Effect of Trace La on Microstructure and Thermal Conductivity of Hypoeutectic Al-7Si Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. Microstructural Characterization and Phase Analysis
2.3. Heat Storage Performance Analysis
3. Results and Discussion
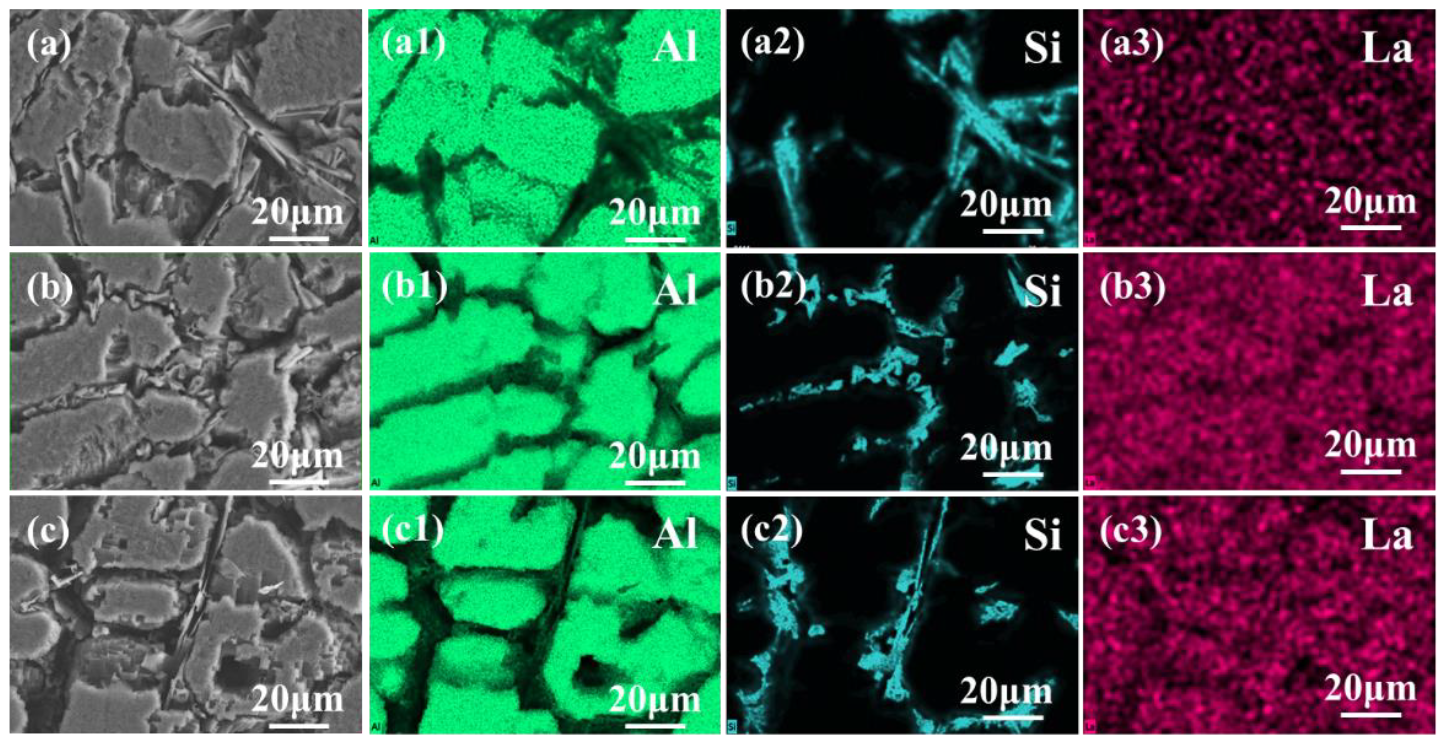
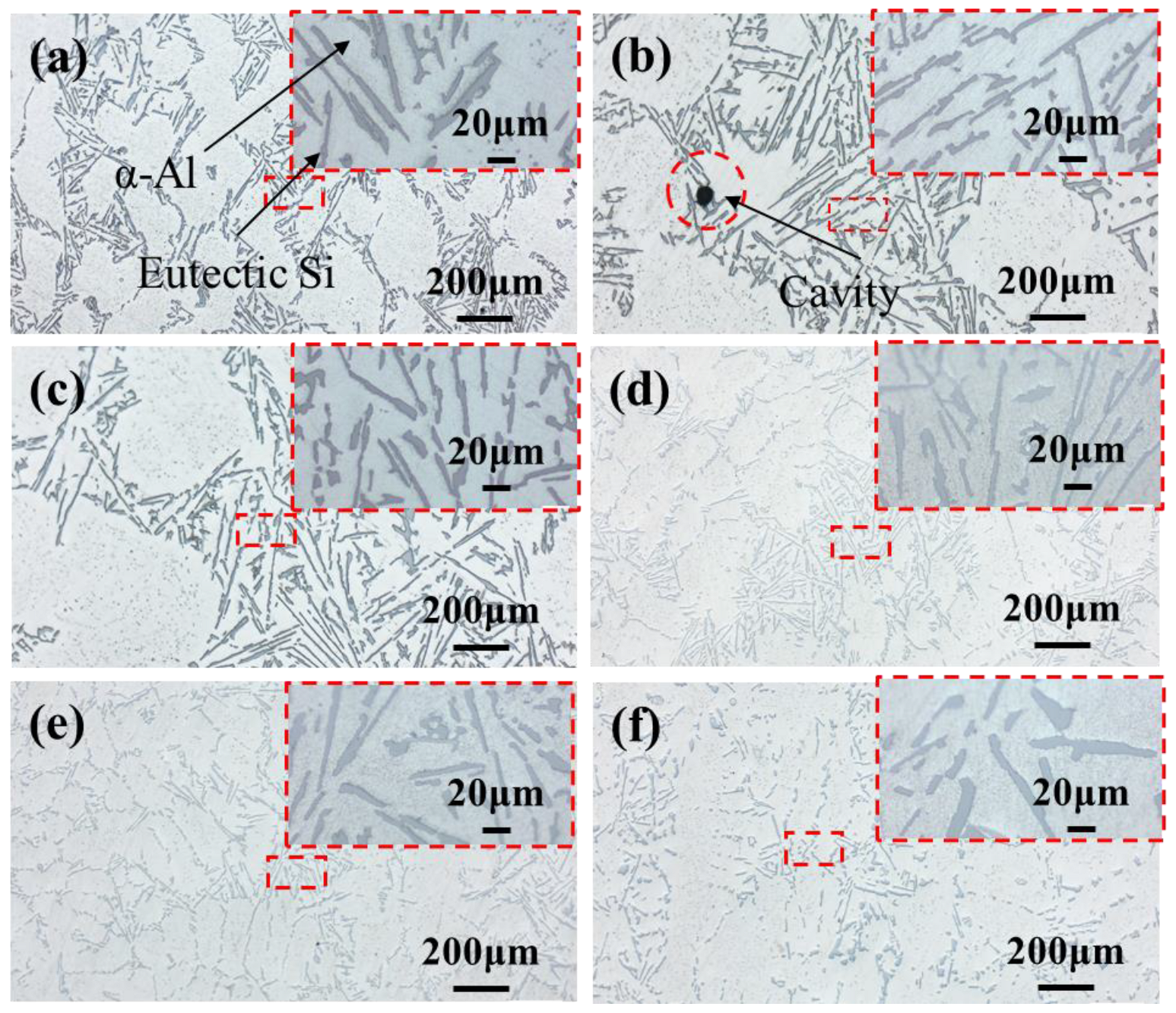
3.1. Influence of Trace La on the Microstructure in Al-7Si Alloy
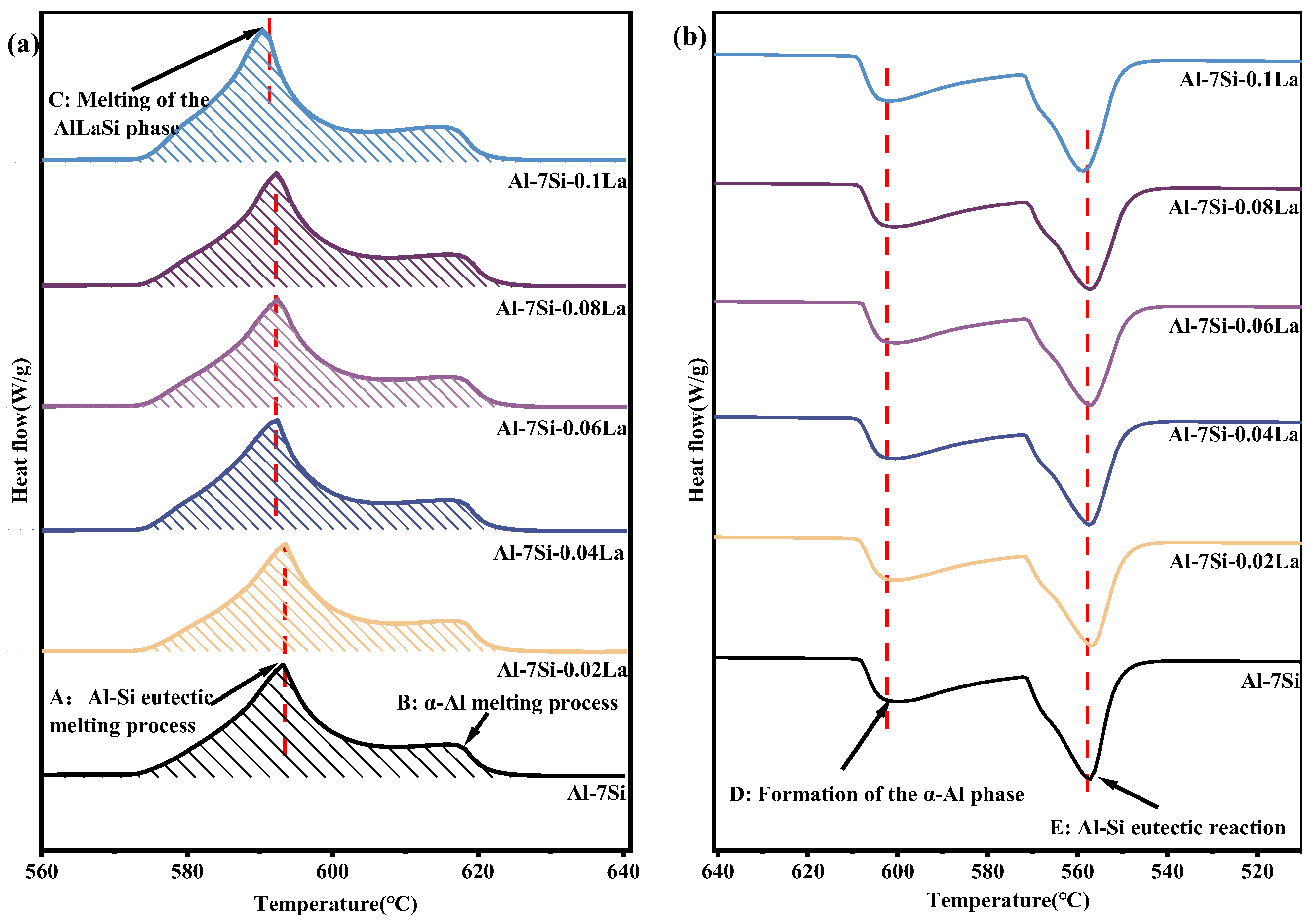
3.2. Influence of Trace La Additions on the Solidification Characteristics in Al-7Si Alloy
3.3. Influence of Trace La on Thermal Conductibility in Al-7Si Alloy
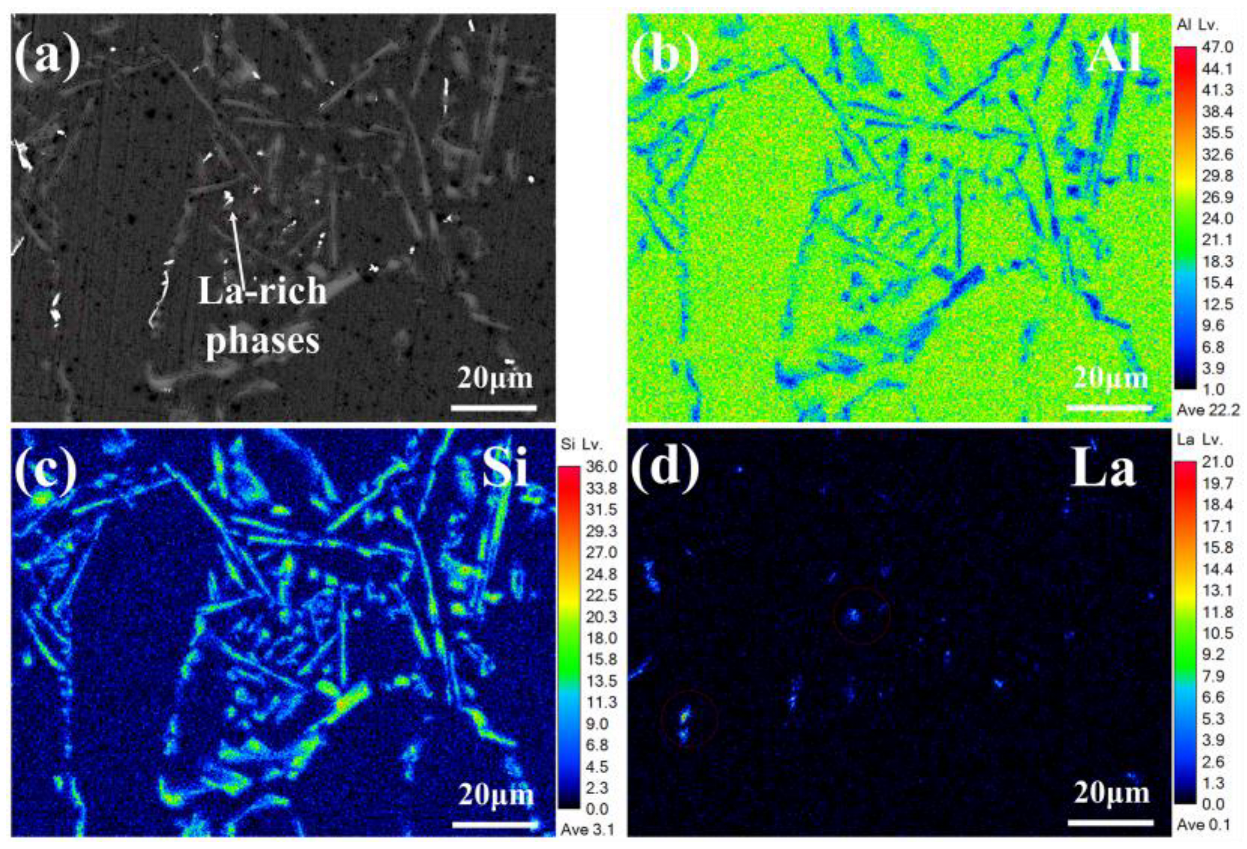
3.4. Microstructure Development in Al-7Si Alloy Doped with Trace Lanthanum Following 100 Thermal Cycles
3.5. The Effect of Trace La on the Latent Heat of Phase Transformation in Al-7Si Alloy After 100 Thermal Cycles
4. Mechanisms
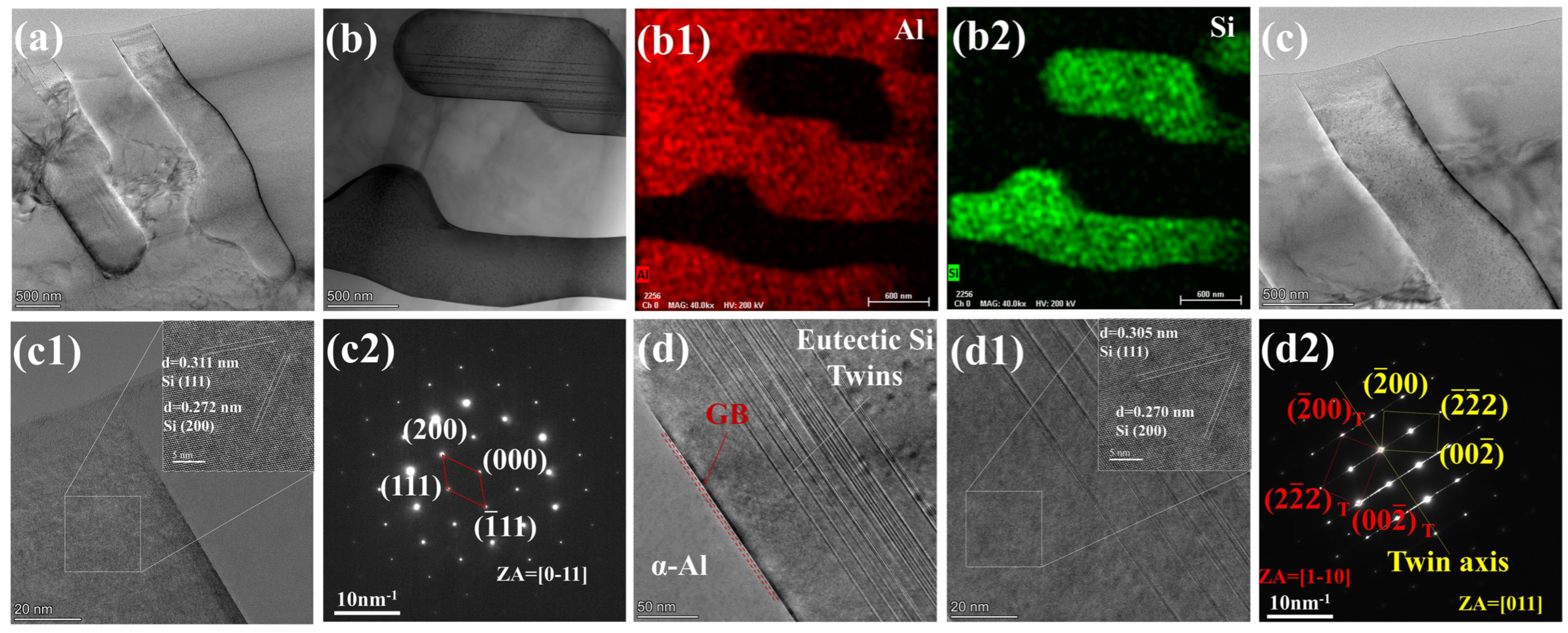
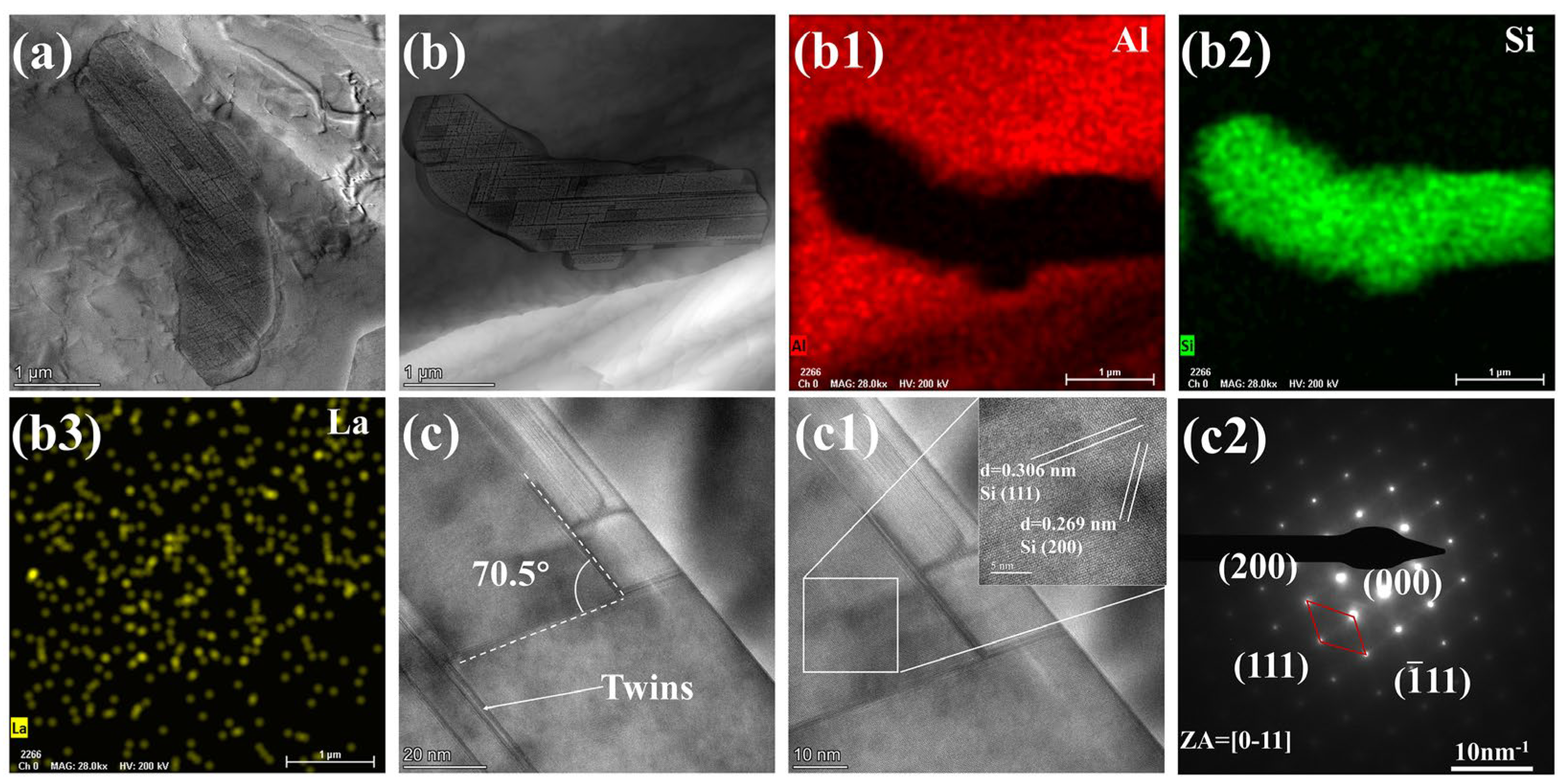
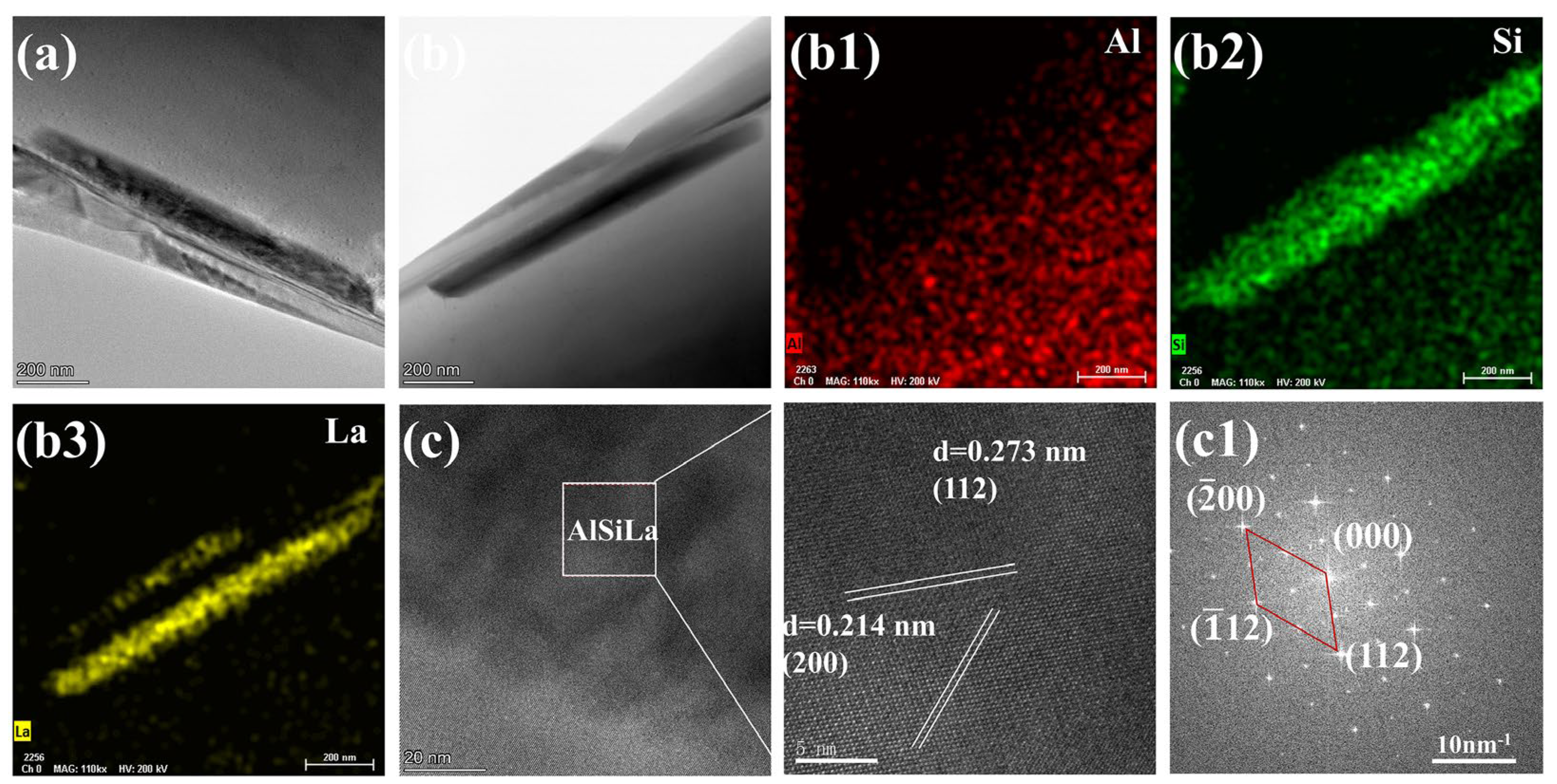
4.1. The Modification Mechanism of La on Eutectic Si in Al-7Si Alloy
4.2. Influence of La on the Thermal Conduction Mechanism of Eutectic Si in Al-7Si Alloys
5. Conclusions
- (1)
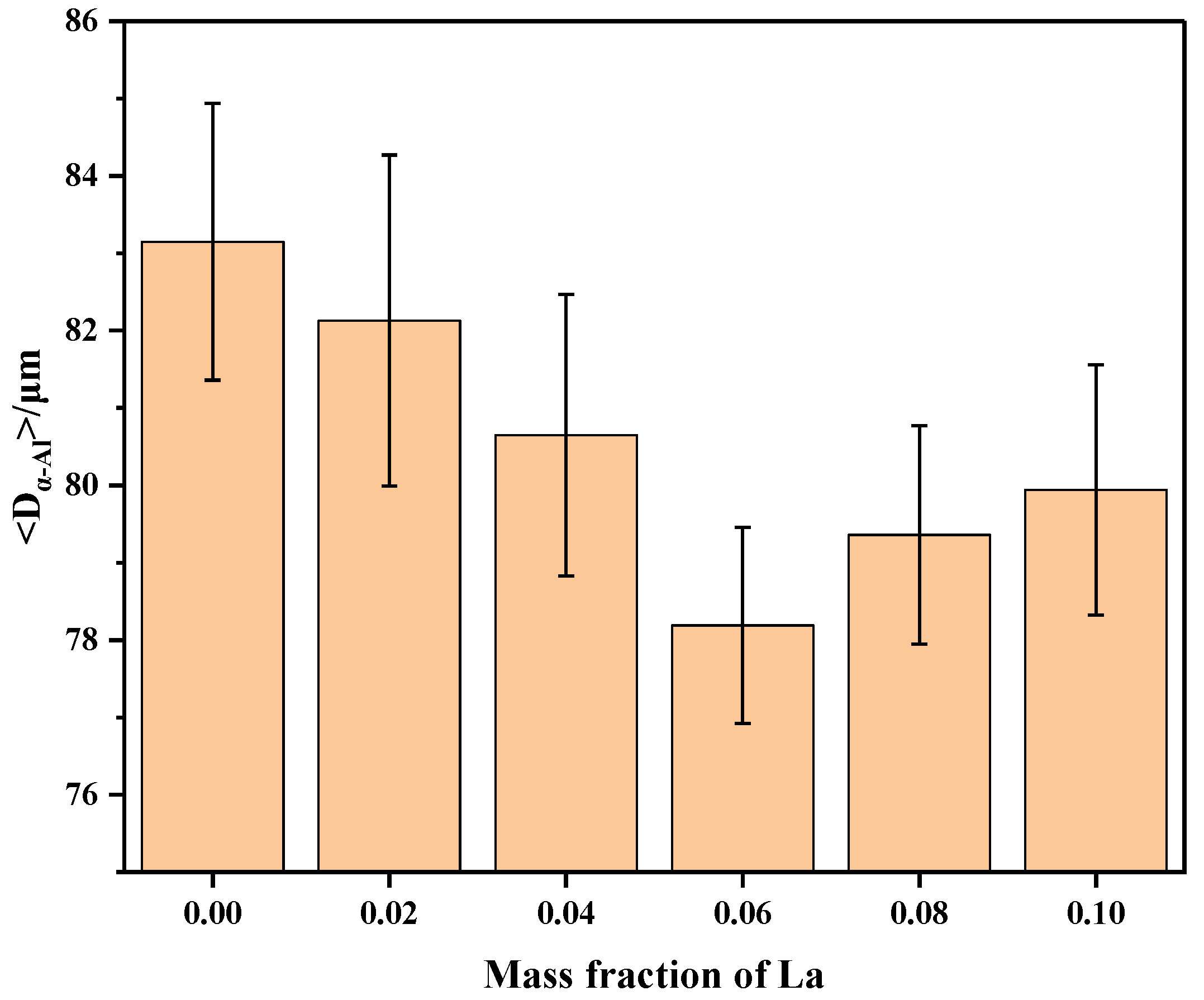
- Adding La transformed eutectic Si particles from plate-like to short rod-like structures, with a marked reduction in size; the modification effect was optimal at 0.06 wt.% La. Meanwhile, primary α-Al’s grain size gradually decreased as La content increased. At 0.06 wt.% La, it decreased by 35.33% compared with the original alloy. Trace amounts of La can induce the generation of high-density interlaced twins on eutectic Si surface, with the twin plane being the {111}Si plane and the twin direction being <112>Si. In addition, La will also form the AlSiLa phase in the alloy, which is distributed near the eutectic Si and wraps around the eutectic Si.
- (2)
- Adding La can significantly enhance the thermal conductivity of the Al-7Si alloy. At a La content of 0.06 wt.%, the alloy’s thermal conductivity peaked at 179.259 W·m−1·K−1, 15.36% higher than the original alloy. Excess La (≥0.06 wt.%) promotes the formation of AlSiLa intermetallic compounds, impedes free electron movement, and diminishes thermal conductivity.
- (3)
- Adding La can significantly improve the alloy’s high-temperature oxidation resistance. After oxidizing the original Al-7Si alloy at a constant 700 °C for 96 h, its oxidation weight gain per unit of area amounted to 3.908 × 10−3 g·cm−2. When 0.1 wt.% La was added, the oxidative gain per unit area decreased to 1.931 × 10−3 g·cm−2, a reduction of 50.59%. The alloy’s oxidation rate gradually slows as oxidation time increases.
- (4)
- After 100 thermal cycles, the phase transition temperature in the alloy stays largely unaltered. However, the metamorphic effect of La diminishes, the quantity of plate-like eutectic Si increases, and when the La content is ≥ 0.06 wt.%, the latent heat of phase transition decreases compared with that before thermal cycling. Among them, the latent heat of phase transition of the original Al-7Si alloy increases from 336.9 J/g to 342.7 J/g; the latent heat of phase transition of the Al-7Si-0.06La alloy decreases from 340.4 J/g to 328.6 J/g; and the latent heat of phase transition of the Al-7Si-0.1La alloy decreases from 364.9 J/g to 328.2 J/g. La can effectively restrain the growth of primary α-Al grains during thermal cycles. At a La content of 0.06 wt.%, the size of primary α-Al grains was 78.19 μm, 5.97% smaller than that of the original alloy. As La content increased to 0.1 wt.%, the alloy’s dendrite spacing was 79.94 μm, 3.86% smaller than the original Al-7Si alloy.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, Z.; Zou, B.Y.; Cong, L.; Xie, C.P.; Li, C.; Jiao, G.; Zhao, Y.Q.; Nei, B.J.; Zhang, T.T.; Ge, Z.W.; et al. Recent progress and outlook of thermal energy storage technologies. Energy Storage Sci. Technol. 2022, 11, 2746–2771. [Google Scholar]
- Liu, W.; Li, Z.M.; Liu, M.Y.; Yang, C.Y.; Mei, C.; Li, Y. Review of high-temperature phase change heat storage material preparation and applications. Energy Storage Sci. Technol. 2023, 12, 398–430. [Google Scholar]
- Feng, Y.F.; Jiang, S.J.; Fu, X.; Chen, Y. Current status of heat storage technology and research progress of phase change thermal storage materials. Inf. Rec. Mater. 2023, 24, 32–36. [Google Scholar]
- Xiao, J.B.; Zou, B.; Zhuang, Y.J.; Liu, C.Z.; Li, C.C.; Chen, J. Research and application on thermal conduction performance of metal foam composite phase change system. J. Cent. South Univ. (Sci. Technol.) 2022, 53, 4687–4699. [Google Scholar]
- Abd El-Hamid, M.; Wei, G. Effect of varying the thermophysical properties of phase change material on the performance of photovoltaic/thermal-PCM hybrid module numerically. J. Energy Storage 2024, 86, 111326. [Google Scholar] [CrossRef]
- Sadiq, H.H.; Mussa, M.A. Numerical study of thermal conductivity effect on the performance of thermal energy storage. J. Eng. 2022, 28, 57–77. [Google Scholar] [CrossRef]
- Bie, Y.; Li, M.; Malekian, R.; Chen, F.; Feng, Z.K.; Li, Z.X. Effect of phase transition temperature and thermal conductivity on the performance of Latent Heat Storage System. Appl. Therm. Eng. 2018, 135, 218–227. [Google Scholar] [CrossRef]
- Deng, T.X.; Luan, D.C.; Hu, Z.H.; Hu, Z.H.; Ren, Y.; Zuo, C.M.; Li, Y.; Zhuo, X.Y.; Wang, Z.Y. Research Progress of Aluminum Based Phase Change Metal Materials for Thermal Energy Storage. Mater. China 2022, 41, 653–660. [Google Scholar]
- Chen, Z.G.; Zhao, J.W.; Chen, X.S.; Dai, G.Z.; Han, J. High Temperature Oxidation Resistance of Al-Cu-Si Based Alloy Heat Storage Material. Hot Work. Technol. 2021, 50, 76–79. [Google Scholar]
- Zhao, Y.; Liu, H.; Zhao, C. Experimental study on the cycling stability and corrosive property of Al-Si alloys as phase change materials in high-temperature heat storage. Sol. Energy Mater. Sol. Cells 2019, 203, 110165. [Google Scholar] [CrossRef]
- Powell, R. Correlation of metallic thermal and electrical conductivities for both solid and liquid phases. Int. J. Heat Mass Transf. 1965, 8, 1033–1045. [Google Scholar] [CrossRef]
- Lu, L.; Nogita, K.; Dahle, A. Combining Sr and Na additions in hypoeutectic Al-Si foundry alloys. Mater. Sci. Eng. A 2005, 399, 244–253. [Google Scholar] [CrossRef]
- Cui, X.L.; Cui, H.W.; Wu, Y.Y.; Liu, X.F. The improvement of electrical conductivity of hypoeutectic Al-Si alloys achieved by composite melt treatment. J. Alloys Compd. 2019, 788, 1322–1328. [Google Scholar] [CrossRef]
- Gan, J.Q.; Huang, Y.J.; Wen, C.; Du, J. Effect of Sr modification on microstructure and thermal conductivity of hypoeutectic Al-Si alloys. Trans. Nonferrous Met. Soc. China 2020, 30, 2879–2890. [Google Scholar] [CrossRef]
- Liu, D.Y.; Yu, X.Y.; Gao, W.L.; Mao, G.L.; Gao, Y.L. Role of Sb Element in Casting Al-Si Alloy. Hot Work. Technol. 2022, 51, 1–5+12. [Google Scholar]
- Elgallad, E.M.; Ibrahim, M.F.; Doty, H.W.; Samuel, F.H. Microstructural characterisation of Al-Si cast alloys containing rare earth additions. Philos. Mag. 2018, 98, 1337–1359. [Google Scholar] [CrossRef]
- Jiang, W.M.; Fan, Z.T.; Dai, Y.C.; Chi, L. Effects of rare earth elements addition on microstructures, tensile properties and fractography of A357 alloy. Mater. Sci. Eng. A 2014, 597, 237–244. [Google Scholar] [CrossRef]
- Li, Q.L.; Xia, T.D.; Lan, Y.F.; Zhao, W.J.; Fan, L.; Li, P.F. Effect of rare earth cerium addition on the microstructure and tensile properties of hypereutectic Al–20% Si alloy. J. Alloys Compd. 2013, 562, 25–32. [Google Scholar] [CrossRef]
- Mao, G.L.; Liu, S.G.; Wu, Z.; Zhu, C.C.; Gao, W.L. The effects of Y on primary α-Al and precipitation of hypoeutectic Al-Si alloy. Mater. Lett. 2020, 271, 127795. [Google Scholar] [CrossRef]
- Mao, F.; Wei, S.Z.; Ou, L.Y.; Zhang, C.; Wang, X.D.; Cao, Z.Q. Different influences of rare earth Eu addition on primary Si refinement in hypereutectic Al-Si alloys with varied purity. Materials 2019, 12, 3505. [Google Scholar] [CrossRef]
- Bhatt, B.; Martucci, A.; Virgillito, E.; Gobber, F.; Bondioli, F.; Manfredi, D.; Lombardi, M.; Fino, P. Deciphering Microstructures and Phases of Gas-Atomised Novel Al-Fe-Si-Cr-Ni Alloys. Metals 2024, 14, 17. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.R.; Xiao, Z.; Mingyang Du, M.Y.; Zhang, Z.; Liu, M.J.; Li, P.N. The effect of Si content on the microstructure and thermal conductivity of Al-XSi-3Cu-5Mg alloys produced by laser additive manufacturing. J. Alloys Compd. 2025, 1034, 181341. [Google Scholar] [CrossRef]
- Jeyaprakash, N.; Moinuddin, S.Q.; Alnamasi, K.; Alsharif, A. Effect of post-heat treatment cooling strategies on Si morphology and nano-mechanical behavior of additively manufactured AlSi10Mg alloy. J. Mater. Res. Technol. 2025, 36, 8308–8324. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Chao, P.; Luke, S.; Lien, H.H.; Hunter, A.H.; Misra, A.; Shahani, A.J. Three-dimensional morphology of an ultrafine Al-Si eutectic produced via laser rapid solidification. Scr. Mater. 2023, 232, 115471. [Google Scholar] [CrossRef]
- Zhu, W.Q.; Yu, M.Z.; Tang, X.; Chen, X.Y.; Xu, Z.B.; Zeng, J.M. Effect of Er and Si on Thermal Conductivity and Latent Heat of Phase Transformation of Aluminum-Based Alloy. Acta Metall. Sin. 2020, 56, 1485–1494. [Google Scholar]
- Chen, Z.Q.; Jia, J.Y.; Hu, W.X.; Wang, W.; Wang, X.Q. Combining effect of Er and Sr on microstructure and mechanical properties of as-casted A356 alloy. Rare Met. Mater. Eng. 2020, 49, 3388–3394. [Google Scholar]
- Zheng, Q.; Zhang, L.; Jiang, H.; Zhao, J.Z.; He, J. Effect mechanisms of micro-alloying element La on microstructure and mechanical properties of hypoeutectic Al-Si alloys. J. Mater. Sci. Technol. 2020, 47, 142–151. [Google Scholar] [CrossRef]
- Lu, M.; Ye, Z.F.; Zheng, Q.J.; Jiang, H.X.; Zhao, J.Z.; He, J.; Zhang, L.L. Effects of Micro-alloying Element La on Microstructures and Properties of Al-0.6Mg-0.6Si Alloy. Spec. Cast. Nonferrous Alloys 2021, 41, 1494–1499. [Google Scholar]
- Pourbahari, B.; Emamy, M. Effects of La intermetallics on the structure and tensile properties of thin section gravity die-cast A357 Al alloy. Mater. Des. 2016, 94, 111–120. [Google Scholar] [CrossRef]
- Wu, X.Y.; Zhang, H.R.; Jiang, H.T.; Mi, Z.L.; Zhang, H. Multi-refinement effect of rare earth lanthanum on α-Al and eutectic Si phase in hypoeutectic Al-7Si alloy. Metals 2020, 10, 621. [Google Scholar] [CrossRef]
- GB/T 43589-2023; Gold Alloys for Adornment—Determination of Multi-Element Contents by Laser Ablation–Inductively Coupled Plasma Mass Spectrometry. China Standards Press: Beijing, China, 2023.
- Li, J.H.; Albu, M.; Hofer, F.; Schumacher, P. Solute adsorption and entrapment during eutectic Si growth in A-Si-based alloys. Acta Mater. 2015, 83, 187–202. [Google Scholar] [CrossRef]
- Zhang, L.L.; Ji, Z.W.; Zhao, J.Z.; He, J.; Jiang, H.X. Key Factors influencing eutectic Si modification in Al-Si hypoeutectic alloy by trace La. Acta Metall. Sin. 2023, 59, 1541–1546. [Google Scholar]
- Li, Y.X.; Liu, J.Y.; Zhang, J.C.; Li, H.F. Microstructure evolution of hypereutectic Al-Si alloy with high Si content during semi-solid remelting. Chin. J. Nonferrous Met. 2014, 24, 2287–2294. [Google Scholar] [CrossRef]
- Du, C.; Jin, S.; Fang, Y.; Li, J.; Hu, S.Y.; Yang, T.T.; Zhang, Y.; Huang, J.Y.; Sha, G.; Wang, Y.G.; et al. Ultrastrong nanocrystalline steel with exceptional thermal stability and radiation tolerance. Nat. Commun. 2018, 9, 5389. [Google Scholar] [CrossRef]
- Liu, X.R.; Zhang, Y.D.; Beausir, B.; Liu, F.; Esling, C.; Yu, F.X.; Zhao, X.; Zuo, L. Twin-controlled growth of eutectic Si in unmodified and Sr-modified Al-12.7%Si alloys investigated by SEM/EBSD. Acta Mater. 2015, 97, 338–347. [Google Scholar] [CrossRef]
- Li, J.H.; Wang, X.D.; Ludwig, T.H.; Tsunekawa, Y.; Arnberg, L.; Jiang, J.Z.; Schumacher, P. Modification of eutectic Si in Al-Si alloys with Eu addition. Acta Mater. 2015, 84, 153–163. [Google Scholar] [CrossRef]
- Chen, J.; Wen, F.; Liu, C.; Li, W.; Zhou, Q.; Zhu, W.; Zhang, Y.; Guan, R. The microstructure and property of Al-Si alloy improved by the Sc-microalloying and Y2O3 nano-particles. Sci. Technol. Adv. Mater. 2021, 22, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.F.; Gu, W.L.; Lu, C.X.; Yang, S.G.; Sun, Y. Interfacial Stability and Branch Growth of Eutectic Si Under Different Solidification Conditions. Foundry 2022, 71, 1095–1100. [Google Scholar]
- Nafisi, S.; Ghomashchi, R.; Vali, H. Eutectic nucleation in hypoeutectic Al-Si alloys. Mater. Charact. 2008, 59, 1466–1473. [Google Scholar] [CrossRef]
- He, Y.; Zhang, H.L.; Li, T.; Wang, X.T. Calculation of Jackson’s factor of Mg2Si in Mg melt using coordination polyhedron. J. Alloys Compd. 2013, 581, 494–497. [Google Scholar] [CrossRef]
- Pineau, A.; Guillemot, G.; Reinhart, G.; Nguyen, T.H.; Dabo, Y.; Mangelinck, N.N. Three-dimensional cellular automaton modeling of silicon crystallization with grains in twin relationships. Acta Mater. 2020, 191, 230–244. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, Y.; Casari, D.; Li, Y.; Zhao, D.; Niu, X. In-situ X-radiographic study of nucleation and growth behaviour of primary silicon particles during solidification of a hypereutectic Al-Si alloy. J. Alloys Compd. 2020, 832, 154948. [Google Scholar] [CrossRef]
- Li, J.; Barrirero, J.; Engstler, M.; Hötzel, R.; Garcia, P.; Renner, M.; Schmidt, M.; Weygand, D.; Wagner, J.; Hahn, H. Nucleation and Growth of Eutectic Si in Al-Si Alloys with Na Addition. Metall. Mater. Trans. A 2015, 46, 1300–1311. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, S.; Li, B.; Rong, Y.; Zhang, L. Modification of multi-component Al–Si casting piston alloys by addition of rare earth yttrium. Mater. Res. Express 2019, 6, 106525. [Google Scholar] [CrossRef]
- Mao, F.; Qiao, Y.F.; Zhang, P.; Ou, L.M.; Chen, C.; Zhang, C.; Wang, Y. Modification Mechanism of Rare Earth Eu on Eutectic Si in Hypoeutectic Al-Si Alloy. Int. J. Met. 2021, 16, 634–645. [Google Scholar] [CrossRef]
- Lu, S.; Hellawell, A. The mechanism of silicon modification in aluminum-silicon alloys: Impurity induced twinning. Metall. Trans. A 1987, 18, 1721–1733. [Google Scholar] [CrossRef]
- Mao, F.; Wei, S.; Chong, C.; Zhang, C.; Wang, X.D.; Cao, Z.Q. Modification of the silicon phase and mechanical properties in Al-40Zn-6Si alloy with Eu addition. Mater. Des. 2020, 186, 108268. [Google Scholar] [CrossRef]
- Mao, G.L.; Yan, H.; Zhu, C.C.; Zhen, W.; Gao, W.L. The varied mechanisms of yttrium (Y) modifying a hypoeutectic Al–Si alloy under conditions of different cooling rates. J. Alloys Compd. 2019, 806, 909–916. [Google Scholar] [CrossRef]
- Li, L.F.; Li, D.Q.; Luo, M.; Zhang, Y.Z.; Kang, Y.T.; Zhu, Q.; Midson, S.P. Influence of Rare Earth Additions on the Microstructure and Mechanical Properties of Al7Si0.3Mg Alloys Processed by Semi-Solid Die Casting and Gravity Die Casting. Solid State Phenom. 2019, 285, 69–74. [Google Scholar] [CrossRef]
- De Giovanni, M.; Kaduk, J.; Srirangam, P. Modification of Al-Si Alloys by Ce or Ce with Sr. JOM 2018, 71, 426–434. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, M.W.; Xu, C.; Xiao, W.L.; Yamagata, H.; Xie, S.H.; Ma, C.L. Effects of Sr, Ce and P on the microstructure and mechanical properties of rapidly solidified Al 7Si alloys. Mater. Charact. 2018, 140, 290–298. [Google Scholar] [CrossRef]
- Madelung, O.; White, G.K. Thermal Conductivity of Pure Metals and Alloys. In Landolt-Börnstein—Group III Condensed Matter; Springer: Berlin/Heidelberg, Germany, 1991; Volume 15c. [Google Scholar] [CrossRef]
- Takeuchi, H. Quantum Elliptic Vortex in a Nematic-Spin Bose-Einstein Condensate. Phys. Rev. Lett. 2021, 12, 126. [Google Scholar] [CrossRef]
- Vandersluis, E.; Emadi, P.; Andilab, B.; Gruzleski, J.; Chen, X.G. The role of silicon morphology in the electrical conductivity and mechanical properties of as-cast B319 aluminum alloy. Metall. Mater. Trans. A 2020, 51, 1874–1886. [Google Scholar] [CrossRef]
- Stadler, F.; Antrekowitsch, H.; Fragner, W.; Kaufmann, H.; Uggowitzer, P.J. The effect of main alloying elements on the physical properties of Al-Si foundry alloys. Mater. Sci. Eng. A 2013, 560, 481–491. [Google Scholar] [CrossRef]
- Tian, L.; Anderson, I.E.; Riedemann, T.; Russell, A.M. Modeling the electrical resistivity of deformation processed metal-metal composites. Acta Mater. 2014, 77, 151–161. [Google Scholar] [CrossRef]
- Li, L.F.; Li, D.Q.; Mao, F.; Feng, J.; Zhang, Y.Z.; Kang, Y.L. Effect of cooling rate on eutectic Si in Al-7.0Si-0.3Mg alloys modified by La additions. J. Alloys Compd. 2020, 826, 154206. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zheng, Q.J.; Jiang, H.X.; He, J.; Zhao, J.Z. Effect of La addition on microstructure evolution of hypoeutectic Al–6Si alloys. J. Mater. Sci. 2020, 55, 7546–7554. [Google Scholar] [CrossRef]
- Qi, Z.Y.; Wang, B.; Jiang, H.X.; Zhang, H.; Li, B.; Zhai, G. Effect of trace rare earth La on microstructure and properties of Al-7Si-0.6 Fe alloy. Acta Phys. Sin. 2024, 73, 076401. [Google Scholar] [CrossRef]






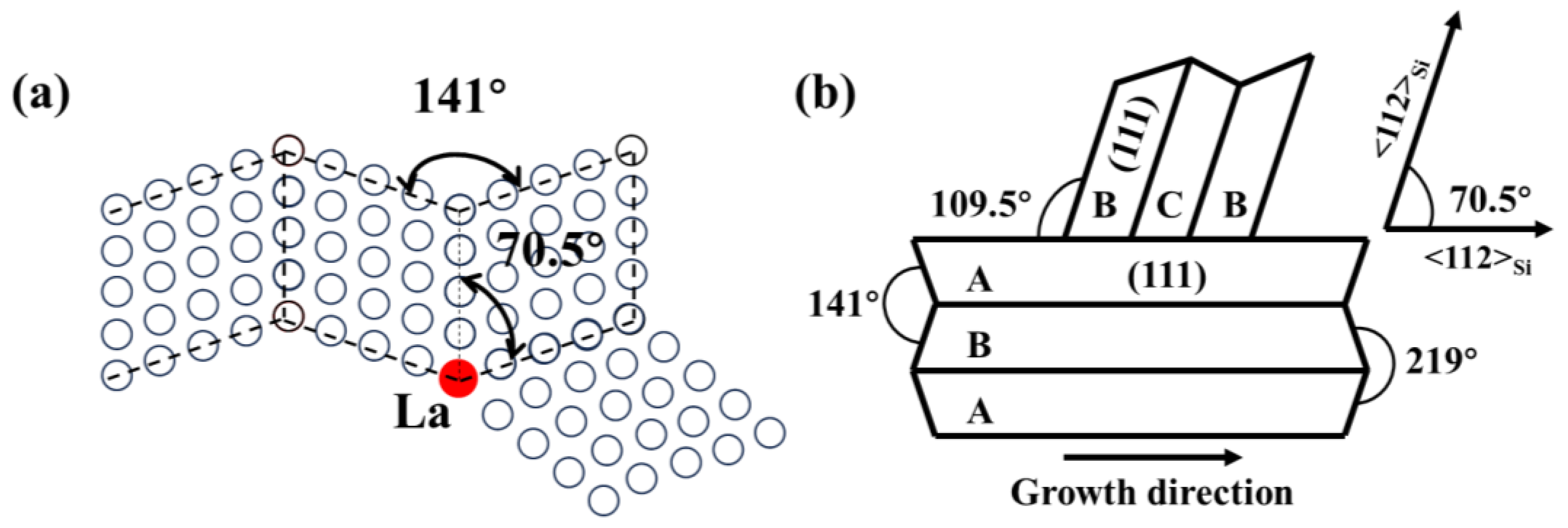
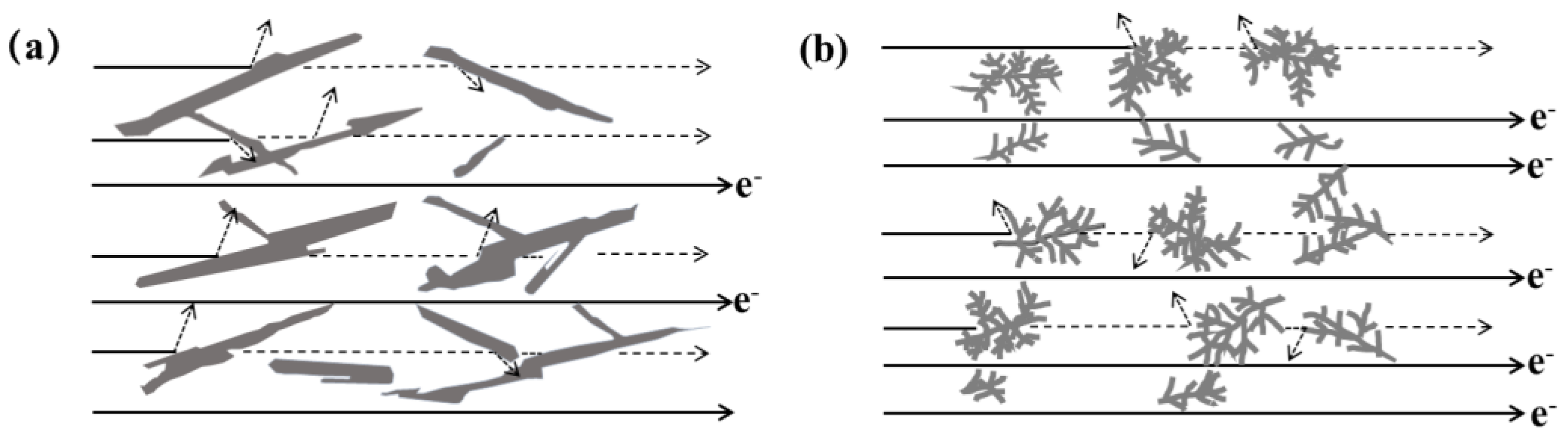
| Alloy | Al-7Si | Al-7Si-0.02La | Al-7Si-0.04La | Al-7Si-0.06La | Al-7Si-0.08La | Al-7Si-0.1La |
|---|---|---|---|---|---|---|
| As-cast (La content) | 0.0010 | 0.022 | 0.038 | 0.058 | 0.075 | 0.090 |
| Post-cycling (La content) | <0.0010 | 0.020 | 0.038 | 0.050 | 0.063 | 0.087 |
| ρ | Cp | α | κ | Δκ | Δκ/κunmd | |
|---|---|---|---|---|---|---|
| (g·cm−3) | (J·g−1·K−1) | (mm2·s−1) | (W·m−1·K−1) | (W·m−1·K−1) | % | |
| Al-7Si | 2.651 | 0.889 | 65.961 | 155.397 | - | - |
| Al-7Si-0.02La | 2.656 | 0.968 | 65.975 | 169.614 | 14.217 | 9.15 |
| Al-7Si-0.04La | 2.663 | 0.941 | 68.889 | 172.566 | 17.169 | 11.05 |
| Al-7Si-0.06La | 2.671 | 0.957 | 70.159 | 179.259 | 23.862 | 15.36 |
| Al-7Si-0.08La | 2.665 | 0.825 | 74.265 | 163.362 | 7.965 | 5.13 |
| Al-7Si-0.1La | 2.742 | 0.839 | 70.290 | 161.645 | 6.248 | 4.02 |
| Sample | Al-7Si | Al-7Si-0.02La | Al-7Si-0.04La | Al-7Si-0.06La | Al-7Si-0.08La | Al-7Si-0.1La |
|---|---|---|---|---|---|---|
| Latent Heat of Melting (As-Cast) (J/g) | 336.9 | 327.8 | 330.1 | 340.4 | 341.9 | 364.9 |
| Latent Heat of Melting (Post-Cycling) (J/g) | 342.7 | 353.7 | 343.7 | 328.6 | 338.9 | 328.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, J.-Y.; Li, J.-C.; Sui, Y.; Wen, L.; Zhang, R.-Y. Effect of Trace La on Microstructure and Thermal Conductivity of Hypoeutectic Al-7Si Alloy. Metals 2025, 15, 1087. https://doi.org/10.3390/met15101087
Yue J-Y, Li J-C, Sui Y, Wen L, Zhang R-Y. Effect of Trace La on Microstructure and Thermal Conductivity of Hypoeutectic Al-7Si Alloy. Metals. 2025; 15(10):1087. https://doi.org/10.3390/met15101087
Chicago/Turabian StyleYue, Jun-Yu, Ji-Cheng Li, Yi Sui, Lei Wen, and Rui-Ying Zhang. 2025. "Effect of Trace La on Microstructure and Thermal Conductivity of Hypoeutectic Al-7Si Alloy" Metals 15, no. 10: 1087. https://doi.org/10.3390/met15101087
APA StyleYue, J.-Y., Li, J.-C., Sui, Y., Wen, L., & Zhang, R.-Y. (2025). Effect of Trace La on Microstructure and Thermal Conductivity of Hypoeutectic Al-7Si Alloy. Metals, 15(10), 1087. https://doi.org/10.3390/met15101087






