The Leaching of Valuable Metals (Li, Co, Ni, Mn, Cu) from Black Mass from Spent Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.1.1. Feed Batch 1
2.1.2. Feed Batch 2
2.2. Solution Assays
2.3. Determination of Acid Consumption During Leaching
2.4. Solid Analyses
2.4.1. Solid Assays
2.4.2. X-Ray Diffraction
2.5. Leaching Kinetics Tests
2.6. Leaching Tests on Roasted Batch 2 Material
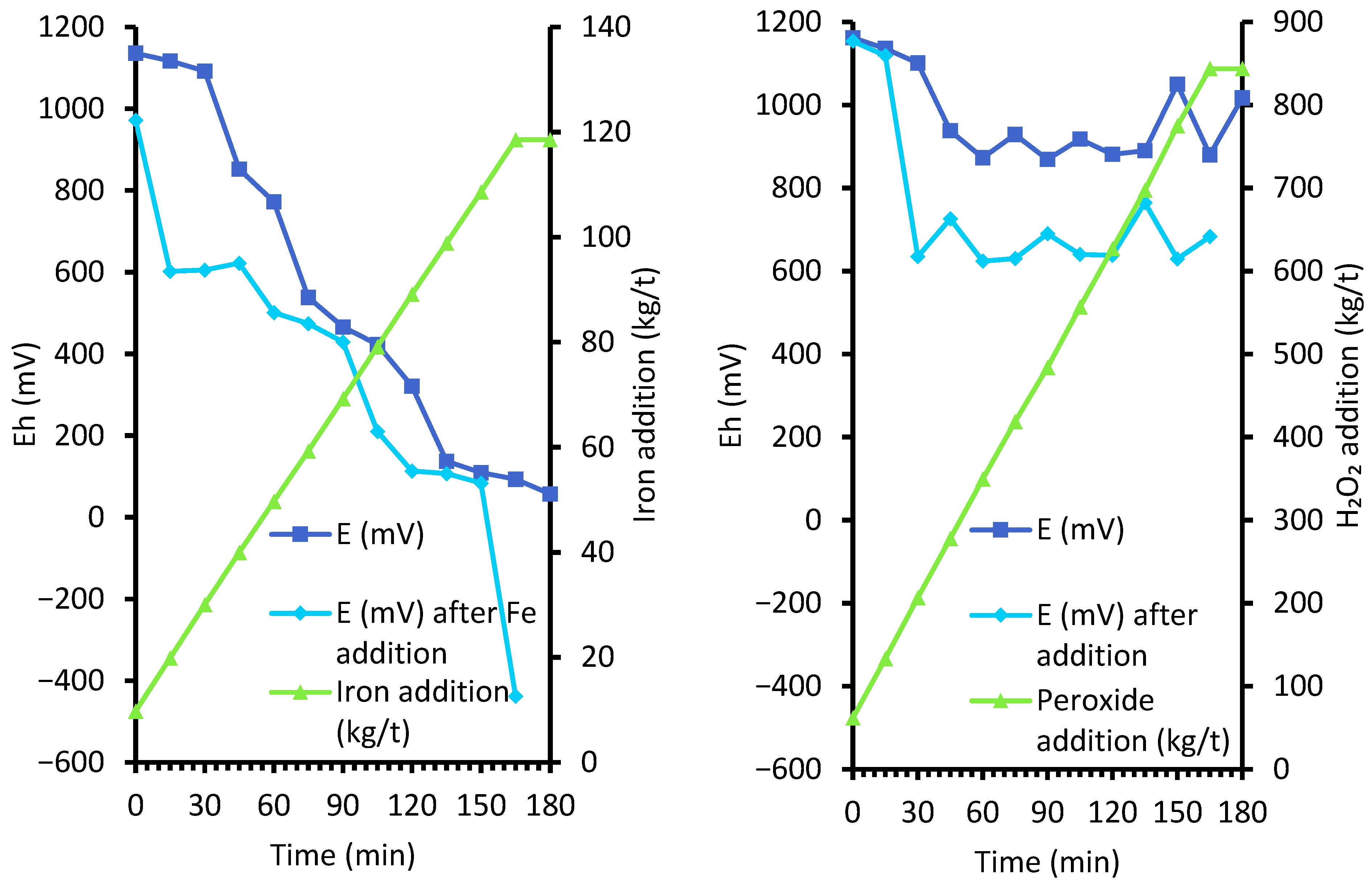
2.6.1. Controlled Potential Leach Tests
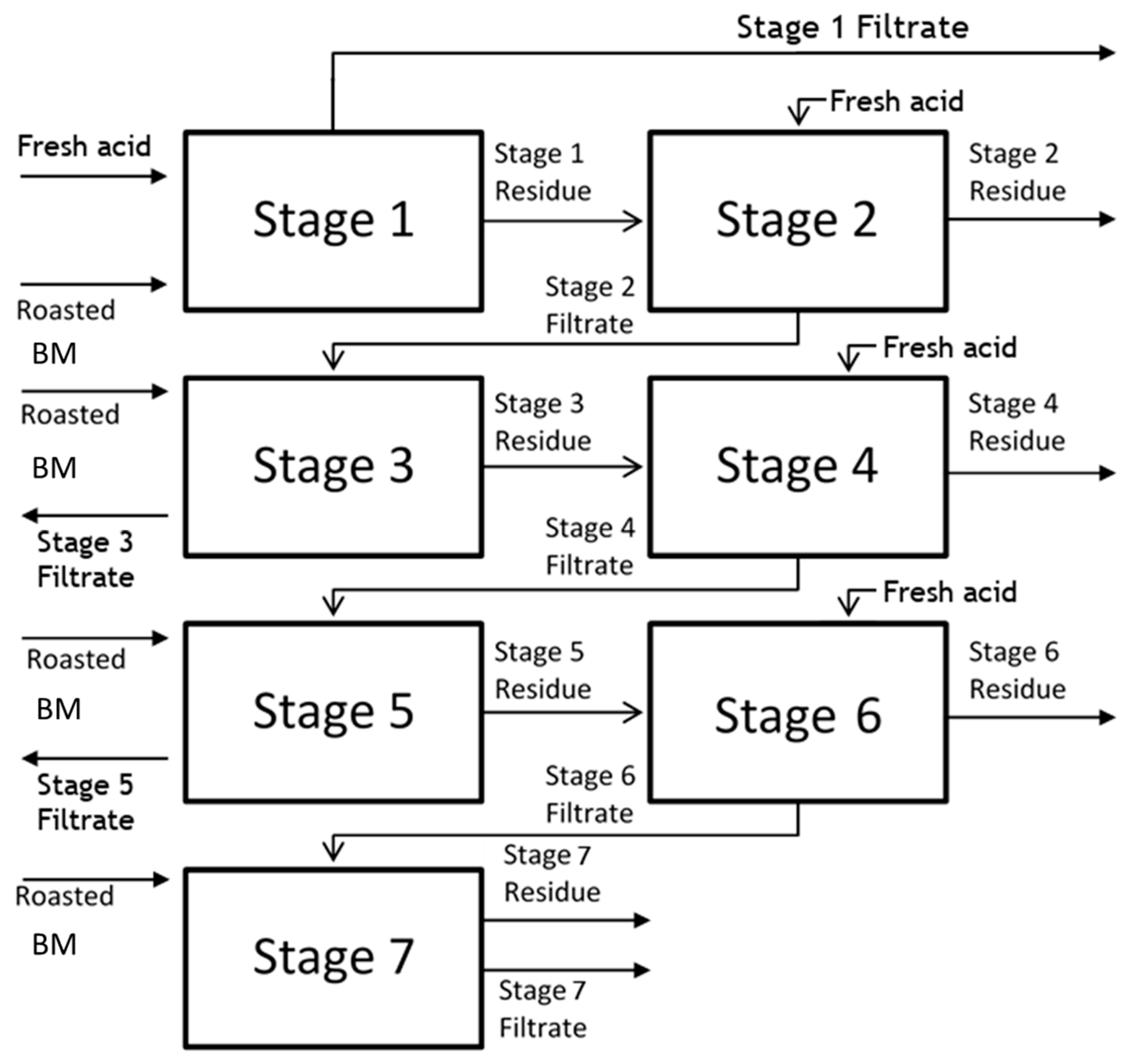
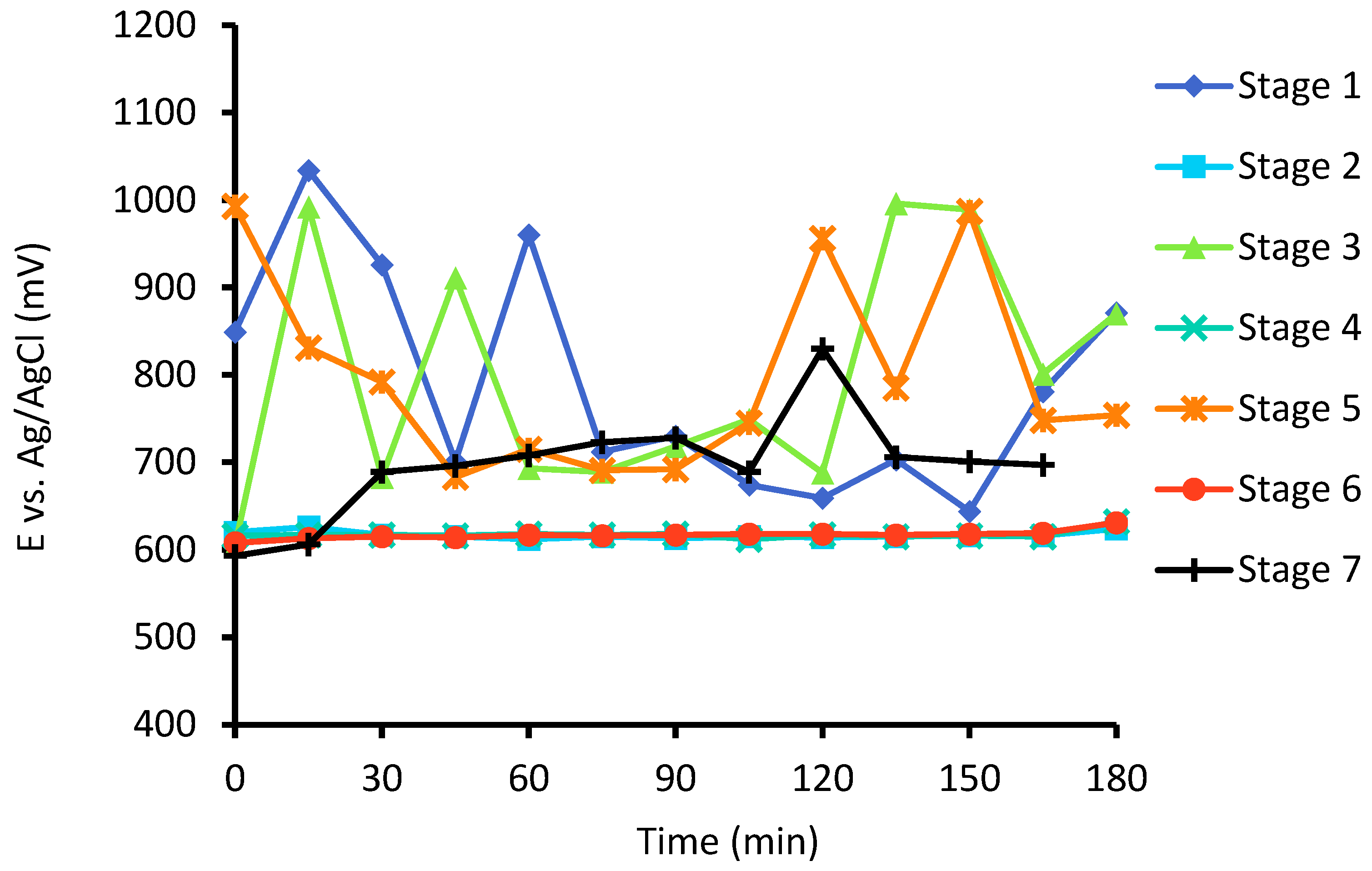
2.6.2. Seven-Stage Semi-Continuous Test
3. Results and Discussion
3.1. Leaching Kinetics Study
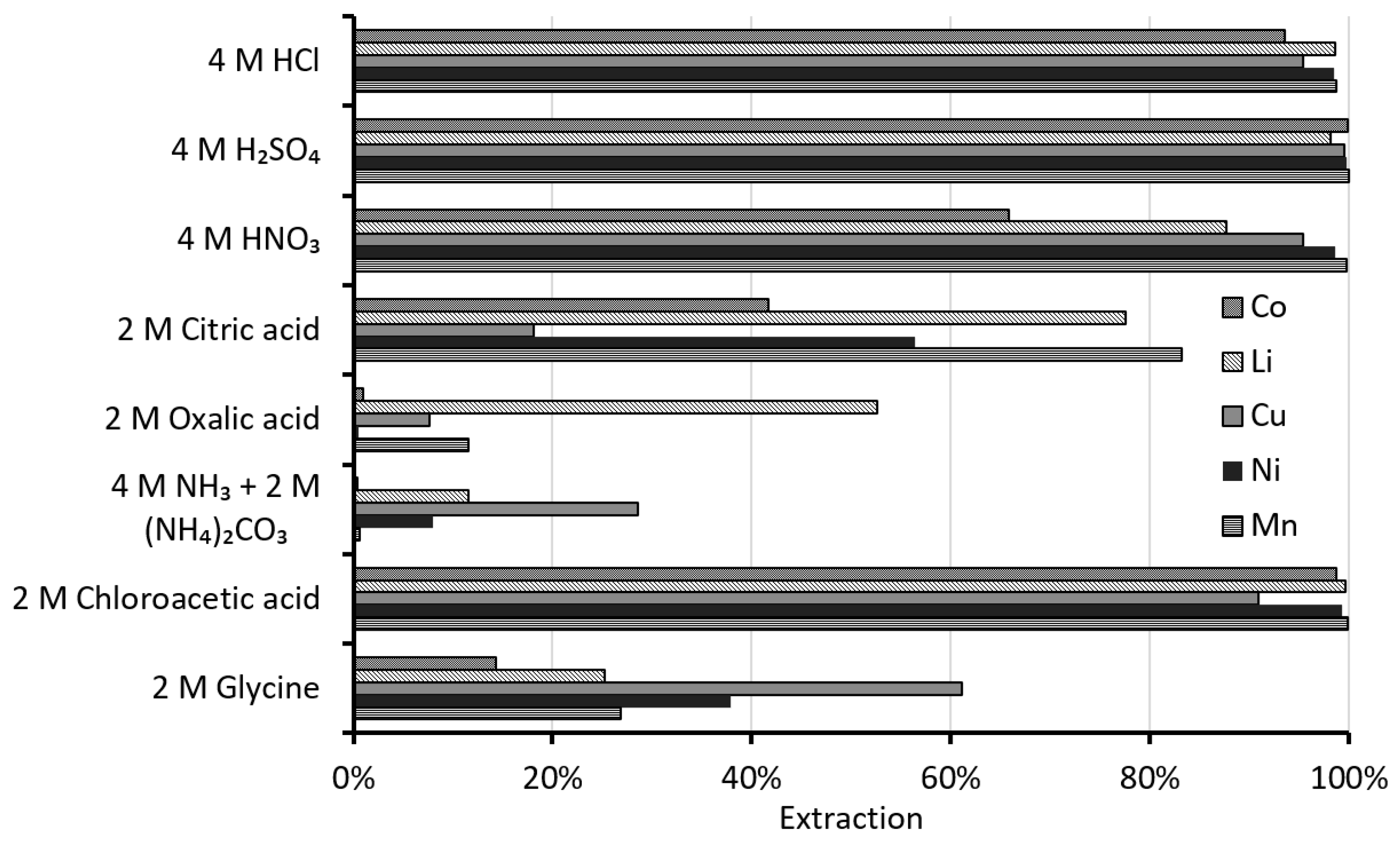
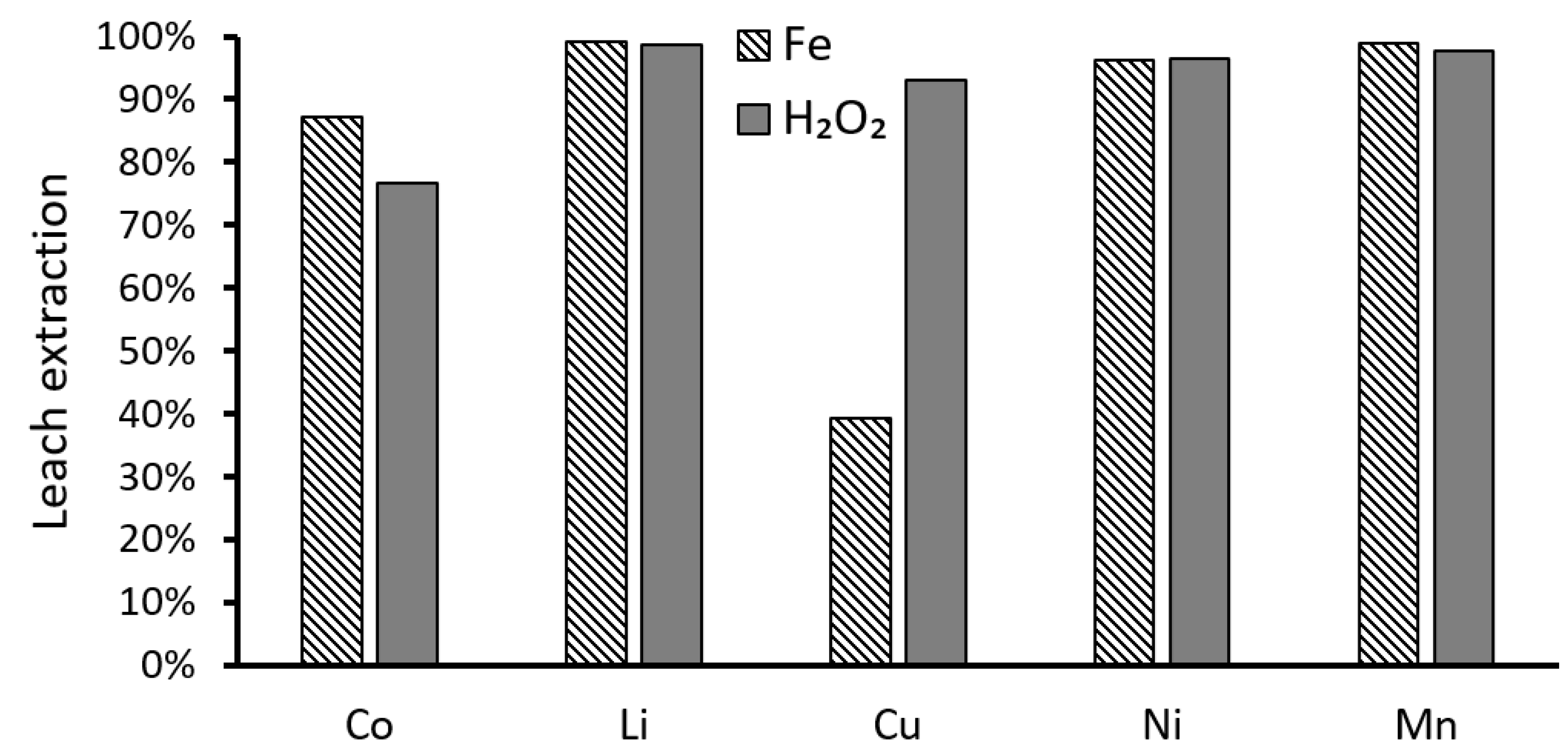
3.1.1. Comparisons of Different Lixiviants
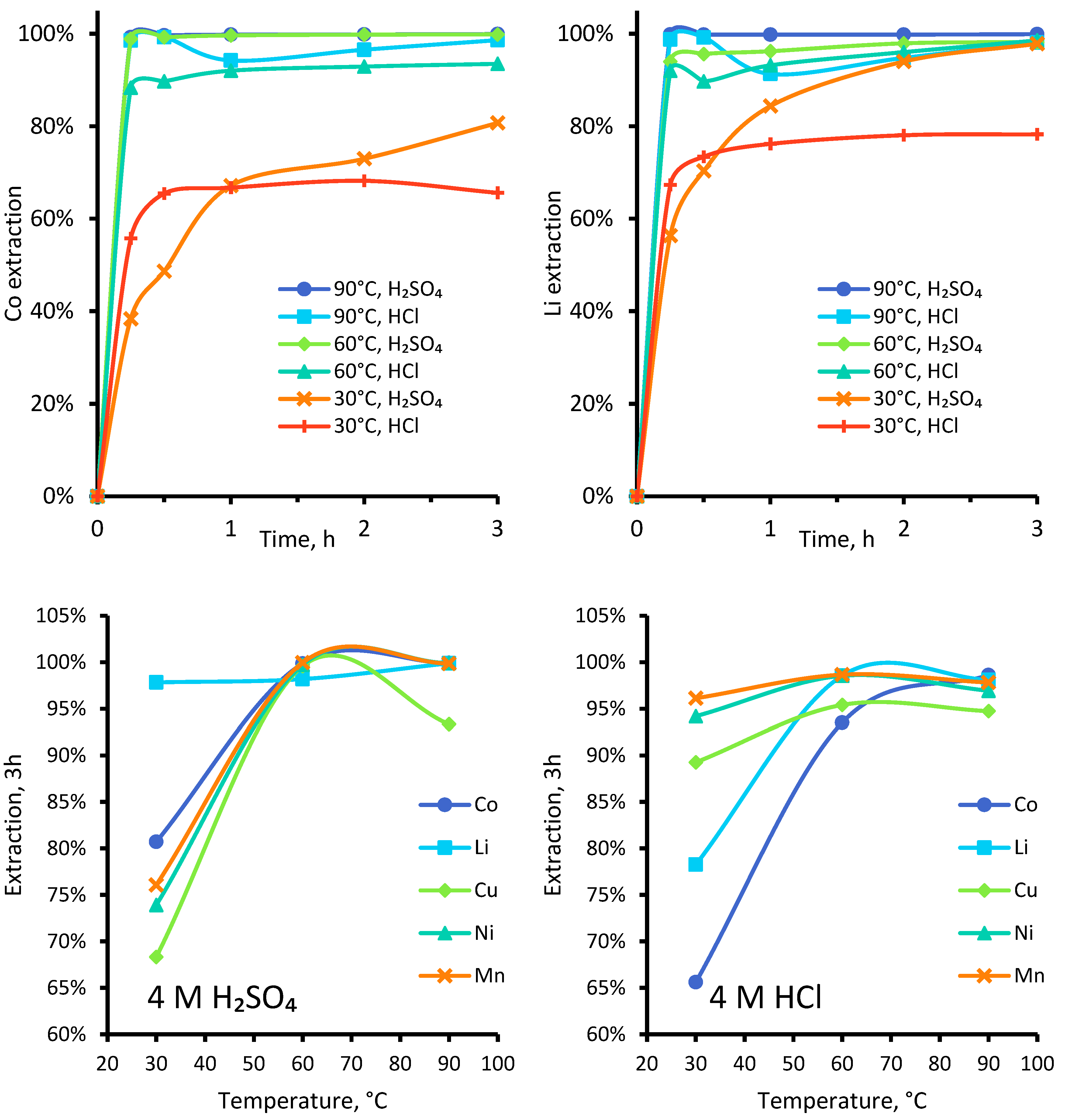
3.1.2. Effect of Varied Temperature, 4 M Sulphuric or Hydrochloric Acid
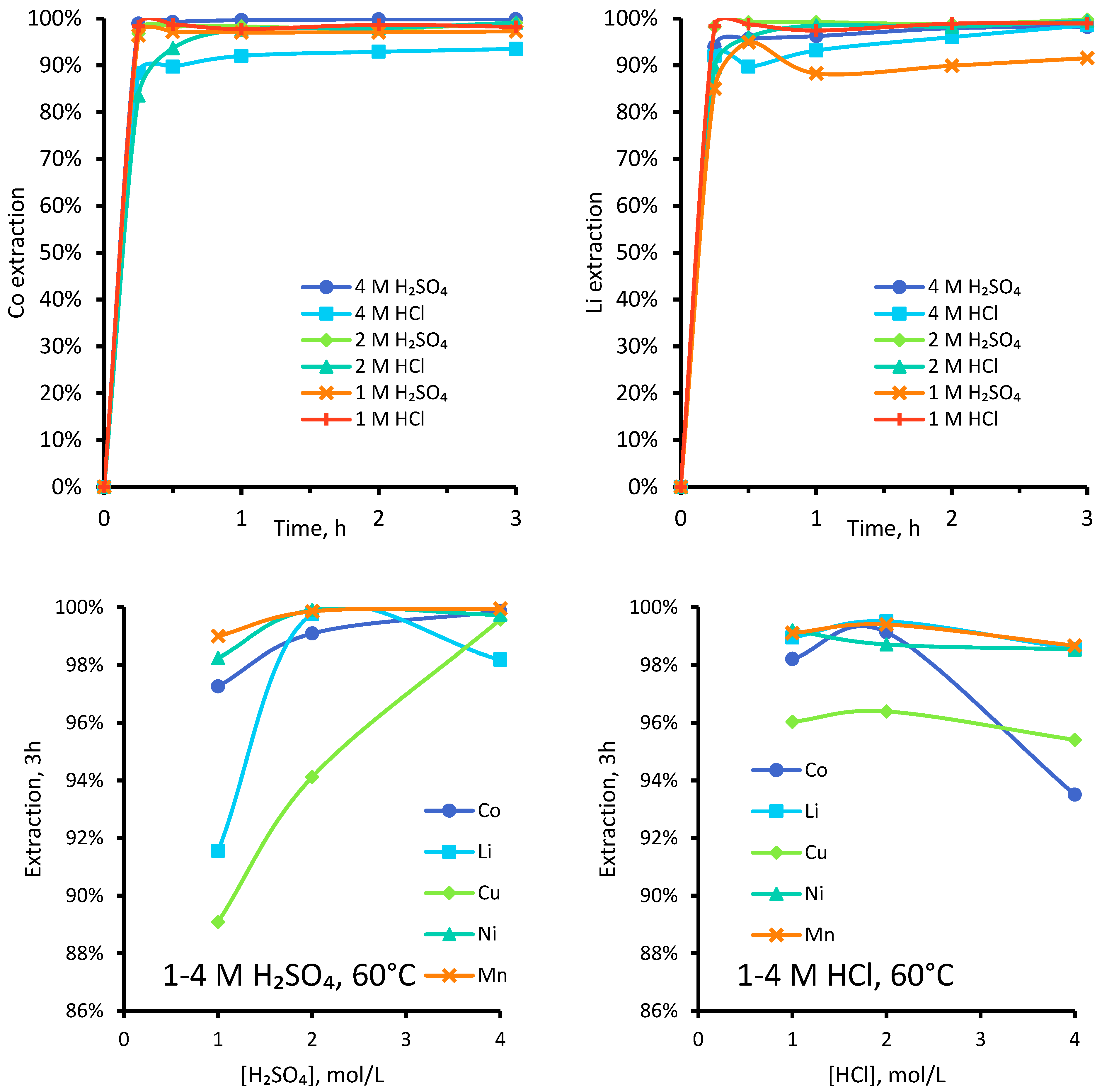
3.1.3. Effect of Varied Concentrations of Sulphuric and Hydrochloric Acid
3.1.4. Effect of Varied Reductant Concentration
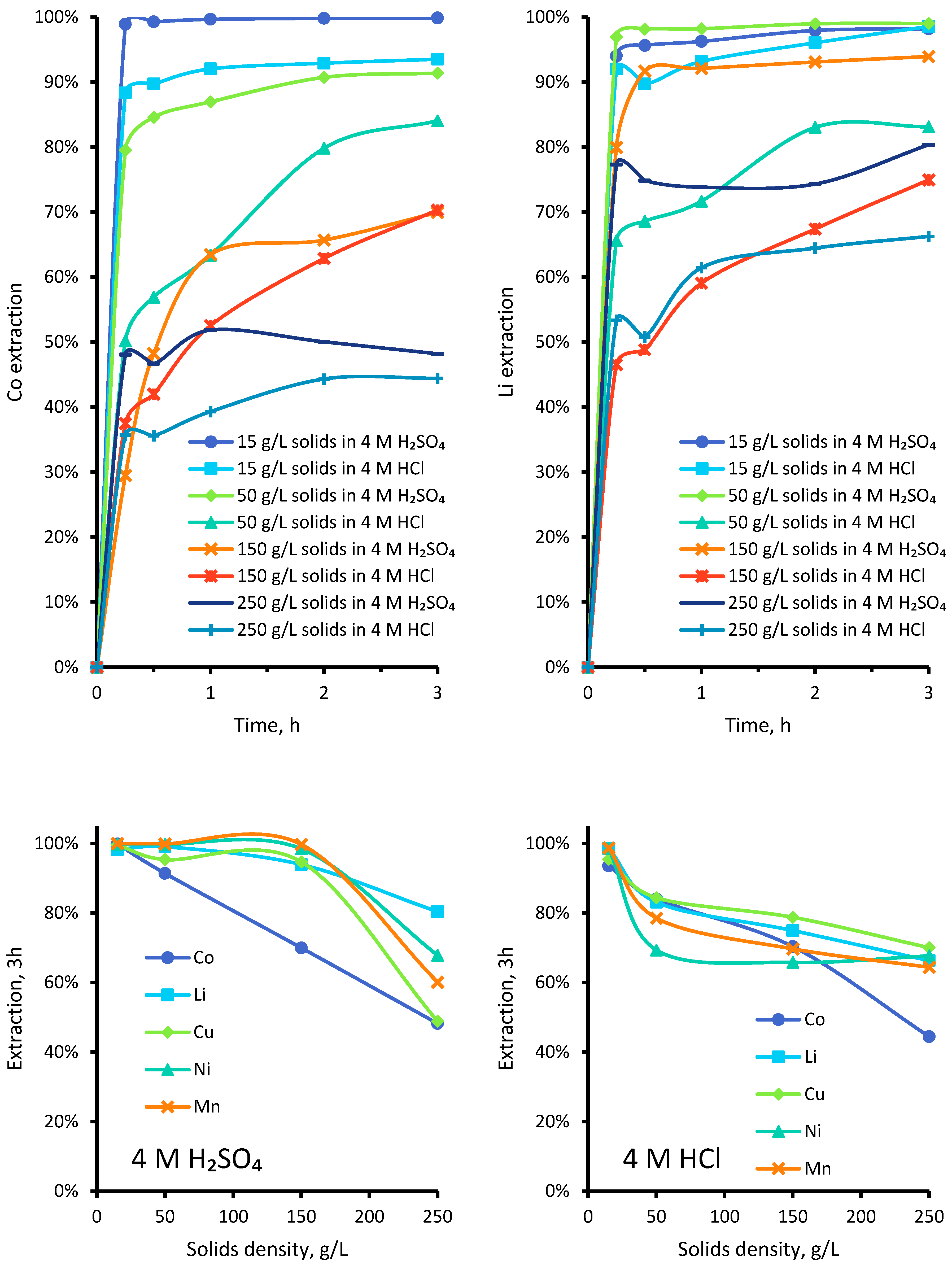
3.1.5. Effect of Varied Pulp Densities
3.2. Controlled Potential Leaches
3.3. Semi-Continuous Leach Test
3.3.1. Leach Results
3.3.2. Leach Residue Characterisation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, L.P.; Sun, S.Y.; Song, X.F.; Yu, J.G. Leaching process for recovering valuable metals from the LiNi1/3Co1/3Mn1/3O2 cathode of lithium-ion batteries. Waste Manag. 2017, 64, 171–181. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Singh, N. Recycling of Spent Lithium-Ion Battery: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Amalia, D.; Singh, P.; Zhang, W.; Nikoloski, A.N. A Review of Pretreatment Methods for Spent Lithium-Ion Batteries to Produce Black Mass—Comparison of Processes of Asia Pacific Recyclers. Miner. Process. Extr. Met. Rev. 2024, 46, 626–643. [Google Scholar] [CrossRef]
- Bhar, M.; Ghosh, S.; Krishnamurthy, S.; Kaliprasad, Y.; Martha, S.K. A review on spent lithium-ion battery recycling: From collection to black mass recovery. RSC Sustain. 2023, 1, 1150. [Google Scholar] [CrossRef]
- Klemettinen, L.; Biswas, J.; Klemettinen, A.; Zhang, J.; O’Brien, H.; Partinen, J.; Jokilaakso, A. Towards integration of pyro- and hydrometallurgical unit operations for efficient recovery of battery metals from waste lithium-ion batteries. In Proceedings of the 12th International Conference on Molten Slags, Fluxes and Salts (MOLTEN 2024), Brisbane, Australia, 17–19 June 2024; pp. 1571–1584. [Google Scholar] [CrossRef]
- Moon, S.; Chae, W.; Jun, B.Y.; Yoon, Y.; Rho, H. Comparative study on the leaching behavior of lithium-ion battery black mass using sulfuric and organic acids combined with hydrogen peroxide. Sep. Purif. Technol. 2025, 376, 133991. [Google Scholar] [CrossRef]
- Partinen, J.; Halli, P.; Varonen, A.; Wilson, B.P.; Lundström, M. Investigating battery black mass leaching performance as a function of process parameters by combining leaching experiments and regression modelling. Miner. Eng. 2024, 215, 108828. [Google Scholar] [CrossRef]
- Gilligan, R.; O’Malley, G.P.; Nikoloski, A.N. Characterisation & pre-treatment of spent lithium-ion batteries and investigative leaching of the contained metals. Extractive Metallurgy Hub, Harry Butler Institute, Murdoch University: Rockingham, WA, Australia, 2026; under review. [Google Scholar]
- Ross, B.J.; LeResche, M.; Liu, D.; Durham, J.D.; Dahl, E.U.; Lipson, A.L. Mitigating the Impact of Thermal Binder Removal for Direct Li-Ion Battery Recycling. ACS Sustain. Chem. Eng. 2020, 8, 12511–12515. [Google Scholar] [CrossRef]
- Vieceli, N.; Benjamasutin, P.; Promphan, R.; Hellström, P.; Paulsson, M.; Petranikova, M. Recycling of Lithium-Ion Batteries: Effect of Hydrogen Peroxide and a Dosing Method on the Leaching of LCO, NMC Oxides, and Industrial Black Mass. ACS Sustain. Chem. Eng. 2023, 11, 9662–9673. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, H.; Xie, Y.; Ning, P.; An, H.; You, H.; Nawaz, F. A closed-loop process for recycling LiNi1/3Co1/3Mn1/3O2 from the cathode scraps of lithium-ion batteries: Process optimization and kinetics analysis. Sep. Purif. Technol. 2015, 150, 186–195. [Google Scholar] [CrossRef]
- Ku, H.; Jung, Y.; Jo, M.; Park, S.; Kim, S.; Yang, D.; Rhee, K.; An, E.M.; Sohn, J.; Kwon, K. Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching. J. Hazard. Mater. 2016, 313, 138–146. [Google Scholar] [CrossRef]
- Vieceli, N.; Nogueira, C.A.; Guimarães, C.; Pereira, M.F.C.; Durão, F.O.; Margarido, F. Hydrometallurgical recycling of lithium-ion batteries by reductive leaching with sodium metabisulphite. Waste Manag. 2018, 71, 350–361. [Google Scholar] [CrossRef]
- Zheng, X.; Gao, W.; Zhang, X.; He, M.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Spent lithium-ion battery recycling—Reductive ammonia leaching of metals from cathode scrap by sodium sulphite. Waste Manag. 2017, 60, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Chernayev, A.; Zou, Y.; Wilson, B.P.; Lundström, M. The interference of copper, iron and aluminum with hydrogen peroxide and its effects on reductive leaching of LiNi1/3Mn1/3Co1/3O2. Sep. Purif. Technol. 2022, 281, 119903. [Google Scholar] [CrossRef]
- Qi, Y.; Meng, F.; Yi, X.; Shu, J.; Chen, M.; Sun, Z.; Sun, S.; Xiu, F.R. A novel and efficient ammonia leaching method for recycling waste lithium ion batteries. J. Clean. Prod. 2020, 251, 119665. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Lai, F.; Yan, F.; Zhang, Z. Reduction-ammoniacal leaching to recycle lithium, cobalt, and nickel from spent lithium-ion batteries with a hydrothermal method: Effect of reductants and ammonium salts. Waste Manag. 2020, 102, 122–130. [Google Scholar] [CrossRef]
- Yang, J.; Lai, Y.; Liu, F.; Jia, M.; Jiang, L. Countercurrent leaching of Ni, Co, Mn, and Li from spent lithium-ion batteries. Waste Manag. Res. 2020, 38, 1358–1366. [Google Scholar] [CrossRef]
- Yan, K.; Chen, Q.; Xiong, Z.; Wu, J.; Zhang, Z.; Xu, Z.; Wang, R.; Li, J.; Zhong, S. A Novel Method for the Recovery of Li from Spent Lithium-Ion Batteries Using Reduction Roasting–Countercurrent Leaching. JOM 2022, 74, 3821–3832. [Google Scholar] [CrossRef]
- Zhao, T.; Traversy, M.; Choi, Y.; Ghahreman, A. A novel process for multi-stage continuous selective leaching of lithium from industrial-grade complicated lithium-ion battery waste. Sci. Total Environ. 2014, 909, 168533. [Google Scholar] [CrossRef]
- Gilligan, R.; O’Malley, G.P.; Nikoloski, A.N. Impurities separation from spent lithium-ion batteries leach solution and recovery of the valuable elements. Extractive Metallurgy Hub, Harry Butler Institute, Murdoch University: Rockingham, WA, Australia, 2026; under review. [Google Scholar]
- Yang, Y.; Xu, S.; He, Y. Lithium recycling and cathode material regeneration from acid leach liquor of spent lithium-ion battery via facile co-extraction and coprecipitation processes. Waste Manag. 2017, 64, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Šulcek, Z.; Povondra, P. Methods of Decomposition in Inorganic Analysis; CRC Press: Boca Raton, FI, USA, 1989. [Google Scholar]
- Conte, V.; Floris, B. Vanadium catalyzed oxidation with hydrogen peroxide. Inorganica Chim. Acta 2010, 363, 1935–1946. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Acute Exposure Guideline Levels; National Research Council (US) Committee on Toxicology. 3, Monochloroacetic Acid Acute Exposure Guideline Levels. In Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 7; National Academies Press (US): Washington, DC, USA, 2009; Available online: https://www.ncbi.nlm.nih.gov/books/NBK214910/ (accessed on 13 February 2023).
- Çuhadar, I.E.; Mennik, F.; Dinç, N.I.; Gül, A.; Burat, F. Characterization and recycling of lithium nickel manganese cobalt oxide type spent mobile phone batteries based on mineral processing technology. J. Mater. Cycles Waste Manag. 2023, 25, 1746–1759. [Google Scholar] [CrossRef]


| Element | Mass % | Element | Mass % | Element | Mass % | Element | Mass % |
|---|---|---|---|---|---|---|---|
| Co | 30.7% | Al | 2.01% | Cu | 1.54% | Mn | 1.23% |
| Li | 3.78% | P | 1.90% | Ni | 1.24% | Fe | 1.09% |
| Element | ppm | Element | ppm | Element | ppm | Element | ppm |
|---|---|---|---|---|---|---|---|
| Zn | 5137 | Na | 530 | Cr | 184 | Nd | 28 |
| Pb | 4219 | Cd | 499 | La | 122 | Pr | 14 |
| As | 815 | Ca | 271 | Ce | 82 |
| Element | Mass % | Element | Mass % | Element | Mass % | Element | Mass % |
|---|---|---|---|---|---|---|---|
| Co | 24.0% | Ni | 3.81% | Mn | 2.80% | Na | 1.77% |
| Cu | 4.49% | Li | 3.54% | Al | 2.65% |
| Element | ppm | Element | ppm | Element | ppm | Element | ppm |
|---|---|---|---|---|---|---|---|
| Fe | 5217 | Cd | 1240 | Cr | 294 | Nd | 68 |
| Pb | 3829 | Ca | 1038 | P | 226 | As | 55 |
| Zn | 3433 | La | 323 | Ce | 193 | Pr | 29 |
| Sample | Co | Li | Cu | Ni | Mn | Al | Fe |
|---|---|---|---|---|---|---|---|
| Batch 2 Roast 2 | 34.0% | 5.07% | 3.66% | 5.26% | 4.09% | 3.65% | 0.354% |
| Sample | Co | Li | Cu | Ni | Mn | Al | Fe |
|---|---|---|---|---|---|---|---|
| Batch 2 Roasts 3+4 digest 1 | 36.3% | 5.09% | 2.88% | 5.40% | 4.21% | 3.79% | 0.268% |
| Batch 2 Roasts 3+4 digest 2 | 36.7% | 5.17% | 4.02% | 5.69% | 4.36% | 3.80% | 0.303% |
| Batch 2 Roasts 3+4 avg. | 36.5% | 5.13% | 3.45% | 5.54% | 4.28% | 3.79% | 0.285% |
| Leaching | Co | Li | Cu | Ni | Mn |
|---|---|---|---|---|---|
| Stage 1 | 86.0% | 99.1% | 97.2% | 97.6% | 99.2% |
| Stage 1 + 2 | 93.0% | 99.8% | 99.8% | 98.9% | 99.8% |
| Stage 3 | 84.6% | 98.9% | 98.6% | 97.2% | 98.8% |
| Stage 3 + 4 | 92.5% | 99.7% | 99.8% | 98.6% | 99.8% |
| Stage 5 | 85.7% | 99.0% | 98.8% | 97.2% | 98.8% |
| Stage 5 + 6 | 93.0% | 99.7% | 99.8% | 98.9% | 99.8% |
| Stage 7 | 84.8% | 98.6% | 98.6% | 96.4% | 98.7% |
| Stage | Filtrate pH | Filtrate E, mV vs. Ag/AgCl |
|---|---|---|
| 1 | −0.16 | 725 |
| 2 | −0.29 | 611 |
| 3 | −0.21 | 701 |
| 4 | −0.48 | 609 |
| 5 | −0.22 | 718 |
| 6 | −0.29 | 612 |
| 7 | −0.02 | 735 |
| Stage | Acid Consumption, g/100 mL | Acid Consumption, kg/t |
|---|---|---|
| 1 | 28.84 | 1353 |
| 2 | 4.54 | 962 |
| 3 | 31.53 | 1405 |
| 4 | 5.34 | 1074 |
| 5 | 32.32 | 1430 |
| 6 | 5.28 | 1111 |
| 7 | 33.04 | 1536 |
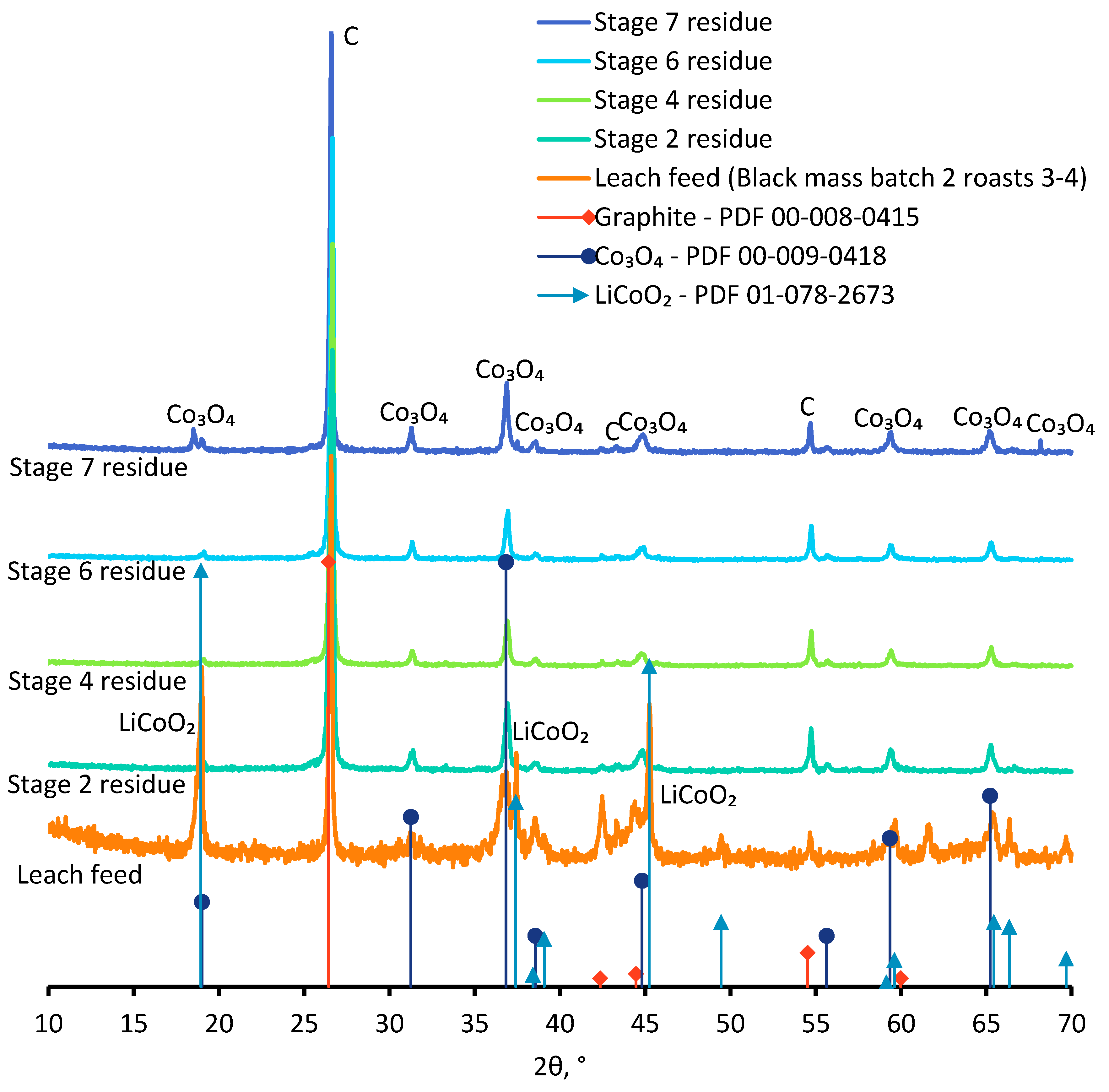
| Residue | Co | Li | Cu | Ni | Mn | Al | Fe | Phases Identified |
|---|---|---|---|---|---|---|---|---|
| Stage 1 | 21.6 | 0.2 | 0.4 | 0.6 | 0.2 | 0.5 | 0.4 | Not analysed |
| Stage 2 | 13.4 | 0.0 | 0.0 | 0.3 | 0.0 | 0.3 | 0.2 | Graphite, Co3O4 |
| Stage 3 | 22.8 | 0.2 | 0.2 | 0.6 | 0.2 | 0.6 | 0.4 | Not analysed |
| Stage 4 | 14.2 | 0.1 | 0.0 | 0.4 | 0.0 | 0.5 | 0.2 | Graphite, Co3O4 |
| Stage 5 | 22.0 | 0.2 | 0.2 | 0.7 | 0.2 | 0.5 | 0.3 | Not analysed |
| Stage 6 | 13.8 | 0.1 | 0.0 | 0.3 | 0.0 | 0.5 | 0.1 | Graphite, Co3O4 |
| Stage 7 | 22.4 | 0.3 | 0.2 | 0.8 | 0.2 | 0.8 | 0.5 | Graphite, Co3O4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilligan, R.; O’Malley, G.P.; Nikoloski, A.N. The Leaching of Valuable Metals (Li, Co, Ni, Mn, Cu) from Black Mass from Spent Lithium-Ion Batteries. Metals 2025, 15, 1155. https://doi.org/10.3390/met15101155
Gilligan R, O’Malley GP, Nikoloski AN. The Leaching of Valuable Metals (Li, Co, Ni, Mn, Cu) from Black Mass from Spent Lithium-Ion Batteries. Metals. 2025; 15(10):1155. https://doi.org/10.3390/met15101155
Chicago/Turabian StyleGilligan, Rorie, Glen P. O’Malley, and Aleksandar N. Nikoloski. 2025. "The Leaching of Valuable Metals (Li, Co, Ni, Mn, Cu) from Black Mass from Spent Lithium-Ion Batteries" Metals 15, no. 10: 1155. https://doi.org/10.3390/met15101155
APA StyleGilligan, R., O’Malley, G. P., & Nikoloski, A. N. (2025). The Leaching of Valuable Metals (Li, Co, Ni, Mn, Cu) from Black Mass from Spent Lithium-Ion Batteries. Metals, 15(10), 1155. https://doi.org/10.3390/met15101155






