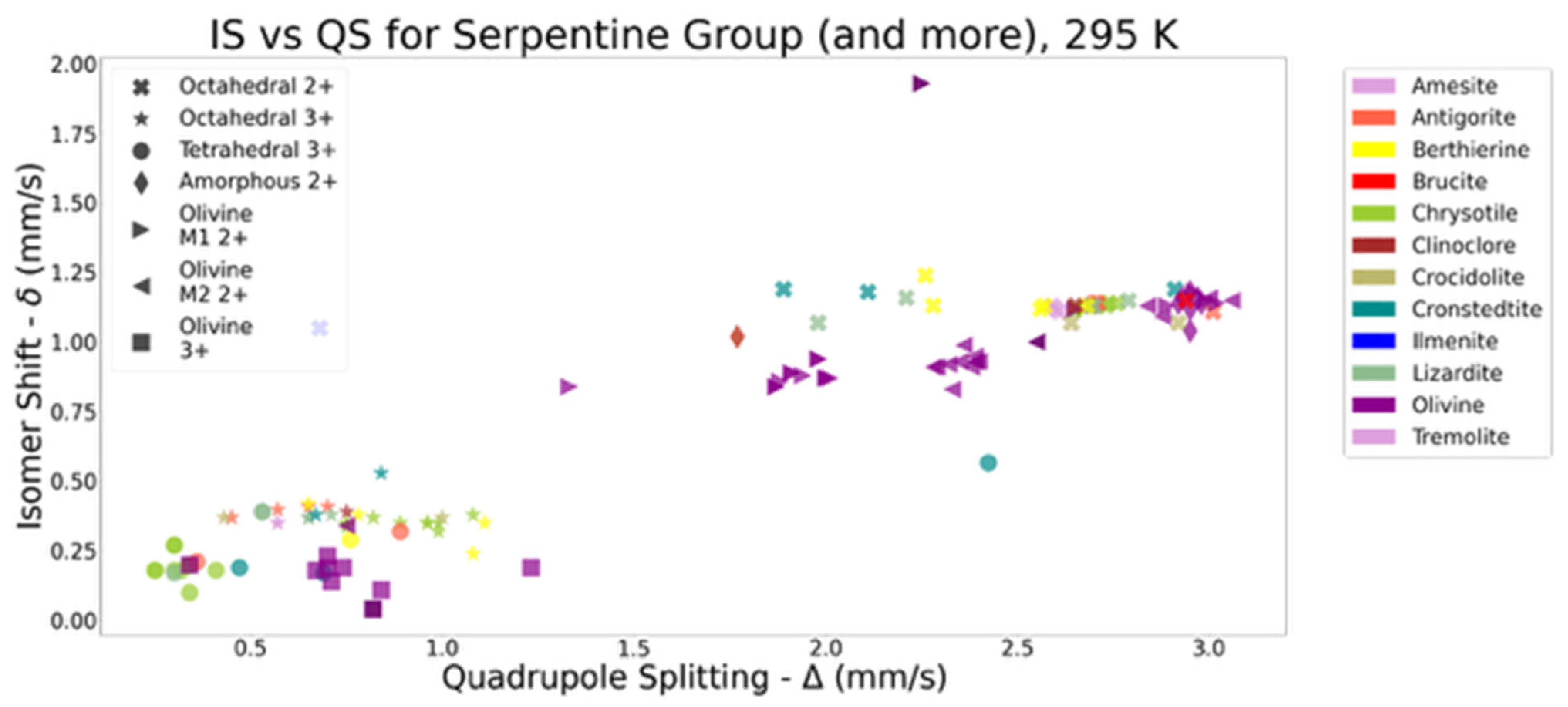
| Mineral | Crystal Site | Fe Valency | δ (mm/s) | Δ (mm/s) | Bhf (T) | Reference |
|---|---|---|---|---|---|---|
| Chrysotile | O | Fe2+ | 1.13 | 2.75 | [82] | |
| O | Fe3+ | 0.31 | 0.86 | |||
| T | Fe3+ | 0.18 | 0.33 | |||
| Lizardite | O | Fe2+ | 1.14 | 2.70 | [83] | |
| O | Fe3+ | 0.40 | 0.70 | |||
| T | Fe3+ | 0.24 | 0.39 | |||
| Antigorite | O | Fe2+ | 1.13 | 2.69 | [81,82] | |
| O | Fe3+ | 0.41 | 0.70 | |||
| T | Fe3+ | 0.21 | 0.36 | |||
| Magnetite | 0.28 | 49.0 | [83] | |||
| 0.66 | 45.9 |
Appendix A.2.2. Mössbauer Spectra and Complementary Data on a Selection of Hand-Picked Grains from Separated Fractions (“Chrysotile-Free”, Mag. And Non-Mag. Fractions from the Head-Sample
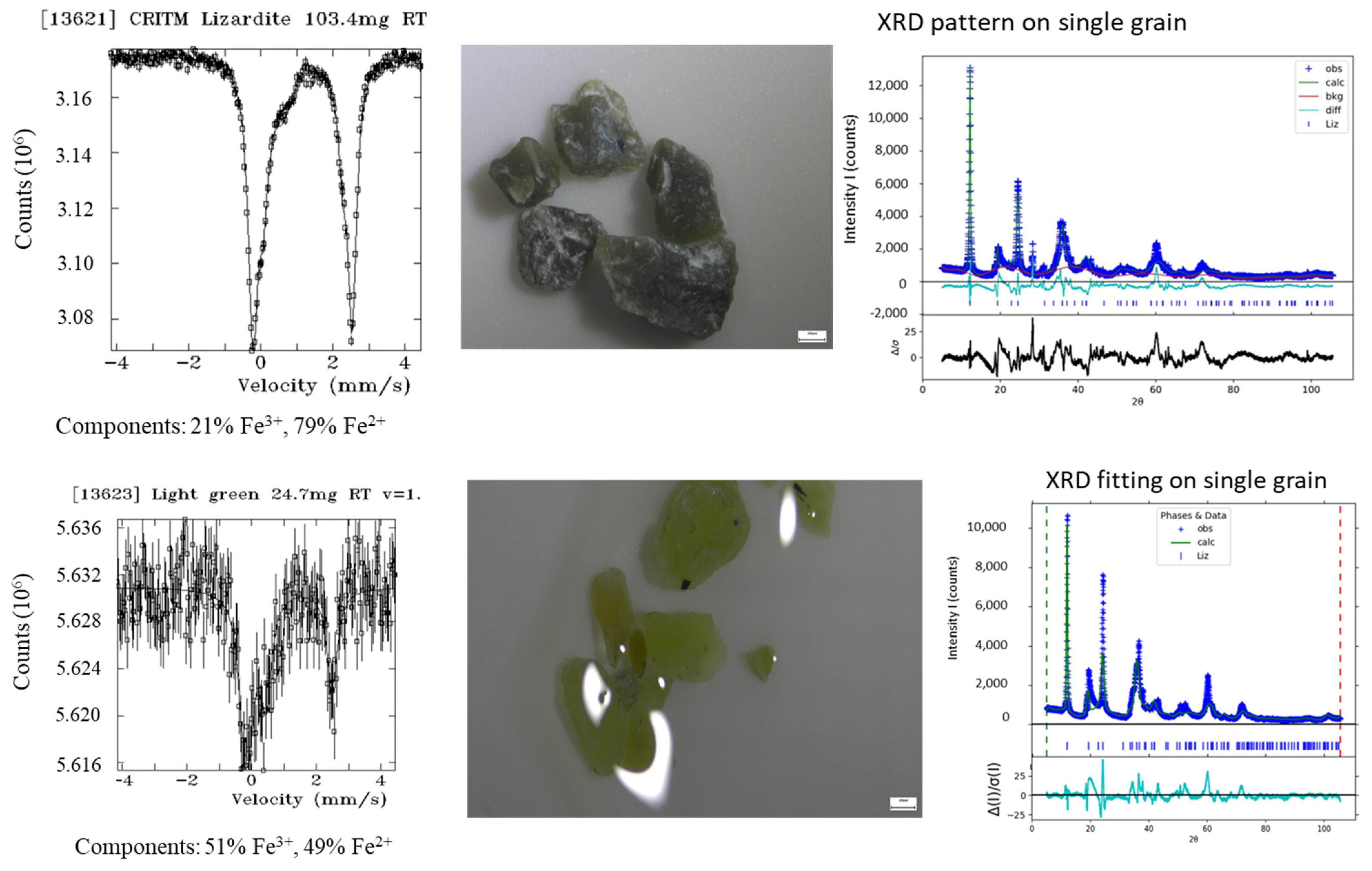

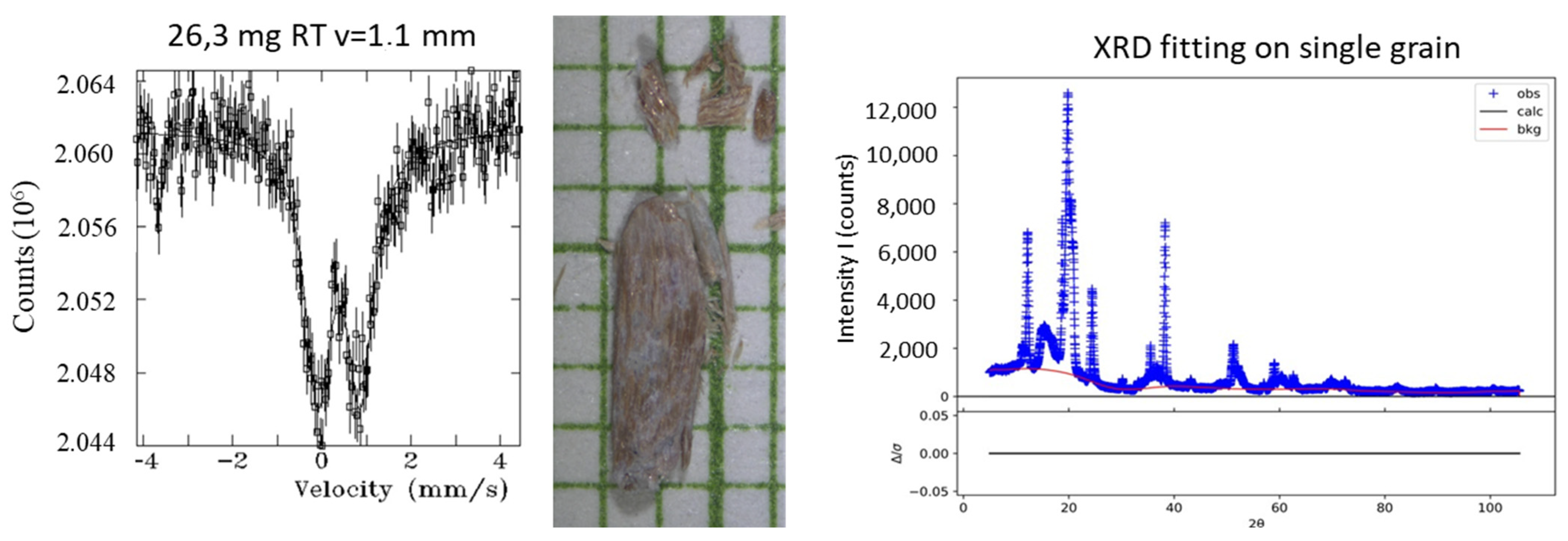

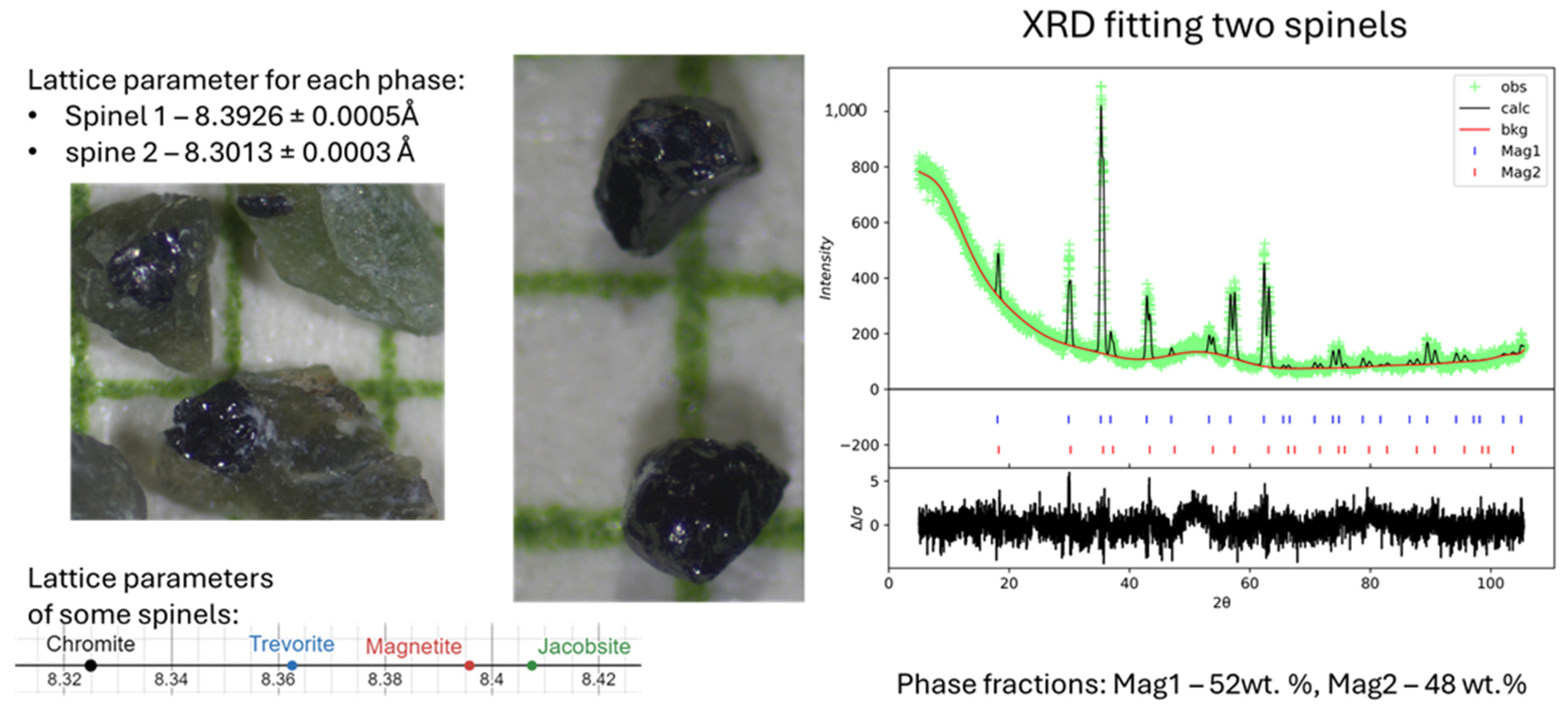
| Instrument | Cameca Sx100 | |||||||
|---|---|---|---|---|---|---|---|---|
| Accelerating Voltage | 15 kV | |||||||
| Beam Currents | 20 nA | |||||||
| Beam Size | 10 um | |||||||
| Mg | Cr | Fe | Ni | Si | Co | |||
| Counting Time (seconds) | 20 | 20 | 20 | 20 | 20 | 20 | ||
| Analytical Crystals | TAP | LPET | LLiF | LLiF | LTAP | LLiF | ||
| Standards | Olivine | Chromite | Hematite | NiO | Olivine | Metal | ||
| Standard Sources | CM Taylor | CM Taylor | CM Taylor | Cameca | CM Taylor | Cameca | ||
| Oxide % | ||||||||
| Name | Point# | MgO | Cr2O3 | FeO | NiO | SiO2 | CoO | Total |
| Pale Green-G1-1 | 13 | 40.50 | 0.003 | 1.601 | 0.035 | 42.39 | 0.018 | 84.55 |
| Pale Green-G2-2 | 14 | 40.39 | 0.055 | 2.010 | 0.034 | 41.63 | 0.000 | 84.12 |
| Pale Green-G3-3 | 15 | 38.39 | 0.000 | 3.072 | 0.051 | 40.19 | 0.000 | 81.70 |
| Antigorite-G1-1 | 16 | 37.99 | 0.000 | 3.579 | 0.025 | 43.56 | 0.009 | 85.17 |
| Antigorite-G2-2 | 17 | 38.37 | 0.000 | 3.499 | 0.000 | 43.26 | 0.030 | 85.16 |
| Antigorite-G3-3 | 18 | 37.79 | 0.002 | 4.045 | 0.402 | 42.83 | 0.000 | 85.06 |
| (Fe-OX) Antigorite-Mag-1 | 19 | 0.077 | 0.000 | 91.54 | 0.012 | 0.073 | 0.022 | 91.72 |
| (Fe-OX) Antigorite-Mag-2 | 20 | 0.442 | 0.000 | 90.48 | 0.010 | 0.429 | 0.063 | 91.42 |
| Lizardite-G1-1 | 21 | 39.97 | 0.003 | 2.054 | 0.137 | 42.16 | 0.032 | 84.36 |
| Lizardite-G2-2 | 22 | 39.85 | 0.049 | 2.140 | 0.549 | 42.74 | 0.050 | 85.38 |
| Lizardite-G3-3 | 23 | 38.43 | 0.002 | 3.448 | 0.154 | 43.98 | 0.000 | 86.01 |
| YB+LG-G1-1 | 24 | 38.69 | 0.519 | 2.106 | 0.088 | 40.50 | 0.000 | 81.90 |
| YB+LG-G2-2 | 25 | 38.65 | 0.491 | 1.940 | 0.011 | 40.11 | 0.026 | 81.22 |
| YB+LG-G3-3 | 26 | 39.43 | 0.566 | 2.192 | 0.004 | 40.70 | 0.001 | 82.90 |
| YB+LG-G4-4 | 27 | 39.06 | 0.068 | 2.994 | 0.067 | 42.12 | 0.026 | 84.34 |
| YB+LG-G5-5 | 28 | 39.82 | 0.000 | 2.246 | 0.000 | 42.31 | 0.017 | 84.40 |
| YB+LG-G6-6 | 29 | 40.34 | 0.000 | 1.628 | 0.029 | 42.03 | 0.004 | 84.04 |
| Mineral | SiO2 | Al2O3 | MgO | FeO | Fe2O3 | TiO2 | Cr2O3 | MnO | CaO | Na2O | H2O |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chrysotile | 43.63 | 43.63 | 13.00 | ||||||||
| Lizardite | 43.36 | 43.63 | 13.00 | ||||||||
| Antigorite | 39.95 | 30.15 | 17.92 | 11.98 | |||||||
| Tremolite | 59.17 | 24.81 | 13.81 | 2.22 | |||||||
| Actinolite | 54.86 | 2.63 | 16.11 | 10.61 | 0.47 | 0.19 | 0.17 | 12.03 | 0.8 | 2.11 | |
| Chlorite | 43.19 | 26.18 | 8.28 | 1.59 | 20.35 | ||||||
| Forsterite | 42.71 | 57.26 | |||||||||
| Brucite | 69.11 | 30.89 | |||||||||
| Magnetite | 31.03 | 68.97 | |||||||||
| Chromite | 32.10 | 67.90 | |||||||||
| Quartz | 100 |
Appendix A.3. Whole-Rock Geochemistry of the Head-Sample
| Sample Code | Standard JA-1 | Standard JA-1 | 29400A-E1 | 29400A-E2 | 29400A-E3 | 29400A-E4 | |
|---|---|---|---|---|---|---|---|
| Date of Preparation | 8 March 2024 | 8 March 2024 | 8 March 2024 | 8 March 2024 | |||
| Identification | Unit | Obtained | Expected | Head-Sample | Head-Sample | Head-Sample | Head-Sample |
| Na2O | % | 5.055 | 3.84 | 0.51 | 0.49 | 0.30 | 0.34 |
| MgO | % | 1.508 | 1.57 | 42.46 | 42.35 | 42.93 | 43.31 |
| Al2O3 | % | 15.058 | 15.22 | 1.03 | 1.00 | 0.69 | 0.63 |
| SiO2 | % | 61.844 | 63.97 | 41.06 | 42.35 | 42.20 | 41.83 |
| P2O5 | % | 0.088 | 0.165 | ||||
| SO3 | % | 0.019 | 0.13 | 0.17 | 0.08 | 0.07 | |
| K2O | % | 0.692 | 0.77 | 0.13 | 0.13 | 0.06 | 0.05 |
| CaO | % | 5.367 | 5.7 | 0.47 | 0.40 | 0.24 | 0.30 |
| TiO2 | % | 0.791 | 0.85 | ||||
| Cr2O3 | % | 0.48 | 0.40 | 0.30 | 0.28 | ||
| MnO | % | 0.194 | 0.14 | 0.15 | 0.15 | 0.16 | |
| Fe2O3 | % | 7.541 | 7.07 | 11.73 | 10.88 | 10.90 | 10.88 |
| NiO | % | 5.055 | 3.84 | 0.40 | 0.36 | 0.38 | 0.43 |
| CuO | % | ||||||
| ZnO | % | 0.33 | 0.19 | 0.32 | 0.24 | ||
| Total | % | 98.87 | 98.87 | 98.54 | 98.53 |
| Sample # | 29400A | 29400B | 29400C | |
|---|---|---|---|---|
| Date of Preparation | 8 March 2024 | 8 March 2024 | 8 March 2024 | |
| Identification | Unit | Head-Sample Test #9HP-0.5 gH3BO3 | Head-Sample Test #9HP-0.5 gH3BO3 | Head-Sample Test #9HP-0.5 gH3BO3 |
| Na | mg/Kg | 12 | 12 | 12 |
| Mg | mg/Kg | 211,082 | 207,132 | 208,894 |
| Al | mg/Kg | 2400 | 2221 | 3669 |
| K | mg/Kg | 450 | 420 | 1349 |
| Ca | mg/Kg | 7391 | 8979 | 6222 |
| Ti | mg/Kg | 39 | 47 | 50 |
| V | mg/Kg | 13 | 13 | 12 |
| Cr | mg/Kg | 557 | 582 | 640 |
| Mn | mg/Kg | 828 | 829 | 904 |
| Fe | mg/Kg | 63,913 | 59,430 | 65,454 |
| Co | mg/Kg | 100 | 99 | 93 |
| Ni | mg/Kg | 227 | 2162 | 2043 |
| Cu | mg/Kg | 6 | 6 | 37 |
| Zn | mg/Kg | 106 | 112 | 90 |
| As | mg/Kg | 4 | 6 | 1 |
| Se | mg/Kg | <1 | <1 | <1 |
| Mo | mg/Kg | <0.2 | 0.4 | <0.2 |
| Cd | mg/Kg | <0.1 | <0.1 | <0.1 |
| Sn | mg/Kg | <0.1 | <0.1 | <0.1 |
| Sb | mg/Kg | <0.1 | <0.1 | <0.1 |
| Te | mg/Kg | <0.1 | <0.1 | <0.1 |
| Ba | mg/Kg | 10 | 11 | 27 |
| Pb | mg/Kg | <0.1 | 0.3 | 2.7 |
| Bi | mg/Kg | <0.1 | <0.1 | <0.1 |
| U | mg/Kg | <0.1 | 0.1 | <0.1 |
Appendix B. Leaching Section

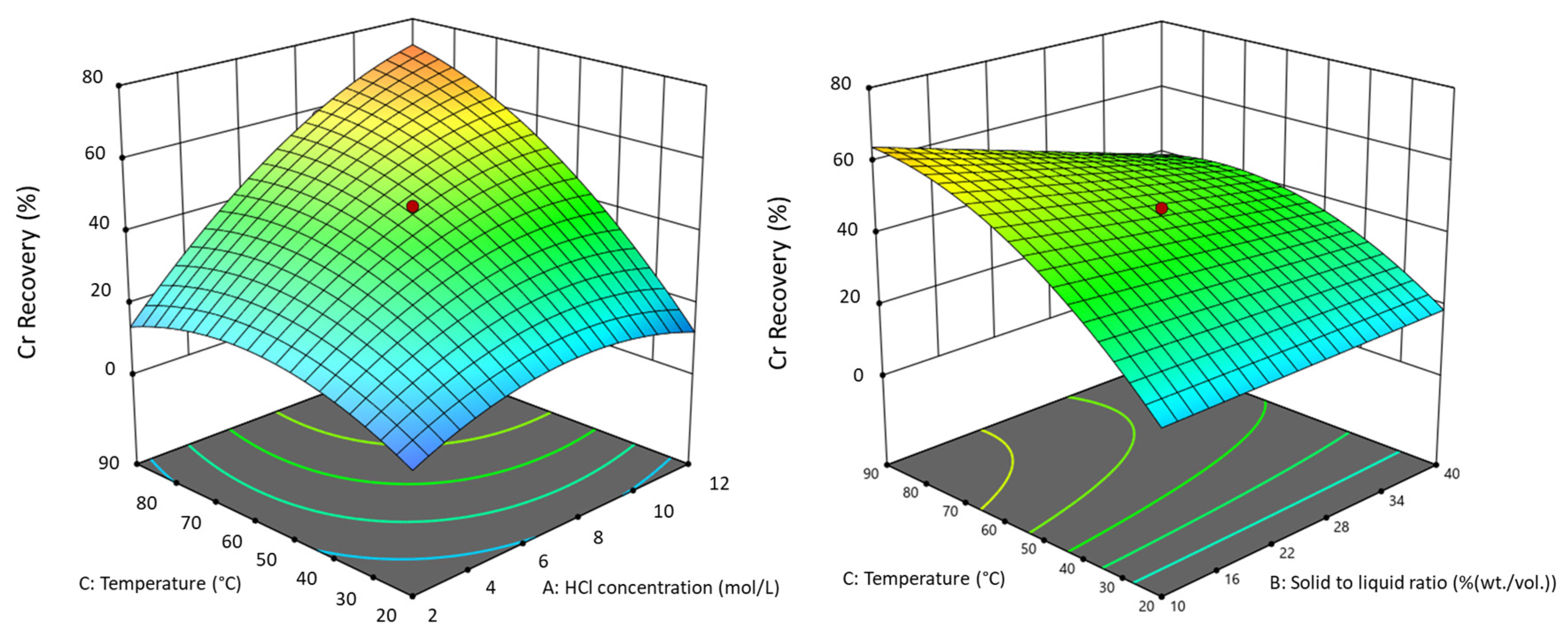
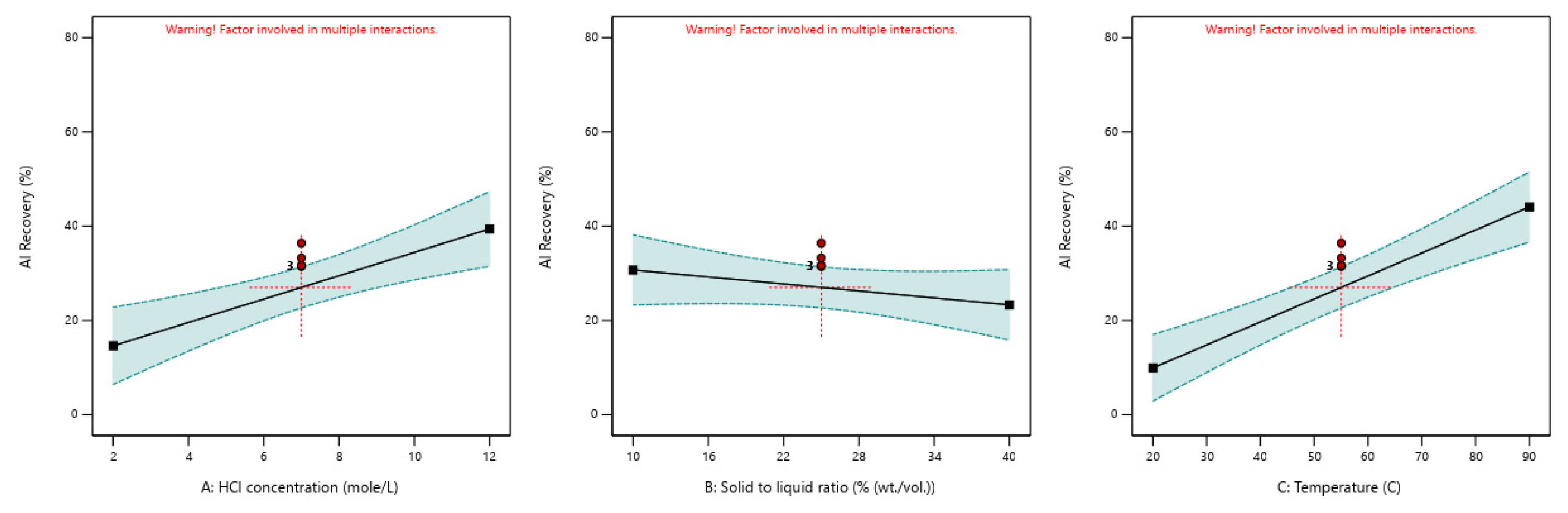
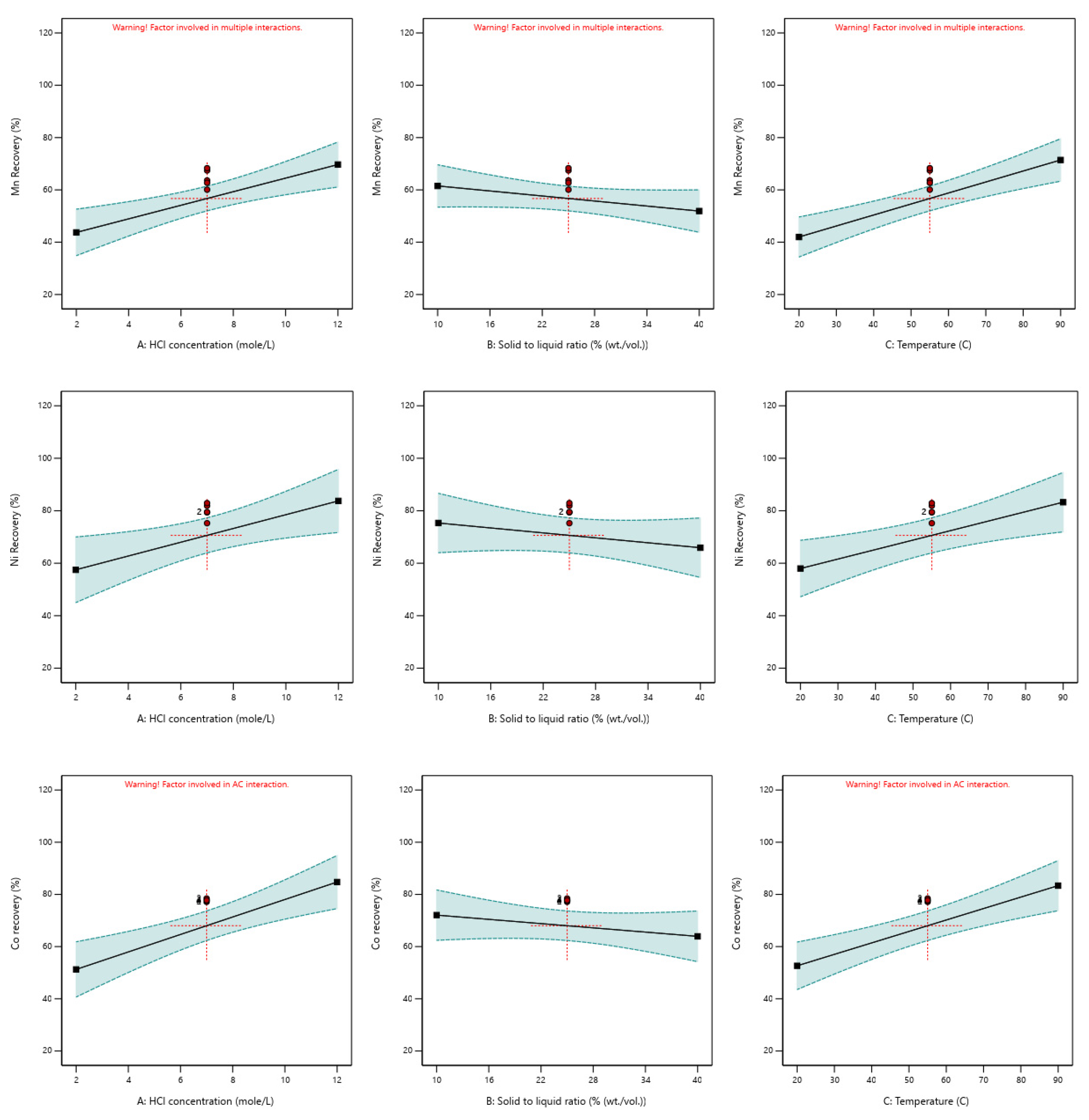
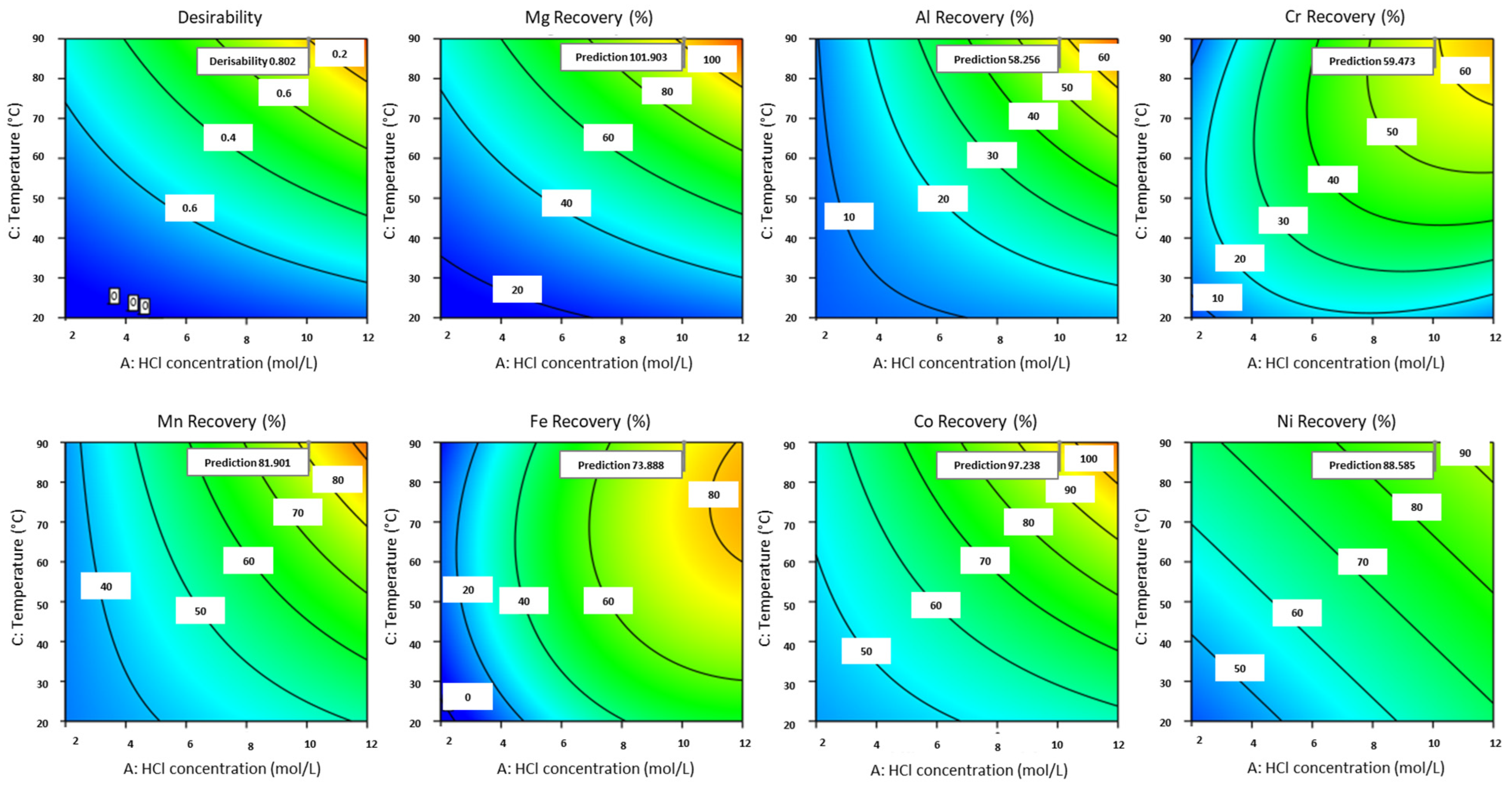

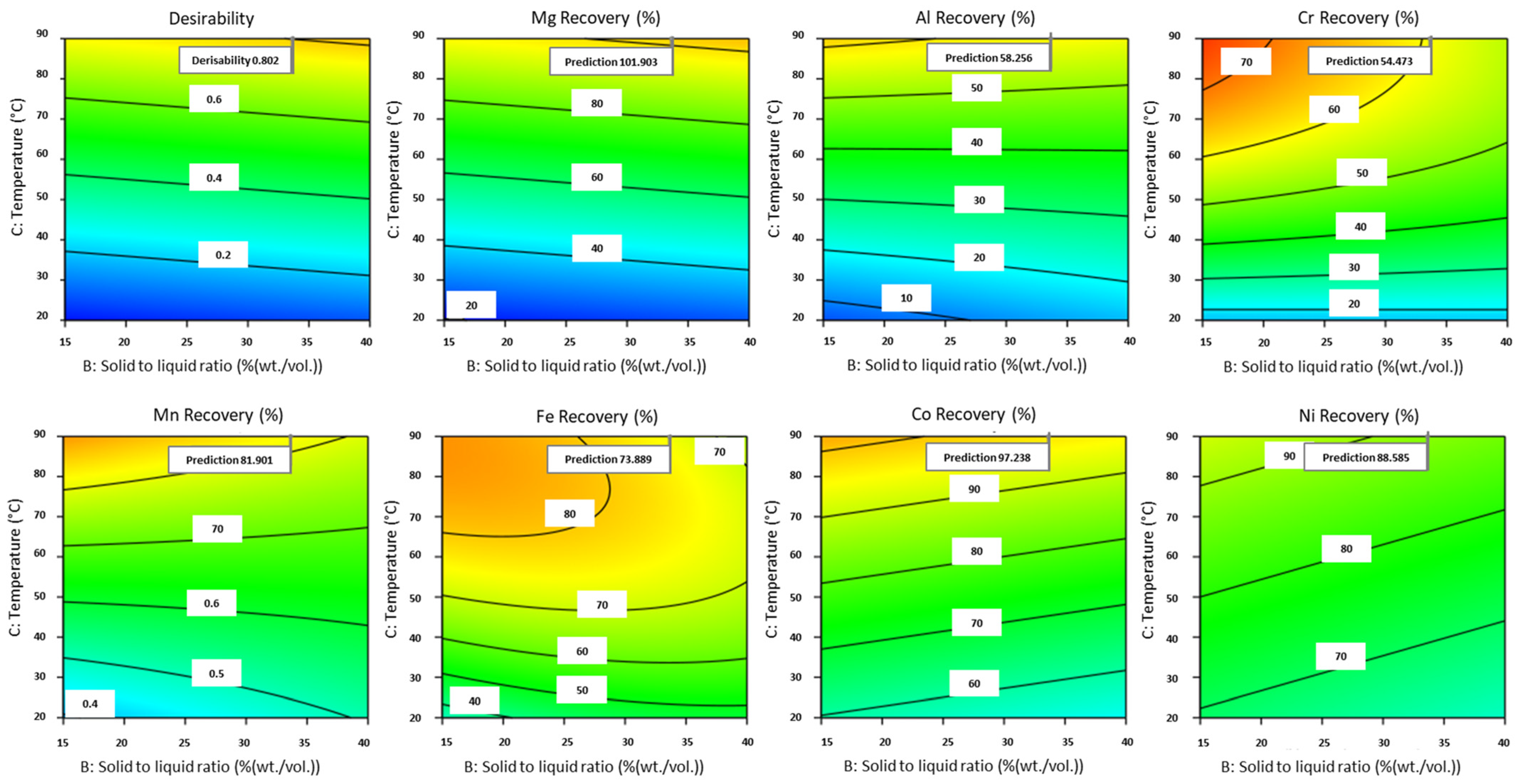
| Element | Significant Factors | Model p-Value | Factor p-Value | Actual Equation | R2 |
|---|---|---|---|---|---|
| Mg | [HCl] | <0.0001 | 0.0031 | 59.5716 − 6.582 × [HCl] | 0.9225 |
| [S/L] | 0.0525 | −1.6775 × [S/L] | |||
| T | <0.0001 | +0.3260 × T | |||
| [HCl] × [S/L] | 0.0228 | +0.0193 × [HCl] × [S/L] | |||
| [HCl] × T | 0.0112 | +0.0781 × [HCl] × T | |||
| Ni | [HCl] | 0.0076 | 0.0037 | 41.7971 + 2.7266 × [HCl] | 0.7560 |
| [S/L] | 0.0366 | −0.3259 × [S/L] | |||
| T | 0.0071 | +0.3751 × T | |||
| Cr | [HCl] | <0.0001 | <0.0001 | −17.3530 + 2.9614 × [HCl] | 0.8657 |
| [S/L] | 0.0495 | −0.2017 × [S/L] | |||
| T | <0.0001 | +0.8469 × T | |||
| [HCl] × T | 0.0036 | +0.0559 × [HCl] × T | |||
| [S/L] × T | 0.0749 | −0.0121 × [S/L] × T | |||
| [HCl]2 | 0.0665 | −0.0089 × [HCl]2 | |||
| T2 | 0.0380 | −0.0060 × T2 | |||
| Co | [HCl] | 0.0002 | <0.0001 | 25.2560 + 3.3626 × [HCl] | 0.8688 |
| [S/L] | 0.0390 | −0.1962 × [S/L] | |||
| T | 0.0002 | +0.4561 × T | |||
| Fe | [HCl] | 0.0001 | <0.0001 | −74.1267 + 10.7060 × [HCl] | 0.8791 |
| [S/L] | 0.2121 | +1.2700 × [S/L] | |||
| T | 0.0005 | +2.1591 × T | |||
| [HCl] × [S/L] | 0.3679 | +0.0909 × [HCl] × [S/L] | |||
| [HCl] × T | 0.4028 | +0.0365 × [HCl] × T | |||
| [S/L] × T | 0.0422 | −0.0197 × [S/L] × T | |||
| [HCl]2 | 0.0286 | −0.5914 × [HCl]2 | |||
| [S/L]2 | 0.4755 | −0.0226 × [S/L]2 | |||
| T2 | 0.0368 | −0.0127 × T2 | |||
| Mn | [HCl] | 0.0001 | 0.0018 | 46.1771 − 0.8993 × [HCl] | 0.7821 |
| [S/L] | 0.1196 | −0.2866 × [S/L] | |||
| T | 0.0001 | +0.0464 × T | |||
| [HCl] × T | 0.0426 | +0.0612[HCl] × T | |||
| Al | [HCl] | <0.0001 | 0.0017 | 29.6489 − 3.6024 × [HCl] | 0.8211 |
| [S/L] | 0.2583 | +0.7849 × [S/L] | |||
| T | <0.0001 | −0.02145 × T | |||
| [HCl] × [S/L] | 0.0862 | +0.0852 × [HCl] × [S/L] | |||
| [HCl] × T | 0.0055 | +0.06314 × [HCl] × T |
References
- Girotto, C.P.; De Campos, S.D.; De Campos, É.A. Chrysotile asbestos treated with phosphoric acid as an adsorbent for ammonia nitrogen. Heliyon 2020, 6, e03397. [Google Scholar] [CrossRef]
- Virta, R.L. Asbestos: Geology, Mineralogy, Mining, and Uses; US Department of the Interior, US Geological Survey: Washington, DC, USA, 2002; p. 149. [Google Scholar]
- Flanagan, D.M. Mineral Commodity Summaries 2025; US Geological Survey: Washington, DC, USA, 2025. Available online: https://pubs.usgs.gov/publication/mcs2025 (accessed on 15 October 2025).
- Spasiano, D.; Pirozzi, F. Treatments of asbestos containing wastes. J. Environ. Manag. 2017, 204, 82–91. [Google Scholar] [CrossRef]
- MacLaren, J.F. National Inventory of Sources and Emissions of Asbestos (1970). Air Pollution Control Directorate Environmental Protection Service, Report APCD, 73-4, Ottawa. 1973. Available online: https://publications.gc.ca/site/fra/9.868627/publication.html (accessed on 15 October 2025).
- Bernstein, D.M.; Toth, B.; Rogers, R.A.; Sepulveda, R.; Kunzendorf, P.; Phillips, J.I. Evaluation of the dose-response and fate in the lung and pleura of chrysotile-containing brake dust compared to chrysotile or crocidolite asbestos in a 28-day quantitative inhalation toxicology study. Toxicol. Appl. Pharmacol. 2018, 351, 74–92. [Google Scholar] [CrossRef]
- Baigenzhenov, O.S.; Chepushtanova, T.A.; Altmyshbayeva, A.Z.; Temirgali, I.A.; Maldybayev, G.; Sharipov, R.H. Investigation of thermodynamic and kinetic regularities of asbestos waste leaching processes. Results Eng. 2024, 21, 102000. [Google Scholar] [CrossRef]
- CNESST. Asbestos. Commission des Normes, de L’équité, de la Santé et de la Sécurité du Travail. Available online: https://www.cnesst.gouv.qc.ca/en/prevention-securite/identifier-corriger-risques/liste-informations-prevention/asbestos (accessed on 1 January 2025).
- Beaudoin, G.; Hébert, R.; Constantin, M.; Duchesne, J.; Cecchi, E.; Huot, F.; Vigneau, S.; Fiola, R. Spontaneous carbonation of Serpentine in milling and mining waste, southern Québec and Italy. In Proceedings of the ACEME08, 2nd International Conference on Accelerated Carbonation for Environmental and Materials Engineering, Rome, Italy, 1–3 October 2008. [Google Scholar]
- Lévesque, A.; Bélanger, N.; Poder, T.G.; Filotas, É.; Dupras, J. From white to green gold: Digging into public expectations and preferences for ecological restoration of asbestos mines in southeastern Quebec, Canada. Extr. Ind. Soc. 2020, 7, 1411–1423. [Google Scholar] [CrossRef]
- Grenier, F. L’industrie canadienne de l’amiante. Cah. Géographie Qué 1959, 3, 329. [Google Scholar] [CrossRef]
- Kuyek, J. Asbestos Mining in Canada. In Proceedings of the International Ban Asbestos Conference, Ottawa, ON, Canada, 13 September 2003. [Google Scholar]
- State Land Hazards and Contaminated Sites. Available online: https://www.wa.gov.au/government/document-collections/state-land-hazards-and-contaminated-sites (accessed on 15 June 2025).
- Riordon, P.H. Geology of The Asbestos Deposits of Southern Québec; Report DP-186; Ministère des Ressources Naturelles. 1973. Available online: https://gq.mines.gouv.qc.ca/documents/examine/dp186/DP186.pdf (accessed on 15 June 2025).
- Schroetter, J.; Tremblay, A.; Bédard, J.H. Structural evolution of the Thetford Mines Ophiolite Complex, Canada: Implications for the southern Québec ophiolitic belt. Tectonics 2005, 24, TC1001, Erratum in Tectonics 2005, 24 https://doi.org/10.1029/2005TC001799. [Google Scholar] [CrossRef]
- Dana, J.D. A System of Mineralogy: Including an Extended Treatise on Crystallography: With an Appendix, Containing the Application of Mathematics to Crystallographic Investigation, and a Mineralogical Bibliography; Durrie & Peck and Herrick & Noyes, Ed.; Original from New York Public Library: New York, NY, USA, 1837; p. 571. Available online: https://catalog.hathitrust.org/Record/008631200 (accessed on 15 June 2025).
- Serpentine: The Mineral Serpentine Information and Pictures. Available online: https://www.minerals.net/mineral/serpentine.aspx (accessed on 15 June 2025).
- Lizardite Mineral Data. Available online: https://webmineral.com/data/Lizardite.shtml (accessed on 15 June 2025).
- Baigenzhenov, O.; Khabiyev, A.; Mishra, B.; Aimbetova, I.; Yulusov, S.; Temirgali, I. Asbestos Waste Treatment—An Effective Process to Selectively Recover Gold and Other Nonferrous Metals. Recycling 2022, 7, 85. [Google Scholar] [CrossRef]
- Chrysotile Mineral Data. Available online: https://webmineral.com/data/Chrysotile.shtml (accessed on 15 June 2025).
- Riebeckite Mineral Data. Available online: https://webmineral.com/data/Riebeckite.shtml (accessed on 15 June 2025).
- Grunerite Mineral Data. Available online: https://webmineral.com/data/Grunerite.shtml (accessed on 15 June 2025).
- Actinolite Mineral Data. Available online: https://webmineral.com/data/Actinolite.shtml (accessed on 15 June 2025).
- Anthophyllite Mineral Data. Available online: https://webmineral.com/data/Anthophyllite.shtml (accessed on 15 June 2025).
- Tremolite Mineral Data. Available online: https://webmineral.com/data/Tremolite.shtml (accessed on 15 June 2025).
- Maletaškić, J.; Stanković, N.; Daneu, N.; Babić, B.; Stoiljković, M.; Yoshida, K. Acid leaching of natural chrysotile asbestos to mesoporous silica fibers. Phys. Chem. Min. 2018, 45, 343–351. [Google Scholar] [CrossRef]
- Preiner, M.; Xavier, J.C.; Sousa, F.L.; Zimorski, V.; Neubeck, A.; Lang, S.Q. Serpentinization: Connecting Geochemistry, Ancient Metabolism and Industrial Hydrogenation. Life 2018, 8, 41. [Google Scholar] [CrossRef]
- Bédard, J.H.; Schroetter, J.-M.; Pagé, P.; Tremblay, A.; Bécu, V. Overview of the geology and Cr-PGE potential of the Southern Québec Ophiolite Belt. Geol. Assoc. Can. 2007, 5, 433–448. [Google Scholar]
- Li, X.; Chen, Y.; Li, X.; Wang, M.; Xie, W.; Ding, D. Asbestos-Environment Pollution Characteristics and Health-Risk Assessment in Typical Asbestos-Mining Area. Toxics 2023, 11, 494. [Google Scholar] [CrossRef]
- Jacques, O.; Pienitz, R. Asbestos mining waste impacts on the sedimentological evolution of the Bécancour chain of lakes, southern Quebec (Canada). Sci. Total Environ. 2022, 807, 151079. [Google Scholar] [CrossRef]
- Ministère de l’Environnement et de la Lutte Contre les Changements Climatiques (MELCC); Ministère de l’Énergie et des Ressources Naturelles (MERN). L’état des Lieux et la Gestion de l’Amiante et des Résidus Miniers Amiantés. Bureau D’audiences Publiques Sur L’environnement. 2020. Available online: https://www.bape.gouv.qc.ca/fr/dossiers/etat-des-lieux-et-gestion-de-l-amiante-et-residus-miniers-amiantes/ (accessed on 15 October 2025).
- Paolini, V.; Tomassetti, L.; Segreto, M.; Borin, D.; Liotta, F.; Torre, M. Asbestos treatment technologies. J. Mater. Cycles Waste Manag. 2019, 21, 205–226. [Google Scholar] [CrossRef]
- Talbi, G. Caractérisation, Destruction et Recyclage des Déchets Amiantés. Ph.D. Dissertation, Université Montpellier, Montpellier, France, 2018. [Google Scholar]
- Patents Assigned to Les Sables Olimag, Inc.—Justia Patents Search. Available online: https://patents.justia.com/assignee/les-sables-olimag-inc (accessed on 16 June 2025).
- Nkuna, R.; Ijoma, G.N.; Matambo, T.S.; Chimwani, N. Accessing Metals from Low-Grade Ores and the Environmental Impact Considerations: A Review of the Perspectives of Conventional versus Bioleaching Strategies. Minerals 2022, 12, 506. [Google Scholar] [CrossRef]
- Pasquier, L.C.; Mercier, G.; Blais, J.F.; Cecchi, E.; Kentish, S. Technical & economic evaluation of a mineral carbonation process using southern Québec mining wastes for CO2 sequestration of raw flue gas with by-product recovery. Int. J. Greenh. Gas. Control 2016, 50, 147–157. [Google Scholar]
- Wang, F.; Dreisinger, D.; Xiao, Y. Accelerated CO2 mineralization and utilization for selective battery metals recovery from olivine and laterites. J. Clean. Prod. 2023, 393, 136345. [Google Scholar] [CrossRef]
- Nowamooz, A.; Dupuis, J.C.; Beaudoin, G.; Molson, J.; Lemieux, J.M.; Horswill, M. Atmospheric Carbon Mineralization in an Industrial-Scale Chrysotile Mining Waste Pile. Environ. Sci. Technol. 2018, 52, 8050–8057. [Google Scholar] [CrossRef]
- Lin, X.; Li, X.; Liu, H.; Boczkaj, G.; Cao, Y.; Wang, C. A review on carbon storage via mineral carbonation: Bibliometric analysis, research advances, challenges, and perspectives. Sep. Purif. Technol. 2024, 338, 126558. [Google Scholar] [CrossRef]
- Pronost, J.; Beaudoin, G.; Tremblay, J.; Larachi, F.; Duchesne, J.; Hébert, R. Carbon Sequestration Kinetic and Storage Capacity of Ultramafic Mining Waste. Environ. Sci. Technol. 2011, 45, 9413–9420. [Google Scholar] [CrossRef]
- Exterra. Available online: https://exterracarbon.com/ (accessed on 15 September 2025).
- Nagamori, M.; Boivin, J.A. Technico-Economic Simulation for the Hcl-Leaching of Hybrid Serpentine and Magnesite Feeds. Can. Met. Q. 2001, 40, 47–60. [Google Scholar] [CrossRef]
- Chouinard, S. Valorisation des Résidus de Serpentine par Extraction du Magnésium au Moyen de Procédés Hydrométallurgiques. Master’s Dissertation, Université du Québec, Institut National de la Recherche Scientifique Centre Eau, Terre et Environnement, Québec, QC, Canada, 2006. [Google Scholar]
- Yoo, K.; Kim, B.S.; Kim, M.S.; Lee, J.C.; Jeong, J. Dissolution of Magnesium from Serpentine Mineral in Sulfuric Acid Solution. Mater. Trans. 2009, 50, 1225–1230. [Google Scholar] [CrossRef]
- Valouma, A.; Verganelaki, A.; Tetoros, I.; Maravelaki-Kalaitzaki, P.; Gidarakos, E. Magnesium oxide production from chrysotile asbestos detoxification with oxalic acid treatment. J. Hazard. Mater. 2017, 336, 93–100. [Google Scholar] [CrossRef]
- Talbi, G.; Cambon, M.; Cambon, O. Virtuous cycle of destruction and total recycling of pure asbestos and asbestos-containing waste. J. Mater. Cycles Waste Manag. 2019, 21, 1167–1176. [Google Scholar] [CrossRef]
- Shayakhmetova, R.A.; Mukhametzhanova, A.A.; Akbayeva, D.N.; Terlikbaeva, A.Z.h.; Osipov, P.A.; Alimzhanova, A.M. Magnesium and silicon recovery from chrysotile asbestos waste of the deposit Zhitikara, Kazakhstan. Sci. Rep. 2024, 14, 31866. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Wu, L.; Tong, L.; Zhu, J. Recovery of Mg from H2SO4 Leaching Solution of Serpentine to Precipitation of High-Purity Mg(OH)2 and 4MgCO3·Mg(OH)2·4H2O. Minerals 2023, 13, 318. [Google Scholar] [CrossRef]
- Cheng, T.C.; Morissette, C.L.; Mines, T. Deportments of Magnesium and Silicon in a Two-part HCl-NaOH Leaching of Asbestos Tailings. In Proceedings of the Tailings & Mine Waste, Denver, CO, USA, 10–13 November 2024; pp. 1891–1901. [Google Scholar]
- Balagh, Z.; Ait-khouia, Y.; Benzaazoua, M.; Taha, Y. Magnesium and calcium extraction from phosphate mine waste rock using phosphoric acid: Thermodynamics, parameter optimization, kinetics, and reaction mechanism. J. Ind. Eng. Chem. 2025, 146, 812–825. [Google Scholar] [CrossRef]
- Taheri, B.; Larachi, F. Mineral-Based Magnesium Extraction Technologies: Current and Future Practices. Processes 2025, 13, 2945. [Google Scholar] [CrossRef]
- Celik, C.; Peacey, J.; Bishop, G.; White, C.; Giasson, E.; Avedesian, M. Magnola—An innovative process for magnesium production, Materials Science; Metallurgy & Metallurgical Engineering. In Proceedings of the 3rd International Magnesium Conference, Manchester, UK, 10–12 April 1996; Available online: https://www.webofscience.com/wos/WOSCC/full-record/A1997BJ62T00003 (accessed on 16 June 2025).
- Telgerafchi, A.E.; Rutherford, M.; Espinosa, G.; McArthur, D.; Masse, N.; Perrin, B. Magnesium production by molten salt electrolysis with liquid tin cathode and multiple effect distillation. Front. Chem. 2023, 11, 1192202. [Google Scholar] [CrossRef]
- Fournier, J.; Gauthier, L. Process to Produce Magnesium Compounds, and Various by-Products Using Sulfuric Acid in a HCl Recovery Loop. U.S. Patent EP3221479A1, 30 October 2018. Available online: https://patents.google.com/patent/EP3221479A1/en?oq=US+Patent+EP3221479A1 (accessed on 16 June 2025).
- Dutrizac, J.E.; Chen, T.T.; White, C.W. Fundamentals of Serpentine Leaching in Hydrochloric Acid Media. In Magnesium Technology, 1st ed.; Wiley: Hoboken, NJ, USA, 2000; pp. 40–51. [Google Scholar]
- Baigenzhenov, O.S.; Kozlov, V.A.; Luganov, V.A.; Mishra, B.; Shayahmetova, R.A.; Aimbetova, I.O. Complex Processing of Wastes Generated in Chrysotile Asbestos Production. Min. Process Extr. Met. Rev. 2015, 36, 242–248. [Google Scholar] [CrossRef]
- Abdel-Aal, E.A.; El-Sayed, D.; Ismail, A.K.; El-Hosieny, F.I. Hydrometallurgical processing of serpentine ore. IOP Conf. Ser. Mater. Sci. Eng. 2018, 427, 012028. [Google Scholar] [CrossRef]
- Morgan, A. Acid leaching studies of chrysotile asbestos from mines in the Coalinga region of California and from Quebec and British Columbia. Ann. Occup. Hyg. 1997, 41, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Faraji, F.; Golmohammadzadeh, R.; Rashchi, F.; Alimardani, N. Fungal bioleaching of WPCBs using Aspergillus niger: Observation, optimization and kinetics. J. Environ. Manag. 2018, 217, 775–787. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, P.; Chao, Q.; Jiang, M. Efficient acid leaching of high-magnesium boron tailings and the low-cost recovery of siliceous residues with good adsorption capacity. Hydrometallurgy 2022, 209, 105827. [Google Scholar] [CrossRef]
- Tian, B.; Cui, Y.; Qin, Z.; Wen, L.; Li, Z.; Chu, H. Indirect bioleaching recovery of valuable metals from electroplating sludge and optimization of various parameters using response surface methodology (RSM). J. Environ. Manag. 2022, 312, 114927. [Google Scholar] [CrossRef] [PubMed]
- Kolbadinejad, S.; Ghaemi, A. Optimization of atmospheric leaching parameters for cadmium and zinc recovery from low-grade waste by response surface methodology (RSM). Sci. Rep. 2024, 14, 1490. [Google Scholar] [CrossRef]
- Kumari, M.; Gupta, S.K. Response surface methodological (RSM) approach for optimizing the removal of trihalomethanes (THMs) and its precursors by surfactant modified magnetic nanoadsorbents (sMNP)—An endeavor to diminish probable cancer risk. Sci. Rep. 2019, 9, 18339. [Google Scholar] [CrossRef]
- Gavras, D.F. Mössbauer Study of Hand-Picked Minerals from Natural Asbestos Tailings—Summer Report; Physics Department, McGill University: Montreal, QC, Canada, 2024; p. 36. [Google Scholar]
- Marzini, L.; Osticioli, I.; Ciofini, D.; Agresti, J.; Bellagamba, S.; Paglietti, F. Identification, mapping, and quantification of asbestos minerals in ACM and NOA using NIR-SWIR hyperspectral scan imaging: Preliminary study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 333, 125893. [Google Scholar] [CrossRef]
- Kabombo, D.; Azizi, D.; Hébert, R.; Larachi, F. Multistep concentration of lizardite/antigorite from chrysotile mine tailings—case of the Carey Mine site in East-Broughton (Québec). Int. J. Chem. React. Eng. 2021, 19, 483–498. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Yan, W.; He, M.; Li, L.; Sui, X. Identify key serpentines antigorite, lizardite and chrysotile with various compositions and crystallographic orientations using micro-Raman spectroscopy. Solid. Earth Sci. 2023, 8, 295–304. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Larachi, F. Mössbauer Spectroscopy and Catalytic Reaction Studies of Chrysotile-Catalyzed Steam Reforming of Benzene. J. Phys. Chem. C 2011, 115, 6841–6848. [Google Scholar] [CrossRef]
- Rinaudo, C.; Gastaldi, D. Characterization of Chrysotile, Antigorite and Lizardite By FT-RAMAN spectroscopy. Can. Mineral. 2003, 41, 883–890. [Google Scholar] [CrossRef]
- Laboratoire Silica Determination and Quantification of Asbestos Fibers by Microscopy. Private Report 2023, 2.
- Activation Laboratories Ltd. X-Ray Diffraction Analysis of Four Samples; A24-04494; Activation Laboratories Ltd.: Ancaster, ON, Canada, 2024; 7p. [Google Scholar]
- Shi, L. McGill Microprobe Laboratory. Available online: https://www.eps.mcgill.ca/~lang/Web/Probe/main.html (accessed on 16 June 2025).
- Ancey, M.; Batenaire, F.; Tixier, R. Application of Statistical Methods in Microanalysis. Chapter 7. In Microanalysis and Scanning Electron Microscopy; Summer School; St-Martin-d’Héres; 38401; France; Maurice, F., Meny, L., Tixier, R., Eds.; Les Editions de Physique: Orsay, France, 1978; pp. 319–342. [Google Scholar]
- Okai, T. Preparation of new GSJ geochemical reference material JA-1a (Andesite) and collaborative analysis of JA-1a and JSO-1 (Soil). In Proceedings of the 50th Annual Meeting of the Geochemical Society of Japan; 2003; p. 49. Available online: https://cir.nii.ac.jp/crid/1571135650999739904?lang=en (accessed on 16 June 2025).
- El-Sayed, D.; Ismail, A.K.; El-Hosiny, F.I. Magnesium Chloride Crystals with Studying Mechanism and Leaching Kinetics of Serpentinite Ore by Hydrochloric Acid. Trans. Indian. Inst. Met. 2023, 76, 1439–1446. [Google Scholar] [CrossRef]
- Beglaryan, H.; Isahakyan, A.; Zulumyan, N.; Melikyan, S.; Terzyan, A. A study of magnesium dissolution from serpentinites composed of different serpentine group minerals. Min. Eng. 2023, 201, 108171. [Google Scholar] [CrossRef]
- Dastkhoon, M.; Ghaedi, M.; Asfaram, A.; Goudarzi, A.; Langroodi, S.M.; Tyagi, I. Ultrasound assisted adsorption of malachite green dye onto ZnS: Cu-NP-AC: Equilibrium isotherms and kinetic studies—Response surface optimization. Sep. Purif. Technol. 2015, 156, 780–788. [Google Scholar] [CrossRef]
- Daval, D.; Hellmann, R.; Martinez, I.; Gangloff, S.; Guyot, F. Lizardite serpentine dissolution kinetics as a function of pH and temperature, including effects of elevated pCO2. Chem. Geol. 2013, 351, 245–256. [Google Scholar] [CrossRef]
- Yardley, E. Enhancing the Dissolution of Lizardite with Oxalate. Available online: https://digitalcommons.library.umaine.edu/etd/1736 (accessed on 15 June 2025).
- Zhao, D.; Sun, H.; Peng, T.; Zeng, L. Ionic dissolution and structural evolution of chrysotile and lizardite at the mineral–water interface: Reactions in sulfuric acid solution. Clays Clay Min. 2024, 72, e32. [Google Scholar] [CrossRef]
- Lacinska, A.M.; Styles, M.T.; Bateman, K.; Wagner, D.; Hall, M.R.; Gowing, C. Acid-dissolution of antigorite, chrysotile and lizardite for ex situ carbon capture and storage by mineralisation. Chem. Geol. 2016, 437, 153–169. [Google Scholar] [CrossRef]
- Mellini, M.; Fuchs, Y.; Viti, C.; Lemaire, C.; Linarè, S.J. Insights into the antigorite structure from Mössbauer and FTIR spectroscopies. Eur. J. Miner. 2002, 14, 97–104. [Google Scholar] [CrossRef]
- O’Hanley, D.S.; Dyar, M.D. The composition of lizardite 1T and the formation of magnetite in serpentinites. Am. Miner. 1993, 78, 391–404. [Google Scholar]
- Delina, R.E.G.; Perez, J.P.H.; Stammeier, J.A.; Bazarkina, E.F.; Benning, L.G. Partitioning and Mobility of Chromium in Iron-Rich Laterites from an Optimized Sequential Extraction Procedure. Environ. Sci. Technol. 2024, 58, 6391–6401. [Google Scholar] [CrossRef]
- Doriguetto, A.C.; Fernandes, N.G.; Persiano, A.I.C.; Filho, E.N.; Grenèche, J.M.; Fabris, J.D. Characterization of a natural magnetite. Phys. Chem. Min. 2003, 30, 249–255. [Google Scholar] [CrossRef]
- Viti, C.; Mellini, M. Contrasting chemical compositions in associated lizardite and chrysotile in veins from Elba, Italy. Eur. J. Miner. 1997, 9, 585–596. [Google Scholar] [CrossRef]
- Dyar, M.D.; Agresti, D.G.; Schaefer, M.W.; Grant, C.A.; Sklute, E.C. Mössbauer spectroscopy of earth and planetary materials. Annu. Rev. Earth Planet. Sci. 2006, 34, 83–125. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016; Available online: https://www.taylorfrancis.com/books/9781498754293 (accessed on 20 June 2025).
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2005; p. 643. [Google Scholar]
- Sagbas, A. Analysis and optimization of surface roughness in the ball burnishing process using response surface methodology and desirability function. Adv. Eng. Softw. 2011, 42, 992–998. [Google Scholar] [CrossRef]
- Somot, S. Radium, Uranium et Métaux Dans Les Résidus de Traitement Dynamique, Acide et Alcalin, de Minerais d’Uranium. Sciences de la Terre. Doctoral Dissertation, Université Henri Poincaré, Nancy, France, 1997. [Google Scholar]
- Ndlovu, B.N.; Forbes, E.; Becker, M.; Deglon, D.A.; Franzidis, J.P.; Laskowski, J.S. The effects of chrysotile mineralogical properties on the rheology of chrysotile suspensions. Miner. Eng. 2011, 24, 1004–1009. [Google Scholar] [CrossRef]
- Hintikka, V.V.; Kalapudas, R.P.; Viitanen, P.I. Effect of Rheology of Grinding Efficiency in the Laboratory Scale Continuous Classifying Mill. Miner. Process. Extr. Metall. Rev. 2000, 20, 133–154. [Google Scholar] [CrossRef]
- Uddin, S.; Rao, S.R.; Mirnezami, M.; Finch, J.A. Processing an ultramafic ore using fiber disintegration by acid attack. Int. J. Miner. Process. 2012, 102, 38–44. [Google Scholar] [CrossRef]
- Radziszewski, P. Energy recovery potential in comminution processes. Miner. Eng. 2013, 46, 83–88. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.; Xu, Z. Microwave Treatment of Ultramafic Nickel Ores: Heating Behavior, Mineralogy, and Comminution Effects. Minerals 2018, 8, 524. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.; Xu, Z. Effect of microwave pre-treatment on ultramafic nickel ore slurry rheology. Miner. Eng. 2014, 61, 97–104. [Google Scholar] [CrossRef]



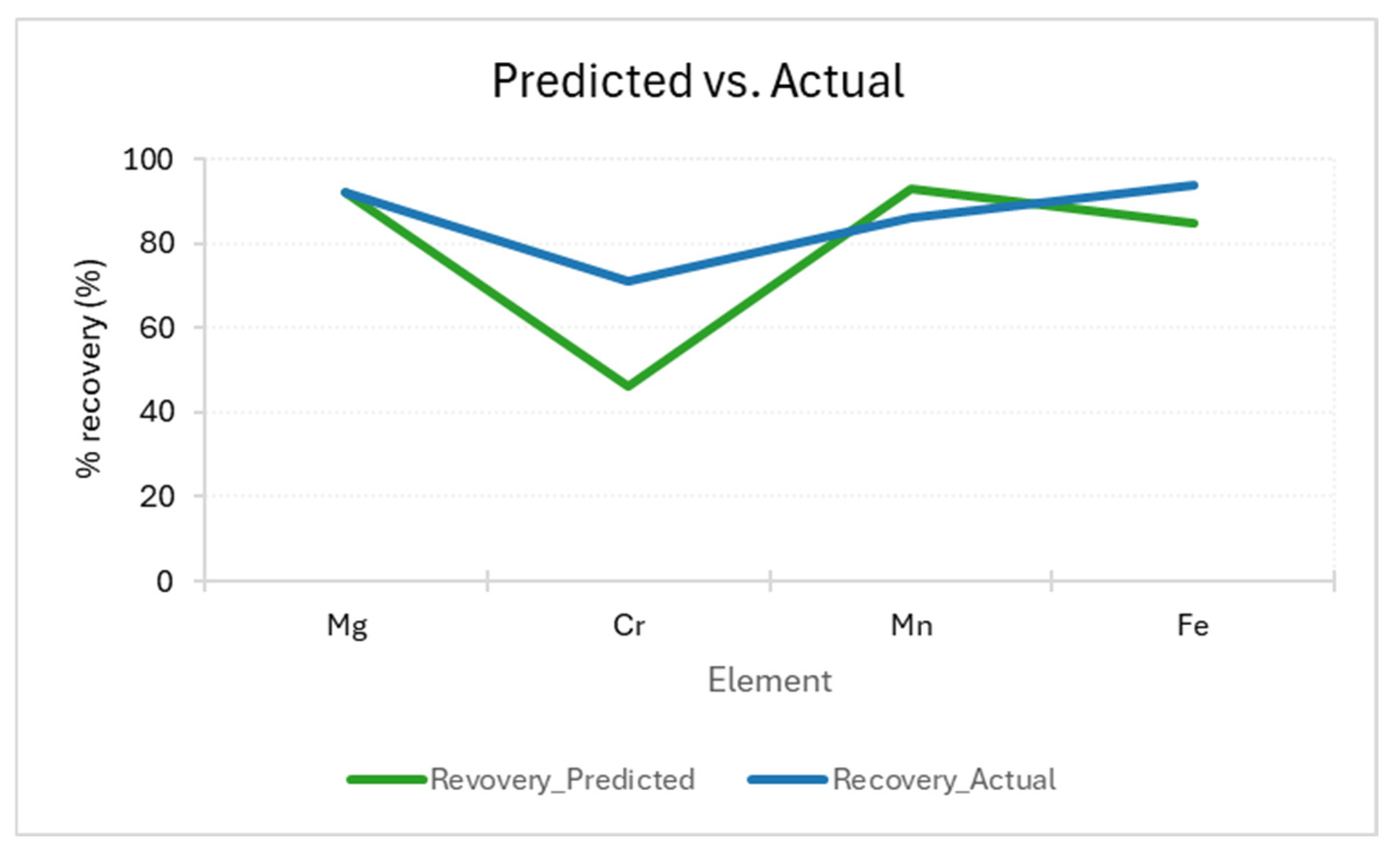
| Identified Minerals [64,71] | Estimated Amounts |
|---|---|
| serpentine (lizardite, chrysotile mostly) | Most of the sample |
| chlorite | Few percents |
| forsterite (Mg-olivine) | Few percents |
| brucite (Mg (OH)2) | Few percents |
| magnetite (Fe3O4, and other spinels) | Minor |
| quartz (SiO2) | Accessory |
| Mineral | δ (mm/s) | Δ (mm/s) | Bhf (T) | Area | |
|---|---|---|---|---|---|
| Lizardite 1 | This study | ||||
| Component 1 (Fe2+) | 1.15(1) | 2.75(1) | 0 | 60.0 | |
| Component 2 (Fe2+) | 1.16(1) | 2.20(1) | 0 | 19.3 | |
| Component 3 (Fe3+) | 0.32(2) | 0.59(4) | 0 | 20.7 | |
| Antigorite | This study | ||||
| Component 1 (Fe2+) | 1.14(1) | 2.71(1) | 0 | 90 | |
| Component 2 (Fe3+) | 0.25(2) | 0.42(3) | 0 | 10 | |
| Magnetic fraction of the head sample | This study | ||||
| Component 1 | 0.28(1) | −0.02(1) | 48.7(1) | 24 | Assigned to magnetite |
| Component 2 | 0.64(1) | +0.02(2) | 45.7(1) | 37 | Assigned to magnetite |
| Component 3 (Fe2+) | 1.15(1) | 2.84(1) | 0 | 25 | Assigned to antigorite |
| Component 4 (Fe3+) | 0.38(1) | 0.46(1) | 0 | 8 | - |
| Component 4 (Fe3+) | 0.45(1) | 1.05(1) | 0 | 6 | - |
| Oxide | Unit | 1 | 2 | 2 | 4 | 5 | Mean | Std. Dev. |
|---|---|---|---|---|---|---|---|---|
| SiO2 | % | 36.37 | 36.08 | 35.79 | 35.83 | 36.43 | 36.1 | 0.3 |
| Al2O3 | % | 0.86 | 0.81 | 0.78 | 0.70 | 0.67 | 0.76 | 0.07 |
| CaO | % | 0.21 | 0.34 | 0.20 | 0.16 | 0.21 | 0.22 | 0.06 |
| MgO | % | 40.27 | 40.00 | 40.32 | 40.99 | 40.42 | 40.4 | 0.3 |
| Na2O | % | - | - | - | - | - | - | - |
| K2O | % | 0.02 | 0.05 | 0.05 | 0.02 | 0.04 | 0.04 | 0.01 |
| Fe2O3 | % | 8.47 | 8.91 | 9.08 | 8.50 | 8.28 | 8.7 | 0.3 |
| TiO2 | % | - | - | - | - | - | - | - |
| MnO | % | 0.12 | 0.11 | 0.11 | 0.10 | 0.12 | 0.11 | 0.01 |
| Cr2O3 | % | 0.28 | 0.30 | 0.32 | 0.28 | 0.40 | 0.31 | 0.04 |
| NiO | % | 0.28 | 0.29 | 0.31 | 0.33 | 0.32 | 0.31 | 0.02 |
| LOI | % | 12.96 | 12.96 | 12.96 | 12.96 | 12.96 | - | - |
| Run | Mg (ppm) | Al (ppm) | Cr (ppm) | Mn (ppm) | Fe (ppm) | Co (ppm) | Ni (ppm) |
|---|---|---|---|---|---|---|---|
| W-series | 210,000 | 2345 | 670 | 885 | 73,242 | 98 | 2227 |
| An. Series (n = 3) | 209,036 ± 1364 (0.7%) | 2763 ± 604 (22%) | 593 ± 31 (5%) | 854 ± 34 (4%) | 62,932 ± 2335 (4%) | 97 ± 3 (3%) | 2144 ± 67 (3%) |
| D-series (n = 5) | 236,038 ± 4991 (2%) | 2748 ± 231 (8%) | 706 ± 41 (6%) | 814 ± 20 (3%) | 60,286 ± 1750 (3%) | 95 ± 3 (3%) | 1868 ± 97 (5%) |
| Run | Acid Concentration (mol/L) | Solid to Liquid Ratio (%) | Temperature (°C) | Mg (%) | Al (%) | Cr (%) | Mn (%) | Fe (%) | Co (%) | Ni (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.0 | 10 | 60 | 69 | 26 | 25 | 59 | 27 | 65 | 79 |
| 2 | 5.5 | 40 | 90 | 64 | 26 | 25 | 58 | 46 | 71 | 76 |
| 3 | 12.0 | 13 | 30 | 31 | 13 | 21 | 51 | 68 | 65 | 78 |
| 4 | 5.5 | 10 | 20 | 18 | 7 | 9 | 36 | 14 | 42 | 53 |
| 5 | 7.0 | 25 | 55 | 68 | 31 | 31 | 65 | 71 | 78 | 85 |
| 6 | 2.0 | 40 | 50 | 17 | 3 | 7 | 27 | 5 | 30 | 37 |
| 7 | 12.0 | 36 | 83 | 123 | 65 | 58 | 104 | 115 | 118 | 124 |
| 8 | 7.0 | 25 | 55 | 66 | 27 | 32 | 62 | 67 | 79 | 86 |
| 9 | 5.5 | 10 | 20 | 21 | 7 | 12 | 42 | 17 | 50 | 62 |
| 10 | 8.6 | 25 | 20 | 20 | 7 | 12 | 41 | 41 | 56 | 64 |
| 11 | 7.0 | 25 | 55 | 55 | 27 | 33 | 66 | 68 | 79 | 78 |
| 12 | 10.2 | 10 | 90 | 85 | 44 | 48 | 84 | 86 | 100 | 95 |
| 13 | 8.7 | 13 | 30 | 19 | 8 | 16 | 43 | 49 | 60 | 58 |
| 14 | 8.7 | 40 | 55 | 63 | 27 | 31. | 66 | 74 | 79 | 77 |
| 15 | 8.5 | 25 | 90 | 90 | 45 | 53 | 78 | 82 | 81 | 82 |
| 16 | 2.0 | 23 | 90 | 28 | 0 | 1 | 34 | 7 | 37 | 37 |
| 17 | 12.0 | 36 | 85 | 80 | 39 | 38 | 71 | 74 | 80 | 74 |
| 18 | 5.1 | 40 | 20 | 19 | 6 | 10 | 40 | 17 | 49 | 54 |
| 19 | 7.0 | 25 | 55 | 70 | 28 | 35 | 71 | 83 | 79 | 83 |
| 20 | 10.4 | 40 | 20 | 21 | 8 | 15 | 46 | 55 | 59 | 59 |
| 21 | 7.0 | 25 | 55 | 67 | 27 | 32 | 70 | 78 | 78 | 82 |
| 22 | 5.0 | 10 | 90 | 110 | 49 | 51 | 96 | 96 | 100 | 106 |
| 23 | 2.0 | 27 | 20 | 18 | 7 | 9 | 41 | 13 | 47 | 54 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 18,705.92 | 5 | 3741.18 | 14.71 | <0.0001 | Significant |
| A: HCl concentration | 1573.45 | 1 | 1573.45 | 6.19 | 0.0235 | |
| B: Solid to liquid ratio | 283.08 | 1 | 283.08 | 1.11 | 0.3062 | |
| C: Temperature | 12,073.9 | 1 | 12,073.9 | 47.48 | <0.0001 | |
| AB | 1199.19 | 1 | 1199.19 | 4.72 | 0.0443 | |
| AC | 1081.88 | 1 | 1081.88 | 4.25 | 0.0548 | |
| Residual | 4323.02 | 17 | 254.3 | |||
| Lack of Fit | 3177.61 | 10 | 317.76 | 1.94 | 0.1954 | Not significant |
| Pure Error | 1145.41 | 7 | 163.63 | |||
| Cor Total | 23,028.93 | 22 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 18,885.06 | 9 | 2098.34 | 9.23 | 0.0002 | Significant |
| A: HCl concentration | 10,558.25 | 1 | 10,558.25 | 46.45 | <0.0001 | |
| B: Solid to liquid ratio | 251.43 | 1 | 251.43 | 1.11 | 0.3121 | |
| C: Temperature | 4187.34 | 1 | 4187.34 | 18.42 | 0.0009 | |
| AB | 262.69 | 1 | 262.69 | 1.16 | 0.3019 | |
| AC | 234.32 | 1 | 234.32 | 1.03 | 0.3285 | |
| BC | 843.73 | 1 | 843.73 | 3.71 | 0.0762 | |
| A2 | 904.44 | 1 | 904.44 | 3.98 | 0.0675 | |
| B2 | 109.57 | 1 | 109.57 | 0.482 | 0.4997 | |
| C2 | 1009.37 | 1 | 1009.37 | 4.44 | 0.0551 | |
| Residual | 2955.09 | 13 | 227.31 | |||
| Lack of Fit | 1753.6 | 6 | 292.27 | 1.7 | 0.2507 | Not significant |
| Pure Error | 1201.49 | 7 | 171.64 | |||
| Cor Total | 21,840.15 | 22 |
| % Recovery | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mg | Al | Cr | Mn | Fe | Co | Ni | ||
| Analysed Feed | 90 | 62 | 66 | 81 | 92 | 88 | 92 | |
| (σ = 2) | (σ = 3) | (σ = 3) | (σ = 10) | (σ = 2) | (σ = 2) | (σ = 2) | ||
| Calculated Feed | 88 | 59 | 64 | 83 | 91 | 87 | 91 | |
| (σ = 3) | (σ = 2) | (σ = 3) | (σ = 8) | (σ = 3) | (σ = 2) | (σ = 2) | ||
| Mean | 89 | 61 | 65 | 82 | 92 | 88 | 92 | |
| Sigma tot | 4 | 4 | 4 | 13 | 4 | 3 | 3 | |
| % Recovery | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mg | Al | Cr | Mn | Fe | Co | Ni | ||
| Analysed Feed | 92 | 65 | 71 | 90 | 94 | 90 | 93 | |
| (σ = 1) | (σ = 2) | (σ = 1) | (σ = 1) | (σ = 1) | (σ = 1) | (σ = 2) | ||
| Calculated Feed | 91 | 61 | 69 | 90 | 93 | 92 | 92 | |
| (σ = 2) | (σ = 1) | (σ = 1) | (σ = 1) | (σ = 1) | (σ = 3) | (σ = 2) | ||
| Mean | 92 | 63 | 70 | 90 | 94 | 91 | 93 | |
| Sigma tot | 2 | 2 | 1 | 1 | 1 | 3 | 3 | |
| % Recovery | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mg | Al | Cr | Mn | Fe | Co | Ni | ||
| Analysed Feed | 88 | 64 | 69 | 82.5 | 91.5 | 87.5 | 91 | |
| (σ = 1) | (σ = 3) | (σ = 1) | (σ = 0.5) | (σ = 0.5) | (σ = 0.5) | (σ = 1) | ||
| Calculated Feed | 86 | 56 | 66 | 83.5 | 90.5 | 86.5 | 91 | |
| (σ = 1) | (σ = 1) | (σ = 2) | (σ = 0.5) | (σ = 0.5) | (σ = 0.5) | (σ = 1) | ||
| Mean | 87 | 60 | 68 | 83 | 91 | 87 | 91 | |
| Sigma tot | 1 | 3 | 2 | 1 | 1 | 1 | 1 | |
| % Recovery | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mg | Al | Cr | Mn | Fe | Co | Ni | ||
| Analysed Feed | 92 | 77 | 71 | 86 | 94 | 91 | 93 | |
| (σ = 1) | (σ = 15) | (σ = 3) | (σ = 2) | (σ = 1) | (σ = 0.5) | (σ = 1) | ||
| Calculated Feed | 90 | 72 | 68 | 86 | 93 | 90 | 93 | |
| (σ = 1) | (σ = 18) | (σ = 3) | (σ = 2) | (σ = 1) | (σ = 1) | (σ = 1) | ||
| Mean | 92 | 75 | 70 | 86 | 94 | 91 | 93 | |
| Sigma tot | 1 | 23 | 4 | 3 | 1 | 1 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajah, Z.; Gavras, D.F.; Andrianandraina, H.; Faraji, F.; Traoré, M.; Somot, S.; Larachi, F.; Ryan, D.; Bouajila, A. Optimizing the Leaching Parameters of Asbestos Tailings for Maximizing the Recovery of Critical Metals. Metals 2025, 15, 1215. https://doi.org/10.3390/met15111215
Rajah Z, Gavras DF, Andrianandraina H, Faraji F, Traoré M, Somot S, Larachi F, Ryan D, Bouajila A. Optimizing the Leaching Parameters of Asbestos Tailings for Maximizing the Recovery of Critical Metals. Metals. 2025; 15(11):1215. https://doi.org/10.3390/met15111215
Chicago/Turabian StyleRajah, Zouhour, Daphne Freda Gavras, Herizo Andrianandraina, Fariborz Faraji, Mahamadou Traoré, Stéphanie Somot, Faïçal Larachi, Dominic Ryan, and Ahmed Bouajila. 2025. "Optimizing the Leaching Parameters of Asbestos Tailings for Maximizing the Recovery of Critical Metals" Metals 15, no. 11: 1215. https://doi.org/10.3390/met15111215
APA StyleRajah, Z., Gavras, D. F., Andrianandraina, H., Faraji, F., Traoré, M., Somot, S., Larachi, F., Ryan, D., & Bouajila, A. (2025). Optimizing the Leaching Parameters of Asbestos Tailings for Maximizing the Recovery of Critical Metals. Metals, 15(11), 1215. https://doi.org/10.3390/met15111215







