Enhanced Mechanical and Corrosion Properties of As-Extruded Mg-12Gd-2Zn-0.4Zr Alloy by Nd Additions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Microstructure Characterization
2.3. Mechanical Properties and Corrosion Resistance
2.4. Statistical Analysis
3. Results
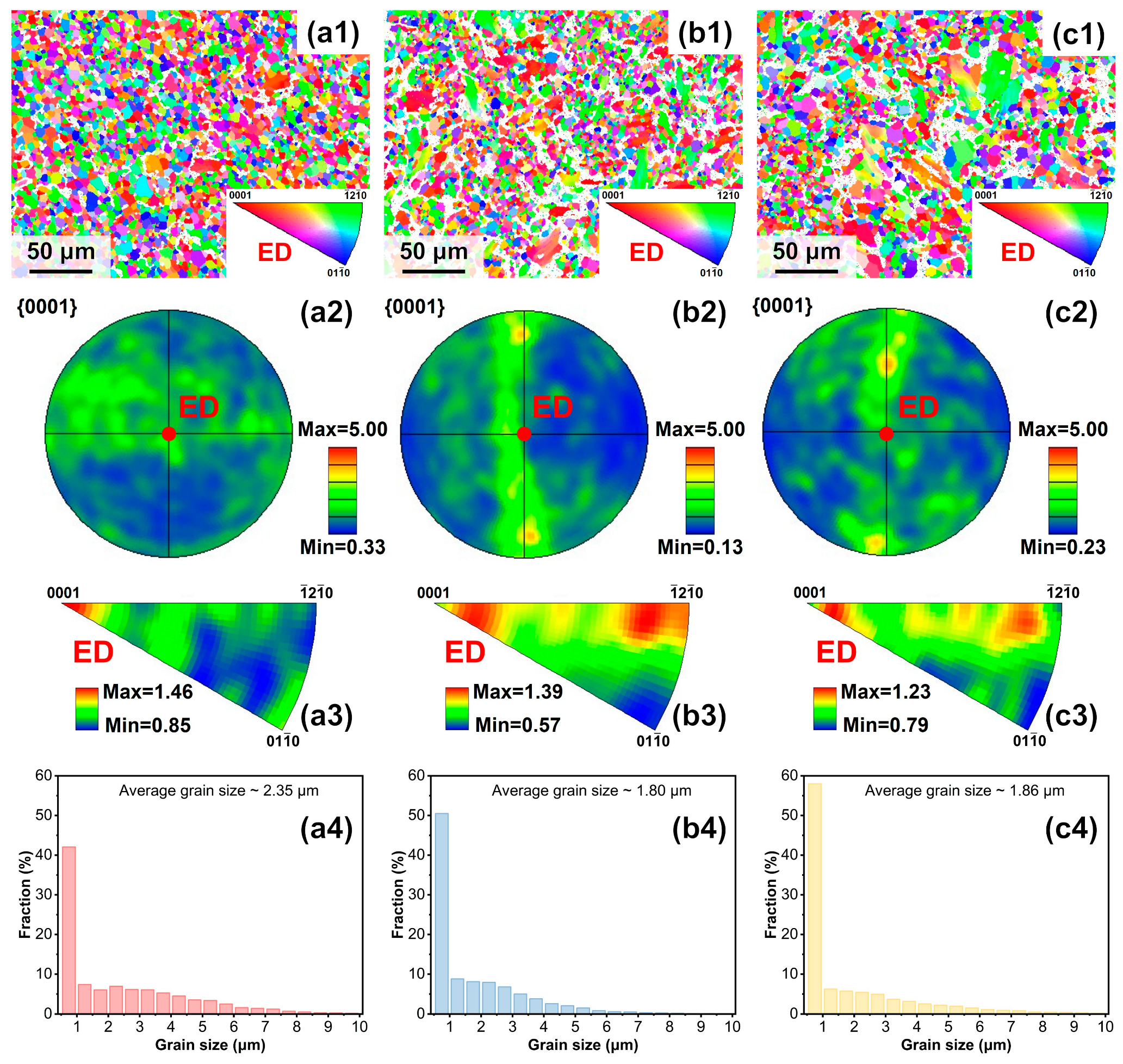
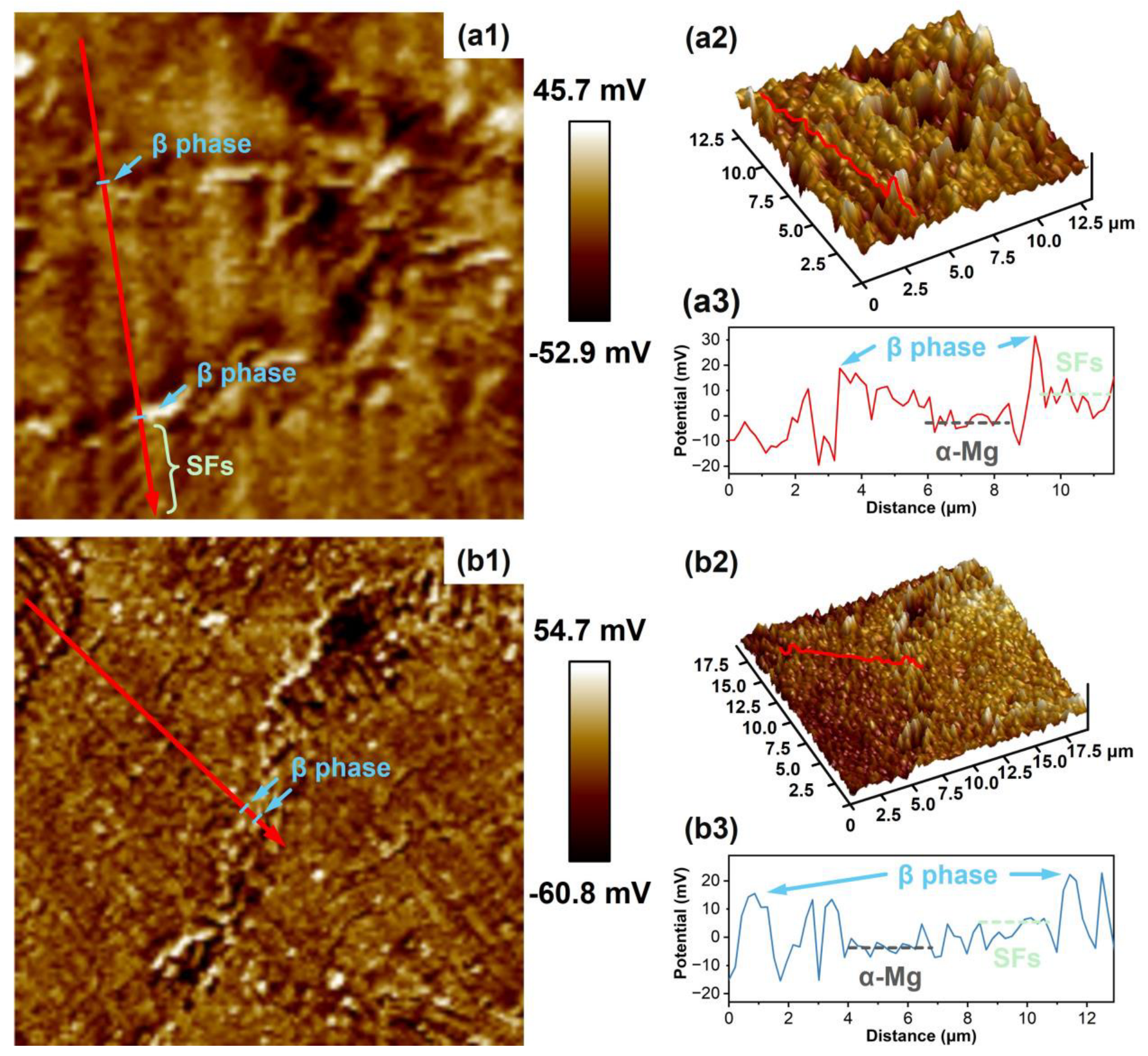
3.1. Microstructures and Phases
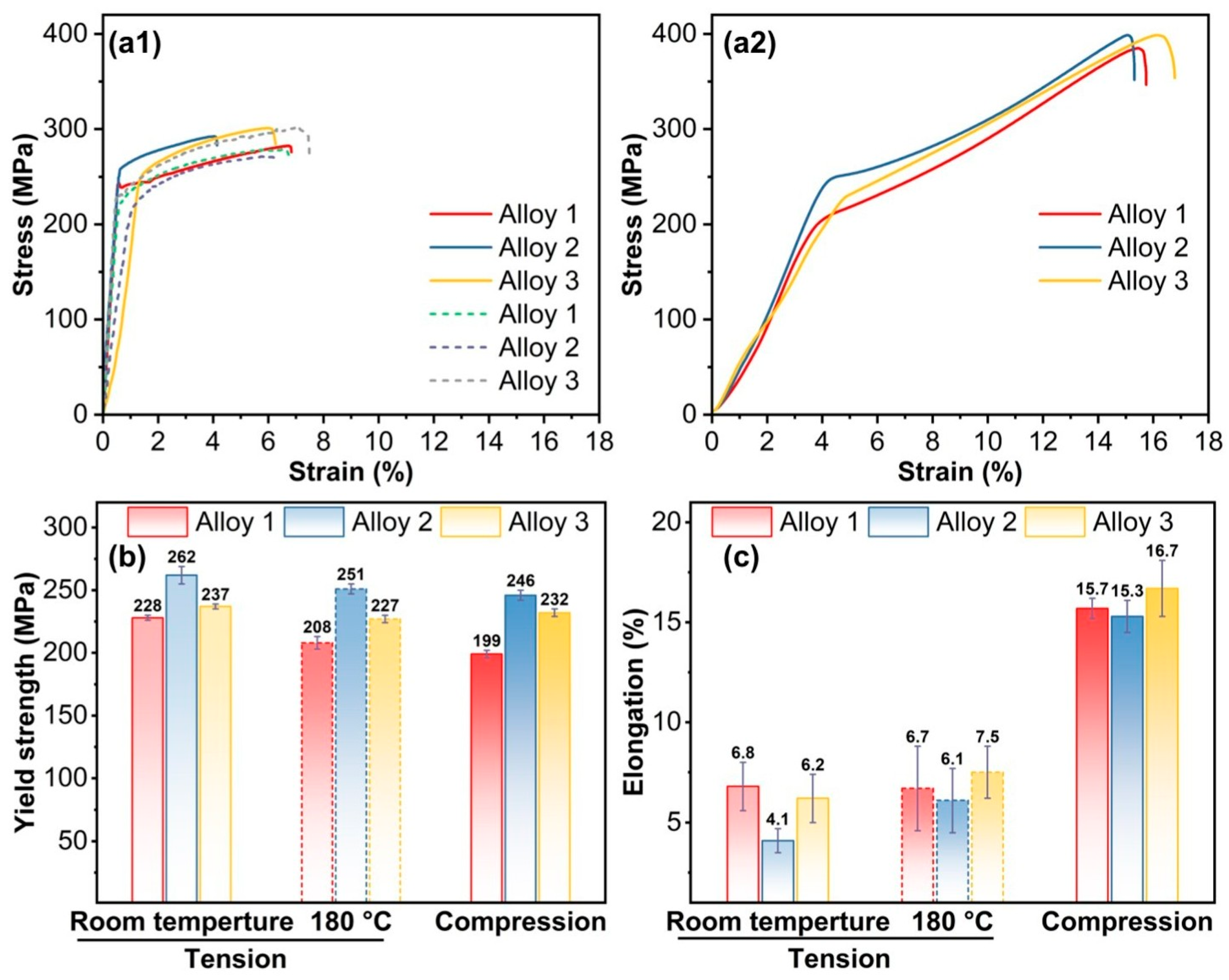
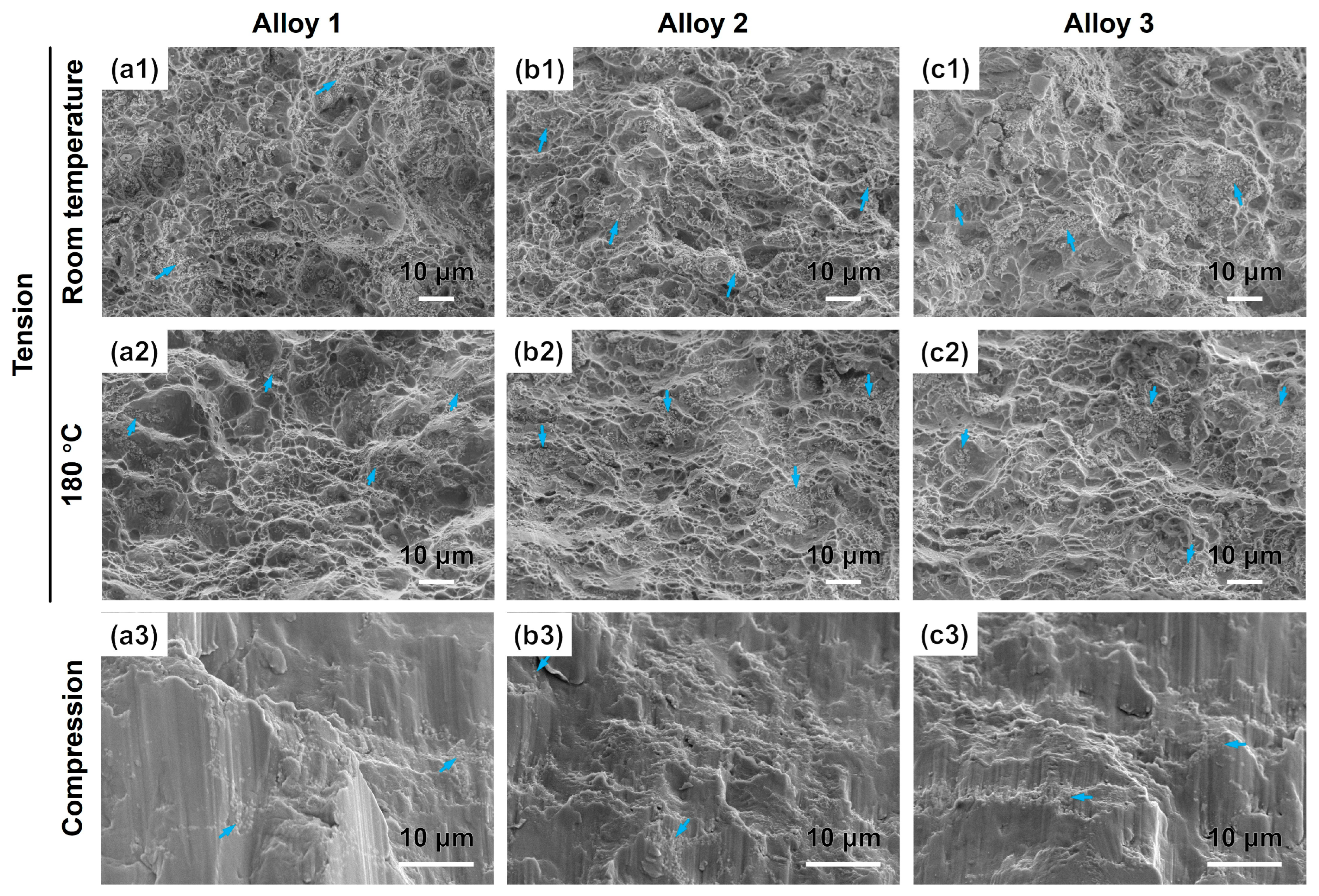
3.2. Mechanical Properties
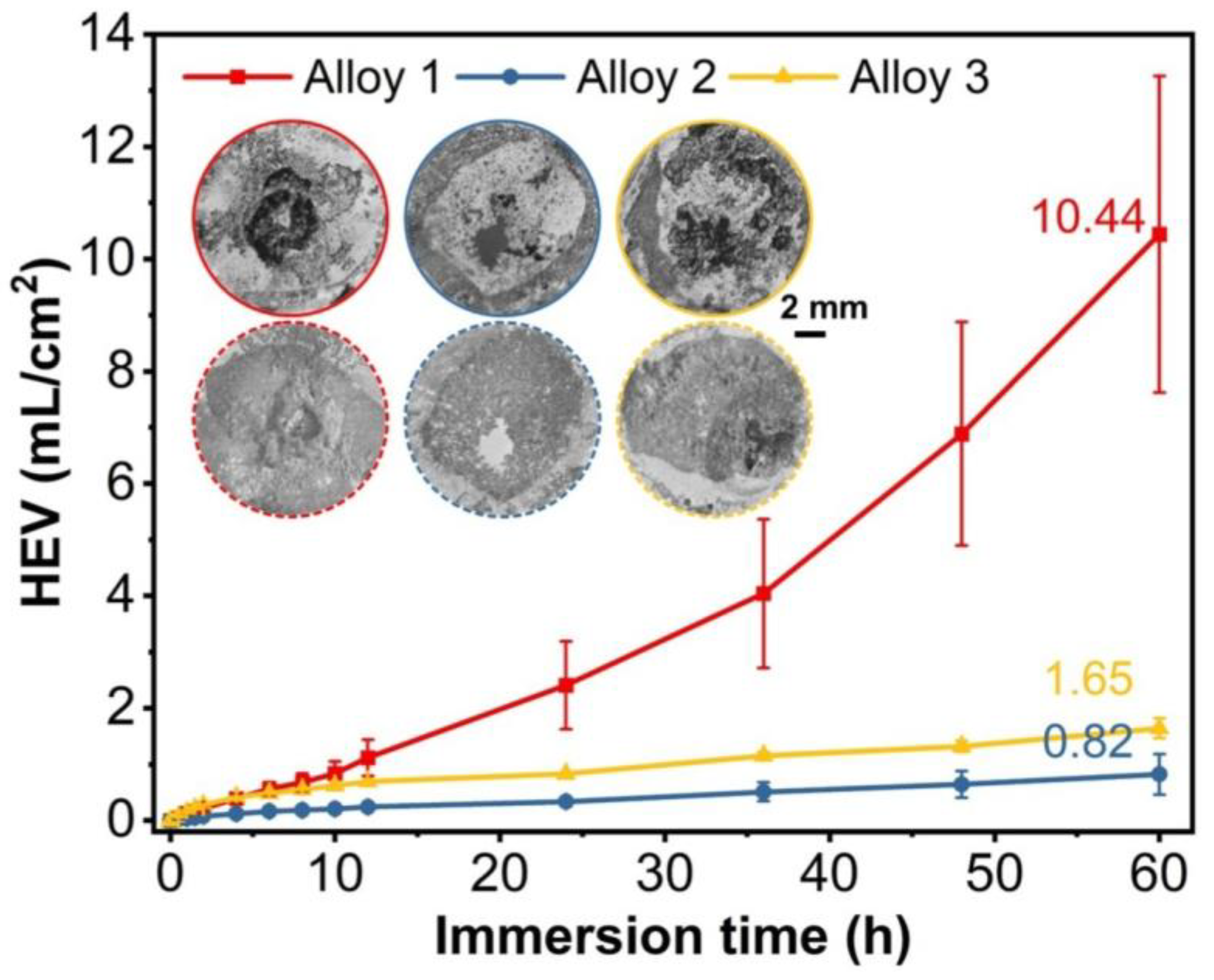
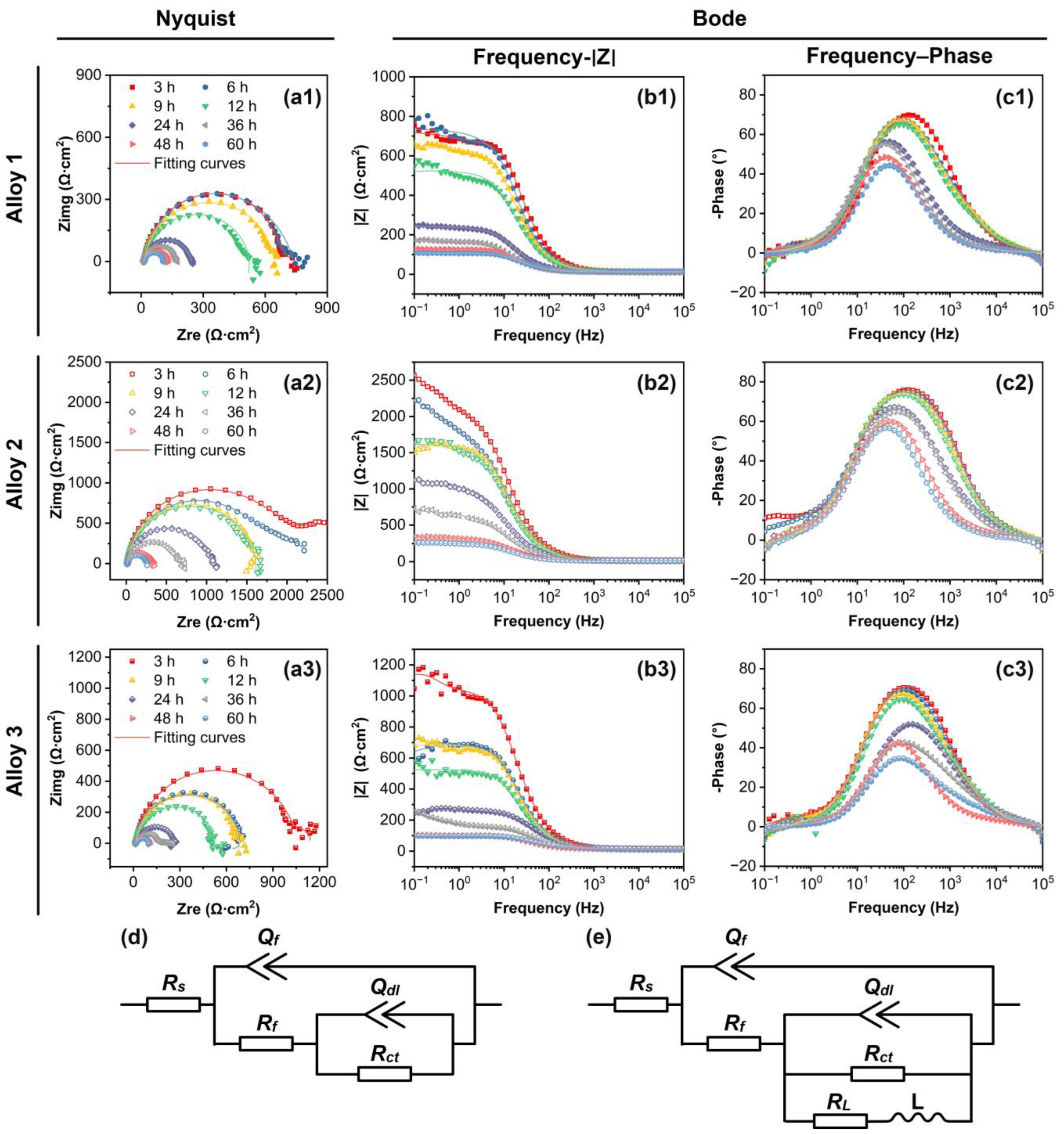
3.3. Corrosion Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Xiong, X.M.; Chen, J.; Peng, X.D.; Chen, D.L.; Pan, F.S. Research advances of magnesium and magnesium alloys worldwide in 2022. J. Magnes. Alloys 2023, 11, 2611–2654. [Google Scholar] [CrossRef]
- Zhan, L.; Kong, C.H.; Zhao, C.Y.; Cui, X.H.; Zhang, L.H.; Song, Y.; Lu, Y.; Xia, L.; Ma, K.; Yang, H.Y.; et al. Recent advances on magnesium alloys for automotive cabin components: Materials, applications, and challenges. J. Mater. Res. Technol. 2025, 36, 9924–9961. [Google Scholar] [CrossRef]
- Jing, C.H.; Sheng, L.Y.; Xu, J.H.; Xu, Z.F.; Wu, D.; Jiao, J.K. Improved CFRTP/AZ31B alloy hybrid joint by surface micro-texture and laser rotational welding technique. Mater. Des. 2025, 255, 114155. [Google Scholar] [CrossRef]
- Dai, J.W.; Dong, Q.S.; Nie, Y.J.; Jia, Y.Q.; Chu, C.L.; Zhang, X.B. Insight into the role of Y addition in the microstructures, mechanical and corrosion properties of as-cast Mg-Gd-Y-Zn-Ca-Zr alloys. Mater. Des. 2022, 221, 110980. [Google Scholar] [CrossRef]
- Behera, M.; Shabadi, R.; Gruescu, C. Engineering Corrosion Resistance in Magnesium Alloys for Biomedical Applications: A Synergy of Zn/Ca Atomic Ratio and Texture-Based Approach. Metals 2024, 14, 1002. [Google Scholar] [CrossRef]
- Zhang, Y.X. Regulating the microstructures and creep behaviors of an LPSO-containing Mg alloy via heat treatment. Mater. Sci. Eng. A 2024, 914, 147153. [Google Scholar] [CrossRef]
- Kan, Z.Y.; Yang, G.Y.; Xiao, L.; Wang, C.H.; Qin, H.; Wang, B.S.; Jie, W.Q. Asymmetric tension-compression mechanical behaviors of extruded Mg-4.58Zn-2.6Gd-0.18Zr alloy with double-peak fiber texture. J. Alloys Compd. 2024, 989, 174291. [Google Scholar] [CrossRef]
- Anyanwu, I.A.; Kamado, S.; Kojima, Y. Aging characteristics and high temperature tensile properties of Mg–Gd–Y–Zr alloys. Mater. Trans. 2001, 42, 1206–1211. [Google Scholar]
- Ouyang, S.X.; Yang, G.Y.; Qin, H.; Wang, C.H.; Luo, S.F.; Jie, W.Q. Effect of the precipitation state on high temperature tensile and creep behaviors of Mg-15Gd alloy. J. Magnes. Alloys 2022, 10, 3459–3469. [Google Scholar] [CrossRef]
- Liu, X.Q.; Qiao, X.G.; Zhang, X.K.; Zhang, D.D.; Xiao, L.; Zhong, W.J.; Zhu, X.R.; Lian, J.C.; Zheng, M.Y. Achieving low tension-compression yield asymmetry and ultrahigh strength in Mg–1Al–1Ca–0.2Mn (wt%) alloy via sub-micron grain refinement. J. Mater. Res. Technol. 2024, 28, 2235–2246. [Google Scholar] [CrossRef]
- Li, J.L.; Chen, H.C.; Wu, D.; Chen, R.S.; Song, J.; Yi, X. Strain rate-dependent tension-compression asymmetry in cast Mg-Gd-Y alloy: Insights into slip and twinning mechanisms. J. Mater. Sci. Technol. 2025, 219, 134–146. [Google Scholar] [CrossRef]
- Xie, D.S.; Pan, H.C.; Pan, Z.; Zhang, D.D.; Tang, W.N.; Yang, C.B.; Xie, H.B.; Ren, Y.P.; Qin, G.W. Achieving high heat-resistant property in dilute Mg-0.2Ce wrought alloy by retarding recrystallization process. J. Alloy. Compd. 2023, 958, 170410. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Hong, Y.; Li, H.Y.; Zhang, D.P. Creep behavior and dynamic precipitation of highly heat-resistant Mg alloy with low rare-earth content. J. Alloy. Compd. 2024, 997, 174960. [Google Scholar] [CrossRef]
- Qi, M.F.; Wei, L.Y.; Xu, Y.Z.; Wang, J.; Liu, A.S.; Hao, B.; Wang, J.C. Effect of trace yttrium on the microstructure, mechanical property and corrosion behavior of homogenized Mg-2Zn-0.1Mn-0.3Ca-xY biological magnesium alloy. Int. J. Min. Met. Mater. 2022, 29, 1746–1754. [Google Scholar] [CrossRef]
- Chang, L.L.; Guo, J.; Su, X.J. Effect of Y on microstructure evolution and mechanical properties of Mg−4Li−3Al alloys. Trans. Nonferr. Met. Soc. China 2021, 31, 3691–3702. [Google Scholar] [CrossRef]
- Su, N.; Wu, Q.Y.; Ding, C.Y.; Wu, Y.J.; Peng, L.M. Effect of Y and Gd solutes on grain refinement of the as-extruded Mg-Gd(-Y)-Zn-Mn alloys. J. Alloy. Compd. 2023, 968, 171804. [Google Scholar] [CrossRef]
- Dong, N.N.; Wang, J.H.; Ma, H.B.; Jin, P.P. Effects of Nd content on thermal expansion behavior of Mg-Nd alloys. Mater. Today Commun. 2021, 29, 102894. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.R.; Guo, E.Y.; Wang, X.J.; Kang, H.J.; Chen, Z.N.; Wang, T.M. The effect of Gd on the microstructure evolution and mechanical properties of Mg-4Zn-0.6Ca alloy. Mater. Sci. Eng. A 2023, 868, 144756. [Google Scholar] [CrossRef]
- Nie, Y.J.; Dai, J.W.; Li, X.; Zhang, X.B. Recent developments on corrosion behaviors of Mg alloys with stacking fault or long period stacking ordered structures. J. Magnes. Alloys 2021, 9, 1123–1146. [Google Scholar] [CrossRef]
- Dong, Q.S.; Jiang, J.H.; Zhang, J.H.; Hu, Z.; Zhang, X.B. Clarifying stress corrosion cracking behavior of biomedical Mg-Gd-Zn-Zr alloy. J. Magnes. Alloys 2025, 13, 3450–3465. [Google Scholar] [CrossRef]
- Dai, C.N.; Pei, S.L.; Ma, K.; Wang, Y.; Wang, D.Q.; Wang, J.X.; Ma, J.F. Rapid corrosion rates and high mechanical properties of as-extruded Mg–Er–Ni alloys by introducing LPSO and γʹ phases. J. Mater. Res. Technol. 2023, 24, 6246–6263. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, X.; Chen, A.Q.; Liu, B.S.; Zhang, H.; Li, S.S.; Qin, G.W. Deformation behavior and strengthening mechanism of a gradient nanostructured WE43 Mg alloy. J. Magnes. Alloys, 2025; online. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.X.; Li, M.; Tang, S.X.; Huang, Y.C.; Liu, Y.; Huang, C.Q. Achieving enhanced mechanical properties of extruded Mg-Gd-Y-Zn-Zr alloy by regulating the initial LPSO phases. Mater. Sci. Eng. A 2024, 917, 147411. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, F.L.; Wu, Y.J.; Peng, L.M.; Li, L.; He, C.; Chen, Q.; Liu, Y.J.; Wang, Q.Y. Outstanding fatigue performance of Mg-Gd-Zn-Zr alloy enriched with SFs rather than LPSO Structure. J. Magnes. Alloys 2025, 13, 90–100. [Google Scholar] [CrossRef]
- Koji, H.; Xuan, L.Z.; Michiaki, Y.; Yoshihito, K.; Takayoshi, N. Strengthening mechanisms acting in extruded Mg–based long–period stacking ordered (LPSO)–phase alloys. Acta Mater. 2019, 163, 226–239. [Google Scholar]
- Nie, Z.; Wang, F.L.; Li, J.Y.; Liu, C.L.; Zhao, C.Y.; Sanandiya, S.C.; Calvat, M.; Dhruv Anjaria, D.; Zeng, J.; Dong, S.; et al. Strain localization induced by closely spaced lamellae structure in a Mg alloy containing long period stacking ordered structure. Int. J. Plast. 2025, 193, 104423. [Google Scholar] [CrossRef]
- Xie, J.S.; Zhang, J.H.; Zhang, Z.; Yang, Q.; Guan, K.; He, Y.Y.; Wang, R.; Zhang, H.; Qiu, X.; Wu, R.Z. New insights on the different corrosion mechanisms of Mg alloys with solute-enriched stacking faults or long period stacking ordered phase. Corros. Sci. 2022, 198, 110163. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, F.L.; Zeng, J.; Zhou, H.T.; Wang, F.H.; Dong, S.; Jin, L.; Dong, J. Nd microalloying significantly enhances precipitation strengthening of Mg-Gd-Y-Zn-Zr alloy. J. Alloys Compd. 2025, 1025, 180324. [Google Scholar] [CrossRef]
- Bao, R.R.; Zhang, J.H.; He, Y.Y.; Li, Z.H.; Liu, S.J.; Dong, H.; Yang, Q.; Zhang, X.B.; Qiu, X. Exploring low-alloyed as-extruded Mg alloy with high strength and high corrosion resistance. J. Magnes. Alloys, 2025; online. [Google Scholar] [CrossRef]
- Gao, S.P.; Lyu, S.Y.; Zhao, Q.; Chen, M.F. Effect of Extrusion Temperature on the Microstructure and Properties of Biomedical Mg-1Zn-0.4Ca-1MgO Composite. Metals 2025, 15, 337. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Huang, W.Y.; Liu, Y.; Zhao, J.G.; Ma, Q.; Ren, Z.Z.; Yang, X.Y.; Wu, Y.Z.; Wang, L.J.; Zhou, S.; et al. Influence of different extrusion temperatures on microstructure and mechanical properties of Mg-Bi-Zn-Ca alloy. Vacuum 2025, 238, 114297. [Google Scholar] [CrossRef]
- Nie, Q.D.; Liu, J.Z.; Ge, Z.B.; Wang, Y.Q.; Zou, Y.; Wang, S.Z. High-strength Mg-Gd-Y-Zn-Zr alloy with large size produced by differential temperature and small extrusion ratio process. J. Alloy. Compd. 2025, 1036, 181790. [Google Scholar] [CrossRef]
- Kang, X.K.; Nie, K.B.; Deng, K.K.; Guo, Y.C. Effect of extrusion parameters on microstructure, texture and mechanical properties of Mg-1.38Zn-0.17Y-0.12Ca (at. %) alloy. Mater. Charact. 2019, 151, 137–145. [Google Scholar] [CrossRef]
- Lu, X.Z.; Chen, Z.J.; Zou, X.J.; Zhang, J.; Tu, Y.; Zhou, X.J.; Chen, X.M.; Lai, C.P.; Chan, L.; Zeng, G. Synergistic Improvement of Mechanical and Corrosion Properties of Mg-9.1Y-1.8Zn Alloys by Hot Extrusion. Met. Mater. Int. 2024, 30, 3141–3154. [Google Scholar] [CrossRef]
- Liu, Y.H.; Liu, B.Y.; Li, B.; Wang, J.H.; Li, H.X.; Ding, N.; Zhang, J.S. The microstructure and corrosion properties of as-extruded Mg-Gd-Y-Zn-Cu-Ni alloy by altering the preheat treatment. J. Mater. Res. Technol. 2025, 37, 4925–4935. [Google Scholar] [CrossRef]
- Hong, L.X.; Wang, R.X.; Zhang, X.B. Effects of Nd on microstructure and mechanical properties of as-cast Mg-12Gd-2Zn-xNd-0.4Zr alloys with stacking faults. Int. J. Min. Met. Mater. 2022, 29, 1570–1577. [Google Scholar] [CrossRef]
- Hong, L.X.; Wang, R.X.; Zhang, X.B. The Role of Nd in Corrosion Properties of Mg-12Gd-2Zn-0.4Zr Alloys. J. Mater. Eng. Perform. 2021, 30, 6000–6008. [Google Scholar] [CrossRef]
- Panchal, M.; Ravi, K.R.; Kaushik, L.; Khatirkar, R.; Choi, S.; Singh, J. Texture Control Techniques for Improving Room Temperature Formability of Mg Alloys including Pre-twinning: A Review. J. Magnes. Alloys 2023, 29, 3471–3489. [Google Scholar] [CrossRef]
- Xu, Z.H.; Zhang, J.H.; He, Y.Y.; Yang, Q.; Liu, S.J.; Zhao, T.F.; Dong, H.; Wu, R.Z.; Zhang, X.B.; Qiu, X. Revealing anti-corrosion mechanism of low-alloyed Mg-Gd-Zn-Zr alloy with ultra-low corrosion rate. Corros. Sci. 2025, 251, 112931. [Google Scholar] [CrossRef]
- Liu, K.; Liu, J.X.; Li, S.B.; Wang, Z.H.; Du, W.B.; Wang, Q.F. Effects of secondary phases on texture and mechanical properties of as-extruded Mg–Zn–Er alloys. Trans. Nonferr. Met. Soc. China 2018, 28, 890–895. [Google Scholar] [CrossRef]
- Yang, Q.; Lv, S.H.; Guan, K.; Xie, Z.F.; Qiu, X. Extra-conventional strengthening mechanisms in non-recrystallized grains of an extruded Mg-Gd-Zr alloy. J. Magnes. Alloys 2025, 12, 4561–4573. [Google Scholar] [CrossRef]
- Li, S.C.; Wang, Z.; Zhao, X.; Wang, X.D.; Yu, J.M. Study on the plasticity improvement mechanism and grain refinement of AZ80 Mg alloy under cryogenic multi-directional forging. J. Mater. Res. Technol. 2024, 33, 384–397. [Google Scholar] [CrossRef]
- Medina, J.; Garces, G.; Pérez, P.; Stark, A.; Schell, N.; Adeva, P. High temperature mechanical behaviour of Mg–6Zn–1Y alloy with 1 wt.% calcium addition: Reinforcing effect due to I-(Mg3Zn6Y1) and Mg6Zn3Ca2 phases. J. Magnes. Alloys 2020, 8, 1047–1060. [Google Scholar] [CrossRef]
- Leng, Z.; Zhang, J.H.; Zhang, M.L.; Liu, X.H.; Zhan, H.B.; Wu, R.Z. Microstructure and high mechanical properties of Mg–9RY–4Zn (RY: Y-rich misch metal) alloy with long period stacking ordered phase. Mater. Sci. Eng. A 2012, 540, 38–45. [Google Scholar] [CrossRef]
- Su, N.; Wu, Y.J.; Deng, Q.C.; Chang, Z.Y.; Wu, Q.Y.; Xue, Y.T.; Yang, K.; Chen, Q.; Peng, L.M. Synergic effects of Gd and Y contents on the age-hardening response and elevated-temperature mechanical properties of extruded Mg–Gd(-Y)-Zn-Mn alloys. Mater. Sci. Eng. A 2021, 810, 141019. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, J.X.; Yang, C.M.; Chen, Y.X.; Cao, S.C.; Zhao, Z.X.; Li, X.L.; Lu, C. Mechanical Properties and Deformation Mechanisms of Mg-Gd-Y-Zr Alloy at Cryogenic and Elevated Temperatures. J. Mater. Eng. Perform. 2016, 26, 590–600. [Google Scholar] [CrossRef]
- Li, X.Y.; Xia, W.J.; Chen, J.H.; Yan, H.G.; Li, Z.Z.; Su, B.; Song, M. Dynamic recrystallization, texture and mechanical properties of high Mg content Al–Mg alloy deformed by high strain rate rolling. Trans. Nonferrous Met. Soc. China 2021, 31, 2885–2898. [Google Scholar] [CrossRef]
- Li, B.; Wu, J.; Teng, B.G. Influences of the Texture Characteristic and Interdendritic LPSO Phase Distribution on the Tensile Properties of Mg–Gd–Y–Zn–Zr Sheets Through Hot Rolling. Acta Metall. Sin. (Engl. Lett.) 2021, 34, 1051–1064. [Google Scholar] [CrossRef]
- Papanastasiou, P.; Durban, D. Singular crack-tip plastic fields in Tresca and Mohr-Coulomb solids. Int. J. Solids Struct. 2018, 136–137, 250–258. [Google Scholar] [CrossRef]
- Dong, Q.S.; Dai, J.W.; Qian, K.; Liu, H.; Zhou, X.X.; Yao, Q.Q.; Lu, M.M.; Chu, C.L.; Xue, F.; Bai, J. Dual self-healing inorganic-organic hybrid coating on biomedical Mg. Corros. Sci. 2022, 200, 110230. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, B.C.; Li, M.X.; Wang, H.Y.; Ma, Y.L. Effect of Microstructures and Textures on Different Surfaces on Corrosion Behavior of an as-Extruded ATZ411 Magnesium Alloy Sheet. Acta Metall. Sin. (Engl. Lett.) 2021, 34, 1029–1041. [Google Scholar] [CrossRef]
- Djebari, K.; Türen, Y.; Elen, L.; Ahlatçı, H. A Comparative Study of As-Cast Alloys of ZW21 with Varied Element Additions, Investigating Dry and Corrosive Wear in Body Fluid. JOM 2025, 77, 4344–4362. [Google Scholar] [CrossRef]
- Yi, S.Y.; Zhao, S.C.; Feng, Y.C.; Wang, L.; Guo, E.J.; Sun, Q.Y. Effect of heat treatment on microstructure, mechanical properties and corrosion behavior of Mg–Sm–Zn–Zr–xNd alloys. J. Mater. Res. Technol. 2025, 36, 5341–5354. [Google Scholar] [CrossRef]
- Zhang, X.B.; Dai, J.W.; Zhang, R.F.; Ba, Z.X.; Birbilis, N. Corrosion behavior of Mg–3Gd–1Zn–0.4Zr alloy with and without stacking faults. J. Magnes. Alloys 2019, 7, 240–248. [Google Scholar] [CrossRef]
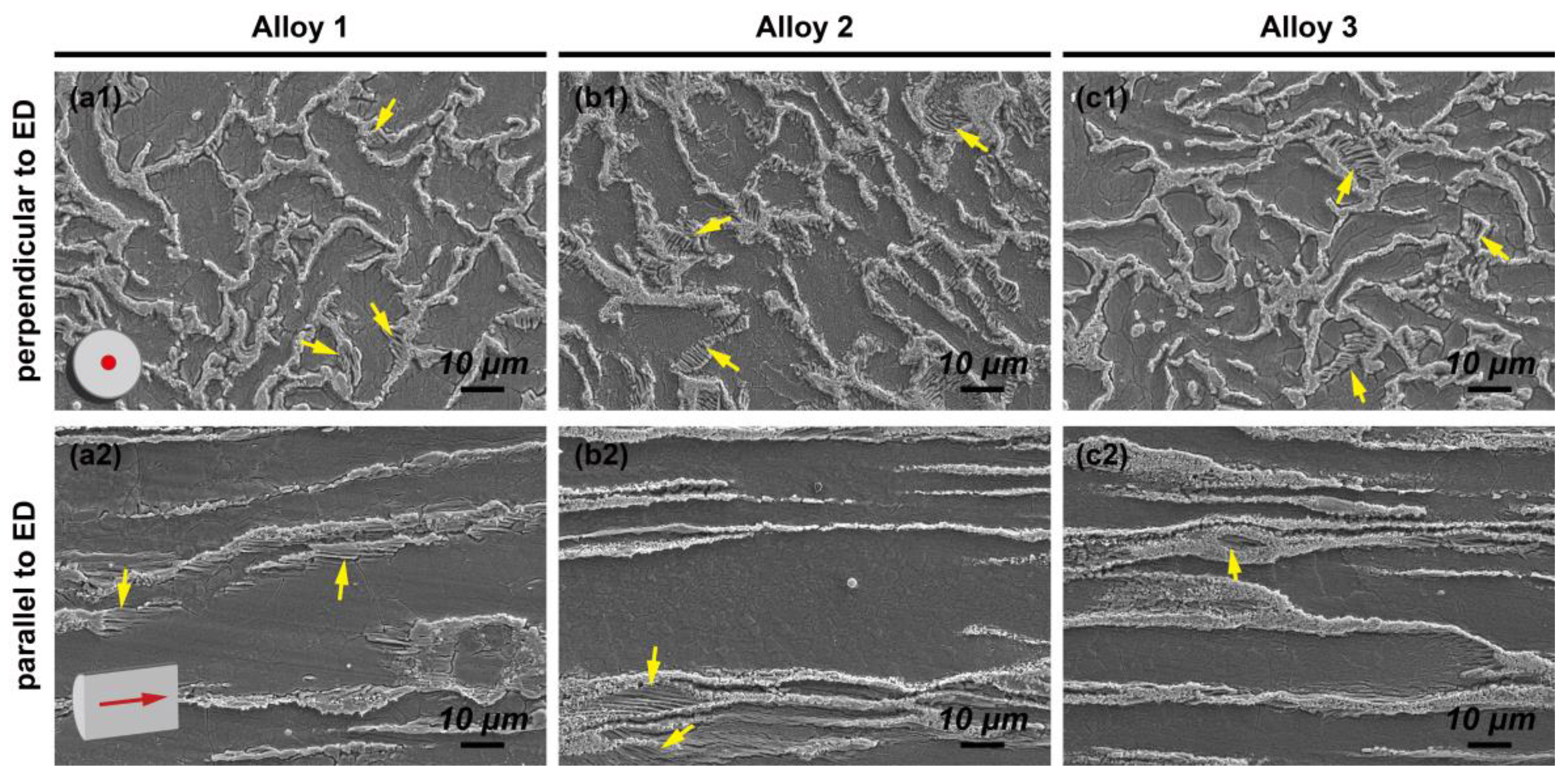
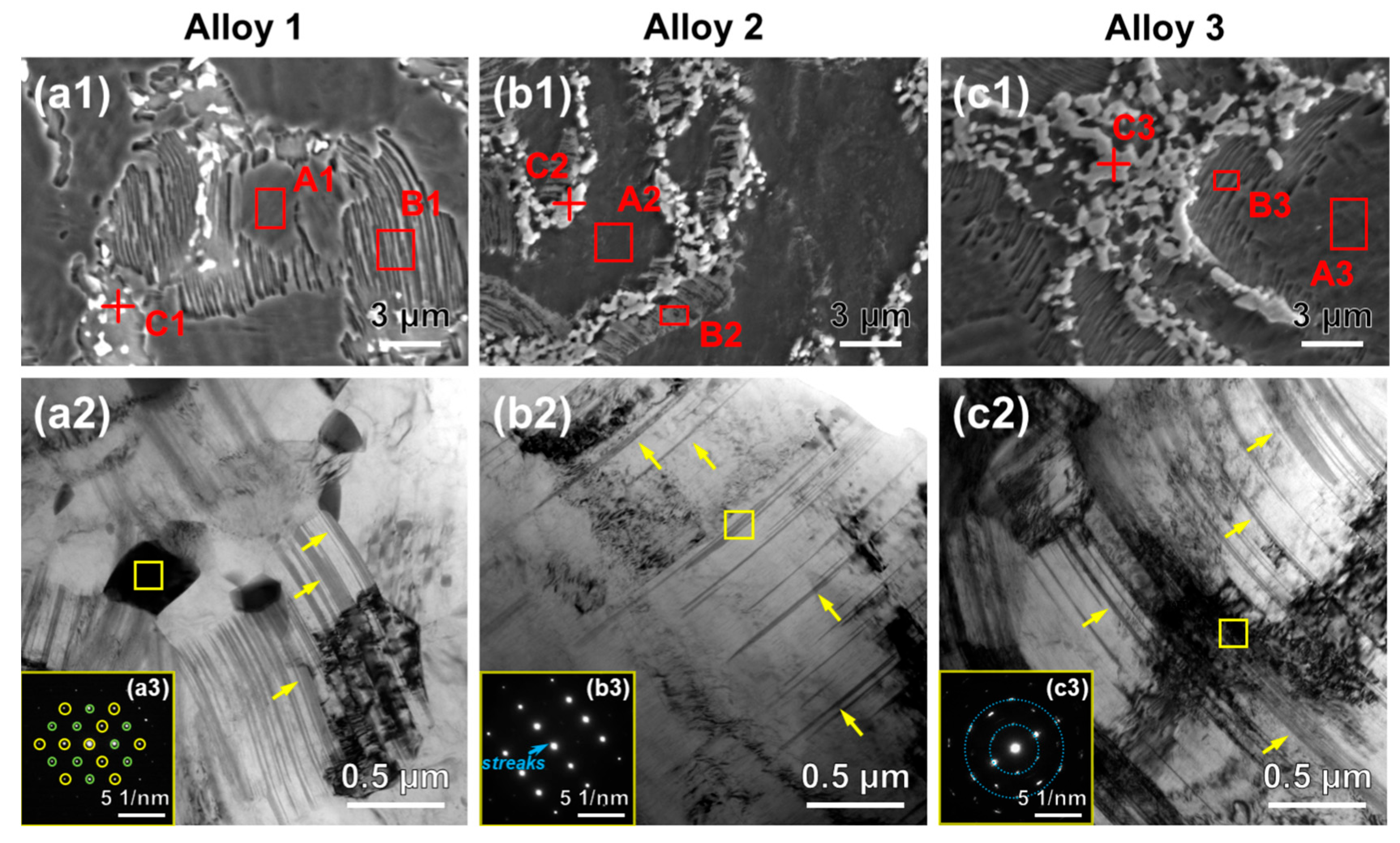


| Alloys | Areas | Gd | Zn | Zr | Nd | Mg |
|---|---|---|---|---|---|---|
| Alloy 1 | A1 | 4.96 | 0.52 | 0.26 | - | Bal. |
| B1 | 5.89 | 1.67 | 0.24 | - | Bal. | |
| C1 | 40.64 | 10.20 | 0.20 | - | Bal. | |
| Alloy 2 | A2 | 6.72 | 0.74 | 0.68 | 0.91 | Bal. |
| B2 | 11.36 | 2.00 | 0.47 | 0.97 | Bal. | |
| C2 | 55.80 | 14.65 | 0.21 | 2.87 | Bal. | |
| Alloy 3 | A3 | 7.72 | 1.27 | 0.87 | 0.99 | Bal. |
| B3 | 12.75 | 3.00 | 0.27 | 1.61 | Bal. | |
| C3 | 50.02 | 15.53 | 0.35 | 5.79 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Hong, L.; Dai, J.; Zhang, X. Enhanced Mechanical and Corrosion Properties of As-Extruded Mg-12Gd-2Zn-0.4Zr Alloy by Nd Additions. Metals 2025, 15, 1077. https://doi.org/10.3390/met15101077
He J, Hong L, Dai J, Zhang X. Enhanced Mechanical and Corrosion Properties of As-Extruded Mg-12Gd-2Zn-0.4Zr Alloy by Nd Additions. Metals. 2025; 15(10):1077. https://doi.org/10.3390/met15101077
Chicago/Turabian StyleHe, Jiahuan, Lixin Hong, Jianwei Dai, and Xiaobo Zhang. 2025. "Enhanced Mechanical and Corrosion Properties of As-Extruded Mg-12Gd-2Zn-0.4Zr Alloy by Nd Additions" Metals 15, no. 10: 1077. https://doi.org/10.3390/met15101077
APA StyleHe, J., Hong, L., Dai, J., & Zhang, X. (2025). Enhanced Mechanical and Corrosion Properties of As-Extruded Mg-12Gd-2Zn-0.4Zr Alloy by Nd Additions. Metals, 15(10), 1077. https://doi.org/10.3390/met15101077







