Hydride-Induced Responses in the Mechanical Behavior of Zircaloy-4 Sheets
Abstract
1. Introduction
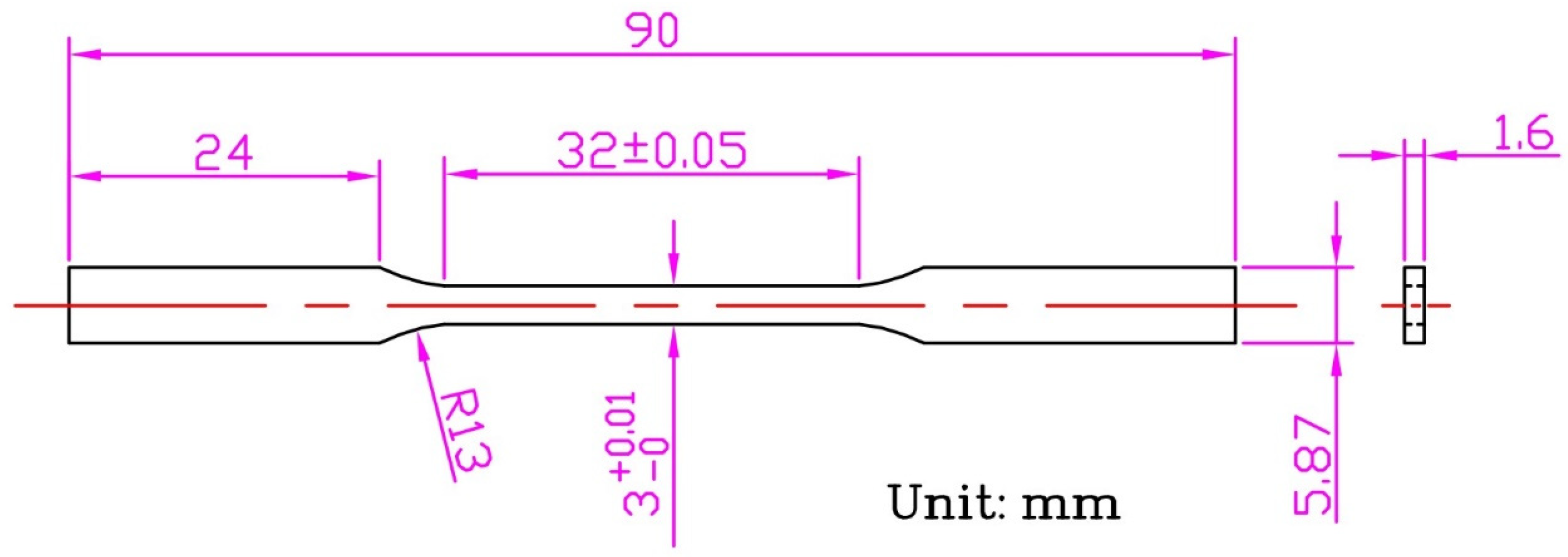
2. Materials and Methods
3. Results
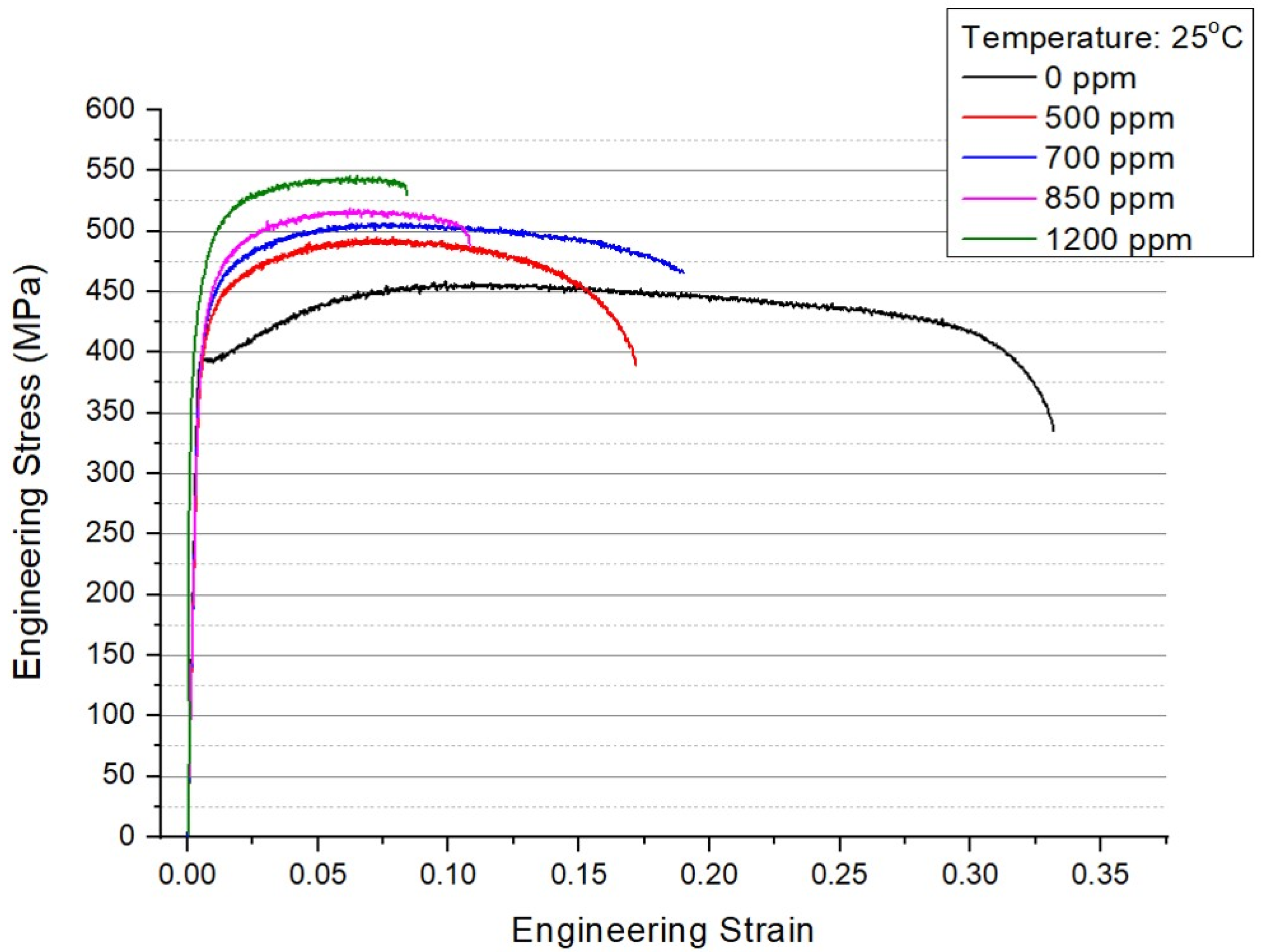
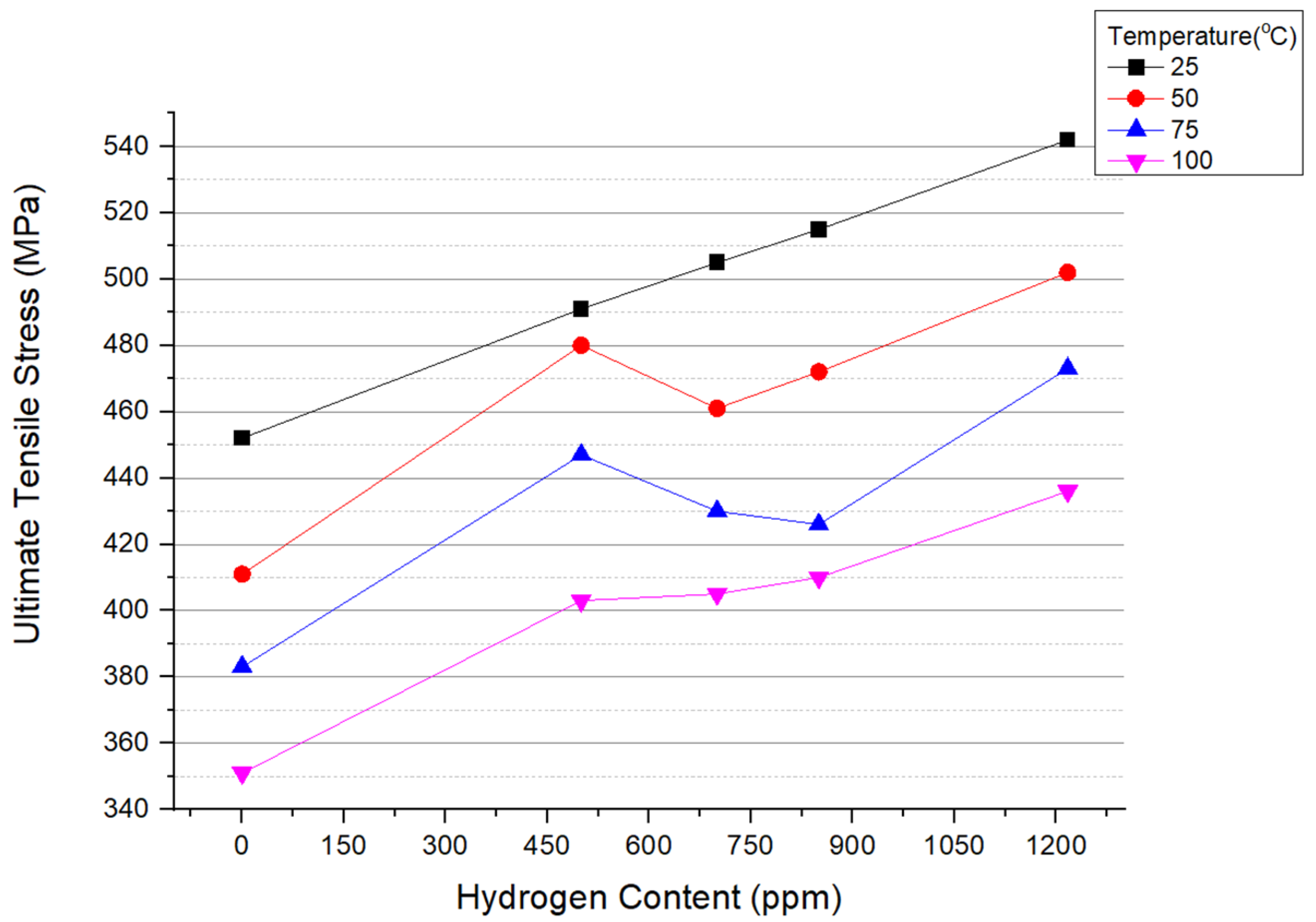
3.1. Ultimate Tensile Strength
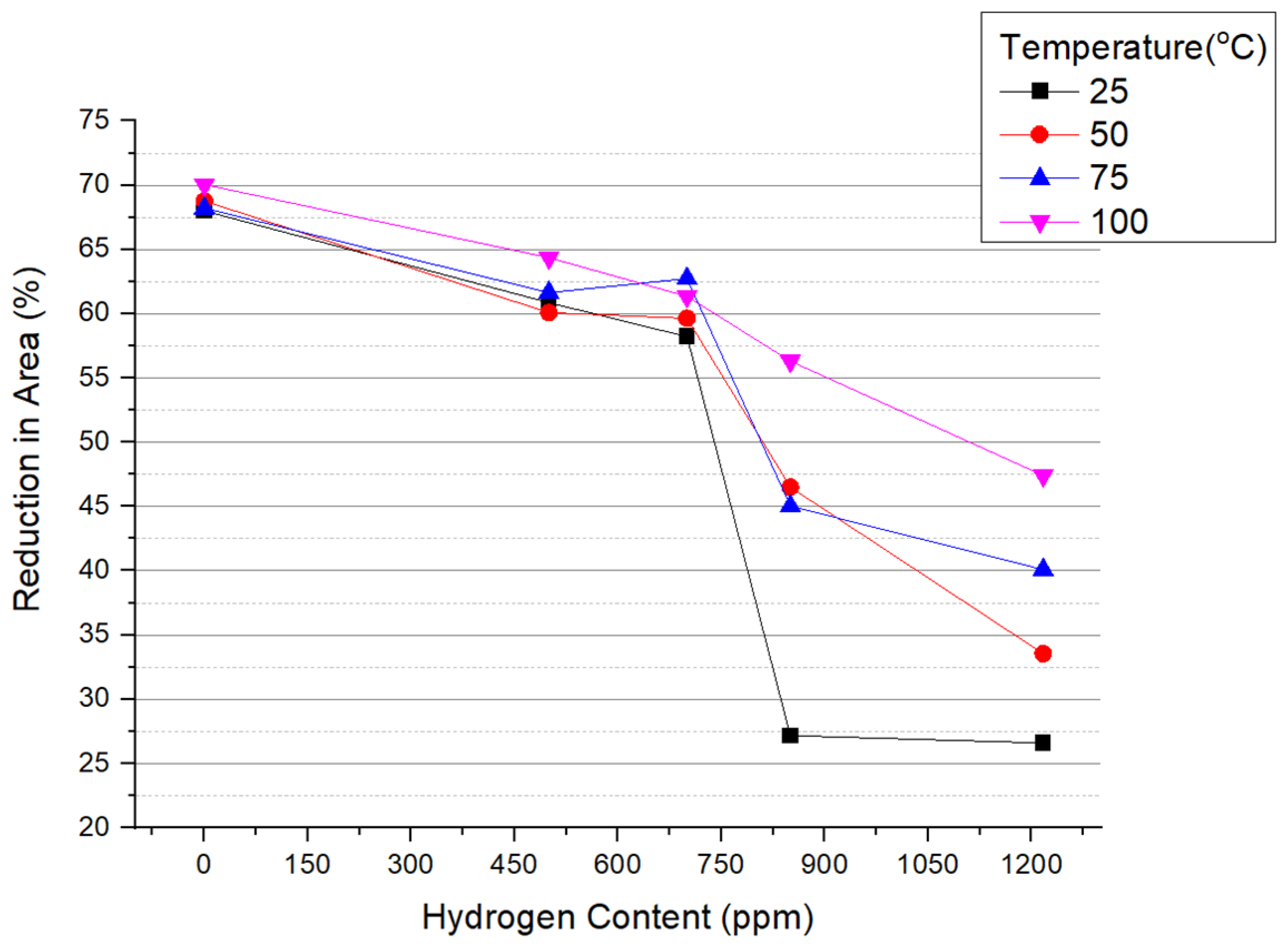
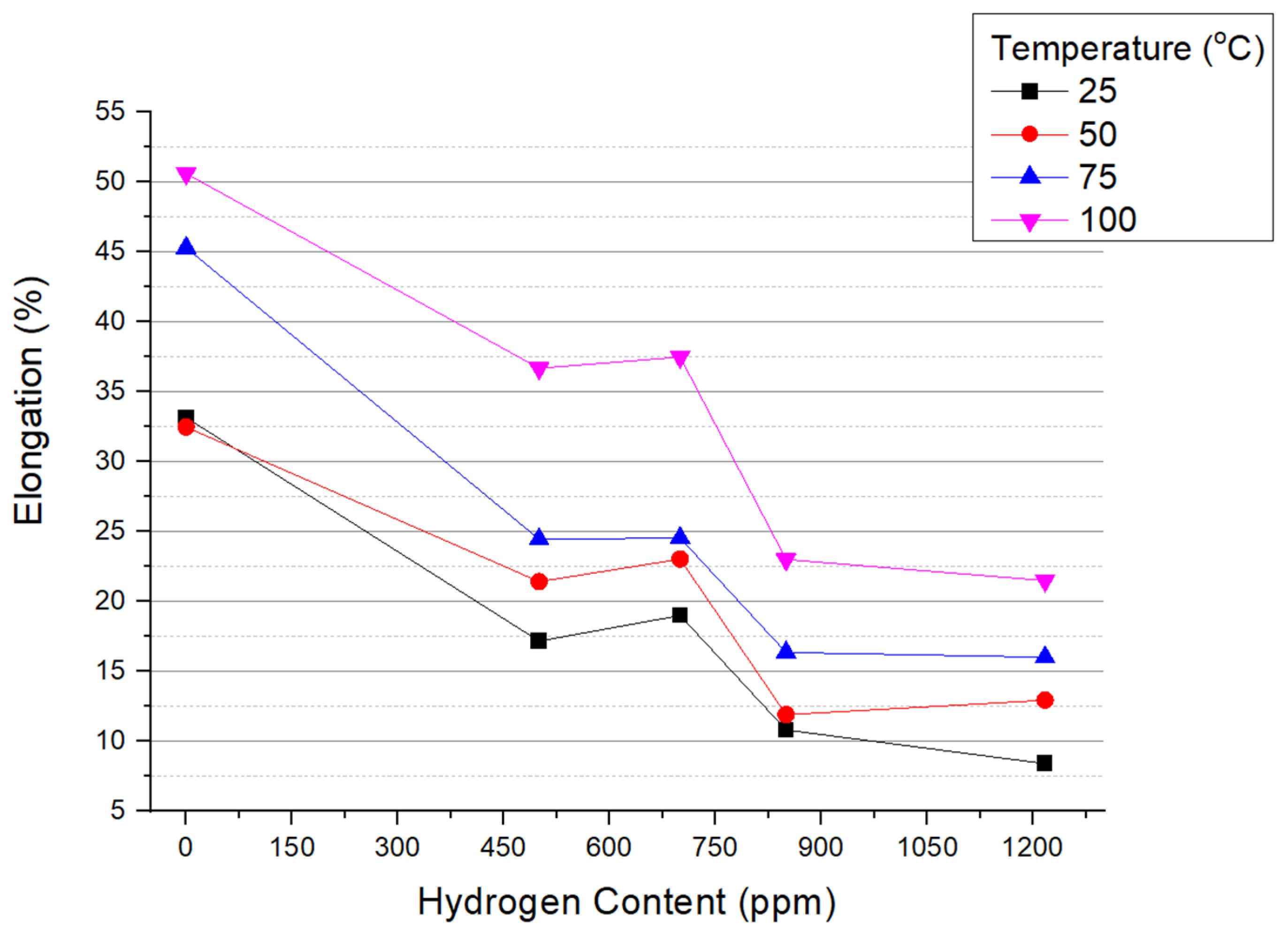
3.2. Reduction in Area and Elongation
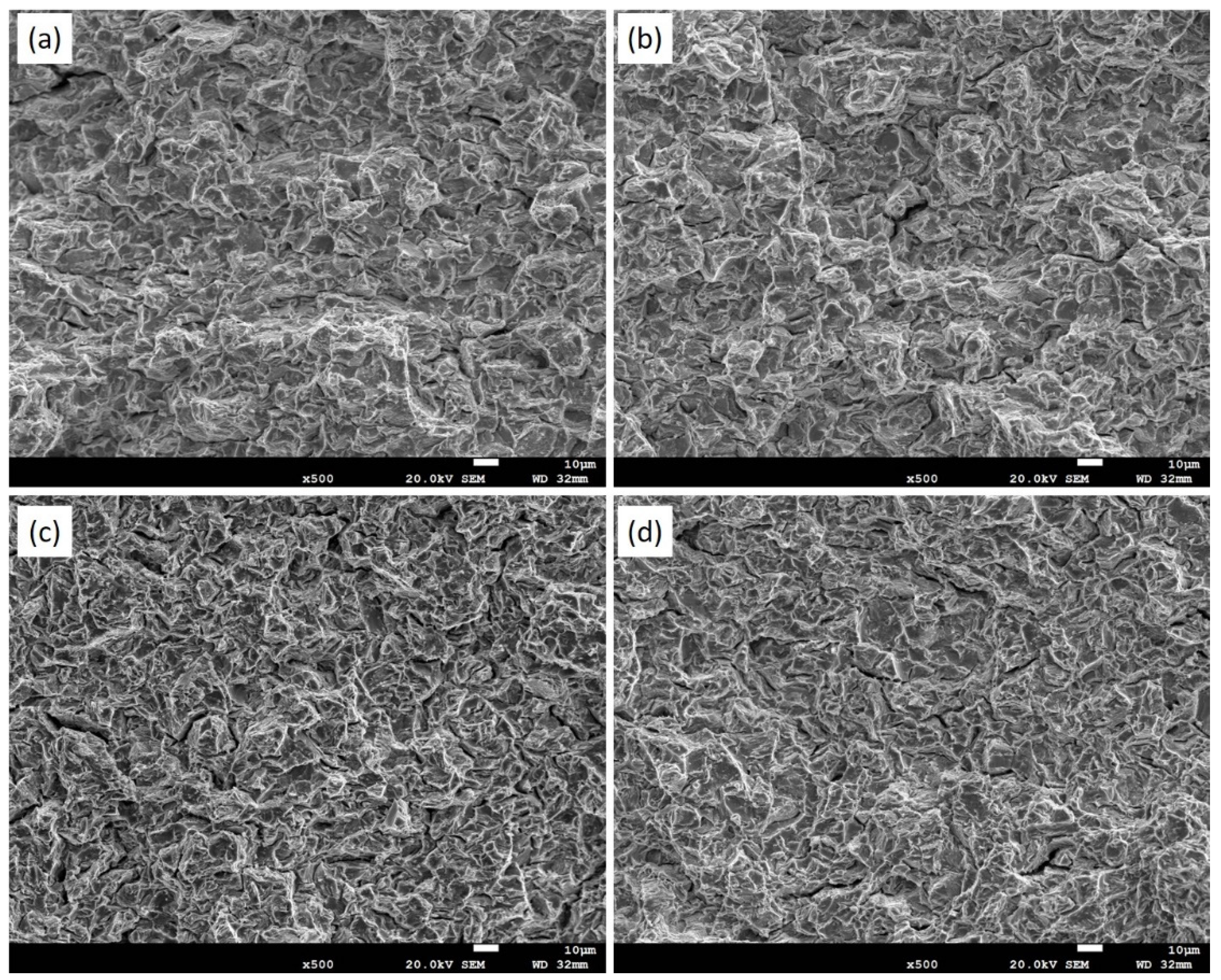
3.3. Optical Micrographs and Fractographs
4. Discussion
4.1. Tensile Behavior of Zricaloy-4
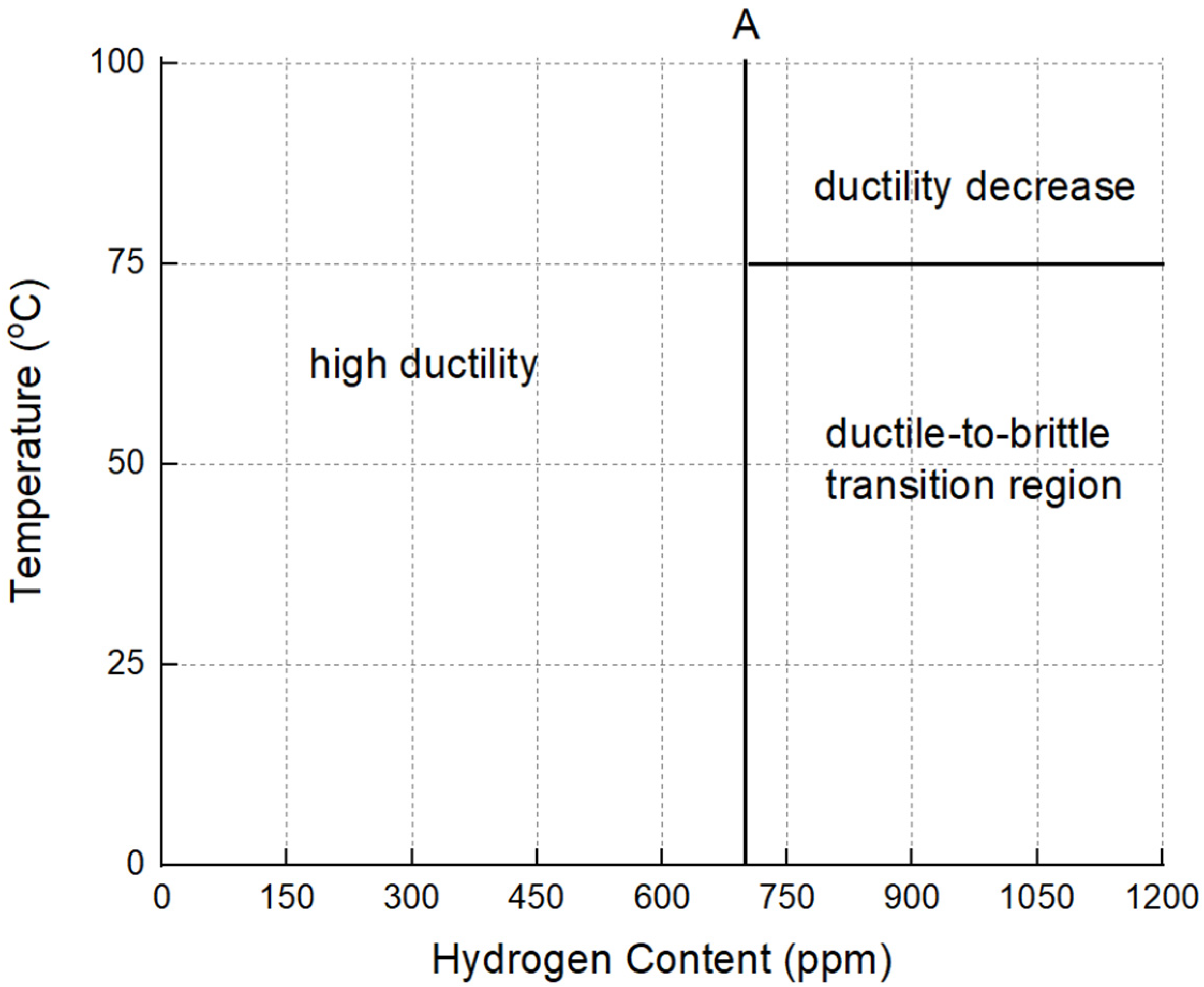
4.2. Ductile-to-Brittle Transition of Zricaloy-4
4.3. Elongation Behavior of Zricaloy-4
5. Conclusions
- (1)
- A ductile-to-brittle transition was observed in specimens with hydrogen content ranging between 700 and 850 ppm when tested at temperatures of 25 °C, 50 °C, and 75 °C. For tests conducted at 100 °C, there was a gradual decrease in the reduction in area as hydrogen content increased from 0 to 1217 ppm H.
- (2)
- At 25 °C, the ultimate tensile strength (UTS) of Zircaloy-4 shows a linear increase as hydrogen concentration rises from 0 to 1217 ppm H. However, at higher temperatures, the UTS behavior becomes more complex, with initial increases, followed by reductions, and then subsequent increases again, particularly in the hydrogen concentration ranges of 500–700 ppm H and 500–850 ppm H.
- (3)
- Elongation (EL) in hydrided specimens is affected by both temperature and hydrogen concentration. As the hydrogen concentration rises, uniform EL experiences a discernible decline, whereas non-uniform EL undergoes even more pronounced reductions. With the increase in testing temperatures, uniform EL remains relatively stable, but non-uniform EL markedly increases, indicating enhanced strain localization.
- (4)
- Fractography results revealed that quasi-cleavage features on the fracture surface became evident when the hydrogen content reached 850 ppm H, regardless of the testing temperatures ranging from 25 °C to 100 °C.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.; Morniroli, J.; Legris, A.; Ambard, A.; Khin, Y.; Legras, L.; Blat-Yrieix, M. Identification and characterization of a new zirconium hydride. J. Microsc. 2008, 232, 410–421. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, R.; Shu, G.; Wu, P.; Xiao, H. First-Principles Study of Different Polymorphs of Crystalline Zirconium Hydride. J. Phys. Chem. C 2010, 114, 22361–22368. [Google Scholar] [CrossRef]
- Hu, J.; Liu, J.; Lozano-Perez, S.; Grovenor, C.R.; Christensen, M.; Wolf, W.; Wimmer, E.; Mader, E.V. Hydrogen pickup during oxidation in aqueous environments: The role of nano-pores and nano-pipes in zirconium oxide films. Acta Mater. 2019, 180, 105–115. [Google Scholar] [CrossRef]
- Suman, S. Impact of hydrogen on rupture behaviour of Zircaloy-4 nuclear fuel cladding during loss-of-coolant accident: A novel observation of failure at multiple locations. Nucl. Eng. Technol. 2020, 53, 474–483. [Google Scholar] [CrossRef]
- Neogy, S.; Srivastava, D.; Tewari, R.; Singh, R.; Dey, G.; Banerjee, S. Microstructural study of hydride formation in Zr–1Nb alloy. J. Nucl. Mater. 2003, 322, 195–203. [Google Scholar] [CrossRef]
- Varias, A.; Massih, A. Hydride-induced embrittlement and fracture in metals—Effect of stress and temperature distribution. J. Mech. Phys. Solids 2002, 50, 1469–1510. [Google Scholar] [CrossRef]
- Birch, R.; Wang, S.; Tong, V.S.; Britton, T.B. The effect of cooling rate and grain size on hydride microstructure in Zircaloy-4. J. Nucl. Mater. 2018, 513, 221–225. [Google Scholar] [CrossRef]
- Michler, T.; Schweizer, F.; Wackermann, K. Review on the Influence of Temperature upon Hydrogen Effects in Structural Alloys. Metals 2021, 11, 423. [Google Scholar] [CrossRef]
- Jia, Y.-J.; Han, W.-Z. Mechanisms of Hydride Nucleation, Growth, Reorientation, and Embrittlement in Zirconium: A Review. Materials 2023, 16, 2419. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Truster, T.; Senor, D.J.; Devanathan, R. A Crystal Plasticity Finite Element Method Modeling of Zircaloy with Hydride Phases Based on Scanning Electron Micrographs. In Proceedings of the ASME 2022 17th International Manufacturing Science and Engineering Conference, Virtual, 27 June–1 July 1 2022. [Google Scholar]
- Mcconnell, P.; Hanson, B.; Lee, M.; Sorenson, K. Extended Dry Storage of Used Nuclear Fuel: Technical Issues: A USA Perspective. Nucl. Eng. Technol. 2011, 43, 405–412. [Google Scholar] [CrossRef]
- Waldrop, K. Extended Storage Collaboration Program International Subcommittee Report: International Perspectives on Technical data Gaps Associated with Extended Storage and Transportation of Used Spent Nuclear Fuel; EPRI: Palo Alto, CA, USA, 2012. [Google Scholar]
- Camper, L.W. US NRC Interim Staff Guidance (ISG)–11, Revision 3; U.S. Nuclear Regulatory Commission: Washington, DC, USA, 2003.
- Savchuk, E.; Sokolenko, V.; Karaseva, E.; Mats, A.; Frolov, V.; Pylypenko, M. Effect of Hydrogenation on Creep and Structure Evolution of Nanocrystalline Zr1nb Alloy. Probl. At. Sci. Technol. 2023, 64–68. [Google Scholar] [CrossRef]
- Bai, J.; Prioul, C.; Lansiart, S.; François, D. Brittle fracture induced by hydrides in zircaloy-4. Scr. Met. Mater. 1991, 25, 2559–2563. [Google Scholar] [CrossRef]
- Bai, J.B.; Francois, D. Some evidence of a brittle-ductile transition of zirconium hybride between 20 and 350degC. J. Nucl. Mater. 1992, 187, 186–189. [Google Scholar] [CrossRef]
- Huang, J.-H.; Huang, S.-P. Effect of hydrogen contents on the mechanical properties of Zircaloy-4. J. Nucl. Mater. 1994, 208, 166–179. [Google Scholar] [CrossRef]
- Hsu, H.-H. An evaluation of hydrided Zircaloy-4 cladding fracture behavior by X-specimen test. J. Alloys Compd. 2006, 426, 256–262. [Google Scholar] [CrossRef]
- Robertson, I.M.; Sofronis, P.; Nagao, A.; Martin, M.L.; Wang, S.; Gross, D.W.; Nygren, K.E. Hydrogen Embrittlement Understood. Met. Mater. Trans. B 2015, 46, 1085–1103. [Google Scholar] [CrossRef]
- Birnbaum, H.; Sofronis, P. Hydrogen-enhanced localized plasticity—A mechanism for hydrogen-related fracture. Mater. Sci. Eng. A 1994, 176, 191–202. [Google Scholar] [CrossRef]
- Gajowiec, G.; Bartmański, M.; Majkowska-Marzec, B.; Zieliński, A.; Chmiela, B.; Derezulko, M. Hydrogen Embrittlement and Oxide Layer Effect in the Cathodically Charged Zircaloy-2. Materials 2020, 13, 1913. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Kanezaki, T.; Mine, Y.; Matsuoka, S. Hydrogen Embrittlement Mechanism in Fatigue of Austenitic Stainless Steels. Met. Mater. Trans. A 2008, 39, 1327–1339. [Google Scholar] [CrossRef]
- Mohamed, I.; Hasan, T.; Zikry, M.A. Thermomechanical Microstructural Predictions of Fracture Nucleation of Zircaloy-4 Alloys with δ and ɛ Hydride Distributions. J. Eng. Mater. Technol. 2021, 144, 011008. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Santisteban, J.; Vizcaíno, P.; Ribárik, G.; Ungar, T. Quantification of dislocations densities in zirconium hydride by X-ray line profile analysis. Acta Mater. 2016, 117, 1–12. [Google Scholar] [CrossRef]
- Qin, W.; Szpunar, J.; Kumar, N.K.; Kozinski, J. Microstructural criteria for abrupt ductile-to-brittle transition induced by δ-hydrides in zirconium alloys. Acta Mater. 2014, 81, 219–229. [Google Scholar] [CrossRef]
- Vazquez, C.; Zelaya, E.; Fortis, A.M.; Bozzano, P.B. Irradiation Hardening and Microstructure Characterization of Zr-1% Nb During Low Dose Neutron Irradiation. J. Mater. Appl. 2021, 10, 63–72. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kook, D.-H.; Kim, T.-H.; Kim, J.-S. Stress and temperature-dependent hydride reorientation of Zircaloy-4 cladding and its effect on the ductility degradation. J. Nucl. Sci. Technol. 2014, 52, 717–727. [Google Scholar] [CrossRef]
- Cinbiz, M.N.; Koss, D.A.; Motta, A.T. The influence of stress state on the reorientation of hydrides in a zirconium alloy. J. Nucl. Mater. 2016, 477, 157–164. [Google Scholar] [CrossRef]
- Aomi, M.; Baba, T.; Miyashita, T.; Kamimura, K.; Yasuda, T.; Shinohara, Y.; Takeda, T.; Limback, M.; Kammenzind, B.; Dean, S.W. Evaluation of Hydride Reorientation Behavior and Mechanical Properties for High-Burnup Fuel-Cladding Tubes in Interim Dry Storage. J. ASTM Int. 2008, 5, 1–21. [Google Scholar] [CrossRef]
- Saeidi, N.; Ashrafizadeh, F.; Niroumand, B.; Barlat, F. Evaluation of Fracture Micromechanisms in a Fine-Grained Dual Phase Steel during Uniaxial Tensile Deformation. Steel Res. Int. 2014, 85, 1386–1392. [Google Scholar] [CrossRef]


| Charging temperature range (°C) | 200~300 | |||
| Number of charging cycles | 14 | 28 | 60 | 85 |
| Targeted hydrogen content (ppm) | 600 | 900 | 1200 | 1500 |
| Measured hydrogen content (ppm) | 500 ± 13 | 700 ± 32 | 850 ± 76 | 1217 ± 237 |
| Hydrogen Content (ppm) | Temperature (°C) | Total EL (%) | Uniform EL (%) | Non-Uniform EL (%) |
|---|---|---|---|---|
| 0 | 25 | 33.2 | 11.5 | 21.7 |
| 50 | 32.5 | 11.9 | 20.6 | |
| 75 | 45.3 | 10.1 | 35.2 | |
| 100 | 50.6 | 9.9 | 40.7 | |
| 700 | 25 | 19 | 7.2 | 11.8 |
| 50 | 23 | 7.1 | 15.9 | |
| 75 | 24.5 | 7.3 | 17.2 | |
| 100 | 37.5 | 5.9 | 31.6 | |
| 1217 | 25 | 8.4 | 7.1 | 1.3 |
| 50 | 12.9 | 6.2 | 6.7 | |
| 75 | 16 | 6.7 | 9.3 | |
| 100 | 21.5 | 5.9 | 15.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tung, H.-M.; Chen, T.-C. Hydride-Induced Responses in the Mechanical Behavior of Zircaloy-4 Sheets. Metals 2024, 14, 177. https://doi.org/10.3390/met14020177
Tung H-M, Chen T-C. Hydride-Induced Responses in the Mechanical Behavior of Zircaloy-4 Sheets. Metals. 2024; 14(2):177. https://doi.org/10.3390/met14020177
Chicago/Turabian StyleTung, Hsiao-Ming, and Tai-Cheng Chen. 2024. "Hydride-Induced Responses in the Mechanical Behavior of Zircaloy-4 Sheets" Metals 14, no. 2: 177. https://doi.org/10.3390/met14020177
APA StyleTung, H.-M., & Chen, T.-C. (2024). Hydride-Induced Responses in the Mechanical Behavior of Zircaloy-4 Sheets. Metals, 14(2), 177. https://doi.org/10.3390/met14020177







