Treatment of Na2SO4-Containing Wastewater Generated During the Recycling of Spent Lithium-Ion Batteries: Comparative Study on the Operating Modes of Bipolar Membrane Electro-Dialysis
Abstract
1. Introduction
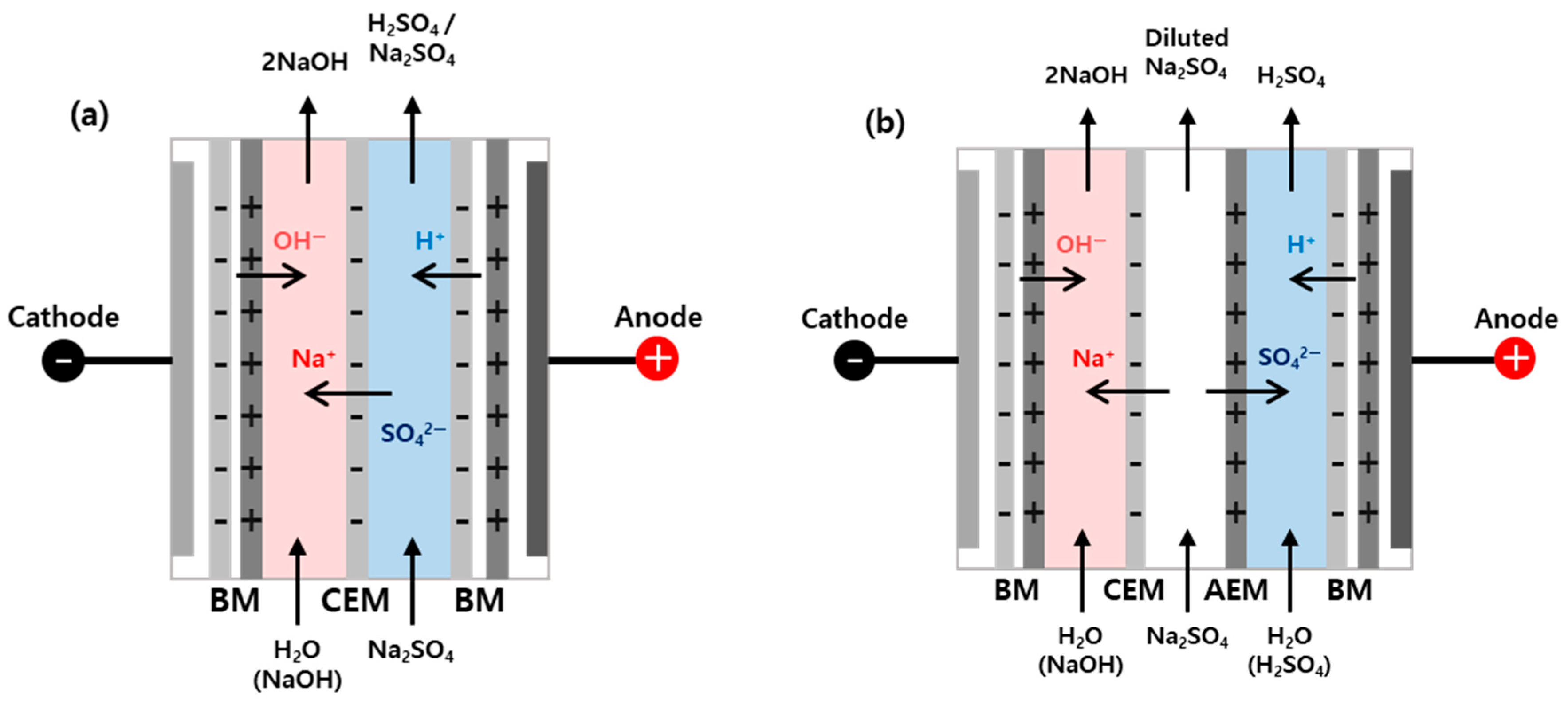
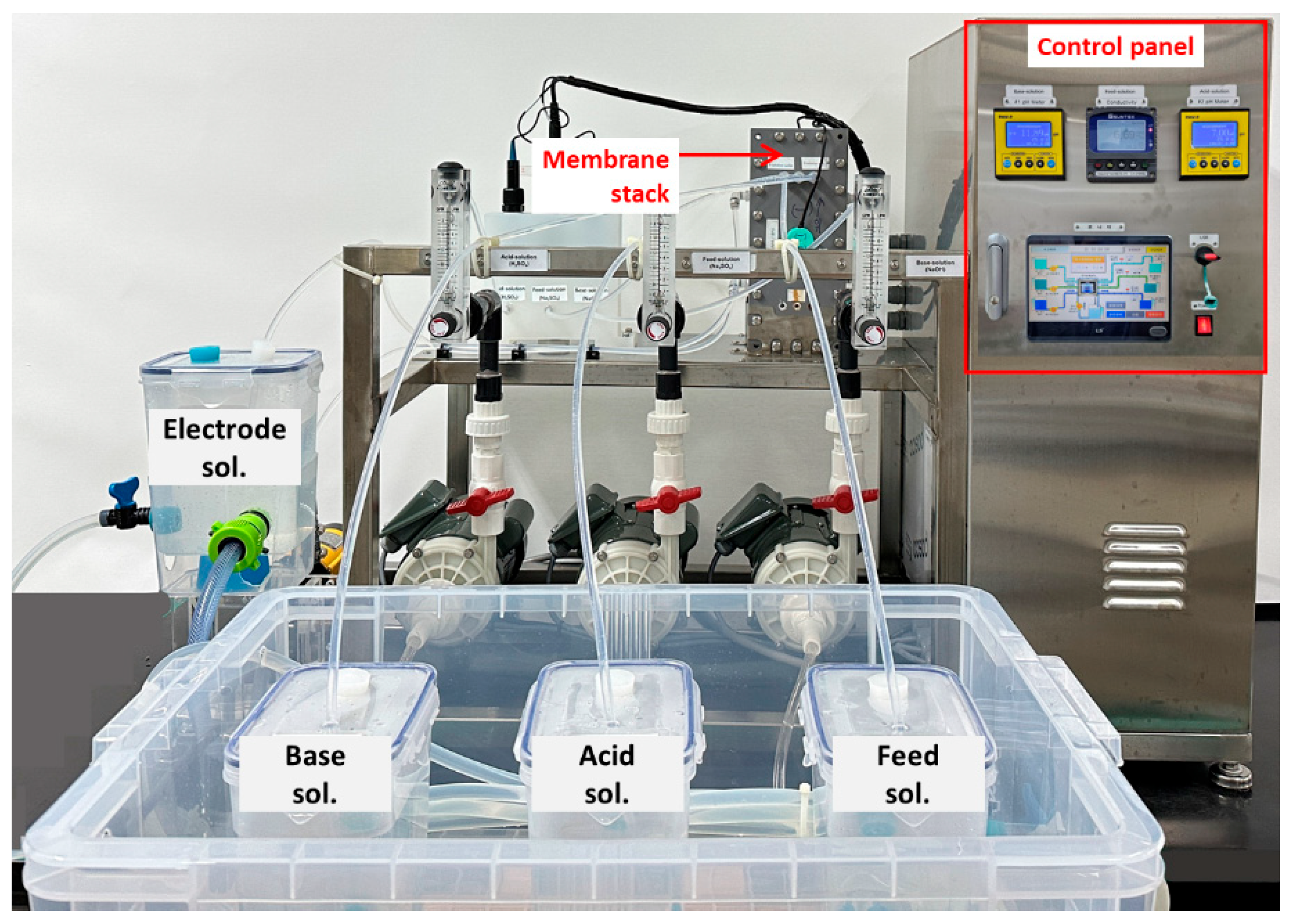
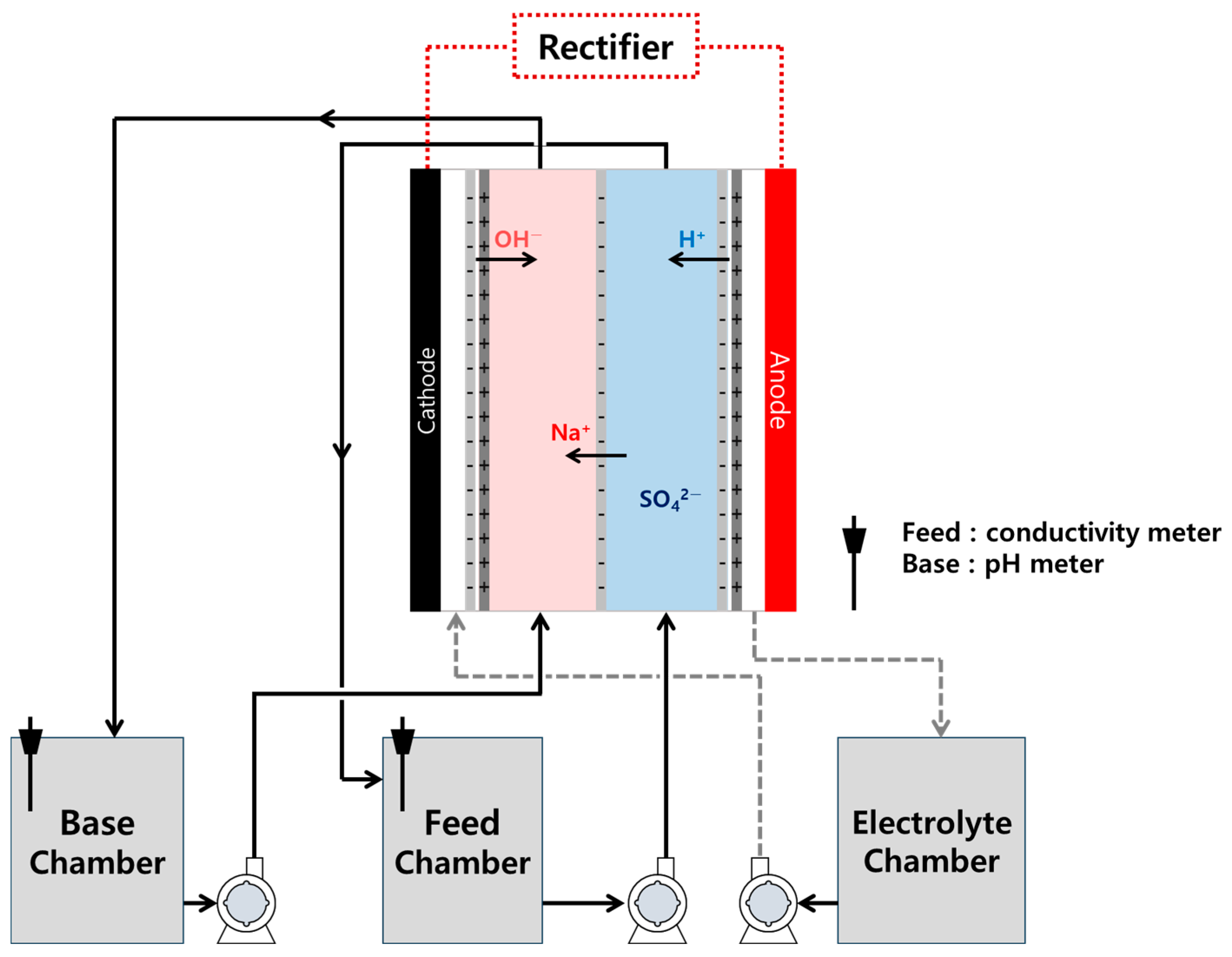
2. Materials and Methods
3. Results and Discussion
3.1. Comparison Between the Acid and Base Recovery Efficiencies of the Two- and Three-Compartment Systems
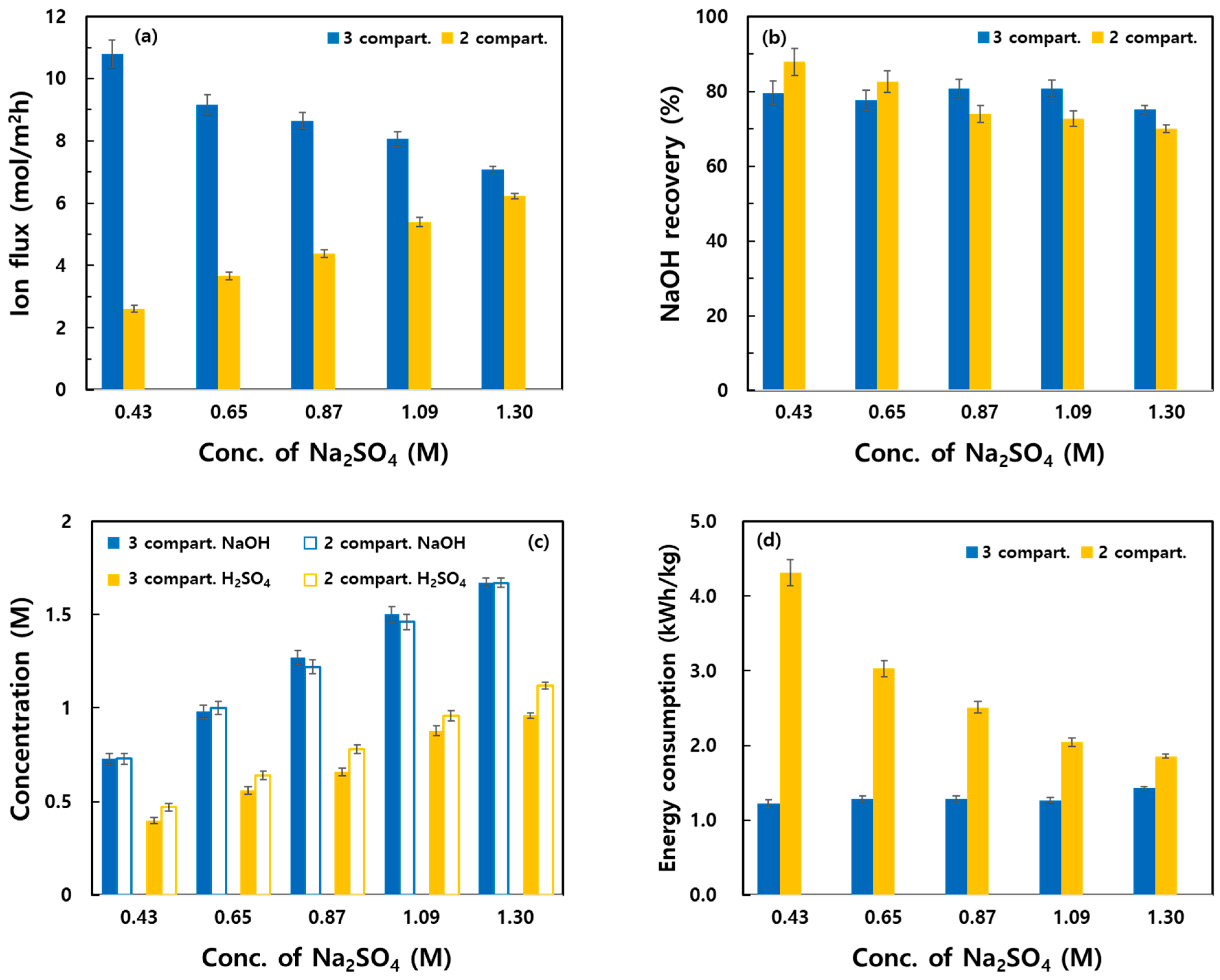
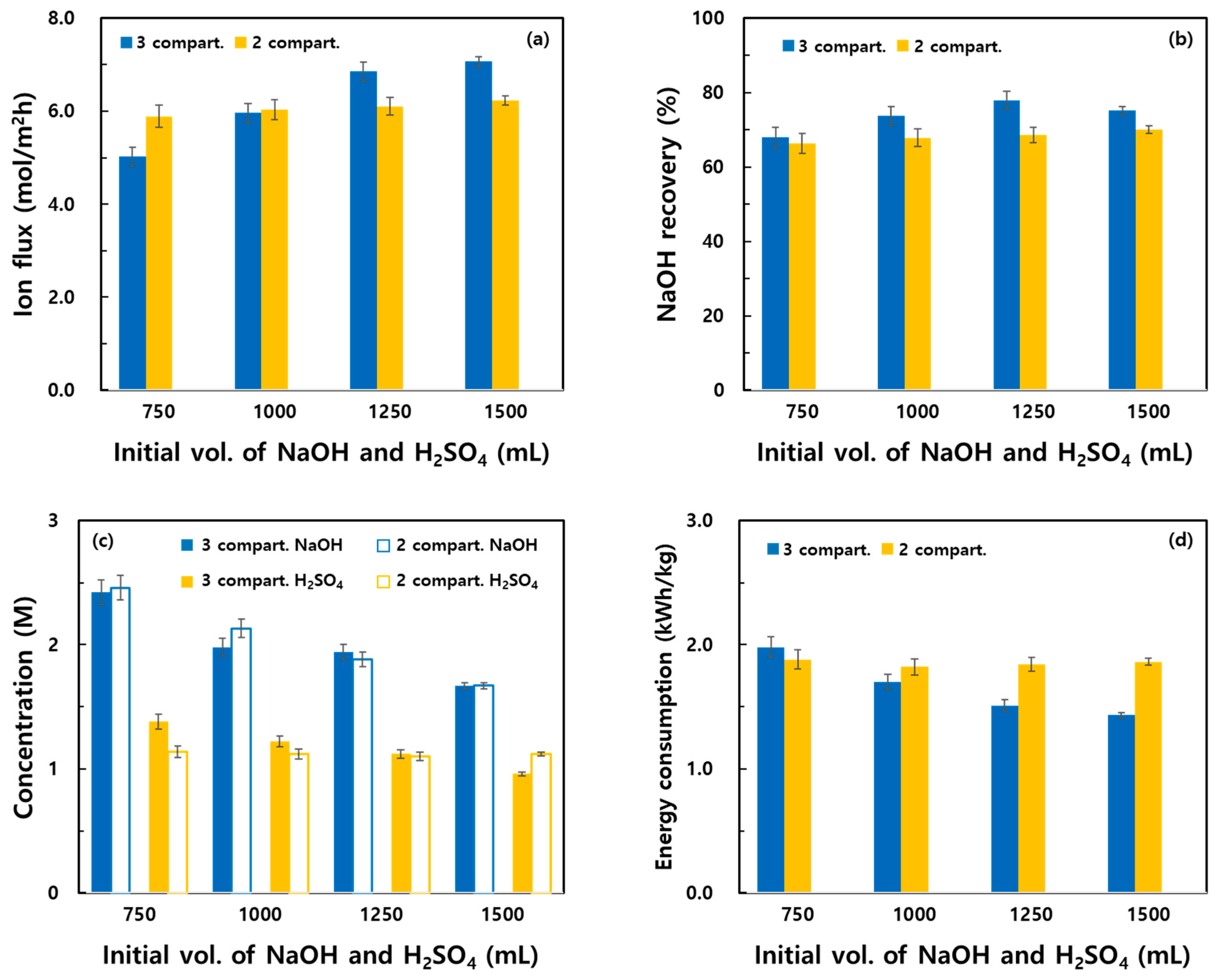
3.2. Effect of Process Parameters on the Recovery Characteristics of the Two Systems
3.3. Techno-Economic Feasibility Analysis
3.4. Comparison of Operational Stability
4. Conclusions
- The two-compartment system initially showed higher Na+ flux and NaOH recovery rate. However, after 240 min, the three-compartment system demonstrated superior performance. The three-compartment system enabled the recovery of a high-purity acid and base and showed moderately stable long-term performance in terms of CE and energy consumption.
- With increasing current density, the Na+ flux, NaOH recovery rate, and energy consumption increased. In most cases, the three-compartment system outperformed the two-compartment system. The CE continuously decreased in the two-compartment system, while it increased up to 360 A/m2 and then declined in the three-compartment system. This was attributed to different heat accumulation and concentration polarization effects in each system. Although the two-compartment system exhibited lower energy consumption, additional thermal control may be required for extended operations.
- With increasing Na2SO4 concentration, both systems exhibited higher concentrations of recovered NaOH and H2SO4 as well as improved CE. In the three-compartment system, the Na+ flux decreased owing to reduced voltage at high Na2SO4 concentrations, whereas in the two-compartment system, the flux increased owing to the weakening of the concentration polarization effect. The two-compartment system was less energy efficient at low concentrations, but it showed improved energy efficiency at high concentrations.
- With increasing initial concentrations of NaOH and H2SO4, the three-compartment system showed a decline in the Na+ flux and recovery rate as well as increased energy consumption. In contrast, the two-compartment system exhibited an improved flux and recovery rate beyond a certain concentration threshold, with a corresponding decrease in the energy consumption. These results were attributed to the differences between the concentration gradients, back-diffusion effects, and water transport rates of the two systems, highlighting that recovery characteristics depend on the concentration conditions.
- Increasing the initial volumes of NaOH and H2SO4 in the recovery compartments increased the Na+ flux and NaOH recovery rates in both systems. At volumes of ≥1250 mL, the three-compartment system demonstrated superior recovery performance. Low initial volumes increased the concentrations of the recovered species; however, differences in the water transport and recovery rates caused variations in the final concentrations. Energy consumption substantially decreased in the three-compartment system with the increasing volumes, suggesting that volume control is critical for process optimization.
- Both configurations successfully recovered NaOH and H2SO4 from the Na2SO4-containing wastewater, with estimated annual process costs of USD 4.43 and USD 6.43 for the two- and three-compartment systems, respectively. Despite the lower processing cost of the two-compartment system, its capital investment is expected to be higher because it may require thermal management and post-treatment. Under future stricter environmental regulations, BMED can be an effective strategy for the recovery of resources and treatment of Na2SO4-containing wastewater.
- The operational stability assessment confirmed the higher voltage stability and electrical reliability of the two-compartment system and its susceptibility to membrane degradation due to heat accumulation and repeated acid or base contact. In contrast, the three-compartment system exhibited better structural resistance to thermal and chemical stress but experienced voltage increases in long-term operations, which can compromise membrane integrity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEM | Anion exchange membrane |
| C.E | Current efficiency |
| CEM | Cation exchange membrane |
| BPM | Bipolar membrane |
| BMED | Bipolar membrane electrodialysis |
| LIBs | Lithium-ion batteries |
References
- Giza, K.; Pospiech, B.; Gęga, J. Future technologies for recycling spent lithium-ion batteries (LIBs) from electric vehicles—Overview of latest trends and challenges. Energies 2023, 16, 5777. [Google Scholar] [CrossRef]
- Yoo, K. Lithium ion battery recycling industry in South Korea. Resour. Recycl. 2023, 32, 13–20. [Google Scholar] [CrossRef]
- Ahn, J.-W.; Cho, Y.-C. Current status and prospect of waste lithium ion battery (LIB) recycling technology by hydrometallurgical process. Resour. Recycl. 2023, 32, 3–17. [Google Scholar] [CrossRef]
- Verbaan, N.; Naidoo, R. A Review of Hydrometallurgical Flowsheets Considered for the Treatment of Black Mass. Presented via iQHub. Available online: https://www.slideshare.net/slideshow/review-of-hydrometallurgical-flowsheets-for-treatment-of-black-mass/256586490 (accessed on 9 September 2025).
- Ding, Z.; Li, J.; Huang, Y.; Sun, Y.; Lin, H.; Li, J.; Zhuge, X.; Ren, Y. The Green Recycling and Reuse of the Spent Lithium-Ion Battery with Nickel Cobalt Manganate as the Cathode. Preprint at SSRN. Available online: https://ssrn.com/abstract=4739651 (accessed on 18 August 2025).
- Yu, K.S.; Yu, H.Y. Method for Preparing a Precursor for Cathode Active Material of Secondary Battery and Apparatus Thereof. KR Patent 10-2023063, 19 September 2019. [Google Scholar]
- Ekholm, P.; Lehtoranta, J.; Taka, M.; Sallantaus, T.; Riihimäki, J. Diffuse sources dominate the sulfate load into Finnish surface waters. Sci. Total Environ. 2020, 748, 141297. [Google Scholar] [CrossRef]
- Reinsel, M.A. A new process for sulfate removal from industrial waters. In Proceedings of the National Meeting of American Society for Surface Mining and Reclamation, Scottadale, AZ, USA, 13–19 August 1999; pp. 546–550. [Google Scholar] [CrossRef]
- Kuldeep, B.; Badenhorst, W.D.; Kauranen, P.; Pajari, H.; Ruismäki, R.; Mannela, P.; Murtomäki, L. Bipolar membrane electrodialysis for sulfate recycling in the metallurgical industries. Membranes 2021, 11, 718. [Google Scholar] [CrossRef]
- Shin, H.S.; Jung, J.Y.; Bae, B.U.; Paik, B.C. Phase-separated anaerobic toxicity assays for sulfate and sulfide. Water Environ. Res. 1995, 67, 802–807. [Google Scholar] [CrossRef]
- Joo, J.; Moon, J.-K.; Jang, Y. A study on the desalination process for high-concentration Na2SO4 using pilot-scale bipolar membrane electrodialysis. Desalination Water Treat. 2025, 322, 101222. [Google Scholar] [CrossRef]
- Atia, T.A.; Elia, G.; Hahn, R.; Altimari, P.; Pagnanelli, F. Closed-loop hydrometallurgical treatment of end-of-life lithium ion batteries: Towards zero-waste process and metal recycling in advanced batteries. J. Energy Chem. 2019, 35, 220–227. [Google Scholar] [CrossRef]
- Parnamae, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar membranes: A review on principles, latest developments, and applications. J. Membr. Sci. 2021, 617, 118538. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Lee, B.; Kim, M.; Kim, G.; Kim, C. Valorization of Na2SO4 in wastewater from spent lithium-ion battery recycling via BMED. Chem. Eng. J. 2025, 504, 158834. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis and related processes. In Membrane Separations Technology: Principles and Applications; Noble, R.D., Stern, S.A., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 2, pp. 213–278. [Google Scholar]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Cho, Y.-C. A study on Na2SO4 Treatment Using Bipolar Electrodialysis System. Ph.D. Thesis, Daejin University, Pocheon-si, Republic of Korea, 2024. [Google Scholar]
- Xue, S.; Wu, C.; Wu, Y.; Chen, J.; Li, Z. Bipolar membrane electrodialysis for treatment of sodium acetate waste residue. Sep. Purif. Technol. 2015, 154, 193–203. [Google Scholar] [CrossRef]
- Lee, Y.; Seo, M.; Ahn, J. Regeneration of NaOH from the spent Na2SO4 solution by two-compartment bipolar membrane electrodialysis. Korean J. Met. Mater. 2025, 63, 281–290. [Google Scholar] [CrossRef]
- Kroupa, J.; Kinčl, J.; Cakl, J. Recovery of H2SO4 and NaOH from Na2SO4 by electrodialysis with heterogeneous bipolar membrane. Desalination Water Treat. 2015, 56, 3238–3246. [Google Scholar] [CrossRef]
- Wang, D.; Meng, W.; Lei, Y.; Li, C.; Cheng, J.; Qu, W.; Wang, G.; Zhang, M.; Li, S. The novel strategy for increasing the efficiency and yield of the bipolar membrane electrodialysis by the double conjugate salts stress. Polymers 2020, 12, 343. [Google Scholar] [CrossRef]
- Giesbrecht, P.K.; Freund, M.S. Recent Advances in Bipolar Membrane Design and Applications. Chem. Mater. 2020, 32, 8060–8090. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Y.; Shen, J.; Van der Bruggen, B. Application of Bipolar Membrane Electrodialysis in Environmental Protection: A Review. Membranes 2022, 12, 864. [Google Scholar] [CrossRef]
- Tanaka, Y. Ion exchange membranes: Preparation, properties, and applications. In Ion Exchange Membranes: Fundamentals and Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 12, pp. 217–268. [Google Scholar]
- Tanaka, Y. Concentration polarization in ion-exchange membrane electrodialysis: The events arising in an unforced flowing solution in a desalting cell. J. Membr. Sci. 2003, 217, 65–80. [Google Scholar] [CrossRef]
- Venugopal, K.; Dharmalingam, S. Desalination efficiency of a novel bipolar membrane based on functionalized polysulfone. Desalination 2012, 296, 37–45. [Google Scholar] [CrossRef]
- Moon, S.H. Electrochemical Processes of Ion Exchange Membranes; GIST PRESS: Gwangju, Republic of Korea, 2021; pp. 1–296. [Google Scholar]
- León, T.; Shah, S.A.; López, J.; Culcasi, A.; Jofre, L.; Cipollina, A.; Cortina, J.L.; Tamburini, A.; Micale, G. Electrodialysis with bipolar membranes for the generation of NaOH and HCl solutions from brines: An inter-laboratory evaluation of thin and ultrathin non-woven cloth-based ion-exchange membranes. Membranes 2022, 12, 1204. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.; Khanarian, G. Water dissociation in bipolar membranes: Experiments and theory. J. Membr. Biol. 1978, 38, 11–30. [Google Scholar] [CrossRef]
- Strathmann, H. Ion-Exchange Membrane Separation Processes; Membrane Science and Technology Series; Elsevier: Amsterdam, The Netherlands, 2004; Volume 9, pp. 89–146. [Google Scholar]
- Zhu, S.; Kingsbury, R.S.; Call, D.F.; Coronell, O. Impact of Solution Composition on the Resistance of Ion Exchange Membranes. J. Membr. Sci. 2018, 554, 39–47. [Google Scholar] [CrossRef]
- Bazinet, L.; Geoffroy, T.R. Electrodialytic Processes: Market Overview, Membrane Phenomena, Recent Developments and Sustainable Strategies. Membranes 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Tian, B.; Luo, S.; Chi, Y.; Aishajiang, D.; Zhang, Y.; Yang, M. High-value conversion of waste Na2SO4 by a bipolar membrane electrodialysis metathesis system. Resour. Conserv. Recycl. 2022, 186, 106556. [Google Scholar] [CrossRef]
- Brauns, E. Finite elements-based 2D theoretical analysis of the effect of IEX membrane thickness and salt solution residence time on the ion transport within a salinity gradient power reverse electrodialysis half cell pair. Desalination Water Treat. 2013, 51, 6429–6443. [Google Scholar] [CrossRef]
- Gao, W.; Fang, Q.; Yan, H.; Wei, X.; Wu, K. Recovery of acid and base from sodium sulfate containing lithium carbonate using bipolar membrane electrodialysis. Membranes 2021, 11, 152. [Google Scholar] [CrossRef]
- Huang, X.; Song, M.; Wang, H.; Lv, S.; Qi, S.; Wang, C.; Zhang, H.; Ruan, X. Waste salt separation by antisolvent crystallization process: Mechanism study of Na2SO4–NaCl–solvent ternary phase diagrams and its life cycle assessment. Desalination 2025, 560, 118985. [Google Scholar] [CrossRef]
- Tufa, R.A.; Blommaert, M.A.; Chanda, D.; Li, Q.; Vermaas, D.A.; Aili, D. Bipolar Membrane and Interface Materials for Electrochemical Energy Systems. ACS Appl. Energy Mater. 2021, 4, 7419–7439. [Google Scholar] [CrossRef]
- Blommaert, M.A.; Aili, D.; Tufa, R.A.; Li, Q.; Smith, W.A.; Vermaas, D.A. Insights and Challenges for Applying Bipolar Membranes in Advanced Electrochemical Energy Systems. ACS Energy Lett. 2021, 6, 2539–2548. [Google Scholar] [CrossRef]
- Jeong, J.H.; Shin, E.K.; Jeong, J.J.; Na, I.C.; Chu, C.H.; Park, K.P. Degradation of Electrode and Membrane in Proton Exchange Membrane Fuel Cell After Water Electrolysis. Korean Chem. Eng. Res. 2014, 52, 695–700. [Google Scholar] [CrossRef]
- Tanaka, Y. Ion Exchange Membrane Electrodialysis: Fundamentals, Desalination, Separation; Nova Science Publishers: New York, NY, USA, 2010; pp. 1–308. [Google Scholar]





| Time (min) | Operating Mode | Na+ Flux (mol/m2h) | Recovery (%) | Recovered Concentration (M) | C.E (%) | Energy Consumption (kWh/kg) * | ||
|---|---|---|---|---|---|---|---|---|
| NaOH | H2SO4 | NaOH | H2SO4 | |||||
| 30 | 2 compart. | 14.19 | 9.97 | 9.55 | 0.35 | 0.13 | 99.99 | - |
| 3 compart. | 11.50 | 8.08 | 6.72 | 0.30 | 0.18 | 84.74 | - | |
| 60 | 2 compart. | 13.28 | 18.67 | 18.02 | 0.57 | 0.25 | 97.88 | - |
| 3 compart. | 10.99 | 15.44 | 13.15 | 0.48 | 0.26 | 80.96 | - | |
| 120 | 2 compart. | 11.35 | 31.91 | 31.96 | 0.89 | 0.45 | 83.67 | - |
| 3 compart. | 10.05 | 28.25 | 25.43 | 0.78 | 0.41 | 74.07 | - | |
| 240 | 2 compart. | 8.95 | 50.32 | 49.90 | 1.31 | 0.73 | 65.97 | - |
| 3 compart. | 8.96 | 50.34 | 45.95 | 1.25 | 0.64 | 66.00 | - | |
| 360 | 2 compart. | 7.02 | 59.22 | 57.15 | 1.49 | 0.88 | 51.76 | - |
| 3 compart. | 8.05 | 67.85 | 63.88 | 1.56 | 0.82 | 59.30 | - | |
| 480 | 2 compart. | 5.72 | 64.29 | 59.88 | 1.58 | 0.97 | 42.14 | 1.52 |
| 3 compart. | 6.74 | 75.18 | 77.35 | 1.67 | 0.96 | 49.69 | 1.43 | |
| Current Density (A/m2) | Operating Mode | Na+ Flux (mol/m2h) | Recovery (%) | Recovered Concentration (M) | C.E (%) | Energy Consumption (kWh/kg) * | ||
|---|---|---|---|---|---|---|---|---|
| NaOH | H2SO4 | NaOH | H2SO4 | |||||
| 250 | 2 compart. | 6.64 | 37.35 | 37.21 | 1.02 | 0.54 | 69.94 | 0.87 |
| 3 compart. | 6.32 | 35.65 | 32.80 | 0.96 | 0.50 | 60.09 | 0.97 | |
| 310 | 2 compart. | 7.62 | 42.87 | 42.52 | 1.14 | 0.63 | 66.11 | 0.96 |
| 3 compart. | 7.78 | 43.72 | 40.02 | 1.14 | 0.57 | 60.69 | 1.03 | |
| 360 | 2 compart. | 9.00 | 50.57 | 49.66 | 1.31 | 0.73 | 66.30 | 1.00 |
| 3 compart. | 9.01 | 50.66 | 46.78 | 1.27 | 0.65 | 66.42 | 1.11 | |
| 400 | 2 compart. | 9.51 | 53.46 | 50.67 | 1.36 | 0.78 | 61.73 | 1.09 |
| 3 compart. | 10.27 | 57.72 | 51.18 | 1.40 | 0.70 | 61.91 | 1.11 | |
| 450 | 2 compart. | 10.61 | 59.63 | 51.64 | 1.50 | 0.81 | 62.54 | 1.13 |
| 3 compart. | 11.11 | 62.44 | 57.25 | 1.48 | 0.75 | 58.93 | 1.25 | |
| Conc. of Na2SO4 (M) | Operating Mode | Na+ Flux (mol/m2h) | Recovery (%) | Recovered Concentration (M) | C.E (%) | Energy Consumption (kWh/kg) * | ||
|---|---|---|---|---|---|---|---|---|
| NaOH | H2SO4 | NaOH | H2SO4 | |||||
| 0.43 | 2 compart. | 2.61 | 87.98 | 79.08 | 0.73 | 0.47 | 15.38 | 4.31 |
| 3 compart. | 10.80 | 79.62 | 76.51 | 0.73 | 0.40 | 69.58 | 1.23 | |
| 0.65 | 2 compart. | 3.67 | 82.61 | 72.52 | 1.00 | 0.64 | 21.66 | 3.03 |
| 3 compart. | 9.15 | 77.62 | 78.82 | 0.98 | 0.56 | 61.36 | 1.29 | |
| 0.87 | 2 compart. | 4.38 | 73.91 | 70.20 | 1.22 | 0.78 | 25.84 | 2.51 |
| 3 compart. | 8.65 | 80.82 | 73.64 | 1.27 | 0.66 | 59.69 | 1.29 | |
| 1.09 | 2 compart. | 5.39 | 72.73 | 68.60 | 1.46 | 0.96 | 31.78 | 2.05 |
| 3 compart. | 8.06 | 80.85 | 83.75 | 1.50 | 0.88 | 56.38 | 1.27 | |
| 1.30 | 2 compart. | 6.23 | 70.00 | 63.78 | 1.67 | 1.12 | 36.39 | 1.86 |
| 3 compart. | 7.07 | 75.18 | 77.35 | 1.67 | 0.96 | 49.69 | 1.43 | |
| Initial Conc. of NaOH and H2SO4 (M) | Operating Mode | Na+ Flux (mol/m2h) | Recovery (%) | Recovered Concentration (M) | Water Migration (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| NaOH | H2SO4 | NaOH | H2SO4 | Base | Acid | Feed | |||
| 0.05 | 2 compart. | 5.90 | 66.39 | 63.42 | 1.59 | 1.02 | 20.30 | - | −20.30 |
| 3 compart. | 7.50 | 79.41 | 77.78 | 1.72 | 0.91 | 23.34 | 16.69 | −40.04 | |
| 0.10 | 2 compart. | 6.23 | 70.00 | 63.78 | 1.67 | 1.12 | 17.13 | - | −17.13 |
| 3 compart. | 7.07 | 75.18 | 77.35 | 1.67 | 0.96 | 27.40 | 16.65 | −44.05 | |
| 0.30 | 2 compart. | 6.24 | 70.14 | 63.32 | 1.83 | 1.08 | 16.16 | - | −16.16 |
| 3 compart. | 6.95 | 76.37 | 78.39 | 2.03 | 1.14 | 13.20 | 16.25 | −29.45 | |
| 0.50 | 2 compart. | 6.25 | 70.33 | 63.38 | 1.93 | 1.09 | 15.68 | - | −15.68 |
| 3 compart. | 6.39 | 71.91 | 74.62 | 2.13 | 1.26 | 12.98 | 16.69 | −29.67 | |
| Initial Vol. of NaOH and H2SO4 (mL) | Operating Mode | Na+ Flux (mol/m2h) | Recovery (%) | Recovered Concentration (M) | Water Migration (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| NaOH | H2SO4 | NaOH | H2SO4 | Base | Acid | Feed | |||
| 750 | 2 compart. | 5.89 | 66.32 | 62.01 | 2.46 | 1.14 | 44.47 | - | −22.23 |
| 3 compart. | 5.02 | 67.90 | 66.46 | 2.42 | 1.38 | 50.55 | 31.97 | −41.04 | |
| 1000 | 2 compart. | 6.03 | 67.85 | 63.55 | 2.13 | 1.12 | 28.80 | - | −19.20 |
| 3 compart. | 5.96 | 73.75 | 73.01 | 1.98 | 1.22 | 50.11 | 25.16 | −50.48 | |
| 1250 | 2 compart. | 6.10 | 68.60 | 63.65 | 1.88 | 1.10 | 20.30 | - | −16.92 |
| 3 compart. | 6.85 | 77.93 | 76.44 | 1.94 | 1.12 | 35.89 | 19.76 | −44.52 | |
| 1500 | 2 compart. | 6.23 | 70.00 | 63.78 | 1.67 | 1.12 | 15.68 | - | −15.68 |
| 3 compart. | 7.07 | 75.18 | 77.35 | 1.67 | 0.96 | 27.40 | 16.65 | −44.05 | |
| Techno-Economic Factors | Two-Compartment System | Three-Compartment System |
|---|---|---|
| Membrane used | Cation exchange membrane and bipolar membrane | Cation exchange membrane, anion exchange membrane, and bipolar membrane |
| Bipolar membrane reaction | H2O → H+ + OH− | |
| Recovery of Na2SO4 to NaOH | 66.32% | 67.90% |
| Recovery of Na2SO4 to H2SO4 | 62.01% | 66.46% |
| Concentration of recovered NaOH | 2.46 M | 2.42 M |
| Concentration of recovered H2SO4 | 1.14 M (H2SO4 + Na2SO4 mixed solution) | 1.38 M |
| NaOH purity | Comparably to commercial NaOH obtained from membrane plants | |
| H2SO4 purity | 63.3% (1.14 M H2SO4 + 0.66 M Na2SO4) | Comparable to commercial H2SO4 obtained from membrane plants |
| Operating temperature | 27–30 °C | 25–28 °C |
| Average voltage (V) | 17.34 | 18.92 |
| CE (%) | 34.74 | 36.09 |
| Process time (h) | 9.00 | 9.87 |
| Energy consumption (kWh/kg) #1 | 1.88 | 1.98 |
| Process capacity (kg/year) #2,3 | 162.01 | 131.31 |
| Total energy consumption (kWh/year) | 305 | 260 |
| Electricity charge (USD/kWh) #4 | 0.10 | 0.10 |
| Total energy cost (USD/year) | 30.5 | 26.0 |
| Supplied Na2SO4 price (USD/year) #5 | 14.0 | 11.4 |
| Produced NaOH price (USD/year) #6 | 27.88 | 21.08 |
| Produced H2SO4 price (USD/year) #7 | 12.15 | 9.87 |
| Total process cost (USD/year) | 4.43 | 6.43 |
| Stability Factor | Two-Compartment System | Three-Compartment System |
|---|---|---|
| Membrane durability | The CEM is exposed to the acid and base, which adversely affect membrane durability | As the acid and base are separated, membrane durability is improved compared to the two-compartment system |
| Voltage stability | Owing to a reduced operating voltage, the system is more stable than the three-compartment system | The rapid increase in the voltage may affect membrane durability |
| Temperature stability | Higher than that of the three-compartment system | System stable at 25–28 °C |
| Operational stability | The two-compartment system exhibits superior voltage stability and electrical durability during long-term operation, whereas the three-compartment system offers better structural resistance to thermal and chemical stress | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, M.; Lee, Y.; Kim, J.; Chang, J.; Cho, Y.; Ahn, J. Treatment of Na2SO4-Containing Wastewater Generated During the Recycling of Spent Lithium-Ion Batteries: Comparative Study on the Operating Modes of Bipolar Membrane Electro-Dialysis. Metals 2025, 15, 1067. https://doi.org/10.3390/met15101067
Seo M, Lee Y, Kim J, Chang J, Cho Y, Ahn J. Treatment of Na2SO4-Containing Wastewater Generated During the Recycling of Spent Lithium-Ion Batteries: Comparative Study on the Operating Modes of Bipolar Membrane Electro-Dialysis. Metals. 2025; 15(10):1067. https://doi.org/10.3390/met15101067
Chicago/Turabian StyleSeo, Minhyuk, Youngjae Lee, Junhee Kim, Jaehyuk Chang, Yeonchul Cho, and Jaewoo Ahn. 2025. "Treatment of Na2SO4-Containing Wastewater Generated During the Recycling of Spent Lithium-Ion Batteries: Comparative Study on the Operating Modes of Bipolar Membrane Electro-Dialysis" Metals 15, no. 10: 1067. https://doi.org/10.3390/met15101067
APA StyleSeo, M., Lee, Y., Kim, J., Chang, J., Cho, Y., & Ahn, J. (2025). Treatment of Na2SO4-Containing Wastewater Generated During the Recycling of Spent Lithium-Ion Batteries: Comparative Study on the Operating Modes of Bipolar Membrane Electro-Dialysis. Metals, 15(10), 1067. https://doi.org/10.3390/met15101067







