Ultrasound-Assisted Synthesis of High-Entropy Materials for Enhanced Oxygen Evolution Electrocatalysis
Abstract
1. Introduction
2. Experimental Section
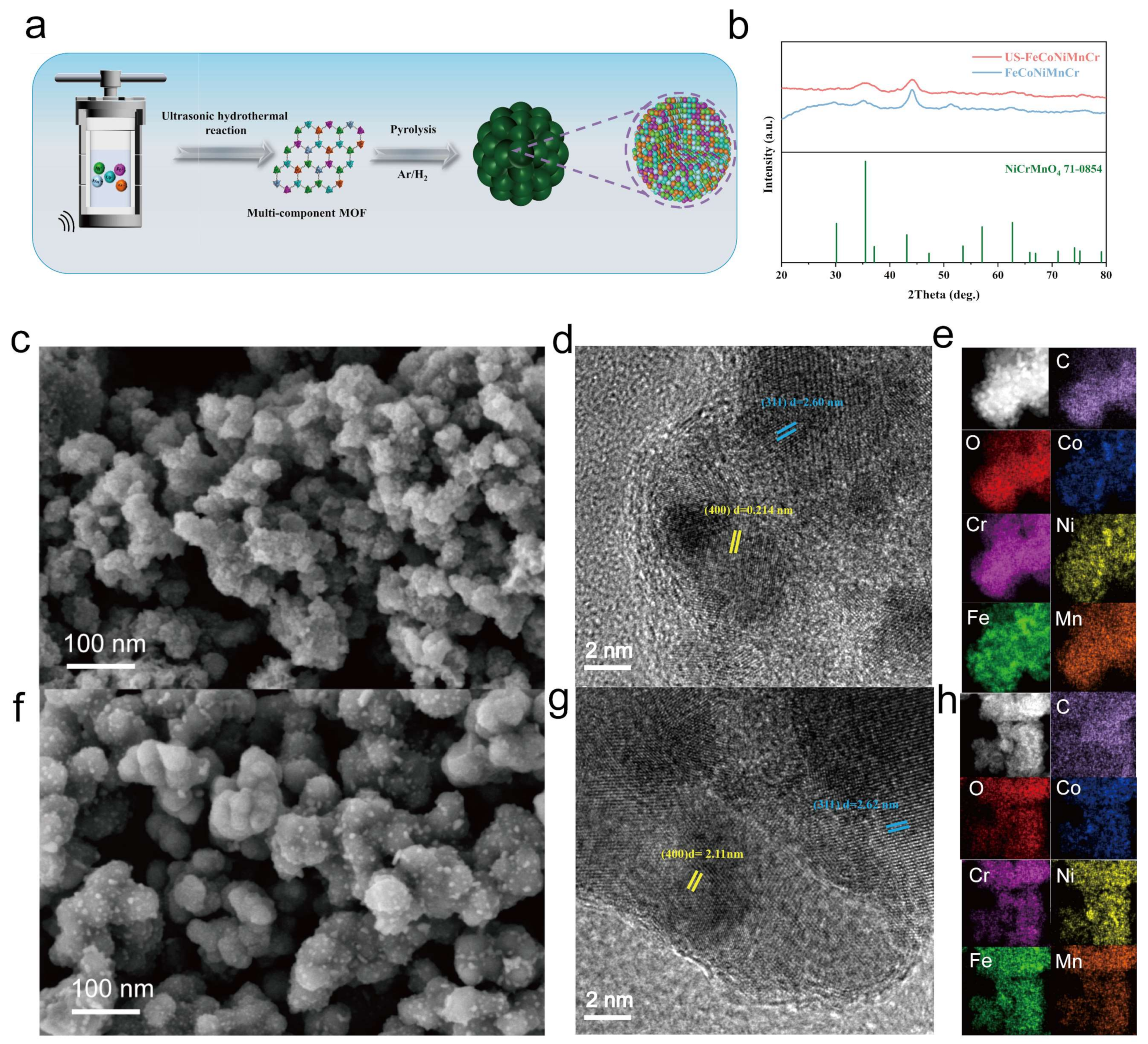
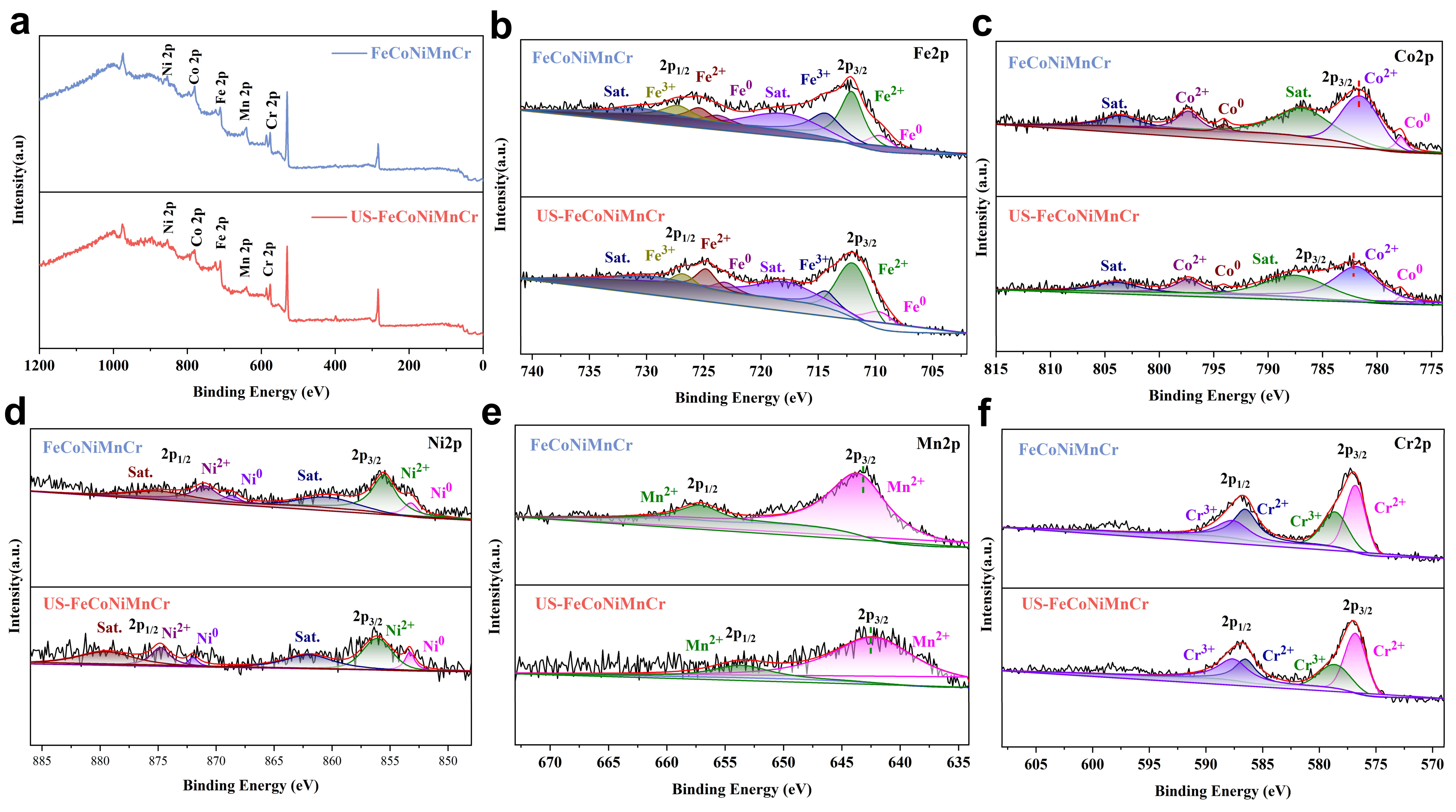
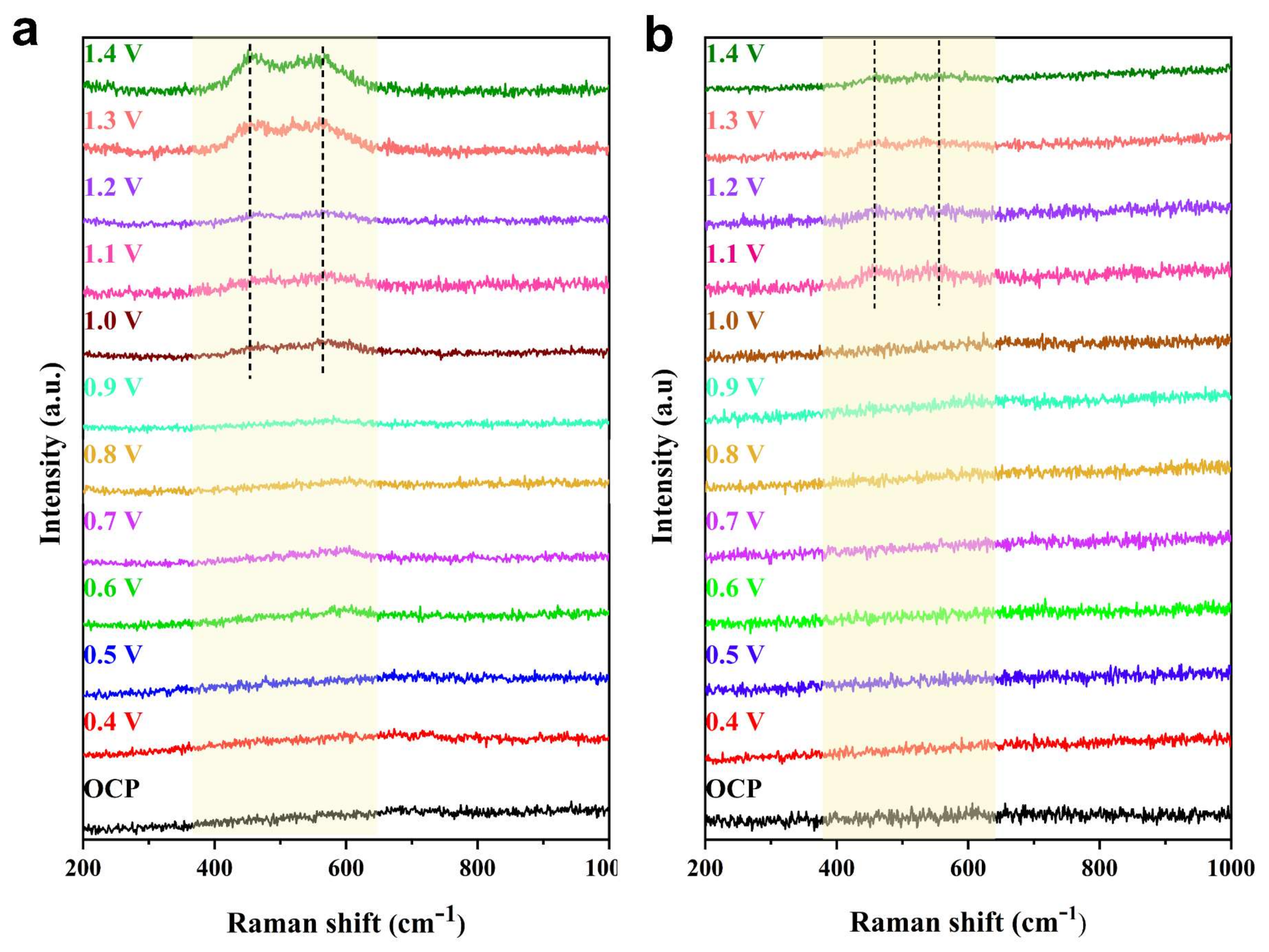
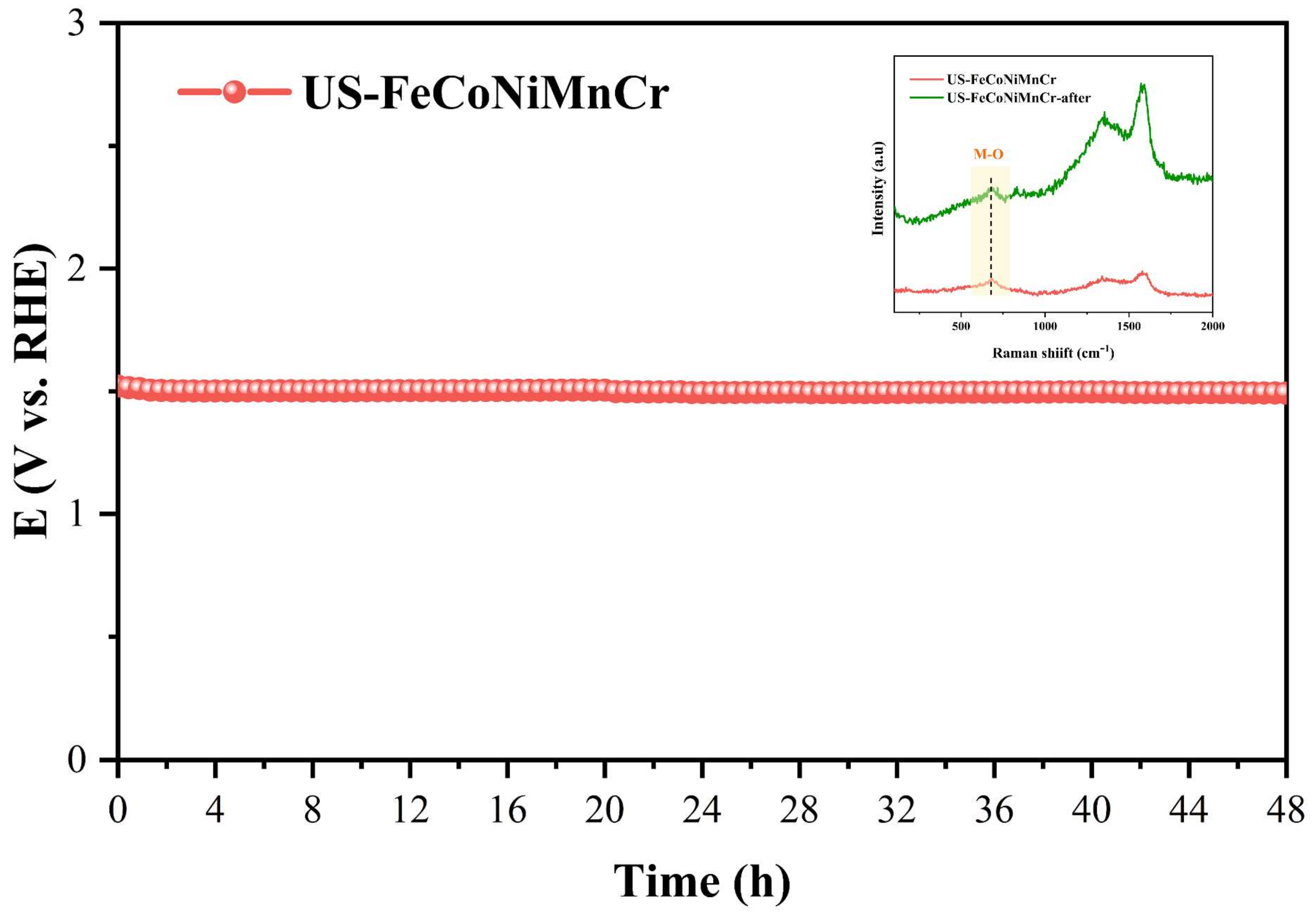
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, H.H.; Cao, J.D.; Wang, F.H.; Di, S.X.; Zhu, H.; Pu, M.; Bulanova, A. Composition-tunable PtCu porous nanowires as highly active and durable catalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2021, 46, 18284–18293. [Google Scholar] [CrossRef]
- Cerchier, P.; Dabala, M.; Brunelli, K. Green synthesis of copper nanoparticles with ultrasound assistance. Green Process. Synth. 2017, 6, 311–316. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Mei, Y.; Le, T.; Shao, H.; Jiang, H.; Feng, Y.; Hu, J. NiFe layered double hydroxide as an efficient bifunctional catalyst for electrosynthesis of hydrogen peroxide and oxygen. Int. J. Hydrogen Energy 2022, 47, 36831–36842. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, C.; Wang, Z.; Zhang, Y.; Le, T.; Mei, Y.; Feng, Y.; Hu, J. Enhanced two-electron oxygen reduction for hydrogen peroxide production via fine-tuning the concentration of oxygen vacancies in MoO3−x. Appl. Catal. A Gen. 2023, 661, 119242. [Google Scholar] [CrossRef]
- Qi, Q.; Tai, J.; Hu, J.; Zhang, Z.; Dai, L.; Song, H.; Shao, M.; Zhang, C.; Zhang, L. Ligand Functionalized Iron-Based Metal-Organic Frameworks for Efficient Electrocatalytic Oxygen Evolution. ChemCatChem 2021, 13, 4976–4984. [Google Scholar] [CrossRef]
- Lim, D.; Oh, E.; Lim, C.; Shim, S.E.; Baeck, S.-H. Bimetallic NiFe alloys as highly efficient electrocatalysts for the oxygen evolution reaction. Catal. Today 2020, 352, 27–33. [Google Scholar] [CrossRef]
- Ning, H.; Li, G.; Chen, Y.; Zhang, K.; Gong, Z.; Nie, R.; Hu, W.; Xia, Q. Porous N-Doped Carbon-Encapsulated CoNi Alloy Nanoparticles Derived from MOFs as Efficient Bifunctional Oxygen Electrocatalysts. ACS Appl. Mater. Interfaces 2019, 11, 1957–1968. [Google Scholar] [CrossRef]
- Jiang, J.; Chang, L.; Zhao, W.; Tian, Q.; Xu, Q. An advanced FeCoNi nitro-sulfide hierarchical structure from deep eutectic solvents for enhanced oxygen evolution reaction. Chem. Commun. 2019, 55, 10174–10177. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Lu, X.; Hu, Y.; Zhu, G.; Chen, R.; Ma, L.; Zhu, H.; Tie, Z.; Liu, J.; et al. The effects of Al substitution and partial dissolution on ultrathin NiFeAl trinary layered double hydroxide nanosheets for oxygen evolution reaction in alkaline solution. Nano Energy 2017, 35, 350–357. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Jiang, L.; Langan, T.; Wood, T.; Sanders, P.; Dorin, T. Isotropy of precipitate distribution in pre-stretched Al-Cu-(Sc)-(Zr) alloys. Scr. Mater. 2022, 210, 114452. [Google Scholar] [CrossRef]
- Sun, S.; Bai, J.; Gu, J.; Guo, K.; Morley, N.; Gao, Q.; Zhang, Y.; Esling, C.; Zhao, X.; Zuo, L. Extraordinary mechanical properties and room-temperature magnetocaloric effects in spark plasma sintered all-d-metal Ni-Co-Mn-Ti alloy. J. Alloys Compd. 2024, 976, 173406. [Google Scholar] [CrossRef]
- Mei, Y.; Feng, Y.; Zhang, C.; Zhang, Y.; Qi, Q.; Hu, J. High-Entropy Alloy with Mo-Coordination as Efficient Electrocatalyst for Oxygen Evolution Reaction. ACS Catal. 2022, 12, 10808–10817. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Hu, J.; Li, B.; Qi, Q.L.; Zhang, Y.T.; Chen, J.; Dong, P.; Zhang, C.X.; Zhang, Y.J.; Leung, M.K.H. Recent research progress on high-entropy alloys as electrocatalytic materials. J. Alloys Compd. 2022, 918, 165585. [Google Scholar] [CrossRef]
- Brechtl, J.; Feng, R.; Liaw, P.K.; Beausir, B.; Jaber, H.; Lebedkina, T.; Lebyodkin, M. Mesoscopic-scale complexity in macroscopically-uniform plastic flow of an Al0.3CoCrFeNi high-entropy alloy. Acta Mater. 2023, 242, 118445. [Google Scholar] [CrossRef]
- Xing, Y.; Li, C.J.; Mu, Y.K.; Jia, Y.D.; Song, K.K.; Tan, J.; Wang, G.; Zhang, Z.Q.; Yi, J.H.; Eckert, J. Strengthening and deformation mechanism of high-strength CrMnFeCoNi high entropy alloy prepared by powder metallurgy. J. Mater. Sci. Technol. 2023, 132, 119–131. [Google Scholar] [CrossRef]
- Wang, Z.; You, J.; Zhao, Y.; Yao, R.; Liu, G.; Lu, J.; Zhao, S. Research progress on high entropy alloys and high entropy derivatives as OER catalysts. J. Environ. Chem. Eng. 2023, 11, 109080. [Google Scholar] [CrossRef]
- Zhang, G.; Ming, K.; Kang, J.; Huang, Q.; Zhang, Z.; Zheng, X.; Bi, X. High entropy alloy as a highly active and stable electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2018, 279, 19–23. [Google Scholar] [CrossRef]
- Dai, W.; Lu, T.; Pan, Y. Novel and promising electrocatalyst for oxygen evolution reaction based on MnFeCoNi high entropy alloy. J. Power Sources 2019, 430, 104–111. [Google Scholar] [CrossRef]
- Löffler, T.; Meyer, H.; Savan, A.; Wilde, P.; Garzón Manjón, A.; Chen, Y.-T.; Ventosa, E.; Scheu, C.; Ludwig, A.; Schuhmann, W. Discovery of a Multinary Noble Metal–Free Oxygen Reduction Catalyst. Adv. Energy Mater. 2018, 8, 1802269. [Google Scholar] [CrossRef]
- Nellaiappan, S.; Katiyar, N.K.; Kumar, R.; Parui, A.; Malviya, K.D.; Pradeep, K.G.; Singh, A.K.; Sharma, S.; Tiwary, C.S.; Biswas, K. High-Entropy Alloys as Catalysts for the CO2 and CO Reduction Reactions: Experimental Realization. ACS Catal. 2020, 10, 3658–3663. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, H.; Wu, X.; Deng, Y.; Wang, Z.; Han, Y.; Li, H.; Shi, Y.; Chen, X.; Li, S.; et al. Multi-Site Electrocatalysts Boost pH-Universal Nitrogen Reduction by High-Entropy Alloys. Adv. Funct. Mater. 2021, 31, 2006939. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, Z.; Xie, P.; Lacey, S.D.; Jacob, R.J.; Xie, H.; Chen, F.; Nie, A.; Pu, T.; Rehwoldt, M.; et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science 2018, 359, 1489–1494. [Google Scholar] [CrossRef]
- Akhlaghi, P.; Amirjan, M.; Parvin, N. The effect of processing parameters and heat-treatment on the microstructure and mechanical properties of PM CoCrFeMnNiTi0.1 high-entropy alloy. Mater. Chem. Phys. 2021, 257, 123722. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Zhang, C.; Feng, Y.; Shao, H.; Chen, J.; Hu, J.; Zhang, L. Fabricating carbon quantum dots of graphitic carbon nitride vis ultrasonic exfoliation for highly efficient H2O2 production. Ultrason. Sonochemistry 2023, 99, 106582. [Google Scholar] [CrossRef] [PubMed]
- Pollet, B.G. A Short Introduction to Sonoelectrochemistry. Electrochem. Soc. Interface 2018, 27, 41. [Google Scholar] [CrossRef]
- Hinman, J.J.; Suslick, K.S. Nanostructured Materials Synthesis Using Ultrasound. Top. Curr. Chem. 2017, 375, 12. [Google Scholar] [CrossRef] [PubMed]
- Sancheti, S.V.; Gogate, P.R. A review of engineering aspects of intensification of chemical synthesis using ultrasound. Ultrason. Sonochemistry 2017, 36, 527–543. [Google Scholar] [CrossRef]
- Lu, Z.P.; Wang, H.; Chen, M.W.; Baker, I.; Yeh, J.W.; Liu, C.T.; Nieh, T.G. An assessment on the future development of high-entropy alloys: Summary from a recent workshop. Intermetallics 2015, 66, 67–76. [Google Scholar] [CrossRef]
- Miracle, D.B.; Miller, J.D.; Senkov, O.N.; Woodward, C.; Uchic, M.D.; Tiley, J. Exploration and Development of High Entropy Alloys for Structural Applications. Entropy 2014, 16, 494–525. [Google Scholar] [CrossRef]
- Sivalingam, S.; Sen, S. Valorization of coal fly ash into nanozeolite by sonication-assisted hydrothermal method. J. Environ. Manag. 2019, 235, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Z.; Du, S.; Zhang, Y.; Li, H.; Xiao, Z.; Chen, W.; Chen, R.; Wang, Y.; Zou, Y.; et al. Low-temperature synthesis of small-sized high-entropy oxides for water oxidation. J. Mater. Chem. A 2019, 7, 24211–24216. [Google Scholar] [CrossRef]
- Chen, S.; Huang, H.; Jiang, P.; Yang, K.; Diao, J.; Gong, S.; Liu, S.; Huang, M.; Wang, H.; Chen, Q. Mn-Doped RuO2 Nanocrystals as Highly Active Electrocatalysts for Enhanced Oxygen Evolution in Acidic Media. ACS Catal. 2020, 10, 1152–1160. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Zhou, L.; Dai, S. Enhanced OER performance of composite Co–Fe-based MOF catalysts via a one-pot ultrasonic-assisted synthetic approach. Sustain. Energy Fuels 2021, 5, 1095–1102. [Google Scholar] [CrossRef]
- Vijay, A.; Ramanujachary, K.V.; Lofland, S.E.; Vaidya, S. Role of crystal structure and electrical polarization of an electrocatalyst in enhancing oxygen evolution performance: Bi-Fe-O system as a case study. Electrochim. Acta 2022, 407, 139887. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, B.; De Luna, P.; Liang, Y.; Comin, R.; Voznyy, O.; Han, L.; García de Arquer, F.P.; Liu, M.; Dinh, C.T.; et al. Theory-driven design of high-valence metal sites for water oxidation confirmed using in situ soft X-ray absorption. Nat. Chem. 2018, 10, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Duan, H.; Yu, J.; Qiu, C.; Yu, R.; Gao, J.; Li, S.; Du, X.; Si, Z.; Yang, S. Strong Electron Coupling Effect at the CoO/CeO2 Interface Enables Efficient Oxygen Evolution Reaction. ACS Mater. Lett. 2022, 4, 2572–2578. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, B.; Zeng, C.; Guo, S. Electrocatalytic activity of high-entropy alloys toward oxygen evolution reaction. MRS Commun. 2018, 8, 1230–1235. [Google Scholar] [CrossRef]
- Ding, Z.; Bian, J.; Shuang, S.; Liu, X.; Hu, Y.; Sun, C.; Yang, Y. High Entropy Intermetallic–Oxide Core–Shell Nanostructure as Superb Oxygen Evolution Reaction Catalyst. Adv. Sustain. Syst. 2020, 4, 1900105. [Google Scholar] [CrossRef]
- Qiu, H.-J.; Fang, G.; Gao, J.; Wen, Y.; Lv, J.; Li, H.; Xie, G.; Liu, X.; Sun, S. Noble Metal-Free Nanoporous High-Entropy Alloys as Highly Efficient Electrocatalysts for Oxygen Evolution Reaction. ACS Mater. Lett. 2019, 1, 526–533. [Google Scholar] [CrossRef]
- Yan, W.; Jiang, H.; Yi, W.; Zhao, C.; Xia, Y.; Cong, H.; Tang, L.; Cheng, G.J.; He, J.; Deng, H. High-entropy-alloy nanoparticles synthesized by laser metallurgy using a multivariate MOF. Mater. Chem. Front. 2022, 6, 2796–2802. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.; Zhang, B.; Wang, G.; Jiang, K.; Zheng, Z.; Shen, J. High-entropy alloy CuCrFeNiCoP film of Cu-based as high-efficiency electrocatalyst for water splitting. J. Alloys Compd. 2023, 969, 172439. [Google Scholar] [CrossRef]
- Zhao, X.; Xue, Z.; Chen, W.; Bai, X.; Shi, R.; Mu, T. Ambient fast, large-scale synthesis of entropy-stabilized metal–organic framework nanosheets for electrocatalytic oxygen evolution. J. Mater. Chem. A 2019, 7, 26238–26242. [Google Scholar] [CrossRef]
- Li, P.; Wan, X.; Su, J.; Liu, W.; Guo, Y.; Yin, H.; Wang, D. A Single-Phase FeCoNiMnMo High-Entropy Alloy Oxygen Evolution Anode Working in Alkaline Solution for over 1000 h. ACS Catal. 2022, 12, 11667–11674. [Google Scholar] [CrossRef]
- Wang, H.; Wei, R.; Li, X.; Ma, X.; Hao, X.; Guan, G. Nanostructured amorphous Fe29Co27Ni23Si9B12 high-entropy-alloy: An efficient electrocatalyst for oxygen evolution reaction. J. Mater. Sci. Technol. 2021, 68, 191–198. [Google Scholar] [CrossRef]
- Liu, L.-H.; Li, N.; Han, M.; Han, J.-R.; Liang, H.-Y. Scalable synthesis of nanoporous high entropy alloys for electrocatalytic oxygen evolution. Rare Met. 2022, 41, 125–131. [Google Scholar] [CrossRef]
- Ma, P.; Zhang, S.; Zhang, M.; Gu, J.; Zhang, L.; Sun, Y.; Ji, W.; Fu, Z. Hydroxylated high-entropy alloy as highly efficient catalyst for electrochemical oxygen evolution reaction. Sci. China Mater. 2020, 63, 2613–2619. [Google Scholar] [CrossRef]
- Kim, J.-H.; Son, B.-R.; Yoon, D.-H.; Hwang, K.-T.; Noh, H.-G.; Cho, W.-S.; Kim, U.-S. Characterization of blue CoAl2O4 nano-pigment synthesized by ultrasonic hydrothermal method. Ceram. Int. 2012, 38, 5707–5712. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Xi, S.; Song, J.; Dou, S.; Li, X.; Du, Y.; Diao, C.; Xu, Z.J.; Wang, X. Tuning of lattice oxygen reactivity and scaling relation to construct better oxygen evolution electrocatalyst. Nat. Commun. 2021, 12, 3992. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Cheng, Y.; Xing, H.; Li, J.; Zhu, X.; Ma, L.; Li, Y.; Liu, D. S-Doping Triggers Redox Reactivities of Both Iron and Lattice Oxygen in FeOOH for Low-Cost and High-Performance Water Oxidation. Adv. Funct. Mater. 2022, 32, 2112674. [Google Scholar] [CrossRef]
- Wang, F.; Zou, P.; Zhang, Y.; Pan, W.; Li, Y.; Liang, L.; Chen, C.; Liu, H.; Zheng, S. Activating lattice oxygen in high-entropy LDH for robust and durable water oxidation. Nat. Commun. 2023, 14, 6019. [Google Scholar] [CrossRef] [PubMed]
- Tomar, A.K.; Pan, U.N.; Kim, N.H.; Lee, J.H. Enabling Lattice Oxygen Participation in a Triple Perovskite Oxide Electrocatalyst for the Oxygen Evolution Reaction. ACS Energy Lett. 2023, 8, 565–573. [Google Scholar] [CrossRef]
- Hu, J.; Guo, T.; Zhong, X.; Li, J.; Mei, Y.; Zhang, C.; Feng, Y.; Sun, M.; Meng, L.; Wang, Z.; et al. In Situ Reconstruction of High-Entropy Heterostructure Catalysts for Stable Oxygen Evolution Electrocatalysis under Industrial Conditions. Adv. Mater. 2024, 2310918. [Google Scholar] [CrossRef]
- Ting, N.-H.; Nguyen, T.X.; Lee, C.-H.; Chen, Y.-C.; Yeh, C.-H.; Chen, H.-Y.T.; Ting, J.-M. Composition-controlled high entropy metal glycerate as high-performance electrocatalyst for oxygen evolution reaction. Appl. Mater. Today 2022, 27, 101398. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhang, C.; Zhang, Y.; Hu, J. Ultrasound-Assisted Synthesis of High-Entropy Materials for Enhanced Oxygen Evolution Electrocatalysis. Metals 2024, 14, 384. https://doi.org/10.3390/met14040384
Wang Z, Zhang C, Zhang Y, Hu J. Ultrasound-Assisted Synthesis of High-Entropy Materials for Enhanced Oxygen Evolution Electrocatalysis. Metals. 2024; 14(4):384. https://doi.org/10.3390/met14040384
Chicago/Turabian StyleWang, Zhiyuan, Chengxu Zhang, Yue Zhang, and Jue Hu. 2024. "Ultrasound-Assisted Synthesis of High-Entropy Materials for Enhanced Oxygen Evolution Electrocatalysis" Metals 14, no. 4: 384. https://doi.org/10.3390/met14040384
APA StyleWang, Z., Zhang, C., Zhang, Y., & Hu, J. (2024). Ultrasound-Assisted Synthesis of High-Entropy Materials for Enhanced Oxygen Evolution Electrocatalysis. Metals, 14(4), 384. https://doi.org/10.3390/met14040384




