Effect of Copper Content on the Microstructure and Properties of the Sintered Porous Aluminum Wick
Abstract
1. Introduction
2. Experimental Procedure
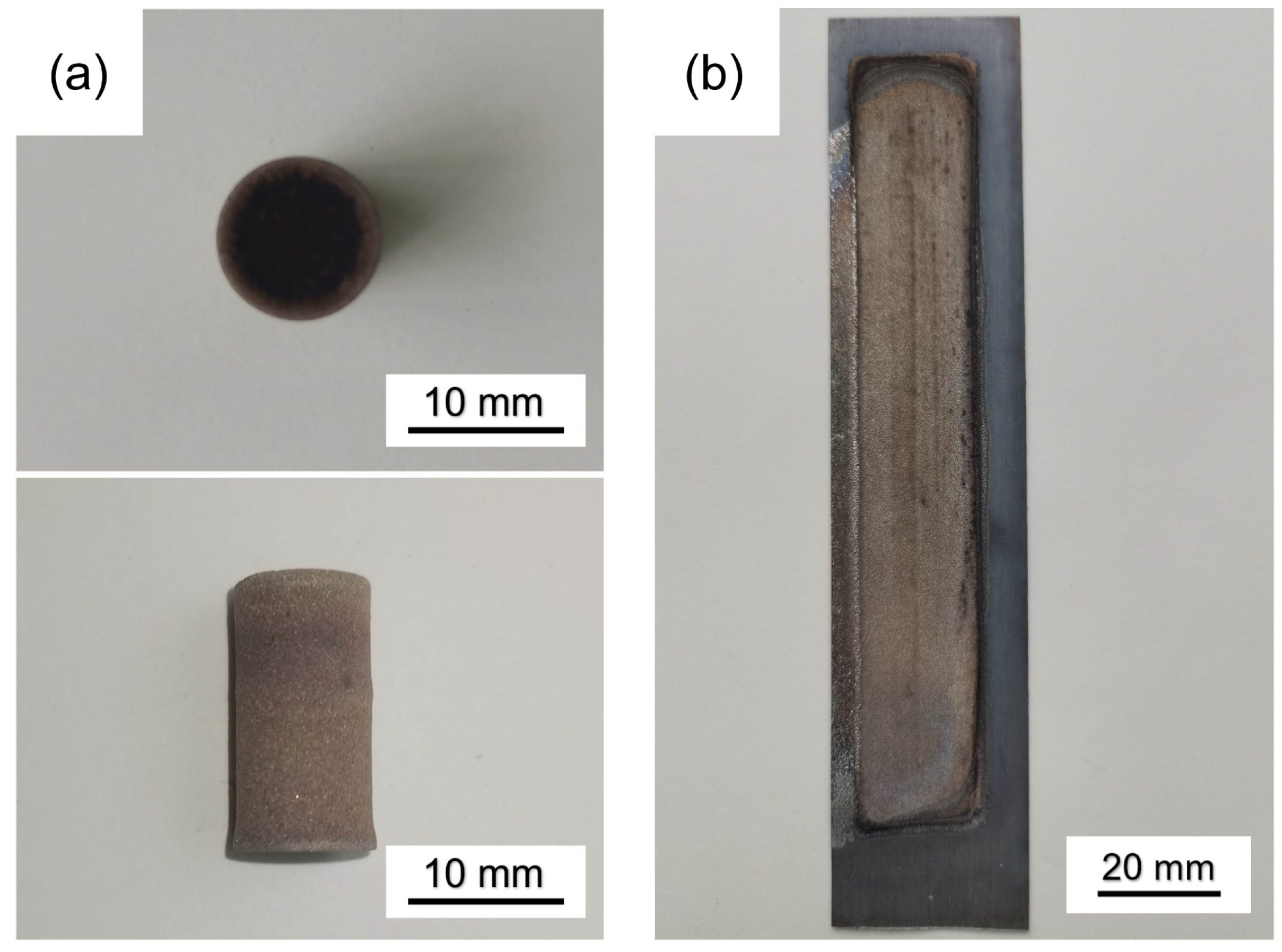
2.1. Experimental Process
2.2. Characterization
2.2.1. Characterization of Porous Al Wick
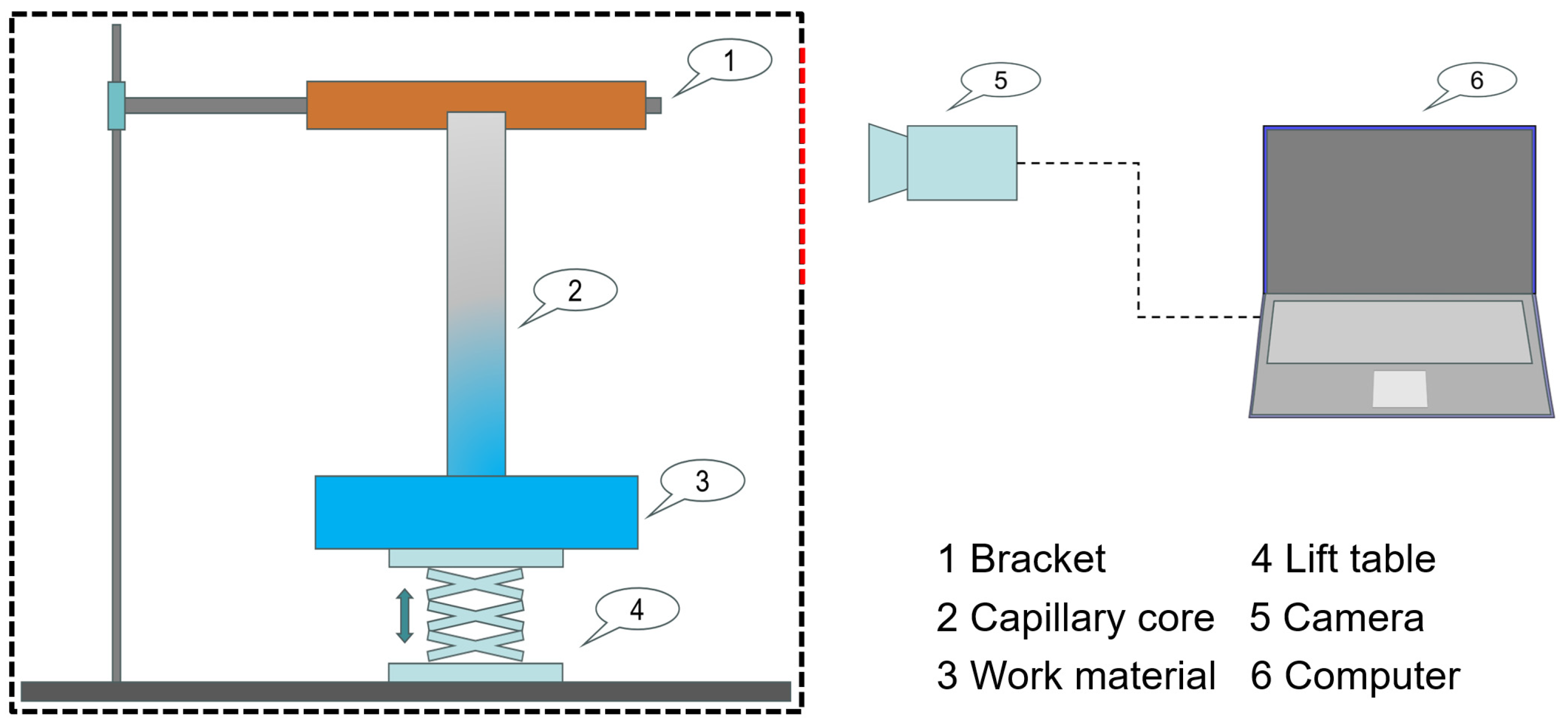
2.2.2. Capillary Rise Experiments
3. Results and Discussion
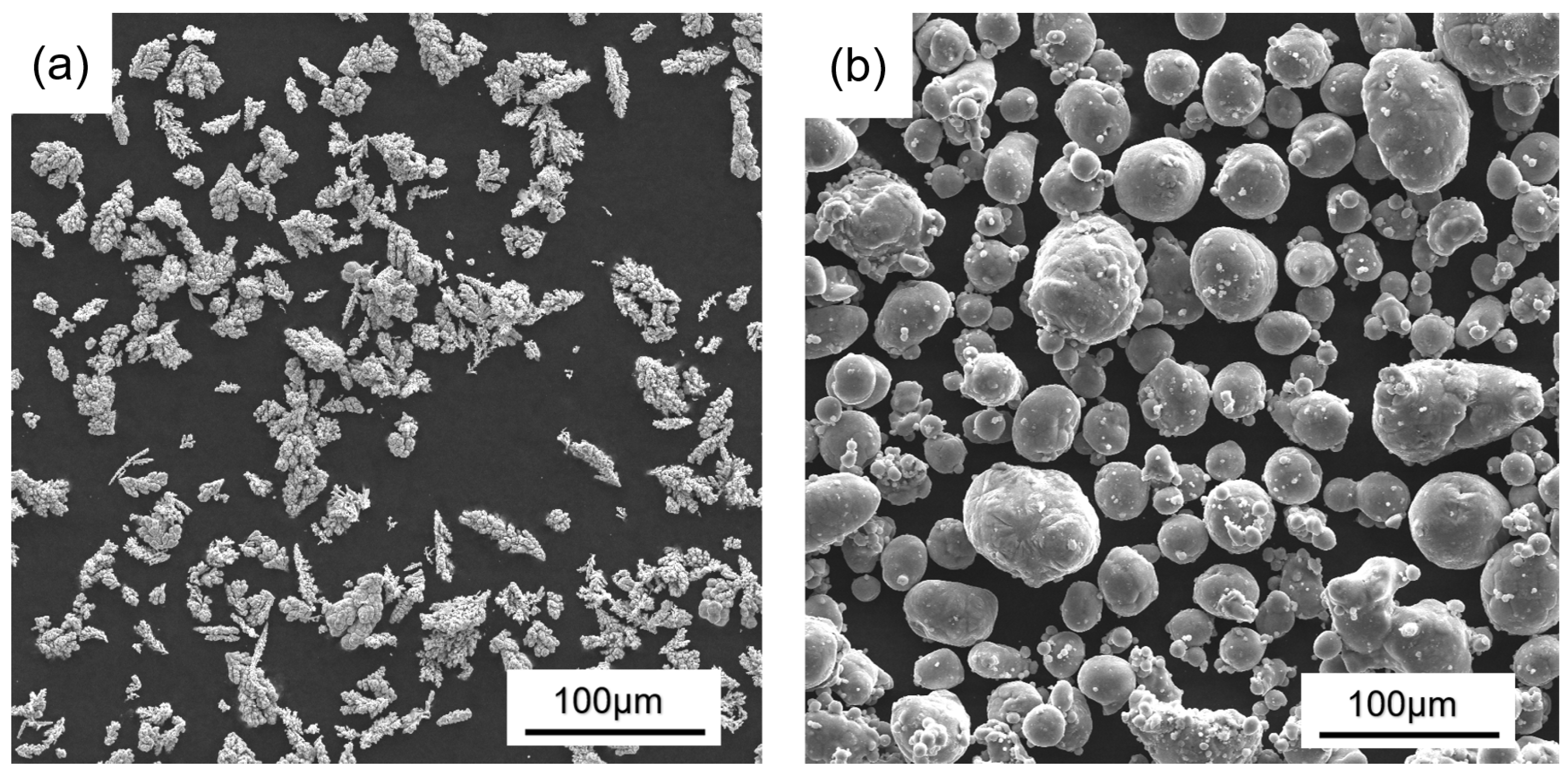
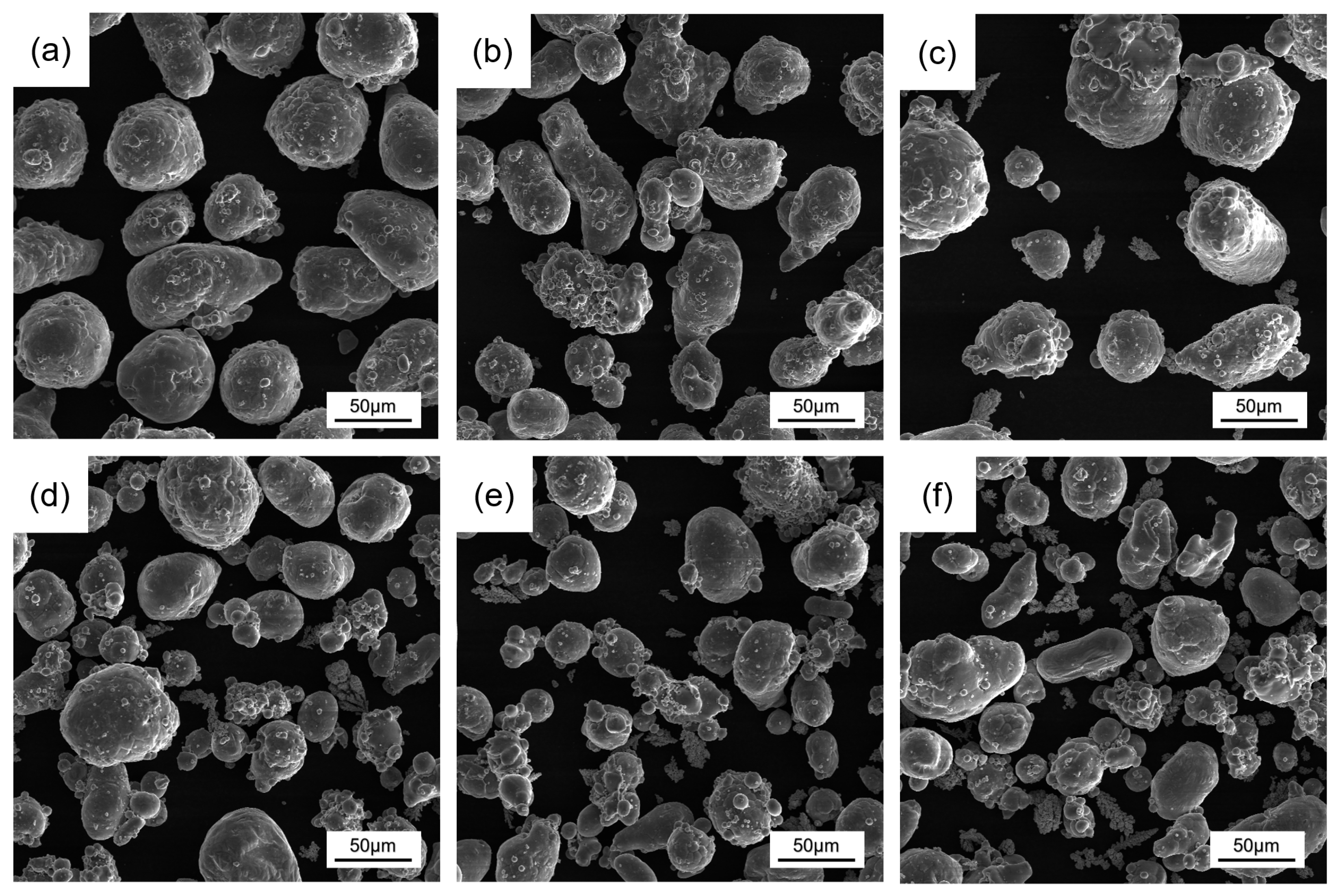
3.1. Powder Composition
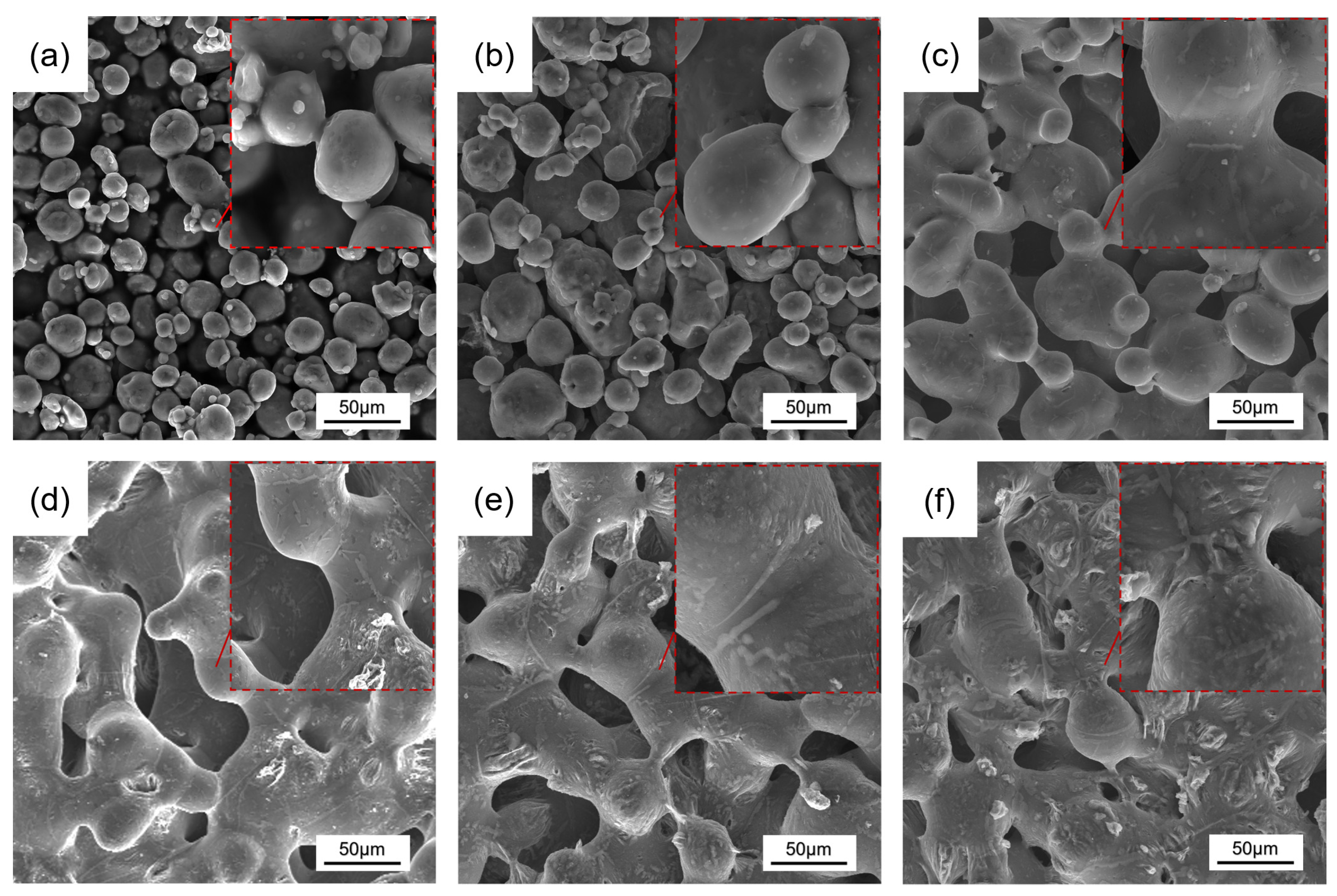
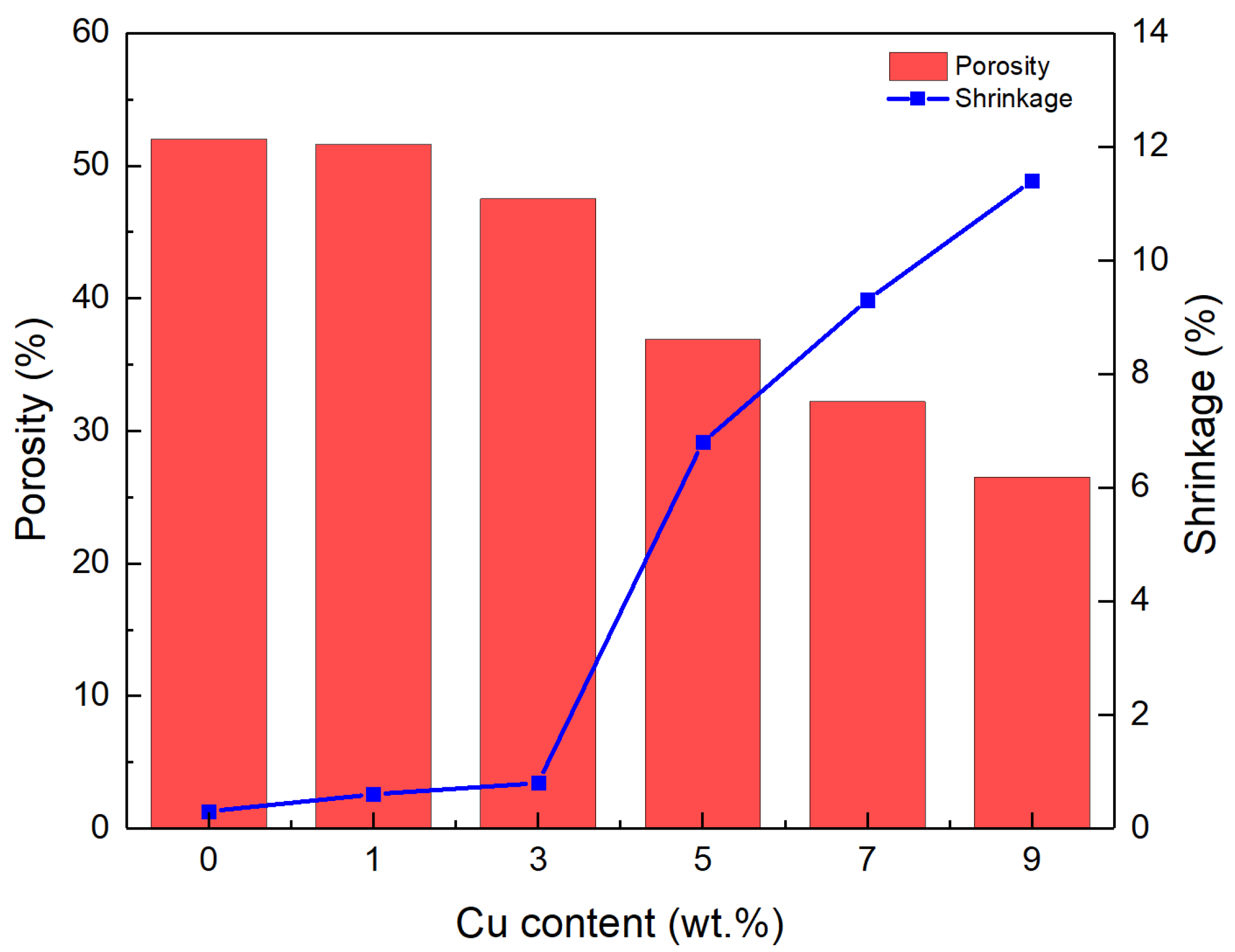
3.2. Pore Structure
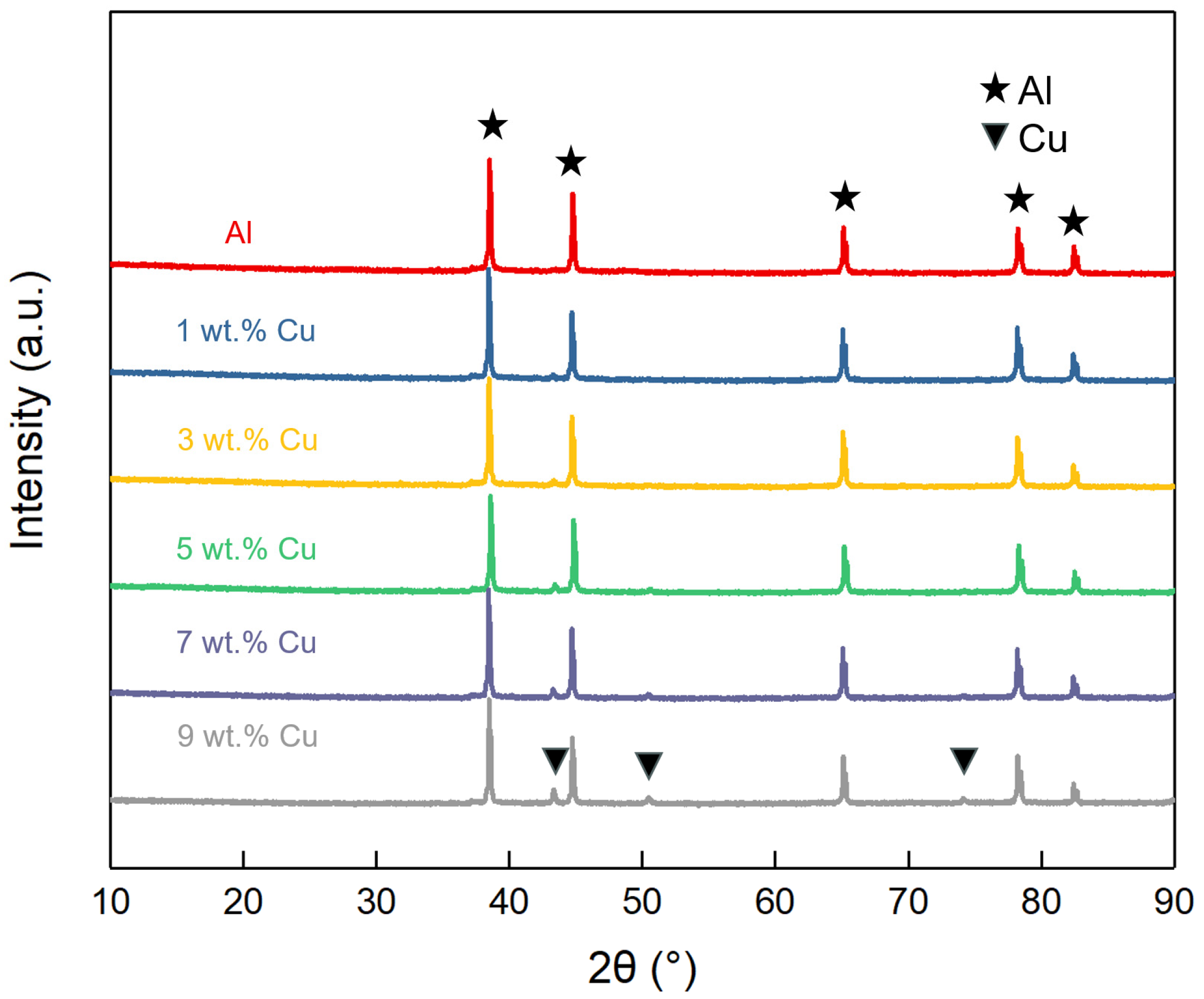
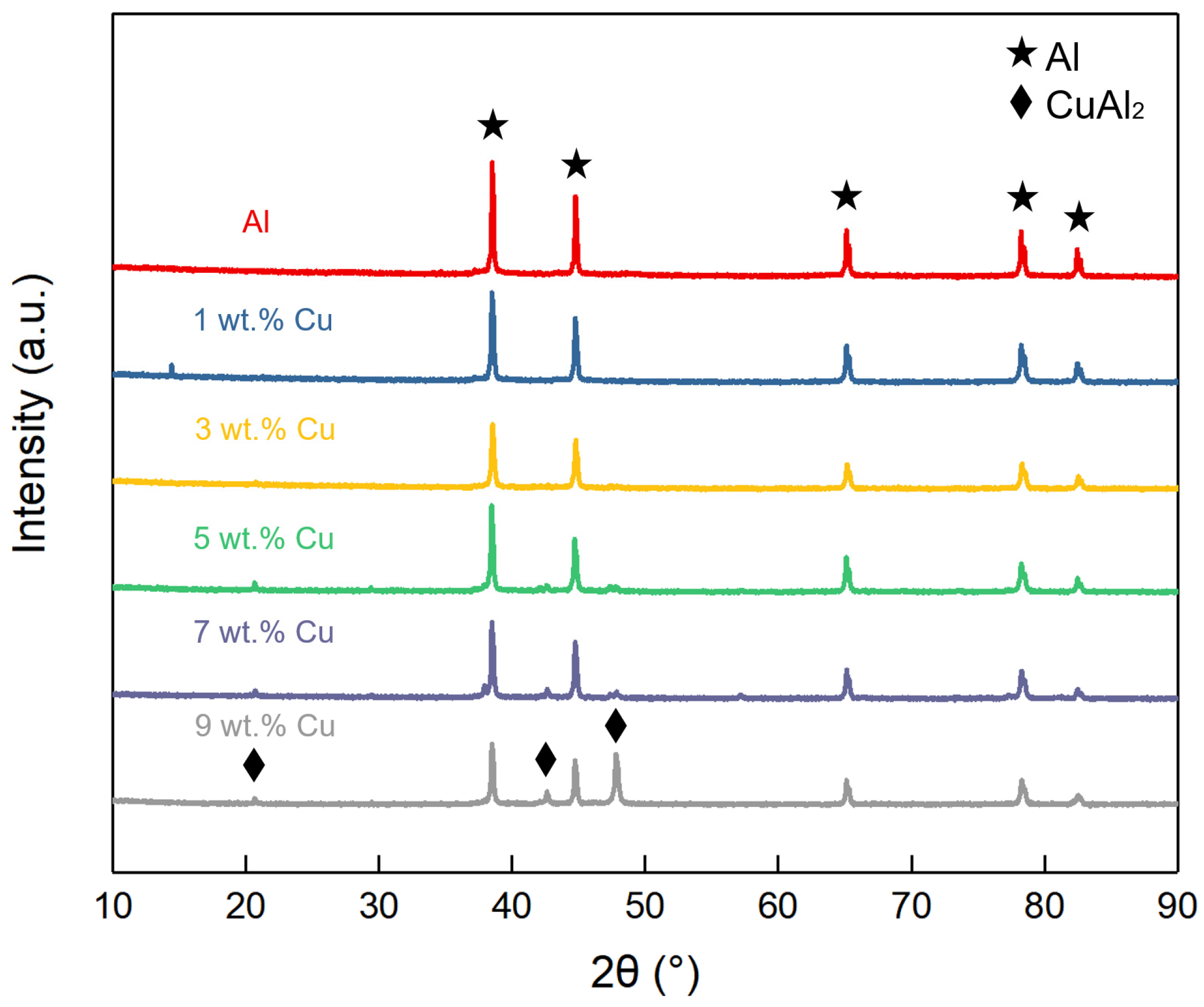
3.3. Phase Composition
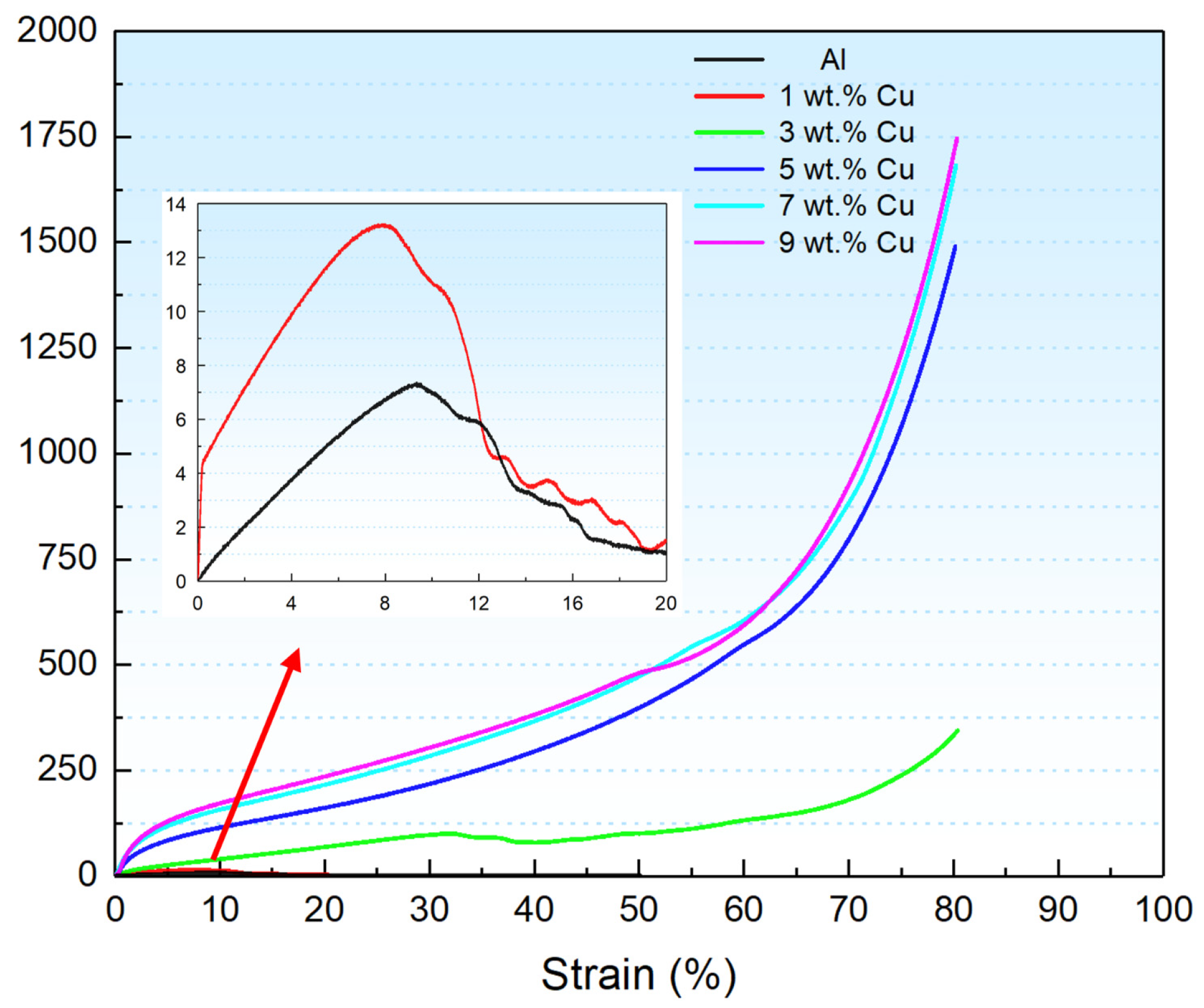
3.4. Mechanical Properties
3.5. Capillary Properties
4. Conclusions
- With increasing Cu content, the formation of sintered necks was favored, and the pore morphology became smooth and regular, while the shrinkage increased.
- During the sintering process, Cu and Al tend to form CuAl2, which breaks the oxide film on the surface and accelerates the densification of Al powder.
- The compressive strength is gradually enhanced with increasing Cu content, while the capillary characteristic parameter and permeability of porous wicks are reduced.
- At the optimal Cu amount of 3 wt.%, a porous Al wick with a porosity of 47.5%, a plateau stress of 11.82 MPa, and a capillary characteristic parameter of 6.72 × 10−8 N was obtained, meeting the requirements for wick applications.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, D.; Sun, Y.; Wang, G.; Chen, S.; Ding, G. Significant Factors Affecting Heat Transfer Performance of Vapor Chamber and Strategies to Promote It: A Critical Review. Int. J. Heat Mass Transf. 2021, 175, 121132. [Google Scholar] [CrossRef]
- Yan, C.; Li, H.; Tang, Y.; Ding, X.; Yuan, X.; Liang, Y.; Zhang, S. A Novel Ultra-Thin Vapor Chamber with Composite Wick for Portable Electronics Cooling. Appl. Therm. Eng. 2023, 226, 120340. [Google Scholar] [CrossRef]
- He, G.; Sheng, Y.; Ye, G.; He, B.; Yu, B.; Tian, M.; Jian, Q.; Yu, X. Heat Transfer Performance Analysis of an Ultra-Thin Aluminum Vapor Chamber with Serrated Microgrooves. Appl. Therm. Eng. 2023, 229, 120604. [Google Scholar] [CrossRef]
- Ušakovs, I.; Mishkinis, D.; Galkin, I.A.; Bubovich, A.; Podgornovs, A. Experimental Thermal Characterization of the in-Wheel Electric Motor with Loop Heat Pipe Thermal Management System. Case Stud. Therm. Eng. 2023, 47, 103069. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, G.; Wu, J. Recent Advances in the Optimization of Evaporator Wicks of Vapor Chambers: From Mechanism to Fabrication Technologies. Appl. Therm. Eng. 2021, 188, 116611. [Google Scholar] [CrossRef]
- Zhou, F.; Zhou, J.; Huai, X. Advancements and Challenges in Ultra-Thin Vapor Chambers for High-Efficiency Electronic Thermal Management: A Comprehensive Review. Int. J. Heat Mass Transf. 2023, 214, 124453. [Google Scholar] [CrossRef]
- Deng, D.; Liang, D.; Tang, Y.; Peng, J.; Han, X.; Pan, M. Evaluation of Capillary Performance of Sintered Porous Wicks for Loop Heat Pipe. Exp. Therm. Fluid Sci. 2013, 50, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, Z.; Yu, Y.; Rhamdhani, M.A.; Gao, Y.; Guo, C. Research on the Effective Thermal Conductivity of Nickel-Based Bi-Porous Capillary Wicks: Modeling and Validation. Int. J. Heat Mass Transf. 2024, 218, 124776. [Google Scholar] [CrossRef]
- Li, Q.; Lan, Z.; Chun, J.; Lian, S.; Wen, R.; Ma, X. Fabrication and Capillary Characterization of Multi-Scale Micro-Grooved Wicks with Sintered Copper Powder. Int. Commun. Heat Mass Transf. 2021, 121, 105123. [Google Scholar] [CrossRef]
- Wang, J.; Tang, Y.; Huang, H.; Xi, X.; Li, H.; Yan, C.; Zhang, S. Rice-Inspired Oriented Copper Fiber Wick with Excellent Capillary Performance for Ultra-Thin Vapor Chamber. Appl. Therm. Eng. 2024, 236, 121573. [Google Scholar] [CrossRef]
- Wan, Z.; Deng, J.; Li, B.; Xu, Y.; Wang, X.; Tang, Y. Thermal Performance of a Miniature Loop Heat Pipe Using Water–Copper Nanofluid. Appl. Therm. Eng. 2015, 78, 712–719. [Google Scholar] [CrossRef]
- Zhang, J.; Lian, L.; Liu, Y. Liquid Phase Enhanced Sintering of Porous Aluminum for Cylindrical Al-Acetone Heat Pipe. Int. J. Heat Mass Transf. 2020, 152, 119512. [Google Scholar] [CrossRef]
- Tian, W.; He, S.; Liu, Z.; Liu, W. Experimental Investigation of a Miniature Loop Heat Pipe with Eccentric Evaporator for Cooling Electronics. Appl. Therm. Eng. 2019, 159, 113982. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wang, J.; Luan, T.; Chen, H.; Xue, H. Experimental Study on the Characteristics of Loop Heat Pipe with Modified Carbon Fiber Felt Wick. Appl. Therm. Eng. 2023, 234, 121239. [Google Scholar] [CrossRef]
- Selivanov, V.V.; Silnikov, M.V.; Markov, V.A.; Popov, Y.V.; Pusev, V.I. Using Highly Porous Aluminum Alloys and Honeycomb Structures in Spacecraft Landing Gear. Acta Astronaut. 2021, 180, 105–109. [Google Scholar] [CrossRef]
- Aperador, W.; Delgado, A.; Bautista, J. Improved Corrosion Protection Properties in Anodic Films Type Porous on 2024 T3 Aluminium Alloys Obtained by Pulse Reverse Plating. Int. J. Electrochem. Sci. 2013, 8, 9607–9617. [Google Scholar] [CrossRef]
- Bang, S.; Ryu, S.; Ki, S.; Song, K.; Kim, J.; Kim, J.; Nam, Y. Superhydrophilic Catenoidal Aluminum Micropost Evaporator Wicks. Int. J. Heat Mass Transf. 2020, 158, 120011. [Google Scholar] [CrossRef]
- Jiang, G.; Tian, Z.; Luo, X.; Chen, C.; Hu, X.; Wang, L.; Peng, R.; Zhang, H.; Zhong, M. Ultrathin Aluminum Wick with Dual-Scale Microgrooves for Enhanced Capillary Performance. Int. J. Heat Mass Transf. 2022, 190, 122762. [Google Scholar] [CrossRef]
- Zhong, G.; Tang, Y.; Ding, X.; Chen, G.; Li, Z. Experimental Investigation on Wettability and Capillary Performance of Ultrasonic Modified Grooved Aluminum Wicks. Int. J. Heat Mass Transf. 2021, 179, 121642. [Google Scholar] [CrossRef]
- Wu, L.; Yu, Z.; Liu, C.; Ma, Y.; Huang, Y.; Wang, T.; Yang, L.; Yan, H.; Liu, W. Microstructure and Tensile Properties of Aluminum Powder Metallurgy Alloy Prepared by a Novel Low-Pressure Sintering. J. Mater. Res. Technol. 2021, 14, 1419–1429. [Google Scholar] [CrossRef]
- Chai, G.; Lu, H.; Nie, Z.; Jia, E.; Wang, J.; Guo, F. Strengthening Mechanism of Porous Aluminum Foam by Micro-Arc Discharge. Tribol. Int. 2024, 191, 109169. [Google Scholar] [CrossRef]
- Paramore, J.D.; Dunstan, M.K.; Butler, B.G.; Lewis, D.O. Powder Casting: Producing Bulk Metal Components from Powder without Compaction. JOM 2020, 72, 3112–3120. [Google Scholar] [CrossRef]
- Torres, Y.; Lascano, S.; Bris, J.; Pavón, J.; Rodriguez, J.A. Development of Porous Titanium for Biomedical Applications: A Comparison Between Loose Sintering and Space-Holder Techniques. Mater. Sci. Eng. C 2014, 37, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.H.; Shen, T.; Wang, D.Z. Effects of Solid Loading on Pore Structure and Properties of Porous Feal Intermetallics by Gel Casting. Powder Technol. 2019, 344, 169–176. [Google Scholar] [CrossRef]
- Jiang, B.; Zhao, N.Q.; Shi, C.S.; Li, J.J. Processing of Open Cell Aluminum Foams with Tailored Porous Morphology. Scr. Mater. 2005, 53, 781–785. [Google Scholar] [CrossRef]
- Jiang, B.; Zhao, N.Q.; Shi, C.S.; Du, X.W.; Li, J.J.; Man, H.C. A Novel Method for Making Open Cell Aluminum Foams by Powder Sintering Process. Mater. Lett. 2005, 59, 3333–3336. [Google Scholar] [CrossRef]
- Yang, S.; Luo, H.; Wang, L.; Guang, Z.; Zhang, P.; Liu, Y. Interface Structure and Bonding Strength of Metallurgical Bonded Aluminum Foam Sandwich (Afs) Fabricated by Hot-Pressing. Vacuum 2023, 211, 111987. [Google Scholar] [CrossRef]
- Jafari, M.J.; Madvari, R.F.; Ebadzadeh, T. Optimized Design and Experimental Validation of Sound Absorption Coefficient Performance in Aluminium Metal Foam by Spark Plasma Sintering. Heliyon 2023, 9, e16428. [Google Scholar] [CrossRef] [PubMed]
- MacAskill, I.A.; Hexemer, R.L.; Donaldson, I.W.; Bishop, D.P. Effects of Magnesium, Tin and Nitrogen on the Sintering Response of Aluminum Powder. J. Mater. Process. Technol. 2010, 210, 2252–2260. [Google Scholar] [CrossRef]
- Liu, R.; Xiang, D. Low-Temperature Synthesis of Mullite by Molten Molybdenum Trioxide Assisted Mesoporous Aluminum Source Dissolution. Ceram. Int. 2023, 49, 27688–27696. [Google Scholar] [CrossRef]
- Sonoda, T.; Katou, K.; Asahina, T.; Banno, T.; Yamada, Y. Improvement of Porous Structure of Sintered Highly Porous Aluminum Materials by Surface Modification of Aluminum Particles with Tin. Surf. Interface Anal. 2008, 40, 1736–1740. [Google Scholar] [CrossRef]
- Gulevskiy, V.A.; Antipov, V.I.; Vinogradov, L.V.; Tsurikhin, S.N.; Kolmakov, A.G.; Gulevskiy, V.V.; Prutskov, M.E. Study of a Highly Porous Composite Material Based on an Aluminum Matrix with an Ordered Cellular Structure Formed by Hollow Copper–Graphite Spherical Granules. Inorg. Mater. Appl. Res. 2022, 13, 480–484. [Google Scholar] [CrossRef]
- Tang, Y.; Deng, D.; Lu, L.; Pan, M.; Wang, Q. Experimental Investigation on Capillary Force of Composite Wick Structure by IR Thermal Imaging Camera. Exp. Therm. Fluid Sci. 2010, 34, 190–196. [Google Scholar] [CrossRef]
- Tang, Y.; Tang, H.; Li, J.; Zhang, S.; Zhuang, B.; Sun, Y. Experimental Investigation of Capillary Force in a Novel Sintered Copper Mesh Wick for Ultra-Thin Heat Pipes. Appl. Therm. Eng. 2017, 115, 1020–1030. [Google Scholar] [CrossRef]
- Lago, M.; Araujo, M. Capillary Rise in Porous Media. Phys. A Stat. Mech. Appl. 2001, 289, 1–17. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, K.; Chan, S.; Tang, Y. Fabrication of Nano CuAl2O4 Spinel for Copper Stabilization and Antibacterial Application. J. Hazard. Mater. 2019, 371, 550–557. [Google Scholar] [CrossRef]
- Jacob, K.T.; Alcock, C.B. Thermodynamics of CuAlO2 and CuAl2O4 and Phase Equilibria in the System Cu2O-CuO-Al2O3. J. Am. Ceram. Soc. 1975, 58, 192–195. [Google Scholar] [CrossRef]
- Massalski, T.B. The Al−Cu (Aluminum-Copper) System. Bull. Alloy Phase Diagr. 1980, 1, 27–33. [Google Scholar] [CrossRef]
- Duarte, I.; Fiedler, T.; Krstulović-Opara, L.; Vesenjak, M. Brief Review on Experimental and Computational Techniques for Characterization of Cellular Metals. Metals 2020, 10, 726. [Google Scholar] [CrossRef]
- Suzuki, A.; Kosugi, N.; Takata, N.; Kobashi, M. Microstructure and Compressive Properties of Porous Hybrid Materials Consisting of Ductile Al/Ti and Brittle Al3Ti Phases Fabricated by Reaction Sintering with Space Holder. Mater. Sci. Eng. A 2020, 776, 139000. [Google Scholar] [CrossRef]

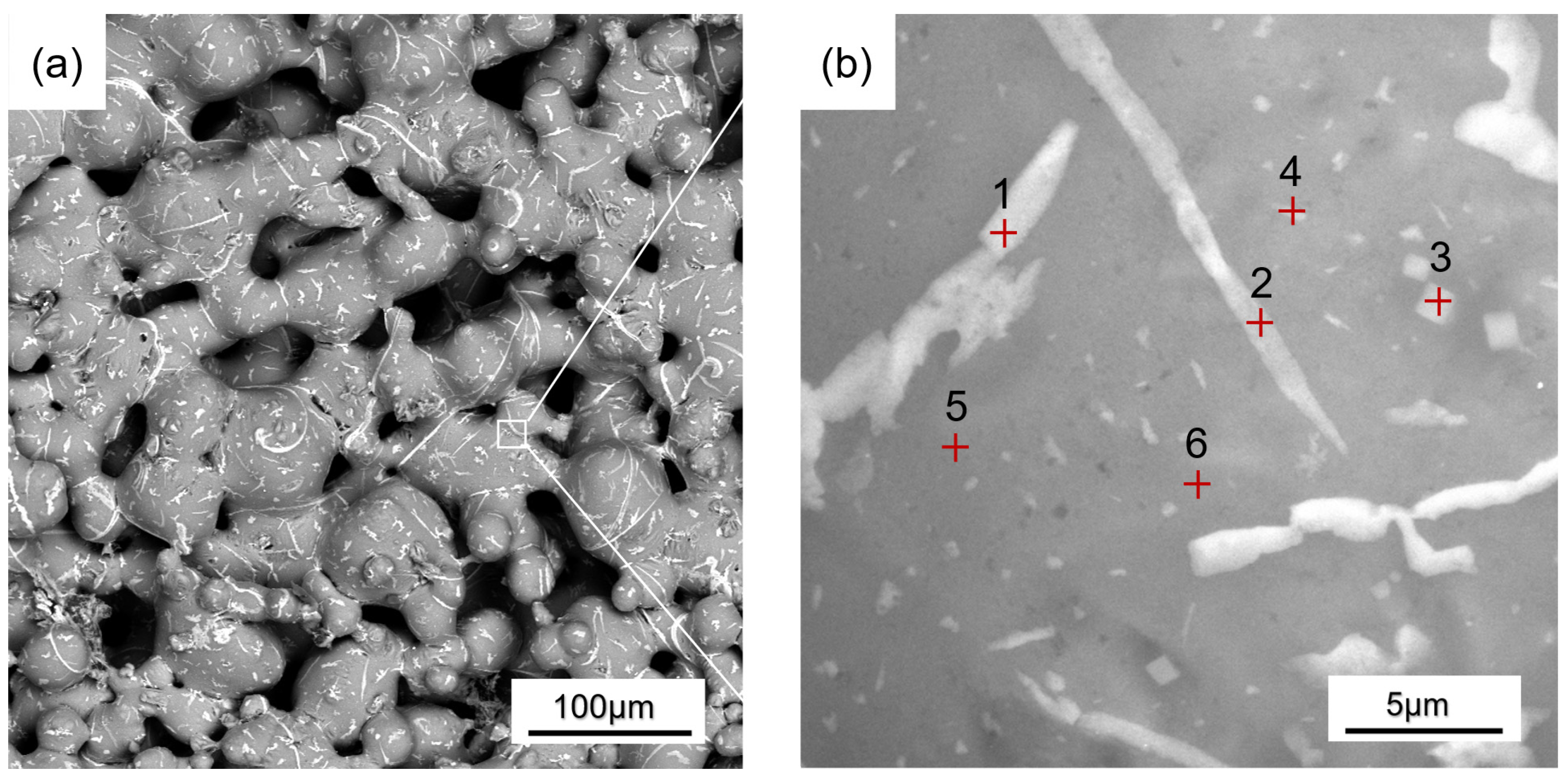

| Point | Al/at.% | Cu/at.% | Total/at.% |
|---|---|---|---|
| 1 | 81.5 | 18.5 | 100 |
| 2 | 85.6 | 14.4 | 100 |
| 3 | 93.1 | 6.9 | 100 |
| 4 | 93.2 | 6.8 | 100 |
| 5 | 99.1 | 0.9 | 100 |
| 6 | 99 | 1 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Duan, B. Effect of Copper Content on the Microstructure and Properties of the Sintered Porous Aluminum Wick. Metals 2024, 14, 386. https://doi.org/10.3390/met14040386
Cai Y, Duan B. Effect of Copper Content on the Microstructure and Properties of the Sintered Porous Aluminum Wick. Metals. 2024; 14(4):386. https://doi.org/10.3390/met14040386
Chicago/Turabian StyleCai, Yanbo, and Bohua Duan. 2024. "Effect of Copper Content on the Microstructure and Properties of the Sintered Porous Aluminum Wick" Metals 14, no. 4: 386. https://doi.org/10.3390/met14040386
APA StyleCai, Y., & Duan, B. (2024). Effect of Copper Content on the Microstructure and Properties of the Sintered Porous Aluminum Wick. Metals, 14(4), 386. https://doi.org/10.3390/met14040386






