Physical–Mechanical and Corrosion Resistance Characterization of a Water-Based Epoxy Primer Applied to Galvanized Steel
Abstract
1. Introduction
2. Materials and Methods
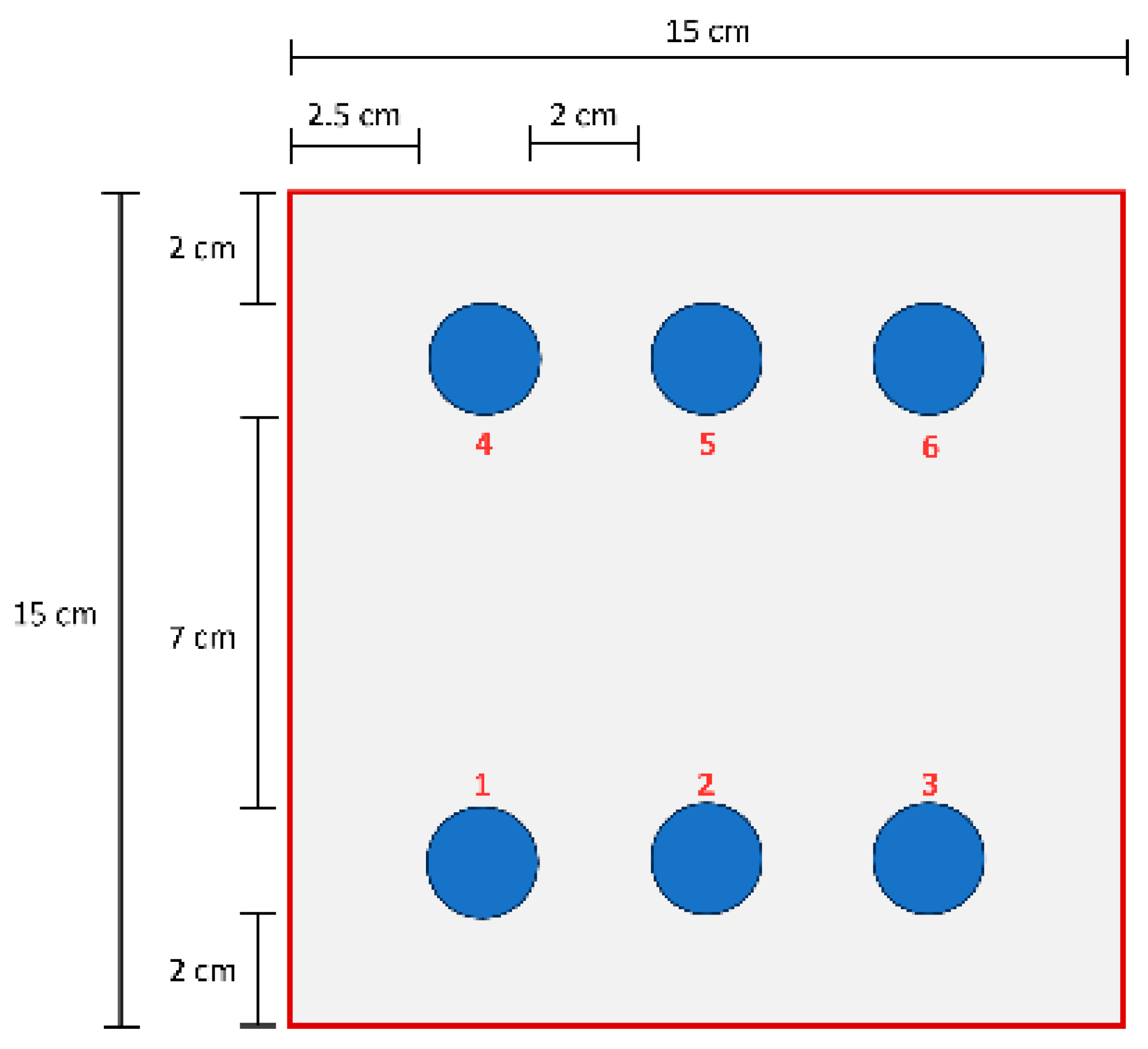
2.1. Epoxy Coating Application
2.2. Coating Characterization Methods
2.3. Accelerated Aging and Corrosion Tests Methods
- Natural outdoor exposure monitored from July 2023 to February 2024;
- Thermal shock (IR lamp) cycles on specimens with reduced PUR foam, following the program in Table 1;
- Alternating hot/cold cycles: 8 cycles of 80 °C × 2 h and −20 °C × 2 h;
- Blister test: specimens (foam removed) placed on cups half-filled with water and held at 70 °C for 1 month;
- Neutral salt spray: 5% NaCl at 35 °C for 500 h.
2.4. Evaluation of Mechanical Properties
3. Results
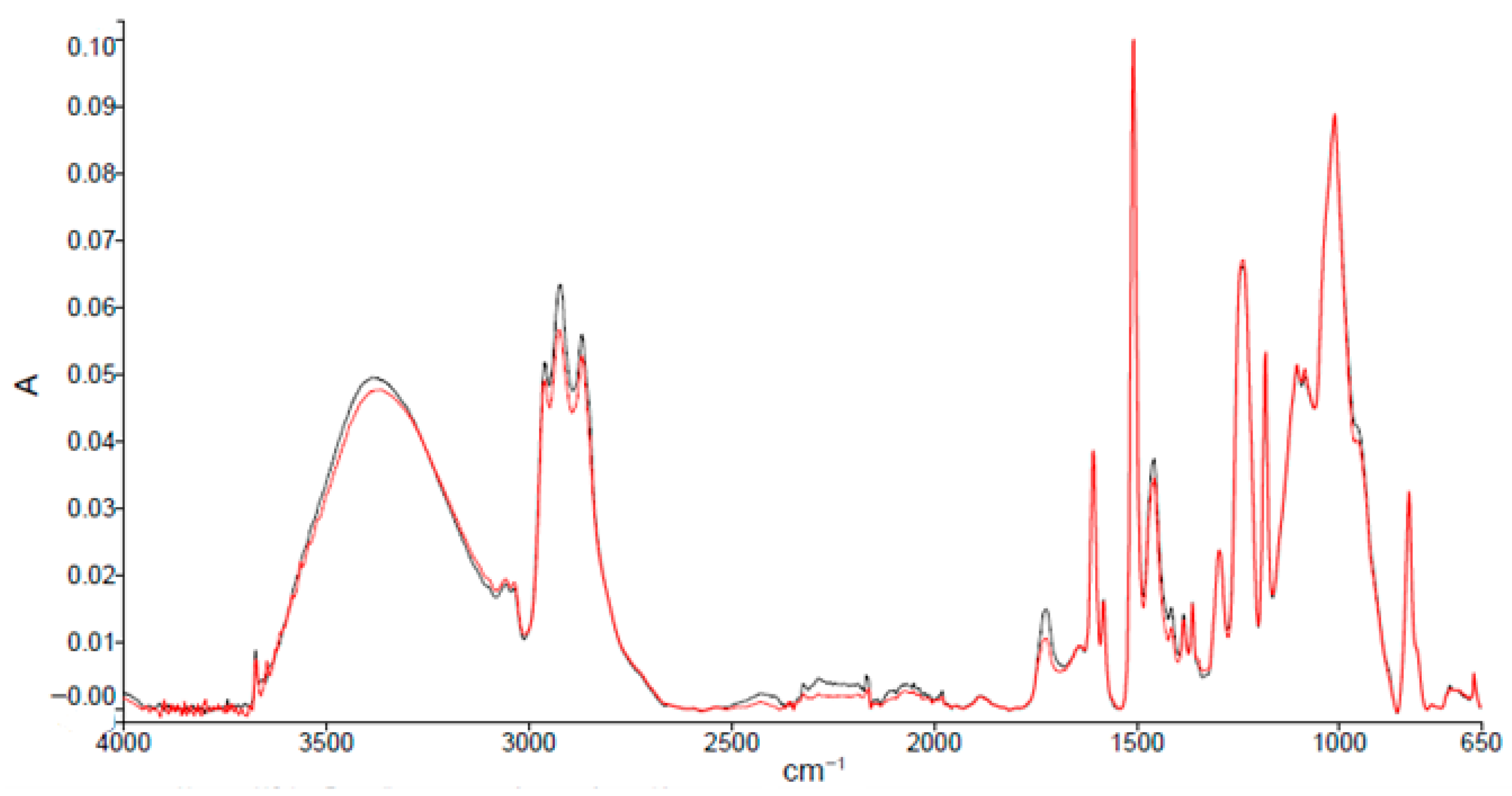
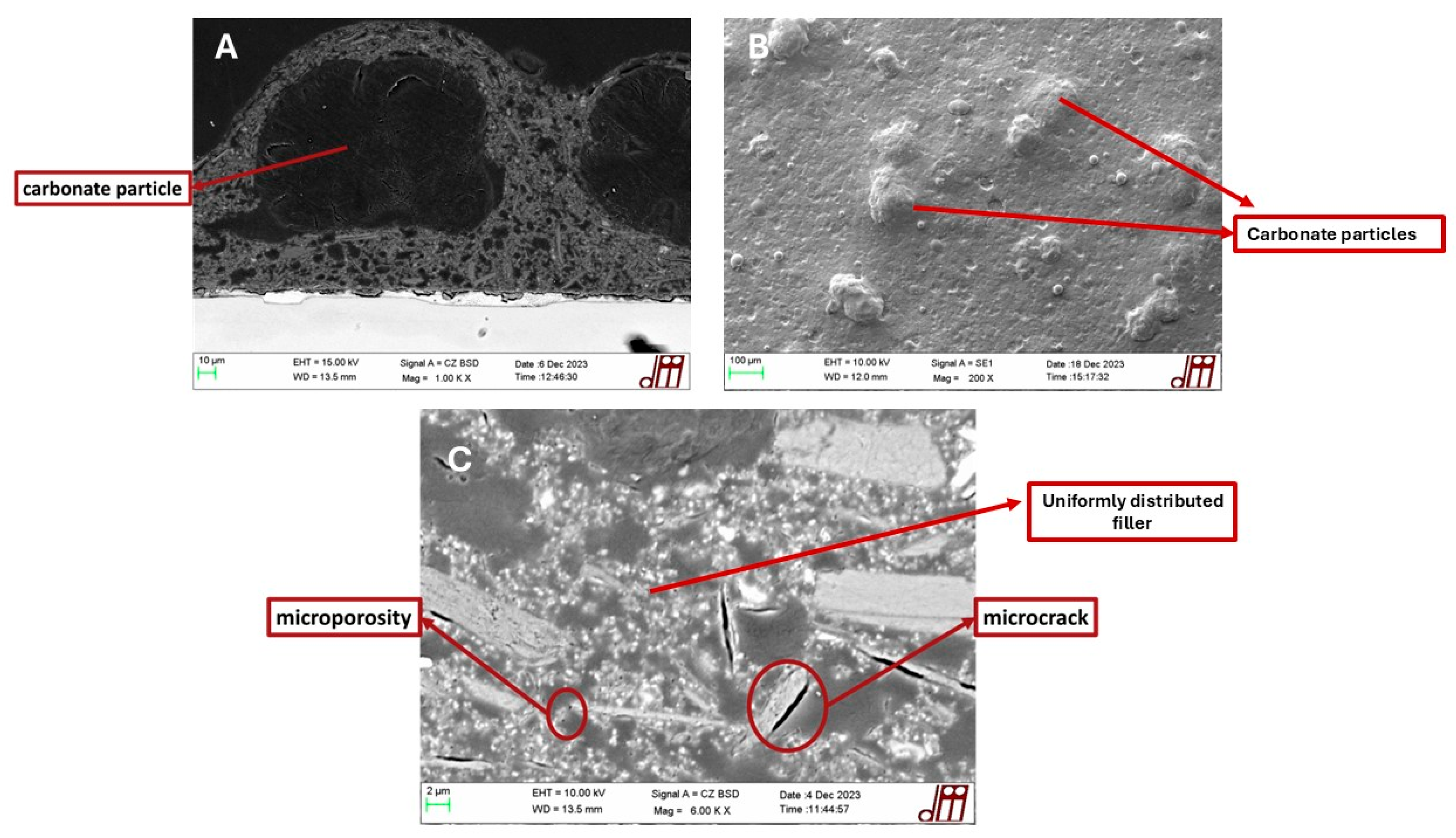
3.1. Coating Characterization
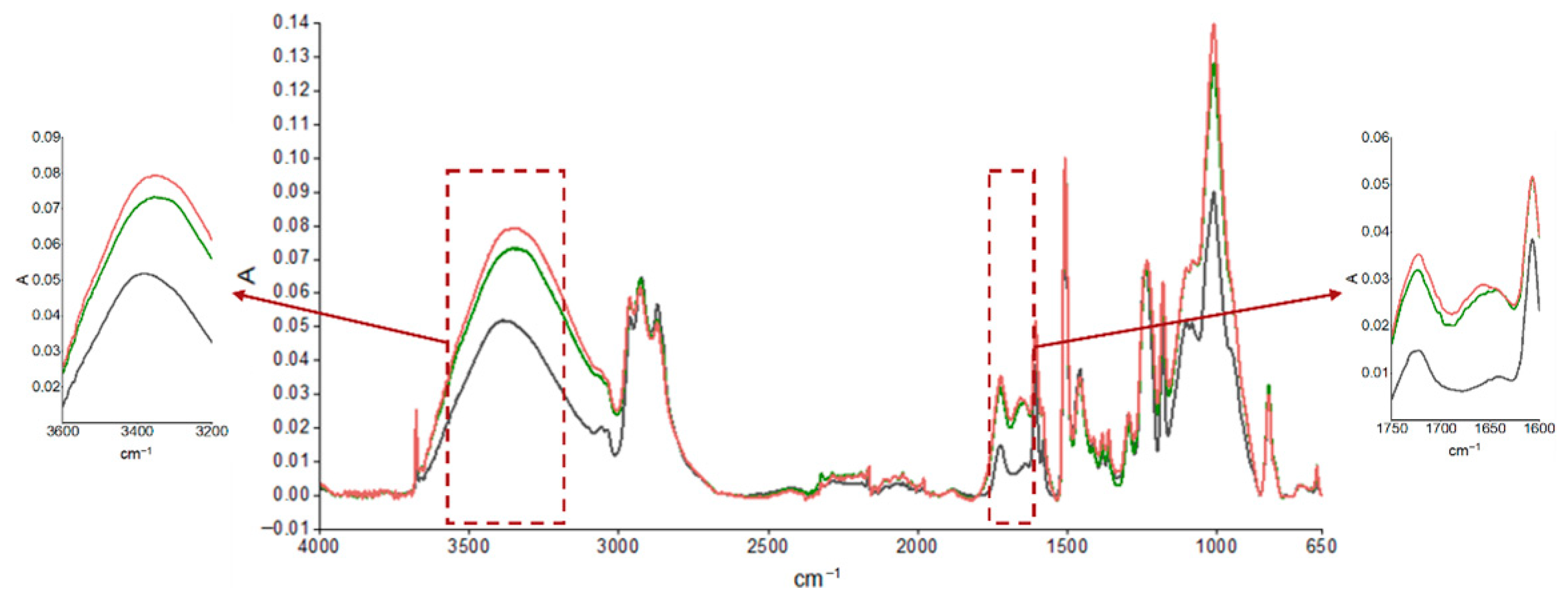
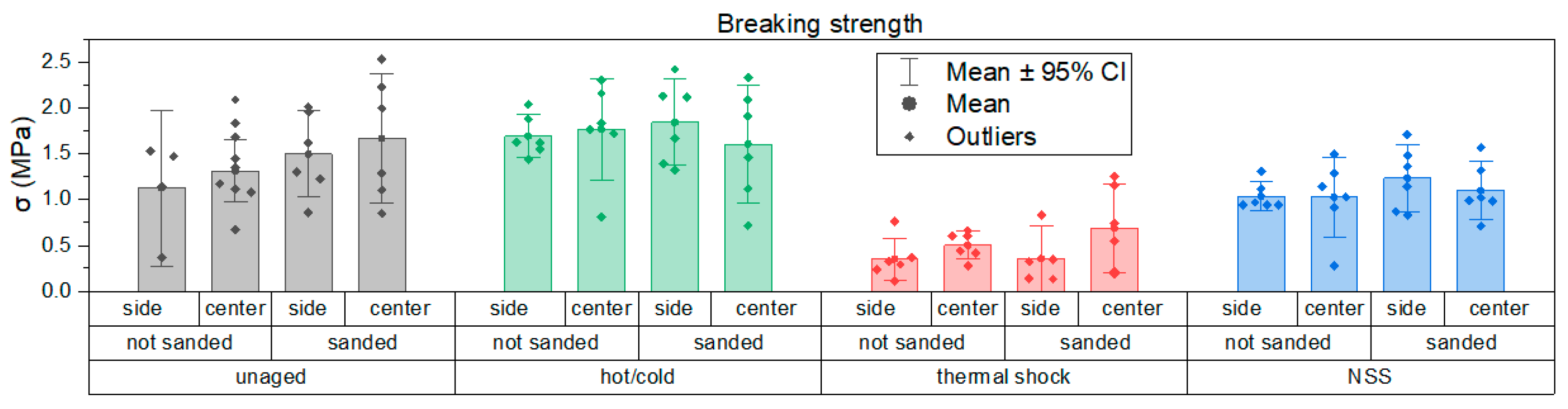
3.2. Accelerated Aging and Corrosion Tests
3.3. Evaluation of Mechanical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davies, J.M. (Ed.) Lightweight Sandwich Construction; Wiley-Blackwell: Oxford, UK, 2001; ISBN 9780632040278. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Du, X.; Du, Z.; Cheng, X.; Wang, H. A self-healing polyurethane-based composite coating with high strength and anti-corrosion properties for metal protection. Compos. Part B Eng. 2021, 225, 109273. [Google Scholar] [CrossRef]
- Pezzato, L.; Settimi, A.G.; Cerchier, P.; Gennari, C.; Dabalà, M.; Brunelli, K. Microstructural and corrosion properties of PEO coated zinc-aluminized (ZA) steel. Coatings 2020, 10, 448. [Google Scholar] [CrossRef]
- Pezzato, L.; Akbarzadeh, S.; Settimi, A.G.; Moschin, E.; Moro, I.; Olivier, M.; Brunelli, K.; Dabalà, M. Corrosion and antifouling properties of copper-containing PEO coatings produced on steels. Surf. Coat. Technol. 2024, 482, 130631. [Google Scholar] [CrossRef]
- Pezzato, L.; Settimi, A.G.; Fanchin, D.; Moschin, E.; Moro, I.; Dabalà, M. Effect of Cu addition on the corrosion and antifouling properties of PEO coated zinc-aluminized steel. Materials 2022, 15, 7895. [Google Scholar] [CrossRef]
- National Physical Laboratory. Coating for the Protection of Structural Steelwork. Available online: https://www.npl.co.uk/getattachment/08d520bd-e6ce-4178-896a-f062570809e2/No-3_CPSSGuide_Templated_V3.pdf?lang=en-US (accessed on 26 November 2024).
- Sienel, G.; Rieth, R.; Rowbottom, K.T. Epoxides. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Vidil, T.; Tournilhac, F.; Musso, S.; Robisson, A.; Leibler, L. Control of reactions and network structures of epoxy thermosets. Prog. Polym. Sci. 2016, 62, 126–179. [Google Scholar] [CrossRef]
- Zafar, S. Waterborne epoxy based coating materials. Am. Sci. Res. J. Eng. Technol. Sci. 2018, 50, 133–154. [Google Scholar]
- Elmore, J.D.; Kincaid, D.S.; Komar, P.C.; Nielsen, J.E. Waterborne epoxy protective coatings for metal. J. Coat. Technol. 2002, 74, 63–72. [Google Scholar] [CrossRef]
- Miller, T.; Zhang, Y. Performance comparison of waterborne and solvent-borne epoxy primers. In Proceedings of the SSPC Conference, Tampa, FL, USA, 26 January–3 February 2012. [Google Scholar]
- Jomy, J.; Prabhu, D.; Prabhu, P.R. Inhibitors Incorporated into Water-Based Epoxy Coatings on Metals for Corrosion Protection: A Review. J. Bio- Tribo-Corros. 2022, 8, 44. Available online: https://link.springer.com/content/pdf/10.1007/s40735-022-00643-7.pdf (accessed on 26 November 2024). [CrossRef]
- Hao, J.; Yang, K.; Wu, J.; Wu, M.; Li, Y. Overview of recent developments in composite epoxy resin in organic coating on steel (2020–2024). Materials 2025, 18, 1531. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Haddadi, S.A.; Labbani Motlagh, A.; Ghaderi, M.; Ramezanzadeh, B.; Mahdavian, M.; Arjmand, M.; Jalili, M. Epoxy nanocomposite coating based on calcium zinc phosphate with dual active/barrier corrosion mitigation properties. Prog. Org. Coat. 2022, 163, 106677. [Google Scholar] [CrossRef]
- Wang, J.; Qi, Y.; Zhao, X.; Zhang, Z. Electrochemical investigation of corrosion behavior of epoxy modified silicate zinc-rich coatings in 3.5% NaCl solution. Coatings 2020, 10, 444. [Google Scholar] [CrossRef]
- Langer, E.; Zubielewicz, M.; Królikowska, A.; Komorowski, L.; Krawczyk, K.; Wanner, M.; Aktas, L.; Hilt, M. Zinc-reduced anticorrosive primers—Water-based versus solvent-based. Coatings 2025, 15, 64. [Google Scholar] [CrossRef]
- Deflorian, F.; Rossi, S.; Fedel, M. Organic coatings degradation: Comparison between natural and artificial weathering. Corros. Sci. 2008, 50, 2360–2366. [Google Scholar] [CrossRef]
- UNI EN ISO 4624:2016; Pitture e Vernici—Test di Trazione (Pull-Off Test) per Adesione. UNI Ente Italiano di Normazione: Milano, Italy, 2016.
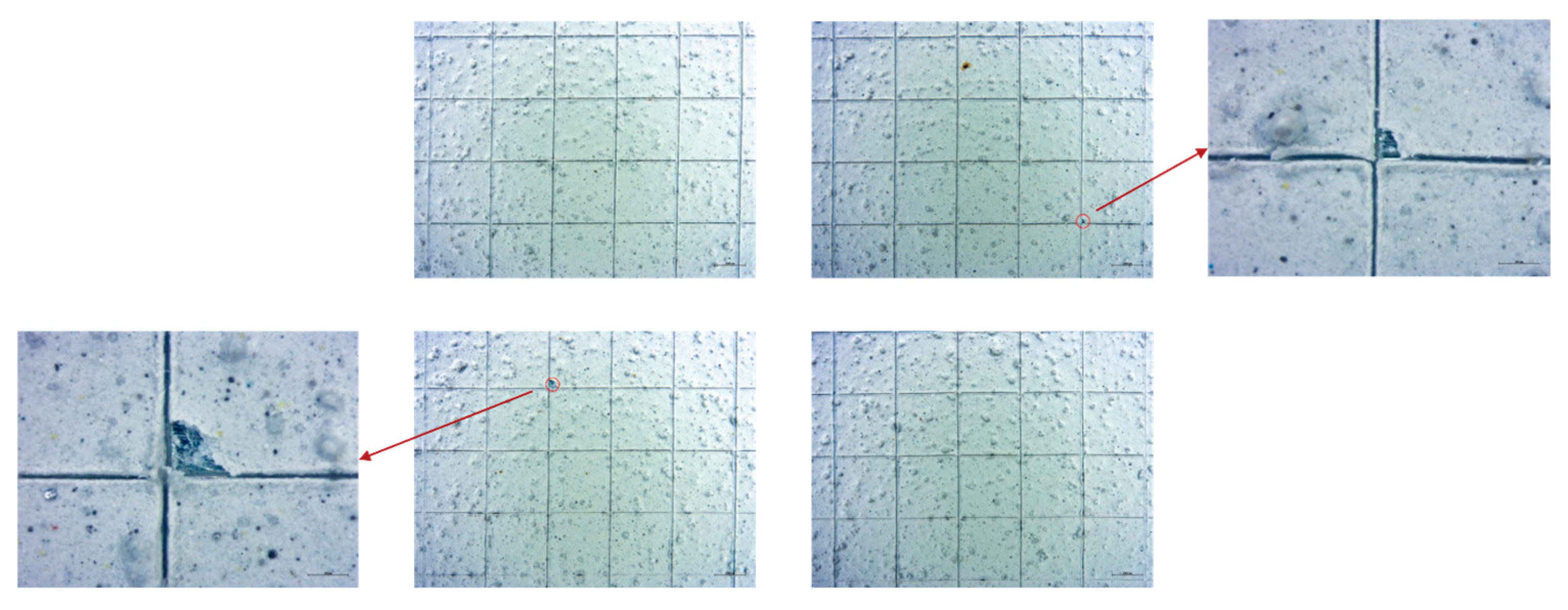
- UNI EN ISO 2409:2020; Pitture e Vernici—Prova di Quadrettatura. UNI Ente Italiano di Normazione: Milano, Italy, 2020.
- Stojanović, I.; Logar, M.; Turkalj, L.; Cindrić, I.; Kurtela, M.; Franjić, H. Influence of catalytic infrared radiation on the protective properties of industrial epoxy primers. Materials 2023, 16, 6551. [Google Scholar] [CrossRef]
- Shahidi, S.; Mohammadi, B.; Mohammadi, S.; Vessally, E. The effect of the hybrid multi-layered graphene oxide/talc as a hydrophobic agent in epoxy coating. Plast. Rubber Compos. 2022, 51, 13–34. [Google Scholar] [CrossRef]
- Manni, A.; Matadi Boumbimba, R.; Mikdam, A.; El Bouari, A.; Addiego, F.; Meziani, J.; Wary, M. Magnesite and dolomite micro-particles: Preparation, physical properties and application in bio-based polymer composite. Polym. Bull. 2022, 79, 2149–2171. [Google Scholar] [CrossRef]
- Mailhot, B.; Morlat-Thérias, S.; Bussière, P.; Le Pluart, L.; Duchet, J.; Sautereau, H.; Gérard, J.; Gardette, J. Photoageing behaviour of epoxy nanocomposites: Comparison between spherical and lamellar nanofillers. Polym. Degrad. Stab. 2008, 93, 1786–1792. [Google Scholar] [CrossRef]
- Mailhot, B.; Morlat-Thérias, S.; Bussière, P. Study of the degradation of an epoxy/amine resin, 2. Macromol. Chem. Phys. 2005, 206, 585–591. [Google Scholar] [CrossRef]
- Galant, C.; Fayolle, B.; Kuntz, M.; Verdu, J. Thermal and radio-oxidation of epoxy coatings. Prog. Org. Coat. 2010, 69, 322–329. [Google Scholar] [CrossRef]
- Li, S.; Bi, H.; Weinell, C.E.; Dam-Johansen, K. Non-destructive evaluations of water uptake in epoxy coating. J. Appl. Polym. Sci. 2023, 141, e54777. [Google Scholar] [CrossRef]
- Mohammadkhah, S.; Ramezanzadeh, M.; Eivaz Mohammadloo, H.; Ramezanzadeh, B.; Ghamsarizade, R. Construction of a nano-micro nacre-inspired 2D-MoS2-MOF-glutamate carrier toward designing a high-performance smart epoxy composite. J. Ind. Eng. Chem. 2023, 121, 358–377. [Google Scholar] [CrossRef]
- Xing, C.; Wang, W.; Qu, S.; Tang, Y.; Zhao, X.; Zuo, Y. Degradation of zinc-rich epoxy coating in 3.5% NaCl solution and evolution of its EIS parameters. J. Coat. Technol. Res. 2021, 18, 843–860. [Google Scholar] [CrossRef]
- Bierwagen, G.; Tallman, D.; Li, J.; He, L.; Jeffcoate, C. EIS studies of coated metals in accelerated exposure. Prog. Org. Coat. 2003, 46, 149–158. [Google Scholar] [CrossRef]
- RIPOL. Liquid or Powder Coating: Which One to Prefer and Why? RIPOL. The Power of Colour. 2023. Available online: https://www.ripol.com/en/liquid-or-powder-coating-which-one-to-prefer-and-why/ (accessed on 26 November 2024).
- Prajapati, S.C.; Kamani, P.K. Acrylic-based monocoat eco-friendly, anticorrosive coating for cathodic electrodeposition. Surf. Eng. 2022, 38, 618–632. [Google Scholar] [CrossRef]


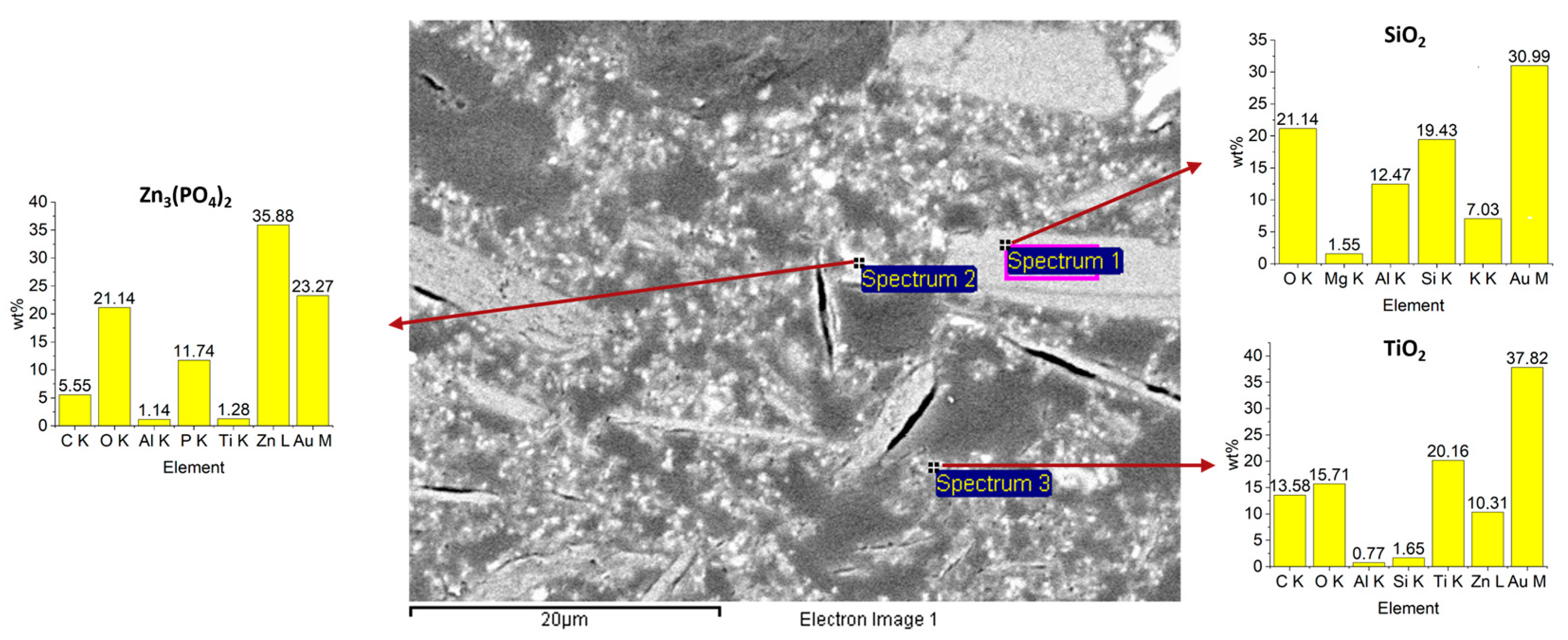
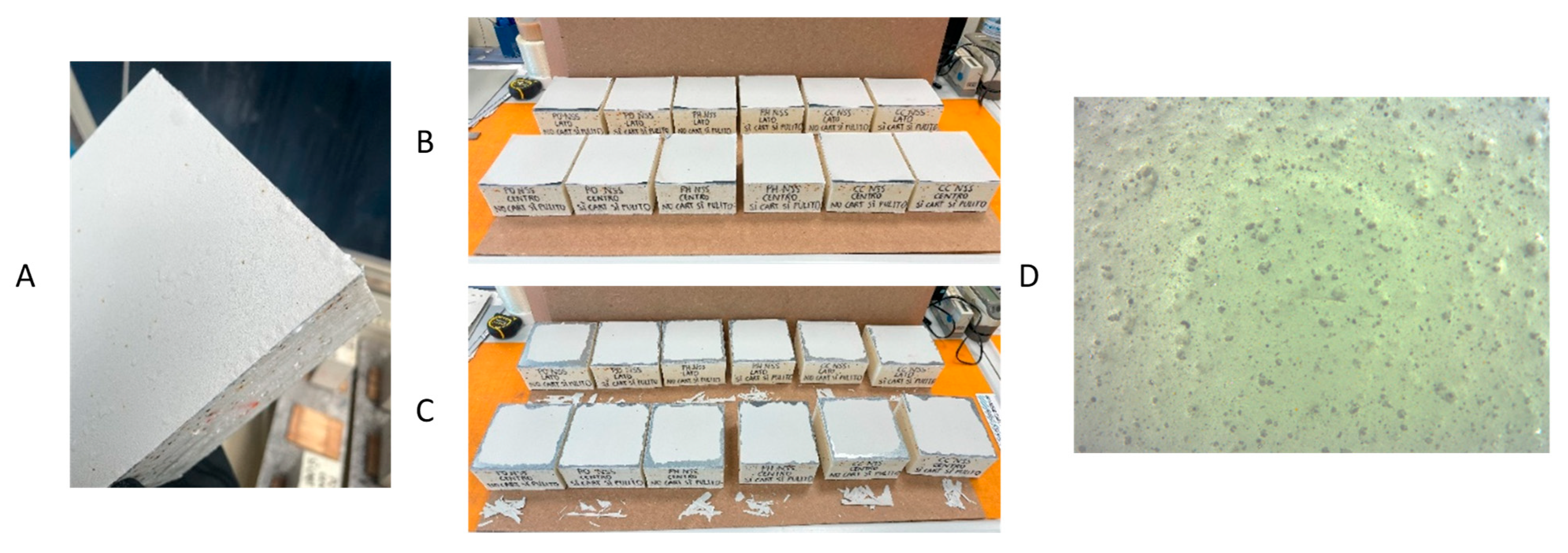
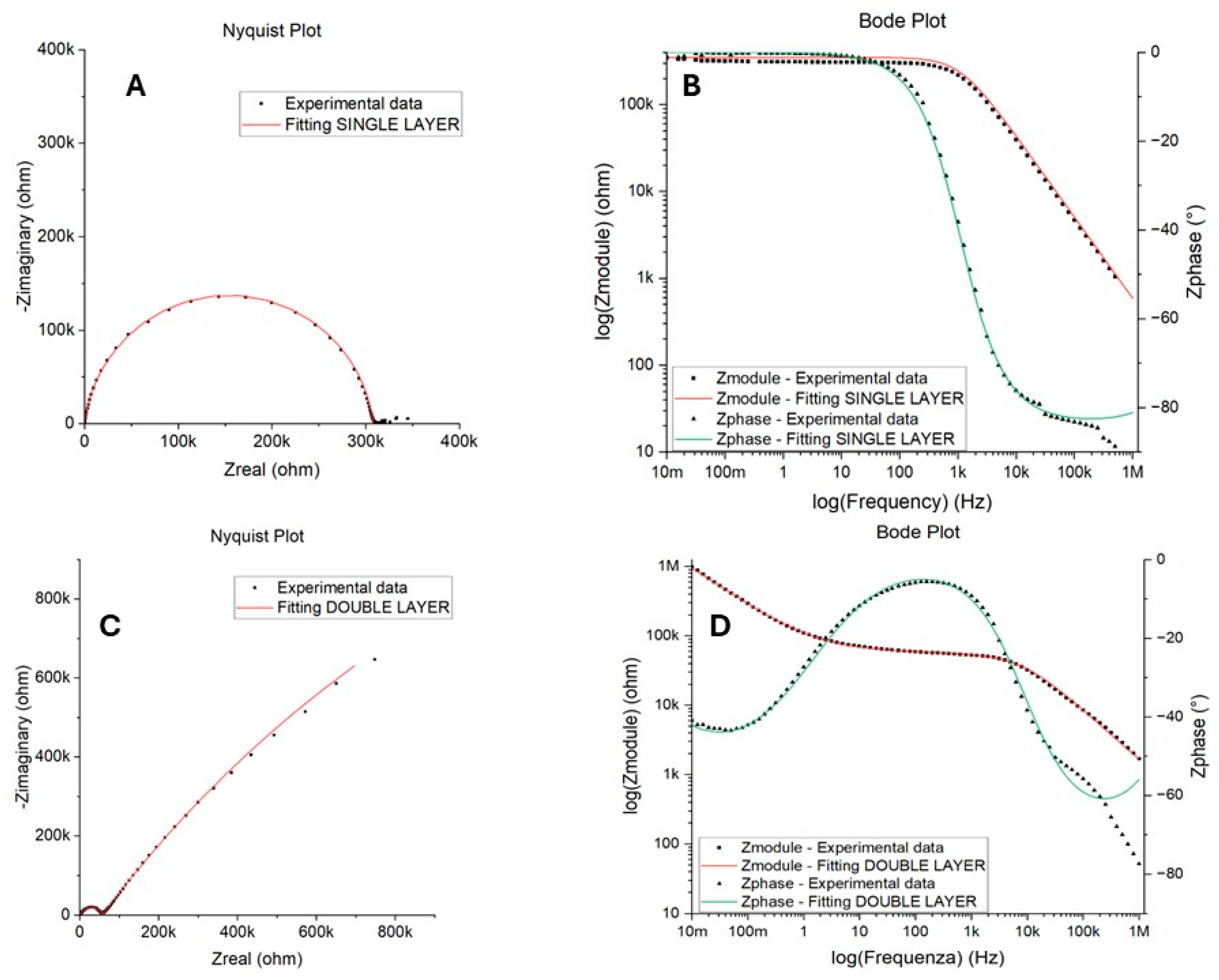



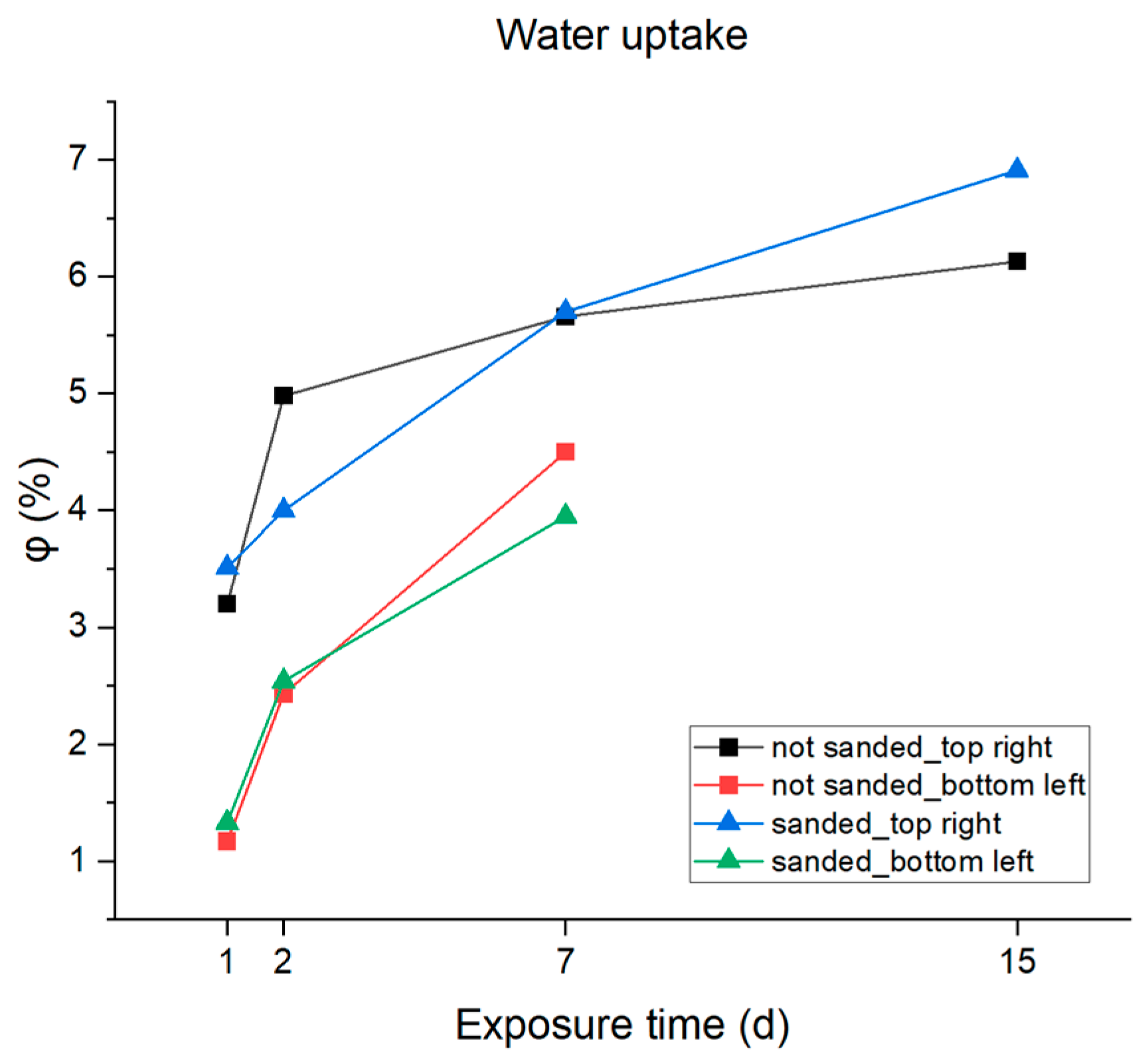
| Day | Temperature [°C] | Time |
|---|---|---|
| 1 | 70 | 30′ + 30′ (on; off) × 9 h |
| 2 | 80 | 30′ + 30′ (on; off) × 9 h |
| 3 | 80 | 9 h |
| 4 | 90 | 9 h |
| 5 | 100 | 9 h |
| 6 | ||
| 7 |
| Absorption Peak Wave Number (cm−1) | Characteristic Bond and Movement |
|---|---|
| ≈3500 | O-H stretching |
| 3057 | Stretching C-H bonding oxirane ring |
| 2965–2873 | Stretching C-H and CH2 bonding and aromatic and aliphatic CH |
| 1608 | Stretching C=C aromatic |
| 1509 | Stretching C-C aromatic |
| 1250 | Bending C-H bonding |
| 1036 | Stretching C-O-C ether bonding |
| 915 | Stretching C-O bond oxirane group |
| 831 | Stretching C-O-C bond oxirane group |
| 772 | Rocking CH2 group |
| 574 | Vibration out of the bond plane O-H bond |
| Circuit Elements | |
|---|---|
| Rs | solution Resistance |
| Rp | polarization Resistance |
| Rpore | pore Resistance |
| Qc | CPE of coating Capacitance |
| Rct | charge transfer Resistance |
| Qdl | CPE of double layer Capacitance |
| Rs (Ω) | CPEc (Ssa) | Rp or Rpore * (Ω) | CPEdl (Ssa) | Rct (Ω) | |||
|---|---|---|---|---|---|---|---|
| Qc | a | Qdl | a | ||||
| Unsanded upper right | |||||||
| T0 | 6.70 × 101 | 7.01 × 10−10 | 9.36 × 10−1 | 2.13 × 108 | - | - | - |
| 1 day | 3.30 × 102 | 7.24 × 10−10 | 9.51 × 10−1 | 4.79 × 105 | - | - | - |
| 2 days | 2.84 × 101 | 9.65 × 10−10 | 9.34 × 10−1 | 1.21 × 106 | - | - | - |
| 7 days | 1.25 × 101 | 9.84 × 10−10 | 9.37 × 10−1 | 1.01 × 105 | - | - | - |
| 15 days | 3.04 × 101 | 1.37 × 10−9 | 9.14 × 10−1 | 2.26 × 105 | - | - | - |
| Unsanded bottom left | |||||||
| T0 | 8.82 × 101 | 6.04 × 10−10 | 9.36 × 10−1 | 8.30 × 107 | - | - | - |
| 1 day | 1.40 × 101 | 8.32 × 10−10 | 9.18 × 10−1 | 2.49 × 106 | - | - | - |
| 2 days | 2.39 × 101 | 7.57 × 10−10 | 9.31 × 10−1 | 1.00 × 106 | - | - | - |
| 7 days | 2.20 × 101 | 8.89 × 10−10 | 9.27 × 10−1 | 3.08 × 103 | - | - | - |
| 15 days | 3.68 × 102 | 3.97 × 10−9 | 7.68 × 10−1 | 5.68 × 104 | 5.01 × 10−6 | 5.90 × 10−1 | 4.84 × 106 |
| Sanded upper right | |||||||
| T0 | 4.00 × 101 | 9.10 × 10−10 | 9.45 × 10−1 | 9.54 × 107 | - | - | - |
| 1 day | 2.05 × 102 | 9.86 × 10−10 | 9.57 × 10−1 | 4.20 × 105 | - | - | . |
| 2 days | 1.80 × 101 | 1.15 × 10−9 | 9.46 × 10−1 | 3.88 × 105 | - | - | - |
| 7 days | 1.10 × 101 | 1.2 × 10−9 | 9.50 × 10−1 | 4.79 × 104 | - | - | - |
| 15 days | 2.00 × 101 | 1.81 × 10−9 | 9.24 × 10−1 | 4.43 × 104 | - | - | - |
| Sanded bottom left | |||||||
| T0 | 1.46 × 102 | 1.12 × 10−9 | 9.17 × 10−1 | 5.88 × 107 | - | - | - |
| 1 day | 1.28 × 101 | 9.58 × 10−10 | 9.42 × 10−1 | 9.60 × 105 | - | - | - |
| 2 days | 2.00 × 101 | 1.33 × 10−9 | 9.20 × 10−1 | 1.51 × 106 | - | - | - |
| 7 days | 2.27 × 101 | 1.17 × 10−9 | 9.38 × 10−1 | 2.74 × 105 | - | - | - |
| 15 days | 3.23 × 102 | 3.57 × 10−9 | 7.96 × 10−1 | 3.04 × 105 | 5.35 × 10−6 | 6.86 × 10−1 | 2.40 × 106 |
| Cc (F) | Cdl (F) | |
|---|---|---|
| Unsanded upper right | ||
| T0 | 2.21 × 10−10 | - |
| 1 g. | 3.30 × 10−10 | - |
| 2 gg. | 2.82 × 10−10 | - |
| 7 gg. | 2.89 × 10−10 | - |
| 15 gg. | 2.77 × 10−10 | - |
| Unsanded bottom left | ||
| T0 | 1.92 × 10−10 | - |
| 1 g. | 1.63 × 10−10 | - |
| 2 gg. | 2.02 × 10−10 | - |
| 7 gg. | 2.20 × 10−10 | - |
| 15 gg. | 6.86 × 10−11 | 6.31 × 10−8 |
| Sanded upper right | ||
| T0 | 3.36 × 10−10 | - |
| 1 g. | 4.93 × 10−10 | - |
| 2 gg. | 4.19 × 10−10 | - |
| 7 gg. | 4.62 × 10−10 | - |
| 15 gg. | 4.42 × 10−10 | - |
| Sanded bottom left | ||
| T0 | 2.72 × 10−10 | - |
| 1 g. | 3.12 × 10−10 | - |
| 2 gg. | 2.92 × 10−10 | - |
| 7 gg. | 3.69 × 10−10 | - |
| 15 gg. | 1.07 × 10−10 | 2.91 × 10−7 |
| Water Absorption φ (%) | ||||
|---|---|---|---|---|
| 1 d | 2 d | 7 d | 15 d | |
| 3.5%NaCl | ||||
| not sanded_top right | 3.2 | 4.98 | 5.66 | 6.13 |
| not sanded_bottom left | 1.17 | 2.43 | 4.50 | - |
| sanded_top right | 3.51 | 0.69 | 5.70 | 6.91 |
| sanded_bottom left | 1.33 | 2.54 | 3.95 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galiotto, R.; Zanetti, S.; Traini, R.; Pezzato, L. Physical–Mechanical and Corrosion Resistance Characterization of a Water-Based Epoxy Primer Applied to Galvanized Steel. Metals 2025, 15, 1196. https://doi.org/10.3390/met15111196
Galiotto R, Zanetti S, Traini R, Pezzato L. Physical–Mechanical and Corrosion Resistance Characterization of a Water-Based Epoxy Primer Applied to Galvanized Steel. Metals. 2025; 15(11):1196. https://doi.org/10.3390/met15111196
Chicago/Turabian StyleGaliotto, Rosalia, Solidea Zanetti, Rocco Traini, and Luca Pezzato. 2025. "Physical–Mechanical and Corrosion Resistance Characterization of a Water-Based Epoxy Primer Applied to Galvanized Steel" Metals 15, no. 11: 1196. https://doi.org/10.3390/met15111196
APA StyleGaliotto, R., Zanetti, S., Traini, R., & Pezzato, L. (2025). Physical–Mechanical and Corrosion Resistance Characterization of a Water-Based Epoxy Primer Applied to Galvanized Steel. Metals, 15(11), 1196. https://doi.org/10.3390/met15111196








