Investigation of Surface Stability and Behavior of Diamalloy 2002 Hard Coatings Under High-Temperature Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Substrates and Coatings
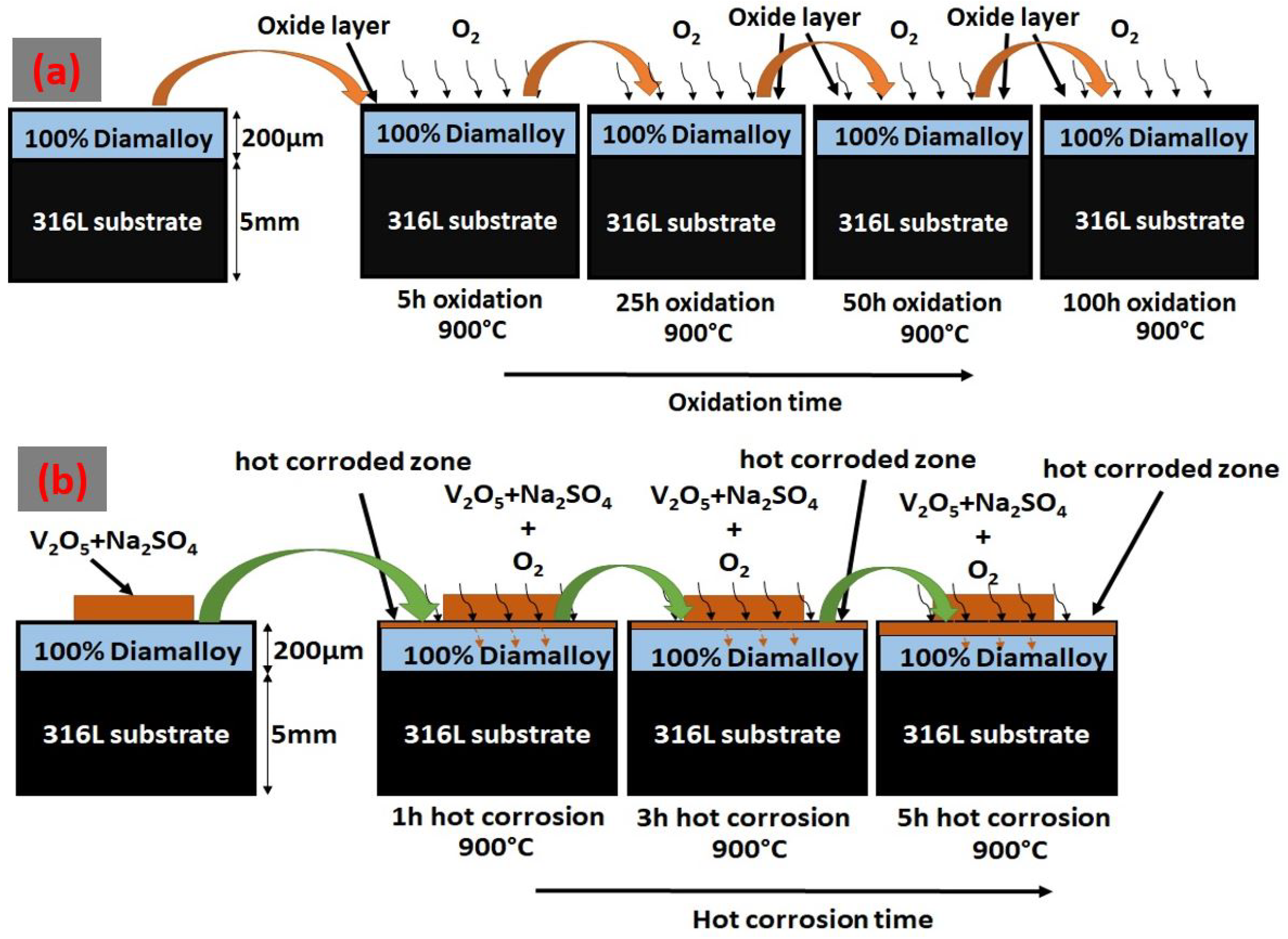
2.2. Investigations on Oxidation and Hot Corrosion
3. Results and Discussion
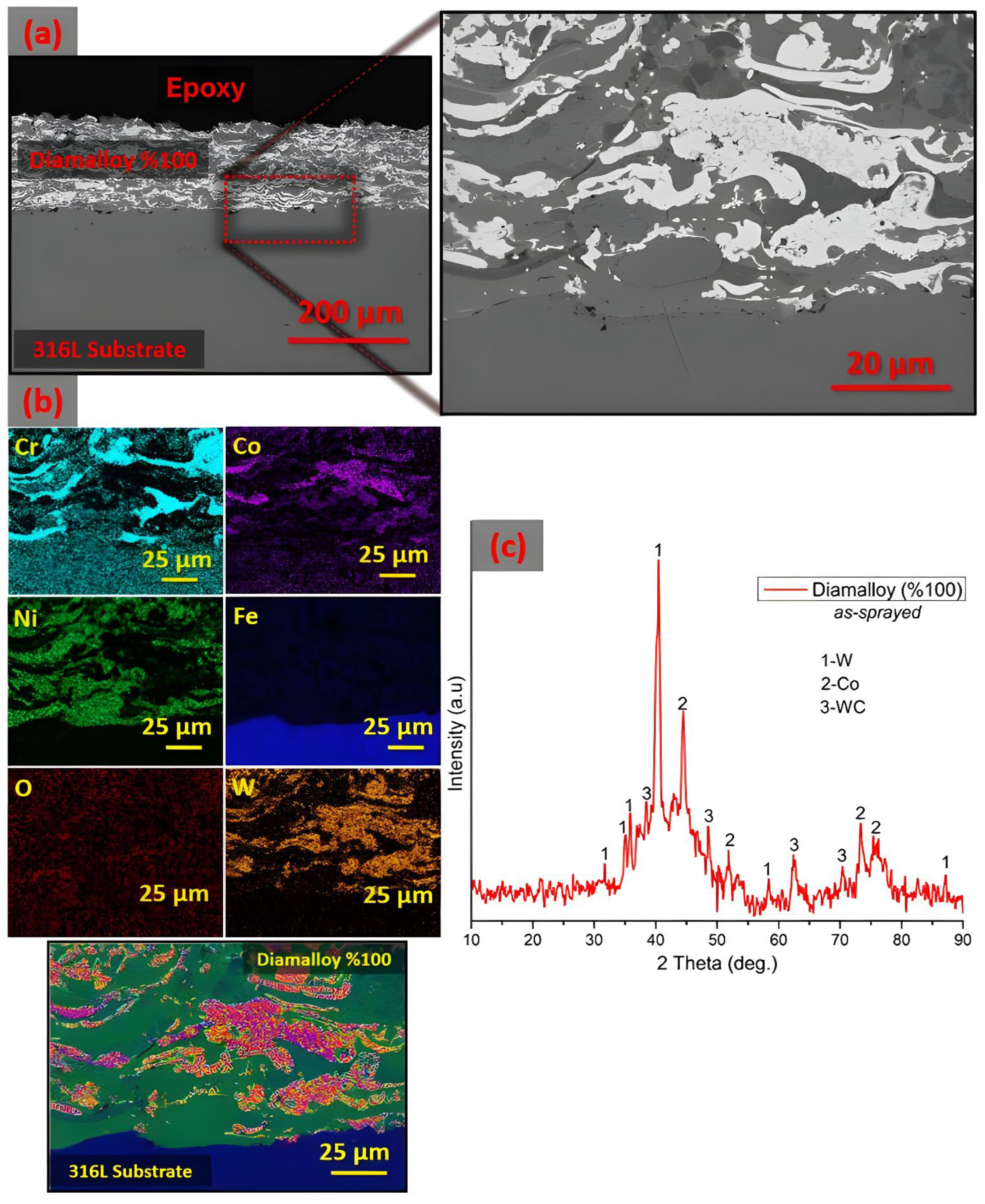
3.1. Characterization of As-Sprayed Coating System
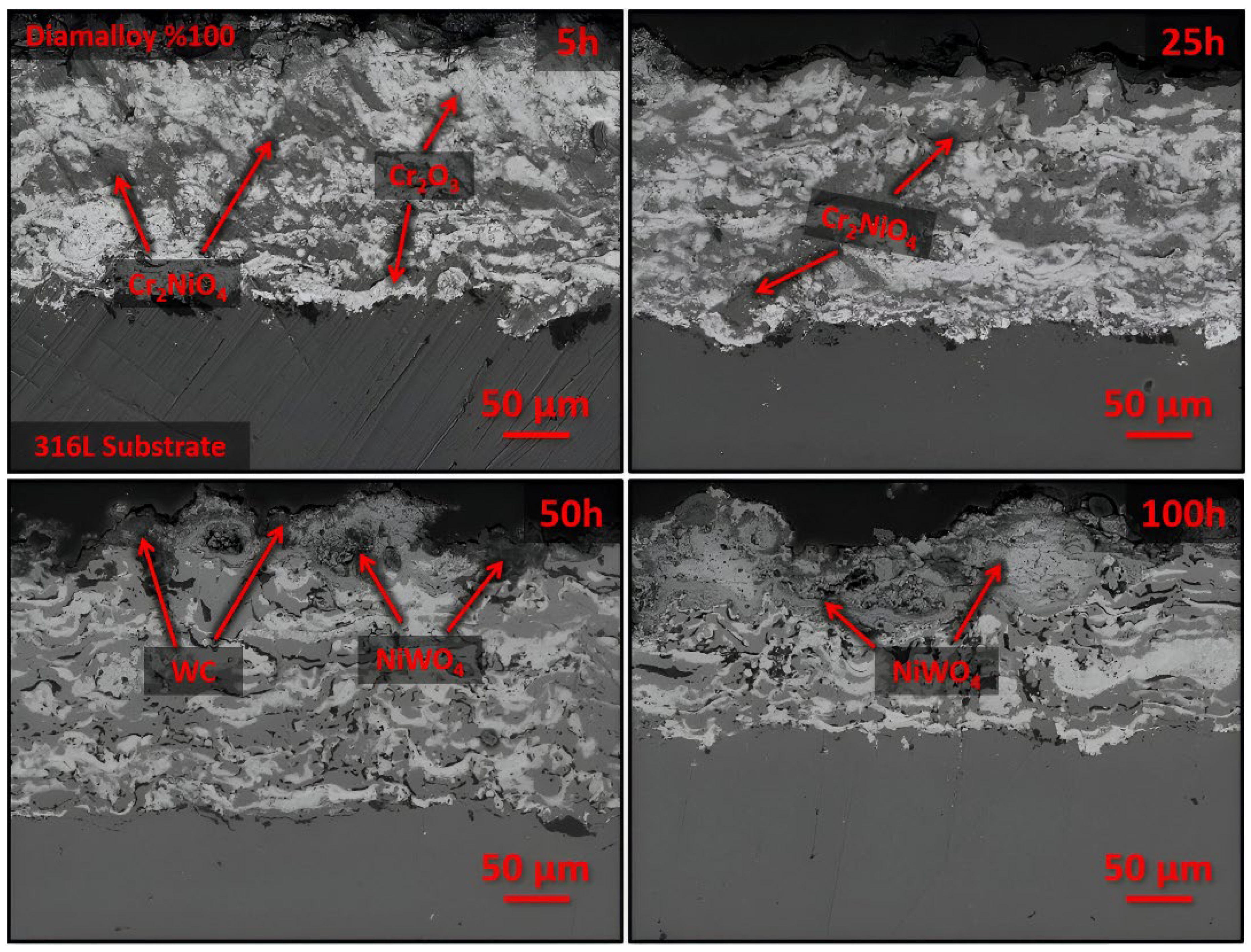
3.2. Oxidation Effect on Coating Systems
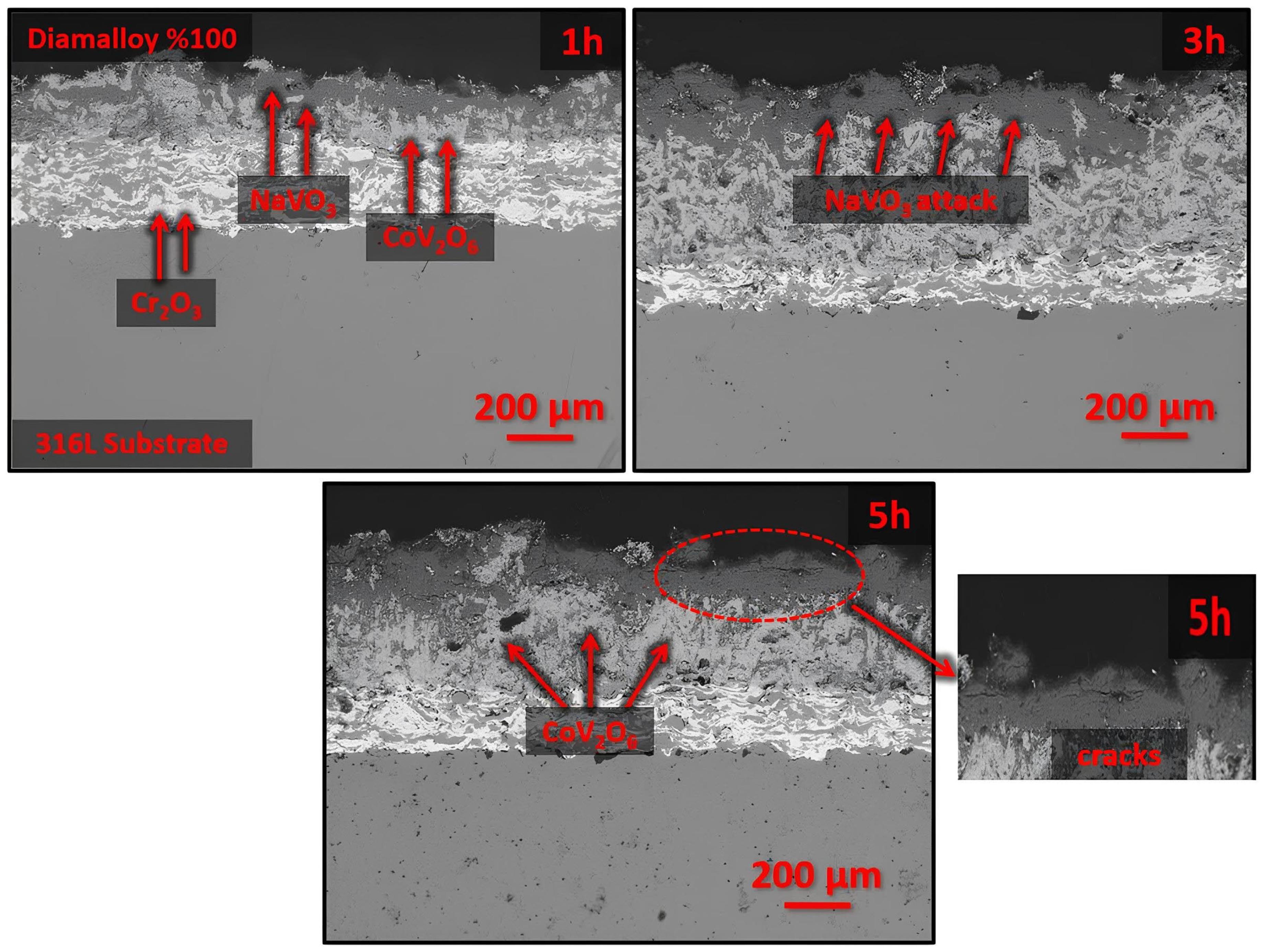
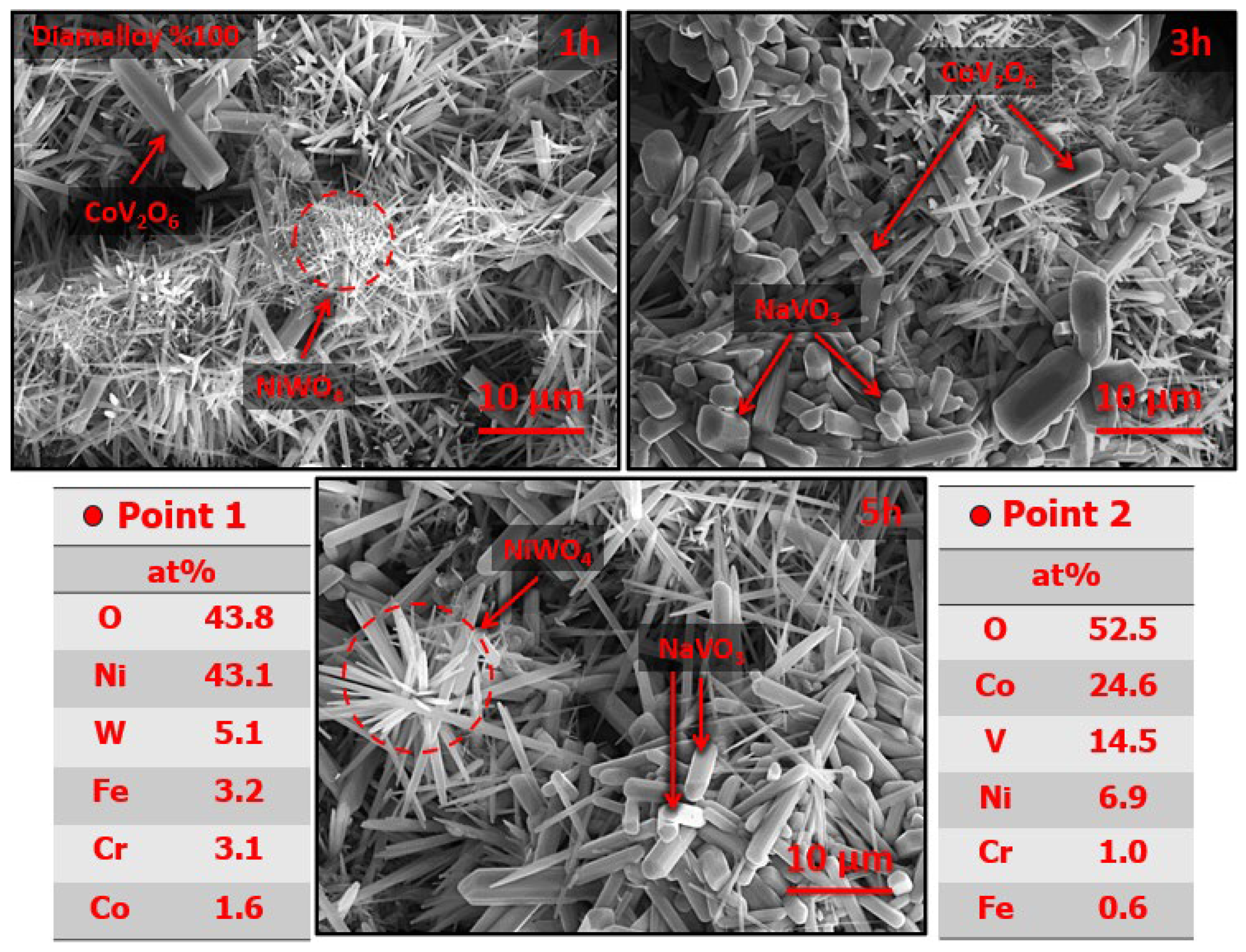
3.3. Effects of Hot Corrosion on the Coating System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sivakumar, R.; Mordike, B.L. High Temperature Coatings For Gas Turbine Blades: A Review. Surf. Coat. Technol. 1989, 37, 139–160. [Google Scholar] [CrossRef]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Sanz, Y.E.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef]
- Lakkannavar, V.; Yogesha, K.B.; Prasad, C.D.; Phanden, R.K.; Srinivasa, G.; Prasad, S.C. Thermal spray coatings on high-temperature oxidation and corrosion applications—A comprehensive review. Results Surf. Interfaces 2024, 16, 100250. [Google Scholar] [CrossRef]
- Al-Mutairi, S.; Hashmi, M.S.J.; Yilbas, B.S.; Stokes, J. Microstructural characterization of HVOF/plasma thermal spray of micro/nano WC-12%Co powders. Surf. Coat. Technol. 2015, 264, 175–186. [Google Scholar] [CrossRef]
- Lima, R.S.; Khor, K.A.; Li, H.; Cheang, P.; Marple, B.R. HVOF spraying of nanostructured hydroxyapatite for biomedical applications. Mater. Sci. Eng. A 2005, 396, 181–187. [Google Scholar] [CrossRef]
- Jadidi, M.; Moghtadernejad, S.; Dolatabadi, A. A comprehensive review on fluid dynamics and transport of suspension/liquid drop lets and particles in High-Velocity Oxygen-Fuel (HVOF) thermal spray. Coatings 2015, 5, 576–645. [Google Scholar] [CrossRef]
- Odabas, O.; Karaoglanli, A.C. Comparison of microstructural evolution and high temperature oxidation behavior of AlCoCrFeNiTi and AlCoCrFeNiZr high-entropy alloy coatings. Surf. Coat. Technol. 2024, 494, 131529. [Google Scholar] [CrossRef]
- Odabas, O.; Ozgurluk, Y.; Ozkan, D.; Binal, G.; Calis, I.; Karaoglanli, A.C. Investigation of vermiculite infiltration effect on microstructural properties of thermal barrier coatings (TBCs) produced by electron beam physical vapor deposition method (EB-PVD). Surf. Coat. Technol. 2022, 443, 128645. [Google Scholar] [CrossRef]
- Sharma, J.; Bhandari, A.; Jangra, S.; Goyat, M.S. Sol–gel derived highly hydrophobic Polystyrene/SiO2 spray coatings on polished stainless steel and textured aluminium substrates. Trans. Inst. Met. Finish. 2024, 102, 77–82. [Google Scholar] [CrossRef]
- Kong, Z.; Wang, X.; Hu, N.; Jin, Y.; Tao, Q.; Xia, W.; Lin, X.M.; Vasdravellis, G. Mechanical properties of SLM 316 L stainless steel plate before and after exposure to elevated temperature. Constr. Build. Mater. 2024, 444, 137786. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, S.; Ma, J.; Li, J.; Mou, L. Understanding the effect of decreasing C contents and increasing solid-solution time on intergranular corrosion resistance of 304 austenitic stainless steel. J. Mater. Res. Technol. 2024, 30, 47504761. [Google Scholar] [CrossRef]
- Lakkannavar, V.; Yogesha, K.B.; Prasad, C.D.; Suresh, R.; Lakshmikanthan, A.; Hanumanthlal, S. Investigation of cyclic oxidation and corrosion behavior of plasma-sprayed NiCrAlY/Cr3C2/h-BN coatings on T22 boiler steel alloy. Surf. Coat. Technol. 2024, 492, 131205. [Google Scholar] [CrossRef]
- Bogdan, M.; Peter, I. A Comprehensive Understanding of Thermal Barrier Coatings (TBCs): Applications, Materials, Coating Design and Failure Mechanisms. Metals 2024, 14, 575. [Google Scholar] [CrossRef]
- Sun, H.; Zou, B.; Wang, X.; Chen, W.; Zhang, G.; Quan, T.; Huang, C. Advancements in multi-material additive manufacturing of advanced ceramics: A review of strategies, techniques and equipment. Mater. Chem. Phys. 2024, 319, 129337. [Google Scholar] [CrossRef]
- Tuli, N.T.; Khatun, S.; Rashid, A.B. Unlocking the future of precision manufacturing: A comprehensive exploration of 3D printing with fiber-reinforced composites in aerospace, automotive, medical, and consumer industries. Heliyon 2024, 10, e27328. [Google Scholar] [CrossRef]
- Özorak, C.; Islak, S. Microstructure, wear and corrosion properties of Cu–SiC/WCCo composite coatings on the Cu substrate surface by plasma spray method. Mater. Chem. Phys. 2024, 314, 128903. [Google Scholar] [CrossRef]
- Stanford, M.K.; Jain, V.K. Friction and wear characteristics of hard coatings. Wear 2001, 251, 990–996. [Google Scholar] [CrossRef]
- Gisario, A.; Barletta, M.; Veniali, F. Laser surface modification (LSM) of thermally-sprayed Diamalloy 2002 coating. Opt. Laser Technol. 2012, 44, 1942–1958. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, W.; He, D.; Huang, Y.; Cai, Z.; Zhou, L.; Xing, Z.; Wang, H. Study on preparation and wear resistance of NiCrBSi-WC/Co composite coatings by pulsed magnetic field assisted supersonic plasma spraying. Surf. Coat. Technol. 2022, 448, 128897. [Google Scholar] [CrossRef]
- Pawlowski, L. The Science and Engineering of Thermal Spray Coatings, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Tucker, R.C., Jr. (Ed.) Handbook of Thermal Spray Technology; ASM International: Materials Park, OH, USA, 2013. [Google Scholar]
- Mayrhofer, P.H.; Mitterer, C.; Hultman, L.; Clemens, H. Microstructural design of hard coatings. Prog. Mater. Sci. 2006, 51, 1032–1114. [Google Scholar] [CrossRef]
- Fraser, R.; Girtan, M. A Selective Review of Ceramic, Glass and Glass–Ceramic Protective Coatings: General Properties and Specific Characteristics for Solar Cell Applications. Materials 2023, 16, 3906. [Google Scholar] [CrossRef]
- Raoelison, R.N.; Xie, Y.; Sapanathan, T.; Planche, M.P.; Kromer, R.; Costil, S.; Langlade, C. Cold gas dynamic spray technology: A comprehensive review of processing conditions for various technological developments till to date. Addit. Manuf. 2018, 19, 134–159. [Google Scholar] [CrossRef]
- Veselá, K. Atomic Layer Deposition. Bachelor’s Thesis, Czech Technical University in Prague, Prague, Czechia, 2021. [Google Scholar]
- Nalwa, H.S. Deposition and Processing of Thin Films; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Yao, Z.; Li, W. Microstructure and thermal analysis of APS nano PYSZ coated aluminum alloy piston. J. Alloys Compd. 2020, 812, 152162. [Google Scholar] [CrossRef]
- Hui, R.; Wang, Z.; Kesler, O.; Rose, L.; Jankovic, J.; Yick, S.; Maric, R.; Ghosh, D. Thermal plasma spraying for SOFCs: Applications, potential advantages, and challenges. J. Power Sources 2007, 170, 308–323. [Google Scholar] [CrossRef]
- Odhiambo, J.G.; Li, W.G.; Zhao, Y.T.; Li, C.L. Porosity and its significance in plasma-sprayed coatings. Coatings 2019, 9, 460. [Google Scholar] [CrossRef]
- Ren, X.; Wang, F. High-temperature oxidation and hot-corrosion behavior of a sputtered NiCrAlY coating with and without aluminizing. Surf. Coat. Technol. 2006, 201, 30–37. [Google Scholar] [CrossRef]
- Cai, J.; Li, X.; Lyu, P.; Guan, Q.; Lu, J.; Xue, W. Hot corrosion behavior of NiCoCrAlYSi laser cladding coating modified using high-current pulsed electron beam in different corrosive salt environments. Mater. Charact. 2024, 208, 113565. [Google Scholar] [CrossRef]
- Huang, H.; Wang, W.; Yi, G.; Shan, Y.; Wan, S.; Zhang, G.; Du, X.; Wan, M. Influences of oxidation and hot corrosion on the tribological properties of CoCrNiWMo alloy and the preliminary exploration of the interaction between hot corrosion and wear. Mater. Chem. Phys. 2024, 318, 129308. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Wang, J. High-temperature oxidation behavior of HVOF-sprayed Diamalloy 2002 coatings. Surf. Coat. Technol. 2018, 349, 123–132. [Google Scholar]
- Kumar, R.; Singh, P.; Verma, K. Oxidation resistance of APS-sprayed Diamalloy 2002 coatings at 850–950 °C. J. Therm. Spray Technol. 2020, 29, 987–996. [Google Scholar]
- Yang, S.; Gao, S.; Xue, W.; Wu, B.; Duan, D. Oxidation and hot corrosion behaviors of NiAlTa protective material for turbine single crystal blade tips. Corros. Sci. 2024, 230, 111899. [Google Scholar] [CrossRef]
- Rahimi, J.; Choupani, N.; Poursaeidi, E.; Montakhabi, F.; Sigaroodi, M.J.; Jamalabad, Y.Y. Investigation of the hot corrosion effect son the lifetime of TBCs with and without TC. J. Alloys Compd. 2024, 1004, 175840. [Google Scholar] [CrossRef]
- Gao, Y.; Chong, K.; Liu, C.; Cao, Y.; Wu, D.; Zou, Y. Electrochemical corrosion and high temperature hot corrosion behavior of NbTaTiV and CrNbTaTiV high entropy alloy. J. Mater. Res. Technol. 2024, 28, 216–234. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Toor, I.H.; Patel, F.; Al-Shehri, Y.; Baig, M.A. HVOF Diamalloy 2002 coating of steel surface: Electrochemical corrosion resistance. Ind. Lubr. Tribol. 2015, 67, 119–123. [Google Scholar] [CrossRef]
- Yuan, J.; Zhu, Y.; Zheng, X.; Ruan, Q.; Ji, H. Improvement in tribological properties of atmospheric plasma-sprayed WC-Co coating followed by Cu electrochemical impregnation. Appl. Surf. Sci. 2009, 255, 7959–7965. [Google Scholar] [CrossRef]
- De, H.L.; Lovelock, V. Powder/Processing/Structure Relationships in WC-Co Thermal Spray Coatings: A Review of the Published Literature. J. Therm. Spray Technol. 1998, 7, 357–373. [Google Scholar]
- El Rayes, M.M.; Abdo, H.S.; Khalil, K.A. Erosion—Corrosion of Cermet Coating. Int. J. Electrochem. Sci. 2013, 8, 1117–1137. [Google Scholar] [CrossRef]
- Sampath, S.; Schulz, U.; Jarligo, M.O.; Kuroda, S. Processing science of advanced thermal-barrier systems. MRS Bull. 2012, 37, 903–910. [Google Scholar] [CrossRef]
- Martena, M.; Botto, D.; Fino, P.; Sabbadini, S.; Gola, M.M.; Badini, C. Modelling of TBC system failure: Stress distribution as a function of TGO thickness and thermal expansion mismatch. Eng. Fail. Anal. 2006, 13, 409–426. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Zhang, W.W.; Yi, P. Simulation of thermal barrier coating spallation induced by the initiation/growth/coalescence of internal crack and interfacial crack based on a real image model. Ceram. Int. 2022, 48, 24888–24897. [Google Scholar] [CrossRef]
- Gao, R.; Ye, X.X.; Yan, S.; Lu, Y.; Jiang, L.; Li, Z.; Zhou, X. Effects of tungsten content on the high-temperature oxidation behavior of Ni-xW-6Cr alloys. Corros. Sci. 2019, 149, 87–99. [Google Scholar] [CrossRef]
- Schlieter, A.; Shakhverdova, I.; Leyens, C. Fabrication of Riblet Structures on a Ni-based Superalloy (PWA1483) for Potential Drag Reduction in High Temperature Applications Based on Laser Optimization. Adv. Eng. Mater. 2015, 17, 1008–1016. [Google Scholar] [CrossRef]
- Solecka, M.; Kusiński, J.; Kopia, A.; Rozmus-Górnikowska, M.; Radziszewska, A. High-Temperature Corrosion of Ni-Base Alloys by Waste Incineration Ashes. Acta Phys. Pol. A 2016, 130, 1045–1048. [Google Scholar] [CrossRef]
- Lee, D.B.; Ko, J.H.; Kwon, S.C. Oxidation of Ni-W coatings at 700 and 800 °C in air. Surf. Coat. Technol. 2005, 193, 292–296. [Google Scholar] [CrossRef]
- Yun, D.W.; Seo, S.M.; Jeong, H.W.; Yoo, Y.S. Effect of refractory elements and Al on the high temperature oxidation of Ni-base superalloys and modelling of their oxidation resistance. J. Alloys Compd. 2017, 710, 8–19. [Google Scholar] [CrossRef]
- Rapp, R.A.; Zhang, Y.S. Hot Corrosion of Materials: Fundamental Studies. JOM 1994, 46, 47–55. [Google Scholar] [CrossRef]
- Sidhu, H.S.; Sidhu, B.S.; Prakash, S. The role of HVOF coatings in improving hot corrosion resistance of ASTM-SA210 GrA1 steel in the presence of Na2SO4-V2O5 salt deposits. Surf. Coat. Technol. 2006, 200, 5386–5394. [Google Scholar] [CrossRef]
- Habibi, M.H.; Wang, L.; Liang, J.; Guo, S.M. An investigation on hot corrosion behavior of YSZ-Ta2O5 in Na2SO4+V2O5 salt at 1100 °C. Corros. Sci. 2013, 75, 409–414. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Zou, B.; Zhou, X.; Dong, S.; Li, L.; Jiang, J.; Deng, L.; Cao, X. Hot corrosion behaviour of nanostructured zirconia in molten NaVO3 salt. Ceram. Int. 2017, 43, 10415–10427. [Google Scholar] [CrossRef]
- Khan, I.; Gu, Y.; Wooh, S. Shape-Controlled First-Row Transition Metal Vanadates for Electrochemical and Photoelectrochemical Water Splitting. Chem. Rec. 2024, 24, e202300127. [Google Scholar] [CrossRef]
- Saxena, N.; Kumar, P. ABO4 and AB2O6 structured metal oxide-based gas sensors. In Complex and Composite Metal Oxides for Gas, VOC and Humidity Sensors; Volume 2: Technology and New Trends; Elsevier: Amsterdam, The Netherlands, 2024; pp. 385–404. [Google Scholar] [CrossRef]
- Lenertz, M.; Alaria, J.; Stoeffler, D.; Colis, S.; Dinia, A. Magnetic properties of low-dimensional α and γ CoV2O6. J. Phys. Chem. C 2011, 115, 17190–17196. [Google Scholar] [CrossRef]
- Kurzawa, M.; Bosacka, M. Phase Relations in the Subsolidus Area of the Cov2O6-ComoO4-Coo Subsystem Included by the Ternary Coo-V2O5-MoO3 System. J. Therm. Anal. Calorim. 2001, 65, 451–455. [Google Scholar] [CrossRef]


| Current | 500 A |
|---|---|
| Voltage | 65 V |
| Ar flow | 55 (L.min−1) |
| H2 flow | 8 (L.min−1) |
| Powder feed rate | 25 g/min |
| Stand-off distance | 200 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozbek, Y.Y.; Odabas, O.; Binal, G.; Ozgurluk, Y.; Karaoglanli, A.C. Investigation of Surface Stability and Behavior of Diamalloy 2002 Hard Coatings Under High-Temperature Conditions. Metals 2025, 15, 1169. https://doi.org/10.3390/met15111169
Ozbek YY, Odabas O, Binal G, Ozgurluk Y, Karaoglanli AC. Investigation of Surface Stability and Behavior of Diamalloy 2002 Hard Coatings Under High-Temperature Conditions. Metals. 2025; 15(11):1169. https://doi.org/10.3390/met15111169
Chicago/Turabian StyleOzbek, Yildiz Yarali, Okan Odabas, Gulfem Binal, Yasin Ozgurluk, and Abdullah Cahit Karaoglanli. 2025. "Investigation of Surface Stability and Behavior of Diamalloy 2002 Hard Coatings Under High-Temperature Conditions" Metals 15, no. 11: 1169. https://doi.org/10.3390/met15111169
APA StyleOzbek, Y. Y., Odabas, O., Binal, G., Ozgurluk, Y., & Karaoglanli, A. C. (2025). Investigation of Surface Stability and Behavior of Diamalloy 2002 Hard Coatings Under High-Temperature Conditions. Metals, 15(11), 1169. https://doi.org/10.3390/met15111169







