Enhancing Biomedical Metal 3D Printing with AI and Nanomaterials Integration
Abstract
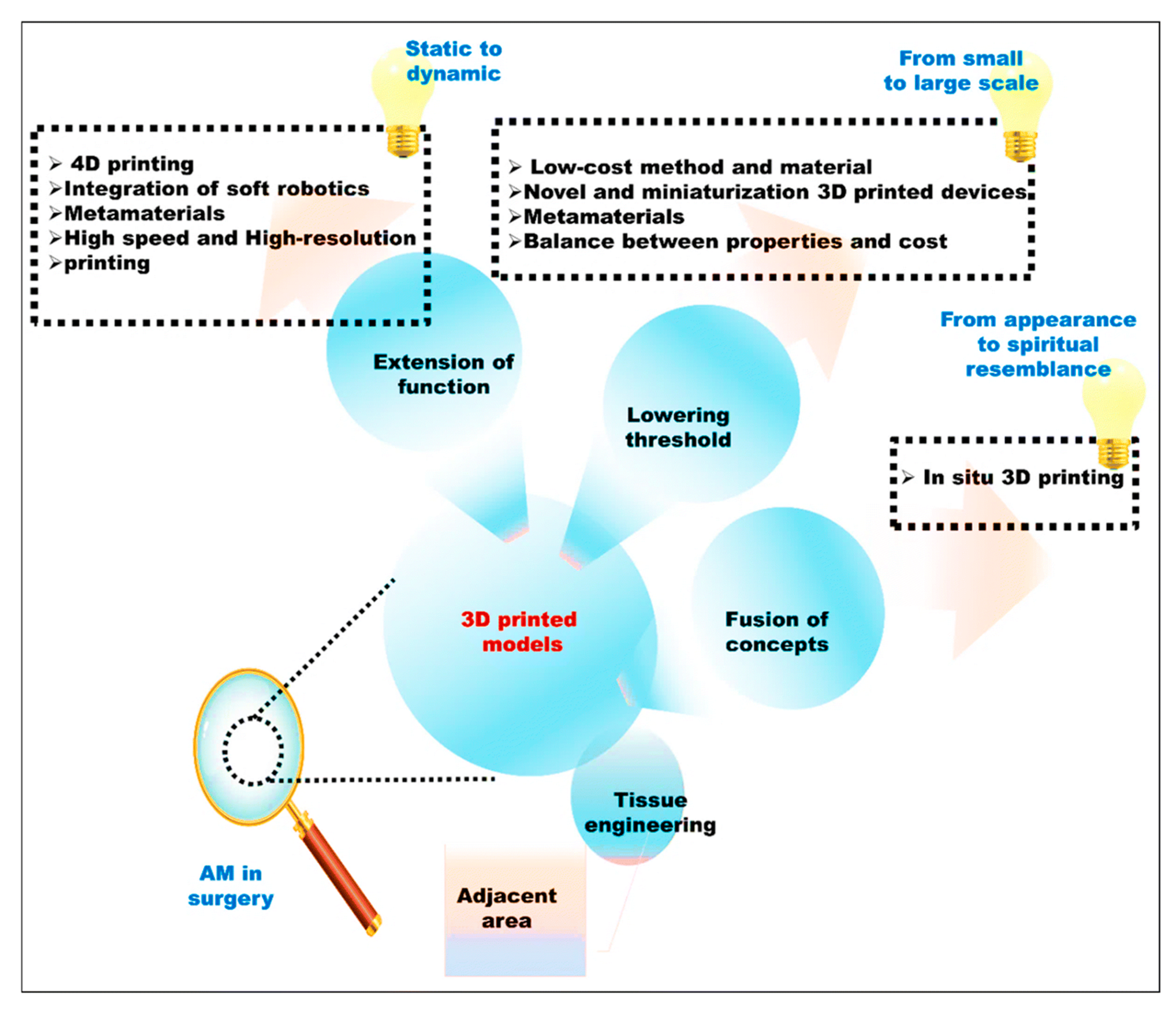
1. Introduction
2. Role of Artificial Intelligence in Metal 3D Printing Application
2.1. Design Optimization
2.1.1. Topology Optimization Techniques
2.1.2. Generative Design
2.1.3. Machine Learning (ML)
2.2. Process Monitoring and Control
2.3. Predictive Analysis
2.4. Limitations and Future Outlook
2.5. Biomedical Relevance of Metal Additive Manufacturing Process Using AI
3. Nanomaterials in Metal 3D Printing Applications
3.1. Mechanical Strength and Wear Resistance
3.2. Biocompatibility and Antimicrobial Properties
3.3. Controlled Degradation
4. AI-Guided Material Discovery and Process Optimization
4.1. Identification of Novel Metal Matrix Nanocomposites
4.2. Optimization of Sintering and Laser Parameters
4.2.1. Modeling of Physical Processes
4.2.2. Real-Time Monitoring and Adaptive Control
4.3. Reducing Trial and Error Experimentation
4.3.1. Digital Twins for Closed-Loop Process Prediction
4.3.2. Transfer Learning and Generative Optimization
5. Biomedical Application of Metal 3D Printing with AI and Nanomaterials Integration
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karunakar, K.K.; Cheriyan, B.V.; Nandhini, J.; Kataria, K.; Yabase, L.; Devan, P.; Kannan, M.S. Comprehensive Review of Biomedical Metals and Strategies for Advancing Regenerative Medicine. Biomed. Mater. Devices 2025, 1–33. [Google Scholar] [CrossRef]
- Dixit, S.; Gupta, S.; Sharma, A. Surgical Devices for Biomedical Implants. In Additive Manufacturing for Biomedical Applications: Recent Trends and Challenges; Springer: Berlin/Heidelberg, Germany, 2024; pp. 195–218. [Google Scholar]
- Ashraf, M.; Gibson, I.; Rashed, M.G. Challenges and prospects of 3D printing in structural engineering. In Proceedings of the 13th International Conference on Steel, Space and Composite Structures, Perth, Australia, 31 January–2 February 2018. [Google Scholar]
- Kumar, A.; Kumar, D.; Faisal, N.; Sharma, A.; Kumar Ansu, A.; Goyal, A.; Saxena, K.K.; Prakash, C.; Kumar, D. Application of 3D printing technology for medical implants: A state-of-the-art review. Adv. Mater. Process. Technol. 2024, 10, 357–372. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Chandra, L. AI-Enhanced Additive Manufacturing: Intelligent 3d Printing for Complex Designs. J. Primeasia 2024, 5, 1–9. [Google Scholar] [CrossRef]
- Ji, Z.; Wan, Y.; Zou, Y.; Wang, H. Enhancement of osteogenic and antibacterial properties of 3D-printed porous Ti-6Al-4V implants using multi-scale composite structures and ZnO nanoparticles. J. Alloys Compd. 2025, 1021, 179620. [Google Scholar] [CrossRef]
- Ng, W.L.; Goh, G.L.; Goh, G.D.; Ten, J.S.J.; Yeong, W.Y. Progress and opportunities for machine learning in materials and processes of additive manufacturing. Adv. Mater. 2024, 36, 2310006. [Google Scholar] [CrossRef]
- Kumar, S.; Gopi, T.; Harikeerthana, N.; Gupta, M.K.; Gaur, V.; Krolczyk, G.M.; Wu, C. Machine learning techniques in additive manufacturing: A state of the art review on design, processes and production control. J. Intell. Manuf. 2023, 34, 21–55. [Google Scholar] [CrossRef]
- Nogueira, R.; Eguchi, M.; Kasmirski, J.; de Lima, B.V.; Dimatos, D.C.; Lima, D.L.; Glatter, R.; Tran, D.L.; Piccinini, P.S. Machine learning, deep learning, artificial intelligence and aesthetic plastic surgery: A qualitative systematic review. Aesthetic Plast. Surg. 2025, 49, 389–399. [Google Scholar] [CrossRef]
- Fallah Madvari, R. Artificial intelligence (AI), machine learning (ML) and deep learning (DL) on health, safety and environment (HSE). Arch. Occup. Health 2022, 6, 1321–1322. [Google Scholar] [CrossRef]
- Soori, M.; Jough, F.K.G.; Dastres, R.; Arezoo, B. Additive manufacturing modification by artificial intelligence, machine learning, and deep learning: A review. Addit. Manuf. Front. 2025, 4, 200198. [Google Scholar] [CrossRef]
- Boretti, A. A perspective on 3D printing in the medical field. Ann. 3D Print. Med. 2024, 13, 100138. [Google Scholar] [CrossRef]
- Živković, M.; Žujović, M.; Milošević, J. Architectural 3D-Printed structures created using artificial intelligence: A review of techniques and applications. Appl. Sci. 2023, 13, 10671. [Google Scholar] [CrossRef]
- Rojek, I.; Mikołajewski, D.; Dostatni, E.; Macko, M. AI-optimized technological aspects of the material used in 3D printing processes for selected medical applications. Materials 2020, 13, 5437. [Google Scholar] [CrossRef]
- Han, F.; Huang, X.; Wang, X.; Chen, Y.f.; Lu, C.; Li, S.; Lu, L.; Zhang, D.W. Artificial Intelligence in Orthopedic Surgery: Current Applications, Challenges, and Future Directions. MedComm 2025, 6, e70260. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Jung, Y.; Kim, S.; Lee, I.; Kang, N. Deep generative design: Integration of topology optimization and generative models. J. Mech. Des. 2019, 141, 111405. [Google Scholar] [CrossRef]
- Senhora, F.V.; Chi, H.; Zhang, Y.; Mirabella, L.; Tang, T.L.E.; Paulino, G.H. Machine learning for topology optimization: Physics-based learning through an independent training strategy. Comput. Methods Appl. Mech. Eng. 2022, 398, 115116. [Google Scholar] [CrossRef]
- Müller, L.; Schumacher, N.; Steffen, L.; Haubelt, C. Generative Design of the Architecture Platform in Multiprocessor System Design. Electronics 2024, 13, 1404. [Google Scholar] [CrossRef]
- Peto, M.; García-Ávila, J.; Rodriguez, C.A.; Siller, H.R.; da Silva, J.V.L.; Ramírez-Cedillo, E. Review on structural optimization techniques for additively manufactured implantable medical devices. Front. Mech. Eng. 2024, 10, 1353108. [Google Scholar] [CrossRef]
- Jhunjhunwala, P.; Kishor, A.; Burela, R.G.; Singh, R.; Gupta, A. Finite element analysis and topology optimization of Ti-6Al-4V hip implant fabricated by laser powder bed fusion process. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2022, 239, 1607–1615. [Google Scholar] [CrossRef]
- Sharma, N.; Ostas, D.; Rotar, H.; Brantner, P.; Thieringer, F.M. Design and additive manufacturing of a biomimetic customized cranial implant based on voronoi diagram. Front. Physiol. 2021, 12, 647923. [Google Scholar] [CrossRef]
- Guo, K.; Yang, Z.; Yu, C.-H.; Buehler, M.J. Artificial intelligence and machine learning in design of mechanical materials. Mater. Horiz. 2021, 8, 1153–1172. [Google Scholar] [CrossRef]
- Tang, T.; Wang, L.; Zhu, M.; Zhang, H.; Dong, J.; Yue, W.; Xia, H. Topology Optimization: A Review for Structural Designs Under Statics Problems. Materials 2024, 17, 5970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Khandelwal, K.; Guo, T. Finite strain topology optimization with nonlinear stability constraints. Comput. Methods Appl. Mech. Eng. 2023, 413, 116119. [Google Scholar] [CrossRef]
- Moscatelli, E.; Gonçalves, J.F.; Lino, C.E.; da Silva, A.L.F.; de Sá, L.F.N.; Silva, E.C.N. Topology optimization with pressure loads and finite deformation. Int. J. Mech. Sci. 2025, 303, 110603. [Google Scholar] [CrossRef]
- Zhou, Y.; Nomura, T.; Saitou, K. Anisotropic multicomponent topology optimization for additive manufacturing with build orientation design and stress-constrained interfaces. J. Comput. Inf. Sci. Eng. 2021, 21, 011007. [Google Scholar] [CrossRef]
- Wenzhi, G.; Yongtao, S.; Lu, N.; Gang, C. Fluid-structure interaction simulation for multi-body flexible morphing structures. Chin. J. Aeronaut. 2024, 37, 137–147. [Google Scholar]
- Kamat, H.; Pai, A.; Vernekar, N.K.; Kini, C.R.; Shenoy, S.B. Topological optimization and fatigue life prediction of a single pad externally adjustable fluid film bearing. Sci. Rep. 2024, 14, 13346. [Google Scholar] [CrossRef]
- Pramanik, R.; Verstappen, R.; Onck, P. Computational fluid–structure interaction in biology and soft robots: A review. Phys. Fluids 2024, 36, 101302. [Google Scholar] [CrossRef]
- Saadi, J.I.; Yang, M.C. Generative design: Reframing the role of the designer in early-stage design process. J. Mech. Des. 2023, 145, 041411. [Google Scholar] [CrossRef]
- Koul, P. A review of generative design using machine learning for additive manufacturing. Adv. Mech. Mater. Eng. 2024, 41, 145–159. [Google Scholar] [CrossRef]
- Regenwetter, L.; Nobari, A.H.; Ahmed, F. Deep generative models in engineering design: A review. J. Mech. Des. 2022, 144, 071704. [Google Scholar] [CrossRef]
- Danhaive, R.; Mueller, C.T. Design subspace learning: Structural design space exploration using performance-conditioned generative modeling. Autom. Constr. 2021, 127, 103664. [Google Scholar] [CrossRef]
- Urquhart, L.; Wodehouse, A.; Loudon, B.; Fingland, C. The application of generative algorithms in human-centered product development. Appl. Sci. 2022, 12, 3682. [Google Scholar] [CrossRef]
- Zhang, Y.; Wong, L.N.Y. A review of numerical techniques approaching microstructures of crystalline rocks. Comput. Geosci. 2018, 115, 167–187. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Kang, N. Generative AI-driven design optimization: Eight key application scenarios. JMST Adv. 2025, 7, 105–111. [Google Scholar] [CrossRef]
- Ladani, L.J. Applications of artificial intelligence and machine learning in metal additive manufacturing. J. Phys. Mater. 2021, 4, 042009. [Google Scholar] [CrossRef]
- Equbal, A.; Equbal, A.; Khan, Z.A.; Badruddin, I.A. Machine learning in additive manufacturing: A comprehensive insight. Int. J. Lightweight Mater. Manuf. 2025, 8, 264. [Google Scholar] [CrossRef]
- Zhang, R.; Strickland, J.; Hou, X.; Yang, F.; Li, X.; de Oliveira, J.A.; Li, J.; Zhang, S. Rapid residual stress simulation and distortion mitigation in laser additive manufacturing through machine learning. Addit. Manuf. 2025, 102, 104721. [Google Scholar] [CrossRef]
- Gan, Z.; Kafka, O.L.; Parab, N.; Zhao, C.; Heinonen, O.; Sun, T.; Liu, W. Universal low-dimensional scaling laws in 3d printing of metals. arXiv 2020, arXiv:2005.00117. [Google Scholar]
- Herzog, T.; Brandt, M.; Trinchi, A.; Sola, A.; Molotnikov, A. Process monitoring and machine learning for defect detection in laser-based metal additive manufacturing. J. Intell. Manuf. 2024, 35, 1407–1437. [Google Scholar] [CrossRef]
- Khanafer, K.; Cao, J.; Kokash, H. Condition monitoring in additive manufacturing: A critical review of different approaches. J. Manuf. Mater. Process. 2024, 8, 95. [Google Scholar] [CrossRef]
- Mahamud, Z.H.; Khan, M.R.; Amin, J.M.; Islam, M.S. AI For Defect Detection in Additive Manufacturing: Applications In Renewable Energy And Biomedical Engineering. Strateg. Data Manag. Innov. 2025, 2, 1–20. [Google Scholar] [CrossRef]
- Gan, Y.; He, K.; Yu, B.; Dong, H.; Zhou, Z.; Dong, C. Influence of process parameters on the quality of laser powder bed fusion of Alnico. J. Alloys Compd. 2025, 1022, 179791. [Google Scholar] [CrossRef]
- Rastak, M.; Vanaei, S.; Vanaei, S.; Moezzibadi, M. Machine Learning in 3D Printing. Ind. Strateg. Solut. 3D Print. Appl. Optim. 2024, 273–294. [Google Scholar] [CrossRef]
- Wang, R.; Standfield, B.; Dou, C.; Law, A.C.; Kong, Z.J. Real-time process monitoring and closed-loop control on laser power via a customized laser powder bed fusion platform. Addit. Manuf. 2023, 66, 103449. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Zhu, Q.; Cao, Y. Closed-Loop Control of Melt Pool Temperature during Laser Metal Deposition. Sensors 2024, 24, 5020. [Google Scholar] [CrossRef]
- Gunasegaram, D.R.; Barnard, A.S.; Matthews, M.J.; Jared, B.H.; Andreaco, A.M.; Bartsch, K.; Murphy, A.B. Machine learning-assisted in-situ adaptive strategies for the control of defects and anomalies in metal additive manufacturing. Addit. Manuf. 2024, 81, 104013. [Google Scholar] [CrossRef]
- Syafrudin, M.; Alfian, G.; Fitriyani, N.L.; Rhee, J. Performance analysis of IoT-based sensor, big data processing, and machine learning model for real-time monitoring system in automotive manufacturing. Sensors 2018, 18, 2946. [Google Scholar] [CrossRef]
- Chinchanikar, S.; Shaikh, A.A. A review on machine learning, big data analytics, and design for additive manufacturing for aerospace applications. J. Mater. Eng. Perform. 2022, 31, 6112–6130. [Google Scholar] [CrossRef]
- Bendoly, E.; Chandrasekaran, A.; Lima, M.d.R.F.; Handfield, R.; Khajavi, S.H.; Roscoe, S. The role of generative design and additive manufacturing capabilities in developing human–AI symbiosis: Evidence from multiple case studies. Decis. Sci. 2024, 55, 325–345. [Google Scholar] [CrossRef]
- Leng, Y.; Sun, R. Multifunctional Biomedical Devices with Closed-Loop Systems for Precision Therapy. Adv. Healthc. Mater. 2025, 2500860. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.; Parvizi, S.; Baghbanijavid, H.; Tan, A.T.; Nematollahi, M.; Ramazani, A.; Fang, N.X.; Elahinia, M. Computational modelling of process–structure–property–performance relationships in metal additive manufacturing: A review. Int. Mater. Rev. 2022, 67, 1–46. [Google Scholar] [CrossRef]
- Babu, S.S.; Mourad, A.-H.I.; Harib, K.H.; Vijayavenkataraman, S. Recent developments in the application of machine-learning towards accelerated predictive multiscale design and additive manufacturing. Virtual Phys. Prototyp. 2023, 18, e2141653. [Google Scholar] [CrossRef]
- Zhao, S.; Shi, Y.; Huang, C.; Li, X.; Lu, Y.; Wu, Y.; Li, Y.; Wang, L. Integrating Machine Learning into Additive Manufacturing of Metallic Biomaterials: A Comprehensive Review. J. Funct. Biomater. 2025, 16, 77. [Google Scholar] [CrossRef]
- Ayvaz, S.; Alpay, K. Predictive maintenance system for production lines in manufacturing: A machine learning approach using IoT data in real-time. Expert Syst. Appl. 2021, 173, 114598. [Google Scholar] [CrossRef]
- Luo, J.; Wu, M.; Gopukumar, D.; Zhao, Y. Big data application in biomedical research and health care: A literature review. Biomed. Inform. Insights 2016, 8, BII-S31559. [Google Scholar] [CrossRef]
- Monostori, L. AI and machine learning techniques for managing complexity, changes and uncertainties in manufacturing. Eng. Appl. Artif. Intell. 2003, 16, 277–291. [Google Scholar] [CrossRef]
- Gachago, J. The Transformative Impact of Artificial Intelligence on the Digital Maturity of Hospitals. In Digital Maturity in Hospitals: Strategies, Frameworks, and Global Case Studies to Shape Future Healthcare; Springer: Berlin/Heidelberg, Germany, 2025; pp. 291–321. [Google Scholar]
- Plathottam, S.J.; Rzonca, A.; Lakhnori, R.; Iloeje, C.O. A review of artificial intelligence applications in manufacturing operations. J. Adv. Manuf. Process. 2023, 5, e10159. [Google Scholar] [CrossRef]
- Deneault, J.R.; Chang, J.; Myung, J.; Hooper, D.; Armstrong, A.; Pitt, M.; Maruyama, B. Toward autonomous additive manufacturing: Bayesian optimization on a 3D printer. MRS Bull. 2021, 46, 566–575. [Google Scholar] [CrossRef]
- Tripathi, S.; Ansari, A.A.; Singh, M.; Dash, M.; Kumar, P.; Singh, H.; Panda, B.; Nukavarapu, S.; Camci-Unal, G.; Li, B. Transforming surgical planning and procedures through the synergistic use of additive manufacturing, advanced materials and artificial intelligence: Challenges and opportunities. Mater. Horiz. 2025, 12, 7814–7864. [Google Scholar] [CrossRef] [PubMed]
- Filardi, V. The Evolution and Impact of Customized Implants with Intricate Designs. In Biomaterials in Orthopaedics & Trauma: Current Status and Future Trends in Revolutionizing Patient Care; Springer: Berlin/Heidelberg, Germany, 2025; pp. 291–307. [Google Scholar]
- Ashebir, D.A.; Hendlmeier, A.; Dunn, M.; Arablouei, R.; Lomov, S.V.; Di Pietro, A.; Nikzad, M. Detecting multi-scale defects in material extrusion additive manufacturing of fiber-reinforced thermoplastic composites: A review of challenges and advanced non-destructive testing techniques. Polymers 2024, 16, 2986. [Google Scholar] [CrossRef]
- Al-Zyoud, W.; Haddadin, D.; Hasan, S.A.; Jaradat, H.; Kanoun, O. Biocompatibility testing for implants: A novel tool for selection and characterization. Materials 2023, 16, 6881. [Google Scholar] [CrossRef]
- Ali, H. Artificial intelligence in multi-omics data integration: Advancing precision medicine, biomarker discovery and genomic-driven disease interventions. Int J Sci Res Arch 2023, 8, 1012–1030. [Google Scholar]
- Gracia Martínez, J.L.; Pfang, B.; Morales Coca, M.Á.; Caramés Sánchez, C.; del Olmo Rodríguez, M.; Villegas García, M.A.; Short Apellaniz, J.; Arcos Campillo, J.; Álvaro de la Parra, J.A.; Manzano Lorefice, F. Implementing a closed loop clinical decision support system for sustainable preoperative care. npj Digit. Med. 2025, 8, 6. [Google Scholar] [CrossRef]
- Ahmed, Z.; Mohamed, K.; Zeeshan, S.; Dong, X. Artificial intelligence with multi-functional machine learning platform development for better healthcare and precision medicine. Database 2020, 2020, baaa010. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Islam, K.S.; Dip, T.M.; Chowdhury, M.F.M.; Debnath, S.R.; Hasan, S.M.M.; Sakib, M.S.; Saha, T.; Padhye, R.; Houshyar, S. A review on nanomaterial-based additive manufacturing: Dynamics in properties, prospects, and challenges. Prog. Addit. Manuf. 2024, 9, 1197–1224. [Google Scholar] [CrossRef]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Sehrt, J.T.; Kleszczynski, S.; Notthoff, C. Nanoparticle improved metal materials for additive manufacturing. Prog. Addit. Manuf. 2017, 2, 179–191. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Ramakrishna, S. Surface engineering of biomaterials in orthopedic and dental implants: Strategies to improve osteointegration, bacteriostatic and bactericidal activities. Biotechnol. J. 2021, 16, 2000116. [Google Scholar] [CrossRef]
- Liu, H.; Webster, T.J. Nanomedicine for implants: A review of studies and necessary experimental tools. Biomaterials 2007, 28, 354–369. [Google Scholar] [CrossRef]
- Alam, M.I.; Kashyap, S.; Balaji, P.G.; Yadav, A.K.; Flora, S. 3D-Printed Medical Implants: Recent Trends and Challenges. Biomed. Mater. Devices 2025, 3, 750–770. [Google Scholar] [CrossRef]
- Aston, D.; Bow, J.; Gangadean, D. Mechanical properties of selected nanostructured materials and complex bio-nano, hybrid and hierarchical systems. Int. Mater. Rev. 2013, 58, 167–202. [Google Scholar] [CrossRef]
- Alli, Y.A.; Anuar, H.; Manshor, M.R.; Bankole, O.M.; Abd Rahman, N.A.; Olatunde, S.K.; Omotola, E.O.; Oladoye, P.O.; Ejeromedoghene, O.; Suhr, J. Influence of nanocomposites in extrusion-based 3D printing: A review. Hybrid Adv. 2023, 3, 100069. [Google Scholar] [CrossRef]
- Liang, W.; Zhou, C.; Zhang, H.; Bai, J.; Jiang, B.; Jiang, C.; Ming, W.; Zhang, H.; Long, H.; Huang, X. Recent advances in 3D printing of biodegradable metals for orthopaedic applications. J. Biol. Eng. 2023, 17, 56. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Sangarimotlagh, Z.; Karbasi, M.; Dikici, B. Enhancing corrosion resistance in Mg-based alloys through MOF-incorporated coatings: A comprehensive review. Appl. Surf. Sci. Adv. 2024, 21, 100607. [Google Scholar] [CrossRef]
- Rao, J.; Li, Y.; Sun, J.; Gao, H.; Yang, C.; Ma, Y.; Yu, R.; Zheng, X.; Ding, Y. Biodegradable Zn–Li–Sr alloy implants with antioxidant properties for improved bone osseointegration under diabetic conditions. J. Mater. Chem. B 2025, 13, 6414–6428. [Google Scholar] [CrossRef]
- Velu, R.; Calais, T.; Jayakumar, A.; Raspall, F. A comprehensive review on bio-nanomaterials for medical implants and feasibility studies on fabrication of such implants by additive manufacturing technique. Materials 2019, 13, 92. [Google Scholar] [CrossRef]
- Ali, H.; Ali, S.; Ali, K.; Ullah, S.; Ismail, P.M.; Humayun, M.; Zeng, C. Impact of the Nanoparticle Incorporation in Enhancing Mechanical Properties of Polymers. Results Eng. 2025, 27, 106151. [Google Scholar] [CrossRef]
- Feng, X.; Chen, J.; Lin, X.; Zhang, X.; Wang, Y.; Sun, C. Microdeformation and strengthening mechanism of 3D printed TC4/TC11 gradient titanium alloy subjected to tensile loading. Mater. Sci. Eng. A 2024, 915, 147265. [Google Scholar] [CrossRef]
- Kotteda, T.K.; Kumar, M.; Kumar, P.; Chekuri, R.B.R. Metal matrix nanocomposites: Future scope in the fabrication and machining techniques. Int. J. Adv. Manuf. Technol. 2022, 1–19. [Google Scholar] [CrossRef]
- Baltzis, D.; Orfanidis, S.; Lekatou, A.; Paipetis, A. Stainless steel coupled with carbon nanotube-modified epoxy and carbon fibre composites: Electrochemical and mechanical study. Plast. Rubber Compos. 2016, 45, 95–105. [Google Scholar] [CrossRef]
- Mantripragada, V.P.; Lecka-Czernik, B.; Ebraheim, N.A.; Jayasuriya, A.C. An overview of recent advances in designing orthopedic and craniofacial implants. J. Biomed. Mater. Res. Part A 2013, 101, 3349–3364. [Google Scholar] [CrossRef] [PubMed]
- Rouf, S.; Raina, A.; Haq, M.I.U.; Naveed, N.; Jeganmohan, S.; Kichloo, A.F. 3D printed parts and mechanical properties: Influencing parameters, sustainability aspects, global market scenario, challenges and applications. Adv. Ind. Eng. Polym. Res. 2022, 5, 143–158. [Google Scholar] [CrossRef]
- Mubarak, S.; Dhamodharan, D.B.; Kale, M.; Divakaran, N.; Senthil, T.; P, S.; Wu, L.; Wang, J. A novel approach to enhance mechanical and thermal properties of SLA 3D printed structure by incorporation of metal–metal oxide nanoparticles. Nanomaterials 2020, 10, 217. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, D.; Zhu, R.; Yang, C.; Wang, L.; Zhang, L.-C. Revolutionizing medical implant fabrication: Advances in additive manufacturing of biomedical metals. Int. J. Extrem. Manuf. 2024, 7, 022002. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Y.; Qian, Z. Nanostructured surface modification to bone implants for bone regeneration. J. Biomed. Nanotechnol. 2018, 14, 628–648. [Google Scholar] [CrossRef]
- Pietrocola, G.; Campoccia, D.; Motta, C.; Montanaro, L.; Arciola, C.R.; Speziale, P. Colonization and infection of indwelling medical devices by Staphylococcus aureus with an emphasis on orthopedic implants. Int. J. Mol. Sci. 2022, 23, 5958. [Google Scholar] [CrossRef]
- Narayana, P.; Srihari, P. Biofilm resistant surfaces and coatings on implants: A review. Mater. Today Proc. 2019, 18, 4847–4853. [Google Scholar] [CrossRef]
- Liaw, K.; Delfini, R.H.; Abrahams, J.J. Dental implant complications. In Seminars in Ultrasound, CT and MRI; WB Saunders: Philadelphia, PA, USA, 2015; pp. 427–433. [Google Scholar]
- Mishra, A.; Aggarwal, A.; Khan, F. Medical device-associated infections caused by biofilm-forming microbial pathogens and controlling strategies. Antibiotics 2024, 13, 623. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rath, B.; Tingart, M.; Eschweiler, J. Role of implants surface modification in osseointegration: A systematic review. J. Biomed. Mater. Res. Part A 2020, 108, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, G.; Li, J.J. Advances in implant surface modifications to improve osseointegration. Mater. Adv. 2021, 2, 6901–6927. [Google Scholar] [CrossRef]
- Gaviria, J.; Alcudia, A.; Begines, B.; Beltrán, A.M.; Rodríguez-Ortiz, J.A.; Trueba, P.; Villarraga, J.; Torres, Y. Biofunctionalization of porous Ti substrates coated with Ag nanoparticles for potential antibacterial behavior. Metals 2021, 11, 692. [Google Scholar] [CrossRef]
- Clarissa, W.H.-Y.; Chia, C.H.; Zakaria, S.; Evyan, Y.C.-Y. Recent advancement in 3-D printing: Nanocomposites with added functionality. Prog. Addit. Manuf. 2022, 7, 325–350. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable materials and metallic implants—A review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Heydari, Z.; Lami, G.H.; Saberi, A.; Baltatu, M.S.; Vizureanu, P. A comprehensive review of the current research status of biodegradable zinc alloys and composites for biomedical applications. Materials 2023, 16, 4797. [Google Scholar] [CrossRef]
- Zhao, S.; Tayyebi, M.; Yarigarravesh, M.; Hu, G. A review of magnesium corrosion in bio-applications: Mechanism, classification, modeling, in-vitro, and in-vivo experimental testing, and tailoring Mg corrosion rate. J. Mater. Sci. 2023, 58, 12158–12181. [Google Scholar] [CrossRef]
- Vojtech, D.; Kubasek, J.; Capek, J.; Pospisilova, I. Comparative mechanical and corrosion studies on magnesium, zinc and iron alloys as biodegradable metals. Mater. Teh. 2015, 49, 877–882. [Google Scholar] [CrossRef]
- Khan, A.R.; Grewal, N.S.; Zhou, C.; Yuan, K.; Zhang, H.-J.; Jun, Z. Recent advances in biodegradable metals for implant applications: Exploring in vivo and in vitro responses. Results Eng. 2023, 20, 101526. [Google Scholar] [CrossRef]
- Odeyemi, O.O.; Alaba, P.A. Efficient and reliable corrosion control for subsea assets: Challenges in the design and testing of corrosion probes in aggressive marine environments. Corros. Rev. 2025, 43, 79–126. [Google Scholar] [CrossRef]
- Ayyagari, A.; Hasannaeimi, V.; Grewal, H.S.; Arora, H.; Mukherjee, S. Corrosion, erosion and wear behavior of complex concentrated alloys: A review. Metals 2018, 8, 603. [Google Scholar] [CrossRef]
- Walter, N.; Stich, T.; Docheva, D.; Alt, V.; Rupp, M. Evolution of implants and advancements for osseointegration: A narrative review. Injury 2022, 53, S69–S73. [Google Scholar] [CrossRef]
- Ma, B.; Martín, C.; Kurapati, R.; Bianco, A. Degradation-by-design: How chemical functionalization enhances the biodegradability and safety of 2D materials. Chem. Soc. Rev. 2020, 49, 6224–6247. [Google Scholar] [CrossRef]
- Mayakrishnan, V.; Murugan, P.A. Degradation Studies of Resorbable Materials for Biomedical Applications. In Nanomanufacturing Techniques in Sustainable Healthcare Applications; CRC Press: Boca Raton, FL, USA, 2024; pp. 258–277. [Google Scholar]
- Meng, J.; Liu, X.; Niu, C.; Pang, Q.; Li, J.; Liu, F.; Liu, Z.; Mai, L. Advances in metal–organic framework coatings: Versatile synthesis and broad applications. Chem. Soc. Rev. 2020, 49, 3142–3186. [Google Scholar] [CrossRef]
- Wazeer, A.; Das, A.; Sinha, A.; Inaba, K.; Ziyi, S.; Karmakar, A. Additive manufacturing in biomedical field: A critical review on fabrication method, materials used, applications, challenges, and future prospects. Prog. Addit. Manuf. 2023, 8, 857–889. [Google Scholar] [CrossRef]
- Kumar, N.V.; Chakradhar, D.; Abhilash, P. Advancing bioimplant manufacturing through artificial intelligence. In Bioimplants Manufacturing; CRC Press: Boca Raton, FL, USA, 2024; pp. 284–312. [Google Scholar]
- Frączek, W.; Kotela, A.; Kotela, I.; Grodzik, M. Nanostructures in orthopedics: Advancing diagnostics, targeted therapies, and tissue regeneration. Materials 2024, 17, 6162. [Google Scholar] [CrossRef]
- Wei, A.; Mehtala, J.G.; Patri, A.K. Challenges and opportunities in the advancement of nanomedicines. J. Control. Release 2012, 164, 236–246. [Google Scholar] [CrossRef]
- Murzin, S.P. Artificial intelligence-driven innovations in laser processing of metallic materials. Metals 2024, 14, 1458. [Google Scholar] [CrossRef]
- Casati, R.; Vedani, M. Metal matrix composites reinforced by nano-particles—A review. Metals 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Zong, Z.; Guan, Y. AI-driven intelligent data analytics and predictive analysis in Industry 4.0: Transforming knowledge, innovation, and efficiency. J. Knowl. Econ. 2025, 16, 864–903. [Google Scholar] [CrossRef]
- Amirtharaj Mosas, K.K.; Chandrasekar, A.R.; Dasan, A.; Pakseresht, A.; Galusek, D. Recent advancements in materials and coatings for biomedical implants. Gels 2022, 8, 323. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Mo, X.; Chen, M.; Yao, C. Machine-learning-assisted microstructure–property linkages of carbon nanotube-reinforced aluminum matrix nanocomposites produced by laser powder bed fusion. Nanotechnol. Rev. 2021, 10, 1410–1424. [Google Scholar]
- Wu, X.; Zhou, Y.; Zhang, J.; Liang, J. Data driven performance prediction of titanium-based matrix composites. Alex. Eng. J. 2023, 85, 300–306. [Google Scholar] [CrossRef]
- Del Guercio, G.; Bosio, F.; Phutela, C.; Robertson, S.; Aboulkhair, N.T. Additive manufacturing of novel aluminium matrix composites with enhanced strength and processability via boron nitride functionalization. Addit. Manuf. Lett. 2024, 11, 100237. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, Z.; Yan, J. Machine learning for metal additive manufacturing: Predicting temperature and melt pool fluid dynamics using physics-informed neural networks. Comput. Mech. 2021, 67, 619–635. [Google Scholar] [CrossRef]
- Jiang, F.; Xia, M.; Hu, Y. Physics-informed machine learning for accurate prediction of temperature and melt pool dimension in metal additive manufacturing. 3D Print. Addit. Manuf. 2024, 11, e1679–e1689. [Google Scholar] [CrossRef]
- Ajenifujah, O.T.; Ogoke, F.; Wirth, F.; Beuth, J.; Farimani, A.B. Integrating multi-physics simulations and machine learning to define the spatter mechanism and process window in laser powder bed fusion. arXiv 2024, arXiv:2405.07823. [Google Scholar] [CrossRef]
- Ogoke, F.; Farimani, A.B. Thermal control of laser powder bed fusion using deep reinforcement learning. Addit. Manuf. 2021, 46, 102033. [Google Scholar] [CrossRef]
- Hemmasian, A.; Ogoke, F.; Akbari, P.; Malen, J.; Beuth, J.; Farimani, A.B. Surrogate modeling of melt pool temperature field using deep learning. Addit. Manuf. Lett. 2023, 5, 100123. [Google Scholar] [CrossRef]
- Liu, N.; Li, X.; Rajanna, M.R.; Reutzel, E.W.; Sawyer, B.; Rao, P.; Lua, J.; Phan, N.; Yu, Y. Deep neural operator enabled digital twin modeling for additive manufacturing. arXiv 2024, arXiv:2405.09572. [Google Scholar] [CrossRef]
- Chen, L.; Yao, X.; Liu, K.; Tan, C.; Moon, S.K. Multisensor fusion-based digital twin in additive manufacturing for in-situ quality monitoring and defect correction. Proc. Des. Soc. 2023, 3, 2755–2764. [Google Scholar]
- Luo, Q.; Shimanek, J.D.; Simpson, T.W.; Beese, A.M. An image-based transfer learning approach for using in situ processing data to predict laser powder bed fusion additively manufactured Ti-6Al-4V mechanical properties. 3D Print. Addit. Manuf. 2025, 12, 48–60. [Google Scholar]
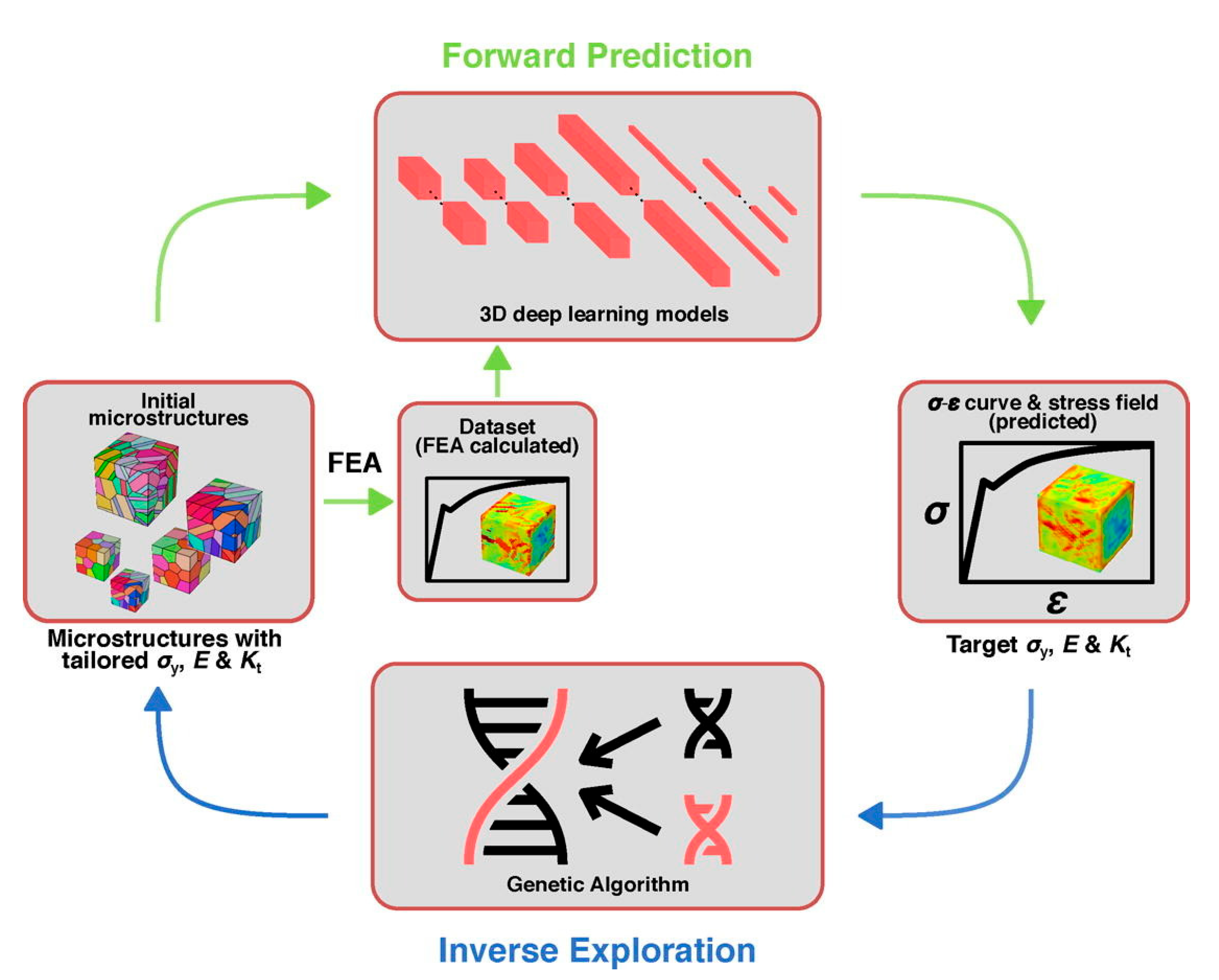
- Shang, X.; Liu, Z.; Zhang, J.; Lyu, T.; Zou, Y. Tailoring the mechanical properties of 3D microstructures: A deep learning and genetic algorithm inverse optimization framework. Mater. Today 2023, 70, 71–81. [Google Scholar] [CrossRef]
- Sarmah, P.; Gupta, K. Recent advancements in fabrication of metal matrix composites: A systematic review. Materials 2024, 17, 4635. [Google Scholar] [CrossRef]
- Karuppusamy, M.; Thirumalaisamy, R.; Palanisamy, S.; Nagamalai, S.; Massoud, E.E.S.; Ayrilmis, N. A review of machine learning applications in polymer composites: Advancements, challenges, and future prospects. J. Mater. Chem. A 2025, 13, 16290–16308. [Google Scholar] [CrossRef]
- Nayak, M.; Narayana, A.S.S. Machine Learning for Nano Process Optimization. Edge Intell. Explor. Front. AI Edge 2025, 11, 307–325. [Google Scholar]
- Zubair, M.; Hussai, M.; Al-Bashrawi, M.A.; Bendechache, M.; Owais, M. A Comprehensive Review of Techniques, Algorithms, Advancements, Challenges, and Clinical Applications of Multi-modal Medical Image Fusion for Improved Diagnosis. arXiv 2025, arXiv:2505.14715. [Google Scholar] [CrossRef]
- Li, N.; Liu, W.; Wang, Y.; Zhao, Z.; Yan, T.; Zhang, G.; Xiong, H. Laser additive manufacturing on metal matrix composites: A review. Chin. J. Mech. Eng. 2021, 34, 38. [Google Scholar] [CrossRef]
- Ali, H.; Ghadbeigi, H.; Mumtaz, K. Residual stress development in selective laser-melted Ti6Al4V: A parametric thermal modelling approach. Int. J. Adv. Manuf. Technol. 2018, 97, 2621–2633. [Google Scholar] [CrossRef]
- Wu, H.; Chen, X.; Kong, L.; Liu, P. Mechanical and biological properties of titanium and its alloys for oral implant with preparation techniques: A review. Materials 2023, 16, 6860. [Google Scholar] [CrossRef]
- Omidian, H. AI-powered breakthroughs in material science and biomedical polymers. J. Bioact. Compat. Polym. 2025, 40, 161–174. [Google Scholar] [CrossRef]
- Chang, C.-W.; Dinh, N.T. Classification of machine learning frameworks for data-driven thermal fluid models. Int. J. Therm. Sci. 2019, 135, 559–579. [Google Scholar] [CrossRef]
- Masinelli, G.; Shevchik, S.A.; Pandiyan, V.; Quang-Le, T.; Wasmer, K. Artificial intelligence for monitoring and control of metal additive manufacturing. In International Conference on Additive Manufacturing in Products and Applications; Springer: Cham, Switzerland, 2020; pp. 205–220. [Google Scholar]
- Samsonovich, A.V.; Kitsantas, A.; Wahidi, S.; Dolgikh, A.A. Self-Regulated Learning (SRL) with AI in Problem-Based Learning. In Biologically Inspired Cognitive Architectures Meeting; Springer: Cham, Switzerland, 2024; pp. 345–357. [Google Scholar]
- Yang, H.; Fang, H.; Wang, C.; Wang, Y.; Qi, C.; Zhang, Y.; Zhou, Q.; Huang, M.; Wang, M.; Wu, M. 3D printing of customized functional devices for smart biomedical systems. SmartMat 2024, 5, e1244. [Google Scholar] [CrossRef]
- Khakbiz, M.; Esteghza, A. AI-Integrated Surface Topography and Experimental Investigation of PEO-Coated Mg-Zn-Si Alloys for Next-Generation Biodegradable Medical Implants. Colloids Surf. A Physicochem. Eng. Asp. 2025, 726, 137800. [Google Scholar] [CrossRef]
- Oulefki, A.; Amira, A.; Foufou, S. Digital twins and AI transforming healthcare systems through innovation and data-driven decision making. Health Technol. 2025, 15, 299–321. [Google Scholar] [CrossRef]
- Qian, C.; Liu, X.; Ripley, C.; Qian, M.; Liang, F.; Yu, W. Digital twin—Cyber replica of physical things: Architecture, applications and future research directions. Future Internet 2022, 14, 64. [Google Scholar] [CrossRef]
- Goswami, R.; Saxena, D.; Kumari, P. Precision In Practice: The Evolution Of Patient-Specific Implants In Modern Surgery. Int. J. Environ. Sci. 2025, 11, 425–430. [Google Scholar]
- Chen, A.; Wang, Z.; Vidaurre, K.L.L.; Han, Y.; Ye, S.; Tao, K.; Wang, S.; Gao, J.; Li, J. Knowledge-reuse transfer learning methods in molecular and material science. arXiv 2024, arXiv:2403.12982. [Google Scholar] [CrossRef]
- da Silva, R.G.L. The advancement of artificial intelligence in biomedical research and health innovation: Challenges and opportunities in emerging economies. Glob. Health 2024, 20, 44. [Google Scholar] [CrossRef]
- Nandipati, M.; Fatoki, O.; Desai, S. Bridging nanomanufacturing and artificial intelligence—A comprehensive review. Materials 2024, 17, 1621. [Google Scholar] [CrossRef]
- Chitraju Gopal Varma, S. From Experimentation to Production: Streamlining the ML Model Lifecycle for Faster Insights. SSRN 2024. [Google Scholar] [CrossRef]
- Singh, A.B.; Khandelwal, C.; Dangayach, G.S. Revolutionizing healthcare materials: Innovations in processing, advancements, and challenges for enhanced medical device integration and performance. J. Micromanuf. 2024. [Google Scholar] [CrossRef]
- Ortis, G.B.; Zapparoli, F.C.; Dantas, L.R.; Suss, P.H.; Soni, J.F.; Mendonça, C.J.A.; Loesch, G.H.; Loesch, M.d.M.O.N.; Tuon, F.F. Additive Manufacturing, Thermoplastics, CAD Technology, and Reverse Engineering in Orthopedics and Neurosurgery–Applications to Preventions and Treatment of Infections. Antibiotics 2025, 14, 565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Malekahmadi, O.; Sajadi, S.M.; Li, Z.; Abu-Hamdeh, N.H.; Rawa, M.J.; Al-Ebrahim, M.A.; Karimipour, A.; Viet, H. Thermomechanical properties of coated PLA-3D-printed orthopedic plate with PCL/Akermanite nano-fibers: Experimental procedure and AI optimization. J. Mater. Res. Technol. 2023, 27, 1307–1316. [Google Scholar] [CrossRef]
- Kashwani, R.; Ahuja, G.; Narula, V.; Jose, A.T.; Kulkarni, V.; Hajong, R.; Gupta, S. Future of dental care: Integrating AI, metaverse, AR/VR, teledentistry, CAD & 3D printing, blockchain and CRISPR innovations. Community Pract. 2024, 21, 123–137. [Google Scholar]
- Zarei, A.; Farazin, A. Synergizing additive manufacturing and machine learning for advanced hydroxyapatite scaffold design in bone regeneration. J. Aust. Ceram. Soc. 2025, 61, 797–813. [Google Scholar]
- Dai, Z.; An, J.; Huang, X. Arginine-loaded mesoporous silica nanoparticles modified 3D-printed nanocomposite denture base resin with improved mechanical and antimicrobial properties. BMC Oral Health 2025, 25, 1–11. [Google Scholar] [CrossRef]
- De Santis, R.; Russo, T.; Rau, J.V.; Papallo, I.; Martorelli, M.; Gloria, A. Design of 3D additively manufactured hybrid structures for cranioplasty. Materials 2021, 14, 181. [Google Scholar] [CrossRef]
- He, H.; Fan, G.; Saba, F.; Tan, Z.; Su, Z.; Xiong, D.; Li, Z. Enhanced distribution and mechanical properties of high content nanoparticles reinforced metal matrix composite prepared by flake dispersion. Compos. Part B Eng. 2023, 252, 110514. [Google Scholar] [CrossRef]
- Cao, C.; Killips, A.; Li, X. Advances in the science and engineering of metal matrix nanocomposites: A review. Adv. Eng. Mater. 2024, 26, 2400217. [Google Scholar] [CrossRef]
- Marasli, C.; Katifelis, H.; Gazouli, M.; Lagopati, N. Nano-based approaches in surface modifications of dental implants: A literature review. Molecules 2024, 29, 3061. [Google Scholar] [CrossRef] [PubMed]
- Harale, A.A.; Jadhav, D.; Jadhav, P.; Mohite, D.D.; Unde, S.S. Revolutionizing Orthopedics through Integration of Artificial Intelligence and 3D Printing for Enhanced Patient Care. Indian J. Orthop. 2025, 59, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, R.; Regondi, S. Artificial intelligence-empowered 3D and 4D printing technologies toward smarter biomedical materials and approaches. Polymers 2022, 14, 2794. [Google Scholar] [CrossRef] [PubMed]
- Podutwar, A.A.; Chandorkar, P.U.; Chabukswar, A.R.; Polshettiwar, S.A.; Jagdale, S.C. The intersection of nanotechnology and biotechnology implications for human health. In Nanotechnology in Societal Development; Springer: Berlin/Heidelberg, Germany, 2024; pp. 271–305. [Google Scholar]
- Kunrath, M.F.; Campos, M.M. Metallic-nanoparticle release systems for biomedical implant surfaces: Effectiveness and safety. Nanotoxicology 2021, 15, 721–739. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Webster, T.J.; Zhang, L.G. How Can 3D Printing be a Powerful Tool in Nanomedicine? Taylor & Francis: Abingdon, UK, 2018; Volume 13, pp. 251–253. [Google Scholar]

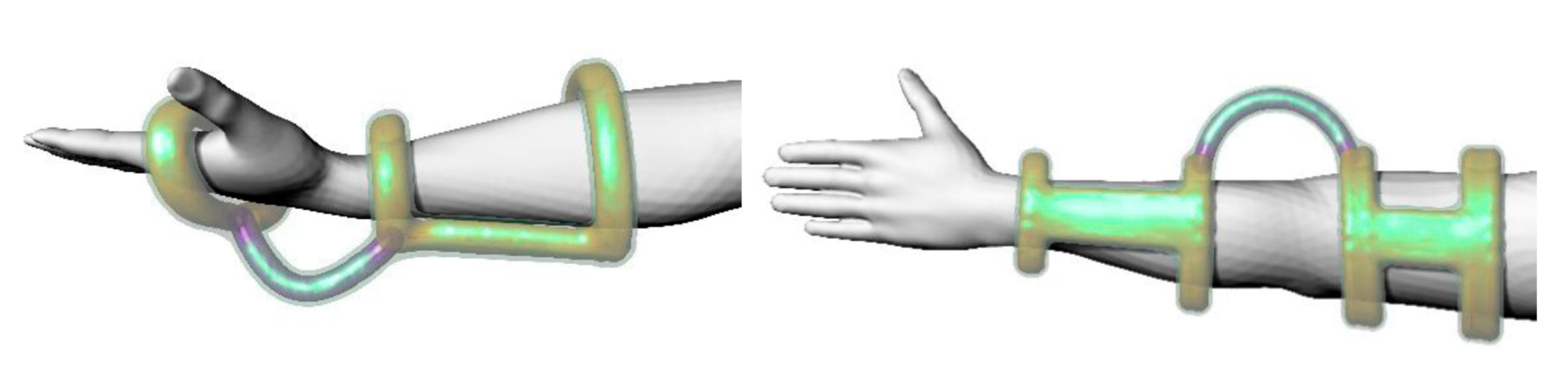

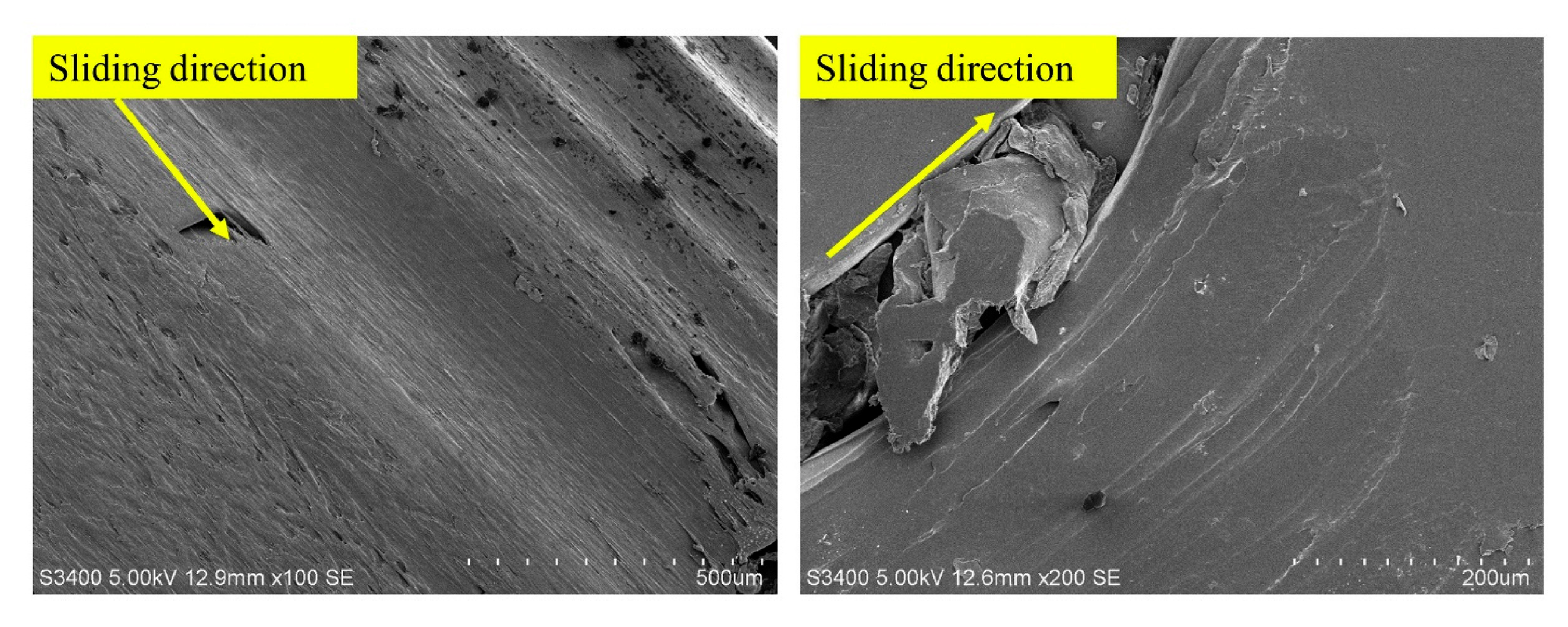
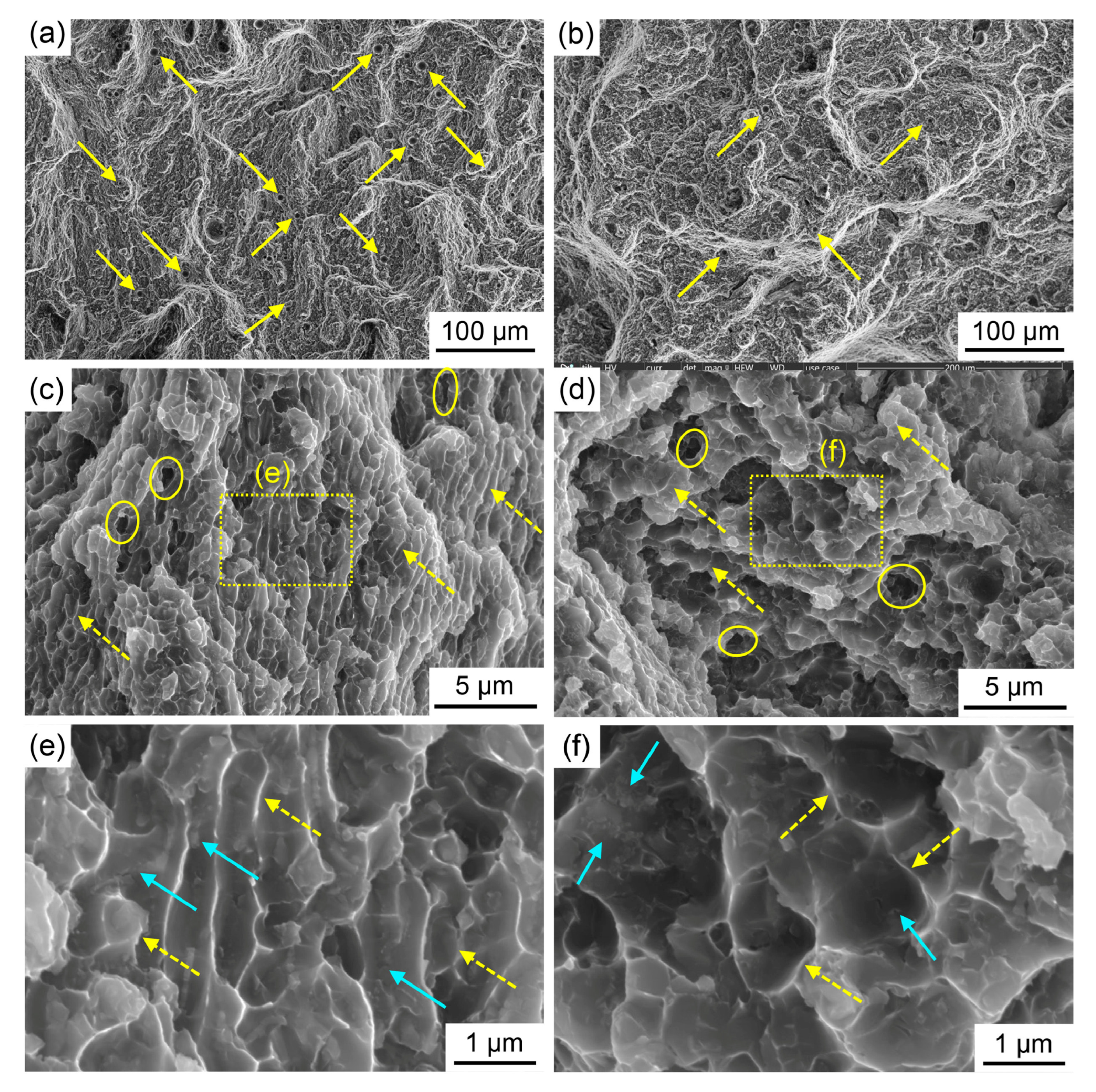


| AI Method | Key Function | Example Application |
|---|---|---|
| Topology optimization | Optimizing material layout for weight and strength | Lightweight orthopedic [19] implants, dental prosthetics [20] |
| Generative design | Automated generation of design alternatives | Customized cranial implants [21], bone scaffolds |
| Machine learning | Predicting mechanical properties and optimizing parameters | Predicting implant fatigue life, optimizing surface roughness |
| Process | Beam Type | Laser/ Electron Power | Scan Speed | Layer Thickness | Powder/ Material | Nanoparticle Additives | Key Outcomes |
|---|---|---|---|---|---|---|---|
| LPBF/SLM | Laser | 195–225 W [44] | 1250 mm/s [44] | Not reported | Ti, CoCr, stainless steel [44] | Ag, Ti, Cu [6,7] | Defect reduction, improved precision, and enhanced stability are critical for high-precision biomedical implants [44,45,46,47]. |
| LMD/Melt Pool Control | Laser | Not reported | Not reported | Not reported | Ti alloys [46] | Not reported | Melt pool control, reduced cracks and porosity, supports stable fabrication of load-bearing implants [44,45,46,47]. |
| Predictive Analysis | N/A | N/A | N/A | N/A | N/A | N/A | ML predicts residual stress, porosity, and microstructural features; optimizes printing parameters to improve reproducibility and patient-specific implant performance [49,50,51]. |
| Material/System | Nanostructure Approach | Degradation/Mechanical Impact | Benefits |
|---|---|---|---|
| Zn-0.8 Li porous scaffold [77] | SLM and salt leaching for controlled porosity | 7–12% weight loss in 28 days | Biodegradable support; enhanced osteointegration |
| Zn + β-TCP composite [78] | LPBF with ceramic nanoparticle reinforcement | Uniform degradation over 12 weeks | pH buffering; improved osteoconductivity. |
| Mg-MOF coated surface [79] | MOF coating forms a protective barrier | Significantly reduced corrosion rates | Stimuli-responsive degradation; antimicrobial benefits |
| Zn-Li-Sr alloy [80] | Alloying with Li and Sr for targeted enhancement | Tensile strength increases over 6 times compared to pure Zn; uniform degradation in diabetic models | Enhanced strength, osteoblast proliferation, and antioxidant response in diabetic bone healing application. |
| Aspect | Details | References |
|---|---|---|
| Identification of new MMNCs | AI models predict mechanical properties based on nanoparticle type, size, and dispersion, and optimize loading to balance strength and risk of agglomeration. | [118,119,120] |
| Optimization of sintering parameters | Physics-informed models predict melt pool dynamics and temperature gradients; adaptive control reduces defects and residual stresses. | [121,122,123] |
| Real-time monitoring and adaptive control | Deep learning models analyze melt pool images, dynamically adjust laser power/scan speed, reduce porosity, and improve accuracy. | [124,125] |
| Reducing trial-and-error experimentation | Digital twins and transfer learning enable the virtual replication and reuse of models, reducing the need for experimental iterations. | [126,127,128,129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Jeevanandam, J.; Danquah, M.K. Enhancing Biomedical Metal 3D Printing with AI and Nanomaterials Integration. Metals 2025, 15, 1163. https://doi.org/10.3390/met15101163
Liu J, Jeevanandam J, Danquah MK. Enhancing Biomedical Metal 3D Printing with AI and Nanomaterials Integration. Metals. 2025; 15(10):1163. https://doi.org/10.3390/met15101163
Chicago/Turabian StyleLiu, Jackie, Jaison Jeevanandam, and Michael K. Danquah. 2025. "Enhancing Biomedical Metal 3D Printing with AI and Nanomaterials Integration" Metals 15, no. 10: 1163. https://doi.org/10.3390/met15101163
APA StyleLiu, J., Jeevanandam, J., & Danquah, M. K. (2025). Enhancing Biomedical Metal 3D Printing with AI and Nanomaterials Integration. Metals, 15(10), 1163. https://doi.org/10.3390/met15101163







