Abstract
TC4 alloy is widely used in dental implantation due to its excellent biocompatibility and low density. However, it is necessary to further improve the corrosion resistance and surface hardness of the titanium alloy to prevent surface damage that could result in the release of metal ions into the oral cavity, potentially affecting oral health. In this study, Ti-N-O layers were fabricated on the surface of TC4 alloy using a two-step hollow cathode plasma source oxynitriding technique. This resulted in the formation of TiN, Ti2N, TiO2, and nitrogen-stabilized α(N)-Ti phases on the TC4 alloy, forming a Ti-N-O modified layer. The microhardness of the samples treated with plasma oxynitriding (PNO) was found to be 300–400% higher than that of untreated (UN) samples. The experimental conditions were set at 520 °C, and the corrosion current density of the PNO sample was measured to be 7.65 × 10−8 A/cm2, which is two orders of magnitude lower than that of the UN sample. This indicates that the PNO-treated TC4 alloy exhibited significantly improved corrosion resistance in the artificial saliva solutions.
1. Introduction
Titanium alloys are widely used for manufacturing medical implants in humans due to their exceptional properties, such as high specific strength, fatigue strength, and biocompatibility [1,2]. These properties make them an ideal choice for implants that require durability and the ability to withstand the corrosive environment of the human body. When exposed to air at ambient temperatures, titanium and its alloys form a surface oxide layer. However, this naturally occurring oxide layer can degrade in certain corrosive environments, leading to its dissolution [3]. The natural oxide film is susceptible to dissolution in reducing or complex media and can rapidly decompose in acidic or fluoride solutions [4,5]. Increased acidity of the medium or the presence of ions (F−, Cl−, ) can increase the corrosiveness of the medium, reduce the protective properties of the oxide passivation layer, or accelerate its degradation rate [6,7]. While titanium alloys are vulnerable to fluoride ions, other active anions, such as Cl−, can also harm the oxide layer of titanium alloys. For instance, Cl− present in artificial saliva can be adsorbed on the oxide film, leading to its decomposition [8]. In successful cases involving surgical and medical instruments, various surface engineering technologies, such as physical vapor deposition (PVD), chemical vapor deposition (CVD), and plasma nitriding (PN), have been employed to enhance biocompatibility, antibacterial effects, and high corrosion resistance in the human body [9,10]. Among these techniques, PN is extensively used as a surface modification method capable of forming relatively thick, complex, adherent films with corrosion resistance [11,12,13]. TiN coating, in particular, exhibits good biocompatibility and holds promise for various applications in human implants [14]. However, in the harsh oral corrosion environment, a TiN protective layer alone cannot effectively prevent oral bacterial infection, consequently impacting its antibacterial activity. Wang et al. demonstrated that TiN-coated samples on pure titanium experienced erosion and damage when exposed to fluoride-containing artificial saliva [15]. Therefore, further improvements in the performance of the TiN-modified layer are necessary.
Wierzchoń et al. [16] conducted a study in which they utilized a plasma-assisted oxidation-nitridation process to prepare a TiO2 + Ti2N + Ti(N) diffusion layer, resulting in improved corrosion and wear resistance of the substrate material. Furthermore, the presence of nano-crystalline titanium oxide (rutile) demonstrated enhanced biological performance when titanium and its alloys came into contact with blood. The formation of TiNxOy metal compounds involves the replacement of nitrogen atoms with oxygen atoms in the face-centered cubic (fcc) lattice of TiN, causing lattice distortion and the introduction of defects, ultimately improving the properties of the material [17]. The incorporation of oxygen into the cationic fcc sublattice plays a key role in this transformation. Banakh et al. [18] found that titanium nitride coatings were deposited on commercially pure titanium and observed significant bioactivity. In another study, Albayrak et al. [19] investigated the nitriding and oxynitride behavior of CP-Ti samples through the anodization of the TiO2 layer after the plasma nitriding process. The porous oxynitride samples showed better corrosion resistance.
The hollow cathode discharge effect plays a crucial role in enhancing the efficiency of ionization and creating a denser plasma through multiple electron–gas collisions [20,21,22]. This effect is particularly advantageous for the nitriding of titanium alloys, which require high diffusion temperatures. The use of high-density plasma allows higher and more controlled processing temperatures to be achieved in a shorter period [23]. In the nitriding furnace, a hollow cathode device is employed to generate a hollow cathode discharge, serving as a powerful working medium and an effective heat source for heating the workpiece [24]. Plasma nitriding technology offers numerous benefits, including rapid nitriding speed, high efficiency, energy saving, and the production of high-quality, corrosion-resistant nitride layers at low temperatures [25,26]. In this study, a two-step hollow cathodic plasma source oxynitride was employed to prepare a Ti-N-O modified layer on a TC4 alloy substrate. The surface treatment involved plasma nitriding followed by plasma oxynitride, resulting in the formation of a nitride layer that subsequently transformed into a Ti-N-O modified layer. By utilizing this duplex technology, it is anticipated that high-quality composite modified layers can be achieved, leading to improved corrosion resistance of titanium alloys in artificial saliva solutions.
2. Materials and Methods
2.1. Preparation of Ti-N-O Compound Layer
The material used for the experiments was TC4 alloy bolts, size M8 × 13 mm. The samples were acid washed with HF-HNO3 (45 mL HF (49%) + 205 mL HNO3 (65%) + 750 mL deionized water) solution to wash off the oil stains and oxide on the surface. The duration of the acid wash was ten minutes. Finally, the samples were cleaned separately using an ultrasonic treatment for 10 min with acetone and anhydrous ethanol. The samples were dried and loaded into the nitriding equipment.
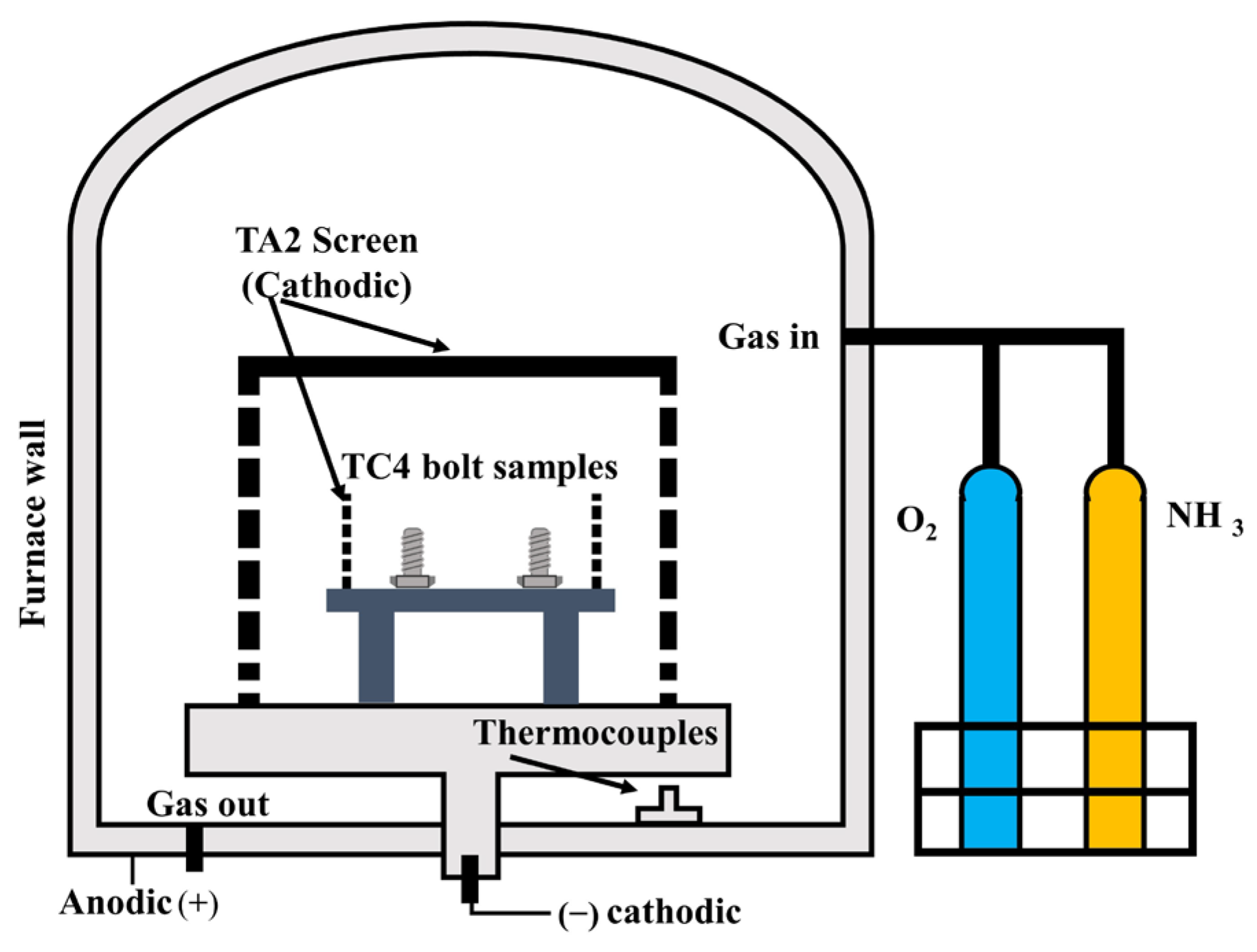
The self-developed experimental hollow cathode device is shown in Figure 1. This hollow cathode device intensified the collision between the plasma and the TA2 screen, thereby increasing the plasma concentration. This enabled the sample to achieve plasma nitriding rapidly. The parameters for the surface treatment of samples are shown in Table 1.
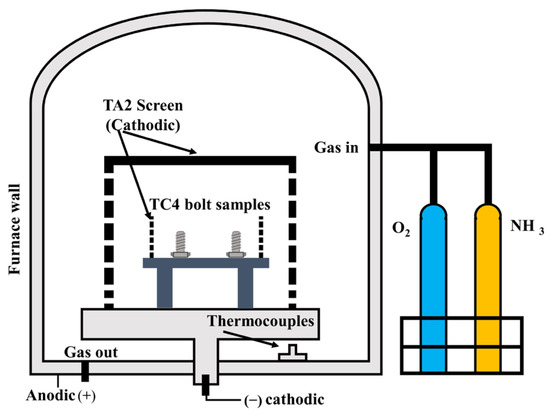
Figure 1.
Structure diagram of hollow cathode device.

Table 1.
Parameters for surface treatment of samples.
2.2. Performance Test and Organization Observation
X-ray diffraction (XRD) analysis was performed using a D8ADVANCE X-ray diffractometer system made by BRUKER with Cu-Kα radiation. The analysis involved scanning in the 2θ range of 20°–90° to evaluate the crystal structure of UN and PNO samples. The microstructure of the sample section was observed using a Zeiss Axio Observer 3M metallographic microscope, and the surface hardness was measured with an HXD-1000TM/LCD microhardness tester. X-ray photoelectron spectroscopy (XPS) analysis of the surface chemical composition was conducted using a PHI Quantra II system from JAPAN, and data were processed using CasaXPS software. The analysis used peak calibration with energy C1s (284.8 eV). For electrochemical testing, a CS310 electrochemical workstation was used with a three-electrode system. An artificial saliva composition was used (see Table 2) and maintained at 37 °C and pH 6.65 ± 0.01. The titanium bolt was connected to a silver wire and immersed in the artificial saliva as a working electrode. The contact area between the silver wire and the bolt was excluded from the analysis (illustrated in Figure 1), and the surface area of the electrode was approximately 215 mm2. A reference electrode of Ag/AgCl was used, and a platinum sheet was the auxiliary electrode. An open circuit potential test was performed on the sample for 10 min. Electrochemical impedance spectroscopy (EIS) measurement was conducted using a sinusoidal potential perturbation of 10 mV in the frequency range of 10−2–105 Hz. On the basis of the EIS results, Nyquist and Bode plots of the sample were obtained, and a dynamic potential polarization test was performed in the range of 0.6–1.5 V (relative to the reference electrode) at a scanning rate of 0.5 mV/s. Three replicate experiments were carried out, and the experimental data were fitted using Zview-2 and Origin 2018 software.

Table 2.
Chemical compositions of artificial saliva.
3. Results and Analysis
3.1. Analysis of Organization and Physical Structure
3.1.1. The Thickness of The Composite Layer of The PNO Samples
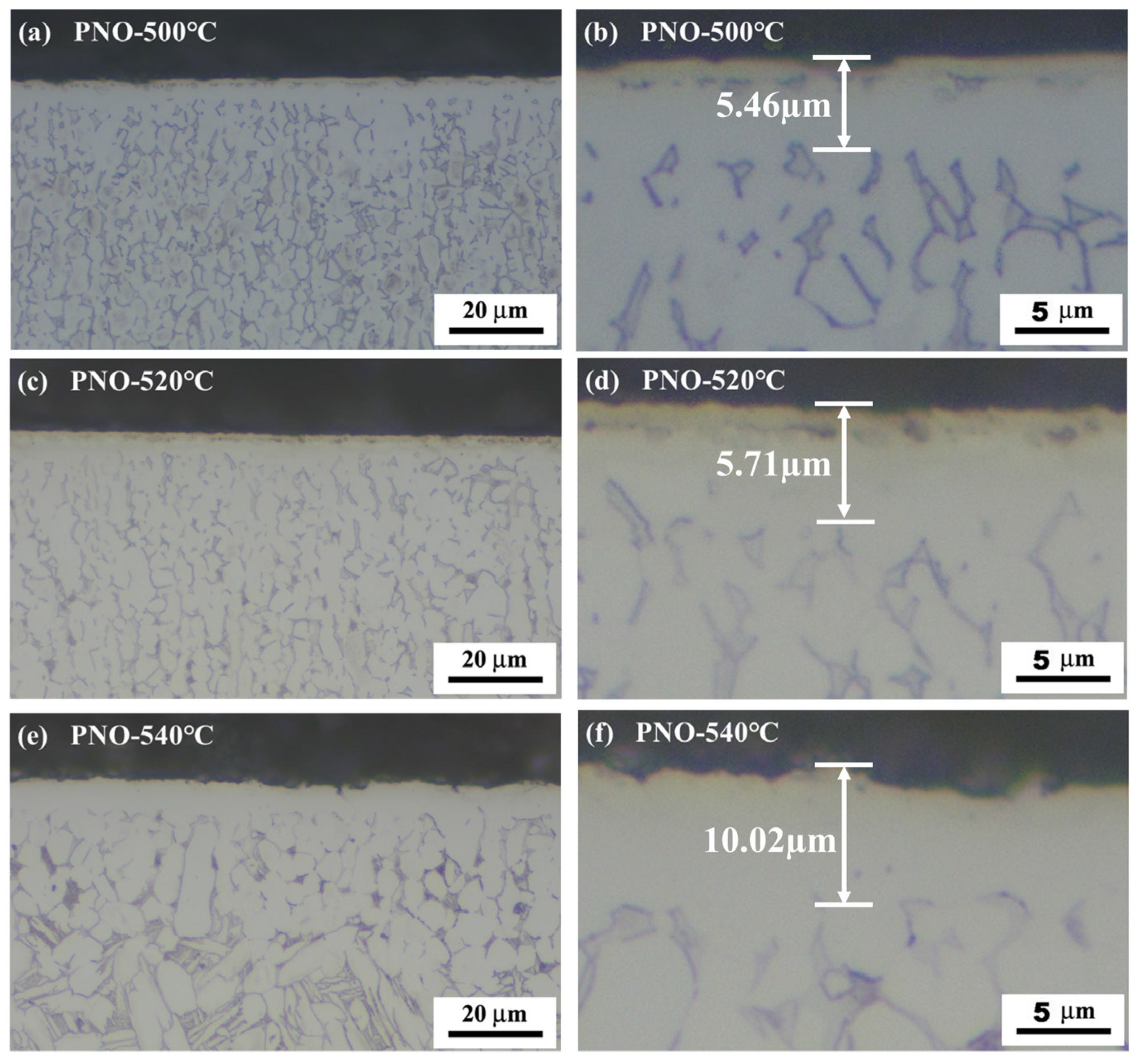
The cross-sectional morphology of the PNO samples after corrosion with the Kroll solution is shown in Figure 2. The PNO samples had a prominent bright white layer on the surface, which was not affected by the corrosive solution. The Ti-N-O composite layer of titanium was superior to the substrate in terms of corrosion resistance. Observations revealed a light-yellow layer above the white and bright layer on the PNO sample. The reason behind this phenomenon is the formation of TiN, which presents a characteristic golden-yellow hue resulting from the diffusion of nitrogen into the TC4 alloy [27]. For the PNO samples, the thickness of the composite layer was approximately 5.5 μm, 5.7 μm, and 10.0 μm, respectively.
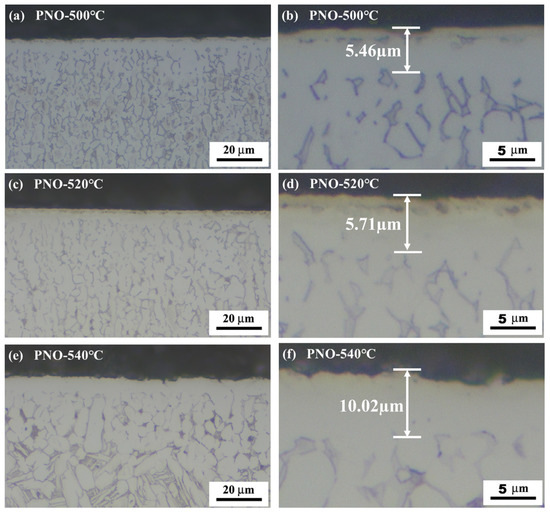
Figure 2.
Cross-sectional micrographs of UN and PNO samples.
3.1.2. Phase Determination
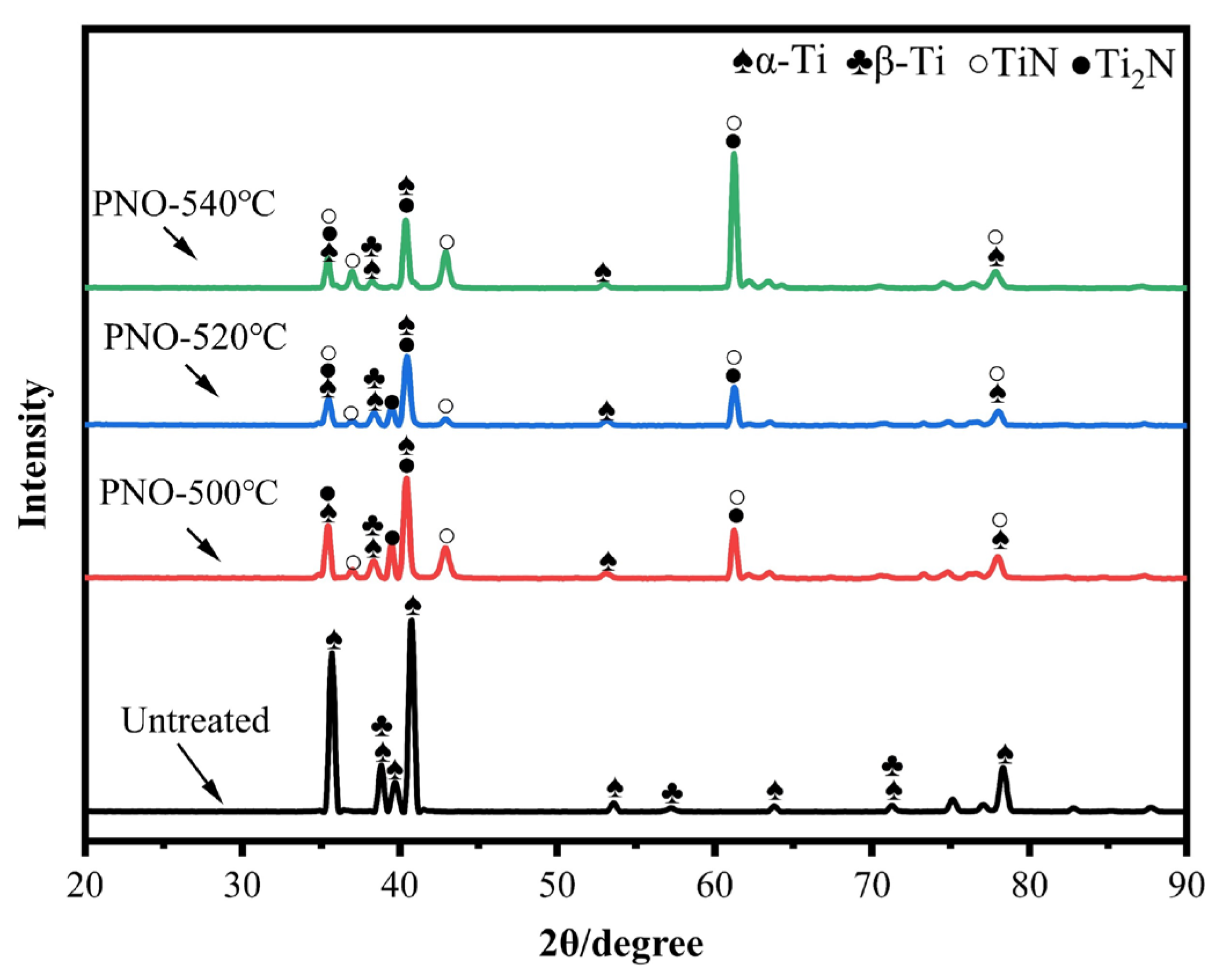
Figure 3 shows the XRD patterns of the UN and the PNO samples. The UN sample consisted of α-Ti with a close-packed hexagonal lattice (hcp) (100) orientation and β-Ti with a body-centered cubic structure (bcc) (101) orientation. For the PNO samples compared with the original sample, the α-Ti peak was weakened. The β-Ti was unchanged, while the diffraction peaks of TiN (PDF#38-1420) and Ti2N (PDF#76-0198) and some TiNx peaks appeared. In addition, both the α-Ti (100) and (101) diffraction peaks (PDF # 89-3725) shifted to a low angle, which indicates that the crystal lattice was distorted during the process of oxynitride [28,29]. The Ti2N phase was generated at a lower temperature of 500 °C, while the intensity of the TiN peak increased at higher temperatures of 520 and 540 °C. The main phases of the PNO samples were TiN and Ti2N.
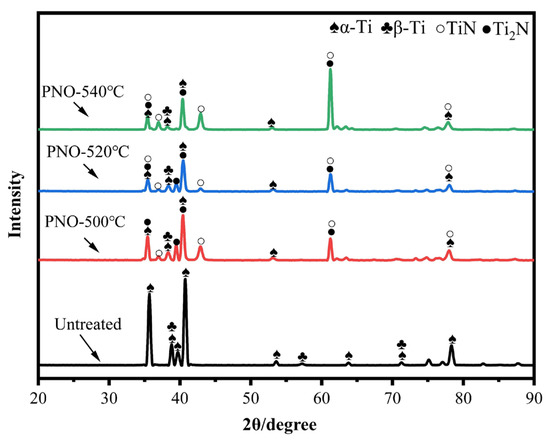
Figure 3.
X-ray diffraction patterns of UN and PNO samples.
3.1.3. XPS Analysis of PNO-520 Samples before Artificial Saliva Corrosion
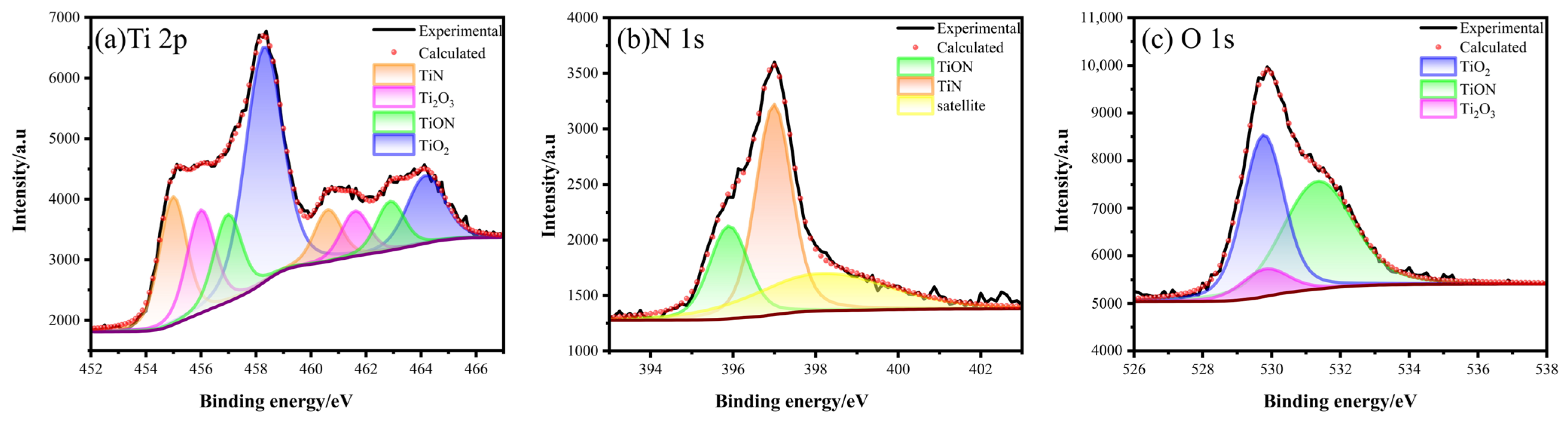
As shown in Figure 4, the Ti2p peak spectrum suggested that several components were present, including TiO2, TiN, TiON, and Ti2O3. By analyzing the binding energy, we can distinguish between the different components. Specifically, the presence of TiN can be identified by the binding energies of 454.98 and 460.61 eV, while TiON in TiNxOy can be represented by the binding energies of 455.99 and 461.60 eV [30,31]. The binding energy at 458.28 (standard value 458.3) and 464.16 eV (average value 464.19 eV) correspond to TiO2. This could be attributed to the distortion of the lattice structure in TiO2 caused by N-doping during plasma source oxynitride, which shifts the energy levels of the orbitals [32]. The binding energies of Ti2O3 were 456.96 and 462.87 eV [33]. The binding energy points of 395.8 and 396.98 eV in N1s corresponded to TiON and TiN, respectively. Three peaks were found in O1s, namely TiO2, Ti2O3, and TiON, with binding energies of 529.76, 529.8, and 531.34 eV, respectively [34].
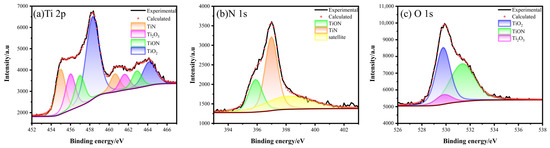
Figure 4.
XPS spectra of Ti2p, N1s, and O1s of PNO−520 sample.
3.2. Analysis of Mechanical Properties
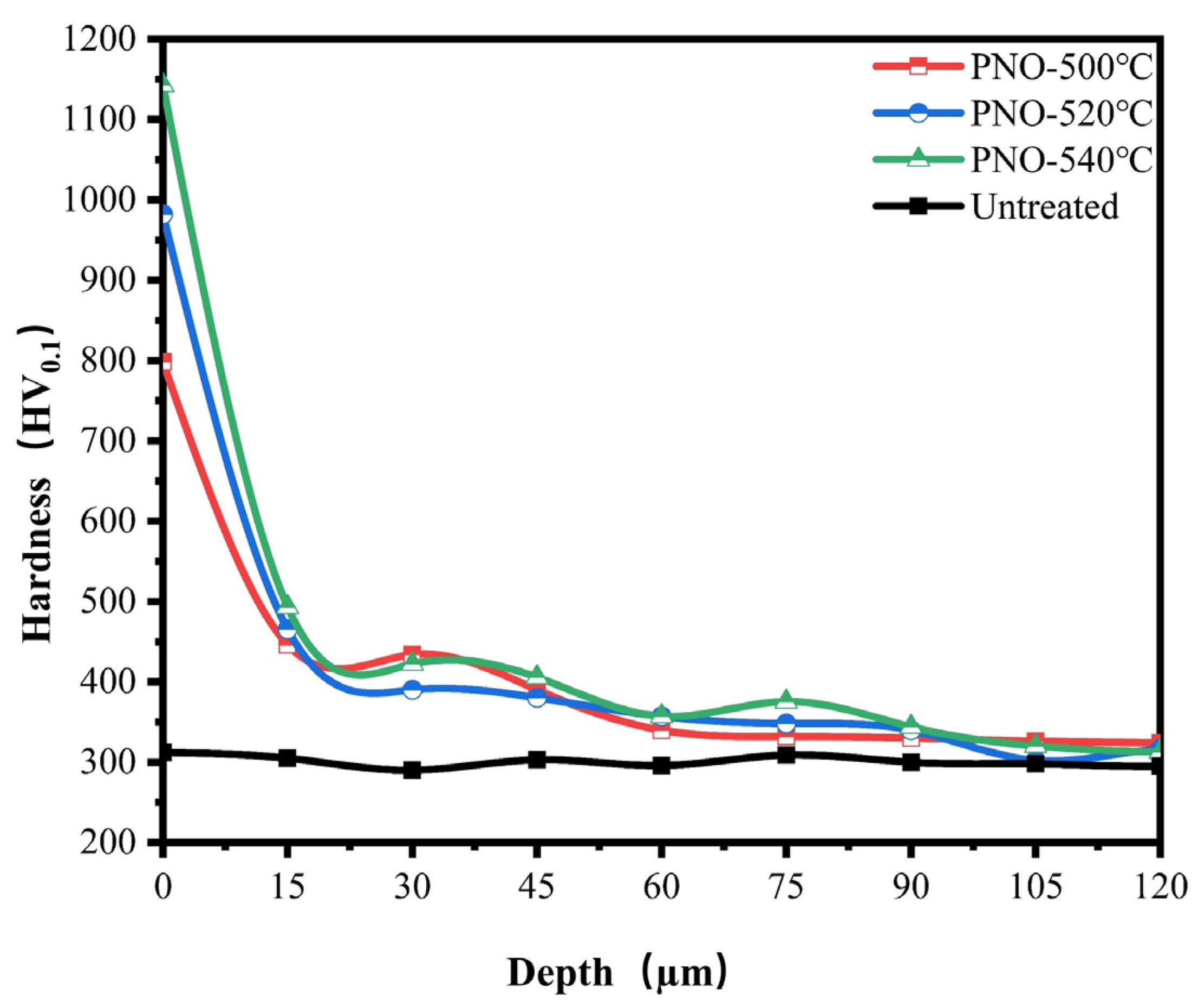
As shown in Figure 5, the surface hardness of the PNO samples was significantly improved compared with the matrix hardness of TC4 alloy (300.0 HV0.1). The surface hardness values of the PNO samples were 798.3 HV0.1, 982.1 HV0.1, and 1142.7 HV0.1, respectively. The results obtained from XRD indicated that TiN and Ti2N phases were on the surface of the PNO samples. The primary factor behind the increased surface hardness was the presence of the TiN and Ti2N phases [35,36]. As their content increases, so does the hardness of the surface. The microhardness change is associated with the treatment temperature. With the rise in the oxynitride temperature, the depth of the diffusion layer increased gradually. The diffusion layers of the PNO-500, PNO-520, and PNO-540 samples were 45 μm, 48 μm, and 75 μm, respectively. Nitrogen and titanium form an interstitial solid solution in the diffusion layer, improving the substrate’s surface hardness.
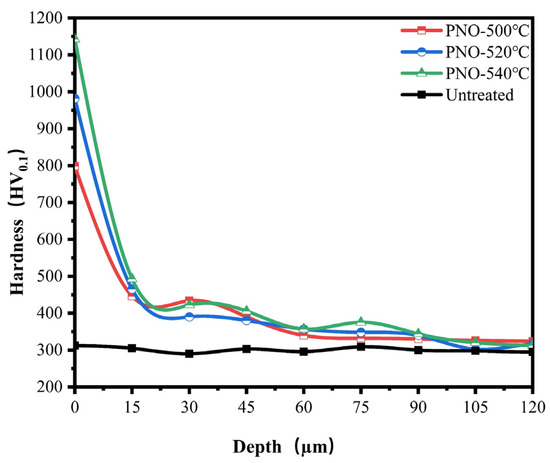
Figure 5.
Microhardness depth distribution of UN and PNO samples.
3.3. Analysis of Corrosion Resistance Properties
3.3.1. Analysis of Polarization Curves
When titanium alloys are implanted into the human body as dental implants, the saliva in the human mouth can corrode and degrade implanted titanium alloy teeth. The corrosion of this process is simulated through an electrochemical corrosion test. In the electrochemical corrosion experiment of artificial saliva, bubbles can be observed on the sample’s surface. This phenomenon indicates that a hydrogen evolution reaction occurs during the corrosion process (1) [37,38].
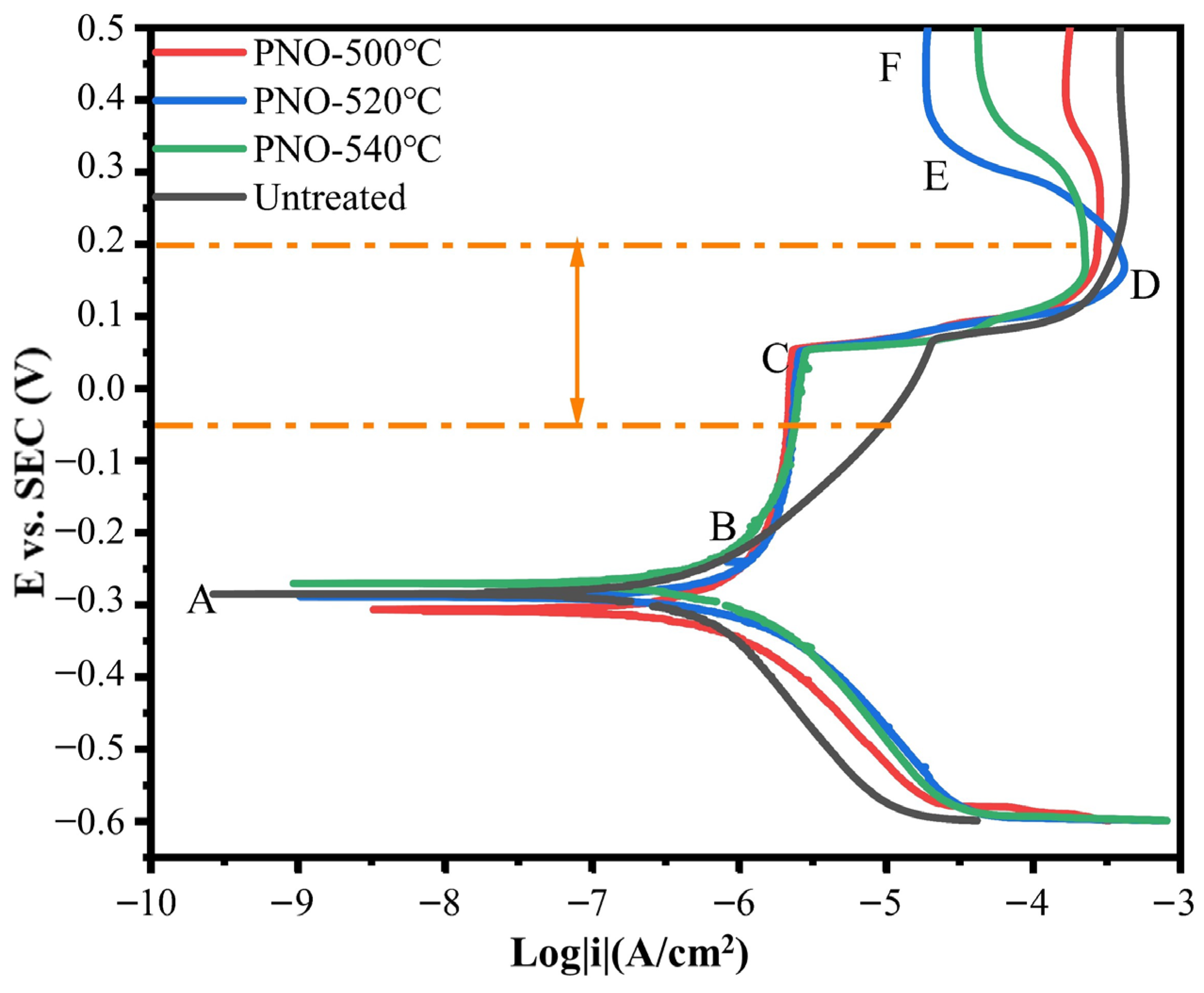
As shown in Figure 6, in the AB anodic region, the titanium alloy dental implant sample oxide began to dissolve, and the activation polarization affected the dissolution rate. The current density of the PNO sample changed very little at a potential of −0.2–0.1 V and hardly changed with the potential. In addition, the BC region was the active–passive transition region, where the formation of TiO2 passivation film could prevent the further dissolution of the base material [39]. The PNO samples had a common feature: the passivation film was generated at almost the same current density. By contrast, the UN sample generated passivation film at a much higher current density [40]. At 0.1–0.2 V, the passivation film dissolved, at which time the current density and potential increased. At point D, there was a sharp decrease in current density and a corresponding increase in potential, leading to the regeneration of the passivation film [41]. The potential for further growth in EF regional voltage is still significant, while the current remains relatively unchanged. The surface of the sample generated a dense passivation film, indicating that it was in a stable passivation stage. The anodic polarization suggested that the passivation film on the PNO samples underwent a repassivation process involving forming, dissolving, and regenerating the indicated morphology of the anode region, suggesting the presence of a protective layer. By contrast, the cathode region produced predominantly hydrogen. Electrochemical polarization characteristic tests showed that the corrosion resistance of the PNO samples was better than that of the UN sample in the human oxidation potential (−58–212 mV) range.
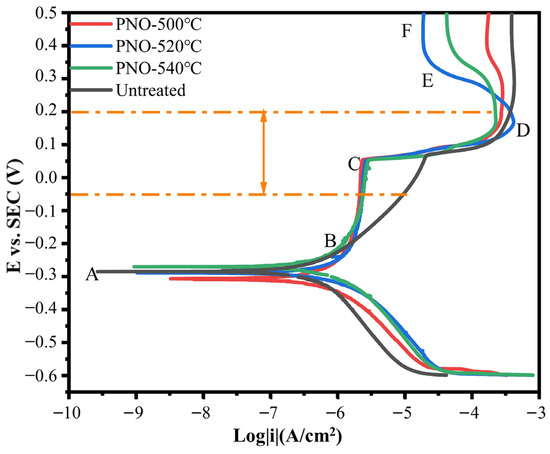
Figure 6.
Potentiodynamic polarization curves of UN and PNO samples in artificial saliva.
The polarization voltage, corrosion current density, and polarization resistance were obtained through fitting using Cview-2 software. As shown in Table 3, the corrosion current density calculated by the software was based on the Stern–Geary Equation (2) [42,43]:

Table 3.
The Icorr, Ecorr, and Rp of different samples.
Table 3 shows the data polarization potential (Ecorr), corrosion current density (Icorr), and polarization resistance (Rp) corresponding to the polarization curves. Ecorr reflects the thermodynamic tendency for corrosion to occur [44]. The higher the value of Ecorr, the lower the corrosion tendency of the sample [45]. According to the Ecorr, the UN sample was −0.289 V. As the temperature increased, the Ecorr of PNO-540 was −0.265 V. It can be seen that temperature had a particular influence on the Ecorr of the TC4 alloy dental implant bolts. The corrosion resistance of a material can be judged by its Icorr. The lower the Icorr value, the better the corrosion resistance of the material [46]. After the treatment, the Icorr and Rp of the PNO-520 sample were 7.65 × 10−8 A/cm2 and 3.40 × 105 Ω·cm2, respectively. The Icorr and Rp of the PNO-540 sample were 9.35 × 10−7 A/cm2 and 2.78 × 104 Ω·cm2, respectively. The Icorr value of the PNO-520 sample was lower than the Icorr values of the PNO-500 and PNO-540 samples. Similarly, the Rp value of the PNO-520 sample was one order of magnitude higher than those of the PNO-500 and PNO-540 samples. A sufficiently thick Ti-N-O layer was found to reduce the Icorr values of the substrate. However, if the nitride layer is too thin, it reduces the resistance to Cl− ions in the solution, thereby reducing the Icorr values [47]. Moreover, when the thickness of Ti-N-O was high enough to prevent the adsorption of Cl− ions in artificial saliva, the surface roughness became the main factor that affected the corrosion resistance of the sample. During the process of hollow cathodic plasma source oxynitride, the sample was bombarded by the plasma. The higher the temperature, the more active the plasma, and the rougher the surface morphology of the sample [48]. Thus, it was observed that the corrosion resistance of the PNO-540 sample deteriorated as the surface roughness increased. Therefore, the PNO-520 sample exhibited the best corrosion resistance. Bao et al. [49] prepared TiNxOy coating on the TC4 surface through physical vapor deposition. The Icorr of the coating sample in an acidic solution (pH = 5.2) was only 6.0 × 10−8 A/cm2. Du et al. [50] produced various Ti-N-O coatings with an Icorr range of 100–500 nA/cm2 and an Rp range of 70–300 kΩ∙cm2. Meanwhile, Subramanian et al. [51] manufactured titanium oxynitride coatings with an Icorr of 0.67 × 10−7 A/cm2 and a Vcorr of 3.1 × 10−3 mm/a. The Icorr of the PNO-520 sample prepared by our process was 7.65 × 10−8 A/cm2, and the Rp was 3.40 × 105 Ω·cm2. The above results are similar, indicating that the Ti-N-O modified layer prepared by our process improved the corrosion resistance of the sample.
3.3.2. XPS Analysis of PNO-520 Samples after Artificial Saliva Corrosion
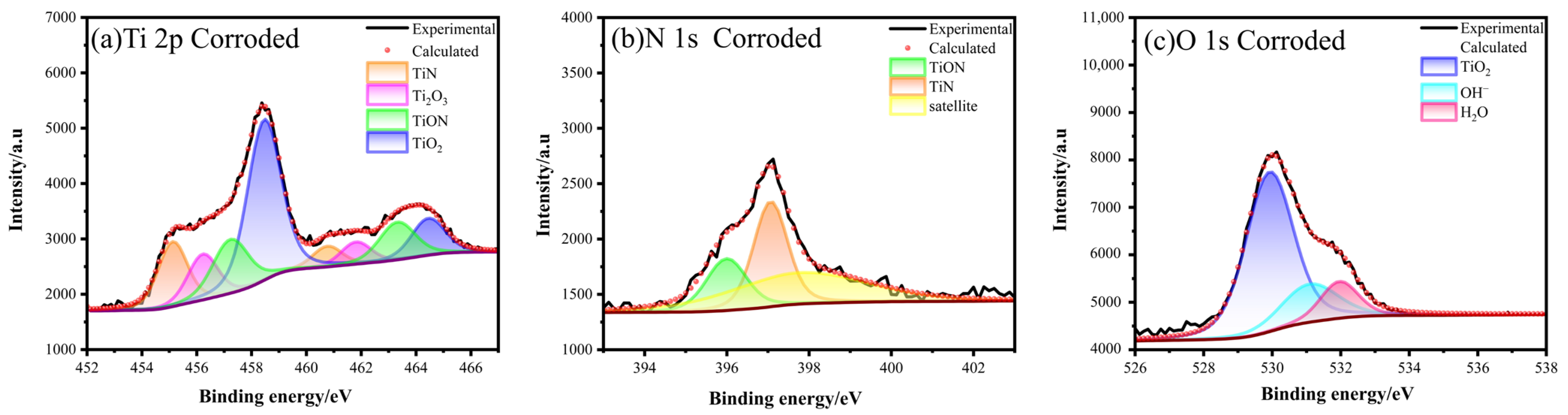
To elucidate the factors affecting the corrosion resistance of PNO samples in artificial saliva and their passivation film repassivation mechanism, XPS analysis was conducted on PNO-520 corrosion specimens (Figure 7). A Ti2O3 peak observed at the 456.2 and 461.81 eV binding energies in the Ti2p spectrum suggested that oxidation reactions occurred on the surface of the titanium alloy. The fitted peaks of Ti2p were all paired. The observed binding energies of Ti2p3/2 = 455.12, 457.2, and 458.46 eV, and Ti2p1/2 = 460.76, 463.31, and 464.48 eV indicated the presence of TiN, TiON, and TiO2. N1s correspond to TiON and TiN with binding energies of 395.99 and 397.06 eV (standard binding energy: 397.00 eV) with an overall decrease in the area of the peaks. The O1s orbital exhibited three closely fitted peaks. The binding energy of 529.92 eV is a prominent characteristic of TiO2. The binding energy of 531.09 eV meant that the hydration reaction of TiO2 in artificial saliva generated OH−. The binding energy at 531.96 eV represented H2O as the bound water absorbed by the sample oxide film [52]. The O1s peak showed that the passivation film mainly comprised TiO2, a small amount of Ti(OH)x, and an oxide film combined with water. The presence of these oxides improves the microstructure’s uniformity and improves the sample’s corrosion resistance [53].
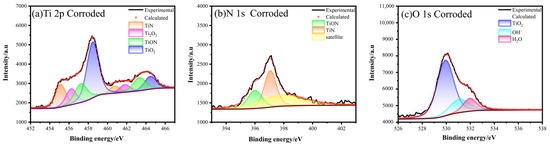
Figure 7.
XPS Spectra of Ti2p, N1s, and O1s after corrosion of PNO-520 sample in artificial saliva.
3.3.3. Electrochemical Impedance Spectroscopy Analysis of Different Samples
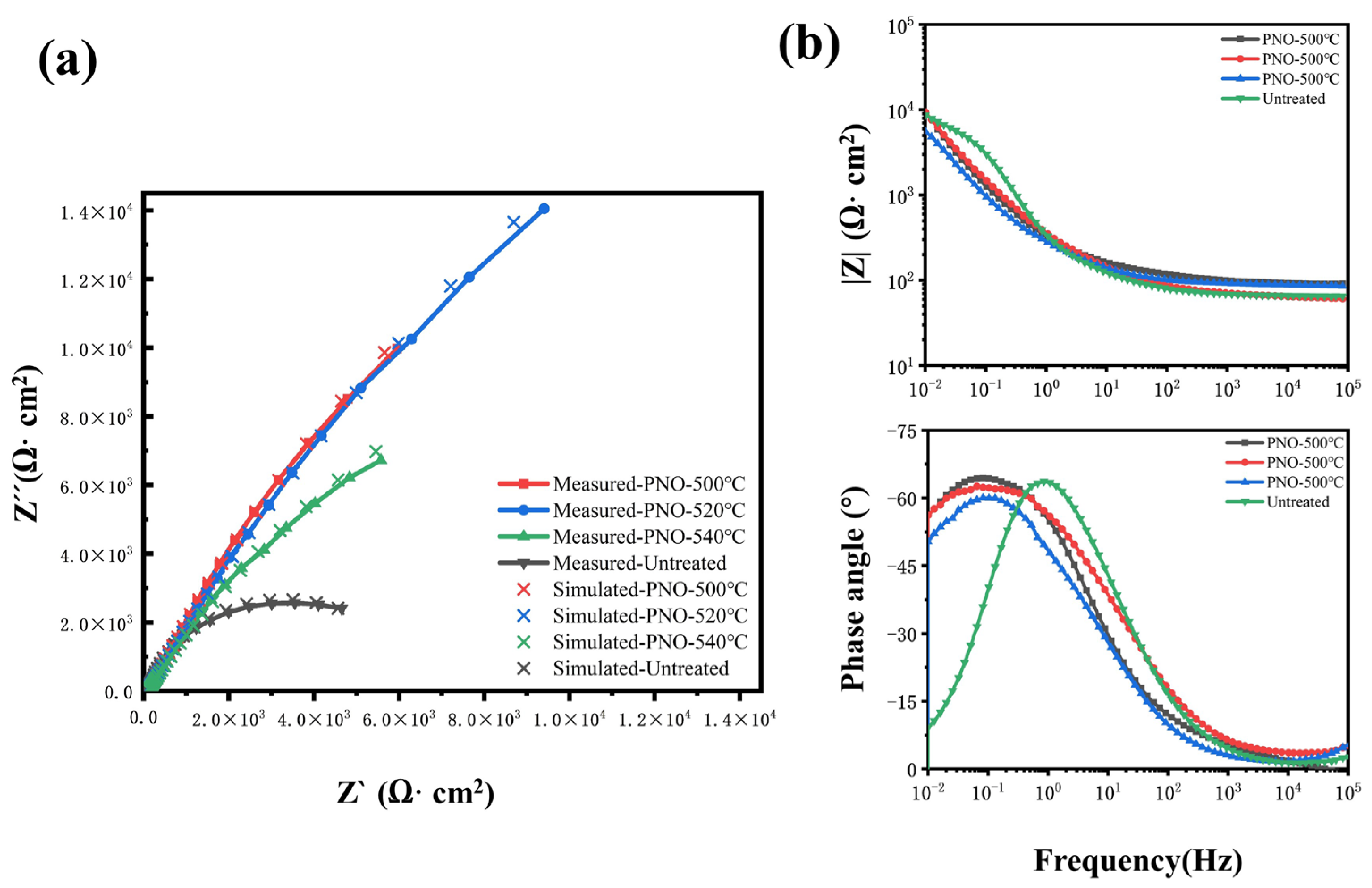
Figure 8 shows the Nyquist and Bode plots of TC4 alloy in human artificial saliva. The Nyquist curve represents the variation of impedance among different titanium alloy samples. The capacitance vs. frequency curve exhibited a flat, incomplete resistance arc across the entire frequency range. Moreover, the shape of the arc suggested the involvement of electrochemical charge transfer [49]. With an increase in temperature, the transfer of charges at the surface of the electrode exhibits greater intricacy, causing an increase in resistance and an enhancement in corrosion resistance [11,54]. As a consequence, a greater scope of the capacitive arch arises. This tendency holds ultimately. The convex peak on the Bode phase angle diagram and the linear change in the Bode impedance diagram in the low-frequency region range (10−2–101 Hz) with a slope close to −1 reflect the capacitive behavior at the interface between the passivated film and the artificial saliva with the typical characteristics of an ideally polarized electrode [49,55]. In comparison with the PNO samples, the UN sample showed considerably lower values for the capacitive arc radius, as well as for the Bode phase angle and impedance at low and medium frequencies. This suggests that the UN sample exhibits more pronounced ion transport and electrode reaction processes [6,56]. The correlation between measured data and simulated data is shown in Figure 8a. The measured data matched well with the simulated data. The corrosion resistance of the UN sample was worse than that of the PNO samples. This showed that the corrosion resistance of the specimens was in the order of PNO-520 > PNO-500 > PNO-540 > UN.
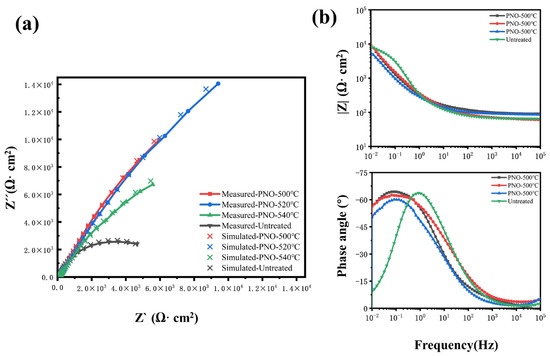
Figure 8.
Electrochemical impedance spectroscopy analysis of different samples in artificial saliva: (a) Nyquist diagram, (b) Bode diagram.
3.3.4. The Mechanism of The Unique Repassivation Process Phenomenon in PNO Samples
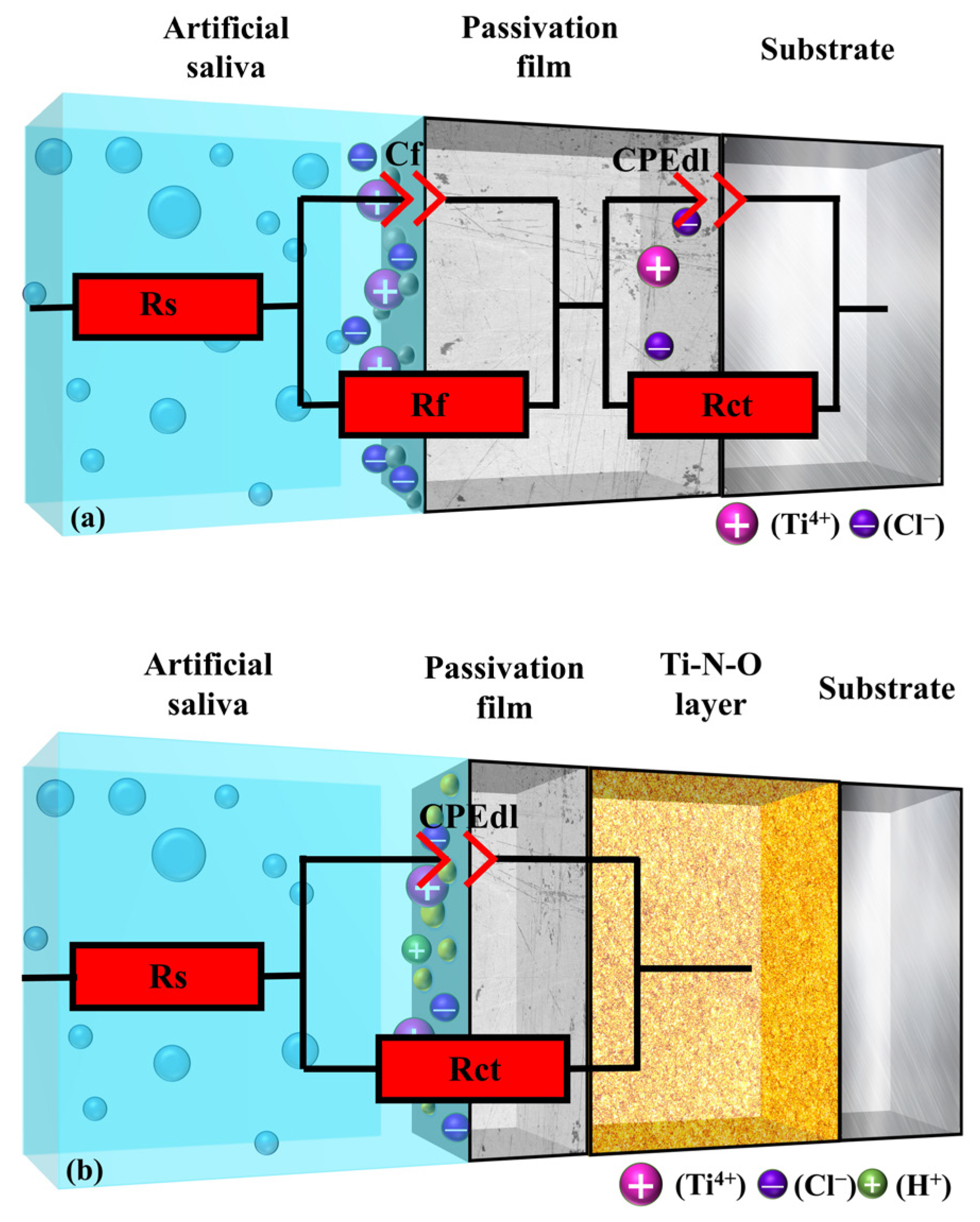
According to the EIS curve data, the equivalent circuit was fitted to the sample with Zview-2 software. The corrosion state of the model in the artificial saliva was explained with a three-dimensional stereogram, as shown in Figure 9, in which the UN and PNO samples corresponded to the Rs((CfRf) (CPEdl Rct)), and Rs (CPEdl Rct) models, respectively.
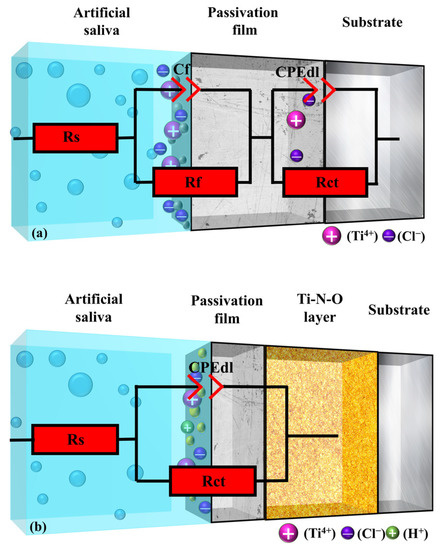
Figure 9.
The equivalent circuit diagram in artificial saliva: (a) UN sample, (b) PNO sample.
The CPE impedance is defined as ZC = 1/[C(jω)n], where n represents the deviation from the actual capacitive behavior. It is the CPE exponent with values between 0 and 1. When n = 1, the normal phase angle element is an ideal capacitor. On the other hand, when n = 0, the normal-phase angle element is pure resistance.
The size of the circuit parameters was simulated using Zview-2 software. The correlation between the measured data and the calculated data is shown in Figure 8a. The measured data matched well with the simulated data. The UN sample was a non-dense porous electrode circuit with a double-time constant that denoted the solution resistance. Rf represents the charge transfer reaction resistance. Cf denotes the bilayer capacitance. Rct and CPEdl represent the resistance and capacitance of the passivated film [57]. As can be seen in Table 4, the passivation film Rct (6.50 × 103 Ω·cm2) of the UN sample was more significant than the charge transfer resistance Rf (86.98 Ω·cm2). This indicates that the passivation film’s generation hindered the charge transfer from the artificial saliva to the titanium alloy substrate. At this time, corrosion mainly occurred in the interface between the artificial saliva and the multilayer pores of the passivation film (Figure 9a). The above behavior (Figure 9a) is called crevice corrosion, in which the artificial saliva can penetrate the TC4 alloy substrate through defects, crevices, and pores [58].

Table 4.
Electrochemical EIS circuit fitting parameters for UN and PNO samples in artificial saliva.
Studies have shown that the soluble chlorine compounds formed in various types of artificial body fluids due to the presence of Cl− reduce the stability of the passivation film on the surface of a TC4 alloy, accelerate the dissolution of the anode, and reduce the corrosion resistance [59,60]. When titanium alloy is implanted as a dental implant bolt in the human mouth, the presence of Cl− in the saliva accelerates the reaction under the corrosion of oral saliva. The artificial saliva chosen for this experiment was a weak acidic solution with a pH of 6.65 ± 0.01. The following crevice corrosion reactions (3) and (4) [60] occurred:
The PNO samples were single-time constant dense multiphase structured electrode circuits. Rs represented the solution resistance, and Rct and CPEdl represented the nitrogen oxide resistance and the double-layer capacitance, respectively. The fitted parameters are shown in Table 4, which indicates that the PNO-520 sample resistance Rct (1.36 × 105 Ω·cm2) was two orders of magnitude larger than the initial sample resistance Rct (6.43 × 103 Ω·cm2). The PNO-500 (8.47 × 104 Ω·cm2) and PNO-540 (6.18 × 104 Ω·cm2) sample resistance values were one order of magnitude higher than the initial resistance value. Figure 7c depicts the XPS detection analysis results, which show that upon exposure to an artificial environment, the material’s surface undergoes corrosion. This process leads to the formation of bound water and hydroxyl groups on its surface. The growth of TiO2 was also observed to occur concomitantly with hydration. Ti-OH groups were formed on the oxidized surface OH− groups, which could attract cations (Mg2+, Ca2+, K1+, Na+), and the following reaction occurred (5) [59,61]:
The experiments demonstrated that within a given solution, the titanium alloy’s corrosion byproduct Ti3+ can be converted to Ti4+, which serves as a substance that decelerates corrosion, thereby prompting the resumption of passivation [40].
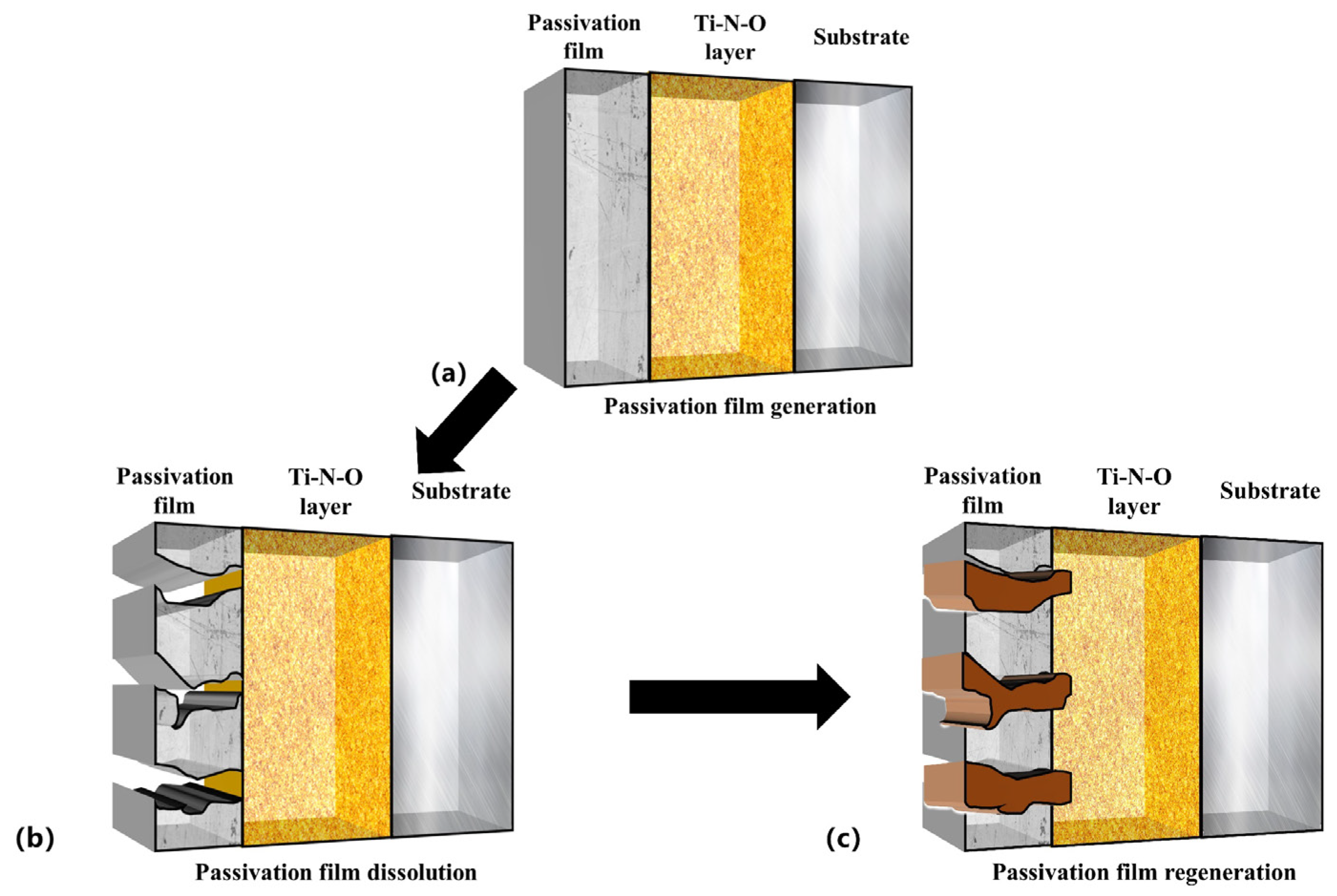
The repassivation process of the passive film is shown in Figure 10 and can be divided into three steps: (6), (7), and (8) [8,62]:
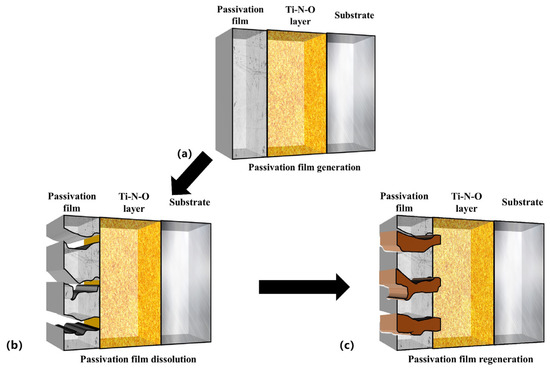
Figure 10.
Three-dimensional schematic diagram of dissolution and regeneration of Ti-N-O modified layer passivation film in artificial saliva.
Passivation was observed in the PNO samples exposed to a corrosive artificial saliva environment (Figure 6, −0.3 V–0.05 V). A passivation film was formed on their surfaces (Figure 10a). The analysis (Figure 7) indicated that the film was composed of TiO2 and Ti2O3.
Secondly, the chemical stability of Ti-N-O, the intermediate product of the titanium-nitrogen compound and titanium-oxygen compound, is not as good as titanium oxide. Since the Ti-N bond dissociation energies are lower than those of the Ti-O bond, the intermediate product TiNxOy partially dissolves. Additionally, according to reaction (6), the passivation film undergoes a hydration reaction, leading to the further dissolution of the film (see Figure 10b).
Finally, the PNO samples showed secondary passivation behavior (0.2–0.5 V). The repassivation mechanism of the passivation film of the Ti-N-O modified layer gave the sample durable and adequate corrosion resistance under the artificial saliva. The PNO samples formed a layer of oxide and TiN + Ti2N + α-Ti(N) nitrides. The nitrides and oxides became a corrosion-resistant barrier layer, effectively protecting the substrate [63,64,65,66].
The above analysis shows that the PNO samples have a more vital ability to inhibit the penetration of corrosion ions than the UN sample. The repassivation process of the PNO sample’s passive film improves its corrosion resistance in artificial saliva. This is attributed to the dense structures of the nitrides Ti2N and TiN, which prevent the invasion of saliva, and the superior corrosion resistance of the oxide TiO2, which hampers active ion diffusion. As a result, the electrochemical corrosion effect was reduced, and contact between the substrate and artificial saliva was effectively blocked, reducing charge transfer [67]. The Ti-N-O modified layer with a dense structure, fast passivation ability, and high chemical stability was responsible for the improved corrosion resistance of the samples [68].
4. Conclusions
The corrosion resistance of TC4 titanium alloy in artificial saliva was evaluated by comparing UN and PNO samples in terms of the phase type, surface hardness, depth of diffusion layer, and EIS testing. The key findings of this study are as follows:
(1) By employing a two-step hollow cathodic plasma source oxynitride technique at temperatures of 500, 520, and 540 °C, a modified Ti-N-O layer was successfully formed on the surface of the TC4 alloy, enhancing its properties. The PNO sample consisted of TiO2, TiN, and Ti2N layers and a-Ti(N) phase, forming the Ti-N-O modified layer.
(2) The composite layer thickness of the PNO samples ranged from 5.5 to 10.0 μm, with corresponding hardness values of 798.3 to 1142.7 HV0.1. Specifically, for the PNO-520 sample, the composite layer had a thickness of approximately 6.0 μm, and the diffusion layer extended beyond 48 μm. The surface hardness of this sample was measured at 982.1 HV0.1, which was approximately three times higher than the hardness of the substrate.
(3) The PNO sample exhibited a significantly stronger ability to inhibit the penetration of corrosion ions in the artificial saliva solutions compared with the UN sample. The impedance measurement of the PNO-520 sample yielded a value of 1.36 × 105 Ω·cm2, which was two orders of magnitude higher than that of the UN sample (6.50 × 103 Ω·cm2). Furthermore, the corrosion current density of the PNO-520 sample was 7.65 × 10−8 A/cm2, lower than that of the UN sample (2.78 × 10−6 A/cm2). The unique repassivation process of the passivating film in the PNO sample, involving generation, dissolution, and regeneration, effectively enhanced its corrosion resistance in harsh environments.
Author Contributions
Conceptualization, Y.H. and Y.L.; methodology, J.Y.; software, M.S. and M.W. (Wang Mufan); validation, J.Y., Z.Z. (Zelong Zhou) and Z.Z. (Zhehao Zhang); formal analysis, X.Y. and B.X.; investigation, J.Y., D.F. and Z.Z. (Zelong Zhou); resources, Z.Z. (Zhehao Zhang); data curation, M.W. (Mingjia Wang) and C.W.; writing—original draft preparation, J.Y.; writing—review and editing, Y.L.; visualization, J.Y. and X.Y.; supervision, Y.L.; project administration, M.W. (Mingjia Wang); funding acquisition, Y.L. and M.W. (Mingjia Wang). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 52175192, and the Shandong Natural Science Foundation, grant number ZR2022ME211.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siony, N.; Vuong, L.; Lundaajamts, O.; Kadkhodaei, S. Computational design of corrosion-resistant and wear-resistant titanium alloys for orthopedic implants. Mater. Today Commun. 2022, 33, 104465. [Google Scholar] [CrossRef]
- Gurel, S.; Yagci, M.B.; Bal, B.; Canadinc, D. Corrosion behavior of novel Titanium-based high entropy alloys designed for medical implants. Mater. Chem. Phys. 2020, 254, 123377. [Google Scholar] [CrossRef]
- Omarov, S.; Nauryz, N.; Talamona, D.; Perveen, A. Surface Modification Techniques for Metallic Biomedical Alloys: A Concise Review. Metals 2022, 13, 82. [Google Scholar] [CrossRef]
- Krishnan, V.; Krishnan, A.; Remya, R.; Ravikumar, K.K.; Nair, S.A.; Shibli, S.M.; Varma, H.K.; Sukumaran, K.; Kumar, K.J. Development and evaluation of two PVD-coated beta-titanium orthodontic archwires for fluoride-induced corrosion protection. Acta Biomater 2011, 7, 1913–1927. [Google Scholar] [CrossRef]
- Atapour, M.; Pilchak, A.L.; Shamanian, M.; Fathi, M.H. Corrosion behavior of Ti–8Al–1Mo–1V alloy compared to Ti–6Al–4V. Mater. Des. 2011, 32, 1692–1696. [Google Scholar] [CrossRef]
- Lario, J.; Vicente Escuder, Á.; Segovia, F.; Amigó, V. Electrochemical corrosion behavior of Ti–35Nb–7Zr–5Ta powder metallurgic alloys after Hot Isostatic Process in fluorinated artificial saliva. J. Mater. Res. Technol. 2022, 16, 1435–1444. [Google Scholar] [CrossRef]
- Yu, F.; Addison, O.; Davenport, A. Temperature-Dependence Corrosion Behavior of Ti6Al4V in the Presence of HCl. Front. Mater. 2022, 9, 880702. [Google Scholar] [CrossRef]
- Pohrelyuk, I.M.; Fedirko, V.M.; Tkachuk, O.V.; Proskurnyak, R.V. Corrosion resistance of Ti–6Al–4V alloy with nitride coatings in Ringer’s solution. Corros. Sci. 2013, 66, 392–398. [Google Scholar] [CrossRef]
- Grigoriev, S.; Sotova, C.; Vereschaka, A.; Uglov, V.; Cherenda, N. Modifying Coatings for Medical Implants Made of Titanium Alloys. Metals 2023, 13, 718. [Google Scholar] [CrossRef]
- Naeem, M.; Awan, S.; Shafiq, M.; Raza, H.A.; Iqbal, J.; Díaz-Guillén, J.C.; Sousa, R.R.M.; Jelani, M.; Abrar, M. Wear and corrosion studies of duplex surface-treated AISI-304 steel by a combination of cathodic cage plasma nitriding and PVD-TiN coating. Ceram. Int. 2022, 48, 21473–21482. [Google Scholar] [CrossRef]
- Adachi, S.; Ueda, N. Wear and Corrosion Properties of Cold-Sprayed AISI 316L Coatings Treated by Combined Plasma Carburizing and Nitriding at Low Temperature. Coatings 2018, 8, 456. [Google Scholar] [CrossRef]
- Hoja, S.; Steinbacher, M.; Zoch, H.W. Compound layer design for deep nitrided gearings. Metals 2020, 10, 455. [Google Scholar] [CrossRef]
- Sharifahmadian, O.; Zhai, C.; Hung, J.; Shineh, G.; Stewart, C.A.C.; Fadzil, A.A.; Ionescu, M.; Gan, Y.; Wise, S.G.; Akhavan, B. Mechanically robust nitrogen-rich plasma polymers: Biofunctional interfaces for surface engineering of biomedical implants. Mater. Today Adv. 2021, 12, 100188. [Google Scholar] [CrossRef]
- Liu, J.; Liu, D.; Li, S.; Deng, Z.; Pan, Z.; Li, C.; Chen, T. The effects of graphene oxide doping on the friction and wear properties of TiN bioinert ceramic coatings prepared using wide-band laser cladding. Surf. Coat. Technol. 2023, 458, 129354. [Google Scholar] [CrossRef]
- Wang, X.; Bai, S.; Li, F.; Li, D.; Zhang, J.; Tian, M.; Zhang, Q.; Tong, Y.; Zhang, Z.; Wang, G.; et al. Effect of plasma nitriding and titanium nitride coating on the corrosion resistance of titanium. J. Prosthet. Dent. 2016, 116, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Wierzchoń, T.; Czarnowska, E.; Grzonka, J.; Sowińska, A.; Tarnowski, M.; Kamiński, J.; Kulikowski, K.; Borowski, T.; Kurzydłowski, K.J. Glow discharge assisted oxynitriding process of titanium for medical application. Appl. Surf. Sci. 2015, 334, 74–79. [Google Scholar] [CrossRef]
- Behzadi, P.; Badr, M.; Zakeri, A. Duplex surface modification of pure Ti via thermal oxidation and gas nitriding: Preparation and electrochemical studies. Ceram. Int. 2022, 48, 34374–34381. [Google Scholar] [CrossRef]
- Banakh, O.; Moussa, M.; Matthey, J.; Pontearso, A.; Cattani-Lorente, M.; Sanjines, R.; Fontana, P.; Wiskott, A.; Durual, S. Sputtered titanium oxynitride coatings for endosseous applications: Physical and chemical evaluation and first bioactivity assays. Appl. Surf. Sci. 2014, 317, 986–993. [Google Scholar] [CrossRef]
- Albayrak, C.; Hacisalihoglu, I.; Vangolu, S.Y.; Alsaran, A. Tribocorrosion behavior of duplex treated pure titanium in Simulated Body Fluid. Wear 2013, 302, 1642–1648. [Google Scholar] [CrossRef]
- Zhang, C.W.; Wen, K.; Gao, Y. Columnar and nanocrystalline combined microstructure of the nitrided layer by active screen plasma nitriding on surface-nanocrystalline titanium alloy. Appl. Surf. Sci. 2023, 617, 156614. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, M.H.; Wang, Z.W.; Zhang, Z.H.; He, Y.Y.; Yan, J.W.; Lu, J.P.; Qiu, J.X.; Li, Y. Comparison of tribological properties of nitrided Ti-N modified layer and deposited TiN coatings on TA2 pure titanium. Tribol. Int. 2022, 174, 107712. [Google Scholar] [CrossRef]
- de Abreu, L.H.; Naeem, M.; Monção, R.M.; Costa, T.H.C.; Díaz-Guillén, J.C.; Iqbal, J.; Sousa, R.R. The Effect of Cathodic Cage Plasma TiN Deposition on Surface Properties of Conventional Plasma Nitrided AISI-M2 Steel. Metals 2022, 12, 961. [Google Scholar] [CrossRef]
- Domínguez-Meister, S.; Ibáñez, I.; Dianova, A.; Brizuela, M.; Braceras, I. Nitriding of titanium by hollow cathode assisted active screen plasma and its electro-tribological properties. Surf. Coat. Technol. 2021, 411, 126998. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Bacci, T. Surface Modification of Austenitic Stainless Steel by Means of Low Pressure Glow-Discharge Treatments with Nitrogen. Coatings 2019, 9, 604. [Google Scholar] [CrossRef]
- Adachi, S.; Yamaguchi, T.; Ueda, N. Formation and Properties of Nitrocarburizing S-Phase on AISI 316L Stainless Steel-Based WC Composite Layers by Low-Temperature Plasma Nitriding. Metals 2021, 11, 1538. [Google Scholar] [CrossRef]
- Wang, Z.W.; Li, Y.; Zhang, Z.H.; Zhang, S.Z.; Ren, P.; Qiu, J.X.; Wang, W.W.; Bi, Y.J.; He, Y.Y. Friction and wear behavior of duplex-treated AISI 316L steels by rapid plasma nitriding and (CrWAlTiSi)N ceramic coating. Results Phys. 2021, 24, 104132. [Google Scholar] [CrossRef]
- Sun, F.; Liu, X.-L.; Luo, S.-Q.; Xiang, D.-D.; Ba, D.-C.; Lin, Z.; Song, G.-Q. Duplex treatment of arc plasma nitriding and PVD TiN coating applied to dental implant screws. Surf. Coat. Technol. 2022, 439, 128449. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Wang, L. Surface properties of nitrided layer on AISI 316L austenitic stainless steel produced by high temperature plasma nitriding in short time. Appl. Surf. Sci. 2014, 298, 243–250. [Google Scholar] [CrossRef]
- Cherenda, N.N.; Basalai, A.V.; Shymanski, V.I.; Uglov, V.V.; Astashynski, V.M.; Kuzmitski, A.M.; Laskovnev, A.P.; Remnev, G.E. Modification of Ti-6Al-4V alloy element and phase composition by compression plasma flows impact. Surf. Coat. Technol. 2018, 355, 148–154. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Lu, M.Y.; Fan, Z.Q.; Huang, S.Q.; Huang, H. Laser deposition of wear-resistant titanium oxynitride/titanium composite coatings on Ti-6Al-4V alloy. Appl. Surf. Sci. 2020, 531, 147212. [Google Scholar] [CrossRef]
- Ye, Q.W.; Li, Y.; Zhang, M.Y.; Zhang, S.Z.; Bi, Y.J.; Gao, X.P.; He, Y.Y. Electrochemical behavior of (Cr, W, Al, Ti, Si)N multilayer coating on nitrided AISI 316L steel in natural seawater. Ceram. Int. 2020, 46, 22404–22418. [Google Scholar] [CrossRef]
- Jaeger, D.; Patscheider, J. A complete and self-consistent evaluation of XPS Spectra of TiN. J. Electron Spectrosc. Relat. Phenom. 2012, 185, 523–534. [Google Scholar] [CrossRef]
- Wang, Z.G.; Zu, X.T.; Xiang, X.; Zhu, S.; Wang, L.M. Surface modification of Ti-4Al-2V alloy by nitrogen implantation. J. Mater. Sci. 2006, 41, 3363–3367. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L. Study of oxidized layer formed on aluminium alloy by plasma oxidation. Thin Solid Film. 2009, 517, 3208–3210. [Google Scholar] [CrossRef]
- Hong, X.; Feng, K.; Tan, Y.F.; Wang, X.L.; Tan, H. Effects of process parameters on microstructure and wear resistance of TiN coatings deposited on TC11 titanium alloy by electrospark deposition. Trans. Nonferrous Met. Soc. China 2017, 27, 1767–1776. [Google Scholar] [CrossRef]
- Mohammadi, M.; Akbari, A.; Warchomicka, F.; Pichon, L. Depth profiling characterization of the nitride layers on gas nitrided commercially pure titanium. Mater. Charact. 2021, 181, 111453. [Google Scholar] [CrossRef]
- Guo, Y.; Fang, Y.; Dai, G.; Sun, Z.; Wang, Y.; Yuan, Q. The effect of hydrogen treatment on microstructures evolution and mechanical properties of titanium alloy fabricated by selective laser melting. J. Alloy. Compd. 2022, 890, 161642. [Google Scholar] [CrossRef]
- Adam, D.B.; Tsai, M.-C.; Awoke, Y.A.; Huang, W.-H.; Lin, C.-H.; Alamirew, T.; Ayele, A.A.; Yang, Y.-W.; Pao, C.-W.; Su, W.-N.; et al. Engineering self-supported ruthenium-titanium alloy oxide on 3D web-like titania as iodide oxidation reaction electrocatalyst to boost hydrogen production. Appl. Catal. B Environ. 2022, 316, 121608. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Tseng, C.C.; Cheng, T.C.; Nguyen, V.; Chang, Y.H. Formation and characterization of calcium phosphate ceramic coatings on Ti-6Al-4V alloy. Mater. Today Commun. 2022, 31, 103686. [Google Scholar] [CrossRef]
- Wei, Y.; Pan, Z.M.; Fu, Y.; Yu, W.; He, S.L.; Yuan, Q.Y.; Luo, H.; Li, X.G. Effect of annealing temperatures on microstructural evolution and corrosion behavior of Ti-Mo titanium alloy in hydrochloric acid. Corros. Sci. 2022, 197, 110079. [Google Scholar] [CrossRef]
- Borgioli, F. From Austenitic Stainless Steel to Expanded Austenite-S Phase: Formation, Characteristics and Properties of an Elusive Metastable Phase. Metals 2020, 10, 187. [Google Scholar] [CrossRef]
- Karimi, S.; Nickchi, T.; Alfantazi, A. Effects of bovine serum albumin on the corrosion behaviour of AISI 316L, Co–28Cr–6Mo, and Ti–6Al–4V alloys in phosphate buffered saline solutions. Corros. Sci. 2011, 53, 3262–3272. [Google Scholar] [CrossRef]
- Li, Q.; Wei, M.; Yang, J.; Zhao, Z.; Ma, J.; Liu, D.; Lan, Y. Effect of Ca addition on the microstructure, mechanical properties and corrosion rate of degradable Zn-1Mg alloys. J. Alloy. Compd. 2021, 887, 161255. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, M.; Zhu, J.; Tu, J.; Jiao, S. Microstructure and Corrosion Resistance of Ti6Al4V Manufactured by Laser Powder Bed Fusion. Metals 2023, 13, 496. [Google Scholar] [CrossRef]
- Shahmohammadi, M.; Sun, Y.N.; Yuan, J.C.C.; Mathew, M.T.; Sukotjo, C.; Takoudis, C.G. In vitro corrosion behavior of coated Ti6Al4V with TiO2, ZrO2, and TiO2/ZrO2 mixed nanofilms using atomic layer deposition for dental implants. Surf. Coat. Technol. 2022, 444, 128686. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Shao, M.; Zhang, Z.; Wang, C.; Yan, J.; Lu, J.; Zhang, L.; Xie, B.; He, Y.; et al. Characterization and electrochemical behavior of a multilayer-structured Ti–N layer produced by plasma nitriding of electron beam melting TC4 alloy in Hank’s solution. Vacuum 2023, 208, 111737. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, M.; Zhang, Z.; Yi, X.; Yan, J.; Zhou, Z.; Fang, D.; He, Y.; Li, Y. Corrosion Behavior of Nitrided Layer of Ti6Al4V Titanium Alloy by Hollow Cathodic Plasma Source Nitriding. Materials 2023, 16, 2961. [Google Scholar] [CrossRef] [PubMed]
- She, D.S.; Yue, W.; Fu, Z.Q.; Wang, C.B.; Yang, X.K.; Liu, J.J. Effects of nitriding temperature on microstructures and vacuum tribological properties of plasma-nitrided titanium. Surf. Coat. Technol. 2015, 264, 32–40. [Google Scholar] [CrossRef]
- Bao, Y.C.; Wang, W.L.; Cui, W.F.; Qin, G.W. Corrosion resistance and antibacterial activity of Ti-N-O coatings deposited on dental titanium alloy. Surf. Coat. Technol. 2021, 419, 127296. [Google Scholar] [CrossRef]
- Du, J.W.; Chen, L.; Chen, J.; Yue, J.L. Effects of additional oxygen on the structural, mechanical, thermal, and corrosive properties of TiN coatings. Ceram. Int. 2022, 48, 14432–14441. [Google Scholar] [CrossRef]
- Subramanian, B.; Muraleedharan, C.V.; Ananthakumar, R.; Jayachandran, M. A comparative study of titanium nitride (TiN), titanium oxy nitride (TiON) and titanium aluminum nitride (TiAlN), as surface coatings for bio implants. Surf. Coat. Technol. 2011, 205, 5014–5020. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, F.; Cui, Y.-T.; Yoshida, H.; Wen, L.; Jin, Y. Influences of formation potential on oxide film of TC4 in 0.5 M sulfuric acid. Appl. Surf. Sci. 2021, 544, 148888. [Google Scholar] [CrossRef]
- Rao, X.; Du, L.; Zhao, J.J.; Tan, X.D.; Fang, Y.X.; Xu, L.Q.; Zhang, Y.P. Hybrid TiO2/AgNPs/g-C3N4 nanocomposite coatings on TC4 titanium alloy for enhanced synergistic antibacterial effect under full spectrum light. J. Mater. Sci. Technol. 2022, 118, 35–43. [Google Scholar] [CrossRef]
- Hussein, M.A.; Yilbas, B.; Kumar, A.M.; Drew, R.; Al-Aqeeli, N. Influence of Laser Nitriding on the Surface and Corrosion Properties of Ti-20Nb-13Zr Alloy in Artificial Saliva for Dental Applications. J. Mater. Eng. Perform. 2018, 27, 4655–4664. [Google Scholar] [CrossRef]
- Yang, X.J.; Du, C.W.; Wan, H.X.; Liu, Z.Y.; Li, X.G. Influence of sulfides on the passivation behavior of titanium alloy TA2 in simulated seawater environments. Appl. Surf. Sci. 2018, 458, 198–209. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Bacci, T. Surface Modification of a Nickel-Free Austenitic Stainless Steel by Low-Temperature Nitriding. Metals 2021, 11, 1845. [Google Scholar] [CrossRef]
- Liu, C.L.; Wang, Y.J.; Wang, M.; Huang, W.J.; Chu, P.K. Electrochemical stability of TiO2 nanotubes with different diameters in artificial saliva. Surf. Coat. Technol. 2011, 206, 63–67. [Google Scholar] [CrossRef]
- Wu, J.; Li, M.; Lin, C.C.; Gao, P.F.; Zhang, R.; Li, X.; Zhang, J.X.; Cai, K.Y. Moderated crevice corrosion susceptibility of Ti6Al4V implant material due to albumin-corrosion interaction. J. Mater. Sci. Technol. 2022, 109, 209–220. [Google Scholar] [CrossRef]
- Li, J.; Bai, Y.; Fan, Z.D.; Li, S.J.; Hao, Y.L.; Yang, R.; Gao, Y.B. Effect of fluoride on the corrosion behavior of nanostructured Ti-24Nb-4Zr-8Sn alloy in acidulated artificial saliva. J. Mater. Sci. Technol. 2018, 34, 1660–1670. [Google Scholar] [CrossRef]
- Chenghao, L.; Li’nan, J.; Chuanjun, Y.; Naibao, H. Crevice Corrosion Behavior of CP Ti, Ti-6Al-4V Alloy and Ti-Ni Shape Memory Alloy in Artificial Body Fluids. Rare Met. Mater. Eng. 2015, 44, 781–785. [Google Scholar] [CrossRef]
- Mareci, D.; Chelariu, R.; Gordin, D.M.; Ungureanu, G.; Gloriant, T. Comparative corrosion study of Ti-Ta alloys for dental applications. Acta Biomater 2009, 5, 3625–3639. [Google Scholar] [CrossRef]
- Oliveira, V.M.C.A.; Aguiar, C.; Vazquez, A.M.; Robin, A.; Barboza, M.J.R. Improving corrosion resistance of Ti–6Al–4V alloy through plasma-assisted PVD deposited nitride coatings. Corros. Sci. 2014, 88, 317–327. [Google Scholar] [CrossRef]
- Azumi, K.; Seo, M. Changes in electrochemical properties of the anodic oxide film formed on titanium during potential sweep. Corros. Sci. 2001, 43, 533–546. [Google Scholar] [CrossRef]
- Shen, H.Y.; Wang, L. Corrosion resistance and electrical conductivity of plasma nitrided titanium. Int. J. Hydrog. Energy 2021, 46, 11084–11091. [Google Scholar] [CrossRef]
- Fossati, A.; Borgioli, F.; Galvanetto, E.; Bacci, T. Corrosion resistance properties of plasma nitrided Ti–6Al–4V alloy in nitric acid solutions. Corros. Sci. 2004, 46, 917–927. [Google Scholar] [CrossRef]
- Tarnowski, M.; Borowski, T.; Skrzypek, S.; Kulikowski, K.; Wierzchoń, T. Shaping the structure and properties of titanium and Ti6Al7Nb titanium alloy in low-temperature plasma nitriding processes. J. Alloy. Compd. 2021, 864, 158896. [Google Scholar] [CrossRef]
- Liu, J.M.; Lou, Y.X.; Zhang, C.; Yin, S.; Li, H.M.; Sun, D.Q.; Sun, X.H. Improved corrosion resistance and antibacterial properties of composite arch-wires by N-doped TiO2 coating. Rsc Adv. 2017, 7, 43938–43949. [Google Scholar] [CrossRef]
- Cui, W.F.; Niu, F.J.; Tan, Y.L.; Qin, G.W. Microstructure and tribocorrosion performance of nanocrystalline TiN graded coating on biomedical titanium alloy. Trans. Nonferrous Met. Soc. China 2019, 29, 1026–1035. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).