A Comparative Investigation of Duplex and Super Duplex Stainless Steels Processed through Laser Powder Bed Fusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Materials
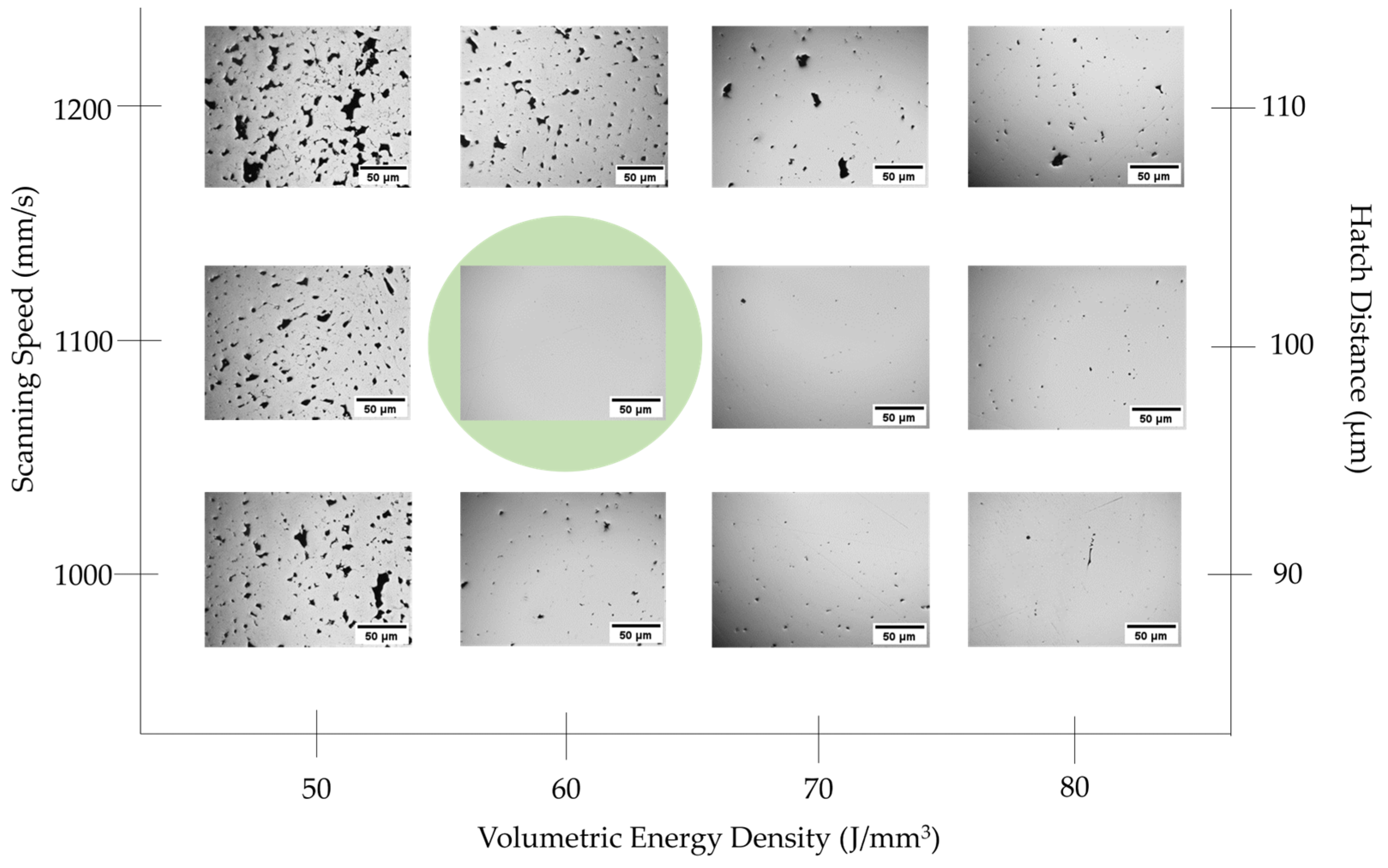
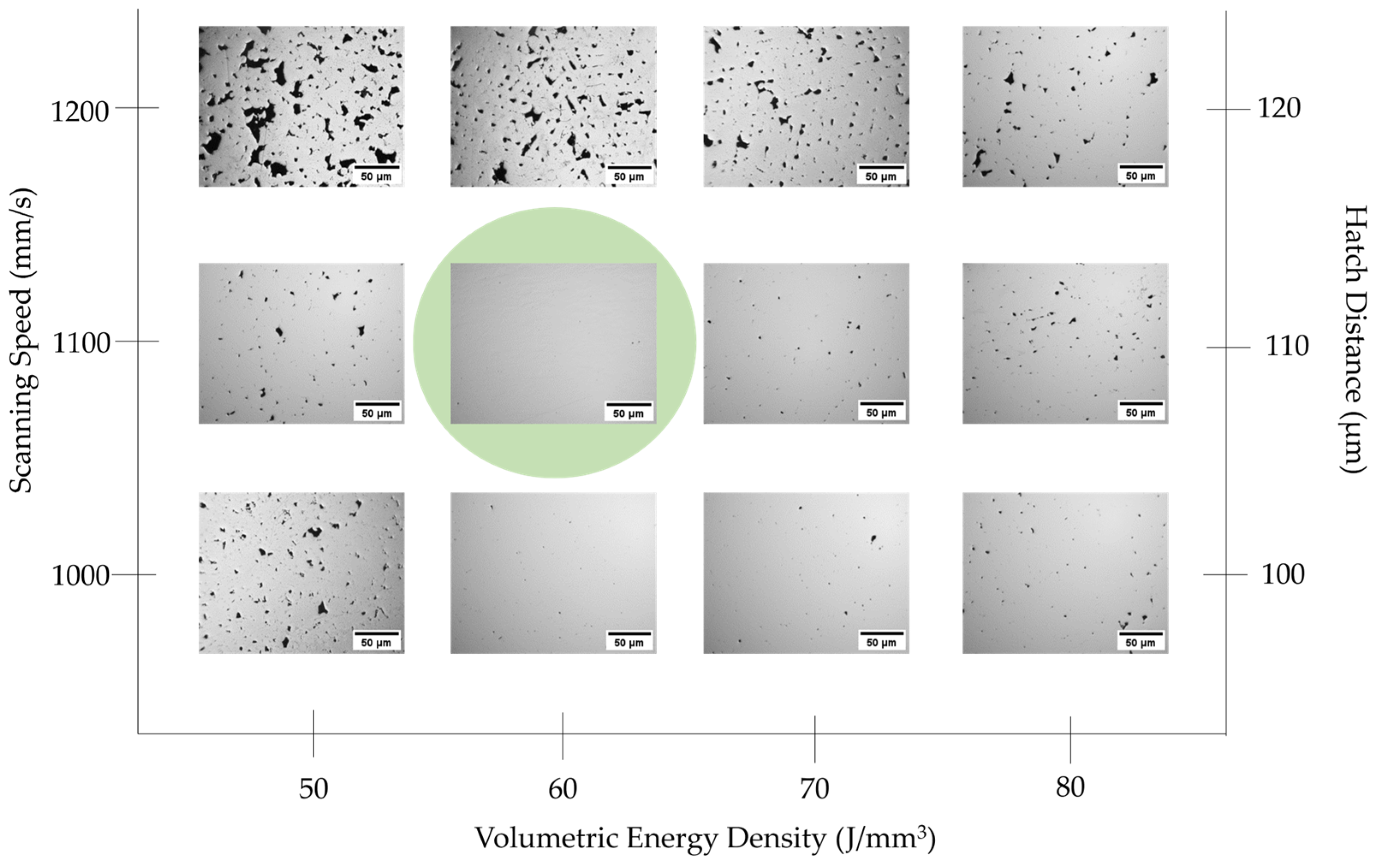
2.2. LPBF Parameter Optimization and Post-AM Heat Treatment
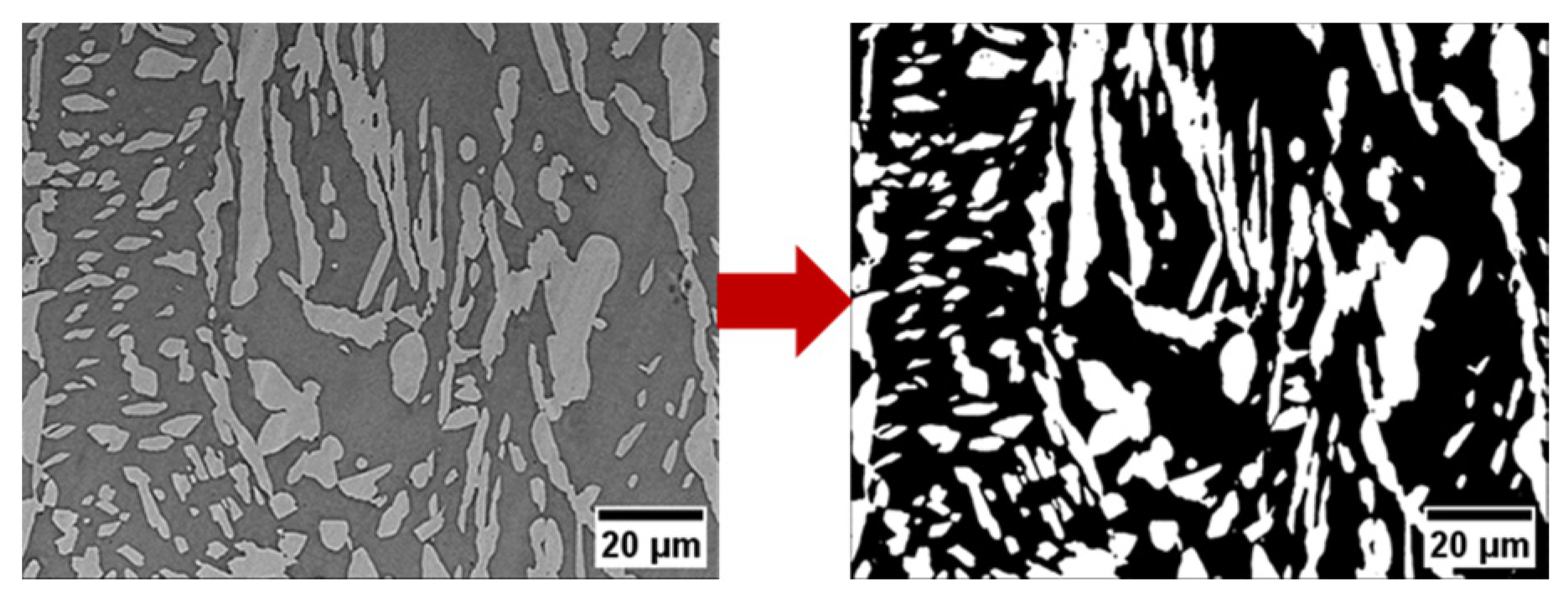
2.3. Density and Microstructure of LPBF-Processed As-Built and Heat-Treated Samples
2.4. Nanoindentation Testing of LPBF-Processed As-Built and Heat-Treated Samples
3. Results
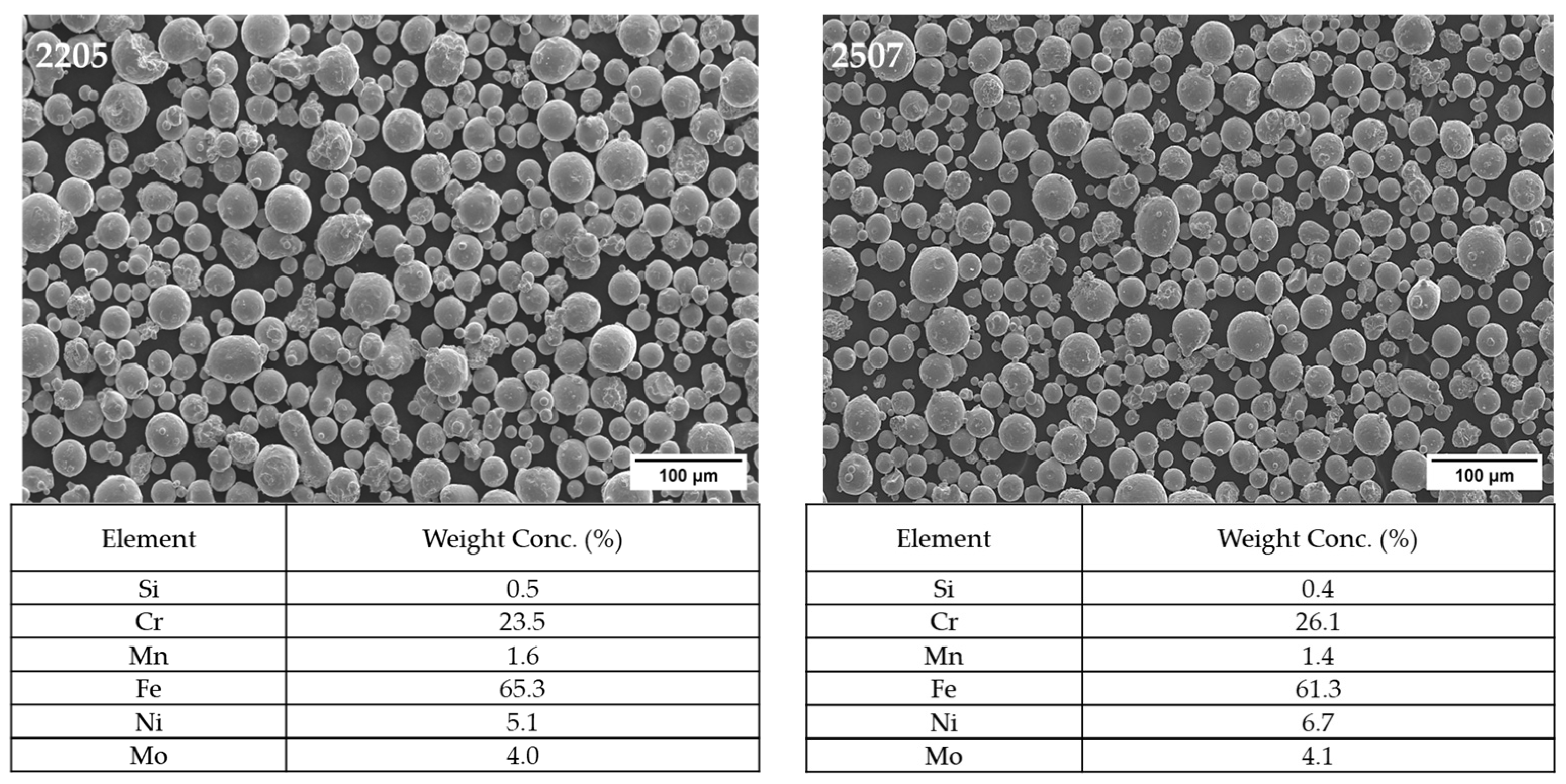
3.1. Powder Characterization
3.2. Density–Porosity Analysis of LPBF Samples
3.3. DSS and SDSS Microstructural Analysis
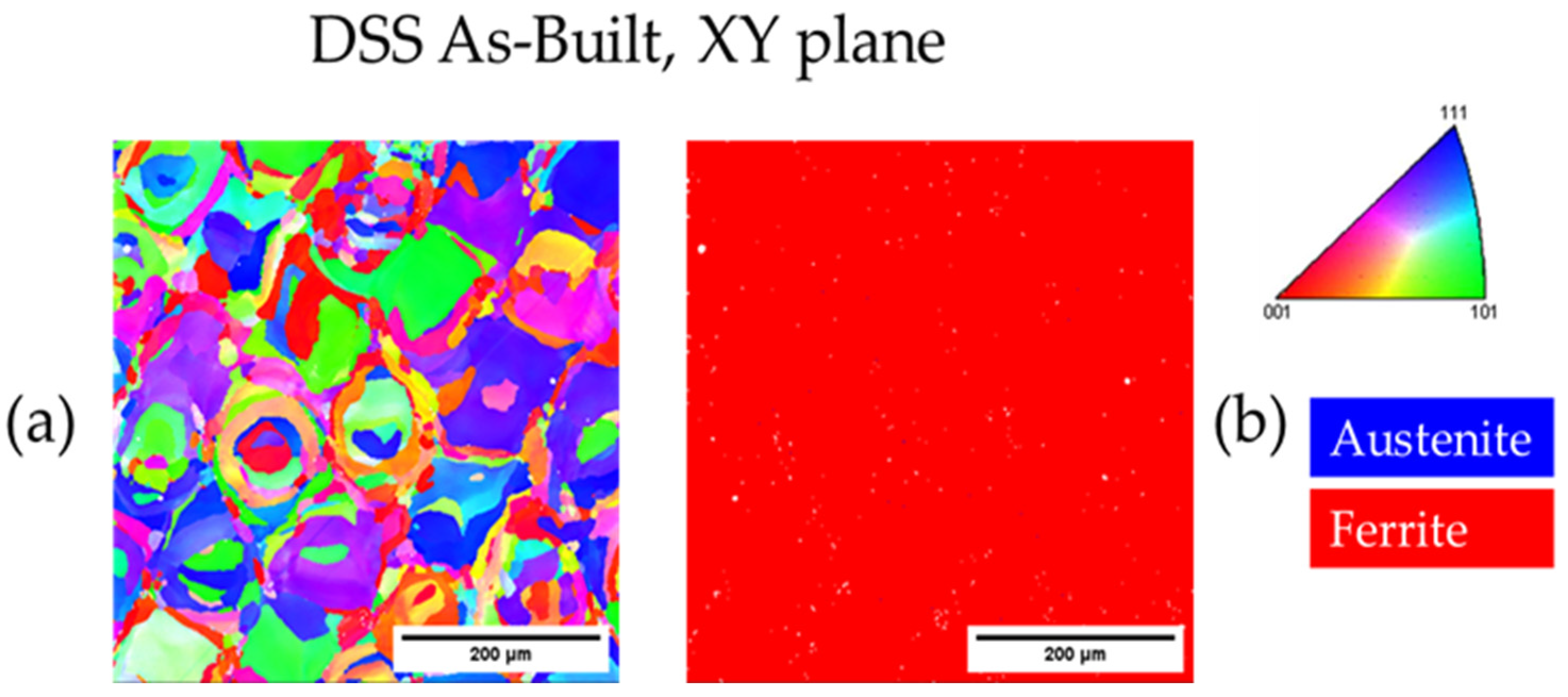
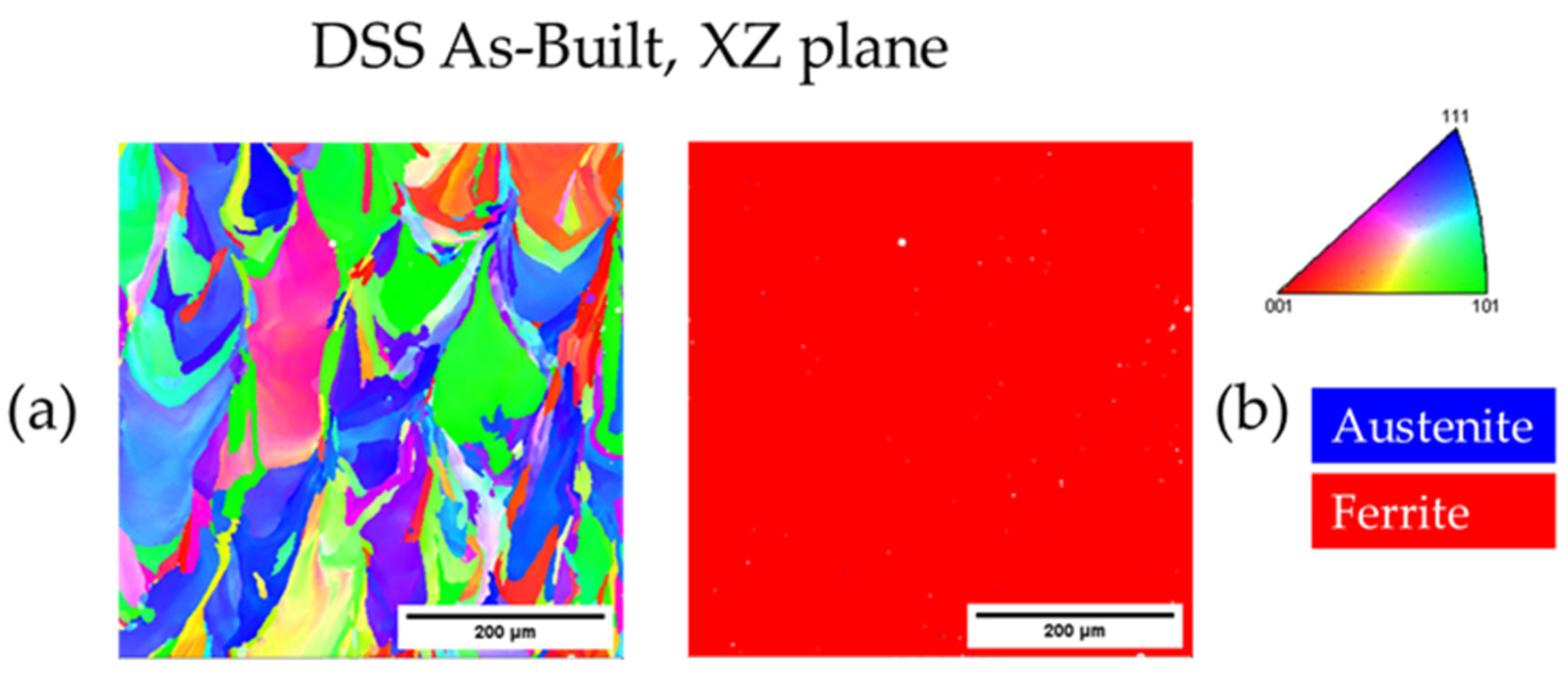
3.3.1. As-Built DSS 2205
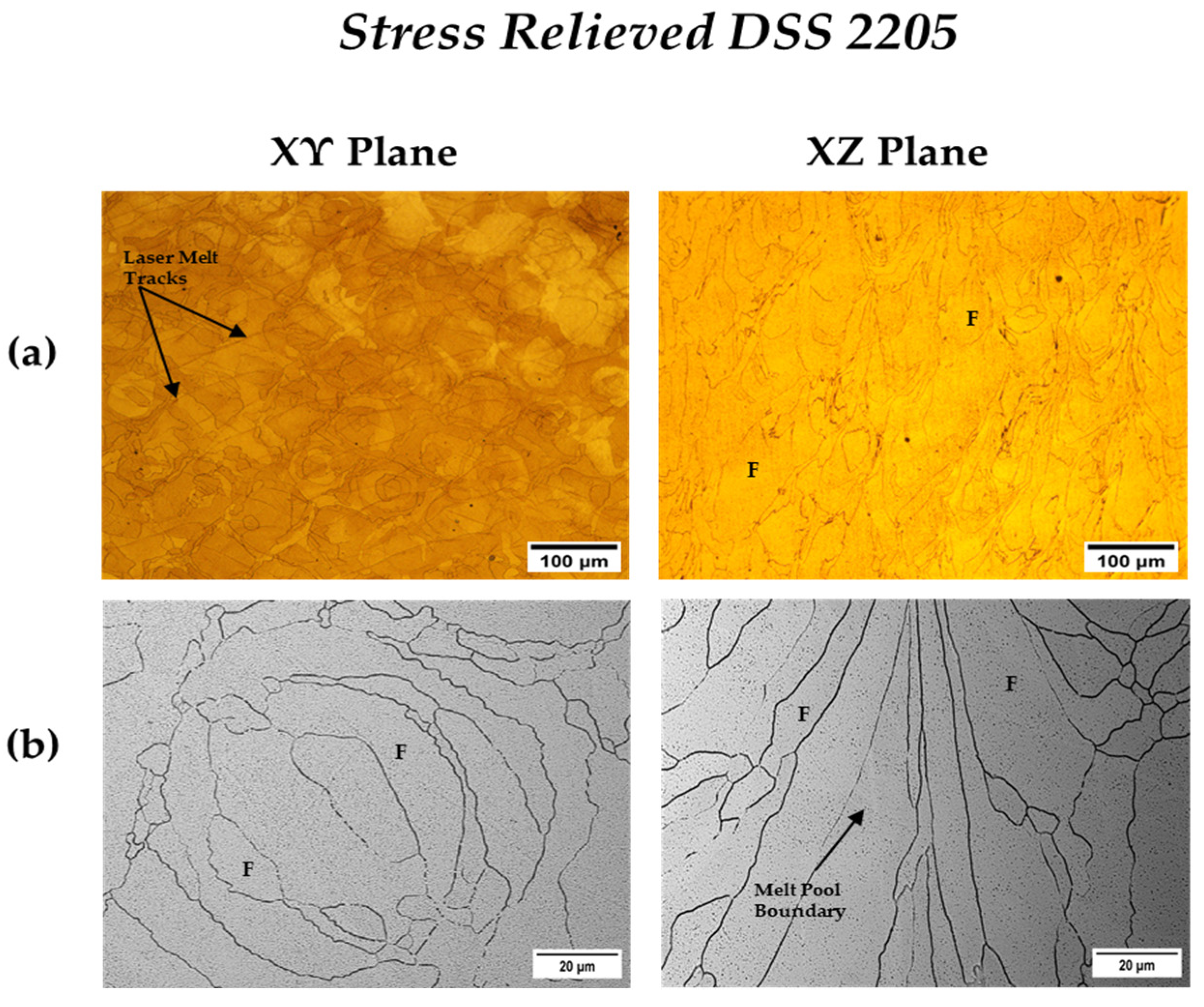
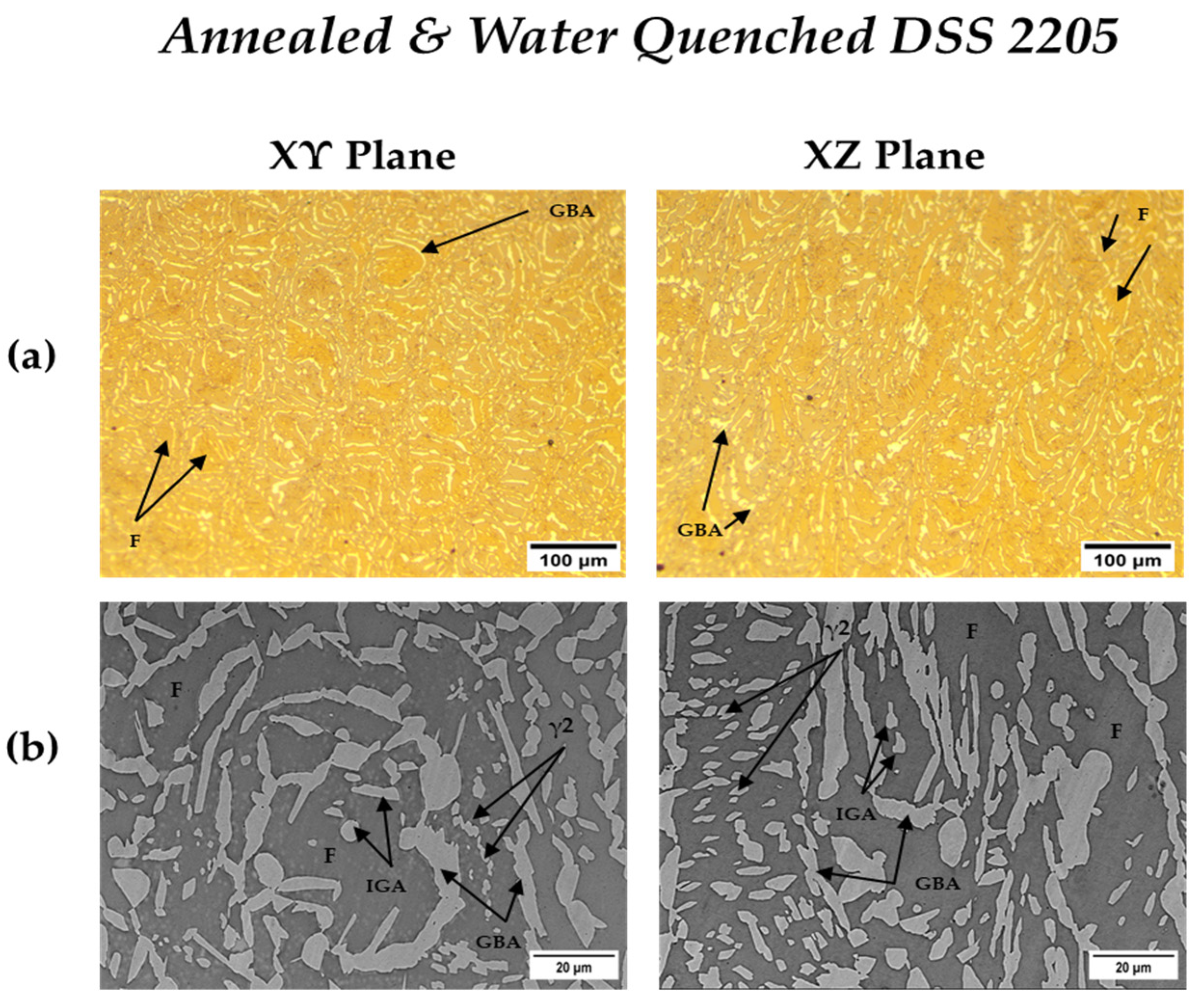
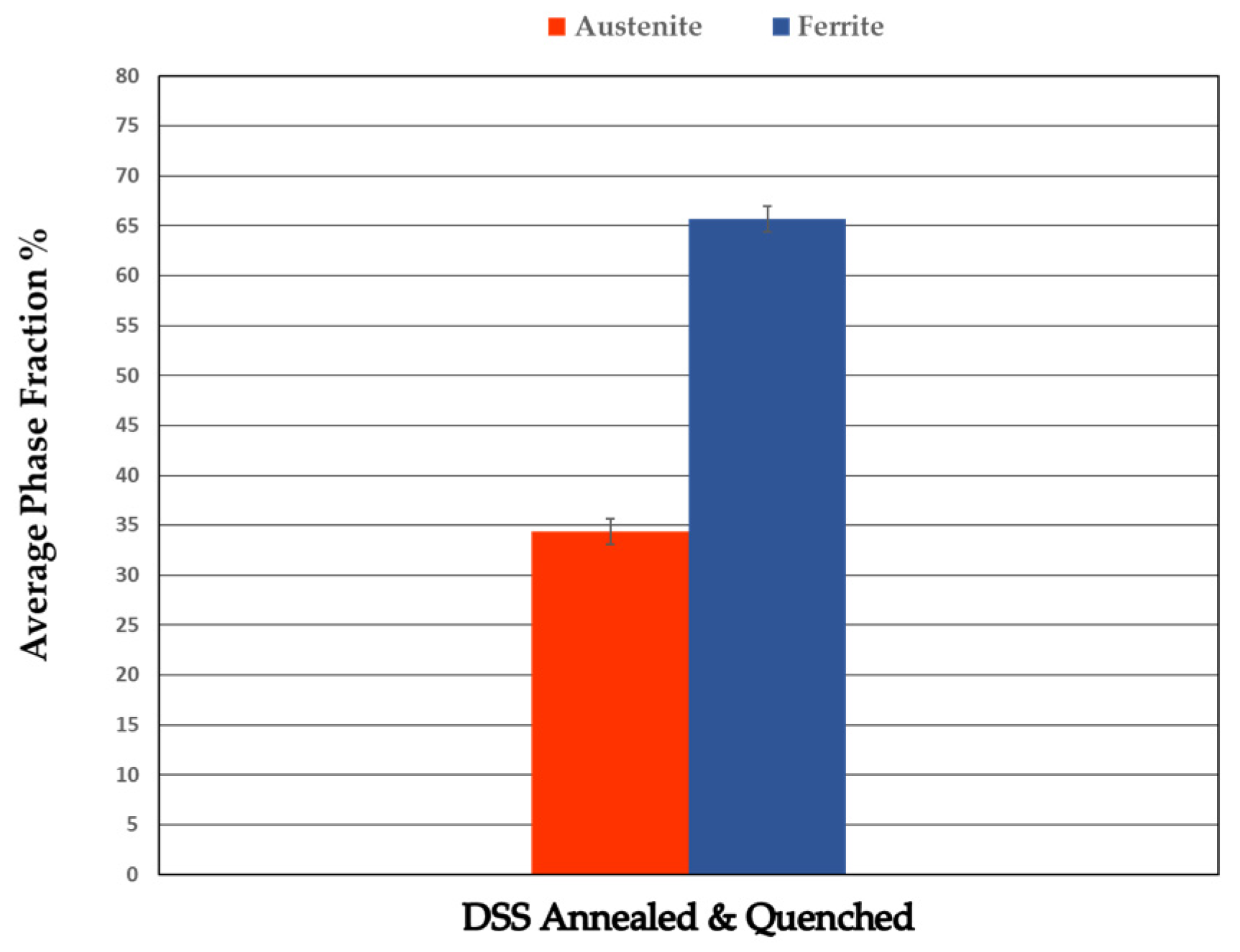
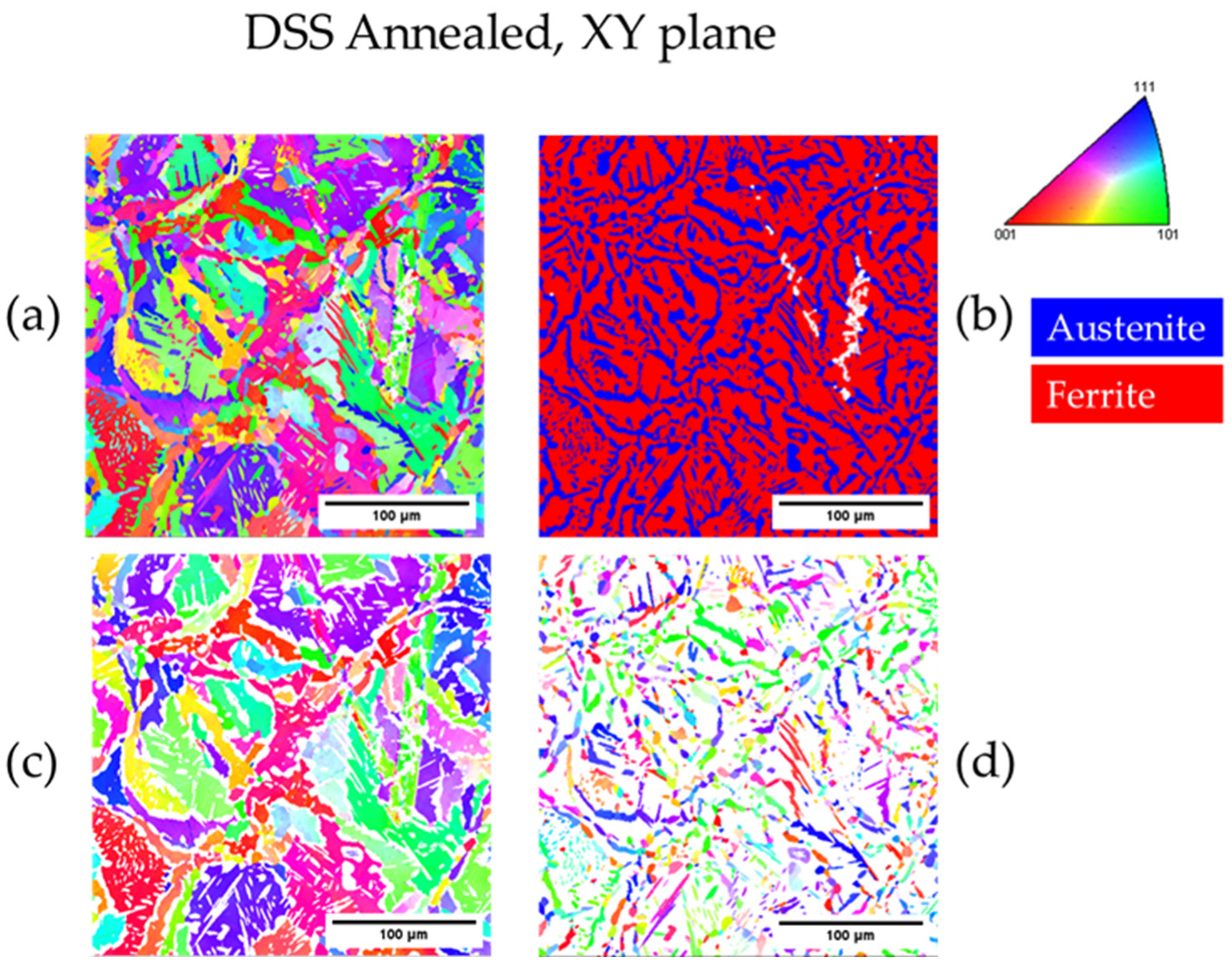
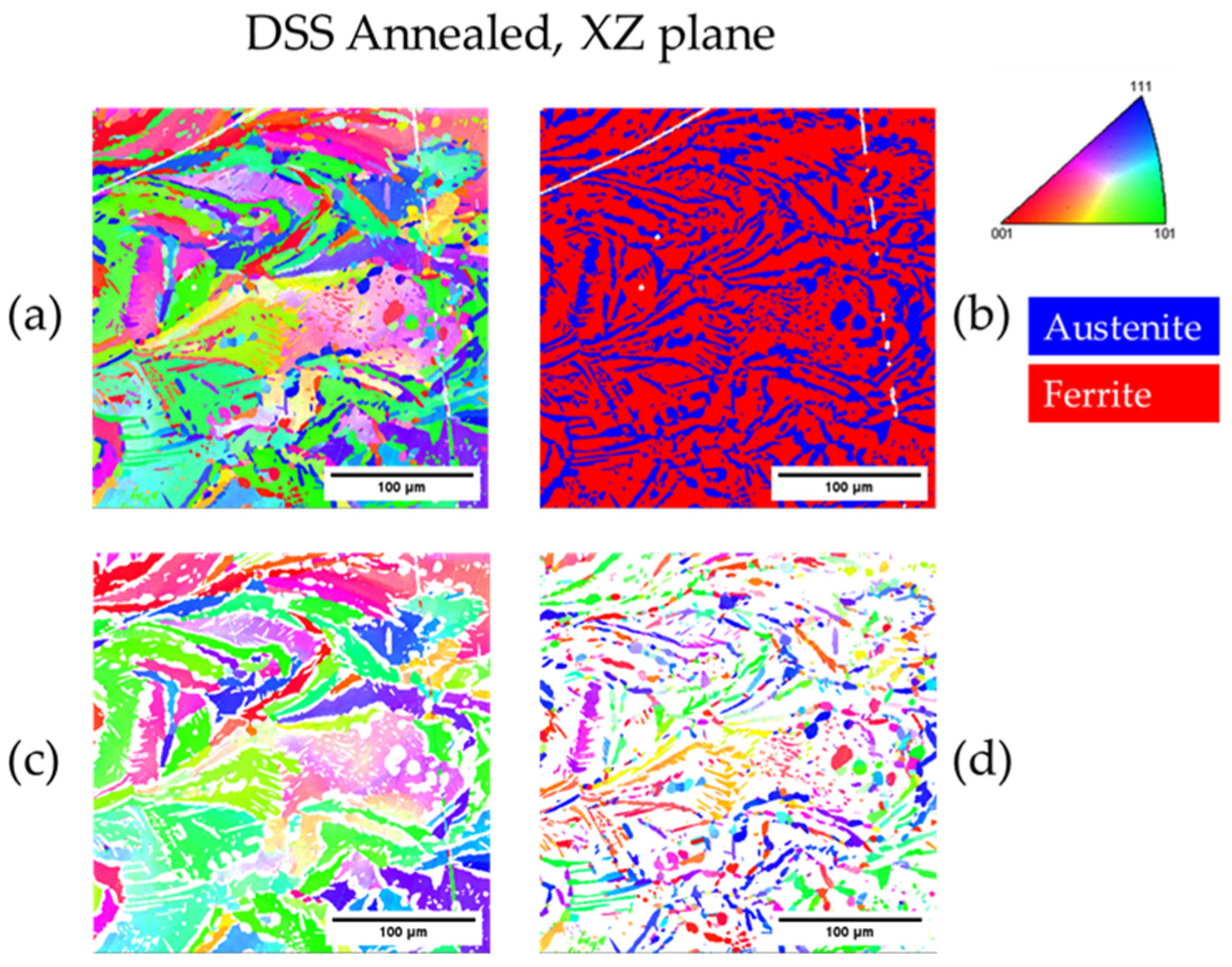
3.3.2. Heat-Treated DSS 2205
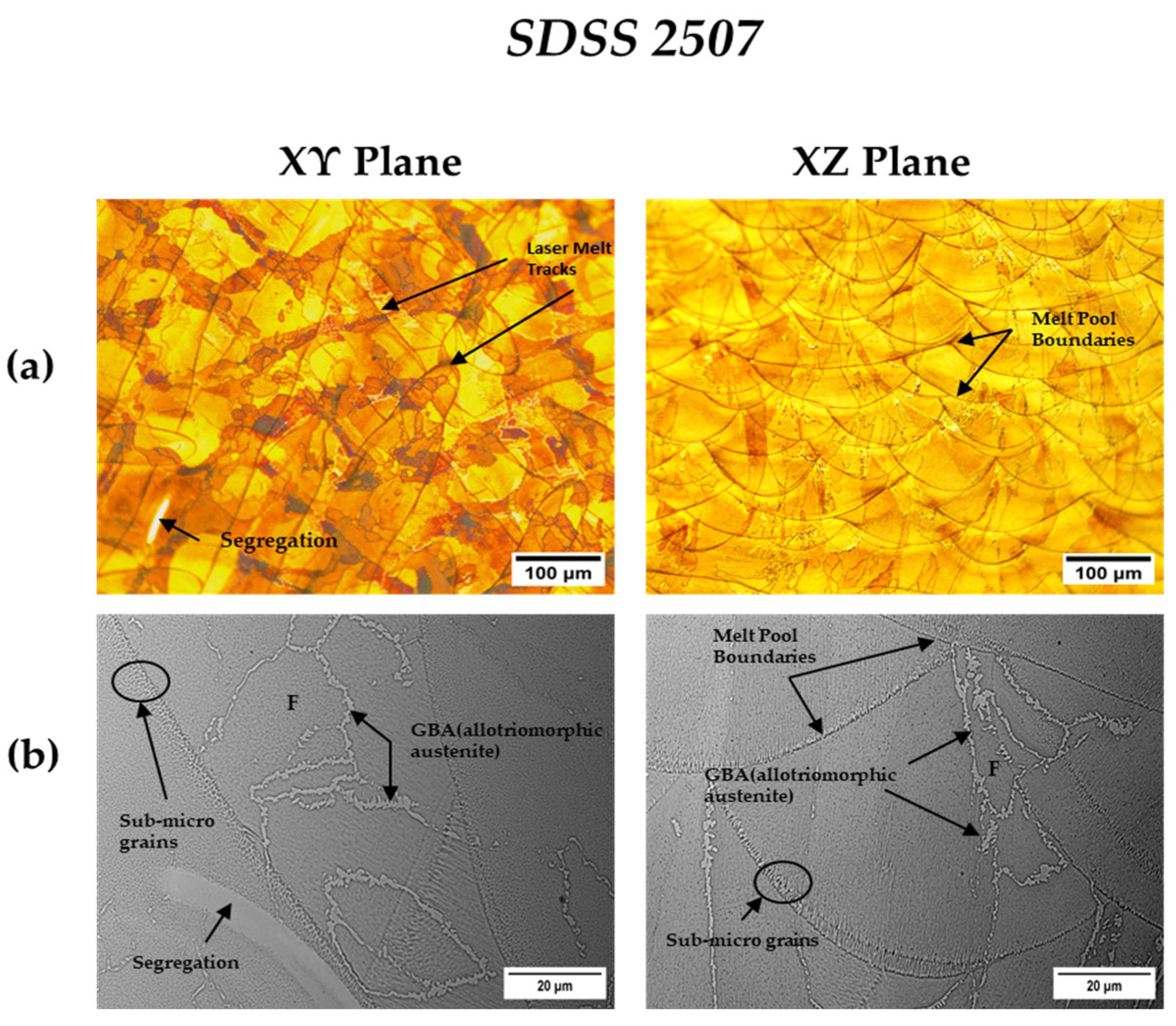
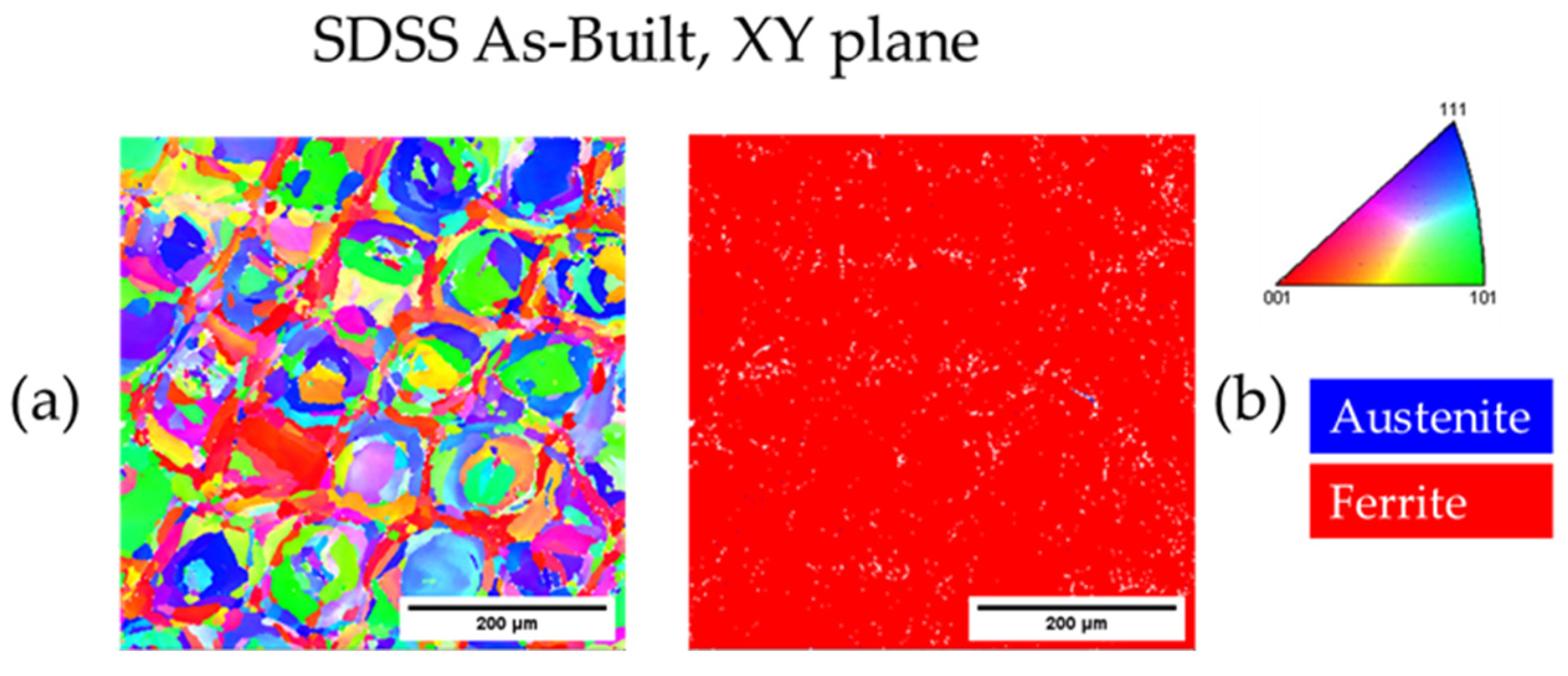
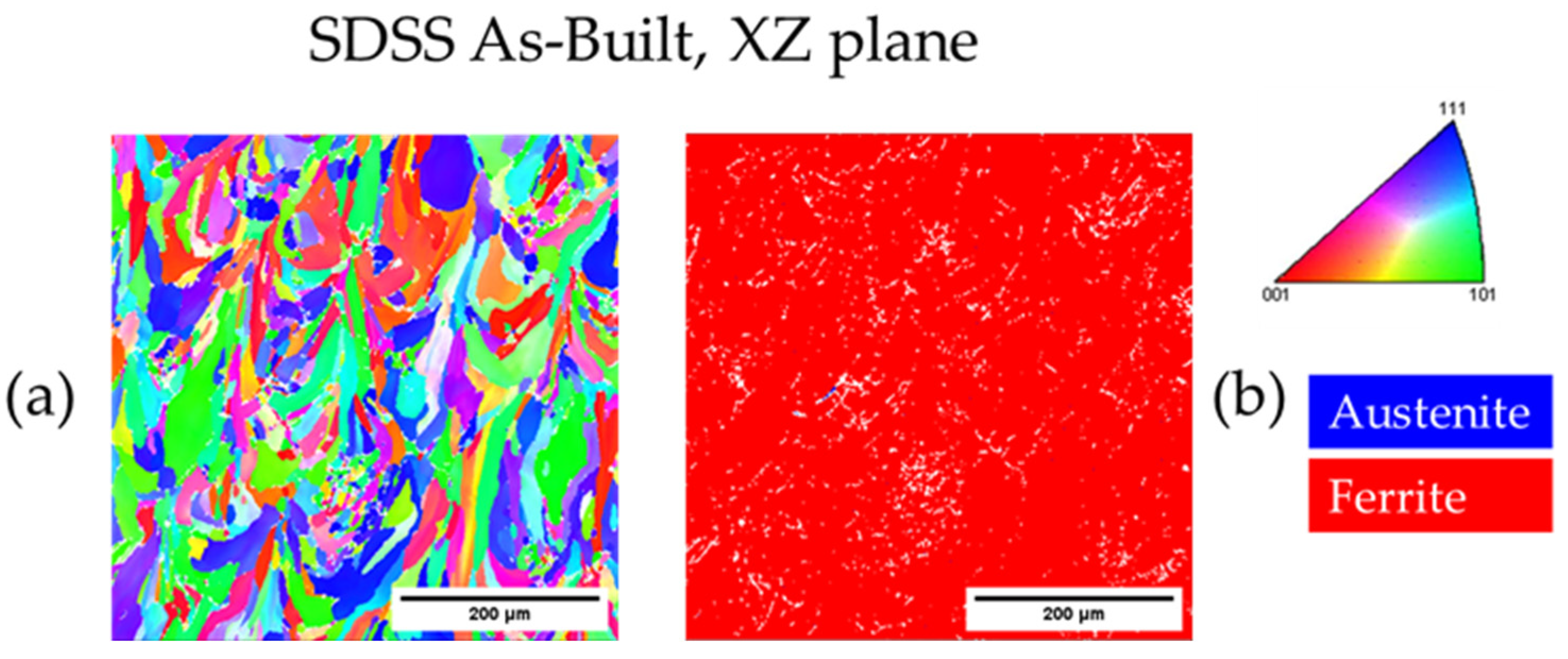
3.3.3. As-Built SDSS 2507
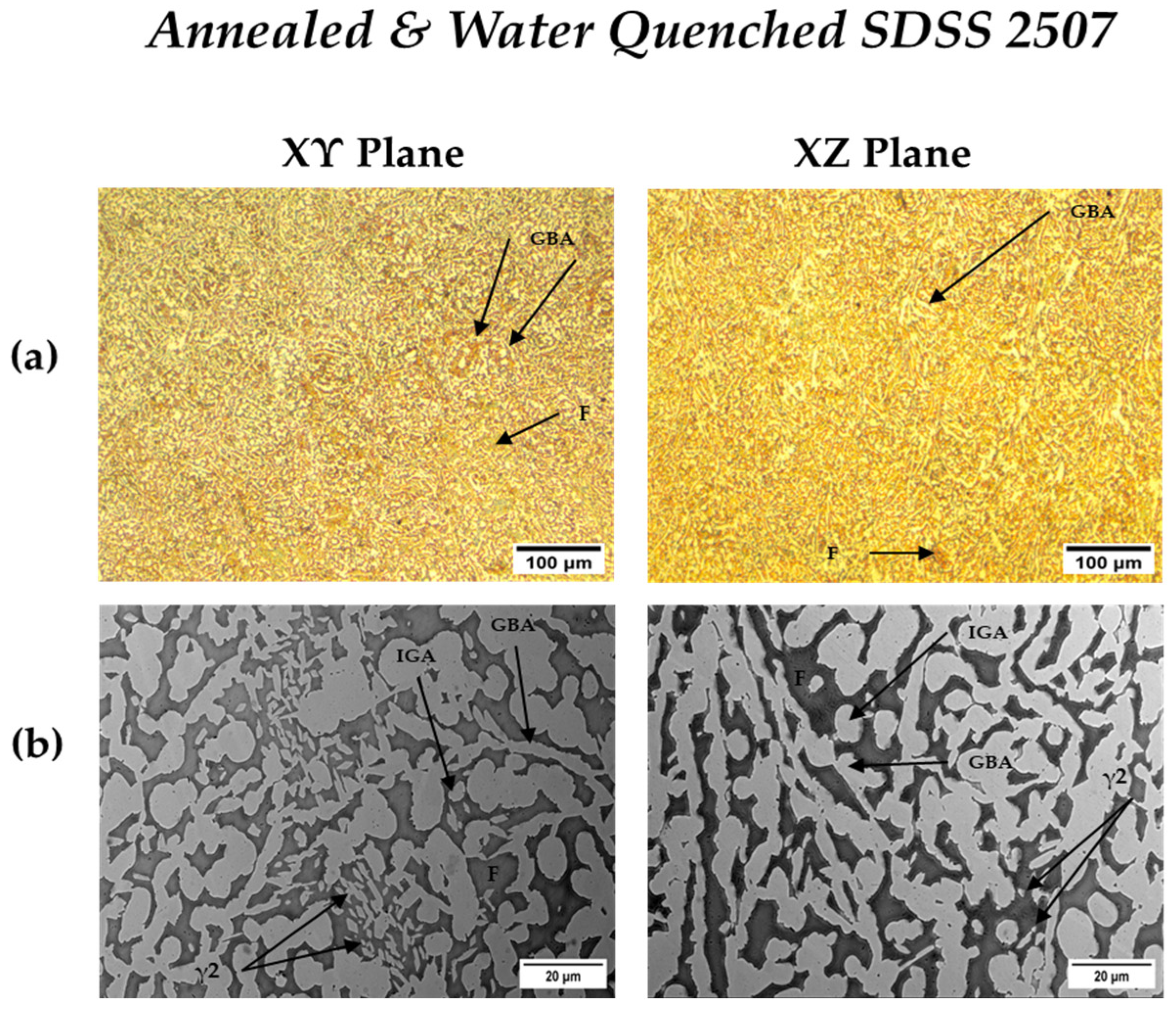
3.3.4. Heat-Treated SDSS 2507
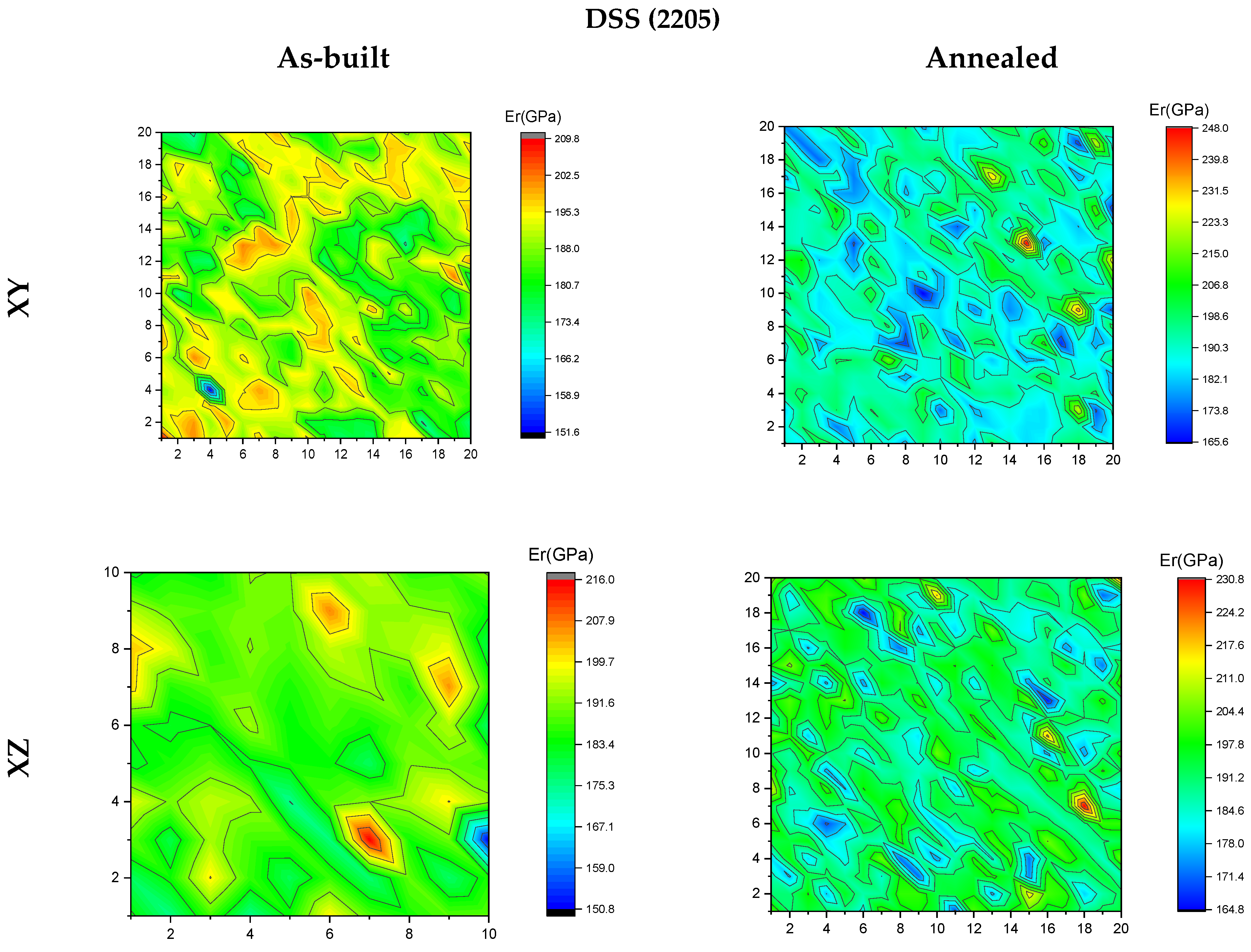
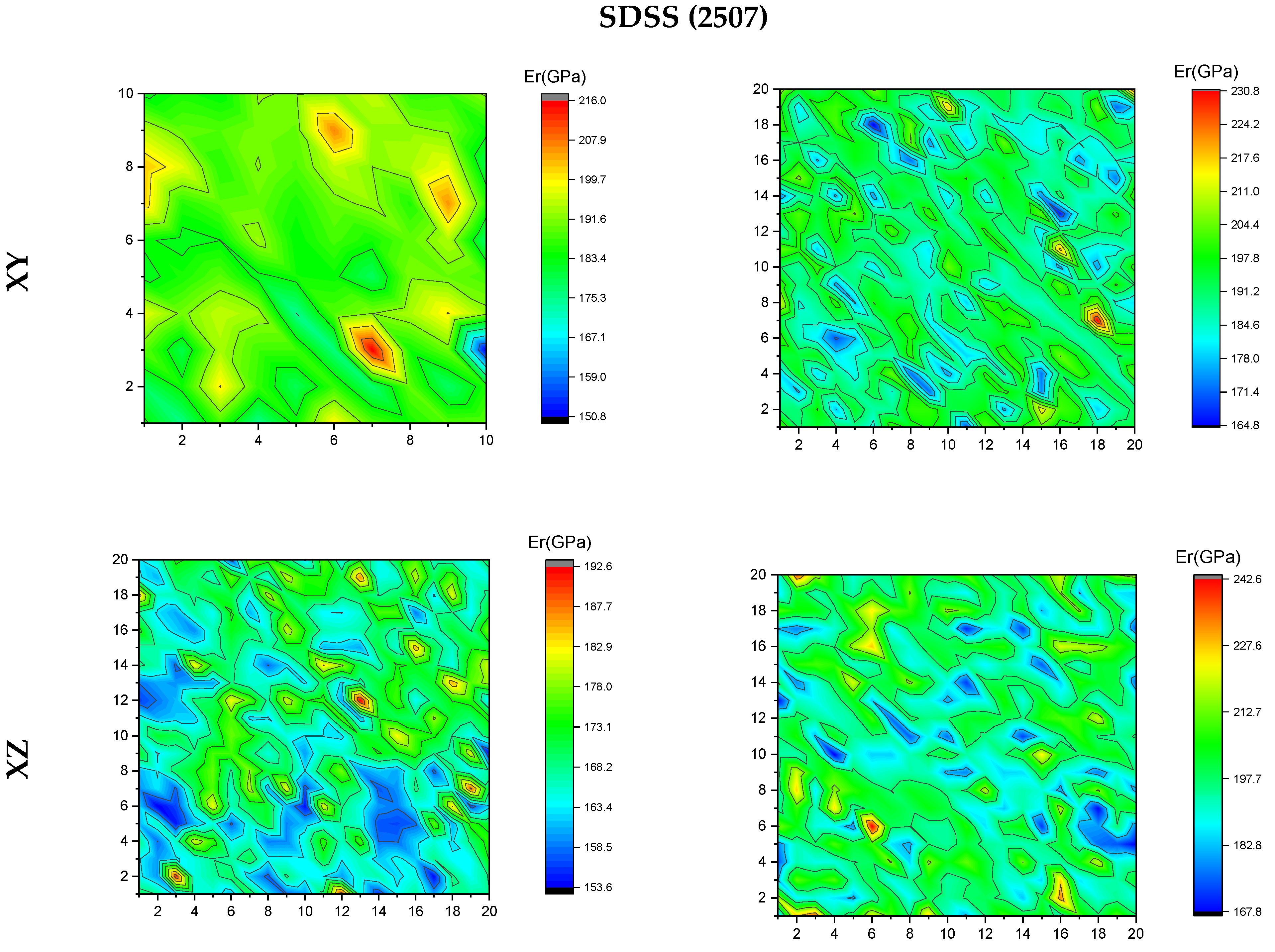
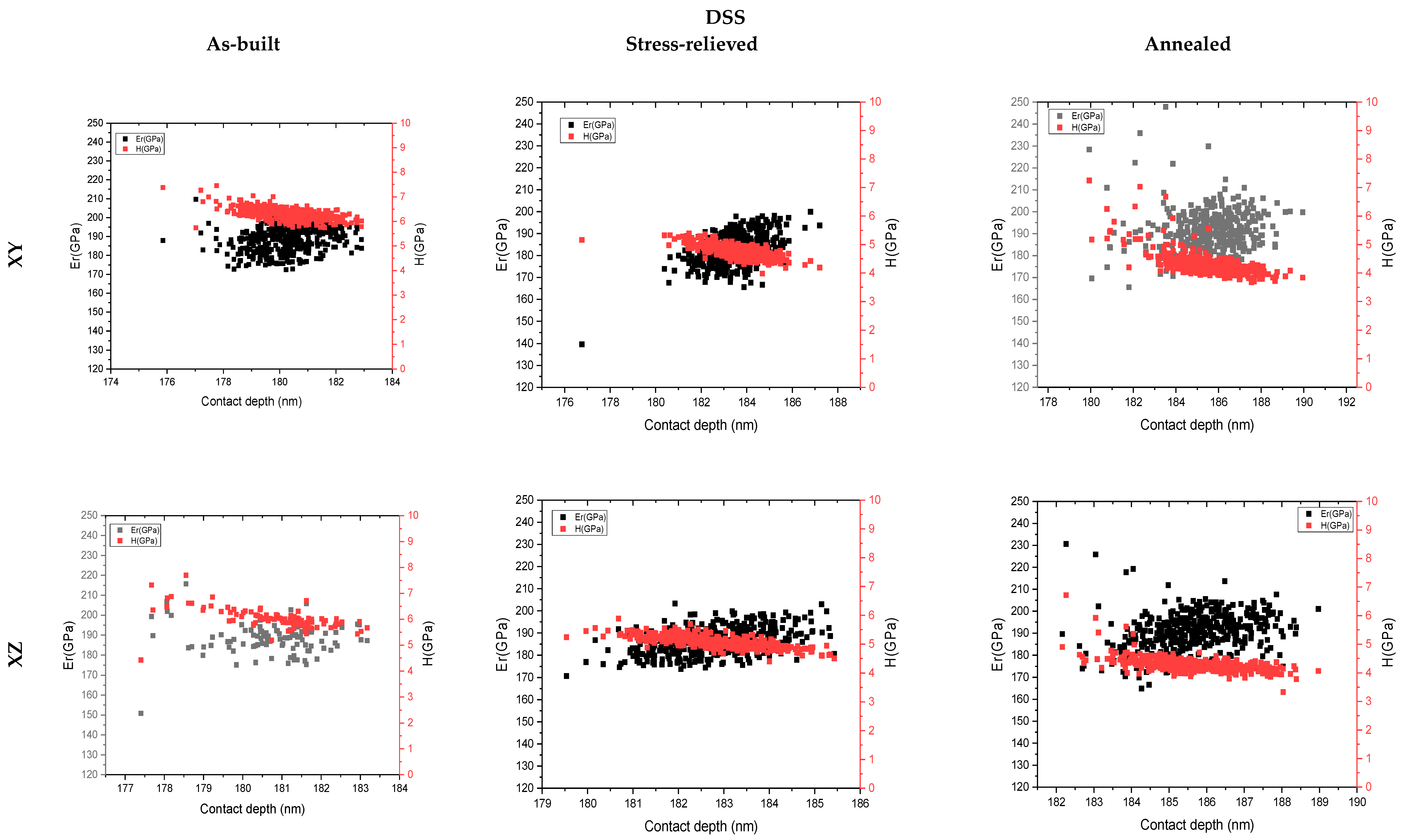
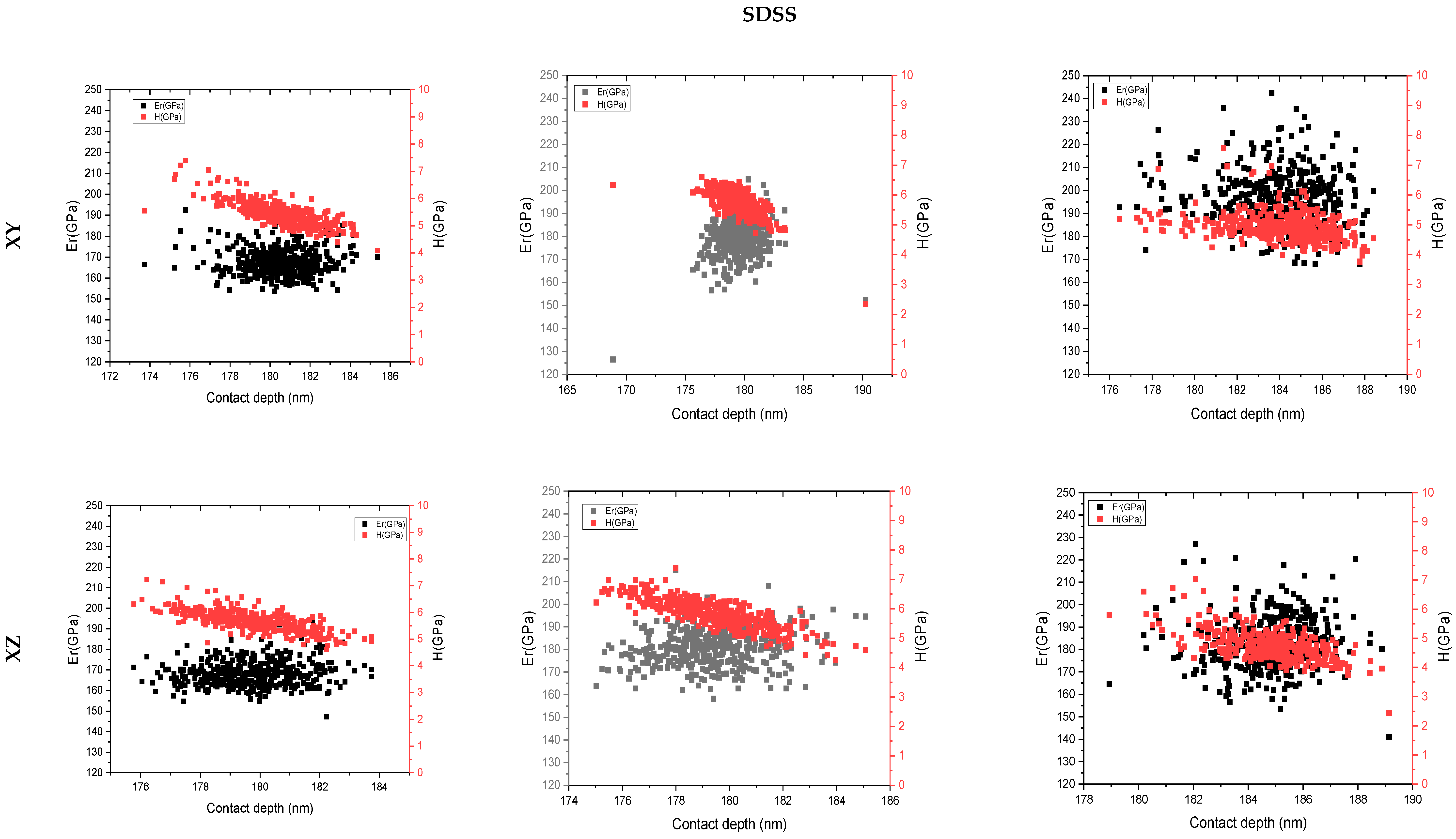
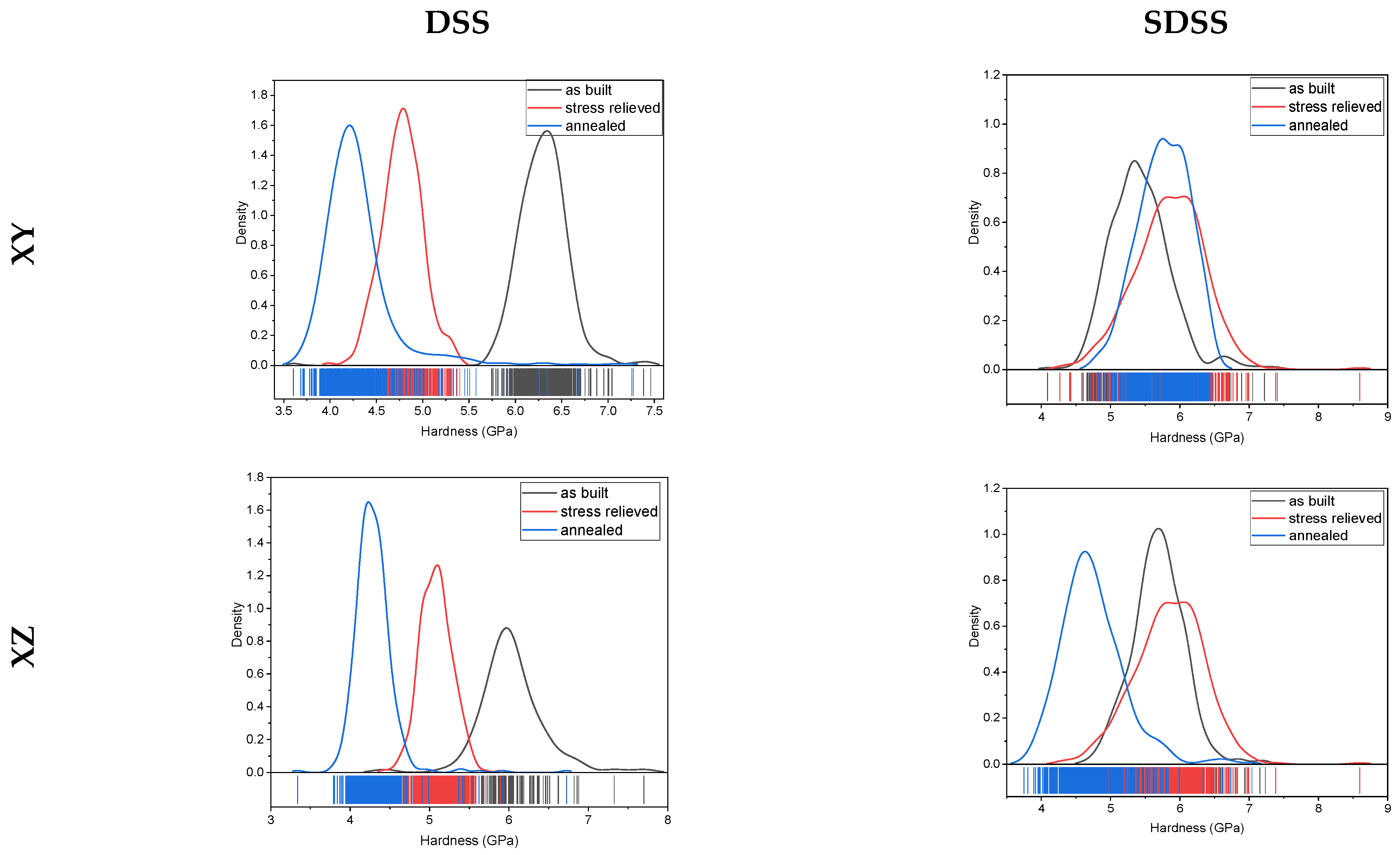
3.4. Nanoindentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Francis, R.; Byrne, G. Duplex Stainless Steels—Alloys for the 21st Century. Metals 2021, 11, 836. [Google Scholar] [CrossRef]
- Sharma, L.; Sharma, K. Dissimilar welding of super duplex stainless steel (SDSS) and pipeline steel—A brief overview. Mater. Today Proc. 2022. [Google Scholar] [CrossRef]
- Khan, W.N.; Chhibber, R. Effect of filler metal on solidification, microstructure and mechanical properties of dissimilar super duplex/pipeline steel GTA weld. Mater. Sci. Eng. A 2021, 803, 140476. [Google Scholar] [CrossRef]
- Gatto, M.L.; Cerqueni, G.; Groppo, R.; Tognoli, E.; Santoni, A.; Cabibbo, M.; Mattioli-Belmonte, M.; Mengucci, P. On the Biomechanical Performances of Duplex Stainless Steel Graded Scaffolds Produced by Laser Powder Bed Fusion for Tissue Engineering Applications. J. Funct. Biomater. 2023, 14, 489. [Google Scholar] [CrossRef]
- Gatto, M.L.; Santoni, A.; Santecchia, E.; Spigarelli, S.; Fiori, F.; Mengucci, P.; Cabibbo, M. The Potential of Duplex Stainless Steel Processed by Laser Powder Bed Fusion for Biomedical Applications: A Review. Metals 2023, 13, 949. [Google Scholar] [CrossRef]
- Alvarez-Armas, I.; Degallaix-Moreuil, S. Duplex Stainless Steels; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Gunn, R.N. (Ed.) 1—Developments, grades and specifications. In Duplex Stainless Steels—Microstructure, Properties and Applications; Woodhead Publishing: Sawston, UK, 1997; pp. 1–13. [Google Scholar]
- Chail, G.; Kangas, P. Super and hyper duplex stainless steels: Structures, properties and applications. Procedia Struct. Integr. 2016, 2, 1755–1762. [Google Scholar] [CrossRef]
- Davidson, K.; Singamneni, S. Selective Laser Melting of Duplex Stainless Steel Powders: An Investigation. Mater. Manuf. Process. 2016, 31, 1543–1555. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, S.; Cheng, F. A specially-designed super duplex stainless steel with balanced ferrite:austenite ratio fabricated via flux-cored wire arc additive manufacturing: Microstructure evolution, mechanical properties and corrosion resistance. Mater. Sci. Eng. A 2022, 854, 143809. [Google Scholar] [CrossRef]
- Pantelakis, S.; Eriksson, M.; Lervåg, M.; Sørensen, C.; Robertstad, A.; Brønstad, B.M.; Nyhus, B.; Aune, R.; Ren, X.; Akselsen, O.M.; et al. Additive manufacture of superduplex stainless steel using WAAM. MATEC Web Conf. 2018, 188, 03014. [Google Scholar] [CrossRef]
- Sayyar, N.; Hansen, V.; Tucho, W.M.; Minde, M.W. Directed laser deposition of super duplex stainless steel: Microstructure, texture evolution, and mechanical properties. Heliyon 2023, 9, e15144. [Google Scholar] [CrossRef]
- Zitelli, C.; Folgarait, P.; Di Schino, A. Laser Powder Bed Fusion of Stainless Steel Grades: A Review. Metals 2019, 9, 731. [Google Scholar] [CrossRef]
- Cobbinah, P.V.; Nzeukou, R.A.; Onawale, O.T.; Matizamhuka, W.R. Laser Powder Bed Fusion of Potential Superalloys: A Review. Metals 2020, 11, 58. [Google Scholar] [CrossRef]
- Hengsbach, F.; Koppa, P.; Duschik, K.; Holzweissig, M.J.; Burns, M.; Nellesen, J.; Tillmann, W.; Tröster, T.; Hoyer, K.-P.; Schaper, M. Duplex stainless steel fabricated by selective laser melting—Microstructural and mechanical properties. Mater. Des. 2017, 133, 136–142. [Google Scholar] [CrossRef]
- Mulhi, A.; Dehgahi, S.; Waghmare, P.; Qureshi, A.J. Process Parameter Optimization of 2507 Super Duplex Stainless Steel Additively Manufactured by the Laser Powder Bed Fusion Technique. Metals 2023, 13, 725. [Google Scholar] [CrossRef]
- Xiang, H.; Chen, G.; Zhao, W.; Wu, C. Densification Behavior and Build Quality of Duplex Stainless Steel Fabricated by Laser Powder Bed Fusion. Metals 2023, 13, 741. [Google Scholar] [CrossRef]
- Raffeis, I.; Vroomen, U.; Adjei-Kyeremeh, F.; Großmann, D.; Hammelrath, H.; Westhoff, E.; Bremen, S.; Boscolo Bozza, D.; Bhrig-Polaczek, A. Comparative investigations into microstructural and mechanical properties of as-cast and laser powder bed fusion (LPBF) fabricated duplex steel (1.4517). Mater. Und Werkst. 2020, 51, 432–444. [Google Scholar] [CrossRef]
- Davidson, K.P.; Singamneni, S.B. Metallographic evaluation of duplex stainless steel powders processed by selective laser melting. Rapid Prototyp. J. 2017, 23, 1146–1163. [Google Scholar] [CrossRef]
- Haghdadi, N.; Ledermueller, C.; Chen, H.; Chen, Z.; Liu, Q.; Li, X.; Rohrer, G.; Liao, X.; Ringer, S.; Primig, S. Evolution of microstructure and mechanical properties in 2205 duplex stainless steels during additive manufacturing and heat treatment. Mater. Sci. Eng. A 2022, 835, 142695. [Google Scholar] [CrossRef]
- Freitas, B.J.M.; Rodrigues, L.C.M.; Claros, C.A.E.; Botta, W.J.; Koga, G.Y.; Bolfarini, C. Ferritic-induced high-alloyed stainless steel produced by laser powder bed fusion (L-PBF) of 2205 duplex stainless steel: Role of microstructure, corrosion, and wear resistance. J. Alloy. Compd. 2022, 918, 165576. [Google Scholar] [CrossRef]
- Freitas, G.C.L.D.; Fonseca, G.S.d.; Moreira, L.P.; Leite, D.N.F. Phase transformations of the duplex stainless steel UNS S31803 under non-isothermal conditions. J. Mater. Res. Technol. 2021, 11, 1847–1851. [Google Scholar] [CrossRef]
- Kashiwar, A.; Vennela, N.P.; Kamath, S.L.; Khatirkar, R.K. Effect of solution annealing temperature on precipitation in 2205 duplex stainless steel. Mater. Charact. 2012, 74, 55–63. [Google Scholar] [CrossRef]
- Mampuya, M.B.; Umba, M.C.; Mutombo, K.; Olubambi, P.A. Effect of heat treatment on the microstructure of duplex stainless steel 2205. Mater. Today Proc. 2021, 38, 1107–1112. [Google Scholar] [CrossRef]
- Gargalis, L.; Karavias, L.; Graff, J.S.; Diplas, S.; Koumoulos, E.P.; Karaxi, E.K. Novel Powder Feedstock towards Microstructure Engineering in Laser Powder Bed Fusion: A Case Study on Duplex/Super Duplex and Austenitic Stainless-Steel Alloys. Metals 2023, 13, 1546. [Google Scholar] [CrossRef]
- Cui, C.; Becker, L.; Gärtner, E.; Boes, J.; Lentz, J.; Uhlenwinkel, V.; Steinbacher, M.; Weber, S.; Fechte-Heinen, R. Laser Additive Manufacturing of Duplex Stainless Steel via Powder Mixture. J. Manuf. Mater. Process. 2022, 6, 72. [Google Scholar] [CrossRef]
- Köhler, M.L.; Kunz, J.; Herzog, S.; Kaletsch, A.; Broeckmann, C. Microstructure analysis of novel LPBF-processed duplex stainless steels correlated to their mechanical and corrosion properties. Mater. Sci. Eng. A 2021, 801, 140432. [Google Scholar] [CrossRef]
- Nilsson, J.O. Super duplex stainless steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Steiner Petrovič, D.; Pirnat, M.; Klančnik, G.; Mrvar, P.; Medved, J. The effect of cooling rate on the solidification and microstructure evolution in duplex stainless steel. J. Therm. Anal. Calorim. 2012, 109, 1185–1191. [Google Scholar] [CrossRef]
- Narasimharaju, S.R.; Zeng, W.; See, T.L.; Zhu, Z.; Scott, P.; Jiang, X.; Lou, S. A comprehensive review on laser powder bed fusion of steels: Processing, microstructure, defects and control methods, mechanical properties, current challenges and future trends. J. Manuf. Process. 2022, 75, 375–414. [Google Scholar] [CrossRef]
- David, S.A.; Vitek, J.M.; Reed, R.W.; Hebble, T.L. Effect of Rapid Solidification on Stainless Steel Weld Metal Microstructures and Its Implications on the Schaeffler Diagram; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 1987. [Google Scholar]
- Saeidi, K.; Kevetkova, L.; Lofaj, F.; Shen, Z. Novel ferritic stainless steel formed by laser melting from duplex stainless steel powder with advanced mechanical properties and high ductility. Mater. Sci. Eng. A 2016, 665, 59–65. [Google Scholar] [CrossRef]
- Xie, C.; Li, B.; Liu, G.; Liu, J.; Ying, H.; Li, D.; Wang, S.; Wang, L. Study on the effect of solution treatment on mechanical and corrosion properties of SAF 2507DSS produced by LPBF. J. Mater. Res. Technol. 2023, 26, 2070–2081. [Google Scholar] [CrossRef]
- Nigon, G.N.; Burkan Isgor, O.; Pasebani, S. The effect of annealing on the selective laser melting of 2205 duplex stainless steel: Microstructure, grain orientation, and manufacturing challenges. Opt. Laser Technol. 2021, 134, 106643. [Google Scholar] [CrossRef]
- Llorca-Isern, N.; López-Luque, H.; López-Jiménez, I.; Biezma, M.V. Identification of sigma and chi phases in duplex stainless steels. Mater. Charact. 2016, 112, 20–29. [Google Scholar] [CrossRef]
- Knyazeva, M.; Pohl, M. Duplex Steels. Part II: Carbides and Nitrides. Metallogr. Microstruct. Anal. 2013, 2, 343–351. [Google Scholar] [CrossRef]
- Di Cocco, V.; Iacoviello, F.; Ischia, G. Duplex stainless steels “475 °C embrittlement”: Influence of the chemical composition on the fatigue crack propagation. Procedia Struct. Integr. 2017, 3, 299–307. [Google Scholar] [CrossRef]
- Papula, S.; Song, M.; Pateras, A.; Chen, X.B.; Brandt, M.; Easton, M.; Yagodzinskyy, Y.; Virkkunen, I.; Hanninen, H. Selective Laser Melting of Duplex Stainless Steel 2205: Effect of Post-Processing Heat Treatment on Microstructure, Mechanical Properties, and Corrosion Resistance. Materials 2019, 12, 2468. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Zhao, W.; Lu, Y. Effect of solution temperature on microstructure and mechanical properties of selective laser melted Fe–22Cr–5Ni-0.26N duplex stainless steel. J. Mater. Res. Technol. 2022, 19, 1379–1389. [Google Scholar] [CrossRef]
- Hosseini, A.; Karlsson, V.; Örnek, L.; Reccagni, C.; Wessman, P.; Engelberg, S.D. Microstructure and functionality of a uniquely graded super duplex stainless steel designed by a novel arc heat treatment method. Mater. Charact. 2018, 139, 390–400. [Google Scholar] [CrossRef]
- Moallemi, M.; Zarei-Hanzaki, A.; Kim, S.-J.; Alimadadi, H. On the microstructural-textural characterization and deformation analysis of a nano/ultrafine grained Fe-20Cr-8Mn-0.3N duplex alloy with superior mechanical properties. Mater. Charact. 2019, 156, 109878. [Google Scholar] [CrossRef]
- Wang, X.F.; Yang, X.P.; Guo, Z.D.; Zhou, Y.C.; Song, H.W. Nanoindentation Characterization of Mechanical Properties of Ferrite and Austenite in Duplex Stainless Steel. Adv. Mater. Res. 2007, 26–28, 1165–1170. [Google Scholar] [CrossRef]
- ISO 13322-1:2014; Particle Size Analysis Image Analysis Methods Part 1: Static Image Analysis Methods. ISO: London, UK, 2014.
- A Hosseini, V.; Hurtig, K.; Eyzop, D.; Östberg, A.; Janiak, P.; Karlsson, L. Ferrite content measurement in super duplex stainless steel welds. Weld. World 2019, 63, 551–563. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Tao, P.; Gong, J.-m.; Wang, Y.-f.; Jiang, Y.; Li, Y.; Cen, W.-w. Characterization on stress-strain behavior of ferrite and austenite in a 2205 duplex stainless steel based on nanoindentation and finite element method. Results Phys. 2018, 11, 377–384. [Google Scholar] [CrossRef]
- Vincent, C.; Silvain, J.F.; Heintz, J.M.; Chandra, N. Effect of porosity on the thermal conductivity of copper processed by powder metallurgy. J. Phys. Chem. Solids 2012, 73, 499–504. [Google Scholar] [CrossRef]
- Yi, F.; Zhou, Q.; Wang, C.; Yan, Z.; Liu, B. Effect of powder reuse on powder characteristics and properties of Inconel 718 parts produced by selective laser melting. J. Mater. Res. Technol. 2021, 13, 524–533. [Google Scholar] [CrossRef]
- Akram, J.; Chalavadi, P.; Pal, D.; Stucker, B. Understanding grain evolution in additive manufacturing through modeling. Addit. Manuf. 2018, 21, 255–268. [Google Scholar] [CrossRef]
- Haghdadi, N.; Laleh, M.; Moyle, M.; Primig, S. Additive manufacturing of steels: A review of achievements and challenges. J. Mater. Sci. 2021, 56, 64–107. [Google Scholar] [CrossRef]
- Charitidis, C.A.; Dragatogiannis, D.A.; Koumoulos, E.P.; Kartsonakis, I.A. Residual stress and deformation mechanism of friction stir welded aluminum alloys by nanoindentation. Mater. Sci. Eng. A 2012, 540, 226–234. [Google Scholar] [CrossRef]
- Ferro, P.; Meneghello, R.; Savio, G.; Berto, F. A modified volumetric energy density–based approach for porosity assessment in additive manufacturing process design. Int. J. Adv. Manuf. Technol. 2020, 110, 1911–1921. [Google Scholar] [CrossRef]
- Snell, R.; Tammas-Williams, S.; Chechik, L.; Lyle, A.; Hernández-Nava, E.; Boig, C.; Panoutsos, G.; Todd, I. Methods for Rapid Pore Classification in Metal Additive Manufacturing. Jom 2019, 72, 101–109. [Google Scholar] [CrossRef]
- Ghayoor, M.; Lee, K.; He, Y.; Chang, C.-h.; Paul, B.K.; Pasebani, S. Selective laser melting of 304L stainless steel: Role of volumetric energy density on the microstructure, texture and mechanical properties. Addit. Manuf. 2020, 32, 101011. [Google Scholar] [CrossRef]
- Chowdhury, S.; Yadaiah, N.; Prakash, C.; Ramakrishna, S.; Dixit, S.; Gupta, L.R.; Buddhi, D. Laser powder bed fusion: A state-of-the-art review of the technology, materials, properties & defects, and numerical modelling. J. Mater. Res. Technol. 2022, 20, 2109–2172. [Google Scholar] [CrossRef]
- Zhang, Z.; Jing, H.; Xu, L.; Han, Y.; Zhao, L. Investigation on microstructure evolution and properties of duplex stainless steel joint multi-pass welded by using different methods. Mater. Des. 2016, 109, 670–685. [Google Scholar] [CrossRef]
- Zhang, B.; Li, H.; Zhang, S.; Jiang, Z.; Lin, Y.; Feng, H.; Zhu, H. Effect of nitrogen on precipitation behavior of hyper duplex stainless steel S32707. Mater. Charact. 2021, 175, 111096. [Google Scholar] [CrossRef]
- Xianfeng, X.; Cong, L.; Yanshu, F.; Xiaojun, Y.; Lijun, S. Progress on Experimental Study of Melt Pool Flow Dynamics in Laser Material Processing. In Liquid Metals; Samson Jerold Samuel, C., Gnanasekaran, S., Suresh, M., Eds.; IntechOpen: Rijeka, Croatia, 2021; p. Ch.3. [Google Scholar]
- Shang, C.; Wu, H.; Pan, G.; Zhu, J.; Wang, S.; Wu, G.; Gao, J.; Liu, Z.; Li, R.; Mao, X. The Characteristic Microstructures and Properties of Steel-Based Alloy via Additive Manufacturing. Materials 2023, 16, 2696. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zhou, K.; Kuang, M.; Ma, W.; Kuang, T. Microstructural characterization and properties of selective laser melted maraging steel with different build directions. Sci. Technol. Adv. Mater. 2018, 19, 746–758. [Google Scholar] [CrossRef]
- Wang, Y.M.; Voisin, T.; McKeown, J.T.; Ye, J.; Calta, N.P.; Li, Z.; Zeng, Z.; Zhang, Y.; Chen, W.; Roehling, T.T.; et al. Additively manufactured hierarchical stainless steels with high strength and ductility. Nat. Mater. 2018, 17, 63–71. [Google Scholar] [CrossRef]
- Verma, J.; Taiwade, R.V. Effect of welding processes and conditions on the microstructure, mechanical properties and corrosion resistance of duplex stainless steel weldments—A review. J. Manuf. Process. 2017, 25, 134–152. [Google Scholar] [CrossRef]
- Khan, W.N.; Mahajan, S.; Chhibber, R. Investigations on reformed austenite in the microstructure of dissimilar super duplex/pipeline steel weld. Mater. Lett. 2021, 285, 129109. [Google Scholar] [CrossRef]
- Kotechi, D.J.; Siewert, T.A. WRC-1992 Constitution Diagram for Stainless Steel Weld Metals: A sodification of the WRC-1988 Diagram. Welding 1992, 71, 172. [Google Scholar]
- Mercelis, P.; Kruth, J.P. Residual stresses in selective laser sintering and selective laser melting. Rapid Prototyp. J. 2006, 12, 254–265. [Google Scholar] [CrossRef]
- Brown, D.W.; Adams, D.P.; Balogh, L.; Carpenter, J.S.; Clausen, B.; King, G.; Reedlunn, B.; Palmer, T.A.; Maguire, M.C.; Vogel, S.C. In Situ Neutron Diffraction Study of the Influence of Microstructure on the Mechanical Response of Additively Manufactured 304L Stainless Steel. Metall. Mater. Trans. A 2017, 48, 6055–6069. [Google Scholar] [CrossRef]
- Wu, A.S.; Brown, D.W.; Kumar, M.; Gallegos, G.F.; King, W.E. An Experimental Investigation into Additive Manufacturing-Induced Residual Stresses in 316L Stainless Steel. Metall. Mater. Trans. A 2014, 45, 6260–6270. [Google Scholar] [CrossRef]
- Shamsujjoha, M.; Agnew, S.R.; Fitz-Gerald, J.M.; Moore, W.R.; Newman, T.A. High Strength and Ductility of Additively Manufactured 316L Stainless Steel Explained. Metall. Mater. Trans. A 2018, 49, 3011–3027. [Google Scholar] [CrossRef]
- Chen, W.; Voisin, T.; Zhang, Y.; Forien, J.-B.; Spadaccini, C.M.; McDowell, D.L.; Zhu, T.; Wang, Y.M. Microscale residual stresses in additively manufactured stainless steel. Nat. Commun. 2019, 10, 4338. [Google Scholar] [CrossRef]
- Wang, H.; Wang, A.; Li, C.; Yu, X.; Xie, J.; Liu, C. Effect of Secondary-Phase Precipitation on Mechanical Properties and Corrosion Resistance of 00Cr27Ni7Mo5N Hyper-Duplex Stainless Steel during Solution Treatment. Materials 2022, 15, 7533. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, A.; Tabbakh, T.; Basak, A.K. Microstructural and Nanoindentation Investigation on the Laser Powder Bed Fusion Stainless Steel 316L. Materials 2023, 16, 5933. [Google Scholar] [CrossRef]





| Duplex Stainless Steel 2205 | ||||||||||
| C | S | N | Cr | Fe | Mn | Mo | Ni | P | Si | Cu |
| 0.022 | 0.005 | 0.13 | 22.1 | Bal. | 1.03 | 3.2 | 5.2 | 0.01 | 0.51 | - |
| Super Duplex Stainless Steel 2507 | ||||||||||
| 0.013 | 0.005 | 0.29 | 24.7 | Bal. | 0.77 | 3.6 | 8.0 | 0.011 | 0.45 | 0.01 |
| No. of Sample | Laser Power (W) | Scan Speed (mm/s) | Hatch Distance (μm) | Laser Beam Spot Size (μm) | Layer Thickness (μm) | Volumetric Energy Density (J/mm3) |
|---|---|---|---|---|---|---|
| 1 | 113 | 357 | 120 | 80 | 40 | 65.94 |
| 2 | 204 | 769 | 100 | 80 | 40 | 66.32 |
| 3 | 226 | 940 | 110 | 80 | 40 | 54.64 |
| 4 | 269 | 1058 | 100 | 80 | 40 | 63.56 |
| 5 | 296 | 1130 | 100 | 80 | 40 | 65.49 |
| 6 | 304 | 946 | 110 | 80 | 40 | 73.03 |
| 7 | 317 | 1189 | 100 | 80 | 40 | 66.65 |
| 8 | 334 | 963 | 120 | 80 | 40 | 72.25 |
| 9 | 347 | 1242 | 100 | 80 | 40 | 69.84 |
| 10 | 363 | 1056 | 120 | 80 | 40 | 71.61 |
| 11 | 425 | 1329 | 110 | 80 | 40 | 72.68 |
| 12 | 467 | 1346 | 120 | 80 | 40 | 72.28 |
| No. of Sample | Laser Power (W) | Scan Speed (mm/s) | Hatch Distance (μm) | Laser Beam Spot Size (μm) | Layer Thickness (μm) | Volumetric Energy Density (J/mm3) |
|---|---|---|---|---|---|---|
| 1 | 95 | 331 | 120 | 80 | 40 | 59.79 |
| 2 | 125 | 408 | 110 | 80 | 40 | 69.63 |
| 3 | 169 | 590 | 120 | 80 | 40 | 59.67 |
| 4 | 182 | 723 | 100 | 80 | 40 | 62.93 |
| 5 | 187 | 566 | 100 | 80 | 40 | 82.59 |
| 6 | 189 | 781 | 120 | 80 | 40 | 50.41 |
| 7 | 192 | 661 | 120 | 80 | 40 | 60.51 |
| 8 | 262 | 794 | 110 | 80 | 40 | 74.99 |
| 9 | 267 | 894 | 110 | 80 | 40 | 67.87 |
| 10 | 305 | 1174 | 110 | 80 | 40 | 59.04 |
| 11 | 336 | 1230 | 100 | 80 | 40 | 68.29 |
| 12 | 347 | 1016 | 110 | 80 | 40 | 77.62 |
| Bulk Powder Characteristics | DSS 2205 | SDSS 2507 |
|---|---|---|
| Apparent density (g/cm3) | 3.9 | 4.0 |
| Tapped density (g/cm3) | 4.3 | 4.4 |
| Flowability (s/50 g) | 18.9 | 18.4 |
| Particle Size Measurements | DSS 2205 | SDSS 2507 |
|---|---|---|
| Min. Equivalent Circle Diameter (μm) | 0.52 | 0.52 |
| Max. Equivalent Circle Diameter (μm) | 84.8 | 88.2 |
| Equivalent Circle Diameter Mean (μm) | 21.5 | 25.4 |
| Equivalent Circle D10 (μm) | 21.9 | 23.4 |
| Equivalent Circle D50 (μm) | 40.4 | 39.4 |
| Equivalent Circle D90 (μm) | 62.5 | 59.1 |
| Elements (wt.%) | ||||||
|---|---|---|---|---|---|---|
| Annealed DSS | Si | Mn | Cr | Fe | Ni | Mo |
| Ferrite | 0.700 | - | 24.500 | 66.300 | 4.100 | 4.400 |
| Austenite | - | 1.205 | 21.586 | 68.474 | 6.225 | 2.510 |
| Elements (wt.%) | ||||||
|---|---|---|---|---|---|---|
| As-Built SDSS | Si | Mn | Cr | Fe | Ni | Mo |
| Ferrite | 1.7 | - | 26.4 | 58.8 | 6.2 | 6.9 |
| Grain Boundary Austenite | - | 0.9 | 25.8 | 62.4 | 7.0 | 4.1 |
| Segregation | - | - | 23.9 | 46.7 | 23.1 | 6.4 |
| Elements (wt.%) | ||||||
|---|---|---|---|---|---|---|
| Annealed SDSS | Si | Mn | Cr | Fe | Ni | Mo |
| Ferrite | 0.6 | - | 27.2 | 62.0 | 5.4 | 4.8 |
| Austenite | - | 0.9 | 23.4 | 62.5 | 10.1 | 3.1 |
| Sample | Phase Fraction, % | Grain Size (ECD/µm) | ||
|---|---|---|---|---|
| Austenite | Ferrite | Austenite | Ferrite | |
| As-Built DSS XY | - | 100.0 | - | 12.6 |
| As-Built DSS XZ | - | 100.0 | - | 15.9 |
| As-Built SDSS XY | - | 100.0 | - | 10.5 |
| As-Built SDSS XZ | - | 100.0 | - | 11.0 |
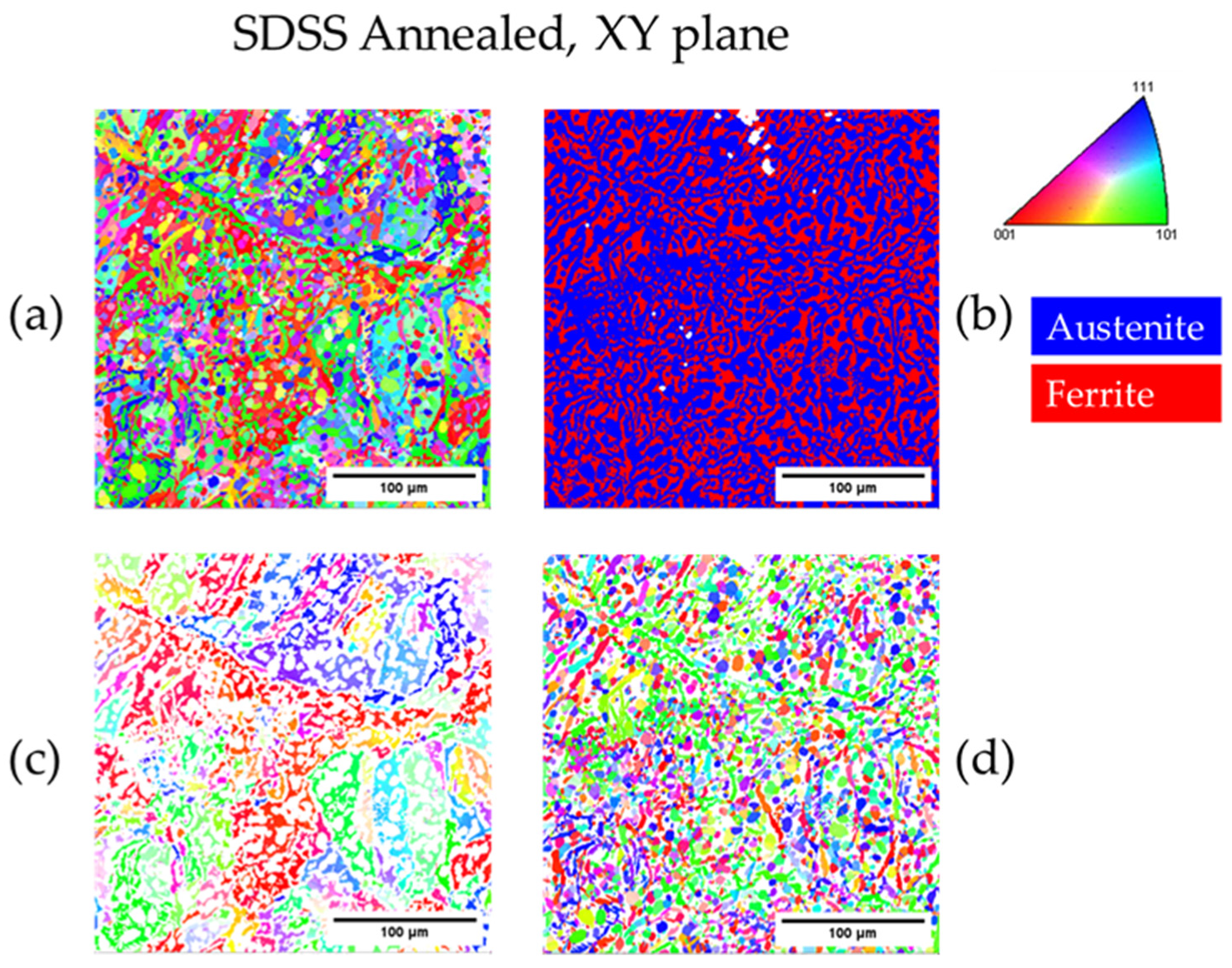
| Annealed DSS XY | 31.1 | 68.9 | 3.3 | 22 |
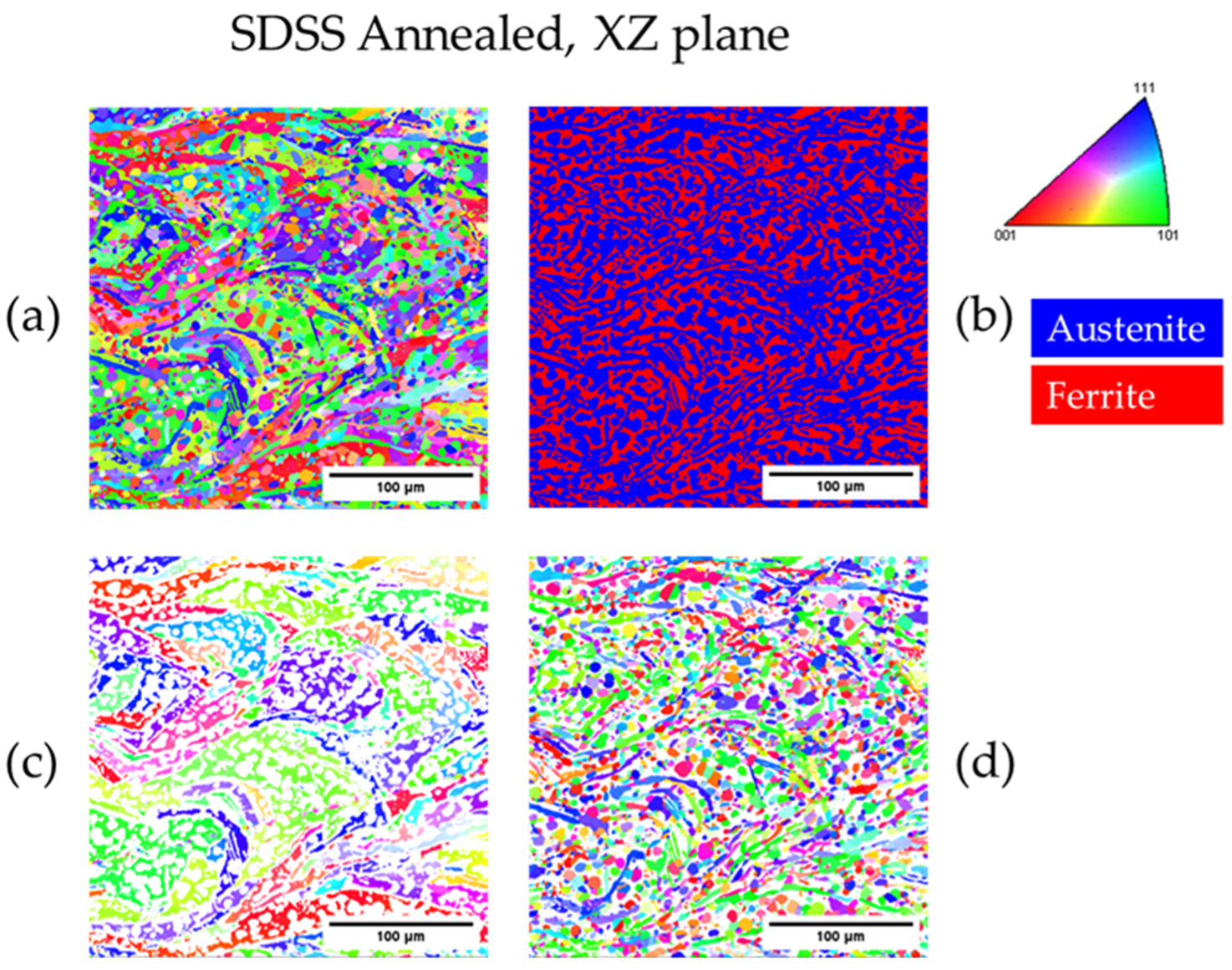
| Annealed DSS XZ | 31.6 | 68.4 | 3.4 | 25 |
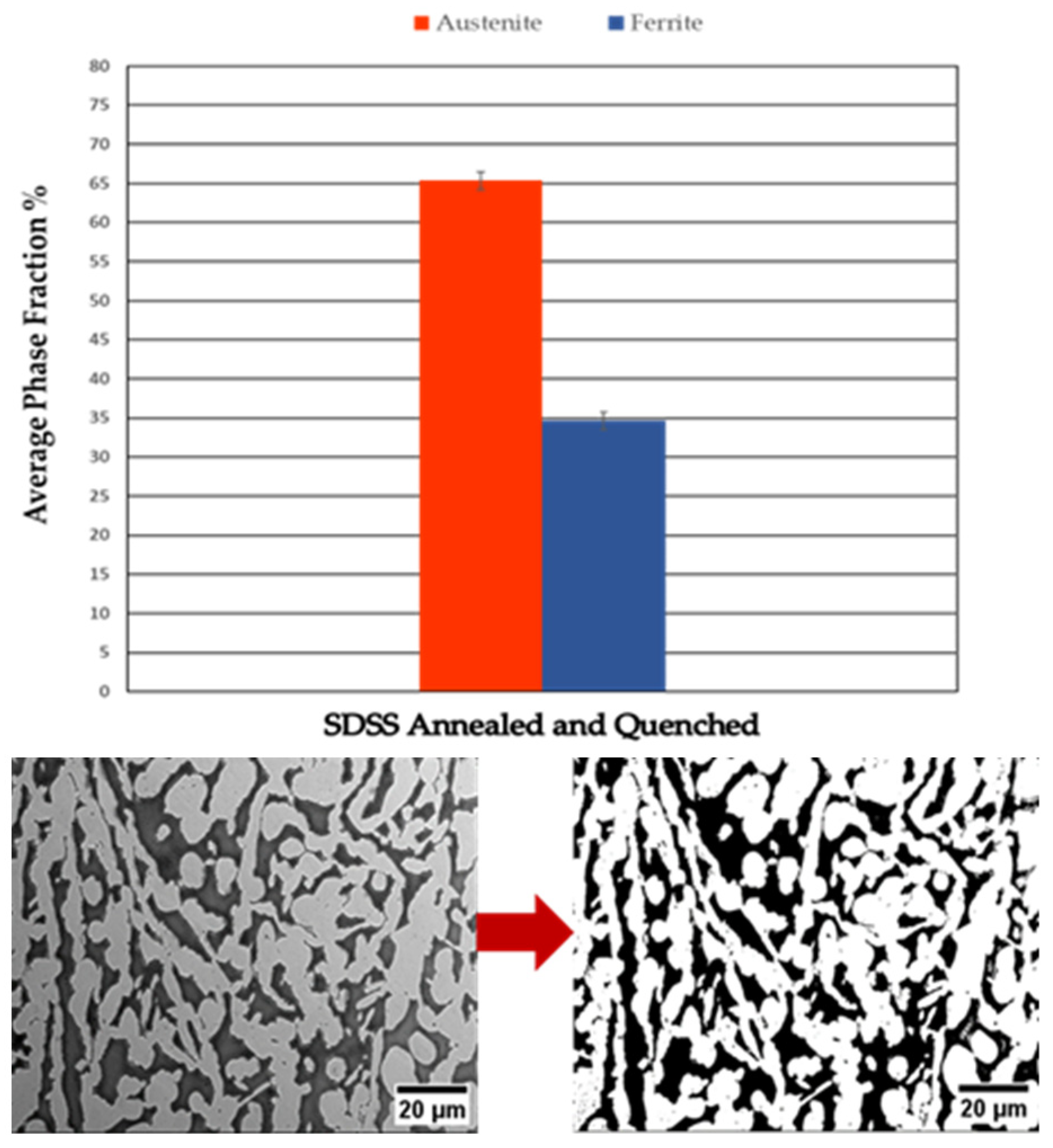
| Annealed SDSS XY | 62.8 | 37.2 | 3.1 | 18 |
| Annealed SDSS XZ | 61.8 | 38.2 | 3.3 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gargalis, L.; Karavias, L.; Graff, J.S.; Diplas, S.; Koumoulos, E.P.; Karaxi, E.K. A Comparative Investigation of Duplex and Super Duplex Stainless Steels Processed through Laser Powder Bed Fusion. Metals 2023, 13, 1897. https://doi.org/10.3390/met13111897
Gargalis L, Karavias L, Graff JS, Diplas S, Koumoulos EP, Karaxi EK. A Comparative Investigation of Duplex and Super Duplex Stainless Steels Processed through Laser Powder Bed Fusion. Metals. 2023; 13(11):1897. https://doi.org/10.3390/met13111897
Chicago/Turabian StyleGargalis, Leonidas, Leonidas Karavias, Joachim S. Graff, Spyros Diplas, Elias P. Koumoulos, and Evangelia K. Karaxi. 2023. "A Comparative Investigation of Duplex and Super Duplex Stainless Steels Processed through Laser Powder Bed Fusion" Metals 13, no. 11: 1897. https://doi.org/10.3390/met13111897
APA StyleGargalis, L., Karavias, L., Graff, J. S., Diplas, S., Koumoulos, E. P., & Karaxi, E. K. (2023). A Comparative Investigation of Duplex and Super Duplex Stainless Steels Processed through Laser Powder Bed Fusion. Metals, 13(11), 1897. https://doi.org/10.3390/met13111897







