Hydrothermal Leaching Kinetics of Vanadium from an Iron Vanadate Mineral Using Oxalic Acid
Abstract
:1. Introduction
2. Experimental Section
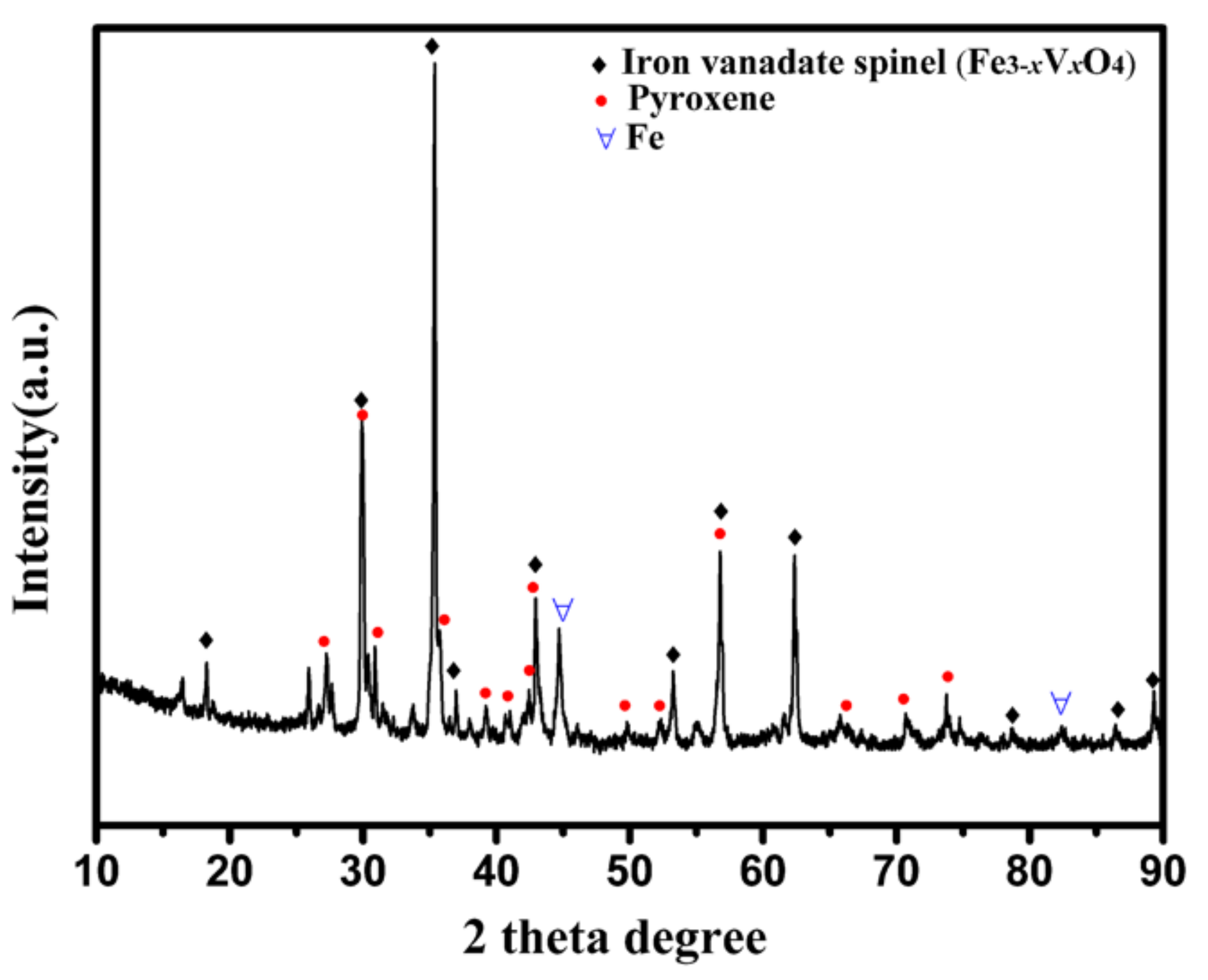
2.1. Raw Materials
2.2. Hydrothermal Leaching and Analytical
3. Results and Discussion
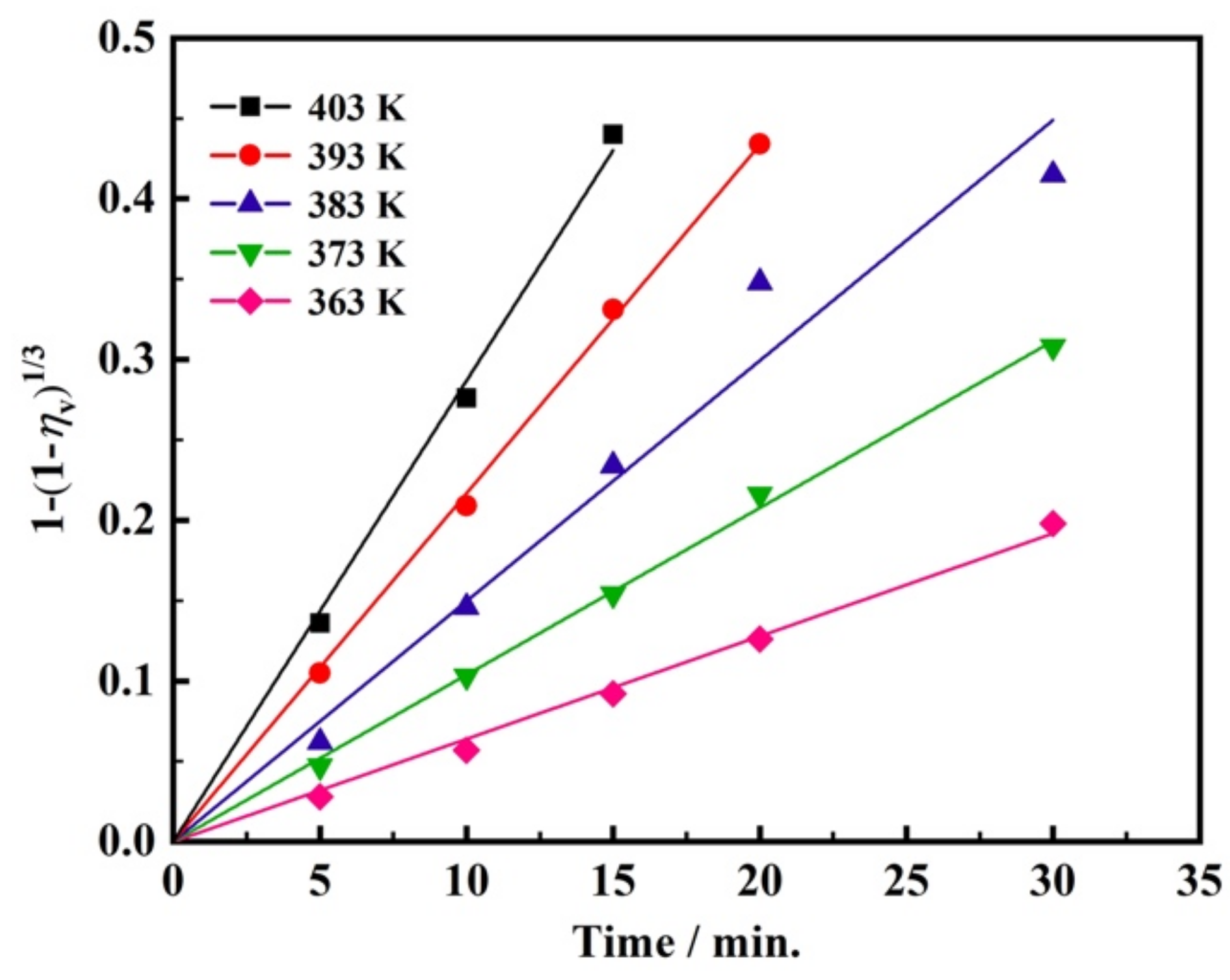
3.1. Effect of Temperature on the Leaching Kinetics
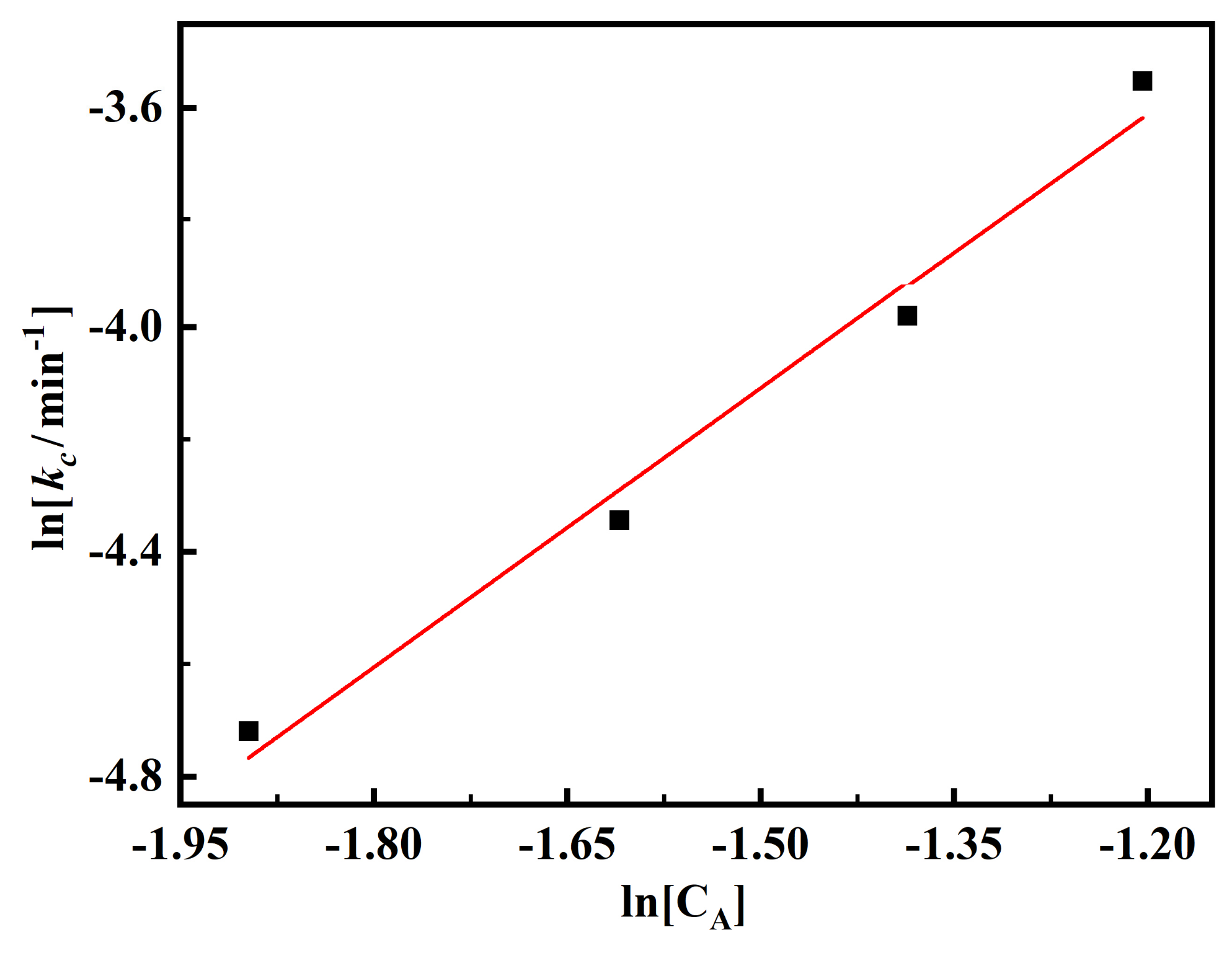
3.2. Effect of the Concentration of Leaching Agent on the Leaching Kinetics
3.3. Establishment of the Kinetic Model of the Hydrothermal Leaching Process
4. Conclusions
- An interfacial chemical reaction was the rate-controlling step of the hydrothermal leaching process within the temperature ranging from 363 to 403 K and the leaching efficiency less than 85%;
- The apparent activation energy of the interfacial chemical reaction was 45.6 kJ/mol;
- The order of the interfacial chemical reaction to the concentration of oxalis acid was around 1.66;
- The hydrothermal leaching behavior of vanadium by oxalic acid can be described well by the following model, where denotes the concentration of oxalic acid, expressed by its mass fraction relative to water, and represents the leaching time, expressed by min.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Imtiaz, M.; Rizwan, M.S.; Xiong, S.; Li, H.; Ashraf, M.; Shahzad, S.M.; Shahzad, M.; Tu, S. Vanadium, recent advancements and research prospects: A review. Environ. Int. 2015, 80, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Moskalyk, R.R.; Alfantazi, A.M. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Bao, S.-X.; Liu, T.; Chen, T.-J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Radwany, M.R.; Barton, I.F. The process mineralogy of leaching sandstone-hosted uranium-vanadium ores. Miner. Eng. 2022, 187, 107811. [Google Scholar] [CrossRef]
- Drexler, M.; Barton, I.; Zanetta, P.M. Vanadium in phyllosilicate ores: Occurrence, crystal chemistry, and leaching behavior. Miner. Eng. 2023, 201, 108205. [Google Scholar] [CrossRef]
- Kerr, A.; Walsh, J.A.; Sparkes, G.W.; Hinchey, J.G. Vanadium potential in Newfoundland and Labrador: A review and assessment. In Current Research Newfoundland and Labrador Department of Natural Resources Geological Survey; Geological Survey: Reston, VA, USA, 2013; pp. 137–165. [Google Scholar]
- Lee, J.-C.; Kurniawan; Kim, E.-Y.; Chung, K.W.; Kim, R.; Jeon, H.-S. A review on the metallurgical recycling of vanadium from slags: Towards a sustainable vanadium production. J. Mater. Res. Technol. 2021, 12, 343–364. [Google Scholar] [CrossRef]
- An, Y.; Ma, B.; Li, X.; Chen, Y.; Wang, C.; Wang, B.; Gao, M.; Feng, G. A review on the roasting-assisted leaching and recovery of V from vanadium slag. Process Saf. Environ. Prot. 2023, 173, 263–276. [Google Scholar] [CrossRef]
- Liu, L.; Kauppinen, T.; Tynjälä, P.; Liu, T. Water leaching of roasted vanadium slag: Desiliconization and precipitation of ammonium vanadate from vanadium solution. Hydrometallurgy 2023, 215, 105989. [Google Scholar] [CrossRef]
- Dong, Z.H.; Zhang, J.; Yan, B.J. Co-extraction of vanadium titanium and chromium from vanadium slag by oxalic acid hydrothermal leaching with synergy of Fe powder. Metall. Mater. Trans. B 2021, 52, 3961–3969. [Google Scholar] [CrossRef]
- Hu, Y.-J.; Zhang, Y.-M.; Bao, S.-X.; Liu, T. Effects of the mineral phase and valence of vanadium on vanadium extraction from stone coal. Int. J. Miner. Metall. Mater. 2012, 19, 893–898. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, Z.; Jin, J.; Li, Y.; Han, Y. Vanadium extraction from V-bearing shale using oxidation roasting and acid leaching. Miner. Eng. 2023, 192, 107985. [Google Scholar] [CrossRef]
- Gao, F.; Olayiwola, A.U.; Liu, B.; Wang, S.; Du, H.; Li, J.; Wang, X.; Chen, D.; Zhang, Y. Review of vanadium production part I: Primary resources. Miner. Process. Extr. Metall. Rev. 2021, 43, 466–488. [Google Scholar] [CrossRef]
- Yan, B.; Wang, D.; Wu, L.; Dong, Y. A novel approach for pre-concentrating vanadium from stone coal ore. Miner. Eng. 2018, 125, 231–238. [Google Scholar] [CrossRef]
- Chen, X.; Yan, B. A novel method to extract V3+ from iron vanadate spinel minerals by one leaching step. Hydrometallurgy 2020, 198, 105517. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, L.; Wang, L.; Li, S.; Yang, G.; Cao, C.; Liu, S.; Nie, Y.; Jia, L. Optimization and kinetic analysis of direct acid leaching of vanadium from converter vanadium slag under atmospheric pressure. Miner. Eng. 2023, 198, 108091. [Google Scholar] [CrossRef]
- Hui, X.; Zhang, J.; Liang, Y.; Chang, Y.; Zhang, W.; Zhang, G. Comparison and evaluation of vanadium extraction from the calcification roasted vanadium slag with carbonation leaching and sulfuric acid leaching. Sep. Purif. Technol. 2022, 297, 121466. [Google Scholar] [CrossRef]
- Nayl, A.A.; Awwad, N.S.; Aly, H.F. Kinetics of acid leaching of ilmenite decomposed by KOH Part 2. Leaching by H2SO4 and C2H2O4. J. Hazard. Mater. 2009, 168, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Zafar, Z.I.; Ashraf, M. Selective leaching kinetics of calcareous phosphate rock in lactic acid. Chem. Eng. J. 2007, 131, 41–48. [Google Scholar] [CrossRef]
- Li, W.; Yan, X.; Niu, Z.; Zhu, X. Selective recovery of vanadium from red mud by leaching with using oxalic acid and sodium sulfite. J. Environ. Chem. Eng. 2021, 9, 105669. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, G.; Xiang, P.; Tao, Y.; Tang, Y.; Hu, Y. Effect of activator on kinetics of direct acid leaching of vanadium from clay vanadium ore. Sep. Purif. Technol. 2022, 281, 119937. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Z.; Li, Y.; Zhu, Y.; Xie, K. Selective sulfuric acid cyclic leaching of vanadium from the calcification roasting pellets of vanadium titanomagnetite. J. Mater. Res. Technol. 2023, 23, 778–790. [Google Scholar] [CrossRef]
- Ashraf, M.; Zafar, Z.I.; Ansari, T.M. Selective leaching kinetics and upgrading of low-grade calcareous phosphate rock in succinic acid. Hydrometallurgy 2005, 80, 286–292. [Google Scholar] [CrossRef]





| Elements | Contents (Mass%) | Elements | Contents (Mass%) |
|---|---|---|---|
| Fe | 23.2 | V | 9.7 |
| Si | 11.9 | Ca | 4.7 |
| Mg | 3.8 | Al | 3.3 |
| S | 1.2 | K | 1.9 |
| Ba | 0.2 | Ti | 0.1 |
| P | 0.1 | Na | 0.1 |
| Cr | 0.1 | O | 39.7 |
| Temp. (K) | 5 min | 10 min | 15 min | 20 min | 30 min |
|---|---|---|---|---|---|
| 363 | 8.1% | 16.1% | 25.1% | 33.3% | 48.4% |
| 373 | 13.5% | 27.9% | 39.5% | 51.9% | 66.9% |
| 383 | 17.5% | 37.8% | 55.1% | 72.3% | 80.0% |
| 393 | 28.4% | 50.5% | 70.1% | 81.9% | 85.1% |
| 403 | 35.6% | 62.0% | 82.4% | 85.6% | 87.1% |
| Temp. (K) | ||||
|---|---|---|---|---|
| /min−1 | /min−1 | |||
| 363 | 0.00639 | 0.998 | 0.00089 | 0.906 |
| 373 | 0.01038 | 0.999 | 0.00211 | 0.942 |
| 383 | 0.01496 | 0.989 | 0.00395 | 0.959 |
| 393 | 0.02168 | 0.999 | 0.00587 | 0.959 |
| 403 | 0.02869 | 0.999 | 0.00789 | 0.950 |
| Concentration of Oxalic Acid (wt %) | 5 min | 10 min | 15 min | 20 min | 30 min |
|---|---|---|---|---|---|
| 15 | 12.9% | 24.6% | 34.8% | 44.0% | 61.0% |
| 20 | 21.7% | 35.3% | 49.8% | 60.6% | 75.6% |
| 25 | 28.4% | 46.6% | 62.3% | 74.8% | 85.1% |
| 30 | 35.6% | 62.0% | 82.4% | 85.6% | 87.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, B.; Chen, X.; Yan, B. Hydrothermal Leaching Kinetics of Vanadium from an Iron Vanadate Mineral Using Oxalic Acid. Metals 2023, 13, 1629. https://doi.org/10.3390/met13091629
Shen B, Chen X, Yan B. Hydrothermal Leaching Kinetics of Vanadium from an Iron Vanadate Mineral Using Oxalic Acid. Metals. 2023; 13(9):1629. https://doi.org/10.3390/met13091629
Chicago/Turabian StyleShen, Biao, Xuexin Chen, and Baijun Yan. 2023. "Hydrothermal Leaching Kinetics of Vanadium from an Iron Vanadate Mineral Using Oxalic Acid" Metals 13, no. 9: 1629. https://doi.org/10.3390/met13091629
APA StyleShen, B., Chen, X., & Yan, B. (2023). Hydrothermal Leaching Kinetics of Vanadium from an Iron Vanadate Mineral Using Oxalic Acid. Metals, 13(9), 1629. https://doi.org/10.3390/met13091629







