Thermodynamics of Aluminothermic Processes for Ferrotitanium Alloy Production from Bauxite Residue and Ilmenite
Abstract
1. Introduction
2. Materials
3. Theoretical Calculations
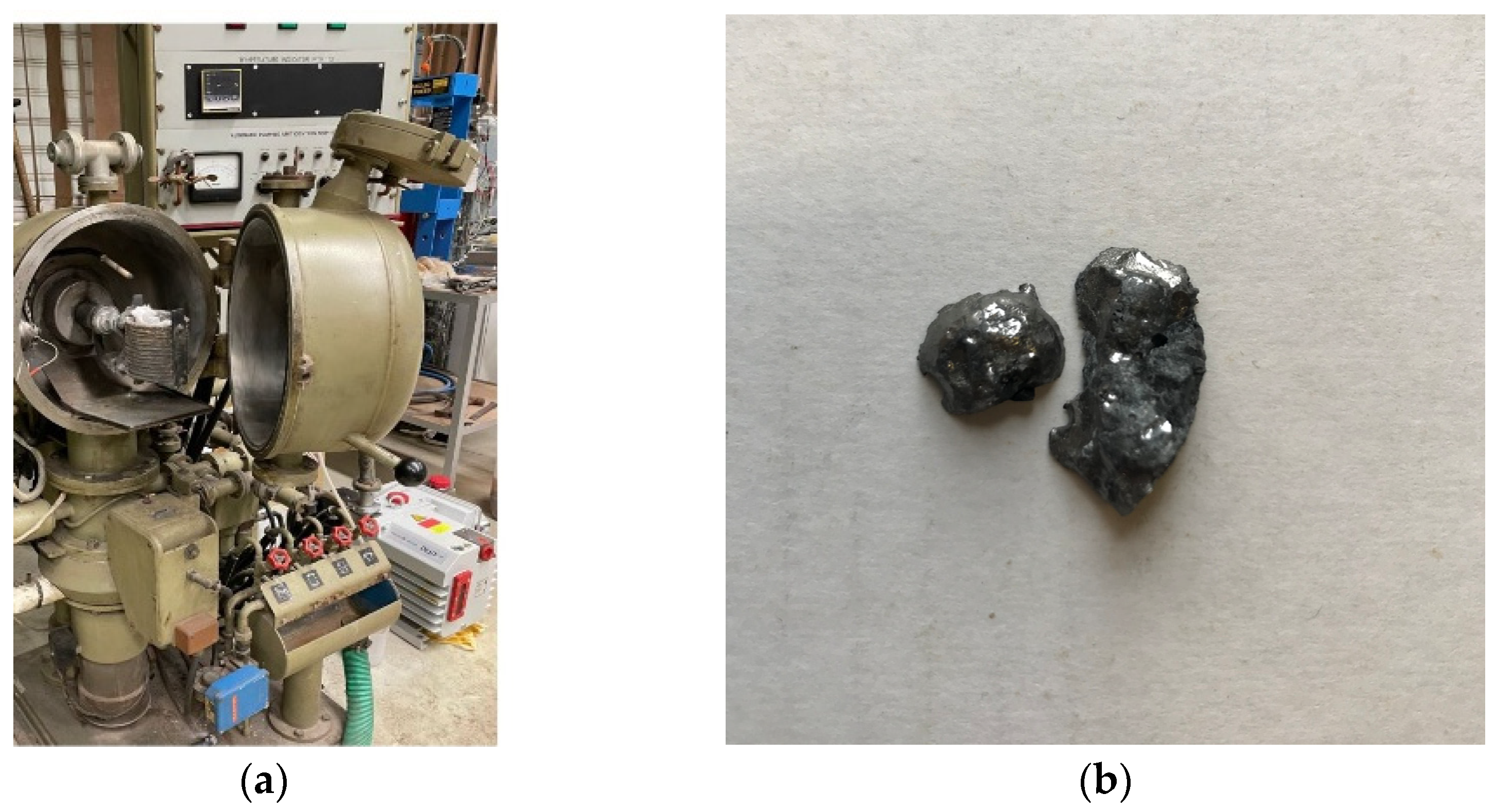
4. Experimental Method
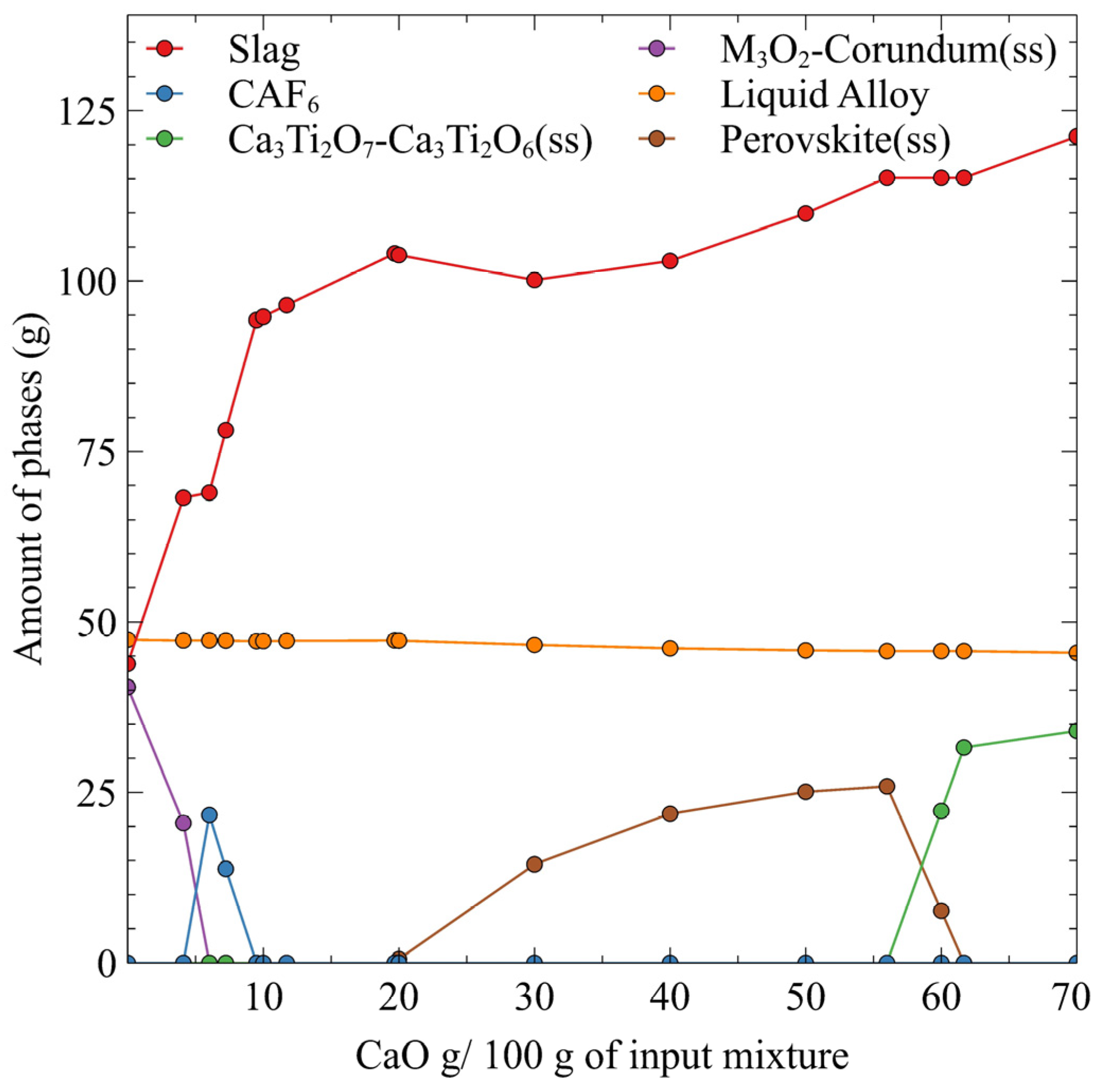
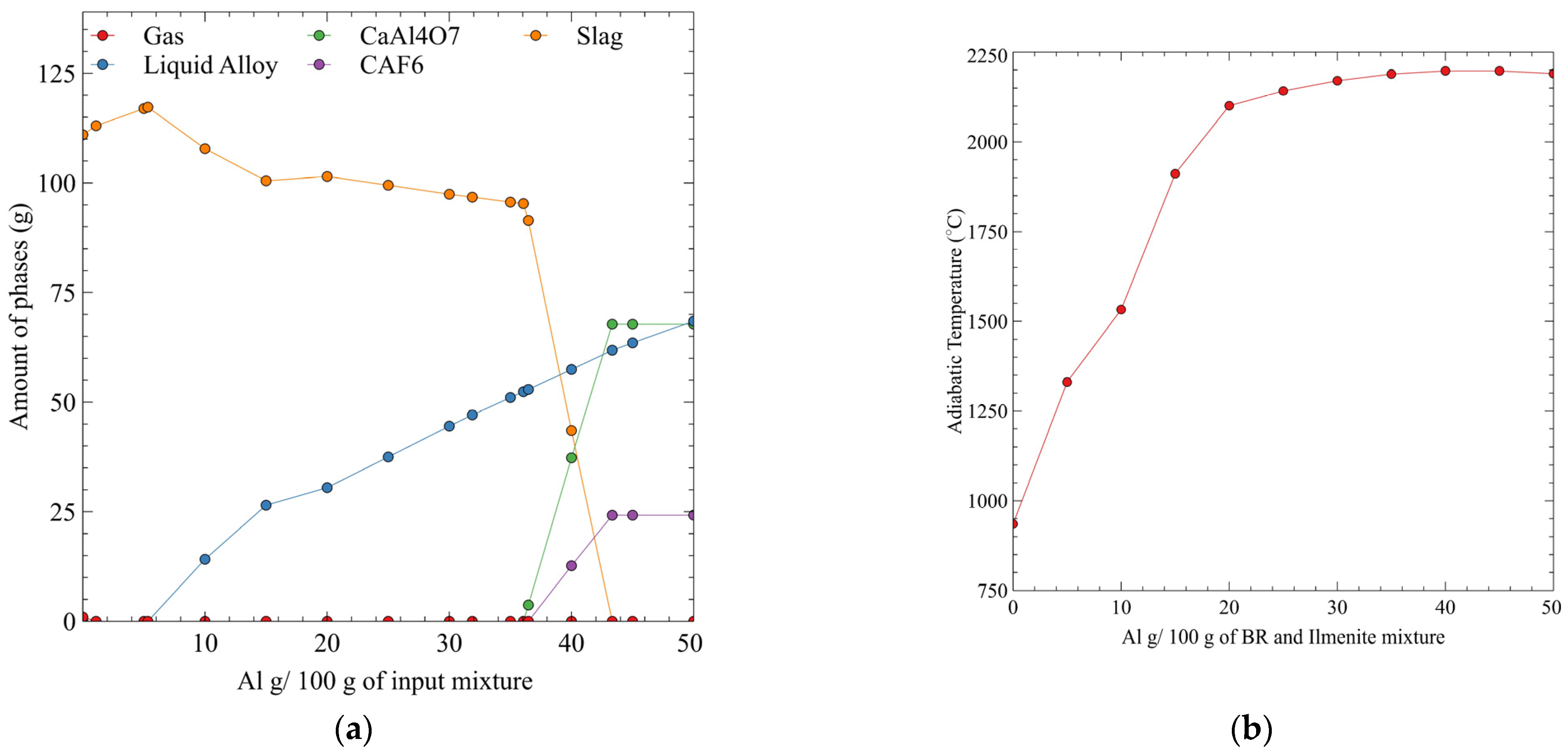
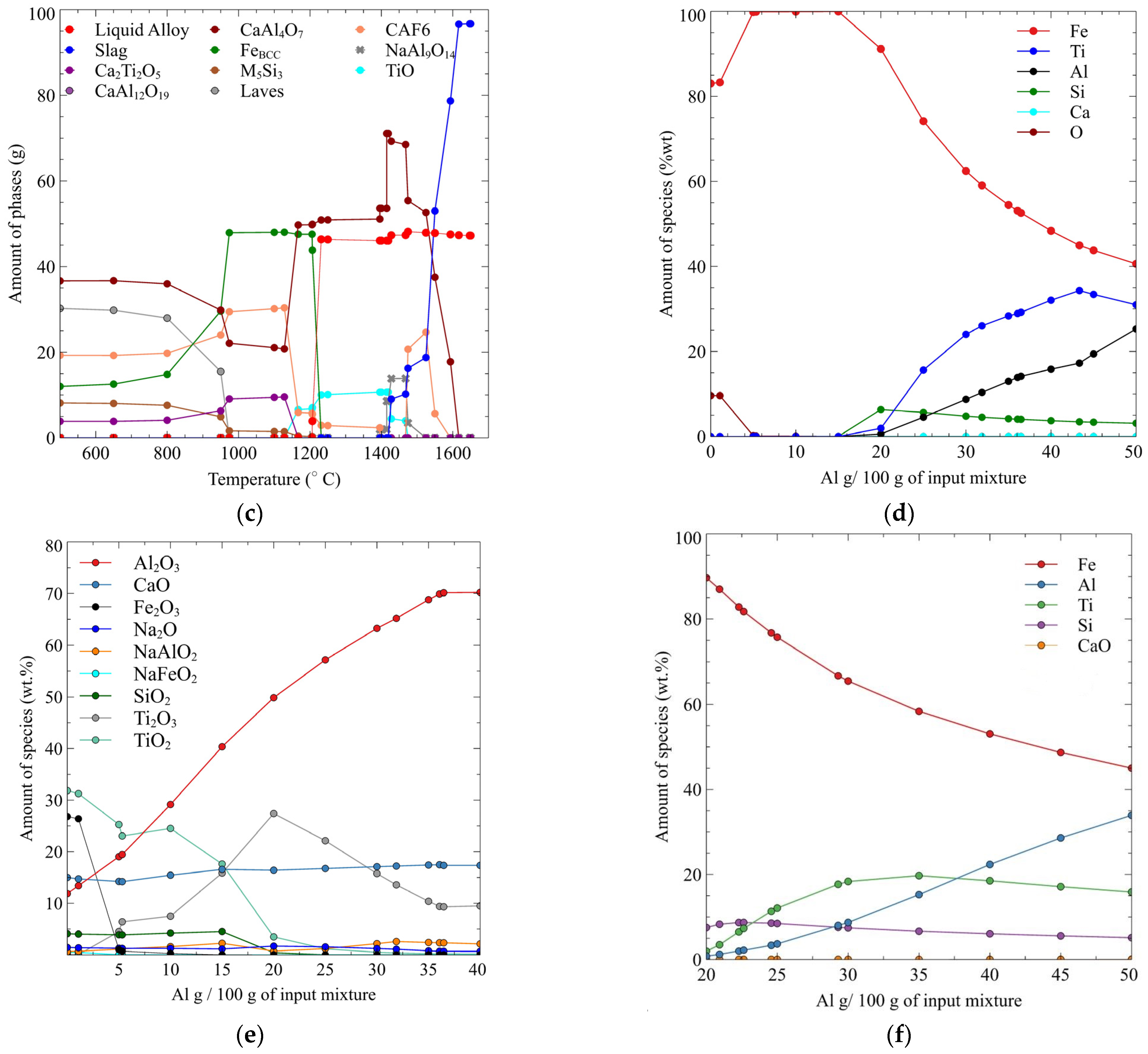
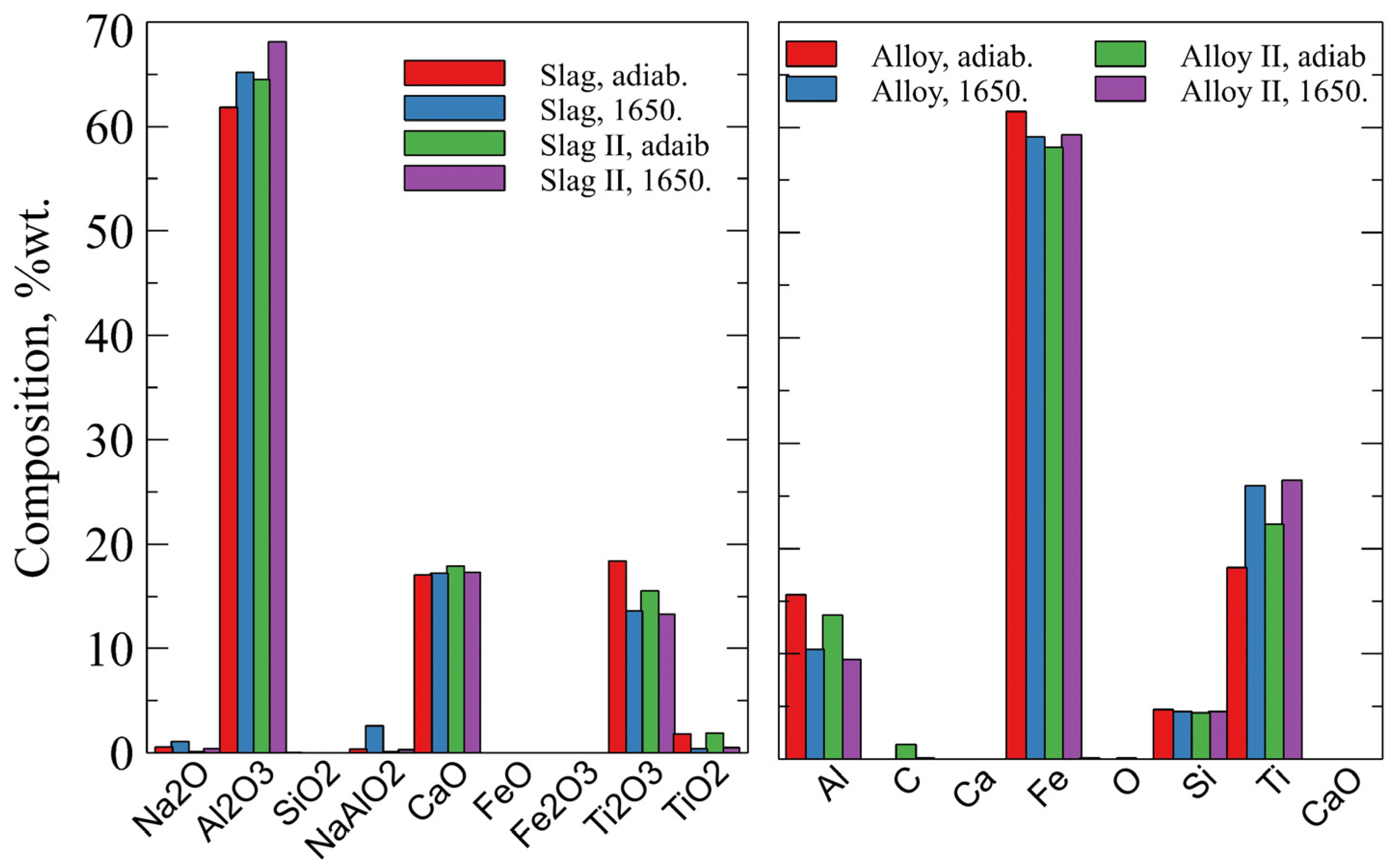
5. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reddy, P.S.; Reddy, N.G.; Serjun, V.Z.; Mohanty, B.; Das, S.K.; Reddy, K.R.; Rao, B.H. Properties and assessment of applications of red mud (bauxite residue): Current status and research needs. Waste Biomass Valorization 2021, 12, 1185–1217. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Bucher, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Balomenos, E.; Davris, P.; Pontikes, Y.; Panias, D. Mud2Metal: Lessons learned on the path for complete utilization of bauxite residue through industrial symbiosis. J. Sustain. Metall. 2017, 3, 551–560. [Google Scholar] [CrossRef]
- Chiara, C.; Balomenos, E.; Panias, D. Optimization of microwave reductive roasting process of bauxite residue. Metals 2020, 10, 1083. [Google Scholar]
- Agatzini-Leonardou, S.; Oustadakis, P.; Tsakiridis, P.E.; Markopoulos, C. Titanium Leaching from Red Mud by Diluted Sulfuric Acid at Atmospheric Pressure. J. Hazard. Mater. 2008, 157, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Alkan, G.; Yagmurlu, B.; Cakmakoglu, S.; Hertel, T.; Kaya, Ş.; Gronen, L.; Stopic, S.; Friedrich, B. Novel Approach for Enhanced Scandium and Titanium Leaching Efficiency from Bauxite Residue with Suppressed Silica Gel Formation. Sci. Rep. 2018, 8, 5676. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, C.; Alexandri, A.; Vind, J.; Panagiotopoulou, A.; Tsakiridis, P.; Panias, D. Scandium and Titanium Recovery from Bauxite Residue by Direct Leaching with a Brønsted Acidic Ionic Liquid. Metals 2018, 8, 834. [Google Scholar] [CrossRef]
- Gasik, M.; Dashevskii, V.; Bizhanov, A. Metallurgy of Ferrotitanium. In Ferroalloys. Topics in Mining, Metallurgy and Materials Engineering; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Yang, W.; Zhang, Y.; Zhang, L.-F.; Duan, H.-J.; Wang, L. Population evolution of oxide inclusions in Ti-stabilized ultra-low carbon steels after deoxidation. J. Iron. Steel Res. Int. 2015, 22, 1069–1077. [Google Scholar] [CrossRef]
- Pande, M.M.; Guo, M.; Blanpain, B. Inclusion formation and interfacial reactions between FeTi alloys and liquid steel at an early stage. ISIJ Int. 2013, 53, 629–638. [Google Scholar] [CrossRef]
- Bale, C.W.; Chartrand, P.; Degterov, S.A.; Eriksson, G.; Hack, K.; Mahfoud, R.B.; Melançon, J.; Pelton, A.; Petersen, S. FactSage thermochemical software and databases. Calphad 2002, 26, 189–228. [Google Scholar] [CrossRef]
- ISO 5454:1980; International Organization for Standardization. Ferrotitanium-Specification and Conditions of Delivery. ISO: Geneva, Switzerland, 1980.
- Vafeias, M.; Bempelou, A.; Georgala, E.; Davris, P.; Balomenos, E.; Panias, D. Leaching of Ca-Rich Slags Produced from Reductive Smelting of Bauxite Residue with Na2CO3 Solutions for Alumina Extraction: Lab and Pilot Scale Experiments. Minerals 2021, 11, 896. [Google Scholar] [CrossRef]
| BR/Ilmenite (%wt/%wt) | Fe (%wt) | Ti (%wt) | Al (%wt) | Si (%wt) |
|---|---|---|---|---|
| 80/20 | 76.5 | 11.5 | 3.6 | 8.6 |
| 70/30 | 69.6 | 17.3 | 6 | 7 |
| 60/40 | 63.7 | 22.2 | 8.3 | 5.8 |
| 50/50 | 58.9 | 26.2 | 10.5 | 4.4 |
| BR/Ilmenite (%wt/%wt) | Al2O3 (%wt) | CaO (%wt) | Na2O (%wt) | SiO2 (%wt) | TiO2 (%wt) | Ti2O3 (%wt) |
|---|---|---|---|---|---|---|
| 80/20 | 65.5 | 22.4 | 2.8 | 0.2 | 0.6 | 8.6 |
| 70/30 | 67.8 | 19.1 | 2.4 | - | 0.4 | 10.3 |
| 60/40 | 67.6 | 18.2 | 2.1 | - | 0.4 | 11.7 |
| 50/50 | 66.9 | 17.2 | 2. | - | 0.4 | 13.4 |
| BR/Ilmenite (%wt/%wt) | Fe (wt.%) | Ti (wt.%) | Si (wt.%) | Al (wt.%) |
|---|---|---|---|---|
| 80/20 | 67.35 | 24.24 | 7.46 | 2.84 |
| 70/30 | 63.36 | 27.36 | 6.78 | 3.47 |
| 60/40 | 59.35 | 31.32 | 5.93 | 4.34 |
| 50/50 | 54.01 | 35.82 | 5.04 | 5.47 |
| BR/Ilmenite (%wt/%wt) | Al2O3 (%wt) | CaO (%wt) | Na2O (%wt) | SiO2 (%wt) | Fe2O3 (%wt) | TiO2 (%wt) |
|---|---|---|---|---|---|---|
| 80/20 | 69.45 | 10.76 | 4.37 | 2.51 | 2.13 | 1.93 |
| 70/30 | 68.12 | 10.18 | 4.12 | 2.27 | 2.81 | 2.37 |
| 60/40 | 68.73 | 11.35 | 4.83 | 2.38 | 2.58 | 2.11 |
| 50/50 | 70.2 | 12.24 | 5.04 | 2.12 | 3.47 | 2.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sparis, D.; Lazou, A.; Balomenos, E.; Panias, D. Thermodynamics of Aluminothermic Processes for Ferrotitanium Alloy Production from Bauxite Residue and Ilmenite. Metals 2024, 14, 200. https://doi.org/10.3390/met14020200
Sparis D, Lazou A, Balomenos E, Panias D. Thermodynamics of Aluminothermic Processes for Ferrotitanium Alloy Production from Bauxite Residue and Ilmenite. Metals. 2024; 14(2):200. https://doi.org/10.3390/met14020200
Chicago/Turabian StyleSparis, Dimitris, Adamantia Lazou, Efthymios Balomenos, and Dimitrios Panias. 2024. "Thermodynamics of Aluminothermic Processes for Ferrotitanium Alloy Production from Bauxite Residue and Ilmenite" Metals 14, no. 2: 200. https://doi.org/10.3390/met14020200
APA StyleSparis, D., Lazou, A., Balomenos, E., & Panias, D. (2024). Thermodynamics of Aluminothermic Processes for Ferrotitanium Alloy Production from Bauxite Residue and Ilmenite. Metals, 14(2), 200. https://doi.org/10.3390/met14020200





