2. Experimental Procedures
The Ni50Mn28Ga22 (at.%) alloy ingots were prepared using arc-melting high purity elements of Ni (99.99%), Mn (99.7%), and Ga (99.99%) four times to ensure uniform composition. After the alloy ingot was broken into powder, the alloy powder of 20–30 μm was sieved and mixed with NaCl. To control the pore size of the porous Ni50Mn28Ga22 alloy, the 50 vol.% sieved pure NaCl powders were mixed into the Ni–Mn–Ga powder as the space holders, where the NaCl powders were sieved into 20–30 μm, 50–75 μm, 112–200 μm, 200–355 μm, respectively. The mixed powder was obtained using ball milling mixtures consisting of 50 vol.% mechanically ground Ni50Mn28Ga22 powder and 50 vol.% NaCl powders of various sizes (>99.5% purity) for 5 h. Then, the mixed powder was placed into a steel mold and a unidirectional pressure of approximately 500 MPa was applied for 1 min. During the sintering process, the crucible containing the green billets was placed into a vacuum furnace and heated to 1023 K for 1 h and then heated to 1373 K and kept for 2 h to make NaCl volatilize from the compact to achieve complete sintering between the powders. It was then cooled to room temperature with a cooling rate of 5 K/min. The remaining NaCl was dissolved by immersing the sample into water for 48 h.
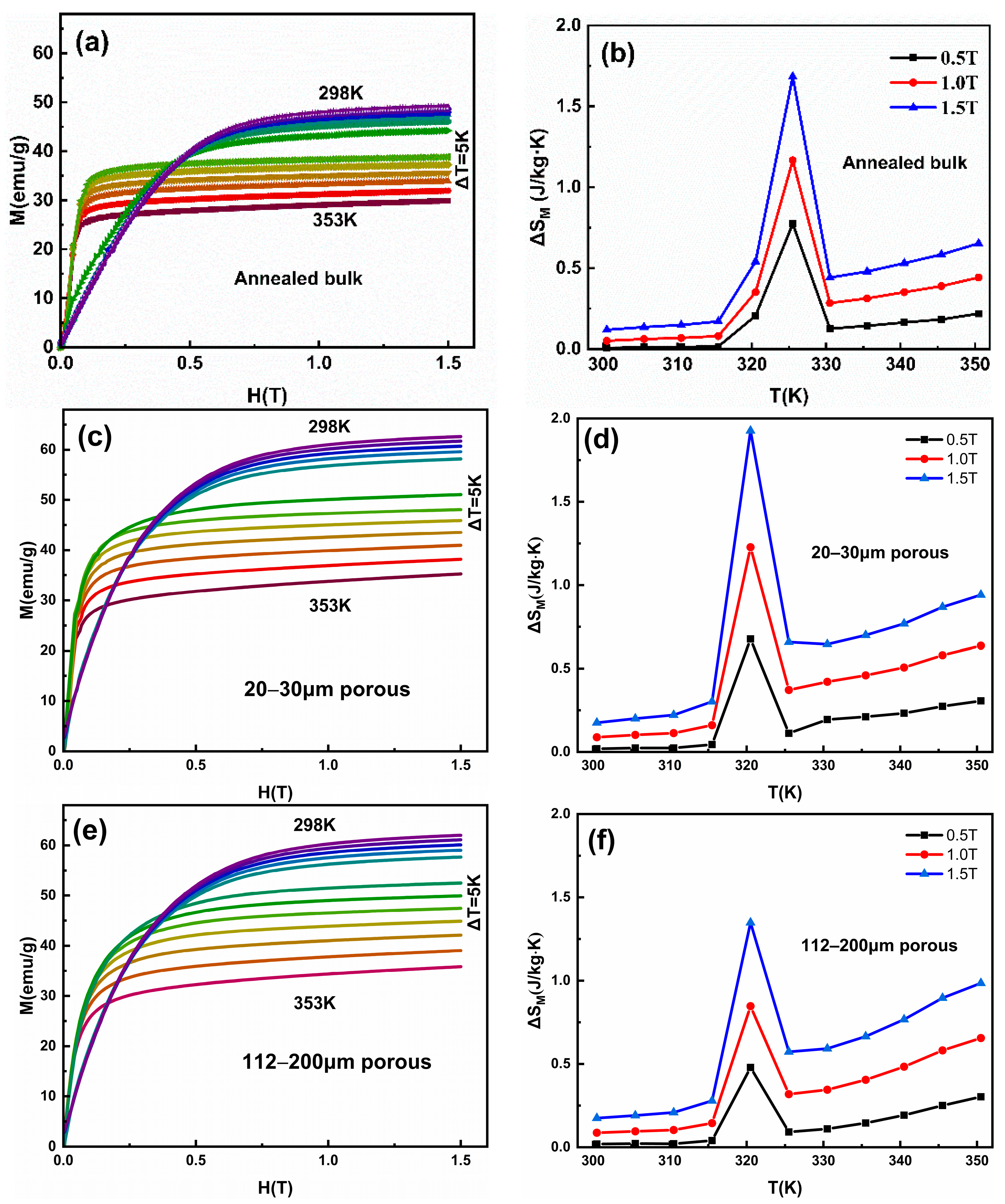
The porous alloys were characterized using an SEM (Carl Zeiss, Oberkochen, Germany) equipped with an EDS system to analyze the microstructure, fracture, and composition. To further examine the grain morphology, the corroded surface was observed using an optical microscope (Carl Zeiss, Oberkochen, Germany). The sample underwent mechanical polishing and etching using Kalling’ II solution (100 mL alcohol, 100 mL hydrochloric acid, and 5 g CuCl2) to prepare the porous surface for analysis. The phase analysis of Cu–Kα radiation (λ = 1.5406 Å) at room temperature was conducted using an X-ray diffractometer (XRD, Rigaku, Tokyo, Japan). The martensitic transformation was measured with a heating/cooling rate of 10 K/min using DSC (TA Instruments, New Castle, DE, USA). Isothermal magnetization M(H) curves within a temperature range of 298–353 K were obtained using a vibrating sample magnetometer (VSM) of PPMS (Quantum Design, San Diego, CA, USA) at a heating and cooling rate of 3 K/min, under magnetic fields up to 1.5 T. Specifically, to measure the M(H) curves, the sample was equilibrated at 353 K to achieve a full austenite state before being cooled to the test temperature and maintained for 1 min before starting measurements. Additionally, the magnetic field was applied perpendicular to the direction of green bodies pressing. The magnetocaloric effect (MCE), characterized by magnetic entropy change (ΔSm), was then calculated from the M(H) curves based on the Maxwell relation.
To investigate the mechanical properties of porous alloys, incremental cyclic compression tests were conducted to assess both their shape memory effect and superelasticity at room temperature and 353 K. Uniaxial loading and unloading experiments were carried out using a universal testing machine (DDL-50), with the axial displacement of the sample being monitored through an extensometer (YUU-25/5). The samples, with a dimension of diameter 3 × 6 mm, were subjected to a temperature of 353 K (Af + 15 K) for 10 min during the assessment of the superelasticity effect, ensuring a fully austenitic state and complete grain recovery. Additionally, to investigate the dependence of the recoverable shape memory effect on the pre-strain, the samples were immediately unloaded at the set strain value upon reaching the specified strain, under the same strain rate.
3. Results and Discussion
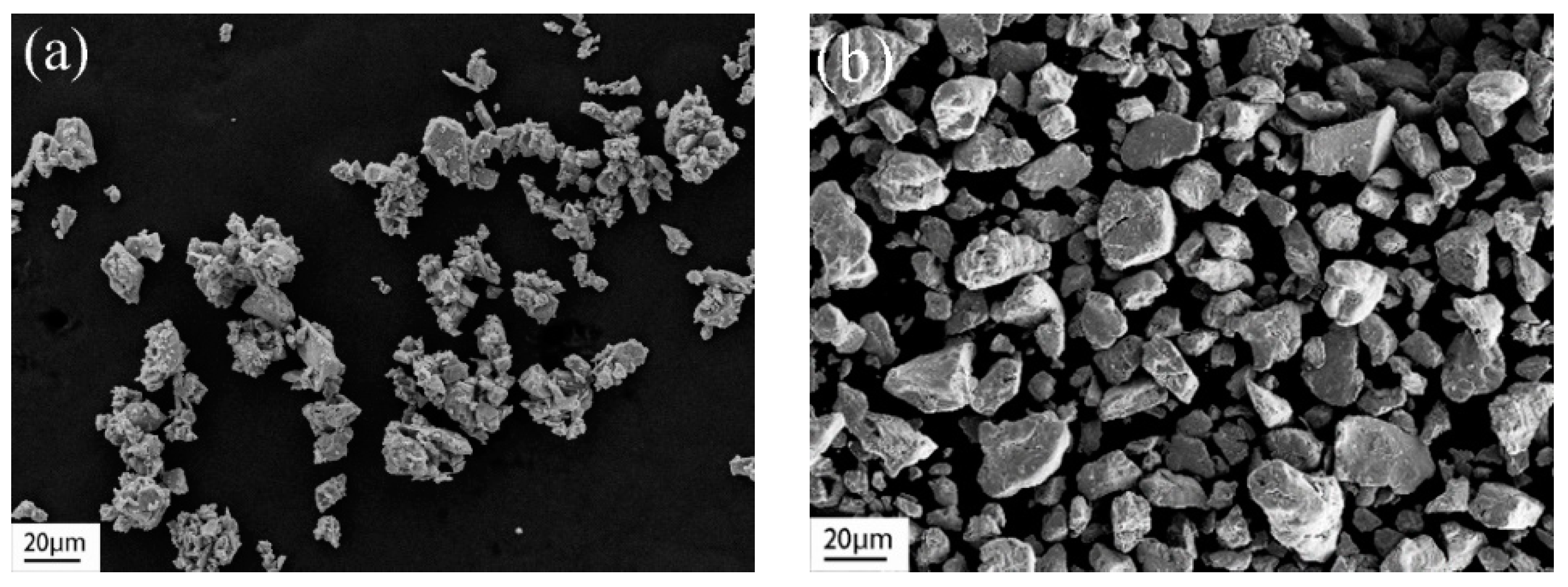
The microstructures of NaCl and Ni
50Mn
28Ga
22 alloy powder are depicted in
Figure 1a and
Figure 1b, respectively. The NaCl powder displays an irregular shape with a size range of 20–30 μm after grinding and screening, with a few particles being smaller than 10 μm. Similarly, the alloy powder exhibits irregularity at a size of 20–30 μm.
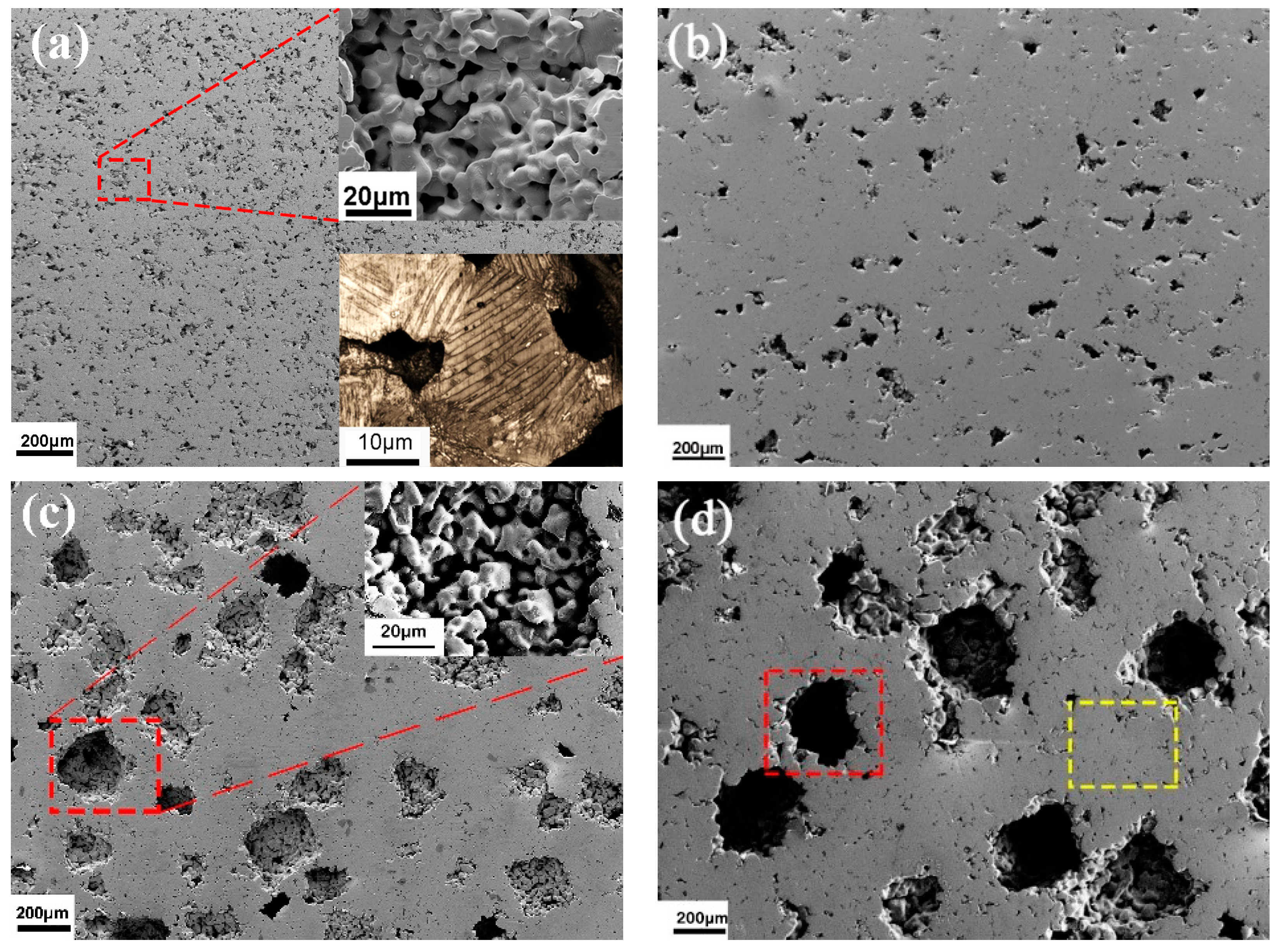
The morphologies of porous alloys with varying pore sizes are presented in
Figure 2. The dual pore structure consisted of a regular pore formed using a pore-forming agent and small sintered pores (<10 μm). When NaCl with pore sizes of 20–30 μm (a) and 50–75 μm (b) were added, the porous alloy exhibited an irregular polygonal pore morphology with sharp corners. Conversely, for larger NaCl pore sizes of 112–200 μm (c) and 200–355 μm (d), the pore morphology was relatively regular and the shape was similar to the added NaCl. The magnified view in
Figure 2a reveals a complete through-hole structure in the prepared porous alloy, with well-formed sintering necks between the alloy particles, and a uniform distribution and relatively consistent size of pores. The pillar of the porous sample, with a grain size of approximately 25 μm, was composed of multiple martensitic twins arranged in different directions and formed a well-sintered neck. The illustration in the lower right corner of
Figure 1 shows a microscopic image of a corroded porous alloy pillar captured using an optical microscope. It can be seen that an obvious lamellar martensite runs through the entire grain and sintering neck, suggesting that coarse martensite twins were achieved through pressing sintering without requiring further heat treatment. Moreover, this structural configuration effectively reduces the number of grain boundaries in the alloy, while the arrangement of martensitic plates perpendicular to the pillar direction facilitates the release of individual grain strain. It can be seen from the enlarged image in
Figure 2c that the macropores were caused by the occupation of NaCl particles, but there were still small pores in the matrix. This is caused by the gaps between alloy particles during the green billet pressing process, and most of these pores are closed pores. Similarly, in
Figure 2d, the larger pores (red box) in the porous alloy are attributed to the pore-forming agent, whereas the formation of smaller pores (yellow box) occurred between the alloy particles during the sintering process.
Table 1 lists the content and porosity of Ni
50Mn
28Ga
22 porous alloy elements obtained using sintering at 1373 K. The actual porosity of the porous alloys was calculated using the following formula:
where
P represents the porosity, and
ρ0 and
ρ denote the density of porous Ni
50Mn
28Ga
22 alloy and bulk Ni
50Mn
28Ga
22 alloy, respectively [
22]. From the table, it can be seen that the composition of porous alloys with different sizes was similar, indicating that the pores did not affect the alloy composition. Compared to the experimental design, the composition of porous alloys with different pore sizes showed a small amount of Mn volatilization, which occurred during the sintering. In addition, the porosity was slightly lower than 50%, which is due to the slight melting of the alloy surface during sintering, causing alloy size shrinkage.
The DSC curves and room temperature XRD patterns of the Ni
50Mn
28Ga
22 porous alloy with different pore sizes are depicted in
Figure 3a and
Figure 3b, respectively. The DSC curve reveals a singular endothermic peak during heating and a separate exothermic peak during cooling for samples with various pore sizes, indicating a one-step transformation consistent with the as-cast samples [
23]. The phase transition start and finish temperatures (
Ms,
Mf,
As,
Af), as well as the Curie point (
Tc) of the porous alloy, were determined using the tangent method and are presented in
Table 2 for evaluation. The thermal hysteresis Δ
T was calculated using
. It is noteworthy that the positions of the endothermic and exothermic peaks and the phase transition temperature across various pore sizes exhibited no significant changes. In addition, as the pore size increased, the height of the phase transition peak decreased and widened, and this change in peak shape was caused by lattice distortion, which was caused by stress [
24]. The larger pores cause severe deformation of the alloy powder during the pressing process, resulting in a stress concentration. The transformation of late heat ΔH calculated using the phase transition peak was 10.23, 9.62, 9.62, and 9.48 J/g, in order of pore size from small to large, with no significant difference. This indicates that the pore size does not affect the amount of grains involved in phase transformation in porous alloys. The XRD pattern in
Figure 3b illustrates that the Ni
50Mn
28Ga
22 porous alloy possesses a non-modulation martensite structure (NM) with a tetragonal arrangement at room temperature. Furthermore, all diffraction peaks can be attributed to the NM martensite phase, implying that the porous alloy retains a uniform composition and does not undergo second-phase precipitation subsequent to sintering [
25,
26,
27,
28]. The lattice constants and cell volumes of porous alloys with different pore sizes were calculated from XRD curves and are listed in
Table 3. It can be seen that the pore size had no significant effect on the lattice constant and cell volume.
3.1. Shape Memory Effect
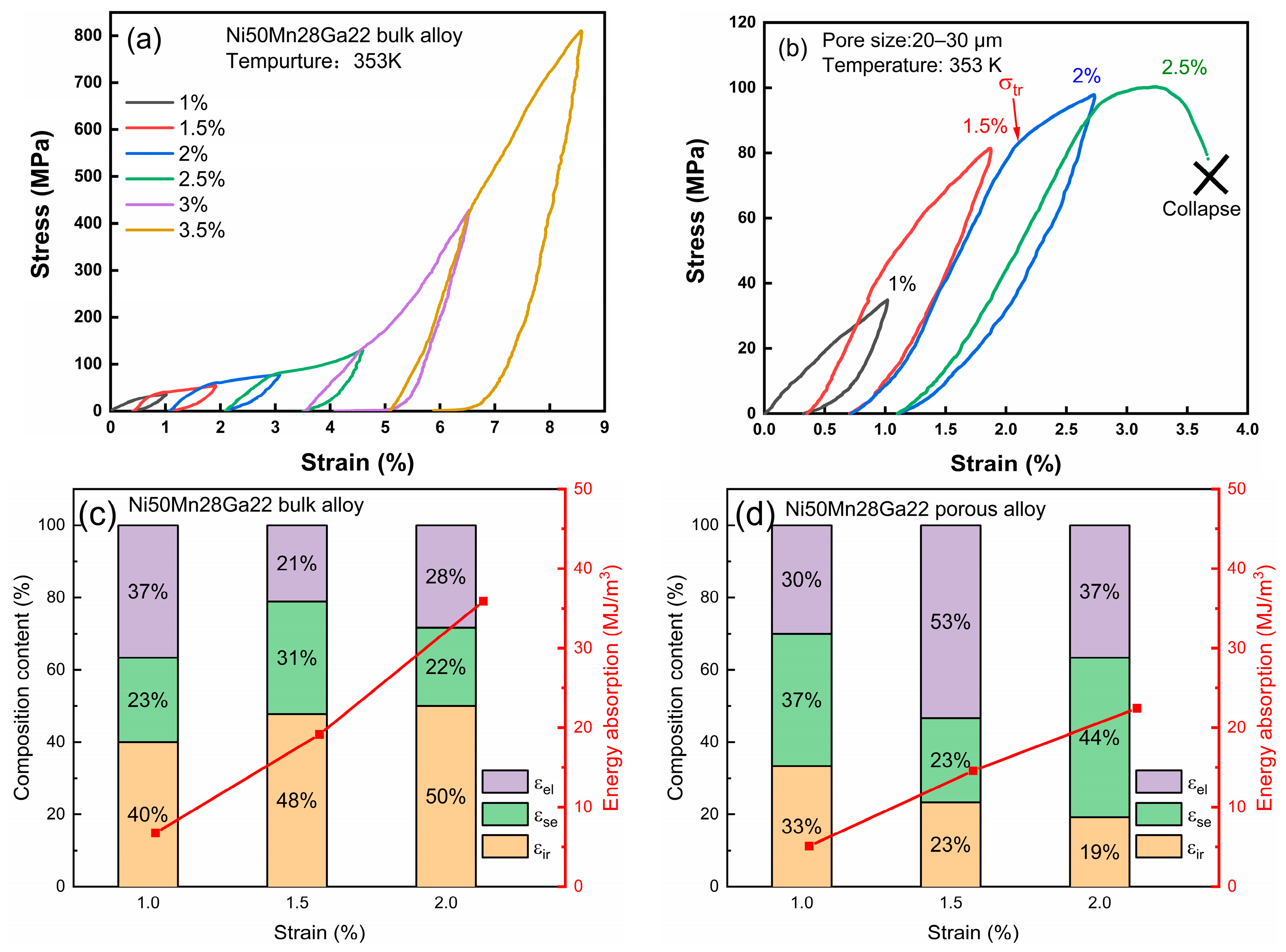
The mechanical properties of magnetocaloric materials are of crucial importance for their potential applications. The
Af temperature of the samples, as depicted in
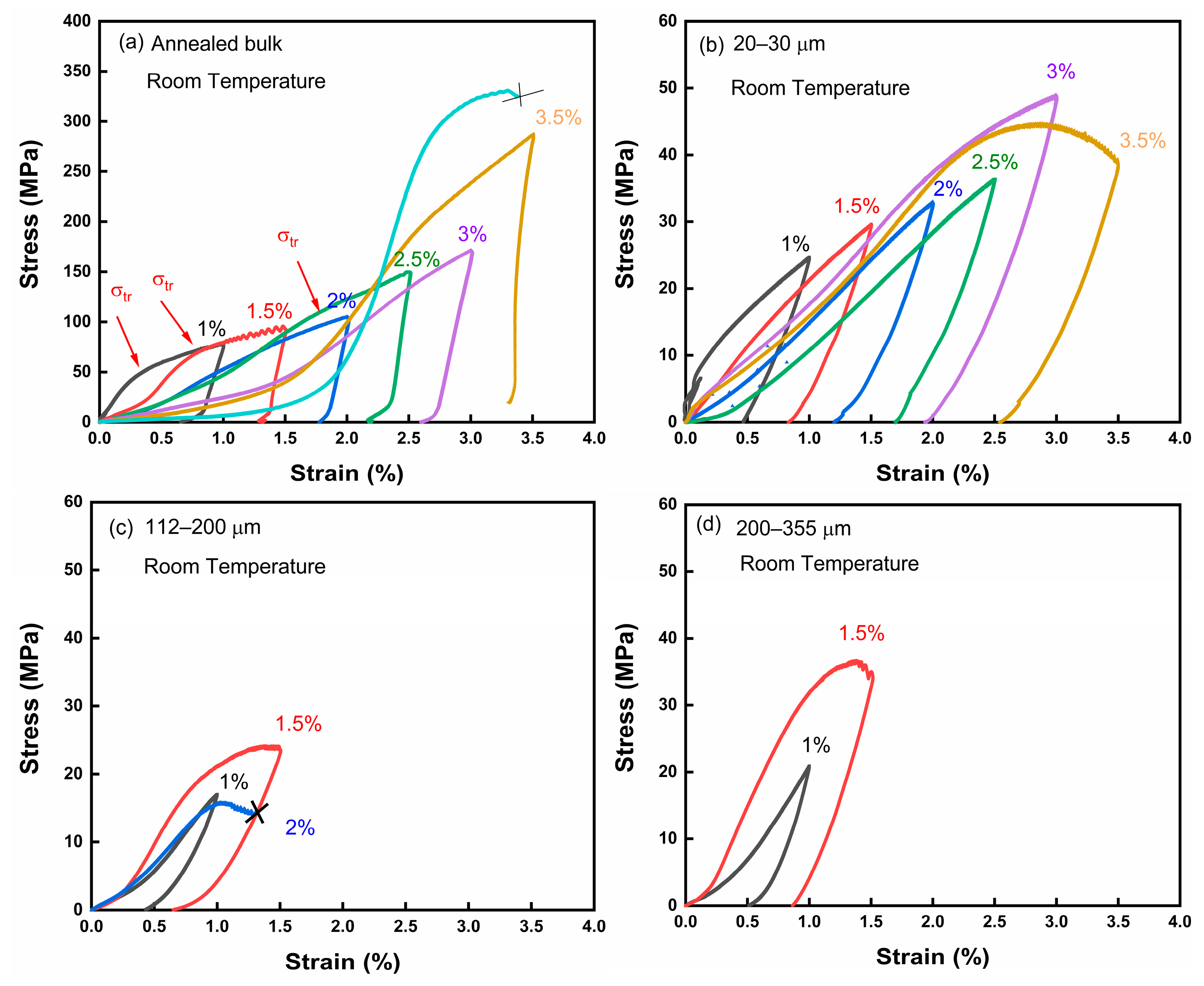
Figure 3a, was approximately 340 K. Therefore, shape memory effect tests were conducted at room temperature (295 K), wherein the samples were in a completely martensitic state. The comparison between the annealed bulk alloy and porous alloys with different pore sizes (20–30 μm, 112–200 μm, and 200–355 μm) is illustrated in the compression experiment results shown in
Figure 4. The sample underwent compression experiments using a cyclic increment method, wherein it was first subjected to a compression recovery of 1%, followed by heating at 473 K for 10 min to achieve shape memory recovery. Subsequently, after the sample cooled down, the next compression process was initiated. Each compression process increased the compression amount by 0.5% compared to the previous one until the sample eventually collapsed.
In
Figure 4a, the annealed bulk alloy exhibits an observable inflection point during the compression process, marked by the red arrow, indicating the de-twinning process of the martensite variant. It is evident from
Figure 4b that the porous sample experienced a significant reduction in maximum stress when subjected to the same compression force as the bulk alloy. This reduction can be attributed to the decrease in the equivalent cross-sectional area resulting from the presence of numerous pores. Simultaneously, the abundance of pores led to a decrease in the number of grain boundaries, thereby lowering the resistance of the martensite twin movement. Notably, the 20–30 μm porous alloy demonstrated an enhanced elastic recovery compared to the bulk material. This enhancement can be attributed to the pores serving as a buffer layer during the deformation process, absorbing some strain and augmenting the elastic deformation of the material.
At the same time, the critical stress of martensite variant detwinning gradually decreased with an increase in the compression time. This can be attributed to the path effect resulting from the repeated compression process, which also exists in bulk alloys [
5,
29,
30,
31,
32,
33,
34]. The process leads to the reorientation of dislocations, causing some disadvantageously oriented dislocations to move outside the twin motion path [
35]. Consequently, this results in a decreased resistance to twinning motion. Due to the difference in porosity between porous and block structures, when stress is applied to the porous structure, the actual stress borne by the structure is greater than that stress. In addition, if the stress is divided by the relative density of the sample, the actual force acting on the porous alloy pillar node can be obtained. It is worth noting that under a 1% pre-strain, the equivalent stress of porous alloys is
[
36], which is significantly lower than that of bulk alloys (76.24 MPa), which also confirms that pores, as elastic layers, store some stress. The increase in twin stress caused by a 3% compression is attributed to work-hardening resulting from the accumulation of a large number of dislocations due to extensive deformation. During a 3.5% compression, the sample experienced a partial pillar fracture. As the pore size increased, the brittleness of samples with pore sizes of 112–200 μm and 200–355 μm also increased, leading to premature fracture (
Figure 4c,d). This is because the larger pores reduced the overall structural strength of the alloy, causing significant local stress during the stress process, leading to the early collapse of the specimens.
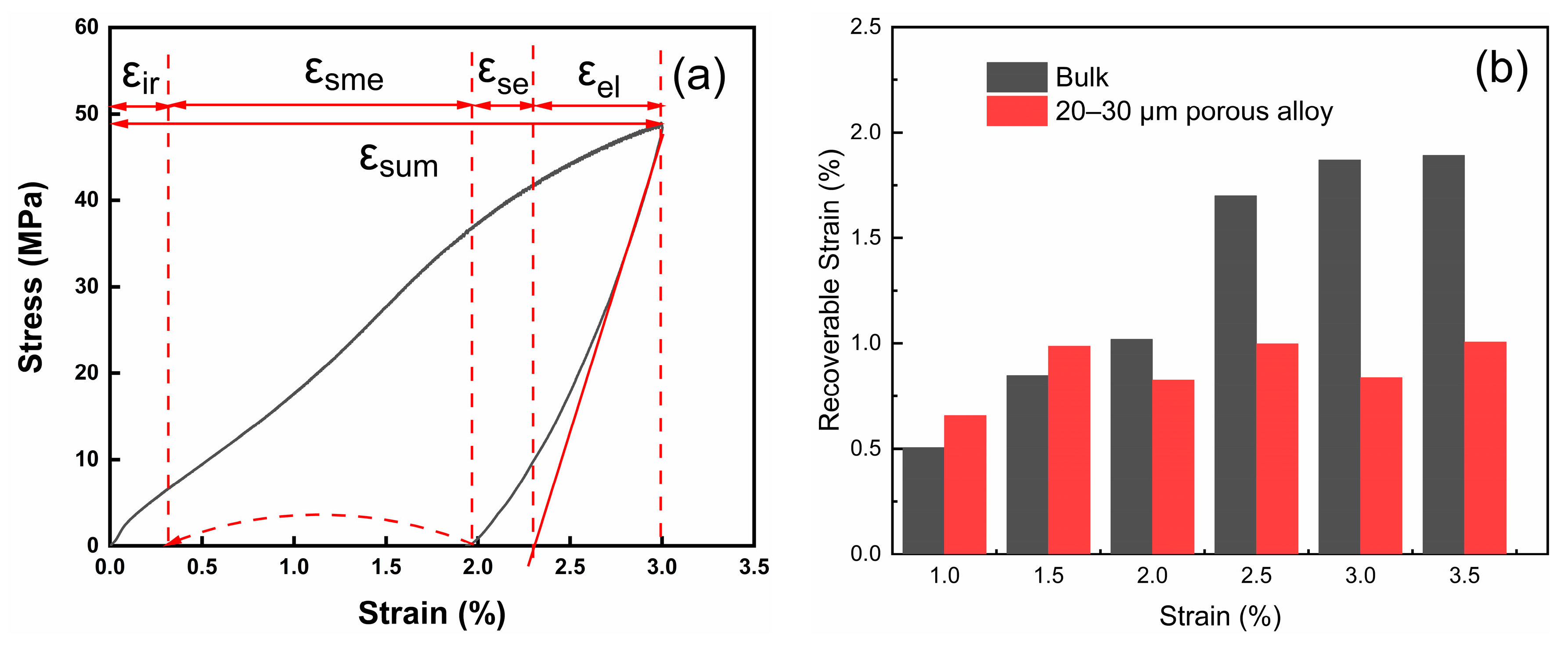
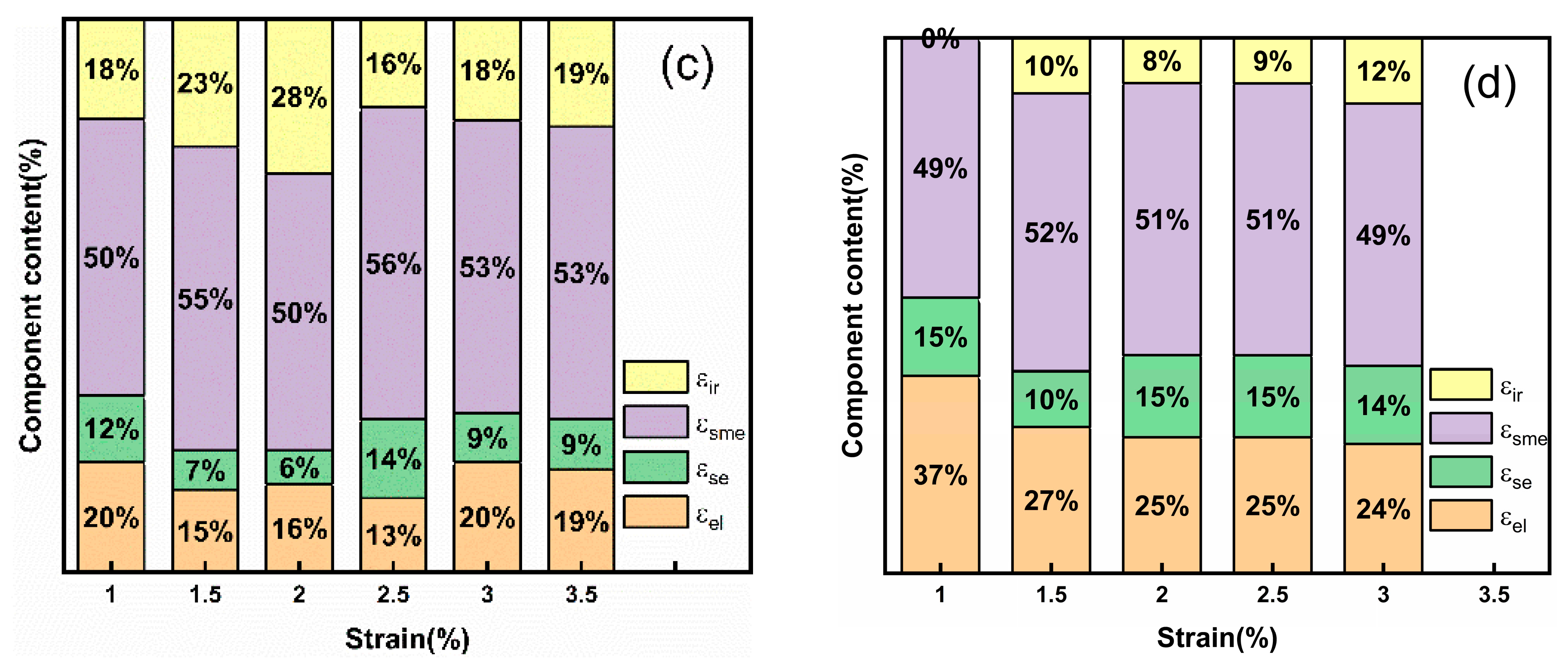
To better analyze the phase transition process during the sample recovery stage, the recovery rate of each strain component of the annealed bulk and 20–30 μm porous alloy specimen are illustrated in
Figure 5. The compression strain during each load–unload–heat recovery cycle comprises four components: elastic recovery strain
εel, superelastic recovery strain
εse, shape memory effect (SME) strain
εsme, and irrecoverable strain
εir, where the total strain
εsum is the sum of these individual components, as shown in
Figure 5a [
18] and the following formula:
Figure 5b shows the shape memory strain variation with pre-strain of bulk alloys and porous alloys with pore sizes of 20–30 μm. It can be seen that the shape memory strain of the bulk alloys gradually increased with the increase in pre-strain, while the shape memory strain of porous alloys remained constant. This is because the Ni
50Mn
28Ga
22 bulk alloy can conduct stress between adjacent grains without loss during deformation. However, due to the addition of pores, stress can only be transmitted along the pillars or nodes, and pores act as elastic layers, absorbing a large amount of strain, resulting in fewer grains in porous alloys where stress reaches the critical stress of martensitic twin reorientation, resulting in lower shape memory strain and higher elastic recovery. It is worth noting from
Figure 5c,d that the presence of superelastic strains in both bulk and porous alloys can be attributed to the austenitic phases in the alloys. Notably, the porous alloy exhibited a markedly higher elastic strain than the bulk alloy due to the abundance of pores in the former, effectively serving as a buffer layer. Despite this difference, both types of alloys displayed the same proportion of shape memory strain. However, it is important to highlight that the pores lead to a significant reduction in the irrecoverable strain of the alloy. This phenomenon is attributed to the presence of a large number of dislocations in bulk alloys, which tend to concentrate stress and form cracks at their sites. Conversely, the introduction of pores in porous alloys substantially decreases the number of grain boundaries, making it challenging for stress to concentrate in dislocations, and thereby minimizing the occurrence of grain boundary fractures. Nevertheless, the incorporation of pores also introduces structural drawbacks to the alloy. Owing to the reduced strength of the sintered neck area, the sample is unable to withstand considerable stress, resulting in a lower maximum deformation capacity for the porous alloy compared to the bulk alloy [
37].
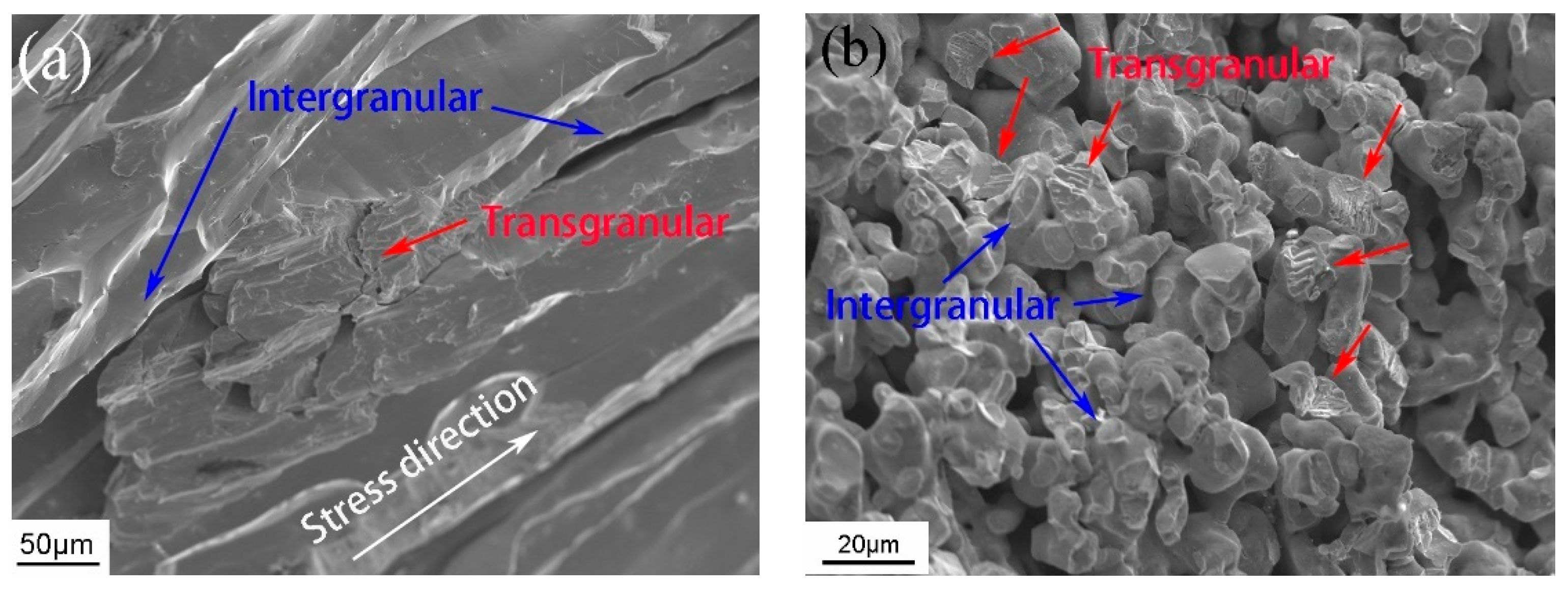
To further analyze the fracture process of the alloy, the fracture of the annealed bulk alloy and the 20–30 μm porous alloy were examined. Due to the inherent brittleness of the bulk NiMnGa alloy, most of the fracture surface was an intergranular fracture (
Figure 6a), which aligned along the stress direction. There was also a small amount of transgranular fracture. In contrast,
Figure 6b reveals a combination of intergranular and transgranular fractures on the porous alloy’s fracture surface. Notably, most of the intergranular fractures resulted from weak sintering necks, while well-sintered sintering necks exhibited transgranular fractures. It is obvious that the stress threshold for an intergranular fracture is lower than that for a transgranular fracture. Consequently, stress initially triggers the fracture of weak sintering necks in porous alloys and then concentrates on the grain of the alloy pillar, causing a transgranular fracture. Therefore, enhancing the strength of the sintering neck in porous alloys can significantly enhance their overall strength.
Author Contributions
Conceptualization, X.L. and J.Z.; methodology, K.W.; software, Y.L.; validation, K.W., Y.L. and Z.W.; formal analysis, Z.W.; investigation, Y.Z.; resources, Y.Z.; data curation. X.L.; writing—original draft preparation, X.L.; writing—review and editing, K.W.; visualization, K.W.; supervision, J.Z.; project administration, J.Z.; funding acquisition, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by State Key Laboratory for Advanced Metals and Materials, grant number [2018Z-26].
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Villa, F.; Morlotti, A.; Fanciulli, C.; Passaretti, F.; Albertini, F.; Villa, E. Anomalous mechanical behavior in NiMnGa alloy sintered through open die pressing method. Mater. Today Commun. 2023, 34, 105391. [Google Scholar] [CrossRef]
- de Souza Silva, F.; Correa, M.A.; Bohn, F.; Bezerra da Silva, R.; dos Passos, T.A.; Torquato, R.A.; de Lima, B.A.G.; Cordeiro, C.H.N.; Oliveira, D.F.d. Effect of Nb doping on NiMnSn Heusler alloys: Mechanical, structural, and magnetic properties modifications. J. Mater. Res. Technol. 2023, 26, 5167–5176. [Google Scholar] [CrossRef]
- Gao, P.; Tian, B.; Xu, J.; Tong, Y.; Chen, F.; Li, L. Investigation on porous NiMnGa alloy and its composite with epoxy resin. J. Alloys Compd. 2022, 892, 162248. [Google Scholar] [CrossRef]
- Gao, P.; Liu, Z.-X.; Tian, B.; Tong, Y.-X.; Chen, F.; Li, L. Microstructure, phase transformation and mechanical properties of NiMnGa particles/Cu composites fabricated by SPS. Trans. Nonferrous Met. Soc. China 2022, 32, 3291–3300. [Google Scholar] [CrossRef]
- Li, D.; Li, Z.; Zhang, X.; Liu, C.; Zhang, G.; Yang, J.; Yang, B.; Yan, H.; Cong, D.; Zhao, X.; et al. Giant Elastocaloric Effect in Ni-Mn-Ga-Based Alloys Boosted by a Large Lattice Volume Change upon the Martensitic Transformation. ACS Appl. Mater. Interfaces 2022, 14, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bai, J.; Sun, S.; Xu, J.; Jiang, X.; Guan, Z.; Gu, J.; Cong, D.; Zhang, Y.; Esling, C.; et al. A multielement alloying strategy to tune magnetic and mechanical properties in NiMnTi alloys via Co and B. J. Appl. Phys. 2022, 132, 095101. [Google Scholar] [CrossRef]
- Mishra, A.; Dubey, P.; Chandra, R.; Srivastava, S.K. Observation of large exchange bias field in Mn rich NiMnAl Heusler alloy thin film. J. Magn. Magn. Mater. 2021, 532, 167964. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, D.; Qi, Q.; Zhang, J.; Yao, Y.; Zhang, Y.; Cong, D.; Zhu, J. Multistep superelasticity of Ni-Mn-Ga and Ni-Mn-Ga-Co-Cu microwires under stress-temperature coupling. Acta Mater. 2017, 140, 326–336. [Google Scholar] [CrossRef]
- Hong, S.H.; Park, H.J.; Song, G.; Liaw, P.K.; Kim, K.B. Ultrafine shape memory alloys synthesized using a metastable metallic glass precursor with polymorphic crystallization. Appl. Mater. Today 2021, 22, 100961. [Google Scholar] [CrossRef]
- Lynch, C.S.; Sozinov, A.; Likhachev, A.A.; Ullakko, K. Magnetic and magnetomechanical properties of Ni-Mn-Ga alloys with easy axis and easy plane of magnetization. In Smart Structures and Materials 2001: Active Materials: Behavior and Mechanics; SPIE: Bellingham, WA, USA, 2001. [Google Scholar]
- Sozinov, A.; Likhachev, A.A.; Lanska, N.; Söderberg, O.; Koho, K.; Ullakko, K.; Lindroos, V.K. Stress-induced variant rearrangement in Ni-Mn-Ga single crystals with nonlayered tetragonal martensitic structure. J. De Phys. IV 2004, 115, 121–128. [Google Scholar] [CrossRef]
- Straka, L.; Heczko, O. Superelastic response of Ni-Mn-Ga martensite in magnetic fields and a simple model. ITM 2003, 39, 3402–3404. [Google Scholar] [CrossRef]
- Huang, L.; Cong, D.Y.; Suo, H.L.; Wang, Y.D. Giant magnetic refrigeration capacity near room temperature in Ni40Co10Mn40Sn10 multifunctional alloy. Appl. Phys. Lett. 2014, 104, 132407. [Google Scholar] [CrossRef]
- Chen, F.; Tong, Y.-X.; Tian, B.; Li, L.; Zheng, Y.-F. Martensitic transformation and magnetic properties of Ti-doped NiCoMnSn shape memory alloy. Rare Met. 2013, 33, 511–515. [Google Scholar] [CrossRef]
- Chen, L.; Hu, F.X.; Wang, J.; Bao, L.F.; Zheng, X.Q.; Pan, L.Q.; Yin, J.H.; Sun, J.R.; Shen, B.G. Magnetic entropy change and transport properties in Ni45Co5Mn36.6In13.4 melt-spun ribbons. J. Alloys Compd. 2013, 549, 170–174. [Google Scholar] [CrossRef]
- Dey, S.; Roy, R.K.; Ghosh, M.; Basu Mallick, A.; Mitra, A.; Panda, A.K. Enhancement in magnetocaloric properties of NiMnGa alloy through stoichiometric tuned phase transformation and magneto-thermal transitions. J. Magn. Magn. Mater. 2017, 439, 305–311. [Google Scholar] [CrossRef]
- Hu, F.-X.; Sun, J.-R.; Wu, G.-H.; Shen, B.-G. Magnetic entropy change in Ni50.1Mn20.7Ga29.6 single crystal. J. Appl. Phys. 2001, 90, 5216–5219. [Google Scholar] [CrossRef]
- Wang, K.; Hou, R.; Xuan, J.; Li, X.; Zhu, J. Shape memory effect and superelasticity of Ni50Mn30Ga20 porous alloy prepared by imitation casting method. Intermetallics 2022, 149, 107668. [Google Scholar] [CrossRef]
- Otaru, A.J. Review on the Acoustical Properties and Characterisation Methods of Sound Absorbing Porous Structures: A Focus on Microcellular Structures Made by a Replication Casting Method. Met. Mater. Int. 2019, 26, 915–932. [Google Scholar] [CrossRef]
- Mohd Razali, R.N.; Abdullah, B.; Ismail, M.H.; Muhamad, N. Characteristic of Modified Geometrical Open-Cell Aluminum Foam by Casting Replication Process. Mater. Sci. Forum 2016, 846, 37–41. [Google Scholar] [CrossRef]
- Boonyongmaneerat, Y.; Chmielus, M.; Dunand, D.C.; Mullner, P. Increasing magnetoplasticity in polycrystalline Ni-Mn-Ga by reducing internal constraints through porosity. Phys. Rev. Lett. 2007, 99, 247201. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Z.; Zhang, L.; Wei, S. Pore/skeleton structure and compressive strength of porous Mo3Si-Mo5Si3-Mo5SiB2 intermetallic compounds prepared by spark plasma sintering and homogenization treatment. J. Alloys Compd. 2021, 856, 158150. [Google Scholar] [CrossRef]
- Laszcz, A.; Hasiak, M.; Kaleta, J. Temperature Dependence of Anisotropy in Ti and Gd Doped NiMnGa-Based Multifunctional Ferromagnetic Shape Memory Alloys. Materials 2020, 13, 2906. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Kim, J.T.; Park, H.J.; Kim, Y.S.; Suh, J.Y.; Na, Y.S.; Lim, K.R.; Shim, C.H.; Park, J.M.; Kim, K.B. Influence of Zr content on phase formation, transition and mechanical behavior of Ni-Ti-Hf-Zr high temperature shape memory alloys. J. Alloys Compd. 2017, 692, 77–85. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Z.; Chen, F.; Lin, N.Y.; Wang, X.Y.; Zhang, B.Y.; Li, L. Ferromagnetic shape memory alloy Ni52.5Mn22.5Ga25: Phase transformation at high pressure and effects on natural gas hydration. Mater. Lett. 2023, 343, 134371. [Google Scholar] [CrossRef]
- Cheng, P.; Zhang, G.; Li, Z.; Yang, B.; Zhang, Z.; Wang, D.; Du, Y. Combining magnetocaloric and elastocaloric effects to achieve a broad refrigeration temperature region in Ni43Mn41Co5Sn11 alloy. J. Magn. Magn. Mater. 2022, 550, 169082. [Google Scholar] [CrossRef]
- Lázpita, P.; Pérez-Checa, A.; Barandiarán, J.M.; Ammerlaan, A.; Zeitler, U.; Chernenko, V. Suppression of martensitic transformation in Ni-Mn-In metamagnetic shape memory alloy under very strong magnetic field. J. Alloys Compd. 2021, 874, 159814. [Google Scholar] [CrossRef]
- Hosoda, H.; Lazarczyk, J.; Sratong-on, P.; Tahara, M.; Chernenko, V. Elaboration of magnetostrain-active NiMnGa particles/polymer layered composites. Mater. Lett. 2021, 289, 129427. [Google Scholar] [CrossRef]
- Chen, P.; Cai, X.; Liu, Y.; Wang, Z.; Jin, M.; Jin, X. Combined effects of grain size and training on fatigue resistance of nanocrystalline NiTi shape memory alloy wires. Int. J. Fatigue 2023, 168, 107461. [Google Scholar] [CrossRef]
- Xuan, J.; Gao, J.; Ding, Z.; Li, X.; Zhu, J. Improved superelasticity and fatigue resistance in nano-precipitate strengthened Ni50Mn23Ga22Fe4Cu1 microwire. J. Alloys Compd. 2021, 877, 160296. [Google Scholar] [CrossRef]
- Tong, W.; Liang, L.; Xu, J.; Wang, H.J.; Tian, J.; Peng, L.M. Achieving enhanced mechanical, pseudoelastic and elastocaloric properties in Ni-Mn-Ga alloys via Dy micro-alloying and isothermal mechanical cyclic training. Scr. Mater. 2022, 209, 114393. [Google Scholar] [CrossRef]
- Bai, J.; Wang, J.; Shi, S.; Liang, X.; Yang, Y.; Yan, H.; Zhao, X.; Zuo, L.; Zhang, Y.; Esling, C. Theoretical prediction of structural stability, elastic and magnetic properties for Mn2NiGa alloy. Mod. Phys. Lett. B 2021, 35, 2150231. [Google Scholar] [CrossRef]
- Gao, J.; Ding, Z.; Fu, S.; Wang, K.; Ma, L.; Zhu, J. The magnetization and magnetoresistance of Ni46Mn23Ga22Co5Cu4 shape memory microwires after mechanical training. J. Mater. Res. Technol. 2023, 23, 1120–1129. [Google Scholar] [CrossRef]
- Chiu, W.-T.; Goto, A.; Tahara, M.; Inamura, T.; Hosoda, H. Investigation of the martensite variant reorientation of the single crystal Ni-Mn-Ga alloy via training processes and a modification with a silicone rubber. Mater. Chem. Phys. 2023, 297, 127390. [Google Scholar] [CrossRef]
- Gaitzsch, U.; Pötschke, M.; Roth, S.; Rellinghaus, B.; Schultz, L. Mechanical training of polycrystalline 7M Ni50Mn30Ga20 magnetic shape memory alloy. Scr. Mater. 2007, 57, 493–495. [Google Scholar] [CrossRef]
- Imran, M.; Zhang, X.; Qian, M.; Geng, L. Enhancing the Elastocaloric Cooling Stability of Ni Fe Ga Alloys via Introducing Pores. Adv. Eng. Mater. 2020, 22, 1901140. [Google Scholar] [CrossRef]
- Roth, S.; Gaitzsch, U.; Pötschke, M.; Schultz, L. Magneto-Mechanical Behaviour of Textured Polycrystals of NiMnGa Ferromagnetic Shape Memory Alloys. Adv. Mater. Res. 2008, 52, 29–34. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Xing, D.; Shen, H.; Qian, M.; Liu, J.; Chen, D.; Sun, J. Martensite transformation and superelasticity in polycrystalline Ni–Mn–Ga–Fe microwires prepared by melt-extraction technique. Mater. Sci. Eng. A 2015, 636, 157–163. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, D.; Li, Z.; Cong, J.; Yu, Z.; Zhao, S.; Jiang, P.; Cong, D.; Zheng, X.; Qiao, K.; et al. Large enhancement of magnetocaloric effect induced by dual regulation effects of hydrostatic pressure in Mn0.94Fe0.06NiGe compound. J. Mater. Sci. Technol. 2022, 114, 73–80. [Google Scholar] [CrossRef]
- Cheng, P.; Zhou, Z.; Chen, J.; Li, Z.; Yang, B.; Xu, K.; Li, Z.; Li, J.; Zhang, Z.; Wang, D.; et al. Combining magnetocaloric and elastocaloric effects in a Ni45Co5Mn37In13 alloy. J. Mater. Sci. Technol. 2021, 94, 47–52. [Google Scholar] [CrossRef]
- Guan, Z.; Jiang, X.; Gu, J.; Bai, J.; Liang, X.; Yan, H.; Zhang, Y.; Esling, C.; Zhao, X.; Zuo, L. Large magnetocaloric effect and excellent mechanical properties near room temperature in Ni-Co-Mn-Ti non-textured polycrystalline alloys. Appl. Phys. Lett. 2021, 119, 051904. [Google Scholar] [CrossRef]
- Bai, J.; Liu, D.; Gu, J.; Jiang, X.; Liang, X.; Guan, Z.; Zhang, Y.; Esling, C.; Zhao, X.; Zuo, L. Excellent mechanical properties and large magnetocaloric effect of spark plasma sintered Ni-Mn-In-Co alloy. J. Mater. Sci. Technol. 2021, 74, 46–51. [Google Scholar] [CrossRef]
- Huang, X.-M.; Zhao, Y.; Yan, H.-L.; Jia, N.; Yang, B.; Li, Z.; Zhang, Y.; Esling, C.; Zhao, X.; Zuo, L. Giant magnetoresistance, magnetostrain and magnetocaloric effects in a Cu-doped<001>-textured Ni45Co5Mn36In13.2Cu0.8 polycrystalline alloy. J. Alloys Compd. 2021, 889, 161652. [Google Scholar] [CrossRef]
- Díaz-García, Á.; Law, J.Y.; Moreno-Ramírez, L.M.; Giri, A.K.; Franco, V. Deconvolution of overlapping first and second order phase transitions in a NiMnIn Heusler alloy using the scaling laws of the magnetocaloric effect. J. Alloys Compd. 2021, 871, 159621. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, X.; Gan, W.; Ding, C.; Liu, C.; Geng, L.; Yan, Y. Large rotating magnetocaloric effects in polycrystalline Ni-Mn-Ga alloys. J. Alloys Compd. 2021, 874, 159755. [Google Scholar] [CrossRef]
- Aydogmus, T.; Bor, S. Superelasticity and compression behavior of porous TiNi alloys produced using Mg spacers. J. Mech. Behav. Biomed. Mater. 2012, 15, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Aydoğmuş, T.; Bor, Ş. Processing of porous TiNi alloys using magnesium as space holder. J. Alloys Compd. 2009, 478, 705–710. [Google Scholar] [CrossRef]
- Golub, V.; L’Vov, V.A.; Salyuk, O.; Barandiaran, J.M.; Chernenko, V.A. Magnetism of nanotwinned martensite in magnetic shape memory alloys. J. Phys. Condens. Matter 2020, 32, 313001. [Google Scholar] [CrossRef]
- Li, Z.; Yang, B.; Zhang, Y.; Esling, C.; Zou, N.; Zhao, X.; Zuo, L. Crystallographic insights into the intermartensitic transformation in Ni–Mn–Ga alloys. Acta Mater. 2014, 74, 9–17. [Google Scholar] [CrossRef]
- Huang, L.; Cong, D.Y.; Wang, Z.L.; Nie, Z.H.; Dong, Y.H.; Zhang, Y.; Ren, Y.; Wang, Y.D. Direct evidence for stress-induced transformation between coexisting multiple martensites in a Ni–Mn–Ga multifunctional alloy. J. Phys. D Appl. Phys. 2015, 48, 265304. [Google Scholar] [CrossRef]
Figure 1.
Microstructure of (a) NaCl powder and (b) Ni50Mn28Ga22 alloy powder.
Figure 2.
Microstructure of Ni50Mn28Ga22 porous alloy with pore sizes of (a) 20–30 μm, (b) 50–75 μm, (c) 112–200 μm, and (d) 200–355 μm.
Figure 3.
(a) DSC curves and (b) XRD patterns of Ni50Mn28Ga22 porous alloy.
Figure 4.
The shape memory effect curves of the Ni50Mn28Ga22 (a) annealed bulk and porous alloys with different pore sizes of (b) 20–30 μm, (c) 112–200 μm, and (d) 200–355 μm.
Figure 5.
(a) Schematic of strain division during the recovery process. (b) Shape memory strain varies with pre-strain of bulk and porous alloys. Recovery ratio of each strain component curve of (c) Ni50Mn28Ga22 bulk alloy, (d) 20–30 μm porous alloy.
Figure 6.
Fracture morphology of (a) annealed bulk alloy and (b) 20–30 μm porous alloy.
Figure 7.
Cyclic compressive stress–strain curve at 353 K of (a) Ni50Mn28Ga22 bulk alloy and (b) Ni50Mn28Ga22 porous alloy with a pore size of 20–30 μm. Corresponding recovery ratio of each strain component curve of (c) annealed bulk alloy and (d) porous alloys.
Figure 8.
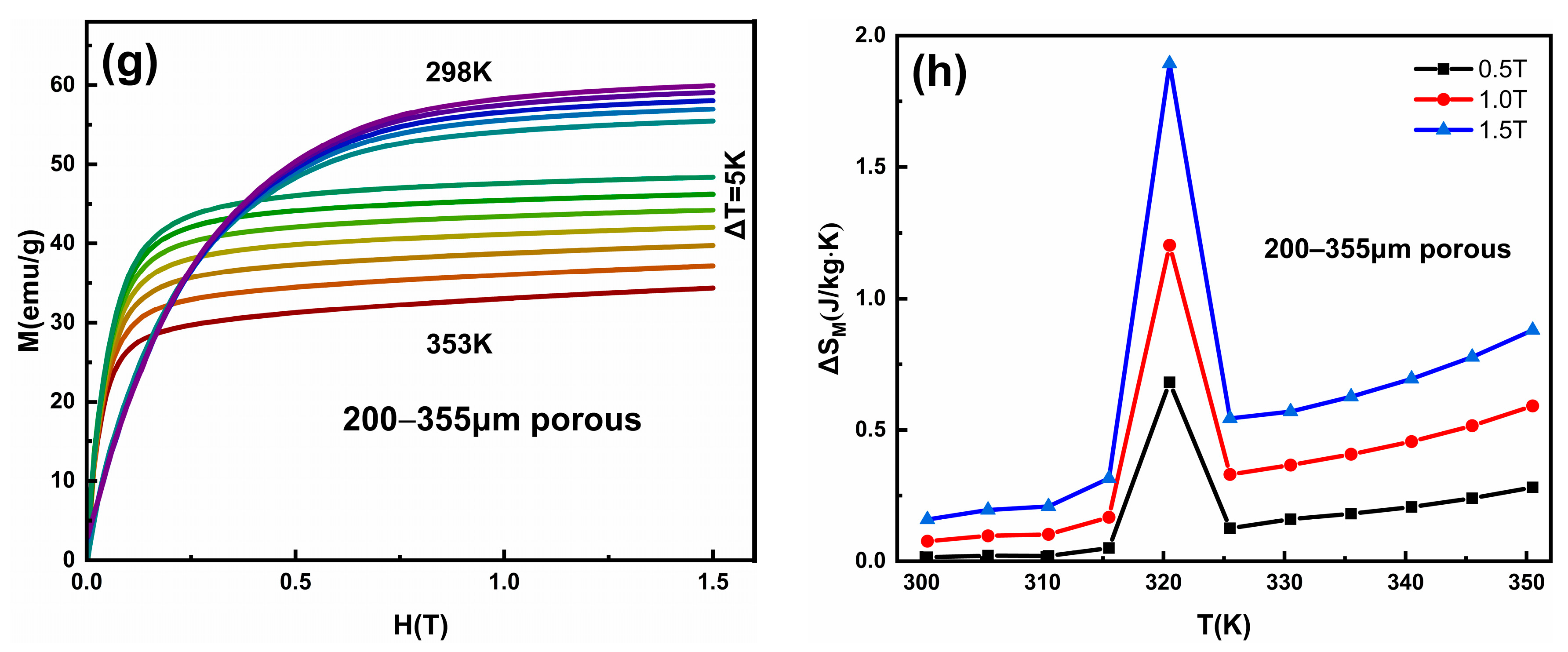
Isothermal magnetization M(H) curves and temperature-dependent magnetic field-induced entropy change ΔSM in the Ni50Mn28Ga22 (a,b) annealed bulk alloy, and (c,d) 20–30 μm, (e,f) 112–200 μm, and (g,h) 200–355 μm porous alloy.
Table 1.
Content and porosity of Ni50Mn28Ga22 porous alloys with different pore forming agent size.
| Pore Forming Agent Size (μm) | Ni
(at.%) | Mn
(at.%) | Ga
(at.%) | Porosity (vol.%) |
|---|
| 20–30 | 47.10 | 26.72 | 26.18 | 41.24 |
| 50–75 | 50.20 | 25.34 | 24.46 | 43.94 |
| 112–200 | 47.14 | 25.66 | 27.20 | 46.64 |
| 200–355 | 47.38 | 26.77 | 25.85 | 51.22 |
Table 2.
The phase transformation temperatures (Ms, Mf, As, Af), Curie point (Tc), and hysteresis ΔT of the Ni50Mn28Ga22 porous alloy.
| Pore-Forming Agent Size (μm) | Ms
(K) | Mf
(K) | As
(K) | Af
(K) | Tc
(K) | ΔT
(K) |
|---|
| 20–30 | 320.93 | 311.41 | 321.49 | 333.16 | 364.63 | 14.88 |
| 50–75 | 323.52 | 307.45 | 320.37 | 338.13 | 365.16 | 16.08 |
| 112–200 | 320.17 | 309.14 | 319.93 | 333.96 | 365.04 | 11.28 |
| 200–355 | 320.74 | 309.18 | 318.61 | 334.29 | 366.19 | 13.48 |
Table 3.
Lattice parameters and cell volume of calculated according to the X-ray diffraction data of Ni50Mn28Ga22 porous alloys.
| Pore-Forming Agent Size (μm) | Lattice Constant (Å) | Cell Volume (Å3) |
|---|
| a | b | c |
|---|
| 20–30 | 3.83 | 3.83 | 6.66 | 97.89 |
| 50–75 | 3.87 | 3.87 | 6.58 | 99.06 |
| 112–200 | 3.86 | 3.86 | 6.59 | 98.52 |
| 200–355 | 3.88 | 3.88 | 6.57 | 99.16 |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).