The Influence of Electrostatic Separation Parameters on the Recovery of Metals from Pre-Crushed PCBs
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Output Flow Rates
2.2. Chemical and Physical Characterizations
3. Results and Discussions
3.1. Raw Material Characterization
3.2. Effect of Feed Flow Rates in the Electrostatic Separation
3.3. Effect of Electrode Voltage in the Electrostatic Separation
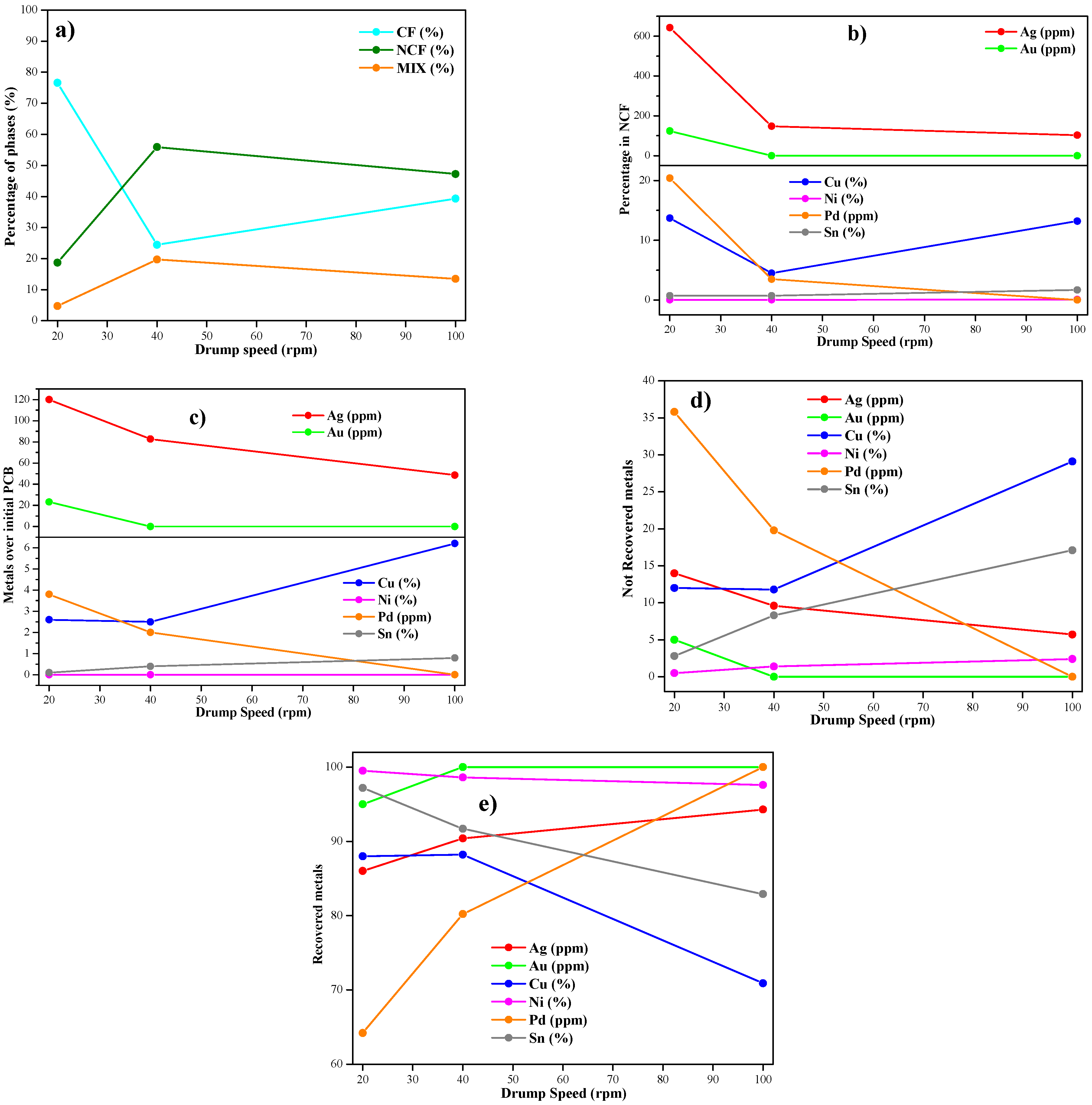
3.4. Effect of Drum Speed in the Electrostatic Separation
4. Conclusions
- Electrostatic separation tests were successfully carried out on pre-crushed printed circuit boards (PCBs) at different feed flow rates, electrode voltages, and drum speeds.
- The determination of different output fractions suggests that the tests separated according to conductive fractions (CFs) and non-conductive fractions (NCFs), with an intermediate non-correctively separated fraction (mixed fraction, MIX), but in all cases with a low percentage (always less than 3.5 wt.%).
- In terms of the recovery of metals from the initial PCBs, the maximum values are obtained for the specimen FR120-EV35-DS20, i.e., for a feed flow rate of 120 kg/h, a 35 kV electrode voltage, and a drum speed of 20 rpm. In those conditions, more than 90% of metal recovery was reached for all of them, except CU, where an 85% recovery was reached.
- Specifically, if the maximum percentage of Cu is required, as a key metal in the industry, using a low drum speed is the key parameter, at 20–40 rpm, corresponding to the samples FR30-EV40-DS20 and FR30-EV40-DS40. Opposite, if the maximum recovered amount is required for noble metals (Au and Ag, mainly), the drum speed has less influence, suggesting the use of intermediate values of the feed flow rate and electrode voltage to recover up to 95 wt.% of both noble metals.
- Although no single set of operating conditions can achieve perfect separation, the primary objective of these processes is to isolate the metal fraction as effectively as possible. The resulting metal-rich mixture typically requires further refining and/or purification through methods such as hydrometallurgy, smelting, or vacuum distillation, if necessary. In the case of the NCF (i.e., the plastic-rich stream), additional separation techniques are required to achieve complete metal recovery. Processes such as flotation, plastic distillation, or incineration with CO2 capture may be necessary to enable full material recovery and contribute to closing the loop of the circular economy.
- Furthermore, although not explored in this study, the management and potential reuse or disposal of the separated fractions represent an important area for future research, given their variability depending on the goals and capabilities of the processing entity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cui, J.; Forssberg, E. Mechanical recycling of waste electric and electronic equipment: A review. J. Hazard. Mater. 2003, 99, 243–263. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Fray, D.J. Recovery of high purity precious metals from printed circuit boards. J. Hazard. Mater. 2009, 164, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.G.; Spottiswood, D.J. The theory of electrostatic separations: A review part II. Particle charging. Miner. Eng. 1989, 2, 193–205. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, K. A new technology for recycling materials from waste printed circuit boards. J. Hazard. Mater. 2010, 175, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhu, J.; Huang, X.; Ruan, J.; Xu, Z. Assessment of precious metals positioning in waste printed circuit boards and the economic benefits of recycling. Waste Manag. 2022, 139, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Veit, H.M.; Bernardes, A.M.; Ferreira, J.Z.; Tenório, J.A.S.; de Fraga Malfatti, C. Recovery of copper from printed circuit boards scraps by mechanical processing and electrometallurgy. J. Hazard. Mater. 2006, 137, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Ogunniyi, I.O.; Vermaak, M.K.G.; Groot, D.R. Chemical composition, and liberation characterization of printed circuit board comminution fines for beneficiation investigations. Waste Manag. 2009, 29, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Chicardi, E.; Lopez-Paneque, A.; García-Orta, V.H.G.; Sepúlveda-Ferrer, R.E.; Gallardo, J.M. Enrichment Methods for Metal Recovery from Waste from Electrical and Electronic Equipment: A Brief Review. Metals 2025, 15, 140. [Google Scholar] [CrossRef]
- Kumar, A.; Holuszko, M.; Espinosa, D.C.R. E-waste: An overview on generation, collection, legislation, and recycling practices. Resour. Conserv. Recycl. 2017, 122, 32–42. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Xu, Z. Electrostatic separation for recovering metals and nonmetals from waste printed circuit board: Problems and improvements. Environ. Sci. Technol. 2008, 42, 5272–5276. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.M.; Bellopede, R.; Tori, A.; Zanetti, G.; Marini, P. Gravity and Electrostatic Separation for Recovering Metals from Obsolete Printed Circuit Board. Materials 2022, 15, 1874. [Google Scholar] [CrossRef] [PubMed]
- EU Parliament and Council. Directive 2012/19/EU on Waste Electrical and Electronic Equipment (WEEE). Off. J. Eur. Union 2012. Available online: https://eur-lex.europa.eu/eli/dir/2012/19/2024-04-08 (accessed on 23 June 2025).
- Guo, C.; Wang, H.; Liang, W.; Fu, J.; Yi, X. Liberation characteristic and physical separation of printed circuit board (PCB). Waste Manag. 2011, 31, 2161–2166. [Google Scholar] [CrossRef] [PubMed]
- Suponik, T.; Franke, D.M.; Nuckowski, P.M.; Matusiak, P.; Kowol, D.; Tora, B. Impact of Grinding of Printed Circuit Boards on the Efficiency of Metal Recovery by Means of Electrostatic Separation. Minerals 2021, 11, 281. [Google Scholar] [CrossRef]
- ISO 3310-1:2016; Test Sieves—Technical Requirements and Testing—Part 1: Test Sieves of Metal Wire Cloth. AENOR: Madrid, Spain, 2016.
- Holgersson, S.; Steenari, B.M.; Björkman, M.; Cullbrand, K. Analysis of the metal content of small-size Waste Electric and Electronic Equipment (WEEE) printed circuit boards—Part 1: Internet routers, mobile phones and smartphones. Resour. Conserv. Recycl. 2018, 133, 300–308. [Google Scholar] [CrossRef]
- Luyima, A.; Shi, H.; Zhang, L. Leaching studies for metals recovery from waste printed wiring boards. JOM 2011, 63, 38–41. [Google Scholar] [CrossRef]
- Sheng, P.P.; Etsell, T.H. Recovery of gold from computer circuit board scrap using aqua regia. Waste Manag. Res. 2007, 25, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Lindley, K.S.; Rowson, N.A. Feed preparation factors affecting the efficiency of electrostatic separation. Phys. Sep. Sci. Eng. 1997, 8, 161–173. [Google Scholar] [CrossRef]
- Gehringer, S.; Luckeneder, C.; Flachberger, H. Systematic Separation Studies on Finely Dispersed Raw Magnesite by Using Triboelectrostatic Belt Separation. BHM Berg- Und Hüttenmänn. Monatsh. 2020, 165, 364–368. [Google Scholar] [CrossRef]




| Operating Conditions * | Feed Flow Rate (kg/h) | Electrode Voltage (kV) | Drumm Speed (rpm) |
|---|---|---|---|
| FR30-EV35-DS20 | 30 | 35 | 20 |
| FR75-EV35-DS20 | 75 | ||
| FR120-EV35-DS20 | 120 | ||
| FR30-EV25-DS40 | 30 | 25 | 40 |
| FR30-EV30-DS40 | 30 | ||
| FR30-EV40-DS40 | 40 | ||
| FR30-EV40-DS20 | 30 | 40 | 20 |
| FR30-EV40-DS40 | 40 | ||
| FR30-EV40-DS100 | 100 |
| Sample | Al (wt.%) | Ag (ppm) # | Au (ppm) # | Cu (wt.%) | Fe (wt.%) | Ni (wt.%) | Pd (ppm) # | Si (wt.%) | Sn (wt.%) |
|---|---|---|---|---|---|---|---|---|---|
| Crushed PCBs | 12.7 ± 1.2 | 858 ± 23 | 467 ± 14 | 21.4 ± 1.2 | 0.7 ± 0.3 | 1.6 ± 0.3 | 9.9 ± 1.0 | 3.8 ± 0.4 | 4.7 ± 0.9 |
| Operating Conditions * | Output Fraction ** | Mass (wt.%) | Density (g/cm3) | Plastics (wt.%) |
|---|---|---|---|---|
| FR30-EV35-DS20 | CF | 64.6 ± 2.2 | 5.7 | 2.8 ± 0.2 |
| NCF | 31.0 ± 2.3 | 2.1 | 44.1 ± 1.2 | |
| MIX | 1.4 ± 0.1 | 3.1 | 7.3 ± 0.7 | |
| FR75-EV35-DS20 | CF | 69.5 ± 3.2 | 7.7 | 1.9 ± 0.7 |
| NCF | 27.7 ± 2.3 | 2.0 | 32.2 ± 2.3 | |
| MIX | 2.8 ± 1.0 | 2.2 | 16.7 ± 3.0 | |
| FR120-EV35-DS20 | CF | 78.9 ± 2.3 | 8.1 | 2.1 ± 0.6 |
| NCF | 16.1 ± 2.2 | 1.9 | 39.6 ± 2.5 | |
| MIX | 3.0 ± 0.5 | 2.9 | 12.1 ± 1.8 |
| Operating Conditions * | Fraction | Ag (ppm) | Au (ppm) # | Cu (wt.%) | Ni (wt.%) | Pd (ppm) # | Sn (wt.%) |
|---|---|---|---|---|---|---|---|
| FR30-EV35-DS20 | NCF ** | 627 ± 21 | 123.0 ± 15.5 | 12.3 ± 1.1 | 0.05 ± 0.01 | 5.8 ± 0.5 | 0.9 ± 0.1 |
| AP ## | 194 ± 14 | 38.1 ± 8.0 | 3.8 ± 0.6 | 0.02 ± 0.01 | 1.8 ± 0.2 | 0.3 ± 0.1 | |
| MP @ | 22.6 ± 3.2 | 8.2 ± 2.0 | 17.8 ± 2.6 | 1.0 ± 0.2 | 18.2 ± 2.2 | 5.9 ± 0.3 | |
| FR75-EV35-DS20 | NCF | 431 ± 16 | 121.0 ± 10.3 | 11.1 ± 0.6 | 0.07 ± 0.01 | 6.6 ± 1.4 | 1.6 ± 0.1 |
| AP | 119 ± 11 | 33.5 ± 3.6 | 3.1 ± 0.2 | 0.02 ± 0.01 | 1.8 ± 0.1 | 0.4 ± 0.1 | |
| MP | 13.9 ± 1.3 | 7.2 ± 1.3 | 14.4 ± 0.4 | 1.2 ± 0.1 | 18.4 ± 0.5 | 9.4 ± 0.2 | |
| FR120-EV35-DS20 | NCF | 445 ± 22 | 122 ± 12 | 16.7 ± 1.2 | 0.06 ± 0.01 | 3.3 ± 0.2 | 0.9 ± 0.1 |
| AP | 72 ± 16 | 19.6 ± 0.6 | 2.7 ± 0.1 | 0.01 ± 0.01 | 0.5 ± 0.1 | 0.2 ± 0.1 | |
| MP | 8.4 ± 3.0 | 4.2 ± 0.2 | 12.6 ± 1.0 | 0.6 ± 0.1 | 5.4 ± 0.2 | 3.1 ± 0.1 |
| Operating Conditions * | Output Fraction ** | Mass (wt.%) | Density (g/cm3) | Plastics (wt.%) |
|---|---|---|---|---|
| FR30-EV25-DS40 | CF | 31.4 ± 2.2 | 4.8 | <1 |
| NCF | 52.2 ± 3.2 | 2.3 | 29.1 ± 3.3 | |
| MIX | 16.4 ± 1.9 | 2.4 | 24.7 ± 2.3 | |
| FR30-EV30-DS40 | CF | 24.6 ± 1.9 | 5.8 | <1 |
| NCF | 57.8 ± 5.8 | 2.3 | 30.5 ± 4.0 | |
| MIX | 17.7 ± 3.1 | 2.5 | 16.1 ± 2.7 | |
| FR30-EV40-DS40 | CF | 22.2 ± 1.2 | 5.3 | <1 |
| NCF | 57.5 ± 4.0 | 2.2 | 33.4 ± 1.9 | |
| MIX | 20.2 ± 1.6 | 3.0 | <1 |
| Operating Conditions * | Fraction | Ag (ppm) # | Au (ppm) # | Cu (wt.%) | Ni (wt.%) | Pd (ppm) # | Sn (wt.%) |
|---|---|---|---|---|---|---|---|
| FR30-EV25-DS40 | NCF ** | 431 ± 13 | 121 ± 12 | 10.1 ± 0.9 | 0.07 ± 0.01 | 7.6 ± 0.2 | 1.6 ± 0.2 |
| AP ## | 225 ± 12 | 63 ± 5 | 5.3 ± 1.2 | 0.04 ± 0.01 | 4.0 ± 0.4 | 0.8 ± 0.1 | |
| MP @ | 26.2 ± 3.2 | 13.5 ± 3.6 | 24.6 ± 3.2 | 2.3 ± 0.1 | 40.1 ± 4.3 | 17.8 ± 1.2 | |
| FR30-EV30-DS40 | NCF | 484 ± 25 | 80 ± 2.7 | 13.9 ± 1.8 | 0.07 ± 0.01 | 9.7 ± 2.1 | 1.3 ± 0.1 |
| AP | 279 ± 23 | 46 ± 5.5 | 8.0 ± 1.0 | 0.04 ± 0.01 | 5.6 ± 1.0 | 0.8 ± 0.1 | |
| MP | 32.6 ± 6.6 | 9.9 ± 1.7 | 37.5 ± 3.0 | 2.5 ± 0.3 | 56.6 ± 5.6 | 16.0 ± 1.3 | |
| FR30-EV40-DS40 | NCF | 444 ± 18 | 123 ± 16 | 16.7 ± 2.5 | 0.06 ± 0.02 | 3.3 ± 0.8 | 0 |
| AP | 255 ± 29 | 71 ± 10 | 9.6 ± 0.8 | 0.03 ± 0.01 | 1.9 ± 0.3 | 0 | |
| MP | 29.8 ± 4.3 | 15.1 ± 2.2 | 44.9 ± 2.9 | 2.2 ± 0.1 | 19.2 ± 3.9 | 0 |
| Operating Conditions * | Output Flow Type ** | Mass (wt.%) | Density (g/cm3) | Plastics (wt.%) |
|---|---|---|---|---|
| FR30-EV40-DS20 | CF | 76.6 ± 9.1 | 5.6 | <1 |
| NCF | 18.7 ± 3.4 | 2.0 | 48.8 ± 5.2 | |
| MIX | 4.7 ± 1.2 | 3.0 | 38.5 ± 3.0 | |
| FR30-EV40-DS40 | CF | 24.4 ± 2.5 | 5.2 | <1 |
| NCF | 55.9 ± 6.0 | 2.3 | 33.4 ± 2.7 | |
| MIX | 19.7 ± 3.0 | 3.0 | <1 | |
| FR30-EV40-DS100 | CF | 39.3 ± 2.1 | 5.6 | <1 |
| NCF | 47.2 ± 3.6 | 2.0 | 43.0 ± 4.0 | |
| MIX | 13.5 ± 1.9 | 2.4 | 18.9 ± 3.3 |
| Operating Conditions* | Fraction | Ag (ppm) # | Au (ppm) # | Cu (wt.%) | Ni (wt.%) | Pd (ppm) # | Sn (wt.%) |
|---|---|---|---|---|---|---|---|
| FR30-EV40-DS20 | NCF ** | 643 ± 23 | 124 ± 16 | 13.7 ± 1.5 | 0.04 ± 0.01 | 20.4 ± 3.2 | 0.7 ± 0.1 |
| AP ## | 120 ± 8 | 23.2 ± 4.0 | 2.6 ± 0.2 | 0 | 3.8 ± 0.3 | 0.1 ± 0.1 | |
| MP @ | 14.0 ± 1.3 | 5.0 ± 0.5 | 12.0 ± 2.0 | 0.5 ± 0.1 | 38.5 ± 4.9 | 2.8 ± 0.2 | |
| FR30-EV40-DS40 | NCF | 148 ± 17 | 0 | 4.5 ± 1.1 | 0.04 ± 0.01 | 3.5 ± 0.2 | 0.7 ± 0.1 |
| AP | 82.7 ± 11.0 | 0 | 2.5 ± 0.6 | 0 | 2.0 ± 0.2 | 0.4 ± 0.1 | |
| MP | 9.6 ± 1.6 | 0 | 11.8 ± 0.8 | 1.4 ± 0.2 | 19.8 ± 3.5 | 8.3 ± 0.3 | |
| FR30-EV40-DS100 | NCF | 103 ± 8.2 | 0 | 13.2 ± 1.9 | 0.08 ± 0.01 | 0 | 1.7 ± 0.2 |
| AP | 48.6 ± 5.0 | 0 | 6.2 ± 1.2 | 0 | 0 | 0.8 ± 0.1 | |
| MP | 5.7 ± 1.7 | 0 | 29.1 ± 2.2 | 2.4 ± 1.0 | 0 | 17.1 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Paneque, A.M.; García-Orta, V.H.G.; Gallardo, J.M.; Sepúlveda-Ferrer, R.E.; Chicardi, E. The Influence of Electrostatic Separation Parameters on the Recovery of Metals from Pre-Crushed PCBs. Metals 2025, 15, 826. https://doi.org/10.3390/met15080826
Lopez-Paneque AM, García-Orta VHG, Gallardo JM, Sepúlveda-Ferrer RE, Chicardi E. The Influence of Electrostatic Separation Parameters on the Recovery of Metals from Pre-Crushed PCBs. Metals. 2025; 15(8):826. https://doi.org/10.3390/met15080826
Chicago/Turabian StyleLopez-Paneque, Antonio Manuel, Victoria Humildad Gallardo García-Orta, Jose Maria Gallardo, Ranier Enrique Sepúlveda-Ferrer, and Ernesto Chicardi. 2025. "The Influence of Electrostatic Separation Parameters on the Recovery of Metals from Pre-Crushed PCBs" Metals 15, no. 8: 826. https://doi.org/10.3390/met15080826
APA StyleLopez-Paneque, A. M., García-Orta, V. H. G., Gallardo, J. M., Sepúlveda-Ferrer, R. E., & Chicardi, E. (2025). The Influence of Electrostatic Separation Parameters on the Recovery of Metals from Pre-Crushed PCBs. Metals, 15(8), 826. https://doi.org/10.3390/met15080826







