The Influence of a Corrosive Environment on Fatigue and Mechanical Properties of An Al-Cast Alloy with Higher Fe Content
Abstract
1. Introduction
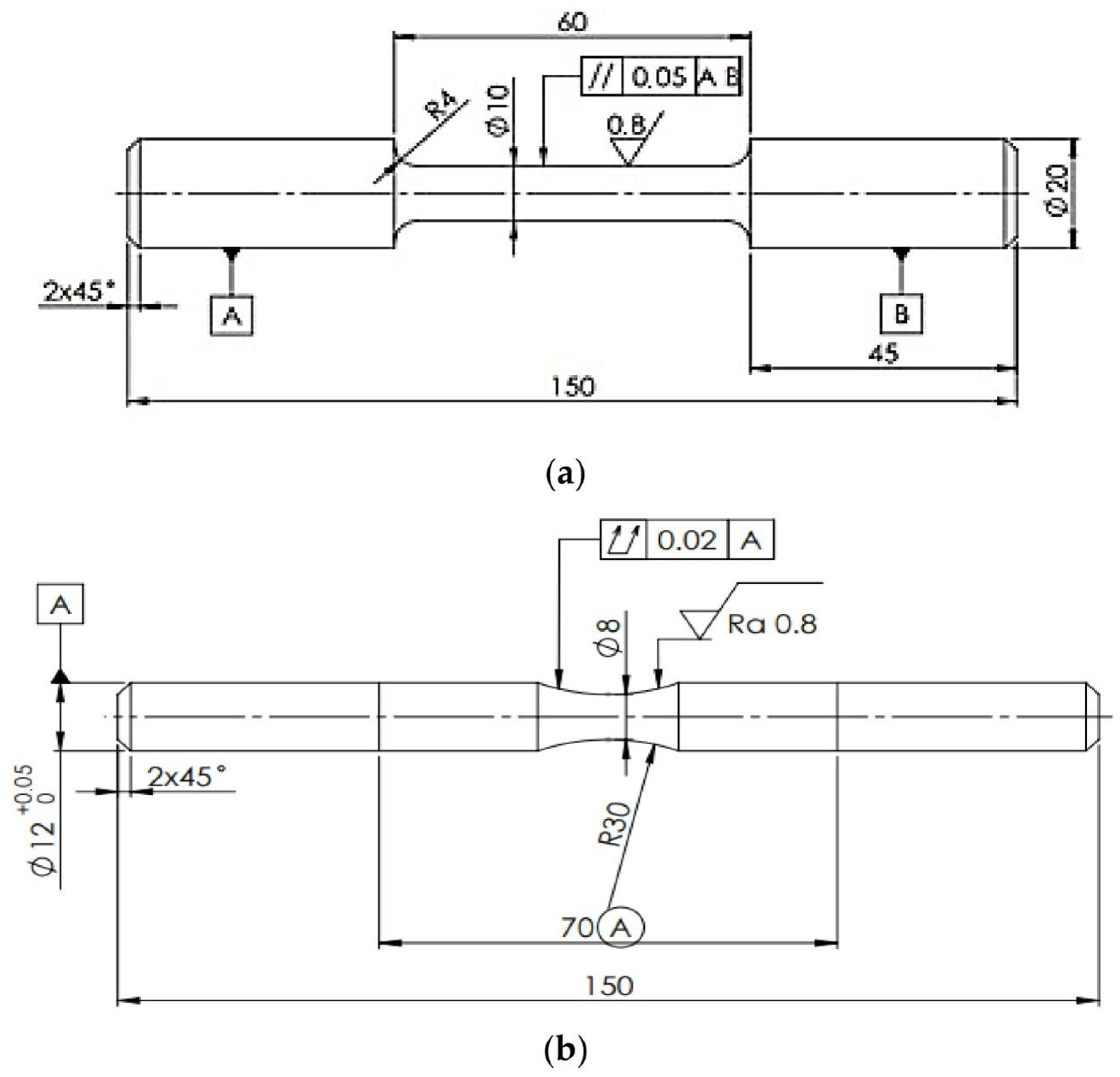
2. Materials and Methods
3. Results and Discussion
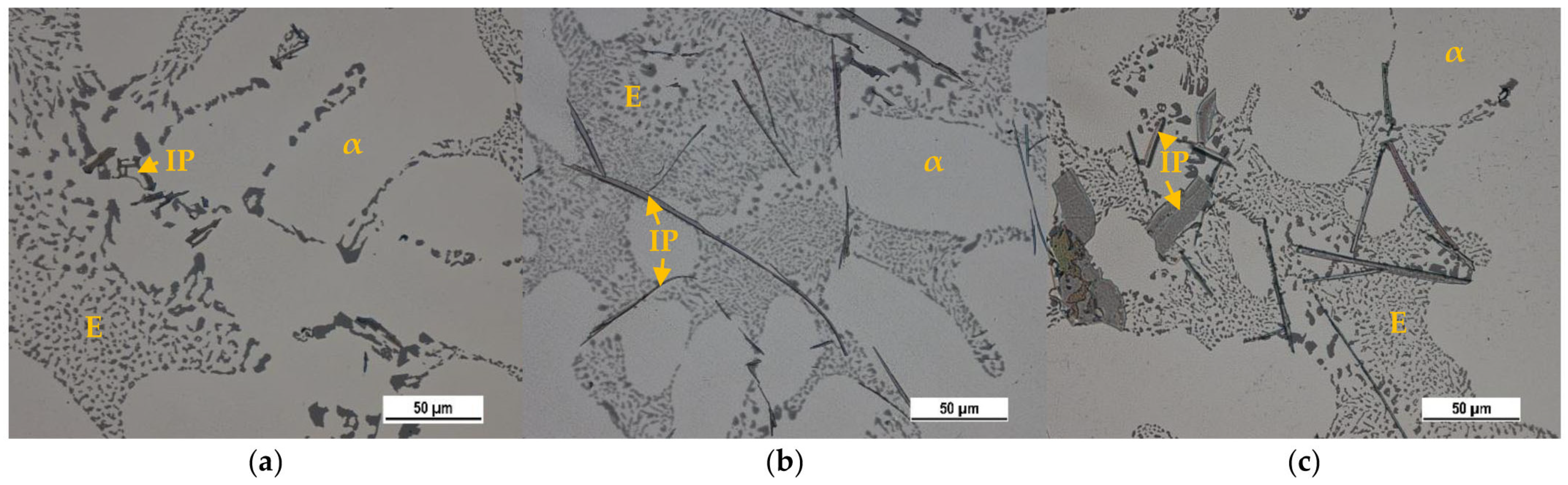
3.1. Basic Microstructure
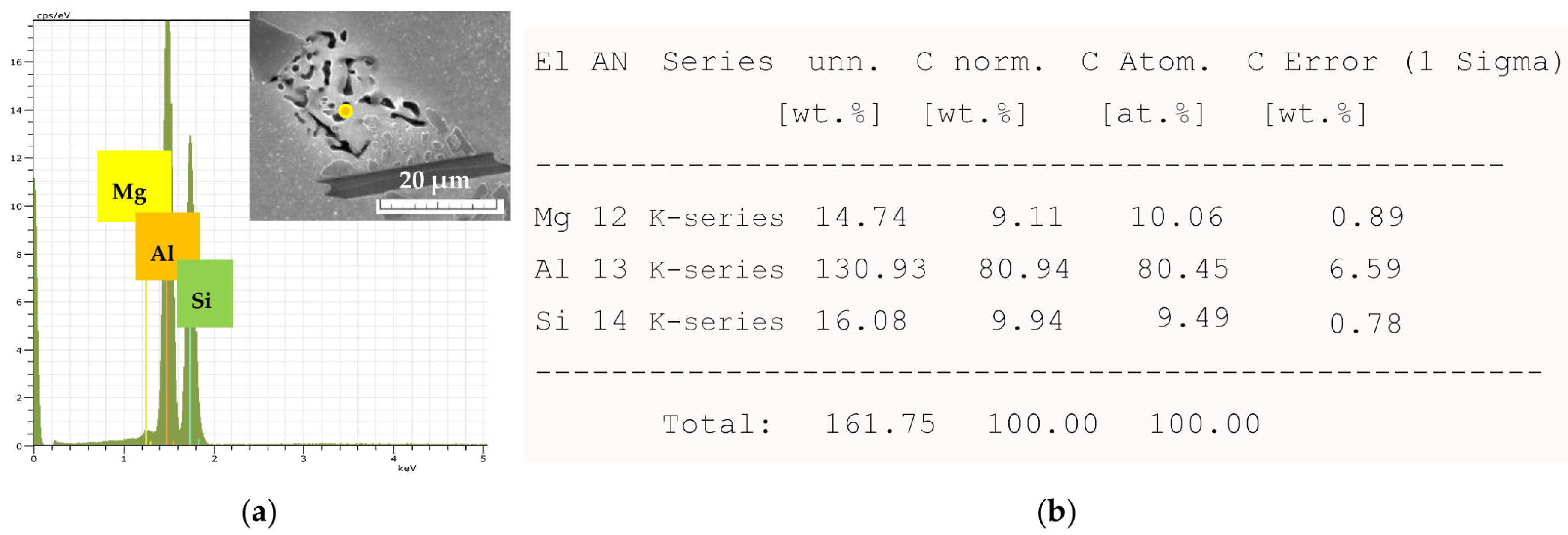
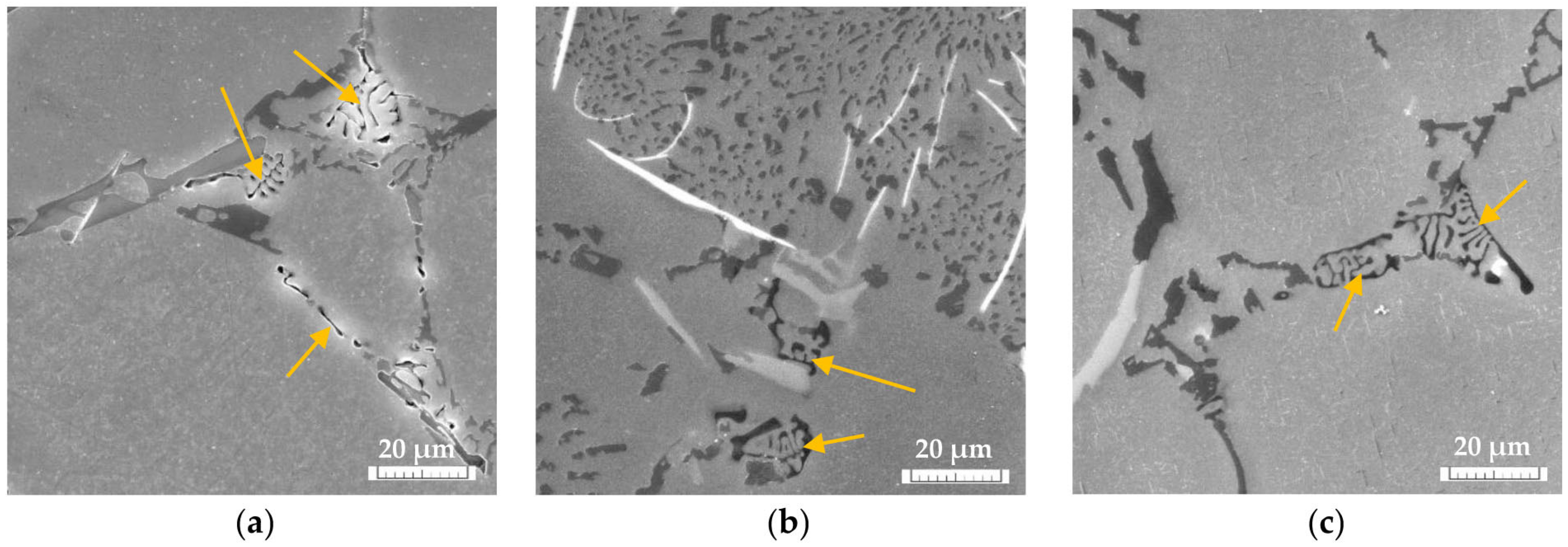
The Effect of Fe on the Mg2Si Intermetallic Phases
3.2. Effects of the Corrosive Environment on Mechanical Properties
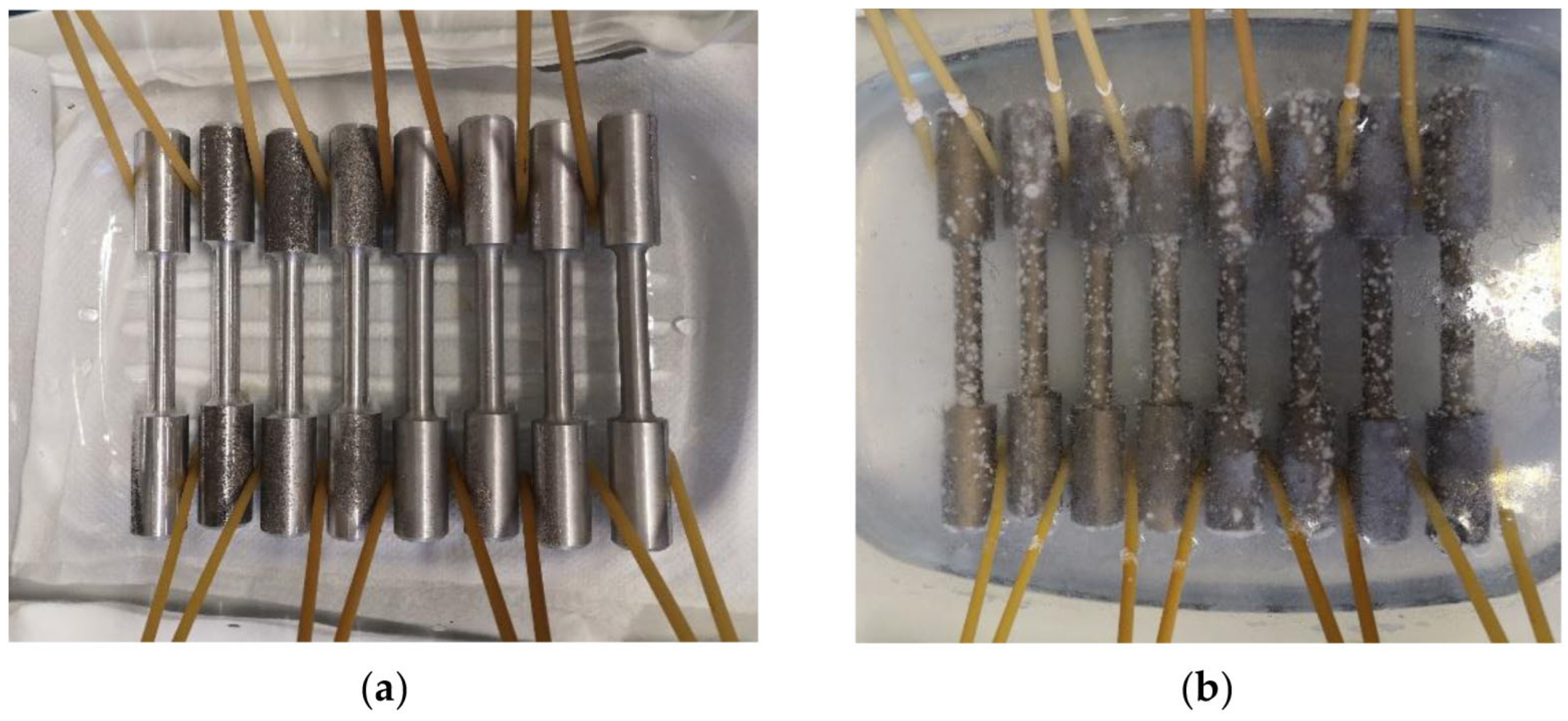
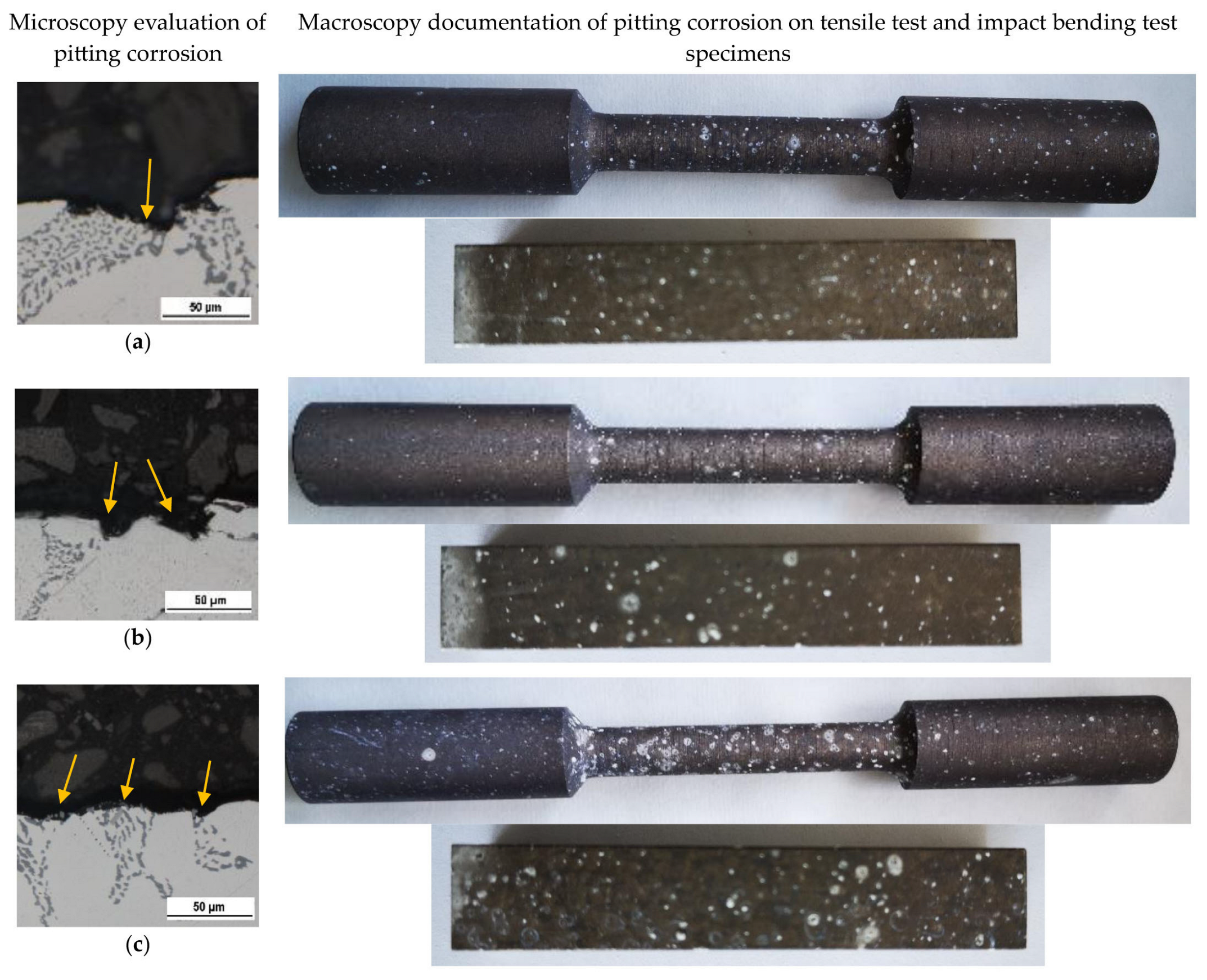
Evaluation of the Corrosion Attack on Tested Specimens
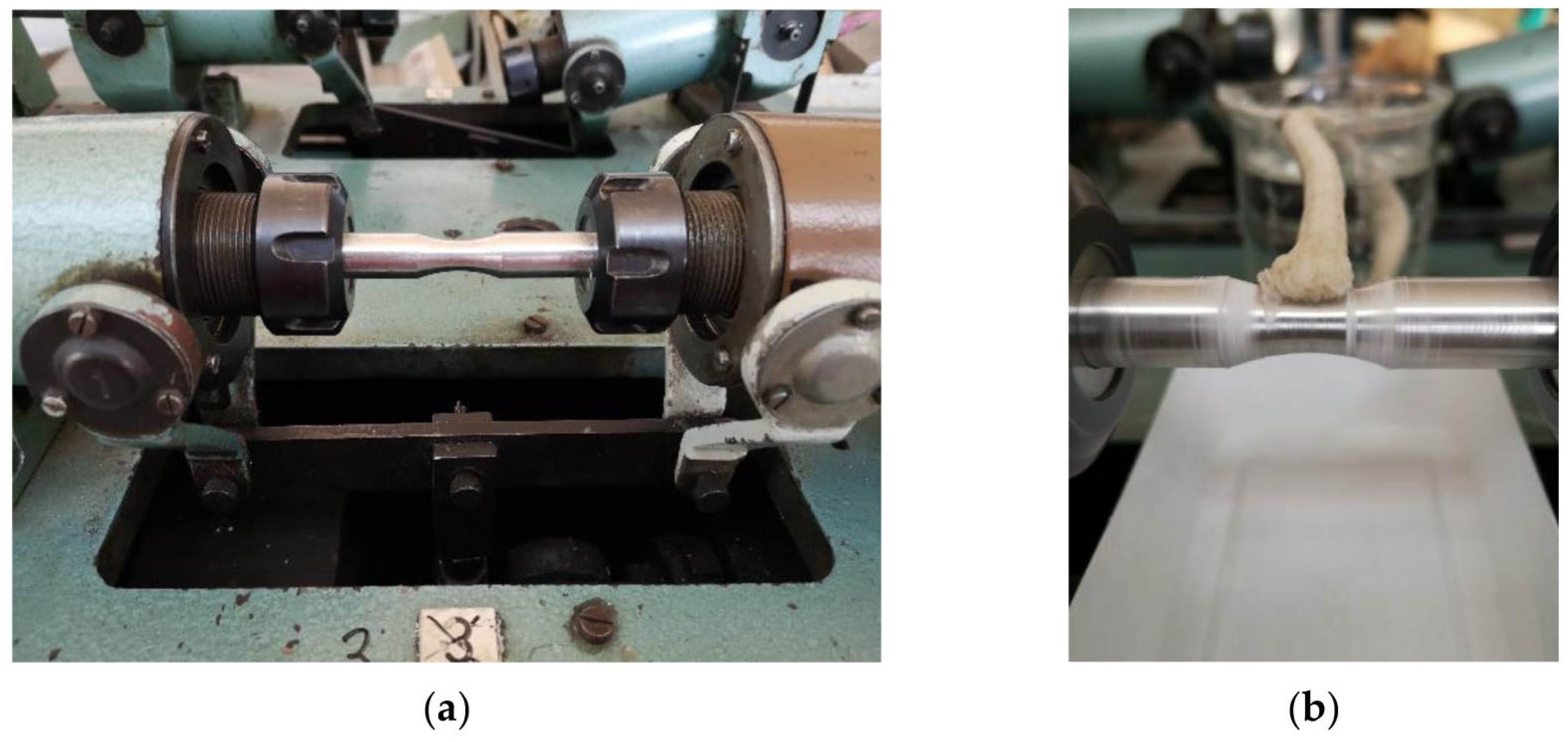
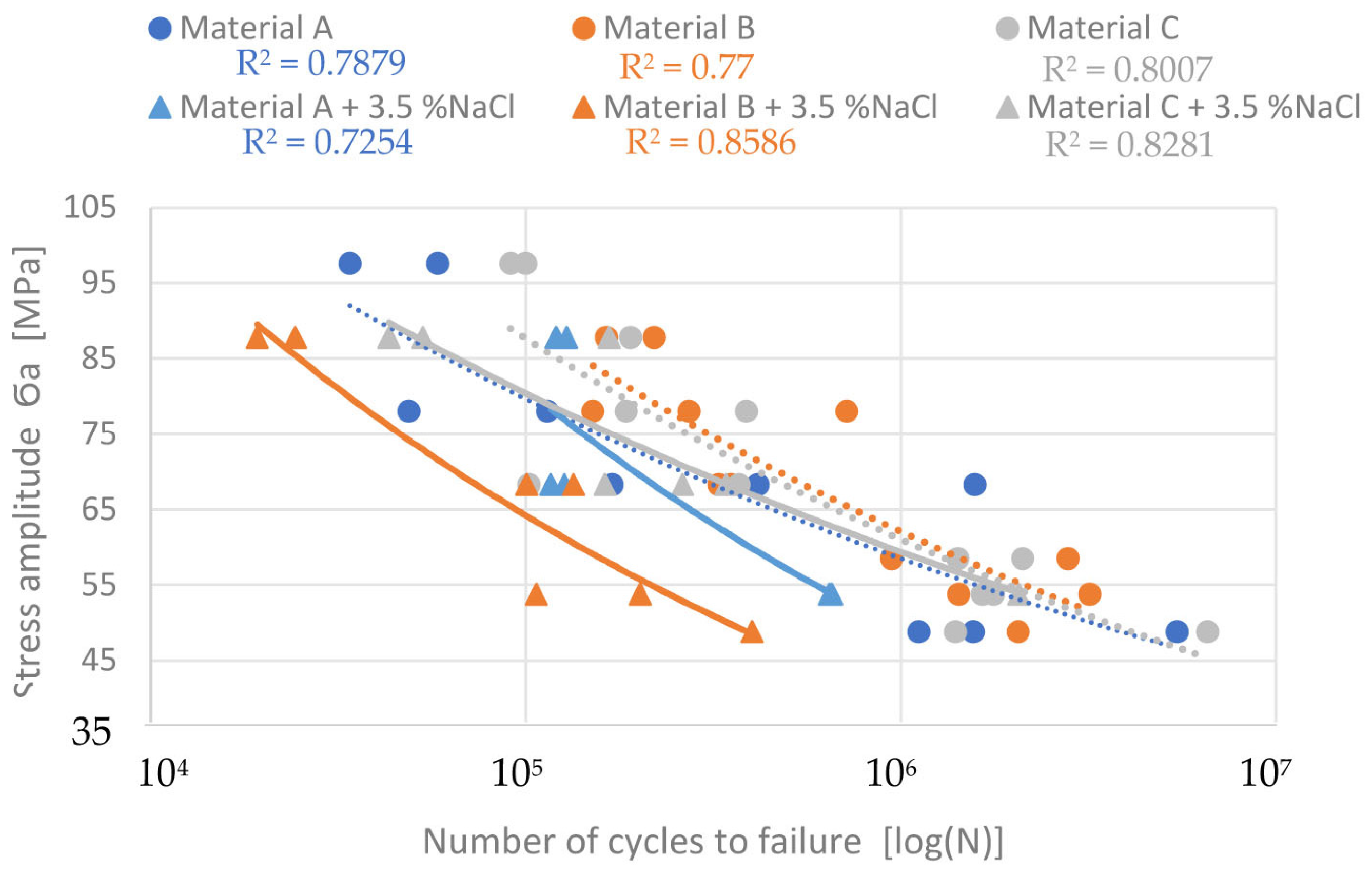
3.3. Effects of the Corrosive Environment on Fatigue Properties
3.4. Effects of the Corrosive Environment on Fracture Surfaces
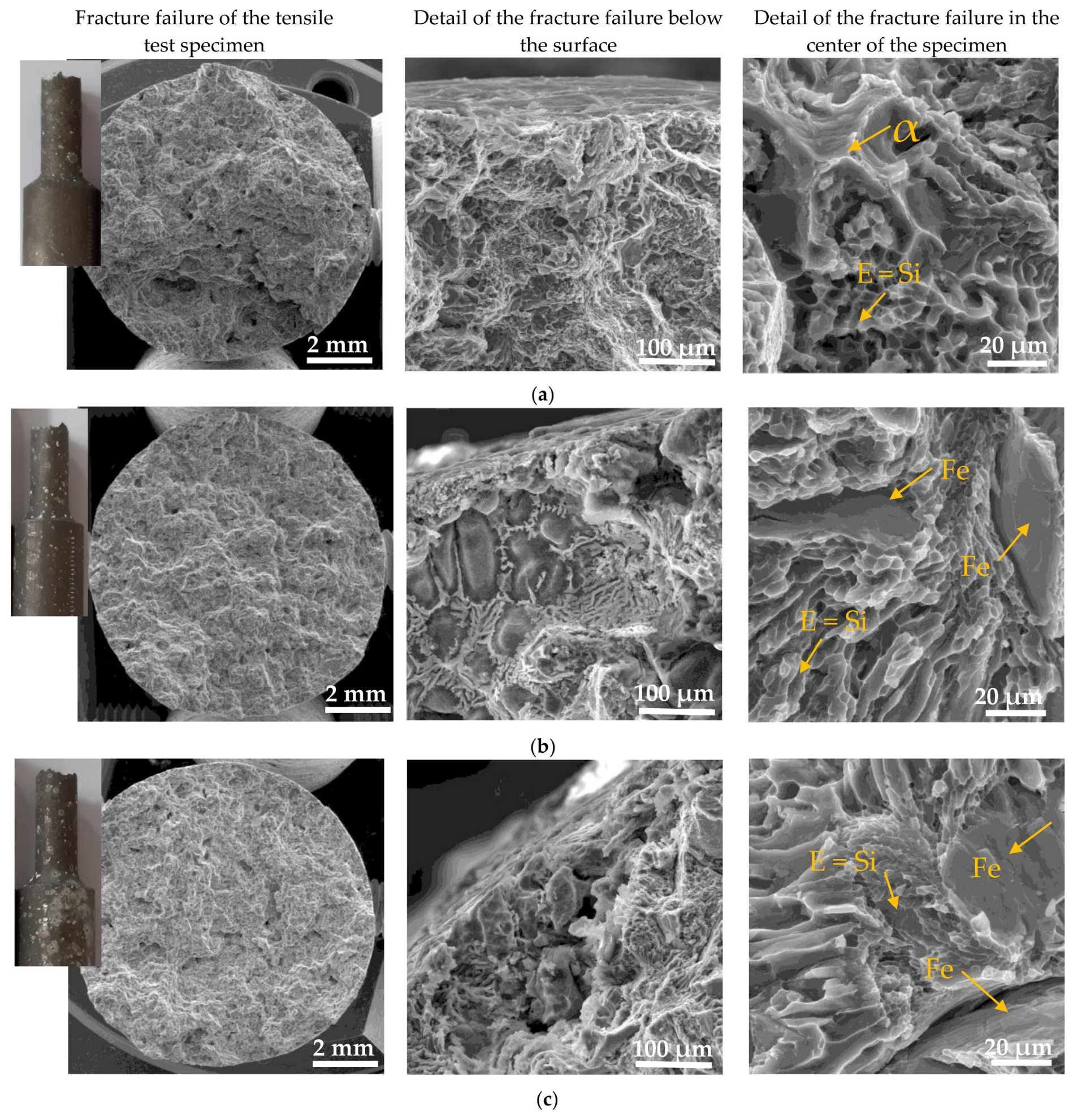
3.4.1. Fracture Surfaces after Tensile Strength and Charpy Impact Tests
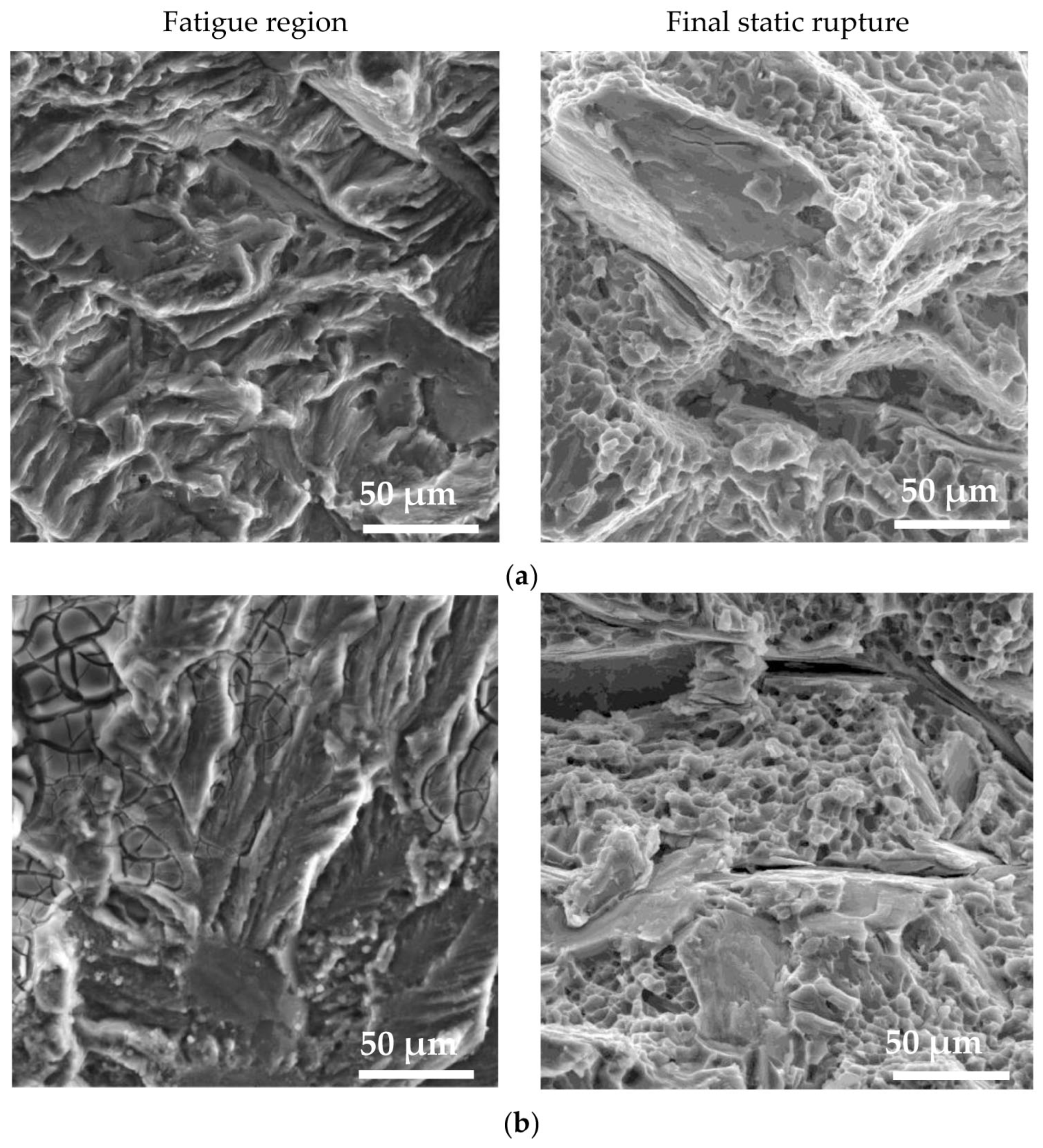
3.4.2. Fracture Surfaces after Fatigue Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schlesinger, M. Aluminum Recycling, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; p. 282. [Google Scholar] [CrossRef]
- Nicoletto, G.; Anzelotti, G.; Konečná, R. X-ray Computed Tomography vs. Metallography for Pore Sizing and Fatigue of Cast Al-alloys. Procedia Eng. 2010, 2, 547–554. [Google Scholar] [CrossRef]
- Senčáková, L.; Virčíková, E. Life cycle assessment of primary aluminium production. Acta Metall. Slovaca 2007, 13, 412–419. [Google Scholar]
- Das, K.S. Designing Aluminum Alloys for a Recycling Friendly World. Mater. Sci. Forum 2006, 519–521, 1239–1244. [Google Scholar] [CrossRef]
- Cui, J.; Roven, H.R. Recycling of automotive aluminium. Trans. Nonferrous Met. Soc. China 2010, 20, 2057–2063. [Google Scholar] [CrossRef]
- Bakedano, A.; Niklas, A.; Fernández-Calvo, A.I.; Plata, G.; Lozares, J.; Berlanga-Labari, C. Comparative Study of the Metallurgical Quality of Primary and Secondary AlSi10MnMg Aluminium Alloys. Metals 2021, 11, 1147. [Google Scholar] [CrossRef]
- Tillová, E.; Chalupová, M.; Hurtalová, L. Evolution of the Fe-rich phases in recycled AlSi9Cu3 cast alloy during solution treatment. Communications 2010, 12, 95–101. [Google Scholar] [CrossRef]
- Svobodova, J.; Lunak, M.; Lattner, M. Analysis of the Increased Iron Content on the Corrosion Resistance of the AlSi7Mg0.3 Alloy Casting. Manuf. Technol. 2019, 19, 1041–1046. [Google Scholar] [CrossRef]
- Wu, J.; Djavanroodi, F.; Gode, C.; Attarilar, S.; Ebrahimi, M. Melt refining and purification processes in Al alloys: A comprehensive study. Mater. Res. Express 2022, 9, 032001. [Google Scholar] [CrossRef]
- Boromei, L.; Ceschini, L.; Morri, A.; Nicoletto, G.; Riva, E. Influence of the solidification microstructure and porosity on the fatigue strength of Al-Si-Mg casting alloys. Metall. Sci. Technol. 2010, 2, 18–24. [Google Scholar]
- Samuel, A.M.; Samuel, F.H.; Doty, H.W.; Valtierra, S. Beta Al5FeSi phase platelets-porosity formation relationship in A319.2 type alloys. Int. J. Met. Cast. 2018, 12, 55–70. [Google Scholar] [CrossRef]
- Taylor, J. Iron-containing intermetallic phases in Al-Si based casting alloys. Procedia Mater. Sci. 2012, 1, 19–33. [Google Scholar] [CrossRef]
- Hadzima, B.; Liptáková, T. Fundamentals of Electrochemical Corrosion of Metals, 1st ed.; Edis: Žilina, Slovakia, 2008; p. 116. (In Slovak) [Google Scholar]
- Ricker, R.; Duquette, D. Corrosion Fatigue of Aluminum Alloys. In Proceedings of the First Joint DoD/FAA/NASA Conference on Aging Aircraft, Ogden, UT, USA, 8–10 July 1997. [Google Scholar]
- Esmaily, M.; Blüche, D.B.; Svensson, J.E.; Halvarsson, M.; Johansson, L.G. New insights into the corrosion of magnesium alloys—The role of aluminum. Scr. Mater. 2016, 115, 91–95. [Google Scholar] [CrossRef]
- Vargel, C. Corrosion of Aluminium, 1st ed.; Elsevier: Paris, France, 2004; p. 626. [Google Scholar]
- Torbati-Sarraf, H.; Stannard, T.; Callagon La Plante, E.; Sant, G.N.; Chawla, N. Direct observations of microstructure-resolved corrosion initiation in AA7075-T651 at the nanoscale using vertical scanning interferometry (VSI). Mater. Charact. 2020, 161, 110166. [Google Scholar] [CrossRef]
- Winkler, S.L.; Ryan, M.; Flower, H.M. Pitting corrosion in cast 7XXX aluminium alloys and fibre reinforced MMCs. Corros. Sci. 2004, 46, 893–902. [Google Scholar] [CrossRef]
- Roodbari, M.K. Effect of Microstructure on the Performance of Corrosion Resistant Alloys. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2015; p. 74. Available online: http://hdl.handle.net/11250/2377272 (accessed on 10 May 2022).
- Ambat, R.; Davenport, A.J.; Scamans, G.M.; Afseth, A. A Effect of iron-containing intermetallic particles on the corrosion behaviour of aluminium. Corros. Sci. 2006, 48, 3455–3471. [Google Scholar] [CrossRef]
- Liang, W.J.; Rometsch, P.A.; Cao, L.F.; Birbilis, N. General aspects related to the corrosion of 6xxx series aluminium alloys: Exploring the influence of Mg/Si ratio and Cu. Corros. Sci. 2013, 76, 119–128. [Google Scholar] [CrossRef]
- Gebril, M.A.; Omar, M.Z.; Othman, N.K.; Mohamed, I.F. Microstructural Refinement and Corrosion Resistance Improvement of Heat-Treated A356 Alloy Processed by Equal Channel Angular Pressing. Sains Malays. 2019, 48, 2749–2757. [Google Scholar] [CrossRef]
- Lange, G.; Pohl, M. Systematische Beurteilung Technischer Schadensfälle, 1st ed.; Wiley–VCH Verlag GmbH: Weinheim, Germany, 2014; p. 548. [Google Scholar]
- Činčala, M. High Cycle Fatigue of Bearing Steel 14 109. Ph.D. Thesis, University of Žilina, Žilina, Slovakia, 2005; p. 91. [Google Scholar]
- Caton, M.J.; Jones, J.W.; Mayer, H.; Stanzl-Tschegg, S.E.; Allison, J.E. Demonstration of an endurance limit in cast 319 aluminum. Metall. Mater. Trans. A 2003, 34, 33–41. [Google Scholar] [CrossRef]
- Zhu, X.; YI, J.Z.; Jones, J.W.; Allison, J.E. A Probabilistic Model of Fatigue Strength Controlled by Porosity Population in a 319-Type Cast Aluminum Alloy: Part I. Model Development. Miner. Met. Mater. Soc. ASM Int. 2007, 38, 1111–1122. [Google Scholar] [CrossRef]
- Yi, J.Z. Effect of Microstructure and Defects on the Fatigue Behavior of Cast A356- T6 Aluminium-Silicon Alloy. Ph.D. Thesis, Department of Materials, Imperial College, London, UK, 2004. [Google Scholar]
- Wang, Q.G.; Apelian, D.; Lados, D.A. Fatigue behavior of A356/357 aluminum cast alloys. Part II-Effect of microstructural constituents. J. Light Met. 2001, 1, 85–97. [Google Scholar] [CrossRef]
- Markovičová, L.; Hurtalová, L.; Zatkalíková, V.; Garbacz, T. Evolution of composite structures by light microscopy and image analysis. Manuf. Technol. 2014, 14, 351–355. [Google Scholar] [CrossRef]
- Liu, M.; Uggowitzer, P.J.; Nagasekhar, A.V.; Schmutz, P.; Easton, M.; Song, G.; Atrens, A. Calculated phase diagrams and the corrosion of die-cast Mg–Al alloys. Corros. Sci. 2009, 51, 602–619. [Google Scholar] [CrossRef]
- ISO 6892-1A; Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. ISO: Geneva, Switzerland, 2004.
- Czekaj, E.; Zych, J.; Kwak, Z.; Garbacz-Klempka, A. Quality Index of the AlSi7Mg0.3 Aluminium Casting Alloy Depending on the Heat Treatment Parameters. Arch. Foundry Eng. 2016, 16, 25–28. [Google Scholar] [CrossRef]
- Tiryakioglu, M.; Staley, J.T.; Campbell, J. Evaluating structural integrity of cast Al–7%Si–Mg alloys via work hardening characteristics II. A new quality index. Mater. Sci. Eng. 2004, 368, 231–238. [Google Scholar] [CrossRef]
- STN EN ISO 6506–1; Metallic Materials: Brinell Hardness Test. Part 1, Test Method. ISO: Geneva, Switzerland, 2005.
- STN 42 0363; Metal Testing. Fatigue Testing of Metals. Methodology of Testing. Úrad pre normalizáciu, metrológiu a skúšobníctvo SR: Bratislava, Slovakia, 1986.
- Kuchariková, L.; Medvecká, D.; Tillová, E.; Belan, J.; Kritikos, M.; Chalupová, M.; Uhríčik, M. The Effect of the β-Al5FeSi Phases on Microstructure. Mechanical and Fatigue Properties in A356.0 Cast Alloys with Higher Fe Content without Additional Alloying of Mn. Materials 2021, 14, 1943. [Google Scholar] [CrossRef] [PubMed]
- Irizalp, S.G.; Saklakoglu, N. Effect of Fe-rich intermetallic on the microstructure and mechanical properties of thixoformed A380 aluminium alloy. Eng. Sci. Technol. Int. J. 2014, 17, 58–62. [Google Scholar] [CrossRef]
- Mathew, J.; Remy, G.; Williams, M.A.; Tang, F.; Srirangam, P. Effect of Fe Intermetallics on Microstructure and Properties of Al-7Si Alloys. J. Miner. Met. Mater. Soc. 2019, 71, 4362–4369. [Google Scholar] [CrossRef]
- Taylor, J.A. The effect of iron in Al-Si casting alloys. In Proceedings of the 35th Australian Foundry Institute National Conference, Adelaide, Australia, 31 October–3 November 2004; pp. 148–157. [Google Scholar]
- Seifeddine, S. The Influence of Fe on the Microstructure and Mechanical Properties of Cast Al-Si Alloys; Literature Review-Vilmer Project; Jönköping University: Jönköping, Sweden, 2007. [Google Scholar]
- Yasakau, K.A.; Zheludkevich, M.L.; Ferreira, M.G.S. Role of Intermetallics in Corrosion of Aluminum Alloys; Smart Corrosion Protection; Intermetallic Matrix Composites, Woodhead Publishing: Duxford, UK, 2018; pp. 425–462. [Google Scholar] [CrossRef]
- Kuchariková, L.; Liptáková, T.; Tillová, E.; Kajánek, D.; Schmidová, E. Role of Chemical Composition in Corrosion of Aluminum Alloys. Metals 2018, 8, 581. [Google Scholar] [CrossRef]
- Osório, W.R.; Garcia, L.R.; Goulart, P.R.; Garcia, A. Effects of eutectic modification and T4 heat treatment on mechanical properties and corrosion resistance of an Al–9 wt. % Si casting alloy. Mater. Chem. Phys. 2007, 106, 343–349. [Google Scholar] [CrossRef]
- Osório, W.R.; Goulart, P.R.; Garcia, A. Effect of silicon content on microstructure and electrochemical behavior of hypoeutectic Al–Si alloys. Mater. Lett. 2008, 62, 365–369. [Google Scholar] [CrossRef]
- Trško, L.; Hadzima, B.; Fintová, S. Influence of Nano-Grained Surface Layer on Fatigue Properties of Structural Materials; Pearson Publishing House: London, UK, 2020. [Google Scholar]
- Trško, L.; Guagliano, M.; Bokůvka, O.; Nový, F. Fatigue life of AW 7075 Aluminium Alloy after Severe Shot Peening Treatment with Different Intensities. Procedia Eng. 2014, 74, 246–252. [Google Scholar] [CrossRef]
- Li, Y.; Retraint, D.; Gao, P.; Xue, H.; Gao, T.; Sun, Z. Effect of Surface Mechanical Attrition Treatment on Torsional Fatigue Properties of a 7075 Aluminum Alloy. Metals 2022, 12, 785. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; LI, B.; Cai, W. Characterization of tensile fracture in heavily alloyed Al−Si piston alloy. Prog. Nat. Sci. 2011, 21, 380–385. [Google Scholar] [CrossRef]
- Maceka, W.; Owsińskib, R.; Trembaczc, J.; Branco, R. Three-dimensional fractographic analysis of total fracture areas in 6082 aluminium alloy specimens under fatigue bending with controlled damage degree. Mech. Mater. 2020, 147, 103410. [Google Scholar] [CrossRef]
- Pedersen, M. Introduction to Metal Fatigue; Department of Engineering, Aarhus University: Denmark, Denmark, 2018; p. 91. [Google Scholar]
- Wang, Q.G.; Caceres, C.H.; Griffiths, J.R. Damage by eutectic particle cracking in aluminum casting alloys A356/357. Met. Mater. Trans. 2003, 34, 2901–2912. [Google Scholar] [CrossRef]
- Caceres, C.; Griffiths, J. Damage by the cracking of silicon particles in an Al-7Si-0.4Mg casting alloy. Acta Mater. 1996, 44, 25–33. [Google Scholar] [CrossRef]
- Cao, X.; Campbell, J. Morphology of β-Al5FeSi Phase in Al-Si Cast Alloys. Mater. Trans. 2006, 47, 1303–1312. [Google Scholar] [CrossRef]
- Hurtalová, L.; Tillová, E.; Chalupová, M. The Changes of Fatigue Properties in Aluminium Cast Alloy during Solution Treatment. Key Eng. Mater. 2013, 592–593, 433–436. [Google Scholar] [CrossRef]
- Tillová, E.; Chalupová, M.; Borko, K.; Kuchariková, L. Changes of Fracture Surface in Recycled A356 Cast Alloy. Mater. Today Proc. 2016, 3, 1183–1188. [Google Scholar] [CrossRef]
- Hurtalová, L.; Tillová, E.; Chalupová, M.; Belan, J.; Uhríčik, M. The Influence of Two Different Casting Moulds on the Fatigue Properties of the Al–Si–Cu Cast Alloy. Adv. Struct. Mater. 2016, 61, 61–70. [Google Scholar] [CrossRef]





| Type | Material | Si | Fe | Cu | Mn | Mg | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| primary | A | 7.028 | 0.123 | 0.013 | 0.009 | 0.354 | 0.036 | 0.123 | 92.253 |
| secondary | B | 7.340 | 0.454 | 0.021 | 0.009 | 0.302 | 0.020 | 0.118 | 91.673 |
| secondary | C | 7.315 | 0.655 | 0.030 | 0.010 | 0.292 | 0.028 | 0.120 | 91.486 |
| Measured Properties | Before Corrosion | After Corrosion | ||||
|---|---|---|---|---|---|---|
| A | B | C | A | B | C | |
| UTS [MPa] | 141 | 150 | 147 | 130 | 140 | 144 |
| HBW 5/250/15 | 52 | 55 | 54 | 58 | 59 | 59 |
| Ductility [%] | 1.45 | 1.91 | 1.55 | 1.7 | 1.5 | 1.3 |
| Impact energy [J] | 14 | 12 | 10.5 | 10.5 | 8 | 7.8 |
| Quality Index | Before Corrosion | After Corrosion | ||||
|---|---|---|---|---|---|---|
| A | B | C | A | B | C | |
| QI | 165.21 | 192.16 | 175.55 | 164.57 | 166.41 | 161.09 |
| Material A | Material B | Material C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A [mm2] | D [mm] | P [mm] | A [mm2] | D [mm] | P [mm] | A [mm2] | D [mm] | P [mm] | |
| Minimum | 48.66 | 0.25 | 0.78 | 48.66 | 0.25 | 0.78 | 24.83 | 0.18 | 0.56 |
| Maximum | 339.91 | 0.66 | 2.07 | 397.26 | 0.71 | 2.23 | 437.98 | 0.75 | 2.35 |
| Average value | 115.99 | 0.402 | 1.21 | 161.34 | 0.434 | 1.39 | 144.73 | 0.403 | 1.34 |
| Mean deviation | 71.74 | 0.120 | 0.33 | 105.31 | 0.15 | 0.44 | 91.09 | 0.15 | 0.42 |
| Fatigue Life | Before Corrosion | After Corrosion | ||||
|---|---|---|---|---|---|---|
| A | B | C | A | B | C | |
| σ3×105 [MPa] | 68.5 | 75.5 | 73.5 | 63.5 | 51.5 | 69.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuchariková, L.; Pastierovičová, L.; Tillová, E.; Uhríčik, M.; Zatkalíková, V.; Šajgalík, M. The Influence of a Corrosive Environment on Fatigue and Mechanical Properties of An Al-Cast Alloy with Higher Fe Content. Metals 2023, 13, 1019. https://doi.org/10.3390/met13061019
Kuchariková L, Pastierovičová L, Tillová E, Uhríčik M, Zatkalíková V, Šajgalík M. The Influence of a Corrosive Environment on Fatigue and Mechanical Properties of An Al-Cast Alloy with Higher Fe Content. Metals. 2023; 13(6):1019. https://doi.org/10.3390/met13061019
Chicago/Turabian StyleKuchariková, Lenka, Lucia Pastierovičová, Eva Tillová, Milan Uhríčik, Viera Zatkalíková, and Michal Šajgalík. 2023. "The Influence of a Corrosive Environment on Fatigue and Mechanical Properties of An Al-Cast Alloy with Higher Fe Content" Metals 13, no. 6: 1019. https://doi.org/10.3390/met13061019
APA StyleKuchariková, L., Pastierovičová, L., Tillová, E., Uhríčik, M., Zatkalíková, V., & Šajgalík, M. (2023). The Influence of a Corrosive Environment on Fatigue and Mechanical Properties of An Al-Cast Alloy with Higher Fe Content. Metals, 13(6), 1019. https://doi.org/10.3390/met13061019







