Abstract
Despite the increasing interest in enhancing the electrochemical stability of Al alloys through protective coatings, the role of electron donor agents during coating formation remains poorly understood in terms of morphological control and anticorrosion properties in aqueous environments. In this context, 1H-Benzotriazole (BTA) was utilized as a proof of concept to regulate the in situ reactive integration of V2O5 into the alumina layer via the plasma electrolytic oxidation of a 6061 Al alloy. BTA played a crucial role in chemically incorporating V2O5 into the alumina coating by supplying electrons to VO3− ions, facilitating their reduction. The quantity of BTA added to the electrolyte was found to influence defect morphology and concurrently enhance the chemical incorporation of V2O5. Notably, corrosion measurements revealed that the less porous hybrid film formed with higher corrosion resistance was associated with the utilization of increased concentrations of BTA. These findings highlight the potential of BTA in modifying the structure and improving the ability of alumina coatings to resist corrosion, enabling advanced applications in protecting Al alloys from corrosion.
1. Introduction
Plasma electrolytic oxidation (PEO) is a wet-coating approach that is favored for modifying the compounds and surface structure of various metallic materials. It enables the production of thick, hard, and strongly adherent coatings on valve metals, such as Mg, Al, and Ti, as well as their alloys [1]. The coatings fabricated by PEO are primarily influenced by the conditions associated with the electrolyte and processing parameters [2,3,4,5,6]. Among all the processing variables, the electrolyte’s role has been noted to considerably alter the structure of the coatings, thus affecting their protective characteristics [7]. To date, various inorganic-containing electrolytes have been investigated for their benefits in generating oxide layers on the surfaces of light metals. These electrolytes include silicate, aluminate, phosphate, and borate, each with different chemical additives [8,9,10,11].
Incorporating metallic oxide particles into the PEO coating via electrochemical and electrophoretic mechanisms has been highlighted as an efficient method for boosting their protective attributes [12]. Numerous particles, including SiO2, ZrO2, MoO2, WO3, TiO2, CeO2, VO2, and V2O5, were integrated into coatings applied on Al and its alloys [13,14,15,16,17,18,19,20,21,22]. Among them, it was stated that the inclusion of V2O5 particles would be beneficial to boost the anticorrosive performance of Al alloys. For example, Kwon et al. [21] documented that the introduction of V2O5 into the coating layer of Al alloy led to decreased porosity and enhanced electrochemical stability. These particles, being more electrochemically inert, create barriers against corrosive ions, thus impeding corrosion. In another work, the anticorrosive properties of PEO-coated Al alloy were improved by incorporating V2O5 nanoparticles [22].
On the other hand, incorporating organic additives, such as alcohols, organic acid salts, organic amides, and aromatics, into electrolytes during the PEO process was found to be an effective method to enhance the performance of the coatings [1]. Benzotriazole (BTA) functions as an effective corrosion inhibitor via the formation of a protective layer on metal surfaces [23,24]. This layer serves as a protective barrier, preventing corrosion by shielding the metal from environmental factors. Widely used due to its versatility, BTA is a cost-effective solution in various industries for enhancing metal corrosion resistance. Kaseem et al. [24] showed that the surface modification of an Al alloy via PEO with phosphate and BTA additives led to a reduction in micropore size, thereby limiting the infiltration of aggressive species during corrosion tests.
In earlier investigations, the predominant focus in research on the electrochemical functions of chemical additives in PEO-induced metal oxide formation was on incorporating V2O5. This incorporation entailed two distinct pathways: the initial one involved incorporating V2O5 through the direct addition of V2O5 nanoparticles or utilizing vanadate salts. As far as our understanding extends, there is a notable absence of reports delving into the potential impacts of organic agents on the level of incorporation of V2O5 into the alumina layer. One of the additives that could potentially influence the performance of the PEO coatings is capable of donating electrons during the oxidation process. This capability could contribute to modulating the electrochemical reactions occurring at the alloy–electrolyte interface [25,26]. BTA, as a common electron donor agent, is expected to initiate the plasma-assisted electrochemical reduction in vanadate ions (VO3−) present in the electrolyte. In the present study, an Al alloy underwent PEO treatment in vanadate electrolytes containing varying concentrations of BTA. The resulting hybrid films, primarily composed of V2O5 and Al2O3, were characterized in terms of their structure and composition, and a corrosion assessment.
2. Experimental Procedures
2.1. Fabrication of Hybrid Films
In this study, 6061 Al alloy sheets measuring 25 mm × 20 mm × 2 mm were employed as substrates. Before the PEO process, the samples underwent mechanical grinding with emery papers up to #2400 grit, ultrasonic cleaning in pure ethanol, and drying in warm air. To fabricate the hybrid film through PEO, the Al alloy served as the anode, while a stainless-steel net acted as the cathode. A sequence of PEO processes was carried out using alkaline–phosphate electrolytes with varying concentrations of 1H-benzotriazole (C6H5N3) BTA (soluble organic compound), while keeping the NH4VO3 (soluble salt) concentration consistent. The process was performed for 3 min at a current density of 100 mA/cm2 and a frequency of 60 Hz using an AC power supply (ACP 1010, Gunpo, Republic of Korea), as outlined in Table 1.

Table 1.
Composition of electrolytes with the variation BTA concentration used for hybrid films.
2.2. Characterization
Surface structures of the hybrid films were observed using field emission-scanning electron microscopy (FE-SEM, HITACHI S-4800, Hitachi, Tokyo, Japan) linked with an energy-dispersive X-ray spectroscopy detector (EDS, Horiba Inc., Kyoto, Japan). Before SEM observation, the samples were subjected to a platinum (Pt) sputtering process to enhance their conductivity and improve imaging quality. The size and fraction of micro-pores were measured via SEM observations taken from at least ten different areas for each condition, aided by image analysis software (version 1.47 for Windows, 64 bit, free software, National Institutes of Health, Bethesda, MD, USA). Surface roughness assessments (AFM, ToscaTM Analysis, Anton Paar, Graz, Austria) were performed by taking measurements five times longitudinally and five times laterally across each specimen’s surface. The outcomes are expressed as the arithmetic mean roughness (Ra). Constituent compounds were identified via X-ray diffraction (XRD, RIGAKU, D-MAX 2500, Tokyo, Japan) in a scanning range of 20 to 90 degrees with a step size of 0.05 degrees. The hybrid films also underwent a thorough examination through X-ray photoelectron spectroscopy (XPS, VG Microtech, ESCA 2000, VG Microtech, London, UK) to confirm the correlation between BTA and the incorporation of V2O5.
2.3. Corrosion Tests
The corrosion assessments were performed in a 3.5 wt.% NaCl electrolyte to simulate the salinity of typical seawater. Potentiodynamic polarization (PDP) tests were carried out over the range of −250 to +400 mV relative to the open-circuit potential (OCP) at a scan rate of 1 mV/s, while electrochemical impedance spectroscopy (EIS) tests were conducted in the frequency range of 106 to 0.1 Hz with an AC amplitude of 10 mV over the OCP. All corrosion measurements were performed after stabilizing the OCP for 1 h using a Gamry Potentiostat, Interface 1010, Philadelphia, PA, USA. The experiments were conducted at least five times to guarantee the acquisition of highly precise data.
3. Results and Discussion
3.1. BTA Concentration and Voltage Relationship
The voltage–time plots depicted in Figure 1 illustrate the formation of oxide layers in an alkaline–phosphate–vanadate electrolyte with varying concentrations of BTA. Regardless of the BTA concentrations, the curves exhibit three distinct regions, denoted as regions I, II, and III.
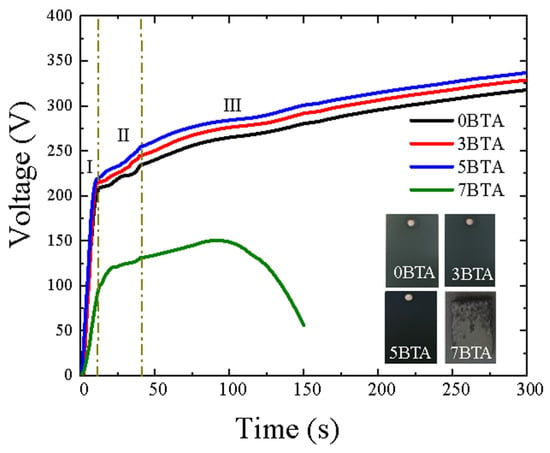
Figure 1.
Voltage–time curves observed during the PEO process of 6061 Al alloy in an alkaline–phosphate–vanadate electrolyte with different concentrations of BTA. The inset displays the color variation among the coated samples, namely 0BTA, 3BTA, 5BTA, and 7BTA samples.
At the onset, in region I, a rapid and sharp linear rise in voltage is observed across all samples, which could be ascribed to the swift formation of a thin passive layer across the entire sample surface [27,28,29]. The breakdown potential is the voltage threshold at which dielectric breakdown occurs in the barrier film during the PEO process. This signifies the point where the barrier film, originally functioning as a resistor to current flow, undergoes breakdown at regions of lower resistance. This transition is marked by the appearance of spark discharges on the surface of the substrate. These spark discharges indicate the initiation of a localized breakdown, marking the transition from uniform barrier film growth to the onset of PEO.
As depicted in Figure 1, the breakdown voltage experiences variations based on the content of BTA in the electrolyte. The breakdown voltage values were ~208 V, ~214 V, ~217 V, and ~100.1 V for 0BTA, 3BTA, 5BTA, and 7BTA samples, respectively. Following the breakdown voltage and during the growth of the PEO coating (region II), the rate of voltage increment diminishes, showing a slower increase with a reduced slope across all samples until the completion of the PEO process. Since the conductivity of the electrolytes remained nearly identical and was unaffected by the concentration of BTA, the variations in breakdown voltage values can be attributed to the increased incorporation of vanadium oxides (as will be proven later) facilitated by higher BTA concentrations. This outcome aligns with the observations made by Tang et al. [30], who found a relationship between the breakdown voltage and the melting point of oxide elements located at the interface of the anode and electrolyte. Thus, it was proposed that the inclusion of nanoparticles with elevated melting points could potentially increase the breakdown voltage.
In stage III, there was a consistent upward trend in the voltage of the 3BTA and 5BTA samples compared to the 0BTA sample, indicating the coating’s augmented resistance to dielectric breakdown. This pattern was characterized by the uniform advancement of the hybrid film across all electrolyte conditions [31]. Nonetheless, an unusual behavior surfaced in the 7BTA sample towards the conclusion of stage III, marked by a substantial drop in voltage, suggesting a partial inhibition of coating growth [32]. Therefore, the opportunity to conduct a microstructural and electrochemical analysis of the surface of the 7BTA sample was not pursued. The observed outcome is likely due to the increasing concentration of BTA, acknowledged as a weak electrolyte, wherein a notable portion of BTA particles may not completely dissociate within the electrolyte solution, thereby disturbing ionic equilibrium and interactions between the electric field and BTA particles [33]. On the other hand, the coating colors of samples with 0BTA, 3BTA, and 5BTA concentrations displayed varying degrees of darkness, with the darkness intensifying as the BTA concentration increased (see inset in Figure 1), ultimately resulting in the 5BTA sample having a black appearance.
3.2. Morphology of the Hybrid Films
Figure 2a,d,g depict the surface morphologies of 0BTA, 3BTA, and 5BTA specimens produced via PEO in electrolytes with varying contents of BTA. Plasma activity during the PEO process is responsible for the creation of a porous structure on the surface of 0BTA, 3BTA, and 5BTA samples [34,35,36].
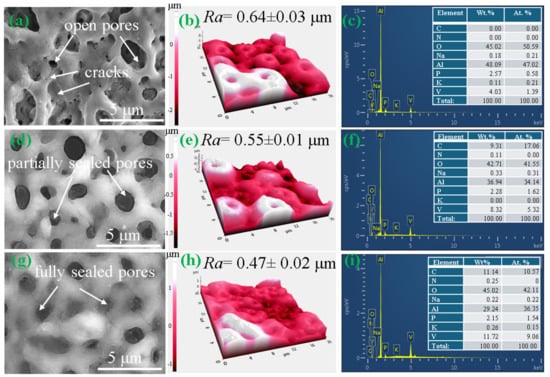
Figure 2.
SEM images, AFM results, and the corresponding EDS analysis conducted on the surfaces of the coated samples, where (a–c) 0BTA, (d–f) 3BTA, and (g–i) 5BTA.
Moreover, some cracks were observed on the 0BTA sample, arising from the swift solidification of molten oxides upon contact with the cold electrolyte. Pore size and porosity level calculations were conducted for the 0BTA, 3BTA, and 5BTA samples, and the outcomes are detailed in Table 2.

Table 2.
The correlation between BTA concentration and the porosity and pore size of the oxide layers produced on 6061 Al alloy through the PEO process.
The introduction of BTA resulted in a decrease in both pore size and porosity, with the 5BTA sample, made in an electrolyte with 5 g/L of BTA, exhibiting the smallest average pore size (~0.87 µm) and the lowest porosity (~6.81%). According to the AFM findings depicted in Figure 2b,e,h, the Ra values for 0BTA, 3BTA, and 5BTA samples were approximately 0.64 ± 0.03, 0.55 ± 0.01 μm, and 0.47 ± 0.02 μm, respectively. This indicates that incorporating BTA in the electrolyte during PEO results in a reduction in Ra values within the oxide films. The reduced surface roughness noticed in the 5BTA samples could be linked to the significant collapse of plasma bubbles, which is likely impeded during the adsorption of BTA molecules. The EDS area findings presented in Figure 2c,f,i show the existence of Al, O, P, K, Na, and V elements in all specimens, while C and traces of N elements were detected in samples coated with electrolytes containing BTA (3BTA and 5BTA). The existence of Al was linked to the Al alloy substrate, whereas the presence of other elements stemmed from the electrolyte solution. Given that BTA in the electrolyte was the sole source of C and N, it was confirmed that BTA successfully participated in plasma-assisted electrochemical reactions. With 5 g/L of BTA present in the electrolyte, the 5BTA sample displayed higher levels of the V element compared to other samples, indicating the induced integration of V-containing compounds (presumably V2O5, as subsequently verified). Hence, the inclusion of BTA in the electrolyte substantially fostered the development of the hybrid film and the assimilation of V oxide. This observation aligns with the EDS mapping shown in Figure 3, unveiling the consistent dispersion of V and O throughout the hybrid film.

Figure 3.
EDS mappings conducted on the surface of the 5BTA sample, as depicted in Figure 2g. The mappings include (a) the EDS layered image, (b) oxygen, (c) aluminum, (d) phosphorus, (e) sodium, (f) potassium, (g) vanadium, (h) nitrogen, and (i) carbon.
Figure 4a–c present cross-sectional morphologies of the 0BTA, 3BTA, and 5BTA samples. The average thickness was found to be consistent (9.15 ± 1.20 μm) across the 0BTA, 3BTA, and 5BTA samples. This indicates that the addition of BTA had minimal impact on the thickness of the samples, likely because they were exposed to identical current densities for a brief duration [37]. The coating layers of each sample revealed a dual structure consisting of inner and outer layers. Remarkably, the inner layer exhibited greater density despite its thinner dimensions compared to the porous outer layer. Interestingly, the compactness of the hybrid films was significantly increased in the 3BTA and 5BTA samples compared to the 0BTA sample. This increase is attributed to the favorable influence of V2O5 incorporation facilitated by the addition of BTA, potentially impeding the formation of micropores. The EDS point analyses conducted at different positions (Figure 4d), such as 1 and 2 from the 0BTA sample, 3 and 4 from the 3BTA sample, and 5 and 6 from the 5BTA sample, showed a notable trend: the incorporation of the V element tended to increase with an increase in the content of BTA. The denser coating layer enriched with stable V2O5 in the 5BTA sample would offer promising protection against substrate corrosion.
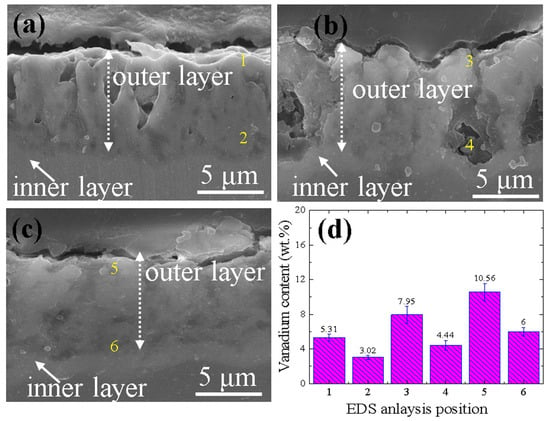
Figure 4.
Cross-sectional images of the (a) 0BTA sample, (b) 3BTA sample, and (c) 5BTA sample. (d) shows the variation in the wt.% content of vanadium element at points 1 and 2 from the 0BTA sample, points 3 and 4 from the 3BTA sample, and points 5 and 6 from the 5BTA sample.
3.3. Compositional Analysis
XRD patterns of 0BTA, 3BTA, and 5BTA specimens resulting from the oxidation of the substrate specimens in BTA-containing electrolytes are presented in Figure 5a. γ-Al2O3, α-Al2O3, AlPO4, α-Al2O3, and V2O5 were identified across all samples. Moreover, peaks originating from the Al substrate were detected in all samples, likely owing to X-ray infiltration through micro-pores within the hybrid films. Notably, the intensities of V2O5-related peaks in the 3BTA and 5BTA samples exceeded those in the 0BTA sample, suggesting the potential influence of BTA in promoting the amount of V2O5 that was incorporated into the hybrid film. No carbon-containing compounds were detected in the 3BTA and 5BTA samples, possibly indicating either the decomposition of BTA under plasma conditions or its incorporation in amorphous forms [38].
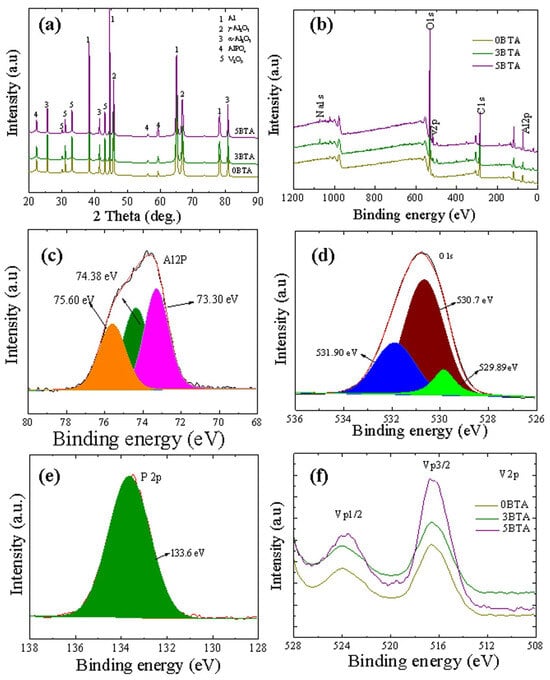
Figure 5.
(a) XRD patterns of the 0BTA, 3BTA, and 5BTA samples. (b) Wide scan spectra of 0BTA, 3BTA, and 5BTA samples. (c–f) The high-resolution spectra of Al2p, O1s, P2p, and V2p for the 5BTA sample.
XPS analysis verified the chemical composition of the 0BTA, 3BTA, and 5BTA samples, as illustrated in the XPS survey spectra presented in Figure 5b. Across all samples, the hybrid films demonstrated a consistent composition, comprising Al, O, Na, K, P, V, and C, consistent with the EDS analysis findings depicted in Figure 2. Given the marginal disparities in the XPS spectra of the hybrid films, the high-resolution spectra of Al 2p, O 1s, P 2p, and V 2p elements are only presented for the 5BTA samples in Figure 5c–f. Specifically, the Al2p spectrum reveals three peaks, corresponding to α-Al2O3, γ-Al2O3, and AlPO4, with binding energies of approximately 74.38, 73.30, and 75.60 eV, respectively [39]. The lowered critical free energy characteristic of the γ-Al2O3 phase leads to the rapid cooling of molten oxides upon interaction with the electrolyte, facilitating the nucleation and subsequent solidification of the γ-Al2O3 phase. Conversely, a slower cooling rate favors the formation of the more enduring α-Al2O3 phase. Under optimal temperature conditions, the metastable γ-Al2O3 phase can transfer into the stable α-Al2O3 phase (Equations (1)–(3)). The O1s spectra fit Al2O3, V2O5, and AlPO4 corresponding to ~530.7, ~529.89 and ~531.9 eV, respectively [40].
In the deconvoluted spectrum of P2p shown in Figure 5e, a solitary peak is noticed at ~133.6 eV, corresponding to AlPO4. In Figure 5b, the high-resolution spectra of V2p, ranging from ~512 eV to ~528 eV, are examined. Specifically, the peak at approximately 523.9 eV corresponds to V2p1/2, while the one at around 516.58 eV is associated with V2p3/2, consistent with the earlier results [41]. It is interesting to note that the V2p1/2 and V2p3/2 peaks were significantly higher for the 5BTA sample compared to the 0BTA and 3BTA samples. This suggests the more substantial incorporation of V2O5 in the BTA sample, consistent with the results obtained from EDS analyses and the XRD patterns presented in Figure 1, Figure 2 and Figure 3.
3.4. Electrochemical Behavior
The micro-pores on the surface of the PEO coating layer provide a short-circuit path for the easier infiltration of the corrosion medium into the inner region, close to the substrate [42,43]. This infiltration leads to a change in the local pH [44], which decreases the energy barrier of the electrical double layer against electrochemical corrosion. Consequently, the dissolution of the oxide coating is triggered [45], leading to surface degradation and a reduction in the overall endurance of the coating in a corrosive environment.
To explore how variations in BTA concentration influence the corrosion tendencies of 0BTA, 3BTA, and 5BTA samples, PDP curves are depicted in Figure 6a. A comparative analysis of their results with those of the uncoated substrate was conducted. The Ecorr and icorr were calculated using the Tafel extrapolation method, whereas the Stern–Geary equation was used to determine the values of polarization resistance (Rp) (Equation (4)). In this equation, βa and βc represent the Tafel slopes (anodic and cathodic), respectively [46]. Table 3 summarizes the results obtained from these investigations.
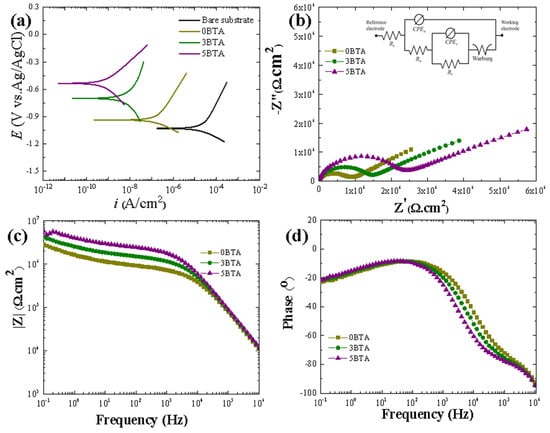
Figure 6.
(a) PDP tests of the 0BTA, 3BTA, and 5BTA samples. (b) Nyquist plots, (c) Bode impedance plots, and (d) Bode phase plots. The inset in Figure 6b shows the circuit model used for fitting the impedance results.

Table 3.
PDP parameters of the bare substrate and hybrid films after 1 h immersion in 3.5 wt.% NaCl electrolyte.
A positive Ecorr and/or a low icorr, indicating a low corrosion rate, typically signify excellent corrosion resistance in a standard polarization curve [47,48].
In Figure 6a, there is a clear trend where the PDP curves move towards more positive potentials and reduced current densities as the BTA content increases from 0 to 5 g/L. The Ecorr of the 0BTA sample shifted in a nobler direction, from −0.94 to −0.53 V Ag/AgCl, upon treatment with 5 g/L of BTA in the solution, as depicted in Figure 6a and Table 3.
As commonly recognized, Ecorr correlates with the thermodynamic aspect, reflecting the susceptibility of the coating to corrosion, and is influenced solely by the composition of the PEO coatings [1,43]. Therefore, we hypothesized that the incorporation of V2O5 into the alumina coating might be responsible for the shift in Ecorr values towards nobler potentials upon PEO treatment in a solution with a BTA additive. Additionally, the 5BTA sample displayed the smallest icorr value compared to the other samples. The results showed a decrease of three orders of magnitude in comparison to the 0BTA sample, roughly one order below the 3 BTA sample. This result suggests the 5BTA sample offers better protection for the Al alloy substrate than the other samples. This difference shows that the 5BTA sample exhibits enhanced corrosion resistance, as also evidenced by its higher Rp value. The improved corrosion protection observed in the 5BTA sample can be attributed to its smaller pore size and reduced porosity, which result from the higher level of V2O5 incorporation compared to the 0BTA and 3BTA samples. V2O5 particles exhibit high chemical stability in neutral electrolytes, such as 3.5 wt. % NaCl solution. The increased presence of V2O5 in the 5BTA sample effectively hinders the transportation of corrosive ions toward the substrate during the corrosion test.
Figure 6b provides insights into the corrosion mechanism through EIS analysis, showcasing Nyquist plots that predominantly display resistive–capacitive characteristics, forming (partial) semi-circular patterns that are inversely linked to the coating dissolution kinetics. Furthermore, the EIS characteristics can be further elucidated through Bode plots (Figure 6c), illustrating impedance values alongside phase angles compared to frequency, contributing to a comprehensive understanding of the corrosion dynamics. In qualitative terms, the impedance modules are in line with the outcomes of the PDP tests, signifying a notable improvement in the anticorrosive properties of the hybrid film with the inclusion of BTA in the electrolyte. Notably, the 5BTA sample displays a superior performance, attributed to the optimal incorporation of V2O5. Moreover, the impedance characteristics of the PEO layer diverge from those of the 5BTA sample at lower frequencies. This indicates that the degree of V2O5 integration has a notable impact on the electrochemical behavior. In a quantitative approach, the EIS responses of samples can be likened to the behavior of circuit components, producing the parameter values systematically detailed in Table 4. Employing Equivalent Electrical Circuit (EEC) modeling enables the disentanglement of various processes occurring in the working electrode. The assigned EEC for all samples’ layers is illustrated as an inset in Figure 6b. In this configuration, Rs denotes the resistance of the corrosive solution; Ro and CPEo indicate the capacitive and resistive properties of the porous outer film. Meanwhile, Ri and CPEi denote the corresponding parameters of the dense inner film [49].

Table 4.
Outcomes of the EIS examinations on the 0BTA, 3BTA, and 5BTA specimens submerged in a 3.5 wt.% NaCl electrolyte.
To address the variation in time constants across the non-uniform surface of the plasma-modified PEO layer, CPE is employed instead of a conventional capacitor. As outlined in Table 4, the values of Ri exceed those of Ro, irrespective of the electrolyte composition, which can be ascribed to the free-defective structure of the inner layer. The values of Ro demonstrated an increase with the increase in BTA concentration to 5 g/L. The extent of the improvement in the values of Ro depends on the amount of V2O5 that is incorporated into the hybrid; thus, the 5BTA sample exhibited the highest Ro value in comparison to the 0BTA and 3BTA samples. Moreover, the high value of Ri in 5BTA, in comparison to the counterparts’ values in other samples, suggests that the incorporation of V2O5 is not only localized in the outer part of the hybrid film but also extends to the inner layer of the film. On the other hand, the parameters of the CPE (Y and n) are correlated with the structure of the hybrid films. A higher value of Y suggests that a larger area is exposed to the corrosive solution, while a higher value of n indicates better uniformity of the oxide layer [50]. The value of Yo in the 0BTA sample exceeded that of the other samples, implying that a larger area is exposed to Cl− ions, aligning with the SEM results in Figure 2, where 0BTA had the largest micropore size. The low value of no in the 0BTA sample also indicated a rougher surface, consistent with AFM results in Figure 2b. In contrast, the 5BTA sample featured a lower value of Yo and a higher value of no, affirming the uniformity observed in the sample in Figure 2.
Figure 7 shows the surface morphologies of the 0BTA, 3BTA, and 5BTA samples after electrochemical corrosion testing in 3.5 wt% NaCl solution. The coatings on all samples exhibited partial damage, characterized by corrosion pits. The surfaces of the 3BTA and 5BTA samples were less affected, displaying a typical PEO morphology. Increasing the BTA concentration to 5BTA resulted in fewer pits appearing on the surface, which could be attributed to the smaller micro-pore size and increased incorporation of V2O5 into the oxide layer. This result confirms that the corrosion resistance of the Mg alloy was significantly enhanced by the incorporation of V2O5 facilitated by the addition of BTA.
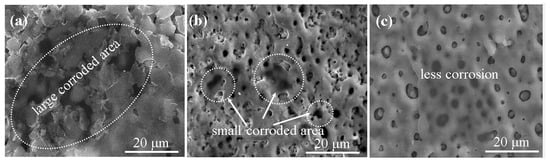
Figure 7.
Surface morphologies showing the levels of degradation by corrosion on the surface of (a) 0BTA, (b) 3BTA, and (c) 5BTA.
3.5. EIS Evaluation for a Long Period of Immersion
To evaluate the corrosion resistance of coatings exposed to a NaCl solution, EIS measurements were conducted over 96 h. The results for 0BTA, 3BTA, and 5BTA samples are shown in Figure 8. Throughout the immersion period, 0BTA exhibited inferior corrosion resistance compared to 3BTA and 5BTA, evident in the smaller capacitive loop diameters in Nyquist plots and decreased impedance modulus at low frequencies, particularly from 24 to 96 h. The more porous structure of 0BTA facilitated the quicker diffusion of corrosive ions, leading to higher corrosion rates. Despite the reduction in capacitive loop diameters in Nyquist plots for 5BTA over time, their corrosion resistance remained superior, attributed to V2O5 particles reducing pore size and inhibiting the infiltration of corrosive anions into the substrate. This superiority was also reflected by the higher impedance values obtained at lower frequencies in Bode impedance plots. Furthermore, in all samples, at least two time constants can be identified from the phase angle plots shown in Figure 8c,f,i.
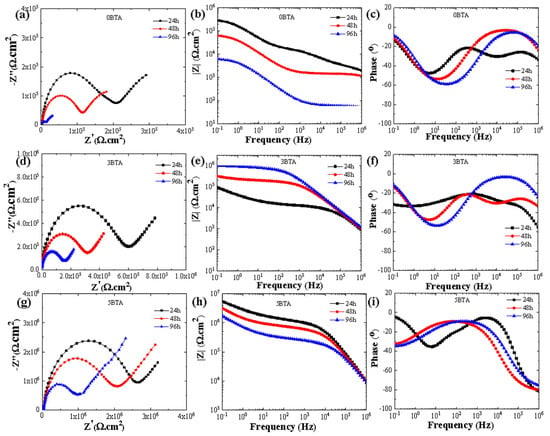
Figure 8.
Nyquist and bode plots obtained for the 0BTA, 3BTA, and 5BTA samples at different immersion times, where (a–c) 0BTA, (d–f) 3BTA, and (g–i) 5BTA.
The impedance responses of each sample were analyzed using the equivalent circuit model depicted in Figure 6b. The parameter values derived from these models are provided in Tables S1–S3. Notably, the 5BTA sample consistently exhibited the highest Ro and Ri values throughout the immersion period. While the Ri values for the 0BTA and 3BTA samples sharply decreased with increasing immersion time, those for the 5BTA sample remained largely unchanged, suggesting that significant protection against erosion for the inner layer of the Al alloy was achieved by the PEO coating containing 5BTA in the electrolyte.
3.6. Incorporation Mechanism of the Particles
Figure 9 depicts a schematic illustration detailing the mechanism underlying the role of BTA in the PEO process. The NH4VO3 salt initially dissociates into NH4+ and VO3− ions initially (Equation (5)). As the electrolyte infiltrates the pores and cools down, it reacts with the molten flux to generate V2O5, as illustrated by Equation (6):
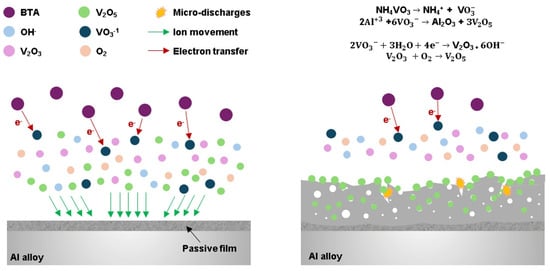
Figure 9.
The mechanism underlying the role of BTA in facilitating the incorporation of V2O5 into the alumina layer.
However, when BTA is introduced into the electrolyte solution, its role as an electron donor significantly alters the mechanism. BTA facilitated the reduction in VO3− species present in the electrolyte solution. This reduction process involves the transfer of electrons from BTA to the VO3− species, leading to the conversion from a higher oxidation state (V5+) to lower oxidation states, such as V3+ in the form of V2O3 (Equation (7)). On the other hand, it is worth mentioning that the addition of BTA to the strongly alkaline electrolyte alters the behavior of vanadium species present in the electrolyte. BTA acts as a complexing agent and electron donor, affecting the speciation and reactivity of vanadium ions. Specifically, BTA can form complexes with vanadium ions, such as VO43− and HVO42−, and facilitate reduction reactions, leading to changes in the oxidation states of vanadium species during the PEO process.
Since the XPS results did not show V2O3, it is suggested that all V2O3 subsequently reacts with oxygen in the electrolyte environment to yield V2O5 (Equation (8)).
This leads to the enhanced incorporation of V2O5 into the coating. The increased availability of reduced VO3− species facilitated by BTA caused the formation of a denser hybrid film. Comparing the effects of using 3 g/L versus 5 g/L of BTA, distinct outcomes were observed. While both concentrations contributed to improved protective properties compared to the case without BTA, the experiment using 5 g/L of BTA notably outperformed its 3 g/L counterpart. The higher concentration of BTA accelerated reduction reactions, resulting in increased V2O5 incorporation and the formation of a denser oxide layer. Therefore, two factors likely contribute to the enhanced chemical stability observed in the 5BTA sample. Firstly, its less porous structure could substantially limit the movement of corrosive anions towards the substrate, thereby improving the corrosion protection properties of the Al alloy. This denser layer offers superior corrosion protection compared to the 3BTA sample. Secondly, the incorporation of stable oxides, such as V2O5, could effectively inhibit the penetration of corrosive anions into the Al alloy substrate. Thus, the choice of BTA concentration significantly impacts the efficiency of the PEO method in enhancing the stability of aluminum alloy coatings in NaCl solution.
4. Conclusions
In this study, our focus centered on the successful integration of V2O5 into the Al2O3 film made on an Al alloy, achieved through a one-step PEO process, where BTA molecules played a substantial role as the electron donor. The breakdown phenomenon inherent to the PEO surface modification process facilitated the electrochemical reduction in NH4VO3, leading to the incorporation of a substantial amount of V2O5 into the Al2O3 layer, a phenomenon catalyzed by the presence of BTA. The electron-donating capabilities of BTA were instrumental in enhancing the reduction in VO3− species, resulting in the more efficient integration of V2O5 into the coating. Notably, the 5BTA sample exhibited a reduction in porosity and surface roughness compared to its counterpart samples, namely 0BTA and 3BTA. This improvement in structural characteristics can be attributed to the role of BTA in promoting the more controlled and effective incorporation of V2O5. Consequently, electrochemical measurements affirmed that the hybrid film made in the presence of 5 g/L BTA demonstrated superior anticorrosive properties. This superiority was primarily attributed to the sealing effect of V2O5 particles, whose activation tendency was found to be higher at increased concentrations of BTA. Thus, the observed structural and corrosion resistance improvements underscore the synergistic influence of BTA in optimizing the incorporation mechanism of V2O5, enhancing the performance of the Al alloy coating.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/met14060606/s1, Table S1: Outcomes of the EIS examinations on the 0BTA specimen submerged in a 3.5 wt.% NaCl electrolyte for different immersion time. Table S2: Outcomes of the EIS examinations on the 3BTA specimen submerged in a 3.5 wt.% NaCl electrolyte for different immersion time. Table S3: Outcomes of the EIS examinations on the 5BTA specimen submerged in a 3.5 wt.% NaCl electrolyte for different immersion time.
Author Contributions
Conceptualization, M.K.; Methodology, M.K.; Investigation, M.K.; Writing—original draft, M.K., A.F.-a. and B.D.; Writing—review & editing, M.K., A.F.-a. and B.D.; Funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF), funded by the Korean government (MSIT; No. 2022R1A2C1006743).
Data Availability Statement
The raw/processed data required to reproduce these findings are not readily available because the data are part of an ongoing study. Requests to access the datasets should be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Toorani, M.; Aliofkhazraei, M.; Golabadi, M.; Rouhaghdam, A.S. Effect of lanthanum nitrate on the microstructure and electrochemical behavior of PEO coatings on AZ31 Mg alloy. J. Alloys Compd. 2017, 719, 242–255. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An investigation of ceramic coating growth mechanisms in plasma electrolytic oxidation (PEO) processing. Electrochim. Acta 2013, 112, 111–119. [Google Scholar] [CrossRef]
- Xia, Q.; Li, X.; Yao, Z.; Jiang, Z. Investigations on the thermal control properties and corrosion resistance of MAO coatings prepared on Mg-5Y-7Gd-1Nd-0.5Zr alloy. Surf. Coat. Technol. 2021, 409, 126874. [Google Scholar] [CrossRef]
- Liu, Z.; Le, Z.; He, X.; Cheng, Y.; Hu, P.; Cheng, Y. Plasma electrolytic oxidation of tantalum in an aluminate electrolyte: Effect of cathodic polarization and frequency. Ceram. Int. 2023, 49, 35042–35062. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Chen, D.; Wang, R.; Li, D.; Guo, C.; Jiang, G.; Chen, D.; Yu, S.; Nash, P. Micro-structures and growth mechanisms of plasma electrolytic oxidation coatings on aluminium at different current densities. Surf. Coat. Technol. 2017, 321, 236–246. [Google Scholar] [CrossRef]
- Orsetti, F.R.; Bukman, L.; Santos, J.S.; Nagay, B.E.; Rangel, E.C.; Cruz, N.C. Methylene blue and metformin photocatalytic activity of CeO2-Nb2O5 coatings is dependent on the treatment time of plasma electrolytic oxidation on titanium. Appl. Surf. Sci. Adv. 2021, 6, 100143. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma electrolytic oxidation (PEO) process—Processing, properties, and applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Gang, X.S.; Xin, S.L.; Gen, Z.R.; Fang, H.X. Properties of aluminum oxide coating on aluminum alloy produced by micro-arc oxidation. Surf. Coat. Technol. 2005, 199, 184–188. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Xue, Z.; Wang, Q.; Wu, X.Q.; Matykina, E.; Skeldon, P.S.; Thompson, G.E. New findings on properties of plasma electrolytic oxidation coatings from study of an Al-Cu-Li alloy. Electrochim. Acta 2013, 107, 358–378. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, L.; Cai, W.; Shan, A.; Jiang, Z. Effects of fluoride on the structure and properties of micro-arc oxidation coating on aluminum alloy. J. Alloys Compd. 2010, 505, 188–193. [Google Scholar] [CrossRef]
- Mohedano, M.; Blawert, C.; Zheludkevich, M.L. Silicate-based Plasma Electrolytic Oxidation (PEO) coatings with incorporated CeO2 particles on AM50 magnesium alloy. Mater. Des. 2015, 86, 735–744. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Molaei, M.; Babaei, K. The effects of nano- and micro-particles on properties of plasma electrolytic oxidation (PEO) coatings applied on titanium substrates: A review. Surf. Interfaces 2020, 21, 100659. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Y.; Blawert, C.; Li, Y.; Zhang, T.; Wang, F.; Kainer, K.U.; Zheludkevich, M. Influence of SiO2 particles on the corrosion and wear resistance of plasma electrolytic oxidation-coated AM50 Mg alloy. Coatings 2018, 8, 306. [Google Scholar] [CrossRef]
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions—A review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Arunnellaiappan, T.; Ashfaq, M.; Krishna, L.R.; Rameshbabu, N. Fabrication of corrosion-resistant Al2O3-CeO2 composite coating on AA7075 via plasma electrolytic oxidation coupled with electrophoretic deposition. Ceram. Int. 2016, 42, 5897–5905. [Google Scholar] [CrossRef]
- Arunnellaiappan, T.; Krishna, L.R.; Anoop, S.; Rani, R.U.; Rameshabu, N. Fabrication of multifunctional black PEO coatings on AA7075 for spacecraft applications. Surf. Coat. Technol. 2016, 307, 735–746. [Google Scholar]
- Hwang, I.J.; Shin, K.R.; Lee, J.S.; Ko, Y.G.; Shin, D.H. Formation of black ceramic layer on aluminum alloy by plasma electrolytic oxidation in electrolyte containing Na2WO4. Mater. Trans. 2012, 53, 559–564. [Google Scholar] [CrossRef]
- Zhu, Z.; Tu, W.; Cheng, Y.; Cheng, Y. The formation of metallic W and amorphous phase in the plasma electrolytic oxidation coatings on an Al alloy from tungstate-containing electrolyte. Surf. Coat. Technol. 2019, 361, 176–187. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Imshinetskiy, I.M.; Nadaraia, K.V.; Gnedenkov, A.S.; Suchkov, S.N.; Opra, D.P.; Pustovalov, E.V.; Ustinov, A.Y.; Sinebryukhov, S.L.; Gnedenkov, S.V. Effect of TiO2 nanoparticles on the photocatalytic properties of PEO coatings on Mg alloy. J. Magnes. Alloys 2023, 11, 735–752. [Google Scholar] [CrossRef]
- Kaseem, M.; Hussain, T.; Rehman, Z.U.; Banisalman, M.; Ko, Y.G. Advantage of an in-situ reactive incorporation over direct particles incorporation of V2O5 for a competitive plasma electrolysis coating. Surf. Coat. Technol. 2020, 399, 126200. [Google Scholar] [CrossRef]
- Kwon, J.H.; Fatimah, S.; Baek, S.H.; Kim, Y.G.; Ko, Y.G. Electrochemical response of VxOy-Al2O3 composite layer with dark-green color achieved by plasma electrolytic oxidation. J. Alloys Compd. 2020, 827, 154367. [Google Scholar] [CrossRef]
- Fan, H.; Ling, N.; Bai, R.; Zhang, J.; Wang, L. Influence of V-containing species on formation and corrosion resistance of PEO coatings developed on AZ31B Mg alloy. Ceram. Int. 2023, 49, 24783–24793. [Google Scholar] [CrossRef]
- Guo, X.; An, M.; Yang, P.; Li, H.; Su, C. Effects of benzotriazole on anodized film formed on AZ31B magnesium alloy in environmental-friendly electrolyte. J. Alloys Compd. 2009, 482, 487–497. [Google Scholar] [CrossRef]
- Kaseem, M.; Hussain, T.; Ko, Y.G. Tailored aluminum coatings for corrosion inhibition considering the synergism between phosphate ions and benzotriazole. J. Alloys Compd. 2020, 822, 153566. [Google Scholar] [CrossRef]
- Jaspard-Mécuson, F.; Czerwiec, T.; Henrion, G.; Belmonte, T.; Dujardin, L.; Viola, A.; Beauvi, J. rTailored aluminium oxide layers by bipolar current adjustment in the plasma electrolytic oxidation (PEO) process. Surf. Coat. Technol. 2007, 201, 8677–8682. [Google Scholar] [CrossRef]
- Kamil, M.P.; Ko, Y.G. Electrochemically stable and catalytically active coatings based on self-assembly of protein-inorganic nanoflowers on plasma-electrolyzed platform. ACS Appl. Mater. Interfaces 2021, 13, 39854–39867. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.W.; Kim, Y.S.; Yang, H.W.; Ko, Y.G.; Shin, D.H. Influence of ZrO2 incorporation into coating layer on electrochemical response of low-carbon steel processed by electrochemical plasma coating. Surf. Coat. Technol. 2015, 269, 314–318. [Google Scholar] [CrossRef]
- Zhu, M.; Song, Y.; Dong, K.; Shan, D.; Han, E.H. Correlation between the transient variation in positive/negative pulse voltages and the growth of PEO coating on 7075 aluminum alloy. Electrochim. Acta 2022, 411, 140056. [Google Scholar] [CrossRef]
- Xiang, N.; Song, R.G.; Zhuang, J.J.; Song, R.X.; Lu, X.Y.; Su, X.P. Effects of current density on microstructure and properties of plasma electrolytic oxidation ceramic coatings formed on 6063 aluminum alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 806–813. [Google Scholar] [CrossRef]
- Tang, M.; Feng, Z.; Li, G.; Zhang, Z.; Zhang, R. High corrosion resistance of the microarc oxidation coatings on magnesium alloy obtained in potassium fluotitanante electrolytes. Surf. Coat. Technol. 2015, 264, 105–113. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Ebrahimi, S.; Yarmand, B. Plasma electrolytic oxidation of monocrystalline silicon using silicate electrolyte containing boric acid. Appl. Surf. Sci. 2018, 462, 913–922. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, R.; Liu, X.; Wang, D.; Zhang, H.; Shen, X.; Shen, D.; Li, D. Influence of different electrolyte additives and structural characteristics of plasma electrolytic oxidation coatings on AZ31 magnesium alloy. Coatings 2020, 10, 817. [Google Scholar] [CrossRef]
- Rong, Z.; Feng, Q. How insoluble particles affect the solutions’ conductivity: A theory and the test in NaCl and chitosan solutions. J. Phys. Chem. B 2011, 115, 12816–12821. [Google Scholar] [CrossRef]
- Ping, W.; Ting, W.; Hao, P.; Yang, G.X. Effect of NaAlO2 concentrations on the properties of micro-arc oxidation coatings on pure titanium. Mater. Lett. 2016, 170, 171–174. [Google Scholar] [CrossRef]
- Narayanan, T.; Song, P.; Lee, M. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Sun, M.; Yerokhin, A.; Bychkova, M.Y.; Shtansky, D.V.; Levashov, E.A.; Matthews, A. Self-healing plasma electrolytic oxidation coatings doped with benzotriazole loaded halloysite nanotubes on AM50 magnesium alloy. Corros. Sci. 2016, 111, 753–769. [Google Scholar] [CrossRef]
- Kaseem, M.; Choe, H.C. The effect of in-situ reactive incorporation of MoOx on the corrosion behavior of Ti-6Al-4V alloy coated via micro-arc oxidation coating. Corros. Sci. 2021, 192, 109764. [Google Scholar] [CrossRef]
- Hsu, C.H.; Teng, T.P.; Lu, H.P. Effects of addition of Al(NO3)3 to electrolytes on alumina coatings by plasma electrolytic oxidation. Surf. Coat. Technol. 2011, 205, 3677–3682. [Google Scholar] [CrossRef]
- Gu, W.C.; Lv, G.H.; Chen, H.; Chen, G.L.; Feng, W.R.; Zhang, G.L.; Yang, S.Z. Investigation of morphology and composition of plasma electrolytic oxidation coatings in systems of Na2SiO3-NaOH and (NaPO3)6-NaOH. J. Mater. Process. Technol. 2007, 182, 28–33. [Google Scholar]
- Ming, H.; Yan, Y.; Ming, J.; Adkins, J.; Li, X.; Zhou, Q.; Zheng, J.J.E.A. Gradient V2O5 surface-coated LiMn2O4 cathode towards enhanced performance in Li-ion battery applications. Electrochem. Acta 2014, 120, 390–397. [Google Scholar] [CrossRef]
- Bayati, M.; Zargar, H.; Molaei, R.; Golestani-Fard, F.; Zanganeh, N.; Kajbafvala, A. MAO-synthesized Al2O3-supported V2O5 nano-porous catalysts: Growth, characterization, and photoactivity. Appl. Surf. Sci. 2010, 256, 3806–3811. [Google Scholar] [CrossRef]
- Sreekanth, D.; Rameshbabu, N.; Venkateswarlu, K. Effect of various additives on morphology and corrosion behavior of ceramiccoatings developed on AZ31 magnesium alloy by plasma electrolytic oxidation. Ceram. Int. 2012, 38, 4607–4615. [Google Scholar] [CrossRef]
- Liang, J.; Srinvasan, P.B.; Blawert, C.; Stormer, M.; Dietzel, W. Influence of pH on the deterioration of plasma electrolytic oxidation coated AM50 magnesium alloy in NaCl solutions. Corros. Sci. 2010, 52, 540–547. [Google Scholar] [CrossRef]
- Malayoglu, U.; Tekin, K.C.; Shrestha, S. Influence of post-treatment on the corrosion resistance of PEO coated AM50B and AM60B Mg alloys. Surf. Coat. Technol. 2010, 205, 1793–1798. [Google Scholar] [CrossRef]
- Kaseem, M.; Min, J.H.; Ko, Y.G. Corrosion behavior of Al-1wt% Mg-0.85 wt% Si alloy coated by micro-arc-oxidation using TiO2 and Na2MoO4 additives: Role of Current density. J. Alloys Compd. 2017, 723, 448–455. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical Polarization I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Mashtalyar, D.; Imshinetskiy, I.; Nadaraia, K.; Gnedenkov, A.; Sinebryukhov, S.; Ustinov, A.; Samokhin, A.; Gnedenkov, S. Influence of ZrO2/SiO2 nanomaterial incorporation on the properties of PEO layers on Mg-Mn-Ce alloy. J. Magnes. Alloys 2022, 10, 513–526. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, Z.; Tang, S.; Dong, W.; Tong, Q.; Li, W. Influence of graphene particles on the micro-arc oxidation behaviors of 6063 alKuminum alloy and the coating properties. Appl. Surf. Sci. 2017, 423, 939–950. [Google Scholar] [CrossRef]
- Yao, Z.; Jiang, Z.; Xin, S.; Sun, X.; Wu, X. Electrochemical impedance spectroscopy of ceramic coatings on Ti–6Al–4V by micro-plasma oxidation. Electrochim. Acta 2005, 50, 3273–3279. [Google Scholar] [CrossRef]
- He, D.; Li, G.; Shen, D.; Guo, C.; Ma, H.; Cai, J. Effect mechanism of ultrasound on growth of micro-arc oxidation coatings on A96061 aluminum alloy. Vacuum 2014, 107, 99–102. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).