Abstract
In order to cleanly and efficiently extract zirconium from zircon sand (the main component is ZrSiO4), sodium hydroxide sub-molten salt was used to decompose ZrSiO4 in this study. When ZrSiO4 reacts with sodium hydroxide sub-molten salt, the formation of Na2ZrSiO5 (a water-insoluble product) considerably affects the separation efficiency of Zr and Si and increases production cost. Thus, it is necessary to control the formation of Na2ZrSiO5. The influence of NaOH content, reaction temperature, reaction time, and NaOH/ore mass ratio on the formation of Na2ZrSiO5 were systematically investigated. The optimum reaction parameters for the inhibition of Na2ZrSiO5 formation were as follows: 80% NaOH content, 245 °C reaction temperature, 4:1 NaOH/ore mass ratio, 10 h reaction time, and 400 r/min agitation speed. These results indicate that ZrSiO4 is decomposed to Na2ZrO3 and Na2SiO3 by reacting with NaOH, realizing the separation of Zr and Si, and then the reactions between Na2ZrO3 and Na2SiO3 result in the formation of Na2ZrSiO5, during the decomposition of ZrSiO4 using NaOH sub-molten salt. The sub-molten salt decomposition process can realize the clean extraction of zirconium, which is conducive to the sustainable development of zirconium resources.
1. Introduction
Zirconium is a type of metal with important practical use. It is widely used in ceramics, chemical and arms industries, electronics, and other fields due to its excellent optical, electrical, thermal and chemical properties [1]. Zircon sand is the main source of zirconium compounds. Zircon sand is the main mineral source of zirconium. The main component of zircon sand is zirconium silicate (ZrSiO4), which belongs to the tetragonal crystal system, has a melting point of up to 2550 °C, and has extremely high chemical stability. The decomposition of ZrSiO4 can only be achieved under extremely harsh reaction conditions, such as thermal decomposition, chlorination, and sintering [2,3].
The most important step of these processes is the separation of Zr and Si in ZrSiO4. During the process of thermal decomposition, ZrSiO4 is decomposed into ZrO2 and SiO2 by high temperature electric furnace. The higher decomposition temperature causes expensive production costs, which limits the use of the process [4,5]. In the chlorination process, ZrSiO4 is chlorinated to form ZrCl4. However, this method requires specific equipment [6,7]. During sintering, ZrSiO4 reacts to produce zirconium oxychloride at a high temperature with sintering agents which include CaCO3, Na2CO3, and NaOH, etc. Compared with NaOH, CaCO3 and Na2CO3 have the disadvantages of needing a high sintering temperature and difficulty in treating their products, resulting in a complex process. Therefore, NaOH sintering is widely used in the industry at present [2,8,9,10,11,12,13,14].
In the sintering process, ZrSiO4 reacts with molten sodium hydroxide and decomposes into sodium zirconate (Na2ZrO3) and sodium silicate (Na2SiO3), which can be easily treated following sintering at high temperatures, so this sintering step is the most important. Zirconium oxychloride (ZrOCl2·8H2O), an important zirconium basic chemical, is obtained by washing, transformation and crystallization of NaZrO3 [8,10]. However, this process has some disadvantages. For example, molten sodium hydroxide reacts with ZrSiO4 in open containers, which can produce a large amount of alkali vapor that threatens the safety of operators. A large amount of liquid produced in the washing process can become a potential pollutant. To solve these problems, it is imperative to develop a new technology to decompose ZrSiO4.
Sub-molten salt medium can effectively improve the extraction efficiency of minerals, and has been widely studied and developed in recent years. It is a medium between molten salt and ordinary electrolytes. It has the advantages of high chemical activity, a high boiling point, excellent fluidity and high activity of oxygen anion [15,16,17,18]. In our previous study, to reduce reaction temperature and pollution, we applied sub-molten salt to decompose ZrSiO4. Compared with the sintering process, the process involving sub-molten salt helps to better recycle NaOH and provides more effective environmental protection. The decomposition kinetics of ZrSiO4 in sodium hydroxide sub-molten salt were studied, and the decomposition flow chart of ZrSiO4 in NaOH sub-molten salt solution was explored, as shown in Figure 1 [19]. The product obtained owing to the decomposition of zircon sand is sodium zirconium silicate. Zirconium oxychloride is obtained by acidolysis, flocculation, filtration, and crystallization. However, in the process involving sub-molten salt, sodium hydroxide will be lost, production cost will be increased, and zirconium will be lost in the flocculation process, thus reducing the total yield of the process. When the decomposition product is sodium zirconate, the reaction product can be washed to remove sodium and silicon, omitting the acidolysis and flocculation process, shortening the process flow and increasing the total yield of the reaction.
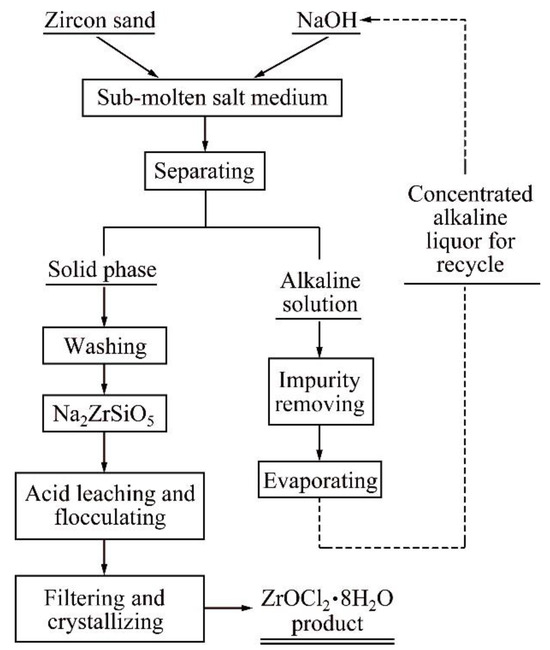
Figure 1.
Schematic flow chart of a cleaner ZrSiO4 production process [19]. Reprinted with permission from ref. [19]. Copyright 2019, Elsevier.
This study aims to investigate the phase change law of ZrSiO4 decomposition products in sodium hydroxide molten salt, to achieve effective separation of Zr and Si, and to explore the reaction process of ZrSiO4 decomposition. The effects of reaction temperature, reaction time, NaOH concentration, stirring speed, and alkali/mineral ratio on the phase of the product were studied using the results of Raman, infrared, and X-ray crystal diffraction analyses. The variation rule of the phase of the product was obtained, which is beneficial to simplify the process of decomposition of zircon sand by NaOH sub-molten salt, and has important theoretical and practical significance for the optimization of a new clean production process of zirconium oxychloride. The reaction process was investigated, enriching the theoretical research of sub-molten salt and providing theoretical guidance for practical production.
2. Experimental Procedure
2.1. Materials
The NaOH employed was of analytical grade and was provided by the Beijing Chemical Plant, China. The water used in the experiment was deionized water, obtained by the water super purification system (Milli-Q, Millipore, Burlington, MA, USA). The main element composition of zircon sand provided by Kingan Hi-tech. Co., Ltd., Nanchang, Jiangxi Province, China was obtained by X-ray fluorescence (XRF) (AXIOS, Arlington, VA, USA). The results are listed in Table 1. The particle size of zircon sand was 22.56 μm (d50), which was measured by laser particle size analyzer (Mastersizer 2000, Malvern, UK), as shown in Figure 2. The zircon sand was characterized by X-ray diffraction (XRD, Philips 1140, Amsterdam, The Netherlands, Cu Kα radiation, 40 mA, and 30 kV) and its crystal structure was determined. The results are shown in Figure 3, and the main crystal phase is ZrSiO4. The scanning electron microscope (SEM, JSM-7610F, Tokyo, Japan) image of the particle is shown in Figure 4. It can be seen from Figure 4 that zircon sand is formed of blocky particles.

Table 1.
Chemical composition of zircon sand (wt%).
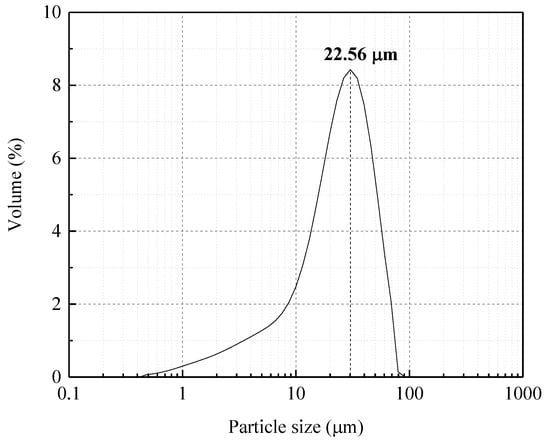
Figure 2.
Size distribution of zircon sand.
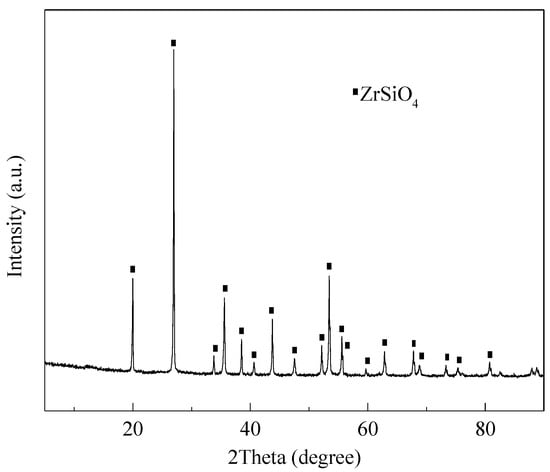
Figure 3.
XRD pattern of the zircon sand.
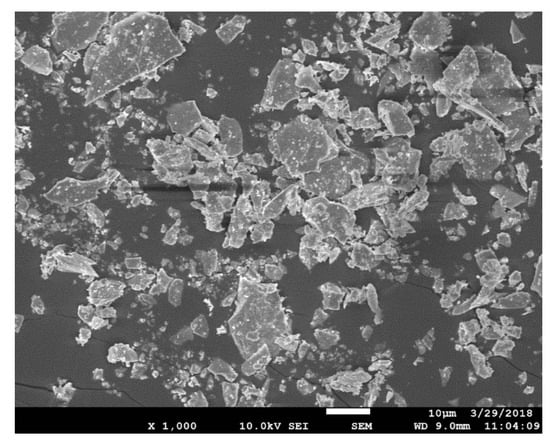
Figure 4.
SEM image of the zircon sand.
2.2. Experimental Device and Process
All experiments were completed in an autoclave, and the structure of the autoclave is shown in Figure 5 [19]. The autoclave was composed of a temperature control system, electric heating system, magnetic stirring system and cooling system. The volume of the inner tank was 1 L. The material of the inner tank in the autoclave was stainless steel lined with nickel. The temperature control system can adjust the heating power to maintain the reaction temperature constant with a precision of ±1 °C.
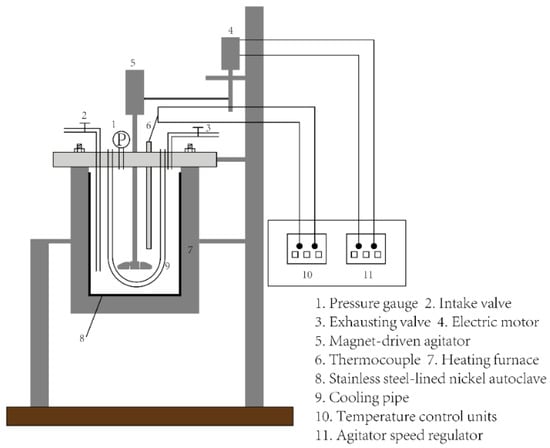
Figure 5.
Experimental device [19] Reprinted with permission from ref. [19]. Copyright 2019, Elsevier.
Sodium hydroxide and deionized water were added to the reactor to form a certain concentration of sub-molten salt, and zircon sand was added according to NaOH/ore ratio. Then, the autoclave was sealed and heated. When the temperature reached the set temperature, the timing started. After the reaction continued for a period of time, the autoclave with the cooling system was used to cool down. When the temperature in the autoclave was reduced to 90 °C, there was no pressure in the autoclave, and the reaction liquid was taken out. After hot filtration, the filter cake was washed five times with deionized water and dried at 105 °C for 12 h. By adding sodium hydroxide to increase the concentration of the filtrate to decompose the zircon sand, the recycling of sodium hydroxide was realized. The products in the reaction process were characterized by Fourier transform infrared (FTIR, Perkin–Elmer Spectrum One, Waltham, MA, USA, sample preparation using potassium bromide) spectroscopy, XRD, Raman spectroscopy (inVia, using a He Ne laser (532 nm)) and X-ray photoelectron spectroscopy (Thermofisher ESCALAB 250Xi, Waltham, MA, USA, Al Kα (hν = 1486.6 eV) as the X-ray source).
The following method was used to obtain the conversion rate of zircon sand. First, the reaction product was washed with hot water to fully remove the soluble substance. Then, the washed solid was added to 3:1 hydrochloric acid (deionized water diluted four times with concentrated hydrochloric acid) (solid–liquid ratio 5:1), stirred at 50 °C for 4 h, and then filtered and washed. The obtained filter cake was unreacted zircon sand. The filter cake was dried and weighed to calculate the conversion rate of zircon sand. All the data in the study were the average of three parallel experiments. The conversion rate of zircon sand was calculated using the following equation:
where m1 is the mass of the residue (unreacted zircon sand), and m2 is the total mass of the reacted zircon sand.
3. Results and Discussion
3.1. Effect of Initial NaOH Concentration
Sodium hydroxide not only acts as a reactant but also affects the properties of the sub-molten salt medium. Therefore, the concentration of initial NaOH is crucial to the decomposition of zircon sand. In order to obtain the influence of initial alkali concentration on the decomposition of zircon sand, the initial alkali concentrations of 50%, 60%, 70%, 75% and 80% by mass fraction were selected as the research object, and the influence of initial sodium hydroxide concentration on the reaction product and conversion rate was studied. In the experiments, the conditions of reaction temperature 245 °C, agitation speed 400 r/min, alkali–ore ratio 3:1 and reaction time 8 h were kept constant.
Figure 6 summarizes the effect of the initial NaOH concentration on the decomposition products of ZrSiO4. Compared with the XRD spectra of the reaction products, when the concentration of NaOH is ≤70%, the reaction products are all Na2ZrSiO5 and unreacted ZrSiO4. As the concentration increases, Na2ZrO3 appears in the product at 75% concentration; however, Na2ZrSiO5 remains, the concentration increases to 80%, and almost all of the products are Na2ZrO3 and Na2SiO3. According to the results shown in Figure 7, the products are extremely sensitive to NaOH concentration. When other conditions are determined, Na2ZrO3 will appear in the reaction products only when the concentration is higher than a specific value. Therefore, considering the applicability, the concentration of NaOH is 80% when other factors are studied later. Figure 6 shows the relationship between concentration and conversion, indicating that the increase of concentration does not increase the conversion evidently. This is because the viscosity of the system increases with concentration, the mass transfer rate slows down, and the reaction rate decreases, which partly offsets the increase of the rate caused by the increase in concentration.
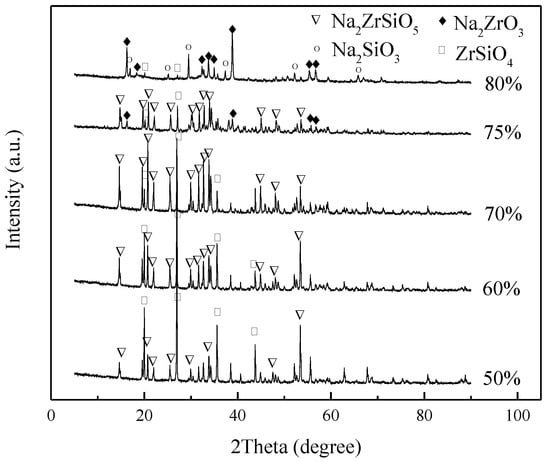
Figure 6.
XRD pattern of products at different NaOH concentrations.
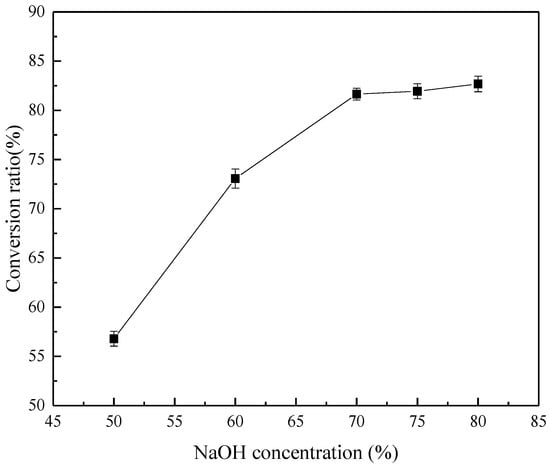
Figure 7.
Effect of initial NaOH concentration on the conversion rate of ZrSiO4.
3.2. Effect of Reaction Temperature
The effect of the reaction temperature on the decomposition rate of ZrSiO4 was investigated at 230 °C, 245 °C, 260 °C, and 275 °C, NaOH/ore mass ratio of 3:1, initial NaOH concentration of 80%, agitation speed of 400 r/min, and reaction time of 4 h, as shown in Figure 8 and Figure 9. The results demonstrate that the conversion of ZrSiO4 increases with temperature. The reaction products are Na2ZrO3, Na2SiO3, and unreacted ZrSiO4 at 245–260 °C. When the temperature increases to 275 °C, most of the ZrSiO4 is decomposed; however, Na2ZrSiO5 appears in the product. Therefore, to avoid the formation of Na2ZrSiO5, the reaction temperature should not be extremely high. In the following investigation, the temperature of 260 °C was selected when the effect of reaction time on the reaction products was investigated.
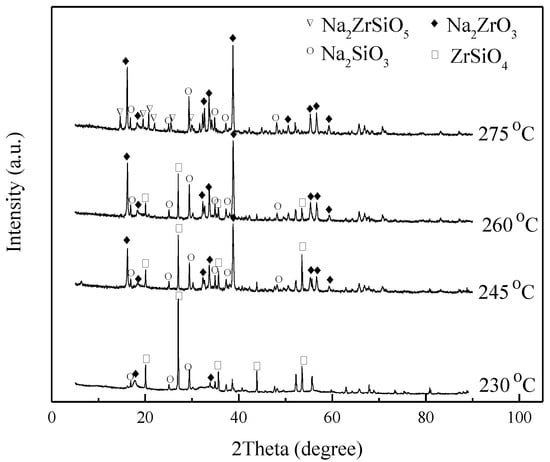
Figure 8.
XRD pattern of products at different temperatures.
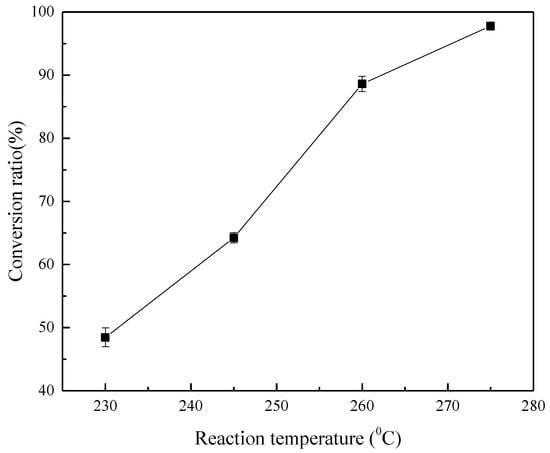
Figure 9.
Effect of reaction temperature on the conversion rate of ZrSiO4.
3.3. Effect of Reaction Time
The effects of reaction time on the reaction products and conversion rate of ZrSiO4 were investigated at the conditions of reaction temperature 260 °C, agitation speed of 400 r/min, NaOH/ore mass ratio of 3:1, and initial NaOH concentration of 80%. The experimental results after reaction times of 2, 4, 5, and 8 h were obtained, as shown in Figure 10 and Figure 11.
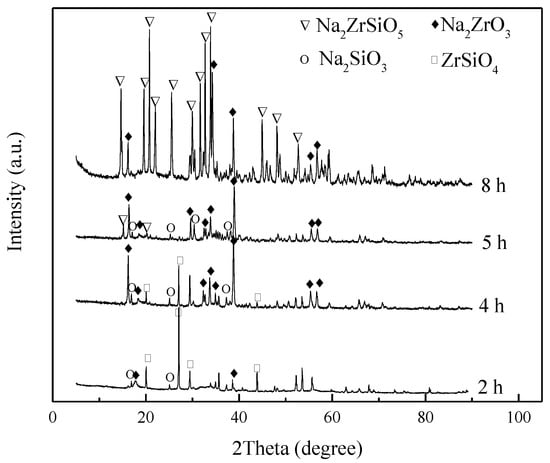
Figure 10.
XRD pattern of products at different reaction times.
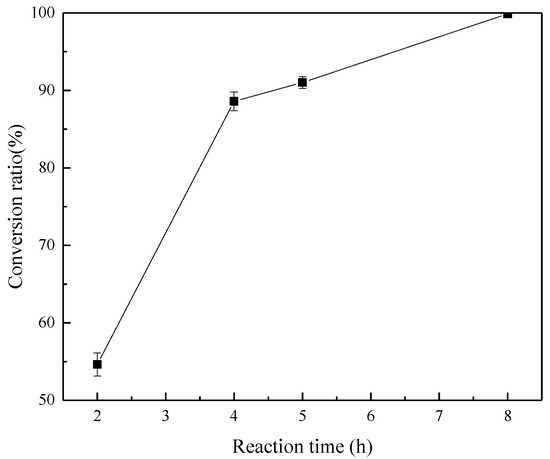
Figure 11.
Effect of reaction time on the conversion rate of ZrSiO4.
Figure 10 shows that the decomposition rate of ZrSiO4 increases gradually with the reaction time. At the initial stage of the reaction, the conversion of ZrSiO4 was low, and only Na2ZrO3 and Na2SiO3 existed in the product. Na2ZrSiO5 gradually appeared in the product as the conversion increased. The concentration of Na2ZrO3 and Na2SiO3 increased with the decomposition rate. Simultaneously, the content of H2O also increases. Therefore, an important relationship between the reaction product and the content of Na2ZrO3, Na2SiO3, and H2O in the system is speculated to exist.
3.4. Effect of NaOH/Ore Mass Ratio
Section 3.3 demonstrates that the content of Na2ZrO3 and Na2SiO3 has an important influence on the reaction products, and the variation in the NaOH/ore mass ratio also affects the content of Na2ZrO3 and Na2SiO3 in the system. Therefore, the effects of NaOH/ore mass ratio on the decomposition products and conversion rate of ZrSiO4 were studied under the conditions of initial NaOH concentration of 80%, reaction temperature of 275 °C, agitation speed of 400 r/min and reaction time of 4 h. In Figure 12, the XRD spectra of reaction products with NaOH/ore mass ratio of 3:1, 3.2:1, 3.5:1, 4:1, and 4.2:1 were obtained. The graph shows that the diffraction peak of Na2ZrSiO5 decreases gradually with the increase of alkali/ore ratio. When the NaOH/ore ratio increases to 4.2:1, there is almost no Na2ZrSiO5 in the product. According to the graph of conversion (Figure 13), ZrSiO4 with a different NaOH/ore mass ratio is almost completely decomposed.
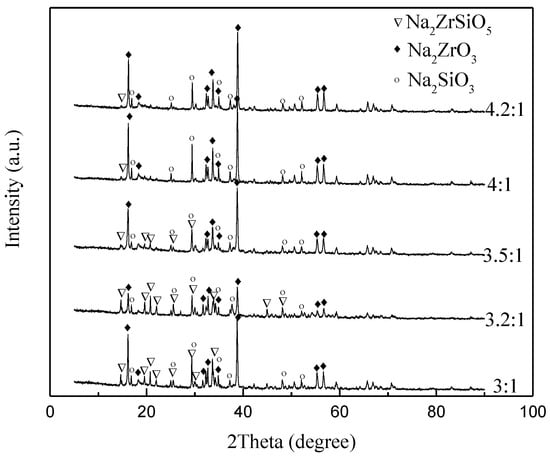
Figure 12.
XRD pattern of products at different NaOH/ore ratio.
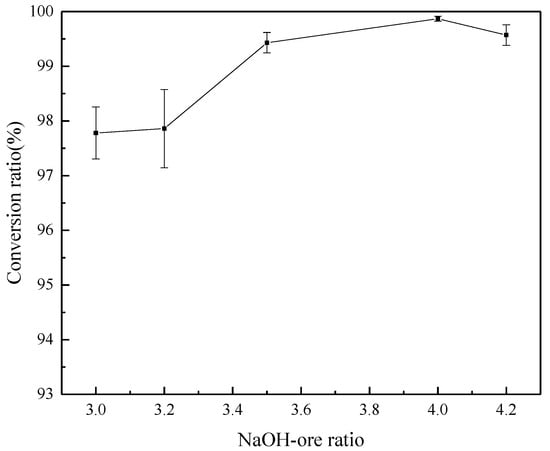
Figure 13.
Effect of NaOH–ore ratio on the conversion rate of ZrSiO4.
3.5. Optimum Technological Condition
According to the analysis of the factors affecting the products obtained during the decomposition of ZrSiO4 in NaOH sub-molten salt, the influence of each factor will be mutually restricted; thus, a series of experiments was conducted, and the reaction products that influence each factor are listed in Table 2. The table indicates that only Na2ZrSiO5 exists in the reaction product when the alkali concentration is 70%, and only when the concentration reaches 80% can Na2ZrO3 be formed in the product. Further, the optimum technological conditions were obtained: alkali concentration 80%, temperature 245 °C, alkali/ore ratio 4:1, reaction time 10 h, and stirring rate 400 r/min.

Table 2.
Phase composition of reaction products with different reaction conditions.
The SEM image and XRD patterns of the product on the optimum technological conditions are shown in Figure 14. It can be seen from the figure that almost all ZrSiO4 is decomposed, and the products are Na2ZrO3 and Na2SiO3. Na2ZrO3 consists of rod-shaped particles.
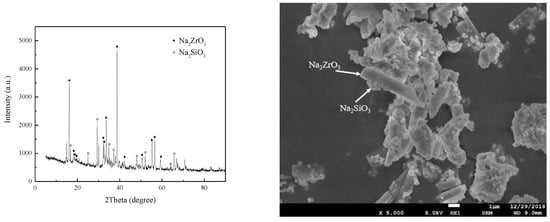
Figure 14.
The SEM image and the X-ray diffraction pattern for the product.
3.6. Mechanism Analysis
3.6.1. Speculation of Reaction Pathway
The main reactions that take place between ZrSiO4 and NaOH are as follows:
According to the previous discussion, under the experimental conditions for the reaction of ZrSiO4 in NaOH sub-molten salt, at the beginning and end of the reaction an excess of NaOH is present. The XRD results of the reaction products demonstrate that the reaction products are Na2ZrO3, Na2SiO3, and Na2ZrSiO5. Therefore, it is speculated that the decomposition reaction of ZrSiO4 in a sub-molten salt system may involve Equations (2) and (3). Na2ZrO3 and Na2SiO3 are the products in the early stage of the reaction. With the reaction proceeding, Na2ZrSiO5 appears in the products. Therefore, it is presumed that Equation (3) represents the first reaction of ZrSiO4 in NaOH sub-molten salt, Na2ZrO3 and Na2SiO3 are produced, and then Na2ZrSiO5 is formed, that is, Equation (6), which is different from the alkali melting process.
3.6.2. Verification of Reaction Pathway
To verify our hypothesis, it is assumed that ZrSiO4 is first decomposed into Na2ZrO3 and Na2SiO3, that is, Equation (3) occurs first. Material proportions were simulated under the conditions of 70% alkali concentration, 4:1 NaOH/ore mass ratio, 260 °C reaction temperature, and 400 r/min stirring rate, as well as 80% alkali concentration, 4:1 NaOH/ore mass ratio, 260 °C reaction temperature, and 400 r/min stirring rate. It was then added to the reactor and continued to react for 4 h to obtain the product as shown in Figure 15. The figure shows that all the products under the condition of 70% alkali concentration are Na2ZrSiO5, while most of the products under the condition of 80% alkali concentration are still Na2ZrO3 and Na2SiO3; only a very small amount of Na2ZrSiO5 is produced.
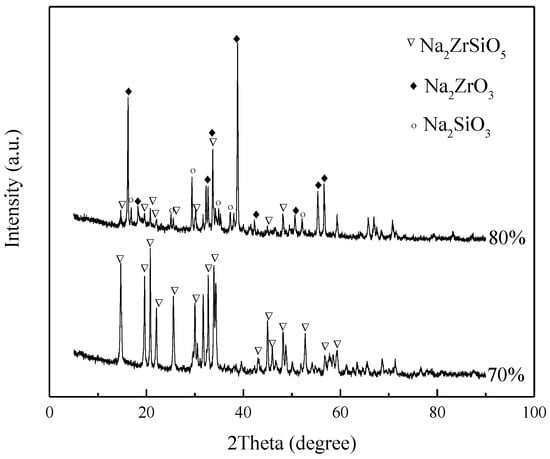
Figure 15.
XRD pattern of products at different conditions.
ZrSiO4 is assumed to decompose into Na2ZrSiO5 at first, that is, Equation (2) occurs first. The final material ratio under the previous two experimental conditions is also simulated. The product obtained after adding NaOH to the reactor is shown in Figure 16. The figure also shows that the products under both conditions are still Na2ZrSiO5, indicating that sodium zirconium silicate does not react in sodium hydroxide sub-molten salt, which is different from the alkali fusion sintering method.
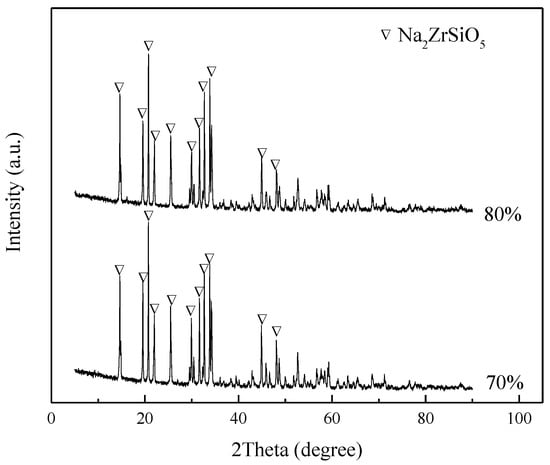
Figure 16.
XRD pattern of products at different conditions.
The discussion indicates that sodium zirconate and sodium silicate can continue to react to produce sodium zirconate in sodium hydroxide molten salt, while sodium silicate zirconate cannot react with sodium hydroxide. The reaction is rapid at low concentrations and slows down as the concentration increases. Thus, the decomposition process of ZrSiO4 in NaOH sub-molten salt is first decomposed into Na2ZrO3 and Na2SiO3, then the two react to produce Na2ZrSiO5, that is, first Equation (3) and then Equation (6).
3.6.3. Infrared Spectroscopy
Figure 17 shows the variations in the infrared spectra of products in sub-molten salts (reaction temperature of 260 °C, NaOH content of 70%, agitation speed of 400 r/min, and NaOH/ore mass ratio of 3:1) at different reaction times. The spectra of A show three characteristic fundamental IR bands of ZrSiO4 at approximately 445, 610, and 890 cm−1 attributed, respectively, to O-Si-O bending vibration, antisymmetric stretching vibration of SiO4 tetrahedron, and antisymmetric stretching vibration of SiO4 tetrahedron [20,21]. The transmission intensity of all the three bands decreases with increasing reaction time, while new vibration peaks appeared at approximately 503, 879, 1043, and 1435 cm−1. The absorption peaks at approximately 503 and 879 cm−1 are attributed to the vibration of Zr-O, and the absorption peaks at approximately 1043 and 1435 cm−1 are attributed to the vibration of Si-O-Si in sodium silicate [21,22,23]. When the reaction time increased to 8 h, the absorption peak at 505 cm−1 disappeared, and at 570, 732, and 930 cm−1, a new absorption peak representing the stretching vibration peak of Si-Onb appeared, which indicated the formation of isolated SiO4 tetrahedra [23].
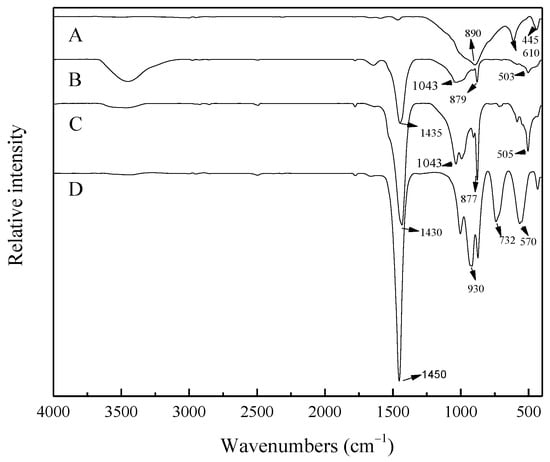
Figure 17.
Infrared spectra of ZrSiO4 and the products in the NaOH sub-molten salt with different reaction time. A: ZrSiO4, B: 1 h, C: 2 h, and D: 8 h.
3.6.4. Raman Spectroscopy
The Raman spectra of the products at different reaction times (reaction temperature of 260 °C, NaOH content of 70%, agitation speed of 400 r/min, and NaOH/ore mass ratio of 3:1) are shown in Figure 18. In the lower wavenumber region, the peaks at 358, 438, and 1009 cm−1 (A1g, ν2, and B1g, symmetric bending) corresponding to Q0 in ZrSiO4 weaken as the reaction time increases [24,25,26,27]. At the initial stage of the reaction, Raman peaks at 305 and 630 cm−1 assigned to ZrO2 are observed, which proves that ZrO2 was separated from ZrSiO4 lattice in the decomposition process of ZrSiO4 in NaOH sub-molten salt [24,26,27]. With time, these peaks gradually disappear. When the reaction lasts for 3 h, a new vibration peak appears at 1050 cm−1, which is the characteristic vibration peak of Si-O-Si. When 1050 characteristic peaks disappear, new vibration peaks at 865 and 930 cm−1 represent the formation of new Q0, which is the same as the result of infrared spectrum analysis [24].
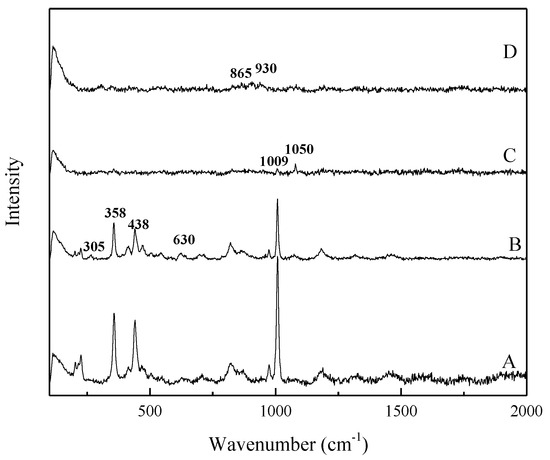
Figure 18.
Raman spectra of ZrSiO4 and the products in the NaOH sub-molten salt with different reaction times. A: ZrSiO4 B: 1 h C: 3 h D: 8 h.
3.6.5. X-ray Photoelectron Spectroscopy
A chemical analysis of the product from ZrSiO4 in NaOH sub-molten salt (reaction temperature of 260 °C, NaOH content of 80%, agitation speed of 400 r/min, NaOH/ore mass ratio of 3:1, and 1 h reaction time) was conducted using XPS, which is known to be a highly surface-sensitive technique. Figure 19 shows that the O 1s spectrum from ZrSiO4 after its reaction in NaOH sub-molten salt leads to the attribution of four distinct chemical species of O atoms, by resolving the O 1s spectra into four spin-orbit pairs with BEs of 530.5, 532.5, 534.5, and 538.8 eV, respectively. Among these four O 1s BE components, the 530.51 eV can be assigned to the O 1s BE of O present in the ZrO2; 532.50 eV, O 1s BE of O in the Si-O-Si; 534.5 eV, O 1s BE of O in the Na2ZrO3; and 538.8 eV, O 1s BE of O in the Na2CO3, which is formed when sodium hydroxide absorbs carbon dioxide from the air [28,29,30].
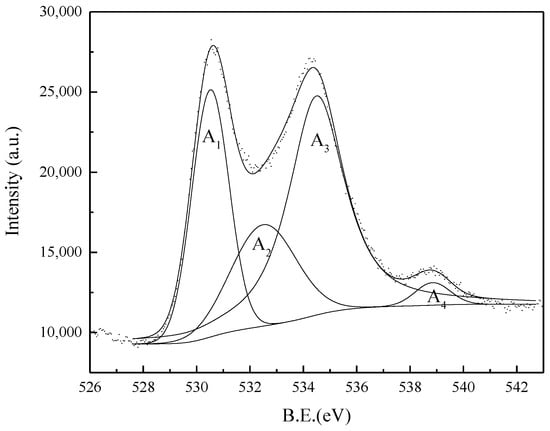
Figure 19.
O 1s HRXPS spectra of the product obtained from ZrSiO4 in the NaOH sub-molten salt with the reaction time of 1 h.
In addition to the O 1s spectrum, the sample was also scanned for a Zr 3d signal. The BE of Zr 3d in Figure 20 can be fitted to two pairs of peaks of A1, A2 and B1, B2. The BEs of A1 and A2 are 188.5 eV and 185.0 eV, respectively, which correspond to the BE of Zr 3d in ZrO2 [28,31]. Further, ZrO2 appears on the surface of ZrSiO4 particles, which is consistent with the results of infrared and Raman analyses. The BEs of B1 and B2 are 183.8 eV and 187.6 eV, respectively, corresponding to the BE of Zr 3d in Na2ZrO3.
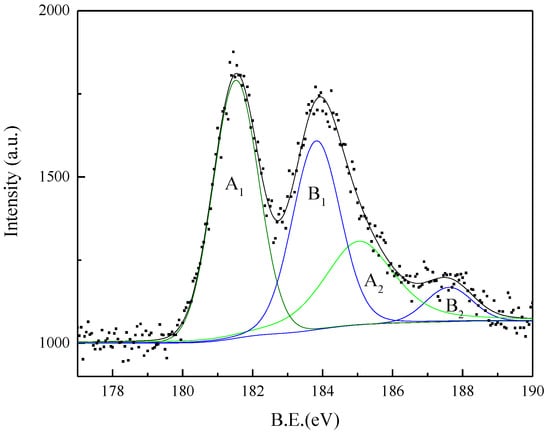
Figure 20.
Zr 3d HRXPS spectra of the product obtained from ZrSiO4 in the NaOH sub-molten salt with the reaction time of 1 h.
The analysis indicates that the decomposition reaction of ZrSiO4 in NaOH sub-molten salt occurs as the zircon–oxygen polyhedron is separated from the silicate lattice under the action of sub-molten salt, which separates zirconium and silicon. Previous studies have demonstrated that there are several stable O2−, which is Lewis alkaline, from the decomposition of OH− in the sub-molten salt system compared with conventional media. Therefore, when decomposing zircon sand in sub-molten salt, the highly active O2− tends to attack the Zr atom in zircon sand, replacing the O in the crystal structure, promoting the formation of new Zr–O bonds, thus breaking the ring crystal structure. The zirconium–oxygen polyhedron is separated from silicate lattice, resulting in the separation of zirconium and silicon. The silicon–oxygen polyhedron and zirconium–oxygen polyhedron form chain-shaped sodium silicate and sodium zirconium, respectively, under the action of Na+. When the water content in the system increases, that is, the alkali concentration decreases, the Na2SiO3 produced will ionize under the action of water molecules to produce SiO32−. SiO32− will attack the Zr atom in Na2ZrO3 and enter the lattice of Na2ZrO3 to form a new island-like silicate (Na2ZrSiO5). With the increase of alkalinity, the solubility of Na2SiO3 decreases, SiO32− in the system decreases, and rate of Na2ZrSiO5 formation slows down, which is the same as the previous analysis results. Therefore, the reaction mechanism of NaOH sub-molten salt decomposition of ZrSiO4 is to decompose Na2ZrO3 and Na2SiO3 sodium under the action of O2− and then combine them to form Na2ZrSiO5 under the action of ionization of SiO32−, as shown in Figure 21 and Figure 22.
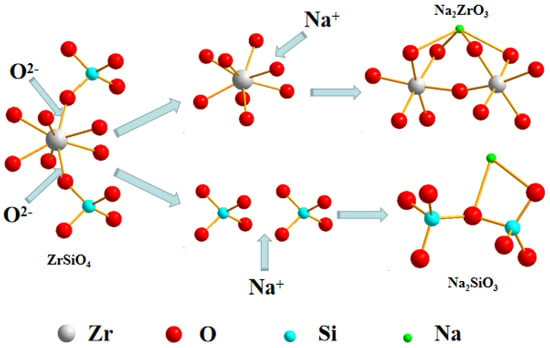
Figure 21.
Schematic pathway of the reaction process to form Na2ZrO3 from ZrSiO4 in NaOH sub-molten salt.
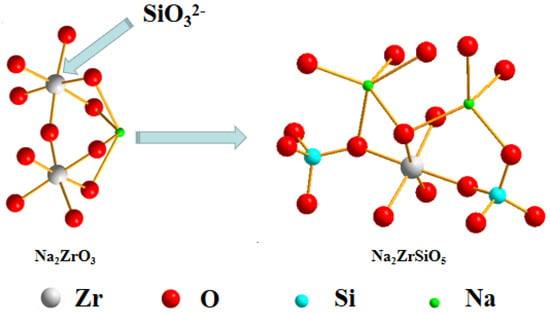
Figure 22.
Schematic pathway of the reaction process to form Na2ZrSiO5 from Na2ZrO3 in NaOH sub-molten salt.
4. Conclusions
The reaction parameters were investigated to control the products formed during the decomposition of ZrSiO4 in the NaOH sub-molten salt. The main conclusions are as follows:
- The effects of alkali concentration, temperature, alkali/ore ratio, and reaction time on the product were explored. The product was extremely dependent on NaOH concentration. It was necessary to coordinate the relationship among NaOH concentration, temperature, alkali/ore ratio, and reaction time to strictly control the formation of Na2ZrSiO5.
- The optimum technological conditions were obtained: alkali concentration 80%, reaction temperature 245 °C, alkali/ore ratio 4:1, reaction time 10 h, stirring rate 400 r/min. Under these conditions, the product obtained was ensured to be Na2ZrO3, achieving effective separation of Zr and Si; further, a high decomposition rate of ZrSiO4 can be ensured.
- The reaction mechanism was investigated and verified. The reaction process of ZrSiO4 in sodium hydroxide sub-molten salt was obtained by XRD spectroscopy infrared spectroscopy, Raman spectroscopy, and XPS analysis. It is elucidated that ZiSiO4 is decomposed to Na2ZrO3 and Na2SiO3 by reacting with NaOH, and then the reactions between Na2ZrO3 and Na2SiO3 result in the formation of Na2ZrSiO5.
Author Contributions
Conceptualization, H.S., J.S. and T.Q.; Methodology, H.S.; Validation, H.S.; Investigation, H.S.; Data curation, H.S. and J.S.; Writing—original draft, H.S.; Writing—review & editing, J.S. and T.Q.; Visualization, H.S.; Project administration, T.Q.; Funding acquisition, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the sub-project of The National Key R&D Program of China [Grant No. 2023YFC2908101].
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nikoofar, K.; Shaddel, N.; Jozi, F. Investigation of the Role of Zirconia and Zirconia-containing Systems as Catalysts in Organic Transformations. Curr. Org. Chem. 2024, 28, 433–462. [Google Scholar] [CrossRef]
- Ran, L.; Qu, J.; Jing, S.; Tao, Q.; Du, A. Analysis of water leaching and transition processes in zirconium oxychloride octahydrate production. Ceram. Int. 2014, 40, 1431–1438. [Google Scholar]
- Liu, J.; Jing, S.; Tao, Q.; Zhang, C.; Qu, J. Controlling the formation of Na2ZrSiO5 in alkali fusion process for zirconium oxychloride production. Adv. Powder Technol. 2015, 27, 1–8. [Google Scholar] [CrossRef]
- Irankhah, R.; Mobasherpour, I.; Alizadeh, M.; Nezhad, S.M.M.; Nikzad, L.; Azar, S.S. Carbothermal reduction of ZrSiO4 for in situ formation of ZrO2-based composites using spark plasma sintering. Ceram. Int. 2023, 49, 2681–2688. [Google Scholar] [CrossRef]
- Fernández-González, D.; Piñuela-Noval, J.; Ruiz-Bustinza, I.; González-Gasca, C.; Gómez-Rodríguez, C.; Quiñonez, L.V.G.; Fernández, A.; Verdejaf, L.F. Solar dissociation of zirconium silicate sand: A clean alternative to obtain zirconium dioxide. J. Clean. Prod. 2023, 420, 138371. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Zhao, Y.; Azhati, A.; Wang, X.; Zhang, Z.; Lv, X. First Principles Investigation of C, Cl2 and CO Co-Adsorption on ZrSiO4 Surfaces for Carbochlorination Reaction. Materials 2024, 17, 1500. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.W.; Zhao, P.; Wu, M.Q.; Yan, P.G.; Gao, X.; Li, J.G.; Huang, J.J.; Lin, X.D.; Jiang, Y.M.; Jin, X.Y.; et al. Effect of H2 addition on the preparation of ZrO2 powder from zircon (ZrSiO4) using a plasma torch. Ceram. Int. 2024, 50, 1360–1369. [Google Scholar] [CrossRef]
- Biswas, R.K.; Habib, M.A.; Karmakar, A.K.; Islam, M.R. A novel method for processing of Bangladeshi zircon: Part I: Baking, and fusion with NaOH. Hydrometallurgy 2010, 103, 124–129. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Jiang, D. Decomposition process of zircon sand concentrate with CaO-NaOH. Rare Met. 2012, 31, 410–414. [Google Scholar] [CrossRef]
- Manhique, A.; Kwela, Z.; Focke, W.W. De Wet Process for the Beneficiation of Zircon: Optimization of the Alkali Fusion Step. Ind. Eng. Chem. Res. 2003, 42, 777–783. [Google Scholar] [CrossRef]
- Silva, R.J.F.D.; Dutra, A.J.B.; Afonso, J.C. Alkali fusion followed by a two-step leaching of a Brazilian zircon concentrate. Hydrometallurgy 2012, 117–118, 93–100. [Google Scholar] [CrossRef]
- Wei, J.; Han, B.; Wei, Y.; Li, N.; Miao, Z. Influence of phase evolution and thermal decomposition kinetics on the properties of zircon ceramic. Ceram. Int. 2021, 47, 27285–27293. [Google Scholar] [CrossRef]
- Wei, J.; Han, B.; Wei, Y.; Li, N.; Wang, Y.; Duan, J.; Fu, J. Decomposition mechanism, sintering process and properties of zircon ceramics: Role of CaO, MnO and Cr2O3. Ceram. Int. 2022, 48, 34289–34297. [Google Scholar] [CrossRef]
- Song, J.; Fan, J.F.; Liu, J.C.; Liu, R.; Qu, J.K.; Qi, T. A two-step zircon decomposition method to produce zirconium oxychloride: Alkali fusion and water leaching. Rare Met. 2020, 39, 448–454. [Google Scholar] [CrossRef]
- Zhu, S.; Mei, J.; Zeng, W.W.; Lu, S.Y.; Su, M.H.; Zhan, H.R.; Huang, Y.S.; Yang, S.L.; Liu, Y.Z. Facile synthesis of TiNb2O7 anode material by KOH sub-molten salt method for lithium-ion batteries. Mater. Lett. 2024, 360, 135980. [Google Scholar] [CrossRef]
- Mohamed, N.; Ornek, C.; Timur, S.; Uergen, M. Anodic behavior of nickel in sub-molten KOH and its relevance for the production of electroactive nickel oxides. Surf. Interfaces 2022, 31, 101963. [Google Scholar] [CrossRef]
- Chen, J.; Guo, S.H.; Omran, M.; Gao, L.; Zheng, H.W.; Chen, G. Microwave-assisted preparation of nanocluster rutile TiO2 from titanium slag by NaOH-KOH mixture activation. Adv. Powder Technol. 2022, 33, 103549. [Google Scholar] [CrossRef]
- Li, S.C.; Kang, C.S.; Kim, S.C.; Kim, C.J.; Kang, C.J. The extraction of Ta, Nb and rare earths from fergusonite by using KOH sub-molten salt leaching. Hydrometallurgy 2021, 201, 105358. [Google Scholar] [CrossRef]
- Sun, H.-Q.; Song, J.; Sun, S.; Qu, J.-K.; Lu, W.; Qi, T. Decomposition kinetics of zircon sand in NaOH sub-molten salt solution. Trans. Nonferrous Met. Soc. China 2019, 29, 1948–1955. [Google Scholar] [CrossRef]
- Ongwandee, M.; Morrison, G.C.; Guo, X.; Chusuei, C.C. Adsorption of trimethylamine on zirconium silicate and polyethylene powder surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2007, 310, 62–67. [Google Scholar] [CrossRef]
- Zhang, M.; Salje, E.K.H.; Wang, A.H.; Li, X.J.; Xie, C.S.; Redfern, S.A.T.; Li, R.X. Vibrational spectroscopy of fast-quenched ZrSiO4 melts produced by laser treatments: Local structures and decomposed phases. J. Phys. Condens. Matter 2005, 17, 6363. [Google Scholar] [CrossRef]
- Zhao, C.M.; Wang, G.C.; Sheng-Li, L.I.; Xin-Gang, A.I.; Wang, Z.R.; Zhai, Y.C. Reaction pathway led by silicate structure transformation on decomposition of CaSiO3 in alkali fusion process using NaOH. Trans. Nonferrous Met. Soc. China 2015, 25, 3827–3833. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Singh, S.; Pandey, O.P. Neutron irradiation effects on optical and structural properties of silicate glasses. Mater. Chem. Phys. 2009, 115, 783–788. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Q.; Xu, M.; Wang, S.; You, J. In situ spectroscopic studies of decomposition of ZrSiO4 during alkali fusion process using various hydroxides. Rsc Adv. 2015, 5, 11658–11666. [Google Scholar] [CrossRef]
- Nasdala, L.; Irmer, G.; Wolf, D. The degree of metamictization in zircon: A Raman spectroscopic study. Eur. J. Mineral. 1995, 7, 471–478. [Google Scholar] [CrossRef]
- Kim, B.K.; Hamaguchi, H.O. Mode Assignments of the Raman Spectrum of Monoclinic Zirconia by Isotopic Exchange Technique. Phys. Status Solidi 1997, 203, 557–563. [Google Scholar] [CrossRef]
- Zhang, M.; Salje, E.K.H. Infrared spectroscopic analysis of zircon: Radiation damage and the metamictstate. J. Phys. Condens. Matter 2001, 13, 3057. [Google Scholar] [CrossRef]
- Guittet, M.J.; Crocombette, J.P.; Gautier-Soyer, M. Bonding and XPS chemical shifts in ZrSiO4 versus SiO2 and ZrO2 Charge transfer and electrostatic effects. Phys. Rev. B 2001, 63, 125117. [Google Scholar] [CrossRef]
- Moon, S.C.; Fujino, M.; Yamashita, H.; Anpo, M. Characterization of Zirconium−Silicon Binary Oxide Catalysts Prepared by the Sol−Gel Method and Their Photocatalytic Activity for the Isomerization of 2-Butene. J. Phys. Chem. B 1997, 101, 444–450. [Google Scholar] [CrossRef]
- Vipul, B.; Asad, S.; Bhargava, S.K.; Absar, A.; Murali, S. Zirconia enrichment in zircon sand by selective fungus-mediated bioleaching of silica. Langmuir 2007, 23, 4993–4998. [Google Scholar]
- Balan, E.; Trocellier, P.; Jupille, J.; Fritsch, E.; Muller, J.P.; Calas, G. Surface chemistry of weathered zircons. Chem. Geol. 2001, 181, 13–22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).