Effect of Overlap Rate on Microstructure and Corrosion Behavior of Laser-Clad Ni60-WC Composite Coatings on E690 Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
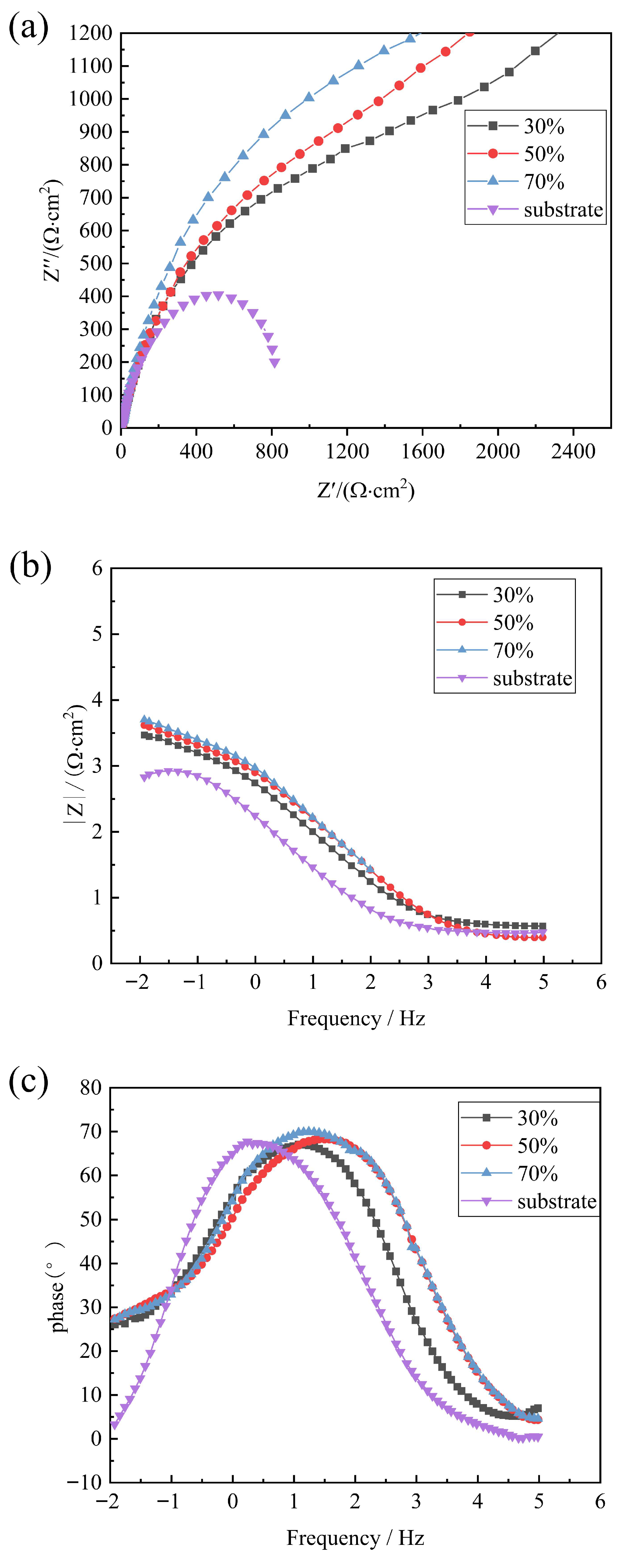
3.1. Analysis of Electrochemical Corrosion Performance of Composite Coating
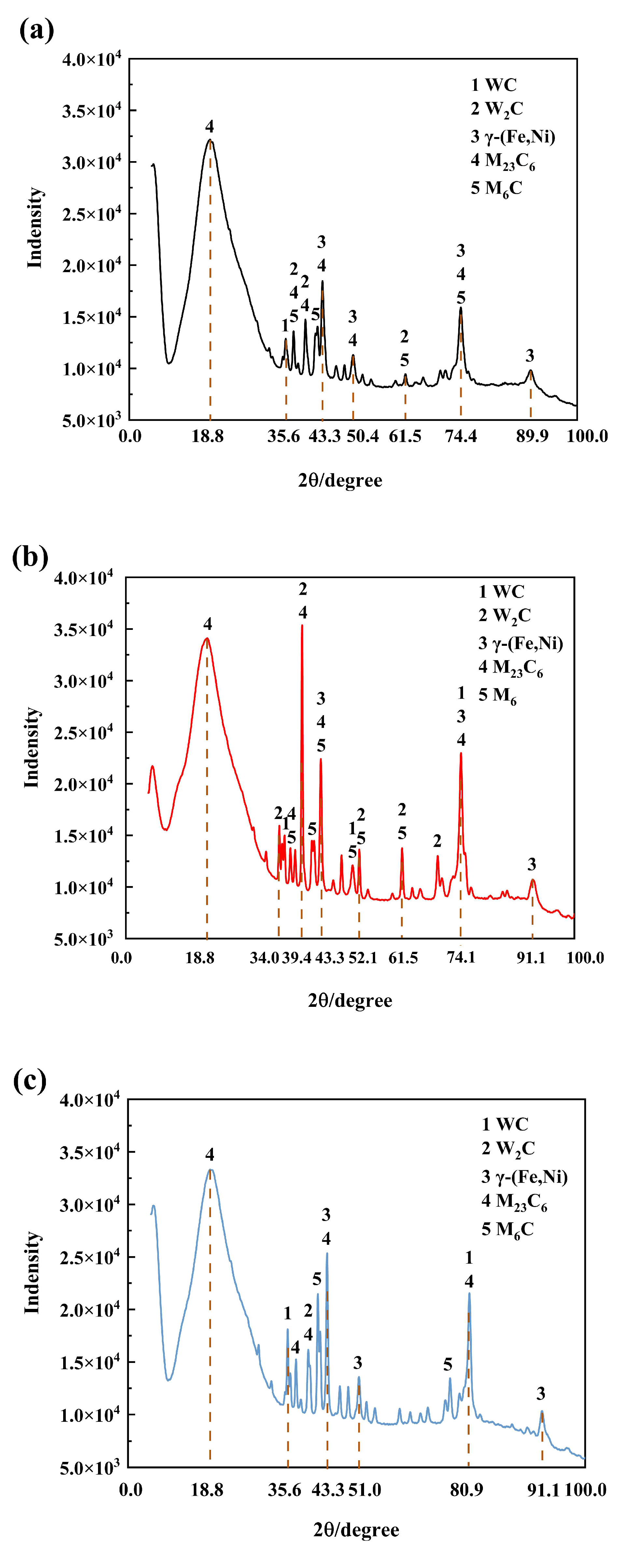
3.2. Microstructure and Performance Analysis of Composite Coatings
3.3. Morphology Analysis After Electrochemical Corrosion
4. Conclusions
- (1)
- The matrix phase of the composite coatings is γ-(Fe, Ni), with reinforcing phases including WC, W2C, and flocculent carbides formed. The overlap rate exerts a significant influence on the microstructure of the cladded layers. The cladded layer fabricated with a 30% overlap rate contains coarse dendrites and strip crystals, accompanied by a small quantity of flocculent carbides. The cladded layer prepared at a 50% overlap rate exhibits grain refinement, consisting of fine dendrites, strip crystals, and flocculent carbides. The microstructure of the cladded layer with a 70% overlap rate is further refined, containing numerous fine strip crystals and a large amount of flocculent carbides.
- (2)
- Laser cladding treatment significantly improves the corrosion resistance of E690 steel surfaces. Electrochemical characterization indicates that the coating with a 70% overlap rate demonstrates excellent corrosion resistance, primarily reflected in three key parameters: ① a notable positive shift in open circuit potential; ② a significant reduction in corrosion current density; and ③ the largest capacitive arc radius in electrochemical impedance spectroscopy analysis.
- (3)
- The enhanced corrosion resistance is mainly attributed to the acquisition of a denser and more uniform microstructure at a 70% overlap rate, which effectively hinders the penetration of corrosive Cl− ions and facilitates the formation of a stable passive film.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OR | overlap rate |
| WC | Tungsten carbide |
| OCP | open circuit potential |
| EIS | electrochemical impedance spectroscopy |
| HSLA | high-strength low-alloy |
| CPE | constant phase element |
| SEM | scanning electron microscopy |
| G | temperature gradient |
| R | solidification rate |
| FWHM | full width at half maximum |
| XRD | X-ray diffraction |
| EDS | energy dispersive spectroscopy |
| FCC | face-centered cubic |
References
- Hu, J.; Lin, G.; Deng, P.; Li, Z.; Tian, Y. Galvanic Corrosion of E690 Offshore Platform Steel in a Simulated Marine Thermocline. Metals 2024, 14, 287. [Google Scholar] [CrossRef]
- Lesyk, D.A.; Mordyuk, B.N.; Martinez, S.; Iefimov, M.O.; Dzhemelinskyi, V.V.; Lamikiz, A. Influence of Combined Laser Heat Treatment and Ultrasonic Impact Treatment on Microstructure and Corrosion Behavior of AISI 1045 Steel. Surf. Coat. Technol. 2020, 401, 126275. [Google Scholar] [CrossRef]
- Shan, B.; Chen, J.; Chen, S.; Ma, M.; Ni, L.; Shang, F.; Zhou, L. Laser Cladding of Fe-Based Corrosion and Wear-Resistant Alloy: Genetic Design, Microstructure, and Properties. Surf. Coat. Technol. 2022, 433, 128117. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, K.; Cao, J.; Meng, G.; Yu, F.; Zhou, Y.; Zhou, H.; La, P.; Xie, H. Effect of Line Energy Density of the Laser Beam on the Microstructure and Wear Resistance Properties of the Obtained Fe3Al Laser Cladding Coatings. Optik 2022, 261, 169256. [Google Scholar] [CrossRef]
- Guo, Y.; Song, S.N.; Huang, Y.B.; Wang, G.L.; Li, Y.B.; Li, Z.Q. Effect of Phase Constituents on Tribological and Corrosion Properties of Fe-Based Laser Clad Carbon Steel. Lasers Eng. 2023, 54, 313–327. [Google Scholar]
- Li, S.; Yi, L.; Jia, X.; Xiang, D.; Liu, T.; Ji, B. A Dense and Compact Laser Cladding Layer with Solid Metallurgical Bonding Significantly Improved Corrosion Resistance of Mg Alloy for Engineering Applications. Mater. Corros. 2020, 71, 1442–1452. [Google Scholar] [CrossRef]
- Yan, Q.; Yin, Q.; Cui, J.; Wang, X.; Qiao, Y.; Zhou, H. Effect of Temperature on Corrosion Behavior of E690 Steel in 3.5 wt.% NaCl Solution. Mater. Res. Express 2021, 8, 016528. [Google Scholar] [CrossRef]
- Wang, J.; Cui, X.; Jin, G.; Zhao, Y.; Wen, X.; Zhang, Y. Effect of In-Situ Ni Interlayer on the Microstructure and Corrosion Resistance of Underwater Wet 316L Stainless Steel Laser Cladding Layer. Surf. Coat. Technol. 2023, 458, 129341. [Google Scholar] [CrossRef]
- Jia, D.; Shi, W.; Zhang, H.; Wu, T.; Diao, Y.; Li, K.; Lu, C. Effects of Y2O3 Content on Wear Resistance and Corrosion Resistance of 316L/TiC Coating Fabricated by Laser Cladding. Coatings 2023, 13, 1348. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, W.; Tian, S.; Jia, Y.; Xiao, Y. Microstructures and High-Temperature Friction and Wear Behavior of High-Velocity Oxygen-Fuel-Sprayed WC-12%Co-6%Cr Coatings before and after Sealing. J. Mater. Eng. Perform. 2022, 31, 448–460. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, T.; Li, G. Research on Wear and Corrosion Resistance of Ni60-WC Coating Fabricated by Laser on the Preheated Copper Alloy. Coatings 2022, 12, 1537. [Google Scholar] [CrossRef]
- Cao, Q.; Fan, L.; Chen, H.; Hou, Y.; Dong, L.; Ni, Z. Wear and Corrosion Mechanisms of Ni-WC Coatings Modified with Different Y2O3 by Laser Cladding on AISI 4145H Steel. Sci. Eng. Compos. Mater. 2022, 29, 364–377. [Google Scholar] [CrossRef]
- Wang, G.; Wang, S.; Yin, Z.; Yang, X.; Wen, D.; Sun, Y. Synthesis of Ni-WC/Al-Ni Functionally Graded Coating with Advanced Corrosion and Wear Resistance on AZ91D Mg Alloy by Laser Cladding. Mater. Lett. 2023, 333, 133645. [Google Scholar] [CrossRef]
- Ge, Y.; Zheng, C.-Y.; Dai, L.-J.; Liu, C.; Kong, D.-J. Effect of Laser Power on the Electrochemical Performance of a Ni-60%WC Alloy Laser Clad Coating on H13 Steel. Laser Eng. 2023, 56, 177–190. [Google Scholar]
- Zeng, M.; Yan, H.; Yu, B.; Hu, Z. Microstructure, Microhardness and Corrosion Resistance of Laser Cladding Ni-WC Coating on AlSi5Cu1Mg Alloy. Trans. Nonferrous Met. Soc. China 2021, 31, 2716–2728. [Google Scholar] [CrossRef]
- Nie, B.; Xue, Y.; Luan, B. Effect of Overlap Rate on the Microstructure and Corrosion Behavior of Pure Copper Laser Cladding. J. Mater. Sci. 2024, 59, 6564–6582. [Google Scholar] [CrossRef]
- Katiyar, P.K.; Randhawa, N.S. Corrosion Behavior of WC-Co Tool Bits in Simulated (Concrete, Soil, and Mine) Solutions with and without Chloride Additions. Int. J. Refract. Met. Hard Mat. 2019, 85, 105062. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Pang, M. Effect of WC Content on Laser Cladding Ni-Based Coating on the Surface of Stainless Steel. Mater. Today Commun. 2022, 31, 103357. [Google Scholar] [CrossRef]
- Human, A.M.; Exner, H.E. Relationship between Electrochemical Behaviour and In-Service Corrosion of WC Based Cemented Carbides. Int. J. Refract. Met. Hard Mater. 1997, 15, 65–71. [Google Scholar] [CrossRef]
- Human, A.M.; Exner, H.E. Electrochemical Behaviour of Tungsten-Carbide Hardmetals. Mater. Sci. Eng. A 1996, 209, 180–191. [Google Scholar] [CrossRef]
- Yuan, W.; Li, R.; Chen, Z.; Gu, J.; Tian, Y. A Comparative Study on Microstructure and Properties of Traditional Laser Cladding and High-Speed Laser Cladding of Ni45 Alloy Coatings. Surf. Coat. Technol. 2021, 405, 126582. [Google Scholar] [CrossRef]
- Guo, N.; Cheng, Q.; Fu, Y.; Tong, J.; Gao, Y.; He, J. Microstructure and Corrosion Behavior of 10CrNi3MoV High-Strength Low-Alloy Steel Coating via Underwater Wire-Feed Laser Cladding. Surf. Coat. Technol. 2023, 473, 129984. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y. Effects of H-BN Content and Heat Treatment Temperature on the Tribological Behavior and Toughness of Laser-Clad Mo2FeB2/WC/h-BN Coatings. Ceram. Int. 2024, 50, 26097–26108. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, W.; Liu, E.; Liu, Y.; Liu, J.; Zhang, B.; Zhou, M.; Xu, Y. Effects of Process Parameters on Microstructure and Properties of In-Situ Synthesized WC-Reinforced Ni-Based Cladding Layer. Mater. Res. 2024, 27, e20230573. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, L.; Xu, Q.; Li, Y.; Li, C.; Li, C. Microstructure and Wear Resistance of Ni-Based WC Coating by Ultra-High Speed Laser Cladding. Acta Metall. Sin. 2020, 56, 1530–1540. [Google Scholar] [CrossRef]
- Xue, S.L.; Li, J.H.; Yao, F.P.; Yang, Y.H.; Li, X.X. Preparation and Structural Properties of Gradient Composite Coatings for Laser Cladding. Laser Eng. 2023, 54, 343–355. [Google Scholar]
- Li, W.; Yang, X.; Xiao, J.; Hou, Q. Effect of WC Mass Fraction on the Microstructure and Friction Properties of WC/Ni60 Laser Cladding Layer of Brake Discs. Ceram. Int. 2021, 47, 28754–28763. [Google Scholar] [CrossRef]
- Wang, H.; Liu, K.; Li, J.; Geng, S.; Jing, L.; Skuratov, V. Reinforcements/Matrix Micro-Interface Evolution and Properties of in-Situ Ni60A/WC Coatings Prepared by Laser Cladding. Surf. Coat. Technol. 2024, 484, 130834. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Y.; Wang, J.; Liu, K. Investigation on Cored-Eutectic Structure in Ni60/WC Composite Coatings Fabricated by Wide-Band Laser Cladding. J. Alloys Compd. 2015, 645, 151–157. [Google Scholar] [CrossRef]
- Parakh, A.; Vaidya, M.; Kumar, N.; Chetty, R.; Murty, B.S. Effect of Crystal Structure and Grain Size on Corrosion Properties of AlCoCrFeNi High Entropy Alloy. J. Alloys Compd. 2021, 863, 158056. [Google Scholar] [CrossRef]
- Man, C.; Dong, C.; Kong, D.; Wang, L.; Li, X. Beneficial Effect of Reversed Austenite on the Intergranular Corrosion Resistance of Martensitic Stainless Steel. Corros. Sci. 2019, 151, 108–121. [Google Scholar] [CrossRef]
- Liu, P.; Hu, L.; Zhang, Q.; Yang, C.; Yu, Z.; Zhang, J.; Hu, J.; Cao, F. Effect of Aging Treatment on Microstructure and Corrosion Behavior of Al-Zn-Mg Aluminum Alloy in Aqueous Solutions with Different Aggressive Ions. J. Mater. Sci. Technol. 2021, 64, 85–98. [Google Scholar] [CrossRef]








| Element | C | Si | Mn | P | S | Cr | Ni | Mo | V | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Substrate (E690) | 0.15 | 0.50 | 1.52 | 0.03 | 0.01 | 1.50 | 3.60 | 0.70 | 0.06 | Bal. | |
| Powder (Ni60) | 8 | 4 | 1 | 60 | 17.5 | 9.5 |
| Pulse Width/ ns | Power/w | Spot Diameter/mm | Powder Feed Rate/(r/min) | Scanning Speed/(mm/min) | |
|---|---|---|---|---|---|
| parameters | 20 | 2200 | 3 | 0.7 | 700 |
| Parameter | 70% | 50% | 30% | Substrate |
|---|---|---|---|---|
| Corrosion Potential/V | −0.39 | −0.43 | −0.36 | −0.69 |
| Corrosion current density/(μA/cm2) | 3.35 | 4.60 | 5.20 | 8.63 |
| Element | C | O | Ni | Cu | Fe | W |
|---|---|---|---|---|---|---|
| A | 31.15 | 4.63 | 7.63 | 1.44 | 9.02 | 46.13 |
| B | 14.39 | 1.90 | 23.75 | 5.47 | 21.78 | 33.71 |
| C | 13.13 | 2.22 | 17.40 | 4.30 | 17.20 | 45.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Guo, G.; Qiu, M.; Zhou, R.; Qin, J. Effect of Overlap Rate on Microstructure and Corrosion Behavior of Laser-Clad Ni60-WC Composite Coatings on E690 Steel. Metals 2025, 15, 1153. https://doi.org/10.3390/met15101153
Cao Y, Guo G, Qiu M, Zhou R, Qin J. Effect of Overlap Rate on Microstructure and Corrosion Behavior of Laser-Clad Ni60-WC Composite Coatings on E690 Steel. Metals. 2025; 15(10):1153. https://doi.org/10.3390/met15101153
Chicago/Turabian StyleCao, Yupeng, Guicang Guo, Ming Qiu, Rui Zhou, and Jiaxin Qin. 2025. "Effect of Overlap Rate on Microstructure and Corrosion Behavior of Laser-Clad Ni60-WC Composite Coatings on E690 Steel" Metals 15, no. 10: 1153. https://doi.org/10.3390/met15101153
APA StyleCao, Y., Guo, G., Qiu, M., Zhou, R., & Qin, J. (2025). Effect of Overlap Rate on Microstructure and Corrosion Behavior of Laser-Clad Ni60-WC Composite Coatings on E690 Steel. Metals, 15(10), 1153. https://doi.org/10.3390/met15101153





