An Investigation on Reduction of Calcium Added Bauxite Residue Pellets by Hydrogen and Iron Recovery through Physical Separation Methods
Abstract
1. Introduction
2. Materials and Methods
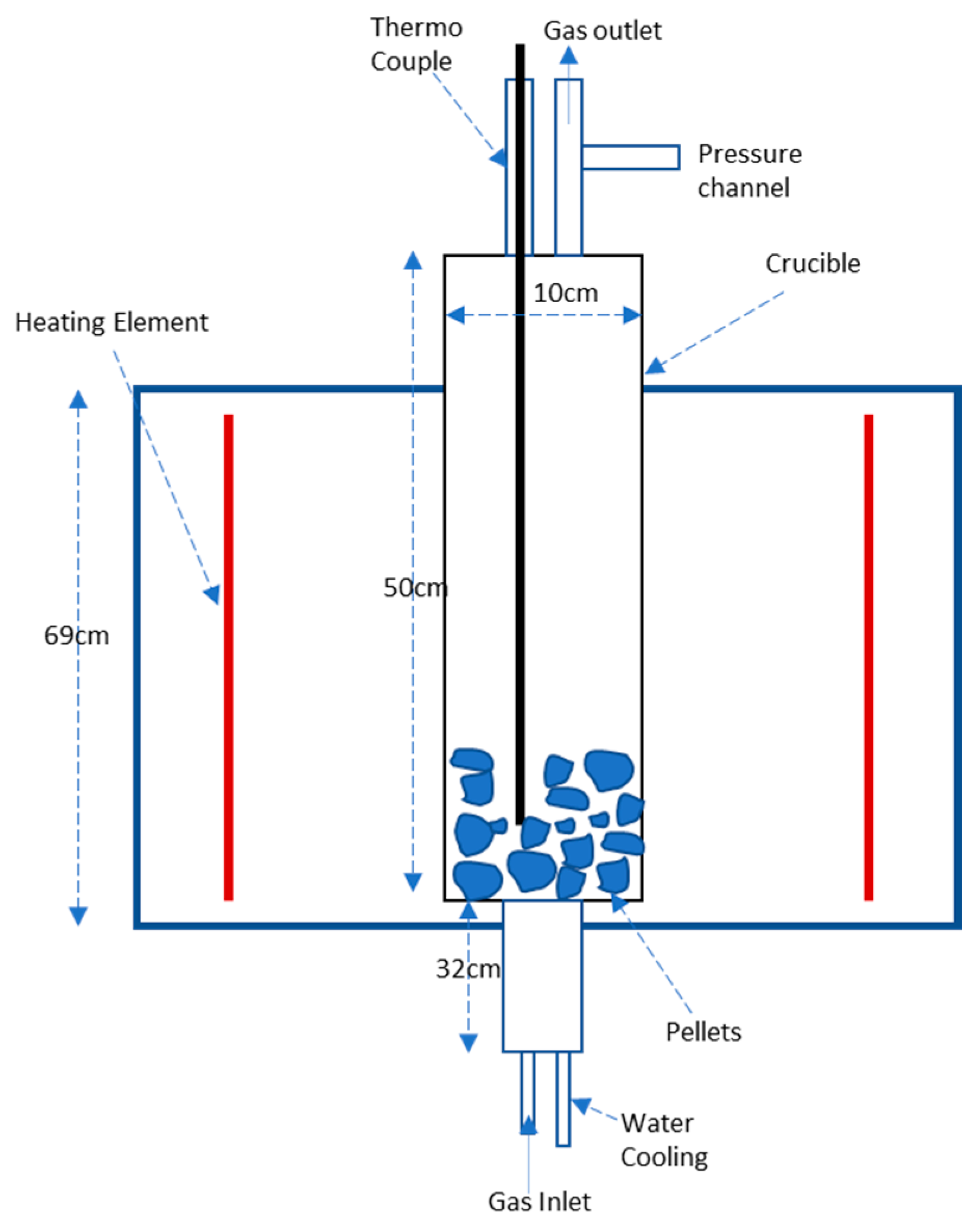
2.1. Pelletizing and Hydrogen Reduction
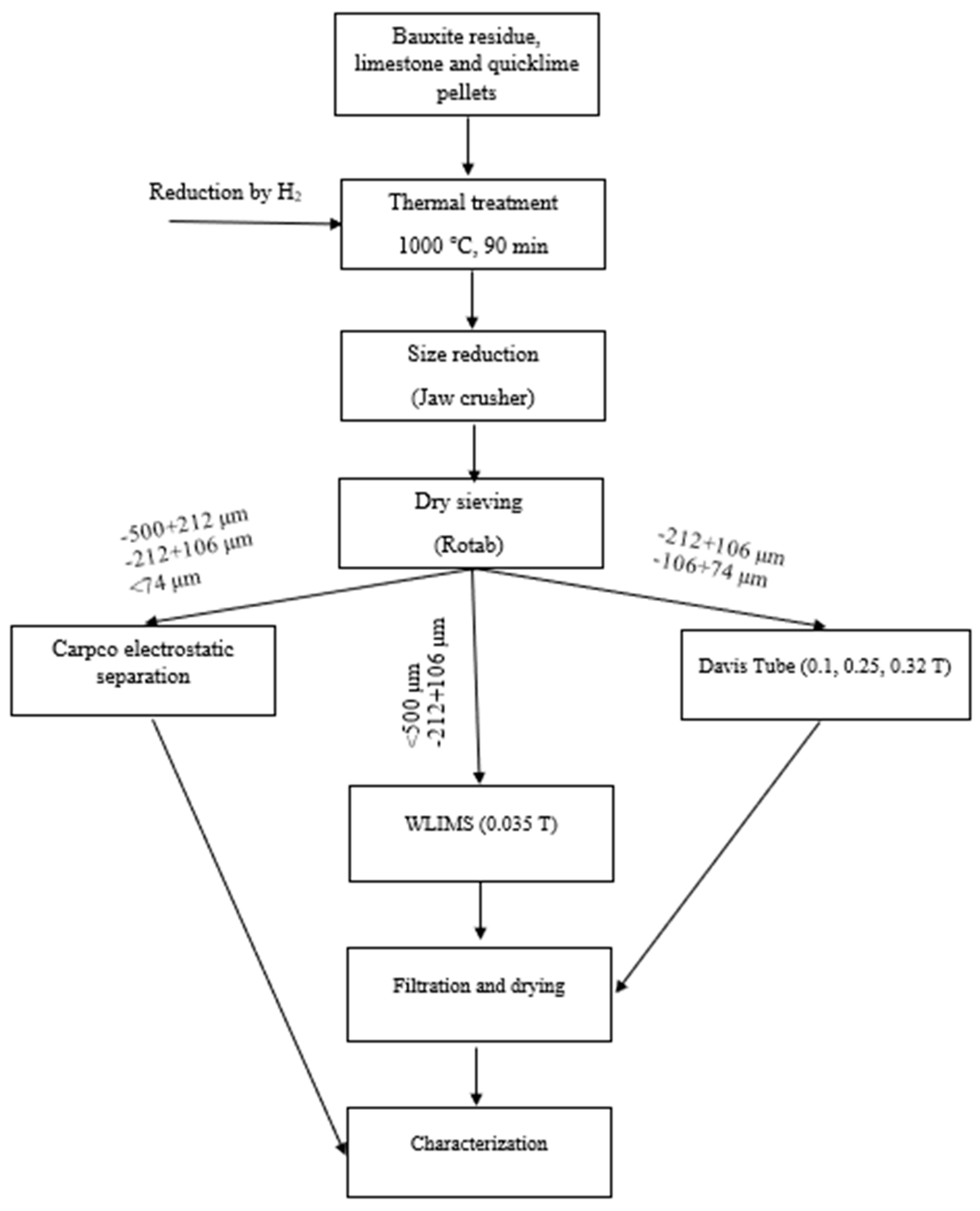
2.2. Sample Preparation and Experimental Methodology
2.2.1. Electrostatic Separation
2.2.2. Wet Magnetic Separations
2.3. Characterization of Materials
3. Results and Discussions
3.1. Mass Changes during Sintering and Reduction
3.2. Properties of Products
3.2.1. Chemical Analysis Results
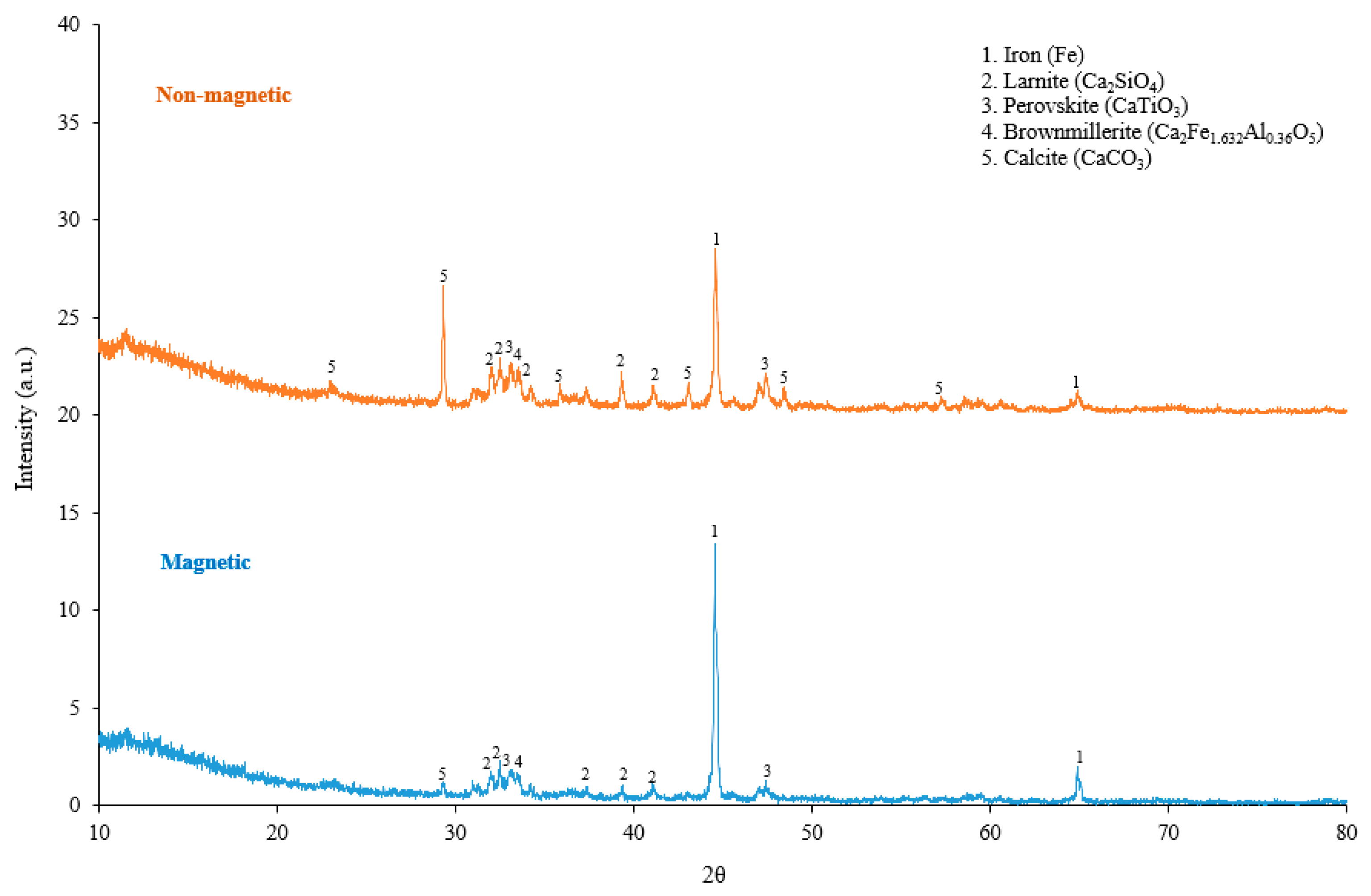
3.2.2. Phase Analysis
3.2.3. Microstructural Analysis
3.2.4. Physical Properties of Products
3.3. Electrostatic Separation
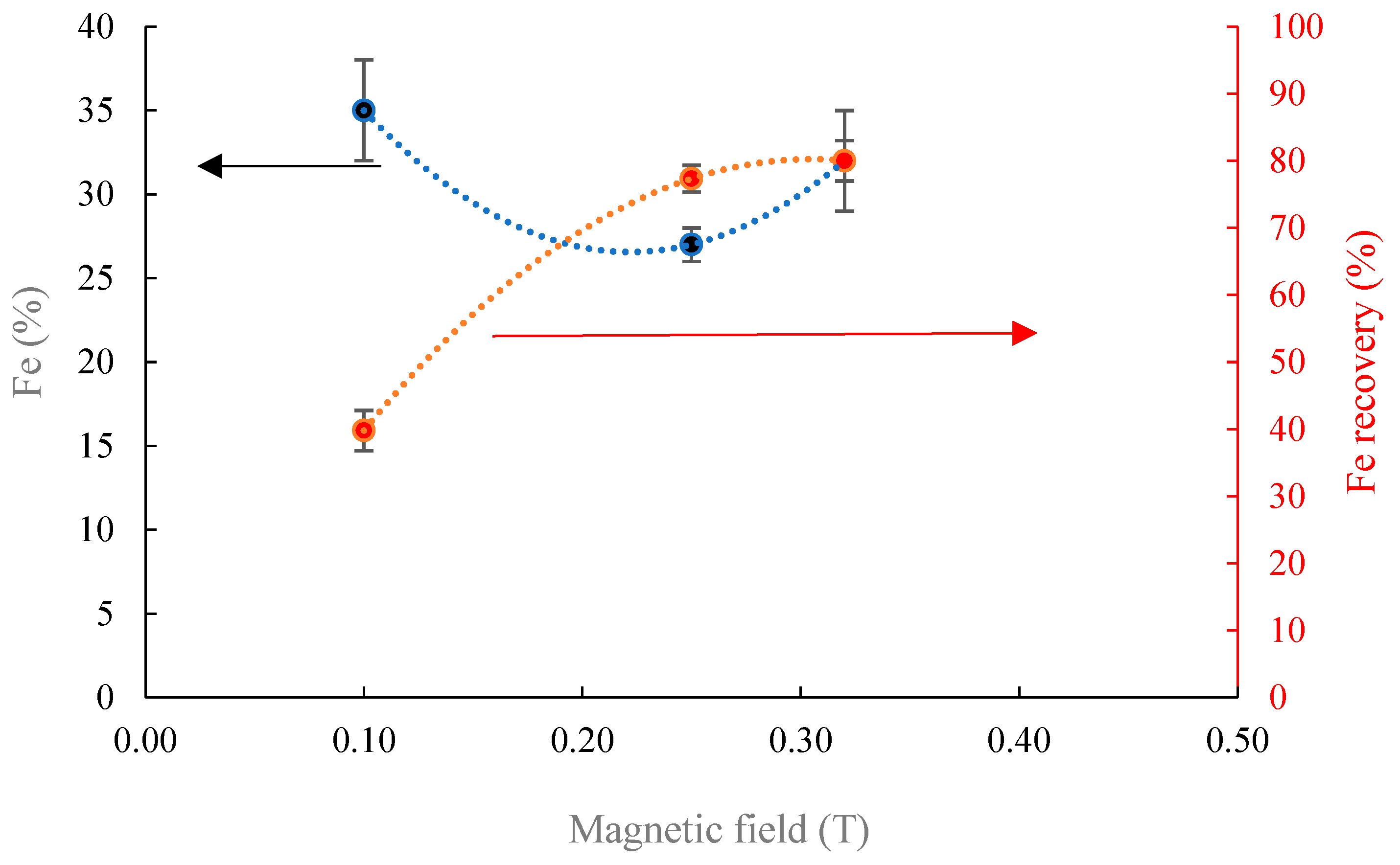
3.4. Magnetic Separation
3.4.1. Wet High-Intensity Magnetic Separation
3.4.2. Wet Low-Intensity Magnetic Separation
4. Conclusions and Future Works
- The iron oxide present in the pellets is converted completely to metallic iron. The mass loss during the reduction of hydrogen, XRD, and SEM analysis indicates all Fe in metallic form in the reduced pellets. The alumina present in the bauxite residue is converted to the mayenite phase during hydrogen reduction and via reacting with adjacent calcium oxide.
- The size of metallic Fe particles in the reduced samples is 5–6 µm on average and they are mostly accumulated in the pellet with the oxide phases in between.
- During the physical separation of the reduced pellets, it is indicated that the electrostatic separation is not a suitable technique to separate iron from the rest of the materials due to an even distribution of Fe throughout the feed’s matrix, fine iron particle sizes (5–6 µm), and the inefficiency of the process. Following the electrostatic experimental results on three different fraction sizes shows that there is only a slight improvement in Fe content, while most of the mass product transfers to the middling part.
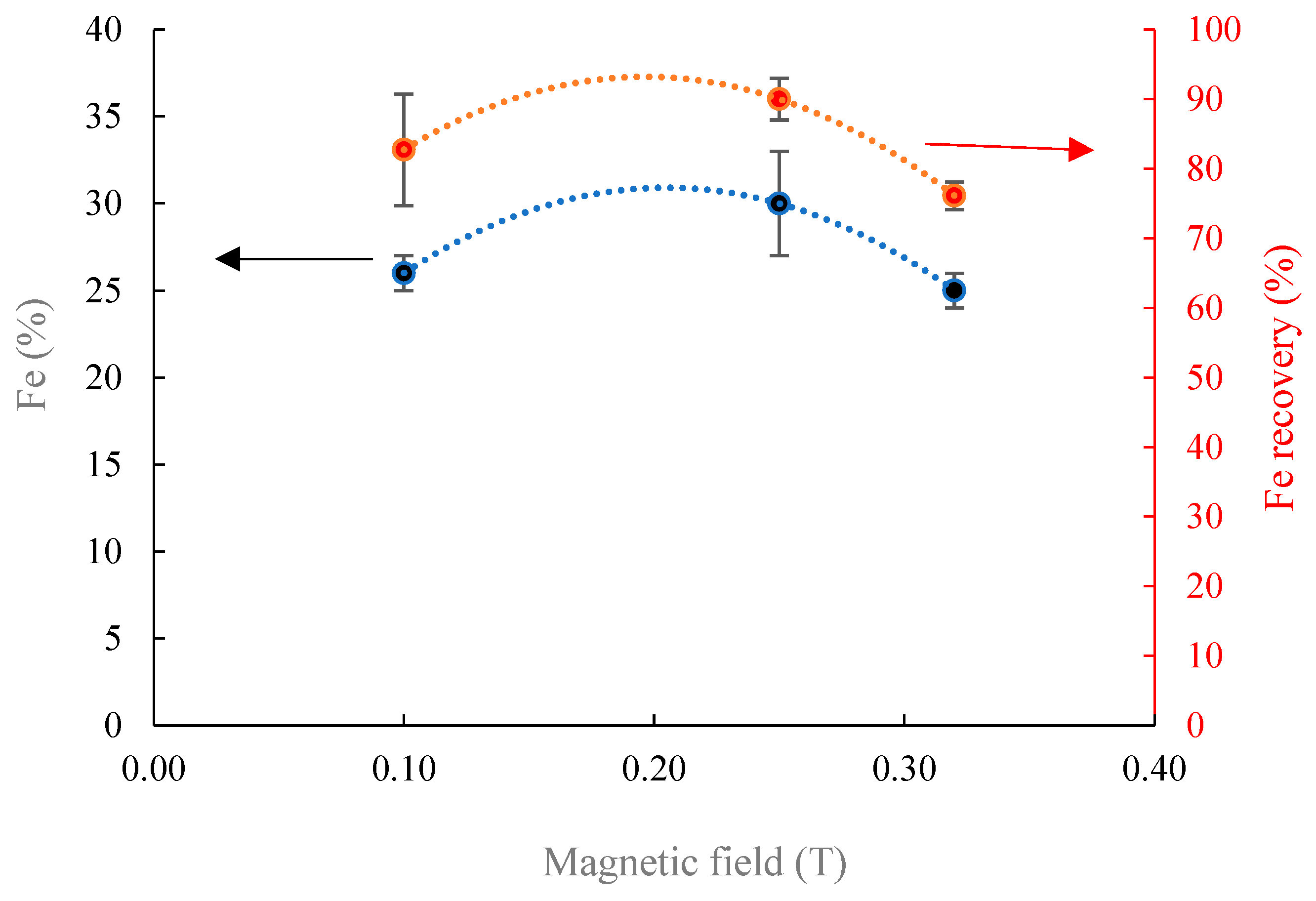
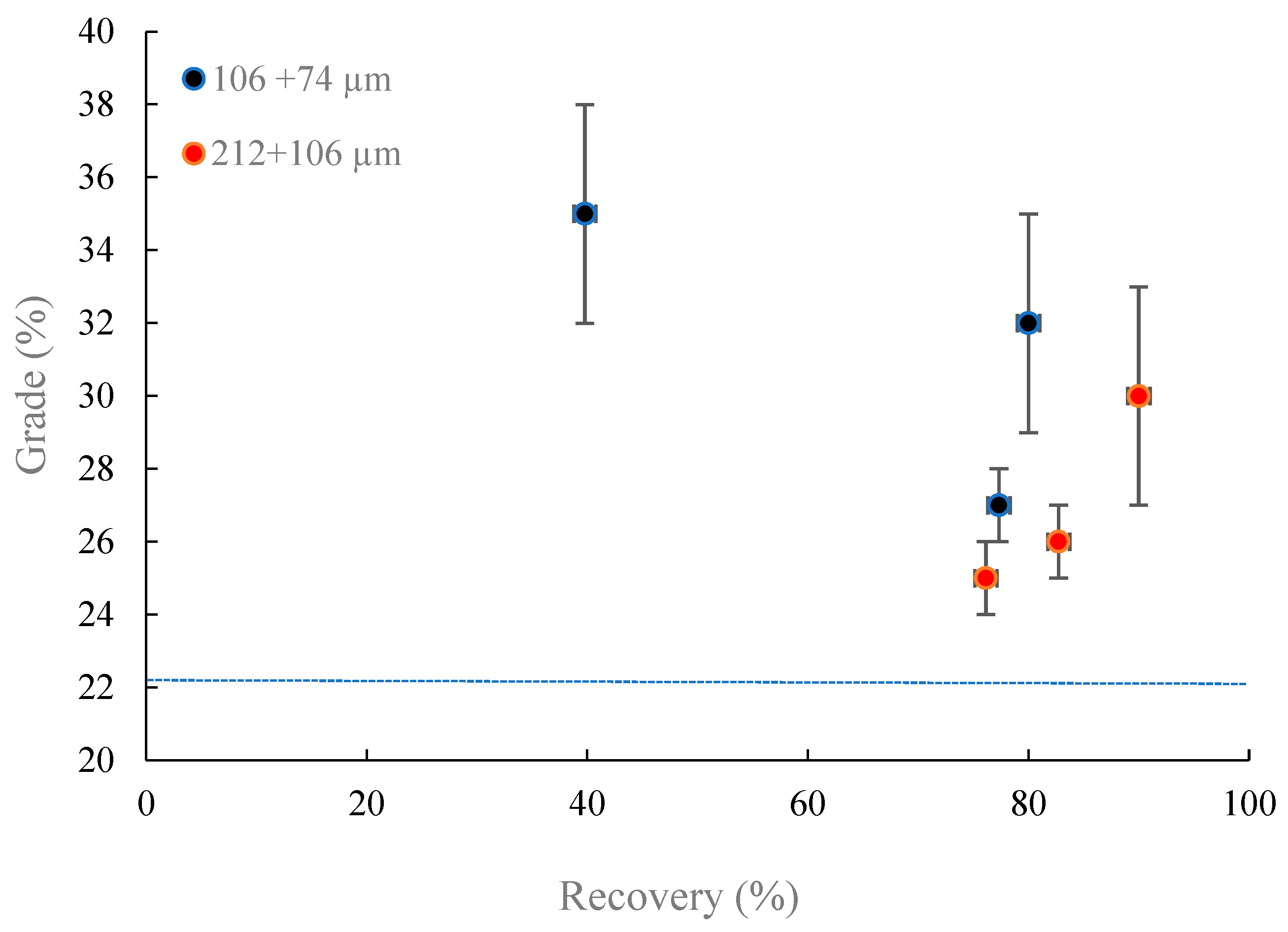
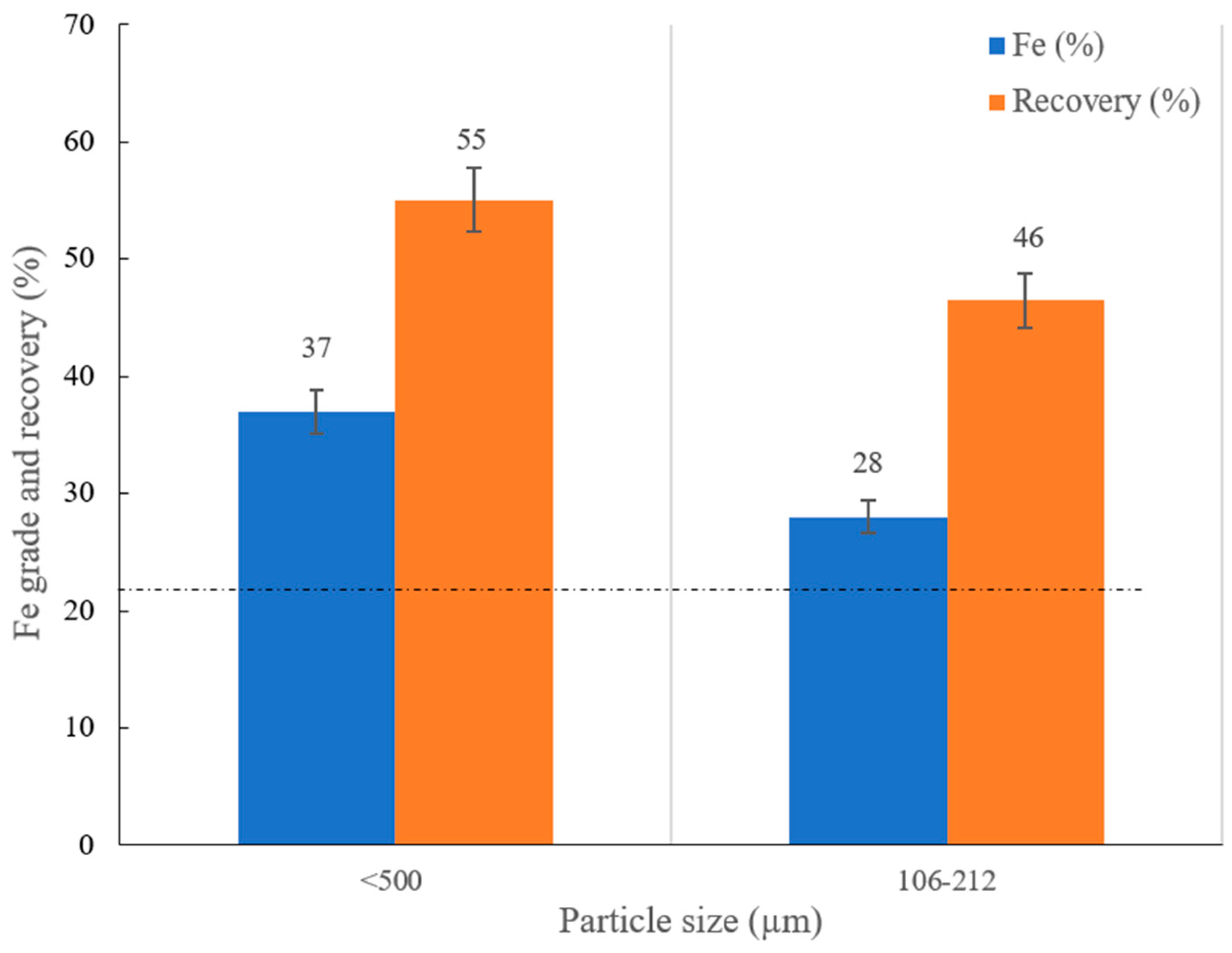
- Magnetic separation outcomes using the Davis Tube under different magnetic fields on two different fraction sizes reveal that Fe magnitude could be improved from 22% to 35% at 0.1 T, which is reduced by increasing the magnetic intensity. Coarsening the particle size from −106 + 74 µm to −212 + 106 µm at the magnetic intensity of 0.1 T induces the attenuation of Fe grade from 35% to 25% and increases the iron recovery from 40% to 83%. Applying WLIMS on −212 + 106 µm induces an increase on Fe content from 22% to 28% with the recovery of 46%.
- Since water-soluble components (e.g., Na-bearing phases) are present in the H2-reduced bauxite residue, water-leaching treatment before magnetic separation tests might be effective and need to be studied in detail.
- A continuous magnetic separation rather than a batch test for enhancing the Fe grade in final product can be considered for future test works.
- Two stages of roughing and cleaning back-to-back on the finer fraction sizes (−106 + 74 µm) can reach higher iron grade and recovery.
- The Mössbauer spectroscopy method is another promising technique for identifying valence state of iron (Fe0 (metallic), Fe2+ and Fe3+) in the studied sample.
- In addition to the XRF, XRD, and SEM-EDX analyses, the standard titrimetric analysis should be considered with the purpose of determining and correlating the content of metallic iron and total iron in the samples.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Products | |||
|---|---|---|---|
| Non-Conductive | Middling | Conductive | |
| Fe (%) | 23 | 22 | 20 |
| Recovery (%) | 65 | 32 | 5 |
References
- Balat, M. Potential importance of hydrogen as a future solution to environmental and transportation problems. Int. J. Hydrogen Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M.; Rosado, P. CO2 and Greenhouse Gas Emissions. Our World Data. 2020. Available online: https://ourworldindata.org/co2-and-greenhouse-gas-emissions (accessed on 3 April 2023).
- Wang, P.; Ryberg, M.; Yang, Y.; Feng, K.; Kara, S.; Hauschild, M.; Chen, W.Q. Efficiency stagnation in global steel production urges joint supply- and demand-side mitigation efforts. Nat. Commun. 2021, 12, 2066. [Google Scholar] [CrossRef] [PubMed]
- Trollip, H.; McCall, B.; Bataille, C. How green primary iron production in South Africa could help global decarbonization. Clim. Policy 2022, 22, 236–247. [Google Scholar] [CrossRef]
- Association, W.S. Climate Change and the Production of Iron and Steel Transforming Steel Production; World Steel Association: Brussels, Belgium, 2021. [Google Scholar]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of reduction of iron oxides by H2: Part I: Low temperature reduction of hematite. Thermochim. Acta 2006, 447, 89–100. [Google Scholar] [CrossRef]
- Skibelid, O.B.; Velle, S.O.; Vollan, F.; Van der Eijk, C.; Hoseinpur-Kermani, A.; Safarian, J. Isothermal hydrogen reduction of a lime-added bauxite residue agglomerate at elevated temperatures for iron and alumina recovery. Materials 2022, 15, 6012. [Google Scholar] [CrossRef]
- Pilla, G.; Kapelari, S.V.; Hertel, T.; Blanpain, B.; Pontikes, Y. Hydrogen reduction of bauxite residue and selective metal recovery. Mater. Today Proc. 2022, 57, 705–710. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Pilla, G.; Hertel, T.; Pontikes, Y.; Kowalczuk, P.B. H2-reduction of bauxite residue and iron recovery through magnetic separation. In Proceedings of the 17th International Mineral Processing Symposium, Istanbul, Türkiye, 15−17 December 2022; pp. 223–243. [Google Scholar]
- Lazou, A.; van der Eijk, C.; Balomenos, E.; Kolbeinsen, L.; Safarian, J. On the direct reduction phenomena of bauxite ore using H2 gas in a fixed bed reactor. J. Sustain. Metall. 2020, 6, 227–238. [Google Scholar] [CrossRef]
- Bahmani-Ghaedi, A.; Hassanzadeh, A.; Sam, A.; Entezari-Zarandi, A. The effect of residual flocculants in the circulating water on dewatering of Gol-e-Gohar iron ore. Miner. Eng. 2022, 179, 107440. [Google Scholar] [CrossRef]
- Kossoff, D.; Dubbin, W.E.; Alfredsson, M.; Edwards, S.J.; Mackline, M.G.; Hudson-Edwards, K.A. Mine tailings dams: Characteristics, failure, environmental impacts, and remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef]
- Mayes, W.M.; Burke, I.T.; Gomes, H.I.; Anton, A.D.; Molnar, M.; Feigl, V.; Ujaczki, E. Advances in understanding environmental risks of red mud after the Ajka spill, Hungary. J. Sustain. Metall. 2016, 2, 332–343. [Google Scholar] [CrossRef]
- Do Carmo, F.F.; Kamino, L.H.Y.; Junior, R.T.; Campos, I.C.; Do Carmo, F.F.; Silvino, G.; Castro, K.J.S.X.; Mauro, M.L.; Rodrigues, N.U.A.; Mirand, M.P.S.; et al. Fundão tailings dam failures: The environment tragedy of the largest technological disaster of Brazilian mining in global context. Perspect. Ecol. Conserv. 2017, 15, 145–151. [Google Scholar] [CrossRef]
- Adamson, A.N.; Bloore, E.J.; Carr, A.R. Basic Principles of Bayer Process Design. In Extractive Metallurgy of Aluminum; Springer: Cham, Switzerland; New York, NY, USA, 1962; Volume 1, pp. 23–57. ISBN 978-3-319-48574-4. [Google Scholar] [CrossRef]
- Klauber, B.C.; Gräfe, M.; Power, G. Bauxite residue issues: II. options for residue utilization. Hydrometallurgy 2011, 108, 11–32. [Google Scholar] [CrossRef]
- Liu, Y.; Naidu, R. Hidden values in bauxite residue (red mud): Recovery of metals. Waste Manag. 2014, 34, 2662–2673. [Google Scholar] [CrossRef]
- Guo, Y.H.; Gao, J.J.; Xu, H.J.; Zhao, K.; Shi, X.F. Nuggets production by direct reduction of high iron red mud. J. Iron Steel Res. Int. 2013, 20, 24–27. [Google Scholar] [CrossRef]
- Uzun, D.; Gulfen, M. Dissolution kinetics of iron and aluminium from red mud in sulphuric acid solution. Indian J. Chem. Technol. 2007, 14, 263–268. [Google Scholar]
- Safarian, J. Extraction of iron and ferrosilicon alloys from low-grade bauxite ores. In The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Cengeloglu, Y.; Kir, E.; Ersoz, M.; Buyukerkek, T.; Gezgin, S. Recovery and concentration of metals from red mud by Donnan dialysis. Colloids Surf. A Physicochem. Eng. Asp. 2003, 223, 95–101. [Google Scholar] [CrossRef]
- Jamieson, E.; Jones, A.; Cooling, D.; Stockton, N. Magnetic separation of Red Sand to produce value. Miner. Eng. 2006, 19, 1603–1605. [Google Scholar] [CrossRef]
- Sadangi, J.K.; Das, S.P.; Tripathy, A.; Biswal, S.K. Investigation into recovery of iron values from red mud dumps. Sep. Sci. Technol. 2018, 53, 2186–2191. [Google Scholar] [CrossRef]
- Habibi, H.; Pirouzan, D.; Shakibania, S.; Pourkarimi, Z.; Mokmeli, M. Physical and chemical separation of Ti, rare earth elements, Fe, and Al from red mud by carbothermal reduction, magnetic separation, and leaching. Environ. Sci. Pollut. Res. 2022, 29, 62952–62972. [Google Scholar] [CrossRef]
- Gotsu, S.; Mishra, B.; Martins, G. Extraction of iron from red mud: Low temperature reduction to magnetite and magnetic separation. In Proceedings of the 2nd International Bauxite Residue Valorization and Best Practices Conference, Athens, Greece, 10 May 2018; pp. 7–10. [Google Scholar]
- Ksiazek, M.; Ringdalen, E.; Hogaas, P.H.; van der Eijk, C. Iron removal from bauxite ores. In Proceedings of the 2nd International Bauxite Residue Valorization and Best Practices Conference, Athens, Greece, 10 May 2018; pp. 39–52. [Google Scholar]
- Samuila, A.; Iuga, A.; Morar, R.; Neamatu, V.; Dascalescu, L. Electrostatic technologies for materials recovery in high-intensity electric fields. Mater. Sci. 2007, 1, 629–687. [Google Scholar]
- Gupta, A.; Yan, D. Mineral Processing Design and Operations, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-0-444-63589-1. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Asimi Neisiani, A. Electrostatic Separation. In Dry Mineral Processing; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Kar, M.K.; van der Eijk, C.; Safarian, J. Hydrogen reduction of high temperature sintered and self-hardened pellets of bauxite residue produced via the addition of limestone and quicklime, TRAVAUX 51. In Proceedings of the 40th International ICSOBA Conference, Athens, Greece, 10–14 October 2022; pp. 823–833. [Google Scholar]
- Monsen, B.E.; Thomassen, E.S.; Bragstad, I.; Ringdalen, E.; Hoegaas, P.H. Characterization of DR pellets for DRI applications. In Proceedings of the AISTech Conference, Cleveland, OH, USA, 3−7 May 2015; Volume 1, pp. 1–11. [Google Scholar]
- Wills, B.A.; Finch, J.A. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 8th ed.; Butterworth-Heinemann: Oxford, UK, 2015; pp. 381–407. [Google Scholar] [CrossRef]
- Dance, A.D.; Morrison, R.D. Quantifying a black art: The electrostatic separation of mineral sands. Miner Eng. 1992, 5, 751–765. [Google Scholar] [CrossRef]
- Dötterl, M.; Wachsmuth, U.; Waldmann, L.; Flachberger, H.; Mirkowska, M.; Brands, L.; Beier, P.M.; Stahl, I. Electrostatic Separation, Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2016; pp. 1–35. [Google Scholar] [CrossRef]
- Pilla, G.; Hertel, T.; Blanpain, B.; Pontikes, Y. Influence of H2 content on Fe, Al and Na Recovery during low-temperature reduction of bauxite residue. In Proceedings of the 8th International Slag Valorisation Symposium, Mechelen, Belgium, 16 November 2023; pp. 146–149. [Google Scholar]
- Kapelari, S.; Gamaletsos, P.N.; Pilla, G.; Pontikes, Y.; Blanpain, B. Developing a Low-Temperature, Carbon-Lean Hybrid Valorisation Process for Bauxite Residue (Red Mud) Towards Metallic Fe and Al Recovery. J. Sustain. Metall. 2023, 9, 1–10. [Google Scholar] [CrossRef]




| Stage Description | Weight (g) |
|---|---|
| Dry pellets | 50 |
| During heating cycle | 16.5 |
| Theoretical mass of O in iron oxide | 4.7 |
| During reduction | 4.6 |
| Fraction reduction (X) | 96.8% |
| Component | Bauxite Residue | Limestone | Quick Lime | Dry Pellets | Reduced Pellets |
|---|---|---|---|---|---|
| Al2O3 | 22 | 0.9 | 0.24 | 13.18 | 20.14 |
| CaO | 8.8 | 52.7 | 96.7 | 27.41 | 39.85 |
| Fe2O3 | 40.71 | 0.15 | 0.08 | 25.03 | - |
| Fe | - | - | - | - | 21.69 |
| K2O | 0.09 | 0.12 | BDL ** | 0.05 | 0.04 |
| MnO | 0.08 | BDL | BDL | BDL | 0.03 |
| MgO | 0.23 | 0.95 | 0.64 | 0.42 | 0.68 |
| Na2O | 3.1 | BDL | BDL | 1.27 | 1.46 |
| P2O5 | 0.11 | 0.01 | 0.02 | 0.05 | 0.10 |
| SO3 | 0.95 | 0.06 | BDL | 0.45 | 1.12 |
| SiO2 | 7.1 | 2.07 | 0.46 | 4.24 | 7.68 |
| TiO2 | 5 | 0.03 | 0.02 | 3.10 | 4.86 |
| Cr2O3 | BDL | BDL | BDL | 0.17 | 0.26 |
| NiO | BDL | BDL | BDL | 0.08 | 0.07 |
| L.O.I. * | 11.83 | 43.01 | 1.84 | 24.55 | 2.13 |
| Fraction Size (µm) | −500 + 212 µm | −212 + 106 µm | <74 µm | |
|---|---|---|---|---|
| Non-conductive | Fe (%) | NA * | 24 | 22 |
| Recovery (%) | NA | 16 | 32 | |
| Middling | Fe (%) | 24 | 23 | 25 |
| Recovery (%) | 98 | 82 | 76 | |
| Conductive | Fe (%) | 20 | 18 | NA |
| Recovery (%) | 0.3 | 5 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassanzadeh, A.; Kar, M.K.; Safarian, J.; Kowalczuk, P.B. An Investigation on Reduction of Calcium Added Bauxite Residue Pellets by Hydrogen and Iron Recovery through Physical Separation Methods. Metals 2023, 13, 946. https://doi.org/10.3390/met13050946
Hassanzadeh A, Kar MK, Safarian J, Kowalczuk PB. An Investigation on Reduction of Calcium Added Bauxite Residue Pellets by Hydrogen and Iron Recovery through Physical Separation Methods. Metals. 2023; 13(5):946. https://doi.org/10.3390/met13050946
Chicago/Turabian StyleHassanzadeh, Ahmad, Manish K. Kar, Jafar Safarian, and Przemyslaw B. Kowalczuk. 2023. "An Investigation on Reduction of Calcium Added Bauxite Residue Pellets by Hydrogen and Iron Recovery through Physical Separation Methods" Metals 13, no. 5: 946. https://doi.org/10.3390/met13050946
APA StyleHassanzadeh, A., Kar, M. K., Safarian, J., & Kowalczuk, P. B. (2023). An Investigation on Reduction of Calcium Added Bauxite Residue Pellets by Hydrogen and Iron Recovery through Physical Separation Methods. Metals, 13(5), 946. https://doi.org/10.3390/met13050946








