Bioleaching of Lithium-Ion Battery Black Mass: A Comparative Study on Gluconobacter oxydans and Acidithiobacillus thiooxidans
Abstract
1. Introduction
2. Scientific Background
2.1. Lithium-Ion Battery Composition and Recycling Challenges
2.2. Biometallurgy as a Sustainable Alternative
- Redoxolysis: Microorganisms catalyze oxidation-reduction reactions to solubilize metal compounds.
- Acidolysis: Microbial production of organic or inorganic acids leads to metal dissolution.
- Complexolysis: Chelating agents produced by microbes form soluble metal complexes.
2.3. Microorganisms in LIB Bioleaching
- Gluconobacter oxydans: A Gram-negative, aerobic bacterium capable of oxidizing glucose into gluconic acid. This organic acid serves as a mild leaching agent, forming stable metal–organic complexes. G. oxydans is particularly effective in mobilizing lithium and cobalt, offering a potential route for selective metal recovery [12,29,30].
- Acidithiobacillus thiooxidans: A chemolithoautotrophic, acidophilic bacterium that oxidizes elemental sulfur and sulfide minerals to generate sulfuric acid. This metabolic process has been widely exploited in the bioleaching of copper and other sulfide ores and is now being investigated for its role in LIB metal dissolution [12,13,30,31,32].
2.4. Research Objectives and Hypotheses
- Assessing the leaching performance of gluconic acid compared to direct bacterial activity: By analyzing the dissolution rates of lithium, cobalt, nickel, and manganese, we aim to determine whether organic acid-mediated leaching is more effective than microbial metabolism alone.
- Evaluating the effectiveness of biologically produced sulfuric acid: The study investigates whether microbially generated sulfuric acid differs in leaching performance compared to chemically synthesized sulfuric acid at an equivalent acidity (pH 1.35).
- Understanding microbial-metal interactions: Since black mass contains a complex mixture of metals, electrolytes, and carbonaceous materials, it is crucial to evaluate how these factors influence microbial growth, metabolism, and leaching efficiency.
3. Materials and Methods
3.1. Black Mass Characterization
3.2. Microorganisms and Culture Conditions
- Gluconobacter oxydans (G. oxydans, DSMZ 3504, Leibniz Institute DSMZ, Braunschweig, Germany): A chemoorganotrophic, Gram-negative bacterium capable of oxidizing glucose to gluconic acid.
- Acidithiobacillus thiooxidans (A. thiooxidans, DSMZ 14887, Leibniz Institute DSMZ, Braunschweig, Germany): A chemolithoautotrophic, acidophilic bacterium that oxidizes sulfur to sulfuric acid.
3.3. Bioleaching Experiments
3.3.1. Direct Bioleaching with G. oxydans
3.3.2. Indirect Bioleaching with Gluconic Acid
3.3.3. Bioleaching with A. thiooxidans
- Non-sterilized cell-free sulfuric acid (containing active enzymes);
- Sterilized microbial sulfuric acid (autoclaved at 121 °C for 20 min).
3.4. Analytical Methods
- cleached is the metal concentration in solution (mg∙L−1);
- cmax is the theoretical maximum concentration based on BM composition.
3.5. Experimental Controls and Reproducibility
4. Results and Discussion
4.1. Leaching Efficiency of Gluconobacter oxydans
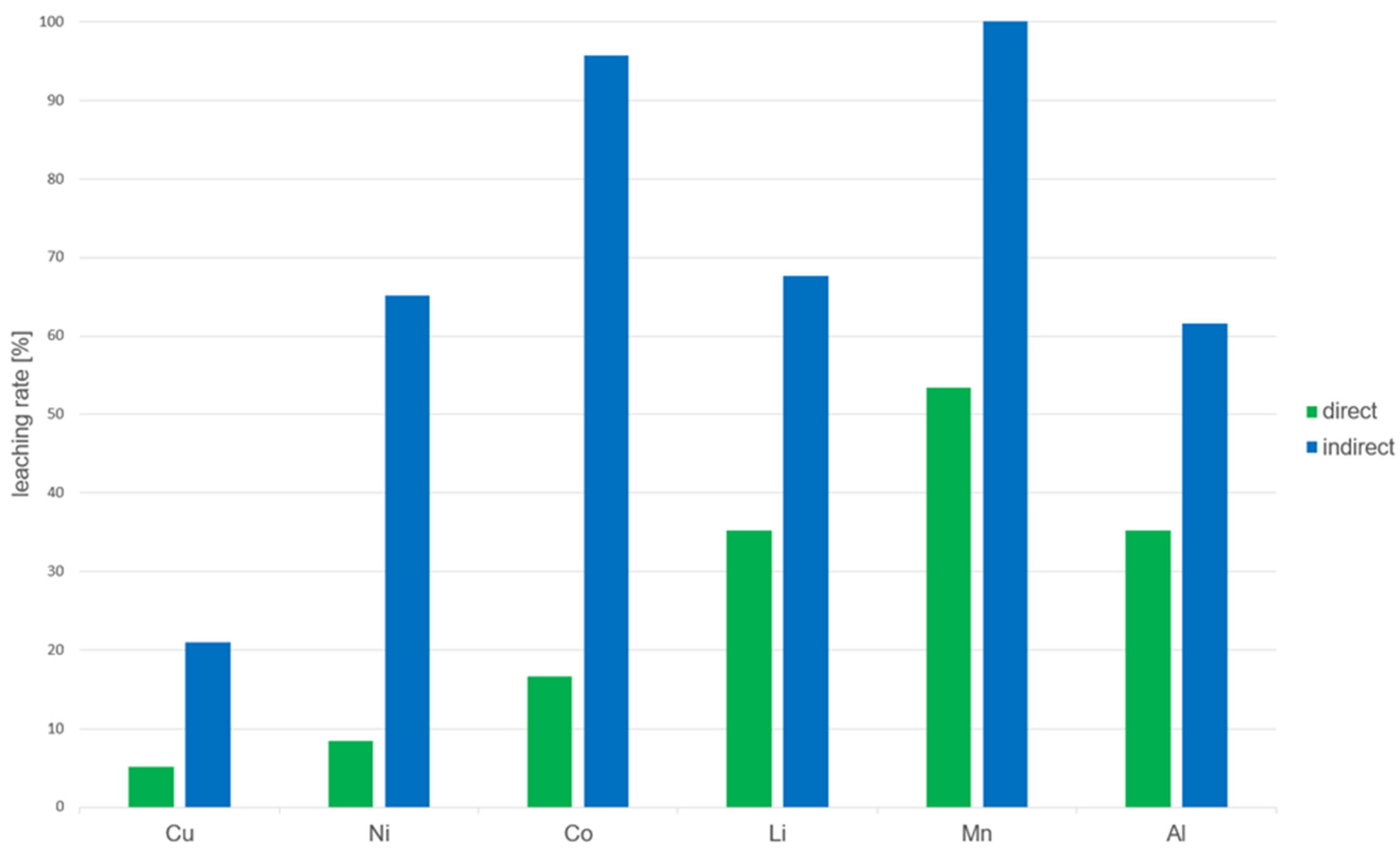
4.2. Comparison of Direct and Indirect Bioleaching
- Cobalt (96%) and manganese (100%) exhibited the highest dissolution rates.
- Nickel (65%), lithium (68%), and aluminum (62%) also showed good solubilization efficiency.
- Due to its noble character, copper was only solubilized to 21%.
- None of the metals showed an extraction efficiency above 55%.
- The solubilization rates for cobalt and nickel were significantly lower (16.7% and 8.5%, respectively).
- For aluminum, the extraction efficiency was approximately 35%, which is half of that achieved in the indirect approach. The partial dissolution of Al observed in the experiments is explained by the oxidative action of gluconic and other organic acids produced by G. oxydans, which have been reported to solubilize aluminum phases in LIB residues [25].
- Copper extraction remained low in both approaches, with only 5% solubilized in the direct bioleaching test.
4.3. Influence of Black Mass on Bioleaching Performance
4.4. Interpretation and Implications of Bioleaching for Industrial LIB Recycling
- Optimized acid production without microbial inhibition;
- Controlled acid application, leading to higher metal selectivity;
- Reduced microbial stress, potentially enabling repeated bioleaching cycles.
4.5. Leaching Efficiency of Different Sulfuric Acid Types
4.6. Comparison of Sulfuric Acid Variants in Bioleaching
- Biologically produced sulfuric acid from A. thiooxidans (green columns);
- Sterilized biologically produced sulfuric acid (red columns);
- Chemically synthesized sulfuric acid (blue columns).
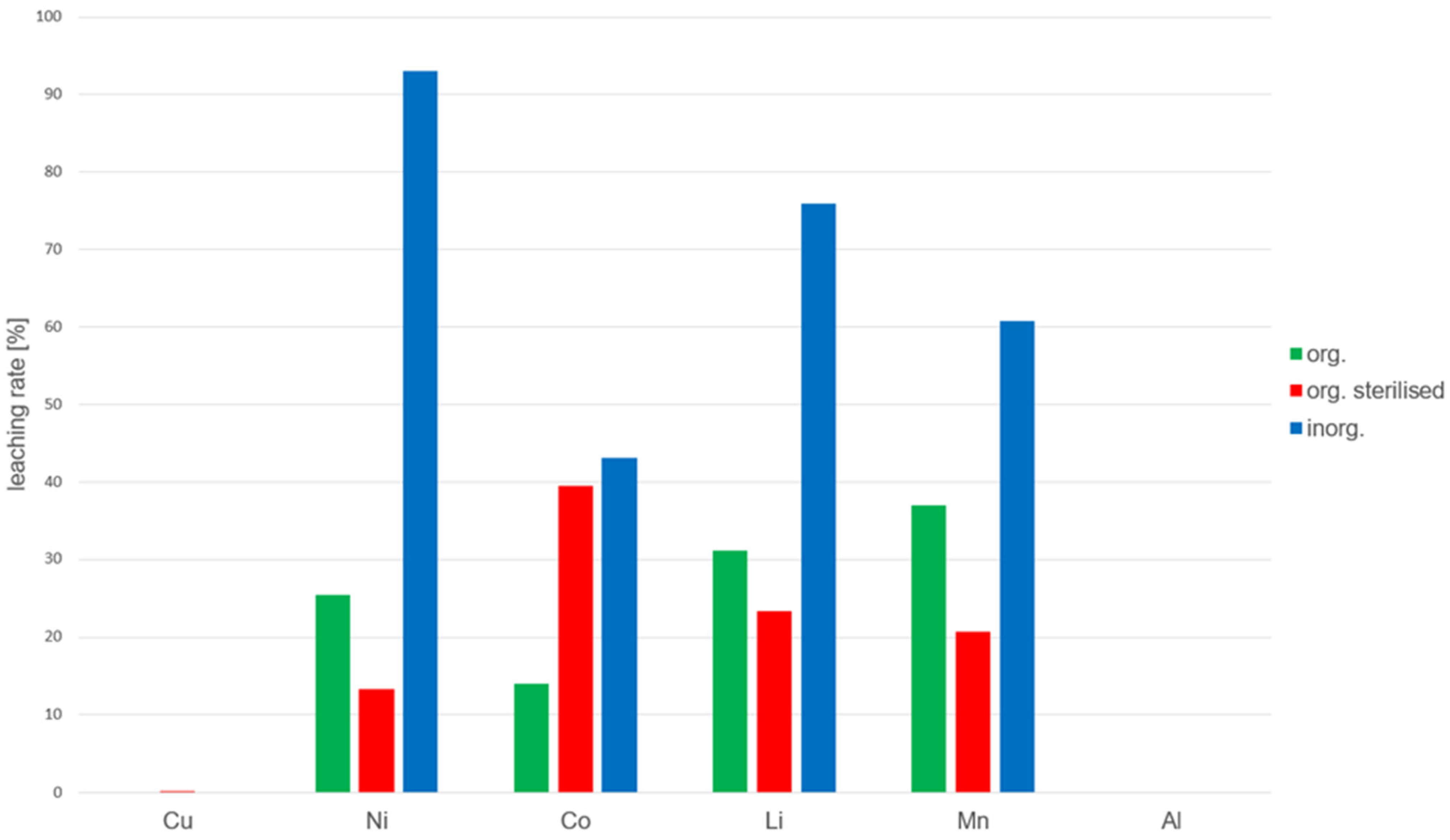
- Poor solubility of copper and aluminum
- 2.
- Superior performance of chemically synthesized sulfuric acid
- 3.
- Variability in cobalt and manganese extraction
4.7. Interpretation of the Overall Results and Industrial Implications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| BM | Black mass |
| LIB | Lithium-ion battery |
| G. oxydans | Gluconobacter oxydans |
| A. thiooxidans | Acidithiobacillus thiooxidans |
| OD | Optical density |
| ICP-OES | Inductively coupled plasma optical emission spectroscopy |
| L/S | Liquid-to-solid ratio |
| Eh | Redox potential |
| SD | Standard deviation |
References
- Xu, C.; Dai, Q.; Gaines, L.; Hu, M.; Tukker, A.; Steubing, B. Future material demand for automotive lithium-based batteries. Commun. Mater. 2020, 1, 99. [Google Scholar] [CrossRef]
- Statista. Distribution of Greenhouse Gas Emissions in the European Union (EU-27) in 2022, by Sector. 2025. Available online: https://www.statista.com/statistics/1325132/ghg-emissions-shares-sector-european-union-eu/ (accessed on 18 February 2025).
- Zechmeister, A. Klimaschutzbericht 2024; Bundesministerium für Klimaschutz, Umwelt, Energie, Mobilität, Innovation und Technologie: Wien, Austria, 2024. [Google Scholar]
- Regulation (EU) 2023/1542 of the European Parliament and of the Council of 12 July 2023 Concerning Batteries and Waste Batteries, Repealing Directive 2006/66/EC and Amending Regulation (EU) No 2019/1020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32023R1542 (accessed on 18 February 2025).
- European Commission: Directorate-General for Research and Innovation. European Green Deal—Research & Innovation Call; Publications Office of the European Union: Brussels, Belgium, 2021. [Google Scholar]
- European Battery Alliance. Securing Raw Materials for Europe’s Battery Production. 2022. Available online: https://single-market-economy.ec.europa.eu/industry/industrial-alliances/european-battery-alliance_en (accessed on 18 February 2025).
- Barman, P.; Dutta, L.; Azzopardi, B. Electric Vehicle Battery Supply Chain and Critical Materials: A Brief Survey of State of the Art. Energies 2023, 16, 3369. [Google Scholar] [CrossRef]
- Windisch-Kern, S.; Gerold, E.; Nigl, T.; Jandric, A.; Altendorfer, M.; Rutrecht, B.; Scherhaufer, S.; Raupenstrauch, H.; Pomberger, R.; Antrekowitsch, H.; et al. Recycling chains for lithium-ion batteries: A critical examination of current challenges, opportunities and process dependencies. Waste Manag. 2022, 138, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhang, B.; Gao, M.; Deng, R.; Zhang, Q. A review on spent Mn-containing Li-ion batteries: Recovery technologies, challenges, and future perspectives. J. Environ. Manag. 2024, 354, 120454. [Google Scholar] [CrossRef] [PubMed]
- Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Schippers, A.; Hetz, S.A.; Ostertag-Henning, C. Laterite ore processing with hydrogen via mild chemical pressure leaching or bioleaching. Hydrometallurgy 2025, 233, 106447. [Google Scholar] [CrossRef]
- Roy, J.J.; Cao, B.; Madhavi, S. A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach. Chemosphere 2021, 282, 130944. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, S. Bioleaching of Electronic Waste Using Extreme Acidophiles. In Electronic Waste Management and Treatment Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 153–174. [Google Scholar]
- Wang, X.-L.; An, K.; Cai, L.; Feng, Z.; Nagler, S.E.; Daniel, C.; Rhodes, K.J.; Stoica, A.D.; Skorpenske, H.D.; Liang, C.; et al. Visualizing the chemistry and structure dynamics in lithium-ion batteries by in-situ neutron diffraction. Sci. Rep. 2012, 2, 747. [Google Scholar] [CrossRef]
- Grey, C.P.; Hall, D.S. Prospects for lithium-ion batteries and beyond-a 2030 vision. Nat. Commun. 2020, 11, 6279. [Google Scholar] [CrossRef]
- Manthiram, A. An Outlook on Lithium Ion Battery Technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef]
- Ciez, R.E.; Whitacre, J.F. Examining different recycling processes for lithium-ion batteries. Nat. Sustain. 2019, 2, 148–156. [Google Scholar] [CrossRef]
- Dobó, Z.; Dinh, T.; Kulcsár, T. A review on recycling of spent lithium-ion batteries. Energy Rep. 2023, 9, 6362–6395. [Google Scholar] [CrossRef]
- Gerold, E.; Schinnerl, C.; Antrekowitsch, H. Critical Evaluation of the Potential of Organic Acids for the Environmentally Friendly Recycling of Spent Lithium-Ion Batteries. Recycling 2022, 7, 4. [Google Scholar] [CrossRef]
- Vest, M.; Georgi-Maschler, T.; Friedrich, B.; Weyhe, R. Recovery of Valuable Metals from Battery Scrap. Chem. Ing. Tech. 2010, 82, 1985–1990. [Google Scholar] [CrossRef]
- Wang, H.; Friedrich, B. Development of a Highly Efficient Hydrometallurgical Recycling Process for Automotive Li–Ion Batteries. J. Sustain. Metall. 2015, 1, 168–178. [Google Scholar] [CrossRef]
- Donnelly, L.; Pirrie, D.; Power, M.; Corfe, I.; Kuva, J.; Lukkari, S.; Lahaye, Y.; Liu, X.; Dehaine, Q.; Jolis, E.M.; et al. The Recycling of End-of-Life Lithium-Ion Batteries and the Phase Characterisation of Black Mass. Recycling 2023, 8, 59. [Google Scholar] [CrossRef]
- Yu, Z.; Han, H.; Feng, P.; Zhao, S.; Zhou, T.; Kakade, A.; Kulshrestha, S.; Majeed, S.; Li, X. Recent advances in the recovery of metals from waste through biological processes. Bioresour. Technol. 2020, 297, 122416. [Google Scholar] [CrossRef]
- Gerold, E.; Kadisch, F.; Lerchbammer, R.; Antrekowitsch, H. Bio-metallurgical recovery of lithium, cobalt, and nickel from spent NMC lithium ion batteries: A comparative analysis of organic acid systems. J. Hazard. Mater. Adv. 2024, 13, 100397. [Google Scholar] [CrossRef]
- Panda, S.; Dembele, S.; Mishra, S.; Akcil, A.; Agcasulu, İ.; Hazrati, E.; Tuncuk, A.; Malavasi, P.; Gaydardzhiev, S. Small-scale and scale-up bioleaching of Li, Co, Ni and Mn from spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2024, 99, 1908–1919. [Google Scholar] [CrossRef]
- Lerchbammer, R.; Gerold, E.; Antrekowitsch, H. High yield organic acid leaching and recovery of valuable metals from end-of-life lithium-ion batteries. Case Stud. Chem. Environ. Eng. 2025, 12, 101271. [Google Scholar] [CrossRef]
- Bahaloo-Horeh, N.; Mousavi, S.M. Enhanced recovery of valuable metals from spent lithium-ion batteries through optimization of organic acids produced by Aspergillus niger. Waste Manag. 2017, 60, 666–679. [Google Scholar] [CrossRef]
- Horeh, N.B.; Mousavi, S.M.; Shojaosadati, S.A. Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger. J. Power Sources 2016, 320, 257–266. [Google Scholar] [CrossRef]
- Junker, A. Untersuchung des Zentralstoffwechsels von Gluconobacter Oxydans Durch die Etablierung Eines Markerfreien Deletionssystems. Ph.D. Thesis, Technische Universität München, Munich, Germany, 2011. [Google Scholar]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Das, A.P. Lithium bioleaching: A review on microbial-assisted sustainable technology for lithium bio-circularity. J. Water Process Eng. 2025, 69, 106744. [Google Scholar] [CrossRef]
- Marín, S.; Acosta, M.; Galleguillos, P.A.; Villegas, Y.; Cautivo, D.; Zepeda, V.J.; Demergasso, C. Transcription Dynamics of CBB-Pathway Genes in Acidithiobacillus thiooxidans Growing under Different CO2 Levels. Solid State Phenom. 2017, 262, 376–380. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, D.; Yang, J.; Wang, W.; Chen, P.; Zhang, S.; Yan, L. Acidithiobacillus thiooxidans and its potential application. Appl. Microbiol. Biotechnol. 2019, 103, 7819–7833. [Google Scholar] [CrossRef]
- Moazzam, P.; Boroumand, Y.; Rabiei, P.; Baghbaderani, S.S.; Mokarian, P.; Mohagheghian, F.; Mohammed, L.J.; Razmjou, A. Lithium bioleaching: An emerging approach for the recovery of Li from spent lithium ion batteries. Chemosphere 2021, 277, 130196. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T.; Jozsa, P.-G.; Schippers, A. Direct versus indirect bioleaching. Process Metall. 1999, 9, 27–49. [Google Scholar]
- Sand, W.; Gehrke, T.; Jozsa, P.-G.; Schippers, A. (Bio)chemistry of bacterial leaching—Direct vs. indirect bioleaching. Hydrometallurgy 2001, 59, 159–175. [Google Scholar] [CrossRef]
- Sand, W.; Rohde, K.; Sobotke, B.; Zenneck, C. Evaluation of metal toxicity in bioleaching processes. Hydrometallurgy 2001, 59, 327–336. [Google Scholar]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage and its impact on microbial communities. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- International Energy Agency (IEA). Ammonia and Sulfuric Acid Technology Roadmap; IEA: Paris, France, 2022; Available online: https://www.iea.org/reports/ammonia-and-sulfuric-acid-technology-roadmap (accessed on 30 August 2025).
- Gładysz-Płaska, A.; Majdan, M.; Skwarek, E. Environmental aspects of sulfuric acid production: A review of energy consumption and CO2 emissions. Environ. Eng. Manag. J. 2021, 20, 713–724. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, S.; Liu, H. Life cycle assessment of industrial sulfuric acid production: Environmental impacts and energy efficiency. J. Clean. Prod. 2019, 238, 117900. [Google Scholar] [CrossRef]
- Chen, X.; Ma, H.; Luo, C.; Zhou, T. Bioleaching of spent lithium-ion batteries by organic acid-producing microorganisms: Dissolution behavior of Al and transition metals. J. Hazard. Mater. 2020, 393, 122390. [Google Scholar]
- Sun, L.; Qiu, K. Organic oxalate as leachant for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag. 2012, 32, 1575–1582. [Google Scholar] [CrossRef]
- Botelho Junior, A.B. Sustainable Mining—Unlocking Resources towards a Circular Economy to Meet Energy Transition through Electrochemistry. J. Environ. Chem. Eng. 2025, 13, 116600. [Google Scholar] [CrossRef]
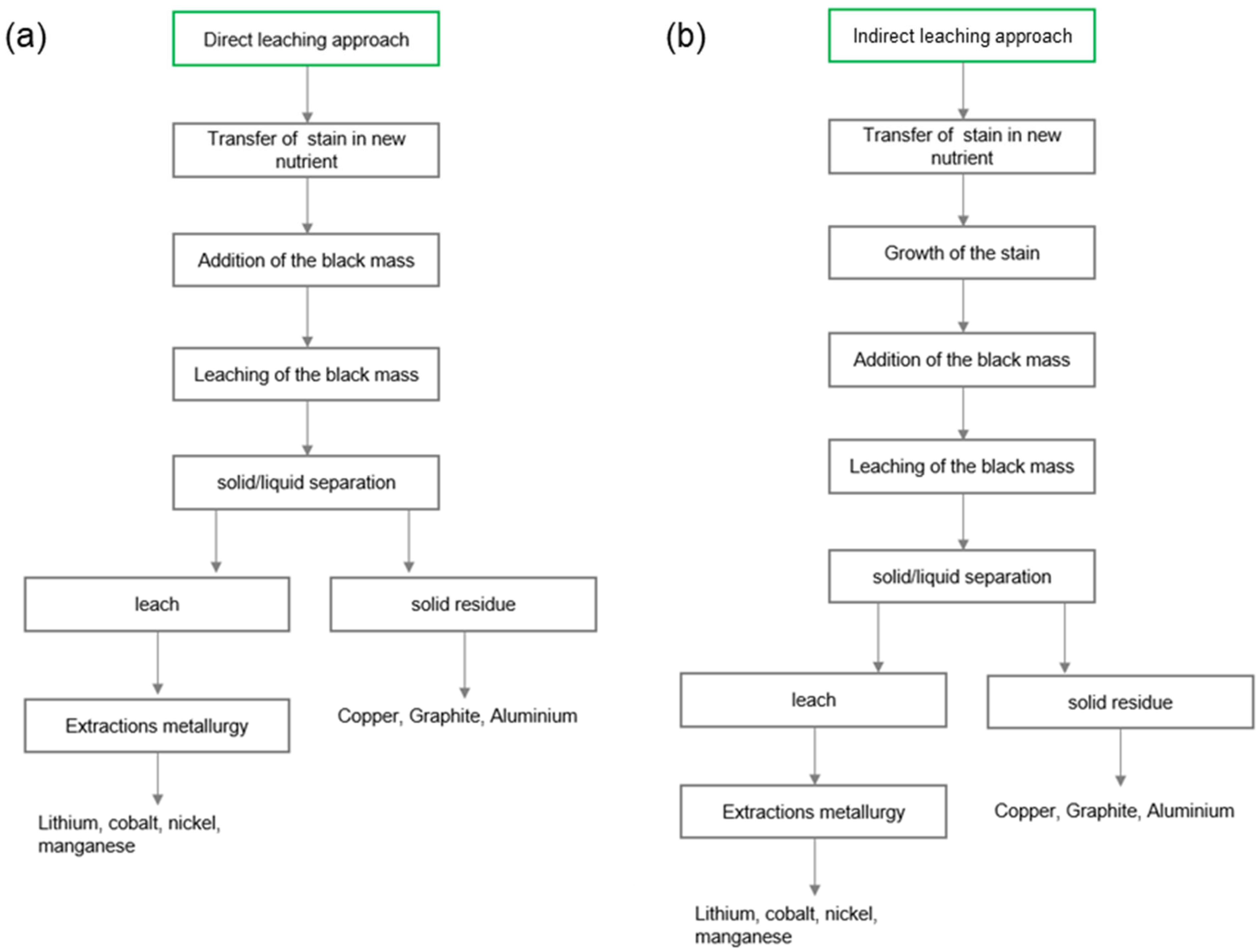
| Test Parameters | Direct Leaching Approach | Indirect Leaching Approach |
|---|---|---|
| Amount of culture medium | 50 mL | 50 mL |
| Amount of black mass | 0.5 g | 0.5 g |
| Incubator temperature | 26 °C | 26 °C |
| Stirred tank temperature | 26 °C | 26 °C |
| Incubation time | 0 h | 1 week |
| Leaching time in the stirred tank | 1 week | 1 week |
| Rotation speed of the stirred tank | 40 U/min | 40 U/min |
| Element | cmax [mg·L−1] | cD [mg·L−1] | cI [mg·L−1] | ηD [%] | ηI [%] |
|---|---|---|---|---|---|
| Copper (Cu) | 480 | 24 | 101 | 5 | 21 |
| Nickel (Ni) | 2200 | 186 | 1433 | 8 | 65 |
| Cobalt (Co) | 650 | 108 | 622 | 17 | 96 |
| Lithium (Li) | 520 | 183 | 352 | 35 | 68 |
| Manganese (Mn) | 730 | 390 | 730 | 53 | 100 |
| Aluminum (Al) | 370 | 130 | 228 | 35 | 62 |
| Element | cmax [mg·L−1] | corg [mg·L−1] | corg,S [mg·L−1] | cinorg [mg·L−1] | ηorg [%] | ηorg,S [%] | ηinorg [%] |
|---|---|---|---|---|---|---|---|
| Copper (Cu) | 480 | 0.07 | 1.33 | 0.02 | 0.01 | 0.28 | 0.00 |
| Nickel (Ni) | 2200 | 122 | 64 | 446 | 26 | 13 | 93 |
| Cobalt (Co) | 650 | 67 | 189 | 207 | 14 | 39 | 43 |
| Lithium (Li) | 520 | 150 | 112 | 364 | 31 | 23 | 76 |
| Manganese (Mn) | 730 | 177 | 99 | 292 | 37 | 21 | 61 |
| Aluminum (Al) | 370 | 0.5 | 0.3 | 0.2 | 0.11 | 0.06 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandl, M.M.; Lerchbammer, R.; Gerold, E. Bioleaching of Lithium-Ion Battery Black Mass: A Comparative Study on Gluconobacter oxydans and Acidithiobacillus thiooxidans. Metals 2025, 15, 1112. https://doi.org/10.3390/met15101112
Mandl MM, Lerchbammer R, Gerold E. Bioleaching of Lithium-Ion Battery Black Mass: A Comparative Study on Gluconobacter oxydans and Acidithiobacillus thiooxidans. Metals. 2025; 15(10):1112. https://doi.org/10.3390/met15101112
Chicago/Turabian StyleMandl, Matthias Markus, Reinhard Lerchbammer, and Eva Gerold. 2025. "Bioleaching of Lithium-Ion Battery Black Mass: A Comparative Study on Gluconobacter oxydans and Acidithiobacillus thiooxidans" Metals 15, no. 10: 1112. https://doi.org/10.3390/met15101112
APA StyleMandl, M. M., Lerchbammer, R., & Gerold, E. (2025). Bioleaching of Lithium-Ion Battery Black Mass: A Comparative Study on Gluconobacter oxydans and Acidithiobacillus thiooxidans. Metals, 15(10), 1112. https://doi.org/10.3390/met15101112







