Emissivity Measurements of Metals Used in Wire-Arc-Directed Energy Deposition Processes
Abstract
1. Introduction
2. Theory
3. Materials and Methods
3.1. Titanium Alloy
3.2. Inconel 718
3.3. Mild Steel
3.4. Aluminum Alloy 2319
3.5. Nickel Aluminum Bronze (NAB)
4. Results
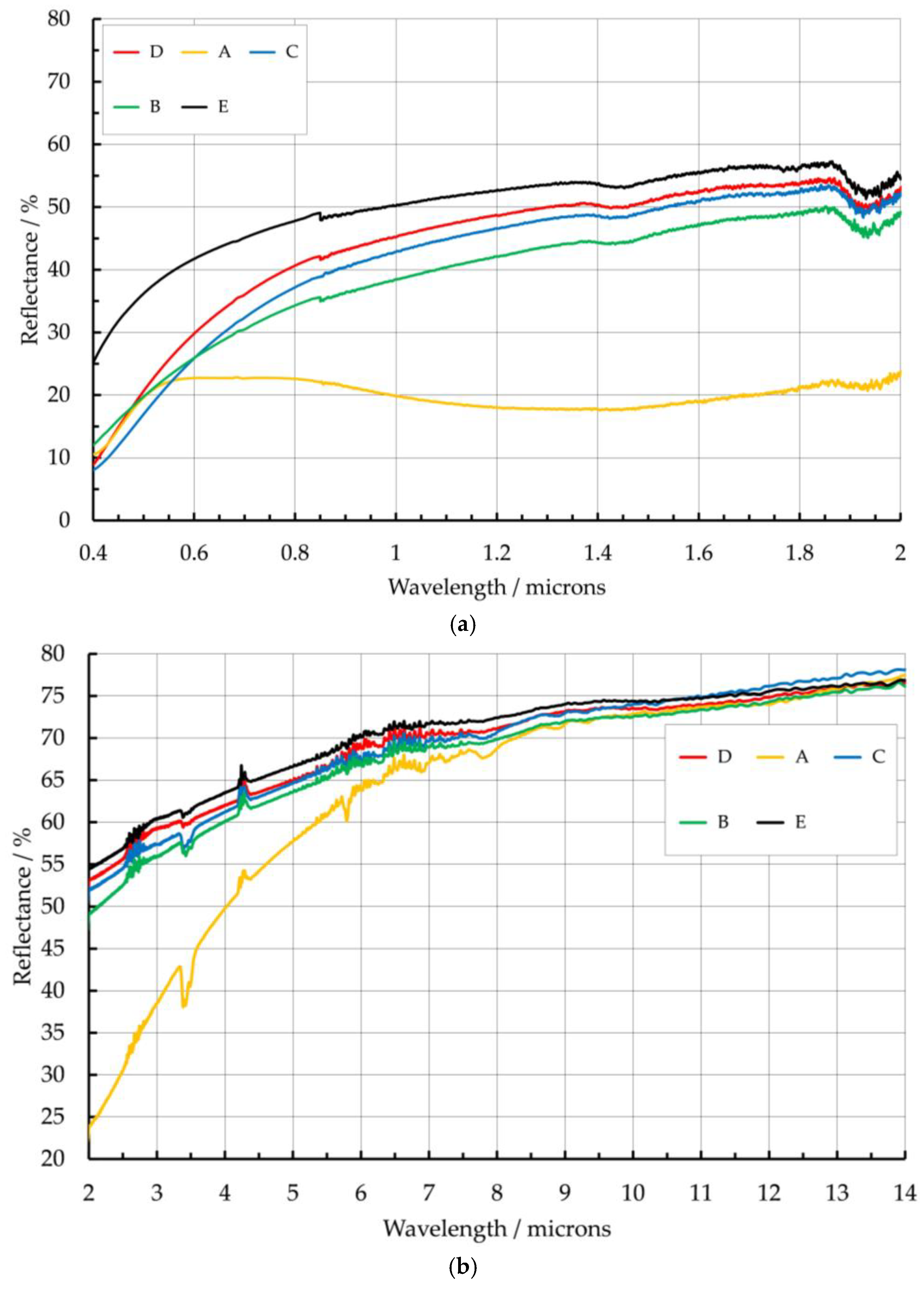
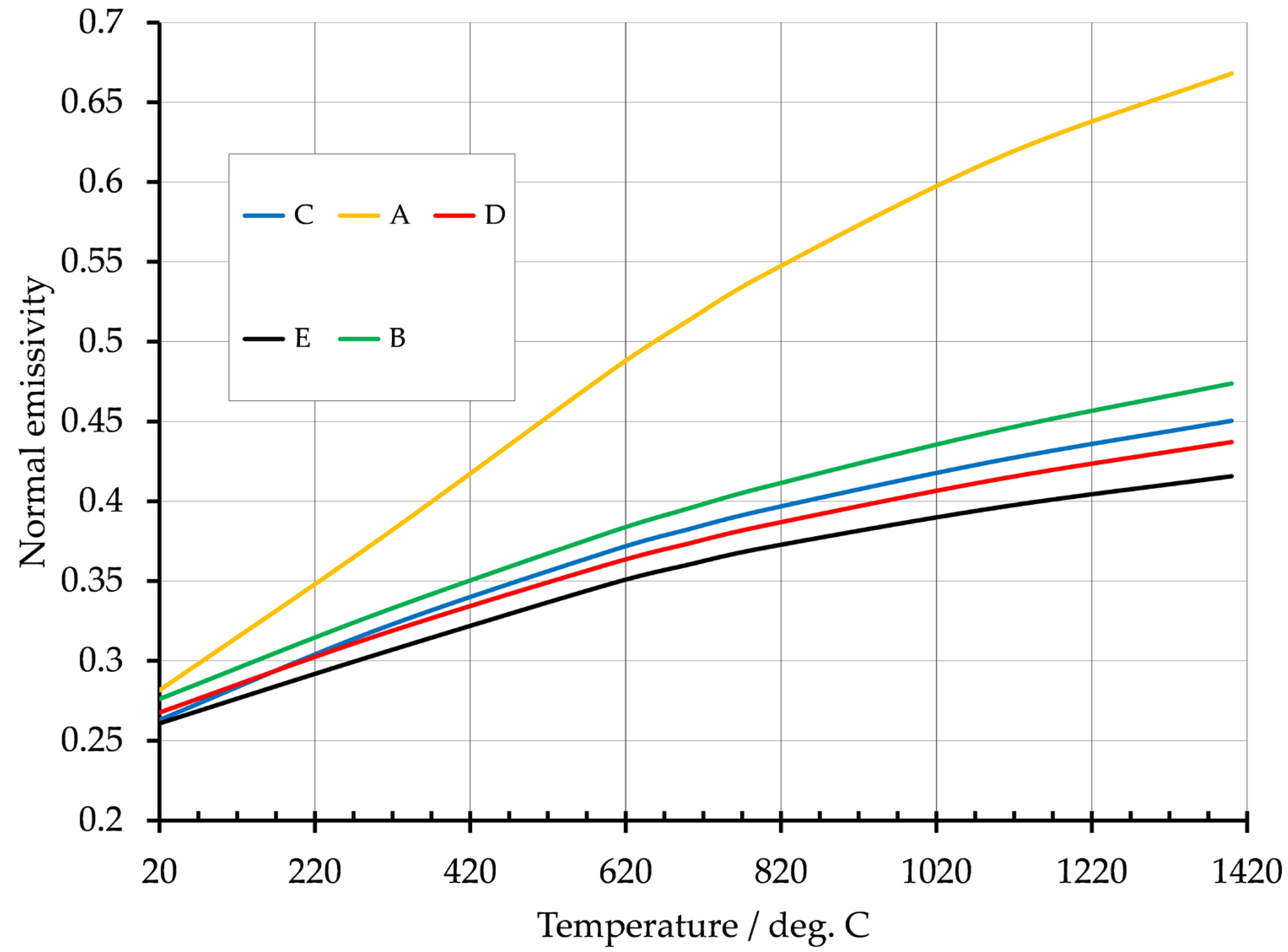
4.1. Titanium Alloy
4.2. Inconel 718, Mild Steel, Aluminum 2319, and NAB
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frazier, W.E. Metal additive manufacturing: A review. J. Mater. Engin. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Williams, S.W.; Martina, F.; Addison, A.C.; Ding, J.; Pardal, G.; Colegrove, P. Wire + Arc additive manufacturing. Mater. Sci. Technol. 2016, 32, 641–647. [Google Scholar] [CrossRef]
- Xu, F.; Dhokia, V.; Colegrove, P.; McAndrew, A.; Williams, S.; Henstridge, A.; Newman, S.T. Realisation of a multi-sensor framework for process monitoring of the wire arc additive manufacturing in producing Ti-6Al-4V parts. Int. J. Comput. Integr. Manuf. 2018, 31, 785–798. [Google Scholar] [CrossRef]
- Wang, C.; Suder, W.; Ding, J.; Williams, S. Wire based plasma arc and laser hybrid additive manufacture of Ti-6Al-4V. J. Mater. Process. Technol. 2021, 293, 117080. [Google Scholar] [CrossRef]
- Ding, J.; Colegrove, P.; Martina, F.; Williams, S.; Wiktorowicz, R.; Palt, M.R. Development of a laminar flow local shielding device for wire+arc additive manufacture. J. Mater. Process. Technol. 2015, 226, 99–105. [Google Scholar] [CrossRef]
- Köhler, M.; Fiebig, S.; Hensel, J.; Dilger, K. Wire and arc additive manufacturing of aluminum components. Metals 2019, 9, 608. [Google Scholar] [CrossRef]
- Seow, C.E.; Coules, H.E.; Wu, G.; Khan, R.H.U.; Xu, X.; Williams, S.W. Wire + Arc Additively Manufactured Inconel 718: Effect of post-deposition heat treatments on microstructure and tensile properties. Mater. Des. 2019, 183, 108157. [Google Scholar] [CrossRef]
- Caballero, A.; Ding, J.; Ganguly, S.; Williams, S. Wire + Arc Additive Manufacture of 17-4 PH stainless steel: Effect of different processing conditions on microstructure, hardness, and tensile strength. J. Mater. Process. Technol. 2019, 268, 54–62. [Google Scholar] [CrossRef]
- WAAMMat, Recapitulation of Mechanical Properties. Available online: https://waammat.com/documents/recap-of-waam-mechanical-properties (accessed on 20 June 2023).
- Lunt, D.; Ho, A.; Davies, A.; Harte, A.; Martina, F.; Quinta da Fonseca, J.; Prangnell, P. The effect of loading direction on strain localization in wire arc additively manufactured Ti-6Al-4V. Mater. Sci. Engin. A 2020, 788, 139608. [Google Scholar] [CrossRef]
- Davis, A.; Kennedy, J.; Ding, J.; Prangnell, P. The effect of processing parameters on rapid-heating β recrystallization in inter-pass deformed Ti-6Al-4V wire-arc additive manufacturing. Mater. Charact. 2020, 163, 110298. [Google Scholar] [CrossRef]
- da Silva, L.J.; Reis, R.P.; Scotti, A. The Potential of IR Pyrometry for Monitoring Interpass Temperature in Wire + Arc Additive Manufacturing. Evol. Mech. Eng. 2019, 3, 1–4. [Google Scholar] [CrossRef]
- Ren, C.G.; Lo, Y.; Tran, H.C.; Lee, M.H. Emissivity calibration method for pyrometer measurement of melting pool temperature in selective laser melting of stainless steel 316L. Int. J. Adv. Manuf. Technol. 2019, 105, 637–649. [Google Scholar] [CrossRef]
- Gulyaev, I.P.; Dolmatov, A.V. Spectral-brightness pyrometry: Radiometric measurements of non-uniform temperature distributions. Int. J. Heat Mass Transf. 2018, 116, 1016–1025. [Google Scholar] [CrossRef]
- Araujo, A. Multi-spectral pyrometry—A review. Meas. Sci. Technol. 2017, 28, 082002. [Google Scholar] [CrossRef]
- Valencia, J.J.; Quested, P.N. Thermophysical Properties. In ASM Handbook, 15: Casting; ASM International: Materials Park, OH, USA, 2008; pp. 468–481. [Google Scholar]
- Teng, S.; Dehgahi, S.; Henein, H.; Wolfe, T.; Qureshi, A. Effect of surface texture, viewing angle, and surface condition on the emissivity of wire arc directed energy deposition manufactured 7075 nano treated aluminium alloy. Int. J. Adv. Manuf. Technol. 2023, 126, 2175–2189. [Google Scholar] [CrossRef]
- Turquais, B.; Sans, J.L.; Davoust, L.; Delacroix, J.; Journeau, C.; Piluso, P.; Chikhi, N. Pyroreflectometry as a technique for the accurate measurement of very high temperatures in molten materials. Rev. Sci. Instrum. 2022, 93, 094901. [Google Scholar] [CrossRef]
- Hagqvist, P.; Sikström, F.; Christiansson, A.K. Emissivity estimation for high temperature radiation pyrometry on Ti-6Al-4V. Measurement 2013, 46, 871–880. [Google Scholar] [CrossRef]
- Shur, B.A.; Peletskii, V.E. The effect of alloying additions on the emissivity of titanium in the neighborhood of polymorphous transformation. High Temp. 2004, 42, 414–420. [Google Scholar] [CrossRef]
- Mohr, M.; Wunderlich, R.; Novakovic, R.; Ricci, E.; Fecht, H.J. Precise Measurements of Thermophysical Properties of Liquid Ti-6Al-4V (Ti64) Alloy On Board the International Space Station. Adv. Eng. Mater. 2020, 22, 2000169. [Google Scholar] [CrossRef]
- Baier, D.; Weckenmann, T.; Wolf, F.; Wimmer, A.; Zaeh, M.F. Underlying Methodology for a Thermal Process Monitoring System for Wire and Arc Additive Manufacturing. J. Manuf. Mater. Process. 2023, 7, 10. [Google Scholar] [CrossRef]
- Keller, B.P.; Nelson, S.E.; Walton, K.L.; Ghosh, T.K.; Tompson, R.V.; Loyalka, S.K. Total hemispherical emissivity of Inconel 718. Nucl. Eng. Des. 2015, 287, 11–18. [Google Scholar] [CrossRef]
- Curry, E.B.; Sahoo, S.; Herrara, C.; Sochnikov, I.; Pamir Alpay, S.; Herbert, R.J.; Willis, B.G.; Qi, J.; Handcock, J.N. Optical response of nickel-based super alloy Inconel-718 for applications in additive manufacturing. J. Appl. Phys. 2020, 127, 245111. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Yu, K.; Liu, Y.; Liu, Y. Emissivity measurement device for manufactured parts based on induction heating. Appl. Therm. Eng. 2025, 279, 127648. [Google Scholar] [CrossRef]
- Jones, J.M.; Mason, P.E.; Williams, A. A compilation of data on the radiant emissivity of some materials at high temperatures. J. Energy Inst. 2019, 92, 523–534. [Google Scholar] [CrossRef]
- Wade, W. Measurements of total hemispherical emissivity of various oxidized metals at high temperature. In National Advisory Committee for Aeronautics; TN 4206; Langley Aeronautical Laboratory: Hampton, VA, USA, 1958. [Google Scholar]
- Touloukian, Y.; DeWitt, D. Thermal radiative properties of metallic elements and alloys. In Thermophysical Properties of Matter—The TPRC Data Series; Defence Technical Information Center: Fort Belvoir, VA, USA, 1970; Volume 7. [Google Scholar]
- Sadiq, H.; Wong, M.B.; Tashan, J.; Al-Mahaidi, R.; Zhao, X.-L. Determination of Steel Emissivity for the Temperature Prediction of Structural Steel Members in Fire. J. Mater. Civ. Eng. 2013, 25, 167–173. [Google Scholar] [CrossRef]
- Dharmendra, C.; Hadadzadeh, A.; Amirkhiz, B.S.; Janaki Ram, G.D.; Mohammadi, M. Microstructural evolution and mechanical behavior of nickel aluminum bronze Cu-9Al-4Fe-4Ni-1Mn fabricated through wire-arc additive manufacturing. Addit. Manuf. 2019, 30, 100872. [Google Scholar] [CrossRef]
- Murray, T.; Thomas, S.; Wu, Y.; Neil, W.; Hutchinson, C. Selective laser melting of nickel aluminum bronze. Addit. Manuf. 2020, 33, 101122. [Google Scholar] [CrossRef]
- Caballero, A.; Ding, J.; Bandari, Y.; Williams, S.W. Oxidation of Ti-6Al-4V during Wire and Arc Additive Manufacture. 3D Print. Addit. Manuf. 2019, 6, 91–98. [Google Scholar] [CrossRef]
- Xu, X.; Ding, J.; Ganguly, S.; Diao, C.; Williams, S.W. Oxide accumulation effects on wire + arc layer-by-layer additive manufacture process. J. Mater. Process. Technol. 2018, 252, 739–750. [Google Scholar] [CrossRef]
- Kauder, L. Spacecraft Thermal Control Coatings References, NASA/TP-2005-212792; NASA Center for Aerospace Information: Hanover, MD, USA, 2005. [Google Scholar]
- Giulietti, N.; Cosoli, G.; Napolitano, R.; Pandarese, G.; Revel, G.M.; Chiariotti, P. Spectral emissivity measurement for high-temperature applications: A systematic review. Acta IMEKO 2025, 14, 1–17. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Del Curto, B.; Pedeferri, M.P. Interference colours of thin oxide layers on titanium. Colour Res. Appl. 2008, 33, 221–228. [Google Scholar] [CrossRef]
- Bauer, W.; Moldenhauer, A.; Rogge, F. Influence of a growing oxide layer on band-emissivities used for optical temperature measurements. In Thermosense XXXI; Proceedings of SPIE; SPIE: Bellingham, WA, USA, 2009; Volume 7299, pp. 1–3. [Google Scholar] [CrossRef]
- Thiessen, R.G.; Bocharova, E.; Mattissen, D.; Sebald, R. Temperature measurement deviation during annealing of multiphase steels. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2010, 41, 857–863. [Google Scholar] [CrossRef]
- Selvi, S.; Vishvaksenan, A.; Rajasekar, E. Cold metal transfer (CMT) technology—An overview. Def. Technol. 2018, 14, 28–44. [Google Scholar] [CrossRef]
- Bento, J.B.; Wang, C.; Ding, J.; Williams, S.W. Process Control Methods in Cold Wire Gas Metal Arc Additive Manufacturing. Metals 2023, 13, 1334. [Google Scholar] [CrossRef]
- Usategui, L.; López-Ferreño, I.; Echániz, T.; Sainz-Menchón, M.; Musi, M.; Clemens, H.; López, G.A. Emissivity measurements conducted on intermetallic γ-TiAl-based alloys for aeronautical applications. J. Mater. Res. Technol. 2023, 27, 3170–3179. [Google Scholar] [CrossRef]
- Hsu, C.P.S. Infrared spectroscopy. In Handbook of Instrumental Techniques for Analytical Chemistry; Settle, F., Ed.; Prentice Hall PTR: Englewood Cliffs, NJ, USA, 1997; pp. 249–277. [Google Scholar]
- Watanabe, M.; Funada, S.; Ohtsuka, M.; Adachi, M.; Fukuyama, H. Density, Normal Spectral Emissivity, Heat Capacity, and Thermal Conductivity of the Ti6Al4V Melt Measured by Electromagnetic Levitation with a Static Magnetic Field. Int. J. Thermophys. 2025, 46, 1–22. [Google Scholar] [CrossRef]
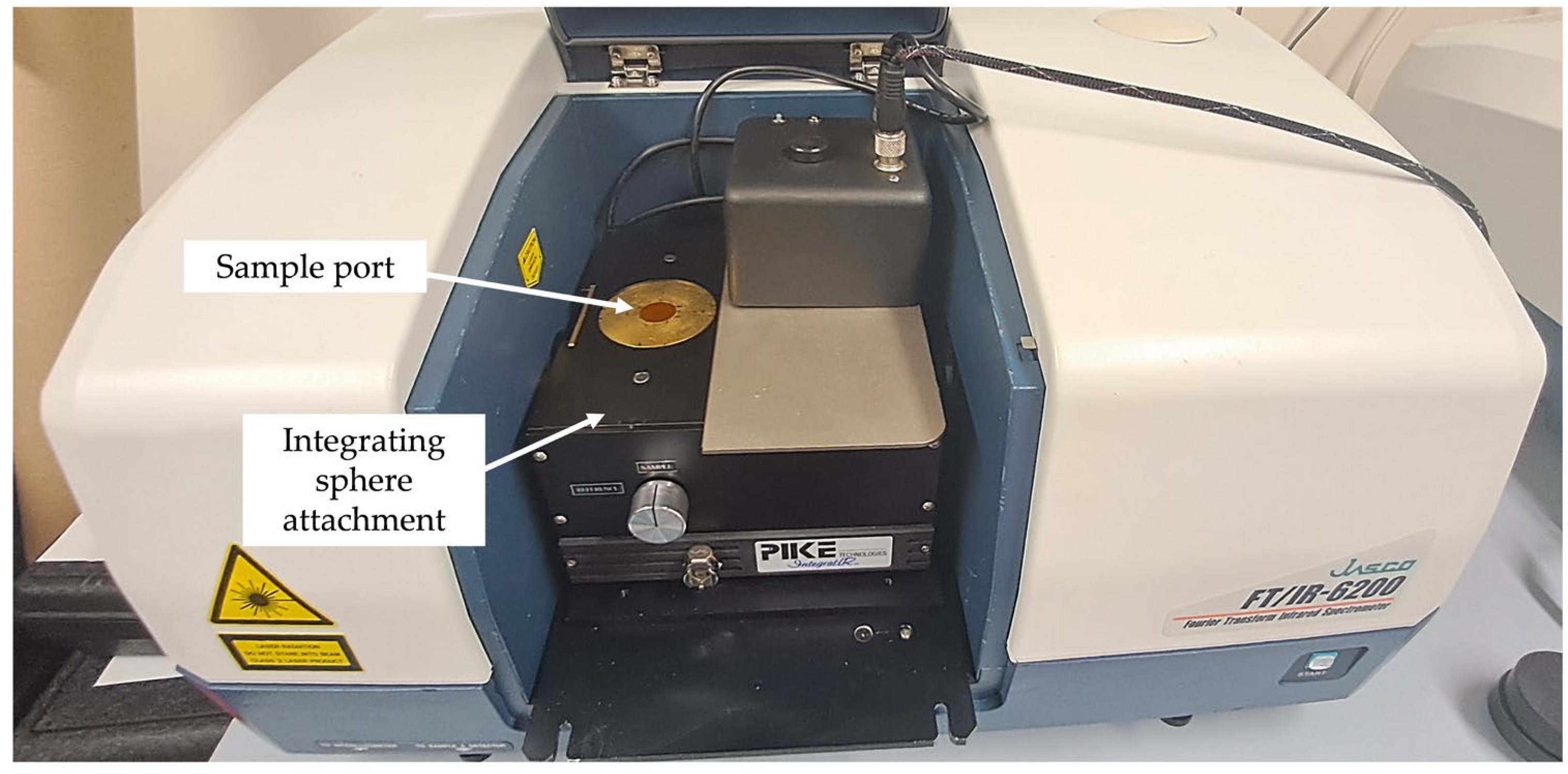


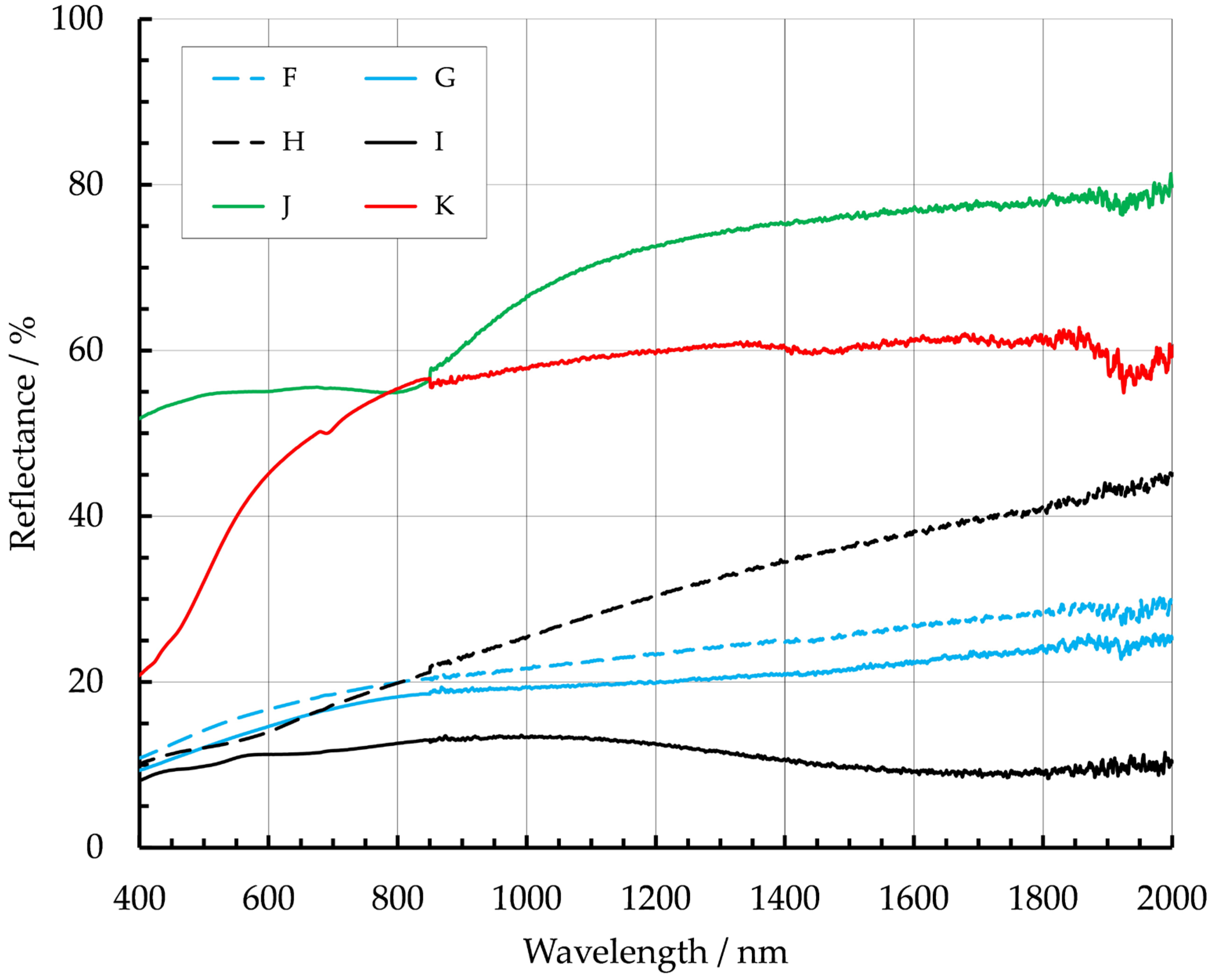
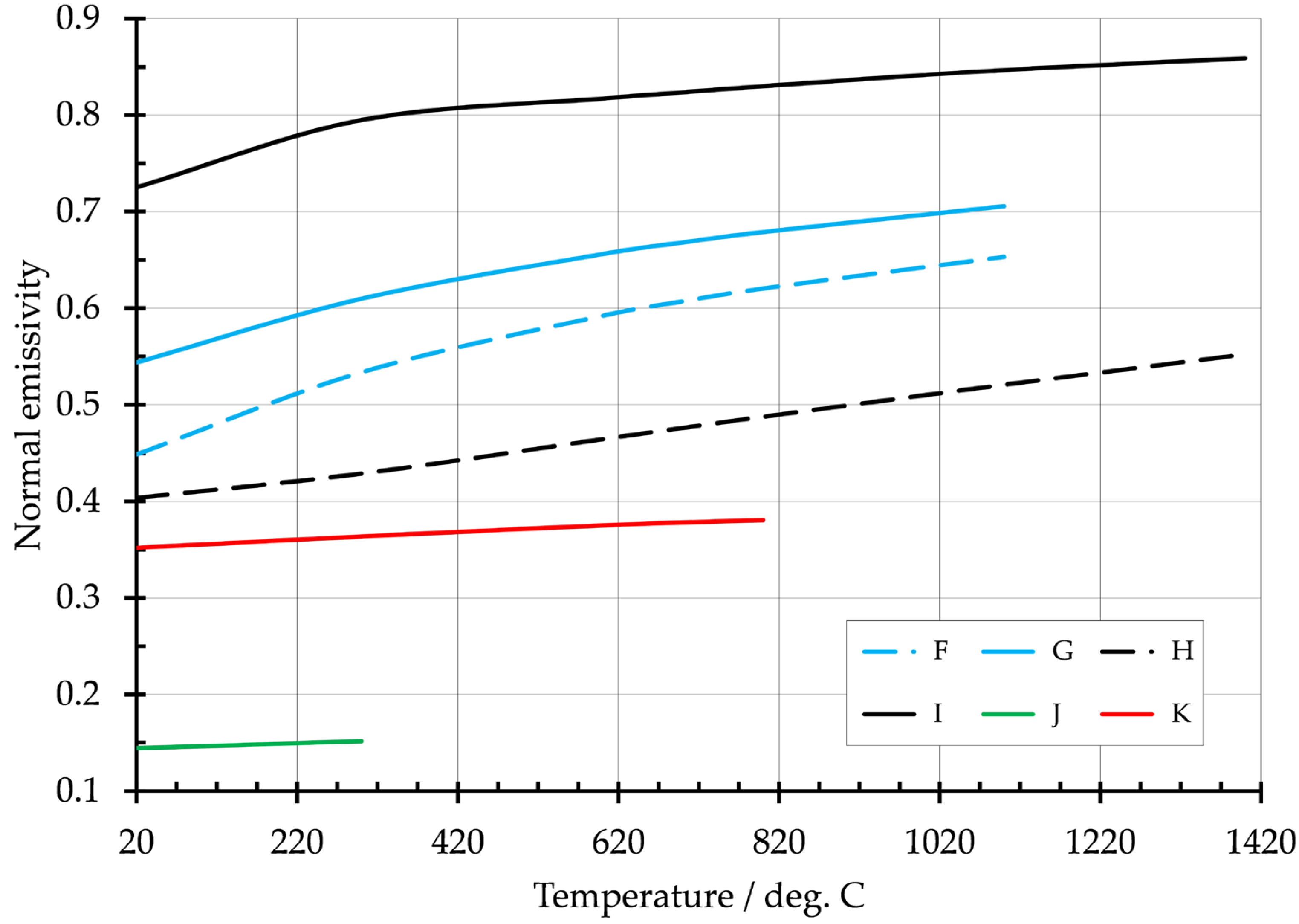
| Metal | Fabrication Process | Reference | Temp. Range/°C | Emissivity |
|---|---|---|---|---|
| Aluminum 2319 | Cold metal transfer DED-Arc | This study | 20–500 | 0.14–0.16 |
| Aluminum | Oxidized sheet | Jones et al. [26] | 200–600 | 0.11–0.19 |
| Inconel 718 | Low O2, DED-Arc | This study | 20–1200 | 0.45–0.66 |
| Inconel 718 | High O2, DED-Arc | This study | 20–1200 | 0.54–0.71 |
| Inconel 718 | Processed sheets | B. Keller et al. [23] | 377–1027 | 0.2–0.7 |
| Inconel 718 | Powder bed fusion, then fine polish | Curry et al. [24] | >1300 | 0.3 (~λ = 1 µm) |
| Inconel 718 | 3D-printed, oxidized component | Li et al. [25] | 700–1000 | 0.55–0.72 |
| Nickel alum. bronze | Cold wire MIG DED-Arc | This study | 20–1000 | 0.35–0.38 |
| Mild steel | Low O2, DED-Arc | This study | 20–1400 | 0.4–0.55 |
| Mild steel | High O2, DED-Arc | This study | 20–1400 | 0.72–0.86 |
| Mild steel | Polish, low oxidation | Wade [27] | 315–427 | 0.3–0.6 |
| Mild Steel | Polish, high oxidation | Touloukian et al. [28] | 316–816 | 0.85–0.945 |
| Carbon steel | Steel rod, low oxidation | Sadiq et al. [29] | 60–600 | 0.28–0.69 |
| Ti-6Al-4V | Low O2, DED-Arc | This study | 20–1400 | 0.26–0.42 |
| Ti-6Al-4V | Medi. O2, DED-Arc | This study | 20–1400 | 0.28–0.47 |
| Ti-6Al-4V | High O2, DED-Arc | This study | 20–1400 | 0.28–0.67 |
| Ti-6Al-4V | Low O2, inductive heating | Hagqvist et al. [19] | 477–1077 | 0.3–0.35 |
| Ti-6Al-4V | Very low O2, polished bars | Shur et al. [20] | 827–1427 | 0.26–0.3 |
| Ti-6Al-4V | Very low O2, heating coil | Mohr et al. [21] | 1327–1487 | 0.3–0.36 |
| Ti-6Al-4V | Medium O2, DED-Arc | Baier et al. [22] | 200 | 0.35 |
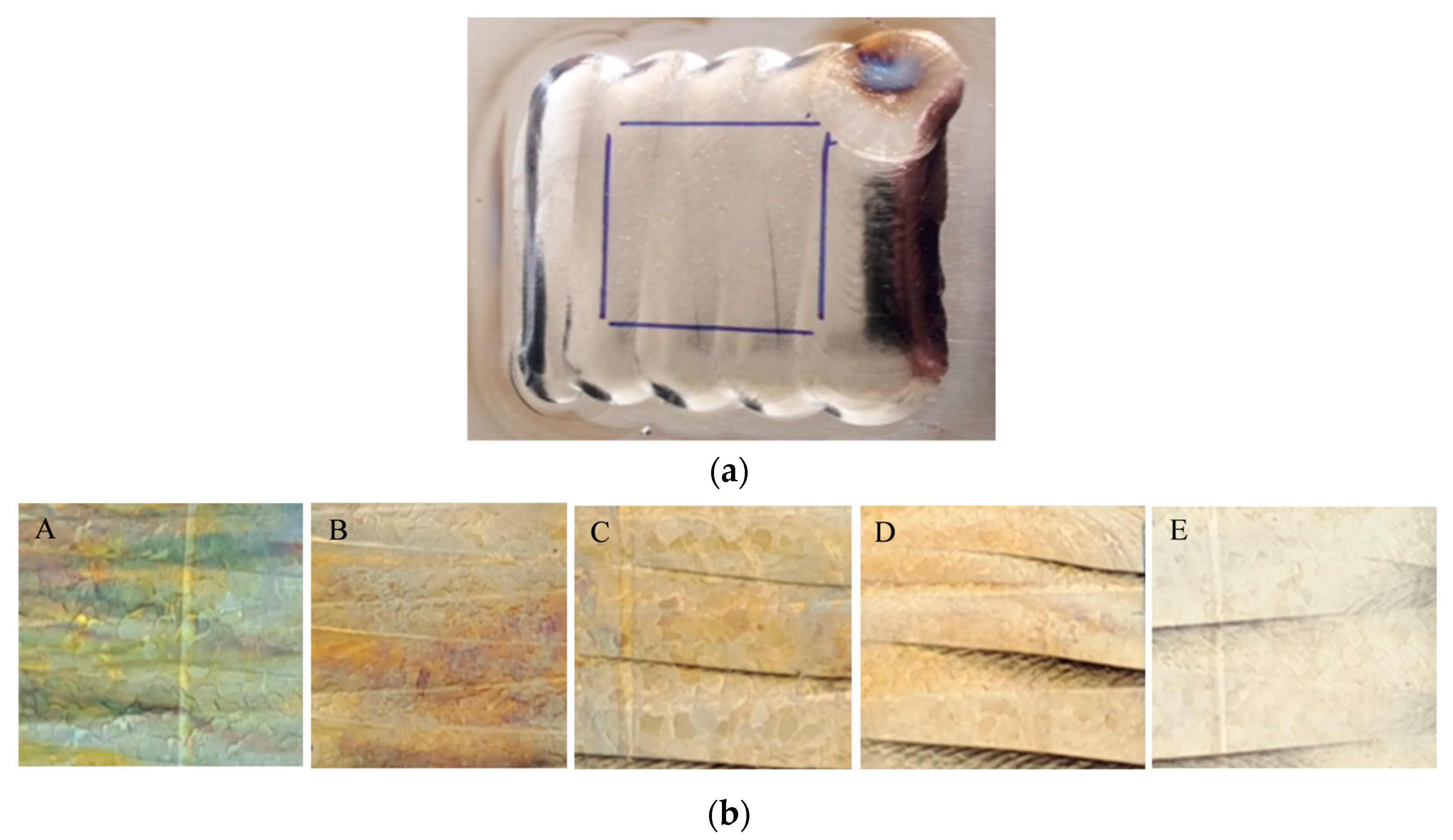
| Sample Ref. | Layer Number, Residual Oxygen Level/ppm. | Visual Appearance |
|---|---|---|
| A | 2 layers, 1850 | Green/blue color |
| B | 2 layers, 85 | Dark straw color |
| C | 2 layers, 12 | Straw color |
| D | 2 layers, 5 | Light straw color |
| E | 3 layers, <5 | Clear, no color |
| Parameter | Ti-6AL-4V | Inconel 718 | Mild Steel | Aluminum 2319 | NAB |
|---|---|---|---|---|---|
| Travel speed, mm/s | 6 | 5 | 4 | 10 | 700 |
| Wire feed speed, m/min. | 1.75 | 1.1 | 1.9 | 7 | 10 and 6 (2 wires) |
| Arc current, A | 190 | 180 | 205 | - | - |
| Argon gas, liter /min | 10 | 10 | 20–70 | 25 | 18 |
| AC plasma | - | - | - | yes | - |
| CW MIG | - | - | - | - | yes |
| Element (%) | Ti-6AL-4V | Inconel 718 | Mild Steel | Aluminum 2319 | NAB |
|---|---|---|---|---|---|
| Mn | - | - | 1.7 | 0.3 | 1.0 |
| Ni | - | balance | - | - | 4.5 |
| Fe | 0.18 | 17.16 | balance | - | 3.5 |
| Al | 6.14 | 0.53 | - | balance | 9.0 |
| Cu | - | - | - | 6.3 | balance |
| Zr | - | - | - | 0.17 | - |
| Ti | balance | 1.03 | - | 0.15 | - |
| C | 0.021 | 0.03 | 0.08 | - | - |
| Si | 0.012 | - | 0.85 | - | - |
| Cr | - | 19.31 | - | - | - |
| Nb | - | 4.88 | - | - | - |
| Mo | - | 3.02 | - | - | - |
| O | 0.15 | - | - | - | - |
| V | 3.94 | - | - | - | - |
| Sample Reference | Residual O2 Atmosphere During Deposition/ppm | Normal Emissivity @ 20 °C |
|---|---|---|
| A | 1850 | 0.28 |
| B | 85 | 0.28 |
| C | 12 | 0.26 |
| D | 5 | 0.27 |
| E | <5 | 0.26 |
| Sample Reference | Metal, (O2 Level)—If Varied | Normal Emissivity @ 20 °C |
|---|---|---|
| F | Inconel 718 (41 ppm O2) | 0.45 |
| G | Inconel 718 (464 ppm O2) | 0.54 |
| H | mild steel (low oxygen) | 0.4 |
| I | mild steel (high oxygen) | 0.72 |
| J | aluminum 2319 | 0.14 |
| K | nickel aluminum bronze | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mullaney, K.; Tatam, R.P. Emissivity Measurements of Metals Used in Wire-Arc-Directed Energy Deposition Processes. Metals 2025, 15, 1078. https://doi.org/10.3390/met15101078
Mullaney K, Tatam RP. Emissivity Measurements of Metals Used in Wire-Arc-Directed Energy Deposition Processes. Metals. 2025; 15(10):1078. https://doi.org/10.3390/met15101078
Chicago/Turabian StyleMullaney, Kevin, and Ralph P. Tatam. 2025. "Emissivity Measurements of Metals Used in Wire-Arc-Directed Energy Deposition Processes" Metals 15, no. 10: 1078. https://doi.org/10.3390/met15101078
APA StyleMullaney, K., & Tatam, R. P. (2025). Emissivity Measurements of Metals Used in Wire-Arc-Directed Energy Deposition Processes. Metals, 15(10), 1078. https://doi.org/10.3390/met15101078






