A Comprehensive and Sustainable Recycling Process for Different Types of Blended End-of-Life Solar Panels: Leaching and Recovery of Valuable Base and Precious Metals and/or Elements
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Initial Preparation of Samples from Different Types of Solar Panels
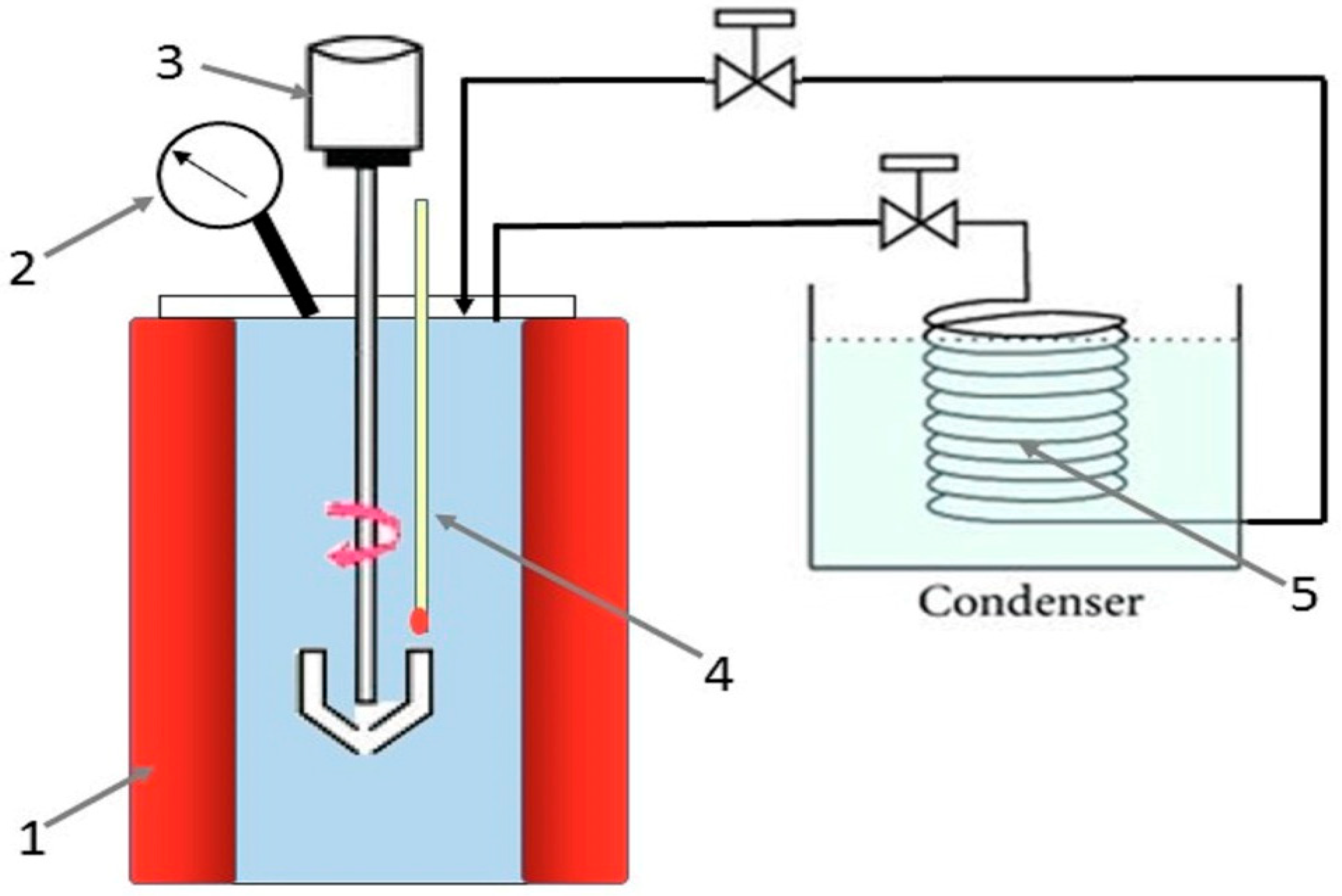
2.3. Methods
2.4. Multivariate Design of Experiment
2.5. Analytical Procedure
3. Results and Discussion
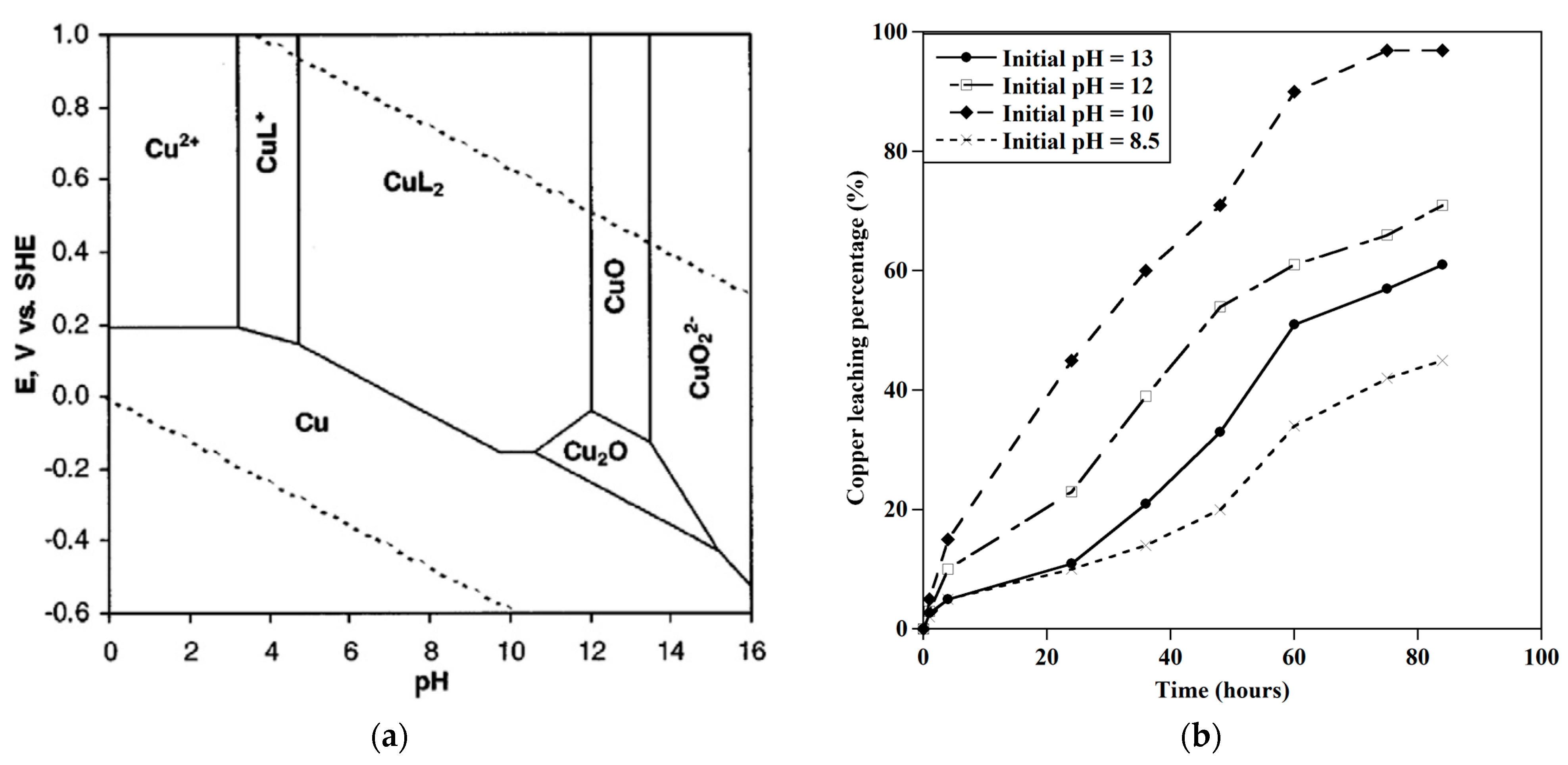
3.1. The Leaching Behavior of Base Metals
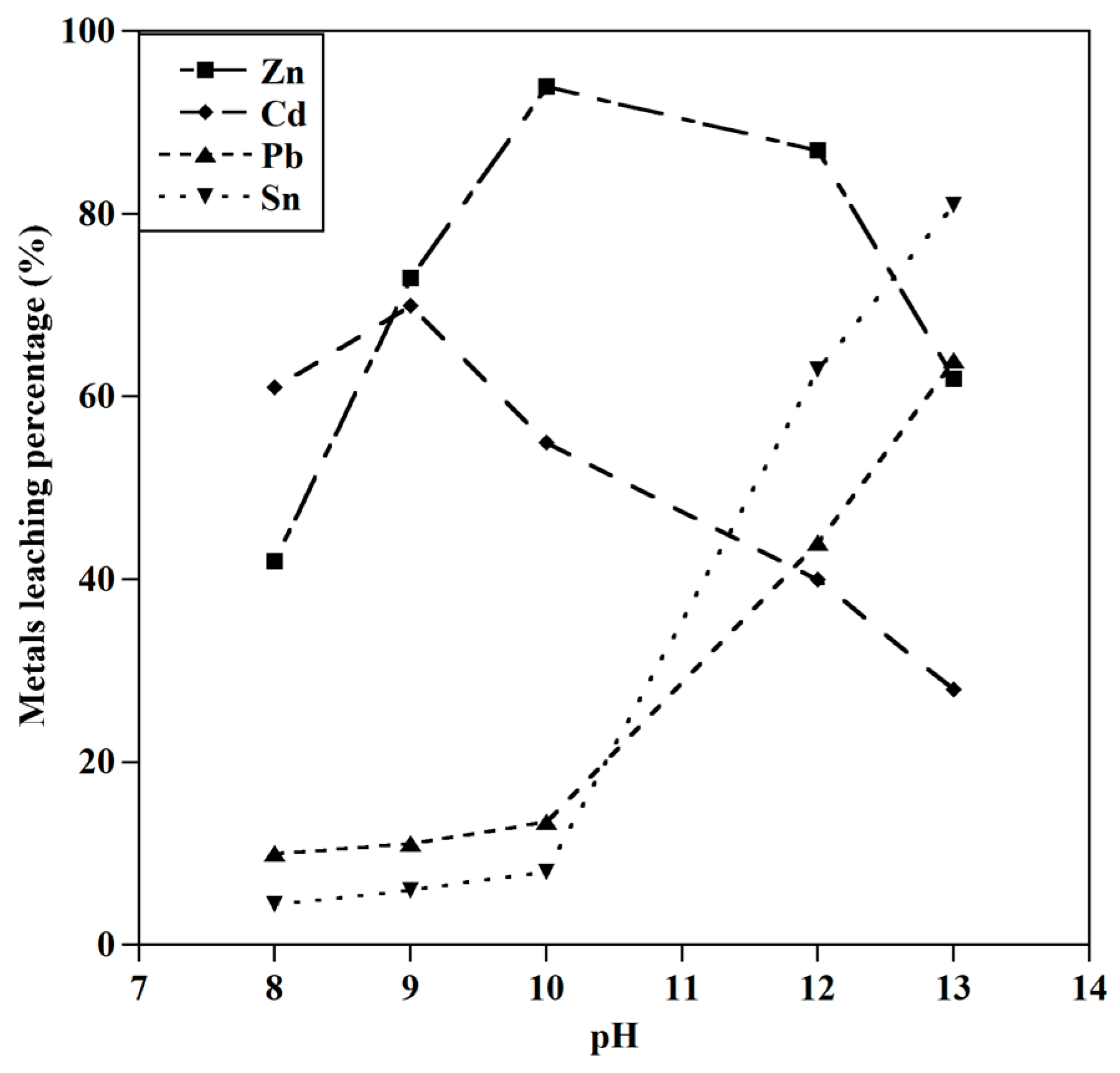
3.1.1. Effect of Initial pH
3.1.2. Effect of Glycine Acid Concentration
3.1.3. Effect of the Solid/Liquid Ratio
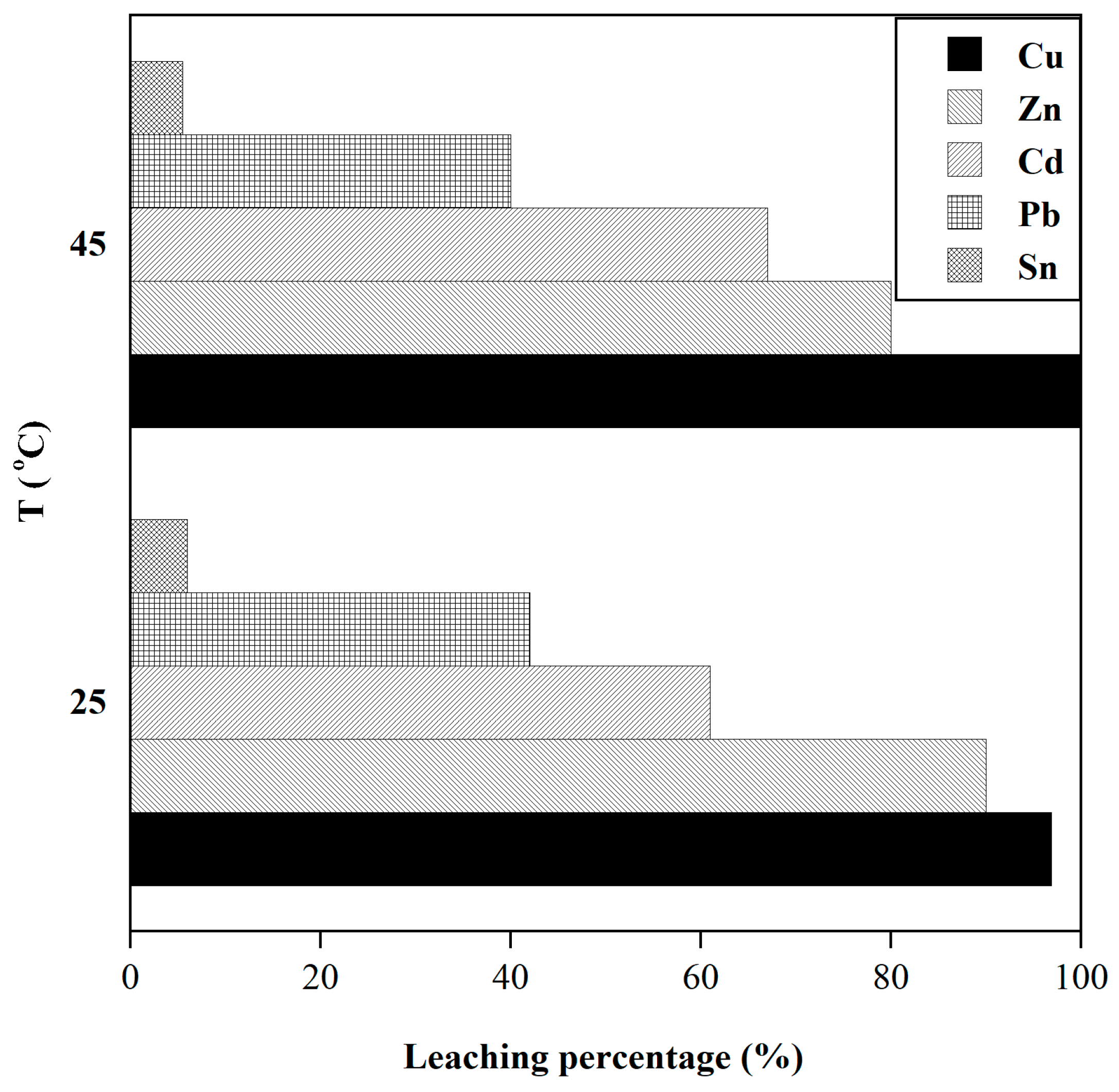
3.1.4. Effect of Temperature
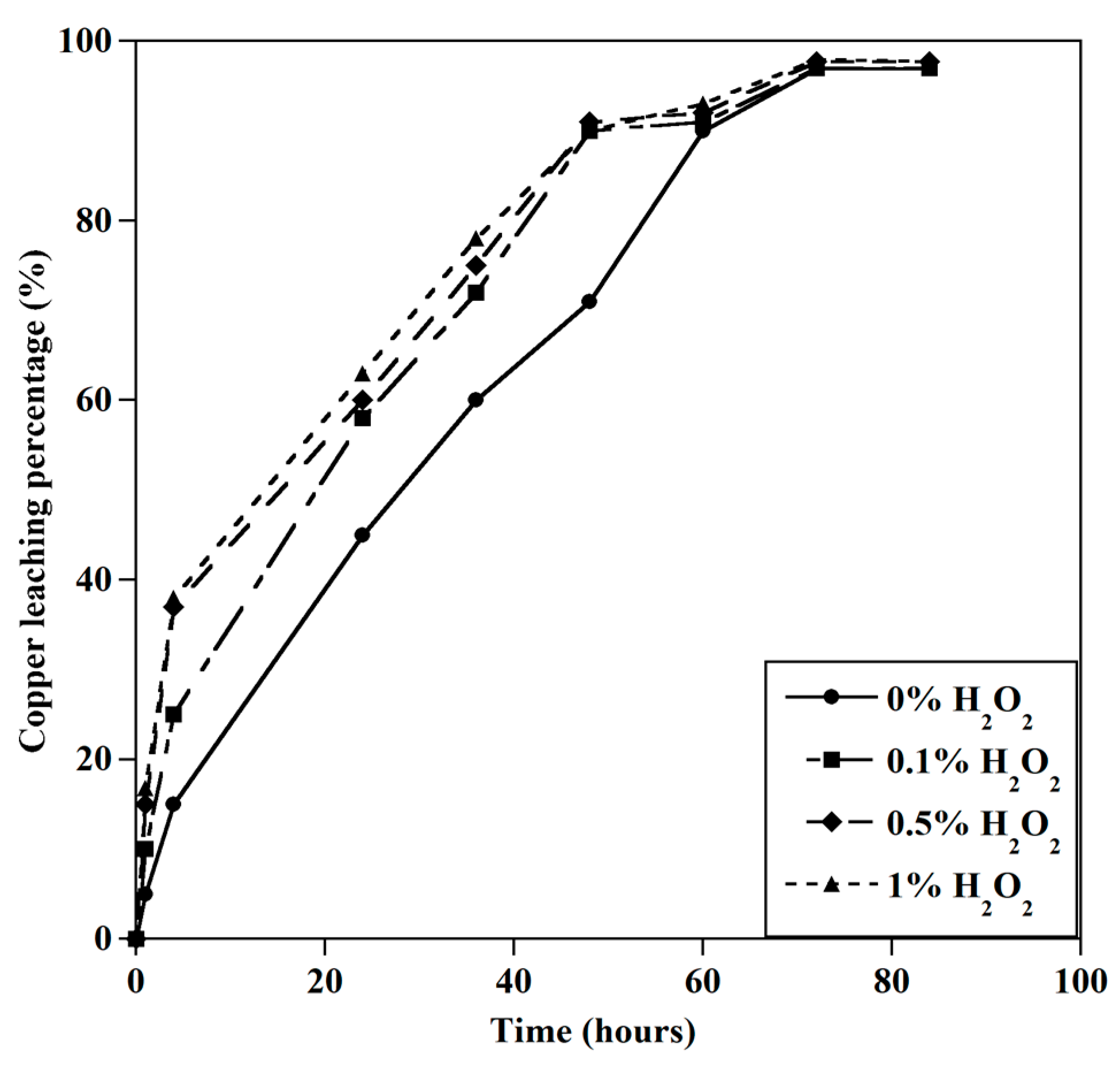
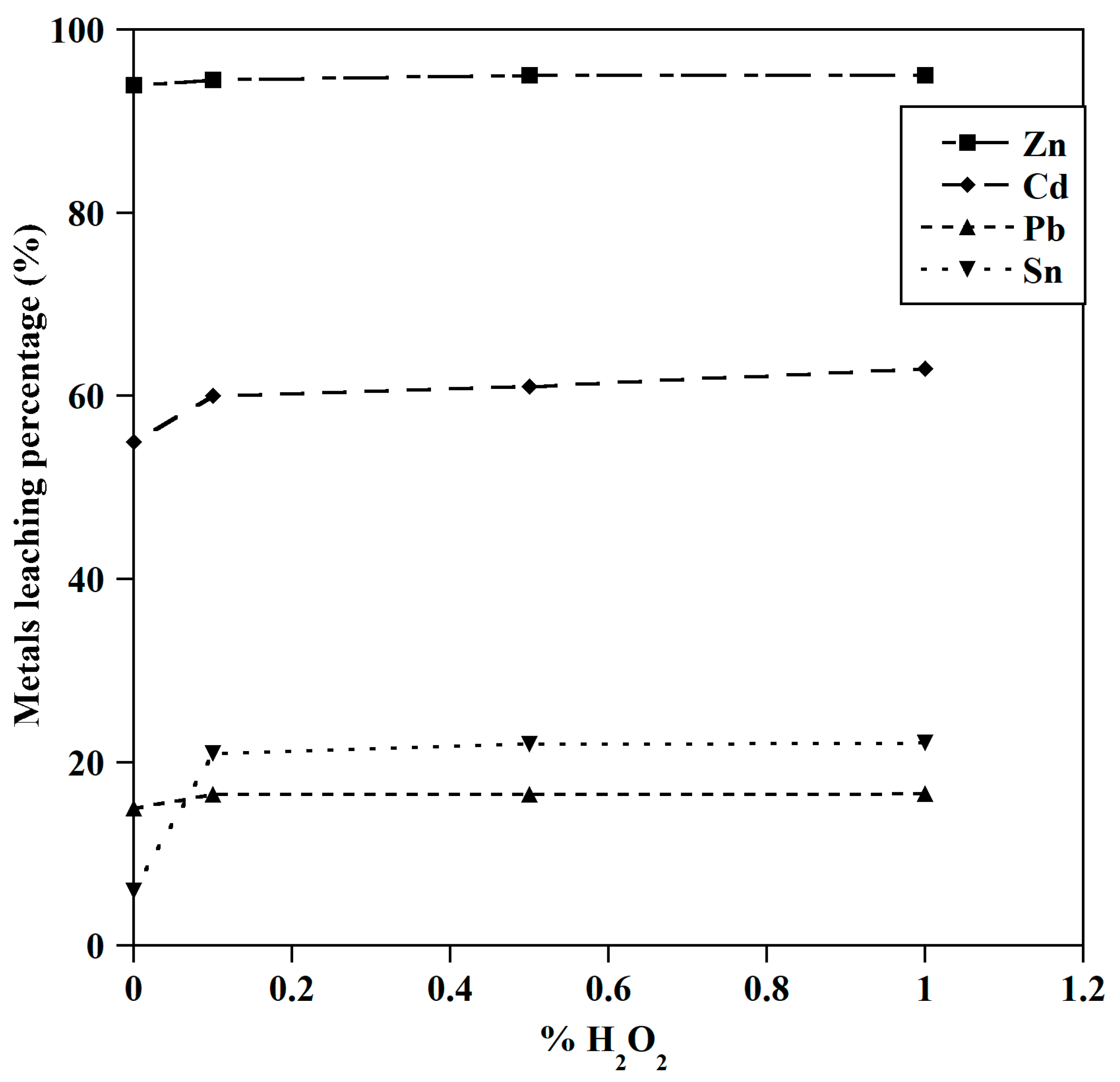
3.1.5. Effect of Hydrogen Peroxide
3.1.6. The Optimization of the Leaching Condition
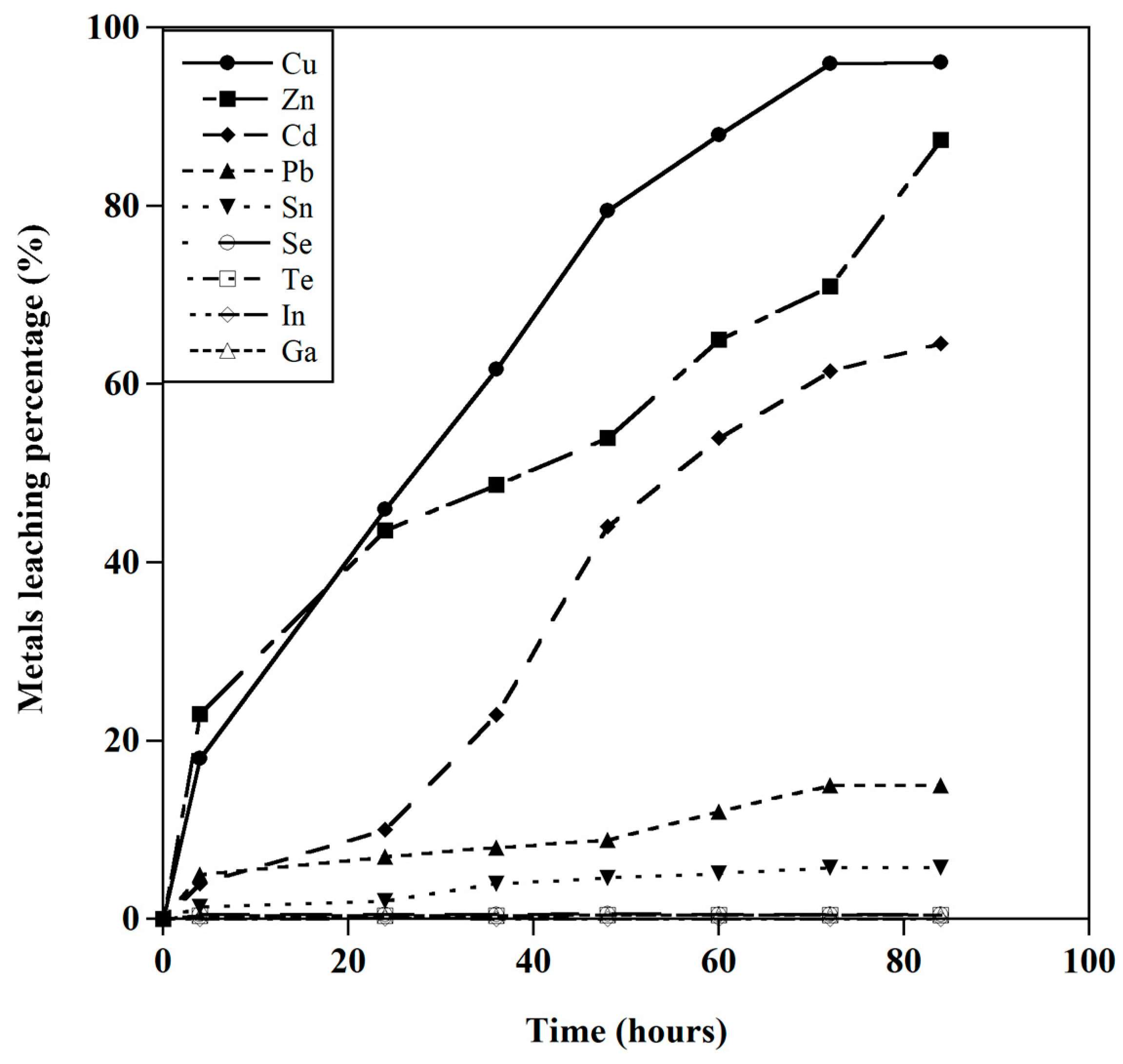
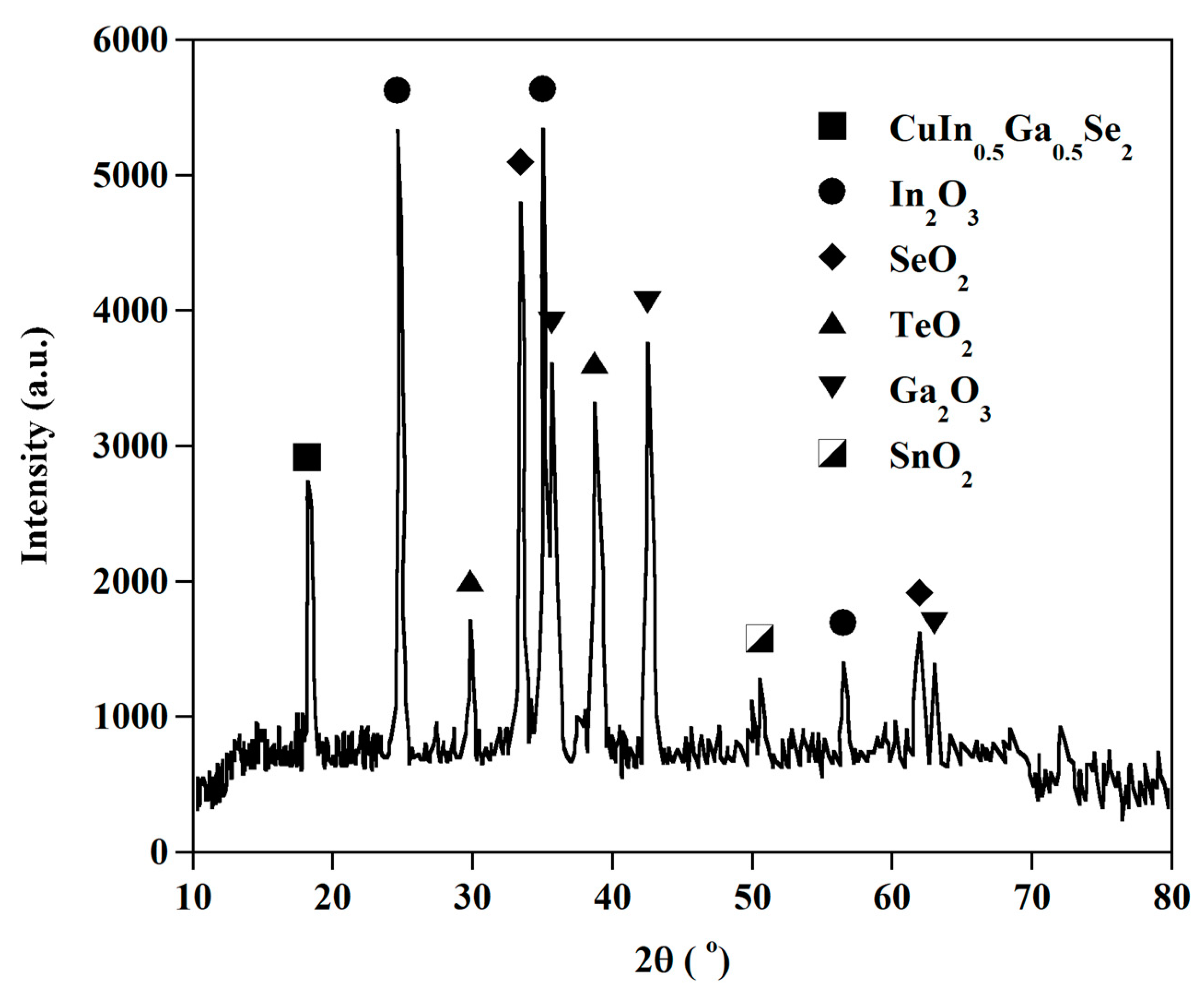
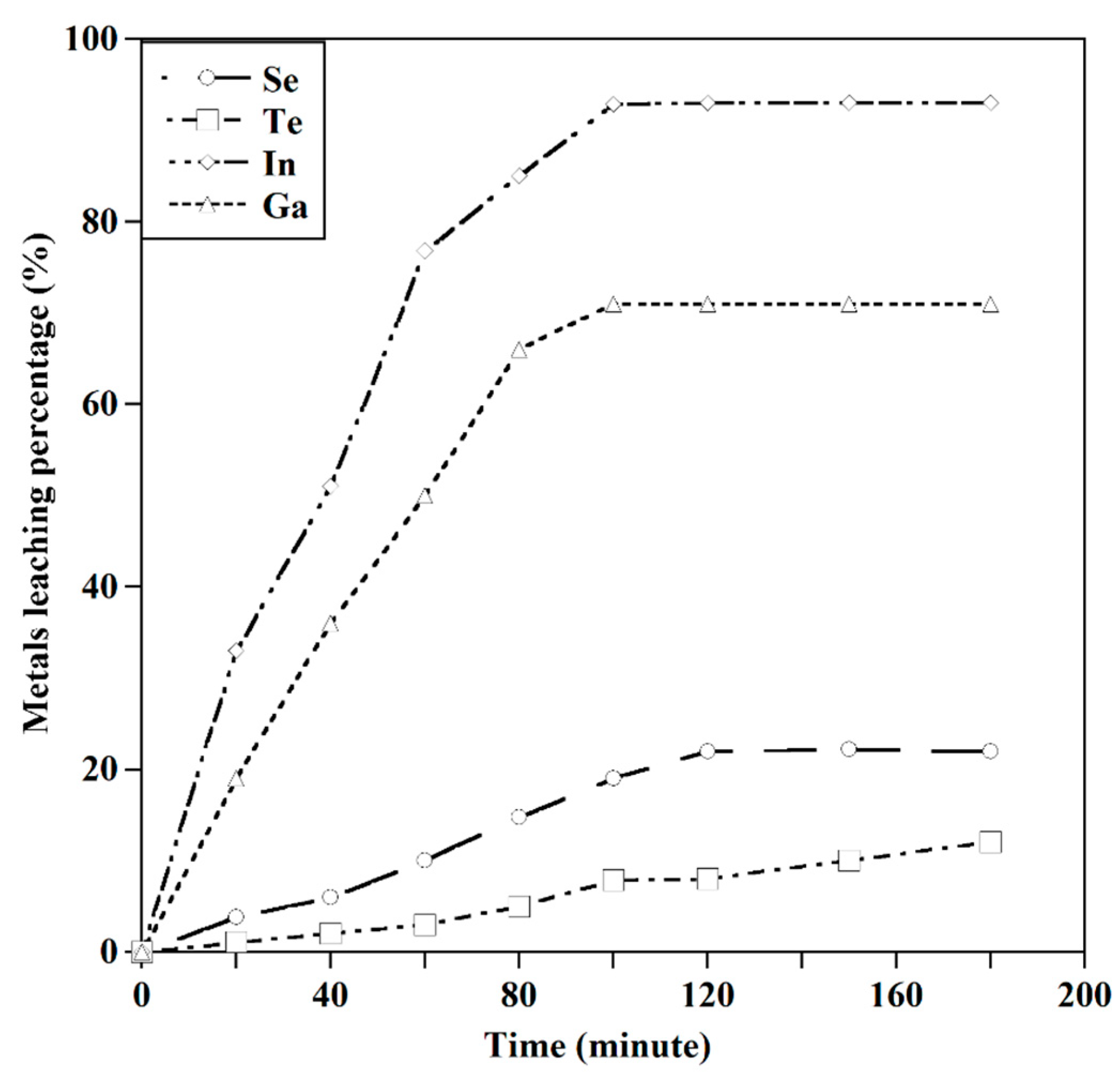
3.2. The Leaching Behavior of Valuable Metals
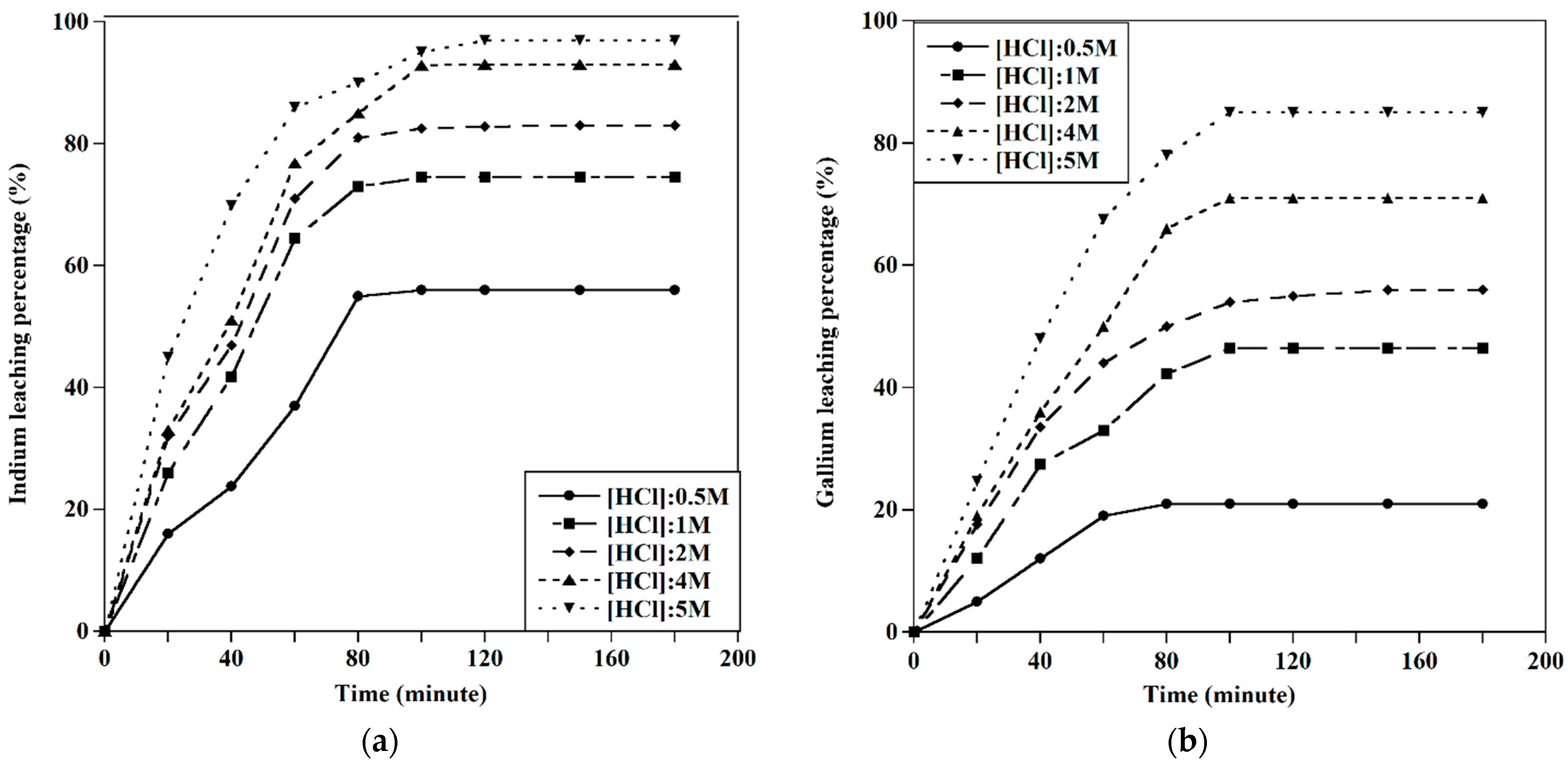
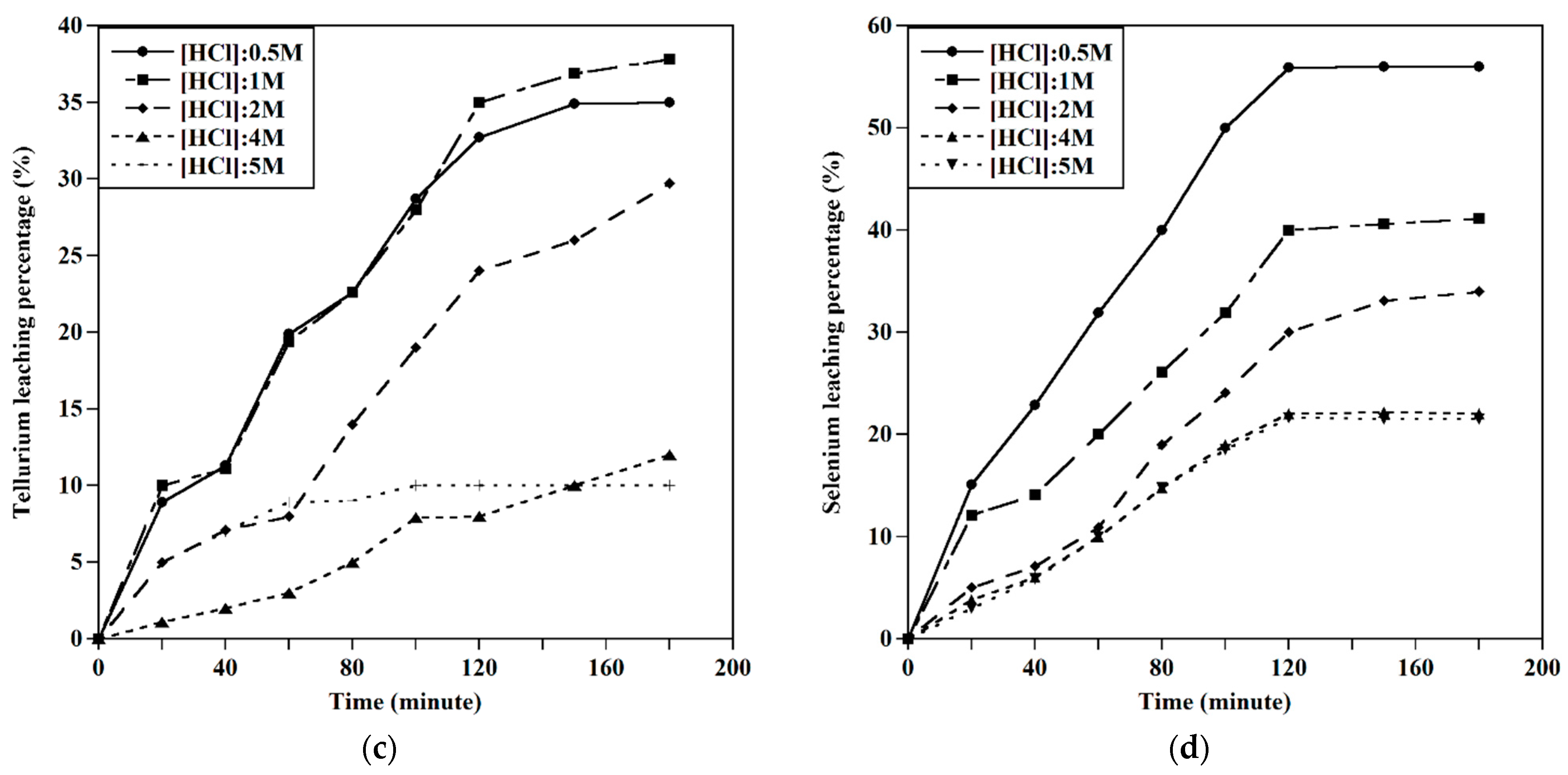
3.2.1. Effect of HCl Concentration
3.2.2. Effect of the S/L Ratio
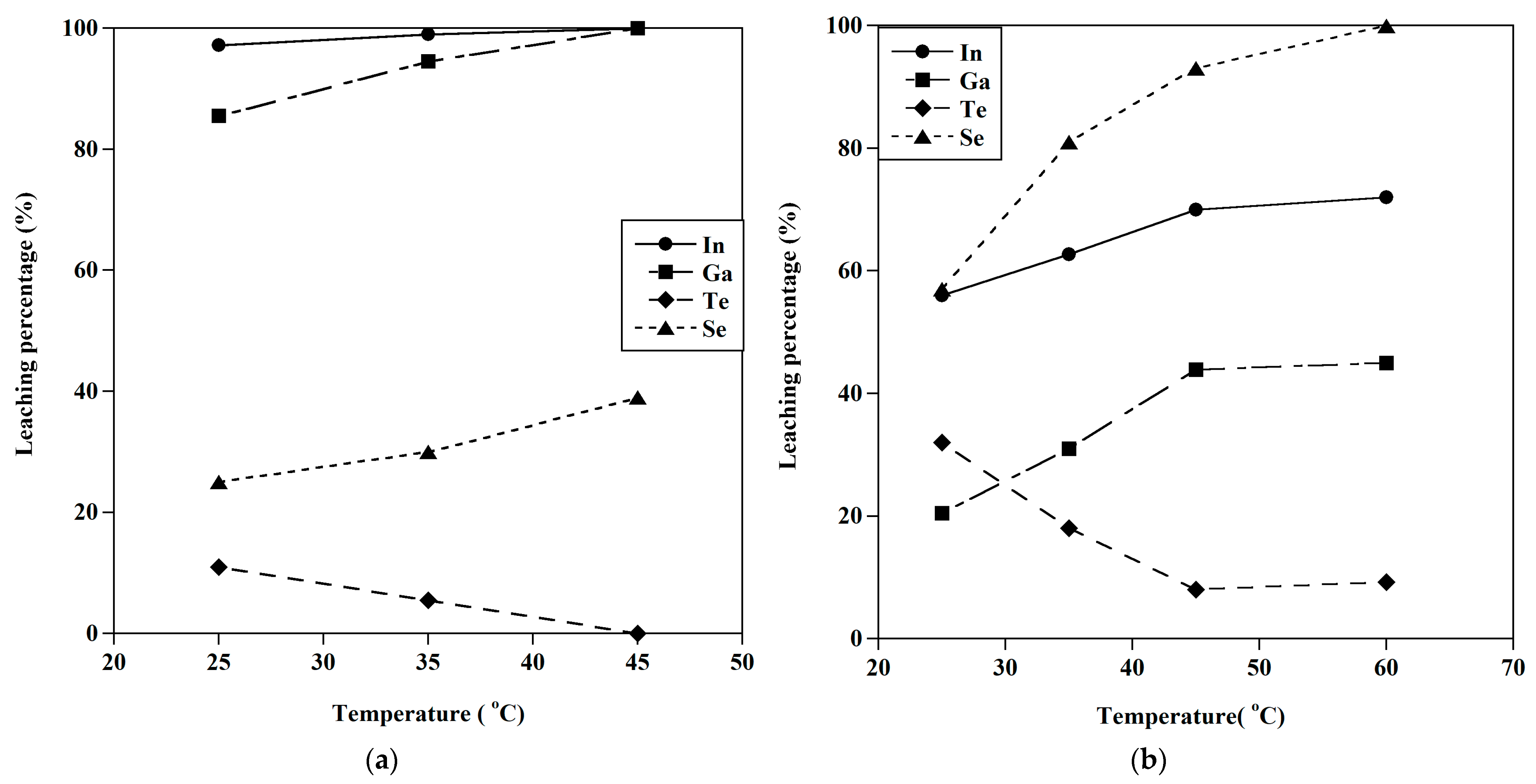
3.2.3. Effect of Temperature
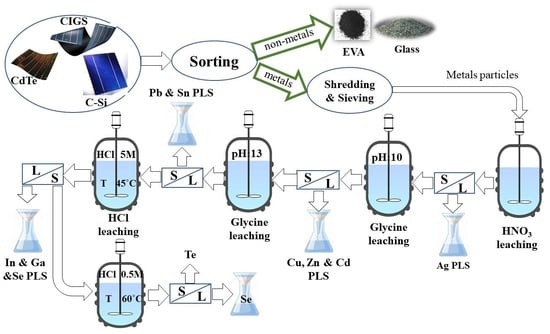
4. Suggested Flowsheet for Recycling All Types of Solar Panels
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chowdhury, M.S.; Kazi, S.R.; Chowdhury, T.; Nuthammachot, N.; Techato, K.; Akhtaruzzaman, M.; Kiong Tiong, S.; Kamaruzzaman, S.; and Nowshad, A. An overview of solar photovoltaic panels’ end-of-life material recycling. Energy Strategy Rev. 2020, 27, 100431. [Google Scholar] [CrossRef]
- Briese, E.; Piezer, K.; Celik, I.; Apul, D. Ecological network analysis of solar photovoltaic power generation systems. J. Clean. Prod. 2019, 223, 368–378. [Google Scholar] [CrossRef]
- Hosseinpour, A.; Tafaghodi Khajavi, L. Slag refining of silicon and silicon alloys: A review. Miner. Process. Extr. Metall. Rev. 2018, 39, 308–318. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. A review on germanium resources and its extraction by hydrometallurgical method. Miner. Process. Extr. Metall. Rev. 2021, 42, 406–426. [Google Scholar] [CrossRef]
- Irena, I.-P. End-of-life management: Solar photovoltaic panels. Int. Renew. Energy Agency Int. Energy Agency Photovolt. Power Syst. 2016. [Google Scholar]
- Fiandra, V.; Sannino, L.; Andreozzi, C.; Graditi, G. End-of-life of silicon PV panels: A sustainable materials recovery process. Waste Manag. 2019, 84, 91–101. [Google Scholar] [CrossRef]
- Yi, Y.K.; Kim, H.S.; Tran, T.; Hong, S.K.; Kim, M.J. Recovering valuable metals from recycled photovoltaic modules. J. Air Waste Manag. Assoc. 2014, 64, 797–807. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Tan, Q.; Peters, A.L.; Yang, C. Waste Management. 2018, 75, 450–458. Int. J. Integr. Waste Manag. Sci. Technol. 2018, 75, 450–458. [Google Scholar]
- Cucchiella, F.; Rosa, P. End-of-Life of used photovoltaic modules: A financial analysis. Renew. Sustain. Energy Rev. 2015, 47, 552–561. [Google Scholar] [CrossRef]
- Monier, V.; Hestin, M. Study on photovoltaic panels supplementing the impact assessment for a recast of the WEEE directive. Final. Rep. 2011, 6. [Google Scholar]
- Evans, A.; Strezov, V.; Evans, T.J. Assessment of sustainability indicators for renewable energy technologies. Renew. Sustain. Energy Rev. 2009, 13, 1082–1088. [Google Scholar] [CrossRef]
- Bhat, I.; Prakash, R. LCA of renewable energy for electricity generation systems—A review. Renew. Sustain. Energy Rev. 2009, 13, 1067–1073. [Google Scholar]
- De Wild-Scholten, M. Energierücklaufzeiten für PV-module und systeme energy payback times of PV modules and systems. Workshop Photovoltaik-Modul. 2009, 26, 27. [Google Scholar]
- Nain, P.; Kumar, A. Understanding manufacturers’ and consumers’ perspectives towards end-of-life solar photovoltaic waste management and recycling. Environ. Dev. Sustain. 2023, 25, 2264–2284. [Google Scholar] [CrossRef]
- Bakhiyi, B.; Labrèche, F.; Zayed, J. The photovoltaic industry on the path to a sustainable future—Environmental and occupational health issues. Environ. Int. 2014, 73, 224–234. [Google Scholar] [CrossRef]
- Płaczek-Popko, E. Top PV market solar cells 2016. Opto-Electron. Rev. 2017, 25, 55–64. [Google Scholar] [CrossRef]
- Duda, J.; Kusa, R.; Pietruszko, S.; Smol, M.; Suder, M.; Teneta, J.; Wójtowicz, T.; Żdanowicz, T. Development of roadmap for photovoltaic solar technologies and market in Poland. Energies 2022, 15, 174. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; You, J.; Diao, H.; Zhao, L.; Wang, W. Back EVA recycling from c-Si photovoltaic module without damaging solar cell via laser irradiation followed by mechanical peeling. Waste Manag. 2022, 137, 312–318. [Google Scholar] [CrossRef]
- Vargas, C.; Chesney, M. End of life decommissioning and recycling of solar panels in the United States. A real options analysis. J. Sustain. Financ. Investig. 2021, 11, 82–102. [Google Scholar] [CrossRef]
- Khawaja, M.K.; Ghaith, M.; Alkhalidi, A. Public-private partnership versus extended producer responsibility for end-of-life of photovoltaic modules management policy. Sol. Energy 2021, 222, 193–201. [Google Scholar] [CrossRef]
- Palaniappan, S.K.; Chinnasamy, M.; Rathanasamy, R.; Kumar, S. Pal. "Recycling of Solar Panels. Recycling of Solar Panels. Mater. Sol. Energy Convers. Mater. Methods Appl. 2021, 47–86. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Moscardini, E.; Abo Atia, T.; Toro, L. Photovoltaic panel recycling: From type-selective processes to flexible apparatus for simultaneous treatment of different types. Miner. Process. Extr. Metall. 2016, 125, 221–227. [Google Scholar] [CrossRef]
- Abdo, D.M.; Mangialardi, T.; Medici, F.; Piga, L. D-Limonene as a Promising Green Solvent for the Detachment of End-of-Life Photovoltaic Solar Panels under Sonication. Processes 2023, 11, 1848. [Google Scholar] [CrossRef]
- Azeumo, M.F.; Germana, C.; Ippolito, N.M.; Medici, F.; Piga, L.; Santilli, S. Photovoltaic module recycling, a physical and a chemical recovery process. Sol. Energy Mater. Sol. Cells 2019, 193, 314–319. [Google Scholar] [CrossRef]
- Ganesan, K.; Valderrama, C. Anticipatory life cycle analysis framework for sustainable management of end-of-life crystalline silicon photovoltaic panels. Energy 2022, 245, 123207. [Google Scholar] [CrossRef]
- Latunussa, C.; Ardente, F.; Blengini, G.A.; Mancini, L. Life Cycle Assessment of an innovative recycling process for crystalline silicon photovoltaic panels. Sol. Energy Mater. Sol. Cells 2016, 156, 101–111. [Google Scholar] [CrossRef]
- Klugmann-Radziemska, E. Current trends in recycling of photovoltaic solar cells and modules waste/Recykling zużytych ogniw i modułów fotowoltaicznych-stan obecny. Chem.-Didact.-Ecol.-Metrol. 2012, 17, 89–95. [Google Scholar] [CrossRef]
- Dias, P.; Javimczik, S.; Benevit, M.; Veit, H. Recycling WEEE: Polymer characterization and pyrolysis study for waste of crystalline silicon photovoltaic modules. Waste Manage. 2017, 60, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Dias, P.; Javimczik, S.; Benevit, M.; Veit, H.; Moura Bernardes, A. Recycling WEEE: Extraction and concentration of silver from waste crystalline silicon photovoltaic modules. Waste Manage. 2016, 57, 220–225. [Google Scholar] [CrossRef]
- Savvilotidou, V.; Antoniou, A.; Gidarakos, E. Toxicity assessment and feasible recycling process for amorphous silicon and CIS waste photovoltaic panels. Waste Manag. 2017, 59, 394–402. [Google Scholar] [CrossRef]
- Dias, P.; Schmidt, L.; Bonan Gomes, L.; Bettanin, A.; Veit, H.; Moura Bernardes, A. Recycling waste crystalline silicon photovoltaic modules by electrostatic separation. J. Sustain. Metall. 2018, 4, 176–186. [Google Scholar] [CrossRef]
- Fthenakis, V.M.; Wang, W. Extraction and separation of Cd and Te from cadmium telluride photovoltaic manufacturing scrap. Prog. Photovolt. Res. Appl. 2006, 14, 363–371. [Google Scholar] [CrossRef]
- Nekouaslazadeh, A. Recycling Waste Solar Panels (c-Si & CdTe) in Sweden; Digitala Vetenskapliga Arkivet: Borås, Sweden, 2021. [Google Scholar]
- Kuczyńska-Łażewska, A.; Klugmann-Radziemska, E.; Witkowska, A. Recovery of Valuable Materials and Methods for Their Management When Recycling Thin-Film CdTe Photovoltaic Modules. Materials 2021, 14, 7836. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, M.; Tsakiridis, P.E.; Kousi, P.; Hatzikioseyian, A.; Zarkadas, I.; Remoundaki, E.; Lyberatos, G. Hydrometallurgical Treatment for the Extraction and Separation of Indium and Gallium from End-of-Life CIGS Photovoltaic Panels. Mater. Proc. 2021, 5, 51. [Google Scholar]
- Gu, S.; Fu, B.; Dodbiba, G.; Fujita, T.; Fang, B. Promising approach for recycling of spent CIGS targets by combining electrochemical techniques with dehydration and distillation. ACS Sustain. Chem. Eng. 2018, 6, 6950–6956. [Google Scholar] [CrossRef]
- Tao, M.; Fthenakis, V.; Ebin, B.; Steenari, B.M.; Butler, E.; Sinha, P.; Corkish, R.; Wambach, K.; Simon, E.S. Major challenges and opportunities in silicon solar module recycling. Prog. Photovolt. Res. Appl. 2020, 28, 1077–1088. [Google Scholar] [CrossRef]
- Lee, C.H.; Chang, Y.W.; Popuri, S.R.; Hung, C.E.; Liao, C.H.; Chang, J.E.; Chen, W.S. Recovery of Silicon, Copper and Aluminum from Scrap Silicon Wafers by Leaching and Precipitation. Environ. Eng. Manag. J. (EEMJ) 2018, 17, 561–568. [Google Scholar]
- Ardente, F.; Latunussa, C.E.; Blengini, G.A. Resource efficient recovery of critical and precious metals from waste silicon PV panel recycling. Waste Manag. 2019, 91, 156–167. [Google Scholar] [CrossRef]
- Berger, W.; Simon, F.G.; Weimann, K.; Alsema, E.A. A novel approach for the recycling of thin film photovoltaic modules. Resour. Conserv. Recycl. 2010, 54, 711–718. [Google Scholar] [CrossRef]
- Mezei, A.; Ashbury, M.; Canizares, R.; Molnar, H.; Given, A.; Meader, K.; Squires, F.; Ojebuoboh, T. Jones, and W. Wang. Hydrometallurgical recycling of the semiconductor material from photovoltaic materials-Part one: Leaching. Hydrometallurgy 2008, 209. [Google Scholar]
- Hosseinipour, S.; Alamdari, E.K.; Sadeghi, N. Avrami Model for the Description of Nucleation and Growth of Tellurium During Cementation by Copper in the Sulfate Media. Metall. Mater. Trans. B 2023, 54, 2670–2679. [Google Scholar] [CrossRef]
- Sasala, R.A.; Bohland, J.; Smigielski, K. Physical and chemical pathways for economic recycling of cadmium telluride thin-film photovoltaic modules. In Proceedings of the Twenty Fifth IEEE Photovoltaic Specialists Conference—1996, Washington, DC, USA, 13–17 May 1996. [Google Scholar]
- Wang, W.; Fthenakis, V. Kinetics study on separation of cadmium from tellurium in acidic solution media using ion-exchange resins. J. Hazard. Mater. 2005, 125, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Dong, Z.; Qu, F.; Ding, F.; Peng, X.; Wang, H.; Gu, H. Removal of CdTe in acidic media by magnetic ion-exchange resin: A potential recycling methodology for cadmium telluride photovoltaic waste. J. Hazard. Mater. 2014, 279, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.-G.; Holm, O.; Berger, W. Resource recovery from urban stock, the example of cadmium and tellurium from thin film module recycling. Waste Manag. 2013, 33, 942–947. [Google Scholar] [CrossRef]
- Amato, A.; Beolchini, F. End-of-life CIGS photovoltaic panel: A source of secondary indium and gallium. Prog. Photovolt. Res. Appl. 2019, 27, 229–236. [Google Scholar] [CrossRef]
- Zimmermann, Y.S.; Niewersch, C.; Lenz, M.; Kül, Z.; Corvini, P.F.X.; Schäffer, A.; Wintgens, T. Recycling of indium from CIGS photovoltaic cells: Potential of combining acid-resistant nanofiltration with liquid–liquid extraction. Environ. Sci. Technol. 2014, 48, 13412–13418. [Google Scholar] [CrossRef]
- Kushiya, K.; Ohshita, M.; Tanaka, M. Development of recycling and reuse technologies for large-area Cu (InGa) Se/sub 2/-based thin-film modules. In Proceedings of the 3rd World Conference on Photovoltaic Energy Conversion, Osaka, Japan, 11–18 May 2003. [Google Scholar]
- Palitzsch, W.; Loser, U. Systematic photovoltaic waste recycling. Green 2013, 3, 79–82. [Google Scholar] [CrossRef]
- Menezes, S. Electrochemical solutions to some thin-film PV manufacturing issues. Thin Solid Film. 2000, 361, 278–282. [Google Scholar] [CrossRef]
- Drinkard, W.F., Jr.; Long, M.O.; Goozner, R.E. Recycling of CIS Photovoltaic Waste. U.S. Patent 5,779,877, 14 July 1998. [Google Scholar]
- Li, X.; Ma, B.; Hu, D.; Zhao, Q.; Chen, Y.; Wang, C. Efficient separation and purification of indium and gallium in spent Copper indium gallium diselenide (CIGS). J. Clean. Prod. 2022, 339, 130658. [Google Scholar] [CrossRef]
- Hu, D.; Ma, B.; Li, X.; Lv, Y.; Chen, Y.; Wang, C. Innovative and sustainable separation and recovery of valuable metals in spent CIGS materials. J. Clean. Prod. 2022, 350, 131426. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. A review on separation of gallium and indium from leach liquors by solvent extraction and ion exchange. Miner. Process. Extr. Metall. Rev. 2018, 40, 278–291. [Google Scholar] [CrossRef]
- Kawatra, S.K. Waste Characterization and Treatment. Min. Metall. Explor. 1998, 15, 65. [Google Scholar] [CrossRef]
- Yang, H.; Liu, J.; Yang, J. Leaching copper from shredded particles of waste printed circuit boards. J. Hazard. Mater. 2011, 187, 393–400. [Google Scholar] [CrossRef]
- Silvas, F.P.C.; Jiménez Correa, M.M.; Caldas, M.P.K.; de Moraes, V.T.; Espinosa, D.C.R.; Tenório, J.A.S. Printed circuit board recycling: Physical processing and copper extraction by selective leaching. Waste Manage. 2015, 46, 503–510. [Google Scholar] [CrossRef]
- Jha, M.K.; Lee, J.C.; Kumari, A.; Choubey, P.K.; Kumar, V.; Jeong, J. Pressure leaching of metals from waste printed circuit boards using sulfuric acid. JOM 2011, 63, 29–32. [Google Scholar] [CrossRef]
- Ríos, G.; Ruiz, O.; Rius, M. Cruells, and Antonio Roca. Leaching of copper from a flash furnace dust using sulfuric acid. Miner. Process. Extr. Metall. Rev. 2022, 43, 411–421. [Google Scholar] [CrossRef]
- Lawson, F.; Cheng, C.-Y.; Lee, L.S.Y. Leaching of copper sulphides and copper mattes in oxygenated chloride/sulphate leachants. Miner. Procesing Extr. Metall. Rev. 1992, 8, 183–203. [Google Scholar] [CrossRef]
- Kavousi, M.; Sattari, A.; Alamdari, E.K.; Firozi, S. Selective separation of copper over solder alloy from waste printed circuit boards leach solution. Waste Manage. 2017, 60, 636–642. [Google Scholar] [CrossRef]
- Agrawal, A.; Kumari, S.; Parveen, M.; Sahu, K.K. Exploitation of copper bleed stream for the extraction and recovery of copper and nickel by bis (2,4,4-trimethylpentyl) phosphinic acid. Miner. Process. Extr. Metall. Rev. 2012, 33, 339–351. [Google Scholar] [CrossRef]
- Kumari, S.; Agrawal, A.; Bagchi, D.; Kumar, V.; Pandey, B.D. Synthesis of copper metal/salts from copper bleed solution of a copper plant. Miner. Process. Extr. Metall. Rev. 2006, 27, 159–175. [Google Scholar] [CrossRef]
- Sahu, S.K.; Agrawal, B.D. Pandey, and Vinay Kumar. Recovery of copper, nickel and cobalt from the leach liquor of a sulphide concentrate by solvent extraction. Miner. Eng. 2004, 17, 949–951. [Google Scholar] [CrossRef]
- Agrawal, A.; Kumari, M.K.; Manoj, B.D.; Kumar, P.V.; Bagchi, D. Seperation & Recovery of Copper & Nickel from Copper Bleed Stream by Solvent Extraction Route. In Proceedings of the International Symposium on Solvent Extraction, Bhubaneswar, India, 26–27 September 2022. [Google Scholar]
- Kokes, H.; Morcali, M.; Acma, E. Dissolution of copper and iron from malachite ore and precipitation of copper sulfate pentahydrate by chemical process. Eng. Sci. Technol. Int. J. 2014, 17, 39–44. [Google Scholar] [CrossRef][Green Version]
- Deng, Z.; Oraby, E.; Eksteen, J. Sulfide precipitation of copper from alkaline glycine-cyanide solutions: Precipitate characterisation. Miner. Eng. 2020, 145, 106102. [Google Scholar] [CrossRef]
- Nassef, E.; El-Taweel, Y.A. Removal of copper from wastewater by cementation from simulated leach liquors. J. Chem. Eng. Process Technol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Oghabi, H.; Haghshenas, D.F.; Firoozi, S. Selective separation of Cd from spent Ni-Cd battery using glycine as an eco-friendly leachant and its recovery as CdS nanoparticles. Sep. Purif. Technol. 2020, 242, 116832. [Google Scholar] [CrossRef]
- Khodaei, H.; Haghshenas, D.F.; Firoozi, S. Selective leaching of zinc from carbonate source using glycine as an ecofriendly lixiviant. Miner. Eng. 2022, 185, 107680. [Google Scholar] [CrossRef]
- Shin, D.; Ahn, J.; Lee, J. Kinetic study of copper leaching from chalcopyrite concentrate in alkaline glycine solution. Hydrometallurgy 2019, 183, 71–78. [Google Scholar] [CrossRef]
- Eksteen, J.; Oraby, E.; Tanda, B. A conceptual process for copper extraction from chalcopyrite in alkaline glycinate solutions. Miner. Eng. 2017, 108, 53–66. [Google Scholar] [CrossRef]
- Fiandra, V.; Sannino, L.; Andreozzi, C.; Corcelli, F.; Graditi, G. Silicon photovoltaic modules at end-of-life: Removal of polymeric layers and separation of materials. Waste Manag. 2019, 87, 97–107. [Google Scholar] [CrossRef]
- Gasnot, L.; Decottignies, V.; Pauwels, J. Kinetics modelling of ethyl acetate oxidation in flame conditions. Fuel 2005, 84, 505–518. [Google Scholar] [CrossRef]
- de Oliveira, L.S.S.; Lima, M.T.W.D.C.; Yamane, L.H.; Ribeiro Siman, R. Silver recovery from end-of-life photovoltaic panels. Detritus 2020, 10, 62–74. [Google Scholar] [CrossRef]
- Rajahalme, J.; Perämäki, S.; Väisänen, A. Separation of palladium and silver from E-waste leachate: Effect of nitric acid concentration on adsorption to Thiol scavenger. Chem. Eng. J. Adv. 2022, 10, 100280. [Google Scholar] [CrossRef]
- Bas, A.D.; Deveci, H.; Yazici, E.Y. Treatment of manufacturing scrap TV boards by nitric acid leaching. Sep. Purif. Technol. 2014, 130, 151–159. [Google Scholar] [CrossRef]
- Aksu, S.; Doyle, F.M. Electrochemistry of copper in aqueous glycine solutions. J. Electrochem. Soc. 2001, 148, B51. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E. Critical Stability Constants: Second Supplement; Springer: Berlin/Heidelberg, Germany, 1989; Volume 6. [Google Scholar]
- Agatino, C.; De Robertis, A.; De Stefano, C.; Gianguzza, A.; Patanè, G.; Rigano, C.; Sammartano, S. Thermodynamic parameters for the formation of glycine complexes with magnesium (II), calcium (II), lead (II), manganese (II), cobalt (II), nickel (II), zinc (II) and cadmium (II) at different temperatures and ionic strengths, with particular reference to natural fluid conditions. Thermochim. Acta 1995, 255, 109–141. [Google Scholar]
- Horsch, W.G. Potentials of the zinc and cadmium electrodes. J. Am. Chem. Soc. 1919, 41, 1787–1800. [Google Scholar] [CrossRef]
- Shen, Y.-S.; Ku, Y.; Wu, M.-H. The cementation of cadmium ion in aqueous solution by a zinc column test. Sep. Sci. Technol. 2003, 38, 3513–3534. [Google Scholar] [CrossRef]
- Ku, Y.; Wu, M.-H.; Shen, Y.-S. A study on the cadmium removal from aqueous solutions by zinc cementation. Sep. Sci. Technol. 2002, 37, 571–590. [Google Scholar] [CrossRef]
- Oraby, E.; Eksteen, J. Gold leaching in cyanide-starved copper solutions in the presence of glycine. Hydrometallurgy 2015, 156, 81–88. [Google Scholar] [CrossRef]
- O’Connor, G.M.; Katerina Lepkova, J.J. Eksteen, and E. A. Oraby. Electrochemical behaviour of copper in alkaline glycine solutions. Hydrometallurgy 2018, 181, 221–229. [Google Scholar] [CrossRef]
- Halpern, J.; Milants, H.; Wiles, D. Kinetics of the Dissolution of Copper in Oxygen-Containing Solutions of Various Chelating Agents. J. Electrochem. Soc. 1959, 106, 647. [Google Scholar] [CrossRef]
- Oraby, E.; Eksteen, J. The selective leaching of copper from a gold–copper concentrate in glycine solutions. Hydrometallurgy 2014, 150, 14–19. [Google Scholar] [CrossRef]
- Tanda, B.; Eksteen, J.; Oraby, E. An investigation into the leaching behaviour of copper oxide minerals in aqueous alkaline glycine solutions. Hydrometallurgy 2017, 167, 153–162. [Google Scholar] [CrossRef]
- Barton, I.F.; Hiskey, J.B. Chalcopyrite leaching in novel lixiviants. Hydrometallurgy 2022, 207, 105775. [Google Scholar] [CrossRef]
- Hao, J.; Wang, X.; Wang, Y.; Wu, Y.; Guo, F. Optimizing the leaching parameters and studying the kinetics of copper recovery from waste printed circuit boards. ACS Omega 2022, 7, 3689–3699. [Google Scholar] [CrossRef]
- Han, Y.; Yi, X.; Wang, R.; Huang, J.; Chen, M.; Sun, Z.; Sun, S.; Shu, J. Copper extraction from waste printed circuit boards by glycine. Sep. Purif. Technol. 2020, 253, 117463. [Google Scholar] [CrossRef]
- Li, H.; Oraby, E.; Eksteen, J. Extraction of copper and the co-leaching behaviour of other metals from waste printed circuit boards using alkaline glycine solutions. Resour. Conserv. Recycl. 2020, 154, 104624. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, H.; Zhang, C.; Liu, B.; Wang, L.; Peng, W.; Cao, Y.; Song, X.; Zhu, X. A novel method for the separation of zinc and cobalt from hazardous zinc–cobalt slag via an alkaline glycine solution. Sep. Purif. Technol. 2021, 273, 119009. [Google Scholar] [CrossRef]
- Prasetyo, E.; Anderson, C.; Nurjaman, F.; Al Muttaqii, M.; Handoko, A.S.; Bahfie, F.; Rofiek Mufakhir, F. Monosodium glutamate as selective lixiviant for alkaline leaching of zinc and copper from electric arc furnace dust. Metals 2020, 10, 644. [Google Scholar] [CrossRef]
- Sato, N.; Daimon, H.; Fujie, K. Decomposition of glycine in high temperature and high pressure water. Kagaku Kogaku Ronbunshu 2002, 28, 113–117. [Google Scholar] [CrossRef]
- Li, J.; Zeng, X.; Chen, M.; Ogunseitan, O.A.; Stevels, A. “Control-Alt-Delete”: Rebooting solutions for the e-waste problem. Environ. Sci. Technol. 2015, 49, 7095–7108. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.F.P.; Oliveira, M.C.; Paterlini, W.C. Simple and fast spectrophotometric determination of H2O2 in photo-Fenton reactions using metavanadate. Talanta 2005, 66, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Chernyaev, A.; Zou, Y.; Wilson, B.P.; Lundström, M. The interference of copper, iron and aluminum with hydrogen peroxide and its effects on reductive leaching of LiNi1/3Mn1/3Co1/3O2. Sep. Purif. Technol. 2022, 281, 119903. [Google Scholar] [CrossRef]
- Oraby, E.; Eksteen, J. The leaching of gold, silver and their alloys in alkaline glycine–peroxide solutions and their adsorption on carbon. Hydrometallurgy 2015, 152, 199–203. [Google Scholar] [CrossRef]
- Paszkowicz, W.; Minikayev, P.; Piszora, D.; Trots, M.; Knapp, T. Wojciechowski, and R. Bacewicz. Thermal expansion of CuInSe 2 in the 11–1073 K range: An X-ray diffraction study. Appl. Phys. A 2014, 116, 767–780. [Google Scholar] [CrossRef]
- Hariskos, D.; Bilger, D.; Braunger, M.R.; Schock, H.W. Investigations on the mechanism of the oxidation of Cu (In, Ga) Se2. In Ternary and Multinary Compounds; CRC Press: Boca Raton, FL, USA, 2020; pp. 707–710. [Google Scholar]
- Balitskii, O.A.; Savchyn, P.P.; Lupshak, Y.; Fiyala, M. Peculiarities of (InxGa1− x) 2Se3 single crystal oxidation. Mater. Lett. 2001, 51, 27–31. [Google Scholar] [CrossRef]
- Ho, T.-L. Fiesers’ Reagents for Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 28. [Google Scholar]
- Swain, B.; Mishra, C.; Kang, L.; Park, K.S.; Lee, C.G.; Hong, H.S.; Park, J.L. Recycling of metal-organic chemical vapor deposition waste of GaN based power device and LED industry by acidic leaching: Process optimization and kinetics study. J. Power Sources 2015, 281, 265–271. [Google Scholar] [CrossRef]
- Chen, W.-S.; Chung, Y.-F.; Tien, K.-W. Recovery of Gallium and Indium from Waste Light Emitting Diodes. Resour. Recycl. 2020, 29, 81–88. [Google Scholar] [CrossRef]
- Rao, S.; Liu, Y.; Wang, D.; Cao, H.; Zhu, W.; Yang, R.; Duan, L.; Liu, Z. Pressure leaching of selenium and tellurium from scrap copper anode slimes in sulfuric acid-oxygen media. J. Clean. Prod. 2021, 278, 123989. [Google Scholar] [CrossRef]
- McPhail, D. Thermodynamic properties of aqueous tellurium species between 25 and 350. Geochim. Cosmochim. Acta 1995, 59, 851–866. [Google Scholar]
- Hosseinipour, S.; Alamdari, E.K.; Sadeghi, N. Selenium and Tellurium Separation: Copper Cementation Evaluation Using Response Surface Methodology. Metals 2022, 12, 1851. [Google Scholar] [CrossRef]





| Cu | Pb | Sn | Cd | Zn | Ag | Se | In | Ga | Te | |
|---|---|---|---|---|---|---|---|---|---|---|
| Metal content (Wt.%) | 38 | 1.9 | 1.1 | 0.81 | 5.9 | 0.56 | 0.12 | 0.09 | 0.08 | 0.51 |
| Factor | Name | Unit | Minimum | Maximum | Coded Low | Coded High |
|---|---|---|---|---|---|---|
| A | [GLY] | M | 0.10 | 1.50 | −1 ↔ 0.10 | +1 ↔ 1.50 |
| B | S/L ratio | gr/L | 5.0 | 200 | −1 ↔ 5.0 | +1 ↔ 200 |
| C | pH | 8.0 | 13.0 | −1 ↔ 8.0 | +1 ↔ 13.0 | |
| D | H2O2 | % | 0.0 | 1.0 | −1 ↔ 0.0 | +1 ↔ 1.0 |
| Reaction | Stability Constant |
|---|---|
| Cu2+ + 2(NH2CH2COO)− = Cu(NH2CH2COO)2 | 15.64 |
| Cu2+ + (NH2CH2COO)− = Cu(NH2CH2COO)+ | 8.57 |
| Cu+ + 2(NH2CH2COO)− = [Cu(NH2CH2COO)2]− | 10.1 |
| Cu(NH2CH2COO)+ + H+ = Cu(NH3CH2COO)2+ | 2.92 |
| (NH2CH2COO)− + H+ = Cu(NH3CH2COO) | 9.778 |
| H(NH2CH2COO) + H+ = H2(NH2CH2COO)+ | 2.350 |
| Std. Run No. | Run | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Response 1 | Response 2 | Response 3 | Response 4 | Response 5 |
|---|---|---|---|---|---|---|---|---|---|---|
| A:[GLY] | B: S/L | C: pH | D: H2O2 | Recovery of Copper | Recovery of Zinc | Recovery of Cadmium | Recovery of Lead | Recovery of Tin | ||
| M | gr/L | % | % | % | % | % | % | % | ||
| 9 | 1 | 0.1 | 100 | 10 | 1 | 25 | 20 | 4.8 | 1.5 | 2 |
| 16 | 2 | 1 | 20 | 10 | 0 | 92 | 88 | 62 | 44 | 5.5 |
| 20 | 3 | 1.5 | 100 | 10 | 0.5 | 36 | 22.8 | 31 | 51 | 2.4 |
| 2 | 4 | 1.5 | 20 | 10 | 0 | 90 | 72.5 | 81 | 67 | 5.6 |
| 14 | 5 | 1 | 10 | 10 | 0 | 92 | 88.5 | 75 | 57 | 5 |
| 7 | 6 | 0.8 | 100 | 10 | 0 | 28 | 17 | 25 | 15 | 4.1 |
| 15 | 7 | 0.8 | 50 | 10 | 0.5 | 54.6 | 49.1 | 37.5 | 21.5 | 9.1 |
| 4 | 8 | 1.5 | 10 | 13 | 0 | 62 | 55 | 72 | 97.8 | 98 |
| 26 | 9 | 1.5 | 5 | 9 | 1 | 43 | 55 | 99 | 60 | 5 |
| 10 | 10 | 1.5 | 5 | 10 | 1 | 93 | 75 | 84 | 62 | 6 |
| 1 | 11 | 0.1 | 5 | 10 | 0 | 40 | 30 | 10 | 5 | 5 |
| 3 | 12 | 0.1 | 200 | 10 | 0.5 | 12 | 11 | 0 | 0 | 0 |
| 22 | 13 | 1.5 | 5 | 13 | 1 | 65 | 56 | 73 | 99.8 | 99.7 |
| 8 | 14 | 0.8 | 100 | 13 | 0 | 14 | 11 | 20 | 77 | 54 |
| 19 | 15 | 0.1 | 100 | 13 | 0.5 | 21 | 18 | 0 | 16 | 24 |
| 18 | 16 | 1.5 | 100 | 8 | 0.5 | 18 | 11.5 | 27 | 14 | 7.4 |
| 25 | 17 | 0.5 | 100 | 10 | 0.5 | 43 | 34 | 17 | 7 | 3 |
| 27 | 18 | 0.8 | 100 | 10 | 0.5 | 33 | 25 | 29.5 | 15 | 2 |
| 12 | 19 | 0.5 | 20 | 10 | 1 | 98.5 | 98.1 | 62 | 16 | 5.8 |
| 28 | 20 | 0.8 | 100 | 10 | 0 | 28 | 17 | 31.7 | 22 | 4.1 |
| 17 | 21 | 0.1 | 100 | 8 | 0.5 | 16 | 15 | 3.2 | 1 | 2 |
| 23 | 22 | 0.8 | 20 | 10 | 1 | 72 | 69 | 29.9 | 24 | 10.3 |
| 6 | 23 | 0.5 | 20 | 13 | 0 | 67 | 62 | 19.5 | 81.9 | 64.9 |
| 5 | 24 | 0.8 | 50 | 10 | 0.5 | 54.6 | 49.1 | 19.5 | 21.5 | 9.1 |
| 24 | 25 | 0.8 | 200 | 10 | 1 | 36 | 28 | 13 | 21 | 8 |
| 11 | 26 | 0.1 | 100 | 10 | 1 | 22.5 | 18.1 | 7.8 | 7 | 7 |
| 13 | 27 | 0.5 | 10 | 10 | 0.5 | 99 | 99 | 60 | 22 | 10 |
| 21 | 28 | 0.5 | 10 | 10 | 1 | 99.9 | 99.5 | 63 | 22.1 | 11 |
| Recovery of Copper | ||||||||
|---|---|---|---|---|---|---|---|---|
| Analyzed Model | Quadratic Model | Reduced Quadratic Model | ||||||
| Coefficient | Sum of Squares | F-Value | p-Value | Coefficient | Sum of Squares | F-Value | p-Value | |
| Intercept | 38.64 | 98.167 | ||||||
| [GLY] | 3.99 | 78.68 | 0.40 | 0.5404 | 92.94 | 109.83 | 0.64 | 0.4346 |
| S/L | −31.18 | 3101.11 | 15.58 | 0.0017 | −0.948 | 6072.01 | 35.23 | <0.0001 |
| pH | −0.45 | 0.51 | 0.00 | 0.9606 | −4.8370 | 36.40 | 0.21 | 0.6510 |
| H2O2 | 1.27 | 7.70 | 0.04 | 0.8471 | −40.319 | 210.62 | 1.22 | 0.2828 |
| [GLY] * S/L | 3.85 | 29.53 | 0.15 | 0.7064 | ||||
| [GLY] * pH | −7.75 | 178.75 | 0.90 | 0.3606 | ||||
| [GLY] * H2O2 | −20.29 | 1826.81 | 9.18 | 0.0097 | −46.35 | 2240.58 | 13.00 | 0.0019 |
| S/L * pH | −7.61 | 42.40 | 0.21 | 0.6521 | ||||
| S/L * H2O2 | −4.01 | 40.75 | 0.20 | 0.6584 | ||||
| pH * H2O2 | 8.70 | 235.23 | 1.18 | 0.2968 | 8.15275 | 407.07 | 2.36 | 0.1408 |
| [GLY]2 | −17.66 | 1056.01 | 5.31 | 0.0384 | −40.279 | 2432.72 | 14.12 | 0.0013 |
| S/L2 | 35.90 | 2183.67 | 10.97 | 0.0056 | 0.003 | 3282.95 | 19.05 | 0.0003 |
| pH2 | −7.81 | 104.79 | 0.53 | 0.4810 | ||||
| H2O22 | −8.74 | 322.93 | 1.62 | 0.2251 | ||||
| Model summary | 21,697.64 | 7.79 | 0.0003 | 21,010.85 | 15.24 | <0.0001 | ||
| Significant | Significant | |||||||
| Residual Lack of Fit | 2587.76 | - | - | 3274.55 | - | - | ||
| Not Significant * | Not Significant * | |||||||
| Model | R2 | Adjusted R2 | Predicted R2 | ANOVA p-Value | |
|---|---|---|---|---|---|
| Recovery of Copper | Quadratic model | 0.893 | 0.779 | −0.674 | 0.0003 |
| Reduced Quadratic model | 0.865 | 0.808 | 0.576 | <0.0001 | |
| Recovery of Zinc | Quadratic model | 0.928 | 0.851 | −0.040 | <0.0001 |
| Reduced Quadratic model | 0.909 | 0.878 | 0.811 | <0.0001 | |
| Recovery of Cadmium | Quadratic model | 0.920 | 0.834 | 0.316 | <0.0001 |
| Reduced Quadratic model | 0.902 | 0.860 | 0.800 | <0.0001 | |
| Recovery of Lead | 2FI model | 0.926 | 0.883 | 0.313 | <0.0001 |
| Reduced 2FI model | 0.906 | 0.879 | 0.670 | <0.0001 | |
| Recovery of Tin | Quadratic model | 0.980 | 0.958 | 0.632 | <0.0001 |
| Reduced Quadratic model | 0.967 | 0.958 | 0.831 | <0.0001 |
| [GLY] (M) | S/L (gr/L) | pH | H2O2 (%) | Recovery (%) | |
|---|---|---|---|---|---|
| Copper | 0.5 | 10 | 10 | 1 | 99.9 |
| Zinc | 0.5 | 10 | 10 | 1 | 99.5 |
| Cadmium | 1.5 | 5 | 9 | 1 | 99 |
| Lead | 1.5 | 5 | 13 | 1 | 99.8 |
| Tin | 1.5 | 5 | 13 | 1 | 99.7 |
| Cu | Pb | Sn | Se | In | Ga | Te | |
|---|---|---|---|---|---|---|---|
| Metal content (Wt.%) | 5 | 1.1 | 3.9 | 23.2 | 10.4 | 9.8 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavousi, M.; Alamdari, E.K. A Comprehensive and Sustainable Recycling Process for Different Types of Blended End-of-Life Solar Panels: Leaching and Recovery of Valuable Base and Precious Metals and/or Elements. Metals 2023, 13, 1677. https://doi.org/10.3390/met13101677
Kavousi M, Alamdari EK. A Comprehensive and Sustainable Recycling Process for Different Types of Blended End-of-Life Solar Panels: Leaching and Recovery of Valuable Base and Precious Metals and/or Elements. Metals. 2023; 13(10):1677. https://doi.org/10.3390/met13101677
Chicago/Turabian StyleKavousi, Maryam, and Eskandar Keshavarz Alamdari. 2023. "A Comprehensive and Sustainable Recycling Process for Different Types of Blended End-of-Life Solar Panels: Leaching and Recovery of Valuable Base and Precious Metals and/or Elements" Metals 13, no. 10: 1677. https://doi.org/10.3390/met13101677
APA StyleKavousi, M., & Alamdari, E. K. (2023). A Comprehensive and Sustainable Recycling Process for Different Types of Blended End-of-Life Solar Panels: Leaching and Recovery of Valuable Base and Precious Metals and/or Elements. Metals, 13(10), 1677. https://doi.org/10.3390/met13101677