Synthesis of TiCx/Al Composites via In Situ Reaction between AlxTi Melt and Dissolvable Solid Carbon
Abstract
1. Introduction
2. Materials and Methods
3. Results
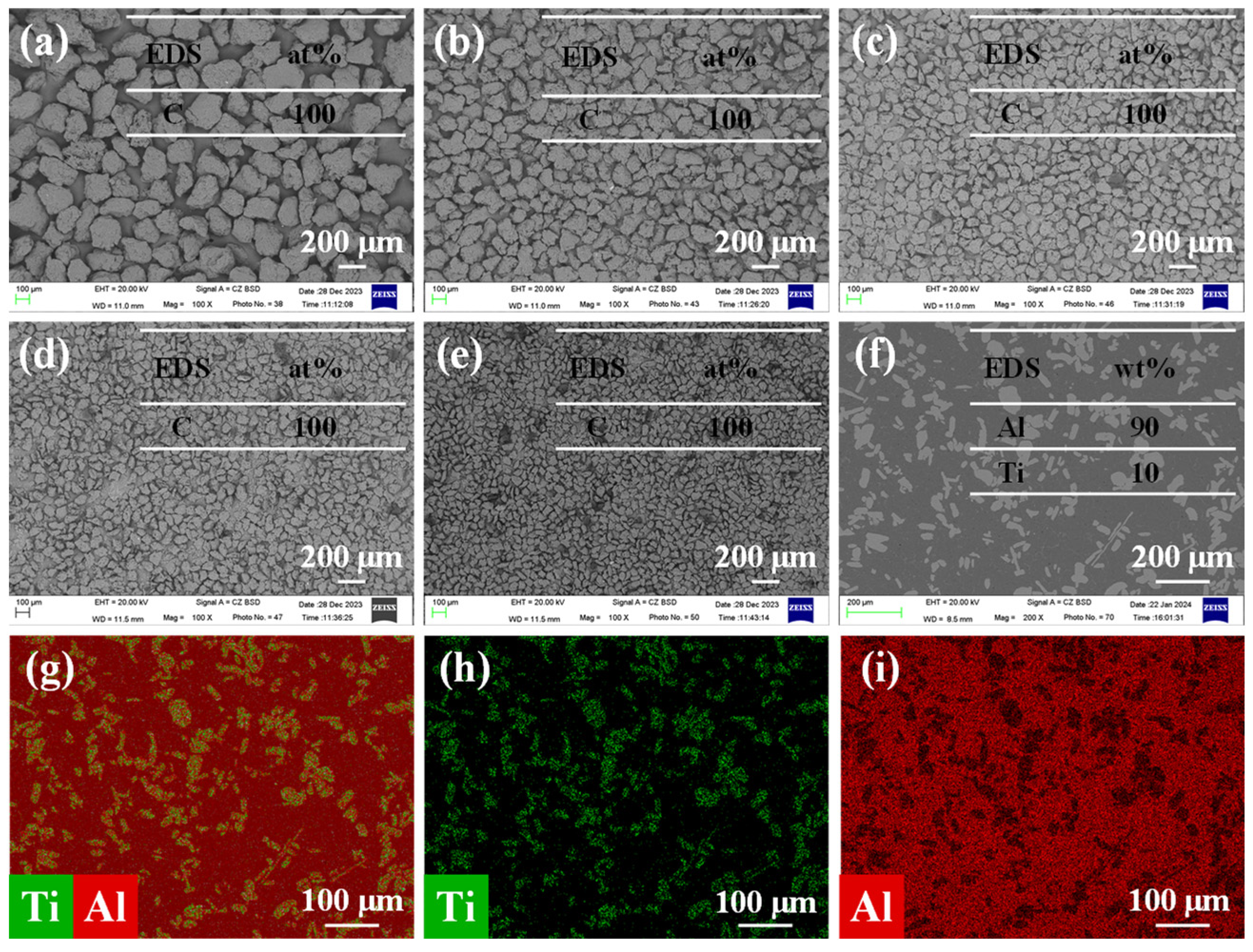
3.1. Raw Material Microstructure
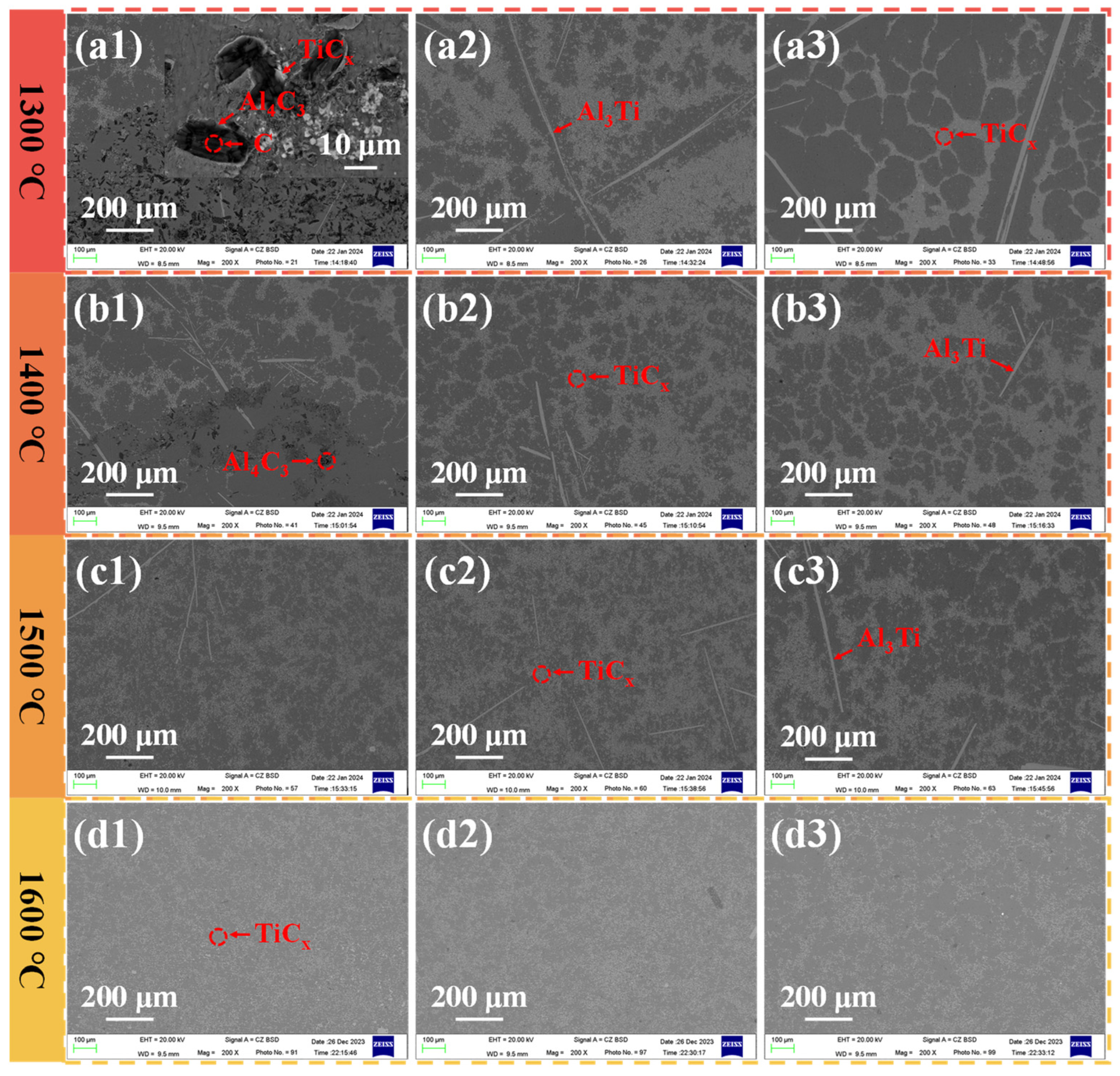
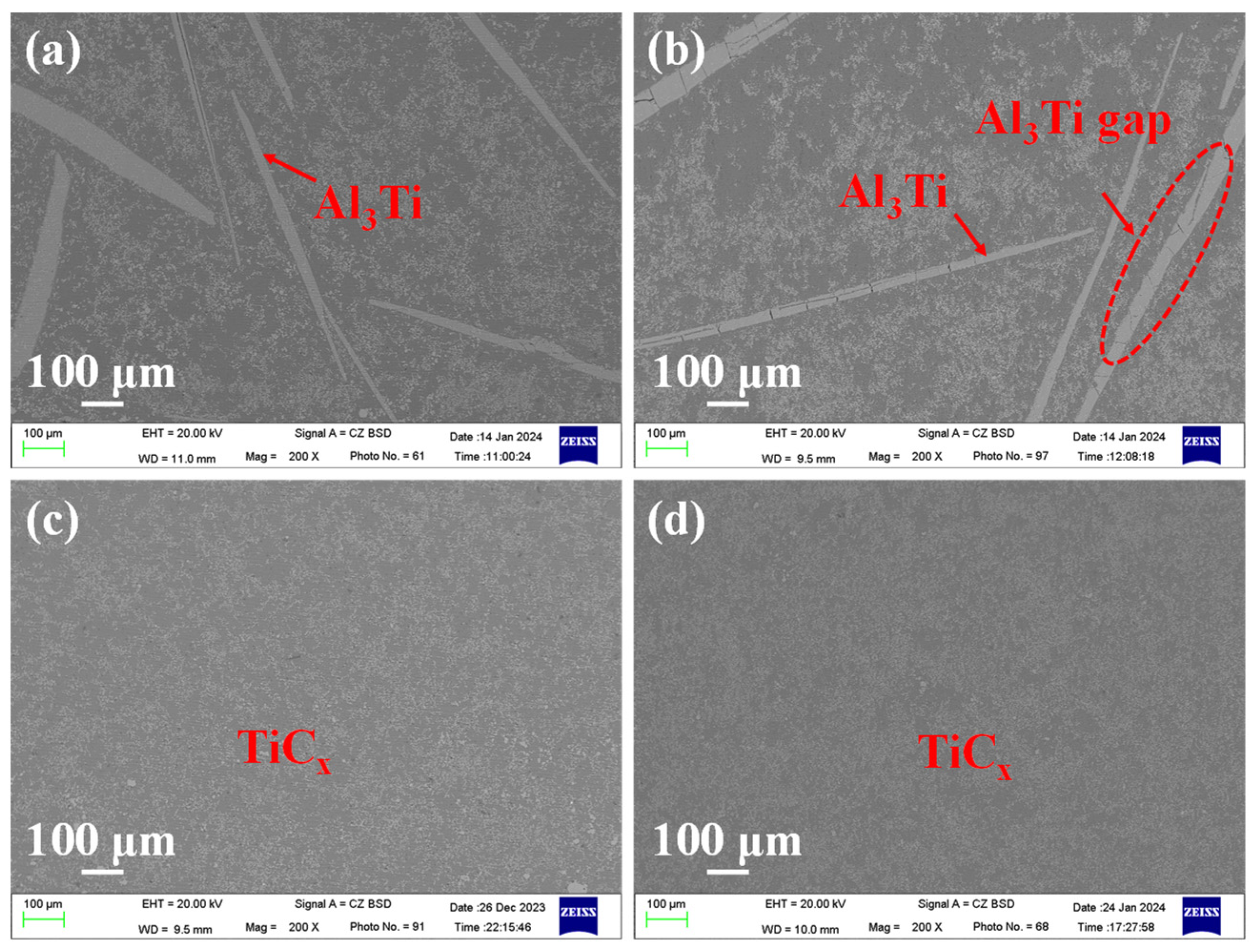
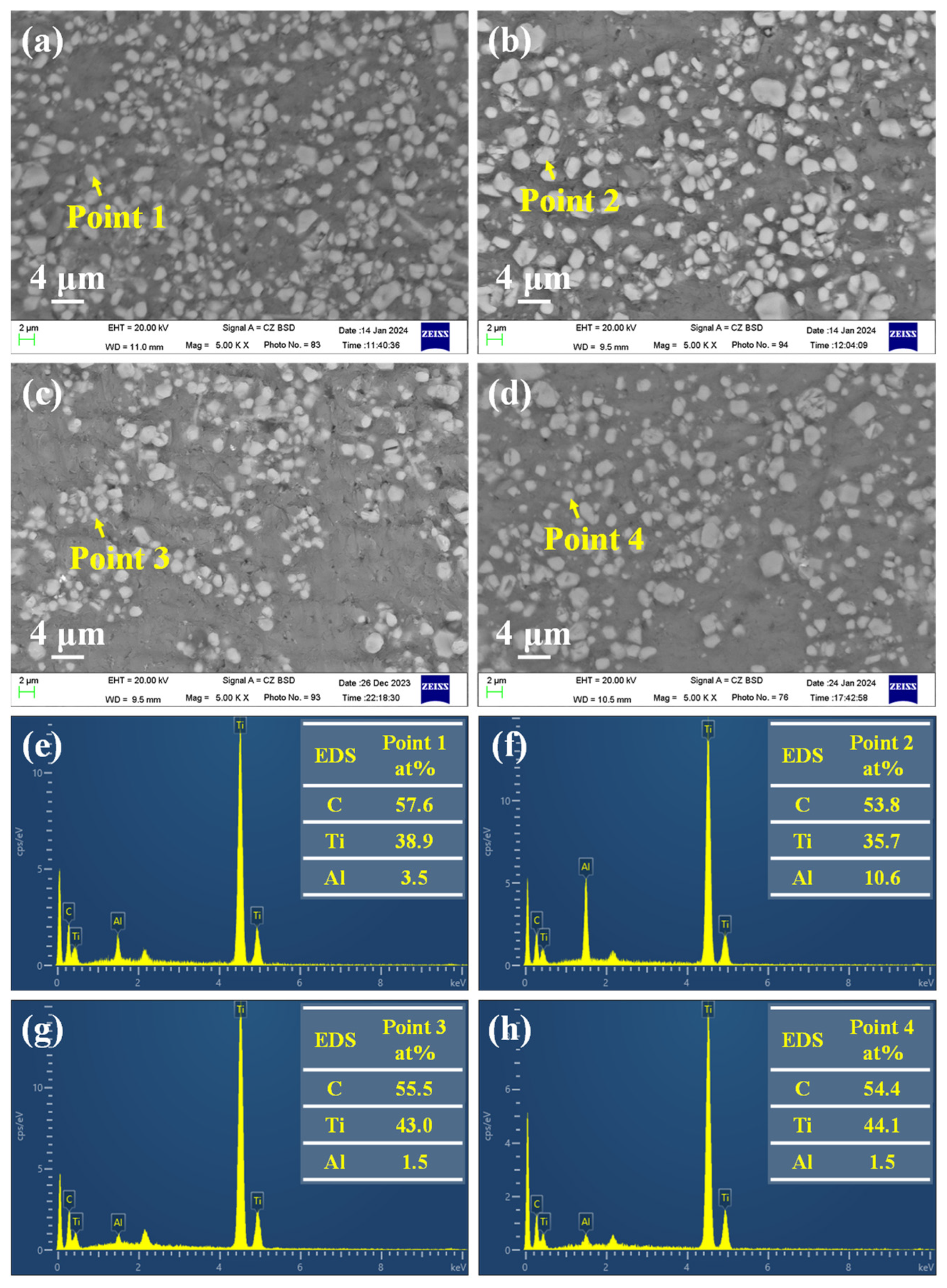
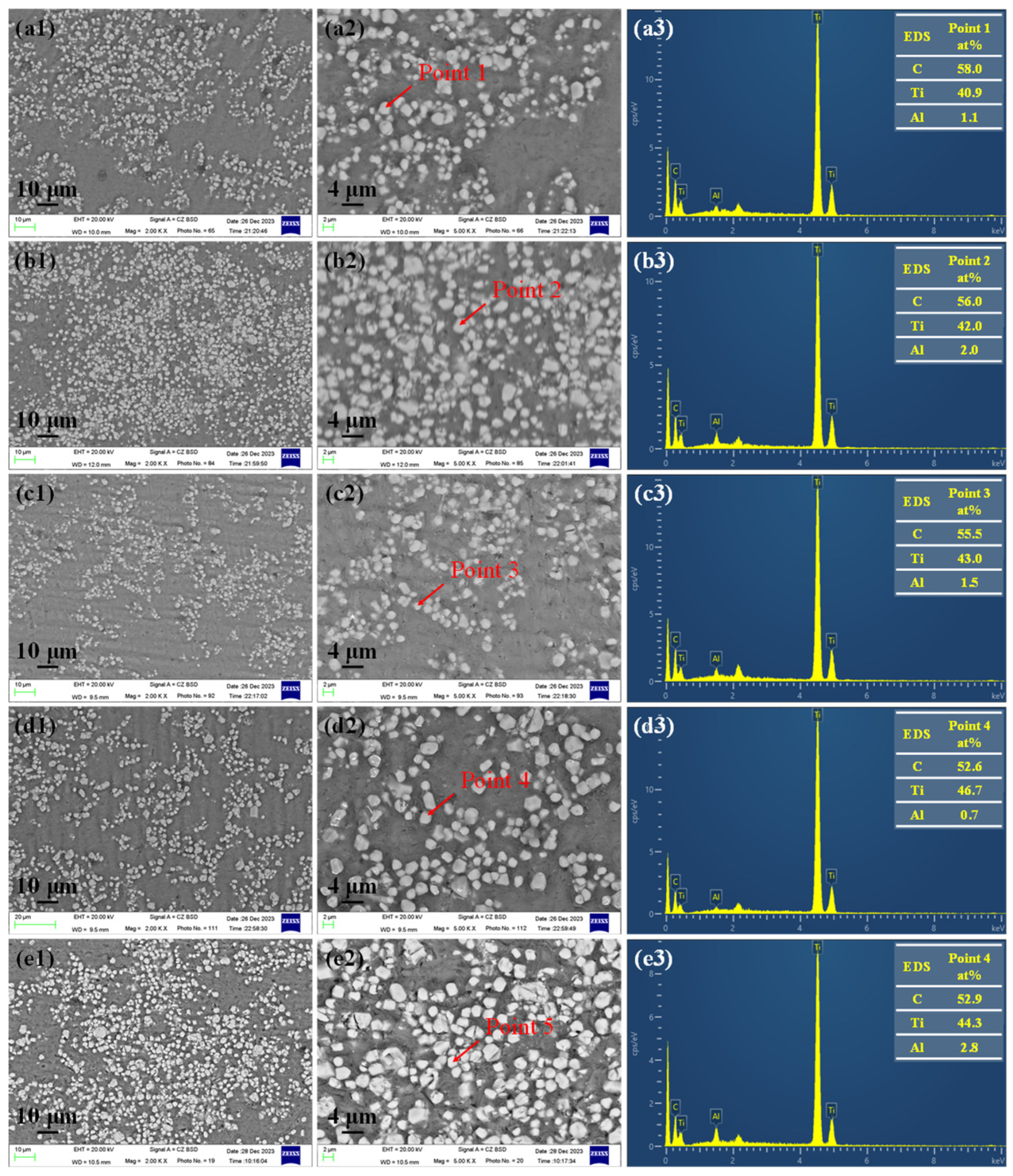
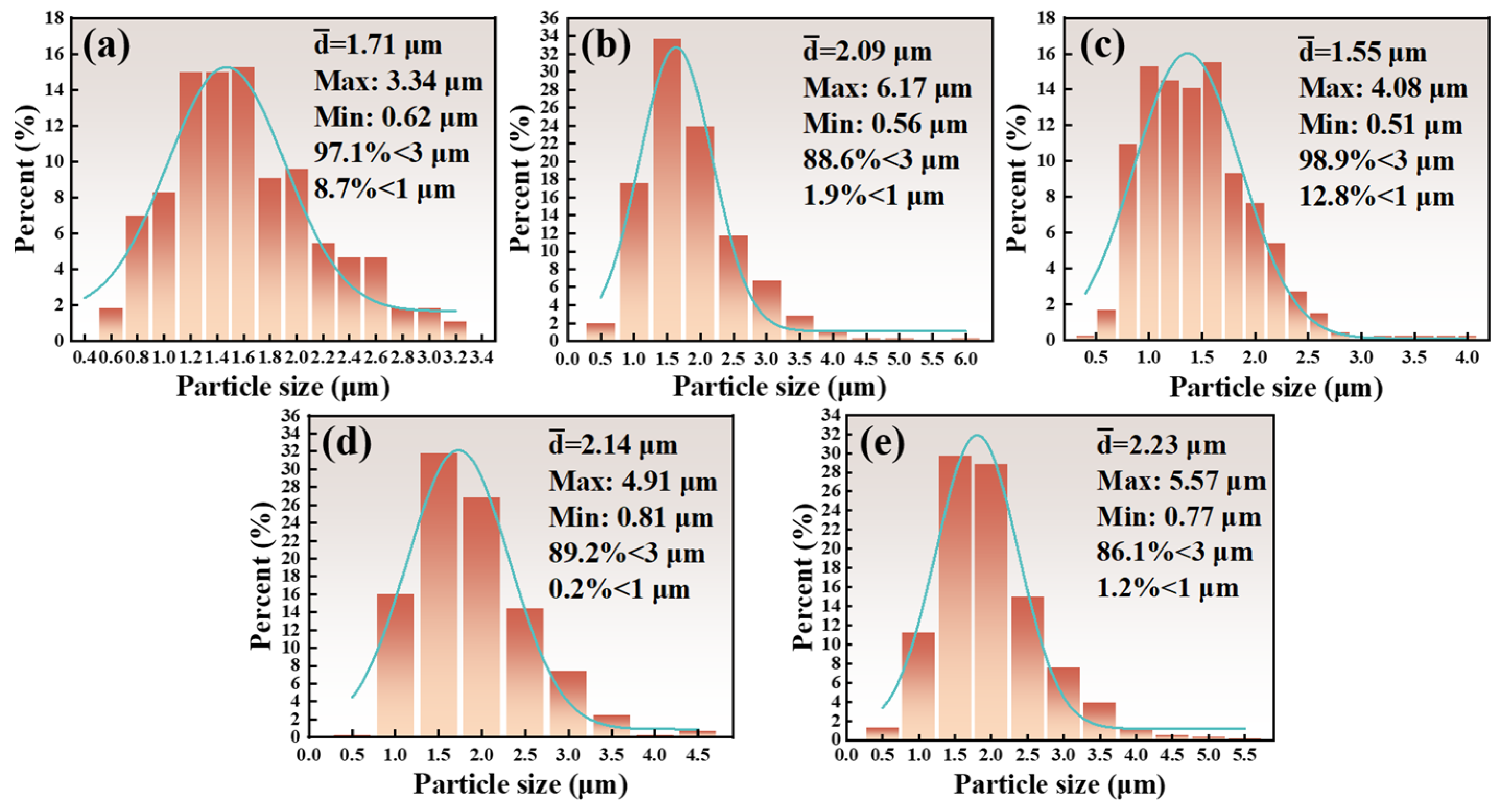
3.2. Effect of Reaction Temperature and Reaction Time on TiCx/Al Composites
3.3. Effect of Graphite Particle Size on TiCx/Al Composites
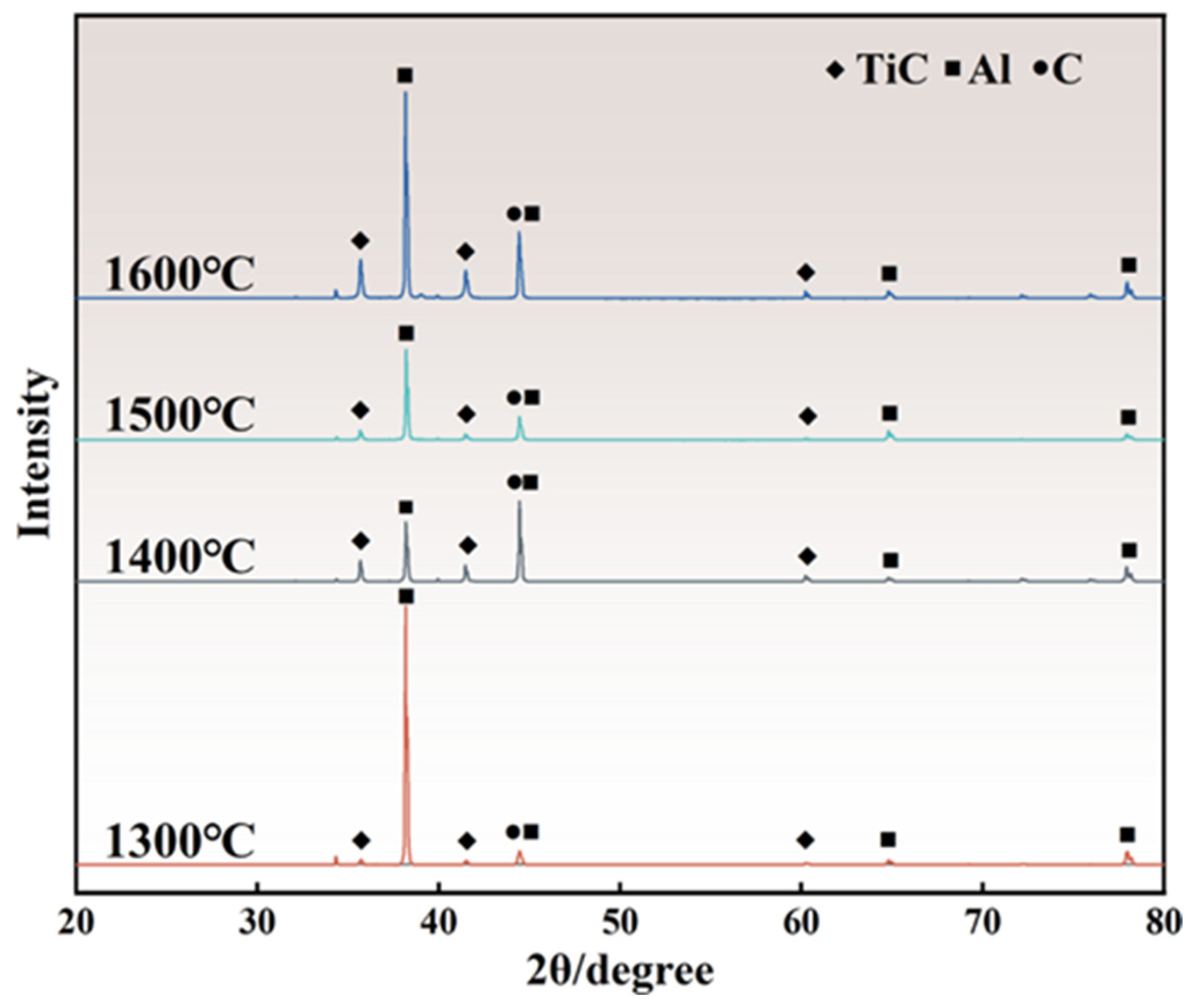
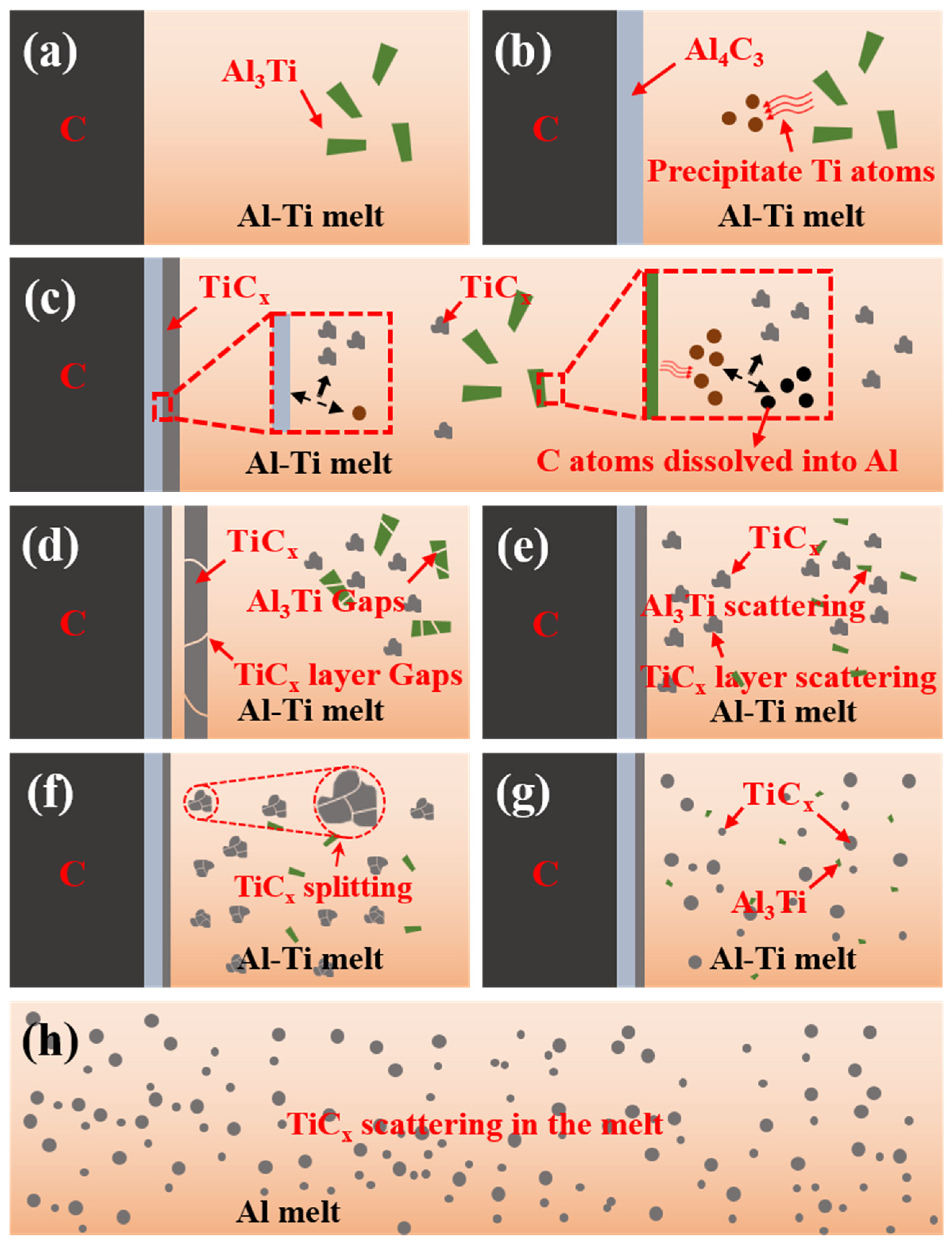
3.4. Synthesis Sequence of TiCx in Al-10Ti-2.5C System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Korkut, M.H. Effect of particulate reinforcement on wear behaviour of aluminium matrix composites. Mater. Sci. Technol. 2004, 20, 73–81. [Google Scholar] [CrossRef]
- Suárez, O.M. Mechanical Properties of a Novel Aluminum Matrix Composite Containing Boron. J. Mech. Behav. Mater. 2001, 12, 225–238. [Google Scholar] [CrossRef]
- Martinez, M.A.; Martin, A.; Llorca, J. Wear of Al-Si alloys and Al-Si/SiC composites at ambient and elevated temperatures. Scr. Metall. Mater. 1993, 28, 207–212. [Google Scholar] [CrossRef]
- Yin, Z.J.; Tao, S.Y.; Zhou, X.M.; Ding, C.X. Microstructure and mechanical properties of Al2O3–Al composite coatings deposited by plasma spraying. Appl. Surf. Sci. 2008, 254, 1636–1643. [Google Scholar] [CrossRef]
- Song, M. Effects of volume fraction of SiC particles on mechanical properties of SiC/Al composites. Trans. Nonferrous Met. Soc. China 2009, 19, 1400–1404. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, A.; Yang, T.; Gao, S.; Liu, S.N.; Guo, B.J.; Jiang, H.Y.; Shi, Y.S.; Yan, C.Z. Microstructure evolution and mechanical properties of Al2O3 foams via laser powder bed fusion from Al particles. Adv. Powder Mater. 2023, 2, 100135. [Google Scholar] [CrossRef]
- Xu, J.; Li, Z.Y.; Zhu, W.H.; Liu, Z.L.; Liu, W.J. Investigation on microstructural characterization of in situ TiB/Al metal matrix composite by laser cladding. Mater. Sci. Eng. A 2007, 447, 307–313. [Google Scholar] [CrossRef]
- Pourkhorshid, E.; Enayati, M.H.; Sabooni, S.; Karimzadeh, F.; Paydar, M.H. Bulk Al-Al3Zr composite prepared by mechanical alloying and hot extrusion for high-temperature applications. Int. J. Miner. Metall. Mater. 2017, 24, 937–942. [Google Scholar] [CrossRef]
- Ding, H.M.; Wu, J.M.; Jia, H.R.; Liu, F.; Wang, J.F. The Influence of Carbon Sources on the Microstructures of In Situ-Synthesized TiC in Al Melts. Materials 2022, 15, 4610. [Google Scholar] [CrossRef]
- Lee, J.C.; Lee, H.I.; Ahn, J.P.; Shi, Z.L.; Kim, Y.M. Modification of the interface in SiC/Al composites. Metall. Mater. Trans. A 2000, 31, 2361–2368. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wang, Z.; Chen, L.Y.; Shu, S.L.; Qiu, F.; Zhang, L.C. Interface formation and bonding control in high-volume-fraction (TiC+ TiB2)/Al composites and their roles in enhancing properties. Compos. Part B Eng. 2021, 209, 108605. [Google Scholar] [CrossRef]
- Jiang, R.S.; Chen, X.F.; Ge, R.W.; Wang, W.H.; Song, G.D. Influence of TiB2 particles on machinability and machining parameter optimization of TiB2/Al MMCs. Chin. J. Aeronaut. 2018, 31, 187–196. [Google Scholar] [CrossRef]
- Sattariand, S.; Atrian, A. Effects of the deep rolling process on the surface roughness and properties of an Al-3vol%SiC nanoparticle nanocomposite fabricated by mechanical milling and hot extrusion. Int. J. Miner. Metall. Mater. 2017, 24, 814–825. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.P.; Wang, X.; Koizumi, Y.; Kenta, Y.; Chiba, A. In-situ fabrication and characterization of ultrafine structured Cu-TiC composites with high strength and high conductivity by mechanical milling. J. Alloys Compd. 2016, 657, 122–132. [Google Scholar] [CrossRef]
- Khorrami, M.S.; Kazeminezhad, M.; Miyashita, Y.; Kokabi, A.H. Improvement in the mechanical properties of Al/SiC nanocomposites fabricated by severe plastic deformation and friction stir processing. Int. J. Miner. Metall. Mater. 2017, 24, 297–308. [Google Scholar] [CrossRef]
- Chak, V.; Chattopadhyay, H.; Dora, T.L. A review on fabrication methods, reinforcements and mechanical properties of aluminum matrix composites. J. Manuf. Process. 2020, 56, 1059–1074. [Google Scholar] [CrossRef]
- Hu, Z.J.; Shen, P.; Jiang, Q.C. Developing high-performance laminated Cu/TiC composites through melt infiltration of Ni-doped freeze-cast preforms. Ceram. Int. 2019, 45, 11686–11693. [Google Scholar] [CrossRef]
- Amosov, A.; Amosov, E.; Latukhin, E.; Kichaev, P.; Umerov, P. Producing TiC-Al Cermet by Combustion Synthesis of TiC Porous Skeleton with Spontaneous Infiltration by Aluminum Melt. In Proceedings of the 2020 7th International Congress on Energy Fluxes and Radiation Effects (EFRE), Tomsk, Russia, 14–26 September 2020. [Google Scholar]
- Huang, X.; Bao, L.K.; Bao, R.; Liu, L.; Tao, J.M.; Wang, J.S.; Zhang, Z.F.; Ge, Z.H.; Tan, S.L.; Yi, J.H.; et al. Reinforced copper matrix composites with highly dispersed nano size TiC in-situ generated from the Carbon Polymer Dots. Adv. Powder Mater. 2023, 2, 2772–2834X. [Google Scholar] [CrossRef]
- Li, W.J.; Zhang, L.; Chen, Z.Y.; Shao, W.Z.; Zhen, L. In Situ Fabrication and Static Contact Resistance of CdMoO4 Reinforced Cu Matrix Composites. Materials 2022, 15, 7206. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.B.; Shen, P.; Zhou, D.S.; Jiang, Q.C. Self-propagating high-temperature synthesis of nano-TiCx particles with different shapes by using carbon nano-tube as C source. Nanoscale Res. Lett. 2011, 6, 515. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Mu, D.K. Rapid dehydrogenation of TiH2 and its effect on formation mechanism of TiC during self-propagation high-temperature synthesis from TiH2–C system. Powder Technol. 2013, 249, 208–211. [Google Scholar] [CrossRef]
- Song, M.S.; Zhang, M.X.; Zhang, S.G.; Huang, B.; Li, J.G. In situ fabrication of TiC particulates locally reinforced aluminum matrix composites by self-propagating reaction during casting. Mater. Sci. Eng. A 2008, 473, 166–171. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, F.; Zhao, Q.L.; Zha, M.; Jiang, Q.C. Superior high creep resistance of in situ nano-sized TiCx/Al-Cu-Mg composite. Sci. Rep. 2017, 7, 4540. [Google Scholar] [CrossRef]
- Tian, W.S.; Zhao, Q.L.; Zhang, Q.Q.; Qiu, F.; Jiang, Q.C. Superior creep resistance of 0.3 wt% nano-sized TiCp/Al-Cu composite. Mater. Sci. Eng. A 2017, 700, 42–48. [Google Scholar] [CrossRef]
- Zhou, D.S.; Qiu, F.; Jiang, Q.C. The nano-sized TiC particle reinforced Al–Cu matrix composite with superior tensile ductility. Mater. Sci. Eng. A 2015, 622, 189–193. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, X.; Zhang, Y.; Wang, J.; Kim, M.J.; Zhang, H. Aluminum carbide hydrolysis induced degradation of thermal conductivity and tensile strength in diamond/aluminum composite. J. Compos. Mater. 2018, 52, 2709–2717. [Google Scholar] [CrossRef]
- Yang, B.; Chen, G.X.; Zhang, J.S. Effect of Ti/C additions on the formation of Al3Ti of in situ TiC/Al composites. Mater. Des. 2001, 22, 645–650. [Google Scholar] [CrossRef]
- Tian, W.S.; Zhao, Q.L.; Zhao, C.J.; Qiu, F.; Jiang, Q.C. The Dry Sliding Wear Properties of Nano-Sized TiCp/Al-Cu Composites at Elevated Temperatures. Materials 2017, 10, 939. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.M.; Li, F.J.; Yang, Q.C.; Zhan, K.; Yang, Z.; Wang, Z.; Zhao, B. A review on interfacial structure optimization and its mechanism on the properties of carbon reinforced metal-matrix composites. Compos. Interfaces 2023, 30, 543–583. [Google Scholar] [CrossRef]
- Jiang, W.H.; Song, G.H.; Han, X.L.; He, C.L.; Ru, H.C. Synthesis of TiC/Al composites in liquid aluminium. Mater. Lett. 1997, 32, 63–65. [Google Scholar] [CrossRef]
- Jin, S.B.; Shen, P.; Lin, Q.L.; Zhan, L.; Jiang, Q.C. Growth Mechanism of TiCx during Self-Propagating High-Temperature Synthesis in an Al-Ti-C System. Cryst. Growth Des. 2010, 10, 1590–1597. [Google Scholar] [CrossRef]
- Chen, T.J.; Wang, R.Q.; Ma, Y.; Hao, Y. Grain refinement of AZ91D magnesium alloy by Al–Ti–B master alloy and its effect on mechanical properties. Mater. Des. 2012, 34, 637–648. [Google Scholar] [CrossRef]


| Sample No. | Sample Component | Graphite Particle Size (μm) | Temperature (°C) | Holding Time (h) |
|---|---|---|---|---|
| A1 | Al-10 wt%Ti + 2.5 wt%C | 75–100 μm | 1300 °C | 4 h |
| A2 | Al-10 wt%Ti + 2.5 wt%C | 75–100 μm | 1400 °C | 4 h |
| A3 | Al-10 wt%Ti + 2.5 wt%C | 75–100 μm | 1500 °C | 4 h |
| A4 | Al-10 wt%Ti + 2.5 wt%C | 75–100 μm | 1600 °C | 4 h |
| B1 | Al-10 wt%Ti + 2.5 wt%C | 75–100 μm | 1600 °C | 0.17 h |
| B2 | Al-10 wt%Ti + 2.5 wt%C | 75–100 μm | 1600 °C | 1 h |
| B3 | Al-10 wt%Ti + 2.5 wt%C | 75–100 μm | 1600 °C | 4 h |
| B4 | Al-10 wt%Ti + 2.5 wt%C | 75–100 μm | 1600 °C | 8 h |
| C1 | Al-10 wt%Ti + 2.5 wt%C | 150–300 μm | 1600 °C | 4 h |
| C2 | Al-10 wt%Ti + 2.5 wt%C | 100–150 μm | 1600 °C | 4 h |
| C3 | Al-10 wt%Ti + 2.5 wt%C | 75–100 μm | 1600 °C | 4 h |
| C4 | Al-10 wt%Ti + 2.5 wt%C | 60–75 μm | 1600 °C | 4 h |
| C5 | Al-10 wt%Ti + 2.5 wt%C | 46–60 μm | 1600 °C | 4 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Sun, H.; Guo, Z. Synthesis of TiCx/Al Composites via In Situ Reaction between AlxTi Melt and Dissolvable Solid Carbon. Metals 2024, 14, 379. https://doi.org/10.3390/met14040379
Guo L, Sun H, Guo Z. Synthesis of TiCx/Al Composites via In Situ Reaction between AlxTi Melt and Dissolvable Solid Carbon. Metals. 2024; 14(4):379. https://doi.org/10.3390/met14040379
Chicago/Turabian StyleGuo, Lei, Hao Sun, and Zhancheng Guo. 2024. "Synthesis of TiCx/Al Composites via In Situ Reaction between AlxTi Melt and Dissolvable Solid Carbon" Metals 14, no. 4: 379. https://doi.org/10.3390/met14040379
APA StyleGuo, L., Sun, H., & Guo, Z. (2024). Synthesis of TiCx/Al Composites via In Situ Reaction between AlxTi Melt and Dissolvable Solid Carbon. Metals, 14(4), 379. https://doi.org/10.3390/met14040379





