Structure Evolution and Mechanical Properties of Sheet Al–2Cu–1.5Mn–1Mg–1Zn (wt.%) Alloy Designed for Al20Cu2Mn3 Disperoids
Abstract
1. Introduction
2. Experimental
3. Experimental Results
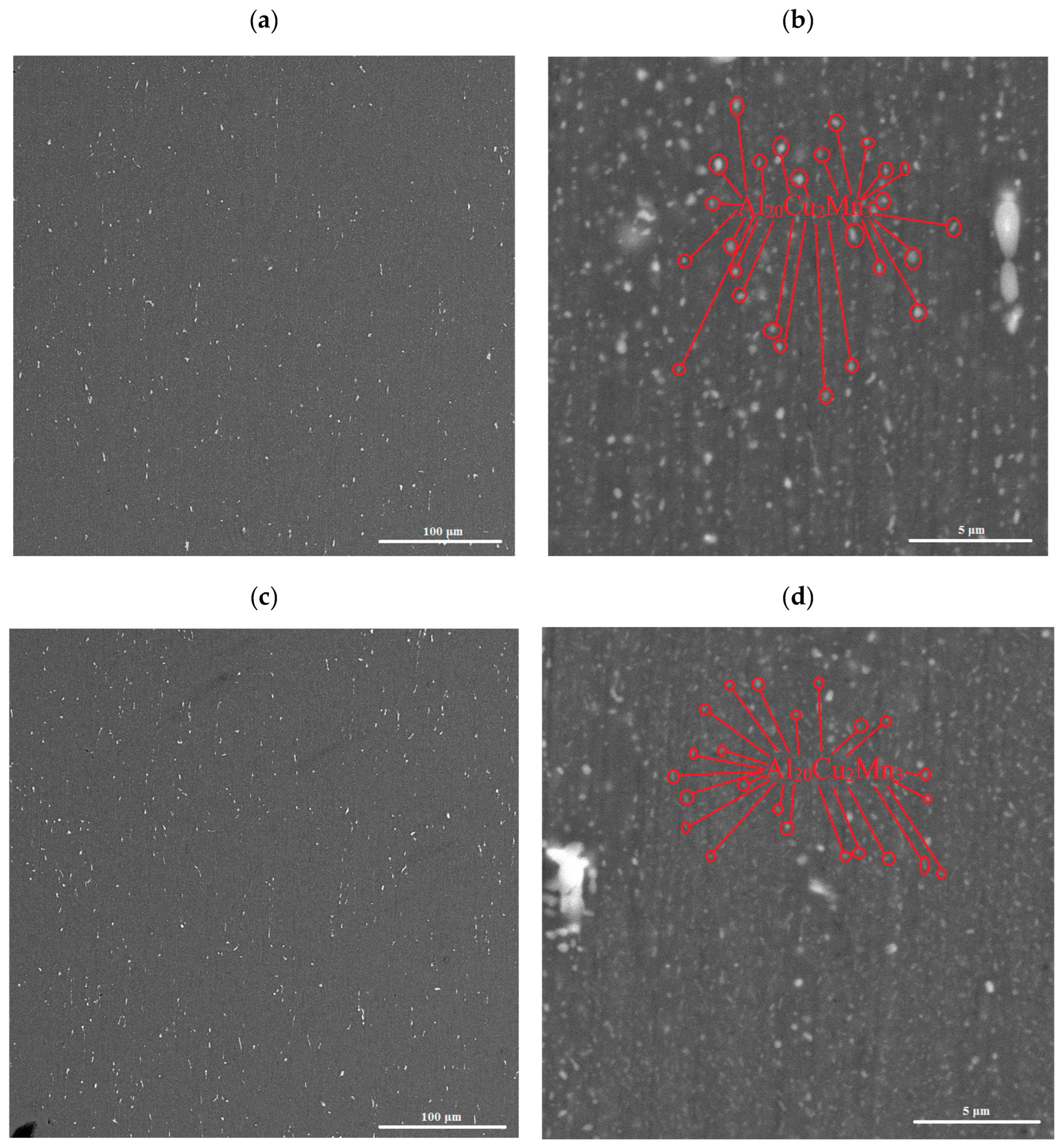
3.1. Phase Composition and Microstructure of the Ingots
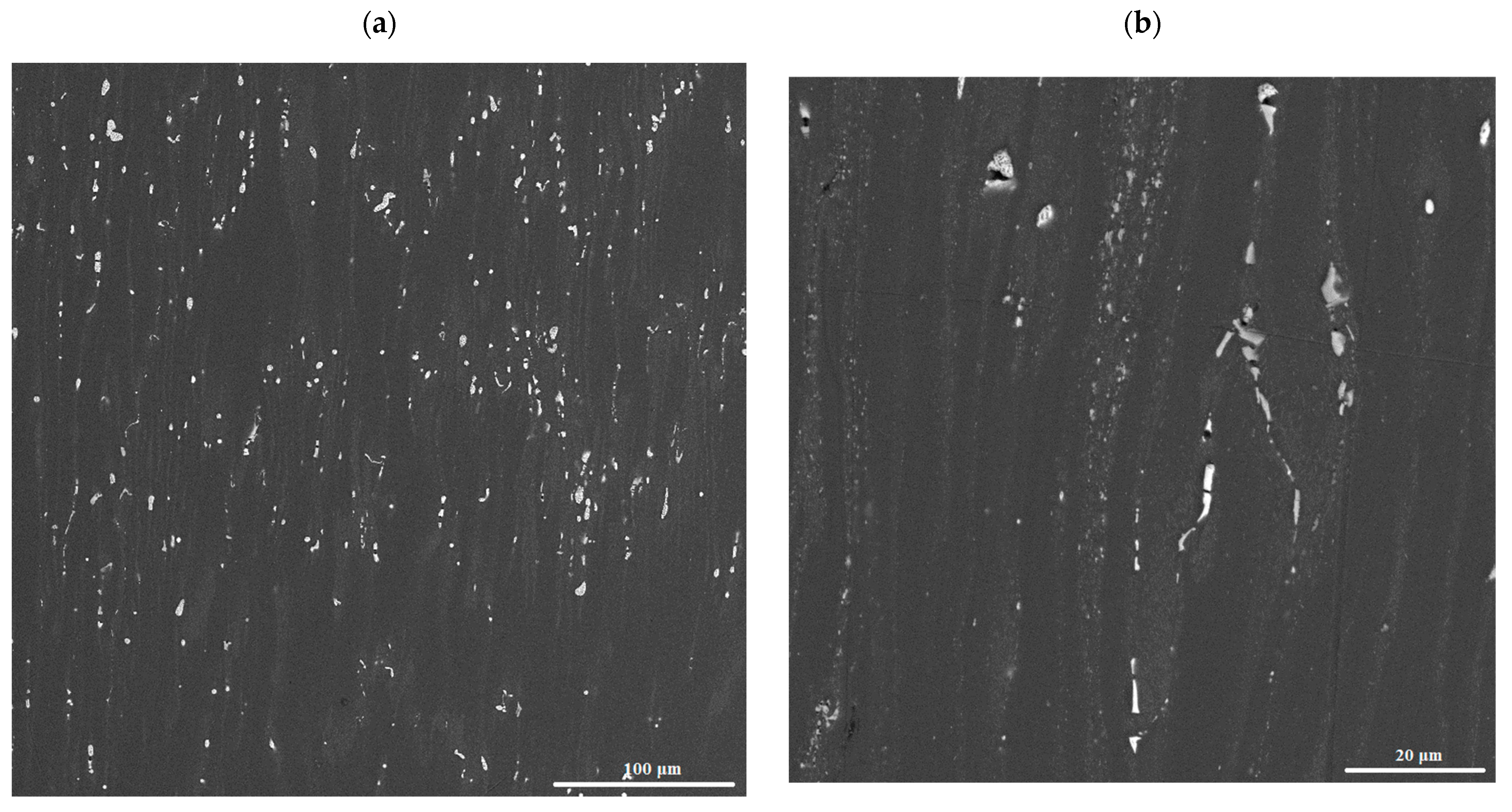
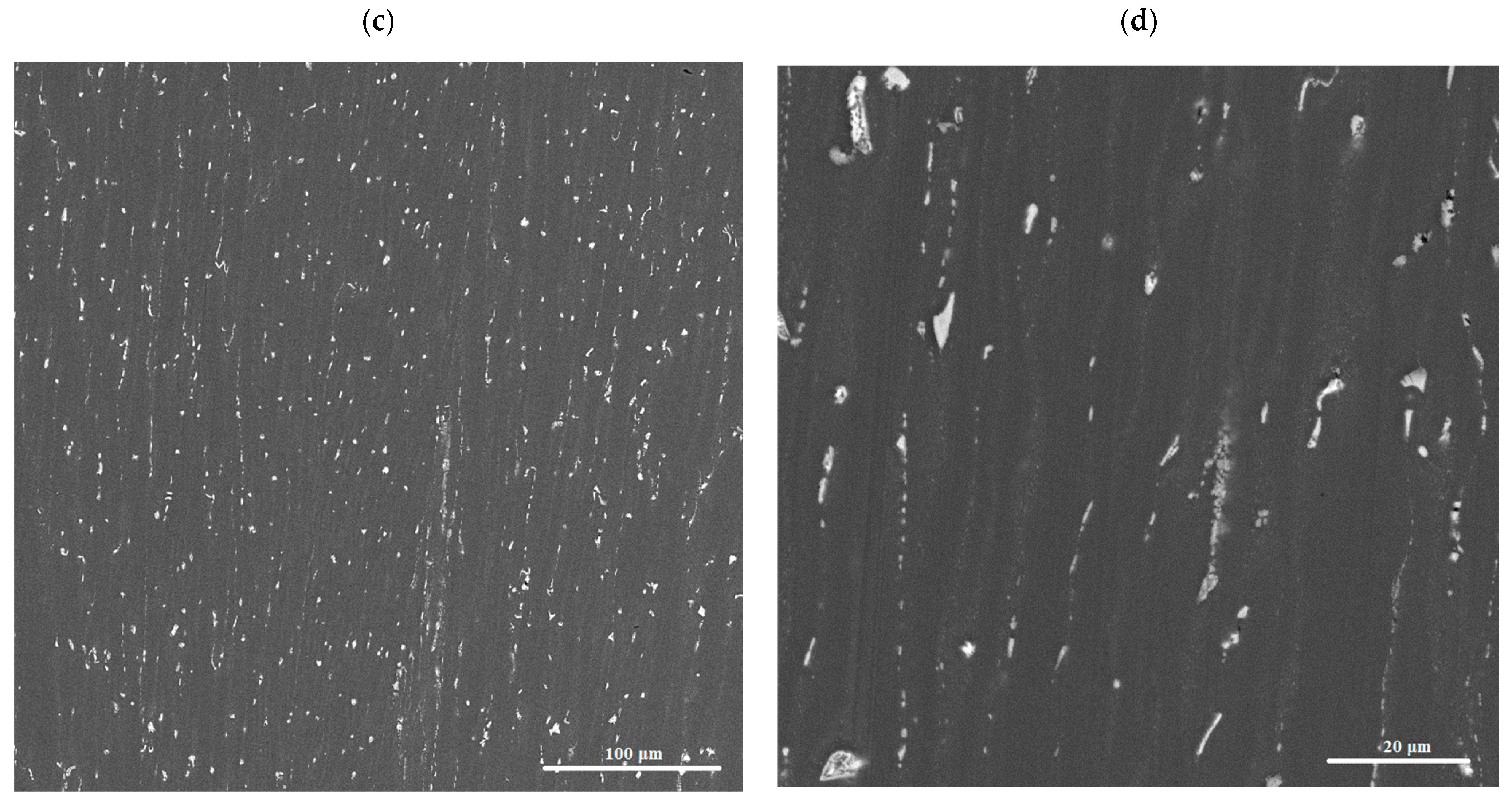
3.2. Microstructure and Phase Composition of Hot-Rolled Sheets
3.3. Hardness and Electrical Resistivity of Hot-Rolled Sheets
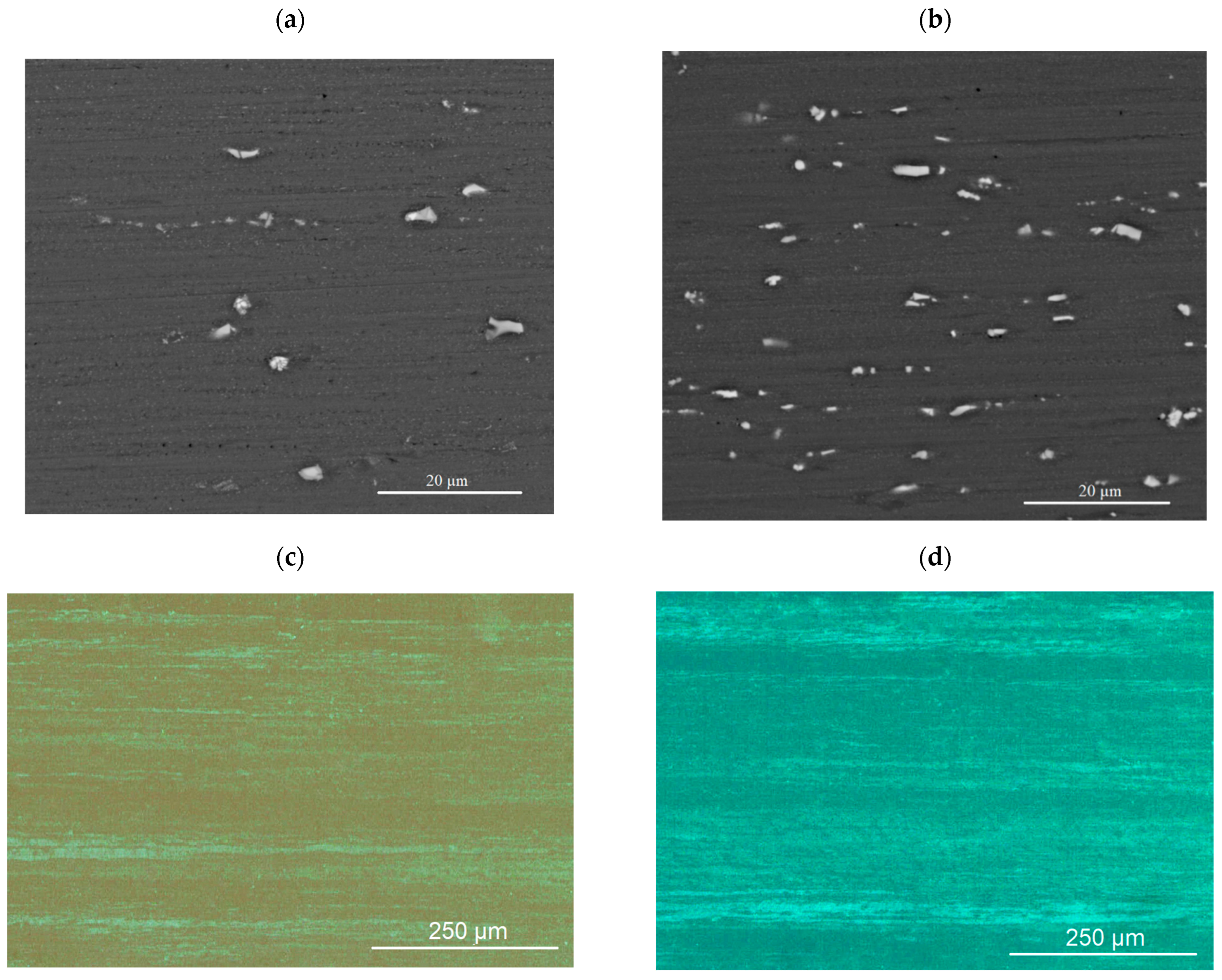
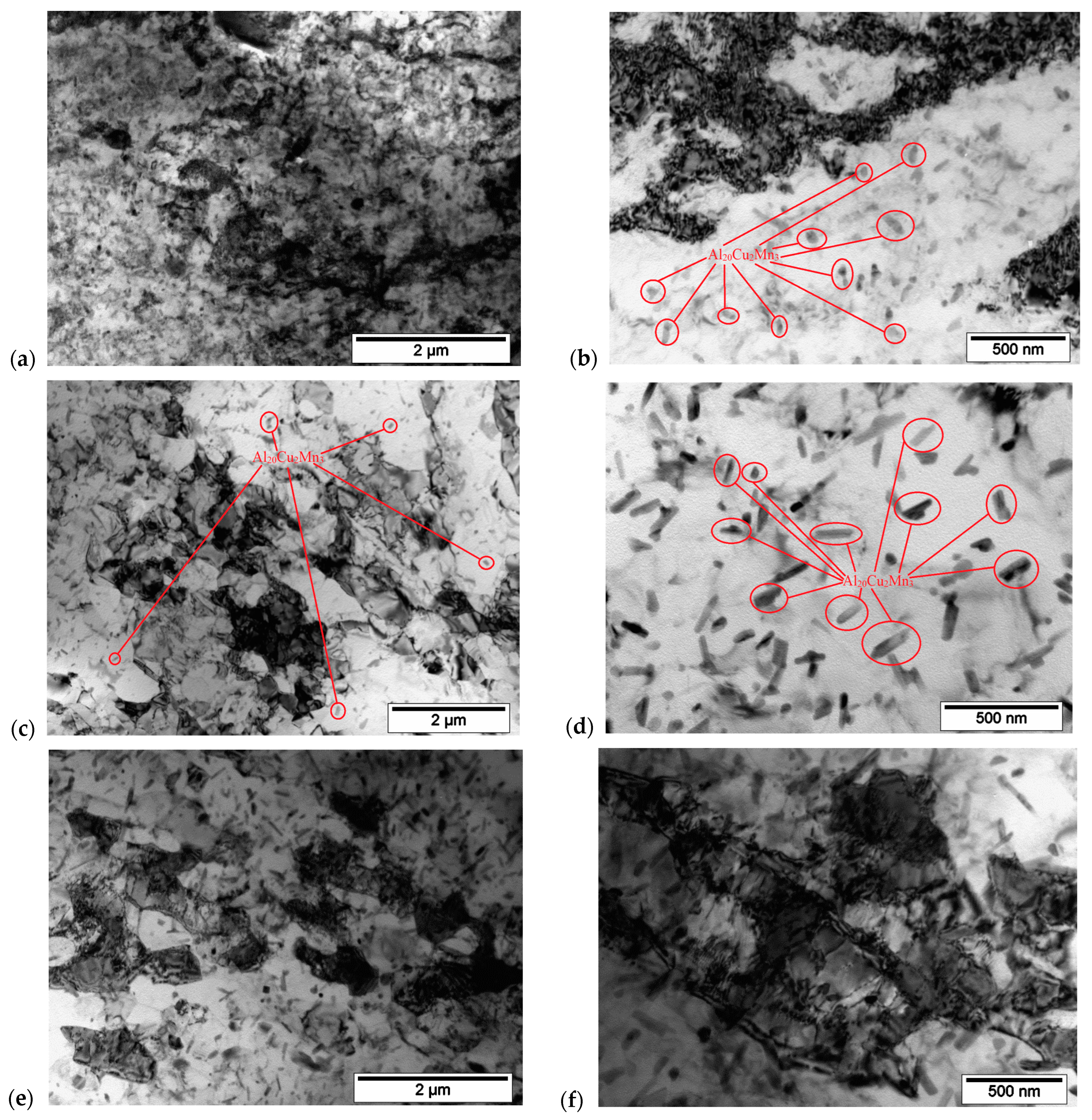
3.4. SEM and TEM Structure of Cold-Rolled Sheets
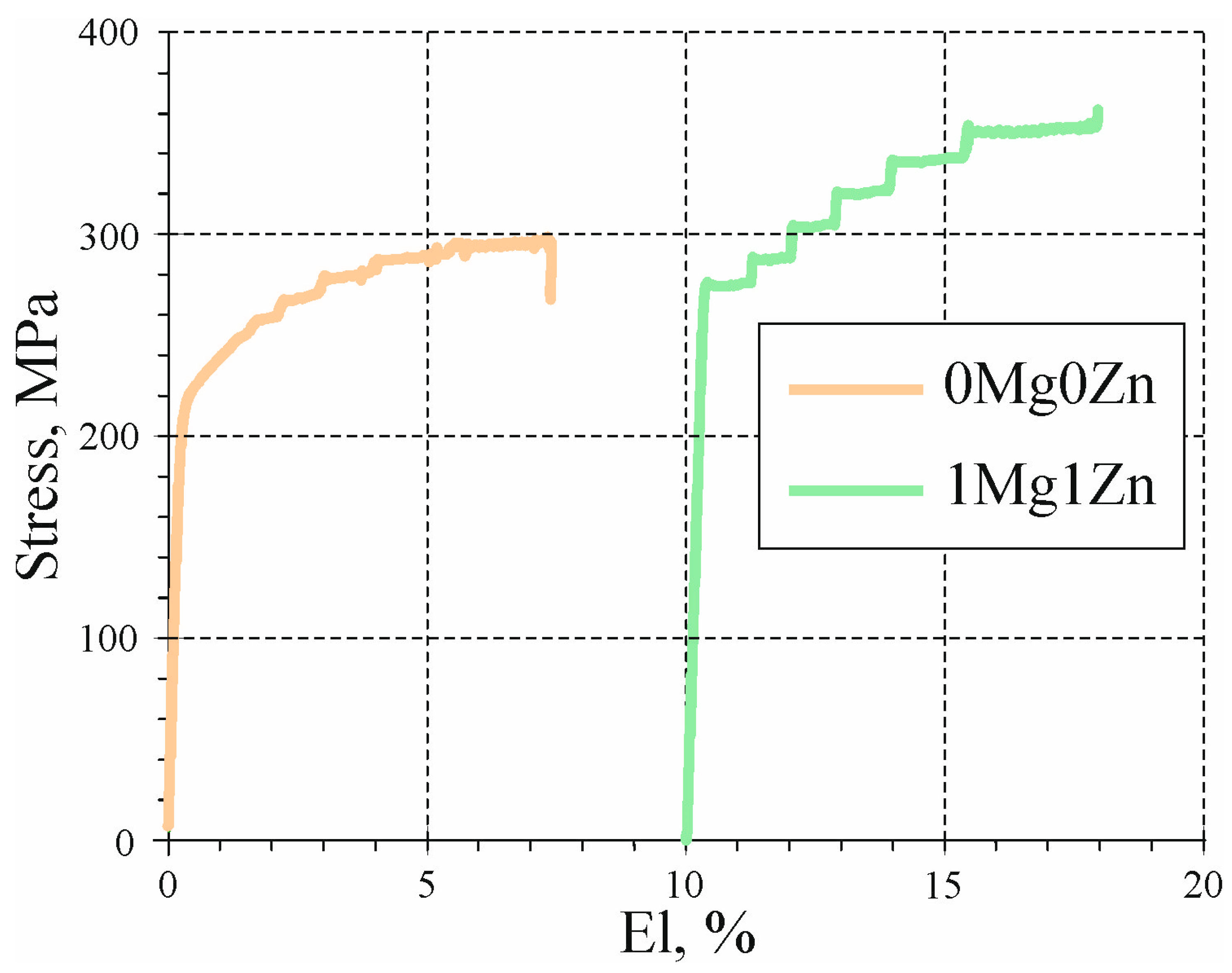
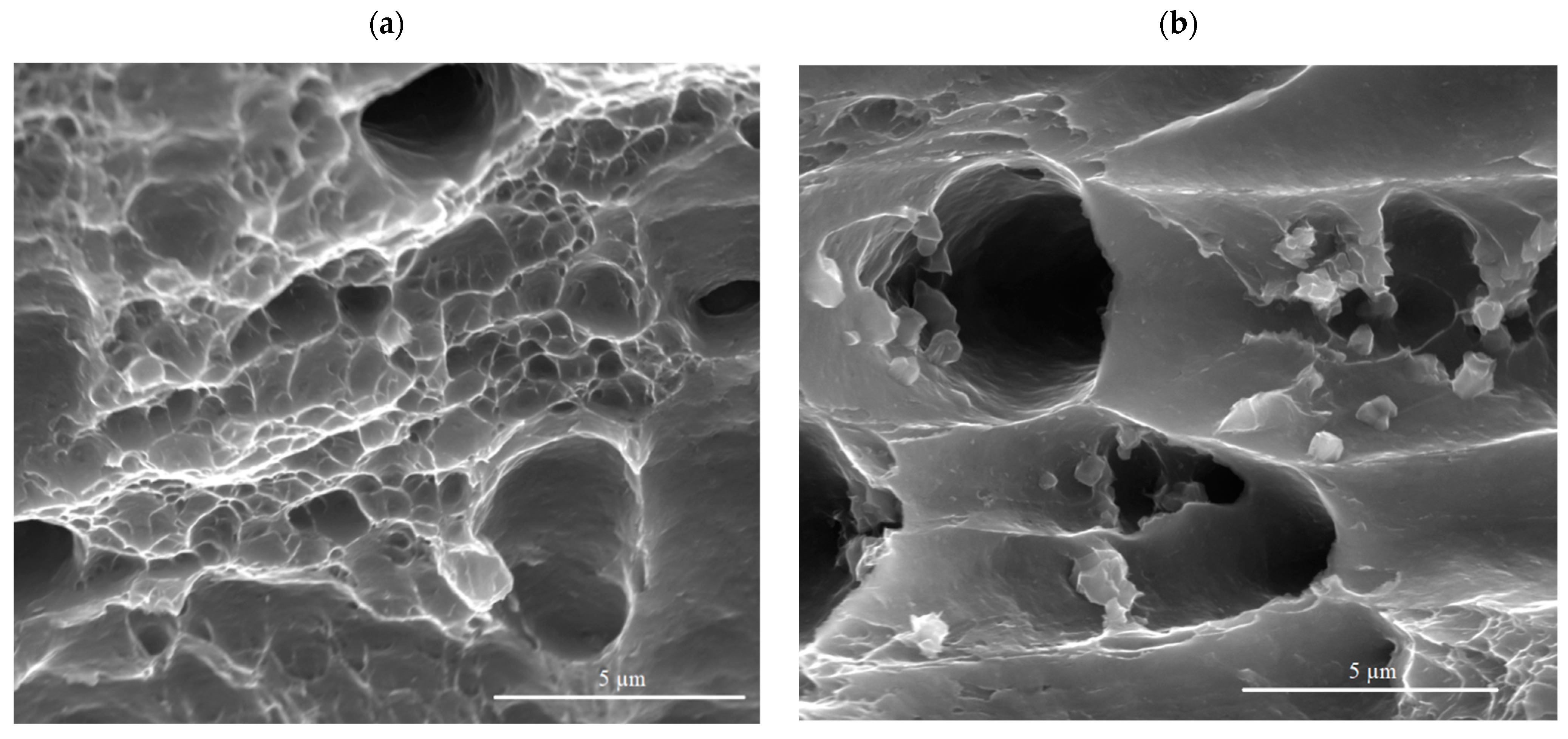
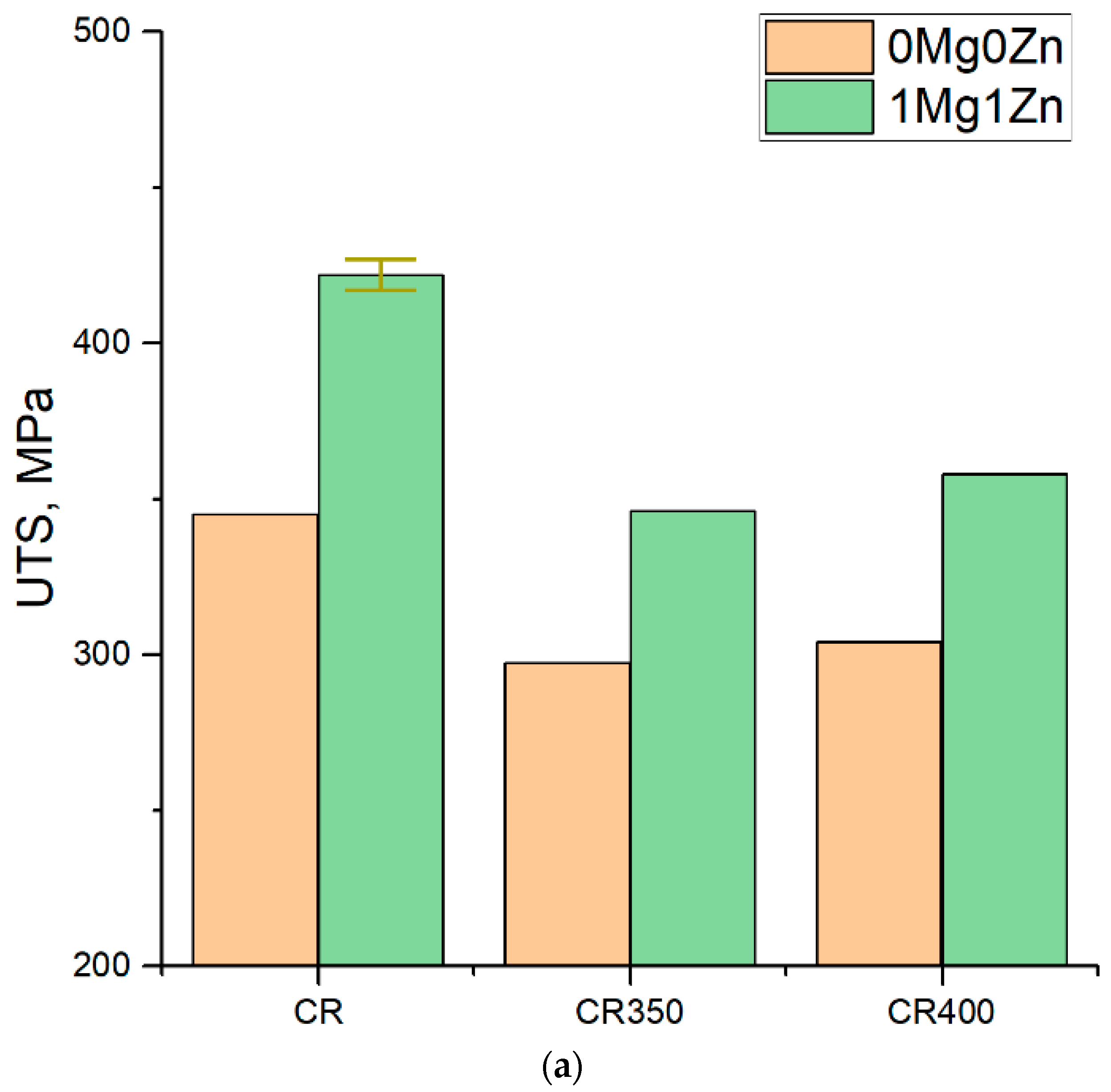
3.5. Mechanical Properties of Cold-Rolled Sheets
4. Discussion
5. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaunky, V.C. Are Shocks to Aluminium Consumption Transitory or Permanent? Rev. Appl. Econ. 2013, 9, 21–37. [Google Scholar] [CrossRef]
- Babcsán, N. Aluminium Infinite Green Circular Economy–Theoretical Carbon Free Infinite Loop, Combination of Material and Energy Cycles; Solutions for Sustainable Development; CRC Press: Boca Raton, FL, USA, 2019; pp. 205–210. [Google Scholar]
- Brough, D.; Jouhara, H. The aluminium industry: A review on state-of-the-art technologies, environmental impacts and possibilities for waste heat recovery. Int. J. Thermofluids. 2020, 1, 100007. [Google Scholar] [CrossRef]
- Ashkenazi, D. How aluminum changed the world: A metallurgical revolution through technological and cultural perspectives. Technol. Forecast. Soc. Chang. 2019, 143, 101–113. [Google Scholar] [CrossRef]
- Pedneault, J.; Majeau-Bettez, G.; Pauliuk, S.; Margni, M. Sector-specific scenarios for future stocks and flows of aluminum: An analysis based on shared socioeconomic pathways. J. Ind. Eco. 2022, 26, 1728–1746. [Google Scholar] [CrossRef]
- Ujah, C.O.; Popoola, A.P.I.; Popoola, O.M. Review on materials applied in electric transmission conductors. J. Mater. Sci. 2022, 57, 1581–1598. [Google Scholar] [CrossRef]
- Zheng, K.; Politis, D.J.; Wang, L.; Lin, J. A review on forming techniques for manufacturing lightweight complex—Shaped aluminium panel components. Int. J. Light. Mater. Manuf. 2018, 1, 55–80. [Google Scholar] [CrossRef]
- Kermanidis, A.T. Aircraft Aluminum Alloys: Applications and Future Trends. In Revolutionizing Aircraft Materials and Processes; Pantelakis, S., Tserpes, K., Eds.; Springer: Cham, Switzerland, 2020; pp. 21–55. [Google Scholar]
- Yang, C.; Zhang, L.; Chen, Z.; Gao, Y.; Xu, Z. Dynamic material flow analysis of aluminum from automobiles in China during 2000-2050 for standardized recycling management. J. Clean. Prod. 2022, 337, 130544. [Google Scholar] [CrossRef]
- Stemper, L.; Tunes, M.A.; Tosone, R.; Uggowitzer, P.J.; Pogatscher, S. On the potential of aluminum crossover alloys. Prog. Mater. Sci. 2022, 124, 100873. [Google Scholar] [CrossRef]
- Asadikiya, M.; Yang, S.; Zhang, Y.; Lemay, C.; Apelian, D.; Zhong, Y. A review of the design of high-entropy aluminum alloys: A pathway for novel Al alloys. J. Mater. Sci. 2021, 56, 12093–12110. [Google Scholar] [CrossRef]
- Raabe, D.; Ponge, D.; Uggowitzer, P.; Roscher, M.; Paolantonio, M.; Liu, C.; Antrekowitsch, H.; Kozeschnik, E.; Seidmann, D.; Gault, B.; et al. Making sustainable aluminium by recycling scrap: The science of “dirty” alloys. Prog. Mater. Sci. 2022, 128, 100947. [Google Scholar] [CrossRef]
- Arowosola, A.; Gaustad, G. Estimating increasing diversity and dissipative loss of critical metals in the aluminum automotive sector. Resour. Conserv. Recycl. 2019, 150, 104382. [Google Scholar] [CrossRef]
- Capuzzi, S.; Timelli, G. Preparation and melting of scrap in aluminum recycling: A review. Metals 2018, 8, 249. [Google Scholar] [CrossRef]
- Niu, G.; Wang, J.; Ye, J.; Mao, J. Enhancing Fe content tolerance in A356 alloys for achieving low carbon footprint aluminum structure castings. J. Mater. Sci. Technol. 2023, 161, 180–191. [Google Scholar] [CrossRef]
- Polmear, I.; StJohn, D.; Nie, J.F.; Qian, M. Light Alloys: Metallurgy of the Light Metals, 5th ed.; Elsevier, Butterworth-Heinemann: Oxford, UK, 2017; pp. 31–107. [Google Scholar] [CrossRef]
- Mondol, S.; Alam, T.; Banerjee, R.; Kumar, S.; Chattopadhyay, K. Development of a high temperature high strength Al alloy by addition of small amounts of Sc and Mg to 2219 alloy. Mater. Sci. Eng. A 2017, 687, 221–231. [Google Scholar] [CrossRef]
- He, H.; Yi, Y.; Huang, S.; Zhang, Y. Effects of cold predeformation on dissolution of second-phase Al2Cu particles during solution treatment of 2219 Al-Cu alloy forgings. Mater. Charact. 2018, 135, 18–24. [Google Scholar] [CrossRef]
- Zuiko, I.; Kaibyshev, R. Aging behavior of an Al–Cu–Mg alloy. J. Alloys Compd. 2018, 759, 108–119. [Google Scholar] [CrossRef]
- de Sousa Araujo, J.V.; Milagre, M.X.; Ferreira, R.O.; de Souza Carvalho Machado, C.; de Abreu, C.P.; Costa, I. Microstructural characteristics of the Al alloys: The dissimilarities among the 2XXX alloys series used in aircraft structures. Metallogr. Microstruct. Anal. 2020, 9, 744–758. [Google Scholar] [CrossRef]
- Kumar, N.S.; Pramod, G.K.; Samrat, P.; Sadashiva, M. A critical review on heat treatment of aluminium alloys. Mater. Today Proc. 2022, 58, 71–79. [Google Scholar] [CrossRef]
- Sirichaivetkul, R.; Limmaneevichitr, C.; Tongsri, R.; Kajornchaiyakul, J. Isothermal Investigation and Deformation Behavior during Homogenization of 6063 Aluminum Alloy. J. Mater. Eng. Perform. 2023, 32, 638–650. [Google Scholar] [CrossRef]
- Rinderer, B. The metallurgy of homogenization. Mater. Sci. Forum. 2011, 693, 264–275. [Google Scholar] [CrossRef]
- Ber, L.B.; Kolobnev, N.I.; Tsukrov, S.L. Heat Treatment of Aluminum Alloys, 1st ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Belov, N.A.; Akopyan, T.K.; Shurkin, P.K.; Korotkova, N.O. Comparative analysis of structure evolution and thermal stability of experimental AA2219 and model Al-2wt.%Mn-2wt.%Cu cold rolled alloys. J. Alloys Compd. 2021, 864, 158823. [Google Scholar] [CrossRef]
- Belov, N.A.; Alabin, A.N.; Matveeva, I.A. Optimization of phase composition of Al–Cu–Mn–Zr–Sc alloys for rolled products without requirement for solution treatment and quenching. J. Alloys Compd. 2014, 583, 206–213. [Google Scholar] [CrossRef]
- Belov, N.A.; Korotkova, N.O.; Akopyan, T.K.; Tsydenov, K.A. Simultaneous Increase of Electrical Conductivity and Hardness of Al–1.5 wt.% Mn Alloy by Addition of 1.5 wt.% Cu and 0.5 wt.% Zr. Metals 2019, 9, 1246. [Google Scholar] [CrossRef]
- Belov, N.A.; Akopyan, T.K.; Korotkova, N.O.; Timofeev, V.N.; Shurkin, P.K. Effect of cold rolling and annealing temperature on structure, hardness and electrical conductivity of rapidly solidified alloy of Al–Cu–Mn–Zr system. Mater. Lett. 2021, 300, 130199. [Google Scholar] [CrossRef]
- Korotkova, N.O.; Shurkin, P.K.; Cherkasov, S.O.; Aksenov, A.A. Effect of Copper Concentration and Annealing Temperature on the Structure and Mechanical Properties of Ingots and Cold-Rolled Sheets of Al–2% Mn Alloy. Russ. J. Non-Ferr. Met. 2022, 63, 190–200. [Google Scholar] [CrossRef]
- Dar, S.M.; Liao, H. Creep behavior of heat resistant Al–Cu–Mn alloys strengthened by fine (θ′) and coarse (Al20Cu2Mn3) second phase particles. Mater. Sci. Eng. A 2019, 763, 138062. [Google Scholar] [CrossRef]
- Mikhaylovskaya, A.V.; Mukhamejanova, A.; Kotov, A.D.; Tabachkova, N.Y.; Prosviryakov, A.S.; Mochugovskiy, A.G. Precipitation Behavior of the Metastable Quasicrystalline I-Phase and θ′-Phase in Al-Cu-Mn Alloy. Metals 2023, 13, 469. [Google Scholar] [CrossRef]
- Belov, N.A.; Cherkasov, S.O.; Korotkova, N.O.; Yakovleva, A.O.; Tsydenov, K.A. Effect of Iron and Silicon on the Phase Composition and Microstructure of the Al–2% Cu–2% Mn (wt%) Cold Rolled Alloy. Phys. Met. Metallogr. 2021, 122, 1095–1102. [Google Scholar] [CrossRef]
- Shelekhov, E.V.; Sviridova, T.A. Programs for X-ray analysis of polycrystals. Met. Sci. Heat Treat. 2000, 42, 309–313. [Google Scholar] [CrossRef]
- Thermo-Calc Software. Available online: http://www.thermocalc.com (accessed on 27 June 2023).
- Tian, A.; Sun, L.; Deng, Y.; Yuan, M. Study of the Precipitation Kinetics, Microstructures, and Mechanical Properties of Al-Zn-Mg-xCu Alloys. Metals 2022, 12, 1610. [Google Scholar] [CrossRef]
- Cinkilic, E.; Yan, X.; Luo, A.A. Modeling Precipitation Hardening and Yield Strength in Cast Al-Si-Mg-Mn Alloys. Metals 2020, 10, 1356. [Google Scholar] [CrossRef]
- Dixit, M.; Mishra, R.S.; Sankaran, K.K. Structure–property correlations in Al 7050 and Al 7055 high-strength aluminum alloys. Mater. Sci. Eng. A 2008, 478, 163–172. [Google Scholar] [CrossRef]
- Thangaraju, S.; Heilmaier, M.; Murty, B.S.; Vadlamani, S.S. On the Estimation of True Hall–Petch Constants and Their Role on the Superposition Law Exponent in Al Alloys. Adv. Eng. Mater. 2012, 14, 892–897. [Google Scholar] [CrossRef]
- Zhu, A.W.; Starke, E.A. Strengthening effect of unshearable particles of finite size: A computer experimental study. Acta Mater. 1999, 47, 3263–3269. [Google Scholar] [CrossRef]
- Starink, M.J.; Wang, S.C. A model for the yield strength of overaged Al–Zn–Mg–Cu alloys. Acta Mater. 2003, 51, 5131–5150. [Google Scholar] [CrossRef]
- Çadırlı, E.; Kaya, H.; Büyük, U.; Üstün, E.; Gündüz, M. Effect of heat treatment on the microstructures and mechanical properties of Al–4Cu–1.5 Mg alloy. Int. J. Metalcast. 2022, 16, 1020–1033. [Google Scholar] [CrossRef]
- Liang, S.S.; Wen, S.P.; Wu, X.L.; Huang, H.; Gao, K.Y.; Nie, Z.R. The synergetic effect of Si and Sc on the thermal stability of the precipitates in AlCuMg alloy. Mater. Sci. Eng. A 2020, 783, 139319. [Google Scholar] [CrossRef]
- Zamani, M.; Toschi, S.; Morri, A.; Ceschini, L.; Seifeddine, S. Optimisation of heat treatment of Al–Cu–(Mg–Ag) cast alloys. J. Therm. Anal. Calorim. 2020, 139, 3427–3440. [Google Scholar] [CrossRef]
- Kairy, S.K.; Rouxel, B.; Dumbre, J.; Lamb, J.; Langan, T.J.; Dorin, T.; Birbilis, N. Simultaneous improvement in corrosion resistance and hardness of a model 2xxx series Al-Cu alloy with the microstructural variation caused by Sc and Zr additions. Corros. Sci. 2019, 158, 108095. [Google Scholar] [CrossRef]
- Mondol, S.; Kashyap, S.; Kumar, S.; Chattopadhyay, K. Improvement of high temperature strength of 2219 alloy by Sc and Zr addition through a novel three-stage heat treatment route. Mater. Sci. Eng. A 2018, 732, 157–166. [Google Scholar] [CrossRef]
- Mondol, S.; Kumar, S.; Chattopadhyay, K. Effect of thermo-mechanical treatment on microstructure and tensile properties of 2219ScMg alloy. Mater. Sci. Eng. A 2019, 759, 583–593. [Google Scholar] [CrossRef]
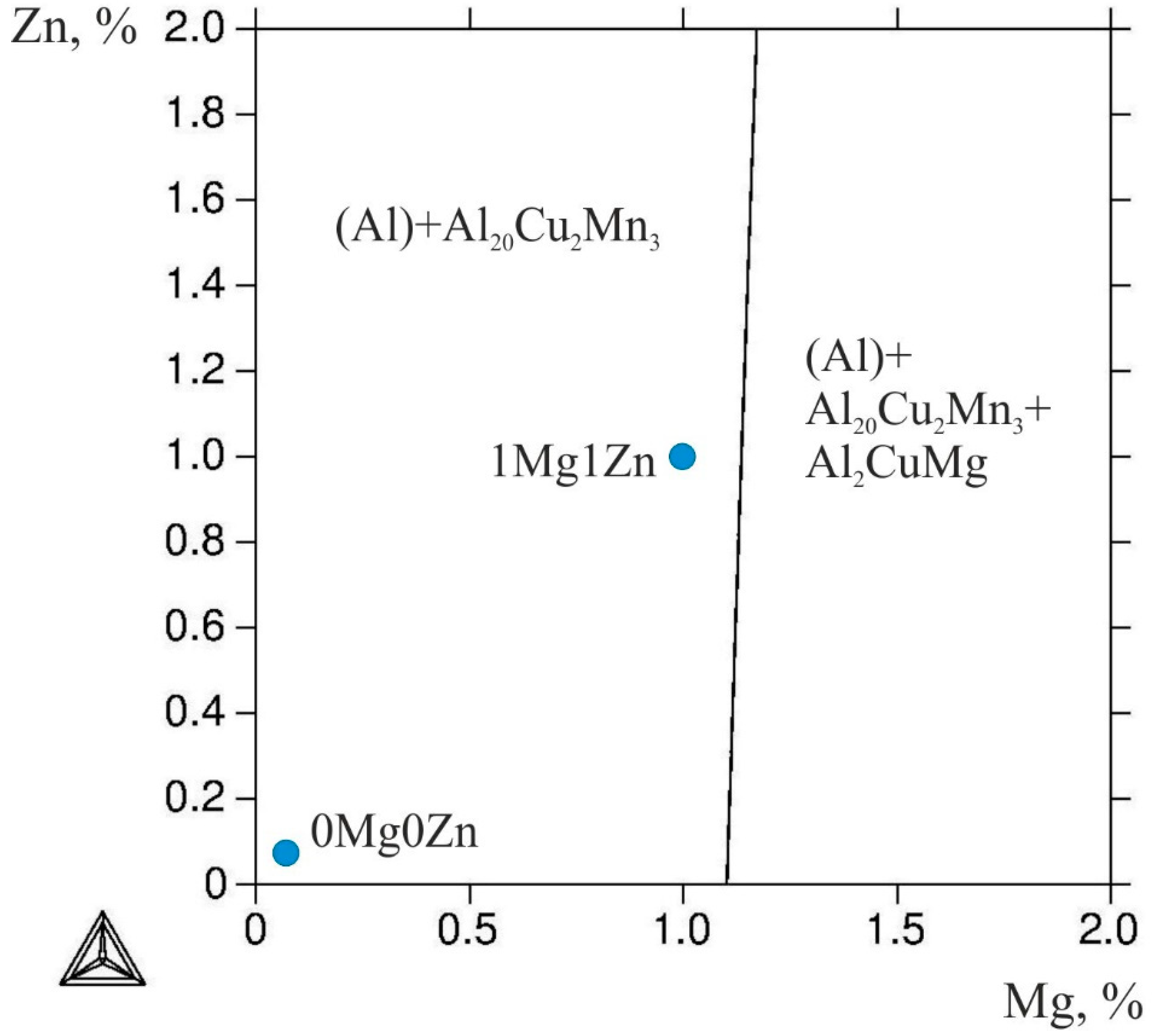







| Alloy Designation | Concentration, wt.% | ||||||
|---|---|---|---|---|---|---|---|
| Cu | Mn | Mg | Zn | Fe | Si | ||
| 0Mg0Zn | 2.06 | 1.46 | 0.03 | 0.03 | 0.11 | 0.08 | balance |
| 1Mg1Zn | 2.07 | 1.44 | 1.02 | 1.11 | 0.12 | 0.08 | balance |
| Process | Obtained Product | Designation |
|---|---|---|
| Casting | Ingot 10 mm × 40 mm × 180 mm | F |
| Hot rolling (at 400 °C) of the foundry ingot (10 mm × 40 mm × 180 mm) | Sheet 2 mm in thickness | HR |
| HR + 300 °C, 3 h | Annealed hot rolled sheet | HR300 |
| HR300 + 350 °C, 3 h | HR350 | |
| HR350 + 400 °C, 3 h | HR400 | |
| HR400 + 450 °C, 3 h | HR450 | |
| HR450 + 500 °C, 3 h | HR500 | |
| Cold rolling of the rolled sheet (from HR350, sheet 0.5 mm) | Sheet 0.5 mm in thickness | CR |
| CR + 350 °C, 3 h | Annealed cold rolled sheet | CR350 |
| CR + 400 °C, 3 h | CR400 |
| Alloy 1 | Heat Treatment Process 2 | HV, MPa | UTS, MPa | YS, MPa | El, % |
|---|---|---|---|---|---|
| 0Mg0Zn | CR | 102.4 | 345 | 339 | 0.6 |
| CR350 | 84.4 | 297 | 238 | 4.8 | |
| CR400 | 84.2 | 304 | 231 | 7.1 | |
| 1Mg1Zn | CR | 122.9 | 422 | 417 | 1.6 |
| CR350 | 96.1 | 346 | 279 | 5.5 | |
| CR400 | 100.6 | 358 | 280 | 6.0 | |
| Average deviations | 1.5 | 8 | 4 | 0.5 | |
| Alloy 1 | T, °C | Fractions of Precipitates, wt.% | |||||
|---|---|---|---|---|---|---|---|
| Al20 | Al15 | Al2Cu | Mg2Si | S | (Al) | ||
| 0Mg0Zn | 350 | 6.63 | 0.99 | 0.45 | – | – | balance |
| 400 | 6.55 | 0.95 | – | – | – | balance | |
| 450 | 6.25 | 0.92 | – | – | – | balance | |
| 1Mg1Zn | 350 | 6.66 | 0.94 | – | 0.04 | 1.30 | balance |
| 400 | 6.51 | 0.97 | – | 0.02 | 0.15 | balance | |
| 450 | 6.18 | 0.99 | – | – | – | balance | |
| Alloy 1 | T, °C | Concentration in (Al), wt.% | |||||
|---|---|---|---|---|---|---|---|
| Cu | Mn | Mg | Zn | Si | Fe | ||
| 0Mg0Zn | 350 | 0.87 | 0.02 | 0.03 | 0.03 | <0.01 | <0.01 |
| 400 | 1.14 | 0.06 | 0.03 | 0.03 | 0.01 | <0.01 | |
| 450 | 1.19 | 0.13 | 0.03 | 0.03 | 0.01 | <0.01 | |
| 1Mg1Zn | 350 | 0.51 | 0.04 | 0.85 | 1.21 | <0.01 | <0.01 |
| 400 | 1.09 | 0.05 | 1.07 | 1.20 | <0.01 | <0.01 | |
| 450 | 1.21 | 0.12 | 1.10 | 1.20 | 0.01 | <0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belov, N.; Akopyan, T.; Tsydenov, K.; Letyagin, N.; Fortuna, A. Structure Evolution and Mechanical Properties of Sheet Al–2Cu–1.5Mn–1Mg–1Zn (wt.%) Alloy Designed for Al20Cu2Mn3 Disperoids. Metals 2023, 13, 1442. https://doi.org/10.3390/met13081442
Belov N, Akopyan T, Tsydenov K, Letyagin N, Fortuna A. Structure Evolution and Mechanical Properties of Sheet Al–2Cu–1.5Mn–1Mg–1Zn (wt.%) Alloy Designed for Al20Cu2Mn3 Disperoids. Metals. 2023; 13(8):1442. https://doi.org/10.3390/met13081442
Chicago/Turabian StyleBelov, Nikolay, Torgom Akopyan, Kirill Tsydenov, Nikolay Letyagin, and Anastasya Fortuna. 2023. "Structure Evolution and Mechanical Properties of Sheet Al–2Cu–1.5Mn–1Mg–1Zn (wt.%) Alloy Designed for Al20Cu2Mn3 Disperoids" Metals 13, no. 8: 1442. https://doi.org/10.3390/met13081442
APA StyleBelov, N., Akopyan, T., Tsydenov, K., Letyagin, N., & Fortuna, A. (2023). Structure Evolution and Mechanical Properties of Sheet Al–2Cu–1.5Mn–1Mg–1Zn (wt.%) Alloy Designed for Al20Cu2Mn3 Disperoids. Metals, 13(8), 1442. https://doi.org/10.3390/met13081442







