Unlocking the Potential of Sebacate: Investigating Its Role in the Inhibition of Filiform Corrosion on Organic Coated Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. SB Electrochemical Characterization in a Near-Neutral Environment
2.2. Electrochemical Simulated Study on the Inhibition of FFC
2.3. Preparation of Acrylic-Coated Steel Samples
2.4. Characterization and Aging of Acrylic-Coated Steel Samples
3. Results
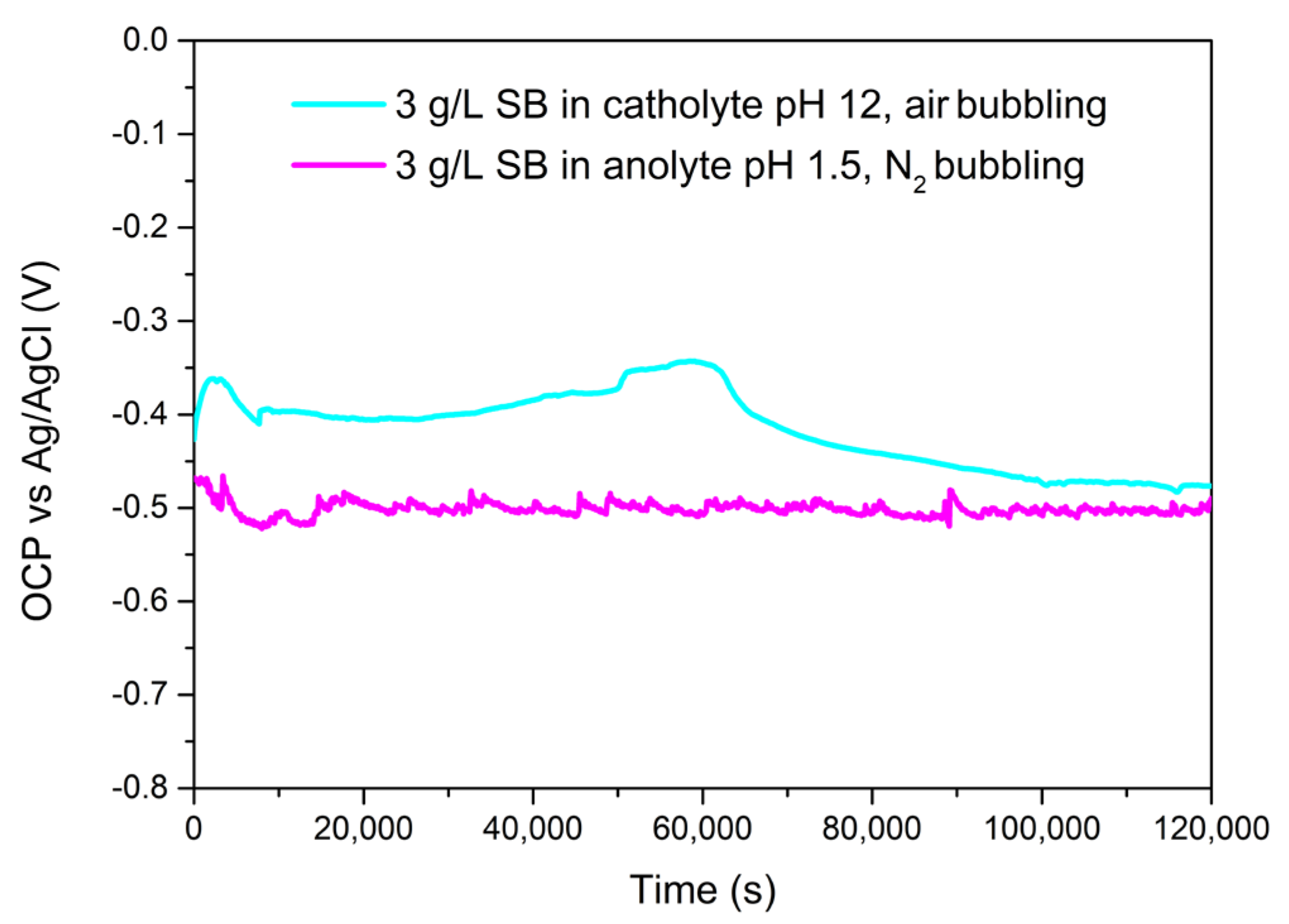
3.1. SB Effect Characterization
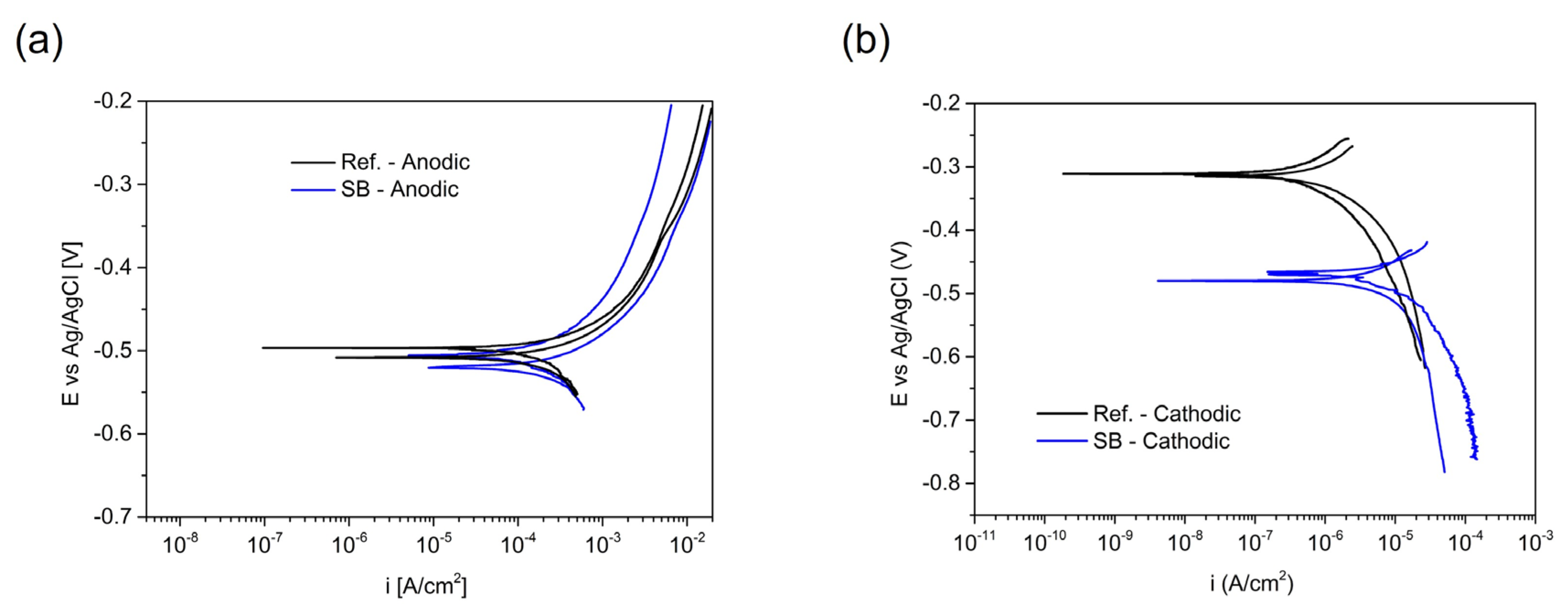
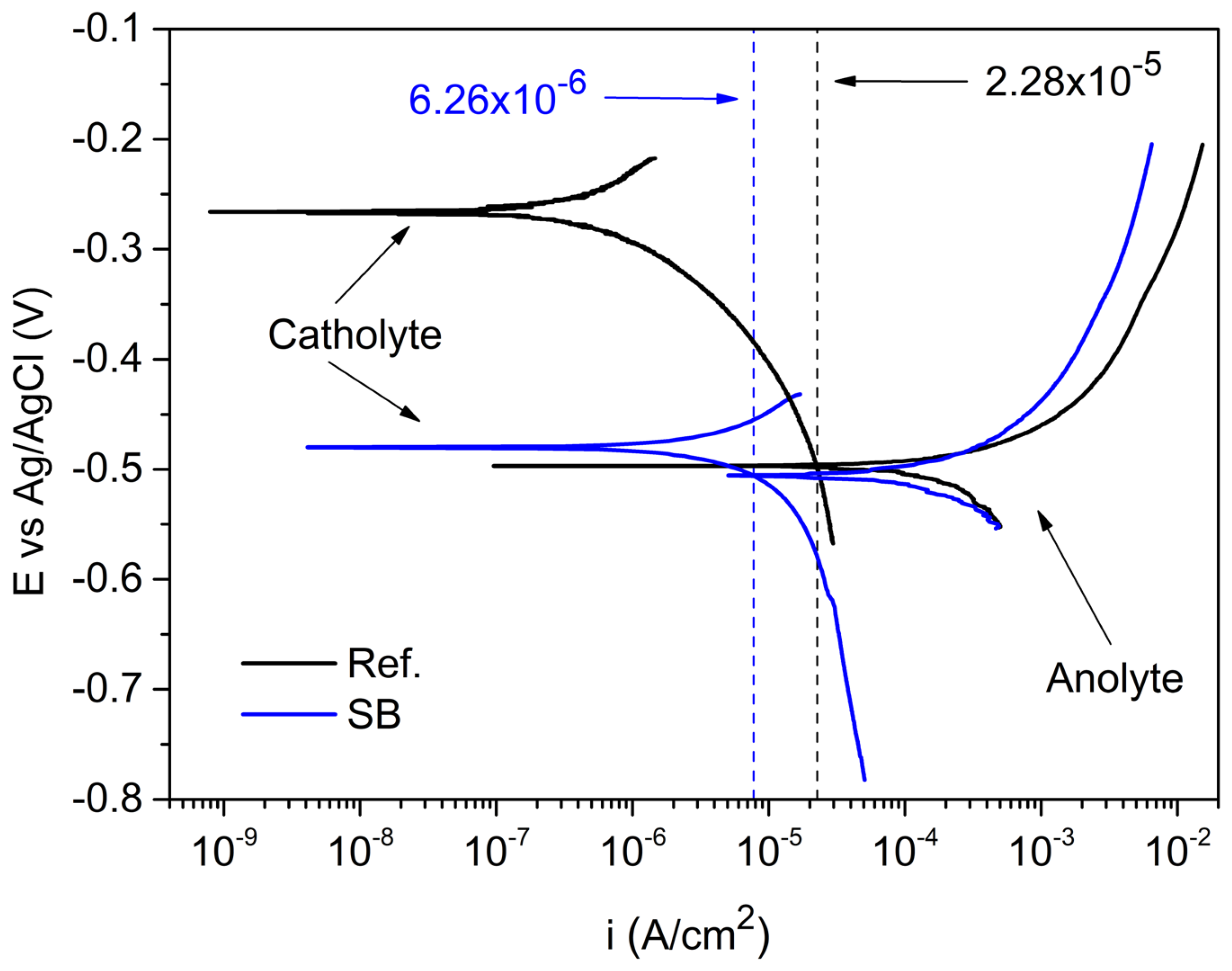
3.2. Prediction of FFC Mitigation Using an Electrochemical Simulated Method
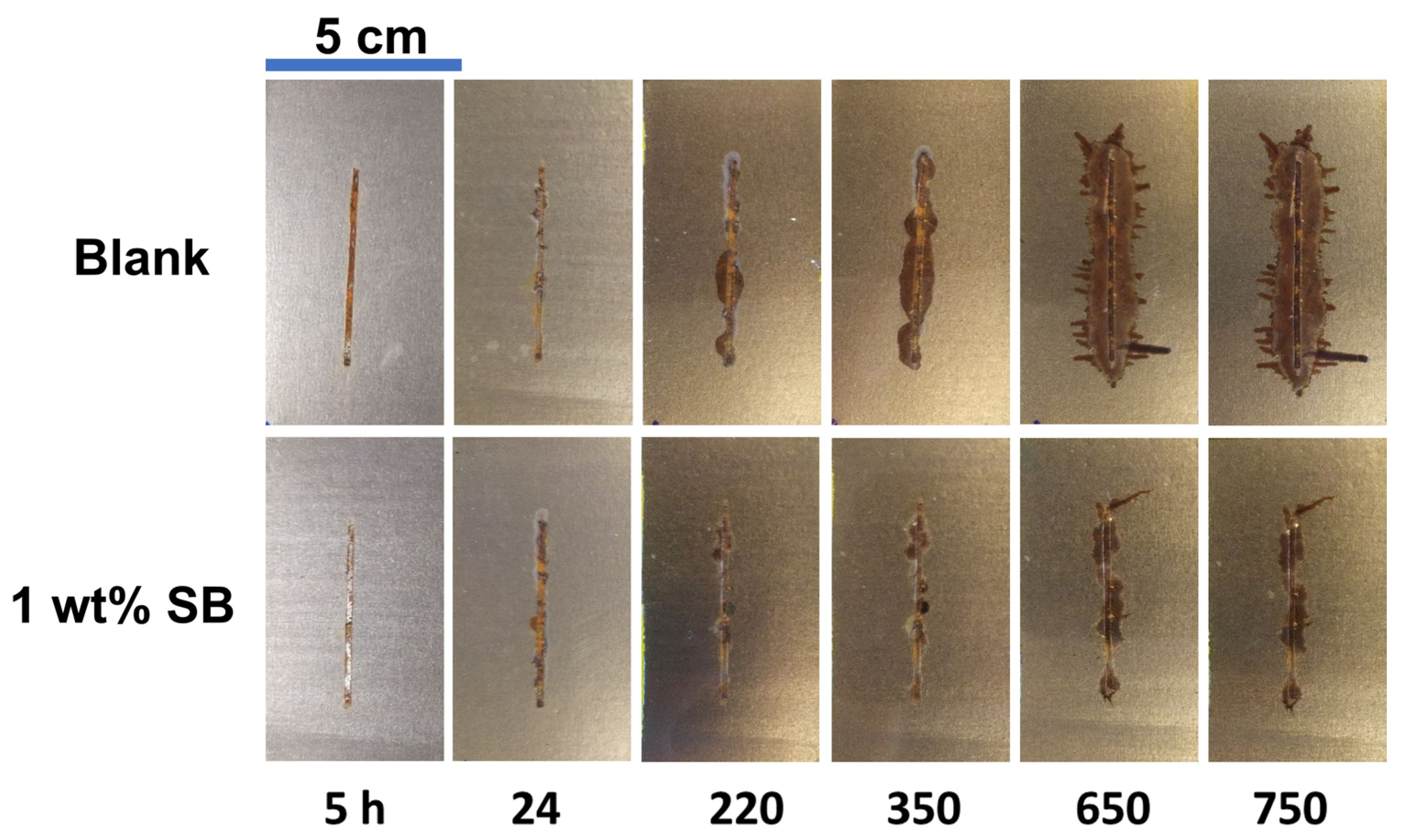
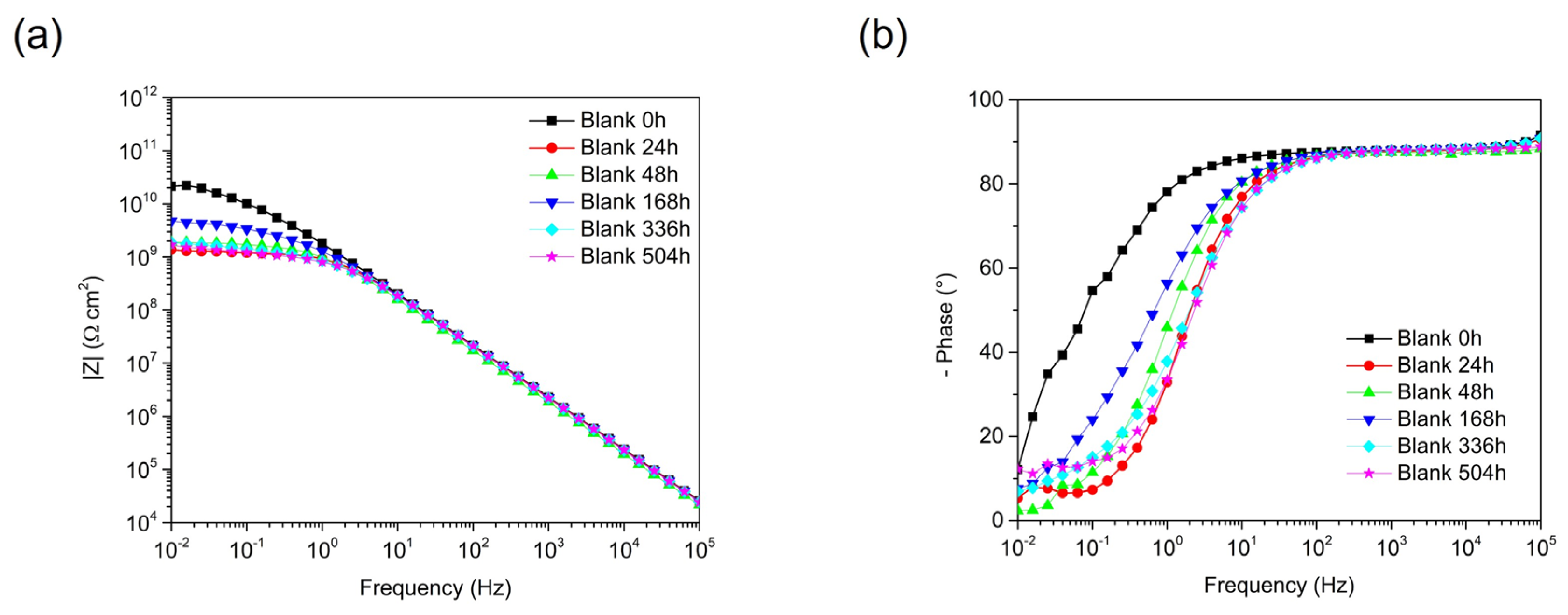
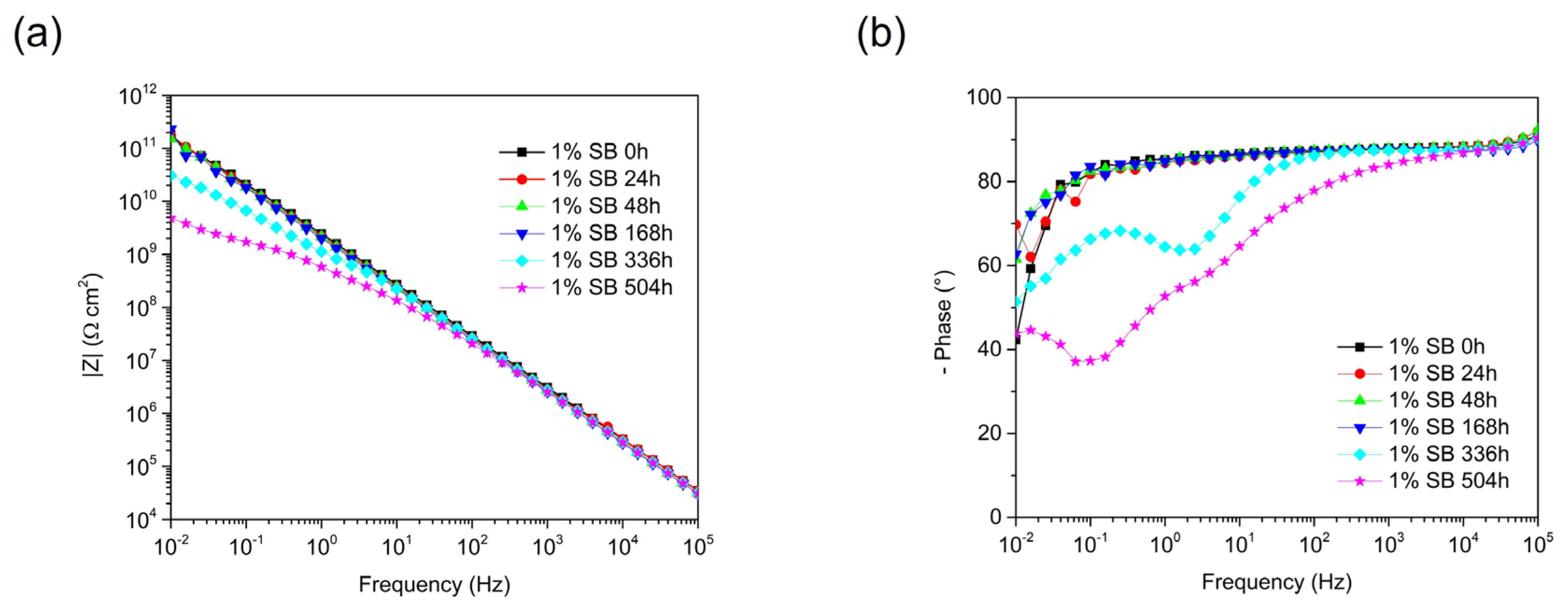
3.3. Acrylic-Coated Steel’s Durability
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Trentin, A.; Pakseresht, A.; Duran, A.; Castro, Y.; Galusek, D. Electrochemical Characterization of Polymeric Coatings for Corrosion Protection: A Review of Advances and Perspectives. Polymers 2022, 14, 2306. [Google Scholar] [CrossRef] [PubMed]
- Lyon, S.B.; Bingham, R.; Mills, D.J. Advances in Corrosion Protection by Organic Coatings: What We Know and What We Would like to Know. Prog. Org. Coat. 2017, 102, 2–7. [Google Scholar] [CrossRef]
- Grundmeier, G.; Schmidt, W.; Stratmann, M. Corrosion Protection by Organic Coatings: Electrochemical Mechanism and Novel Methods of Investigation. Electrochim. Acta 2000, 45, 2515–2533. [Google Scholar] [CrossRef]
- Pélissier, K.; Thierry, D. Powder and High-Solid Coatings as Anticorrosive Solutions for Marine and Offshore Applications? A Review. Coatings 2020, 10, 916. [Google Scholar] [CrossRef]
- Cristoforetti, A.; Rossi, S.; Deflorian, F.; Fedel, M. Exploring the Role of Passivating Conversion Coatings in Enhancing the Durability of Organic-Coated Steel Against Filiform Corrosion Using an Electrochemical Simulated Approach. Prog. Org. Coat. 2024, 189, 108357. [Google Scholar] [CrossRef]
- Milošev, I.; Frankel, G.S. Review—Conversion Coatings Based on Zirconium and/or Titanium. J. Electrochem. Soc. 2018, 165, C127–C144. [Google Scholar] [CrossRef]
- Bastos, A.C.; Ferreira, M.G.; Simões, A.M. Corrosion Inhibition by Chromate and Phosphate Extracts for Iron Substrates Studied by EIS and SVET. Corros. Sci. 2006, 48, 1500–1512. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ghasemi, E.; Mahdavian, M. Sodium Zinc Phosphate as a Corrosion Inhibitive Pigment. Prog. Org. Coat. 2014, 77, 1155–1162. [Google Scholar] [CrossRef]
- Mahdavian, A.M.; Attar, M.M. Investigation on Zinc Phosphate Effectiveness at Different Pigment Volume Concentrations via Electrochemical Impedance Spectroscopy. Electrochim. Acta 2005, 50, 4645–4648. [Google Scholar] [CrossRef]
- Prosek, T.; Thierry, D. A Model for the Release of Chromate from Organic Coatings. Prog. Org. Coat. 2004, 49, 209–217. [Google Scholar] [CrossRef]
- Kalenda, P.; Kalendová, A.; Štengl, V.; Antoš, P.; Šubrt, J.; Kváča, Z.; Bakardjieva, S. Properties of Surface-Treated Mica in Anticorrosive Coatings. Prog. Org. Coat. 2004, 49, 137–145. [Google Scholar] [CrossRef]
- Saurbier, K.; Schultze, J.W.; Geke, J. Temporary Inhibitors of Corrosion in Wet Atmosphere: Electrochemical Investigations of the Mechanism and Efficiency. Electrochim. Acta 1994, 39, 1171–1178. [Google Scholar] [CrossRef]
- Lahem, D.; Poelman, M.; Atmani, F.; Olivier, M.G. Synergistic Improvement of Inhibitive Activity of Dicarboxylates in Preventing Mild Steel Corrosion in Neutral Aqueous Solution. Corros. Eng. Sci. Technol. 2012, 47, 463–471. [Google Scholar] [CrossRef]
- Rocca, E.; Steinmetz, J. Inhibition of Lead Corrosion with Saturated Linear Aliphatic Chain Monocarboxylates of Sodium. Corros. Sci. 2001, 43, 891–902. [Google Scholar] [CrossRef]
- Georges, C.; Rocca, E.; Steinmetz, P. Synergistic Effect of Tolutriazol and Sodium Carboxylates on Zinc Corrosion in Atmospheric Conditions. Electrochim. Acta 2008, 53, 4839–4845. [Google Scholar] [CrossRef]
- Boisier, G.; Lamure, A.; Pébère, N.; Portail, N.; Villatte, M. Corrosion Protection of AA2024 Sealed Anodic Layers Using the Hydrophobic Properties of Carboxylic Acids. Surf. Coat. Technol. 2009, 203, 3420–3426. [Google Scholar] [CrossRef]
- Shulman, G.P.; Bauman, A.J. Organic Acid Sealants for Anodized Aluminum—A New Method for Corrosion Protection. Met. Finish. 1995, 93, 16–19. [Google Scholar] [CrossRef]
- Hefter, G.T.; North, N.A.; Tan, S.H. Organic Corrosion Inhibitors in Neutral Solutions; Part 1—Inhibition of Steel, Copper, and Aluminum by Straight Chain Carboxylates. Corrosion 1997, 53, 657–667. [Google Scholar] [CrossRef]
- Ormellese, M.; Lazzari, L.; Goidanich, S.; Fumagalli, G.; Brenna, A. A Study of Organic Substances as Inhibitors for Chloride-Induced Corrosion in Concrete. Corros. Sci. 2009, 51, 2959–2968. [Google Scholar] [CrossRef]
- Rammelt, U.; Reinhard, G. Application of Electrochemical Impedance Spectroscopy (EIS) for Characterizing the Corrosion-Protective Performance of Organic Coatings on Metals. Prog. Org. Coat. 1992, 21, 205–226. [Google Scholar] [CrossRef]
- Rammelt, U.; Koehler, S.; Reinhard, G. Electrochemical Characterisation of the Ability of Dicarboxylic Acid Salts to the Corrosion Inhibition of Mild Steel in Aqueous Solutions. Corros. Sci. 2011, 53, 3515–3520. [Google Scholar] [CrossRef]
- Godinez-Alvarez, J.M.; Mora-Mendoza, J.L.; Rodriguez-Betancourt, E.; Zavala- Olivares, G.; Gonzalez-Nunez, M.A. Inhibition of Ferrous Metal Corrosion by Carboxylates. In Proceedings of the CORROSION 2004, New Orleans, Louisiana, 28 March 2004. [Google Scholar]
- Mayne, J.; Page, C. Inhibition of the Corrosion of Iron by Benzoate and Acetate Ions. Br. Corros. J. 1974, 9, 223–226. [Google Scholar] [CrossRef]
- Ochoa, N.; Moran, F.; Pébère, N. The Synergistic Effect between Phosphonocarboxylic Acid Salts and Fatty Amines for the Corrosion Protection of a Carbon Steel. J. Appl. Electrochem. 2004, 34, 487–493. [Google Scholar] [CrossRef]
- Caballero, D.; Beltrán-Cobos, R.; Tavares, F.; Cruz-Yusta, M.; Granados, L.S.; Sánchez-Moreno, M.; Pavlovic, I. The Inhibitive Effect of Sebacate-Modified LDH on Concrete Steel Reinforcement Corrosion. ChemEngineering 2022, 6, 72. [Google Scholar] [CrossRef]
- Nguyen, D.T.; To, H.T.X.; Gervasi, J.; Paint, Y.; Gonon, M.; Olivier, M.G. Corrosion Inhibition of Carbon Steel by Hydrotalcites Modified with Different Organic Carboxylic Acids for Organic Coatings. Prog. Org. Coat. 2018, 124, 256–266. [Google Scholar] [CrossRef]
- Bouali, A.C.; Serdechnova, M.; Blawert, C.; Tedim, J.; Ferreira, M.G.S.; Zheludkevich, M.L. Layered Double Hydroxides (LDHs) as Functional Materials for the Corrosion Protection of Aluminum Alloys: A Review. Appl. Mater. Today 2020, 21, 100857. [Google Scholar] [CrossRef]
- Cristoforetti, A.; Deflorian, F.; Rossi, S.; Fedel, M. On the Occurrence of Filiform Corrosion on Organic Coated Carbon Steel Exposed to Cyclic Aging Test. Corrosion 2023, 79, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Cristoforetti, A.; Izquierdo, J.; Souto, R.M.; Deflorian, F.; Fedel, M.; Rossi, S. In-Situ Measurement of Electrochemical Activity Related to Filiform Corrosion in Organic Coated Steel by Scanning Vibrating Electrode Technique and Scanning Micropotentiometry. Corros. Sci. 2024, 227, 111669. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.N. The Mechanism of Group (I) Chloride Initiated Filiform Corrosion on Iron. Electrochem. Commun. 2003, 5, 871–877. [Google Scholar] [CrossRef]
- Watson, T.M.; Coleman, A.J.; Williams, G.; McMurray, H.N. The Effect of Oxygen Partial Pressure on the Filiform Corrosion of Organic Coated Iron. Corros. Sci. 2014, 89, 46–58. [Google Scholar] [CrossRef]
- Bautista, A. Filiform Corrosion in Polymer-Coated Metals. Prog. Org. Coat. 1996, 28, 49–58. [Google Scholar] [CrossRef]
- Ruggeri, R.T.; Beck, T.R. An Analysis of Mass Transfer in Filiform Corrosion. Corrosion 1983, 39, 452–465. [Google Scholar] [CrossRef]
- Mol, J.M.C.; Hinton, B.R.W.; Van Der Weijde, D.H.; De Wit, J.H.W.; Van Der Zwaag, S. A Filiform Corrosion and Potentiodynamic Polarisation Study of Some Aluminium Alloys. J. Mater. Sci. 2000, 35, 1629–1639. [Google Scholar] [CrossRef]
- Cristoforetti, A.; Rossi, S.; Deflorian, F.; Fedel, M. An Electrochemical Study on the Mechanism of Filiform Corrosion on Acrylic-Coated Carbon Steel. Prog. Org. Coat. 2023, 179, 107525. [Google Scholar] [CrossRef]
- Pourbaix, M.; Burbank, J. Atlas D-Equilibres Electrochimiques. J. Electrochem. Soc. 1964, 111, 14C. [Google Scholar] [CrossRef]
- ASTM D2803-09; Standard Guide for Testing Filiform Corrosion Resistance of Organic Coatings on Metal. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM B117-19; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM International: West Conshohocken, PA, USA, 2019.
- Cristoforetti, A.; Parola, F.; Parrino, F.; Izquierdo, J.; Souto, R.M.; Rossi, S.; Deflorian, F.; Fedel, M. Sebacate Intercalated CaAl Layered Double Hydroxide Pigments for Corrosion Protection of Low Carbon Steel: Anion Exchange and Electrochemical Properties. Appl. Clay Sci. 2024, 250, 107300. [Google Scholar] [CrossRef]
- Fedel, M.; Poelman, M.; Olivier, M.; Deflorian, F. Sebacic Acid as Corrosion Inhibitor for Hot-Dip Galvanized (HDG) Steel in 0.1 M NaCl. Surf. Interface Anal. 2019, 51, 541–551. [Google Scholar] [CrossRef]
- Peng, Y.; Hughes, A.E.; Mardel, J.I.; Deacon, G.B.; Junk, P.C.; Catubig, R.A.; Forsyth, M.; Hinton, B.R.W.; Somers, A.E. Dual Function of Rare Earth Carboxylate Compounds on the Barrier Properties and Active Corrosion Inhibition of Epoxy Coatings on Mild Steel. Prog. Org. Coat. 2023, 185, 107870. [Google Scholar] [CrossRef]
- Catubig, R.; Seter, M.; Neil, W.; Forsyth, M.; Hinton, B. Effects of Corrosion Inhibiting Pigment Lanthanum 4-Hydroxy Cinnamate on the Filiform Corrosion of Coated Steel. J. Electrochem. Soc. 2011, 158, C353–C358. [Google Scholar] [CrossRef]
- Glover, C.F.; Williams, G. Inhibition of Corrosion-Driven Organic Coating Delamination and Filiform Corrosion on Iron by Phenyl Phosphonic Acid. Prog. Org. Coat. 2017, 102, 44–52. [Google Scholar] [CrossRef]
- Tokutake, K.; Nishi, H.; Ito, D.; Okazaki, S.; Serizawa, Y. Relationship between Degradation Characteristics of Organic Coating on Internal Bottom Plate of Oil Storage Tank and Constant-Phase Element Parameter Values. Prog. Org. Coat. 2015, 87, 69–74. [Google Scholar] [CrossRef]




| Sample | Rct (Ω·cm2) | Y0 (S·cm−2·sn) | n | Rs (Ω·cm2) | IE% |
|---|---|---|---|---|---|
| Ref. | 1.21 × 103 | 5.45 × 10−4 | 8.14 × 10−1 | 3.75 × 102 | - |
| SB | 6.25 × 104 | 8.99 × 10−5 | 8.08 × 10−1 | 2.97× 102 | 98 |
| Sample | Inhibitor Addition | iCORR FFC (A/cm2) | ∆ECORR (V) |
|---|---|---|---|
| Ref. | - | 2.28 × 10−5 | 0.230 |
| SB | 3 g/L | 6.26 × 10−6 | 0.030 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristoforetti, A.; Rossi, S.; Deflorian, F.; Fedel, M. Unlocking the Potential of Sebacate: Investigating Its Role in the Inhibition of Filiform Corrosion on Organic Coated Steel. Metals 2024, 14, 623. https://doi.org/10.3390/met14060623
Cristoforetti A, Rossi S, Deflorian F, Fedel M. Unlocking the Potential of Sebacate: Investigating Its Role in the Inhibition of Filiform Corrosion on Organic Coated Steel. Metals. 2024; 14(6):623. https://doi.org/10.3390/met14060623
Chicago/Turabian StyleCristoforetti, Andrea, Stefano Rossi, Flavio Deflorian, and Michele Fedel. 2024. "Unlocking the Potential of Sebacate: Investigating Its Role in the Inhibition of Filiform Corrosion on Organic Coated Steel" Metals 14, no. 6: 623. https://doi.org/10.3390/met14060623
APA StyleCristoforetti, A., Rossi, S., Deflorian, F., & Fedel, M. (2024). Unlocking the Potential of Sebacate: Investigating Its Role in the Inhibition of Filiform Corrosion on Organic Coated Steel. Metals, 14(6), 623. https://doi.org/10.3390/met14060623








