Evaluation of a Novel High-Efficiency SHS-EAH Multi-Stage DG-ADP Process for Cleaner Production of High-Quality Ferrovanadium Alloy
Abstract
1. Introduction
2. Experimental Procedure
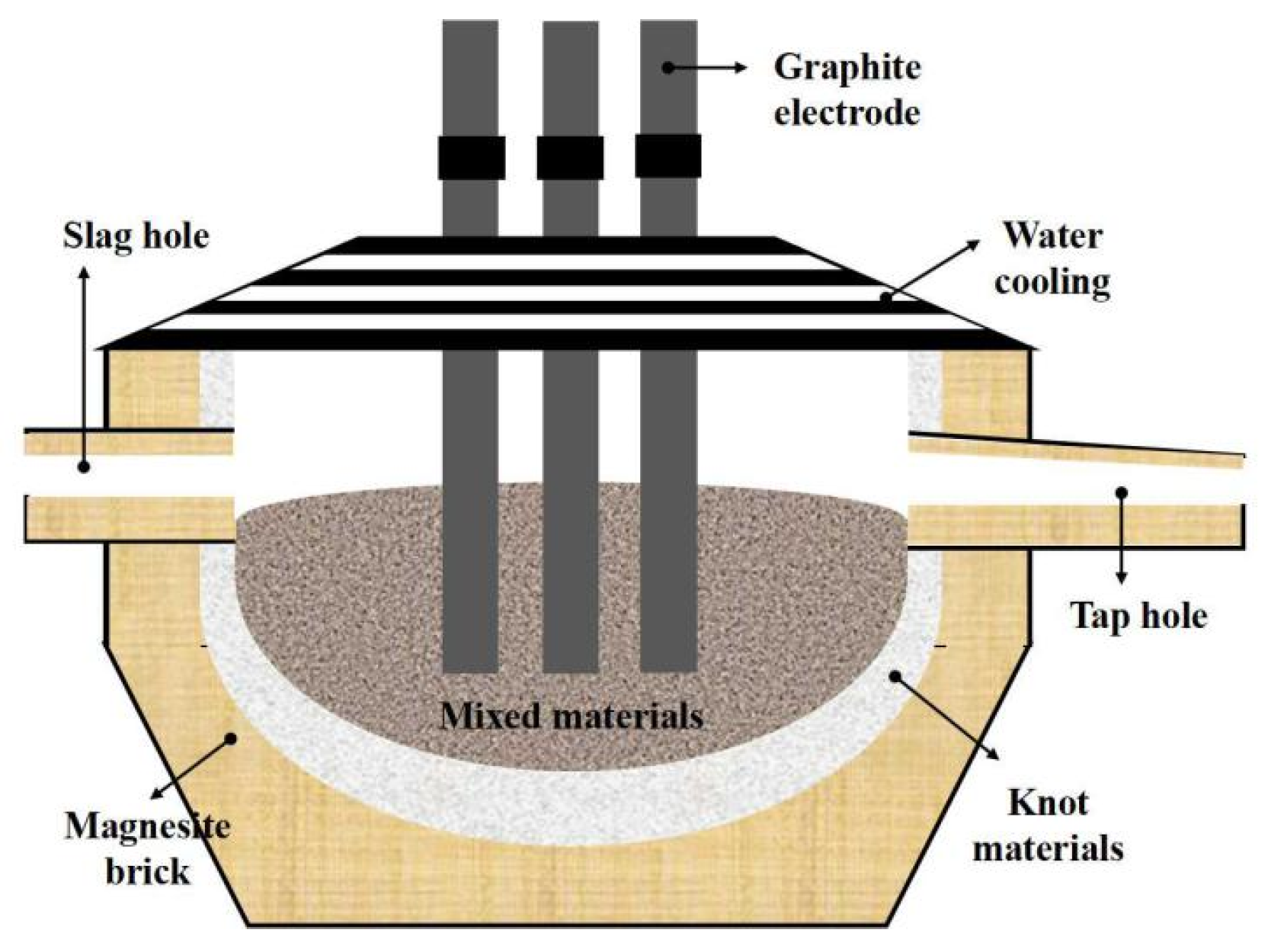
2.1. Materials and Apparatus
2.2. Framework and Methods
2.3. Characteristics
3. Results and Discussion
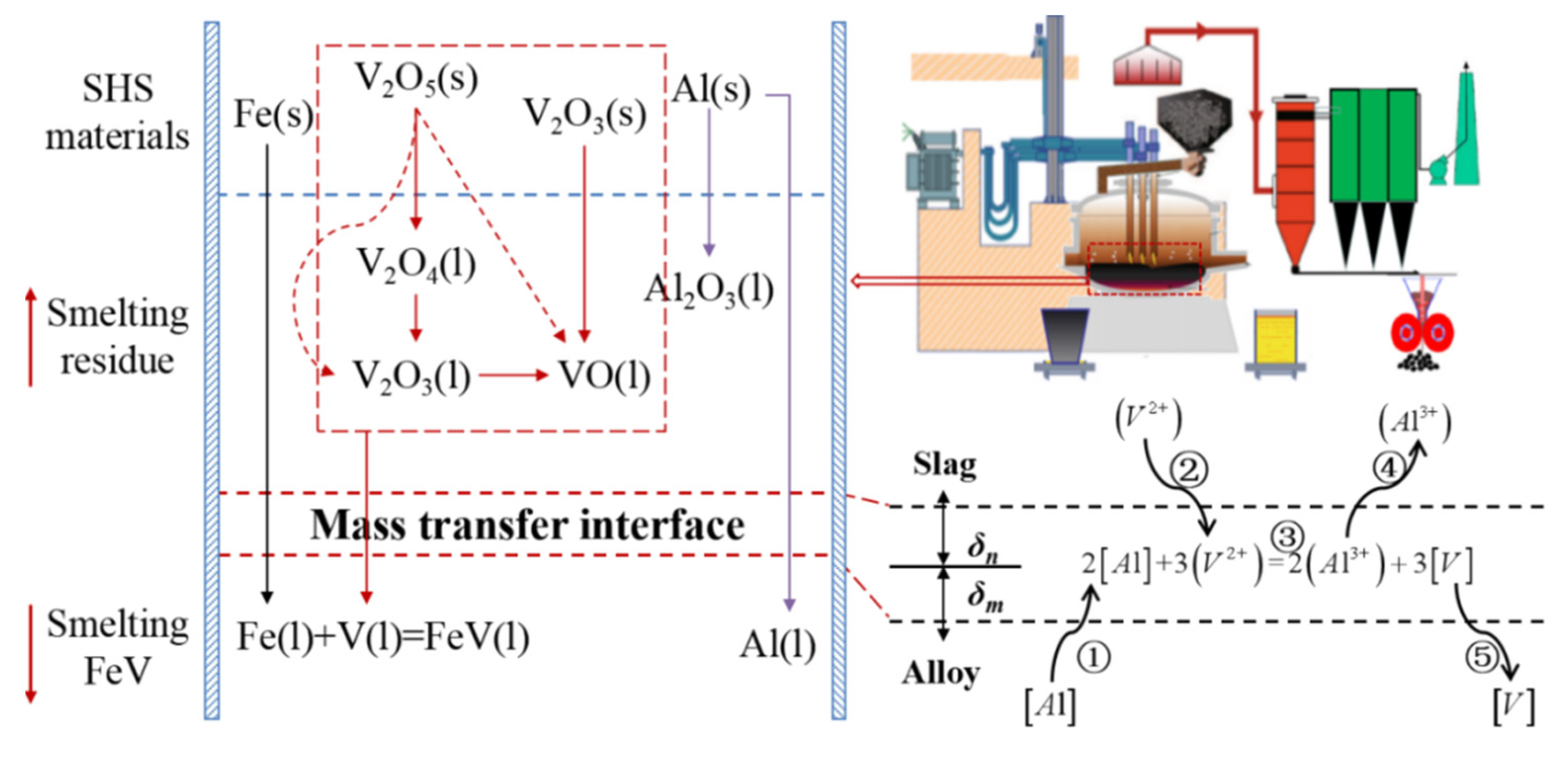
3.1. Thermodynamic Principle of Aluminothermic Reduction
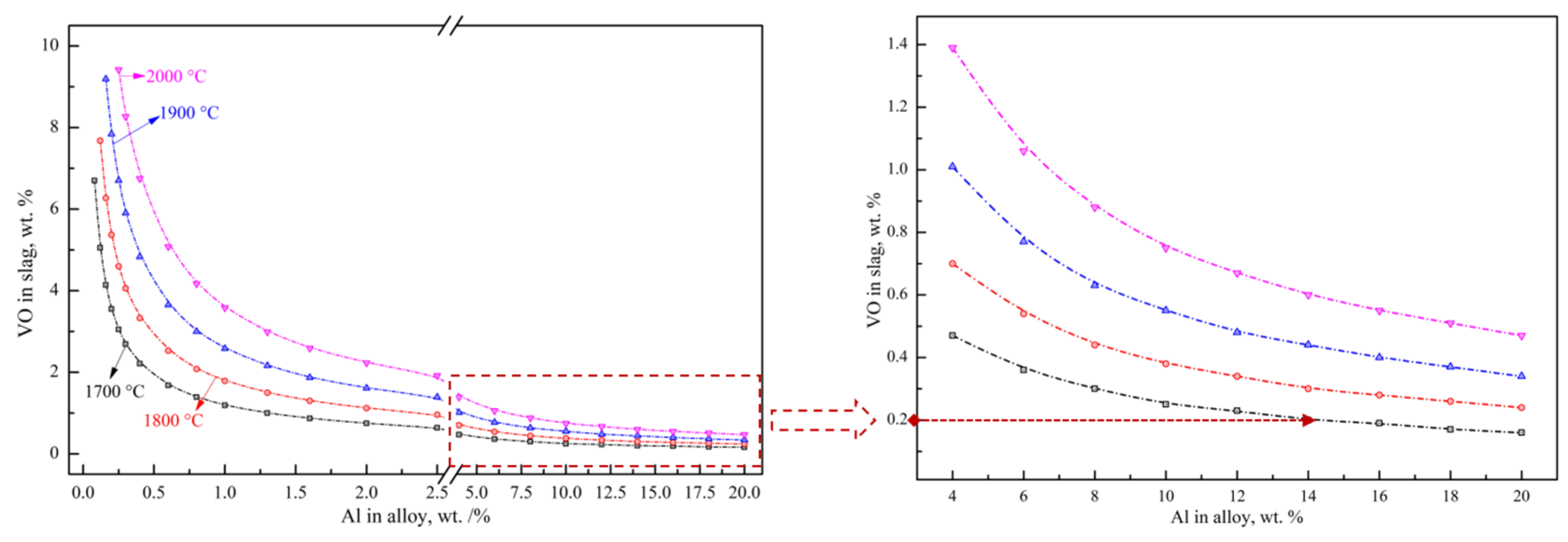
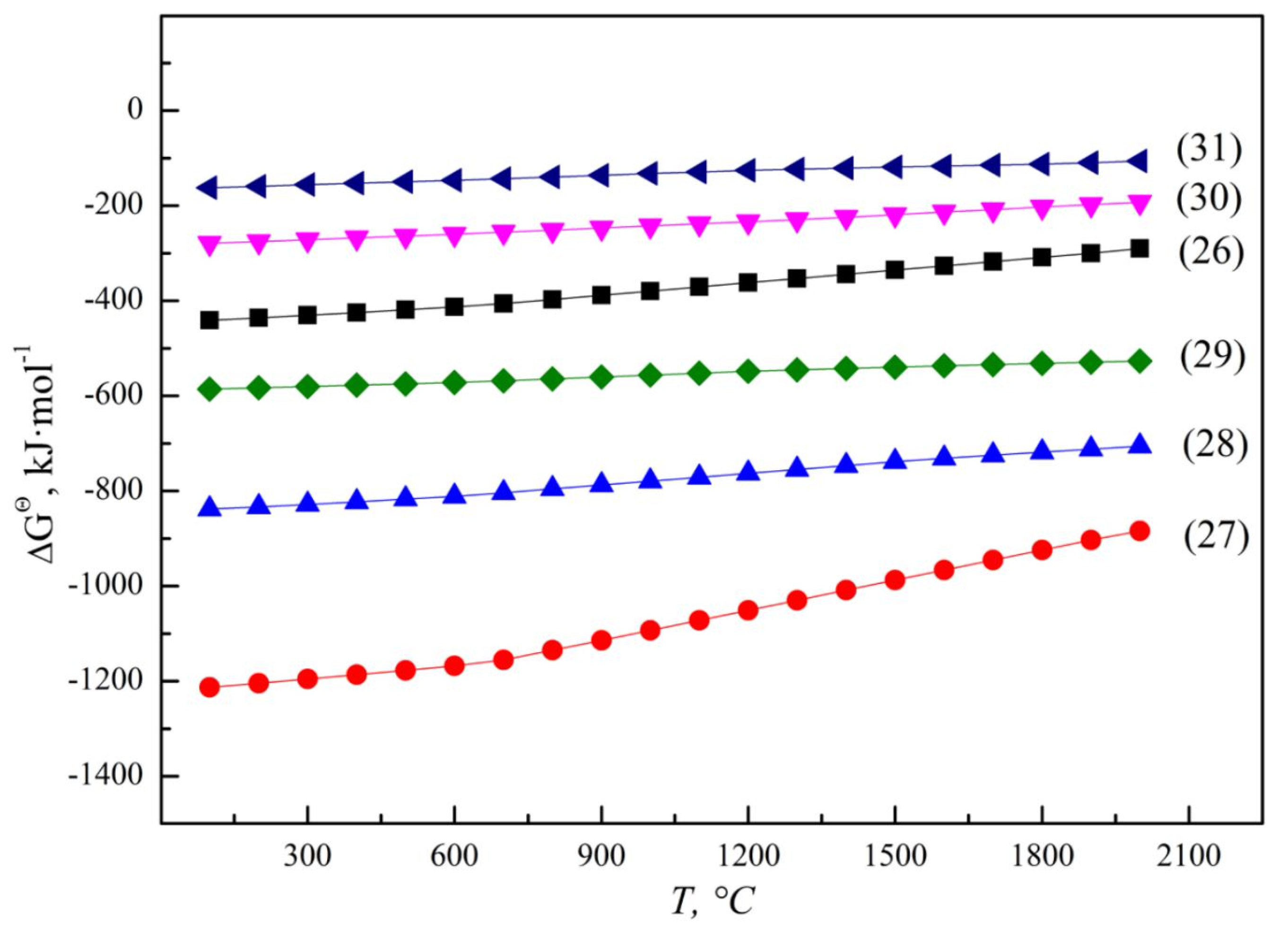
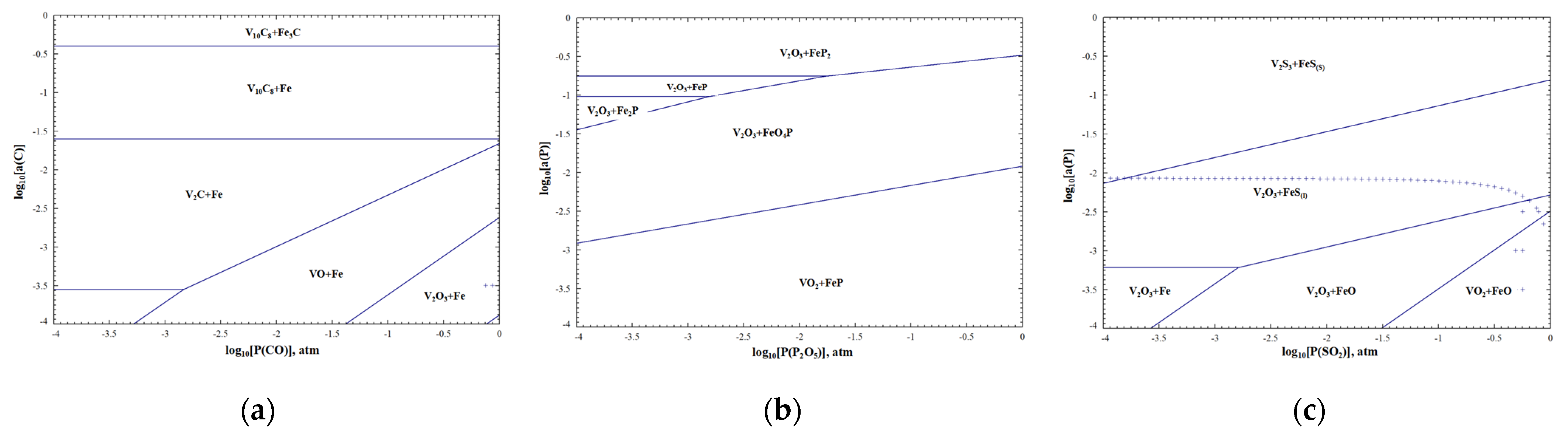
3.1.1. Thermodynamic Equilibrium of V-Al-O
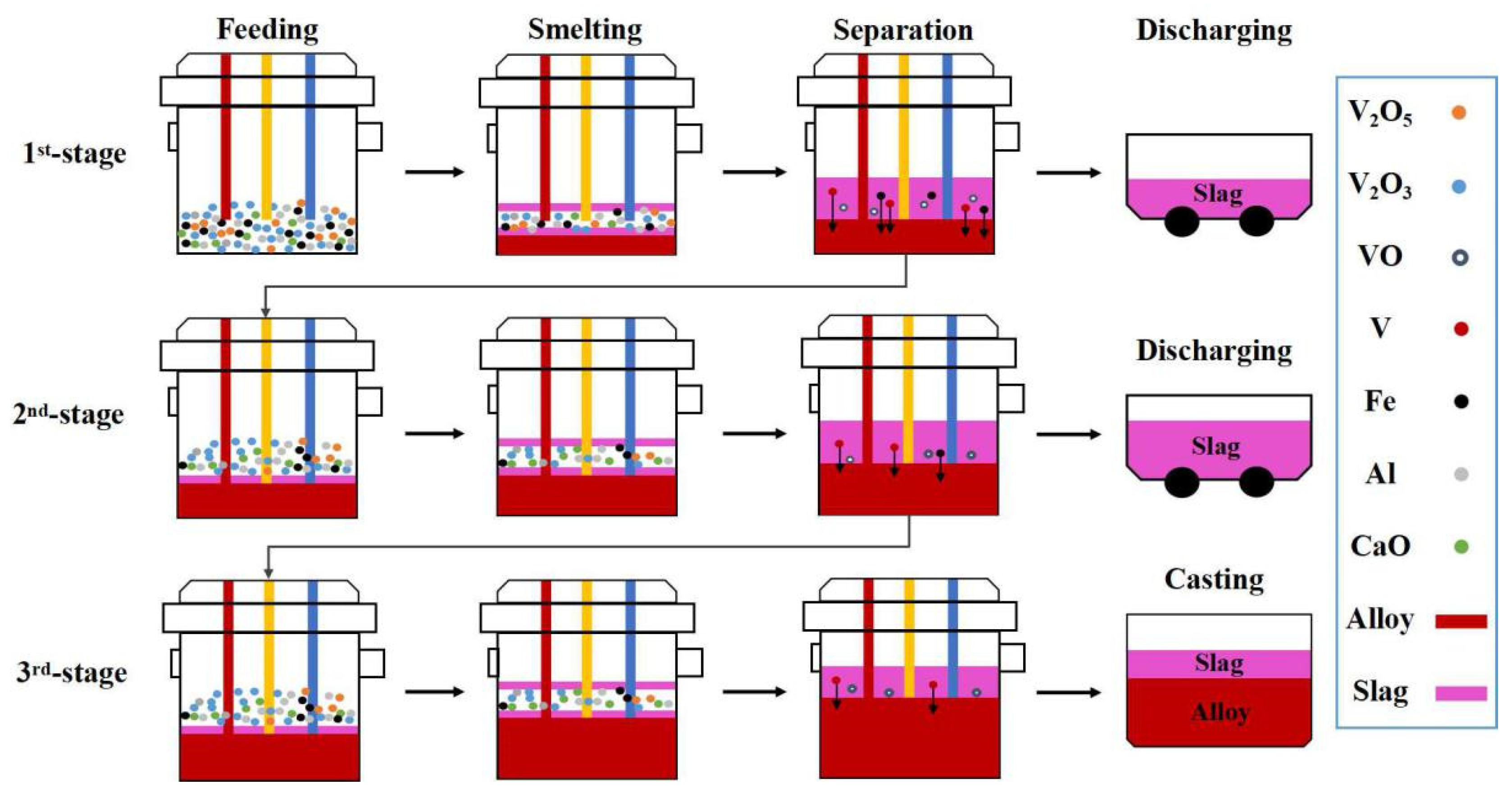
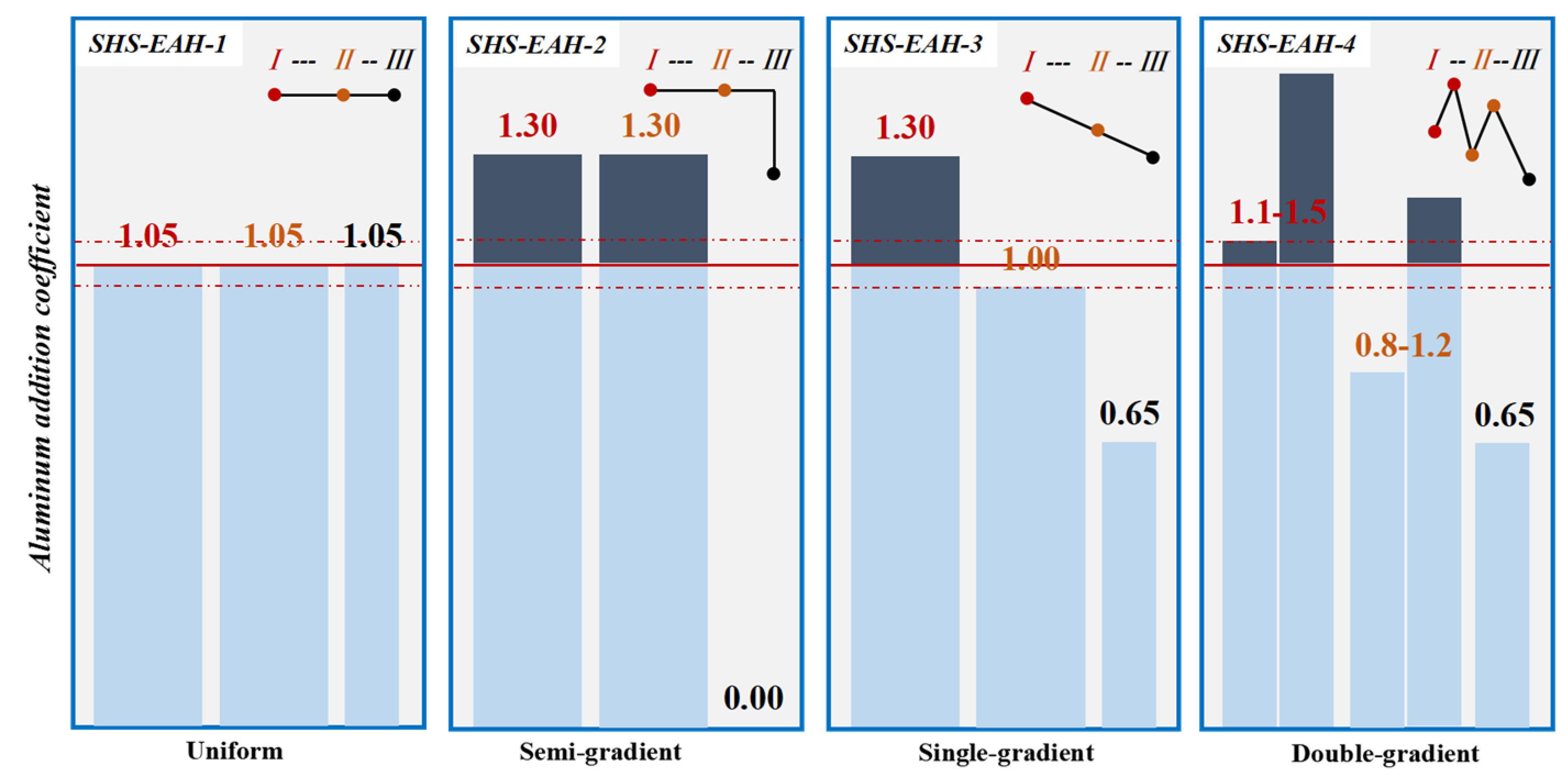
3.1.2. Multi-Stage Gradient Addition for Aluminothermic Reduction
3.2. Efficient Separation of Molten Slag and Alloy
3.2.1. Gravity Settlement during the Reducing Reaction
3.2.2. Efficient Separation of Slag and Alloy
3.3. Impurity Control and Secondary Resources in On-Line Circulation
3.3.1. Impurity Control Principle
3.3.2. Industrialization Control Standard of Raw Material Impurities
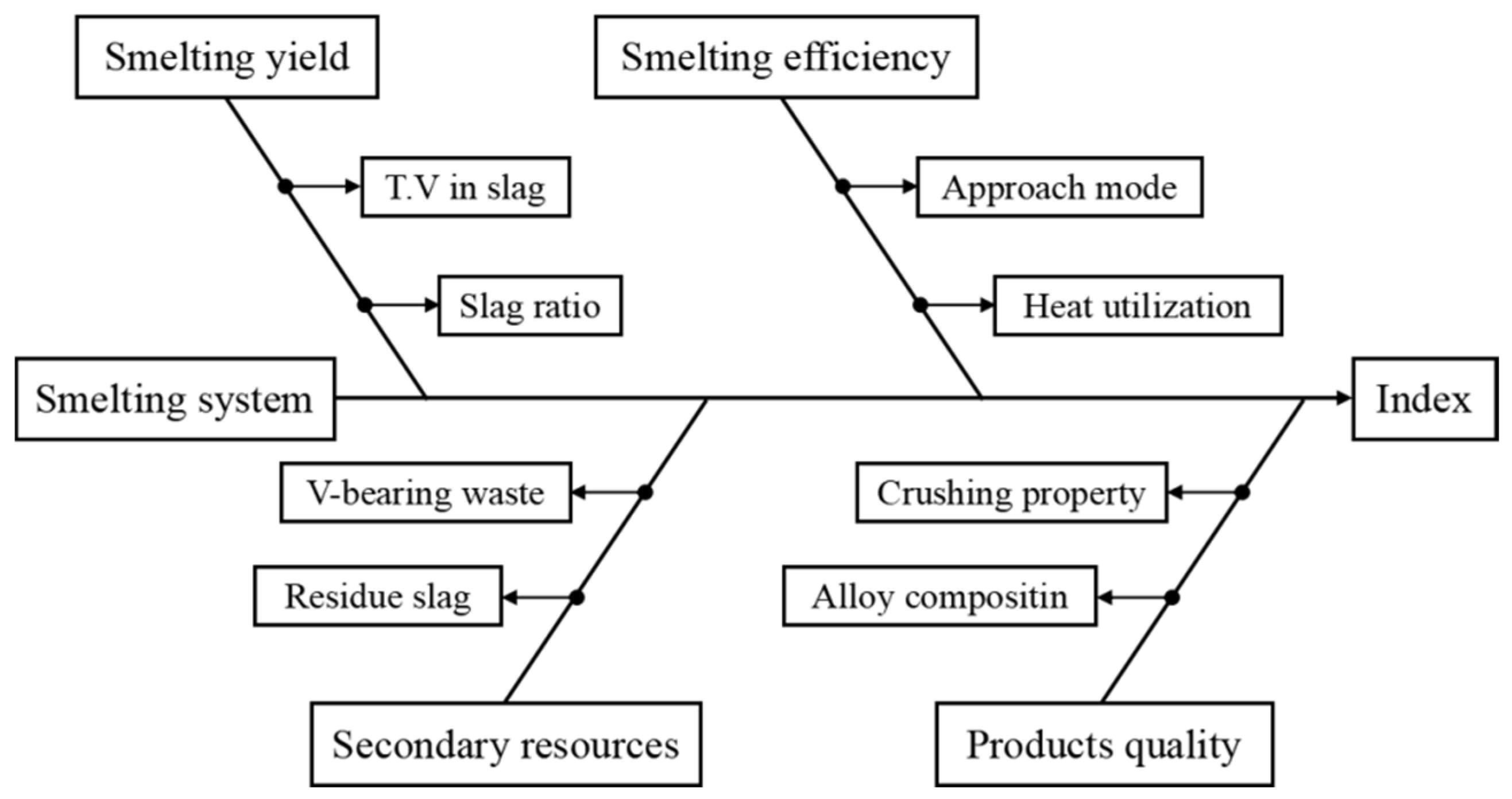
4. Evaluation of the Promoted SHS-EAH Process
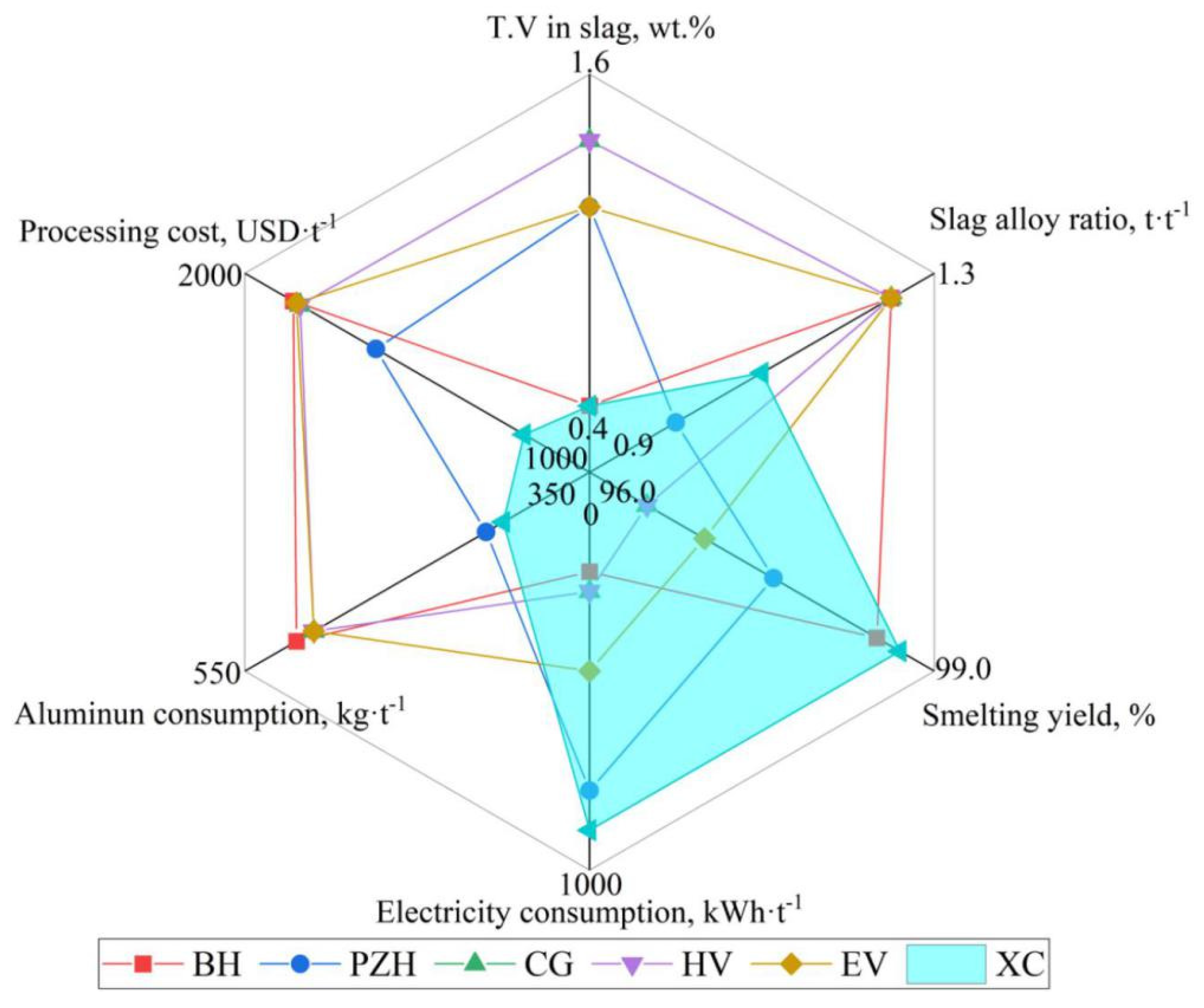
4.1. Evaluation of Technical and Economic Indicators
4.2. Outlook and Challenges
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aarabi, K.M.; Rashchi, F.; Mostoufi, N.; Vahidi, E. Leaching of vanadium from LD converter slag using sulfuric acid. Hydrometallurgy 2010, 102, 14–21. [Google Scholar] [CrossRef]
- Ulmer, U.; Asnao, K.; Patyk, A. Cost reduction possibilities of vanadium-based solid solution-microstructural, thermodynamic, cyclic and environmental effects of ferrovanadium substitution. J. Alloys Compd. 2015, 648, 1024–1130. [Google Scholar] [CrossRef]
- Zhang, X.P.; Kou, G.J.; Wu, C.J. Effect of ferrovanadium inoculation on microstructure and properties of high speed steel. China Foundry 2008, 5, 95. [Google Scholar]
- Dou, T.; Wu, Z.; Mao, J.F. Application of commercial ferrovanadium to reduce cost of Ti-V-based BCC phase hydrogen storage alloys. Mater. Sci. Eng. A 2008, 476, 34. [Google Scholar] [CrossRef]
- Choi, C.; Kim, S.; Kim, R.; Choi, Y.; Kim, S.; Jung, H.; Yang, J.H.; Kim, H.T. A review of vanadium electrolytes for vanadium redox flow batteries. Renew. Sustain. Energy Rev. 2017, 69, 263–274. [Google Scholar] [CrossRef]
- Sestager, K.A.; Alibek, Z.M.; Juan, M.G.; Olga, S.B.; Olga, Y.G.; Natalia, Y.G.; Elena, A.P. Kinetics of the synthesis of aluminum boride by the self-propagating high-temperature synthesis method. Ceramics 2022, 5, 435–446. [Google Scholar]
- Lee, J.C.; Kim, E.Y.; Chung, K.W.; Kim, R.; Jeon, H.S. A review on the metallurgical recycling of vanadium from slags: Towards a sustainable vanadium production. JMR&T 2021, 12, 343–364. [Google Scholar]
- Li, H.W.; Li, X.; Bu, X.P.; Chen, S.J. Prediction of the vanadium content of molten iron in a blast furnace and the optimization of vanadium extraction. Separations 2023, 10, 521. [Google Scholar] [CrossRef]
- Huang, W.J.; Liu, Y.J. Crystallization behavior and growth mechanisms of spinel crystals in vanadium-containing slags. ISIJ Int. 2020, 60, 2183–2190. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Y.F.; Zheng, F.Q.; Chen, F.; Yang, L.-Z.; Jiang, T.; Qiu, G.-Z. Behavior of vanadium during reduction and smelting of vanadium titanomagnetite metallized pellets. Trans. Nonferrous Met. Soc. China 2020, 30, 1687–1696. [Google Scholar] [CrossRef]
- Yang, M.Q.; Yang, J.Y. Vanadium extraction from steel slag: Generation, recycling and management. Environ. Pollut. 2024, 343, 123126. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, K.; Hua, W.H.; Yang, Z.; Zhou, W.; Xie, B. Selective leaching of vanadium in calcification-roasted vanadium slag by ammonium carbonate. Hydrometallurgy 2016, 160, 18–25. [Google Scholar] [CrossRef]
- Han, J.; Zhang, J.; Zhang, J.; Chen, X.; Zhang, L.; Tu, G. Recovery of Fe, V, and Ti in modified Ti-bearing blast furnace slag. Trans. Nonferrous Met. Soc. China 2022, 32, 333–344. [Google Scholar] [CrossRef]
- Liu, S.G.; Ding, E.M.; Ning, P.; Xie, G.; Yang, N. Vanadium extraction from roasted vanadium-bearing steel slag via pressure acid leaching. J. Environ. Chem. Eng. 2021, 9, 105195. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Wang, S.D.; Yang, L.; Luo, J.H.; Shen, B. Mechanisms and kinetics of a new cleaner single cyclic roasting–leaching process for the extraction of vanadium from Linz–Donawitz converter slag using CaCO3 and H2SO4. Clean. Eng. Technol. 2021, 4, 100204. [Google Scholar] [CrossRef]
- Cheng, G.; Han, T.; Zhang, X.; Xue, X.; Yang, H.; Bai, R.; Zhang, W. A novel method of enhancing valuable element recovery for ultra-high-titanium magnetite. J. Clean. Prod. 2023, 410, 137184. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhang, W.; Zhang, L.; Gu, S.Q. Mechanism of vanadium slag roasting with calcium oxide. Int. J. Miner. Process. 2015, 138, 20–29. [Google Scholar] [CrossRef]
- Hu, P.C.; Zhang, Y.M.; Liu, H.; Liu, T.; Li, S.; Zhang, R.B.; Guo, Z.J. High efficient vanadium extraction from vanadium slag using an enhanced acid leaching-coprecipitation process. Sep. Purif. Technol. 2023, 304, 122319. [Google Scholar] [CrossRef]
- Yin, R.T.; Chen, L.; Qing, Z.F.; Xiao, H.B.; Weng, D.S. A novel complexation method for separation and recovery of low valence vanadium, iron and chromium from sulfuric acid solution. J. Clean. Prod. 2022, 373, 133640. [Google Scholar] [CrossRef]
- Yan, J.S.; Dou, Z.H.; Zhang, T.A. Preparation of vanadium by the magnesiothermic self-propagating reduction and process control. Nanotechnol. Rev. 2022, 11, 1237–1247. [Google Scholar]
- Valerii, I.U.; Roman, D.K.; Andrei, O.K. Nanoporous high-temperature filters based on Ti-Al ceramic SHS materials. Ceram. Int. 2020, 46, 23180–23185. [Google Scholar]
- Prokopets, A.D.; Bazhina, P.M.; Konstantinov, A.S.; Chizhikov, A.P.; Antipov, M.S.; Avdeeva, V.V. Structural features of layered composite material TiB2/TiAl/Ti6Al4V obtained by unrestricted SHS-compression. Mater. Lett. 2021, 300, 130165. [Google Scholar] [CrossRef]
- Nikitin, P.Y.; Matveev, A.E.; Zhukov, I.A. Energy-effective AlMgB14 production by self-propagating high-temperature synthesis (SHS) using the chemical furnace as a source of heat energy. Ceram. Int. 2021, 47, 21698–21704. [Google Scholar] [CrossRef]
- Fan, X.Z.; Huang, W.Z.; Zhou, X.; Zou, B.L. Preparation and characterization of NiAl-TiC-TiB2 intermetallic matrix composite coatings by atmospheric plasma spraying of SHS powders. Ceram. Int. 2020, 46, 10512–10520. [Google Scholar] [CrossRef]
- Chen, Z.Y. Development status and prospects of vanadium industrial in the world. Chin. J. Rare Metal 1991, 2, 128. [Google Scholar]
- Song, Y.L.; Dou, Z.H.; Liu, Y.; Zhang, T.A. Study on the preparation process of TiAl alloy by self-propagating metallurgy. J. Mater. Eng. Perform. 2023, 33, 660–669. [Google Scholar] [CrossRef]
- Yu, B.; Sun, Z.H.; Chen, H.J.; Tang, H.J.; Jing, H.; Du, G.C. Preparation and influence factors of FeV50 alloy based on gradient distribution of Al addition. Chin. J. Rare Metal 2017, 41, 1279–1284. [Google Scholar]
- Xian, Y. Effects of Al content on phase transformation in FeV50 and its mechanism. Iron Steel Vanadium Titan. 2012, 33, 14. [Google Scholar]
- Sun, Z.H. Analysis on new vanadium technologies and prospects of vanadium industry. Iron Steel Vanadium Titan. 2012, 33, 1. [Google Scholar]
- Xian, Y.; Zhai, Q.J.; Sun, Z.H. Gradient smelting of ferrovanadium masteralloy by electro-aluminothermic process. Chin. J. Rare Metal 2016, 40, 850. [Google Scholar]
- Li, L.; Ge, W.S.; Chen, Y. Study on Slag Corrosion of the Magnesia Lining during Ferrovanadium Smelting Process. Adv. Mater. Res. 2013, 779, 96–100. [Google Scholar] [CrossRef]
- GB/T 40301-2021; Vanadium Trioxide. Chinese Standards Press: Beijing, China, 2021.
- TB YB/T 5304-2017; Vanadium Pentoxide. Chinese Standards Press: Beijing, China, 2017.
- GB/T 4139-2012; Ferrovanadium. Chinese Standards Press: Beijing, China, 2012.
- QB-2021; Ingredients Standard and Operating Rules for Ferrovanadium. Pangang Group Vanadium & Titanium Resources Co., Ltd.: Panzhihua, China, 2021.
- Yu, B.; Yuan, T.C.; Shi, J.J. Preparation of high-quality FeV50 alloy by an improved SHS-EAH multi-stage process. Ceram. Int. 2023, 49, 15114–15121. [Google Scholar] [CrossRef]
- Jung, I.H.; Decterov, S.A.; Pelton, A.D. Critical thermodynamic evaluation and optimisation of the MgO–Al2O3, CaO–MgO–Al2O3, and MgO–Al2O3–SiO2 systems. J. Phase Equilib. Diffus. 2004, 25, 329–345. [Google Scholar] [CrossRef]
- Yu, B.; Yuan, T.C.; Xiao, D.H. Existing states of vanadium in slag during the preparation of FeV50 by aluminum thermal reduction. Iron Steel Vanadium Titan. 2019, 40, 24–29. [Google Scholar]
- Xian, Y.; Zheng, H.X.; Zhai, Q.J.; Luo, Z.P. A two-dimensional structure map for prediction of the transition-metal Laves phases. Comput. Mater. Sci. 2016, 125, 1–7. [Google Scholar] [CrossRef]
- Yu, B.; Zhou, H.; Sun, Z.H. Theoretical application and factors influencing casting settlement of FeV50 alloy. Chin. J. Eng. 2017, 39, 1822–1826. [Google Scholar]
- Ye, M.F.; Yu, B.; Huang, Y.; Jing, H. Trend and control of P in FeV50 smelting process of large-scale tilting furnace. Iron Steel Vanadium Titan. 2022, 43, 36–41. [Google Scholar]
- Wang, J.; Zhang, P.; Wang, S.D. Evaluation of a green-sustainable industrialized cleaner production for FeV50 and FeV80 alloys from vanadium slag by calcification roasting-ammonia on-line cycle. J. Clean. Prod. 2021, 320, 128896. [Google Scholar] [CrossRef]




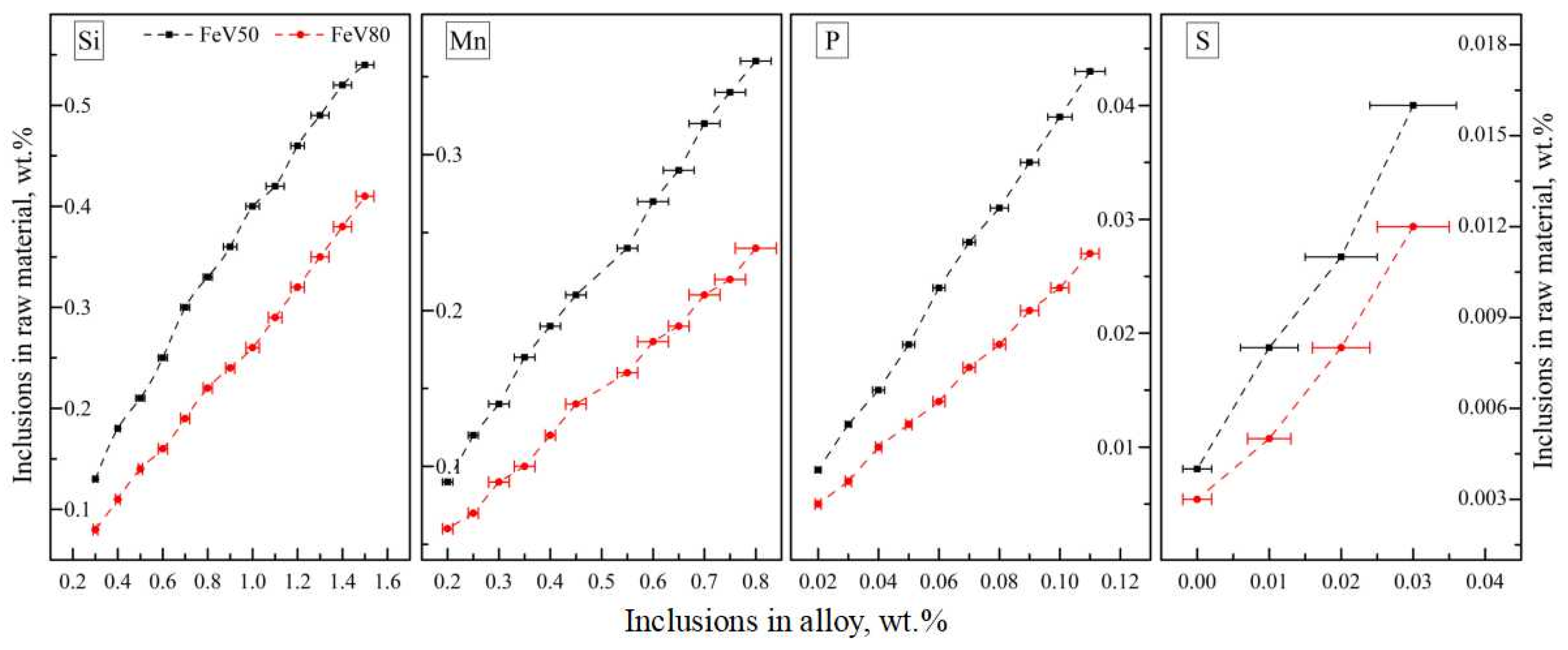

| Original Materials | Purity, ≥wt.% | Impurities Limitation, ≤wt.% | Standards | |||||
|---|---|---|---|---|---|---|---|---|
| Si | Mn | C | P | S | ||||
| Vanadium sources | V2O3 | 62.0 (T.V) | 0.08 | -- | 0.10 | 0.02 | 0.06 | GB/T 40301–2021 [32] |
| V2O5 | 98.0 | 0.11 | -- | 0.04 | 0.05 | 0.03 | YB/T 5304–2017 [33] | |
| FeV | 48.0 (T.V) | 1.50 | 0.50 | 0.30 | 0.08 | 0.06 | GB/T 4139-2012 [34] | |
| Fly ash | 10.0 (T.V) | 2.00 | 0.50 | 0.50 | 0.10 | 0.10 | QB–2021 [35] | |
| Collected dust | 15.0 (T.V) | 2.00 | 0.06 | 0.50 | 0.10 | 0.10 | ||
| VRSS | 3.0 (T.V) | 1.50 | 0.06 | 0.30 | 0.10 | 0.10 | ||
| Iron sources | Scrap iron | 99.0 | 0.10 | 0.10 | 0.10 | 0.05 | 0.05 | |
| BMIG | 95.0 | 0.25 | 0.20 | 0.10 | 0.05 | 0.05 | ||
| Reduction agent | Al | 99.0 | 0.05 | 0.05 | 0.10 | 0.05 | 0.05 | |
| Fluxing medium | CaO | 90.0 | 0.10 | 0.05 | 0.10 | 0.05 | 0.05 | |
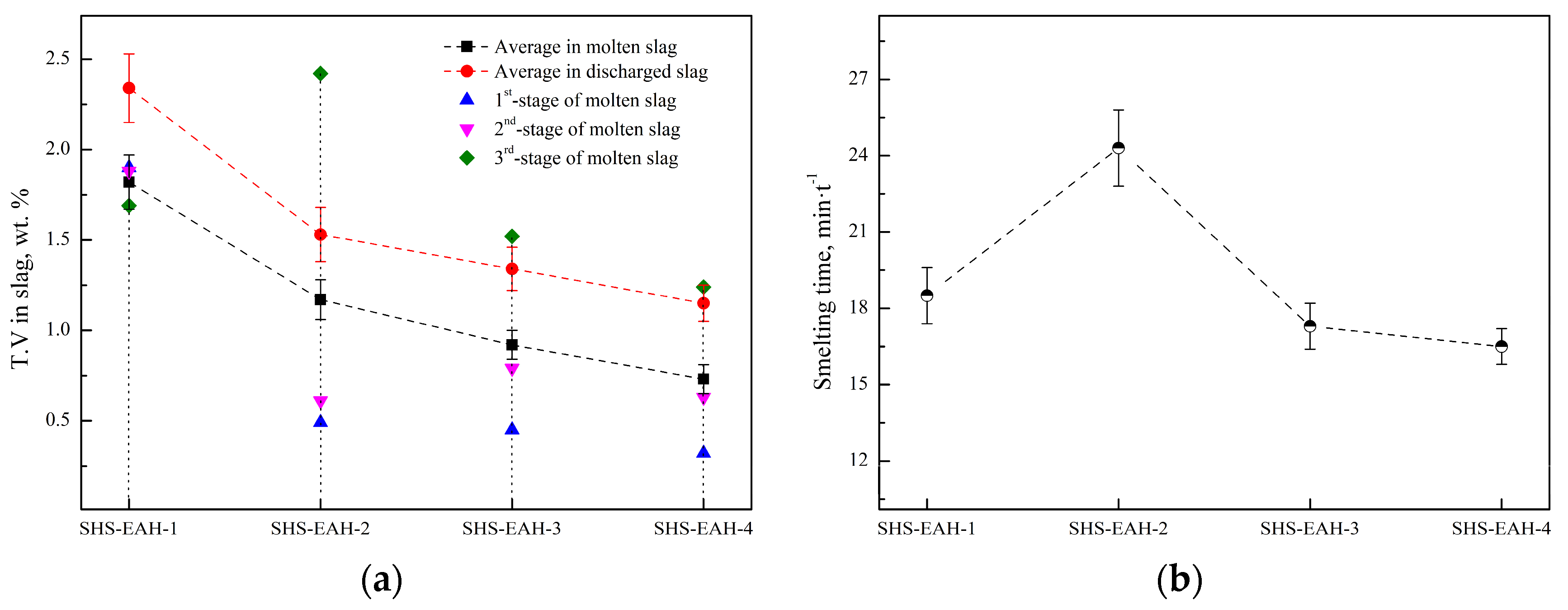
| Al Distribution Pattern | No. | η | Feeding Ratio | η | Average T.V in Molten Slag, wt.% | T.V in Discharged Slag, wt.% | Time, min·t−1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Stage | 2nd Stage | 3rd Stage | 1st Stage | 2nd Stage | 3rd Stage | Ave. | ||||||
| Uniform | SHS-EAH-1 | 1.05 | 1.05 | 1.05 | 4:4:2 | 1.05 | 1.90 | 1.88 | 1.69 | 1.82 | 2.34 | 18.5 |
| Semi-gradient | SHS-EAH-2 | 1.30 | 1.30 | 0.00 | 4:4:2 | 1.05 | 0.49 | 0.61 | 2.42 | 1.17 | 1.53 | 24.3 |
| Single-gradient | SHS-EAH-3 | 1.30 | 1.00 | 0.65 | 4:4:2 | 1.05 | 0.45 | 0.79 | 1.52 | 0.92 | 1.34 | 17.3 |
| Double-gradient | SHE-EAH-4 | 1.1–1.5 | 0.8–1.2 | 0.65 | 2 + 2:2 + 2:2 | 1.05 | 0.32 | 0.63 | 1.24 | 0.73 | 1.15 | 16.5 |
| No. | Elements and Content, wt.% | ||||||
|---|---|---|---|---|---|---|---|
| V | Fe | C | Si | P | S | Al | |
| SHS-EAH-1 | 50.2 | 47.5 | 0.29 | 0.4 | 0.07 | 0.02 | 1.5 |
| SHS-EAH-2 | 50.4 | 47.3 | 0.21 | 0.5 | 0.05 | 0.01 | 1.4 |
| SHS-EAH-3 | 51.3 | 47.0 | 0.16 | 0.6 | 0.05 | 0.01 | 0.8 |
| SHS-EAH-4 | 51.7 | 47.0 | 0.13 | 0.6 | 0.04 | 0.01 | 0.5 |
| FeV50-A | 48.0~55.0 | -- | ≤0.4 | ≤2.0 | ≤0.06 | ≤0.04 | ≤1.5 |
| FeV50-B | 48.0~55.0 | -- | ≤0.6 | ≤2.5 | ≤0.10 | ≤0.05 | ≤2.0 |
| Re0 | Fluid Type | ζ | Settlement Velocity | No. |
|---|---|---|---|---|
| ≤2.0 | Laminar region | 24/Re0 | u0 = d2·(ρm − ρs)·g/18μ | 30 |
| 2.0~500 | Transition region | 18.5/Re00.6 | u0 = 0.27[d·(ρm − ρs)·gRe00.6/ρs]1/2 | 31 |
| 500~2 × 105 | Turbulent region | 0.44 | u0 = 1.74[d·(ρm − ρs)·g/ρs]1/2 | 32 |
| ≥2 × 105 | ― | 0.1 | -- | -- |
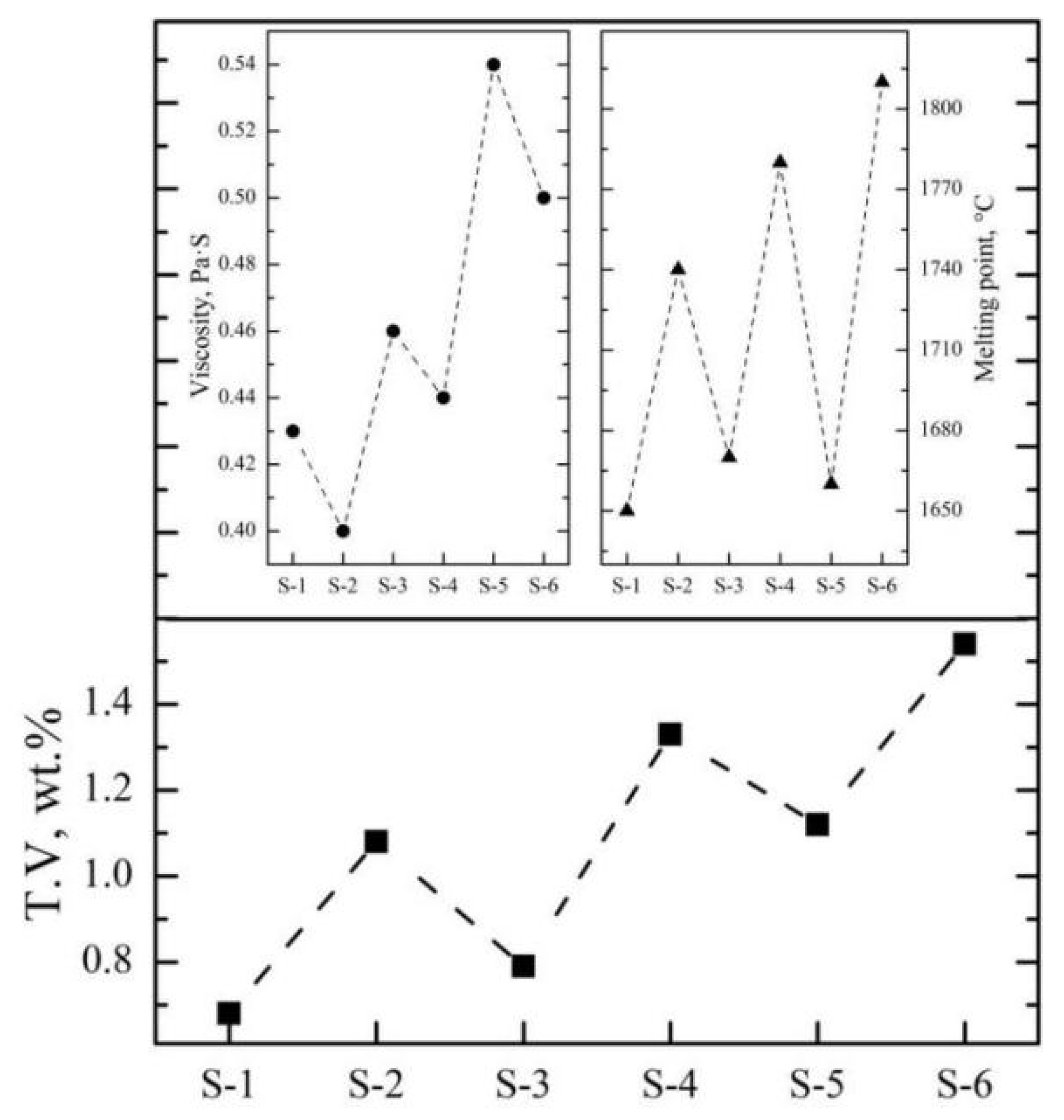
| No. | Slag Composition, wt.% | Viscosity, Pa·s | Melting Point, °C | ||||
|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | MgO | Fe2O3 | |||
| S-1 | 0.6 | 61.5 | 21.3 | 15.5 | 1.1 | 0.43 | 1650 |
| S-2 | 0.8 | 61.0 | 15.3 | 21.2 | 1.7 | 0.40 | 1740 |
| S-3 | 0.5 | 65.2 | 19.8 | 13.0 | 1.5 | 0.46 | 1670 |
| S-4 | 0.6 | 65.4 | 13.1 | 19.5 | 1.4 | 0.44 | 1780 |
| S-5 | 0.5 | 71.3 | 18.7 | 8.1 | 1.4 | 0.54 | 1660 |
| S-6 | 0.6 | 71.6 | 10.8 | 15.4 | 1.6 | 0.50 | 1810 |
| Type | Viscosity, Pa·s | ρ, g·cm−3 | d, mm | u0, m·s−1 | Re0 | t, min |
|---|---|---|---|---|---|---|
| alloy | 0.54 | 7.0 | 0.10 | 3.53 × 10−5 | 2.29 × 10−5 | 141.7 |
| 0.54 | 7.0 | 0.50 | 3.53 × 10−3 | 2.29 × 10−2 | 5.7 | |
| 0.40 | 7.0 | 0.10 | 4.76 × 10−5 | 4.17 × 10−5 | 104.9 | |
| 0.40 | 7.0 | 0.50 | 4.76 × 10−3 | 4.17 × 10−2 | 4.2 | |
| slag | 0.0025 | 3.5 | 0.10 | 7.62 × 10−3 | 1.07 | 0.66 |
| 0.0025 | 3.5 | 0.50 | 0.76 | 1067 | 0.03 |
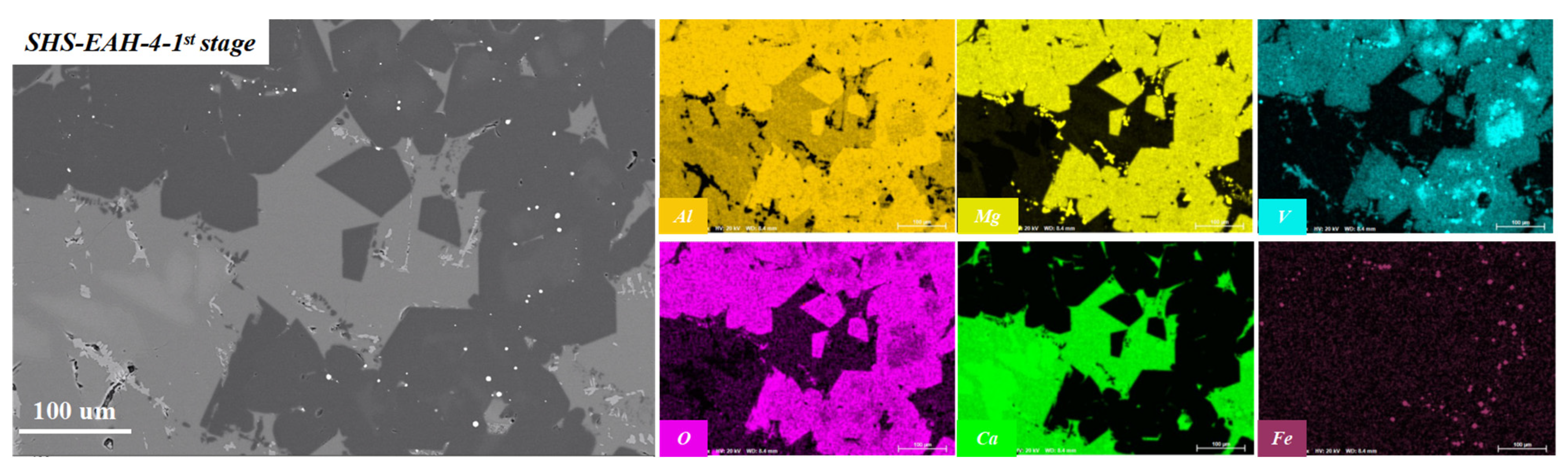
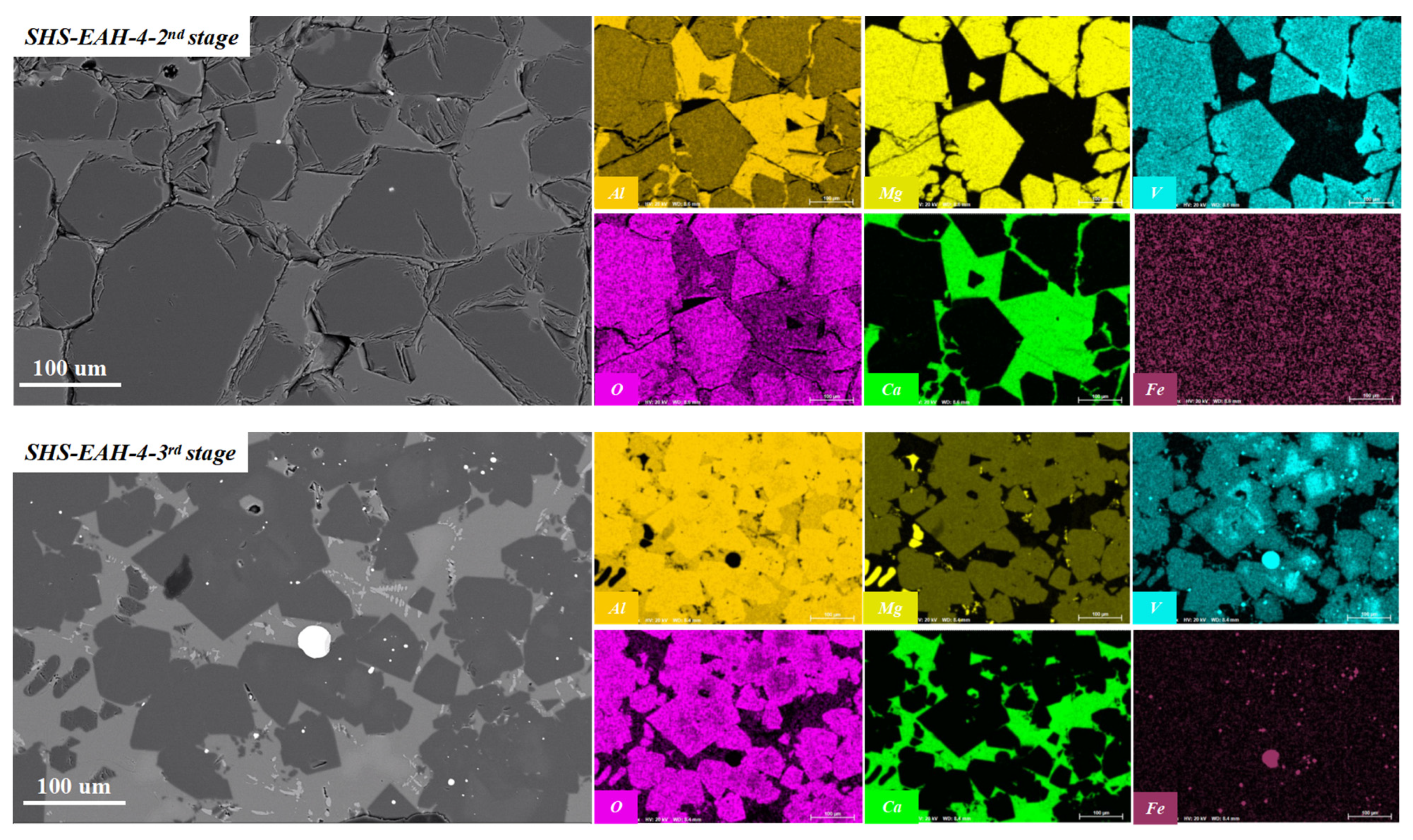
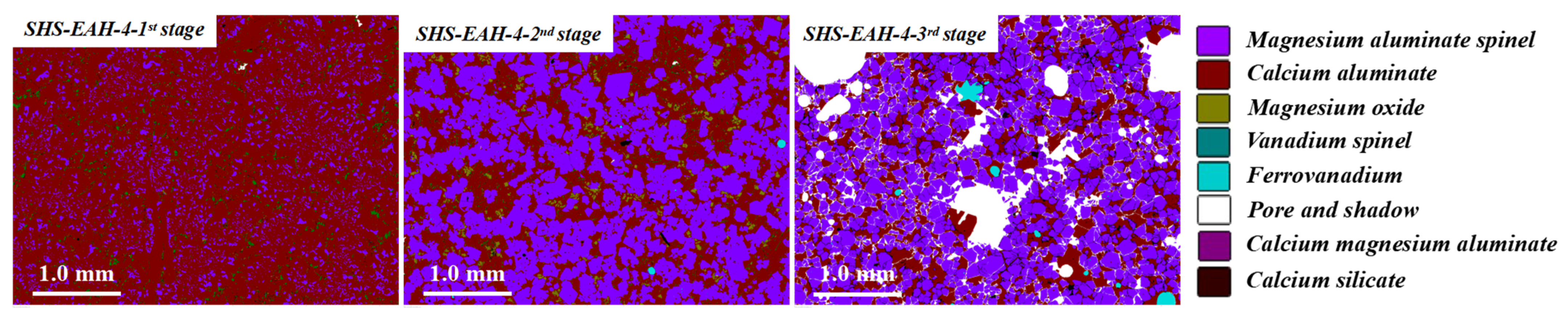
| No. | Mineral Composition and Content, wt.% | |||||
|---|---|---|---|---|---|---|
| MgO·Al2O3 | CaO·Al2O3 | CaO·2Al2O3 | CaO·6Al2O3 | Ferrovanadium | Perovskite | |
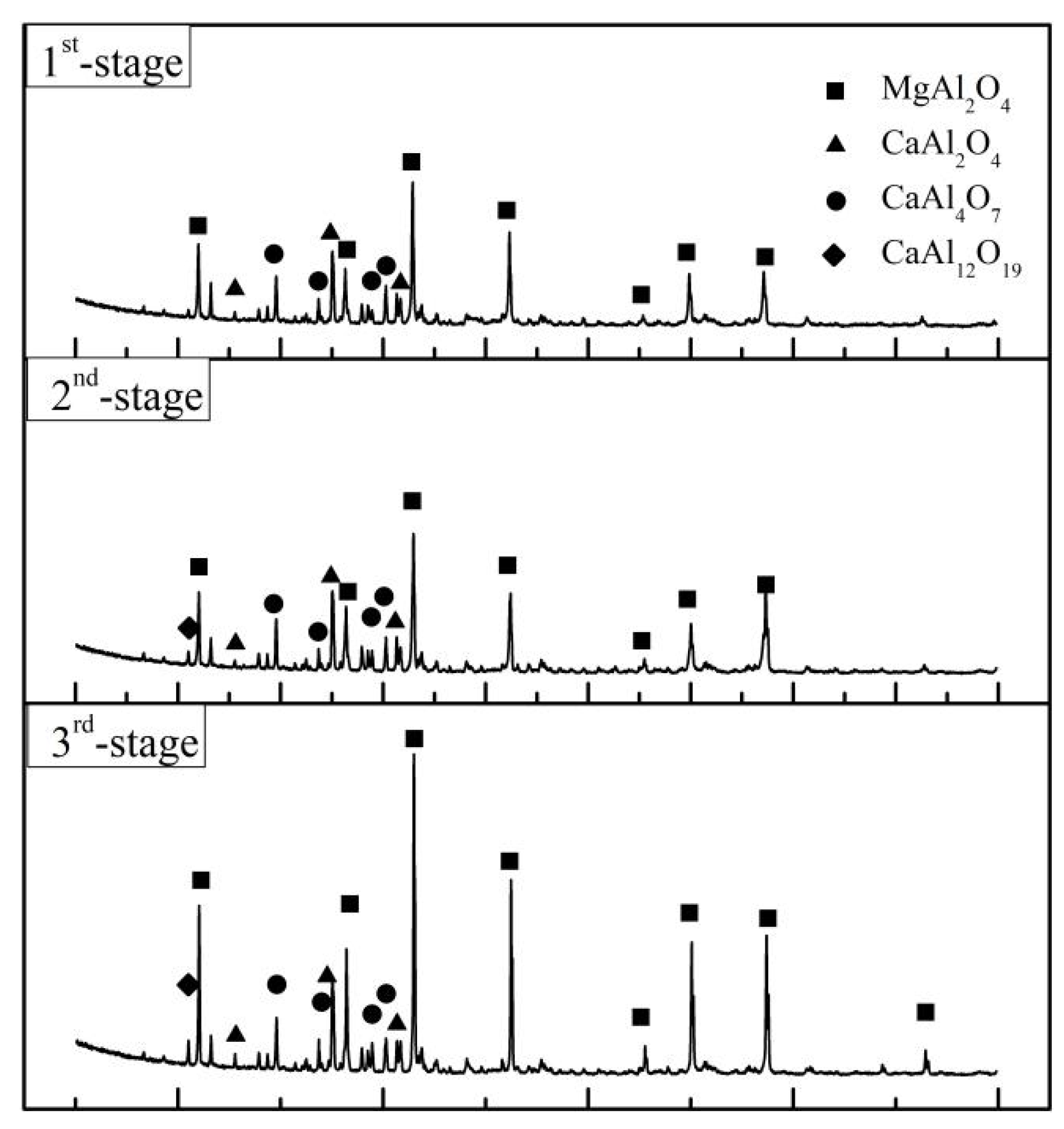
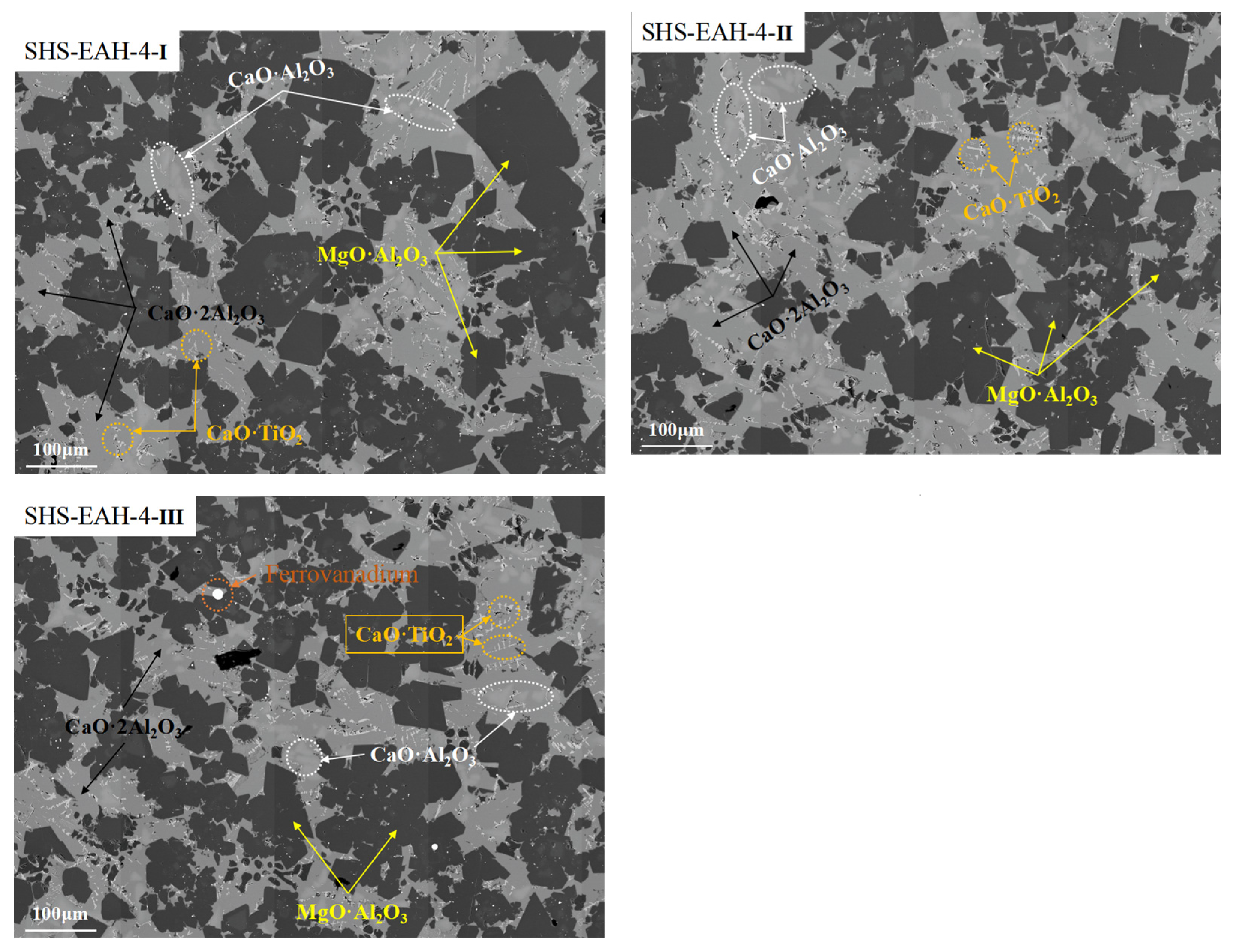
| SHS-EAH-4-I | 53.32 | 9.24 | 35.22 | 0.00 | 0.00 | 2.12 |
| SHS-EAH-4-II | 45.73 | 18.79 | 33.05 | 0.00 | 0.00 | 2.43 |
| SHS-EAH-4-III | 56.25 | 10.40 | 25.82 | 0.00 | 0.73 | 2.89 |
| Grades | Inclusion Composition Control Limitation of Raw Material, wt.% | ||||
|---|---|---|---|---|---|
| Si | Mn | P | S | C | |
| FeV50-A | 0.50 | 0.20 | 0.017 | 0.04 | 0.6 |
| FeV50-B | 0.50 | 0.20 | 0.032 | 0.06 | 0.8 |
| FeV80-A | 0.30 | 0.12 | 0.010 | 0.04 | 0.3 |
| FeV80-B | 0.30 | 0.12 | 0.015 | 0.06 | 0.5 |
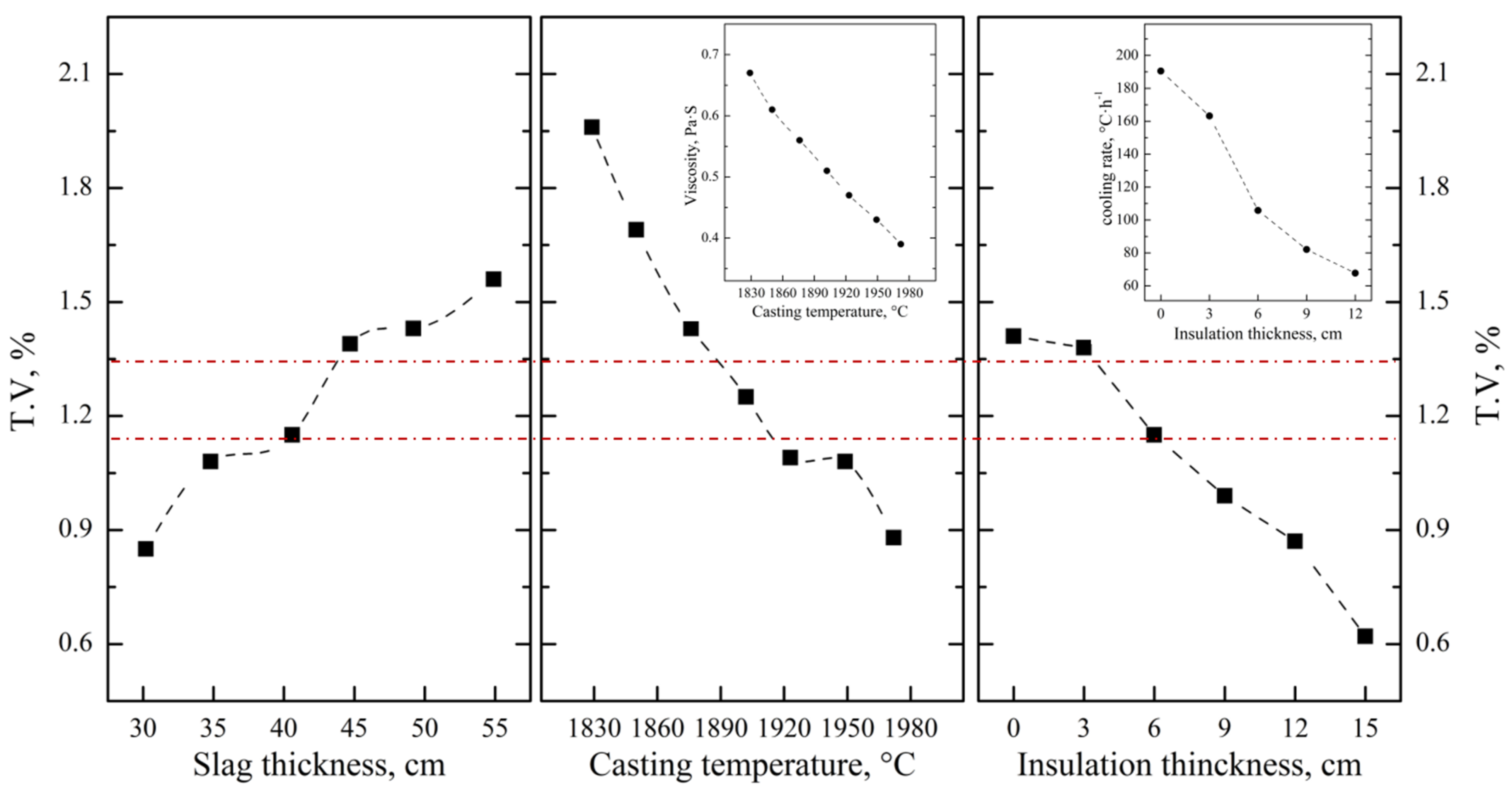
| Distribution ratio, wt.% | 85.1~92.6 | 92.3~95.7 | 72.5~82.3 | 0~42.7 | —— |
| Technical Indicator | Manufacturing Enterprise | |||||
|---|---|---|---|---|---|---|
| PG Group (BH) China | PG Group (PZH) China | CG Group (CG) China | Hayveld (HV) South Africa | Evraz (E) Russia | PG Group (XC) China | |
| Industrialization process | One-step | Multi-stage | ||||
| Al distribution pattern | Uniform | Uniform | Gradient | |||
| Smelting unit | Straight tube furnace | Titling furnace | ||||
| Vanadium source | V2O5 | V2O3 + V2O5 | V2O5 | V2O5 | V2O5 | V2O3 + V2O5 |
| T.V in residue slag, wt.% | 0.6~0.8 | 1.2~1.6 | 1.4~1.6 | 1.4~1.6 | 1.2~1.4 | 0.6~0.9 |
| Slag–alloy ratio (FeV50) | 1.25 | 1.00 | 1.25 | 1.25 | 1.25 | 1.10 |
| Vanadium loss, wt.% | 1.5~2.0 | 2.4~3.2 | 3.5~4.0 | 3.5~4.0 | 3.0~3.5 | 1.3~2.0 |
| Smelting yield, wt.% | 98.0~98.5 | 96.8~97.6 | 96.0~96.5 | 96.0~96.5 | 96.5~97.0 | 98.0~98.7 |
| Elc consumption, kWh·t−1 | 250~300 | 800~900 | 300~350 | 300~350 | 500~600 | 900~1000 |
| Aluminum coefficient | 1.05~1.10 | 1.05~1.10 | 1.05~1.10 | 1.05~1.10 | 1.05~1.10 | 1.05~1.08 |
| Al consumption, kg·t−1 | 520~540 | 410~430 | 510~530 | 510~530 | 510~530 | 400~420 |
| Product controllability | Uncontrollable | Divinable and controllable | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.; Yuan, T.; Shi, J.; Li, R.; Jiang, C.; Ye, M.; Xiao, D.; Chen, H.; Zhang, L.; Wang, N.; et al. Evaluation of a Novel High-Efficiency SHS-EAH Multi-Stage DG-ADP Process for Cleaner Production of High-Quality Ferrovanadium Alloy. Metals 2024, 14, 211. https://doi.org/10.3390/met14020211
Yu B, Yuan T, Shi J, Li R, Jiang C, Ye M, Xiao D, Chen H, Zhang L, Wang N, et al. Evaluation of a Novel High-Efficiency SHS-EAH Multi-Stage DG-ADP Process for Cleaner Production of High-Quality Ferrovanadium Alloy. Metals. 2024; 14(2):211. https://doi.org/10.3390/met14020211
Chicago/Turabian StyleYu, Bin, Tiechui Yuan, Junjie Shi, Ruidi Li, Chenglong Jiang, Mingfeng Ye, Daihong Xiao, Haijun Chen, Lin Zhang, Ning Wang, and et al. 2024. "Evaluation of a Novel High-Efficiency SHS-EAH Multi-Stage DG-ADP Process for Cleaner Production of High-Quality Ferrovanadium Alloy" Metals 14, no. 2: 211. https://doi.org/10.3390/met14020211
APA StyleYu, B., Yuan, T., Shi, J., Li, R., Jiang, C., Ye, M., Xiao, D., Chen, H., Zhang, L., Wang, N., Gao, L., Yin, D., Zhang, L., & Yang, X. (2024). Evaluation of a Novel High-Efficiency SHS-EAH Multi-Stage DG-ADP Process for Cleaner Production of High-Quality Ferrovanadium Alloy. Metals, 14(2), 211. https://doi.org/10.3390/met14020211








