Abstract
The rapid growth of the electric vehicle (EV) market has highlighted the critical importance of securing a stable supply chain for lithium-ion battery (LIB) resources, thereby increasing the need for efficient recycling technologies. Among these, lithium recovery remains a major challenge due to significant losses during conventional processes. In this study, a chlorination roasting process was introduced to convert Li2O in spent LIBs into LiCl, which was subsequently evaporated for selective lithium extraction and recovery. Roasting experiments were conducted under air, vacuum, and N2 conditions at 800–1000 °C for 1–5 h, with Cl/Li molar ratios ranging from 0.5 to 8. The optimal condition for lithium evaporation, achieving 100% recovery, was identified as 1000 °C for 5 h, with a Cl/Li molar ratio of 6 under vacuum. Following lithium removal, residual valuable metals were extracted through H2SO4 leaching, and the effects of acid concentration and H2O2 addition on leaching efficiency were examined. The air-roasted samples exhibited the highest leaching performance, while the vacuum- and N2-roasted samples showed relatively lower efficiency; however, the addition of H2O2 significantly enhanced leaching yields in these cases.
1. Introduction
With growing global awareness of environmental issues, efforts to reduce carbon emissions have intensified worldwide. In response, the demand for electric vehicles (EVs) has expanded rapidly as an alternative to fossil fuel-based transportation. EV sales increased from approximately 10.5 million units in 2022 to 14 million in 2023 and 17 million in 2024, highlighting the remarkable growth of this market [1]. As the EV industry expands, the number of end-of-life EVs is also increasing, leading to a sharp rise in waste lithium-ion battery (LIB) generation. Without proper management, these waste batteries pose serious risks to the environment and human health due to the release of toxic substances, thereby necessitating effective recycling strategies [2,3,4,5].
LIBs, the core component of EVs, consist of a cathode, anode, separator, and electrolyte [6]. Among these, cathodes in NMC-type chemistries contain valuable metals, such as lithium, nickel, cobalt, and manganese, making these LIBs highly recyclable [7,8]. In contrast, cathodes such as lithium iron phosphate (LFP) contain only lithium as the main valuable element, which limits their economic viability as recycling feedstock. According to SNE Research, the global market for waste battery recycling is projected to reach USD 53.6 billion by 2030 and USD 174.1 billion by 2040 [2]. However, the supply chain for critical LIB resources remains unstable, underscoring the urgent need for efficient recycling technologies that can both recover valuable metals and ensure environmental sustainability.
Current recycling technologies for LIBs are generally classified into pyrometallurgical, hydrometallurgical, and direct recycling processes [9,10]. Pyrometallurgical methods such as roasting and smelting [11,12] are effective for large-scale operations but often retain lithium in the slag due to its strong oxygen affinity, making recovery difficult [13,14]. Hydrometallurgical methods, including leaching, precipitation, and solvent extraction, typically recover lithium only at the final stage as a low-quality by-product with low efficiency, while also generating large amounts of wastewater and secondary pollutants. Consequently, the development of simple, high-efficiency processes for selective lithium extraction remains a key challenge.
Recent research has explored the recovery of lithium as lithium carbonate (Li2CO3) through carbothermal reduction or carbonation roasting, followed by water leaching [15,16]. While these processes allow selective separation of lithium, the low solubility of Li2CO3 in water limits recovery efficiency [17]. To overcome this limitation, chlorination roasting has been proposed. In this process, a chlorine donor reacts with metal oxides to form chlorides [18]. Since lithium in waste LIBs primarily exists as lithium oxide (Li2O), it can be converted into lithium chloride (LiCl), which has high solubility in water and can be selectively recovered [17]. Dang et al. [19] reported a lithium evaporation rate of 97.45% under optimal chlorination roasting conditions, demonstrating its potential as a promising method for efficient lithium recovery.
Following lithium separation, the residual valuable metals, such as nickel (Ni), cobalt (Co), manganese (Mn), and copper (Cu), can be further recovered using hydrometallurgical techniques. Sulfuric acid (H2SO4) is widely used in leaching due to its cost-effectiveness and efficiency, while hydrogen peroxide (H2O2) serves as an oxidizing agent that enhances dissolution and reduces environmental impact [20,21,22,23].
In this study, chlorination roasting was applied to black powder to investigate lithium extraction as volatile LiCl was generated during the reaction between Li2O and CaCl2. Under the studied conditions, LiCl volatilization occurs predominantly via sublimation. Furthermore, leaching experiments using H2SO4 and H2O2 were conducted to extract valuable metals from the residue, and the effects of process variables on leaching efficiency were systematically analyzed.
2. Materials and Methods
2.1. Chlorination Roasting Process
Figure 1 shows a schematic of the chlorination roasting and subsequent leaching process. Chlorination roasting experiments were conducted by mixing black powder (8 g) with calcium chloride (CaCl2) as a chlorine donor. The mixture was evenly spread in an alumina crucible and subjected to heat treatment under controlled conditions of air, vacuum, and nitrogen. For the air condition, the gas flow rate was maintained at 5 L/min, while the vacuum condition was maintained at approximately 1 × 10−4 torr, and the nitrogen condition was achieved by introducing N2 gas at the same flow rate (5 L/min) into a vacuum-sealed tube furnace.
To evaluate the effects of temperature and time on lithium evaporation, experiments were carried out at a fixed Cl/Li molar ratio of 2 within the ranges of 800–1000 °C and 1–5 h. The Cl/Li ratio of 2 was selected to provide sufficient chlorine for the reaction between Li2O and CaCl2, ensuring the formation of LiCl. Additional experiments were performed at 1000 °C for 5 h while varying the Cl/Li molar ratio between 0.5 and 8 to determine the optimal conditions for lithium evaporation.
Phase changes before and after roasting were analyzed using X-ray diffraction (XRD, SmartLab 9 kW, Rigaku, Tokyo, Japan) with Cu Kα radiation (λ = 1.5406 Å). Lithium content was quantified by inductively coupled plasma optical emission spectrometry (ICP-OES, Integra XL, GBC Scientific, Braeside, Australia). Based on ICP results, the lithium evaporation rate was calculated according to Equation (1):
where x is the lithium content of the black powder sample subjected to heat treatment, and y is the lithium content of the corresponding sample after chlorination roasting under the same conditions.
2.2. Leaching Process
Leaching tests were conducted on black powder samples subjected to chlorination roasting under optimized conditions for lithium evaporation in air, vacuum, and nitrogen, a solid-to-liquid ratio of 50 g/L, and a reaction temperature of 80 °C. To investigate the effects of sulfuric acid concentration and leaching time, the H2SO4 (97%, MATSUNOEN, Osaka, Japan) molarity was varied between 2 and 4 M, and reaction durations were set to 5, 10, 20, 40, 60, 90, and 120 min. To evaluate the role of oxidizing agents, additional experiments were carried out with 10% H2O2 (34.5%, SAMCHUN, Seoul, Republic of Korea) under 3 M H2SO4 conditions, and the results for the three experimental conditions were compared.
The concentrations of Ni, Co, Mn, and Cu in the pregnant leach solutions (PLSs) were quantified using ICP-OES (Integra XL, GBC Scientific, Australia). Both the absolute concentrations of dissolved metals in the PLS and the leaching efficiencies were calculated according to Equation (2), accounting for evaporation losses and additives:
where MO (mg) is the total mass of the target metal in the solid sample prior to leaching, VM (L) is the volume of leachate, and CM (mg/L) is the concentration of dissolved metal ions in the leachate.
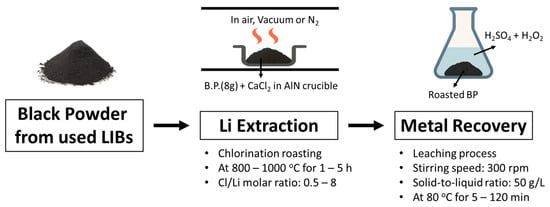
Figure 1.
Schematic diagram of the chlorination roasting and subsequent leaching process.
3. Results and Discussions
3.1. Analysis of Black Powder Sample
The composition of the black powder used in this study was determined by dissolving the sample in aqua regia, followed by ICP-OES analysis. Phase analysis using XRD (Figure S1, Supplementary Materials) revealed that lithium was primarily present in the form of Li2O and Li-NCM oxides (LixNixO2, Lix(NiCoMn)xO2), while the other valuable metals existed as oxides (ICDD card number: Li2O; 01-076-9237, LiNiO2; 01-083-9893, Li0.4Ni1.6O2; 01-081-0095). The total metallic elements quantified by ICP-OES accounted for 28.54 wt.% (Table 1), and the remainder of the sample consisted predominantly of carbon, in agreement with the XRD observations (Figure S1).

Table 1.
Chemical composition of the black powder used in this study, as determined by ICP-OES analysis.
3.2. Selection of the Optimal Chlorine Donor and Lithium Evaporation Behavior Through Thermodynamic Analysis
Figure 2 presents the Gibbs free energy change (ΔG) as a function of temperature for the reactions between Li2O(s) and various chlorine donors, including CaCl2, KCl, NaCl, CuCl2, and AlCl3, explicitly showing the phases of reactants and products (e.g., Li2O (s) + CaCl2 (s) → 2LiCl (g) + CaO (s)). Among these, only CaCl2 exhibits negative ΔG values, indicating that LiCl formation is spontaneous and thermodynamically favorable. In contrast, KCl and NaCl show positive ΔG values, while AlCl3 and CuCl2 are less suitable due to volatility or cost limitations. Therefore, CaCl2 was selected as the most effective chlorine donor in this study. The Gibbs free energy for the formation of 1 mol of LiCl was calculated to compare their thermodynamic feasibility.
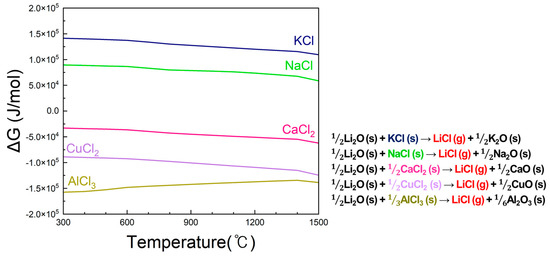
Figure 2.
Gibbs free energy change (ΔG) versus temperature for reactions between Li2O (s) and various chlorine donors (CaCl2, KCl, NaCl, CuCl2, and AlCl3) calculated using FactSage. The phases of reactants and products are indicated (e.g., Li2O (s) + CaCl2 (s) → 2LiCl (g) + CaO (s)).
The results indicated that KCl and NaCl exhibited positive Gibbs free energy values, meaning that LiCl formation is not spontaneous, and thus they are unsuitable as chlorine donors. AlCl3, despite its theoretical reactivity, has a low boiling point (180 °C) and evaporates before LiCl can form, while CuCl2, though feasible, is not cost-effective for large-scale use [24]. In contrast, CaCl2 is inexpensive, widely available, and reacts stably with Li2O to form LiCl spontaneously. Considering thermodynamic stability, cost, and industrial applicability, CaCl2 was selected as the optimal chlorine donor for lithium evaporation in this study.
3.3. Analysis of Lithium Evaporation Efficiency in Chlorination Roasting Under Various Conditions
3.3.1. Air Condition
Effect of Roasting Temperature and Time
Figure 3 presents the XRD patterns of black powder subjected to conventional roasting and chlorination roasting with CaCl2 at 800, 900, and 1000 °C for 5 h in air. After chlorination roasting at 900 and 1000 °C, the Li2O peak intensity decreased significantly. At all roasting temperatures, the LiNiO2 and Li0.4Ni1.6O2 peaks shifted to lower angles with reduced intensity, indicating structural changes. Such peak shifts are typically associated with lattice strain caused by partial lithium removal and cation redistribution within the layered oxide structure. These processes induce contraction or expansion of lattice parameters, leading to observable changes in the diffraction peak positions. In particular, at 1000 °C, the decomposition of Li from LiNiO2 led to the formation of a Li0.45Ni1.05O2 phase. These results suggest that LiCl was generated during chlorination roasting and subsequently evaporated, resulting in lithium depletion.
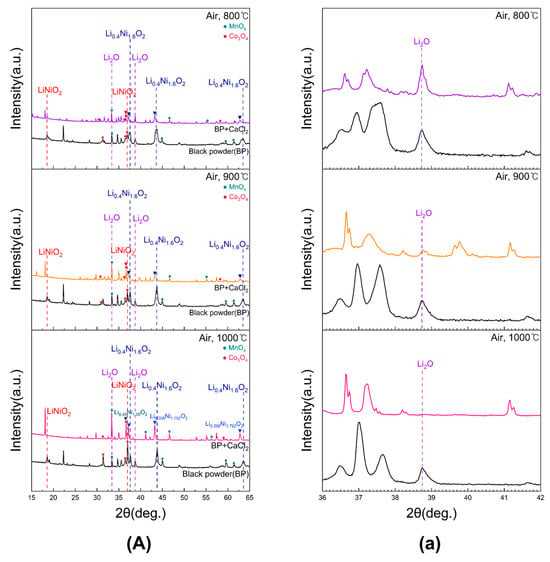
Figure 3.
XRD patterns of black powder samples after chlorination roasting in air ((a) represents the 36–42° range of 2θ for (A)).
Magnified views of the 2θ range of 36–42° for samples in (A) are shown in Figure 3a. The Li2O peak intensity remained nearly unchanged at 800 °C, decreased at 900 °C, and showed a more pronounced reduction at 1000 °C, confirming that lithium evaporation was enhanced with increasing temperature. In addition, the disappearance of the carbon peak after roasting indicated that carbon was removed by volatilization through oxidation. Valuable metals such as Mn and Co were observed as oxides, which is favorable for subsequent leaching.
The Li content before and after chlorination roasting under identical conditions was analyzed by ICP-OES, and the corresponding Li evaporation rates are shown in Figure 4. The evaporation rate increased with longer roasting time at all temperatures, with the effect being most pronounced at 1000 °C compared to 800 and 900 °C. Similarly, at a fixed roasting time, higher temperatures resulted in a distinct increase in Li evaporation. The influence of temperature became more significant as the roasting duration increased. The maximum Li evaporation rate of approximately 82.3% was achieved at 1000 °C for 5 h, indicating that this condition is optimal for promoting lithium evaporation during chlorination roasting in air.
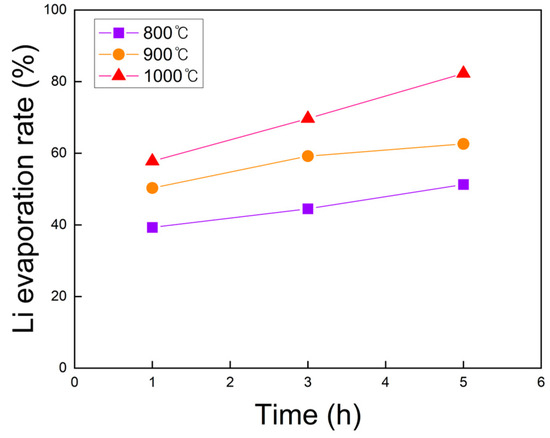
Figure 4.
Lithium evaporation rate of black powder samples after chlorination roasting in air at 800 to 1000 °C for 1 to 5 h, as calculated from ICP-OES results (within ±1% deviation).
Effect of Cl/Li Molar Ratio
Figure 5A shows the XRD patterns of samples obtained after chlorination roasting in air at 1000 °C for 5 h with Cl/Li molar ratios ranging from 0.5 to 8. As the Cl/Li ratio increased, the peak intensities of LiNiO2 and Li0.4Ni1.6O2 decreased and eventually disappeared at ratios above 2. The Li2O peak near 33° overlapped with the manganese oxide peaks, complicating precise identification; however, the comparison of the Li2O peak around 38° revealed the lowest intensities at Cl/Li ratios of 1 and 2, indicating lithium loss due to evaporation during chlorination roasting.
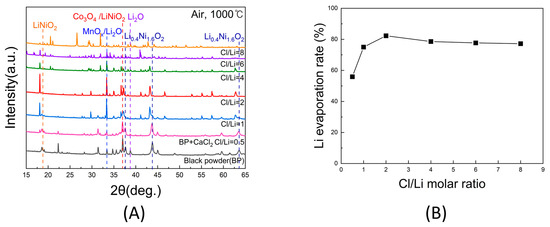
Figure 5.
(A) XRD patterns of black powder samples after chlorination roasting in air at 1000 °C for 5 h with different Cl/Li molar ratios ranging from 0.5 to 8. (B) Lithium evaporation rate of black powder samples after chlorination roasting in air at 1000 °C for 5 h with different Cl/Li molar ratios ranging from 0.5 to 8, as calculated from the ICP-OES results (within ±1% deviation).
To validate these observations, the Li contents before and after roasting were quantified by ICP-OES, and the corresponding evaporation rates are presented in Figure 5B. The Li evaporation rate increased sharply between Cl/Li ratios of 0.5 and 2, reaching a maximum of 82.3% at a ratio of 2. Beyond this value, the evaporation rate slightly decreased, with further increases in the Cl/Li ratio up to 8. Therefore, under air at 1000 °C for 5 h, the optimal Cl/Li molar ratio for lithium evaporation was determined to be 2.
3.3.2. Vacuum Condition
Effect of Roasting Temperature and Time
Figure 6 shows the XRD patterns of black powder subjected to conventional roasting and chlorination roasting with CaCl2 at 800, 900, and 1000 °C for 5 h in vacuum. Lithium was identified in the form of Li2O. Magnified views of the 2θ range of 30–36° for samples in (A) are provided in Figure 6a. At 800 and 900 °C, the Li2O peak intensity exhibited negligible changes after CaCl2 addition. In contrast, at 1000 °C, a marked decrease in Li2O peak intensity was observed, indicating LiCl formation and subsequent evaporation, which resulted in lithium loss. These results confirm that higher roasting temperatures enhance Li evaporation. Unlike in air, carbon peaks were still detected after roasting, suggesting that carbon remained in the samples under vacuum conditions. Furthermore, manganese was identified as MnO2, while Ni and Co appeared in metallic states, implying that an additional conversion step may be necessary during leaching to facilitate their dissolution.
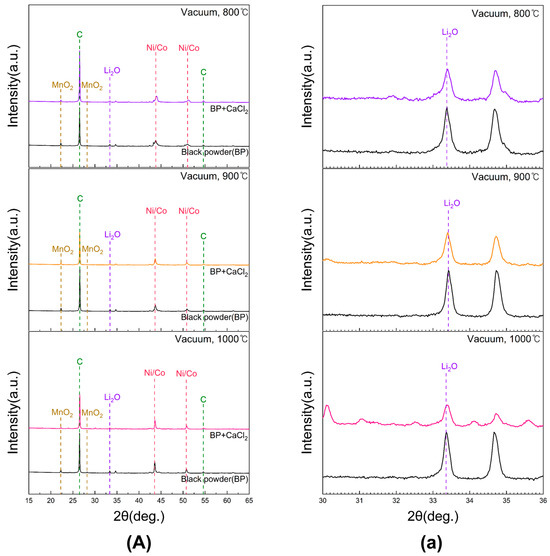
Figure 6.
XRD patterns of black powder samples after chlorination roasting in vacuum ((a) represents the 30–36° range of 2θ for (A)).
The lithium content before and after roasting was quantified by ICP-OES, and the calculated evaporation rates are shown in Figure 7. At all temperatures, longer roasting times increased the Li evaporation rate, with the effect being most significant at 1000 °C. Similarly, at fixed durations, higher roasting temperatures promoted greater Li evaporation, and the influence of temperature became more pronounced with increasing time. The maximum Li evaporation rate of 88.2% was achieved at 1000 °C for 5 h, establishing this condition as optimal for lithium evaporation during chlorination roasting under vacuum.
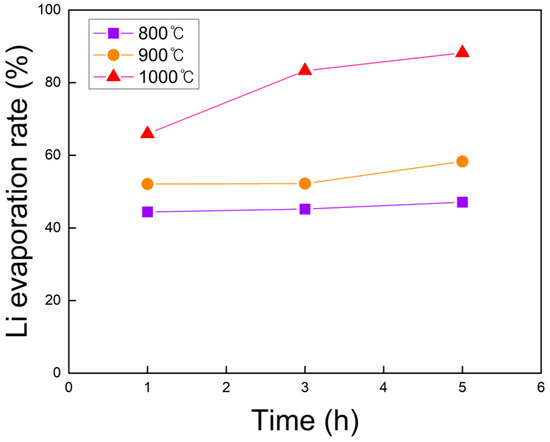
Figure 7.
Lithium evaporation rate of black powder samples after chlorination roasting in vacuum at 800 to 1000 °C for 1 to 5 h, as calculated from ICP-OES results. (within ±1% deviation).
Effect of Cl/Li Molar Ratio
Figure 8A shows the XRD patterns of the samples obtained after chlorination roasting in vacuum at 1000 °C for 5 h with Cl/Li molar ratios ranging from 0.5 to 8. Since differences in Li2O peak intensity were not clearly distinguishable, a magnified view of the 2θ range between 30° and 36° is presented in Figure 8a. The intensities of Li2O peak near 33° gradually decreased with increasing Cl/Li ratio, reaching its minimum at ratios of 4 or higher. The observed shift of Li2O peaks toward higher 2θ indicates lattice contraction due to lithium volatilization. Removal of lithium reduces the lattice parameter, and the consequent decrease in d-spacing leads to higher-angle diffraction peaks according to Bragg’s law. This trend suggests that the lithium content was reduced due to Li evaporation during chlorination roasting.
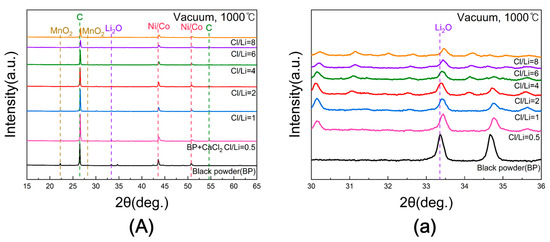
Figure 8.
(A) XRD patterns of black powder samples after chlorination roasting in vacuum at 1000 °C for 5 h with different Cl/Li molar ratios ranging from 0.5 to 8 ((a) represents the 30–36° range of 2θ for (A)).
For quantitative verification, the lithium content before and after roasting was analyzed by ICP-OES, and the corresponding evaporation rates are shown in Figure 9. The Li evaporation rate increased steadily with rising Cl/Li ratio from 0.5 to 6, achieving a maximum of 100% at a ratio of 6. Beyond this point, the evaporation rate declined slightly. Therefore, under vacuum at 1000 °C for 5 h, the optimal Cl/Li molar ratio for lithium evaporation was determined to be 6.
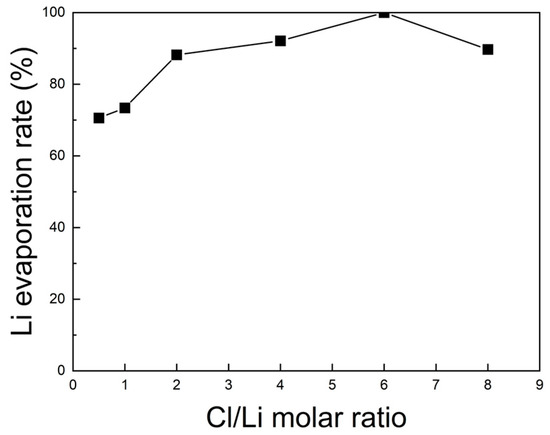
Figure 9.
Lithium evaporation rate of black powder samples after chlorination roasting in vacuum at 1000 °C for 5 h with different Cl/Li molar ratios ranging from 0.5 to 8, as calculated from the ICP-OES results (within ±1% deviation).
3.3.3. Nitrogen Condition
Effect of Roasting Temperature and Time
Figure 10 presents the XRD patterns of black powder subjected to conventional roasting and chlorination roasting with CaCl2 at 800, 900, and 1000 °C for 5 h in N2. Magnified views of the 2θ range between 32° and 46° for samples in (A) are shown in Figure 10a. At 800 and 900 °C, the Li2O peak intensity remained largely unchanged after CaCl2 addition, whereas at 1000 °C the Li2O peak decreased, indicating lithium loss. However, this peak overlapped with Mn4N, making precise quantification difficult. Across all temperatures, LiNiO2 peaks were observed in the samples roasted without CaCl2, while the samples roasted with CaCl2 exhibited Li0.4Ni1.6O2 formation. This transformation suggests that LiCl was generated and subsequently evaporated during chlorination roasting. The presence of Li3N peaks further indicated that lithium oxides reacted with nitrogen to form lithium nitride. Residual carbon peaks showed that carbon did not volatilize under N2 conditions, remaining in the samples after roasting. In addition, Ni, Co, and Mn were detected as nitride phases (Ni3N, Co2N, MnNₓ), implying that further conversion steps may be required to improve leaching efficiency.
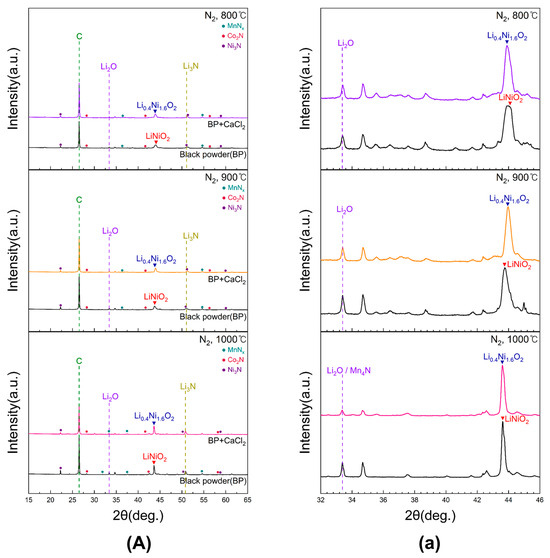
Figure 10.
XRD patterns of black powder samples after chlorination roasting in N2 ((a) represents the 32–46° range of 2θ for (A)).
The lithium content before and after roasting was measured by ICP-OES, and the calculated evaporation rates are shown in Figure 11. At all temperatures, the evaporation rate increased with longer roasting times, although the effect was minor at 800 and 900 °C. At 1000 °C, however, roasting time had a pronounced influence, with higher temperatures consistently promoting greater lithium evaporation. The maximum lithium evaporation rate of 66.9% was achieved at 1000 °C for 5 h, identifying this condition as optimal for lithium evaporation during chlorination roasting in N2.
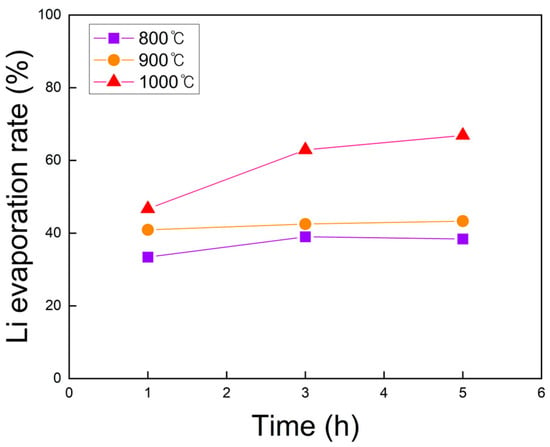
Figure 11.
Lithium evaporation rate of black powder samples after chlorination roasting in N2 at 800 to 1000 °C for 1 to 5 h, as calculated from the ICP-OES results (within ±1% deviation).
Effect of Cl/Li Molar Ratio
Figure 12A shows the XRD patterns of black powder subjected to chlorination roasting at 1000 °C for 5 h in N2 with Cl/Li molar ratios ranging from 0.5 to 8. A magnified view of the 2θ range between 32° and 46° is presented in Figure 12a. With increasing Cl/Li ratio, the Li2O peak near 33° appeared to gradually decrease; however, overlap with the Mn4N peak made accurate evaluation difficult. Across all conditions, the LiNiO2 peak observed in samples without CaCl2 was transformed into Li0.4Ni1.6O2 after CaCl2 addition, reflecting lithium loss due to evaporation. As the variations in peak intensity with respect to Cl/Li ratio were limited, a quantitative analysis was required to confirm these trends.
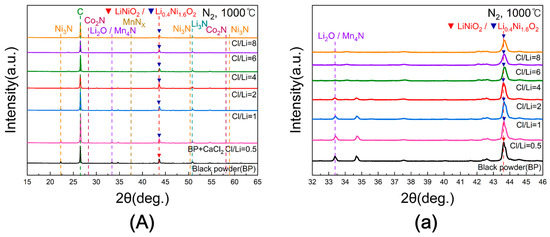
Figure 12.
(A) XRD patterns of black powder samples after chlorination roasting in N2 at 1000 °C for 5 h with different Cl/Li molar ratios ranging from 0.5 to 8 ((a) represents the 32–46° range of 2θ for (A)).
For this purpose, the lithium contents before and after roasting were measured by ICP-OES, and the corresponding evaporation rates are shown in Figure 13. The Li evaporation rate increased steadily as the Cl/Li ratio rose from 0.5 to 2, reaching a maximum of 66.9% at a ratio of 2. Beyond this value, the rate declined slightly. Therefore, under N2 at 1000 °C for 5 h, the optimal Cl/Li molar ratio for lithium evaporation in the chlorination roasting process was determined to be 2.
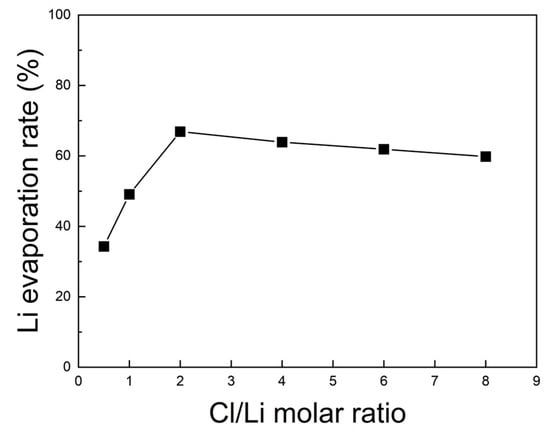
Figure 13.
Lithium evaporation rate of black powder samples after chlorination roasting in N2 at 1000 °C for 5 h with different Cl/Li molar ratios ranging from 0.5 to 8, as calculated from the ICP-OES results (within ±1% deviation).
3.3.4. Discussion
The chlorination roasting study revealed that the optimal roasting condition across all conditions was 1000 °C for 5 h. Under air, the optimal Cl/Li molar ratio for lithium evaporation was 2, whereas under vacuum it was 6, and under nitrogen it was 2. Among these, vacuum roasting exhibited the most favorable performance, achieving complete lithium evaporation (100%). This result can be explained by the reduced sublimation temperature of LiCl under low-pressure conditions, which enhances its volatility. This observation is consistent with the findings of Luo et al. [25], who reported that decreasing pressure lowers the sublimation temperature of metal chlorides, thereby improving chloride separation and purification efficiency in vacuum.
To further support this mechanism, a thermodynamic analysis was performed using FactSage to evaluate the Li2O–CaCl2 system. The pressure–temperature phase diagram (Figure 14) confirmed that gas-phase product formation increased as pressure decreased. This trend can be attributed to the higher vapor pressure of volatile lithium compounds such as LiCl at reduced pressures, which lowers their evaporation temperature and enables gas-phase formation even at relatively low temperatures. Consequently, vacuum conditions are expected to maximize lithium evaporation efficiency.
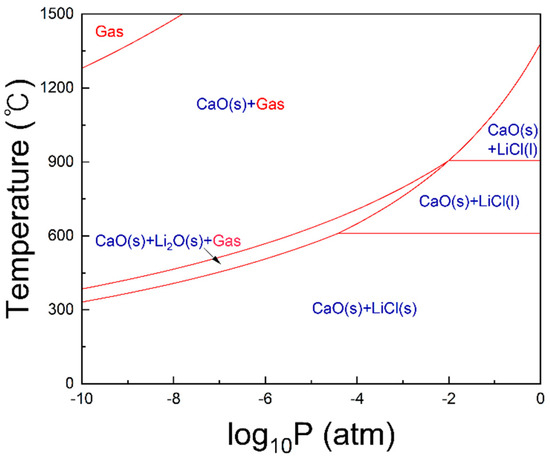
Figure 14.
Phase diagram of the Li2O—CaCl2 system (molar ratio of Li2O/CaCl2 = 1).
In contrast, lithium evaporation under nitrogen was less effective than under air or vacuum. The XRD analysis identified the formation of Li3N after roasting, suggesting that side reactions such as lithium nitride formation occurred. These reactions likely competed with or suppressed LiCl formation, thereby hindering lithium evaporation under N2 conditions.
3.4. Analysis of the Leaching Behavior of Valuable Metals Remaining After Chlorination Roasting Under Various Conditions
3.4.1. Leaching Behavior as a Function of Sulfuric Acid Molarity
Figure 15, Figure 16 and Figure 17 present the leaching results of valuable metals from black powder subjected to chlorination roasting under air, vacuum, and nitrogen. The experiments were carried out at a stirring speed of 300 rpm, a solid-to-liquid ratio of 50 g/L, and a reaction temperature of 80 °C, while the H2SO4 concentration was varied at 2, 3, and 4 M over a reaction time of 0–120 min. The leaching behavior of (a) Ni, (b) Co, (c) Mn, and (d) Cu was evaluated in terms of both leaching efficiency and the corresponding concentrations in the PLS, as a function of acid concentration and reaction time.
Samples Subjected to Chlorination Roasting in Air
Figure 15 shows the leaching efficiencies of Ni, Co, Mn, and Cu from black powder subjected to chlorination roasting under air. Under 2 M H2SO4, the leaching efficiencies of Ni, Co, and Mn increased with reaction time, reaching maximum values of ~77%, ~61%, and ~47%, respectively, at 120 min. At 3 M H2SO4, Ni and Co achieved their highest efficiencies of ~81% and ~66% at 60 min, after which a slight decline was observed, while Mn reached ~52% at 90 min and then stabilized. The leaching behavior at 4 M H2SO4 was similar to that at 3 M, but with overall lower efficiencies, indicating that the reaction limit was occurred above 3 M acid concentration. In contrast, Cu exhibited 100% leaching efficiency across all conditions.
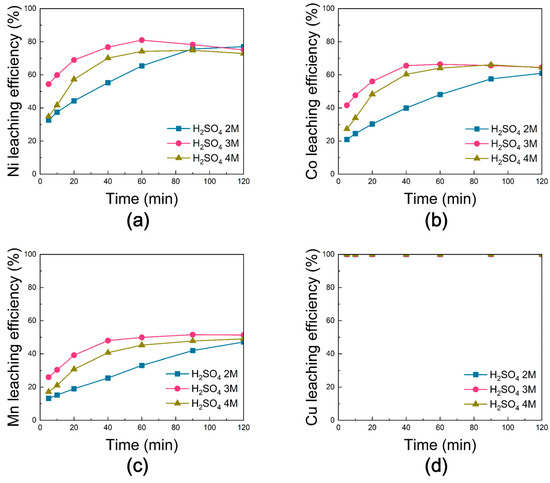
Figure 15.
Leaching behavior of (a) Ni, (b) Co, (c) Mn, and (d) Cu from black powder after chlorination roasting in air as a function of sulfuric acid molarity [H2SO4 molarity: 2–4 M, reaction time: 0–120 min, reaction temperature: 80 °C, solid-to-liquid ratio: 50 g/L, stirring speed: 300 rpm].
Samples Subjected to Chlorination Roasting in Vacuum
Figure 16 shows the leaching efficiencies of Ni, Co, Mn, and Cu from black powder subjected to chlorination roasting under vacuum. For all H2SO4 concentrations, the leaching efficiencies of Ni, Co, and Mn increased with reaction time, reaching maximum values at 120 min. At 3 M H2SO4, Ni, Co, and Mn achieved efficiencies of ~31%, ~48%, and ~31%, respectively, which were slightly higher than those obtained under 2 M and 4 M conditions, suggesting that the reaction limit was reached beyond 3 M. In contrast, Cu exhibited very poor leaching behavior, with efficiencies below 10% across all conditions.
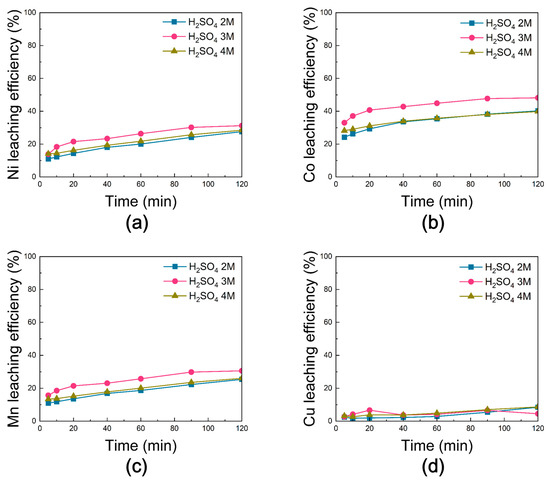
Figure 16.
Leaching behavior of (a) Ni, (b) Co, (c) Mn, and (d) Cu from black powder after chlorination roasting in vacuum as a function of sulfuric acid molarity [H2SO4 molarity: 2–4 M, reaction time: 0–120 min, reaction temperature: 80 °C, solid-to-liquid ratio: 50 g/L, stirring speed: 300 rpm].
Samples Subjected to Chlorination Roasting in N2
Figure 17 shows the leaching efficiencies of Ni, Co, Mn, and Cu from black powder subjected to chlorination roasting under nitrogen. For all H2SO4 concentrations, the efficiencies of Ni, Co, and Mn increased with reaction time and reached their maximum at 120 min. Under 3 M H2SO4, Ni, Co, and Mn exhibited the highest values of ~15%, ~22%, and ~12%, respectively, although the differences compared with 2 M and 4 M were negligible. Cu showed consistently poor leaching behavior, with efficiencies below 10% under all conditions. Overall, compared with the air and vacuum conditions, the nitrogen condition resulted in markedly lower leaching efficiencies for all valuable metals.
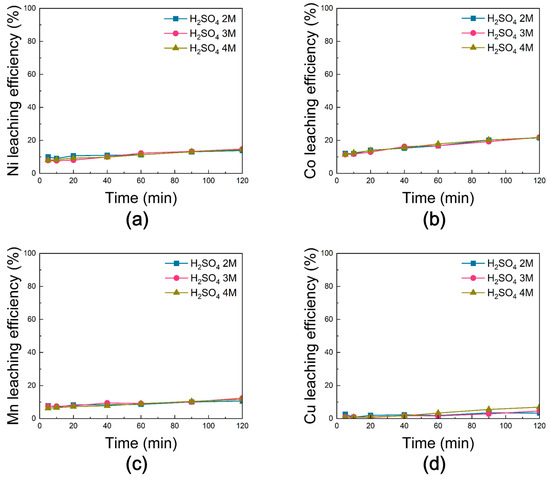
Figure 17.
Leaching behavior of (a) Ni, (b) Co, (c) Mn, and (d) Cu from black powder after chlorination roasting in N2 as a function of sulfuric acid molarity [H2SO4 molarity: 2–4 M, reaction time: 0–120 min, reaction temperature: 80 °C, solid-to-liquid ratio: 50 g/L, stirring speed: 300 rpm].
Discussion on the Leaching Behavior of Residual Valuable Metals with Respect to Their Phases
The leaching behavior of black powder subjected to chlorination roasting under air, vacuum, and nitrogen was examined using H2SO4 leaching. As shown in Figure 15, Figure 16 and Figure 17, the samples roasted in air exhibited the highest leaching efficiency, while those treated under the vacuum and N2 conditions showed considerably lower values. To clarify these differences, XRD analysis was performed (Figure 18).
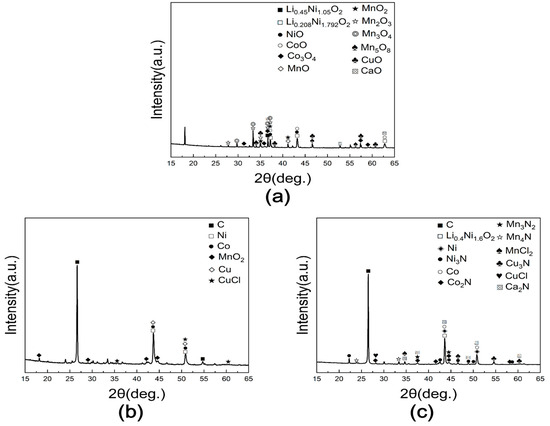
Figure 18.
XRD patterns of valuable metals in black powder obtained after optimized chlorination roasting under (a) air (1000 °C, 5 h, Cl/Li = 2), (b) vacuum (1000 °C, 5 h, Cl/Li = 6), and (c) N2 (1000 °C, 5 h, Cl/Li = 2).
For the sample roasted in air, nickel was present mainly as NiO, cobalt as CoO and Co3O4, manganese as MnO, MnO2, Mn2O3, Mn3O4, and Mn5O8, and copper as CuO. Importantly, these transition metals remained in the form of stable oxides that are not susceptible to chlorination under the applied conditions, whereas lithium existed as Li2O and readily reacted with CaCl2 to form volatile LiCl. This phase stability difference justifies the selectivity of lithium extraction and further validates the effectiveness of CaCl2 as a chlorinating agent. Lithium-containing NCM oxides were also identified, together with CaO generated from the reaction between CaCl2 and oxygen. In the vacuum-roasted sample, residual carbon was detected, and Ni, Co, and Cu were reduced to metallic states due to carbothermal reduction, while Mn remained as MnO2, and copper also appeared as CuCl. In the N2-roasted sample, nitride phases were additionally observed, including Ni3N, Co2N, Mn3N2, Mn4N, and Cu3N, along with metallic phases and Ca2N formed from CaCl2–N2 reactions. MnCl2 and CuCl were also detected.
In general, divalent metal oxides (MOs) react readily with H2SO4 according to Equation (3), forming soluble sulfates and exhibiting high leachability [26]. The air-roasted sample contained NiO, CoO, MnO, and CuO, which facilitated efficient dissolution, explaining its relatively high leaching efficiency. However, the coexistence of higher-valence oxides, such as Co3O4 and MnO2, lowered overall efficiency. In particular, MnO2 (+4 state) is highly insoluble in H2SO4 and tends to precipitate, thereby limiting manganese leaching [26]. In contrast, copper, present mainly as CuO, dissolved completely, leading to nearly 100% leaching.
MO + H2SO4 = MSO4 + H2O
M: divalent metal
In the vacuum- and N2-roasted samples, metals existed predominantly in metallic or nitride phases, which are less reactive toward H2SO4. Therefore, their dissolution required additional oxidation, reducing leaching efficiency. Moreover, significant bubbling was observed during leaching in these samples, attributed to residual carbon. Gas trapped within carbonaceous materials hindered mass transfer and increased resistance to particle movement, while also promoting adsorption of metal ions, collectively reducing efficiency [27,28]. Furthermore, the presence of nitride phases in the N2-roasted sample likely produced secondary by-products upon reaction with H2SO4, further suppressing leaching. These findings suggest that additional pre-treatment to oxidize metallic and nitride phases and remove residual carbon is necessary to improve leaching efficiency.
In addition, it should be noted that the S/L ratio was fixed at 50 g/L in this study. While this condition facilitates controlled comparison of dissolution behaviors, it results in relatively low absolute concentrations of metals in the PLS compared to typical industrial leaching operations. Nonetheless, the reported concentrations provide meaningful insight into the leaching kinetics and selectivity of different metal phases.
3.4.2. Leaching Behavior as a Function of Hydrogen Peroxide Addition
Figure 19 presents the leaching behavior of Ni, Co, Mn, and Cu from black powder subjected to chlorination roasting in (a) air, (b) vacuum, and (c) N2 conditions. The experiments were conducted under fixed conditions of 300 rpm stirring speed, a solid-to-liquid ratio of 50 g/L, a temperature of 80 °C, and 3 M H2SO4, with reaction times of 0–120 min. To evaluate the influence of oxidizing agents, the results obtained without H2O2 were compared with those after the addition of 10% H2O2.
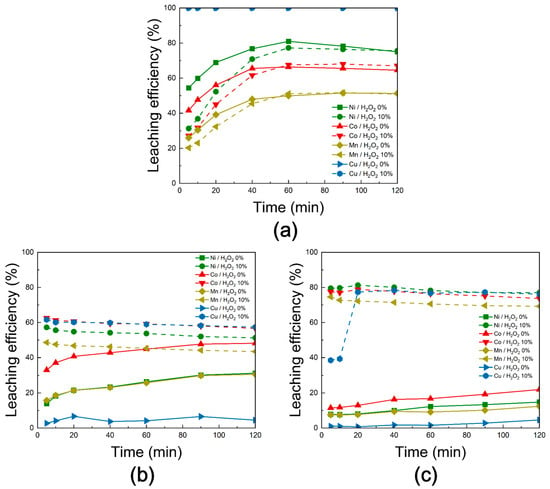
Figure 19.
Leaching behavior of Ni, Co, Mn, and Cu from black powder obtained after chlorination roasting in (a) air, (b) vacuum, and (c) N2 as a function of hydrogen peroxide addition [H2O2 addition: 0% and 10%, reaction time: 0–120 min, H2SO4 molarity: 3 M, reaction temperature: 80 °C, solid-to-liquid ratio: 50 g/L, stirring speed: 300 rpm].
For the air-roasted samples, Ni, Co, and Mn reached their maximum leaching efficiencies at 60 min, after which the values stabilized or slightly declined. Cu was fully leached (100%) under all conditions. The addition of H2O2 had little effect on overall leaching efficiency, and in the case of Ni, efficiency even decreased, likely due to the formation of a passive film in the presence of H2O2 [29]. This limited effect is attributed to reaction supersaturation and the insufficient amount of H2O2 to promote further redox reactions.
For the vacuum-roasted samples, Ni, Co, and Mn showed gradual increases in leaching efficiency without H2O2, reaching maximum values at 120 min. In contrast, with H2O2 addition, all metals achieved their peak efficiencies within the first 5 min, indicating that both the leaching and redox reactions were accelerated. The improvements in efficiency were approximately +26% for Ni, +15% for Co, +18% for Mn, and +55% for Cu. This enhancement is attributed to the oxidation of metallic Ni, Co, and Cu to their more soluble divalent states, and the reduction of MnO2 (+4) to Mn2+, as described in Equations (4) and (5).
For the N2-roasted samples, Ni, Co, Mn, and Cu also exhibited gradual leaching without H2O2, with maximum efficiencies reached at 120 min. However, with H2O2 addition, rapid leaching occurred within 20 min, accompanied by accelerated oxidation-reduction reactions. The efficiency gains were more pronounced than in air or vacuum, with increases of ~66% for Ni, ~57% for Co, ~63% for Mn, and ~73% for Cu. This improvement is attributed not only to the oxidation of metallic phases but also to the conversion of nitride phases into more soluble forms, as outlined in Equations (5)–(8). Overall, the N2-roasted samples exhibited the strongest enhancement in leaching efficiency upon H2O2 addition.
MnO2 + H2O2 + H2SO4 = MnSO4 + 2H2O + O2
M + H2O2 + H2SO4 = MSO4 + 2H2O
2M2N + 4H2O2 + 4H2SO4 = 4MSO4 + 8H2O + N2
2M3N + 6H2O2 + 6 H2SO4 = 6MSO4 + 12H2O + N2
2M4N + 8H2O2 + 8 H2SO4 = 8MSO4 + 16H2O + N2
M: metal
4. Conclusions
In this study, a chlorination roasting process was applied to black powder derived from spent LIBs to selectively extract and recover lithium. Roasting experiments were performed under air, vacuum, and nitrogen conditions, with temperature, duration, and Cl/Li molar ratio as key variables. Subsequent H2SO4 leaching was carried out to recover residual valuable metals, and the effects of acid concentration and H2O2 addition were systematically examined.
The results revealed that lithium evaporation was strongly dependent on both condition and Cl/Li molar ratio. Under air, the optimal condition (1000 °C, 5 h, Cl/Li = 2) yielded a maximum evaporation rate of ~82%. Under vacuum, complete lithium evaporation (100%) was achieved at 1000 °C for 5 h with a Cl/Li ratio of 6, attributed to the reduced sublimation temperature of LiCl at low pressure. In contrast, the nitrogen condition produced lower evaporation rates (~67%) due to side reactions such as Li3N formation, which impeded LiCl generation.
In leaching experiments, the air-roasted sample exhibited the highest efficiencies, reaching ~81% for Ni, ~66% for Co, ~52% for Mn, and 100% for Cu under 3 M H2SO4. This was attributed to the predominance of divalent metal oxides, which dissolve readily in sulfuric acid. By contrast, the vacuum- and nitrogen-roasted samples showed lower leaching efficiencies due to residual carbon and the reduction of metals to less soluble metallic forms. The addition of 10% H2O2 had little effect on the air-roasted sample, but significantly enhanced the leaching of vacuum- and nitrogen-roasted samples, particularly under N2, where efficiencies increased by ~66% for Ni, ~57% for Co, ~63% for Mn, and ~73% for Cu.
This work demonstrates that chlorination roasting enables selective lithium extraction as volatile LiCl, with up to 100% extraction efficiency under optimized conditions. Future studies should address the collection of LiCl vapors and quantify PLS concentrations for practical recovery assessment.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/met15101085/s1; Figure S1: XRD patterns of black powder used in this study.
Author Contributions
Conceptualization, Y.H.K.; methodology, Y.H.K.; software, M.K. and Y.-M.K.; validation, M.K. and S.H.; formal analysis, M.K. and S.H.; investigation, M.K.; resources, M.K.; data curation, M.K.; writing—original draft preparation, M.K.; writing—review and editing, Y.-M.K. and E.P.; visualization, M.K. and E.P.; supervision, Y.H.K. and E.P.; project administration, Y.H.K.; funding acquisition, Y.H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Technology Development Project to Improve Secondary Battery Circulation Usability (Development of Pollutants Reduction Technology Generated in the Lithium-Ion Batteries Recycling Process) through the Korea Environmental Industry & Technology Institute, funded by the Ministry of Environment (RS-2024-00345911).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- International Energy Agency. Global EV Outlook 2024; IEA: Paris, France, 2024. [Google Scholar]
- Research, S. Global Scrapped Battery Recycling Market Expected to Reach US$ 53.6 Bil in 2030 and US$174.1 Bil in 2040; SNE Research: Seongnam-si, Republic of Korea, 2023. [Google Scholar]
- Melchor-Martínez, E.M.; Macias-Garbett, R.; Malacara-Becerra, A.; Iqbal, H.M.N.; Sosa-Hernández, J.E.; Parra-Saldívar, R. Environmental impact of emerging contaminants from battery waste: A mini review. Case Stud. Chem. Environ. Eng. 2021, 3, 100104. [Google Scholar] [CrossRef]
- Chen, M.; Ma, X.; Chen, B.; Arsenault, R.; Karlson, P.; Simon, N.; Wang, Y. Recycling end-of-life electric vehicle lithium-ion batteries. Joule 2019, 3, 2622–2646. [Google Scholar] [CrossRef]
- Costa, C.M.; Barbosa, J.C.; Gonçalves, R.; Castro, H.; Campo, F.J.D.; Lanceros-Méndez, S. Recycling and environmental issues of lithium-ion batteries: Advances, challenges and opportunities. Energy Storage Mater. 2021, 37, 433–465. [Google Scholar] [CrossRef]
- Friedrich, B.; Schwich, L. New science based concepts for increased efficiency in battery recycling. Metals 2021, 11, 533. [Google Scholar] [CrossRef]
- Guo, L.; Thornton, D.B.; Koronfel, M.A.; Stephens, I.E.L.; Ryan, M.P. Degradation in lithium ion battery current collectors. J. Phys. Energy 2021, 3, 032015. [Google Scholar] [CrossRef]
- Vaccari, M.; Parlanti, F.; Manni, F.M.; Orefice, M.; Mathieux, F.; Pannocchia, G.; Tognotti, L.; Bertei, A. Assessing performance in lithium-ion batteries recycling processes: A quantitative modeling perspective. Resour. Conserv. Recycl. 2024, 206, 107643. [Google Scholar] [CrossRef]
- Marchese, D.; Giosuè, C.; Staffolani, A.; Conti, M.; Orcioni, S.; Soavi, F.; Cavalletti, M.; Stipa, P. An Overview of the Sustainable Recycling Processes Used for Lithium-Ion Batteries. Batteries 2024, 10, 27. [Google Scholar] [CrossRef]
- Azimi, G.; Chan, K.H. A review of contemporary and emerging recycling methods for lithium-ion batteries with a focus on NMC cathodes. Resour. Conserv. Recycl. 2024, 209, 107825. [Google Scholar] [CrossRef]
- Park, E.; Kim, M.; Pin, M.-W.; Park, H.; Kim, Y.-H. Precious metal recovery from waste electrical and electronic equipment through oxidative refining. Recycling 2023, 8, 80. [Google Scholar] [CrossRef]
- Park, E.; Kim, M.; Kim, Y.-M.; Kim, Y.H. High-purity copper recovery from copper sludge via oxidative refining using a FeO–CaO–SiO2 slag system. Materials 2025, 18, 4137. [Google Scholar] [CrossRef]
- Dobó, Z.; Dinh, T.; Kulcsár, T. A review on recycling of spent lithium-ion batteries. Energy Rep. 2023, 9, 6362–6395. [Google Scholar] [CrossRef]
- Gerold, E.; Luidold, S.; Antrekowitsch, H. Separation and Efficient Recovery of Lithium from Spent Lithium-Ion Batteries. Metals 2021, 11, 1091. [Google Scholar] [CrossRef]
- Wang, J.; You, X.; She, X.; Xue, Q. Research on the process of carbon thermal reduction for recovery and resynthesis of LiNi0.6Co0.2Mn0.2O2. J. Mater. Cycles Waste Manag. 2024, 26, 346–359. [Google Scholar] [CrossRef]
- Rouquette, L.M.J.; Lemaître, T.; Vieceli, N.; Petranikova, M. Intensification of lithium carbonation in the thermal treatment of spent EV Li-ion batteries via waste utilization and selective recovery by water leaching. Resour. Conserv. Recycl. Adv. 2023, 17, 200125. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.W.; Sohn, I.; Lee, S. Pyrometallurgical recycling of end-of-life lithium-ion batteries. Int. J. Miner. Metall. Mater. 2024, 31, 1554. [Google Scholar] [CrossRef]
- Xing, Z.; Cheng, G.; Yang, H.; Xue, X.; Jian, P. Mechanism and application of the ore with chlorination treatment: A review. Miner. Eng. 2020, 154, 106404. [Google Scholar] [CrossRef]
- Dang, H.; Wang, B.; Chang, Z.; Wu, X.; Feng, J.; Zhou, H.; Li, W.; Sun, C. Recycled Lithium from Simulated Pyrometallurgical Slag by Chlorination Roasting. ACS Sustain. Chem. Eng. 2018, 6, 13160–13167. [Google Scholar] [CrossRef]
- Shuai, J.; Liu, W.; Rohani, S.; Wang, Z.; He, M.; Ding, C.; Lv, X. Efficient extraction and separation of valuable elements from spent lithium-ion batteries by leaching and solvent extraction: A review. Chem. Eng. J. 2025, 503, 158114. [Google Scholar] [CrossRef]
- Davis, K.; Demopoulos, G.P. Hydrometallurgical recycling technologies for NMC Li-ion battery cathodes: Current industrial practice and new R&D developments & trends. RSC Sustain. 2023, 1, 1932–1951. [Google Scholar]
- Targhan, H.; Evans, P.; Bahrami, K. A review of the role of hydrogen peroxide in organic transformations. J. Ind. Eng. Chem. 2021, 104, 295–332. [Google Scholar] [CrossRef]
- Tasawar, A.; Dotto Munchen, D.; Birich, A.; Yeetsorn, R.; Friedrich, B. Effect of oxidative roasting on selective leaching of lithium from industrially shredded lithium iron phosphate blackmass. Metals 2025, 15, 739. [Google Scholar] [CrossRef]
- Deng, P.; Li, L.; Jia, Y.; Liu, D.; Jiang, W.; Kong, L. Chlorination Behavior of Low-Grade Titanium Slag in AlCl3-NaCl Molten Salt. JOM 2022, 74, 213–221. [Google Scholar] [CrossRef]
- Luo, W.; Hu, G.; Ding, J.; Wu, J.; Ma, W. Thermodynamics of Vacuum Chloride Volatilization of Ni, Co, Mn, Li, Al, and Cu in Spent Lithium−Ion Battery. Metals 2022, 12, 2183. [Google Scholar] [CrossRef]
- Gaw, D.C. Manganese Removal from Sulfuric Acid Leach Solutions of Nickel Laterite Ores, in WASM: Minerals, Energy and Chemical Engineering; Curtin University: Perth, Australia, 2020. [Google Scholar]
- Son, S.H.; Kim, J.H.; Kim, H.-J.; Kim, S.J.; Lee, M.S. Leaching of Valuable Metals from NCM Cathode Active Materials in Spent Lithium-Ion Battery by Malic acid. J. Resour. Recycl. 2014, 23, 21–29. [Google Scholar]
- Su, F.; Zhou, X.; Liu, X.; Yang, J.; Tang, J.; Yang, W.; Li, Z.; Wang, H.; Ma, Y.; Zhang, Y. An efficient recovery process of valuable metals from spent lithium-ion batteries in acidic medium assisted with waste areca powder. J. Environ. Chem. Eng. 2022, 10, 108711. [Google Scholar] [CrossRef]
- Bilczuk, D.; Olvera, O.G.; Asselin, E. Kinetic study of the dissolution of metallic nickel in sulphuric acid solutions in the presence of different oxidants. Can. J. Chem. Eng. 2016, 94, 1872–1879. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).