Abstract
Aluminum recycling is a promising solution to environmental and economic issues. Secondary aluminum production will rise in the near future; however, the process is not without challenges. Some of the major concerns during remelting of aluminum are the metal losses due to the oxidation of the molten metal and the removal of impurities from the metal bath. The current study summarizes the latest progress in the use of solid salt fluxes for secondary aluminum production and the treatment of molten metal. The chemistry of solid fluxes has been reviewed, with a correlation to their main chemical and physical characteristics, such as density, fluidity, wettability, and reactivity. An overview of the main types of solid fluxes is also provided, with a particular focus on their functions and applications. The efficiency of solid fluxes relies on several factors, including but not limited to the fluxes’ chemical composition and physical properties, flux amount, processing temperature, and flux morphology. The effect of salt fluxes in delivering satisfactory metal cleanliness and sufficient metal recovery has been summarized according to the main flux’s properties.
1. Introduction
Aluminum is one of the most abundant metals in the Earth’s crust, and it is the second most-used metal in the world after steel. The outstanding specific properties, good corrosion resistance, and electrical and thermal conductivity of aluminum have made it a competitive choice when it comes to different sectors, including the electrical, packaging, building, and construction sectors. However, aluminum finds its most prominent use in the transportation field [1]. The production of lightweight vehicles implies a reduction in fuel consumption and emissions. Thus, aluminum plays an important role in meeting sustainable standards to build a greener future.
Nevertheless, the production process of aluminum still needs considerable efforts to improve its sustainability, as it is estimated to account for up to 3% of all greenhouse gas emissions [2]. The primary production process of aluminum contributes to the emissions to a great extent, given the extremely large amount of energy needed for the electrolytic reduction of alumina through the Hall–Héroult process. Moreover, the mining of the raw materials, i.e., bauxite, and the great number of waste products arising from the Bayer Process, also known as red mud, constitute social and ecological concerns [3].
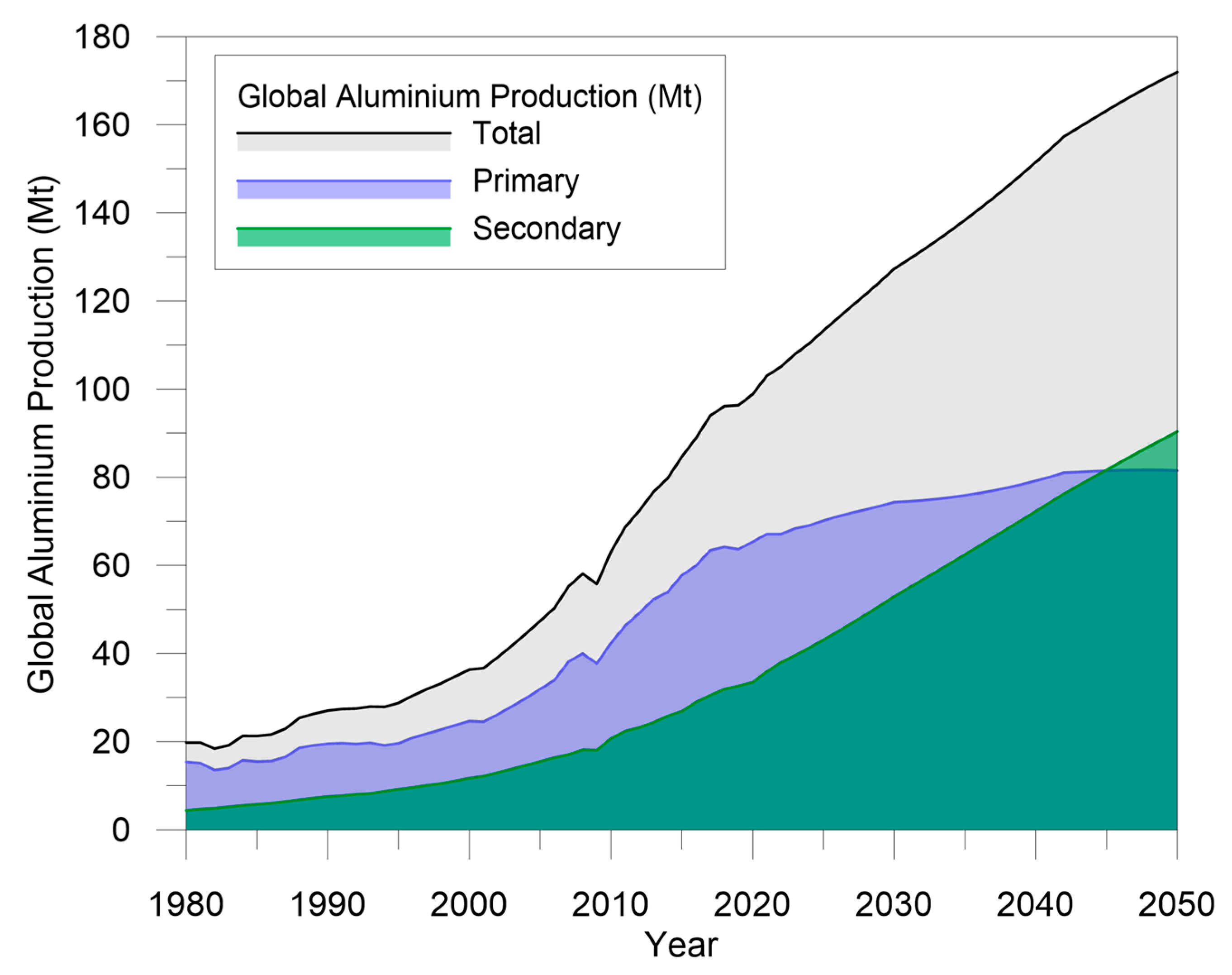
Recent developments in the automotive industry, rapid urban growth, and the innovative uses of aluminum both in the power and construction fields are contributing to aluminum’s rise as a dominant structural and engineering material. For this reason, aluminum production is currently facing a challenge: meeting the growing demand of consumers and, at the same time, improving the sustainability of the production processes to reduce their environmental impact. In this context, aluminum recycling involves significant economic and environmental benefits because it allows reducing emissions and energy requirements by up—to 95% compared to primary production. It is estimated that about one-third of the aluminum produced today originates from scrap [2]. This amount is expected to increase drastically. According to data from the International Aluminum Institute, by 2050, the amount of recycled, also called secondary, aluminum will account for more than 50% of the total aluminum production [4], as shown in Figure 1.
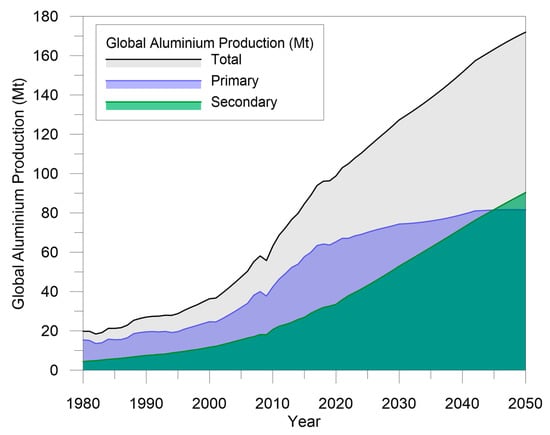
Figure 1.
Global aluminum production from primary and secondary sources. Data elaborated from [4].
A transition from a linear to a circular economy can be facilitated by recycling and improving the quality of secondary aluminum. This makes it crucial to explore methods for improving the sustainability and efficiency of aluminum recycling processes because they can help to reduce waste and promote a more circular economy. Despite the environmental and economic advantages of aluminum recycling, the process is not without challenges. The main issues arising during the scrap remelting phase are the metal losses due to oxidation and the removal of impurities from the metal bath. Several solutions could address such issues, including but not limited to scrap sorting and pre-treatments, dilution with primary aluminum, the use of salt fluxes, filtration, and innovative recycling processes, such as the Hoopes Process and the low-temperature electrolysis [5]. These processes may be used together to obtain better metal quality; however, their efficiency and cost vary to a great extent. Furthermore, aluminum suppliers must meet very demanding metal quality and composition specifications.
Solid salt fluxes are inorganic compounds that are added during the treatment of molten metal to improve the final quality. The main applications of solid salt fluxes are in the aluminum industry, where the processing of molten metal is involved, including aluminum recycling, dross treatment, and molten metal treatment. In the recycling process of aluminum, salt fluxes are added to ensure high metal recovery, decrease oxidation and metal losses, and limit the content of impurities [6]. This can promote a more sustainable and cost-effective approach to secondary aluminum production. In addition to recycling, salt fluxes are used during the treatment of dross, which is a by-product of the aluminum production process. Salt fluxes can help to recover the metal content entrapped in the dross, making the process more cost-effective [7]. Additionally, salt fluxes are used for the treatment of molten aluminum (both primary and secondary) to remove impurities such as alkali and alkali-earth metals as well as oxides. The removal of impurities can significantly improve the quality of the final product [5,8]. Overall, the applications make salt fluxes essential for the effective processing of molten aluminum.
This paper aims to provide an updated overview of the properties of solid fluxes, focusing on their application in the remelting process of aluminum scrap as well as in the treatment of molten aluminum. The review collects and summarizes several aspects which affect the efficiency of fluxing. Consolidated methods of improvement and existing weaknesses in the literature regarding the performance of fluxes are discussed to highlight the need for future developments in the field. Particular attention is dedicated to the impact of solid fluxes on metal cleanliness and metal recovery for their industrial relevance.
2. Functions of Salt Fluxes
Solid fluxing is a common practice used both by operators to improve the quality of molten metal and by recycling plants to process molten aluminum. When aluminum is oxidized, it transforms into Al2O3, leading to metal losses and inclusions. If inclusions remain in the final products, they drastically decrease the performance of components, leading to a reduction of mechanical properties, poor surface properties, and machinability [9]. When fluxes are added to the melt, they create a slag layer over the molten metal bath, thus reducing oxidation. A drawback resulting from the use of salt fluxes is the entrapment of aluminum in the slag, which also leads to metal losses [10]. For this reason, by tailoring the chemical composition, it is necessary to confer satisfactory slag-metal separation and coalescing properties to the flux. Furthermore, salt fluxes are often used to remove impurities from the molten metal by reacting with the unwanted elements and forming compounds that will either join the slag or settle at the bottom of the furnace [5]. The slag can then be easily skimmed off the surface of the bath. The chemical composition of the salt flux can be adjusted according to different variables, including the remelting process, the type and contamination level of the scrap, and the desired final metal quality. According to such variables, one or more salt fluxes can be employed to obtain the desired effect [10,11]. The functions of the salt fluxes will be discussed in more detail in the following sections.
2.1. Protection from Oxidation
Even though aluminum is one of the most common elements in the Earth’s crust, it is never found in a metallic state due to its strong affinity with oxygen according to the reaction [12]
The reaction (1) is exothermic and aluminum oxide, when formed, is thermodynamically stable. At room temperature, a layer of oxide forms and benefits solid aluminum by protecting it from corrosion. However, when the temperature is increased to metallurgical processing levels, the oxide layer’s thickness and the rate of its formation increase significantly. The rate of reaction varies based on several factors, including oxygen availability and atmosphere, but oxidation increases substantially at temperatures above 700 °C [13]. When melting scrap, especially if it has a large specific area, large metal losses occur due to rapid oxidation, resulting in dross formation that floats towards the surface of the metal [14]. The efficiency of the process is reduced as a result. Therefore, molten aluminum operators and recycling plants aim to minimize the oxidation of the liquid metal to avoid such reductions in efficiency.
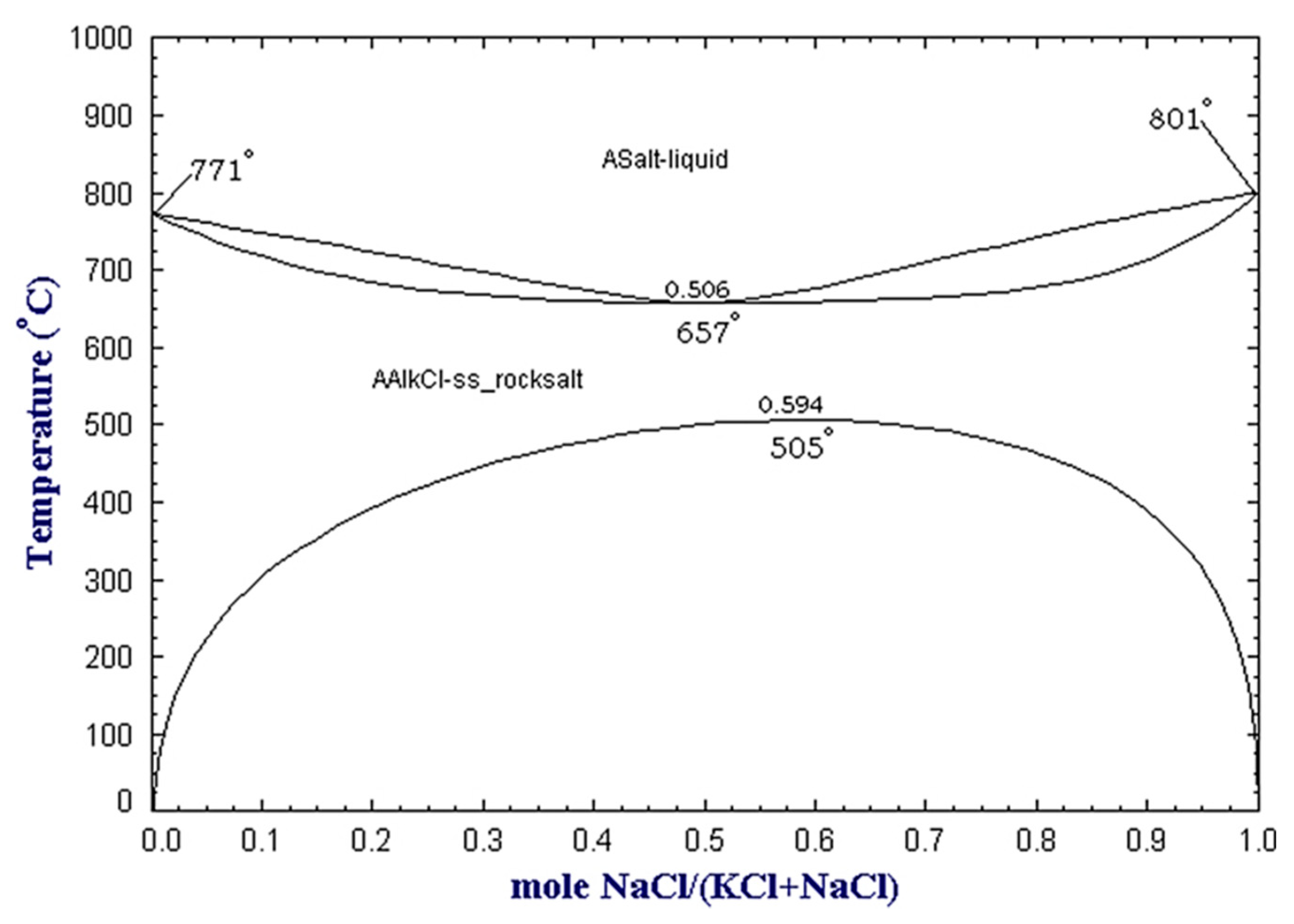
One of the most popular techniques to suppress molten aluminum oxidation is the use of a cover salt flux. The salt is added during the melting process, and it floats over the molten aluminum due to a lower density, protecting it from further oxidation. The most common salt mixtures for this purpose consist of NaCl-KCl mixtures with small additions of fluorides. When melting aluminum, only those chlorides more stable than aluminum chloride can be selected to avoid chemical reactions between the metal and the salt. Thus, the available options are restricted to the chloride salts of alkali and alkali-earth metals such as NaCl, KCl, CaCl2, and MgCl2. Moreover, NaCl and KCl are the most convenient solution as they are cost-effective [12]. At the same time, pure NaCl melts at 801 °C and KCl at 771 °C, and an equimolar mixture forms a eutectic point at 657 °C, as shown in Figure 2. The equimolar mixture is mostly used in the USA and Canada; however, higher NaCl contents, such as 70 wt %, are also widely employed, especially in Europe, due to the higher cost of KCl if compared to NaCl [2,15].
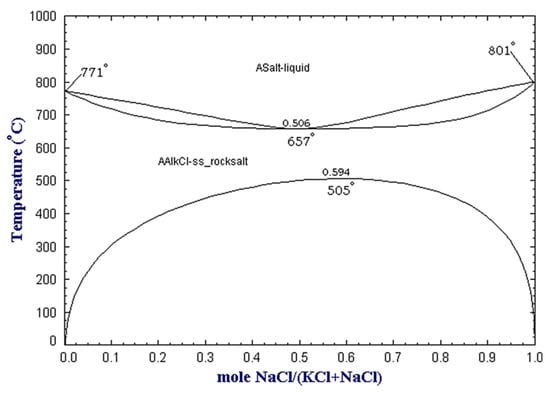
Figure 2.
NaCl-KCl binary phase diagram at atmospheric pressure as obtained from the FactSage™ FTsalt database [16].
Not only do salt fluxes prevent oxidation of molten aluminum, but they also suppress possible inflammations of organic content in the scrap. The organic material in the scrap, if not completely removed during the pre-melting treatments, can cause further oxidation of the metal. Additionally, cover fluxes prevent hydrogen dissolution in the aluminum bath, which would otherwise compromise the mechanical properties of the final products [17].
2.2. Stripping of the Oxide Layer
Even though the addition of salt in the melting process is beneficial because it limits the oxidation of the metal bath, some metal is lost due to the formation of the oxide layer, which entraps molten metal droplets, causing metal losses as the oxides join the slag. The entrapped metal can be recovered by stripping away the oxide layer so that the metal droplets are released and can settle down through the molten flux back into the metal bath. The settling process of the metal droplets through the salt is accelerated when the droplets merge and coagulate, according to Stokes’ law [18]
where v and ρm are the velocity and the density of the metal droplet, respectively, R is the droplet’s radius, ρs and ηs are the density and viscosity of the salt flux, and g is the gravitational acceleration constant. From Equation (2), it is evident how, as the droplets’ radius increases, they move faster through the flux, assisting the coalescence process.
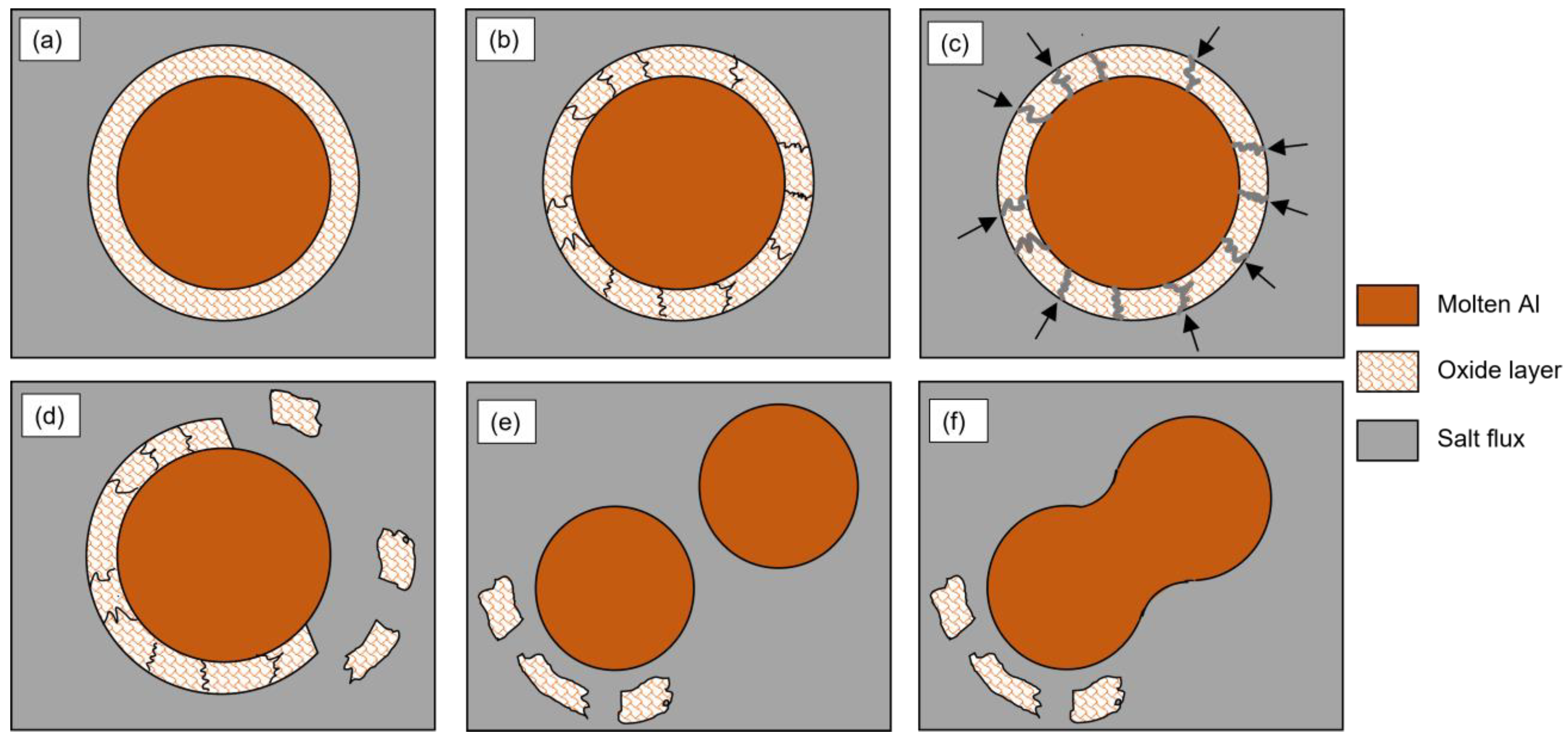
The metal droplets need to be released from the surrounding oxide layer before they can coagulate and settle back into the aluminum melt. The stripping of the oxide layer can be described in three steps: (i) cracks are generated in the oxide layer, which is wetted by the salt flux; (ii) the flux penetrates between the oxide layer and the molten metal; (iii) the oxide layer is detached from the metal droplet [2]. The detached oxides then remain suspended in the slag, leaving the aluminum droplets free to coalesce into larger pieces and re-join the melt, as previously described, according to Equation (2). A schematic representation of the oxide stripping process and coalescence of metal droplets is shown in Figure 3.
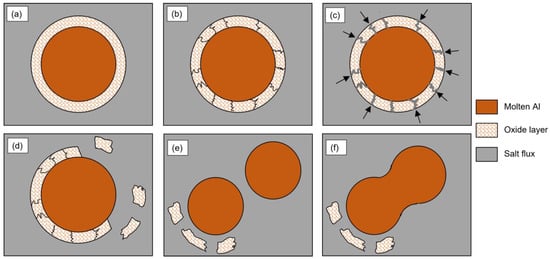
Figure 3.
Sketch showing the stripping process of the oxide layer covering an Al droplet by means of salt flux and coalescence of the Al droplets: (a) Al droplet surrounded by the oxide layer in the molten salt flux, (b) crack generation in the oxide layer, (c) penetration of the flux between the oxide layer and the molten Al, (d) detachment of the oxide layer, (e) Al droplets coming into contact and (f) coalescence of the Al droplets.
Various mechanisms are suggested in the literature to explain the generation of cracks in the oxide layer and the detachment mechanism; however, it is still not clear which one rules the stripping of the oxide layer. It may be a combination of the mechanisms proposed in the literature.
There is general agreement in the literature about the role played by the additions of fluorides to the salt flux in assisting the coalescence of aluminum droplets, thus enhancing the metal recovery of the process. Several studies [11,18,19,20,21] have shown how small additions of fluorides are sufficient to significantly improve the amount of coalesced aluminum when remelting aluminum scrap. This is confirmed by the highly popular practice of using fluoride-containing fluxes in the industry.
Even though the mechanism through which fluorides act is still unclear, two main phenomena are generally identified, i.e., (i) the generation of cracks in the oxide layer and (ii) the detachment of the oxide layer. For the first mechanism, even though the solubility of alumina is quite low in chloride melts with small fluoride additions, the presence of fluorides in the flux could lead to a partial dissolution of the oxide layer, creating a discontinuity in it and facilitating the propagation of cracks. Other theories suggest that the cracks are initiated due to the generation of stresses, which can arise either due to a difference in the thermal expansion coefficient of aluminum and aluminum oxide or to the density difference between α-Al2O3 and γ-Al2O3. Although the transition occurs above 1100 °C, fluorides lower the transition temperature to the range of 700–900 °C. The generation of cracks can also be attributed to the tendency of aluminum to minimize its surface energy by assuming a spherical shape. As this tendency is counteracted by the oxide layer, stresses may arise, causing deformations and, eventually, cracks in the oxide film [15,22].
Regarding the detachment of the oxide layer, it is generally acknowledged in the literature how fluorides exhibit a beneficial effect on the stripping of the oxide layer by adjusting the interfacial tensions between flux and aluminum and between flux and oxide. An improved wettability between the molten salt and the oxide can increase the oxide-stripping ability of the flux. This implies that the interfacial tensions between flux and oxide should be low. Moreover, the interfacial tensions between the flux and the melt should be low enough to facilitate the spreading of the flux on the aluminum melt but not excessively low to facilitate the separation of the flux from the molten aluminum. The interfacial tensions between oxide and molten aluminum should be maximized so that the wettability is minimized to ease the removal of the oxide. Fluorides act over such interfacial tensions by promoting chemical reactions which form tension-active elements. These elements decrease the surface tension of the metal when they are absorbed on its surface [10,11,22].
The ability of oxide stripping is directly related to the coalescence of the metal droplets [11] and thus to the amount of recovered metal from the recycling process. The optimal fluoride type and amount to be added to the salt flux will be discussed in further sections. The use of salt fluxes in the rotary furnaces maximizes the efficiency of the process, as the movement of the melt assists the coalescence. Furthermore, the efficiency is enhanced in tilting rotary furnaces, where coalescence is assisted not only by the rotating movement of the furnace but also by the axial movement [17].
2.3. Impurities Removal
When treating scrap with high levels of contamination and mixed chemical composition, another function of salt fluxes gains more importance, i.e., the possibility of removing harmful impurities from the molten metal. Cleaning the molten metal from impurities is also referred to as refining. The concept of molten metal cleanliness is based on three main parameters: the level of hydrogen in the melt, the dissolved chemical impurities (metallic or not), and solid inclusions. Operators who treat molten aluminum aim to have good control over such parameters and to minimize the number of impurities in the liquid metal. Several refining strategies are available to clean aluminum scrap, and in many cases, they are also used to refine molten primary aluminum [8]. Such practices, the most popular being fluxing, flotation, and filtration [23], can also be employed jointly, depending on the contamination level of the melt and the desired final metal quality. Briefly, the main types of impurities, which compromise the mechanical properties of aluminum alloy products and cause critical issues during the manufacturing processes, are described here.
- Hydrogen in molten aluminum arises mainly from the reaction of Al with moisture. The reaction of magnesium contained in the aluminum melt with water also releases hydrogen in the melt by oxidizing. Although hydrogen has some solubility in molten aluminum, its solubility is much lower in the solid state. Thus, during the solidification process, porosity is formed in the cast products. This leads to severe losses in the mechanical properties of components and issues in the manufacturing processes. The most popular methodology for the removal of hydrogen is degassing. However, cover fluxes also play a role in suppressing humidity absorption, thus preventing hydrogen pick-up by the melt [24].
- Dissolved impurities in molten aluminum may be divided into two sub-categories: (i) accumulated metallic elements, such as Si, Fe, Mn, Cu, Pb, P, Bi, Sn, Zn, and (ii) dissolved alkali and alkali-earth metals such as Mg, Na, Ca, Li, and K [24]. The amount of such elements in the melt not only depends on the scrap origin and type but is destined to increase due to the increasing scrap production and recycling. It is important to monitor the concentration of such impurities in the melt, especially when the required ranges are narrow. The most employed techniques to limit such impurities are either the production of alloys that allow wider ranges of these elements or the dilution of the melt with primary aluminum [8]. Some of them, i.e., Sn, Pb, and Zn, can be removed by selective melting if they are present externally on the aluminum since they have lower melting points than Al [5]. However, this is not possible if they are in a solid solution. Na, Ca, and Mg affect the oxidation kinetics of aluminum by increasing its oxidation rate [8,12], thus increasing the metal losses. Salt fluxes react with the alkali and alkali-earth metal impurities by acting as catalysts for their equilibrium oxidation reactions. The products of the reactions leave the molten metal bath through density separation, either by settling at the bottom of the furnace or by floating to the slag [5].
- Inclusions are typically solid particles, in most cases consisting of non-metallic or intermetallic compounds such as oxides, nitrides, carbides, and borides [25]. Their presence in cast products implies the generation of defects in the aluminum matrix, which can lead to the loss of mechanical properties. Non-metallic inclusions are categorized into exogenous or endogenous based on their origin. Exogenous inclusions arise when the molten metal is contaminated by external sources, such as refractory material and decomposition products, from the burning of coatings and oils. Endogenous inclusions form due to chemical reactions in the molten metal [25]. Bifilms are one of the most dangerous inclusions in cast products, consisting of double oxide films folded on themselves and entrained in castings during solidification. This occurs whenever the molten aluminum bath surface is disturbed; thus, bifilms are easily generated during recycling and foundry operations. Their presence in cast products makes them vulnerable, acting as nucleation sites for porosity during solidification [26]. Along with degassing and filtration, fluxing is one of the most common methods to remove non-metallic inclusions from molten metal. The fluxes react with the inclusions to form slag, which is subsequently removed by skimming [27].
3. Chemical and Physical Properties of Solid Salt Fluxes
An understanding of the chemical composition of the flux is essential for the assessment of the physical and chemical behavior of the flux. The chemical composition of the flux can be tailored to adjust properties such as density, viscosity, reactivity, and wettability. Such properties, in turn, will impart different functions to the flux. The next sections describe the main types of chemical compounds found in the fluxes and how they impact the physical properties and reactivity of the flux.
3.1. Chemistry of Salt Fluxes
The compounds usually found in solid fluxes can be classified into four major groups based on the main effect of the characteristics of the flux. The four major groups are chlorides, fluorides, oxidizing compounds, and solvents of aluminum oxide. They are described in the following sections.
3.1.1. Chlorides
Chlorides usually consist of NaCl and KCl. They mainly act as fillers, given their lower cost compared to other compounds for which chlorides act as carriers, such as fluorides. Chlorides are also used in high percentages for their fluidizing effects. When used individually, they act as passive fluxes, meaning that their reactivity with molten aluminum is negligible, and their effect on the surface tension is trivial if compared to fluorides. As aforementioned, the most common NaCl-KCl ratios are 50–50 and 70–30. Higher NaCl contents lead to a higher melting point of the flux; however, it can be economically convenient given the highest cost for KCl. Furthermore, a limited reduction of the amount of KCl in the flux does not seem to significantly affect the metal recovery [28].
Another binary mixture used as flux is MgCl2-KCl. Sodium-free fluxes are preferred by producers of Al-Mg alloys, especially when the Mg content is high, to avoid the introduction of Na in the aluminum melt. If sodium-containing fluxes are used, the higher the Mg content in the alloy, the more Na is introduced in the melt by fluxes. Furthermore, some flux additives such as NaF or Na3AlF6 can also increase the residual content of Na in the aluminum melt [29]. Nowadays, chloride fluxes such as MgCl2 and CaCl2 are also gaining more attention for the possibility of removing alkali and alkali-earth metals from molten aluminum. Purification of aluminum melt was once carried out through the injection of chlorine gas, but the practice has been abandoned due to environmental concerns for the release of chlorine gas, and it was replaced by solid chloride fluxes [30].
3.1.2. Fluorides
Fluorides, as previously explained, play an essential role in stripping the oxide layer and assisting the coalescence of metal droplets by acting as surfactants for wettability adjustments between salt, oxide, and molten aluminum. Common fluoride compounds include simple fluorides such as CaF2, NaF, KF, MgF2, and AlF3 and double fluoride compounds such as Na3AlF6, KalF4, K3AlF6, Na2SiF6, and K2SiF6 [21,22,24]. Fluorides are usually present in small amounts in flux mixtures, typically around 5 wt %, although their amount varies to a great extent depending on several factors, including the type of scrap, the desired metal quality, the level of inclusions in the melt, as well as the environmental regulations.
Nonetheless, too much fluoride addition can result in a degradation of flux properties because, usually, the fluidity and density increase because of fluoride additions. This results in poor coalescence and reduced metal recovery, as previously mentioned according to Stokes’ law (see Equation (2)). Additionally, an excessive increase in density may also lead to poor metal-slag separation. Thus, the amount of fluoride in the flux should be just enough to improve the wettability properties without excessively compromising the fluidity and density of the salt. Limiting the use of fluorides also implies economic and environmental benefits. As a matter of fact, not are only fluorides more expensive than chlorides, but they also cause health and environmental concerns due to dust and fumes as well as toxic and polluting compounds [31]. Most of them are classified according to EC Regulation No. 1272/2008 [32] as harmful to the environment, toxic, or both. Their amount in the salt flux should be minimized for environmental and health reasons; however, their presence is essential since fluoride-free fluxes do not yield the same efficiency for metal recovery [33].
3.1.3. Oxidizing Agents
Oxidizing agents promote exothermic chemical reactions, which assist the release of entrapped aluminum from the dross back into the molten metal bath. The mechanism is the improvement of fluidity due to the heat released by exothermic reactions [21]. Moreover, when added with fluorides, the heat released facilitates the chemical reactions between fluorides and inclusions [34]. Examples of oxidizing agents include nitrates such as KNO3 and NaNO3, carbonates such as CaCO3 and K2CO3 and sulfates such as K2SO4 and Na2SO4 [24].
3.1.4. Solvents of Aluminum Oxide
Solvents of Al2O3 can also be included in the fluxes. Cryolite is extensively used for the electrolytic conversion of Al2O3 to commercially pure aluminum metal in the Hall–Heroult process because alumina shows good solubility in cryolite melts. However, aluminum oxide has slight solubility in chloride melts with fluoride additions [35]. Some studies [20,36,37] suggested that the alumina solubility in some fluorides plays a key role in the mechanism of stripping the oxide layer, facilitating its dissolution, and improving the wettability [21]. The solvents of aluminum oxide are cryolite (Na3AlF6), borax (Na2B4O7), and potassium borate (K2B4O7) [24]. The chemical compounds used in salt fluxes are presented in Table 1.

Table 1.
Chemical compounds used in salt fluxes and grouped according to the chemical type. The melting point ™ and hazardous classification are also reported for industrial relevance. Data were collected and elaborated from [21,24,38,39,40].
3.2. Physical Properties and Reactivity of Solid Salt Fluxes
The chemical composition of salt fluxes determines their physical properties and reactivity. The key properties of fluxes include density, fluidity, melting temperature, wettability, and reactivity. These features are described in the next sections with a focus on how they are affected by the chemical composition of the fluxes.
3.2.1. Density
The density of the fluxes affects the slag-metal separation and the coalescence of metal droplets as described by Stokes’ law shown in Equation (2). The higher the density difference between metal and slag, the faster the droplets settle through the slag and back into the aluminum melt. The density of aluminum decreases linearly with temperature, and at 800 °C, it ranges between 2.33 and 2.39 g/cm3 [41,42]. At 800 °C, the density of an equimolar NaCl-KCl mixture is approximately 1.53 g/cm3, whereas, if the content of NaCl increases in the mixture up to 90 mol %, the density increases up to 1.55 g/cm3 [43]. Roy et al. [44] investigated the effect of some common fluoride (NaF, LiF, KF, Na3AlF6) additions up to 30 mol % on the density of an equimolar NaCl-KCl mixture at 740 °C. The results showed an increase of the density up to 1.63 g/cm3. Because the density difference between the molten aluminum and the salt mixtures is about 0.7 g/cm3, this is enough to ensure sufficient separation between the two phases [17]. Proper salt-slag separation ensures that the salt floats over the melt without joining it to avoid adhesion of salt granules or lumps which would result in a deleterious effect on the final product and to facilitate the removal of the slag at the end of the melting process.
Nonetheless, the effect of impurities and inclusions in the slag on its density cannot be neglected, as in the case of the effect on viscosity.
3.2.2. Viscosity
The viscosity of fluxes should also be low to assist the settling of metal droplets through the slag layer according to Stokes’ law. Few studies are available regarding the viscosity of molten NaCl-KCl with fluoride additions. Roy et al. [44] found how, after a critical amount of fluoride, the addition of LiF, NaF, and CaF2 increases the kinematic viscosity of an equimolar NaCl-KCl flux. Before reaching this critical amount, the viscosity showed a decreasing trend. Exceptions to these findings include the addition of Na3AlF6 up to 2 mol %, which results in a decrease in viscosity, and KF, which leads to a maximum value of viscosity for 5 mol % addition before declining. Tenorio et al. [45] found how the viscosity of an equimolar mixture decreases with NaF and KF additions, in contrast with the previous study. Milke et al. [46] investigated the solubility of CaF2 in NaCl-KCl at 750 °C and concluded how it decreases drastically, thus increasing the viscosity of the flux when the CaF2 concentration exceeds the solubility limit in the chlorides.
Nevertheless, the viscosity of the salt is only representative of the beginning of the process at the industrial scale. When the salt starts to collect oxides and inclusions, its viscosity increases significantly [10,17]. Xiao et al. [47] found that the presence of non-metallic particles in the slag impacts viscosity more than fluorides, especially when the volume of non-metallic particles in the slag exceeds 10 vol %. The impact of oxides in the flux during the remelting of aluminum chips with a lab-scale rotary furnace was studied by Thoraval and Friedrich [10]. The findings showed a significant decrease in the metal recovery, which was attributed to increased slag viscosity and density resulting from oxides, thus hindering the coalescence and metal-slag separation.
3.2.3. Melting Point
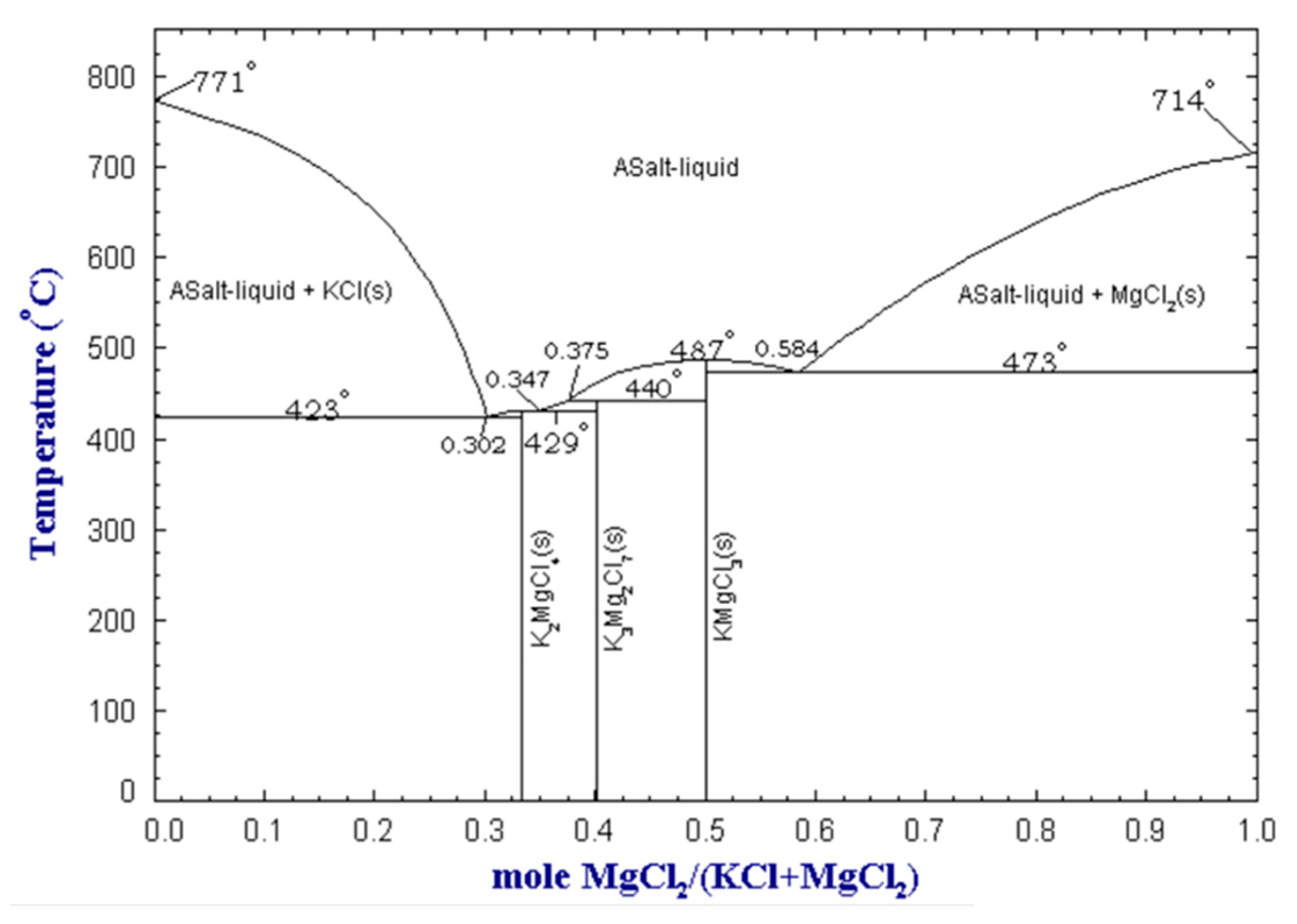
The melting point of the salt is another significant parameter, as the flux should be molten at the processing temperature. Preferably, the melting point of the salt should be close to the melting point of aluminum. If the melting point of the salt is too low, it may result in evaporation of the salt, whereas if it is too high, it may lead to excessive metal oxidation. Furthermore, an excessively high melting temperature leads to increased costs related to higher energy requirements to melt the salt. As previously noted, adding more NaCl to NaCl-KCl mixtures results in a higher melting temperature. However, certain operators, particularly in Europe, choose to prioritize cost savings by using more NaCl, which is less expensive than KCl. Flux mixtures based on KCl and MgCl2 allow obtaining even lower melting points, as the binary system presents eutectic points that melt at temperatures below 500 °C. The KCl-MgCl2 binary phase diagram is shown in Figure 4.
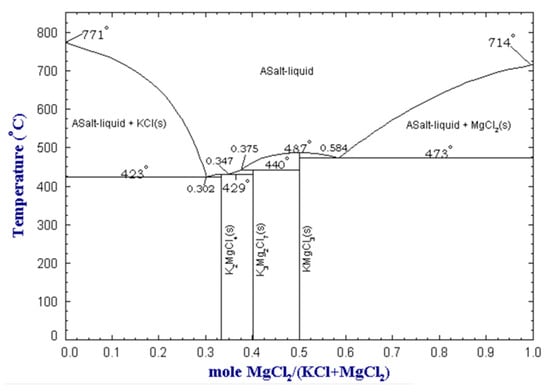
Figure 4.
MgCl2-KCl binary phase diagram at atmospheric pressure, obtained from the FactSage™ FTsalt database [16].
Although the binary systems of NaCl-KCl and KCl-MgCl2 have been extensively studied in the literature, to the author’s best knowledge, there is a lack of experimental evidence of the effect of the fluorides’ addition on the melting point of salt mixtures. Although generally, fluorides show melting points above 1000 °C, some of them show the presence of eutectic points melting below 750 °C when combined with NaCl, as in the case of NaF [16], CaF2 [46], and Na3AlF6 [48]. Even though binary systems of NaCl and fluorides may contribute to the understanding of the melting behavior, they are not representative of common flux mixtures because they are usually constituted by several compounds.
3.2.4. Wetting Properties
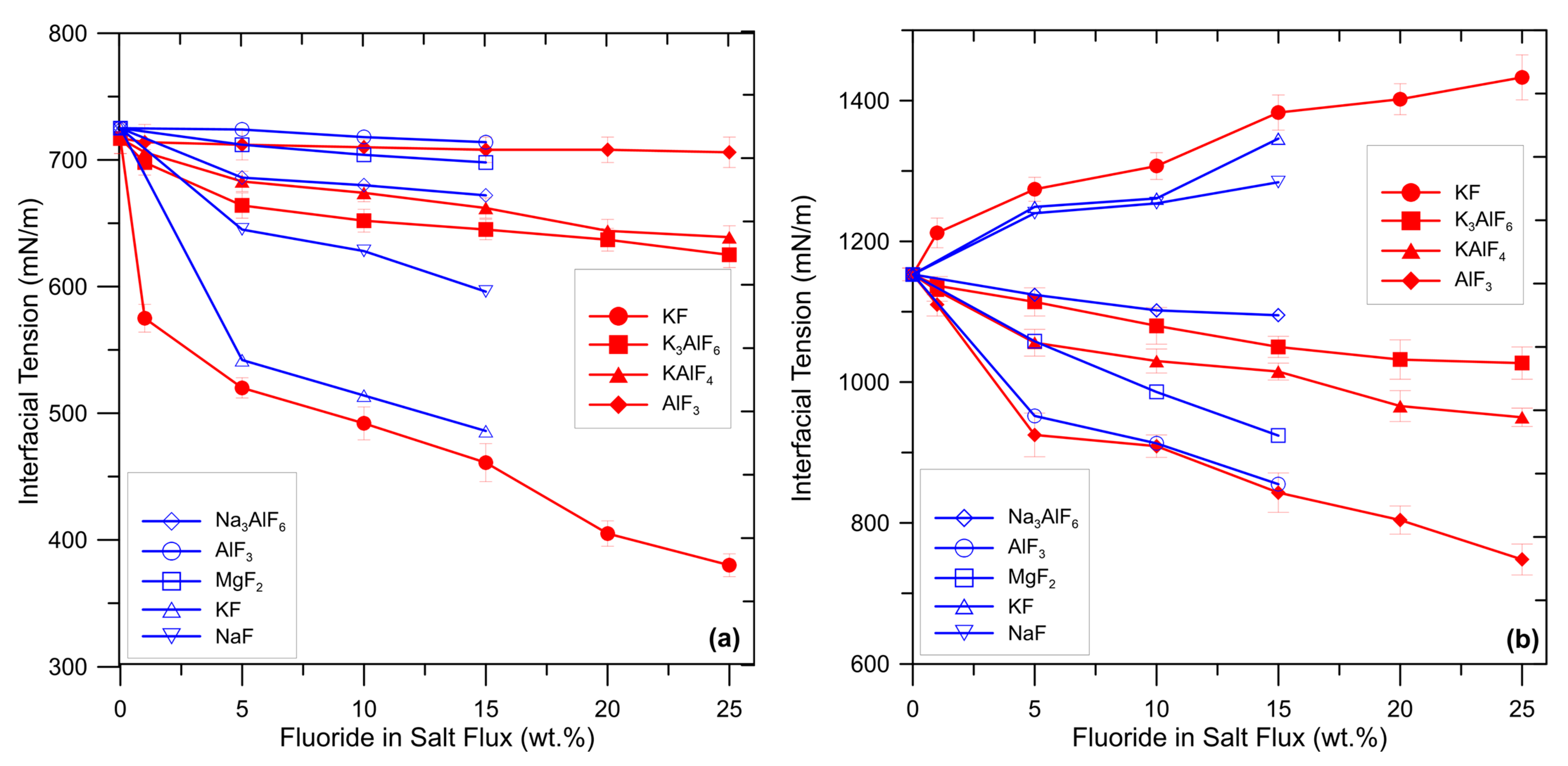
The wetting characteristics of a flux determine its ability to envelop inclusions and transfer them into the slag. It is widely recognized that the ability of flux to strip away and suspend oxides is largely influenced by the degree of wetting between (i) the liquid salt and aluminum melt and (ii) solid oxide and flux. A low value of interfacial tension between flux and oxide assists in the removal of inclusion by wetting. The interfacial tension between flux and aluminum melt should be sufficiently low to allow the spreading of the flux on the melt surface but not excessively low to avoid incomplete separation [11]. As previously explained, fluorides adjust interfacial tensions by promoting chemical reactions which produce surface-active elements (such as Na or K). Enrichment of these surface-active elements at the interface flux/aluminum is responsible for changes in the interfacial tensions. Wan et al. [11] and Shi et al. [22] investigated the effect of several fluoride additions to a commercial salt flux on the interfacial tensions between aluminum melt, oxides, and flux. The results are summarized in Figure 5.
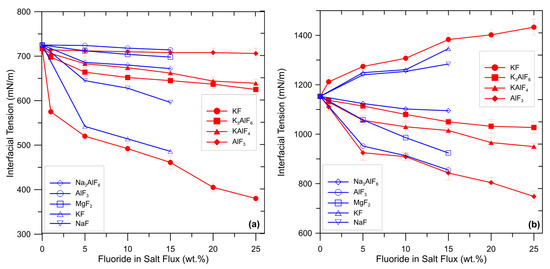
Figure 5.
Effect of some fluoride additions in the flux on the interfacial tension (a) between flux and aluminum melt and (b) between flux and aluminum oxide. Data collected and elaborated from [11,22].
3.2.5. Reactivity
The reactivity of a flux determines the ability to affect the chemical reactions, which lead to the removal of impurities from the aluminum melt. Simulation studies [49,50] based on thermodynamic calculations investigated the possibility of removing dissolved chemical impurities by considering the relevant parameters of the remelting process. The studies have shown how the salt fluxes containing AlCl3 affect the equilibrium constant of elements such as Mg, Ca, Be, Zn, Hg, Cd, Li, and Sr, allowing their removal either by chlorination into the salt flux, oxidation into the slag phase or by evaporation. However, low efficiency in the removal of such impurities and the impossibility of reducing other harmful impurities such as Cu, Si, Fe, and Mn by fluxing was revealed.
In the past, the injection of chlorine gas, i.e., chlorination, was a common strategy to remove alkali and alkali-earth metals. The tramp elements react with Cl2 forming more stable chlorides than aluminum chloride, assisted by the bubbling of the gas. Elements such as Mg, Na, Ca, Li, and K react with fluxes and form compounds which will either settle or float into the slag [51]. The toxicity of chlorine has led to its replacement with other methods, such as the use of fluxes. Nowadays, the most popular technology for the removal of inclusions and alkali from molten aluminum is through the addition of chlorine or fluorine-containing compounds to the salt flux, namely MgCl2 and AlF3, and AlCl3 [30,52]. Replacement of chlorination with solid fluxes can reduce environmental concerns without excessively compromising the efficiency of impurities’ removal; however, to replace the mixing due to bubbles, mechanical agitation is necessary [53]. Even though the use of MgCl2 is effective in the removal of alkali, it is not easily handled due to its high reactivity and tendency to absorb moisture. Thus, it is usually pre-melted in mixtures such as NaCl-KCl to obtain easier handling.
Impurities such as Zn, Si, Fe, Mn, and Cu are extremely difficult to remove from molten aluminum using fluxing [21,50]. Nonetheless, some experimental studies [54,55,56] have shown the possibility of reducing the Fe content in aluminum alloys by treating the melt with a flux mixture containing Na2B4O7. The methodology has shown promising efficiency at the lab scale, even when compared to other strategies, such as filtration and centrifugal separation. Furthermore, the addition of Na2B4O7 not only reduces the Fe content but also shapes Fe intermetallic compounds into less detrimental morphologies and promotes the formation of compounds such as AlB2, which act as grain refiners.
Particular attention should be dedicated to the treatment of Al-Mg alloys, especially for Mg content above 3 wt %, with fluxes containing Na, Ca, or K, as some chemical reactions may lead to the contamination of Al with Na or K. This is due to the exchange reactions of Mg with the impurities which preferentially stabilize Na, Ca, and K in the molten aluminum rather than in the flux [21,30]. Huang et al. [29], by treating an Al-Mg alloy with a NaCl-KCl flux with NaF and Na3AlF6 additions, concluded that the extent of Na contamination increases due to the increasing content of both Na in the flux and Mg in the Al alloy.
4. Different Types of Solid Salt Fluxes
By tailoring the chemical composition, it is possible to obtain several kinds of salt fluxes. The fluxes will perform different functions according to various parameters, including the type and level of contamination of the alloy to be melted, the processing temperature, and the desired final quality. The main categories of salt fluxes for melt treatment are cover fluxes, drossing fluxes, and melt-cleaning (or refining) fluxes. Other categories of salt fluxes which serve specific functions include de-magging fluxes, grain-refining fluxes, and wall-cleaning fluxes. Different fluxes can also be employed jointly, depending on the application. In the case of scrap remelting, one type of salt flux is used to perform simultaneously the functions of covering, refining, and improving the metal recovery. The different types of solid fluxes are widely described in the following sections.
4.1. Cover Fluxes
Cover fluxes suppress the oxidation, humidity absorption, and hydrogen pick-up of molten aluminum. They are typically composed of NaCl and KCl with small fluoride additions to assist coalescence. Usually, simple fluorides, such as NaF, KF, and CaF2, are added [24]. When it is necessary to further improve the coalescing properties of the salt, some cryolite (Na3AlF6) can also be added. Some other additives, such as LiCl or CaCl2, can be added to decrease the melting point of the mixture [21,57]. Cover fluxes are usually spread over the molten bath, and, to fulfill the essential requirements of a cover flux, the salt mixture should be molten at the processing temperature [17].
4.2. Melt-Cleaning Fluxes
Melt-cleaning (or refining) fluxes clean the molten metal from impurities either by wetting them or by chemically reacting with them. To serve these functions, they are composed of simple fluorides to wet the inclusions for easier separation from the melt, and they are richer in chloride compounds. To remove alkali impurities, sometimes they can comprise chloride compounds such as MgCl2 or CaCl2 [24]. NaCl and KCl are present as fillers. They can also include some oxidizing agents to accelerate the chemical reactions between the flux and the impurities. A typical refining flux for purification from alkali and alkali-earth metals consists of 40 wt % KCl—60 wt % MgCl2. A more economical option consists of a tertiary flux among KCl, MgCl2, and NaCl, for example, with the chemical composition of 25 wt % NaCl—15 wt % KCl—60 wt % MgCl2 [30]. The flux is usually either spread over the melt or injected using a lance. Stirring is performed either mechanically or through an impeller, such as in rotary flux injection [9]. Stirring of the melt assists the contact between flux and aluminum, thus accelerating the chemical reactions between them [30].
4.3. Drossing Fluxes
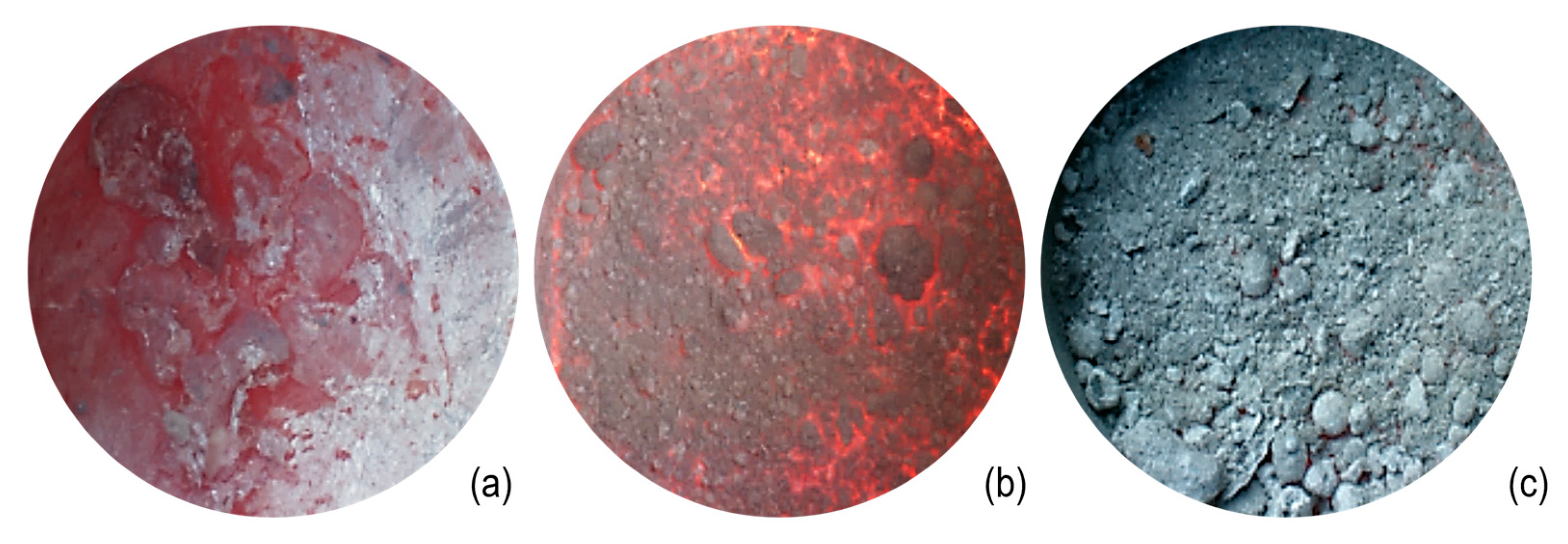
Drossing fluxes are added to ease skimming, that is, the removal of the dross layer, which forms because of metal oxidation. Drossing fluxes should also ensure the release of aluminum metal from the oxides. Thus, such fluxes are composed again mainly of chlorides, but the quantity of fluorides (both simple and double) is greater if compared to cover fluxes. These fluxes are designed to release heat; therefore, oxidizing agents are also present to promote the exothermal reactions that assist the coalescence [21]. Exothermal reactions are also promoted by the presence of double fluorides such as Na2SiF6. Drossing fluxes assist in the removal of metallic aluminum from the dross, which is usually present in contents up to 60–80 wt %. In this way, the dross changes its appearance from wet (light color, with a high metal content) to dry (dark and powdery, with a low residual metal content). These fluxes are usually spread on the molten metal bath and stirred to enhance their reactivity. If the dross is treated properly, its metallic concentration can be reduced to 30 wt % [24], leading to powdery dross, which can be skimmed off more easily. The action of a drossing flux is shown in Figure 6, which highlights the aspect and morphology of the dross before, during, and after treatment with the flux.
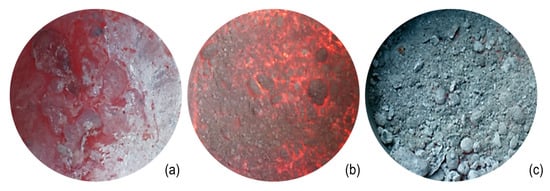
Figure 6.
Evolution of the aspect of the dross layer on aluminum melt, treated with drossing flux: (a) initial wet dross with high residual metal content, (b) treatment of dross with drossing flux, and (c) final dry dross with low residual metal content.
4.4. Fluxes for Scrap Remelting
The term “multi-functional refining flux for scrap recycling” was suggested by Wan et al. [11] to address the flux used for scrap remelting. A combination of the aforementioned types of fluxes (cover, refining, and drossing fluxes) may be used as a scrap remelting flux before the molten metal undergoes further specific treatment. For instance, when melting aluminum scrap in rotary furnaces, a salt flux should ensure proper protection from oxidation while promoting a sufficient degree of coalescence and reducing the impurities content [10]. If the different kinds of fluxes were used one after another, it would result in longer times for melting and treating the molten bath, as well as increased costs and waste production [11]. A multi-functional remelting flux ensures sufficient metal quality and metal recovery from the recycling process. Especially when melting oxidized scrap with a large surface area, the use of a salt flux can significantly improve the metal recovery. When remelting loose aluminum chips using a 50 wt % NaCl-50 wt % KCl with 10 wt % KF additions, Moloodi et al. [58] proved that the metal losses could be reduced by more than 95% if compared to the remelting without the salt flux. Further metal treatment, such as degassing and further refining, can be carried out by specific fluxes in additional steps. A common flux mixture used for scrap remelting includes mainly NaCl and KCl in different ratios, such as 50/50 or 70/30, and fluorides such as KF, NaF, CaF2, Na3AlF6, K3AlF6, NaAlF4, KAlF4 or mixtures of them.
4.5. Wall-Cleaning Fluxes
Wall-cleaning fluxes are necessary to reduce the accumulation of oxides which leads to the formation of α-alumina (corundum) at the side walls of the furnace. If the oxide accumulates excessively, it must be removed mechanically. These fluxes contain compounds that assist the removal of oxides build-up and contain the highest amounts of double fluorides, such as Na3AlF6 and Na2SiF6. Oxidizing compounds are also present and assist the penetration of fluxes in the oxide. They are usually delivered at the refractory walls by means of a gunning device [21,24].
4.6. De-Magging Fluxes
De-magging fluxes are employed with the aim of reducing the Mg content in the Al alloys. Powders of AlF3 or AlCl3 are injected in the melt and react, producing compounds that will be removed by skimming [52]. Chloride solid fluxes containing MgCl2, AlC3, SiCl4, and fluorides such as NaF and KF can also promote the Mg removal; however, the amount of NaF and KF should be kept to a minimum with respect to the MgCl2 content to avoid contamination of the melt with Na and/or K [21].
4.7. Modifying and Grain-Refining Fluxes
Modifying and grain-refining fluxes are employed to introduce an alloying element to the molten aluminum that will modify its microstructure. Some fluxes may be designed to introduce Na in Al-Si alloys to modify the eutectic silicon structure [59]. Such fluxes usually contain sodium carbonate or sodium fluoride. Specific fluxes can also be used to introduce strontium into molten aluminum [60]. Some Ti- and B-containing fluxes (such as KBF4 and K2TiF6) are also employed for the grain refinement of aluminum alloys [61]. Table 2 summarizes the types of fluxes described above.

Table 2.
Classification of solid salt fluxes based on the type and amount of the contained chemical compounds. The chemical and exothermic behaviors and the main type of melting unit of the fluxes are also reported for industrial relevance. Chlorides are not included as they are usually always present in the fluxes.
5. Efficiency of Solid Salt Fluxes
Several methods are available in the literature to assess the efficiency of a flux. Generally, the techniques aim to determine the ability of the salt to deliver either sufficient metal recovery or satisfactory metal cleanliness, depending on the function of the flux.
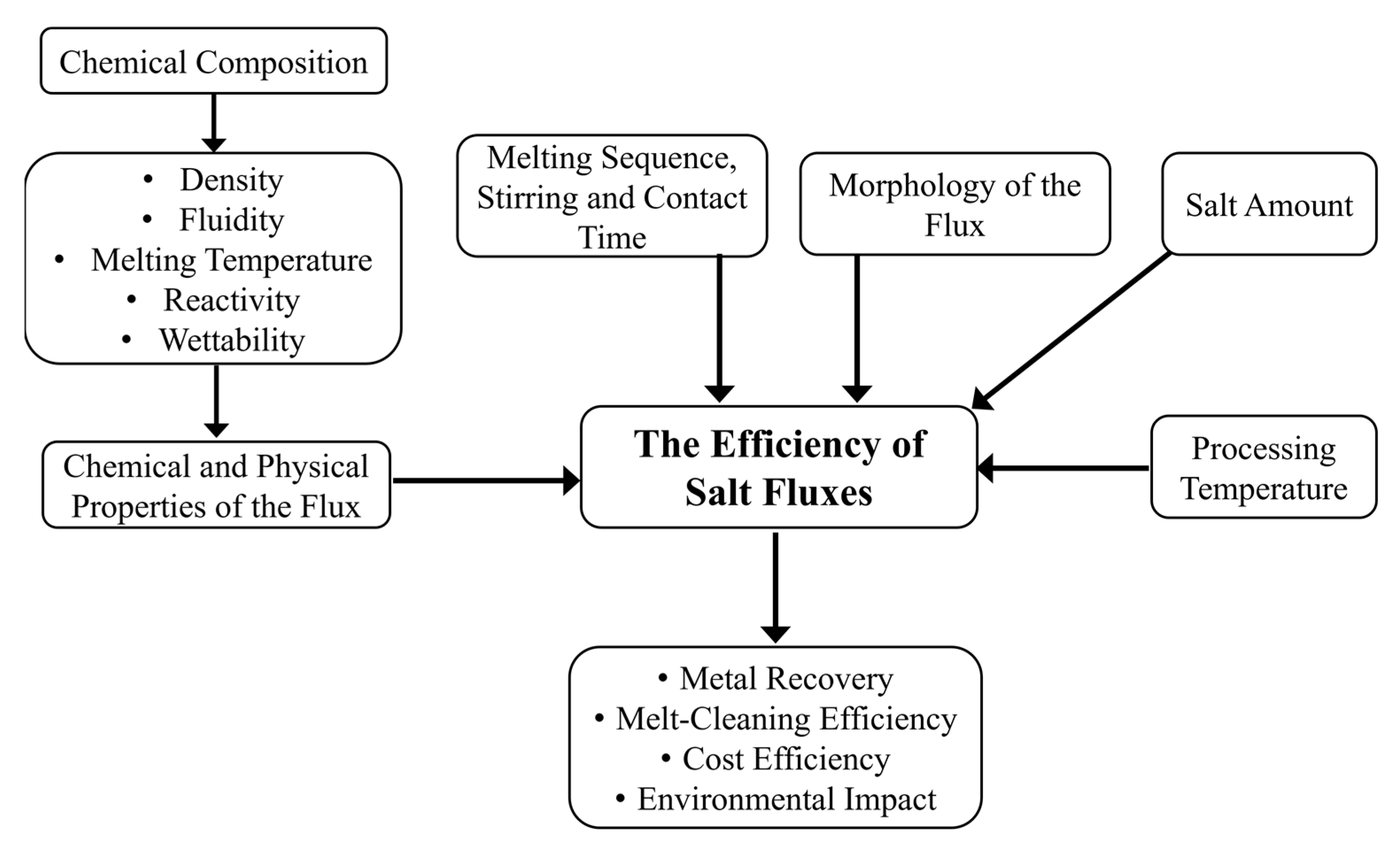
When determining the flux’s efficiency, several parameters must be considered. These include the chemistry of the flux, the amount of added salt and its morphology, and the processing temperature. Another important role in determining the flux’s efficiency is played by the stirring and by the melting sequence. The following sections describe the available methods to assess the efficiency of solid salt fluxes and the parameters involved.
5.1. Assessment of the Efficiency of Salt Fluxes
The methods to estimate the efficiency of salt fluxes can be categorized into two major groups according to the fluxes’ applications. The first group mainly concerns fluxes for scrap remelting. Typically, the evaluation of fluxes for scrap remelting is carried out according to their effectiveness in delivering satisfactory metal recovery. The second category regards fluxes for molten metal treatment, which are evaluated according to their ability to clean the melt from inclusions and impurities. The methods to assess the metal recovery and the melt cleanliness are described as follows.
When remelting aluminum scrap, the aim is to maximize the metal recovery from the process and thus minimize the metal losses due to oxidation and metal entrapment in the slag. The metal recovery can be expressed as [28]
Even though the detailed mechanism through which fluorides act is not fully understood, the addition of fluorides to fluxes is a well-established practice in the industry for scrap treatment. Lab-scale determination of the effectiveness of fluxes is often based on coalescence testing. Since coalescence occurs when the metal droplets coagulate after they are released from the surrounding oxide layer, the coalescence test is a measure of the oxide-stripping ability of the fluxes. There is no standardized procedure to evaluate a flux’s ability to assist coalescence; however, they are usually based on the determination of (i) the time needed for coalescence or (ii) the extent of coalescence after a given time [18]. In both cases, aluminum scrap pieces are molten using a salt flux in a crucible, which is maintained at the processing temperature for an established time. Afterward, the crucible is allowed to cool down and solidify. The aluminum pieces are then recovered from the crucible by leaching the salt in water and sieving. The coalesced pieces are then observed to assess the extent of coalescence, usually according to [62]
The evaluation of the mass of coalesced pieces varies to a great extent in the literature. For example, Vallejo-Olivares et al. [62] used the mass of the largest piece recovered, while Capuzzi et al. [63] considered the fraction of the coalesced droplets and their average diameters to account for their roundness. Besson et al. [18] considered the size of a single molten aluminum chip and chose coalesced pieces that were larger than a certain size limit. The results from coalescence tests are not fully representative of the industrial practice, given the different operating parameters, such as the salt/scrap ratio and type of input material [18]. There are also differences in the types of furnaces, holding times, and processing temperatures. Nonetheless, coalescence tests can help to understand the oxide stripping ability of a flux and the interactions between the metal and the flux [11].
Other studies assessed the metal recovery on a larger scale to improve the representability of the results from an industrial point of view, for example, using lab-scale rotary furnaces [10,28] or industrial-scale furnaces [52,64,65].
Melt cleaning fluxes are typically evaluated based on their ability to deliver improved melt quality and reduce impurities and inclusions. The available strategies to assess the efficiency of fluxes on the cleanliness of the metal bath are numerous and vary to a great extent. They include qualitative and quantitative analysis, which in the industry can include online and offline techniques. One of the most widespread techniques to determine the efficiency of fluxes in removing alkali and alkali-earth metals is the change in the chemical composition of the alloy being treated. As regards inclusions, several techniques are available which allow us to determine the size, concentration, and chemical composition of the inclusions, such as PoDFA (Porous Disc Filtration Analysis) and LIMCA (Liquid Metal Cleanliness Analyzer) [66]. K-mold and RPT (Reduced Pressure Tests) are also common. In particular, Dispinar [27] proposed the Bifilm Index to assess melt cleanliness by measuring the total length of double oxide films on the sectioned surface of the RPT sample. Metallurgical analysis methods such as microstructural investigations and fracture analysis are also found in the literature. Lastly, changes in the mechanical properties, such as UTS and elongation to fracture of the considered alloy before and after flux treatment, are also in use [34,58,67,68,69,70].
5.2. Effect of Processing Parameters on the Efficiency of Salt Fluxes
The efficiency of solid fluxing is affected by several factors. First, the chemical composition determines the chemical and physical behavior of the flux, as described in the previous sections. The type of fluoride and its concentration in the salt flux affect the salt’s ability to recover aluminum. The chemical composition of cleaning fluxes also determines their refining efficiency. Other significant parameters affecting flux properties are the amount of flux added, the processing temperature, the contact time of the flux, the method of delivery of the fluxes, and if stirring is applied. These parameters will be discussed as follows.
Assessments of the fluxes’ efficiency in assisting coalescence usually focus on fluoride concentration in the salt flux and on comparisons between different fluorides at a given concentration. Several studies show that the best coalescence efficiencies are obtained with Na3AlF6, NaF, and KF [11,19,20,21]. Ozer et al. [71] investigated the remelting of used beverage cans (UBC), and their findings showed that a flux amount equal to 1 wt % of the metal charge was enough to significantly improve recycling efficiency. Additionally, 5 wt % fluoride in the flux was sufficient to maximize the recycling efficiency. Sydykov et al. [72] remelted scrap in a rotary kiln by using a 70 % NaCl—30 % KCl mixture to compare the efficiency in metal recovery of CaF2 and Na3AlF6. They found that the fluoride type was not significant in determining the metal recovery. They also concluded that CaF2 concentrations above 3 wt % in the 70 % NaCl—30 % KCl flux are not necessary unless the scrap type is UBC. Thoraval and Friedrich [10] remelted aluminum chips in a lab-scale rotary furnace. They found that the presence of cryolite and oxides in the flux has a conflicting effect on the coalescence efficiency. However, when the flux was free from oxides, a cryolite content of 2 wt % in the equimolar flux was sufficient to maximize the coalescence efficiency of the metal droplets.
Even though the existing literature provides conflicting results regarding the optimal type and concentration of fluoride in the flux for scrap remelting, the most consolidated mixtures for aluminum recycling consist of NaCl-KCl with the addition of either CaF2 or cryolite [47]. The choice is driven by cost considerations and environmental concerns. However, the fluoride amount, especially when using cryolite, should be kept to the minimum necessary to ensure satisfactory metal recovery without aggravating environmental issues. A typical amount of fluoride addition ranges from 3 to 7% of the total weight of the flux [73]. Maintaining the number of added fluorides as low as possible not only implies environmental advantages but also reduces the corrosion of the refractory walls of the furnace. As a matter of fact, fluorides and particularly cryolite, are significantly aggressive and damaging towards the refractory material [74].
Regarding the type of refining flux, Ghanaatian and Raiszadeh [67] investigated the efficiency of bifilm removal from an aluminum melt using a 50 wt % KCl-50 wt % MgCl2 flux and applying stirring with a graphite rotor. By examining the RPT samples, they found that fluxing had a significant effect on bifilm removal and attributed the effect to a physical attachment of the oxides to the flux. Yüksel et al. [69] assessed the melt cleaning efficiency of a NaCl-KCl mixture containing commercial Na3AlF6, different ratios of NaF/AlF3, and Na2SiF6. The efficiency of an MgCl2-KCl mixture with Na2SiF6 was also studied. The fluxes were added as 0.1 wt % of the melt by adding them with a rotary degassing unit. The best melt cleaning efficiency was obtained when Na2SiF6 was added to the MgCl2-KCl mixture, whereas the worst cleaning efficiency was observed when adding commercial cryolite to the NaCl-KCl mixture. Shi et al. [22] also investigated the cleaning efficiency of a NaCl-KCl mixture when adding KF, NaF, Na3AlF6, MgF2, and AlF3. The findings show the best inclusion removal efficiency and significant improvement in the mechanical properties of the samples when using the flux containing KF, whereas the worst results were obtained when adding AlF3. Other studies [25,68,70] also assessed the inclusion removal efficiency of different fluxes; however, the fluxes are reported with the commercial name, and their chemical composition is unknown. Therefore, the correlation between the chemical properties and the melt-cleaning efficiency of the fluxes cannot be fully assessed.
The amount of salt flux necessary for melt treatment strongly depends on the type of process, furnace, input material, and surface area. In foundry applications, the amount of salt flux added will be lower if compared to scrap treatment applications. In transfer ladles and crucible furnaces, the typical amount of powder flux ranges from 0.25 to 0.5 wt % of the total weight of the metal charge. The amount of flux can be halved if using granular flux [33]. Drossing fluxes can be added in amounts up to 1% of the weight of the charged metal [21].
In the case of scrap remelting, the salt flux amount is strongly related to the level of contamination of the scrap. Moreover, it impacts the metal recovery, production and disposal costs, and energy requirements of the process [65]. Generally, the amount of salt added for scrap treatment ranges from 2 to 5% of the total weight of the scrap charge [73]. However, it strongly depends on the type of furnace used to remelt the scrap. When scrap is melted in reverberatory or multi-chamber furnaces, typically, the salt amount is low. Rotary furnaces can also be used with low amounts of salt, but more commonly, they require greater amounts of fluxes since they are used mainly to remelt contaminated and oxidized scrap [2]. The necessary salt amount is usually related to the scrap quality through the salt factor (SF), defined as [65]
where is the amount of scrap, the weight of the added salt and is the metal content in the scrap.
Pirker et al. [28] suggested that it is not possible to precisely determine the salt factor since the feedstock and its impurities also play a role in determining the necessary amount of salt. The treatment of scrap with high organic content requires higher salt amounts to reach satisfactory metal recovery because a significant amount of salt is required to bind the organic material in the slag. Capuzzi et al. [65] attributed the beneficial effect of increased salt amount on the metal recovery to the dilution of the non-metallic particles in the slag, whose effect on viscosity and coalescence has been previously described. However, the salt amount should be kept to the minimum necessary to ensure proper metal recovery. Too high salt amounts lead to increased costs as well as environmental concerns for the treatment of salt cakes. For the fixed-axis rotary furnace, large salt factors ranging from 0.9 to 2 are frequent; more common is the value of 1.8. However, the salt factor in tilting rotary furnaces can be reduced to 0.4 ÷ 0.8, thus implying reduced costs and waste products [58,72]. Drosses are also treated in rotary furnaces to recover the aluminum content, adding salt flux up to 50% of the dross weight [7].
The processing temperature also determines the efficiency of fluxes. If the processing temperature is not sufficient to melt or thermally activate the fluxes, their performance can be compromised. Non-reacted fluxes decrease the efficiency of the molten metal treatment; if they remain solid, they can stick to the final product, thus compromising its quality. Figure 7 shows granules of a drossing flux that remained solid and thus did not properly serve its function of reacting with the dross.

Figure 7.
Non-reacted solid drossing flux granules on a dross lump.
Increasing the processing temperature is beneficial to improve the fluidity of the salt because the viscosity of molten salts generally decreases with increasing temperature [43]. Máté et al. [25] investigated the melt cleaning efficiency of different fluxes, maintaining the furnace temperature between 730 and 750 °C. They concluded that fluxes with lower melting points were more efficient at inclusion removal due to the higher fluidity at the process temperatures. This is in agreement with a previous study from Majidi et al. [75], who studied the effect of fluxing temperature on the inclusion removal efficiency of a salt flux. From their results, an optimal fluxing temperature of 740 °C was found. This temperature was considered the optimum compromise between sufficient fluidity of the flux and limitation of hydrogen absorption. Bhaskar et al. [64] investigated the metal losses as a function of the processing temperature of aluminum chips in different furnaces by using a salt flux. The results show minimum values of metal losses when the temperature is maintained at 750 °C, regardless of the type of furnace. At lower temperatures, the salt is not able to provide sufficient protection, and thus metal is lost in the dross; at higher temperatures, the metal losses are caused by excessive oxidation.
The morphology of fluxes also influences the efficiency. Fluxes are typically available as powders, granulates, pads, or tablets. Powders are either distributed over the melt [17] or, in the case of refining, injected in the melt through a lance or an impeller using a carrier gas [51]. However, powders lead to the release of harmful and hazardous dust. The development of granulated fluxes allows for several advantages; they are handled more easily, their humidity absorption is reduced, and their efficiency is improved. Moreover, they ensure uniformity of the chemical composition of the flux in each grain. The manufacturing process involves melting the salt powders, and after they are solidified, they are ground to produce granules [76]. Despite the additional costs due to their manufacturing process, the amount of flux necessary for the metal treatment is significantly reduced, thus carrying economic benefits [59]. Powder and granulated fluxes, due to their high surface area, are very reactive with the melt, and thus they are consumed quickly. The coarser size of granulated fluxes and fluxes in the form of pads and tablets allows us to prolong the effectiveness of fluxes [77]. Different sizes of granulated fluxes are shown in Figure 8.

Figure 8.
Granulated salt fluxes: (a) medium-sized drossing granulated flux, (b) fine-sized drossing granulated flux, and (c) coarse-sized granulated flux for scrap remelting.
The sequence of melting also determines the effectiveness of the process. According to Schmitz [17], during the recycling of scrap in the rotary furnaces, the salt must be added before melting, whereas, in the reverberatory furnaces, the salt is distributed onto the melt before skimming to reduce further oxidation of the melt. To reclaim swarf and turnings, it is advised to add the flux to a molten metal heel, and after the flux is molten, the scrap is added slowly.
As regards cover fluxes, it was suggested to add the flux in two stages: half of the salt should preferably be added with the solid charge, while the remaining should be introduced inside the furnace when the metal is completely melted [59]. When adding reactive fluxes such as melt-cleaning fluxes, they should be stirred into the melt to ensure a proper reaction between the melt and the flux. Stirring can be carried out mechanically, through bubbling if the flux is carried by a gas, or with the aid of an impeller such as in rotary flux injection. After stirring, the salt should be left in contact with the molten metal to assist the chemical reactions, floatation of inclusions into the salt, and coalescence. By remelting aluminum scrap, Xiao et al. [47] investigated the effect of stirring and found it has an improving effect on coalescence.
Figure 9 summarizes the above-described parameters affecting the efficiency of solid salt fluxes.
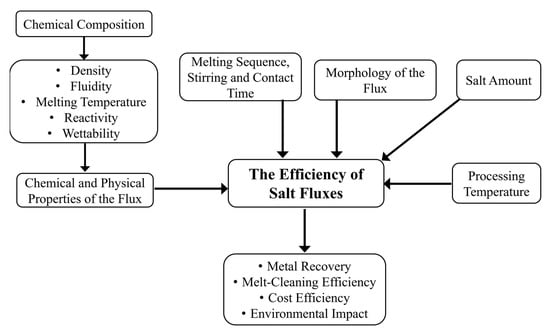
Figure 9.
Schematic flow chart of the main parameters affecting the efficiency of salt fluxes.
6. Conclusions
Secondary aluminum production will increase in the near future because of environmental and economic benefits such as the reduction of emissions, energy requirements, and costs if compared to primary aluminum production. However, the recycling process and the treatment of molten metal still need considerable improvements to ensure the satisfactory quality of the secondary products. Significant issues concern the metal losses due to oxidation of the molten metal and the presence of impurities that compromise the performance of the products.
Salt fluxes are popular media used during the treatment of molten aluminum. They are used in several applications in the aluminum industry; this paper reviews the use of solid fluxes in the recycling of aluminum scrap and in the treatment of molten aluminum to remove impurities and inclusions. Their main functions are the protection of the molten metal from oxidation by forming a liquid layer over the melt, the assistance in promoting the coalescence of Al droplets which would otherwise remain entrapped in the slag, and the removal of impurities and inclusions.
The chemical composition of salt fluxes is essential in determining the physical and chemical properties of the flux. Such properties include the flux’s melting point, density, viscosity, wettability, and reactivity, and they have a remarkable effect on the behavior of the flux and its efficiency. The presence of fluorides in the flux increases the ability to recover aluminum and decrease inclusions in the melt. However, fluoride additions should be minimized for environmental concerns.
The main categories of salt fluxes include cover fluxes, melt-cleaning fluxes, drossing fluxes, fluxes for scrap recycling, wall-cleaning fluxes, and de-magging fluxes. These types of fluxes differ in chemical composition, and they serve specific functions. They may be used separately or jointly according to the application and requirements.
In addition to the chemical composition of the flux, several other parameters affect the efficiency of fluxing. The amount of salt to be added should be optimized to ensure sufficient metal recovery. In the case of recycling, the salt amount strongly depends on the type and level of contamination in the scrap. The processing temperature should ensure proper fluidity of the flux without leading to excessive metal oxidation. The handling and delivery method of the salt depends on the type of remelting process and the kind of flux, and it should be adjusted accordingly.
Author Contributions
V.M., G.T. played a significant role in the conception, design, and writing of the review. V.M., G.T. contributed to collecting and analyzing the data and providing feedback and comments. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- CRU International Limited. Opportunities for Aluminium in a Post-COVID Economy; International Aluminium Institute: London, UK, 2022. [Google Scholar]
- Raabe, D.; Ponge, D.; Uggowitzer, P.J.; Roscher, M.; Paolantonio, M.; Liu, C.; Antrekowitsch, H.; Kozeschnik, E.; Seidmann, D.; Gault, B.; et al. Making sustainable aluminum by recycling scrap: The science of dirty alloys. Prog. Mater. Sci. 2022, 128, 100947. [Google Scholar] [CrossRef]
- Silveira, N.C.G.; Martins, M.L.F.; Bezerra, A.C.S.; Araújo, F.G.S. Red Mud from the Aluminium Industry: Production, Characteristics, and Alternative Applications in Construction Materials—A Review. Sustainability 2021, 13, 12741. [Google Scholar] [CrossRef]
- International Aluminium Institute. “Global Aluminium Cycle”. 9 January 2023. Available online: https://alucycle.international-aluminium.org/public-access/public-global-cycle/ (accessed on 25 January 2023).
- Gaustad, G.; Olivetti, E.; Kirchain, R. Improving aluminum recycling: A survey of sorting and impurity removal technologies. Resour. Conserv. Recycl. 2012, 58, 79–87. [Google Scholar] [CrossRef]
- Capuzzi, S.; Timelli, G. Preparation and melting of scrap in aluminum recycling: A review. Metals 2018, 8, 249. [Google Scholar] [CrossRef]
- Meshram, A.; Singh, K.K. Recovery of valuable products from hazardous aluminum dross: A review. Resour. Conserv. Recycl. 2018, 130, 95–108. [Google Scholar] [CrossRef]
- Schlesinger, M.E. Aluminum Recycling; Taylor & Francis Group: Boca Raton, FL, USA, 2007; pp. 171–192. [Google Scholar]
- Li, C.; Li, J.; Mao, Y.; Ji, J. Mechanism to remove oxide inclusions from molten aluminum by solid fluxes refining method. China Foundry 2017, 14, 233–243. [Google Scholar] [CrossRef]
- Thoraval, M.; Friedrich, B. Metal entrapment in slag during the aluminium recycling process in tilting rotary furnace. In Proceedings of the European Metallurgical Conference, Dusseldorf, Germany, 3–5 June 2015. [Google Scholar]
- Wan, B.; Li, W.; Liu, F.; Lu, T.; Jin, S.; Wang, K.; Yi, A.; Tian, J.; Chen, W. Determination of fluoride component in the multifunctional refining flux used for recycling aluminum scrap. J. Mater. Res. Technol. 2020, 9, 3447–3459. [Google Scholar] [CrossRef]
- Tabereaux, A.T.; Peterson, R.D. Treatise on Process Metallurgy—Volume 3: Industrial Processes; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; pp. 839–917. [Google Scholar]
- Hinton, E.M. The Oxidation of Liquid Aluminium and the Potential for Oxides in Grain Refinement of Aluminium Alloys. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2014. [Google Scholar]
- Vallejo-Olivares, A.; Philipson, H.; Gökelma, M.; Roven, H.J.; Furu, T.; Kvithyld, A.; Tranell, G. Compaction of Aluminium Foil and Its Effect on Oxidation and Recycling Yield. Light Met. 2021, 2021, 735–741. [Google Scholar] [CrossRef]
- Thoraval, M. Understanding Metal Losses in Salt Slags during Aluminium Recycling in Tilting Rotary Furnaces. Ph.D. Thesis, University of Aachen, Aachen, Germany, 2021. [Google Scholar]
- Bale, C.W.; Chartrand, P.; Degterov, S.A.; Eriksson, G.; Hack, K.; Mahfoud, R.B.; Melançon, J.; Pelton, A.D.; Petersen, S. FactSage thermochemical software and databases. Calphad 2002, 26, 189–228. [Google Scholar] [CrossRef]
- Schmitz, C. Handbook of Aluminium Recycling, 2nd ed.; Vulkan-Verlag GmbH: Essen, Germany, 2014. [Google Scholar]
- Besson, S.; Pichat, A.; Xolin, E.; Chartrand, P.; Friedrich, B. Improving coalescence in Al-Recycling by salt optimization. In Proceedings of the European Metallurgical Conference, Dusseldorf, Germany, 26–29 June 2011. [Google Scholar]
- Roy, R.R.; Sahai, Y. Coalescence Behavior of aluminum Alloy Drops in Molten Salts. Mater. Trans. JIM 1997, 38, 995–1003. [Google Scholar] [CrossRef]
- Peterson, R.D. Effect of Salt Flux Additives on Aluminum Droplets Coalescence. In Second International Symposium—Recycling of Metals and Engineered Materials—TMS; TMS: Madison, AL, USA, 1990. [Google Scholar]
- Utigard, T.A.; Friesen, K.; Roy, R.R.; Lim, J.; Silny, A.; Dupuis, C. The properties and uses of fluxes in molten aluminum processing. JOM 1998, 50, 38–43. [Google Scholar] [CrossRef]
- Shi, M.; Li, Y. Performance Improvement in Aluminum Alloy Treated by Salt Flux with Different Fluorides. J. Mater. Eng. Perform. 2022, 32, 3065–3072. [Google Scholar] [CrossRef]
- Wu, J.; Djavanroodi, F.; Gode, C.; Attarilar, S.; Ebrahimi, M. Melt refining and purification processes in Al alloys: A comprehensive study. Mater. Res. Express 2022, 9, ac5b03. [Google Scholar] [CrossRef]
- Gallo, R. “I Have Inclusions! Get Me the Cheapest and Best Flux for Cleaning My Melt!”—Is This the Best Driven, Cost-Saving Approach by a Foundry? In Proceedings of the 121st Metalcasting Congress of the American Foundry Society, Milwauee, WI, USA, 25–27 April 2017. [Google Scholar]
- Máté, M.; Tokár, M.; Fegyverneki, G.; Gyarmati, G. The Comparative Analysis of the Inclusion Removal Efficiency of Different Fluxes. Arch. Foundry Eng. 2020, 20, 53–58. [Google Scholar] [CrossRef]
- Gyarmati, G.; Fegyverneki, G.; Mende, T.; Tokár, M. Characterization of the double oxide film content of liquid aluminum alloys by computed tomography. Mater. Charact. 2019, 157, 109925. [Google Scholar] [CrossRef]
- Dispinar, D. Determination of Metal Quality of Aluminium and Its Alloys. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2005. [Google Scholar]
- Pirker, A.; Antrekowitsch, H.; Fragner, W.; Suppan, H.; Kettner, M. Optimization of the Al-recycling Process for Low Grade Scraps. BHM Berg Hüttenmänn. Mon. 2015, 160, 320–327. [Google Scholar] [CrossRef]
- Huang, C.; Liu, Z.; Huang, J.; Liu, Q.; Li, J. Effect of Sodium-Containing Fluxes on the Residual Sodium Content and Distribution in Al-Mg Alloys. Metals 2021, 11, 1591. [Google Scholar] [CrossRef]
- Tremblay, S.; Desrosiers, L.; Levesque, D. Use of a Tertiary Salt Flux of NaCl, KCl, and MgCl2 for the Purification of Aluminium or Aluminium Alloys, and Method Thereof. U.S. Patent US 2012/0017726, 26 January 2012. [Google Scholar]
- Cusano, G.; Gonzalo, M.R.; Farrell, F.; Remus, R.; Roudier, S.; Sancho, L.D. Best Available Techniques (BAT) Reference Document for the Non-Ferrous Metals Industries, EUR 28648. 2017. Available online: https://eippcb.jrc.ec.europa.eu/sites/default/files/2020-01/JRC107041_NFM_bref2017.pdf (accessed on 27 February 2023).
- European Parliament and Council of European Union. Regulation (EC) 1272/200/on Classification, Labelling and Packaging of Substances and Mixtures; European Parliament and Council of European Union: Brussels, Belgium, 2008.
- Gallo, R. Development, Evaluation and Application of Granular and Powder Fluxes in Transfer Ladles, Crucible, and Reverberatory Furnace. Foundry Pract. 2002, 237, 8–16. [Google Scholar]
- Gyarmati, G.; Fegyverneki, G.; Molnar, D.; Tokár, M. The Melt Cleaning Efficiency of Different Fluxes and Their Effect on the Eutectic Modification Level of AlSi7MgCu Alloy. Livar. Vestn. 2019, 66, 70–87. [Google Scholar]
- Cherginets, V.L.; Baumer, V.N.; Galkin, S.S.; Glushkova, L.V.; Rebrova, T.P.; Shtitelman, Z.V. Solubility of Al2O3 in Some Chloride-Fluoride Melts. Inorg. Chem. 2006, 45, 7367–7371. [Google Scholar] [CrossRef]
- Xiao, Y.; Tang, K. Solubility of alumina in molten chloride-fluoride melts. In Proceedings of the VIII International Conference on Molten Slags, Fluxes and Salts, Santiago, Chile, 18–21 January 2009. [Google Scholar]
- Tenorio, J.A.S.; Espinosa, D.C.R. Effect of salt/oxide interaction on the process of aluminum recycling. J. Light Met. 2002, 2, 89–93. [Google Scholar] [CrossRef]
- Merck KGaA, Sigma Aldrich. Available online: https://www.sigmaaldrich.com (accessed on 27 February 2023).
- National Library of Medicine, PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 27 February 2023).
- Fisher Scientific, Fishersci. Available online: https://www.fishersci.com/us/en/home.html (accessed on 27 February 2023).
- Assael, M.J.; Kakosimos, K.; Banish, R.M.; Brillo, J.; Egry, I.; Brooks, R.; Quested, P.N.; Mills, K.C.; Nagashima, A.; Sato, Y.; et al. Reference Data for the Density and Viscosity of Liquid Aluminum and Liquid Iron. J. Phys. Chem. Ref. Data 2006, 35, 285–300. [Google Scholar] [CrossRef]
- Assael, M.J.; Mihailidou, E.K. Reference Correlation for the Density and Viscosity of Eutectic Liquid Alloys Al+Si, Pb+Bi, and Pb+Sn. J. Phys. Chem. Ref. Data 2012, 41, 3. [Google Scholar] [CrossRef]
- Janz, G.; Tomkins, R.P.T.; Allen, C.B.; Downey, J.R.; Garner, G.L.; Krebs, U.; Singer, S.K. Molten Salts: Volume 4, Part 2, Chlorides and Mixtures. J. Phys. Chem. Ref. Data 1975, 4, 871–1178. [Google Scholar] [CrossRef]
- Roy, R.R.; Ye, J.; Sahai, Y. Viscosity and density of molten salts based on equimolar NaCl-KCl. Mater. Trans. JIM 1997, 38, 6–566. [Google Scholar] [CrossRef]
- Tenorio, J.A.S.; Carboni, M.C.; Espinosa, D.C.R. Recycling of aluminum—Effect of fluoride additions on the salt viscosity and on the alumina dissolution. J. Light Met. 2001, 1, 195–198. [Google Scholar] [CrossRef]
- Milke, E.; Friedrich, B.; Sydykov, A.; Arnold, A. Solubility of CaF2 in NaCl-KCl salt flux for Al-recycling and its effect on Al-loss. In Proceedings of the European Metallurgical Conference, Dusseldorf, Germany, 18–21 September 2005. [Google Scholar]
- Xiao, Y.; Reuter, M.A.; Boin, U. Aluminium Recycling and Environmental Issues of Salt Slag Treatment. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2007, 40, 1861–1875. [Google Scholar] [CrossRef]
- Holm, J.L. Phase investigations of the system Na3AlF6—NaCl. Thermochim. Acta 1988, 133, 283–286. [Google Scholar] [CrossRef]
- Nakajima, K.; Takeda, O.; Miki, T.; Matsubae, K.; Nakamura, S.; Nagasaka, T. Thermodynamic Analysis of Contamination by Alloying Elements in Aluminum Recycling. Environ. Sci. Technol. 2010, 44, 5594–5600. [Google Scholar] [CrossRef]
- Hiraki, T.; Miki, T.; Nakajima, K.; Matsubae, K.; Nakamura, S.; Nagasaka, T. Thermodynamic Analysis for the Refining Ability of Salt Flux for Aluminum Recycling. Materials 2014, 7, 5543–5553. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, X.; Torgerson, A.T.; Long, M. Removal of Impurity Elements from Molten Aluminum: A Review. Miner. Process. Extr. Metall. Rev. 2011, 32, 150–228. [Google Scholar] [CrossRef]
- Velasco, E.; Nino, J. Recycling of aluminium scrap for secondary Al-Si alloys. Waste Manag. Res. 2011, 29, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Bujalski, W.; Kimata, M.; Nayan, N.; Song, J.L.; Jolly, M.R.; Nienow, A.W. Mixing Studies Related to the Cleaning of Molten Aluminium. Chem. Eng. Technol. 2004, 27, 310–314. [Google Scholar] [CrossRef]
- Gao, J.W.; Shu, D.; Wang, J.; Sun, B.D. Effects of Na2B4O7 on the elimination of iron from aluminum melt. Scr. Mater. 2007, 57, 197–200. [Google Scholar] [CrossRef]
- Liu, X.; Liu, T.; Liu, Z.; Xie, H.; Liu, G.; Wu, Y.; Li, J. Effects of B-containing composite flux on the microstructures and mechanical properties of ADC 12 alloys. Mater. Res. Express 2019, 6, 096576. [Google Scholar] [CrossRef]
- Rathinasuriyan, C.; Bharath, A.; Sridhar, K. Reducing iron content from aluminium molten bath through filter bag, centrifugal separation and flux refining method. Mater. Today Proc. 2002, 62, 1026–1032. [Google Scholar] [CrossRef]
- Zhang, Z. Covering Flux for Smelting Aluminum and a Process for Its Preparation. U.S. Patent 5,762,722, 9 June 1998. [Google Scholar]
- Moloodi, A.; Amini, H.; Karimi, E.Z.V.; Golestanipour, M. On the Role of Both Salt Flux and Cold Pressing on Physical and Mechanical Properties of Aluminum Alloy Scraps. Mater. Manuf. Process. 2011, 26, 1207–1212. [Google Scholar] [CrossRef]
- Brown, J. Foseco Non-Ferrous Foundryman’s Handbook, 11th ed.; Butterworth-Heinemann: Woburn, MA, USA, 1999. [Google Scholar]
- Kientzler, P.; Loebbers, K.; Michard, L. Modifying Flux for Molten Aluminium. U.S. Patent 8,603,214, 10 December 2013. [Google Scholar]
- Sofyan, B.T.; Kharistal, D.J.; Trijati, L.; Purba, K.; Susanto, R.E. Grain refinement of AA333 aluminium cast alloy by Al-Ti granulated flux. Mater. Des. 2010, 31, 36–43. [Google Scholar] [CrossRef]
- Vallejo-Olivares, A.; Høgåsen, S.; Kvithyld, A.; Tranell, G. Thermal De-coating Pre-treatment for Loose or Compacted Aluminum Scap and Consequences for Salt-Flux Recycling. J. Sustain. Metall. 2022, 8, 1485–1497. [Google Scholar] [CrossRef]
- Capuzzi, S.; Kvithyld, A.; Timelli, G.; Nordmark, A.; Gumbmann, E.; Engh, T.A. Coalescence of Clean, Coated, and Decoated Aluminum for Various Salts, and Salt-Scrap Ratios. J. Sustain. Metall. 2018, 4, 343–358. [Google Scholar] [CrossRef]
- Bhaskar, M.; Nallusamy, T.; Suresh, P.; Anand, G.; Koilraj, M. Recycling of aluminum chips in die casting foundry. Int. J. Met. 2022, 16, 1575–1583. [Google Scholar] [CrossRef]
- Capuzzi, S.; Timelli, G.; Capra, L.; Roma, L. Study of fluxing in Al refining process by rotary and crucible furnaces. Int. J. Sustain. Eng. 2019, 12, 38–46. [Google Scholar] [CrossRef]
- Dispinar, D.; Campbell, J. A comparison of Methods used to Assess Aluminium Melt Quality. In Proceedings of the Shape Casting, 2nd International Symposium, Orlando, FL, USA, 25 February–1 March 2007. [Google Scholar]
- Ghanaatian, M.H.; Raiszadeh, R. Effect of Different Methods for Removing Bifilm Defects from A356 Aluminum Alloy. Metall. Mater. Trans. B 2022, 53, 503–511. [Google Scholar] [CrossRef]
- Czekaj, E.; Nykiel, J.; Kwak, Z.; Garbacz-Klempka, A.; Nykiel, M. The Influence of Selected Refining Methods of AlSi7Mg0.3 Silumin on its Quality Index. Arch. Foundry Eng. 2018, 18, 72–78. [Google Scholar] [CrossRef]
- Yüksel, Ç.; Dışpınar, D.; Çiğdem, M. An Analytical Approach for the Correlation between Bifilm Index and Tensile Properties of AlSi7Mg0.3 (A356) Aluminum Alloy Cleaned via Rotary Degassing and Different Fluxes. Int. J. Met. 2022, 16, 1–8. [Google Scholar] [CrossRef]
- Okladnikova, N.V.; Uskova, A.L.; Drozdova, T.N.; Orelkina, T.A.; Antonov, M.M. Studies of the effect of nonmetallic inclusions and refining of melt on the quality of ingots and shapes from alloy AD31. Met. Sci. Heat Treat. 2015, 57, 10–14. [Google Scholar] [CrossRef]
- Ozer, G.; Yuksel, C.; Comert, Z.Y.; Guler, K.A. The Effects of Process Parameters on the Recycling Efficiency of Used Aluminium Beverage Cans (UBCs). Mater. Test. 2013, 55, 396–400. [Google Scholar] [CrossRef]
- Sydykov, A.; Friedrich, B.; Arnold, A. Impact of parameter changes on the aluminum recovery in a rotary kiln. In Proceedings of the TMS Annual Meeting, Seattle, WA, USA, 17–21 February 2002. [Google Scholar]
- Ireland, D.T. Method and Composition for Aluminum Recycle Using Salt Flux. U.S. Patent 6053959, 25 April 2000. [Google Scholar]
- Engel, R. Refractory Considerations. Alum. Int. Today 2015, 27, 22–25. [Google Scholar]
- Majidi, O.; Shabestari, S.G.; Aboutalebi, M.R. Study of fluxing temperature in molten aluminum refining process. J. Mater. Process. Technol. 2007, 182, 450–455. [Google Scholar] [CrossRef]
- Murthy, H.S. A Process for the Manufacture of Granular Flux for Addition to Molten Aluminium and Its Alloys. India Patent 205198, 29 June 2007. [Google Scholar]
- Sala, M. Melting Flux for Slagging and Deoxidating Molten Metal. Italy Patent EP3425071B1, 16 December 2020. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).