Valuable Recovery Technology and Resource Utilization of Chromium-Containing Metallurgical Dust and Slag: A Review
Abstract
:1. Introduction
2. Physicochemical Properties of Chromium-Containing Metallurgical Dust and Slag
2.1. Chromium-Containing Metallurgical Dust
2.1.1. Stainless Steel Dust
2.1.2. Ferrochrome Dust
2.2. Chromium-Containing Metallurgical Slag
2.2.1. Stainless Steel Slag
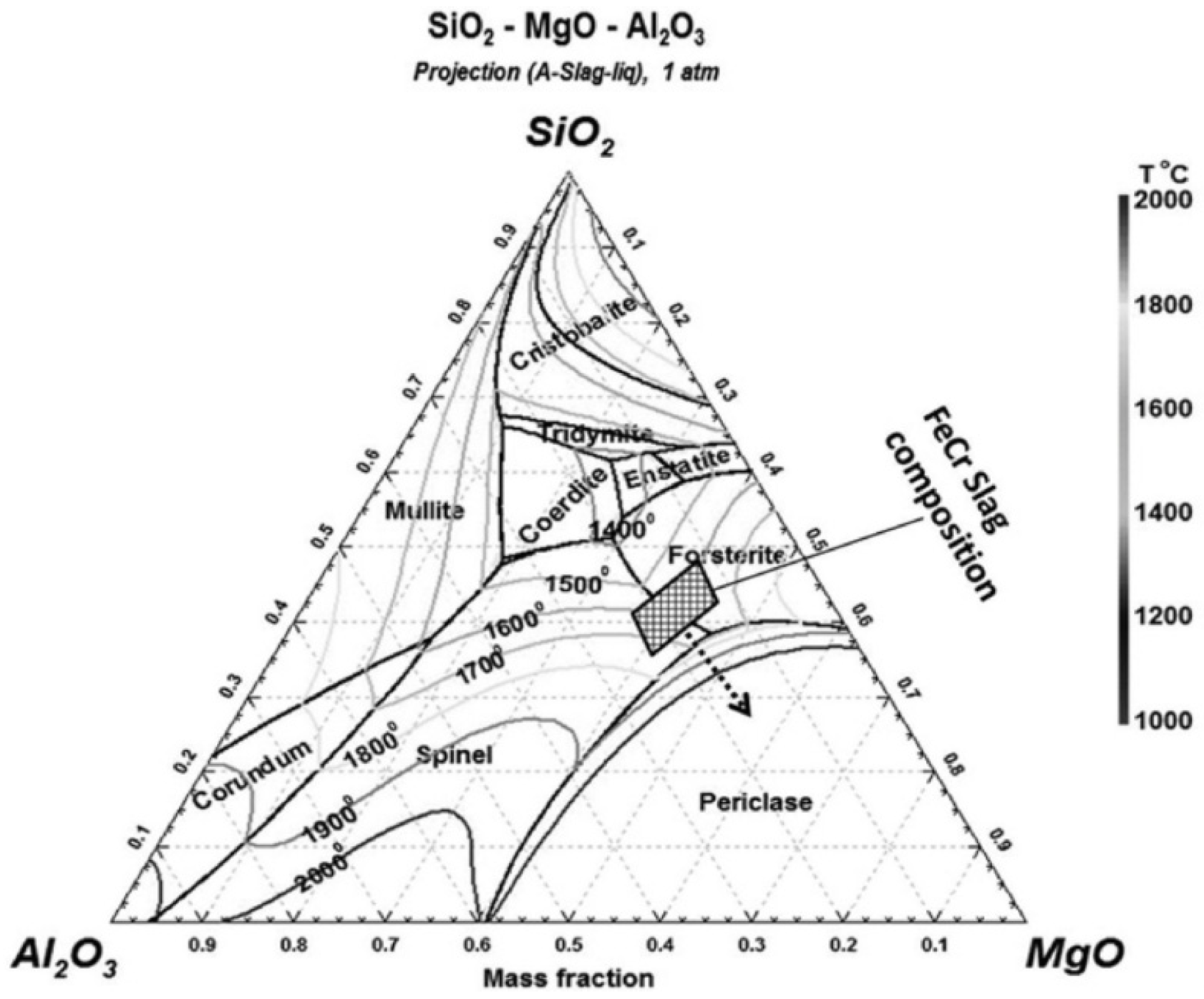
2.2.2. Ferrochrome Slag
3. Valuable Recovery Technology for Chromium-Containing Metallurgical Dust and Slag
3.1. Stainless Steel Dust
- (1)
- Pyrometallurgical process
- (2)
- Hydrometallurgical method
3.2. Ferrochrome Dust
- (1)
- Hydrometallurgical process
- (2)
- Pyrometallurgical process
3.3. Stainless Steel Slag
- (1)
- Pyrometallurgical process
- (2)
- Hydrometallurgical process
3.4. Ferrochrome Slag
4. Resource Utilization of Chromium-Containing Metallurgical Dust and Waste Residue
4.1. Ceramic Pigments
4.2. Construction Industry
4.3. Glass–Ceramic
4.4. Refractory Materials
4.5. Carbonation Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Q.; Liu, K.; Sun, L.F.; Liu, C.J.; Jiang, M.F.; Saxén, H.; Zevenhoven, R. Towards carbon sequestration using stainless steel slag via phase modification and co-extraction of calcium and magnesium. Process Saf. Environ. Prot. 2020, 133, 73–81. [Google Scholar] [CrossRef]
- Habib, A.; Bhatti, H.N.; Iqbal, M. Metallurgical processing strategies for metals recovery from industrial slags. Z. Phys. Chem. 2020, 234, 201–231. [Google Scholar] [CrossRef]
- Liu, P.J.; Liu, Z.G.; Chu, M.S.; Yan, R.J.; Li, F.; Tang, J.; Feng, J. Efficient utilization of stainless steel dust and chromium-containing slag by carbothermal girect reduction: Synergistic mechanism and optimization analysis. J. Sustain. Metall. 2022, 8, 1877–1891. [Google Scholar] [CrossRef]
- Rieger, J.; Schenk, J. Residual processing in the european steel industry: A technological overview. J. Sustain. Metall. 2019, 5, 295–309. [Google Scholar] [CrossRef]
- Guézennec, A.-G.; Huber, J.-C.; Patisson, F.; Sessiecq, P.; Birat, J.-P.; Ablitzer, D. Dust formation in electric arc furnace: Birth of the particles. Powder Technol. 2005, 157, 2–11. [Google Scholar] [CrossRef]
- Kim, E.; Spooren, J.; Broos, K.; Nielsen, P.; Horckmans, L.; Geurts, R.; Vrancken, K.C.; Quaghebeur, M. Valorization of stainless steel slag by selective chromium recovery and subsequent carbonation of the matrix material. J. Clean. Prod. 2016, 117, 221–228. [Google Scholar] [CrossRef]
- Liu, P.J.; Liu, Z.G.; Chu, M.S.; Yan, R.J.; Li, F.; Tang, J. New understanding on metal recovery of Fe, Ni and Cr during carbon-thermal reduction of stainless steel dust. Adv. Powder Technol. 2021, 32, 4273–4285. [Google Scholar] [CrossRef]
- Shen, H.; Forssberg, E.; Nordström, U. Physicochemical and mineralogical properties of stainless steel slags oriented to metal recovery. Resour. Conserv. Recycl. 2004, 40, 245–271. [Google Scholar] [CrossRef]
- Lin, Y.; Yan, B.J.; Fabritius, T.; Shu, Q.F. Immobilization of chromium in stainless steel slag using low zinc electric arc furnace dusts. Metall. Mater. Trans. B 2020, 51, 763–775. [Google Scholar] [CrossRef]
- Bru, K.; Seron, A.; Morillon, A.; Algermissen, D.; Lerouge, C.; Menad, N. Characterization of a chromium-bearing carbon steel electric arc furnace slag after magnetic separation to determine the potential for iron and chromium recovery. Minerals 2021, 12, 47. [Google Scholar] [CrossRef]
- Liu, P.J.; Liu, Z.G.; Chu, M.S.; Yan, R.J.; Li, F.; Tang, J.; Feng, J. Detoxification and comprehensive recovery of stainless steel dust and chromium containing slag: Synergistic reduction mechanism and process parameter optimization. Process Saf. Environ. Prot. 2022, 164, 678–695. [Google Scholar] [CrossRef]
- Niemelä, P.; Kauppi, M. Production, characteristics and use of ferrochromium slags. In Proceedings of the INFACON XI, New Delhi, India, 18–21 February 2007; pp. 171–179. [Google Scholar]
- Ma, G.J.; Ni, H.W.; Craig, A.G. Properties and formation mechanism of ferrochrome electric furnace dust. J. Wuhan Univ. Sci. Technol. (Nat. Sci. Ed.) 2006, 29, 443–445. (In Chinese) [Google Scholar]
- Niemel, P.; Krogerus, H.; Oikarinen, P. Formation, characteristics and utilisation of CO-gas formed in ferrochromium smelting. In Proceedings of the Tenth International Ferro Alloys Congress, Cape Town, South Africa, 1–4 February 2004. [Google Scholar]
- Kurtulus, C.; Kurtulus, R.; Kavas, T. Foam glass derived from ferrochrome slag and waste container glass: Synthesis and extensive characterizations. Ceram. Int. 2021, 47, 24997–25008. [Google Scholar] [CrossRef]
- Fares, A.I.; Sohel, K.M.A.; Al-Jabri, K.; Al-Mamun, A. Characteristics of ferrochrome slag aggregate and its uses as a green material in concrete—A review. Constr. Build. Mater. 2021, 294, 123552. [Google Scholar] [CrossRef]
- Berryman, E.J.; Paktunc, D. Cr(VI) formation in ferrochrome-smelter dusts. J. Hazard. Mater. 2022, 422, 126873. [Google Scholar] [CrossRef]
- Ri, S.; Chu, M.S. Separation of metal nugget from self-reduced product of coal composite stainless steel dust briquette. ISIJ Int. 2015, 55, 1565–1572. [Google Scholar] [CrossRef]
- Zhang, H.W.; Hong, X. An overview for the utilization of wastes from stainless steel industries. Resour. Conserv. Recycl. 2011, 55, 745–754. [Google Scholar]
- Aromaa, J.; Galfi, I.; Stefanova, A.; Forsén, O. Thermal treatment of stainless steel dusts for leaching. Acta Metall. Slovaca 2013, 19, 170–175. [Google Scholar] [CrossRef]
- Capobianco, O.; Costa, G.; Thuy, L.; Magliocco, E.; Hartog, N.; Baciocchi, R. Carbonation of stainless steel slag in the context of in situ Brownfield remediation. Miner. Eng. 2014, 59, 91–100. [Google Scholar] [CrossRef]
- Zhao, Q.Z.; Zeng, Y.N.; Li, J.G.; Wang, Y.J. Selective extraction of chromium from EAF stainless steel slag by pressurized oxidation in a NaOH solution. Mater. Trans. 2020, 61, 2030–2039. [Google Scholar] [CrossRef]
- Golik, V.I.; Klyuev, R.V.; Martyushev, N.V.; Zyukin, D.A.; Karlina, A.I. Technology for nonwaste recovery of tailings of the Mizur Mining and processing plant. Metallurgist 2023, 66, 1476–1480. [Google Scholar] [CrossRef]
- Laudal, D.A.; Benson, S.A.; Addleman, R.S.; Palo, D. Leaching behavior of rare earth elements in Fort Union lignite coals of North America. Int. J. Coal Geol. 2018, 191, 112–124. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhang, X.; Ma, G.J.; Muvunyi, R.A.; Zheng, D.L. Comparison of microwave and conventional processing stainless steelmaking dust to prepare black ceramic pigments. J. Ceram. Process. Res. 2022, 23, 344–349. [Google Scholar]
- Yuan, F.; Zhang, H.N.; Li, H.; Dong, J.H.; Xiong, H.H.; Xu, A.J. Recovery rates of iron, nickel, and chromium via iron-bath reduction of stainless steel dust briquettes based on corundum crucible erosion balance analysis. J. Iron Steel Res. Int. 2018, 25, 320–329. [Google Scholar] [CrossRef]
- Lin, X.L.; Peng, Z.W.; Yan, J.X.; Li, Z.X.; Hwang, J.Y.; Zhang, Y.B.; Li, G.G.; Jiang, T. Pyrometallurgical recycling of electric arc furnace dust. J. Clean. Prod. 2017, 149, 1079–1100. [Google Scholar] [CrossRef]
- Simonyan, L.M.; Zhuravleva, O.E.; Khil’Ko, A. The use of Plasma-arc for extraction of zinc and lead from the steelmaking dust. J. Chem. Sci. Technol. 2015, 4, 1–7. [Google Scholar]
- Liu, P.J.; Liu, Z.G.; Chu, M.S.; Tang, J.; Gao, L.H.; Yan, R.J. Green and efficient utilization of stainless steel dust by direct reduction and self-pulverization. J. Hazard. Mater. 2021, 413, 125403. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Guo, W.M.; Jia, X.L. Reduction of chromium oxides in stainless steel dust. Int. J. Miner. Metall. Mater. 2015, 22, 573–581. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhang, X.; Ma, G.J.; Zheng, D.L.; He, R.X.; Du, T.Y. Effect of impurity components in stainless steel dust on the coloring properties of the prepared black ceramic pigments. J. Ceram. Process. Res. 2023, 24, 1–7. [Google Scholar]
- Ma, G.J.; Garbers-Craig, A.M. Stabilisation of Cr(VI) in stainless steel plant dust through sintering using silica-rich clay. J. Hazard. Mater. 2009, 169, 210–216. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Y.L.; Zhao, Z.; Yuan, F. Effects of Fe2O3 on reduction process of Cr-containing solid waste self-reduction briquette and relevant mechanism. Metals 2019, 9, 51. [Google Scholar] [CrossRef]
- Zhang, H.N.; Hui, L.; Dong, J.H.; Xiong, H.H. Optimization of the stainless steel dust briquette reduction process for iron, chromium, and nickel recovery. High Temp. Mater. Process. 2018, 37, 785–791. [Google Scholar] [CrossRef]
- Kukurugya, F.; Orac, D.; Takacova, Z.; Vindt, T.; Miskufova, A.; Havlik, T.; Kekki, A.; Aromaa, J.; Forsen, O. Chemical and structural characterization of steelmaking dust from stainless steel production. Proc. EMC 2011, 4, 1171–1183. [Google Scholar]
- Peng, B.; Peng, J. Physical and chemical characteristics of dust form electric arc furnace stainless steelmaking and mechanism of its formation. J. N. China Univ. Technol. 2003, 15, 34–40. (In Chinese) [Google Scholar]
- Lobel, J.; Peng, B.; Kozinski, J.; Bourassa, M. Pilot-scale direct recycling of flue dust generated in electric stainless steelmaking. Iron Steelmak. 2000, 27, 41–45. [Google Scholar]
- Ri, S.C.; Chu, M.S.; Chen, S.Y.; Liu, Z.G.; Hong, H. Self-reduction mechanism of coal composite stainless steel dust hot briquette. J. Iron Steel Res. Int. 2016, 23, 314–321. [Google Scholar] [CrossRef]
- Kim, G.; Sohn, I. Selective metal cation concentration during the solidification of stainless steel EAF dust and slag mixtures from high temperatures for increased Cr recovery. J. Hazard. Mater. 2018, 359, 174–185. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Li, Q.J.; Yang, F.X.; Zhang, J.X.; Lu, X.G. Experimental study on stainless steel dust by reduction and enrichment for preparation raw material of powder metallurgy. Trans. Indian Inst. Met. 2020, 74, 119–127. [Google Scholar] [CrossRef]
- Ma, G.J. Cr (VI)-Containing Electri Furnace Dust and Filter Cake-Characteristics, Formation, Leachability and Stabilisation. Doctoral Dissertation, University of Pretoria, Pretoria, South Africa, 2006. [Google Scholar]
- Laforest, G.; Duchesne, J. Characterization and leachability of electric arc furnace dust made from remelting of stainless steel. J. Hazard. Mater. 2006, 135, 156–164. [Google Scholar] [CrossRef]
- Sofilic, T.; Rastovcan-Mioc, A.; Cerjan-Stefanovic, S.; Novosel-Radovic, V.; Jenko, M. Characterization of steel mill electric-arc furnace dust. J. Hazard. Mater. 2004, B109, 59–70. [Google Scholar] [CrossRef]
- Bulut, U.; Ozverdi, A.; Erdem, M. Leaching behavior of pollutants in ferrochrome arc furnace dust and its stabilization/solidification using ferrous sulphate and Portland cement. J. Hazard. Mater. 2009, 162, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Giesekke, E.; Smit, J.; Viljoen, E.; Kruger, A.; Kruger, S.; Maine, C. Evaluation of solid-stabilized products made from Cr (VI)-containing ferrochrome bag-filter dust. In Waste Materials in Construction; Woolley, G.R., Goumans, J.J.J.M., Wainwrigh, P.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 1, pp. 140–150. [Google Scholar]
- Mulange Wa Mulange, D.; Garbers-Craig, A.M. Stabilization of Cr(VI) from fine ferrochrome dust using exfoliated vermiculite. J. Hazard. Mater. 2012, 223–224, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.K.; Patro, S.K. Use of ferrochrome ash (FCA) and lime dust in concrete preparation. J. Clean. Prod. 2016, 131, 237–246. [Google Scholar] [CrossRef]
- Lind, B.B.; FaÈllman, A.M.; Larsson, L.B. Environmental impact of ferrochrome slag in road construction. Waste Manag. 2001, 21, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Das, B. Characterisation and beneficiation studies of charge chrome slag. Scand. J. Metall. 1997, 26, 153–157. [Google Scholar]
- Rosales, J.; Agrela, F.; Diaz-Lopez, J.L.; Cabrera, M. Alkali-activated stainless steel slag as a cementitious material in the manufacture of self-compacting concrete. Materials 2021, 14, 3945. [Google Scholar] [CrossRef] [PubMed]
- Sheen, Y.N.; Wang, H.Y.; Sun, T.H. A study of engineering properties of cement mortar with stainless steel oxidizing slag and reducing slag resource materials. Constr. Build. Mater. 2013, 40, 239–245. [Google Scholar] [CrossRef]
- Kamon, M.; Nontananandh, S.; Katsumi, T. Utilization of stainless-steel slag by cement hardening. J. Jpn. Soc. Soil Mech. Found. Eng. 1993, 33, 118–129. [Google Scholar] [CrossRef]
- Salman, M.; Cizer, Ö.; Pontikes, Y.; Santos, R.M.; Snellings, R.; Vandewalle, L.; Blanpain, B.; Van Balen, K. Effect of accelerated carbonation on AOD stainless steel slag for its valorisation as a CO2-sequestering construction material. Chem. Eng. J. 2014, 246, 39–52. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zeng, Y.N.; Li, J.G.; Zhang, Y.Z.; Wang, W. Properties of ten-year-aged argon oxygen decarburization stainless steel slag. J. Iron Steel Res. Int. 2021, 28, 1233–1242. [Google Scholar] [CrossRef]
- Zhao, H.Q.; Qi, Y.H.; Shi, Y.L.; Na, X.Z.; Feng, H.L. Mechanism and prevention of disintegration of AOD stainless steel slag. J. Iron Steel Res. Int. 2013, 20, 26–30. [Google Scholar] [CrossRef]
- Tao, M.J.; Wang, Y.J.; Li, J.G.; Zeng, Y.N.; Liu, S.H.; Qin, S. Slurry-phase carbonation reaction characteristics of AOD stainless steel slag. Processes 2021, 9, 2266. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zeng, Y.N.; Li, J.G.; Zhang, Y.Z. Cementitious behavior of argon oxygen decarburization stainless steel slag and its stabilization on chromium. Crystals 2020, 10, 876. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, J.L.; Yu, Y.; Zhu, H.Y. Occurrence and leaching behavior of chromium in synthetic stainless steel slag containing FetO. Minerals 2021, 11, 1055. [Google Scholar] [CrossRef]
- Wang, Y.J.; Tao, M.J.; Li, J.G.; Zeng, Y.N.; Qin, S.; Liu, S.H. Carbonation of EAF stainless steel slag and its effect on chromium leaching characteristics. Crystals 2021, 11, 1498. [Google Scholar] [CrossRef]
- Santos, R.M.; Van Bouwel, J.; Vandevelde, E.; Mertens, G.; Elsen, J.; Van Gerven, T. Accelerated mineral carbonation of stainless steel slags for CO2 storage and waste valorization: Effect of process parameters on geochemical properties. Int. J. Greenh. Gas Control 2013, 17, 32–45. [Google Scholar] [CrossRef]
- Liu, M.K.; Ma, G.J.; Zhang, X.; Liu, J.J.; Wang, Q. Preparation of black ceramic tiles using waste copper slag and stainless steel slag of electric arc furnace. Materials 2020, 13, 776. [Google Scholar] [CrossRef]
- Adegoloye, G.; Beaucour, A.L.; Ortola, S.; Noumowe, A. Mineralogical composition of EAF slag and stabilised AOD slag aggregates and dimensional stability of slag aggregate concretes. Constr. Build. Mater. 2016, 115, 171–178. [Google Scholar] [CrossRef]
- Adegoloye, G.; Beaucour, A.L.; Ortola, S.; Noumowé, A. Concretes made of EAF slag and AOD slag aggregates from stainless steel process: Mechanical properties and durability. Constr. Build. Mater. 2015, 76, 313–321. [Google Scholar] [CrossRef]
- Miao, X.W.; Bai, Z.T.; Lu, G.H.; Liu, L.; Guo, M.; Cheng, F.Q.; Zhang, M. Review of comprehensive utilization of typical ferroalloy slags. Chin. J. Eng. 2020, 42, 663–679. (In Chinese) [Google Scholar]
- Jena, S.; Panigrahi, R. Performance assessment of geopolymer concrete with partial replacement of ferrochrome slag as coarse aggregate. Constr. Build. Mater. 2019, 220, 525–537. [Google Scholar] [CrossRef]
- Karhu, M.; Talling, B.; Piotrowska, P.; Matas Adams, A.; Sengottuvelan, A.; Huttunen-Saarivirta, E.; Boccaccini, A.R.; Lintunen, P. Ferrochrome slag feasibility as a raw material in refractories: Evaluation of thermo-physical and high temperature mechanical properties. Waste Biomass Valorization 2020, 11, 7147–7157. [Google Scholar] [CrossRef]
- Bryce-Smith, D. Chromium in the natural and human environments. Endeavour 1989, 13, 45. [Google Scholar] [CrossRef]
- Ma, G.J.; Garbers-Craig, A.M. Cr(VI) containing electric furnace dusts and filter cake from a stainless steel waste treatment plant: Part 2—Formation mechanisms and leachability. Ironmak. Steelmak. 2006, 33, 238–244. [Google Scholar] [CrossRef]
- Zhou, X.T.; Hao, X.T.; Ma, Q.M.; Luo, Z.Q.; Zhang, M.Q.; Peng, J.H. Effects of compound chemical activators on the hydration of low-carbon ferrochrome slag-based composite cement. J. Environ. Manag. 2017, 191, 58–65. [Google Scholar] [CrossRef]
- Bai, Z.T.; Qiu, G.B.; Peng, B.; Guo, M.; Zhang, M. Synthesis and characterization of glass-ceramics prepared from high-carbon ferrochromium slag. RSC Adv. 2016, 6, 52715–52723. [Google Scholar] [CrossRef]
- Hao, X.T. Preparation of Low Temperature Ceramic Cementitious Materials by Activation of Ferrochrome Slag. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2016. (In Chinese). [Google Scholar]
- Cabrera-Real, H.; Romero-Serrano, A.; Zeifert, B.; Hernandez-Ramirez, A.; Hallen-Lopez, M.; Cruz-Ramirez, A. Effect of MgO and CaO/SiO2 on the immobilization of chromium in synthetic slags. J. Mater. Cycles Waste Manag. 2012, 14, 317–324. [Google Scholar] [CrossRef]
- Li, X.M.; Zhao, J.X.; Cui, Y.R.; Yang, J. The comprehensive utilization of EAF dust and pickling sludge of stainless steel works. Mater. Sci. Forum 2009, 620–622, 603–606. [Google Scholar] [CrossRef]
- Doronin, I.; Svyazhin, A. Commercial methods of recycling dust from steelmaking. Metallurgist 2011, 54, 673–681. [Google Scholar] [CrossRef]
- Aromaa, J.; Kekki, A.; Stefanova, A.; Makkonen, H.; Forsén, O. New hydrometallurgical approaches for stainless steel dust treatment. Miner. Process. Extr. Metall. 2016, 125, 242–252. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Cui, K.; Fu, T.; Gao, J.; Hussain, S.; AlGarni, T.S. Pyrometallurgical recovery of zinc and valuable metals from electric arc furnace dust—A review. J. Clean. Prod. 2021, 298, 126788. [Google Scholar] [CrossRef]
- Yakornov, S.A.; Pan’shin, A.M.; Kozlov, P.A.; Ivakin, D.A. Development of charge pelletizing technology based on electric arc furnace dust for pyrometallurgical processing in Rotary Kilns. Metallurgist 2017, 61, 529–534. [Google Scholar] [CrossRef]
- Hara, Y.; Ishiwata, N.; Itaya, H.; Matsumoto, T. Smelting reduction process with a coke packed bed for steelmaking dust recycling. ISIJ Int. 2000, 40, 231–237. [Google Scholar] [CrossRef]
- Shinji, H.; Haruo, K.; Yoshiaki, H. Development of a smelting reduction process for recycling steelmaking dust. Kawasaki Steel Tech. Rep. 1998, 38, 31–37. [Google Scholar]
- Von Billerbeck, E.; Ruh, A.; Kim, D.S. Verarbeitung von filterstäuben aus der elektrostahlerzeugung im wälzprozess. Miner. Nebenprodukte Und Abfälle. Neuruppin TK Verl. Karl J. Thomé-Kozmiensky 2014, 1, 387–398. [Google Scholar]
- Hanewald, R.H.; Dombrowski, D.E. Recovery of metals from steel wastes and production of DRI by the INMETCO process. Iron Steel Eng. 1985, 62, 62–67. [Google Scholar]
- Money, K.; Hanewald, R.; Bleakney, R. Processing Steel Waste Pyrometallurgically at INMETCO. In Proceedings of the 4th International Symposium on Recycling of Metals and Engineered Materials, Pittsburgh, PA, USA, 22–25 October 2000; pp. 397–408. [Google Scholar]
- Gomez, E.; Rani, D.A.; Cheeseman, C.R.; Deegan, D.; Wise, M.; Boccaccini, A.R. Thermal plasma technology for the treatment of wastes: A critical review. J. Hazard. Mater. 2009, 161, 614–626. [Google Scholar] [CrossRef]
- Dutra, A.J.B.; Paiva, P.R.P.; Tavares, L.M. Alkaline leaching of zinc from electric arc furnace steel dust. Miner. Eng. 2006, 19, 478–485. [Google Scholar] [CrossRef]
- Olper, M.; Maccagni, M. Electrolytic Zinc Production from Crude Zinc Oxides with the Ezinex® Process; Recycling of Metals and Engineercd Materials: Warrendale, PA, USA, 2000; pp. 379–397. [Google Scholar]
- Zunkel, A.D. Recovering Zinc and Lead from Electric Arc Furnace Dust: A Technology Status Report; Recycling of Metals and Engineercd Materials: Warrendale, PA, USA, 2000; pp. 227–236. [Google Scholar]
- Nakamura, T.; Shibata, E.; Takasu, T.; Itou, H. Basic consideration on EAF dust treatment using hydrometallurgical processes. Resour. Process. 2008, 55, 46–53. [Google Scholar] [CrossRef]
- Strobos, J.G.; Friend, J.F.C. Zinc recovery from baghouse dust generated at ferrochrome foundries. Hydrometallurgy 2004, 74, 165–171. [Google Scholar] [CrossRef]
- Chen, K.; Li, C.; Gu, J.Q.; He, X.F.; Tian, Z.Q. Experimental studv on resource utilization of chromium in ferrochrome dust. Sichuan Environ. 2022, 41, 1–4. (In Chinese) [Google Scholar]
- Adamczyk, B.; Brenneis, R.; Adam, C.; Mudersbach, D. Recovery of chromium from AOD-converter slags. Steel Res. Int. 2010, 81, 1078–1083. [Google Scholar] [CrossRef]
- Takamitsu, N.; Kunihiko, N.; Katsumi, M. Recovery rate of chromium from stainless slag by iron melts. ISIJ Int. 2007, 44, 665–672. [Google Scholar]
- Kim, E.; Spooren, J.; Broos, K.; Nielsen, P.; Horckmans, L.; Vrancken, K.C.; Quaghebeur, M. New method for selective Cr recovery from stainless steel slag by NaOCl assisted alkaline leaching and consecutive BaCrO4 precipitation. Chem. Eng. J. 2016, 295, 542–551. [Google Scholar] [CrossRef]
- Bai, Z.T.; Zhang, Z.A.; Guo, M.; Hou, X.M.; Zhang, M. Magnetic separation and extraction chrome from high carbon ferrochrome slag. Mater. Res. Innov. 2015, 19, S2-113–S2-118. [Google Scholar] [CrossRef]
- Qiu, W.J.; Gu, Y. Selection of jigging system for ferrochromium in recovered slag. Ferro-Alloys 1988, 39–42. (In Chinese) [Google Scholar]
- Sripriya, R.; Murty, C.V.G.K. Recovery of metal from slag/mixed metal generated in ferroalloy plants-a case study. Int. J. Miner. Process. 2005, 75, 123–134. [Google Scholar] [CrossRef]
- Yao, Z.; Qu, Y.; Luo, H.J.; Sun, X.; Li, J.Z.; Gong, X.Q. Jigging-remelting mothod to recover alloys contained inferrochrome slag. J. Mater. Metall. 2022, 21, 402–407. (In Chinese) [Google Scholar]
- Li, Z.Q.; Zhang, X.; Ma, G.J.; Zheng, D.L.; Xu, J.; Xu, J. Effect of the Fe/Cr molar ratio and calcination temperature on the preparation of black ceramic pigment with stainless steel dust assisted by microwave processing. J. Clean. Prod. 2022, 372, 133751. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhang, X.; Ma, G.J.; Zheng, D.L.; Du, T.Y.; He, R.X. Effect of the nickel molar content on the preparation and properties of spinel-type black ceramic pigment by microwave processing from stainless steelmaking dust. Mater. Today Commun. 2022, 32, 104151. [Google Scholar] [CrossRef]
- Liu, M.K.; Ma, G.J.; Zhang, X.; Zheng, D.L.; Li, Z.Q. Preparation and coloring mechanism of cobalt-free black ceramic pigments from stainless steelmaking dust. Mater. Today Commun. 2022, 33, 104609. [Google Scholar] [CrossRef]
- Rives, V.; Pérez-Bernal, M.E.; Ruano-Casero, R.J.; Nebot-Díaz, I. Development of a black ceramic pigment from non stoichiometric hydrotalcites. J. Eur. Ceram. Soc. 2012, 32, 975–987. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2023.
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef]
- Salman, M.; Dubois, M.; Maria, A.D.; Van Acker, K.; Van Balen, K. Construction materials from stainless steel slags: Technical aspects, environmental benefits, and economic opportunities. J. Ind. Ecol. 2016, 20, 854–866. [Google Scholar] [CrossRef]
- Iacobescu, R.I.; Angelopoulos, G.N.; Jones, P.T.; Blanpain, B.; Pontikes, Y. Ladle metallurgy stainless steel slag as a raw material in Ordinary Portland Cement production: A possibility for industrial symbiosis. J. Clean. Prod. 2016, 112, 872–881. [Google Scholar] [CrossRef]
- Rosales, J.; Agrela, F.; Entrenas, J.A.; Cabrera, M. Potential of stainless steel slag waste in manufacturing self-compacting concrete. Materials 2020, 13, 2049. [Google Scholar] [CrossRef]
- Zhang, S.H.; Liu, L.B.; Tan, K.F.; Zhang, L.H.; Tang, K.J. Influence of burning temperature and cooling methods on strength of high carbon ferrochrome slag lightweight aggregate. Constr. Build. Mater. 2015, 93, 1180–1187. [Google Scholar] [CrossRef]
- Zuginisov, M.T.; Myrzahmetov, M.M.; Sartayev, D.T.; Orynbekov, E.S. Heat-resistant ferrochrome slag based concrete. Mag. Civ. Eng. 2014, 51, 38–45. [Google Scholar] [CrossRef]
- Jena, S.; Panigrahi, R. Feasibility study of the properties of geopolymer concrete with ferrochrome slag and silica fume. Mater. Today Proc. 2021, 38, 2476–2480. [Google Scholar] [CrossRef]
- Islam, M.Z.; Sohel, K.M.A.; Al-Jabri, K.; Al Harthy, A. Properties of concrete with ferrochrome slag as a fine aggregate at elevated temperatures. Case Stud. Constr. Mater. 2021, 15, e00599. [Google Scholar] [CrossRef]
- Zhu, X.P.; Huo, Y.D.; Zhao, R.M.; Wu, H.; Li, F.B.; Sun, S.C.; Liu, C. One-step microcrystalline glass preparation using smelting slag from waste automobile three-way catalysts through iron collection. Appl. Sci. 2022, 12, 11723. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhao, W.G.; Li, F.L.; Duan, H.J.; Zhang, S.W. Preparation and erosion resistance of CaO-Al2O3-MgO-SiO2 microcrystalline glass ceramics. Rare Met. Mater. Eng. 2015, 44, 277–280. [Google Scholar]
- OuYang, S.L.; Zhang, Y.X.; Chen, Y.X.; Zhao, Z.W.; Wen, M.; Li, B.W.; Shi, Y.; Zhang, M.Z.; Liu, S.L. Preparation of glass-ceramics using chromium-containing stainless steel slag: Crystal structure and solidification of heavy metal chromium. Sci. Rep. 2019, 9, 1964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Y.L.; Qu, Z.M. Physicochemical property and chromium leaching behavior in different environments of glass ceramics prepared from AOD stainless steel slag. J. Alloys Compd. 2019, 805, 1106–1116. [Google Scholar] [CrossRef]
- Deng, L.B.; Yun, F.; Jia, R.D.; Li, H.; Jia, X.L.; Shi, Y.; Zhang, X.F. Effect of SiO2/MgO ratio on the crystallization behavior, structure, and properties of wollastonite-augite glass-ceramics derived from stainless steel slag. Mater. Chem. Phys. 2020, 239, 122039. [Google Scholar] [CrossRef]
- Deng, L.B.; Wang, S.; Zhang, Z.; Li, Z.H.; Jia, R.D.; Yun, F.; Li, H.; Ma, Y.H.; Wang, W.C. The viscosity and conductivity of the molten glass and crystallization behavior of the glass ceramics derived from stainless steel slag. Mater. Chem. Phys. 2020, 251, 123159. [Google Scholar] [CrossRef]
- Wang, R.X. Research on Preparation of Glass-Ceramics from Ferrochromium Slag by Sintering. Master’s Thesis, Inner Mongolia University of Science and Technology, Baotou, China, 2022. (In Chinese). [Google Scholar]
- Wang, J.Q.; Han, F.L.; Xin, Z.B.; Yang, B.G.; Zheng, B. Effect of ferrochromium slag doping content on the structure and properties of glass-ceramics from electrolytic manganese slag. China Ceram. 2022, 58, 46–52. (In Chinese) [Google Scholar]
- Sahu, N.; Biswas, A.; Kapure, G.U. Development of refractory material from water quenched granulated ferrochromium slag. Miner. Process. Extr. Metall. Rev. 2016, 37, 255–263. [Google Scholar] [CrossRef]
- Feng, Z.C.; Zhao, Z.H.; Han, H.S.; Li, Y.Y.; Ma, J.H.; Yang, Y. Study on the properties of forsterite-spinel composite materials prepared by high carbon ferrochrome slag. Refractories 2021, 55, 491–497. (In Chinese) [Google Scholar]
- Li, Z.J.; Sun, J.L. The refractory material for ferromanganese ladle lining is made by using carbon ferrochrome slag. Refractories 1999, 33, 37–38, 45. (In Chinese) [Google Scholar]
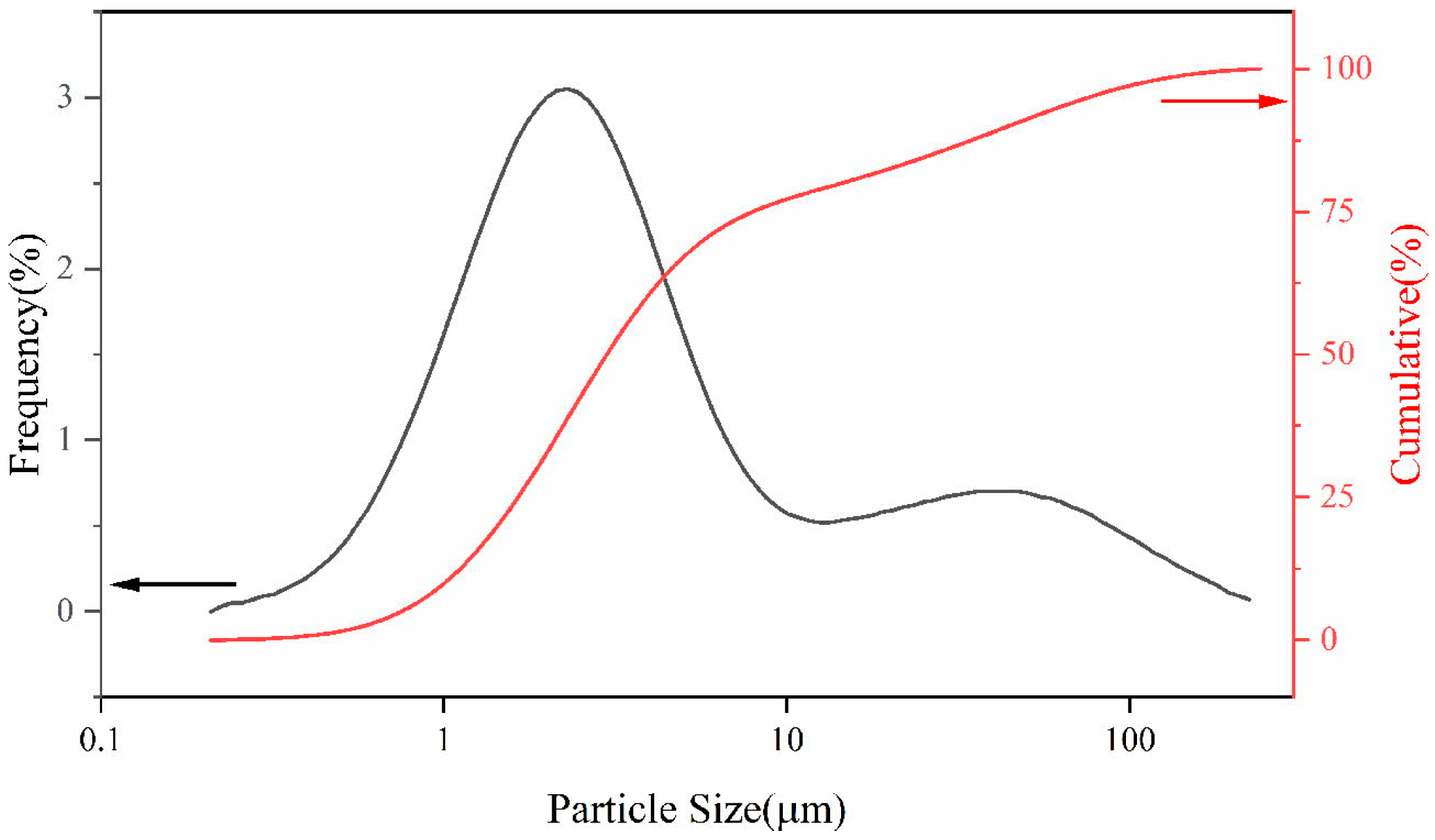
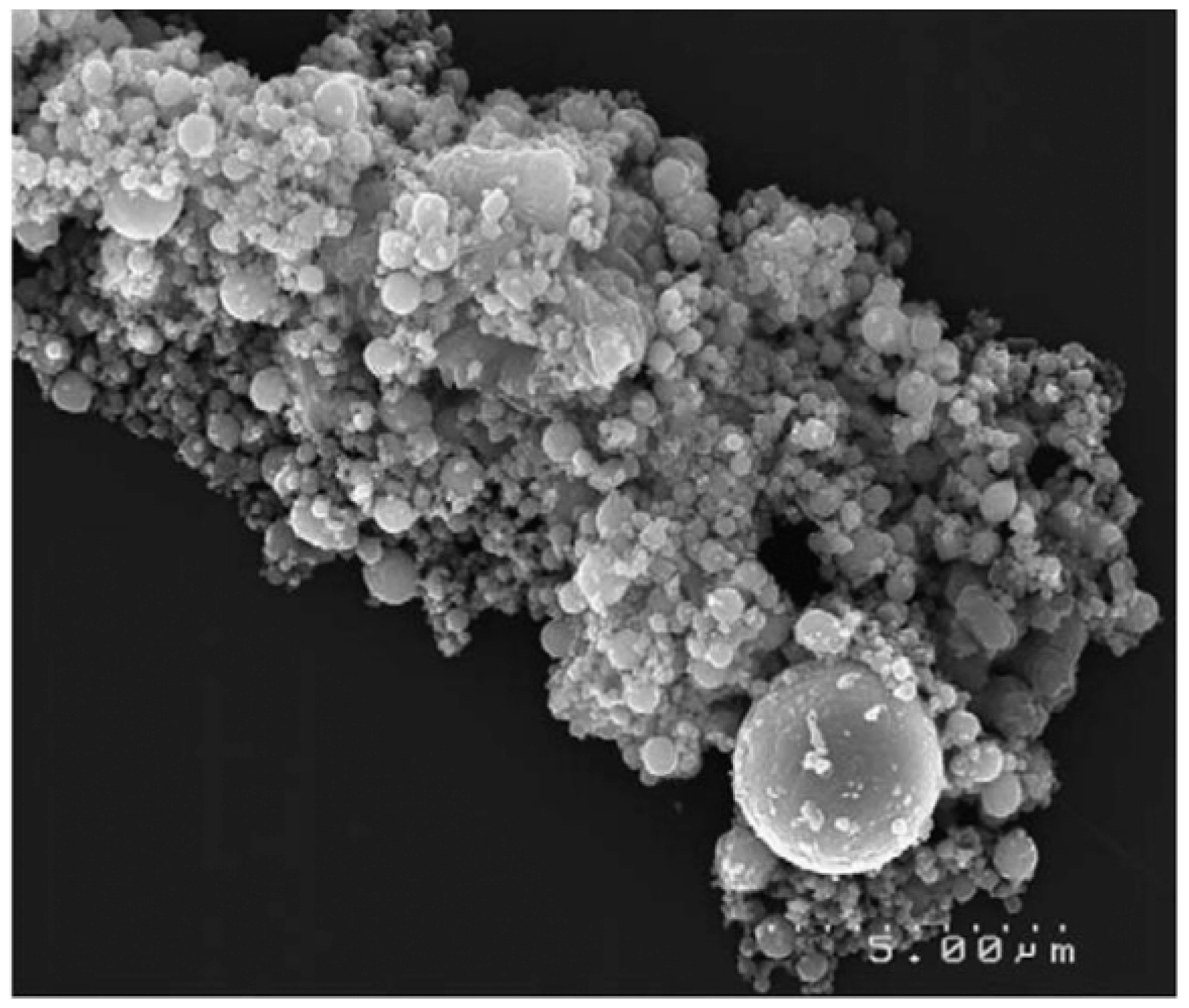
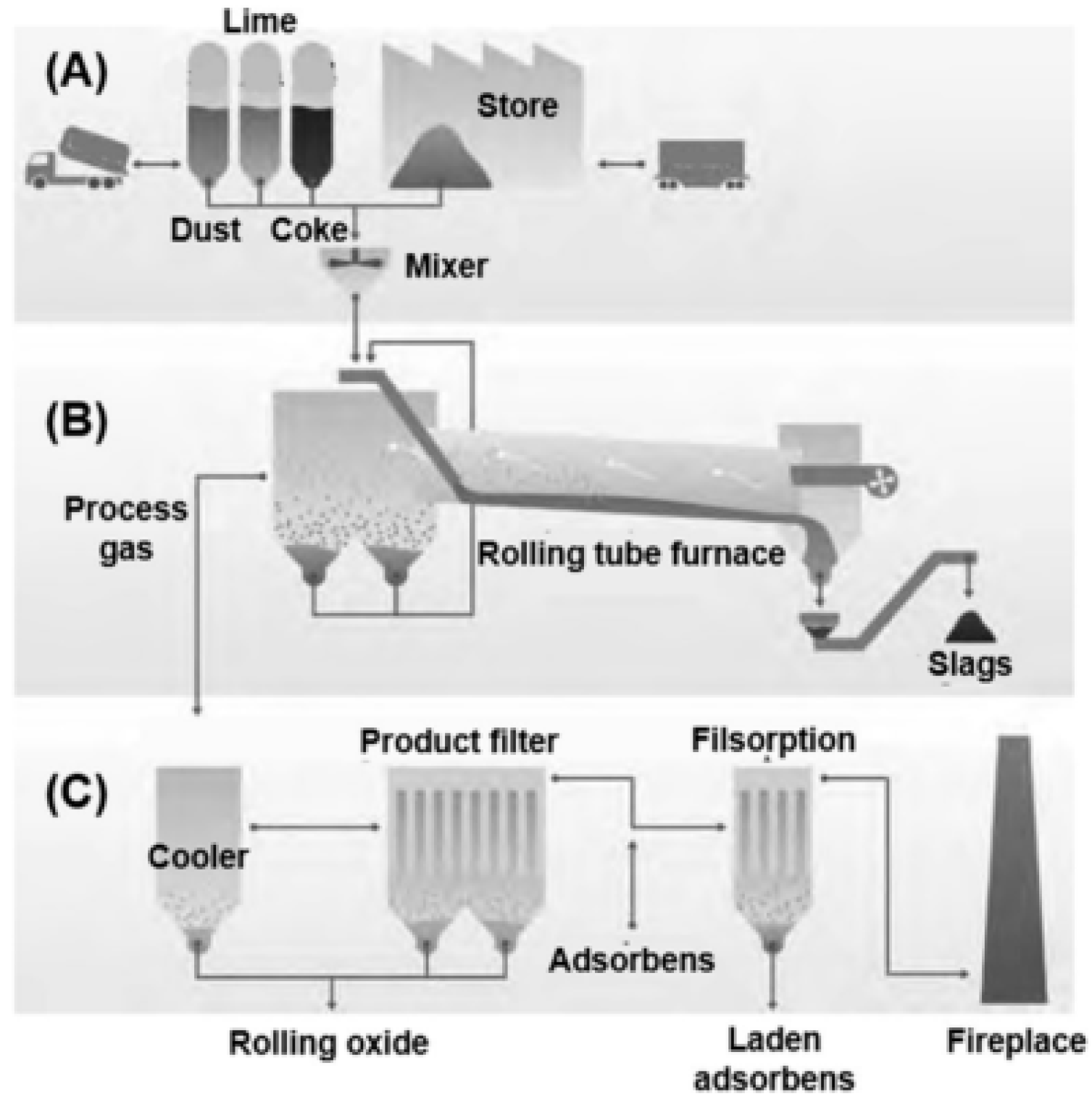

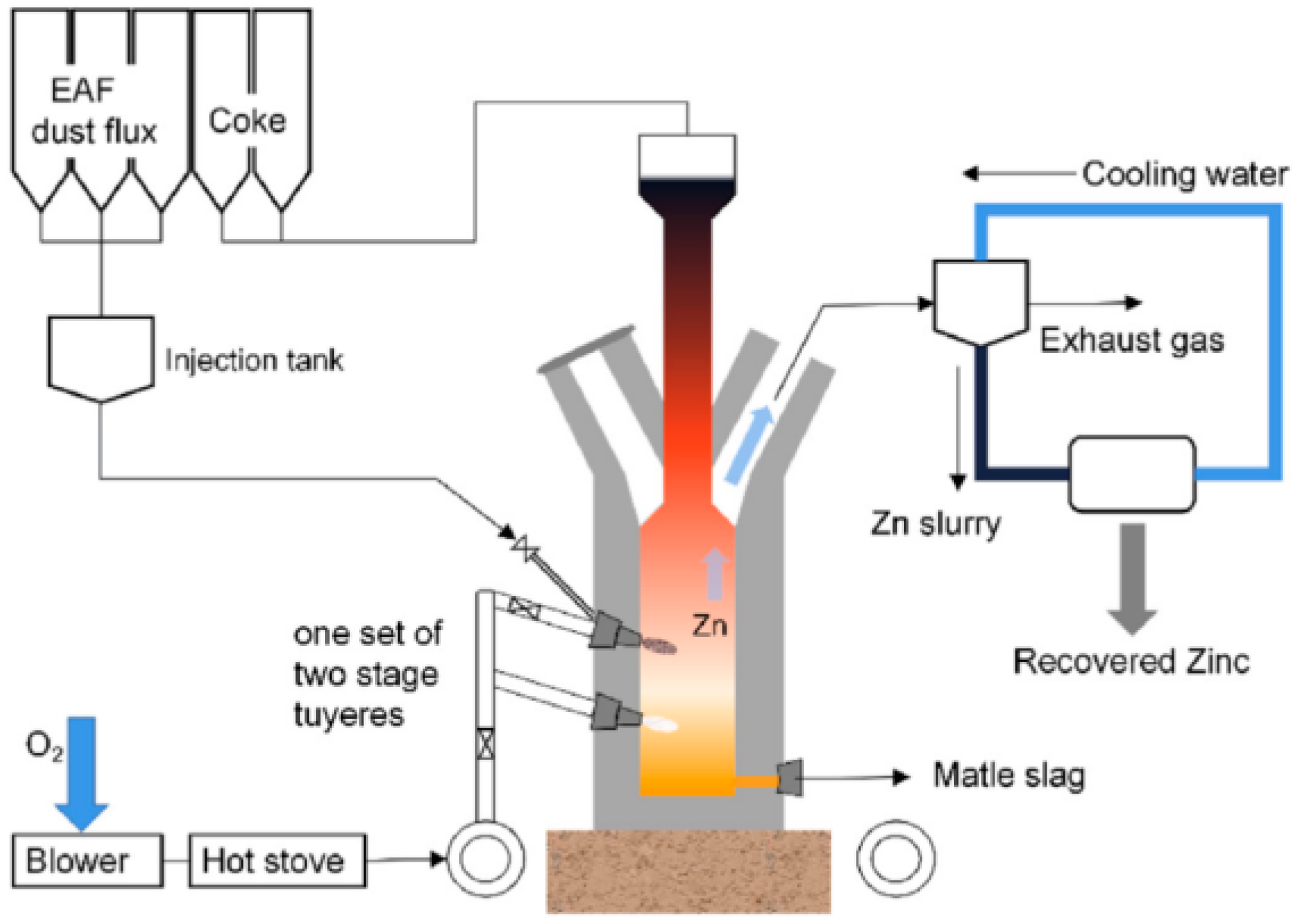





| Elements | Contents (%) | Phase Containing This Element |
|---|---|---|
| Fe | 14.77~53.50 | Fe2O3, Fe3O4, and spinel. |
| Cr | 0.28~35.80 | Spinel, Cr2O3, CrO, and CrCO3. |
| Ni | 0.04~5.42 | Nickel oxide and spinel. |
| Zn | 0.04~12.73 | Zinc, basic zinc chloride, and zinc chloride. |
| Si | 0.09~4.51 | Silicon dioxide, iron olivine, and silicon carbide. |
| Al | 0.16~0.81 | Aluminum oxides and spinel. |
| Mg | 0.04~10.20 | Spinel and magnesium oxide. |
| Ca | 0.83~14.78 | Calcium oxide, fluorite, and limestone. |
| Cr(VI) | 0.14~0.60 | Calcium chromate, CrO3, (K,Na)2Cr2O7, or (K,Na)2CrO4 |
| Ferrochrome Dust | Grain Size d50 (μm) | Moisture (%) | Specific Surface Area (m2/g) | Stack Density (g/cm3) | Water-Soluble Components (%) | pH |
|---|---|---|---|---|---|---|
| Fine dust | 0.71~13.23 | 0.93~1.06 | 5.31~13.2 | 0.49~0.93 | 3.34~11.86 | 8.08~8.48 |
| Coarse dust | 79.76 | 0.48~0.73 | 2.7~3.32 | 1.65~2.11 | 0.30 | 11.18 |
| Element | Content(%) | Phase | ||
|---|---|---|---|---|
| Coarse Ferrochrome Dust | Fine Ferrochrome Dust | Coarse Ferrochrome Dust | Fine Ferrochrome Dust | |
| Cr | 13.14~17.11 | 1.92~7.4 | Chromium spinel | Chromium spinel and FeCr |
| Si | 9.15~13.86 | 16.45~34.2 | Quartz and calcium feldspar | Quartz, magnesia olivine, Mg3Al2Si3O12, and Al2SiO5 |
| Al | 5.61~6.64 | 1.06~5.62 | Chromium spinel and calcium feldspar | Chromium spinel |
| Ca | 0.71~1.72 | 0.14~0.57 | Dolomite and calcium feldspar | - |
| Zn | 0.59~0.64 | 1.37~12.13 | - | ZnO, NaZn4(SO4)Cl(OH)6·6H2O, and Zn4SO4(OH)6·5H2O |
| Fe | 5.37~10.58 | 0.61~3.01 | Chromium spinel and FeCr | Chromium spinel and FeCr |
| Mn | 0.11~0.18 | 0.23~0.58 | - | - |
| Mg | 4.14~7.16 | 1.01~13.92 | Dolomite and chrome spinel | Magnesia olivine, MgO, and aluminum |
| S | 0.28~0.76 | 0.96~3.4 | - | NaZn4(SO4)Cl(OH)6·6H2O and Zn4SO4(OH)6·5H2O |
| Cl | 0.89 | 0.95~3.32 | - | NaCl and NaZn4(SO4)Cl(OH)6·6H2O |
| Na | 1.32~1.89 | 1.71~5.94 | - | NaCl and NaZn4(SO4)Cl(OH)6·6H2O |
| K | 0.84~0.91 | 1.0~7.58 | - | - |
| C | 9.97~15.5 | 1.1~1.58 | Coal, coke, and charcoal | Coal, coke, and charcoal |
| Ga | 0.015 | 0.026~0.39 | - | - |
| Stainless Steel Slag | CaO + MgO | SiO2 | MnO | Al2O3 | FeO | Cr2O3 | P2O5 | Ni |
|---|---|---|---|---|---|---|---|---|
| EAF slag | 40~60 | 20~30 | 2~3 | 3~10 | 0.5~22 | 2~10 | 2~5 | <0.1 |
| AOD slag | 60~70 | 20~30 | <2 | 1~5 | <2 | <1 | - | <0.1 |
| Stainless Steel Slag | Major Minerals | Secondary Minerals | Trace Minerals |
|---|---|---|---|
| EAF slag | Ca2SiO4 and Ca3Mg(SiO4)2 | Ca2MgSi2O7 and Ni-Fe-Cr alloy | Chromium spinel, Fe3O4, Cr2O3, and RO |
| AOD slag | Ca2SiO4 | CaF2, CaCO3, Ca(OH)2, Ca4Si2O7F2, and magnesium silica calcium stone | MgCr2O4, Ca3Mg(SiO4)2, and FeCr2O4 |
| Type | Main Chemical Composition | Main Phases | |||||
|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Cr2O3 | Al2O3 | MgO | Fe2O3 | ||
| High-carbon ferrochrome slag | 0.5~4.8 | 28.6~37 | 1.8~8.73 | 16~32 | 29.2~35 | 0.8~4.0 | MgAl2O4, forsterite, glass phase, metal beads, monticellite, and chromium spinel |
| Low-carbon ferrochrome slag | 20.02~22.2 | 35.54~43.76 | 2.4~5.16 | 9.7~20.13 | 5.86~33.32 | 1.97~2.46 | 2CaO·SiO2, a small amount of 3CaO·MgO·2SiO2 and 2CaO·Al2O3·SiO2 |
| Method | Process Parameters | Recycled Metals | Advantages | Disadvantages |
|---|---|---|---|---|
| Waelz | Temperature at 1100~1200 °C and Carbon thermal reduction in rotary kilns | ZnO | Large processing capacity and mature technology | High cost, wide distribution of product demand sources, and high energy consumption |
| Inmetco | Rotary kilns and electric arc furnaces | Nickel, Chromium, etc. | Large amount of waste treated, short reduction time, wide range of applications, and high metal recovery ratio | Low energy efficiency and increased transportation processes |
| Plasma | Plasma furnace | Chromium, Iron, and Zn | The equipment covers a small area, has high efficiency, has short payback period, and can achieve the separation of different metals with low boiling points while reducing and recovering chromium and iron | Large power consumption, high quality requirements for reducing agents, large consumption of electrodes and refractory materials, and its products also need other equipment to carry out post-processing and other defects |
| Z-Star | Shaft furnace with coke-filled bed; Processing temperature above 1550 °C | Chromium, Iron, Nickel, Zn, and Pb | The slag-iron separation efficiency is high, and the raw material does not need to be blocked; almost all zinc and lead are recovered, and no secondary waste is discharged | Process heat consumption is large, a large amount of sensible heat of the furnace gas cannot be effectively recovered or utilized, and the top wall of the furnace is prone to zinc adherence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Liu, M.; Ma, G.; Zheng, D.; Zhang, X.; Hou, Y. Valuable Recovery Technology and Resource Utilization of Chromium-Containing Metallurgical Dust and Slag: A Review. Metals 2023, 13, 1768. https://doi.org/10.3390/met13101768
Xu J, Liu M, Ma G, Zheng D, Zhang X, Hou Y. Valuable Recovery Technology and Resource Utilization of Chromium-Containing Metallurgical Dust and Slag: A Review. Metals. 2023; 13(10):1768. https://doi.org/10.3390/met13101768
Chicago/Turabian StyleXu, Ju, Mengke Liu, Guojun Ma, Dingli Zheng, Xiang Zhang, and Yanglai Hou. 2023. "Valuable Recovery Technology and Resource Utilization of Chromium-Containing Metallurgical Dust and Slag: A Review" Metals 13, no. 10: 1768. https://doi.org/10.3390/met13101768
APA StyleXu, J., Liu, M., Ma, G., Zheng, D., Zhang, X., & Hou, Y. (2023). Valuable Recovery Technology and Resource Utilization of Chromium-Containing Metallurgical Dust and Slag: A Review. Metals, 13(10), 1768. https://doi.org/10.3390/met13101768







